Abstract
Background:
Coronary artery disease (CAD) is the most common cause of new-onset heart failure (HF). Although guidelines recommend ischemic evaluation in this population, testing has historically been underutilized.
Objectives:
This study aimed to identify contemporary trends in CAD testing for new-onset HF patients, particularly after publication of the Surgical Treatment for Ischemic Heart Failure Extension Study (STICHES), and to characterize geographic and clinician-level variability in testing patterns.
Methods:
We determined the proportion of incident HF patients who received CAD testing from 2004–2019 using an administrative claims database covering commercial insurance and Medicare. We identified demographic and clinical predictors of CAD testing during the 90 days before and after initial diagnosis. Patients were grouped by their county of residence to assess national variation. Patients were then linked to their primary care physician and/or cardiologist to evaluate variation across clinicians.
Results:
Among 558,322 new-onset HF patients, 34.8% underwent CAD testing and 9.3% underwent revascularization. After multivariable adjustment, patients who underwent CAD testing were more likely to be younger, male, diagnosed in an acute care setting, and have systolic dysfunction or recent cardiogenic shock. Incidence of CAD testing remained flat without significant change post-STICHES. Covariate-adjusted testing rates varied from 20–45% across counties. The likelihood of testing was higher among patients co-managed by a cardiologist (adjusted OR 5.12, 95% CI 4.98–5.27) but varied substantially across cardiologists (IQR: 50.9–62.4%).
Conclusions:
Most new-onset HF patients across inpatient and outpatient settings did not receive timely testing for CAD. Substantial variability in testing persists across regions and clinicians.
Keywords: Ischemic CAD, Invasive, Stress testing, Revascularization, Disparity, STICHES
CONDENSED ABSTRACT
Coronary artery disease (CAD) testing in patients with new-onset heart failure (HF) has historically been underutilized. The present study leverages a large administrative claims database to determine contemporary trends in CAD testing between inpatient and outpatient settings, across geographies, and among clinicians. Our results suggest that rates of CAD testing in new-onset HF patients remain low, even after publication of the Surgical Treatment for Ischemic Heart Failure Extension Study in 2016. Most new-onset HF patients across inpatient and outpatient settings did not receive timely testing for CAD. Substantial variability in testing rates persists across clinicians and geographic regions.
INTRODUCTION
The burden of heart failure (HF) in the United States remains high, as rates of hospitalization and mortality have risen over the past decade (1, 2). More than a million patients are diagnosed with new-onset HF every year, and mortality within the first few years exceeds 20 to 40% despite remarkable advances in medical therapy (3). The prevalence of CAD is 60 to 70% in patients with HF with reduced ejection fraction (HFrEF) and approximately 50% in patients with HF with preserved ejection fraction (HFpEF) (4, 5). Since CAD is a common and treatable cause of HF, early identification is an important part of disease management.
In 2013, the American College of Cardiology (ACC) and American Heart Association (AHA) recommended consideration of non-invasive imaging to evaluate for myocardial ischemia or coronary angiography among incident HF patients eligible for revascularization (6). The importance of testing for an ischemic etiology was underscored by the Surgical Treatment for Ischemic Heart Failure (STICH) trial with results from a long-term follow-up study (STICHES) published in 2016 (7). STICHES demonstrated a significant cardiovascular mortality benefit among patients who underwent revascularization via coronary artery bypass graft surgery (8).
Prior analyses have identified the underutilization of testing for CAD in hospitalized patients with incident HF (9–11). However, there are three important gaps in the literature. First, these studies have exclusively focused on the inpatient population, while about half of incident HF is diagnosed in the outpatient setting (12, 13). Second, these studies evaluated CAD testing prior to the publication of STICHES. Finally, a better understanding of the variation in CAD testing may highlight opportunities for improvement. In this large observational study, we aimed to identify the demographic and clinical factors associated with CAD testing among incident HF patients across care settings, as well as temporal, geographic, and clinician-level testing variability.
METHODS
Data Source
Analyses were conducted using the Optum de-identified Clinformatics® DataMart (Optum, Eden Prairie, MN), a database comprising administrative health claims for members of commercial and Medicare Advantage plans across all 50 states. The DataMart included medical and pharmacy claims, enrollment information, inpatient data, and clinician characteristics. Data from approximately 87 million unique individuals were obtained between 2003 and 2020. Data access requests are to be sent to Optum; statistical code will be made available upon request.
Study Population
We identified a cohort of patients aged 18 years or older with an incident HF diagnosis based on the International Classification of Diseases (ICD)-9 and −10 codes (Supplemental Table 1). We defined incident HF as the absence of previous HF diagnoses during a 12-month lookback period with continuous health plan enrollment. Continuous enrollment was necessary to ensure minimal data missingness when determining incident versus prevalent HF and baseline patient characteristics. Similar approaches have been used to define incident HF; shorter lookback periods overestimated HF incidence, whereas longer periods reduced misclassification at the expense of sample size (14–16). To further minimize diagnostic inaccuracy, we restricted our analysis to patients with a HF diagnosis associated with a clinician evaluation and management service or an inpatient admission and required a second HF diagnosis within one year of initial diagnosis (14, 15, 17). For all patients in the database with multiple HF diagnoses, the median and maximum times between consecutive diagnoses were under 12 months for 99% and 70% of patients respectively, consistent with other studies (17, 18).
There were several additional exclusion criteria. First, we excluded patients with documented CAD from 12 months to 3 months before the index date since they might not require a repeat evaluation. Second, we excluded patients on dialysis given the potential inaccuracy of incident HF diagnoses among dialysis-dependent patients. Finally, we excluded patients without at least 90 days of post-index continuous enrollment for outcome ascertainment.
Study Variables
We extracted patient-level demographic and clinical characteristics. Demographic variables included age, sex, race and ethnicity, and geographic region. Race and ethnicity were grouped into mutually exclusive categories: Asian, Black, Hispanic, and White. Geographic region was initially classified using the US Census Bureau divisions and sorted into regions: Midwest, Northeast, South, and West. We determined whether patients received acute care in the emergency department on the day of their first diagnosis or were admitted with HF as a principal or secondary diagnosis within a month. Baseline clinical characteristics were identified from 12 months before to 1 month after the index date using ICD-9 and −10 diagnosis codes (Supplemental Table 1). We also used the presence of any systolic dysfunction code during the lookback period to indicate possible reduced or mid-range EF, which demonstrated a 77% positive predictive value for EF <50% in the Veteran’s Affairs health system (19).
The database used encrypted identifiers for clinicians that represented either individual clinicians or group practices. We linked patients with a new HF diagnosis to a primary care physician (PCP)—internal medicine, family medicine, or general practice—and/or cardiologist. Patients managed by both a PCP and a cardiologist had both clinicians attributed to them. When patients were seen by multiple different PCPs or cardiologists, we attributed the patient to the clinician with the most encounters during the follow-up period. Any remaining ties were resolved using random selection.
The primary outcome was the occurrence of CAD testing. CAD testing was defined as the performance of any of the following during the 90 days before or after the index date: exercise stress test without imaging, stress echocardiogram, nuclear stress imaging, cardiac magnetic resonance imaging, coronary computed tomography angiography, and coronary angiography. Revascularization with percutaneous coronary intervention and coronary artery bypass grafting were also evaluated. Procedures were identified by the presence of relevant ICD-9 and −10 procedural codes and Current Procedure Terminology codes (Supplemental Table 2).
Statistical Analyses
We described baseline patient demographic characteristics and medical comorbidities by the presence of CAD testing using proportions for categorical variables and means with standard deviations for continuous variables. Traditional significance testing is sensitive to large sample sizes, so standardized differences in proportions and means between testing groups were calculated (20). A standardized difference of ≥10% was considered statistically significant (21).
A multivariable logistic regression model was constructed to compute the covariate-adjusted proportion of incident HF patients who were tested for CAD. All baseline characteristics were incorporated in the model. Multilevel categorical covariates with missing data were assigned to a separate “Unknown” category. Other variable transformations included age categorization (< 40, 40–64, 65–79, ≥ 80) and restricted cubic splines with five knots for the index date. Using our fitted model, we calculated the adjusted odds ratios (AOR) and marginal probabilities with covariates conditioned at their means.
To determine the temporal trend in CAD testing rates, our analysis included incident HF patients from 2004 to 2019. We performed three analyses. First, we modeled the index date as a linear continuous variable to determine the annual change in the odds. Second, we used indicator variables for each calendar quarter to model variability in testing across three-month windows. Third, we tested for any impact of the STICHES trial on CAD testing. We performed an interrupted time series analysis using an indicator variable for the STICHES publication on April 21, 2016, and a variable for time after STICHES (slope and level change). In each analysis, we adjusted for patient-level characteristics.
For geographic variation in CAD testing, we mapped five-digit ZIP codes to each patient’s corresponding county and state. Patients in the District of Columbia, Puerto Rico, the Island Areas, and placeholder or legacy ZIP codes were excluded. We created a mixed-effects model with county as a random effect nested under state. We estimated the national variation in testing rates adjusting for underlying patient-level differences. Using open-access data from the Centers for Disease Control and Prevention (CDC), we also obtained the rates of hospitalization for myocardial infarction as a measure of each county’s atherosclerotic cardiovascular disease (ASCVD) burden. A US map was generated using a bivariate color scheme to compare model-estimated testing rates with ASCVD burden.
To evaluate clinician variation, we assigned random identifiers or intercepts for all physicians. We excluded physicians with fewer than 10 new HF diagnoses over the study period to efficiently generate estimates of the intercept variance (22). We also excluded geographic region from the analysis. We created two mixed-effects models to separately predict the variation among PCPs and cardiologists. The primary care model included all patients with an attributed PCP and a variable indicating whether they were also seen by a cardiologist. The cardiology model included all patients with an attributed cardiologist.
We performed several sensitivity analyses. First, we implemented a 24-month lookback requirement. Second, we repeated our analyses on patients with a history of CAD prior to the follow-up period. Third, we limited the cohort to patients with incident acute decompensated inpatient HF by limiting to HF as the primary diagnosis or a HF diagnosis-related group. Finally, we created two scenarios with modified follow-up periods. In the first scenario, we restricted follow-up from index to 90 days post-index, excluding patients tested only in the 3 months preceding their initial HF diagnosis. Second, we extended follow-up to 180 days post-index to account for delayed testing.
All analyses were conducted using R software version 4.0.2 (R Core Team, Vienna, Austria) with Hmisc (v4.4–1), lme4 (v1.1–23), and ggeffects (v0.16.0) packages (23–25). Data access for this project was provided by the Stanford Center for Population Health Sciences Data Core. This study was approved by the Stanford Institutional Review Board.
RESULTS
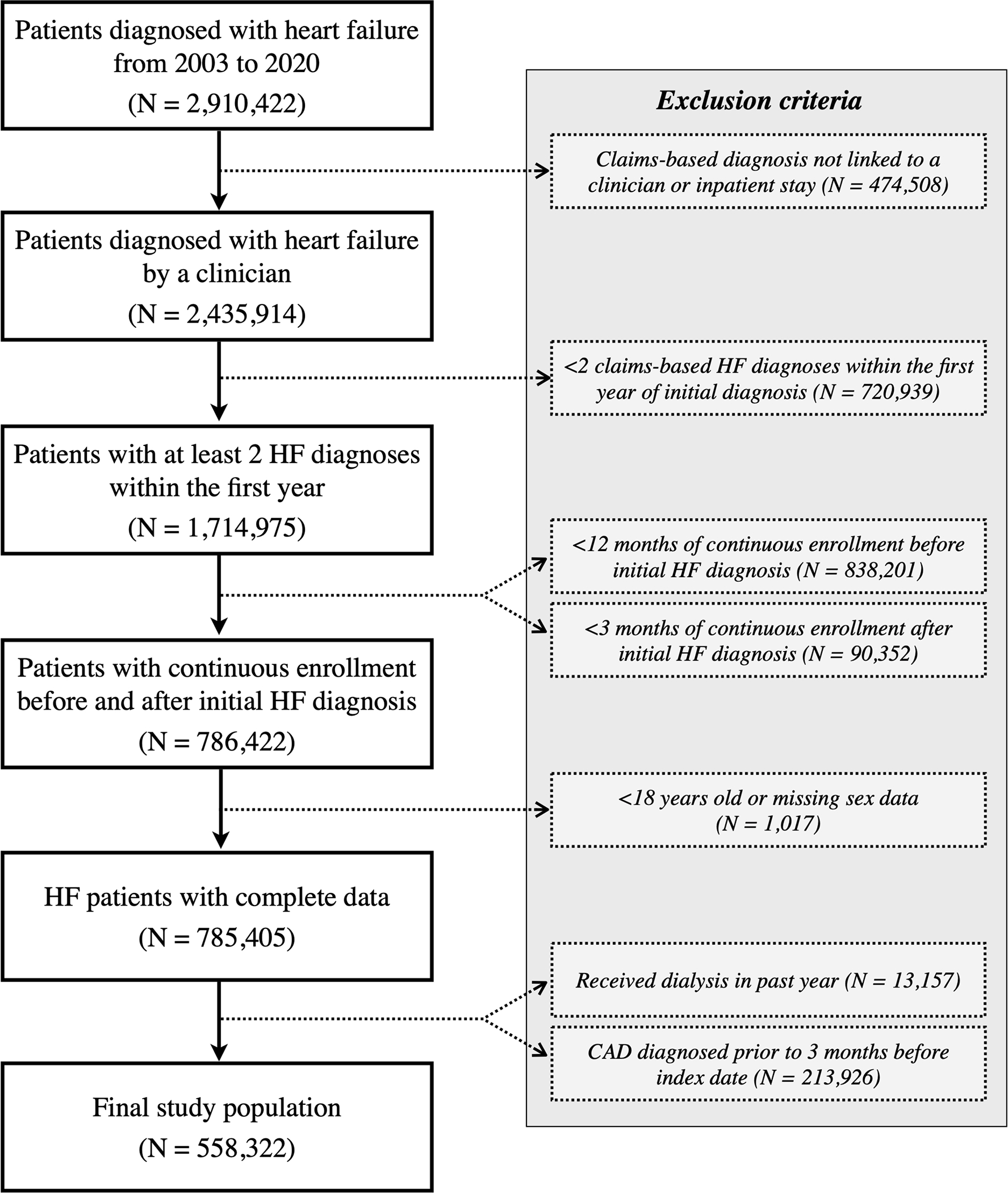
We identified 2,910,422 patients with incident HF from 2003 to 2020 (Figure 1). To minimize diagnostic misclassification and data missingness, we excluded 474,508 (16.3%) patients whose claims-based diagnoses could not be linked to an evaluation and management code or inpatient hospitalization, 720,939 (24.8%) who did not have a second HF diagnosis within one year, and 928,553 (31.9%) who did not have continuous enrollment from the 365 days before to the 90 days after the initial HF diagnosis. Patients who were <18 years of age or had missing sex data were also excluded. There were 785,405 patients with incident heart failure remaining, of which 13,157 (1.7%) were on dialysis and 213,926 (27.2%) had a history of CAD. The final study population included a total of 558,322 patients.
Figure 1. Overview of Cohort Inclusion and Exclusion Criteria.
From the Optum Clinformatics® DataMart containing around 87 million unique patient records, a final cohort of 558,322 incident heart failure patients is included in the study. Patients with continuous enrollment have uninterrupted data collection from 12 months before to 3 months after initial HF diagnosis.
Among the final analytic cohort, 194,214 (34.8%) underwent CAD testing during the 90 days before and after their first HF diagnosis. 121,418 (21.7%) patients were tested for CAD using noninvasive modalities, such as exercise stress testing, nuclear stress imaging, cardiac magnetic resonance imaging, and coronary computed tomography angiography. Coronary angiography was performed in 115,408 (20.7%) patients. Furthermore, 52,153 (9.3%) patients underwent revascularization (Supplemental Figure 1). Baseline demographic and clinical characteristics of the full cohort are shown in Table 1. Based on standardized mean differences, patients who received testing were more likely to be younger, male, have an emergency department visit or inpatient stay within the first month of diagnosis, have systolic dysfunction, and carry cardiovascular risk factors such as hyperlipidemia, obesity, and history of smoking. Negative predictors for CAD testing included a history of dementia and psychotic disorder.
Table 1.
Baseline Patient Demographic and Medical Characteristics by CAD Testing
|
|
||||
|---|---|---|---|---|
| Overall N = 558,322 | CAD testing N = 194,214 | No CAD testing N = 364,108 | Standardized Difference | |
|
| ||||
| Age, mean (SD), yr | 71.7 (11.7) | 68.5 (11.8) | 73.4 (11.3) | 41.9 |
| Female sex, % | 55.5 | 47.2 | 59.9 | 25.6 |
| Race and ethnicity, % | 4.2 | |||
| White | 66.4 | 67.2 | 66.0 | |
| Asian | 2.2 | 2.2 | 2.2 | |
| Black | 12.2 | 12.4 | 12.0 | |
| Hispanic | 9.3 | 8.8 | 9.7 | |
| Unknown | 9.9 | 9.4 | 10.1 | |
| Geographic region, % | 19.8 | |||
| Northeast | 11.0 | 10.9 | 11.0 | |
| Midwest | 22.3 | 24.6 | 21.2 | |
| South | 39.0 | 42.3 | 37.2 | |
| West | 27.6 | 22.1 | 30.5 | |
| Unknown | 0.1 | 0.1 | 0.1 | |
| HF diagnosis identified in ED/inpatient claims a , % | 58.3 | 67.7 | 53.3 | 29.7 |
| Clinical characteristics, % | ||||
| Atrial fibrillation/flutter | 34.5 | 34.6 | 34.5 | 0.3 |
| Alcohol use disorder | 4.3 | 4.9 | 4.0 | 4.0 |
| Cancer | 18.9 | 17.5 | 19.6 | 5.4 |
| Cardiogenic shock | 2.3 | 4.6 | 1.0 | 21.8 |
| Chronic kidney disease | 29.9 | 26.3 | 31.8 | 12.1 |
| Chronic liver disease | 8.0 | 8.3 | 7.8 | 2.0 |
| COPD or asthma | 35.8 | 34.6 | 36.5 | 3.9 |
| Dementia | 10.0 | 3.8 | 13.2 | 34.1 |
| Depression | 20.5 | 18.0 | 21.9 | 9.8 |
| Diabetes | 40.4 | 42.2 | 39.5 | 5.4 |
| Endocarditis | 1.8 | 2.4 | 1.5 | 6.3 |
| Hyperlipidemia | 66.1 | 74.0 | 61.8 | 26.5 |
| Hypertension | 88.1 | 88.7 | 87.8 | 3.0 |
| Myocarditis | 0.3 | 0.6 | 0.1 | 8.0 |
| Noncoronary atherosclerosis | 29.6 | 29.8 | 29.5 | 0.7 |
| Obesity | 24.9 | 28.3 | 23.1 | 11.8 |
| Prior history of CVA/TIA | 23.7 | 24.8 | 23.2 | 3.9 |
| Psychotic disorder | 4.4 | 2.6 | 5.4 | 14.3 |
| Sleep apnea | 14.1 | 16.2 | 13.0 | 9.2 |
| Systolic dysfunction | 25.4 | 18.1 | 39.1 | 47.6 |
| Tobacco use disorder | 27.3 | 33.9 | 23.8 | 22.3 |
| Valvular disorder | 39.7 | 50.0 | 34.2 | 32.4 |
| Ventricular arrhythmia | 8.1 | 13.2 | 5.3 | 27.4 |
Abbreviation: CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident; ED, emergency department; HF, heart failure; TIA, transient ischemic attack; SD, standard deviation
Includes outpatient or ED visits on the index date and inpatient stays within 1 month
In a multivariable logistic regression model adjusted for patient characteristics, several patient-level factors were associated with CAD testing (Central Illustration). Patients aged 40 to 64 years had greater odds of testing (AOR 1.54, 95% confidence interval [CI] 1.47–1.61), while patients 80 or older were less likely to be tested for CAD (AOR 0.51, 95% CI 0.48–0.53). Furthermore, we found evidence of sex and race disparities after covariate adjustment; women were significantly less likely to receive testing compared with men (AOR 0.74, 95% CI 0.73–0.75). Black patients also had lower odds of testing compared with White patients (AOR 0.90, 95% CI 0.88–0.92). Asian patients were more likely to be tested than White patients (AOR 1.06, 95% CI 1.01–1.10). Patients who were seen in an emergency department on the index date or hospitalized within 1 month of their first HF diagnosis had increased AOR of 1.43 (95% CI 1.42–1.45).
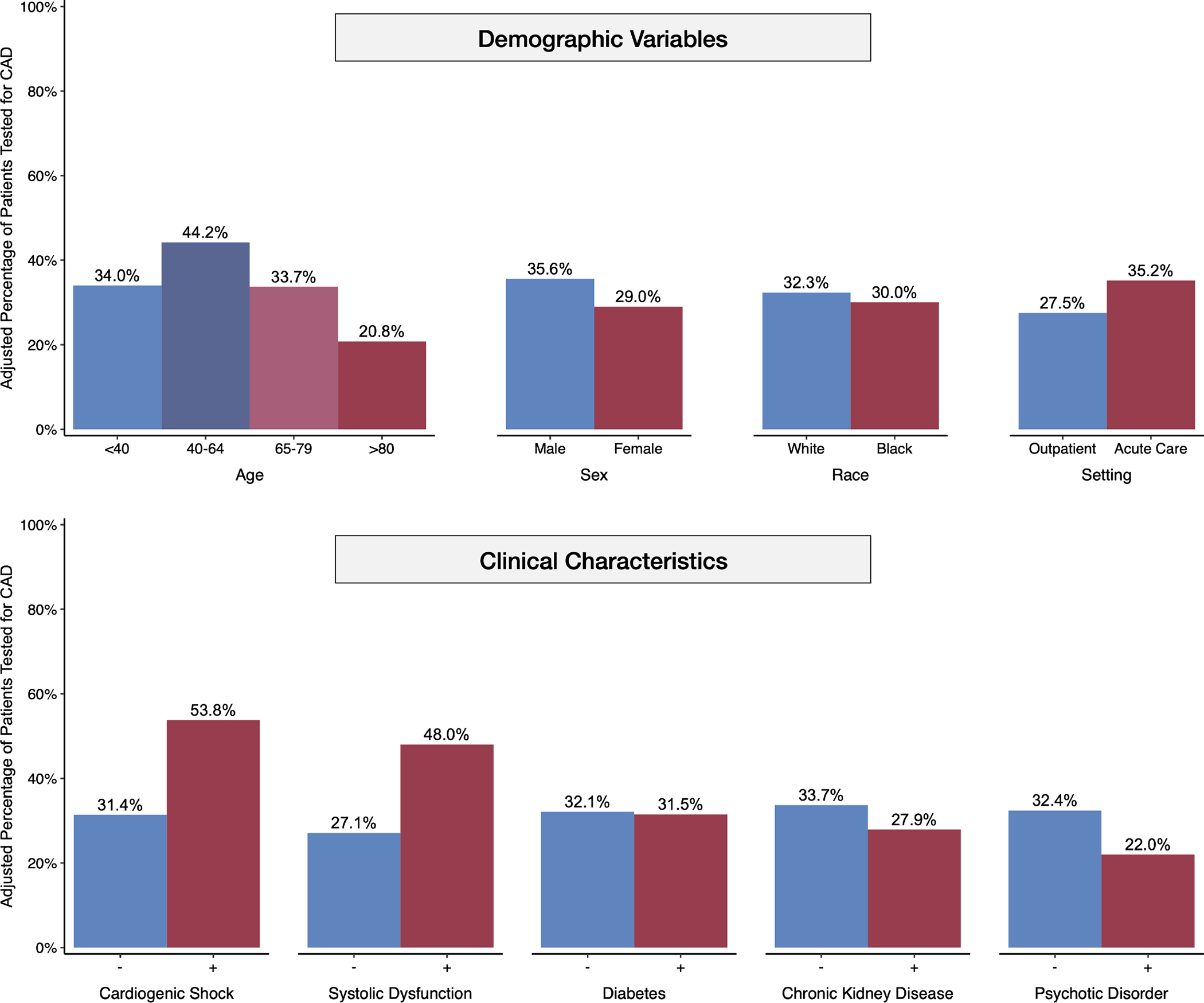
Central Illustration. Adjusted Rates of CAD Testing across Demographic and Clinical Characteristics.
Covariate-adjusted testing rates for CAD are higher for patients of ages 40 to 64, male sex, and White race. Patients seen in an acute care setting around the time of initial diagnosis and those with a history of cardiogenic shock and systolic dysfunction are more likely to undergo testing. CAD testing is underutilized for patients diagnosed only in the outpatient setting and those with a history of diabetes, chronic kidney disease, and severe mental illness.
141,929 (25.4%) patients had a systolic dysfunction code during the 12-month lookback period. These patients had significantly greater odds (AOR 2.49, 95% CI 2.46–2.53) and higher rates (48.0%, 95% CI 47.7–48.3%) of CAD testing compared with those without a systolic dysfunction code (27.5%, 95% CI 27.3–27.7%). Other cardiovascular conditions associated with a higher likelihood of testing included cardiogenic shock diagnosed one week before to one month after the initial HF diagnosis (AOR 2.55, 95% CI 2.44–2.66), myocarditis (AOR 2.79, 95% CI 2.49–3.12), ventricular arrhythmia (AOR 1.92, 95% CI 1.88–1.96), valvular disorders (AOR 1.79, 95% CI 1.76–1.81), and hyperlipidemia (AOR 1.80, 95% CI 1.78–1.83). Weak associations were found in several traditional cardiovascular risk factors, such as noncoronary atherosclerosis, hypertension, obesity, diabetes, and sleep apnea (Table 2). Disproportionately low rates of CAD testing were observed in patients with a history of psychotic disorder (22.0%, 95% CI 21.4–22.6) and dementia (16.3%, 95% CI 16.0–16.7). Other negative predictors included chronic kidney disease, chronic obstructive pulmonary disease, depression, and alcohol use disorder.
Table 2.
Patient Demographic and Clinical Characteristics Associated with CAD Testing
| Demographic Variables | Marginal Probability (95% CI)a | Adjusted OR (95% CI) | Clinical Variables | Marginal Probability (95% CI)a | Adjusted OR (95% CI) |
|---|---|---|---|---|---|
|
| |||||
| Age Group | Heart Failure Type b | ||||
| < 40 | 34.0 (33.0–35.0) | Reference | No systolic dysfunction | 27.1 (26.9–27.2) | Reference |
| 40 – 64 | 44.2 (43.9–44.6) | 1.54 (1.47–1.61) | Systolic dysfunction | 48.0 (47.7–48.3) | 2.49 (2.46–2.53) |
| 65 – 79 | 33.7 (33.5–33.9) | 0.99 (0.94–1.03) | |||
| ≥ 80 | 20.8 (20.5–21.0) | 0.51 (0.48–0.53) | Cardiovascular Conditions | ||
| Atrial fibrillation/flutter | 30.2 (29.9–30.4) | 0.89 (0.87–0.90) | |||
| Sex | Cardiogenic shock | 53.8 (52.8–54.9) | 2.55 (2.44–2.66) | ||
| Male | 35.6 (35.4–35.8) | Reference | Diabetes | 31.5 (31.3–31.8) | 0.97 (0.96–0.99) |
| Female | 29.0 (28.9–29.2) | 0.74 (0.73–0.75) | Endocarditis | 35.2 (34.2–36.2) | 1.17 (1.12–1.22) |
| Hyperlipidemia | 36.4 (36.2–36.5) | 1.80 (1.78–1.83) | |||
| Race and ethnicity | Hypertension | 32.1 (31.9–32.2) | 1.08 (1.06–1.11) | ||
| White | 32.3 (32.1–32.4) | Reference | Myocarditis | 56.5 (53.7–59.3) | 2.79 (2.49–3.12) |
| Asian | 33.5 (32.6–34.4) | 1.06 (1.01–1.10) | Noncoronary atherosclerosis | 33.8 (33.6–34.1) | 1.13 (1.12–1.15) |
| Black | 30.0 (29.6–30.4) | 0.90 (0.88–0.92) | Obesity | 33.0 (32.7–33.3) | 1.07 (1.05–1.09) |
| Hispanic | 32.2 (31.7–32.6) | 0.99 (0.97–1.02) | Prior history of CVA/TIA | 33.9 (33.6–34.2) | 1.13 (1.11–1.15) |
| Unknown | 30.9 (30.4–31.3) | 0.94 (0.92–0.96) | Valvular disorder | 39.9 (39.7–40.1) | 1.79 (1.76–1.81) |
| Ventricular arrhythmia | 46.0 (45.5–46.5) | 1.92 (1.88–1.96) | |||
| Geographic Region | |||||
| Northeast | 31.9 (31.5–32.3) | Reference | Other Conditions | ||
| Midwest | 33.7 (33.4–34.0) | 1.08 (1.06–1.11) | Alcohol use disorder | 27.9 (27.3–28.5) | 0.82 (0.80–0.85) |
| South | 33.3 (33.1–33.5) | 1.06 (1.04–1.09) | Cancer | 29.9 (29.6–30.2) | 0.89 (0.88–0.91) |
| West | 28.5 (28.2–28.7) | 0.85 (0.83–0.87) | Chronic kidney disease | 27.9 (27.6–28.1) | 0.76 (0.75–0.77) |
| Unknown | 32.3 (28.4–36.4) | 1.02 (0.84–1.22) | Chronic liver disease | 29.7 (29.2–30.1) | 0.89 (0.87–0.91) |
| COPD or asthma | 29.2 (28.9–29.4) | 0.82 (0.81–0.83) | |||
| Care Setting – 1st Month after Index Diagnosis c | Dementia | 16.3 (16.0–16.7) | 0.38 (0.37–0.39) | ||
| Depression | 29.3 (29.0–29.6) | 0.86 (0.85–0.87) | |||
| Outpatient only | 27.5 (27.3–27.7) | Reference | Psychotic disorder | 22.0 (21.4–22.6) | 0.59 (0.57–0.61) |
| ED / Inpatient | 35.2 (35.0–35.4) | 1.43 (1.42–1.45) | Sleep apnea | 32.1 (31.7–32.4) | 1.01 (0.99–1.03) |
| Tobacco use disorder | 36.4 (36.1–36.6) | 1.32 (1.30–1.34) | |||
Abbreviation: CI, confidence interval; EF, ejection fraction; OR, odds ratio
Predicted probability of CAD testing adjusted based on mean values for continuous variables and proportions for binary variables
Based on the presence of any systolic dysfunction code over a 12-month lookback period
Includes outpatient or ED visits on the index date and inpatient stays within 1 month
Temporal Variation
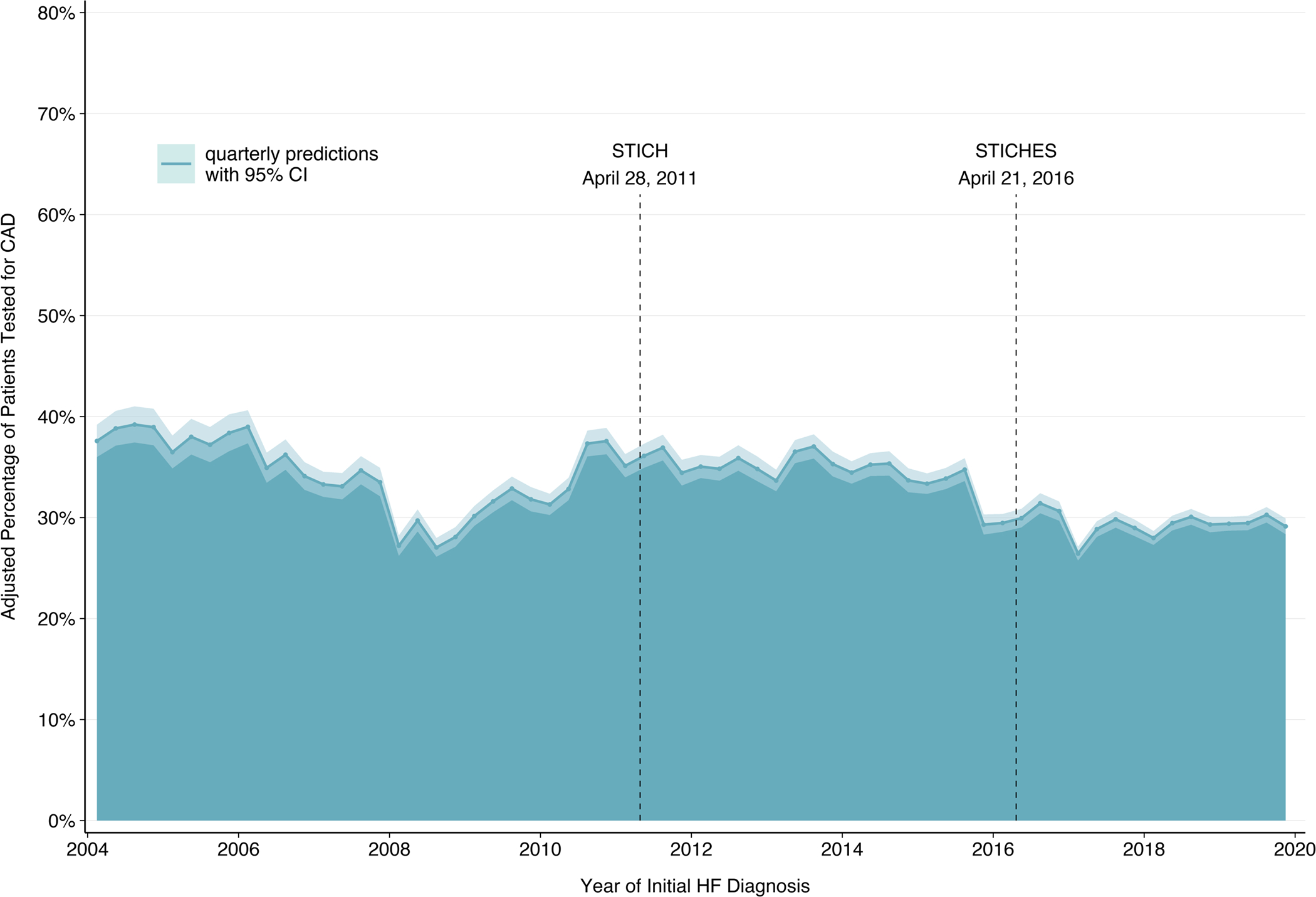
Covariate-adjusted testing rates ranged from 26.4% to 39.2% (Figure 2) throughout the study period. On average, the annual change in the odds of CAD testing from 2004 to 2019 was slightly negative (AOR 0.98, 95% CI 0.98–0.98). An interrupted time series analysis demonstrated minimal slope change in CAD testing from before to after STICHES publication on April 21, 2016 (AOR 0.99, 95% CI 0.99–1.00, to 1.01, 95% CI 1.00–1.02). A significant negative level change was observed post-STICHES (AOR 0.84, 95% CI 0.81–0.86). This trend was similar for the subset of patients with systolic dysfunction.
Figure 2. Variability in CAD Testing Rates Over Time.
Quarterly CAD testing rates for incident heart failure patients, adjusted for patient-level characteristics, vary from 26.4% to 39.2% during the study period. The ribbon width represents the 95% confidence intervals for each three-month period. Vertical markers for STICH and STICHES publications are shown.
Geographic Variation
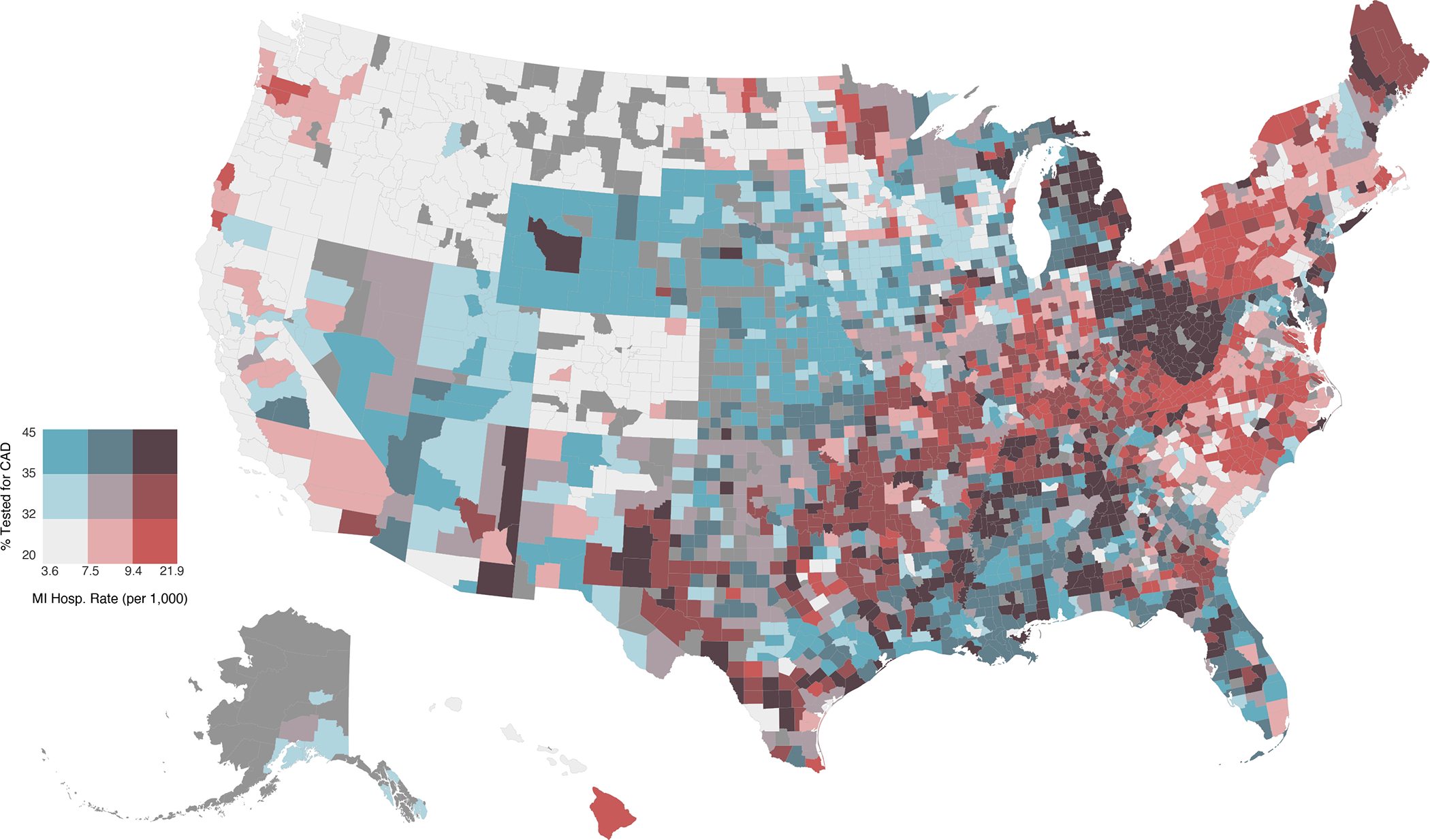
With the addition of county and state as random effects, the covariate-adjusted marginal probability of CAD testing in incident HF ranged from 20.4% in San Luis Obispo County of California to 45.2% in Clay County of Florida. According to CDC data, the average annual rate of hospitalization for myocardial infarction between 2005 and 2018 ranged from 3.6 in Blaine County of Idaho to 21.9 per 1,000 Medicare beneficiaries in Clay County of Kentucky. Figure 3 and Supplemental Figure 2 highlight the heterogeneity in these outcomes across US counties.
Figure 3. National Variability in CAD Testing and ASCVD Burden Across Counties.
This choropleth map visualizes the covariate-adjusted percentage tested for CAD as well as the rates of hospitalization for myocardial infarction across counties using a bivariate color scheme. Terciles are shown for each continuous variable. Breaks for testing percentage are 32.3% and 35.0% (range 20.4–45.1%). Breaks for myocardial infarction hospitalization rate, as a measure of ASCVD burden, are 7.5 and 9.4 events per 1,000 Medicare beneficiaries (range 3.6–21.9). Counties with insufficient data are colored dark gray.
Clinician Variation
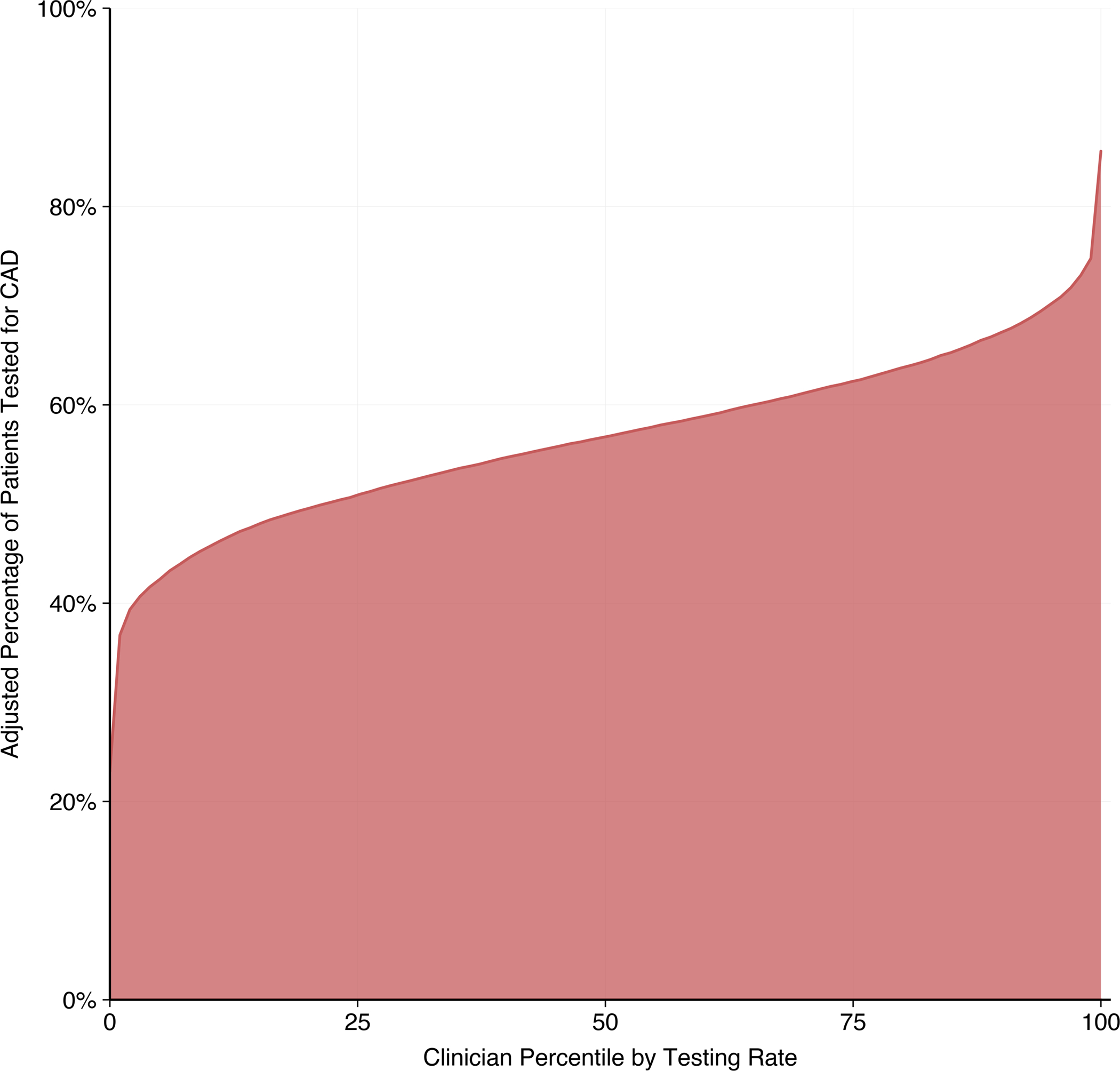
We linked 487,183 (87%) incident HF patients to a physician during the 90 days before and after their initial diagnosis. The CAD testing rate for these patients was 37.6%, slightly higher than that of the full cohort. A PCP was attributed to 94% of these patients, and a cardiologist was attributed to 52%, with 46% seen by both a PCP and cardiologist. We found 16,368 unique clinician or group practices (subsequently referred to as “clinicians”) with 10 or more incident HF patients during the study period, of which 52% were PCPs and 48% were cardiologists. Among those with a PCP, patients co-managed by a cardiologist had significantly greater adjusted odds (AOR 5.12, 95% CI 4.98–5.27) of CAD testing. For an average-risk patient, the median testing rate among PCPs without cardiologist co-management was 15.5% with an interquartile range of 13.6–17.7% (Supplemental Figure 3). In contrast, the median testing rate among cardiologists was 56.8% with an interquartile range of 50.9–62.4% and a median odds ratio of 1.62 (95% CI 1.59–1.64) (Figure 4).
Figure 4. Variability in CAD Testing Rates Across Cardiologists.
This cumulative distribution curve shows 7,921 cardiologists ordered by their CAD testing rates for an average-risk patient. All patient-level covariates are conditioned at their means. The interquartile range of testing rates, as seen by the 25th and 75th clinician percentiles, is 50.9 to 62.4%. Only cardiologists with at least 10 new heart failure diagnoses during the study period are included in this analysis.
Sensitivity Analyses
As previously described, we performed sensitivity analyses to account for several scenarios. We found similar testing rates and effect sizes for patient-level factors with a 24-month lookback requirement and for patients with a history of CAD (Supplemental Table 3). In patients with first-time acute decompensated HF with an inpatient hospitalization, covariate effect sizes were comparable, but the adjusted rate of CAD testing in this inpatient subset was 47.6% (95% CI 47.2–48.0%) compared with 34.5% in the full cohort (95% CI 33.4–35.6%). With a shorter follow-up (90 days post-index), we found a lower adjusted testing rate of 24.6% (95% CI 24.4–24.7%) with similar covariate effect sizes (Supplemental Table 4). Extending the follow-up period from 90 days pre-index to 180 days post-index resulted in a slight increase in the adjusted testing rate to 35.7% (95% CI 35.6–35.9%) with minimal impact on covariates.
DISCUSSION
In this study, we present the largest longitudinal analysis of CAD testing patterns in patients with incident HF. We found that less than 40% of incident HF patients were tested for CAD. Rates of CAD testing have remained flat over our 16-year study period, even after the STICHES trial in 2016 demonstrated long-term mortality benefit with revascularization among patients with ischemic cardiomyopathy. Furthermore, testing rates were significantly lower (<30%) for patients without presentation to an acute care setting around the time of their initial diagnosis. Despite clear recommendations by established guidelines (6, 26), the underutilization of CAD testing has persisted across care settings and over time, underscoring the severe unmet need in this high-risk patient population.
As expected, strong predictors of testing included smoking history, hyperlipidemia, and markers of disease severity such as cardiogenic shock and acute care presentation. Importantly, prominent CAD risk factors such as hypertension, diabetes, obesity, and sleep apnea were not strongly associated with testing, revealing missed opportunities for high-yield CAD work-up. In contrast, negative predictors included alcohol use disorder and chronic obstructive pulmonary disease, both of which increase mortality in patients with ischemic heart disease (27, 28). Patients with severe mental illness, such as depression and schizophrenia, are another critically undertested group, yet some of their specific treatments (e.g., antipsychotics), have been linked to significant ischemic cardiovascular risk (29). In addition, we found notable disparities in demographic factors. Patients of female sex and of black race had lower testing rates after adjusting for patient characteristics. Notably, the odds of testing remained relatively low for women and for blacks even with extended six-month follow-up. These patterns are worthy of further investigation as they could indicate inequities in healthcare access, inaccurate appraisal of cardiovascular risk, misrecognition of ischemic symptoms, and clinician bias (30, 31). Lastly, CAD testing may not be appropriate for patients who are poor candidates for revascularization. Infrequent testing for patients with dementia, for example, may represent sound clinical judgment, as the likelihood of long-term survival benefit may be limited. Likewise, the risks of revascularization in advanced chronic kidney disease may outweigh the benefits given concern for progression to end-stage renal disease. Patient preference and other unmeasured characteristics may also contribute to reduced testing rates.
Our primary outcome was comparable to the CAD testing rates reported by previous observational studies. Of the 5,878 new-onset HF patients in the Cardiovascular Research Network HF study, 37% received testing from 14 days before hospitalization to 6 months after discharge between 2005 and 2008. An analysis of the Truven Health MarketScan database revealed that 27% of the 67,161 hospitalized HF patients underwent ischemic work-up within three months between 2011 and 2013. Finally, 39% of the 17,185 patients in the Get With The Guidelines–Heart Failure registry were tested during the three months before and after index hospitalization between 2009 and 2015. Building on previous work, our study was able to leverage a much larger cohort to evaluate national variability in testing rates. While geographic disparities in cardiovascular disease mortality are well-documented in the US (32), we found relatively low testing rates compared to ASCVD burden in the South and Northeast. The root causes of observed geographic differences are unclear but prior research has identified disparities in resource availability, healthcare access, socioeconomic conditions, and healthy public policies (33). Regional gaps in testing highlight the importance of continued surveillance to uncover underlying drivers.
To our knowledge, this study is the first to characterize clinician-level variability in CAD testing for patients with new-onset HF. The wide discrepancy in testing rates between PCPs and cardiologists is particularly striking, since nearly 50% of our cohort were linked to a PCP without cardiology co-management. One explanation is that incident HF patients treated only in primary care settings have milder disease. Nevertheless, there were observed differences after adjusting for patient-level clinical characteristics across clinicians. Among cardiologists, testing rates were higher but variability was wide; nearly a quarter of cardiologists tested fewer than 50% of their incident HF patients. The substantial variation across clinicians suggests low testing rates cannot be fully explained by characteristics that might limit testing—suitability of revascularization, patient preference, and unmeasured patient-level factors. Disparities in quality of care may influence clinician variability as well. Suboptimal guideline adherence for both cardiologists and PCPs has been noted in the literature, with facility-level constraints, limited clinician awareness, and low patient engagement cited as barriers (34, 35). Heterogeneity in CAD testing across specialties and practices represents an important area for quality improvement campaigns.
Our findings raise concern that new-onset HF patients are not only undertested but also undertreated for CAD. In analyses of the Get With The Guidelines–Heart Failure and Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure registries, HF patients who underwent upfront CAD testing were more likely to receive guideline-directed medical therapy for CAD and revascularization (11, 36). The suboptimal rate of CAD testing across the US is particularly notable as additional data on the benefit of percutaneous revascularization is forthcoming from trials such as REVIVED-BCIS2 (37). Omission of timely testing precludes management of the most common and potentially reversible etiology of HF. Even HFpEF patients may benefit greatly from appropriate testing and treatment given the high co-prevalence of CAD and the paucity of evidence-based therapies (5). The continued underuse of CAD testing in new-onset HF patients leaves much room for improvement.
Study Limitations
First, this analysis relied on administrative claims data for retrieving information on patient covariates and outcomes. Coding standards may vary across practices; therefore, the association between comorbidities and testing rates should be interpreted with caution. Second, claims data are limited in clinical granularity. Detailed information on the severity of HF symptoms and medical comorbidities could further explain the differences in CAD testing rates. Third, we leveraged codes for systolic heart failure to substitute documented EF. Single ICD-9 HF codes have poor sensitivity for discriminating reduced EF, though a recent study using multiple diagnoses over a 1-year period demonstrated 72% sensitivity and 77% positive predictive value for EF <50% (19). Risk of misclassification remains despite the improved accuracy of this approach. Fourth, there may be unmeasured patient-level characteristics contributing to variation across clinicians. Finally, interstate differences in patient enrollment by Optum’s managed care affiliate could also influence observed geographic variation.
CONCLUSION
Among patients with new-onset HF diagnosed between 2004 and 2019, the rates of testing for CAD consistently fell below expectations as set by current guidelines. Testing remained low even after the 10-year outcomes were reported from STICHES in 2016. Substantial variability in testing rates persists across physicians, care settings, and geographic regions. Improved uptake of CAD testing in incident HF may translate to better outcomes in this high-risk patient population.
Supplementary Material
Clinical Perspectives.
Competency in Systems-Based Practice:
In the initial evaluation of patients with incident heart failure (HF), disparities in testing for coronary disease (CAD) involve women, Blacks, those with conventional risk factors like hypertension, diabetes, obesity, sleep apnea, chronic kidney disease, and alcohol use disorders, as well as patients with mental health conditions including depression and schizophrenia.
Translational Outlook:
Further research is required to better understand the drivers of variability across regions, care settings, and clinicians, and to evaluate strategies to improve adherence to guideline-recommended testing for CAD in patients with new-onset HF.
Acknowledgements:
Data for this project were accessed using the Stanford Center for Population Health Sciences Data Core. The Population Health Sciences Data Core is supported by a National Institutes of Health National Center for Advancing Translational Science Clinical and Translational Science Award (UL1 TR001085) and from Internal Stanford funding. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Funding:
ATS is supported by a grant from the NHLBI (1K23HL151672–01).
ABBREVIATIONS
- ACC
American College of Cardiology
- AHA
American Heart Association
- AOR
adjusted odds ratio
- ASCVD
atherosclerotic cardiovascular burden
- CAD
coronary artery disease
- CI
confidence interval
- HF(rEF or pEF)
heart failure (with reduced or preserved ejection fraction)
- ICD-9 / -10
International Classification of Diseases-Ninth Revision / Tenth Revision
- PCP
primary care physician
- STICH(ES)
Surgical Treatment for Ischemic Heart Failure (Extension Study)
Footnotes
Disclosures: None
References
- 1.Glynn P, Lloyd-Jones DM, Feinstein MJ, Carnethon M, Khan SS. Disparities in Cardiovascular Mortality Related to Heart Failure in the United States. J Am Coll Cardiol 2019;73:2354–2355. [DOI] [PubMed] [Google Scholar]
- 2.Roger VL. Epidemiology of Heart Failure. Circ Res 2021;128:1421–1434. [DOI] [PubMed] [Google Scholar]
- 3.Virani SS, Alonso A, Benjamin EJ, et al. Heart Disease and Stroke Statistics—2020 Update: A Report From the American Heart Association. Circulation 2020;141:e139–e596. [DOI] [PubMed] [Google Scholar]
- 4.Gheorghiade M, Sopko G, De Luca L, et al. Navigating the Crossroads of Coronary Artery Disease and Heart Failure. Circulation 2006;114:1202–1213. [DOI] [PubMed] [Google Scholar]
- 5.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in Prevalence and Outcome of Heart Failure with Preserved Ejection Fraction. N Engl J Med 2006;355:251–259. [DOI] [PubMed] [Google Scholar]
- 6.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;62:e147–e239. [DOI] [PubMed] [Google Scholar]
- 7.Velazquez EJ, Lee KL, Deja MA, et al. Coronary-Artery Bypass Surgery in Patients with Left Ventricular Dysfunction. N Engl J Med 2011;364:1607–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Velazquez EJ, Lee KL, Jones RH, et al. Coronary-Artery Bypass Surgery in Patients with Ischemic Cardiomyopathy. N Engl J Med 2016;374:1511–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farmer SA, Lenzo J, Magid DJ, et al. Hospital-Level Variation in Use of Cardiovascular Testing for Adults With Incident Heart Failure: Findings From the Cardiovascular Research Network Heart Failure Study. JACC Cardiovasc Imaging 2014;7:690–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doshi D, Ben-Yehuda O, Bonafede M, et al. Underutilization of Coronary Artery Disease Testing Among Patients Hospitalized With New-Onset Heart Failure. J Am Coll Cardiol 2016;68:450–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Connor KD, Brophy T, Fonarow GC, et al. Testing for Coronary Artery Disease in Older Patients With New-Onset Heart Failure. Circ Heart Fail 2020;13:e006963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ezekowitz JA, Kaul P, Bakal JA, Quan H, McAlister FA. Trends in heart failure care: has the incident diagnosis of heart failure shifted from the hospital to the emergency department and outpatient clinics? Eur J Heart Fail 2011;13:142–147. [DOI] [PubMed] [Google Scholar]
- 13.Sandhu AT, Tisdale RL, Rodriguez F, et al. Disparity in the Setting of Incident Heart Failure Diagnosis. Circ Heart Fail 0:CIRCHEARTFAILURE.121.008538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goyal A, Norton CR, Thomas TN, et al. Predictors of Incident Heart Failure in a Large Insured Population A One Million Person-Year Follow-Up Study. Circ Heart Fail 2010;3:698–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khera R, Pandey A, Ayers C, et al. Contemporary Epidemiology of Heart Failure in Fee-for-service Medicare Beneficiaries Across Healthcare Settings. Circ Heart Fail 2017;10:e004402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prasada S, Rivera A, Nishtala A, et al. Differential Associations of Chronic Inflammatory Diseases with Incident Heart Failure. J Am Coll Cardiol HF 2020;8:489–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Camplain R, Kucharska-Newton A, Cuthbertson C, Wright J, Alonso A, Heiss G. Misclassification of Incident Hospitalized and Outpatient Heart Failure in Administrative Claims Data: The Atherosclerosis Risk in Communities (ARIC) Study. Pharmacoepidemiol Drug Saf 2017;26:421–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bakal JA, McAlister FA, Liu W, Ezekowitz JA. Heart Failure Re-Admission: Measuring the Ever Shortening Gap between Repeat Heart Failure Hospitalizations. PLOS ONE 2014;9:e106494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sandhu AT, Zheng J, Heidenreich PA. Updating The Accuracy of Administrative Claims for Identifying Left Ventricular Ejection Fraction Among Patients with Heart Failure. Cardiovascular Medicine, 2021. Available at: 10.1101/2021.09.15.21263651. Accessed September 20, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mamdani M, Sykora K, Li P, et al. Reader’s guide to critical appraisal of cohort studies: 2. Assessing potential for confounding. BMJ 2005;330:960–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen J Statistical Power Analysis for the Behavioral Sciences. 2nd edition. New York, NY: Routledge, 1988. [Google Scholar]
- 22.Clarke P, Wheaton B. Addressing Data Sparseness in Contextual Population Research: Using Cluster Analysis to Create Synthetic Neighborhoods. Sociol Methods Res 2007;35:311–351. [Google Scholar]
- 23.Lüdecke D ggeffects: Tidy Data Frames of Marginal Effects from Regression Models. J Open Source Softw 2018;3:772. [Google Scholar]
- 24.Harrell FE, with contributions from Charles Dupont and many others. Hmisc: Harrell Miscellaneous. 2021. Available at: https://CRAN.R-project.org/package=Hmisc. Accessed July 30, 2021.
- 25.Bates D, Mächler M, Bolker B, Walker S. Fitting Linear Mixed-Effects Models Using lme4. J Stat Softw 2015;67:1–48. [Google Scholar]
- 26.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129–2200. [DOI] [PubMed] [Google Scholar]
- 27.Roerecke M, Rehm J. Chronic heavy drinking and ischaemic heart disease: a systematic review and meta-analysis. Open Heart 2014;1:e000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sin DD, Man SFP. Chronic obstructive pulmonary disease as a risk factor for cardiovascular morbidity and mortality. Proc Am Thorac Soc 2005;2:8–11. [DOI] [PubMed] [Google Scholar]
- 29.Correll CU, Solmi M, Veronese N, et al. Prevalence, incidence and mortality from cardiovascular disease in patients with pooled and specific severe mental illness: a large-scale meta-analysis of 3,211,768 patients and 113,383,368 controls. World Psychiatry 2017;16:163–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mieres JH, Gulati M, Bairey Merz N, et al. Role of Noninvasive Testing in the Clinical Evaluation of Women With Suspected Ischemic Heart Disease. Circulation 2014;130:350–379. [DOI] [PubMed] [Google Scholar]
- 31.Graham G Disparities in cardiovascular disease risk in the United States. Curr Cardiol Rev 2015;11:238–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh GK, Azuine RE, Siahpush M, Williams SD. Widening Geographical Disparities in Cardiovascular Disease Mortality in the United States, 1969–2011. Int J MCH AIDS 2015;3:134–149. [PMC free article] [PubMed] [Google Scholar]
- 33.Casper M, Kramer MR, Quick H, Schieb LJ, Vaughan AS, Greer S. Changes in the Geographic Patterns of Heart Disease Mortality in the United States. Circulation 2016;133:1171–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McKinlay JB, Link CL, Freund KM, Marceau LD, O’Donnell AB, Lutfey KL. Sources of Variation in Physician Adherence with Clinical Guidelines: Results from a Factorial Experiment. J Gen Intern Med 2007;22:289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoorn CJGM, Crijns HJGM, Dierick-van Daele ATM, Dekker LRC. Review on Factors Influencing Physician Guideline Adherence in Cardiology. Cardiol Rev 2019;27:80–86. [DOI] [PubMed] [Google Scholar]
- 36.Flaherty JD, Rossi JS, Fonarow GC, et al. Influence of coronary angiography on the utilization of therapies in patients with acute heart failure syndromes: Findings from Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF). Am Heart J 2009;157:1018–1025. [DOI] [PubMed] [Google Scholar]
- 37.Perera D, Clayton T, Petrie MC, et al. Percutaneous Revascularization for Ischemic Ventricular Dysfunction: Rationale and Design of the REVIVED-BCIS2 Trial. JACC Heart Fail 2018;6:517–526. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


