Lay Summary
The cost of heart failure care is high due to cost of hospitalization and chronic treatments. Heart failure treatments vary in their benefit and cost. The cost-effectiveness of therapies can be determined by comparing the cost of treatment required to obtain a certain benefit, often defined as an increase in one year of life. This review was sponsored by the Heart Failure Society of America and describes the growing economic burden of heart failure for patients and the health care system in the United States. It also provides a summary of the cost-effectiveness of drugs, devices, diagnostic tests, hospital care, and transitions of care for patients with heart failure. Many medications that are no longer under patent are inexpensive and highly cost-effective. These include ACE inhibitors, beta-blockers and mineralocorticoid receptor antagonists. In contrast, more recently developed medications and devices, vary in cost effectiveness, and often have high out of pocket expenses for patients.
Introduction
Despite remarkable recent advances in the treatment of heart failure, the high cost of care limits delivery of effective care. The Heart Failure Society of America (HFSA) recognizes the important role of cost, cost-effectiveness, and value of diagnosis and treatment in caring for patients with heart failure. This review sponsored by HFSA describes the economic burden of heart failure, providing a summary of evidence for the cost-effectiveness of drugs, devices, diagnostic tests, hospital care, and transitions of care for patients with heart failure.
Formation of Writing Group
The Advocacy Committee of the HFSA requested its members consider developing a manuscript on economic issues in heart failure. This effort was led by the incoming committee Chair (PAH) and included committee members and non-committee members of the HFSA with expertise in this area.
Economic Burden of Heart Failure
Heart failure is a growing burden for the United States and other developed countries due in large part to the aging of their populations. The incidence is approximately 1,000,000 new patients with heart failure per year in the United States. [1] Accordingly, the cost of care for patients with heart failure is substantial. By 2030 it is estimated that over 8 million individuals in the United states will have heart failure for a prevalence rate of 1 in every 33 individuals. [2] The annual cost of caring for a patient with heart failure is near $30,000 in the United States with a wide range of estimates for other countries [3–6]. The majority of this cost is accrued for inpatient care (Figure 1).
Figure 1.
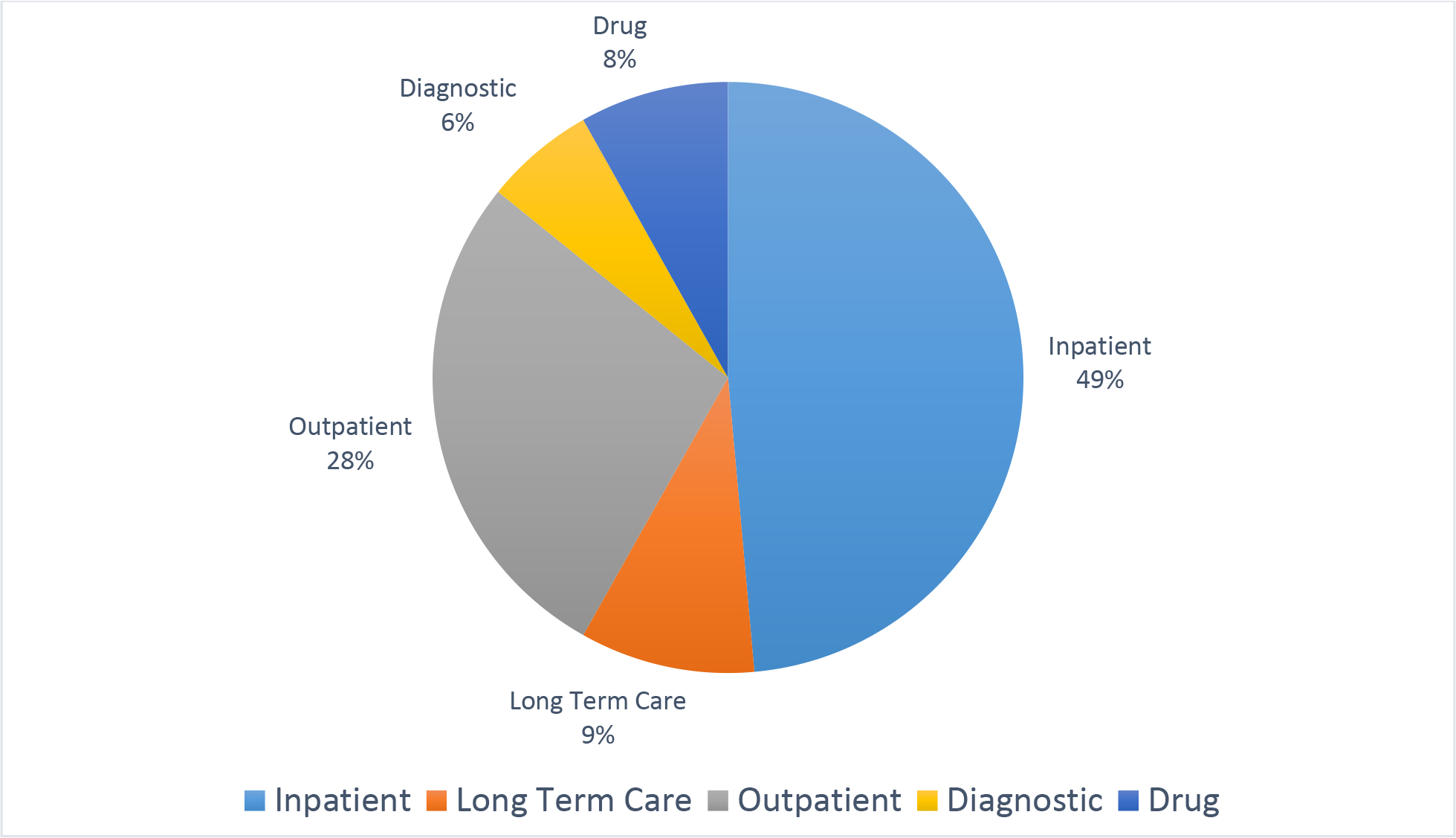
The breakdown of cost of care is shown for care types (2010 resource use). [5] Since this study was performed, it is likely that care has shifted slightly to the outpatient setting.
The economic impact of heart failure is best estimated by the incremental cost due to heart failure and not the entire cost of care. The incremental cost includes both direct treatment of heart failure but also the increase in cost due to heart failure worsening other conditions. In 2012, the American Heart Association estimated the cost attributable to heart failure care to be $3,600 in direct cost and $1,700 in indirect costs per patient per year. [2] Indirect costs include cost of lost employment and are typically included in societal cost analyses. By 2030 US heart failure costs are expected to be at least 70 billion per year ($244 per every US adult) with total cost of caring for patients with heart failure reaching $160 billion. [2] Data from Canada show similar trends with estimated cost of $722 million by 2030 for a principal diagnosis of heart failure and $2.8 billion when secondary diagnoses are included. [3]
Hospitalization Trends
The greatest economic burden related to heart failure results from hospitalizations and rehospitalizations. In many analyses, 75–80% of the direct costs for heart failure are attributable to inpatient hospital stays. [1,2] In 2016, there were 809,000 hospitals discharges with a primary diagnosis of heart failure and another 2–3 million hospitalizations with heart failure as a secondary diagnosis. [1] Heart failure primarily affects older adults, is the second most common inpatient diagnosis billed to Medicare and has among one of the highest 30-day readmission rates of any other medical or surgical condition. [1,2,7] Patients with heart failure requiring inpatient admission are a highly vulnerable population and have a poor prognosis, with 1-year mortality rates exceeding 30%.[1,2,7]
From 2005–2014 hospitalizations for heart failure in the United States increased, largely driven by increased admissions for heart failure with preserved left ventricular ejection fraction. [8] However, after 2014, hospitalizations for heart failure per capita declined in the United States. [1] The reasons for this decline are unclear and likely multifactorial. Per the National Inpatient Sample, heart failure hospitalizations in the United States decreased from 1,000,000 per year in 2002 to 800,000 per year in 2016. [4] Mean hospital length of stay also decreased by 2 days from 8.6 to 6.5 days during this time. Despite the decrease in length of stay, the cost per hospitalization (in constant dollars) has increased 1.4% per year to $19,000 in 2016 dollars.
This increase in cost per hospitalization was associated with more procedures, greater prevalence of cardiogenic shock, and renal failure requiring dialysis. [4] The reduction in length of stay was associated with fewer discharges to home (70% to 65%) and more discharges to long-term care facilities. [4] The reasons for a decline in hospitalizations is likely multifactorial. While improvements in the provision of guideline recommended care is a possible contributor, increased attention to readmission may have prompted providers to attempt outpatient management strategies for patients who would have been previously been hospitalized, though it is not clear financial penalties had a direct effect. [8]
Readmissions
The worldwide prevalence of heart failure (HF) is estimated to be 26 million and is increasing (1). In the United States, 5.7 million adults have been diagnosed with HF, with estimated annual direct costs of $39.2 billion to $60 billion (2, 3). Total HF costs in the United States are expected to exceed $70 billion by 2030 (4).
Heart failure primarily affects older persons and is the second most common inpatient diagnosis billed to Medicare (5). Patients with HF requiring inpatient admission are a vulnerable population and have a poor long-term prognosis, with a 2-year readmission-free survival rate as low as 17% (6). Risks for death and rehospitalization are accentuated immediately after inpatient discharge, with much of the economic burden in HF resulting from costly hospital readmissions. Several groups have identified transition-of-care interventions after acute hospitalization as an important area to improve patient safety and reduce HF costs (4, 7
Several groups have identified targeting reductions in readmissions after a heart failure hospitalization as an important area to reduce heart failure costs. In 2007, the Medicare Payment Advisory Commission (MedPAC) estimated that a substantial portion of Medicare beneficiaries experience a preventable hospital readmission within 30 days of discharge and recommended focusing on readmission reduction. [9] Under the 2010 Patient Protection and Affordable Care Act, the mandatory federal Hospital Readmissions Reduction Program (HRRP) was created to decrease 30-day hospital readmissions. Readmissions reporting started in 2010 and the financial penalty phase began in 2012, with hospitals with higher than expected 30-day all-cause Medicare fee-for-service (FFS) readmissions following initial hospitalization for heart failure, acute myocardial infarction, and pneumonia, penalized up to 3% of their total inpatient Medicare payments. [9] In fiscal year 2020, 83% of Medicare-participating hospitals were penalized, for a total of $563 million dollars. [9] The HRRP has altered the landscape of hospital readmissions and reimbursement within the United States, with 7.7 billion dollars in otherwise owed reimbursement to hospitals budgeted to be withheld in the first 10 years of the program.[9] As hospitals that care for heart failure patients with lower socioeconomic status tend to have higher readmission rates, irrespective of care quality, safety net and other financially vulnerable hospitals have been disproportionately impacted by these penalties.[9] While HRRP was associated with decreases in inpatient 30-day rehospitalization rates for heart failure patients, much of the observable changes in practices after HRRP appear to have resulted from administrative upcoding and inappropriate triage, rather than improvements in transitions of care, outpatient disease management, and use of evidence-based, guideline-directed clinical practices.[9] When adjusted for coding changes observed declines were comparable to hospitals not subject to financial penalties for readmissions, suggesting either no effect or an effect independent of the penalty.[9] Of greater concern, some studies [10,11], though not all [12] have suggested that after the HRRP announcement and penalty phase, patients hospitalized with heart failure have had increases in post-discharge mortality.
Transitions of Care
While the financial penalty based policy approach appears to have been associated with unintended consequences, a number of care transition and heart failure disease management interventions have shown some success in reducing readmission, without compromising patient safety.[1,13,14] The interventions used by these programs include initiating discharge planning early in the course of hospital care, collaborating with pharmacy services in discharge planning, actively involving patients and families or caregivers in the plan of care, providing new processes and systems that ensure patient understanding of education about the plan of care before discharge from the hospital, and improving quality of care by continually monitoring adherence to national evidence-based guidelines. [14] Formal economic analysis of transitional care services after a hospitalization for heart failure, including disease management, nurse home visits and nurse case management, have suggested these are cost-effective strategies.[13] While many care coordination and transitions programs were found to decrease readmissions and costs of heart failure care, not all programs have been shown to be effective.[15]
Outpatient Trends
In contrast to the recent decline in hospitalizations, outpatient care for heart failure has increased. [1] In 2016, there were 1,932,000 office visits and 414,000 emergency department (ED) visits with a primary diagnosis of heart failure.[1] As more ambulatory care systems accept capitation or other increased risk of patient cost there will be more pressure to reduce hospitalizations and ED visits.
Disparities
Racial disparities in heart failure hospitalizations have been demonstrated with higher age-adjusted rates among black patients compared with other races. [16] Data from the Atherosclerosis Risk in Communities (ARIC) study from 2005–2014 demonstrated a higher age-adjusted rate of heart failure hospitalization for Black men (38.1 (36.6 −39.7)) per 1000 per year) than for White men (20.7 (20.0–21.3)) per 1000 per year). [16] Similar differences were noted for Black women (30.5 (29.2–31.8)) compared to White women (15.2 (14.7–15.7)). Furthermore, the trends over time indicate that rates were increasing at a faster rate over 10 years for Black men (+3.7%) and Black women (+4.3%) than for White men (+2.6%) and White women (+1.9%). [16]
Impact of COVID-19 Pandemic on Heart Failure Cost of Care
During the initial phase of the COVID-19 pandemic (through the summer of 2020) the rate of heart failure hospitalizations decreased by 30–40% in many countries. [17–20] A similar decrease in ED visits (44%) [18] was observed. The reasons for this are unclear and may be due in part to patient concerns about seeking care and hospitals being overwhelmed caring for COVID-19 patients. Further surveillance will be needed to assess whether this decline in hospitalizations is associated with an increase in mortality or will lead to a rebound in hospitalizations over time. [21]
The COVID-19 pandemic has also accelerated the use of virtual visits [22] to reduce transmission of COVID-19. These virtual visits do not incur facility or patient transportation costs though patients are often still subject to co-pays. It is likely that the use of such visits will persist when COVID-19 is no longer a significant public health threat. Yet, it is unclear whether the quality of heart failure care provided, and clinical outcomes produced are comparable to those of in-person visits. Currently, compensation for video visits remains comparable to in-person visits in the United States; though it is not clear how long this will last. Telemedicine has also been used for delivery of cardiac rehabilitation for patients for heart failure and this method is likely to continue. [23] Thus, the cost of outpatient heart failure care may have declined during the COVID-19 pandemic, with the potential impact on overall quality, costs, and outcomes requiring further study.
Measuring the Economic Value of Heart Failure Care
Value of care is often measured in units of cost per life-year gained with lower ratios indicating higher value (incremental cost-effectiveness ratio). The American College of Cardiology and American Heart Association have adopted the World Health Organization recommendation of adjusting the threshold for value using the wealth of society as measured by the Gross Domestic Product (GDP). [24] Specifically, a treatment is considered high value if the cost per life year (or quality adjusted life year) gained is less than one GDP per capita. [24] The GDP/capita in the United States in 2019 was approximately $65,000. [25] If the cost per quality adjusted life year gained is over three GDP/capita, then the value is considered poor. A similar threshold for poor value was identified using an opportunity cost approach that estimated how much individuals are willing to pay for health by comparing the amount individuals were willing to pay for private insurance against the clinical harms of not having insurance. [26] The uncertainty in the estimated cost-effectiveness varies and should be considered when evaluating the value of care. Figure 2. shows an estimate both the cost-effectiveness ratio and the uncertainty in the estimate for different heart failure care strategies.
Figure 2.
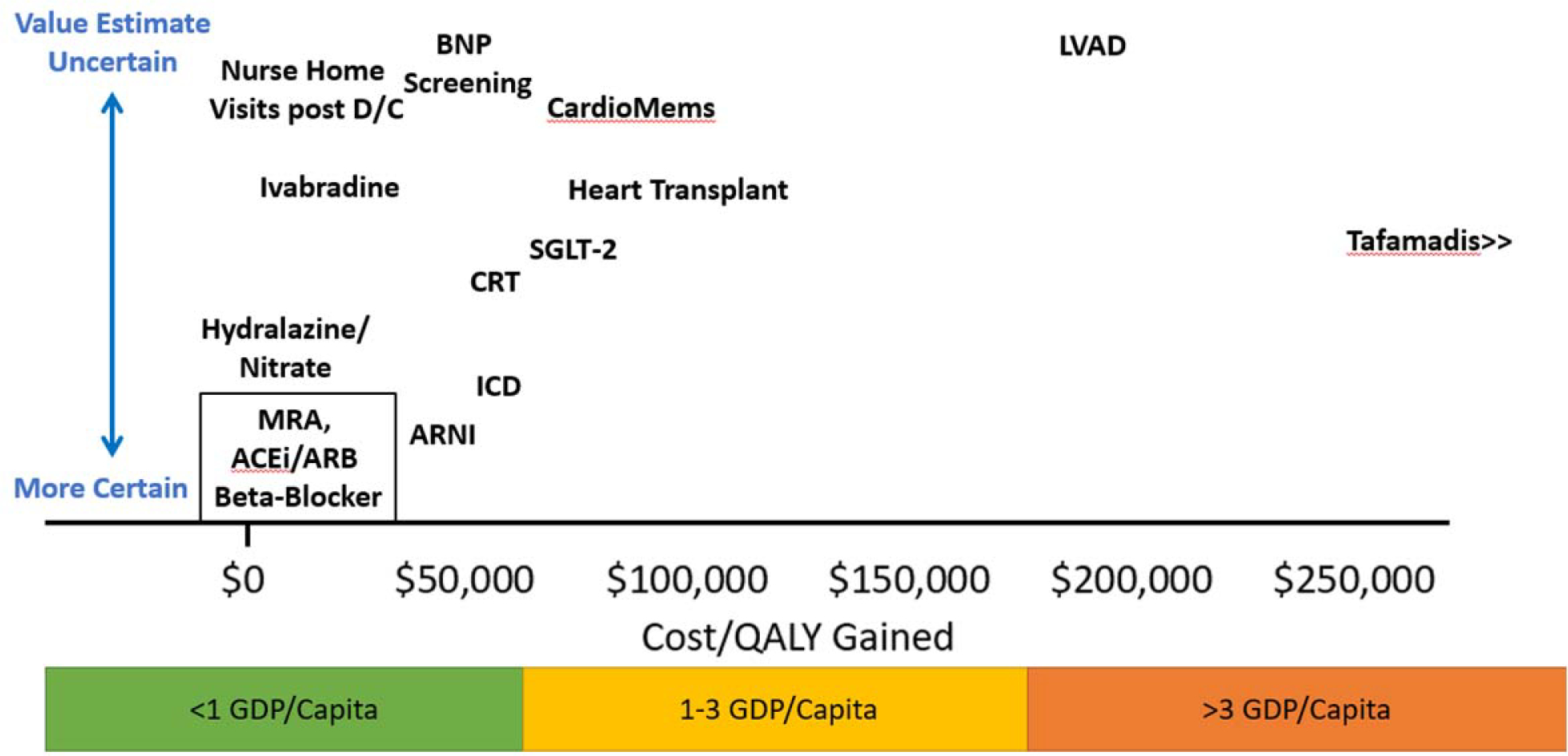
Graphical representation of studies cost-effectiveness for different heart failure therapies. Value estimates are measured on the X axis in terms of cost per quality-adjusted life years gained. The Y axis shows the uncertainty in these estimates. The box outlining MRA, ACE/ARB and Beta-Blockers indicates similar value estimates and certainty of value for these groups. MRA=mineralocorticoid receptor antagonists, ACEi= angiotensin converting enzyme inhibitor, ARB=angiotensin receptor antagonist, ARNI= angiotensin receptor blocker and neprilysin inhibitor, BNP=b-type natriuretic peptide, CRT=cardiac resynchronization therapy, D/C=discharge, GDP=gross domestic product, ICD=implantable cardioverter defibrillator, LVAD=left ventricular assist device, QALY=quality adjusted life years. SGLT-2=sodium glucose co-transporter-2 inhibitors.
Medications
Cost-Effectiveness of Current Heart Failure Therapies
Multiple pharmacologic therapies improve survival among patients with heart failure with reduced ejection fraction [14,27]: selected beta-blockers, angiotensin-converting enzyme inhibitors (ACE-Is), angiotensin receptor blockers (ARBs), mineralocorticoid receptor antagonists (MRAs), hydralazine/nitrate, sacubitril-valsartan, and sodium glucose cotransporter-2 (SGLT-2) inhibitors. [14,27] Ivabradine is an additional therapy that has been shown to improve quality of life and reduce heart failure hospitalizations. [27] Cost-effectiveness studies have evaluated the economic value of these heart failure drugs. [28–41] These studies, described in Table 1, have consistently demonstrated the high value of these therapies at conventional US cost-effectiveness thresholds.[24, 26]
Table.
| Drug (Estimated 2018 Cost)3 | First Author (Year) | Industry Sponsor | CEA Cost | LY; QALY Gain | Cost Difference | ICER ($/QALY or $/LY)4 | Comments |
| Beta-blockers | |||||||
| Bisoprolol ($188) | Gregory (2001) [34] | N | $379 | 1.04; NA | $3,455 | $3,336/LY | Based on CIBIS-II trial; no QALY data |
| Carvedilol ($55) | Delea (1999) [37] | Y | $1,096 | 0.79; NA | $15,735 | $19,918/LY | Based on US Carvedilol Trial; no QALY data |
| Gregory (2001) [34] | N | $2,000 | 2.40; NA | $15,656 | $6,740/LY | Based on US Carvedilol Trial; no QALY data | |
| Metoprolol Succinate ($183) | Gregory (2001) [34] | N | $612 | 1.06; NA | $2,613 | $2,472/LY | Based on MERIT-HF and MDC Trial; no QALY data |
| Any ($55)5 | Banka (2013) [38] | N | $48 | 0.31; 0.24 | $411 | $1,323/QALY | Based on MERIT-CHF trial |
| Angiotensin Converting Enzyme Inhibitors/Angiotensin Receptor Blockers | |||||||
| Captopril ($812) | Tsevat (1995) [29] | Y | $631 | NA; 0.52 | $2,933 | $5,600/QALY | Based on SAVE trial; results displayed for 60yo cohort |
| Enalapril ($192) | Paul (1994) [32] | N | $959 | NA; 0.27 | $2,569 | $9,731/LY | Based on SOLVD and V-HeFT-II trials; only a 10-year time horizon; no QALY data |
| Glick (1995) [35] | Y | $2486 | 0.30; 0.21 | $25 | $115/QALY | Based on SOLVD trial | |
| Any ($40)5 | Banka (2013) [38] | N | $48 | 0.15; 0.12 | -$444 | Dominant Strategy7 | Based on SOLVD trial |
| Shekelle (2003) [30] | N | $5206 | 0.64; 0.66 | $3,718 | $5,644/QALY | Based on SOLVD trial | |
| Mineralocorticoid Receptor Antagonists | |||||||
| Eplerenone ($961) | Weintraub (2005) [28] | Y | $1,1385 | 0.06–0.13; 0.04–0.09 | $1,923-$2,323 | $23,724-$43,301 | Based on EPHESUS trial8 |
| Any ($78)5 | Banka (2013) [38] | N | $48 | 0.10; 0.07 | $47 | $501/QALY | Based on EMPHASIS-HF trial |
| Hydralazine-Nitrates | |||||||
| Hydralazine-Nitrates ($720) | Angus (2005) [39] | Y | $1,971 | 0.26; NA | $10,900 | $44,400/LY | Based on A-HeFT trial; assumed treatment efficacy for only a 2-year duration; no QALY data |
| Sacubitril-Valsartan | |||||||
| Sacubitril-Valsartan ($5,315) | Sandhu (2016) [31] | N | $4,563 | 0.69; 0.62 | $29,204 | $47,053/QALY | Based on PARADIGM-HF trial |
| King (2016) [43] | N | $4,560 | 1.08; 0.76 | $38,633 | $50,959/QALY | Based on PARADIGM-HF trial | |
| Gaziano (2016) [36] | Y | $4,500 | 1.43; 0.78 | $35,200 | $45,017/QALY | Based on PARADIGM-HF trial | |
| Gaziano (2020) [44] | Y | $5,628 | 1.51; 1.24 | $27,353 | $21,532/QALY | Based on PARADIGM-HF and PIONEER-HF; cost-saving when including societal indirect costs | |
| Ivabradine | |||||||
| Ivabradine ($4,706) | Kansal (2016) [33] | Y | $4,500 | 0.16; 0.20 | $4,913 | $24,920/QALY | Based on SHIFT trial; results displayed for Medicare Advantage population; only a 10-year time horizon |
| SGLT-2 Inhibitors | |||||||
| Dapagliflozin ($5,488) | Parizo (2021) [45] | Y | $474 | 0.78; 0.46 | $38,212 | $83,650 QALY | Based on DAPA-HF trial; |
Abbreviations: A-HeFT: African-American Heart Failure; CIBIS-II: Cardiac Insufficiency Bisoprolol Study II; DAPA-HF: Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure; EMPHASIS-HF: Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure; EPHESUS: Eplerenone Post–Acute Myocardial Infarction Heart Failure Efficacy and Survival Study; MDC: Metoprolol in Dilated Cardiomyopathy; MERIT-HF: Metoprolol CR/XL Randomized Intervention Trial in Congestive Heart Failure; NA: not available; PARADIGM-HF: Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure; PIONEER-HF: Comparison of Sacubitril–Valsartan versus Enalapril on Effect on NT-proBNP in Patients Stabilized from an Acute Heart Failure Episode; QALY: quality-adjusted life year; SHIFT: Systolic Heart failure treatment with the If inhibitor Ivabradine Trial; SOLVD: Studies of Left Ventricular Dysfunction; V-HeFT II: Vasodilator-Heart Failure Trial II.
Limited to economic evaluations that include an assessment of clinical benefit via either life-years or quality-adjusted life years and total healthcare costs. Excluded studies without a medium to long-term time horizon.
Estimated cost based on Medicare Part D spending for the drug. Generic costs were utilized when available. For non-specific drugs, the drug with the lowest cost was utilized. This amount does not include proprietary rebates between patented drugs and pharmaceutical plans, which average over 20% of cost across patented drugs. For individual patients, out-of-pocket costs will vary depending on their pharmaceutical plan.
When available, cost per quality-adjusted life years is preferable. For multiple studies, quality-adjusted life years was not calculated. For these studies, results were represented as $/life-year gained.
Estimated cost for the lowest-cost generic in that class.
Approximated based on trial drug costs and trial follow-up duration.
Indicates preferable strategy given lower cost and better clinical outcomes.
Modeled post-trial outcomes using three different patient cohorts leading to range of results.
Most economic analyses were performed soon after the pivotal clinical trials and therapy introduction. Due to the timing of these analyses, there are important considerations that affect their enduring applicability. First, heart failure therapies have been additive with each treatment added to prior therapies, resulting in reduction in heart failure mortality over time.[1] The economic analyses of earlier agents, such as beta-blocker or ACE-I therapy, were based on trials with higher baseline mortality and subsequently greater absolute clinical benefit than observed currently. The non-pharmaceutical costs of treating heart failure have increased over time [42] Critically important is the reduction in drug price following generic availability, particularly for beta-blockers, ACE-Is, and ARBs. A more recent economic analysis demonstrated high value at generic prices.[38]
Three major heart failure drug classes remain under patent in 2021: sacubitril-valsartan, ivabradine, and SGLT-2 inhibitors. Multiple studies have demonstrated the high value of sacubitril-valsartan.[31,36,43] Additionally, a recent analysis based on PIONEER-HF demonstrated sacubitril-valsartan was potentially cost-saving among high-risk patients hospitalized for heart failure when indirect societal costs (costs due to lost employment) were taken into account.[44] Ivabradine has one industry-sponsored cost-effectiveness study that also demonstrated high value.[33] Several SGLT-2 inhibitors are approved by the United States Food and Drug Administration (FDA) for the treatment of heart failure among patients with and without diabetes. There is a published cost-effectiveness evaluation demonstrating intermediate value with dapagliflozin using US prices.[45] Rapid analyses of the economic value of new therapies are critical to inform payer-manufacturer price negotiations and healthcare system supply for novel therapies. Likewise, there must continue to be updated analyses in the setting of changing prices and changes in heart failure epidemiology and costs.
Additional heart failure-related therapies are worth discussion. Tafamadis is an approved therapy for cardiomyopathy due to transthyretin (TTR) amyloidosis that improves survival and quality of life.[46] At its current wholesale acquisition cost of $225,000 annually, it has an incremental cost-effectiveness ratio of $880,000/quality-adjusted life year (QALY), which would be low value based on conventional US thresholds.[47] While wholesale acquisition costs are typically the best estimate of the cost of drug therapy the company may provide discounts which lower the overall cost of care. Cost-effectiveness analyses typically examine a range of drug costs that will include the cost after any discount. Patiromer acetate, a potassium binding agent, is used to enable use of ACE-I/ARB/MRA therapy among patients with hyperkalemia. A single, industry-sponsored analysis found patiromer had an incremental cost-effectiveness ratio of $52,700/QALY.[48] This study made strong assumptions regarding the overall clinical impact of patiromer based on the OPAL-HK trial, a single-arm study of patients with hyperkalemia and chronic kidney disease (including non-heart failure patients) that demonstrated patiromer’s potassium-lowering effect.[49] With limited effectiveness data, the economic value of patiromer remains uncertain.
Budget Impact of New Therapies to the System
Healthcare payers may be more concerned with a therapy’s impact on its short-term total budget than on its long-term cost-effectiveness. The focus on budgetary impact is a product of multiple realities of the healthcare system. First, patients change insurances frequently. Therefore, an insurance company may be more affected by short-term costs than long-term effects. Second, insurance companies must balance their short-term budget. An increase in spending related to a given drug must be offset with other budget adjustments until premiums are adjusted. Finally, United States payers do not have accepted cost-effectiveness thresholds at which therapies are considered reasonable value for coverage. Therefore, effective therapies are “approved” but barriers are erected, such as preauthorization requirements, to limit uptake and minimize the budgetary impact.
There are limited data regarding the budgetary impact of new therapies. The Institute for Clinical and Economic Review (ICER) estimated the cost-effectiveness and budgetary impact of sacubitril-valsartan soon after its approval. It found a similar cost-effectiveness to other analyses.[50,51] Based on a high uptake of sacubitril-valsartan (75% of patients by year 5) given the substantial therapeutic benefit, it estimated a $3.0 billion annual budgetary impact. The report also calculated a value-based price benchmark. This price assumes a drug’s budgetary impact should be proportional to other drugs irrespective of its relative value ($900 million per drug) or disease prevalence. Based on this analysis, sacubitril-valsartan’s estimated price should be at least 9% below the wholesale acquisition cost.
Focusing on budgetary impacts biases against therapies for high-prevalence conditions. New heart failure therapies will have high budgetary impact due to heart failure’s prevalence. Limiting the total spending on a drug independent of its value or disease prevalence ignores the potential to improve clinical outcomes for more patients. Coverage and pricing decisions should focus on the value of therapy rather than on the budgetary impact.
Barriers to Access
Multiple barriers have prevented optimal uptake of heart failure drugs. These include barriers erected by insurance companies – prior authorizations, copays, and deductibles – that are intended to reduce inappropriate utilization in part by forcing patients to share the cost. Unfortunately, these processes also block the adoption of high-value therapies and reduce appropriate utilization.[52]
Conceptually, prior authorization requirements restrict high-cost treatment to scenarios with evidence of clinical benefit.[53] However, the process also exacerbates the challenge of prescribing novel therapies to patients who will benefit. Prior authorization requirements are often applied indiscriminately across high-cost drugs independent of a patient’s clinical characteristics. Even for those patients most likely to benefit from a therapy, gaining authorization is a time-intensive process that increases the barriers to prescribing high-value therapies. For most heart failure drugs, there is little evidence of inappropriate utilization or indication drift, so prior authorization has minimal benefit with potential for significant harm. Prior authorization requests for heart failure drugs should be limited to scenarios where a high-cost therapy is being used for an indication with unclear benefit or where there are clinically equivalent substitutes with lower costs.
The unaffordability of heart failure therapies is a second major barrier to access. Patients are required to pay high out-of-pocket costs via copays and deductibles for many of the new cost-effective heart failure drugs. With guideline-directed heart failure management consisting of multiple therapies in addition to non-heart failure drugs, high total out-of-pocket costs can limit the affordability of heart failure treatment. Multiple studies have found high out-of-pocket costs are associated with lower rates of initial filled prescriptions and adherence to therapy.[54,55] Additionally, randomized trials have demonstrated co-pay waivers can improve therapy adherence.[56,57]
Drug cost sharing has two potential roles. First, it is an additional tool to reduce overutilization of therapies with minimal clinical benefit. Second, cost-sharing may be used for effective therapies that are low-value due to high costs, although, even in this case, cost-sharing would be expected to increase health disparities given low-income patients are less likely to be able to afford the effective therapy.
For high-value drugs, placing the burden of payment on patients may inappropriately decrease therapy rates and worsen clinical outcomes. Sacubitril-valsartan is an example of a cost-effective drug that is unaffordable for many heart failure patients, contributing to inadequate sacubitril-valsartan use and adherence, increasing heart failure morbidity and mortality.[58] Copays and deductibles should be minimal for high-value therapies like sacubitril-valsartan with current out-of-pocket costs covered by the insurance plan. [59] Prioritizing the affordability of high-value drugs is critical to maximize population-health outcomes for diseases such as heart failure.
Devices
Device use in heart failure has increased markedly in the last 40 years. Most devices are tested and approved in patients with heart failure and reduced ejection fraction, but implantable hemodynamic monitoring devices are approved for use in heart failure with preserved ejection fraction as well. The economics of devices are less favorable than those for drug therapy. However, because heart failure is, in general, a highly morbid disease with high mortality and expense, it is possible to show that expensive devices can, in certain circumstances, be economically favorable. Cost of technology implementation is highly dependent on geography and most analyses of device cost effectiveness (CE) come from the US or European perspective. Devices frequently have less robust randomized trial data prior to approval than are available for drug therapy, making uncertainty in CE model inputs higher. Finally, as time passes and/or competition develops in a device market, technology costs may drop. All of these factors increase the uncertainty in CE analyses of heart failure device therapy.
As in all economic assessments, a few factors tend to dominate economic analyses of heart failure devices. These include cost of the device, risk of death, risk of hospitalization, and magnitude of the device’s effect to reduce death, hospitalization, or both. Devices with lower reliability, with significant rates of complication or lower durability, are generally associated with increased costs and this will impact economics unfavorably. Some devices may be most effective when applied to a very ill population due to the magnitude of risk and risk reduction, while others may be most effective applied to a less ill population due to a less dramatic effect that becomes economically more favorable over a longer duration of life.
Defibrillators
Nearly all defibrillator studies show a reduction in sudden cardiac death (SCD) in the implanted arm. [60.61] The overall population-benefit of the reduction, however, can be highly variable from study to study depending on background population risk. As a rule, SCD risk is highest in ischemic cardiomyopathy, and these studies tend to show clear benefit of defibrillator therapy. A paradoxical issue with implantable cardioverter defibrillators (ICD) studies, particularly primary prevention studies, is that of competing risk. In lower risk cardiomyopathy populations with reduced ejection fraction, ICD implantation leads to mortality reduction. However, as patient risk increases, competing risk of heart failure death may overwhelm device-related reductions in SCD risk and reduce value of device implantation.
Primary Prevention Defibrillators
The economic case for primary prevention ICDs is more nuanced. If one assumes a benefit of ICD therapy to prolong good quality life, the longer time horizon in primary prevention analyses (3.5 years to lifetime) tends to make these analyses more favorable. The Multicenter Automatic Defibrillator Implantation Trial (MADIT) in ischemic heart failure showed clear efficacy of ICD implantation, leading to highly cost-effective ICERs based on these criteria from $19,148 – $54,802/QALY [62–65] The case in non-ischemic cardiomyopathy depends on the level of risk reduction assumed, and here variability in randomized trial data makes modeling challenging. Using data from the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT), models provide an ICER in 2011 US dollars between $79,579 – $155,400/QALY.[62–64]
Secondary Prevention Defibrillators
Initial trials of ICD implantation in patients who had had an episode of ventricular arrhythmia or sudden cardiac death showed a profound benefit of the devices to reduce mortality when compared to active anti-arrhythmic therapy.[60] Implantation of an ICD for secondary prevention is a class I, level of evidence B recommendation in survivors of sudden death due to ventricular fibrillation (VF) or hemodynamically unstable sustained ventricular tachycardia (VT) [61] Nevertheless, economic models of ICD therapy show a broad range of results, even in the relatively clear-cut case of secondary prevention. A meta-analysis of the economics of implantable cardioverter defibrillators included 7 studies of CE for secondary prevention ICDs.[62] Cost-effectiveness was maximized by implanting ICDs in patients with higher risk features or lower ejection fractions. In these cases, ICERs ranged from $47,571/QALY to $142,556/QALY in 2011 US dollars.
Resynchronization Therapy
Cardiac resynchronization therapy (CRT) has been shown to reduce risk of death in patients with reduced ejection fraction and prolonged QRS duration. CRT devices cost approximately 1/3 that of defibrillators, but because they are implanted in patients with low ejection fractions, they are often combined with defibrillators, increasing cost of the therapy substantially. Economics of CRT when combined with defibrillator therapy is the subject of a recent systematic review. [66] This review concluded that CRT vs. no therapy was highly cost-effective. The value of CRT-D vs ICD alone was not as high but potentially favorable depending on a society’s willingness to pay to improve outcome.
Using data from the Cardiac Resynchronization in Heart Failure (CARE-HF) trial, the cost-effectiveness of CRT was € 19,319/QALY (US $20,000-$25,000). [67] CRT has also been studied in less ill patients in the REVERSE, MADIT-CRT, and RAFT trials.[68–70] A meta-analysis examining the cost effectiveness of CRT in mild heart failure estimated a cost-effectiveness ratio of $61,700/QALY gained. [71] Recent reports examining long-term efficacy of CRT in mild heart failure have led to more favorable estimates of cost for CRT therapy, even in mild heart failure, with borderline CE when combined with defibrillator therapy if one assumes a sustained mortality reduction.
Implanted Circulatory Support Devices
Data regarding CE of durable mechanical circulatory support (MCS) devices have recently been summarized in a systematic review. [72] Implanted MCS devices increase life expectancy and quality of life in advanced heart failure patients [73–77] but are associated with obligatory upfront technology costs as well as substantial outpatient and readmission costs as well. A few studies have shown ICERs for use of MCS as a bridge to transplant near US$80,000/QALY (2017 dollars),[78–82] but others show ICERs for this strategy in excess of $100,000. [82] or even $200,000 per quality adjusted life-year gained. [83] One study suggested ICERs for long-term, or destination, MCS strategy near $200,000/QALY. [83] One analysis, however, did show a profound drop in post-discharge costs with the newest HeartMate 3 device compared to costs for the HeartWare device (no longer manufactured), driven by a reduction in rehospitalizations, suspected pump thrombosis and stroke. [77]. Ongoing assessment of costs as these technologies evolve is essential. [84,85] Cost effectiveness analyses are not currently available for short term devices utilized as acute therapy in critically ill patients with cardiogenic shock which is partially related to limited data regarding the relative efficacy of different treatment strategies in this scenario.
Cardiac Transplant
Long and colleagues compared heart transplant with destination therapy LVAD and medical therapy among transplant-eligible Inotrope-dependent Stage D heart failure. [86] They used data from ISHLT registry and the REMATCH trial and performed two analyses – a 5.6 month and 12-month waitlist for transplant. They estimated transplant led to improved outcomes and lower cost than DT-LVAD in both scenarios. Compared with medical therapy, they estimated transplant led to 4.12 additional QALYs at a lifetime incremental cost of $398,700 with a 5.6-month waitlist. This led to a cost per QALY of $96,900 of transplant relative to medical therapy with similar results with the 12-month waitlist. The primary caveats are the basing of effectiveness estimates on observational data and the advances in transplant outcomes over time.
Mitral Valve Transcatheter Edge to Edge Repair
An analysis using the COAPT study results [87] found that transcatheter mitral valve transcatheter edge-to-edge repair (TEER) using the Mitraclip device (procedural cost $35,755) among patients with severe secondary mitral regurgitation would improve survival by 1.13 years (0.83 QALYs) and increase cost by $45,648 compared to medial therapy alone for a cost-effectiveness ratio of $55,600/QALY. [88] The benefit noted in the COAPT study has led to a 2A recommendation for TEER in the American College of Cardiology/American Heart Association clinical guidelines. [89] However, a second randomized controlled trial (MITRA-FR) using a similar population found the rate of death or unplanned hospitalization for heart failure at 1 year did not differ significantly between patients who underwent TEER and those who received medical therapy alone. [90] The uncertainty in the benefit makes it difficult to draw conclusions regarding the value of mitral valve TEER.
Diagnostic and Monitoring Tests
While clinical examination and assessment remain the gold standard for screening and diagnosing heart failure, new technological developments have added several options for clinicians managing patients with heart failure.
Brain natriuretic peptide (BNP) and N-terminal (NT) pro-BNP are now routinely used in clinical practice for the diagnosis of heart failure. The use of BNP to diagnosis of heart failure in patients with dyspnea has been shown to be cost effective.[91] More recently, there has been growing interest in using NT pro-BNP-guided management of chronic heart failure, and a 2016 Cochrane review found reduction in heart failure admissions (38% vs. 28%, relative risk 0.70, 95% confidence interval 0.61, 0.80, n=1928 patients, 10 studies, low quality of evidence) though no difference was found in any other clinical outcome.[92] Three of four studies that assessed cost found it to be cost-saving. A more recent NIH-funded RCT, GUIDE-IT, which was published after this Cochrane review, however, did not show any difference in any of the clinical or quality of life outcomes, including heart failure hospitalization.[93] Costs also averaged $5,919 higher in the NT pro-BNP guided arm (95% confidence interval -$1,795, +$13,602). Given the conflicting data, the cost-effectiveness of using natriuretic peptides for management of patients with heart failure remains uncertain.
Community screening with BNP followed by echocardiography was explored in an economic analysis.[94] Performance of BNP in asymptomatic men and women >60 years of age, followed by echocardiography, resulted in increased lifetime cost of care (176,000 US dollars for screening 1000 men, 101,000 US dollars for 1000 women) and improved outcome (7.9 quality-adjusted life years [QALYs] for 1000 men, 1.3 QALYs for 1000 women), resulting in a cost per QALY of $22,300 USD for men and $77,700 USD for women.[93]
There has also been considerable interest in using invasive hemodynamic measures to manage chronic heart failure. CardioMEMS (CardioMEMS Heart Failure System, St Jude Medical Inc, Atlanta, GA) is one such device, which has been approved by the FDA.[95] However, cost effectiveness studies show mixed results ranging from high to intermediate value. [86–98] Sandhu and colleagues found a cost of $71,462 per QALY gained.[88] The most important determinants of the device’s cost-effectiveness were the durability of its effect on hospitalization and mortality over time. A recent trial found less efficacy of CardioMEMS though the population differed. [99] Thus, the cost-effectiveness of CardioMEMS is unclear and requires further study.
Cost and Value in Heart Failure Guidance Documents
As noted above, the ACC/AHA has published recommendations for including statements on cost-effectiveness and value in clinical practice guidelines and performance measure documents. [24] A recent review of 33 clinical guidance documents for heart failure found that 27 (82%) included at least one cost or value statement though most of these focused on the high economic impact of heart failure. [100] Three documents (9%) reported estimated costs of interventions and one estimated out-of-pocket cost.
Summary
The cost of heart failure care is growing due to the aging of the population and the development and new effective but costly therapies. This review has summarized the value of different care strategies and a graphical representation of these is shown in Figure 2. The review focused on high profile heart failure management strategies and published cost-effectiveness data. Thus, other important heart failure care interventions such as rehabilitation and palliative care were not included.
Given limited health care budgets, policy makers must consider the economic value that each treatment or test provides. Policies are needed to minimize out of pocket costs for all high value heart failure treatments. Such policies will directly lead to lives saved and healthier days out of hospital for patients with heart failure.
Acknowledgments
Disclosures / Conflicts of Interest
PAH: None
GCF: Consulting for Abbott, Amgen, AstraZeneca, Bayer, Cytokinetics, Edwards, Janssen, Medtronic, Merck, and Novartis.
YO: None
ATS receives research support from the National Heart, Lung, and Blood Institute (1K23HL151672–01). ATS consults for Acumen, LLC.
HJW: Advisor for Embrace Prevention Care
NKS: None
REFERENCES
- 1.Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Shay CM, Spartano NL, Stokes A, Tirschwell DL, VanWagner LB, Tsao CW; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation. 2020. Mar 3;141(9):e139–e596. [DOI] [PubMed] [Google Scholar]
- 2.Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, Ikonomidis JS, Khavjou O, Konstam MA, Maddox TM, Nichol G, Pham M, Piña IL, Trogdon JG; American Heart Association Advocacy Coordinating Committee; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Stroke Council. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail 2013;6:606–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tran DT, Ohinmaa A, Thanh NX, Howlett JG, Ezekowitz JA, McAlister FA, Kaul P. The current and future financial burden of hospital admissions for heart failure in Canada: a cost analysis. CMAJ Open. 2016. Jul 21;4(3):E365–E370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khan SU, Khan MZ, Alkhouli M. Trends of Clinical Outcomes and Health Care Resource Use in Heart Failure in the United States. J Am Heart Assoc. 2020. Jul 21;9(14):e016782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoon J, Fonarow GC, Groeneveld PW, Teerlink JR, Whooley MA, Sahay A, Heidenreich PA. Patient and Facility Variation in Costs of VA Heart Failure Patients. JACC Heart Fail. 2016;4:551–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lesyuk W, Kriza C, Kolominsky-Rabas P. Cost-of-illness studies in heart failure: a systematic review 2004–2016. BMC Cardiovasc Disord. 2018. May 2;18(1):74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah KS, Xu H, Matsouaka RA, Bhatt DL, Heidenreich PA, Hernandez AF, Devore AD, Yancy CW, Fonarow GC. Heart Failure With Preserved, Borderline, and Reduced Ejection Fraction: 5-Year Outcomes. J Am Coll Cardiol. 2017. Nov 14;70(20):2476–2486. [DOI] [PubMed] [Google Scholar]
- 8.Ody C, Msall L, Dafny LS, Grabowski DC, Cutler DM. Decreases In Readmissions Credited To Medicare’s Program To Reduce Hospital Readmissions Have Been Overstated. Health Aff (Millwood). 2019; 38:6–43. [DOI] [PubMed] [Google Scholar]
- 9.Psotka MA, Fonarow GC, Allen LA, Joynt Maddox KE, Fiuzat M, Heidenreich P, Hernandez AF, Konstam MA, Yancy CW, O’Connor CM. The Hospital Readmissions Reduction Program: Nationwide Perspectives and Recommendations: A JACC: Heart Failure Position Paper. JACC Heart Fail. 2020. Jan;8(1):1–11. [DOI] [PubMed] [Google Scholar]
- 10.Gupta A, Allen LA, Bhatt DL, Cox M, DeVore AD, Heidenreich PA, Hernandez AF, Peterson ED, Matsouaka RA, Yancy CW, Fonarow GC. Association of the Hospital Readmissions Reduction Program Implementation With Readmission and Mortality Outcomes in Heart Failure. JAMA Cardiol. 2018. Jan 1;3(1):44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wadhera RK, Joynt Maddox KE, Wasfy JH, Haneuse S, Shen C, Yeh RW. Association of the Hospital Readmissions Reduction Program With Mortality Among Medicare Beneficiaries Hospitalized for Heart Failure, Acute Myocardial Infarction, and Pneumonia. JAMA. 2018. Dec 25;320(24):2542–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khera R, Wang Y, Nasir K, Lin Z, Krumholz HM. Evaluation of 30-Day Hospital Readmission and Mortality Rates Using Regression-Discontinuity Framework. J Am Coll Cardiol. 2019; 74:219–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blum MR, Øien H, Carmichael HL, Heidenreich P, Owens DK, Goldhaber-Fiebert JD. Cost-Effectiveness of Transitional Care Services After Hospitalization With Heart Failure. Ann Intern Med. 2020. Jan 28. doi: 10.7326/M19-1980. [DOI] [PubMed] [Google Scholar]
- 14.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013; 128:1810–52. [DOI] [PubMed] [Google Scholar]
- 15.Van Spall HGC, Lee SF, Xie F, Oz UE, Perez R, Mitoff PR, Maingi M, Tjandrawidjaja MC, Heffernan M, Zia MI, Porepa L, Panju M, Thabane L, Graham ID, Haynes RB, Haughton D, Simek KD, Ko DT, Connolly SJ. Effect of Patient-Centered Transitional Care Services on Clinical Outcomes in Patients Hospitalized for Heart Failure: The PACT-HF Randomized Clinical Trial. JAMA. 2019. Feb 26;321(8):753–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang PP, Wruck LM, Shahar E, Rossi JS, Loehr LR, Russell SD, Agarwal SK, Konety SH, Rodriguez CJ, Rosamond WD. Trends in Hospitalizations and Survival of Acute Decompensated Heart Failure in Four US Communities (2005–2014): ARIC Study Community Surveillance. Circulation. 2018. Jul 3;138(1):12–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Severino P, D’Amato A, Saglietto A, D’Ascenzo F, Marini C, Schiavone M, Ghionzoli N, Pirrotta F, Troiano F, Cannillo M, Mennuni M, Rognoni A, Rametta F, Galluzzo A, Agnes G, Infusino F, Pucci M, Lavalle C, Cacciotti L, Mather PJ, Grosso Marra W, Ugo F, Forleo G, Viecca M, Morici N, Patti G, De Ferrari GM, Palazzuoli A, Mancone M, Fedele F. Reduction in heart failure hospitalization rate during coronavirus disease 19 pandemic outbreak. ESC Heart Fail 2020. Oct 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toner L, Koshy AN, Ko J, Driscoll A, Farouque O. Clinical Characteristics and Trends in Heart Failure Hospitalizations: An Australian Experience During the COVID-19 Lockdown. JACC Heart Fail 2020. Oct;8(10):872–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frankfurter C, Buchan TA, Kobulnik J, Lee DS, Luk A, McDonald M, Ross HJ, Alba AC. Reduced Rate of Hospital Presentations for Heart Failure During the COVID-19 Pandemic in Toronto, Canada. Can J Cardiol. 2020. Oct;36(10):1680–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hall ME, Vaduganathan M, Khan MS, et al. Reductions in heart failure hospitalizations during the COVID-19 pandemic. J Card Fail 2020;26: 462–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhatt AS, Moscone A, McElrath EE, et al. Fewer hospitalizations for acute cardiovascular conditions during the COVID-19 pandemic. J AM Coll Cardiol 2020;76:280–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gorodeski EZ, Goyal P, Cox ZL, Thibodeau JT, Reay RE, Rasmusson K, Rogers JG, Starling RC. Virtual Visits for Care of Patients with Heart Failure in the Era of COVID-19: A Statement from the Heart Failure Society of America. J Card Fail 2020. Jun;26(6):448–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Charman SJ, Velicki L, Okwose NC, Harwood A, McGregor G, Ristic A, Banerjee P, Seferovic PM, MacGowan GA, Jakovljevic DG. Insights into heart failure hospitalizations, management, and services during and beyond COVID-19. ESC Heart Fail. 2020. Nov 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson JL, Heidenreich PA, Barnett PG, Creager MA, Fonarow GC, Gibbons RJ, Halperin JL, Hlatky MA, Jacobs AK, Mark DB, Masoudi FA, Peterson ED, Shaw LJ; ACC/AHA Task Force on Performance Measures; ACC/AHA Task Force on Practice Guidelines. ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and Task Force on Practice Guidelines. Circulation. 2014. Jun 3;129(22):2329–45. [DOI] [PubMed] [Google Scholar]
- 25.The World Bank, GDP per Capita, United States. https://data.worldbank.org/indicator/NY.GDP.PCAP.CD?end=2019&locations=US&start=2019&view=chart, accessed 12/13/2020.
- 26.Vanness DJ, Lomas J, Ahn H. A Health Opportunity Cost Threshold for Cost-Effectiveness Analysis in the United States. Ann Intern Med. 2020. Nov 3. [DOI] [PubMed] [Google Scholar]
- 27.McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo-Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A; ESC Scientific Document Group. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021; 42:3599–3726. [DOI] [PubMed] [Google Scholar]
- 28.Weintraub WS, Zhang Z, Mahoney EM, et al. Cost-effectiveness of eplerenone compared with placebo in patients with myocardial infarction complicated by left ventricular dysfunction and heart failure. Circulation 2005;111:1106–13. [DOI] [PubMed] [Google Scholar]
- 29.Tsevat J, Duke D, Goldman L, et al. Cost-effectiveness of captopril therapy after myocardial infarction. J Am Coll Cardiol 1995;26:914–9. [DOI] [PubMed] [Google Scholar]
- 30.Shekelle P, Morton S, Atkinson S, et al. Pharmacologic management of heart failure and left ventricular systolic dysfunction: effect in female, black, and diabetic patients, and cost-effectiveness. Evid Rep Technol Assess (Summ) 2003:1–6. [PMC free article] [PubMed] [Google Scholar]
- 31.Sandhu AT, Ollendorf DA, Chapman RH, Pearson SD, Heidenreich PA. Cost-Effectiveness of Sacubitril-Valsartan in Patients With Heart Failure With Reduced Ejection Fraction. Ann Intern Med 2016;165:681–9. [DOI] [PubMed] [Google Scholar]
- 32.Paul SD, Kuntz KM, Eagle KA, Weinstein MC. Costs and effectiveness of angiotensin converting enzyme inhibition in patients with congestive heart failure. Arch Intern Med 1994;154:1143–9. [PubMed] [Google Scholar]
- 33.Kansal AR, Cowie MR, Kielhorn A, et al. Cost-Effectiveness of Ivabradine for Heart Failure in the United States. J Am Heart Assoc 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gregory D, Udelson JE, Konstam MA. Economic impact of beta blockade in heart failure. Am J Med 2001;110 Suppl 7A:74S–80S. [DOI] [PubMed] [Google Scholar]
- 35.Glick HA, Orzol SM, Tooley JF, Remme WJ, Sasayama S, Pitt B. Economic evaluation of the randomized aldactone evaluation study (RALES): treatment of patients with severe heart failure. Cardiovasc Drugs Ther 2002;16:53–9. [DOI] [PubMed] [Google Scholar]
- 36.Gaziano TA, Fonarow GC, Claggett B, et al. Cost-effectiveness Analysis of Sacubitril/Valsartan vs Enalapril in Patients With Heart Failure and Reduced Ejection Fraction. JAMA Cardiol 2016;1:666–72. [DOI] [PubMed] [Google Scholar]
- 37.Delea TE, Vera-Llonch M, Richner RE, Fowler MB, Oster G. Cost effectiveness of carvedilol for heart failure. Am J Cardiol 1999;83:890–6. [DOI] [PubMed] [Google Scholar]
- 38.Banka G, Heidenreich PA, Fonarow GC. Incremental cost-effectiveness of guideline-directed medical therapies for heart failure. J Am Coll Cardiol 2013;61:1440–32. [DOI] [PubMed] [Google Scholar]
- 39.Angus DC, Linde-Zwirble WT, Tam SW, et al. Cost-effectiveness of fixed-dose combination of isosorbide dinitrate and hydralazine therapy for blacks with heart failure. Circulation 2005;112:3745–53. [DOI] [PubMed] [Google Scholar]
- 40.McEwan P, Darlington O, McMurray JJV, Jhund PS, Docherty KF, Böhm M, Petrie MC, Bergenheim K, Qin L. Cost-effectiveness of dapagliflozin as a treatment for heart failure with reduced ejection fraction: a multinational health-economic analysis of DAPA-HF. Eur J Heart Fail. 2020. Nov;22(11):2147–2156. doi: 10.1002/ejhf.1978. Epub 2020 Sep 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Isaza N, Calvachi P, Shen C, Gavin MC, Garan AR, Bellows BK, Kazi DS. Cost-effectiveness of Dapagliflozin in Heart Failure With Reduced Ejection Fraction. Circulation. 2020;142:A15981. (abstract) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Echouffo-Tcheugui JB, Bishu KG, Fonarow GC, Egede LE. Trends in health care expenditure among US adults with heart failure: The Medical Expenditure Panel Survey 2002–2011. Am Heart J 2017;186:63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.King Jordan B, Bellows Brandon K; Shah Rashmee U; Nelson Richard E; Bress Adam P; Cost-Effectiveness of Sacubitril-Valsartan Combination Therapy Compared With Enalapril for the Treatment of Heart Failure With Reduced Ejection Fraction., JACC Heart Fail,2016. May; 4(5):392–402. [DOI] [PubMed] [Google Scholar]
- 44.Gaziano TA, Fonarow GC, Velazquez EJ, Morrow DA, Braunwald E, Solomon SD. Cost-effectiveness of Sacubitril-Valsartan in Hospitalized Patients Who Have Heart Failure With Reduced Ejection Fraction. JAMA Cardiol. 2020; 5:1236–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parizo JT, Goldhaber-Fiebert JD, Salomon JA, Khush KK, Spertus JA, Heidenreich PA, Sandhu AT. Cost-effectiveness of Dapagliflozin for Treatment of Patients With Heart Failure With Reduced Ejection Fraction. JAMA Cardiol. 2021; 6:926–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maurer MS, Schwartz JH, Gundapaneni B, et al. Tafamidis Treatment for Patients with Transthyretin Amyloid Cardiomyopathy. N Engl J Med 2018;379:1007–16. [DOI] [PubMed] [Google Scholar]
- 47.Kazi DS, Bellows BK, Baron SJ, et al. Cost-Effectiveness of Tafamidis Therapy for Transthyretin Amyloid Cardiomyopathy. Circulation 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bounthavong M, Butler J, Dolan CM, et al. Cost-Effectiveness Analysis of Patiromer and Spironolactone Therapy in Heart Failure Patients with Hyperkalemia. Pharmacoeconomics 2018;36:1463–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weir MR, Bakris GL, Bushinsky DA, et al. Patiromer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors. N Engl J Med 2015;372:211–21. [DOI] [PubMed] [Google Scholar]
- 50.Ollendorf DA, Sandhu AT, Chapman R, et al. CardioMEMS™ HF System (St. Jude Medical, Inc.) and Sacubitril/Valsartan (Entresto™, Novartis AG) for Management of Congestive Heart Failure: Effectiveness, Value, and Value-Based Price Benchmarks. Institute for Clinical and Economicc Review. December 1 2015. Available at: https://icer-review.org/wp-content/uploads/2016/01/CHF_Final_Report_120115.pdf. [Google Scholar]
- 51.Ollendorf DA, Sandhu AT, Pearson SD. Sacubitril-Valsartan for the Treatment of Heart Failure: Effectiveness and Value. JAMA Intern Med 2016;176:249–50. [DOI] [PubMed] [Google Scholar]
- 52.Bergethon KE, Wasfy JH. Increasing the Adoption and Diffusion of a Novel Pharmacological Therapy That Is Both Mortality Reducing and Cost-Effective. J Am Heart Assoc 2019;8:e011783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Resneck JS Jr. Refocusing Medication Prior Authorization on Its Intended Purpose. JAMA 2020. [DOI] [PubMed] [Google Scholar]
- 54.Gourzoulidis G, Kourlaba G, Stafylas P, Giamouzis G, Parissis J, Maniadakis N. Association between copayment, medication adherence and outcomes in the management of patients with diabetes and heart failure. Health Policy. 2017;121:363–377. [DOI] [PubMed] [Google Scholar]
- 55.Goldman DP, Joyce GF, Zheng Y. Prescription drug cost sharing: associations with medication and medical utilization and spending and health. JAMA 2007;298:61–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang TY, Kaltenbach LA, Cannon CP, et al. Effect of Medication Co-paymentVouchers on P2Y12 Inhibitor Use and Major Adverse Cardiovascular Events Among Patients With Myocardial Infarction: The ARTEMIS Randomized Clinical Trial. JAMA 2019;321:44–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Choudhry NK, Avorn J, Glynn RJ, et al. Full coverage for preventive medications after myocardial infarction. N Engl J Med 2011;365:2088–97. [DOI] [PubMed] [Google Scholar]
- 58.Sangaralingham LR, Sangaralingham SJ, Shah ND, Yao X, Dunlay SM. Adoption of Sacubitril/Valsartan for the Management of Patients With Heart Failure. Circ Heart Fail 2018;11:e004302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sandhu AT, Heidenreich PA. The Affordability of Guideline-Directed Medical Therapy: Cost Sharing is a Critical Barrier to Therapy Adoption. Circulation. 2021. Mar 16;143(11):1073–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Connolly SJ, Hallstrom AP, Cappato R, Schron EB, Kuck KH, Zipes DP, Greene HL, Boczor S, Domanski M, Follmann D, Gent M and Roberts RS. Meta-analysis of the implantable cardioverter defibrillator secondary prevention trials. AVID, CASH and CIDS studies. Antiarrhythmics vs Implantable Defibrillator study. Cardiac Arrest Study Hamburg. Canadian Implantable Defibrillator Study. Eur Heart J. 2000;21:2071–8. [DOI] [PubMed] [Google Scholar]
- 61.Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, Deal BJ, Dickfeld T, Field ME, Fonarow GC, Gillis AM, Granger CB, Hammill SC, Hlatky MA, Joglar JA, Kay GN, Matlock DD, Myerburg RJ and Page RL. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: Executive summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm. 2018;15:e190–e252. [DOI] [PubMed] [Google Scholar]
- 62.Garcia-Perez L, Pinilla-Dominguez P, Garcia-Quintana A, Caballero-Dorta E, Garcia-Garcia FJ, Linertova R and Imaz-Iglesia I. Economic evaluations of implantable cardioverter defibrillators: a systematic review. Eur J Health Econ. 2015;16:879–93. [DOI] [PubMed] [Google Scholar]
- 63.Sanders GD, Hlatky MA and Owens DK. Cost-effectiveness of implantable cardioverter-defibrillators. N Engl J Med. 2005;353:1471–80. [DOI] [PubMed] [Google Scholar]
- 64.Alcaraz A, González-Zuelgaray J and Augustovski F. [Cost effectiveness of implantable cardioverter-defibrillators for patients who are at risk for sudden death in Argentina]. Value Health. 2011;14:S33–8. [DOI] [PubMed] [Google Scholar]
- 65.Sanders GD, Kong MH, Al-Khatib SM and Peterson ED. Cost-effectiveness of implantable cardioverter defibrillators in patients >or=65 years of age. Am Heart J. 2010;160:122–31. [DOI] [PubMed] [Google Scholar]
- 66.Tomini F, Prinzen F and van Asselt AD. A review of economic evaluation models for cardiac resynchronization therapy with implantable cardioverter defibrillators in patients with heart failure. Eur J Health Econ. 2016;17:1159–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Calvert MJ, Freemantle N, Yao G, Cleland JG, Billingham L, Daubert JC, Bryan S and investigators C-H. Cost-effectiveness of cardiac resynchronization therapy: results from the CARE-HF trial. Eur Heart J. 2005;26:2681–8. [DOI] [PubMed] [Google Scholar]
- 68.Linde C, Abraham WT, Gold MR, St John Sutton M, Ghio S, Daubert C and Group RRrRiSlvdS. Randomized trial of cardiac resynchronization in mildly symptomatic heart failure patients and in asymptomatic patients with left ventricular dysfunction and previous heart failure symptoms. J Am Coll Cardiol. 2008;52:1834–1843. [DOI] [PubMed] [Google Scholar]
- 69.Moss AJ, Hall WJ, Cannom DS, Klein H, Brown MW, Daubert JP, Estes NA, Foster E, Greenberg H, Higgins SL, Pfeffer MA, Solomon SD, Wilber D, Zareba W and Investigators M-CT. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med. 2009;361:1329–38. [DOI] [PubMed] [Google Scholar]
- 70.Tang AS, Wells GA, Talajic M, Arnold MO, Sheldon R, Connolly S, Hohnloser SH, Nichol G, Birnie DH, Sapp JL, Yee R, Healey JS, Rouleau JL and Investigators R-DfAHFT. Cardiac-resynchronization therapy for mild-to-moderate heart failure. N Engl J Med. 2010;363:2385–95. [DOI] [PubMed] [Google Scholar]
- 71.Woo CY, Strandberg EJ, Schmiegelow MD, Pitt AL, Hlatky MA, Owens DK and Goldhaber-Fiebert JD. Cost-Effectiveness of Adding Cardiac Resynchronization Therapy to an Implantable Cardioverter-Defibrillator Among Patients With Mild Heart Failure. Ann Intern Med. 2015;163:417–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schmier JK, Patel JD, Leonhard MJ and Midha PA. A Systematic Review of Cost-Effectiveness Analyses of Left Ventricular Assist Devices: Issues and Challenges. Appl Health Econ Health Policy. 2019;17:35–46. [DOI] [PubMed] [Google Scholar]
- 73.Rose EA, Gelijns AC, Moskowitz AJ, Heitjan DF, Stevenson LW, Dembitsky W, Long JW, Ascheim DD, Tierney AR, Levitan RG, Watson JT, Meier P, Ronan NS, Shapiro PA, Lazar RM, Miller LW, Gupta L, Frazier OH, Desvigne-Nickens P, Oz MC, Poirier VL and Group REoMAftToCHFRS. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med. 2001;345:1435–43. [DOI] [PubMed] [Google Scholar]
- 74.Kirklin JK, Naftel DC, Pagani FD, Kormos RL, Stevenson LW, Blume ED, Miller MA, Baldwin JT, Timothy Baldwin J and Young JB. Sixth INTERMACS annual report: a 10,000-patient database. J Heart Lung Transplant. 2014;33:555–64. [DOI] [PubMed] [Google Scholar]
- 75.Slaughter MS, Rogers JG, Milano CA, Russell SD, Conte JV, Feldman D, Sun B, Tatooles AJ, Delgado RM, Long JW, Wozniak TC, Ghumman W, Farrar DJ, Frazier OH and Investigators HI. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009;361:2241–51. [DOI] [PubMed] [Google Scholar]
- 76.Pagani FD, Miller LW, Russell SD, Aaronson KD, John R, Boyle AJ, Conte JV, Bogaev RC, MacGillivray TE, Naka Y, Mancini D, Massey HT, Chen L, Klodell CT, Aranda JM, Moazami N, Ewald GA, Farrar DJ, Frazier OH and Investigators HI. Extended mechanical circulatory support with a continuous-flow rotary left ventricular assist device. J Am Coll Cardiol. 2009;54:312–21. [DOI] [PubMed] [Google Scholar]
- 77.Mehra MR, Uriel N, Naka Y, Cleveland JC, Yuzefpolskaya M, Salerno CT, Walsh MN, Milano CA, Patel CB, Hutchins SW, Ransom J, Ewald GA, Itoh A, Raval NY, Silvestry SC, Cogswell R, John R, Bhimaraj A, Bruckner BA, Lowes BD, Um JY, Jeevanandam V, Sayer G, Mangi AA, Molina EJ, Sheikh F, Aaronson K, Pagani FD, Cotts WG, Tatooles AJ, Babu A, Chomsky D, Katz JN, Tessmann PB, Dean D, Krishnamoorthy A, Chuang J, Topuria I, Sood P, Goldstein DJ and Investigators M. A Fully Magnetically Levitated Left Ventricular Assist Device - Final Report. N Engl J Med. 2019;380:1618–1627. [DOI] [PubMed] [Google Scholar]
- 78.Clarke A, Pulikottil-Jacob R, Connock M, Suri G, Kandala NB, Maheswaran H, Banner NR and Sutcliffe P. Cost-effectiveness of left ventricular assist devices (LVADs) for patients with advanced heart failure: analysis of the British NHS bridge to transplant (BTT) program. Int J Cardiol 2014;171:338–45. [DOI] [PubMed] [Google Scholar]
- 79.Sutcliffe P, Connock M, Pulikottil-Jacob R, Kandala NB, Suri G, Gurung T, Grove A, Shyangdan D, Briscoe S, Maheswaran H and Clarke A. Clinical effectiveness and cost-effectiveness of second- and third-generation left ventricular assist devices as either bridge to transplant or alternative to transplant for adults eligible for heart transplantation: systematic review and cost-effectiveness model. Health Technol Assess. 2013;17:1–499, v-vi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sharples LD, Dyer M, Cafferty F, Demiris N, Freeman C, Banner NR, Large SR, Tsui S, Caine N and Buxton M. Cost-effectiveness of ventricular assist device use in the United Kingdom: results from the evaluation of ventricular assist device programme in the UK (EVAD-UK). J Heart Lung Transplant. 2006;25:1336–43. [DOI] [PubMed] [Google Scholar]
- 81.Moreno SG, Novielli N and Cooper NJ. Cost-effectiveness of the implantable HeartMate II left ventricular assist device for patients awaiting heart transplantation. J Heart Lung Transplant. 2012;31:450–8. [DOI] [PubMed] [Google Scholar]
- 82.Long EF, Swain GW and Mangi AA. Comparative survival and cost-effectiveness of advanced therapies for end-stage heart failure. Circ Heart Fail. 2014;7:470–8. [DOI] [PubMed] [Google Scholar]
- 83.Baras Shreibati J, Goldhaber-Fiebert JD, Banerjee D, Owens DK and Hlatky MA. Cost-Effectiveness of Left Ventricular Assist Devices in Ambulatory Patients With Advanced Heart Failure. JACC Heart Fail. 2017;5:110–119. [DOI] [PubMed] [Google Scholar]
- 84.Mahr C, McGee E Jr, Cheung A, Mokadam NA, Strueber M, Slaughter MS, Danter MR, Levy WC, Cheng RK, Beckman JA, May DM, Ismyrloglou E, Tsintzos SI, Silvestry SC. Cost-Effectiveness of Thoracotomy Approach for the Implantation of a Centrifugal Left Ventricular Assist Device. ASAIO J 2020. 66:855–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Silvestry SC, Mahr C, Slaughter MS, Levy WC, Cheng RK, May DM, Ismyrloglou E, Tsintzos SI, Tuttle E, Cook K, Birk E, Gomes A, Graham S, Cotts WG. Cost- Effectiveness of a Small Intrapericardial Centrifugal Left Ventricular Assist Device. ASAIO J 2020. 66:862–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Long EF, Swain GW, Mangi AA. Comparative survival and cost-effectiveness of advanced therapies for end-stage heart failure. Circ Heart Fail. 2014;7:470–8. [DOI] [PubMed] [Google Scholar]
- 87.Stone GW, Lindenfeld J, Abraham WT, Kar S, Lim DS, Mishell JM, Whisenant B, Grayburn PA, Rinaldi M, Kapadia SR, Rajagopal V, Sarembock IJ, Brieke A, Marx SO, Cohen DJ, Weissman NJ, Mack MJ; COAPT Investigators. Transcatheter Mitral- Valve Repair in Patients with Heart Failure. N Engl J Med. 2018; 379:2307–2318. [DOI] [PubMed] [Google Scholar]
- 88.Baron SJ, Wang K, Arnold SV, Magnuson EA, Whisenant B, Brieke A, Rinaldi M, Asgar AW, Lindenfeld J, Abraham WT, Mack MJ, Stone GW, Cohen DJ; COAPT Investigators. Cost-Effectiveness of Transcatheter Mitral Valve Repair Versus Medical Therapy in Patients With Heart Failure and Secondary Mitral Regurgitation: Results From the COAPT Trial. Circulation. 2019; 140:1881–1891. [DOI] [PubMed] [Google Scholar]
- 89.Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP 3rd, Gentile F, Jneid H, Krieger EV, Mack M, McLeod C, O’Gara PT, Rigolin VH, Sundt TM 3rd, Thompson A, Toly C. 2020. ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2021 Feb 2;77(4):450–500. [DOI] [PubMed] [Google Scholar]
- 90.Obadia JF, Messika-Zeitoun D, Leurent G, Iung B, Bonnet G, Piriou N, Lefèvre T, Piot C, Rouleau F, Carrié D, Nejjari M, Ohlmann P, Leclercq F, Saint Etienne C, Teiger E, Leroux L, Karam N, Michel N, Gilard M, Donal E, Trochu JN, Cormier B, Armoiry X, Boutitie F, Maucort-Boulch D, Barnel C, Samson G, Guerin P, Vahanian A, Mewton N; MITRA-FR Investigators. Percutaneous Repair or Medical Treatment for Secondary Mitral Regurgitation. N Engl J Med. 2018; 379:2297–2306. [DOI] [PubMed] [Google Scholar]
- 91.Mueller C, Laule-Kilian K, Schindler C, et al. Cost-effectiveness of B-type natriuretic peptide testing in patients with acute dyspnea. Arch Intern Med. 2006;166(10):1081–1087. [DOI] [PubMed] [Google Scholar]
- 92.McLellan J, Heneghan CJ, Perera R, et al. B-type natriuretic peptide-guided treatment for heart failure. Cochrane Database Syst Rev. 2016;12:CD008966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mark DB, Cowper PA, Anstrom KJ, et al. Economic and Quality-of-Life Outcomes of Natriuretic Peptide-Guided Therapy for Heart Failure. J Am Coll Cardiol. 2018;72(21):2551–2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Heidenreich PA, Gubens MA, Fonarow GC, Konstam MA, Stevenson LW, Shekelle PG. Cost-effectiveness of screening with B-type natriuretic peptide to identify patients with reduced left ventricular ejection fraction. J Am Coll Cardiol. 2004;43(6):1019–1026. [DOI] [PubMed] [Google Scholar]
- 95.Givertz MM, Stevenson LW, Costanzo MR, et al. Pulmonary Artery Pressure-Guided Management of Patients With Heart Failure and Reduced Ejection Fraction. J Am Coll Cardiol. 2017;70(15):1875–1886. [DOI] [PubMed] [Google Scholar]
- 96.Sandhu AT, Goldhaber-Fiebert JD, Owens DK, Turakhia MP, Kaiser DW, Heidenreich PA. Cost-Effectiveness of Implantable Pulmonary Artery Pressure Monitoring in Chronic Heart Failure. JACC Heart Fail. 2016;4(5):368–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schmier JK, Ong KL, Fonarow GC. Cost-Effectiveness of Remote Cardiac Monitoring With the CardioMEMS Heart Failure System. Clin Cardiol. 2017;40:430–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Martinson M, Bharmi R, Dalal N, Abraham WT, Adamson PB. Pulmonary artery pressure-guided heart failure management: US cost-effectiveness analyses using the results of the CHAMPION clinical trial. Eur J Heart Fail. 2017;19:652–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lindenfeld J, Zile MR, Desai AS, Bhatt K, Ducharme A, Horstmanshof D, Krim SR, Maisel A, Mehra MR, Paul S, Sears SF, Sauer AJ, Smart F, Zughaib M, Castaneda P, Kelly J, Johnson N, Sood P, Ginn G, Henderson J, Adamson PB Costanzo MR. Haemodynamic-guided management of heart failure (GUIDE-HF): randomised controlled trial. Lancet. 2021;398:991–1001. [DOI] [PubMed] [Google Scholar]
- 100.Ostrominski JW, Hirji S, Bhatt AS, Butler J, Fiuzat M, Fonarow GC, Heidenreich PA, Januzzi JL, Lam CS Maddox TM, O’Connor CM, Vaduganathan M, Cost and Value in Contemporary Heart Failure Clinical Guidance Documents. J Am Coll Cardiol 2021. In Press. [DOI] [PubMed] [Google Scholar]


