Abstract
Long-circulating liposomes (LCL) may be used as targeted antimicrobial drug carriers as they localize at sites of infection. As a result, LCL-encapsulated gentamicin (LE-GEN) has demonstrated superior antibacterial activity over the free drug in a single-dose study of immunocompetent rats with Klebsiella pneumoniae pneumonia. In the present study, the therapeutic efficacy of LE-GEN was evaluated by monitoring rat survival and bacterial counts in blood and lung tissue in clinically relevant models, addressing the issue of impaired host defense and low bacterial antibiotic susceptibility. The results show that in immunocompetent rats infected with the high-GEN-susceptibility K. pneumoniae strain, a single dose of LE-GEN is clearly superior to an equivalent dose of free GEN. Yet complete survival can also be obtained with multiple doses of free GEN. In leukopenic rats infected with the high-GEN-susceptible K. pneumoniae strain, free GEN at the maximum tolerated dose (MTD) was needed to obtain survival. However, with the addition of a single dose of LE-GEN to free-GEN treatment, complete survival can be obtained using a sevenfold-lower cumulative amount of GEN than with free-GEN treatment alone. In leukopenic rats infected with low-GEN-susceptible K. pneumoniae cells, free GEN at the MTD did not result in survival. The use of LE-GEN is needed for therapeutic success. Increasing LE-GEN bilayer fluidity resulted in an increased GEN release from the liposomes and hence improved rat survival, thus showing the importance of the liposome lipid composition for therapeutic efficacy. These results warrant further clinical studies of liposomal formulations of aminoglycosides in immunocompromised patients with severe infections.
Clinical practice shows that failure of antimicrobial treatment is not uncommon. Two major risk factors can be identified: an impaired host defense and a moderate-to-low antibiotic susceptibility of the infectious organism(s) (4, 6, 8, 18). An impaired host defense increases the patients' susceptibility to infections. In addition, a limited ability of the host defense to support antimicrobial treatment increases the chance of treatment failure (15). A low antibiotic susceptibility of bacteria can result in subeffective drug concentrations at the site of infection despite high drug doses (8).
Targeted antibacterial-drug delivery may increase drug concentrations at the infectious focus and therefore help to reduce the treatment failure risks imposed by the impaired host defense or low bacterial susceptibility. Besides, in the case of potentially toxic antibiotics, toxicity to nontarget tissues may be reduced, which allows the use of higher doses. Liposomes have attracted considerable interest as targeted drug carriers in infectious diseases. A number of studies have convincingly demonstrated that so-called long-circulating liposomes tend to localize preferentially at foci of infection or inflammation after intravenous administration (1, 9, 11, 17, 19). The preferential localization appears to be the result of the inflammatory response provoking a locally increased capillary permeability, allowing liposome extravasation. Generally, intravenously administered liposomes that are rapidly opsonized and taken up by the mononuclear phagocyte system (MPS) hardly localize at foci of infection outside the MPS. By coating the liposomal surface with polyethylene glycol (PEG), opsonization and subsequent MPS uptake is reduced, thus prolonging circulation time and interaction with the infectious target site. As a result, these PEG-coated long-circulating liposomes (LCL) show superior target localization characteristics (19). Therefore, LCL have attractive prospects for the site-specific delivery of antimicrobial agents. Previous research has shown that LCL-encapsulated gentamicin (LE-GEN) demonstrates superior antibacterial efficacy compared to free GEN in a single-dose study of rats with intact host defenses and with a unilateral Klebsiella pneumoniae pneumonia (3).
In the present study, the antibacterial efficacy of LE-GEN was evaluated in clinically more relevant models addressing the issue of impaired host defense and low antibiotic susceptibility. Rat survival and bacterial counts in lung tissue and blood were monitored in immunocompetent as well as leukopenic rats with pneumonia caused by high- or low-GEN-susceptible K. pneumoniae strains.
MATERIALS AND METHODS
Liposome preparation and characterization.
Liposomes were prepared as described previously (3). In brief, appropriate amounts of partially hydrogenated egg phosphatidylcholine (PHEPC) (Asahi Chemical Industry Co. Ltd., Ibarakiken, Japan), cholesterol (Sigma Chemical Co., St. Louis, Mo.), and 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[polyethylene glycol-2000] (PEG-DSPE) (Avanti Polar Lipids, Alabaster, Ala.) in a molar ratio of 1.85:1:0.15 were dissolved in a mixture of chloroform and methanol to obtain “rigid” liposomes. To obtain “fluid” liposomes, egg phosphatidylcholine (EPC; Asahi Chemical Industry Co. Ltd.) and PEG-DSPE were dissolved in a molar ratio of 2.85:0.15. After evaporation of the solvent under constant rotation and reduced pressure, the lipid mixture was dried under nitrogen, dissolved in 2-methyl-2-propanol (Sigma Chemical Co.), frozen, and freeze-dried overnight. The resulting lipid film was hydrated for 2 h in 2.5 ml of aqueous GEN (200 mg/ml) (Duchefa Biochemie b.v., Harlem, The Netherlands), and subsequently, 7.5 ml of HEPES-NaCl buffer (10 mM HEPES) (Sigma Chemical Co.) and 135 mM NaCl (Merck, Darmstadt, Germany) (pH 7.4) were added. The hydrated lipids were sonicated for 8 min with an amplitude of 8 μm using a 9.5-mm probe in an MSE Soniprep 150 (Sanyo Gallenkamp PLC, Leicester, United Kingdom). Dynamic light scattering, detected at an angle of 90° to the laser beam on a 4700 system (Malvern Instruments Ltd., Malvern, United Kingdom), was used to evaluate the particle size distribution. Liposomes with a mean particle size of 100 nm were obtained. In addition to the mean particle size, the system reports a polydispersity index (a value between 0 and 1). A polydispersity index of 1 indicates large variations in particle size; a reported value of 0 means that size variation is apparently absent. All liposome preparations used had polydispersity indexes below 0.3. Nonencapsulated GEN was removed by ultracentrifugation in two changes of HEPES-NaCl buffer at 265,000 × g for 2 h at 4°C in an L-70 ultracentrifuge (Beckman, Palo Alto, Calif.). The phosphate concentration was determined spectrophotometrically according to the method of Bartlett (5). Total (encapsulated and free) and unencapsulated (free) GEN was measured with a diagnostic sensitivity test agar (Oxoid, Basingstoke, United Kingdom) diffusion test with Staphylococcus aureus Oxford strain (ATCC 9144) as the indicator organism and standards ranging from 4 to 0.25 μg of GEN/ml, as described previously (3). For total (unencapsulated and encapsulated) GEN measurements, liposomes were destroyed by 0.1% (vol/vol) (final concentration) Triton X-100 (Janssen Chimica, Geel, Belgium). Less than 10% of the GEN in the liposome dispersion was shown to be unencapsulated after ultracentrifugation. The specific activity was between 70 and 80 μg of GEN/μmol of total lipid.
Bacterial strains.
The high-GEN-susceptible K. pneumoniae strain (ATCC 43816; capsular serotype 2; MIC = 0.5 μg/ml) was used. The MIC was determined by plating an inoculum of 104 CFU per spot on Mueller-Hinton (MH) agar (Difco laboratories, Detroit, Mich.) plates containing twofold dilutions of GEN according to the method of Woods and Washington (20). The low-GEN-susceptible K. pneumoniae strain (MIC = 4 μg/ml) was obtained by culturing the high-susceptible strain in MH broth in the presence of increasing concentrations of GEN. The low-GEN-susceptible strain appeared to be stable in vitro after repeated culture in antibiotic-free MH broth.
Unilateral pneumonia.
The animal experiments ethical committee of the Erasmus University Medical Center Rotterdam approved the experiments described in this study. Female RP/AEur/RijHsd strain albino rats (18 to 25 weeks of age; body weight, 185 to 225 g; Harlan, Horst, The Netherlands) with a specified pathogen-free status were used. When indicated, rat host defense was impaired by intraperitoneal injection of 60 mg of cyclophosphamide (Sigma)/kg of body weight every 4 days, starting at 5 days before bacterial inoculation. The leukopenic status of cyclophosphamide-treated animals was ascertained in separate experiments by measuring leukocyte counts in fresh blood samples, obtained by retro-orbital bleeding in EDTA-coated tubes. White blood cells were counted on a Cobas Minos Stex (Roche Haematology, Montpellier, France) using Minotrol 16 standards (Roche Haematology) to verify proper functioning of the instrument. As a result of the cyclophosphamide treatment, the number of leukocytes in the circulation on the day of bacterial inoculation was reduced sixfold from approximately 6 × 109 ± 1 × 109 (buffer-treated controls) to 1 × 109 ± 8 × 108 (means ± standard deviations [SD]; n = 3; P < 0.01). Leukocyte counts remained reduced throughout the study period.
From 5 days to 1 day before bacterial inoculation, the drinking water of the leukopenic rats was supplemented with 1 g of cephalexin monohydrate (Dopharma, Raamsdonksveer, The Netherlands)/liter to prevent superinfections. The drinking water in the remaining study period and the drinking water of immunocompetent rats was autoclaved water, pH 3. At the time of bacterial inoculation, cephalexin concentrations were less than 1 μg/ml in the blood and lung tissue of rats that had received the drinking water supplemented with cephalexin (n = 3 rats), as measured by an agar diffusion test using Escherichia coli as the indicator organism, as described previously (3).
A left-sided unilateral pneumonia was induced as described in detail elsewhere (2). In brief, rats were anesthetized, and the left primary bronchus was intubated. Through the tube, 0.02 ml of a saline suspension of K. pneumoniae was inoculated in the left lung lobe. The inoculated bacteria were in the logarithmic phase of growth. The inoculum was adjusted such that the median survival rates of the rats were comparable in the models. The rats with intact host defenses were inoculated with 106 high-GEN-susceptible K. pneumoniae (ATCC 43816; capsular serotype 2) cells, and the leukopenic rats were inoculated with 105 high-GEN-susceptible or 107 low-GEN-susceptible K. pneumoniae cells. The cephalexin-containing water did not have an effect on rat survival or bacterial outgrowth. The rats were housed individually. The in vivo stability of the phenotype of the low-susceptibility K. pneumoniae strain was checked by culturing dilutions of homogenized left-lung tissue obtained 24 h after bacterial inoculation (the starting point of treatment) on MH plates. Colonies were isolated, and the MIC was determined on MH plates as described above (20). All 100 tested colonies had a stable low-GEN-susceptible phenotype after inoculation in vivo. The same procedure was applied to bacteria isolated at the end of the study period (after the death of the rats or after 14 days). None of the treatments in this study resulted in a change of the MIC of the K. pneumoniae strains pre- and postexposure.
Antimicrobial treatment.
The treatments were started at 24 h after bacterial inoculation. GEN was administered twice daily every 12 h (q12h), and LE-GEN was administered once daily every 24 h (q24h). The formulations were injected intravenously in the tail vein.
Survival.
Ten rats were used per experimental group. The survival of the rats was examined every day until 14 days after bacterial inoculation. The blood of the dead rats was cultured on Columbia III agar supplemented with 5% sheep blood (Beckton-Dickinson) overnight at 37°C. Substantial numbers of exclusively K. pneumoniae cells were recovered in the blood samples from the dead rats.
Quantification of bacterial numbers in blood.
At various time points after bacterial inoculation, blood samples were taken via retro-orbital bleeding in heparinized tubes on ice. Serial dilutions were prepared on ice, and 0.2 ml of each dilution was applied to tryptone-soy-agar plates. The plates were incubated overnight at 37°C, and colonies were counted.
Quantification of bacterial numbers in left-lung tissue.
At various time points after bacterial inoculation, rats were sacrificed by CO2 inhalation. The infected left lung was dissected and homogenized in 20 ml of phosphate-buffered saline (4°C), supplemented with aminoglycoside acetylating enzyme, and 2 mM acetyl coenzyme A (sodium salt) (Sigma Chemical Co.) to inactivate residual GEN present in the tissues, according to Den Hollander et al. (13). Serial dilutions were prepared, and 0.2 ml of each dilution was applied on tryptone-soy-agar plates. The plates were incubated overnight at 37°C, and colonies were counted.
Pharmacokinetics and tissue concentrations of free GEN or LE-GEN.
Free GEN or LE-GEN was injected intravenously in healthy rats at various doses, used in the survival experiments, via the tail vein. Blood samples were taken at various time points after injection from alternate groups of three rats via retro-orbital bleeding under CO2 anaesthesia. The blood samples were collected in heparinized tubes, and after centrifugation, the plasma was collected. Drug concentrations were analyzed using the agar diffusion test as described above. The sample was divided into two portions. One was analyzed directly to determine the free (i.e., nonliposomal) drug concentration. The other was incubated with Triton X-100, as described above, to disrupt the liposomes in order to determine total (free plus encapsulated) drug concentrations. Neither liposome type used in the study showed substantial drug leakage when mixed with plasma and subsequently placed in agar wells and incubated overnight at 37°C. As a result, free-GEN concentrations could be accurately determined separately from the total GEN concentrations.
Total GEN concentrations in lung tissue at different time points after injection were analyzed by dissection of the infected left lung and uninfected right lung, homogenization of the tissue in phosphate-buffered saline, and subsequent incubation with 0.1% Triton X-100 (final concentration) followed by the agar diffusion test as described above.
Statistical analysis.
Survival in different experimental groups and controls was compared by the log rank test (Graph Pad software Inc., San Diego, Calif.).
RESULTS
Rats with intact host defenses infected with high-GEN-susceptible K. pneumoniae.
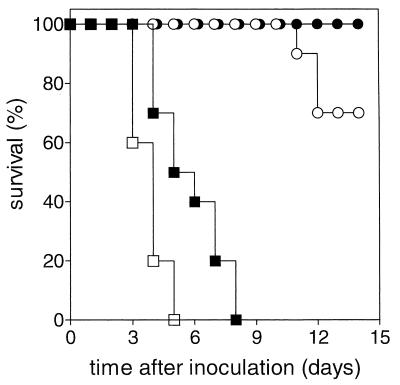
In the first model, rats with intact host defenses were infected with the high-GEN-susceptible K. pneumoniae strain (MIC = 0.5 μg/ml). Treatment with either free GEN or LE-GEN was started 24 h after bacterial inoculation. The survival rates are shown in Fig. 1. Untreated control animals died between day 3 and day 5 after bacterial inoculation. A single dose of free GEN (5 mg/kg) slightly prolonged survival (P < 0.01 compared to controls), but all animals still died before day 8. An equivalent dose of LE-GEN yielded 70% survival after 2 weeks (P < 0.001 compared to a single dose of free GEN [5 mg/kg]). The difference in efficacy between these two treatments is paralleled by the differences in GEN concentrations in the lung tissue after injection. Already at 5 h after a single dose of GEN (5 mg/kg), GEN levels are below 1.5 μg/lung in either the infected left lung or the uninfected right lung. In contrast, an equivalent dose of LE-GEN results in total GEN concentrations of 7.9 ± 0.8 μg/left lung and 4.0 ± 2.1 μg/right lung at 5 h after injection. At 24 h, concentrations of 12.9 ± 4.3 μg/left lung versus 5.5 ± 1.2 μg/right lung were noted, whereas after 48 h, the concentrations were 16.2 ± 3.9 μg/left lung versus 3.3 ± 2.8 μg/right lung (mean ± SD; n = 5 to 9 rats per time point).
FIG. 1.
Effect of free GEN and LE-GEN on survival of rats with intact host defenses infected with a high-GEN-susceptible K. pneumoniae strain. The treatments were control (no drug treatment) (□), free GEN at 5 mg/kg (single dose) (■), free GEN at 5 mg/kg/day q12h for 3 days (●), and LE-GEN at 5 mg/kg (single dose) (○).
Treatment of rats with free GEN (5 mg/kg/day q12h for 3 days) resulted in 100% survival.
Leukopenic rats infected with high-GEN-susceptible K. pneumoniae.
Rat host defense was impaired in the second model by cyclophosphamide injections, resulting in a sixfold reduction in the number of circulating white blood cells. These leukopenic rats were infected with the high-GEN-susceptible K. pneumoniae strain (MIC = 0.5 μg/ml). Treatment with either free GEN, LE-GEN, or a combination of both was started 24 h after bacterial inoculation. The survival rates are shown in Fig. 2 and 3.
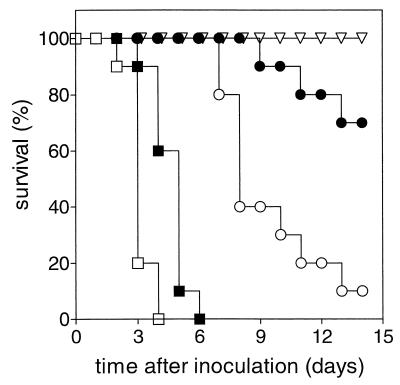
FIG. 2.
Effect of free GEN on survival of leukopenic rats infected with a high-GEN-susceptible K. pneumoniae strain. The treatments were control (no drug treatment) (□), free GEN at 5 mg/kg/day q12h for 3 days (■), free GEN at 5 mg/kg/day q12h for 5 days (○), free GEN at 20 mg/kg/day q12h for 5 days (●), and free GEN at 40 mg/kg/day q12h for 5 days (▿).
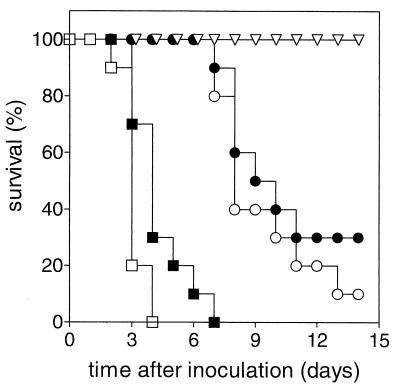
FIG. 3.
Effect of GEN and LE-GEN on survival of leukopenic rats infected with a high-GEN-susceptible K. pneumoniae strain. The treatments were control (no drug treatment) (□), free GEN at 5 mg/kg/day q12h for 5 days (○), free GEN at 5 mg/kg/day q12h for 5 days plus a single dose of LE-GEN at 5 mg/kg on day 1 (▿), free GEN at 5 mg/kg/day q12h for 5 days plus a single dose of free GEN at 5 mg/kg on day 1 (●), and LE-GEN at 5 mg/kg/day q24h for 5 days (■).
Figure 2 shows that, similar to the previous model, untreated control animals died between days 2 and 4 after bacterial inoculation. The therapeutic regimen that resulted in complete survival in the previous model (free GEN at 5 mg/kg/day q12h for 3 days) hardly prolonged survival in leukopenic rats. Free GEN at 5 mg/kg/day q12h for 5 days prolonged survival during treatment, but after termination of treatment only 10% of the rats survived up to 14 days (P < 0.0001 compared to control animals). Increasing the dose of free GEN to 20 mg/kg/day q12h for 5 days showed 70% survival on day 14. A further increase to 40 mg/kg/day q12h for 5 days, which is the maximum tolerated dose (MTD), resulted in complete survival.
Figure 3 shows that addition of a single dose of LE-GEN at 5 mg/kg on day 1 to a 5-day treatment with free GEN at 5 mg/kg/day q12h also resulted in 100% survival. The complete survival obtained with that therapeutic regimen is the result of the addition of liposomal GEN, as the addition of free GEN on day 1 to the 5-day treatment with free GEN showed only 30% survival on day 14 (P < 0.001 for the addition of free GEN versus the addition of LE-GEN). Treatment with LE-GEN only at 5 mg/kg/day q24h for 5 days was not effective, as the majority of rats died during treatment.
The median numbers of bacteria (± range) recovered from the left lung and blood on day 0, 1, 6, 10, and 14 after bacterial inoculation of leukopenic rats infected with the high-GEN-susceptible K. pneumoniae strain are shown in Table 1. The rats received a 5-day treatment with free GEN at 5 mg/kg/day q12h with the addition of a single dose of either LE-GEN (5 mg/kg) or free GEN (5 mg/kg) on day 1. Median bacterial counts 24 h after the last dose (day 6) in the left lungs of rats treated with the combination of free GEN plus LE-GEN were 10-fold lower than those in rats treated with equivalent doses of free GEN alone. In the following days, complete bacterial killing was achieved in rats treated with free GEN plus LE-GEN whereas the majority of rats treated with free GEN alone died. Bacterial counts in blood on day 6 were approximately 100-fold lower for free GEN plus LE-GEN-treated animals than for rats treated with free GEN alone. Median bacterial blood counts stabilized in the following days in the rats that received free GEN plus LE-GEN. Examination of bacterial counts in consecutive blood samples of individual rats revealed that the bacteremia was episodic in nature. The GEN susceptibility of K. pneumoniae recovered from dead or surviving animals was not changed compared to the inoculated bacteria.
TABLE 1.
Number of bacteria in left lung and blood in leukopenic rats infected with the high-GEN-susceptible K. pneumoniae strainsa
| Day | Free GENb + rigid LE-GENc
|
Free GENb + free GENc
|
||||||
|---|---|---|---|---|---|---|---|---|
| Log no. of bacteria/ left lung
|
Log no. of bacteria/ ml of blood
|
Log no. of bacteria/ left lung
|
Log no. of bacteria/ml of blood
|
|||||
| Median | Range | Median | Range | Median | Range | Median | Range | |
| 0 | 5.0 | 0.0 | 5.0 | 0.0 | ||||
| 1 | 8.9 | 8.6–9.3 | 1.7 | 1.0–2.2 | 8.9 | 8.6–9.3 | 1.7 | 1.0–2.2 |
| 6 | 5.7 | 3.0–8.2 | 1.8 | 0.0–2.0 | 6.7 | 3.9–7.7 | 3.5 | 3.1–4.3 |
| 10 | 3.2 | 0.0–5.0 | 2.2 | 0.0–3.4 | —d | —d | ||
| 14 | 0.0 | 0.0–3.1 | 1.2 | 0.0–2.5 | —d | —d | ||
Rats received the indicated combination treatments at different time points after inoculation; 6 animals were used per experimental group.
5 mg/kg/day q12h for 5 days.
5 mg/kg on day 1.
Bacterial counts are not presented, as majority of animals had died.
Leukopenic rats infected with low-GEN-susceptible K. pneumoniae.
The third model combines the clinically encountered problems of an impaired host defense and low bacterial antibiotic susceptibility. Leukopenic rats were infected with the low-GEN-susceptible K. pneumoniae strain (MIC = 4 μg/ml). Treatment consisted of either free GEN or a combination of free GEN and LE-GEN and was started 24 h after bacterial inoculation. The survival rates are shown in Fig. 4.
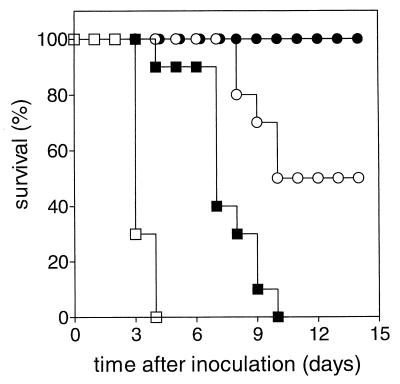
FIG. 4.
Effect of GEN and rigid LE-GEN or fluid LE-GEN on survival of leukopenic rats infected with a low-GEN-susceptible K. pneumoniae strain. The treatments were control (no drug treatment) (□), free GEN at 40 mg/kg/day q12h for 5 days (■), free GEN at 40 mg/kg/day q12h for 5 days plus rigid LE-GEN at 20 mg/kg/day q24h for 5 days (○), and free GEN at 40 mg/kg/day q12h for 5 days plus fluid LE-GEN at 20 mg/kg/day q24h for 5 days (●).
Untreated control animals died, similar to the previous models, on days 3 and 4. Free GEN given at the MTD of 40 mg/kg/day q12h for 5 days, which resulted in complete survival in the previous model, was unsuccessful. Higher doses of free GEN produced acute mortality. Addition of LE-GEN at 20 mg/kg/day q24h for 5 days to 5-day treatment with free GEN at the MTD increased survival up to 50% (P < 0.01 compared to treatment with free GEN alone) without producing acute mortality.
Up to this point, only a rigid LE-GEN formulation had been used, as it proved highly effective. In the present model, the maximum dose of free GEN plus rigid LE-GEN was only partially successful (50% survival). Therefore, a fluid LE-GEN formulation was investigated, as it has been shown that bilayer fluidity can influence the therapeutic efficacy of liposome-encapsulated aminoglycosides (7). A previous study had already demonstrated that the fluid and rigid LE-GEN localize with the same targeting efficiency at the target site (19). Addition of the fluid LE-GEN at 20 mg/kg/day for 5 days instead of equivalent doses of rigid LE-GEN to the 5-day free-GEN treatment at the MTD resulted in complete survival (P < 0.05 compared to free GEN plus rigid LE-GEN).
The median number of bacteria (± range) recovered from the left lung and blood on days 0, 1, 6, 10, and 14 after bacterial inoculation of leukopenic rats infected with the low-GEN-susceptible K. pneumoniae strain is shown in Table 2. Rats received 5-day treatment with free GEN at 40 mg/kg/day q12h with the addition of rigid LE-GEN or fluid LE-GEN at 20 mg/kg/day q24h for 5 days. The median bacterial counts 24 h after the last dose (day 6) in the left lungs of rats treated with free GEN plus fluid LE-GEN were 10-fold lower than those in rats treated with equivalent doses of free GEN plus rigid LE-GEN. In the following days, the median numbers of bacteria in the left lungs of rats treated with free GEN plus fluid LE-GEN were reduced further to zero. Fifty percent of the rats treated with the rigid LE-GEN combination died during this period. The median numbers of K. pneumoniae cells in the blood of rats treated with GEN plus fluid LE-GEN remained zero throughout the study period, whereas bacteria were present in the blood of rats treated with GEN plus rigid LE-GEN. The GEN susceptibility of K. pneumoniae recovered from dead or surviving animals was not changed compared to the inoculated bacteria.
TABLE 2.
Number of bacteria in left lung and blood in leukopenic rats infected with the low-GEN-susceptible K. pneumoniae straina
| Day | Free GENb + rigid LE-GENc
|
Free GENb + fluid LE-GENc
|
||||||
|---|---|---|---|---|---|---|---|---|
| Log no. of bacteria/ left lung
|
Log no. of bacteria/ ml of blood
|
Log no. of bacteria/ left lung
|
Log no. of bacteria/ ml of blood
|
|||||
| Median | Range | Median | Range | Median | Range | Median | Range | |
| 0 | 7.0 | 0.0 | 7.0 | 0.0 | ||||
| 1 | 10.0 | 8.6–10.3 | 1.7 | 1.0–2.2 | 10.0 | 8.6–10.3 | 1.7 | 1.0–2.2 |
| 6 | 7.3 | 3.3–8.3 | 0.7 | 0.0–2.7 | 6.4 | 4.6–7.8 | 0.0 | 0.0–1.2 |
| 10 | —d | —d | 5.4 | 4.1–6.8 | 0.0 | 0.0–1.5 | ||
| 14 | —d | —d | 1.0 | 0.0–4.9 | 0.0 | 0.2–2.4 | ||
Rats received the indicated treatments at different time points after inoculation; 6 animals were used per experimental group.
40 mg/kg/day q12h for 5 days.
20 mg/kg/day q24h for 5 days.
Bacterial counts are not presented as some animals had died.
Pharmacokinetics.
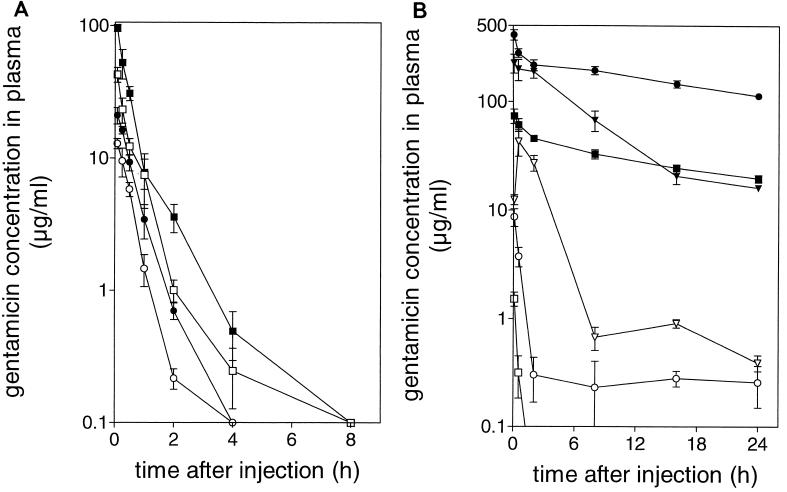
The time courses of blood concentrations of therapeutically available GEN or total (therapeutically available plus liposome-encapsulated) GEN after injection of free GEN, fluid LE-GEN, or rigid LE-GEN are shown in Fig. 5). The pharmacokinetics of free GEN are independent of the dose in the range of 2.5 to 20 mg/kg, as the GEN concentrations in the circulation over time were approximately proportional to the injected dose. Disappearance of free GEN from the bloodstream was relatively rapid, with a half-life of approximately 20 min. At 8 h after injection of the highest dose (20 mg/kg), GEN levels in the blood were already below 0.1 μg/ml.
FIG. 5.
(A) Time course of GEN concentrations in plasma of rats after injection of a single dose of free GEN at 2.5 (○), 5 (●), 10 (□), or 20 (■) mg/kg; There were three animals per experimental group. (B) Time course of GEN concentrations in plasma of rats after injection of a single dose of rigid LE-GEN or fluid LE-GEN. The open symbols indicate microbiologically active (i.e., not liposome-encapsulated) GEN concentrations after a single dose of rigid LE-GEN at 5 mg/kg (□), rigid LE-GEN at 20 mg/kg (○), or fluid LE-GEN at 20 mg/kg (▿). The solid symbols indicate total (i.e., microbiologically active and liposome-encapsulated) GEN concentrations after a single dose of rigid LE-GEN at 5 mg/kg (■), rigid LE-GEN at 20 mg/kg (●), or fluid LE-GEN at 20 mg/kg (▾). There were three animals per experimental group. All points represent means ± SD.
Encapsulation of GEN in rigid liposomes resulted in dramatically increased total GEN concentrations in the circulation after injection. Even at 24 h after injection, 15 to 20% of the injected dose was still present in the bloodstream. Fluid LE-GEN showed a completely different picture. Less than 5% of the injected dose was present in the circulation at 24 h after injection. Since rigid and fluid liposomes exhibit similar blood clearance kinetics, as has been demonstrated previously (19), the difference between the two liposome types is the result of faster drug release from the fluid liposomes, as can also be deduced from the higher free GEN levels after injection of the fluid liposomes. It is not the result of renal impairment, as administration of the highest doses of free GEN, rigid LE-GEN, or fluid LE-GEN used in this experiment did not result in significantly different blood creatinin or blood urea nitrogen levels in these three experimental groups 24 h after administration (data not shown).
DISCUSSION
The preferential localization of liposomes at sites of infection offers an attractive way to selectively increase antibacterial drug concentrations at the target location, with the intention to increase the therapeutic efficacy of antimicrobial treatment. Most studies in this field have been performed in animal models with an intact host defense and with high-antibiotic-susceptible bacteria. However, in clinical practice, two important complicating factors should be taken into account. In particular, patients having impaired host defenses carry a high risk of treatment failure, which further increases when the infectious organism has moderate to low susceptibility to the applied antimicrobial agent. Generally, the studies with animals with intact host defenses report enhanced therapeutic efficacy of the liposomal formulation compared to that of the free drug (3, 7, 16, 21). The same conclusion can be drawn from the present study in the intact host defense model of a high-GEN-susceptible K. pneumoniae pneumonia. As a result of the local delivery of the antibiotic by LE-GEN, a single dose of the liposome-encapsulated drug dramatically improves survival compared to an equivalent dose of free GEN. Increasing the dose of LE-GEN may improve survival to 100%. Yet the clinical relevance of this approach is limited, as a relatively short course of treatment for only 3 days with free GEN at 5 mg/kg/day q12h already yields complete survival. Therefore, the present study was undertaken to investigate the therapeutic potential of LE-GEN in clinically more relevant models of infection that are difficult to treat with conventional antibiotics as a result of impaired host defense and low antibiotic susceptibility of the inoculated bacteria.
In leukopenic rats inoculated with the high-susceptible K. pneumoniae strain, approximately 10 to 100 bacteria per ml of blood were already present 24 h after injection. Thus, in these leukopenic rats, antimicrobial therapy should be directed not only towards the bacteria at the infectious focus (the left lung) but also towards the rapidly occurring bacteremia. The survival experiments in the leukopenic model of a high-GEN-susceptible K. pneumoniae pneumonia show that antimicrobial treatment is far less effective as a result of impaired host defense. Treatment with free GEN at 5 mg/kg/day q12h for 3 days, which produced complete survival in the rats with intact host defenses, hardly prolongs survival in the leukopenic animals. Continuing treatment up to 5 days prolonged survival during treatment. However, after termination of treatment, only 10% survived up to 14 days. Doses of free GEN have to be increased up to 40 mg/kg/day for 5 days (the MTD) to obtain complete survival. These data illustrate why in clinical practice aminoglycosides are used in combination with other classes of antibiotics to increase the therapeutic efficacy under these conditions.
Addition of a single dose of LE-GEN at 5 mg/kg on day 1 to the 5-day treatment with free GEN at 5 mg/kg/day q12h appeared to confer substantial therapeutic benefit. All rats survived, and the cumulative amount of GEN administered was sevenfold lower than the amount of free GEN (40 mg/kg/day for 5 days) needed to obtain complete survival. This reduction in GEN exposure may reduce the risk of the well-known toxicity of GEN on the kidney and audiovestibular apparatus. On the other hand, the altered tissue distribution in general and increased GEN concentrations at the site of infection in particular as a result of the liposome encapsulation might change the toxicity profile. Yet previous studies in beagle dogs with liposome-encapsulated amikacin suggest a favorable safety profile for liposome-encapsulated aminoglycosides, as doses of 20 mg/kg/day for 1 month did not result in adverse effects despite steady-state plasma concentrations of 750 μg/ml (14). Rats treated with only LE-GEN at 5 mg/kg for 5 days already show mortality during treatment. The blood clearance kinetics of free GEN and LE-GEN offer an explanation for the superior efficacy of the combination of free GEN and LE-GEN. Free GEN is therapeutically active in the circulation against the bacteremia but is rapidly cleared after injection, with a half-life of approximately 20 min. Activity in the infected left lung is expected to be limited. When encapsulated in liposomes, on the other hand, the drug only slowly leaves the circulation. LE-GEN hardly releases GEN into the bloodstream and consequently shows limited activity against bacteremia but localizes substantially in the infected left lung (3). Release of GEN from the liposomes localizing in the infected lung leads to the efficient bacterial killing seen. As a result, combination of free GEN and LE-GEN reduces the numbers of bacteria in left-lung tissue and blood 10- and 100-fold more efficiently, respectively, than the treatment with free GEN alone. These numbers were further reduced to zero in the remaining study period. In contrast, the majority of rats treated with free GEN or LE-GEN alone died during this period. The third model addresses an additional factor complicating clinical antimicrobial therapy, i.e., low antibiotic susceptibility of the bacteria. The eightfold increase in the MIC clearly had an effect on the efficacy of treatment, as free GEN at the MTD was no longer effective and all rats died. The addition of LE-GEN at 20 mg/kg/day q24h for 5 days to the 5-day treatment with free GEN at the MTD resulted in 50% survival on day 14 without producing acute toxicity. Probably, as a result of the low GEN susceptibility of the K. pneumoniae strain, the therapeutic availability of GEN released from this rigid liposome formulation in the infected lung is insufficient to obtain 100% survival. As fluid liposomes have been reported to release encapsulated aminoglycosides more easily than their rigid counterparts (7), a fluid LE-GEN formulation was investigated. Cholesterol was omitted from the liposome formulation, as cholesterol has a major rigidifying effect on liposomal bilayers (10). Furthermore, the partially hydrogenated phospholipid PHEPC was replaced by EPC as bilayer rigidity increases with the degree of hydrogenation of the phospholipids in the bilayer. The addition of fluid LE-GEN instead of rigid LE-GEN to 5-day free-GEN treatment at the MTD resulted in an increase in therapeutic effect: complete survival was obtained. The blood clearance kinetics of fluid LE-GEN show that GEN is released into the circulation to a larger extent from this liposome formulation than from rigid LE-GEN. As a result, higher therapeutically active drug concentrations were measured in the bloodstream. Yet, a sufficient amount of GEN remained liposomally encapsulated to be delivered to the left lung to control the local infection, as is supported by the efficient bacterial killing observed in the left lung. These results show that lipid composition is an important determinant of therapeutic efficacy. A careful balance must be sought between release of antibiotic into the circulation to obtain sufficiently high drug levels there versus liposomal retention of the drug in order to achieve sufficiently high levels of (locally released) antibiotic at the infectious focus.
In conclusion, in rats with intact host defenses infected with a high-GEN-susceptible K. pneumoniae strain, LE-GEN is clearly superior to free GEN treatment. Yet the clinical relevance is limited, as complete survival can also be obtained with multiple doses of the free drug. In leukopenic rats infected with high-GEN-susceptible K. pneumoniae cells, the addition of LE-GEN to free GEN treatment shows substantial therapeutic benefit. Complete survival can be obtained by using a sevenfold-lower amount of GEN compared to administration of free GEN alone. In leukopenic rats infected with low-GEN-susceptible K. pneumoniae cells, free GEN at the MTD shows 0% survival. The use of LE-GEN is a strict requirement for achieving therapeutic success. It appears that the increased release of GEN by fluid LE-GEN compared to rigid LE-GEN is more favorable. These results warrant further clinical studies of liposomal formulations of aminoglycosides in immunocompromised patients with severe infections.
ACKNOWLEDGMENTS
This work was supported by grant 902-21-161 from the Dutch Organization for Scientific Research (N.W.O.).
REFERENCES
- 1.Awasthi V, Goins B, Klipper R, Loredo R, Korvick D, Phillips W T. Imaging experimental osteomyelitis using radiolabeled liposomes. J Nucl Med. 1998;39:1089–1094. [PubMed] [Google Scholar]
- 2.Bakker-Woudenberg I A, van den Berg J C, Michel M F. Therapeutic activities of cefazolin, cefotaxime, and ceftazidime against experimentally induced Klebsiella pneumoniae pneumonia in rats. Antimicrob Agents Chemother. 1982;22:1042–1050. doi: 10.1128/aac.22.6.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bakker-Woudenberg I A, ten Kate M T, Stearne-Cullen L E, Woodle M C. Efficacy of gentamicin or ceftazidime entrapped in liposomes with prolonged blood circulation and enhanced localization in Klebsiella pneumoniae-infected lung tissue. J Infect Dis. 1995;171:938–947. doi: 10.1093/infdis/171.4.938. [DOI] [PubMed] [Google Scholar]
- 4.Barker K F. Antibiotic resistance: a current perspective. Br J Clin Pharmacol. 1999;48:109–124. doi: 10.1046/j.1365-2125.1999.00997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartlett G R J. Phosphorus assay in column chromatography. J Biol Chem. 1959;234:466. [PubMed] [Google Scholar]
- 6.Baughman R P. The lung in the immunocompromised patient. Infectious complications. Part 1. Respiration. 1999;66:95–109. doi: 10.1159/000029349. [DOI] [PubMed] [Google Scholar]
- 7.Beaulac C, Clement-Major S, Hawari J, Lagace J. Eradication of mucoid Pseudomonas aeruginosa with fluid liposome-encapsulated tobramycin in an animal model of chronic pulmonary infection. Antimicrob Agents Chemother. 1996;40:665–669. doi: 10.1128/aac.40.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burgess D S. Pharmacodynamic principles of antimicrobial therapy in the prevention of resistance. Chest. 1999;115(Suppl.):19S–23S. doi: 10.1378/chest.115.suppl_1.19s. [DOI] [PubMed] [Google Scholar]
- 9.Corvo M L, Boerman O C, Oyen W J, van Bloois L, Cruz M E, Crommelin D J, Storm G. Intravenous administration of superoxide dismutase entrapped in long circulating liposomes. II. In vivo fate in a rat model of adjuvant arthritis. Biochim Biophys Acta. 1999;1419:325–334. doi: 10.1016/s0005-2736(99)00081-4. [DOI] [PubMed] [Google Scholar]
- 10.Cullis P R, de Kruijff B. Lipid polymorphism and the functional roles of lipids in biological membranes. Biochim Biophys Acta. 1979;559:399–420. doi: 10.1016/0304-4157(79)90012-1. [DOI] [PubMed] [Google Scholar]
- 11.Dams E T, Reijnen M M, Oyen W J, Boerman O C, Laverman P, Storm G, van der Meer J W, Corstens F H, van Goor H. Imaging experimental intraabdominal abscesses with 99mTc-PEG liposomes and 99mTc-HYNIC IgG. Ann Surg. 1999;229:551–557. doi: 10.1097/00000658-199904000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dehpour A R, Samadian T, Rassaee N. Diabetic rats show more resistance to neuromuscular blockade induced by aminoglycoside antibiotics. Gen Pharmacol. 1993;24:1415–1418. doi: 10.1016/0306-3623(93)90428-z. [DOI] [PubMed] [Google Scholar]
- 13.Den Hollander J G, Mouton J W, Bakker-Woudenberg I A, Vleggaar F P, van Goor M P, Verbrugh H A. Enzymatic method for inactivation of aminoglycosides during measurement of postantibiotic effect. Antimicrob Agents Chemother. 1996;40:488–490. doi: 10.1128/aac.40.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fielding R M, Mukwaya G, Sandhaus R A. Clinical and preclinical studies with low-clearance liposomal amikacin (Mikasome®) In: Woodle M C, Storm G, editors. Long-circulating liposomes: old drugs, new therapeutics. Austin, Tex: Landes Bioscience; 1998. pp. 213–226. [Google Scholar]
- 15.Kumana C R, Yuen K Y. Parenteral aminoglycoside therapy. Selection, administration and monitoring. Drugs. 1994;47:902–913. doi: 10.2165/00003495-199447060-00004. [DOI] [PubMed] [Google Scholar]
- 16.Martineau L, Shek P N. Efficacy of liposomal antibiotic therapy in a rat infusion model of Escherichia coli peritonitis. Crit Care Med. 1999;27:1153–1158. doi: 10.1097/00003246-199906000-00041. [DOI] [PubMed] [Google Scholar]
- 17.Oyen W J, Boerman O C, Storm G, van Bloois L, Koenders E B, Claessens R A, Perenboom R M, Crommelin D J, van der Meer J W, Corstens F H. Detecting infection and inflammation with technetium-99m-labeled stealth liposomes. J Nucl Med. 1996;37:1392–1397. [PubMed] [Google Scholar]
- 18.Reynolds H Y. Defense mechanisms against infections. Curr Opin Pulm Med. 1999;5:136–142. doi: 10.1097/00063198-199905000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Schiffelers R M, Bakker-Woudenberg I A, Snijders S V, Storm G. Localization of sterically stabilized liposomes in Klebsiella pneumoniae-infected rat lung tissue: influence of liposome characteristics. Biochim Biophys Acta. 1999;1421:329–339. doi: 10.1016/s0005-2736(99)00139-x. [DOI] [PubMed] [Google Scholar]
- 20.Woods G L, Washington J A. Antibacterial susceptibility tests: dilution and disk diffusion methods. In: Murray P R, editor. Manual of clinical microbiology. 6th ed. Washington, D.C.: American Society for Microbiology; 1995. p. 1327. [Google Scholar]
- 21.Xiong Y Q, Kupferwasser L I, Zack P M, Bayer A S. Comparative efficacies of liposomal amikacin (MiKasome) plus oxacillin versus conventional amikacin plus oxacillin in experimental endocarditis induced by Staphylococcus aureus: microbiological and echocardiographic analyses. Antimicrob Agents Chemother. 1999;43:1737–1742. doi: 10.1128/aac.43.7.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]