Abstract
Mounting research has been performed and published on natural antioxidants, more so than on synthetic ones, as key molecules that control oxidative damage and its pathway to disease. Since the discovery of vitamins, various fully synthetic or natural-identical compounds have been developed as stable small molecules translated into constantly active and completely controlled products which are widely exploited in the food and pharmaceutical industries. There is currently a debate within the literature about their mechanism of action, bioavailability, safety and real benefit for human health. Using a semiquantitative method and eligible criteria of selection, this review aimed to provide a very useful classification of antioxidants and a comprehensive cross-disciplinary description of 32 approved synthetic/natural-identical antioxidants, in terms of regulatory, antioxidant mechanism of action, safety issues, pharmacological properties, effectiveness in human health, timeline and future trends. Enriched interpretation of the data was obtained from summary bibliometrics, useful to portray the “good antioxidant” within the period 1966–2021 and, hopefully, to encourage further research.
Keywords: synthetic antioxidants, natural-identical, antioxidant classification, pharmacological properties, health, disease, oxidative damage
1. Introduction
The word “antioxidant” has become more and more popular in modern society, being widely promoted by mass media, due to the fact that consuming antioxidant compounds through diet provides health benefits.
A simple Google search by typing the keyword “antioxidants” carried out on January 2022 produced 1.56 billion results. However, as noticed by Barry Halliwell, “the term antioxidant is widely used but rarely defined” [1]. A large number of definitions have been given to illustrate what an antioxidant is. Thus, according to the Dictionary of Pharmaceutical Medicine, antioxidants are “substances (e.g., vitamin C, sulphites, ascorbyl palmitate, alkyl gallate, hydrochinone, tocopherols) used in pharmaceutical formulations to inhibit the reaction with oxygen in the surrounding atmosphere; they can react with free radicals to form stable or meta-stable products, thus terminating the oxidation reaction (radical scavenger) in contrast to pro-oxidant agents that increase oxidative stress” [2]. According to the Oxford Dictionary of Science, antioxidants are “substances that slow the rate of oxidation reactions. Various antioxidants are used to preserve foodstuffs and to prevent the deterioration of rubber, synthetic plastics, and many other materials. Some antioxidants act as chelating agents to sequester the metal ions that catalyze oxidation reactions. Others inhibit the oxidation reaction by removing oxygen free radicals” [3]. According to the Handbook of Food Preservation, “in a broad sense, antioxidants are all substances that can protect materials (not only foods) against autoxidation, irrespective of the mechanism of action. More exactly, such compounds should be called oxidation inhibitors, and only those substances that inhibit oxidation by reaction with free radicals should be called antioxidants” [4].
By far, a much larger number of works were performed and published on natural antioxidants than on synthetic ones. A search on the topic “natural antioxidants” or “synthetic antioxidants” from one of the world’s most trusted global citation databases, Web of Science, for the period 2000–2022 (accessed by 21 January 2022) showed 42,577 results on the topic “natural antioxidants” consisting of articles, proceedings papers, books and chapters, and meeting abstracts published in English, of which ~33% were published in Food Science Technology/Nutrition/Dietetics, and ~21% in Pharmacology/Pharmacy/Chemistry Medicinal, mostly published by researchers from China, India and the USA. Regarding the topic “synthetic antioxidants”, it produced only 9692 results of same type of papers published in English (~77% less than for natural antioxidants), of which ~28% were published in Food Science Technology/Nutrition/Dietetics, and ~19% in Pharmacology/Pharmacy/Chemistry Medicinal, mostly published by researchers from India, the USA and China. Despite this, synthetic antioxidants have been successfully used for their high efficacy, low cost and stability in products such as foods, pharmaceuticals or cosmetics; the interest in them has declined because of some safety and health concerns [5].
Vitamins played an essential role in nutrition research and control of the major deficiency syndromes in the 20th century, while the present interest relies on the debate of whether mortality is influenced in the long-term administration of antioxidant vitamins. In this respect, the critical review of Bjelakovic et al. concluded that consuming supplements based on antioxidant vitamin A, C and selenium did not influence mortality neither in healthy individuals, nor in patients; on the contrary, vitamin E was found to increase mortality [6]. Later, some researchers described the effectiveness of the mineral compound zinc sulfate in treating thalassemia and sickle cell diseases, as well as in preventing red blood cell dehydration, by decreasing the number of clinical infections and the number of crises, respectively [7,8].
The process of market authorization of medicines is a laborious one compared to that required for dietary supplements, being the subject of continuous evaluation in terms of safety, efficacy and extended usage. This is the reason why some products have been withdrawn, while others have been found useful in a more extended therapeutic area. Considering the antioxidant compounds, it is questionable if administration depends on evaluating the “oxidative stress status” of individuals or not, because in the absence of an index or a measurable biomarker, the “antioxidant dose” remains unclear [9].
Because new knowledge in the field of free radical biology is a great opportunity for chemists to investigate compounds that might block or activate the function of selected target molecules, there is a high requirement for further development of synthetic antioxidants. However, the current approved antioxidants are not completely portrayed and clearly classified in previous research, and there are disagreements regarding their evidence-based effectiveness in humans. Therefore, in this review, we focus on the chemical and pharmaceutical profiles of 32 principal low-molecular-weight synthetic antioxidants based on the long-held antioxidant hypothesis. Enriched interpretation was given to systematic data by using a semiquantitative method to highlight the scientific interest in the context of strengths and weaknesses of the evidence-based medicine when evaluating the effectiveness of these compounds on human health and estimating future trajectories. Moreover, the impact on public health in terms of epidemiological associations with overall-cause mortality was also discussed. The aim of our multiobjective approach, bridging chemistry with pharmacology, clinical medicine and public health, is to provide insight on synthetic antioxidants as a useful tool for multidisciplinary specialists and possibly health policy actions.
2. Study Design
A semistructured method was used to conduct this study by combining the narrative review [10] with summary bibliometrics—in particular a co-word analysis [11].
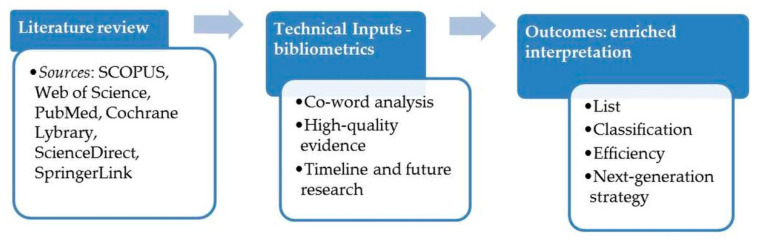
The study started with the identification of approved and experimental synthetic antioxidants using the online databases DrugBank and PubChem, followed by the keyword search using several multidisciplinary databases (SCOPUS, Web of Science), publisher databases (ScienceDirect, SpringerLink) and medicine libraries (PubMed, Cochrane Library) for relevant original articles, review-type articles, books and scientific reports. Search terms such as “antioxidant”, “synthetic antioxidant”, “approved antioxidants”, “antioxidant drugs” and “nano-antioxidants” were mainly used. The results were synthesized as much as possible in tables, charts and graphic design. Systematic data on approved synthetic low-molecular-weight antioxidants, in terms of identification, principal characteristics and biological effects, were translated into a list. Moreover, a comprehensive classification of antioxidant molecules was proposed on the basis of eight eligible criteria: mode of action, mechanism, molecular size, solubility, intended use, occurrence, biological defense and location. Regarding the summary bibliometric analysis, the quality of evidence regarding synthetic antioxidants relevant to health was the main selection criteria applied for the data mining process from systematic reviews and randomized clinical trials, respectively. Analysis was performed on articles published in the 55-year period 1966–2021 and expressed in productivity indicator (number of publications).
The workflow is summarized in Figure 1.
Figure 1.
Overview of the Synthetic Antioxidants Progression Model.
We expect that the two-stage “antioxidant description−antioxidant effects on human health” review matrix will provide a reasonably comprehensive and factual synthesis of knowledge within the context of strengths and limitations of and insight into low-molecular-weight synthetic antioxidants.
3. Approaches to Reduce Oxidative Damage
In biological systems, oxidative stress is produced when reactive oxygen species (ROS) or reactive nitrogen species (RNS) are generated in larger quantities than can be eliminated or stabilized by endogenous antioxidants, a process connected to cellular toxicity and pathologies such as cardiovascular, inflammatory, cancer and neurodegenerative [9].
The ROS, superoxide (O2•−), hydrogen peroxide (H2O2), hydroxyl radical (•OH), singlet oxygen (1O2), RNS, nitric oxide (•NO) and peroxynitrite (ONOO−), generated due to the enzymatic action of xanthine oxidase, monoamine oxidase, NADPH oxidase, nitric oxide synthase, electron transport chains, hemoglobin, auto-oxidation or photochemical reactions, may produce other unstable species called free radicals (e.g., lipid, protein or DNA radicals) [12,13].
The main approach to designing new antioxidants is based on the control of two reactions of radical species [12] by different mechanisms, as follows:
-
(1)
Control of the chain initiation oxidation reactions: (i) preventing O2•− formation through inhibition of xanthine oxidase (allopurinol); (ii) scavenging O2•− (ascorbic acid) or •OH; (iii) chelating metal ions such as Fe2+ (ascorbic acid)—preventive antioxidants;
-
(2)
Control of the chain propagation oxidation reactions: terminating/breaking the auto-oxidative chain reactions (probucol, α-tocopherol and its derivatives)—proper antioxidants/chain-breaking antioxidants.
Other approaches to designing new synthetic antioxidants are based on the fact that radical species mediate signal transduction and gene expression. Therefore, some antioxidants (N-acetyl cysteine) influence gene expression by preventing the activation of a transcription factor, NF-kB, which is triggered by H2O2. Other strategies include searching activators of the activity and expression of endogenous antioxidant enzymes (superoxide dismutase, catalase, glutathione peroxidase) or inhibitors of the activity of pro-oxidative enzymes (NADH oxidase, xanthine oxidase, lipoxygenase). Lipoxygenases are enzymes containing non-hem iron which catalyze the oxidation of polyunsaturated fatty acids, being inhibited by different antioxidants or iron chelators, such as ascorbyl palmitate, α-tocopherol and phenolics. Furthermore, therapeutic antioxidant drugs, in particular lipophilic, may act by multiple mechanisms, e.g., by scavenging ROS plus inducing physical effects on cell membranes [12]. The expression of proteins with antioxidant, anti-inflammatory and other cytoprotective properties induced by the activation of transcription factor nuclear factor-erythroid 2 p45-related factor 2 (NRF2) is one important mechanism exploited in current clinical research on several small-molecule NRF2 activators, e.g., cyclic cyanoenones [14,15].
There are countless research works dealing with the understanding of the mechanism of action of antioxidants in chemical or biological systems which can help findings of new antioxidants, in particular natural ones, generally considered safer than synthetic antioxidants [16,17].
4. Classes of Antioxidants
There is mounting research dealing with different antioxidant molecules, which were classified in several ways, the majority of the studies considering the two most frequently used criteria, the antioxidant mechanism of action (primary and secondary antioxidants) and catalytic issues (enzymatic and non-enzymatic antioxidants) [5,18,19,20,21,22,23,24]. Furthermore, scientists, industry and media representatives, and society/consumers alike often use the words “natural” and “synthetic” when referring to antioxidants. Most people give a negative connotation to the word “synthetic” in relation to a general fear of chemicals in the absence of scientific knowledge. Natural antioxidants are produced by living organisms, such as microorganisms, fungi, plants and animals, generally for their own benefits [5]. Synthetic antioxidants are molecules of various chemical structures produced by experts in the industry, for the benefit of mankind [5]. Both natural and synthetic antioxidants exhibit a certain level of toxicity [5].
Herein, we provide a new classification of antioxidants, based on eight criteria: mode of action, antioxidant mechanism, molecular size, solubility, intended use, origin/occurrence, biological defense and location, as illustrated in Table 1. Furthermore, researchers’ studies concerning antioxidants may be guided by considering the target biomolecule they protect; a great number of studies aim to develop molecules that protect lipids (tocopherols, carotenoids, ascorbate, phenolics, glutathione peroxidase, lipoic acid), proteins (preventive antioxidants, tocopherols, phenolics) or DNA (SOD, glutathione peroxidase, reduced glutathione, cysteine, vitamins) [12].
Table 1.
Complete classification of antioxidant molecules according to mechanistic, physical-chemical, biological and application criteria.
| Mode of Action | Mechanism | Molecular Size | Solubility | Applications | Origin/Occurrence and Others | |||
|---|---|---|---|---|---|---|---|---|
|
True antioxidants ascorbic acid, tocopherols, BHA, BHT, PG |
Primary/Chain-breaking catalase, glutathione peroxidase, SOD, tocopherols, BHA, BHT, TBHQ, PG |
Small ascorbic acid, tocopherols, uric acid, ubiquinol, BHA, BHT, PG, etc. |
Hydrophilic ascorbic acid, flavonoids, glutathione transferins, uric acid |
Health/Multipotent dietary supplements (vitamins), therapeutic drugs intended for various diseases related to oxidative stress (idebenone, acetylcysteine, allopurinol, etc.) |
Natural | Endogenous | Biological defense |
Enzymatic catalase, glutathione peroxidase, SOD |
|
Non-enzymatic β-carotene, Co-enzyme Q10, glutathione, α-lipoic acid, tocopherols, uric acid | ||||||||
| Location |
Intracellular catalase, glutathione peroxidase, SOD1/2 |
|||||||
|
Extracellular ascorbat, β-carotene, reduced glutathione, SOD3, uric acid | ||||||||
|
Membrane-associated α-tocopherol, lutein, zeaxanthin | ||||||||
|
Exogenous various chemical structures, e.g., vitamins (A, D3, E, B3, C), pro-vitamins (carotenoids), minerals (Se, Zn), polyphenols, organosulfur compounds (Cys, Met, glutathione) | ||||||||
|
Antioxidants synergists improve the effects of true antioxidants, e.g., sodium edetate, preservatives (organic acids, lecithin) |
Secondary/Preventive metal chelators, 1O2 quenchers, oxygen scavengers, primary antioxidants regenerators, vit C, EDTA, glutathione reductase, retinol, selenium, thiodipropionate |
Large enzymes, proteins |
Hydrophobic/Lipophilic ascorbyl palmitate, carotenoids, tocopherols, ubiquinol, BHA, BHT, PG, TBHQ |
Additives/Functional agents maintain quality of foods, pharmaceuticals, cosmetics, etc. E306, E310, E311, E312, E319, E320, E321 |
Natural-identical pure substances identical to natural ones, but synthetized by industry; ascorbic acid, β-carotene, tocopherols |
|||
|
Fully synthetic EDTA, BHA, BHT, PG, TBHQ, acetylcysteine | ||||||||
|
Nano-antioxidants various antioxidants delivered by nanoparticles such as oxides (magnetite, zinc oxides, copper oxide), mesoporous silica, chitosan, alginate, poly-D,L-lactide, polybutylcyanoacrylate, polycaprolactone, poly (lactic-co-glycolic acid) | ||||||||
Antioxidants work synergistically, protecting cells against oxidative damage in biological systems [25].
A considerable number of antioxidants are low-molecular-weight molecules, either natural or synthetic, and either hydrophilic or lipophilic, which efficiently scavenge oxygen, nitrogen- and carbon-centered radicals or inhibit chain oxidation reactions; at higher concentrations, such molecules may become pro-oxidants [12].
Generally, synthetic antioxidants are more active and pure than natural ones and possess constant antioxidant activity; on the other hand, they must pass criteria of nontoxicity and safety required by regulatory agencies prior to their marketing [5]. Natural-identical antioxidants combine the advantages of the fully synthetic (cheap, highly active, stable, reproducible properties) and the natural antioxidants (healthy). Some antioxidants provide both biological and antioxidant properties, being called bio-antioxidants, while others show only antioxidant properties without biological activity [26]. In some cases, the biological activity of an antioxidant may not be related to the antioxidant properties, as confirmed in disease models, the main challenge being that these effects (inhibition of cytokine and IL-1β production, antiproliferation) could contribute to an enhanced therapeutic drug [12].
5. Synthetic Low-Molecular-Weight Antioxidants: Properties and Pharmacological Effects
Synthetic small molecules with antioxidant activity may be used either as therapeutic agents, playing key roles as cellular antioxidants, or antioxidant additives. Such additives should be rationally used in pharmaceuticals, not to cover poorly formulated products but play key roles in the retardation of oxidation of active substances and excipients. Antioxidant additives are also used in the food industry with the purpose of retarding the oxidation of nutrients, in particular lipids and proteins. Lately, antioxidants have received attention as essential adjuvants in different types of disease.
Table 2 provides complete and useful data on synthetic and natural-identical antioxidants approved to be used in therapy, pharmaceuticals or foods, including regulatory items (FDA, Pharmacopeia standards) and safety issues related to potential adverse effects. Preservatives were not included in this study; for details, readers are invited to look up to the study of Franco et al. [27].
Table 2.
List of 32 principal approved synthetic low-molecular-weight antioxidants used as technological additives, dietary supplements or therapeutic agents (alphabetical order).
| Common Name/Alternative Names | IUPAC Name (PubChem) |
Applications/Regulatory | Description | Ref. |
|---|---|---|---|---|
| Acetylcarnitine | (R)-3-Acetoxy-4-(trimethylammonio)butyrate | Drug Dietary supplements Antioxidant in dietary supplements /ATC N06BX12 UNII NDW10MX58T |
Pharmacological properties: reduce oxidative stress in patients with Sickle Cell disease, positive effects on neurological disorders (psychostimulant, nootropic), neuropathies, potential antiviral (supportive and therapeutic option in patients with COVID-19 -clinical trial NCT04623619) | [21,28] |
| Antioxidant mechanism: decreases the generation of free radicals, prevents peroxidation of lipids, and oxidation of proteins through a tyrosine kinase A receptor-mediated mechanism; increases intracellular glutathione levels | [29,30] | |||
| Safety issues: LD50 (oral, rat) >5000 mg/kg; nonirritant to skin; nonmutagenic | [31] | |||
| Acetylcysteine (NAC) |
(R)-2-Acetamido-3-sulfanylpropanoic acid | Drug Antioxidant in cosmetics /Eur. Pharmacopoeia |
Pharmacological properties: antioxidant, mucolytic therapeutic agent, reduces the effects of acetaminophen overdose, prevents the contrast nephropathy; immune-modulating properties useful for the treatment and prevention of COVID-19 | [32,33,34,35] |
| Antioxidant mechanism: alteration of intracellular redox reactions, deacetylation to cysteine, which participates in the synthesis of the antioxidant glutathione (stimulates glutathione synthetase), scavenging different types of ROS | [36,37] | |||
| Safety issues: hypersensitivity reactions | [21] | |||
| Allopurinol (International Nonproprietary Name by WHO) |
4-Hydroxypyrazolo[3,4-d]pyrimidine | Therapeutic drug /Eur. and US Pharmacopoeia |
Pharmacological properties: antioxidant, antigout, anticancer (leukemia, lymphoma) | [12,21] |
| Antioxidant mechanism: inhibitor of xanthine oxidase, prevents O2∙− | [12] | |||
| Safety issues: rushes, lymphadenopathy, leucopenia or leukocytosis, eosinophilia, arthralgia, and vasculitis, hepatotoxic | [21] | |||
| Amiloxate (International Nonproprietary Name by WHO) |
3-Methylbutyl (E)-3-(4-methoxyphenyl)prop-2-enoate | Cosmetics /US Pharmacopeia |
Pharmacological properties: antioxidant, UV light absorber, anti-inflammatory, antimicrobial, antiedematous | [21] |
| Antioxidant mechanism of action: free radical scavenger | [38] | |||
| Safety issues: contact and photocontact allergen | [39] | |||
| Ascorbic acid (Vitamin C) | (2R)-2-[(1S)-1,2-Dihydroxyethyl]-3,4-dihydroxy-2H-furan-5-one | Dietary supplements Antioxidant in foods (E300; sodium ascorbate E301; calcium ascorbate E302), pharmaceuticals and cosmetics (ascorbyl palmitate) /FDA GRAS |
Pharmacological properties: antioxidant, scavenger of free radicals, protection of DNA damage, involved in collagen synthesis, increases the intestinal absorption of iron, antiviral/immune-modulating properties useful for treatment and prevention of COVID-19, positive effects in age-related macular degeneration, neurological disorders, atherosclerosis, cancer | [21,40] |
| Antioxidant mechanism: reducing agent, hydrogen donor forming a relatively stable ascorbyl-free radical Asc−∙ (efficient electron donor in biological redox reactions) and dehydroascorbic acid; efficiently recycles other antioxidants (e.g., α-tocopherol, glutathione); ascorbic acid regenerates itself from Asc−∙ with NADH or NADPH-reductases; in the presence of copper and iron becomes pro-oxidant | [41] | |||
| Safety issues: LD50 (oral, rat) 11,900 mg/kg, (IV, mouse) 518 mg/kg; large doses may result in hyperoxaluria and the formation of renal calcium oxalate calculi | [21,31] | |||
| Butylated hydroxyanisole (BHA) |
mixture of 2-t-Butyl-4-methoxyphenol and 3-t-Butyl-4-methoxyphenol |
Antioxidant in foods (E320), food packages, animal feed, pharmaceuticals and cosmetics /FDA (0.02% max. of fat/oil), GRAS |
Properties: high antioxidant activity; increases the levels of liver glutathione and glutathione-S-transferase; not preferred for pharmacological use, due to safety concerns. It acts synergistically with BHT, PG |
[42] |
| Antioxidant mechanism: prevents lipid peroxidation acting as hydrogen donor and interrupting the free radical autoxidative chain reactions (the resulting oxidized phenolic ion is stabilized by the inherent resonance of the benzene ring) | [43] | |||
| Safety issues: LD50 (oral, mouse) 2000 mg/kg; may cause rashes, hyperactivity; confirmed carcinogen (IARC group 2B) | [31,44] | |||
| Butylated hydroxytoluene (BHT) |
2,6-Di-t-butyl-4-methylphenol | Antioxidant in foods (E321), food packages, animal feed, pharmaceuticals and cosmetics /FDA (0.02% max. of fat/oil), GRAS |
Properties: high antioxidant activity; antiviral (inactivates lipid-containing viruses); not preferred for pharmacological use, due to safety concerns. It acts synergistically with BHA |
[42,45] |
| Antioxidant mechanism: prevents lipid peroxidation acting as hydrogen donor and interrupting the free radical autoxidative chain reactions (the resulting oxidized phenolic ion is stabilized by the inherent resonance of the benzene ring) | [43] | |||
| Safety issues: LD50 (oral, rat) 890 mg/kg, (IP, mouse) 138 mg/kg, (IV, mouse) 180 mg/kg; suspected carcinogen (IARC group 3); human skin irritant; eye irritant; may cause rashes, hyperactivity | [31,44] | |||
|
t-Butyl hydroquinone (TBHQ) |
2-t-Butylbenzene-1,4-diol | Antioxidant in foods (E319), pet foods, animal feed, pharmaceuticals and cosmetics /FDA (limitation 0.02% of oil, 0.003% in dry sausage, 0.01% in rendered animal fat, 0.02% in margarine, 0.01% on fat in poultry) |
Properties: antioxidant, antibacterial; not preferred for pharmacological use, due to safety concerns | [46] |
| Antioxidant mechanism: is a nuclear factor E2-related factor 2 (Nrf2) agonist, increases the levels of glutathione | [47] | |||
| Safety issues: LD50 (oral, rat) 700 mg/kg, (IP, rat) 300 mg/kg; tumorigenic and mutagen in experimental animals | [31,44] | |||
| β-Carotene (Provitamin A) |
1,3,3-Trimethyl-2-[(1E,3E,5E,7E,9E,11E,13E,15E,17E)-3,7,12,16-tetramethyl-18-(2,6,6-trimethylcyclohexen-1-yl)octadeca-1,3,5,7,9,11,13,15,17-nonaenyl]cyclohexene | Antioxidant in foods [E160a(i)], animal feed and cosmetics Dietary supplements /FDA GRAS |
Pharmacological properties: antioxidant, precursor of vitamin A, prevents age-related macular degeneration and ischemic heart disease, protects against cancer, antiviral; potential treatment option for COVID-19 with vitamin A | [21,48,49] |
| Antioxidant mechanism: quenching of singlet oxygen 1O2, prevents lipid peroxidation by scavenging peroxide radicals | [50] | |||
| Safety issues: nontoxic on skin; massive doses may cause yellowing of the skin; increase in cancer incidence by administration of high doses; LD50 >5000 mg/kg | [21,31] | |||
| Cholecalciferol (Vitamin D3) |
(3S,5Z,7E)-9,10-Secocholesta-5,7,10-trien-3-ol | Dietary supplements Drug Pharmaceuticals /FDA GRAS |
Pharmacological properties: antioxidant (despite some studies showing controversy), therapeutic potential in diseases related to oxidative stress, anti-ricket, enhances absorption of calcium and phosphorus along the small intestine, anti-inflammatory, cardioprotective, potential in the treatment of COVID-19 (clinical trial phase 1, 2019, NCT04407286) | [51,52,53] |
| Antioxidant mechanism: reduces lipid peroxidation, induces antioxidant enzymes (SOD), stimulates the enzyme sirtuin 1 involved in reduction in oxidative stress and inflammatory response | [51] | |||
| Safety issues: LD50 (oral, rat) 42 mg/kg; experimental teratogen | [31] | |||
| Cysteine | (2R)-2-Amino-3-sulfanylpropanoic acid | Antioxidant in foods (E920), pharmaceuticals and cosmetics Dietary supplements /FDA GRAS, Eur. and US Pharmacopoeia |
Pharmacological properties: antioxidant, prevention of corneal ulceration after chemical burn, skin-whitening | [54] |
| Antioxidant mechanism of action: reducing agent, precursor of reduced glutathione (GSH), chain-breaking antioxidant mechanism; in foods (fruits), L-Cys inhibits the activity of polyphenol oxidase and reduces browning by combination with reactive electrophilic quinones | [55,56] | |||
| Safety issues: LD50 (IP, mouse) 1250 mg/kg, (IV, mouse) 771 mg/kg | [31] | |||
| Dioxybenzone | (2-Hydroxy-4-methoxyphenyl)-(2-hydroxyphenyl)methanone | Dermatological drug (sunscreen in cosmetics) /US Pharmacopeia |
Pharmacological properties: antioxidant, UV light absorber, skin cancer chemopreventive | [57] |
| Antioxidant mechanism: scavenging free radicals and relieving of oxidative stress related to cancer from UV exposure | [57] | |||
| Safety issues: LD50 (oral, rat) >10 g/kg; nontoxic in single oral doses; nonirritating to rabbit eye or skin; | [32] | |||
| Disodium EDTA (disodium edetate) |
Disodium 2-[2-[bis(carboxymethyl)amino]ethyl-(carboxymethyl)amino]acetate | Foods (calcium disodium EDTA E385; Disodium EDTA E386) Drug (Calcium disodium EDTA) Pharmaceuticals Cosmetics /FDA, Eur. and US Pharmacopoeia |
Pharmacological properties: antioxidant, metal chelating, for treatment of lead poisoning (Calcium disodium EDTA) or hypercalcemia (disodium EDTA), potential use in COVID-19 | [31,58] |
| Antioxidant mechanism: reduces metal-induced free radical production, protects against DNA damage and lipid oxidation | [59] | |||
| Safety issues: LD50 (oral, rat) 2 g/kg, (IV, mouse) 56 mg/kg, (IP, mouse) 260 mg/kg; experimental teratogen, reproductive effects; mutagenic data | [32,60] | |||
| Erythorbic acid (isoascorbic acid, isovitamin C) |
(2R)-2-[(1R)-1,2-Dihydroxyethyl]-3,4-dihydroxy-2H-furan-5-one | Antioxidant in foods (E315; sodium erythorbate E316) and pharmaceuticals /FDA GRAS for E315 |
Pharmacological properties: antioxidant, antimicrobial, enhance iron bioavailability, antitumor | [31,61,62,63] |
| Antioxidant mechanism: sodium erythorbate is a reducing agent acting similar to ascorbic acid despite it lacking vitamin C activity, and inhibits nitrite reaction in meat curing | [64] | |||
| Safety issues: Nontoxic; mutagenic; causes DNA damage; LD50 (oral, mouse) 8300 mg/kg and LD50 (oral, rats) 18 g/kg (erythorbic acid); LD50 (oral, rats) >5 g/kg (sodium erythorbate) | [31,62] | |||
| Etidronic acid | (1-Hydroxy-1-phosphonoethyl)phosphonic acid | Drug (etidronate disodium) Antioxidant in pharmaceuticals and cosmetics /FDA, Eur. and US Pharmacopeia |
Pharmacological properties: antioxidant, chelating agent for heavy metal ions, reduces osteoclastic activity (treatment of Paget’s disease, osteoporosis), increase the bone mineral density, inhibition of protein tyrosine phosphatase | [65,66] |
| Antioxidant mechanism: calcium, iron chelator inhibiting the chondrocyte lipid peroxidation, suppress radical formation | [67] | |||
| Safety issues: LD50 (oral, mouse) 1800 mg/kg; impairment of bone mineralization, hypercalcemia, esophageal cancer | [21,32] | |||
| 4-Hexylresorcinol | 4-Hexylbenzene-1,3-diol | Flavoring agent in food (E586) Topical antiseptic Cosmetics /Eur. and US Pharmacopeia |
Pharmacological properties: antioxidant, antiseptic, antihelmintic, local anesthetic, antiviral (against parainfluenza virus type 3) | [21,68] |
| Antioxidant mechanism: inhibits tyrosinase, increases glutathione levels preventing DNA damage, scavenging of peroxyl radicals and oxygen superoxide, reduces lipid and protein peroxidation | [69] | |||
| Safety issues: LD50 (oral, rat) 550 mg/kg; LDLo (IP, mouse) 50 mg/kg, (subcut., mouse) 750 mg/kg; may irritate eyes, skin, respiratory tract; experimental reproductive effects | [31] | |||
| Idebenone | 2-(10-Hydroxydecyl)-5,6-dimethoxy-3-methylcyclohexa-2,5-diene-1,4-dione | Drug (nootropic, antioxidant therapy) /EMA |
Pharmacological properties: antioxidant, stimulates ATP production, neuroprotective (dementia, Alzheimer’s disease), treatment of visual impairment in patients with Leber’s Hereditary Optic Neuropathy; adjuvant for secondary effects of viral infection | [21,70,71] |
| Antioxidant mechanism: scavenger of free radicals (superoxide) being recycled by mitochondrial and cytosolic reductase), inhibits lipid peroxidation in mitochondrial membrane; electron donor to mitochondrial electron transport chain | [70] | |||
| Safety issues: LD50 (oral, rat) 10 g/kg, LD50 (IP, rat) 757–886 mg/kg | [70] | |||
| Lipoic acid | 5-[(3R)-1,2-Dithiolan-3-yl]pentanoic acid | Drug (antioxidant therapy of diabetic neuropathy) Nutraceutical /Eur. and US Pharmacopeia |
Pharmacological properties: antioxidant, analgesic, treatment of diabetic neuropathy, detoxification of mercury in brain cells, treatment of multiple sclerosis (clinical trial 2021) and schizophrenia (clinical trial 2021), potential antiviral including COVID-19 | [21,72,73,74] |
| Antioxidant mechanism: iron, copper chelation, scavenging ROS, the reduced form (dihydrolipoic acid) regenerates endogenous antioxidants (vitamin C, vitamin E, glutathione) repairing oxidative damage | [75] | |||
| Safety issues: cholestatic hepatitis; LD50 (oral, rats) >2000 mg/kg; no mutagenic (Ames assay), not genotoxic (mouse micronucleus assay) | [21,76] | |||
| Melatonin | N-[2-(5-Methoxy-1H-indol-3-yl)ethyl]acetamide | Drug /US Pharmacopeia |
Pharmacological properties: antioxidant, increases GABA and serotonin, efficient in sleep and autistic disorders, anticancer, sunburn prevention, potential positive effect on COVID-19 (clinical trial 2021) | [21,77,78] |
| Antioxidant mechanism: direct free radical scavenging, stimulation of antioxidant enzymes, lowering of free radical generation by increasing oxidative phosphorylation in mitochondria and reducing electron leakage, protects against DNA damage | [79,80] | |||
| Safety issues: LD50 (oral, mouse) 1250 mg/kg | [81] | |||
| Methionine | (2S)-2-Amino-4-methylsulfanylbutanoic acid | Dietary supplements Food and pharmaceutical flavoring agent Feed additive /FDA, Eur. and US, Pharmacopeia, FEMA GRAS |
Pharmacological properties: antioxidant, antihepatotoxic, alternative to acetylcysteine in the treatment of acetaminophen overdose, metal chelating, precursor of cysteine; anticancer (clinical trial phase 2, 2021) | [21,82] |
| Antioxidant mechanism: reduces ROS levels due to Met-sulfoxide reductase, activation of endogenous antioxidant enzymes, stimulating glutathione synthesis, heavy metal chelator | [83] | |||
| Safety issues: LD50 (oral, rat) 36 g/kg, (IP, rat) 4328 mg/kg; as precursor of homocysteine, high doses may result in susceptibility of cardiovascular disease, type-2 diabetes, brain alterations | [31,84,85] | |||
| Niacinamide (Nicotinamide) |
Pyridine-3-carboxylic acid amide | Dietary supplements Food fortification Feed additive (nutritional) Pharmaceutical intermediate Cosmetics /FDA GRAS, Eur., US Pharmacopeia |
Pharmacological properties: antioxidant, prevents pellagra, precursor of coenzymes (NAD) involved in electron transfer reactions in the respiratory chain, skin stimulant, anticancer, reduces LDL, improves HDL, early Alzheimer’s disease treatment (clinical trial phase 2, 2021) | [21,31,32,86] |
| Antioxidant mechanism: scavenging •OH, 1O2 and superoxide O2•−, inhibits the initiation step of lipid peroxidation, increases glutathione levels, protects against both lipid and protein oxidation in brain | [86] | |||
| Safety issues: LD50 (oral, rat) 3500 mg/kg, (IP, mouse) 2050 mg/kg, (subcut., rat) 1680 mg/kg | [31] | |||
| Pentoxifylline (International Nonproprietary Name by WHO) |
3,7-Dimethyl-1-(5-oxohexyl)purine-2,6-dione | Drug /FDA, Eur. and US Pharmacopeia |
Pharmacological properties: antioxidant, anti-inflammatory, immunomodulatory, treatment of peripheral vascular disorders, inhibits the production of the cytokine TNFα, treatment of Diabetic Kidney Disease (clinical trial phase 4, 2021); antiviral potential adjuvant in treatment of COVID-19 (clinical trial) | [21,87,88] |
| Antioxidant mechanism: scavenges free radicals (∙OH), decreases lipid peroxidation | [89] | |||
| Safety issues: LD50 (oral, rat) 1385 mg/kg | [90] | |||
| Probucol (International Nonproprietary Name by WHO) |
2,6-Di-t-butyl-4-[2-(3,5-ditert-butyl-4-hydroxyphenyl)sulfanylpropan-2-ylsulfanyl]phenol | Drug US Pharmacopeia |
Pharmacological properties: antioxidant, anticholesterolemic | [21] |
| Antioxidant mechanism: inhibits lipid peroxidation | [91] | |||
| Safety issues: LD50 (oral, mouse, rat) >5000 mg/kg | [92] | |||
| Propyl gallate (PG) |
Propyl 3,4,5-trihydroxybenzoate | Antioxidant in food (E310), food packages, food-contact coatings, feed, pharmaceuticals and cosmetics /FDA (0.005% migrating from food pkg., 0.02% max. of fat or oil, GRAS, Eur. Pharmacopeia |
Pharmacological properties: antioxidant, hepatoprotector, limited antibacterial and antifungal activity | [21,31] |
| Antioxidant mechanism: hydrogen donor interrupting the free radical autoxidative chain reactions | [43] | |||
| Safety issues: LD50 (oral, rat) 3.8 g/kg, (IP, rat) 0.38 g/kg; skin irritant; questionable carcinogen; experimental tumorigenic, teratogen, reproductive effects | [21,31,44] | |||
| Propylene glycol (PEG) |
Propane-1,2-diol | Food emulsifier (E1520) Pet foods Agent for pharmaceuticals and cosmetics (viscosity control) /FDA GRAS, US and Eur. Pharmacopeia |
Properties: antiseptic, humectant, efficient solvent and extractant of active ingredients including antioxidants | [31] |
| Antioxidant mechanism: propylene glycol mannate sulfate induces the antioxidant enzymes (superoxide dismutase, glutathione peroxidase, catalase) eliminating the oxygen free radicals (study on hyperlipidemic rats) | [93] | |||
| Safety issues: LD50 (oral, rat) 21 g/kg; ocular and skin irritant; no reproductive toxicity; not mutagenic, not carcinogenic | [31,94] | |||
| Retinol (vitamin A) |
(2E,4E,6E,8E)-3,7-Dimethyl-9-(2,6,6-trimethylcyclohexen-1-yl)nona-2,4,6,8-tetraen-1-ol | Dietary supplements Cosmetics /FDA GRAS, Eur. and US Pharmacopeia |
Pharmacological properties: antioxidant, role in vision, epithelial differentiation, growth, bone development, immunity, anticancer, anti-inflammatory, potential immunomodulator in COVID-19 | [95,96] |
| Antioxidant mechanism: scavenges lipid peroxyl radicals (LOO∙) by forming a stable trans-retinol radical intermediate, reduces DNA damage (studies in cancer therapy), stimulates endogenous antioxidant enzymes | [97] | |||
| Safety issues: daily intakes of Vitamin A >50,000 IU in adults and 20,000 IU in infants and young children may cause toxic manifestations; LD50 (oral, rat, 10 day) 7910 mg/kg, (oral, mouse) 6060 mg/kg; experimental reproductive effects; hepatomegaly, visual disturbances | [98,99] | |||
| Selenious acid | Selenous acid | Pharmaceuticals Supplements /US Pharmacopeia |
Pharmacological properties: antioxidant, enzymatic cofactor (glutathione peroxidase), anticancer, stimulates hemoglobin synthesis in erythroleukemia cell lines, immunomodulatory, prevention of atherosclerosis | [21,100] |
| Antioxidant mechanism: selenium is a Cu+ chelator, inhibits DNA damage from •OH radical, maintains the enzymatic activity of glutathione peroxidase | [16] | |||
| Safety issues: LD50 7 mg Se/kg for sodium selenite, 138 mg Se/kg for selenium sulfides (as formulated for anti-dandruff shampoos), and 6700 mg Se/kg for elemental selenium | [101] | |||
| Sitosterol | (3S,8S,9S,10R,13R,14S,17R)-17-[(2R,5R)-5-Ethyl-6-methylheptan-2-yl]-10,13-dimethyl-2,3,4,7,8,9,11,12,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-ol | Dietary supplements Stabilizer in pharmaceuticals, cosmetics /Canadian Provisional DSL |
Pharmacological properties: antioxidant, hypolipidemic, treatment of diaper rash, anti-inflammatory, antiapoptotic, anticancer, potential role as immunostimulant and inhibitor of SARS-CoV-2 spike glycoprotein | [21,31,32,102] |
| Antioxidant mechanism: reduces liver lipid peroxidation in induced cancer colon, maintains the level of antioxidant enzymes (catalase, superoxide dismutase, glutathione peroxidase, glutathione reductase, glutathione S-transferase, reduced glutathione) | [103] | |||
| Safety issues: LD50 (oral, mouse) >25,000 mg/kg; skin and eye irritant; nontoxic | [31] | |||
| Thiodipropionate | 3-(2-Carboxylatoethylsulfanyl)propanoate | Foods (0.02% of fat or oil content of food) Food packages Pharmaceuticals Cosmetics (0.1% and rarely exceed 0.2%) /FDA |
Properties: antioxidant, skin lightening. Acts synergistically with phenols |
[104] |
| Antioxidant mechanism: chain-breaking, decomposes hydrogen peroxide | [105] | |||
|
Safety issues: Dilauryl thiodipropionate: LD50 (oral, rat) >10.3 g/kg; no known toxicity; eye irritant Distearyl thiodipropionate: LD50 (oral, rat) >2500 mg/kg, (IP, rat) >2 g/kg; |
[31] | |||
| α-Tocopherol (vitamin E) |
(2R)-2,5,7,8-Tetramethyl-2-[(4R,8R)-4,8,12-trimethyltridecyl]-3,4-dihydrochromen-6-ol | Dietary supplements Antioxidant in food (E306; α-tocopherol E307; γ-tocopherol E308; δ-tocopherol E309), food packages, animal feed, pharmaceuticals and cosmetics /FDA GRAS |
Pharmacological properties: high antioxidant activity, anticancer, prevents atherosclerosis, cardiovascular diseases and age-related macular degeneration | [106,107] |
| Antioxidant mechanism: directly reacts and neutralizes •OH, alkoxyl and lipid peroxyl (ROO∙) radicals stopping the ROS-induced damage; may be regenerated with vitamin C | [16] | |||
| Safety issues: TDLo (oral, rat) 7500 mg/kg | [31] | |||
| Ubiquinone (ubidecarenona, coenzyme Q 10) |
2-[(2E,6E,10E,14E,18E,22E,26E,30E,34E)-3,7,11,15,19,23,27,31,35,39-decamethyltetraconta-2,6,10,14,18,22,26,30,34,38-decaenyl]-5,6-dimethoxy-3-methylcyclohexa-2,5-diene-1,4-dione | Dietary supplements Antioxidant in pharmaceuticals and cosmetics /US Pharmacopoeia |
Pharmacological properties: antioxidant, cofactor in the mitochondrial electron transport chain, useful in the treatment of cardiovascular diseases, Parkinson’s, fibromyalgia, migraine, diabetes, adjuvant in COVID-19 (clinical trial phase 2, 2021, NCT04960215, 2020-005961-16) | [31,108,109] |
| Antioxidant mechanism: reduces lipid peroxidation, increases antioxidant enzymes (catalase, superoxide dismutase, glutathione peroxidase) | [110] | |||
| Safety issues: lethal dose >2000 mg/kg (oral, rat) | [111] | |||
| Zinc | Zinc | Dietary supplements Antioxidant in pharmaceuticals (zinc glycinate) Cosmetics /FDA FDA GRAS (zinc gluconate) |
Pharmacological properties: antioxidant, enzyme activator, antimicrobial, antidiarrheic, anti-inflammatory, antitumor (clinical trial NCT04488783), antiviral including protection against COVID-19 | [31,112,113] |
| Antioxidant mechanism: induces antioxidant glutathione and enzymes (SOD, glutathione S-transferase, hemeoxygenase-1); protection of protein –SH groups; gene regulation (p53, NF-kB, AP-1) | [114] | |||
|
Safety issues: Zinc acetate: LD50 (oral, rat) 2510 mg/kg Zinc carbonate: TDLo (oral, mouse) 2800 mg/kg; experimental teratogen Zinc chloride: LD50 (oral, rat) 350 mg/kg; irritant, questionable carcinogen; experimental tumorigenic, teratogen, reproductive effects Zinc citrate: LD50 (oral, rat) >5000 mg/kg; experimental reproductive effects Zinc gluconate: LD50 (oral, mouse) 1290 mg/kg; experimental reproductive effects Zinc sulfate: LD50 (oral, rat) 2949 mg/kg; irritant, questionable carcinogen; experimental tumorigenic, teratogen, reproductive effects |
[31] |
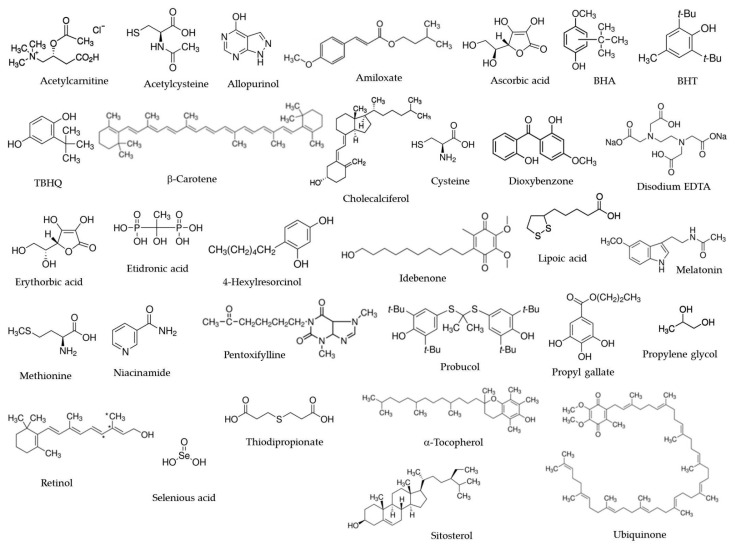
Synthetic low-molecular-weight antioxidants are compounds of different chemical structures, such as alcohols/diols, phenols, benzene derivatives, isoprenoids, aldehydes, amino acid derivatives, indole-amines, fatty acid derivatives, etc., as depicted in Figure 2.
Figure 2.
Chemical structure of approved principal synthetic low-molecular-weight antioxidants (alphabetical order).
There are synthetic drugs designed for treating specific pathological disorders, e.g., dyslipidemias, which demonstrate in vivo or in vitro antioxidant activity, such as probucol. As shown in Table 2, there are several synthetic and natural-identical antioxidants (acetylcarnitine, acetylcysteine, ascorbic acid, β-carotene, cholecalciferol, α-lipoic acid, pentoxifylline, ubiquinone, zinc compounds) that have been considered a supportive and therapeutic option in patients with COVID-19, while other antioxidants such as β-sitosterol were investigated for SARS-CoV-2 infection (for references, see Table 2).
The process of discovery of new synthetic antioxidants as therapeutic drugs is a difficult one, as it requires the identification of bioactive chemical structure, the elucidation of the mechanism of action using different model systems, in vitro and in vivo, and the toxicological evaluation. The identification of new efficient antioxidant additives intended for pharmaceutical use requires complete physical-chemical characterization, solubility and stability studies and analytical strategies for individual pharmaceutical formulation. Next, the process of pharmacokinetics and pharmacodynamics of each drug candidate continues with experimental research (preclinical studies) on cell cultures (in vitro studies) and on lab animals (in vivo tests prior to human testing) to ensure safety for further clinical studies on human subjects (randomized clinical trials); the most accurate evidence has been provided by systematic reviews and meta-analysis of clinical trials. The marketed product will be further evaluated in terms of efficacy and adverse effects, with the updates and decisions being publicly available.
6. Effectiveness of Antioxidant Intervention in Human Health
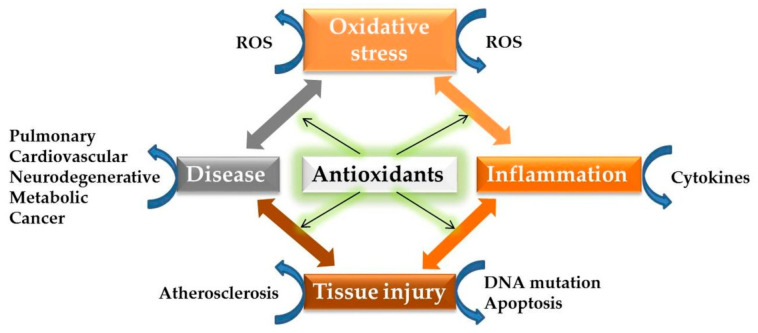
Within the defense system against oxidative stress, most exogenous antioxidants act mainly as radical scavengers that suppress chain initiation, or break chain propagation reactions, so-called primary antioxidants [22]. According to Forman et al., the assumption of the scavenging mechanism cannot be substantiated on a kinetic basis in vivo, thus explaining the limited effectiveness of small molecules in the therapeutic area [115]. An excessive increase in ROS may conduct to several pathological conditions, and finally to disease. This pathway consists of a positive feedback loop between oxidative stress and inflammation, with factors such as ROS and cytokines substantially contributing to inflammation-induced diseases, e.g., atherosclerosis, carcinogenesis, metabolic and neurodegenerative disorders, the process of which is illustrated in Figure 3. Understanding the spatial and temporal features of molecular signaling and cellular consequences within this cycle is helpful for the timing of intervention with targeted therapies [9].
Figure 3.
Effectiveness of antioxidants in the disease-specific pathways.
Figure 3 points out the optimal benefit of antioxidant administration, which is included in the prevention protocol of certain diseases rather than in the therapeutic one. One reasonable argument could be the variable proportion of oxidative stress in the causality of diseases; thus, antioxidants are more often used to ameliorate symptoms.
Another reasonable argument in favor of antioxidant intervention in the first two stages of the disease-specific pathway could be the ability of some antioxidants to reduce inflammation, as confirmed for idebenone, explaining the pleiotropic protective effects via distinct signaling events [116]. In our opinion, the future belongs to a new understanding of the bioactivities of synthetic compounds at a molecular level, which could cut off both oxidative and inflammatory events.
Oxidative stress is not only involved in chronic inflammation [117], but also in the immune response to viral respiratory infections. Recent findings suggested elevated expression of genes involved in ROS production as responsible for the “cytokine storm”—a paradoxical hyper-inflammation in SARS-CoV-2 infection [118]. Early control of the “cytokine storm” is the key to reducing the severity of illness and mortality, as many authors agree [119]. Worldwide, efforts have been made to manage the COVID-19 pandemic, and paramount ongoing research is focused on multiple therapeutics, drug combination and antioxidants, among other targeted drugs. In this respect, N-acetyl cysteine alone or in combination with elastase inhibitors, melatonin, synthetic organoselenium compounds such as Ebselen or high doses of vitamins C or D3 are just a few examples of antioxidant pharmaceuticals proposed to be used in patients with severe COVID-19, and subject of registered clinical trials as well [120,121,122,123,124,125,126,127,128,129].
Time, route (oral/parenteral), and the form of administration (high/maintenance dose) are several key factors to be considered when evaluating the effectiveness and efficacy of these pharmaceuticals. In cancer, for example, concomitant administration of drugs with natural or synthetic antioxidants is not recommended because the effectiveness of chemotherapy is decreased [130]. In preterm infants, an increased risk of bleeding in the brain associated with extra vitamin E given I.V. has been demonstrated, while the risk decreased when the extra vitamin E was administrated by other routes [131]. Moreover, accurate determination of individual’s oxidative stress levels is recommended before prescribing the appropriate antioxidant supplement [132].
6.1. Dietary Antioxidants
Recent systematic reviews concluded that the overall quality of evidence considering antioxidant vitamins was low and few results were positive, as follows: (i) vitamin D3 may reduce the requirement for COVID-19 patients to be put on a ventilator [133]; (ii) vitamin C improved exercise-induced bronchoconstriction [134]; (iii) combination of vitamins C and E and β-carotene proved protection of the macula against age-related deterioration [135]; (iv) combination of vitamin C and β-carotene maintained the cognitive function in the elderly upon long-term use [136]. Furthermore, results from systematic meta-analyses on the relationship between vitamin supplementation and mortality showed no effect of vitamins C, D3 and E on all-cause mortality [137,138,139], while zinc supplementation in children under 5 years old significantly reduced the risk of all-cause mortality [140]. Cancer mortality (total) was significantly reduced by vitamin D3 supplementation, as demonstrated by an updated meta-analysis [141]. From the perspective of public health significance, mortality reduction proves the performance of proper prevention and/or treatment strategies as the major goal of increasing the quality of life.
Research for new adjuvant therapies in rare diseases found the combination of vitamin E, vitamin C, zinc gluconate and selenomethionine to be effective in facioscapulohumeral muscular dystrophy [142]. This combination was approved as medicines under investigation in the European Union. The disease affects less than 1 in 10,000 people and is believed to be linked to oxidative stress.
6.2. Medicines in Use (Internal)
Since oxidative stress plays a crucial role in the pathogenesis of atherosclerosis and subsequently in the onset of cardiovascular disease, anti-atherogenic drugs have been developed to control oxidized low-density-lipoprotein cholesterol and implicitly endothelial damage and plaque formation. In this respect, several antioxidants such as probucol and the metal chelator EDTA were tested or reviewed, but results were inconsistent—no statistical significance was found in the case of probucol in the PROSPECTIVE clinical trial [143], while insufficient evidence was observed to determine the effectiveness or ineffectiveness of the chelation therapy [144].
6.3. Cosmeceuticals
Dietary antioxidants were found to be effective in cosmeceutical applications, in particular some combinations of vitamins C and E intended for skin anti-aging and skin radiance [145,146]. Topically applied melatonin provided effective protection against the harmful effects of UV radiation and skin damage [147], being also recommended for the treatment of androgenic alopecia in the new formulation with lipid nanocarriers [148]. Alongside other advances in this field, the new disciplines, skin chronopharmacology and preventive skin medicine, have been shaped.
6.4. Antioxidant Additives
Synthetic antioxidant additives have been used in food, pharmaceuticals or cosmetics in order to protect nutrients or bioactive compounds against oxidation and thus to extend the shelf life of products. The main approach to develop novel antioxidant additives seems to be the chemical manipulation of natural antioxidants, in particular in terms of lipophilicity (e.g., nonpolar esters of polyphenols/phenolic acids/caffeic acid, substituted by aminophenol analogues) [149]. The synthesis of innovative antioxidant additives is required because of the harmful effects exhibited by some of the most widely used antioxidants, as documented for BHA, BHT and TBHQ [31,44].
A complete review on food antioxidant additives, natural and synthetic, in relation to their properties, mechanism of action, legislation and applications have been published by Carocho et al. [150].
Excipients with antioxidant properties play a fundamental role in pharmaceutical formulations, as it preserves the efficacy, safety and stability of the bioactive compound. However, the added antioxidant itself should prove efficacy and lack of toxicity. The results of recent research suggest that the synthetic additive propylene glycol, used in the fourth generation of e-cigarettes, may induce airway epithelial injury and tissue hypoxia in young tobacco smokers, and also transiently impair arterial oxygen tension in heavy smokers during heating [151]. In our opinion, this issue could be a starting point for more policy action in regulating electronic cigarettes; thus, further evidence is required.
Common synthetic and natural-identical antioxidant additives used in cosmetics are BHA, BHT, TBHQ, propyl gallate, ascorbic acid, β-carotene, retinyl palmitate, tocopherols, cysteine, niacinamide, acetylcysteine, dioxybenzone, EDTA and hexylresorcinol [32].
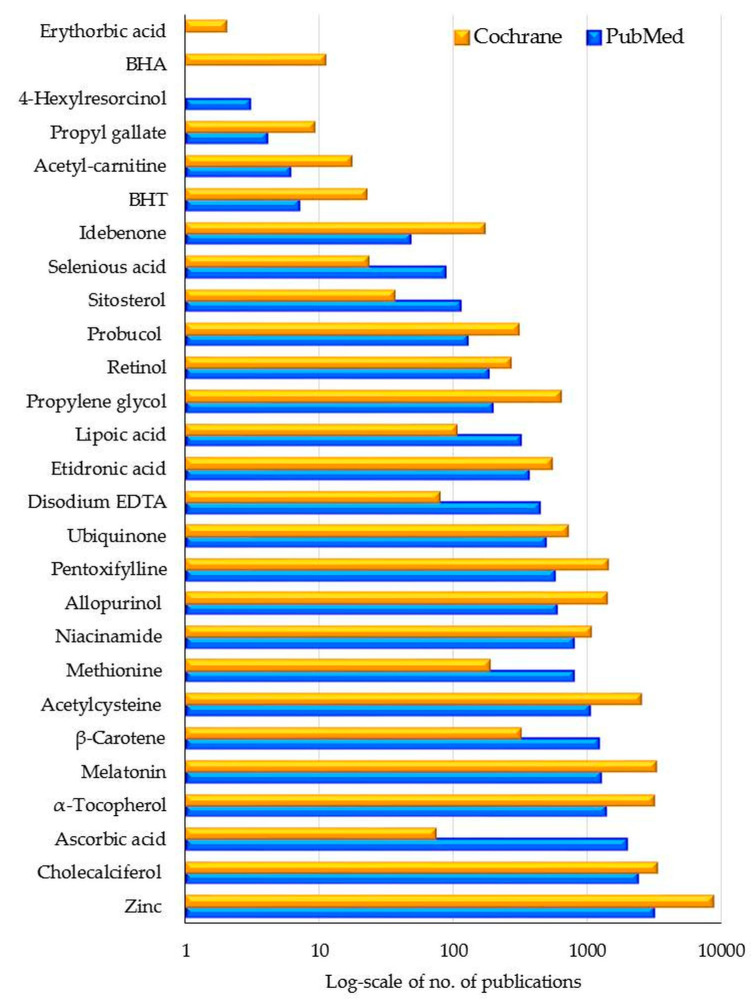
6.5. Summary Bibliometric Data and Future Trends
Bibliometric analysis of the global research output in the field of synthetic antioxidants selected by us and described in Table 2 consisted of 17,260 articles extracted from PubMed, 27,794 articles extracted from Cochrane Library, respectively. Dietary antioxidants were of high interest in human health, particularly zinc and cholecalciferol, as shown in Figure 4. Five of the total 32 antioxidants were not the subjects of medical research dealing with the synthetic approaches and were not included in Figure 4, namely amiloxate, TBHQ, cysteine, dioxybenzone, and thiodipropionate. According to the results by year provided by PubMed, this volume of literature evolved with time as follows: in the last two decades paramount research was dedicated to zinc, cholecalciferol, ascorbic acid, melatonin, acetylcysteine, methionine, niacinamide, allopurinol, ubiquinone, lipoic acid, propylene glycol and sitosterol; between 2000 and 2014, research was representative for α-tocopherol, retinol and etidronic acid; and β-carotene was of high interest until 2004.
Figure 4.
The number of publications on synthetic antioxidants as resulted from the bibliometric analysis of systematic reviews and randomized clinical trials using Cochrane Library and PubMed database (log-scale plot).
Most of the research started in 1966–1975; ubiquinone and retinol raised interest in the 1980s; research on idebenone and selenious acid began in the 1990s, while propyl gallate has been associated with human benefits since 2005.
More than a technical input, this analysis is intended to add contextual value of a „good antioxidant” and their potential utility in health policy, industry, communities and the research field. Looking for scientific literature, it can be concluded that research on the vitamins continues to evolve from their discovery 100 years ago [152].
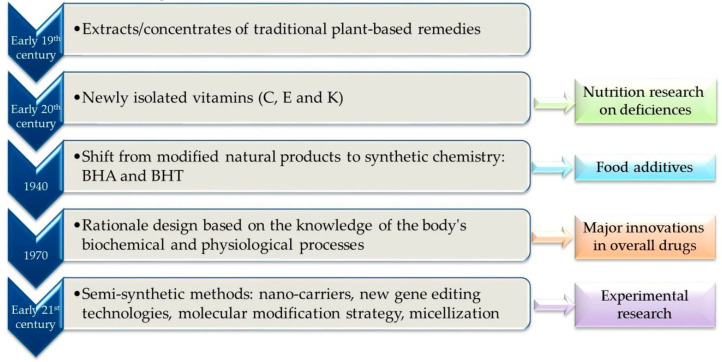
Given the historical interest of direct application of chemical research to medicine [153], our understanding of the rational design of molecular-targeted compounds and the next strategies may be enriched with a timeline of research in the field as illustrated in Figure 5.
Figure 5.
Timeline of important discoveries in the field and ongoing research instruments.
The current research on synthetic antioxidants mainly follows three objectives, as follows: (1) enhancement of the bioavailability and efficacy of oral pharmaceuticals through novel formulations, e.g., nano-antioxidants; (2) modification or improvement of the chemical structures/moieties of existing compounds; and (3) design and synthesis of novel derivatives using a building block molecule, such as the well-known BHT. Despite the potential harmful effects of BHA, BHT and TBHQ due to their cytotoxic metabolites [44], experimental research is challenging because some authors found that BHA, TBHQ and 0.5/2 combination of BHT/BHA act as inhibitors of TNF and protect cells against apoptosis [154,155]. Indolinonic hydroxylamine emerged as a promising synthetic antioxidant [156,157], as shown by in vivo models of diabetic complications [158]. The list of novel compounds is open and may ground future research on effectiveness as well as debatable issues. Some of them could be medicines under evaluation, e.g., omaveloxolone, which is a synthetic oleanane triterpenoid to be used in patients with Friedreich’s ataxia [159], or bardoxolone-methyl for chronic kidney disease, type 2 diabetes mellitus and diabetic nephropathy—both acting as NRF2 activators [160]. The approved dimethyl fumarate and diroximel fumarate as medicines in the European Union have been developed as immunomodulators to treat relapsing forms of multiple sclerosis, but they also work via NRF2, fostering antioxidative pathways and increasing glutathione levels [161,162,163,164].
Several strategies have been developed since the 1980s aiming to find the best fit formulation of bioactive compounds, in terms of stability, solubility and efficacy. In the last decade, nanotechnology appears to be a very promising approach to develop novel antioxidant agents, as nanoparticles can be better controlled in terms of appropriate usage. Nano-antioxidants may refer to nanoparticles functionalized with antioxidants, the encapsulation of antioxidants into nanocarriers or nanoparticles/nanomaterials with intrinsic antioxidant properties such as metals and carbon structures-C60 and their derivatives, carbon nanotube and graphene [165,166,167,168]. For detailed information on nano-antioxidants, readers are invited to look up to the study of Khalil et al. [169]. The formulation of micellar nanoparticles for improving solubility should also be mentioned as another advance in the field, which has been applied to Coenzyme Q10 and silymarin [170,171]. To our knowledge, a single clinical trial was dedicated to nano-antioxidants, regarding the efficacy of an antioxidant nanogel to be used in oral surgery [172]. Nanoparticles are expected to play a beneficial role in medicine, but their safety should be carefully evaluated, and further research is required.
7. Conclusions
Mounting research has been conducted on natural antioxidants as important molecules to control oxidative damage, more so than on synthetic ones. However, a better classification was needed to enable informed interpretation and meaning of natural, natural-identical, fully synthetic and nano-antioxidants. A focus on principal synthetic antioxidants revealed that most (75%) were developed as pharmaceuticals and medicines with pleiotropic effects. Enriched interpretation of efficacy was given by summary bibliometrics, highlighting zinc and cholecalciferol as “good antioxidants” in terms of research productivity; on the basis of high-quality evidence, their effectiveness in human health was particularly by contributing to child and cancer mortality reduction. These findings are of major public health significance regarding the pharmacological success of dietary antioxidants in improving the quality of life.
New research opportunities may come from idebenone and novel synthesized compounds, such as NRF2 activators possessing antioxidant, anti-inflammatory and cytoprotective properties expected to better control the oxidative damage at the molecular level.
With respect to antioxidant additives, the safety of BHA, BHT, TBHQ and PEG poses a potential public health concern; therefore, further investigations are required to substantiate community awareness and even policy action.
We hope that this approach will contribute to a rational pharmaceutical perspective and expect to revive the research on synthetic or natural-identical antioxidants.
Abbreviations
| ATC | Anatomical Therapeutic Chemical (ATC) classification system |
| BHA | Butylated hydroxyanisole |
| BHT | Butylated hydroxytoluene |
| COVID-19 | Coronavirus disease 2019 |
| DNA | Deoxyribonucleic Acid |
| DSL | Domestic Substance List |
| EDTA | Ethylenediamine tetra acetate |
| EMA | European Medicines Agency |
| FEMA | Flavor and Extract Manufacturers Association |
| FDA | The US Food and Drug Administration |
| GRAS | Generally Recognized as Safe |
| HDL | High-density lipoprotein |
| IL-1β | member of the Interleukin-1 family |
| IARC | International Agency for Cancer Research |
| I.P. | Intraperitoneal injection |
| IUPAC | International Union of Pure and Applied Chemistry |
| I.V. | Intravenous medication administration |
| LD50 | 50% Lethal dose |
| LDL | Low-density lipoprotein |
| NAD | Nicotinamide adenine dinucleotide, oxidized form |
| NADH | Nicotinamide adenine dinucleotide, reduced form |
| NRF2 | Nuclear factor erythroid 2-Related Factor 2 |
| PEG | Propylene glycol |
| PG | Propyl gallate |
| RNS | Reactive nitrogen species |
| ROS | Reactive oxygen species |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| SOD | Superoxide dismutase |
| TDLo | Toxic Dose Low (the lowest toxic dose) |
| THBQ | t-Butyl hydroquinone |
| TNF | Tumor necrosis factor |
| UNII | Unique ingredient identifiers, generated by the joint FDA/USP Substance Registration System |
| WHO | World Health Organization |
Author Contributions
Conceptualization, S.O. and M.S.; methodology, S.O. and M.S.; investigation, S.O. and M.S.; resources, S.O. and M.S.; data curation, S.O.; writing—original draft preparation, S.O. and M.S.; writing—review and editing, S.O.; supervision, S.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Halliwell B. How to characterize an antioxidant: An update. Biochem. Soc. Symp. 1995;61:73–101. doi: 10.1042/bss0610073. [DOI] [PubMed] [Google Scholar]
- 2.Nahler G. Dictionary of Pharmaceutical Medicine. 4th ed. Springer; Vienna, Austria: 2017. pp. 18–19. [Google Scholar]
- 3.Daintith J., Martin E. Oxford Dictionary of Science. 6th ed. Oxford University Press; Oxford, UK: 2010. p. 48. [Google Scholar]
- 4.Rahman M.S. Handbook of Food Preservation. 2nd ed. CRC Press; Boca Raton, FL, USA: 2007. p. 264. [DOI] [Google Scholar]
- 5.Pokorný J. Are natural antioxidants better—and safer—than synthetic antioxidants? Eur. J. Lipid Sci. Technol. 2007;109:629–642. doi: 10.1002/ejlt.200700064. [DOI] [Google Scholar]
- 6.Bjelakovic G., Nikolova D., Gluud L.L., Simonetti R.G., Gluud C. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database Syst. Rev. 2012;2012:CD007176. doi: 10.1002/14651858.CD007176.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swe K.M.M., Abas A.B., Bhardwaj A., Barua A., Nair N.S. Zinc supplements for treating thalassaemia and sickle cell disease. Cochrane Database Syst. Rev. 2013;6:CD009415. doi: 10.1002/14651858.CD009415.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagalla S., Ballas S.K. Drugs for preventing red blood cell dehydration in people with sickle cell disease. Cochrane Database Syst. Rev. 2018;10:CD003426. doi: 10.1002/14651858.cd003426.pub6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chatterjee S. Oxidative stress, inflammation, and disease. In: Dziubla T., Butterfield D.A., editors. Oxidative Stress and Biomaterails. Elsevier Academic Press; Amsterdam, The Netherlands: 2016. pp. 35–58. [Google Scholar]
- 10.Snyder H. Literature review as a research methodology: An overview and guidelines. J. Bus. Res. 2019;104:333–339. doi: 10.1016/j.jbusres.2019.07.039. [DOI] [Google Scholar]
- 11.Donthu N., Kumar S., Mukherjee D., Pandey N., Lim W.M. How to conduct a bibliometric analysis: An overview and guidelines. J. Bus. Res. 2021;133:285–296. doi: 10.1016/j.jbusres.2021.04.070. [DOI] [Google Scholar]
- 12.Packer L., Cadenas E. Handbook of Synthetic Antioxidants. CRC Press; Boca Raton, FL, USA: 1997. [Google Scholar]
- 13.Nakai K., Tsuruta D. What are reactive oxygen species, free radicals, and oxidative stress in skin diseases? Int. J. Mol. Sci. 2021;22:10799. doi: 10.3390/ijms221910799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naidu S.D., Dinkova-Kostova A.T. KEAP1, a cysteine-based sensor and a drug target for the prevention and treatment of chronic disease. Open Biol. 2020;10:200105. doi: 10.1098/rsob.200105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cuadrado A., Rojo A.I., Wells G., Hayes J.D., Cousin S.P., Rumsey W.L., Attucks O.C., Franklin S., Levonen A.-L., Kensler T.W., et al. Therapeutic targeting of the NRF2 and KEAP1 partnership in chronic diseases. Nat. Rev. Drug Discov. 2019;18:295–317. doi: 10.1038/s41573-018-0008-x. [DOI] [PubMed] [Google Scholar]
- 16.Lü J.-M., Lin P.H., Yao Q., Chen C. Chemical and molecular mechanisms of antioxidants: Experimental approaches and model systems. J. Cell. Mol. Med. 2010;14:840–860. doi: 10.1111/j.1582-4934.2009.00897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oancea S. A review of the current knowledge of thermal stability of anthocyanins and approaches to their stabilization to heat. Antioxidants. 2021;10:1337. doi: 10.3390/antiox10091337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flieger J., Flieger W., Baj J., Maciejewski R. Antioxidants: Classification, natural sources, activity/capacity measurements, and usefulness for the synthesis of nanoparticles. Materials. 2021;14:4135. doi: 10.3390/ma14154135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kagan V.E., Shvedova A., Serbinova E., Khan S., Swanson C., Powell R., Packer L. Dihydrolipoic acid—A universal antioxidant both in the membrane and in the aqueous phase: Reduction of peroxyl, ascorbyl and chromanoxyl radicals. Biochem. Pharmacol. 1992;44:1637–1649. doi: 10.1016/0006-2952(92)90482-X. [DOI] [PubMed] [Google Scholar]
- 20.Kumar H., Bhardwaj K., Nepovimova E., Kuca K., Dhanjal D.S., Bhardwaj S., Bhatia S.K., Verma R., Kumar D. Antioxidant functionalized nanoparticles: A combat against oxidative stress. Nanomaterials. 2020;10:1334. doi: 10.3390/nano10071334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martindale G.J. The Complete Drug Reference. 38th ed. Pharmaceutical Press; London, UK: 2014. [Google Scholar]
- 22.Pisoschi A.M., Pop A., Iordache F., Stanca L., Predoi G., Serban A.I. Oxidative stress mitigation by antioxidants—An overview on their chemistry and influences on health status. Eur. J. Med. Chem. 2021;209:112891. doi: 10.1016/j.ejmech.2020.112891. [DOI] [PubMed] [Google Scholar]
- 23.Prenzler P.D., Ryan D., Robards K. Introduction to basic principles of antioxidant activity. In: Prenzler P.D., Ryan D., Robards K., editors. Handbook of Antioxidant Methodology: Approaches to Activity Determination. The Royal Society of Chemistry; London, UK: 2021. pp. 1–62. [DOI] [Google Scholar]
- 24.Zhang H.-Y., Yang D.-P., Tang G.-Y. Multipotent antioxidants: From screening to design. Drug Discov. Today. 2006;11:749–754. doi: 10.1016/j.drudis.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 25.Kurutas E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2015;15:71. doi: 10.1186/s12937-016-0186-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kancheva V.D., Kasaikina O.T. Lipid oxidation in homogeneous and micro-heterogeneous media in presence of prooxidants, antioxidants and surfactants. In: Catala A., editor. Lipid Peroxidation. IntechOpen; London, UK: 2012. [DOI] [Google Scholar]
- 27.Franco R., Navarro G., Martinez-Pinilla E. Antioxidants versus food antioxidant additives and food preservatives. Antioxidants. 2019;8:542. doi: 10.3390/antiox8110542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diao B., Wang C., Tan Y., Chen X., Liu Y., Ning L., Chen L., Li M., Liu Y., Wang G., et al. Reduction and functional exhaustion of T cells in patients with Coronavirus disease 2019 (COVID-19) Front. Immunol. 2020;11:827. doi: 10.3389/fimmu.2020.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barhwal K., Hota S., Jain V., Prasad D., Singh S., Ilavazhagan G. Acetyl-l-carnitine (ALCAR) prevents hypobaric hypoxia–induced spatial memory impairment through extracellular related kinase–mediated nuclear factor erythroid 2-related factor 2 phosphorylation. Neuroscience. 2009;161:501–514. doi: 10.1016/j.neuroscience.2009.02.086. [DOI] [PubMed] [Google Scholar]
- 30.Shivalingappa P.C., Jin H., Anantharam V., Kanthasamy A., Kanthasamy A. N-acetyl cysteine protects against methamphetamine-induced dopaminergic neurodegeneration via modulation of redox status and autophagy in dopaminergic cells. Parkinsons Dis. 2012:424285. doi: 10.1155/2012/424285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ash M., Ash I. Handbook of Pharmaceutical Additives. 3rd ed. Synapse Information Resources; New York, NY, USA: 2007. [Google Scholar]
- 32.Ash M., Ash I. Handbook of Cosmetic and Personal Care Additives. 2nd ed. Synapse Information Resources; New York, NY, USA: 2013. [Google Scholar]
- 33.Dekhuijzen P. Antioxidant properties of N-acetylcysteine: Their relevance in relation to chronic obstructive pulmonary disease. Eur. Respir. J. 2004;23:629–636. doi: 10.1183/09031936.04.00016804. [DOI] [PubMed] [Google Scholar]
- 34.Birck R., Krzossok S., Markowetz F., Schnülle P., van der Woude F.J., Braun C. Acetylcysteine for prevention of contrast nephropathy: Meta-analysis. Lancet. 2003;362:598–603. doi: 10.1016/S0140-6736(03)14189-X. [DOI] [PubMed] [Google Scholar]
- 35.Shi Z., Puyo C.A. N-acetylcysteine to combat COVID-19: An evidence review. Ther. Clin. Risk Manag. 2020;16:1047–1055. doi: 10.2147/TCRM.S273700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sadowska A.M. N-acetylcysteine mucolysis in the management of chronic obstructive pulmonary disease. Ther. Adv. Respir. Dis. 2012;6:127–135. doi: 10.1177/1753465812437563. [DOI] [PubMed] [Google Scholar]
- 37.Aruoma O.I., Halliwell B., Hoey B.M., Butler J. The antioxidant action of N-acetylcysteine: Its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Radic. Biol. Med. 1989;6:593–597. doi: 10.1016/0891-5849(89)90066-X. [DOI] [PubMed] [Google Scholar]
- 38.Wypych A., Wypych G. Databook of UV Stabilizers. 2nd ed. ChemTec Publishing; Scarborough, ON, Canada: 2020. [Google Scholar]
- 39.Gunia-Krzyżak A., Słoczyńska K., Popiół J., Koczurkiewicz P., Marona H., Pękala E. Cinnamic acid derivatives in cosmetics: Current use and future prospects. Int. J. Cosmet. Sci. 2018;40:356–366. doi: 10.1111/ics.12471. [DOI] [PubMed] [Google Scholar]
- 40.Milani G., Macchi M., Guz-Mark A. Vitamin C in the treatment of COVID-19. Nutrients. 2021;13:1172. doi: 10.3390/nu13041172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Duarte T.L., Lunec J. Review: When is an antioxidant not an antioxidant? A review of novel actions and reactions of vitamin C. Free Radic. Res. 2005;39:671–686. doi: 10.1080/10715760500104025. [DOI] [PubMed] [Google Scholar]
- 42.Festjens N., Kalai M., Smet J., Meeus A., Van Coster R., Saelens X., Vandenabeele P. Butylated hydroxyanisole is more than a reactive oxygen species scavenger. Cell Death Differ. 2006;13:166–169. doi: 10.1038/sj.cdd.4401746. [DOI] [PubMed] [Google Scholar]
- 43.Brewer M.S. Natural antioxidants: Sources, compounds, mechanisms of action, and potential applications. Compr. Rev. Food Sci. Food Saf. 2011;10:221–247. doi: 10.1111/j.1541-4337.2011.00156.x. [DOI] [Google Scholar]
- 44.Xu X., Liu A., Hu S., Ares I., Martínez-Larrañaga M.-R., Wang X., Martínez M., Anadón A., Martínez M.-A. Synthetic phenolic antioxidants: Metabolism, hazards and mechanism of action. Food Chem. 2021;353:129488. doi: 10.1016/j.foodchem.2021.129488. [DOI] [PubMed] [Google Scholar]
- 45.Snipes W., Person S., Keith A., Cupp J. Butylated hydroxytoluene inactivated lipid-containing viruses. Science. 1975;188:64–66. doi: 10.1126/science.163494. [DOI] [PubMed] [Google Scholar]
- 46.Ooi N., Chopra I., Eady A., Cove J., Bojar R., O’Neill A.J. Antibacterial activity and mode of action of tert-butylhydroquinone (TBHQ) and its oxidation product, tert-butylbenzoquinone (TBBQ) J. Antimicrob. Chemother. 2013;68:1297–1304. doi: 10.1093/jac/dkt030. [DOI] [PubMed] [Google Scholar]
- 47.Zhao Y.-L., Zhao W., Liu M., Liu L., Wang Y. TBHQ-overview of multiple mechanisms against oxidative stress for attenuating methamphetamine-induced neurotoxicity. Oxid. Med. Cell. Longev. 2020:8874304. doi: 10.1155/2020/8874304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Naithani R., Huma L.C., Holland L.E., Shukla D., McCormick D.L., Mehta R.G., Moriarty R.M. Antiviral activity of phytochemicals: A comprehensive review. Mini-Rev. Med. Chem. 2008;8:1106–1133. doi: 10.2174/138955708785909943. [DOI] [PubMed] [Google Scholar]
- 49.Gröber U., Holick M.F. The coronavirus disease (COVID-19)—A supportive approach with selected micronutrients. Int. J. Vitam. Nutr. Res. 2021;92:13–34. doi: 10.1024/0300-9831/a000693. [DOI] [PubMed] [Google Scholar]
- 50.Huang D., Ou B., Prior R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005;53:1841–1856. doi: 10.1021/jf030723c. [DOI] [PubMed] [Google Scholar]
- 51.Kim H.A., Perrelli A., Ragni A., Retta F., De Silva T.M., Sobey C.G., Retta S.F. Vitamin D deficiency and the risk of cerebrovascular disease. Antioxidants. 2020;9:327. doi: 10.3390/antiox9040327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tagliaferri S., Porri D., De Giuseppe R., Manuelli M., Alessio F., Cena H. The controversial role of vitamin D as an antioxidant: Results from randomised controlled trials. Nutr. Res. Rev. 2019;32:99–105. doi: 10.1017/S0954422418000197. [DOI] [PubMed] [Google Scholar]
- 53.Weir E.K., Thenappan T., Bhargava M., Chen Y. Does vitamin D deficiency increase the severity of COVID-19? Clin. Med. 2020;20:e107–e108. doi: 10.7861/clinmed.2020-0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Elias R.J., McClements a.D.J., Decker E.A. Antioxidant activity of cysteine, tryptophan, and methionine residues in continuous phase β-lactoglobulin in oil-in-water emulsions. J. Agric. Food Chem. 2005;53:10248–10253. doi: 10.1021/jf0521698. [DOI] [PubMed] [Google Scholar]
- 55.Miura K., Yazama F., Tai A. Oxidative stress-mediated antitumor activity of erythorbic acid in high doses. Biochem. Biophys. Rep. 2015;3:117–122. doi: 10.1016/j.bbrep.2015.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cilliers J.J.L., Singleton V.L. Caffeic acid autoxidation and the effects of thiols. J. Agric. Food Chem. 1990;38:1789–1796. doi: 10.1021/jf00099a002. [DOI] [Google Scholar]
- 57.Rao G.S., Tokuda H., Ichiishi E., Takasaki M., Iida A., Suzuki N., Konoshima T., Kapadia G.J. Oral chemoprevention of skin cancer in mice by benzophenone sunscreens dioxybenzone and octabenzone in drinking water. Anticancer Res. 2013;33:2535–2540. [PubMed] [Google Scholar]
- 58.Cashman D.P. Why the lower reported prevalence of asthma in patients diagnosed with COVID-19 validates repurposing EDTA solutions to prevent and manage treat COVID-19 disease. Med. Hypotheses. 2020;144:110027. doi: 10.1016/j.mehy.2020.110027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Roussel A.M., Hininger-Favier I., Waters R.S., Osman M., Fernholz K., Anderson R.A. EDTA chelation therapy, without added vitamin C, decreases oxidative DNA damage and lipid peroxidation. Altern. Med. Rev. 2009;14:56–61. [PubMed] [Google Scholar]
- 60.Evstatiev R., Cervenka A., Austerlitz T., Deim G., Baumgartner M., Beer A., Krnjic A., Gmainer C., Lang M., Frick A., et al. The food additive EDTA aggravates colitis and colon carcinogenesis in mouse models. Sci. Rep. 2021;11:5188. doi: 10.1038/s41598-021-84571-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fidler M.C., Davidsson L., Zeder C., Hurrell R.F. Erythorbic acid is a potent enhancer of nonheme-iron absorption. Am. J. Clin. Nutr. 2004;79:99–102. doi: 10.1093/ajcn/79.1.99. [DOI] [PubMed] [Google Scholar]
- 62.Andersen F.A. Final report on the safety assessment of ascorbyl palmitate, ascorbyl dipalmitate, ascorbyl stearate, erythorbic acid, and sodium erythorbate. Int. J. Toxicol. 1999;18:1–26. doi: 10.1177/109158189901800303. [DOI] [Google Scholar]
- 63.Miura Y., Honda S., Masuda A., Masuda T. Antioxidant activities of cysteine derivatives against lipid oxidation in anhydrous media. Biosci. Biotechnol. Biochem. 2014;78:1452–1455. doi: 10.1080/09168451.2014.918496. [DOI] [PubMed] [Google Scholar]
- 64.Ribeiro J., Seifert M., Vinholes J., Rombaldi C., Nora L., Cantillano R. Erythorbic acid and sodium erythorbate effectively prevent pulp browning of minimally processed ‘royal gala’ apples. Ital. J. Food Sci. 2019;31:573–590. doi: 10.14674/IJFS-1451. [DOI] [Google Scholar]
- 65.Dunn C.J., Fitton A., Sorkin E.M. Etidronic acid. A review of its pharmacological properties and therapeutic efficacy in resorptive bone disease. Drugs Aging. 1994;5:446–474. doi: 10.2165/00002512-199405060-00006. [DOI] [PubMed] [Google Scholar]
- 66.Heneberg P. Use of protein tyrosine phosphatase inhibitors as promising targeted therapeutic drugs. Curr. Med. Chem. 2009;16:706–733. doi: 10.2174/092986709787458407. [DOI] [PubMed] [Google Scholar]
- 67.Dombrecht E., De Tollenaere C., Aerts K., Cos P., Schuerwegh A., Bridts C., Van Offel J., Ebo D., Stevens W., De Clerck L. Antioxidant effect of bisphosphonates and simvastatin on chondrocyte lipid peroxidation. Biochem. Biophys. Res. Commun. 2006;348:459–464. doi: 10.1016/j.bbrc.2006.07.075. [DOI] [PubMed] [Google Scholar]
- 68.Shephard A., Zybeshari S. Virucidal action of sore throat lozenges against respiratory viruses parainfluenza type 3 and cytomegalovirus. Antivir. Res. 2015;123:158–162. doi: 10.1016/j.antiviral.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fidalgo J., Deglesne P.A., Arroya R., Ranneva E., Deprez P. 4-hexylresorcinol a new molecule for cosmetic application. J. Biomol. Res. Ther. 2019;8:2. doi: 10.35248/2167-7956.19.8.170. [DOI] [Google Scholar]
- 70.Nagy I.Z. Chemistry, toxicology, pharmacology and pharmacokinetics if idebenone: A review. Arch. Gerontol. Geriatr. 1990;11:177–186. doi: 10.1016/0167-4943(90)90063-C. [DOI] [PubMed] [Google Scholar]
- 71.O’Donovan S.M., Eby H., Henkel N.D., Creeden J., Imami A., Asah S., Zhang X., Wu X., Alnafisah R., Taylor R.T., et al. Identification of new drug treatments to combat COVID19: A signature-based approach using iLINCS. Res. Sq. 2020:rs.3.rs-25643. doi: 10.21203/rs.3.rs-25643/v1. [DOI] [Google Scholar]
- 72.Dragomanova S., Miteva S., Nicoletti F., Mangano K., Fagone P., Pricoco S., Staykov H., Tancheva L. Therapeutic potential of alpha-lipoic acid in viral infections, including COVID-19. Antioxidants. 2021;10:1294. doi: 10.3390/antiox10081294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Uberti F., Ruga S., Farghali M., Galla R., Molinari C. A combination of α-lipoic acid (ALA) and palmitoylethanolamide (PEA) blocks endotoxin-induced oxidative stress and cytokine storm: A possible intervention for COVID-19. J. Diet. Suppl. 2021:1–23. doi: 10.1080/19390211.2021.1966152. [DOI] [PubMed] [Google Scholar]
- 74.Cure E., Cure M.C. Alpha-lipoic acid may protect patients with diabetes against COVID-19 infection. Med. Hypotheses. 2020;143:110185. doi: 10.1016/j.mehy.2020.110185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Biewenga G.P., Haenen G.R.M.M., Bast A. The pharmacology of the antioxidant lipoic acid. Gen. Pharmacol. 1997;29:315–331. doi: 10.1016/S0306-3623(96)00474-0. [DOI] [PubMed] [Google Scholar]
- 76.Cremer D., Rabeler R., Roberts A., Lynch B. Safety evaluation of α-lipoic acid (ALA) Regul. Toxicol. Pharmacol. 2006;46:29–41. doi: 10.1016/j.yrtph.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 77.Cross K.M., Landis D.M., Sehgal L., Payne J.D. Melatonin for the early treatment of COVID-19: A narrative review of current evidence and possible efficacy. Endocr. Pract. 2021;27:850–855. doi: 10.1016/j.eprac.2021.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shneider A., Kudriavtsev A., Vakhrusheva A. Can melatonin reduce the severity of COVID-19 pandemic? Int. Rev. Immunol. 2020;39:153–162. doi: 10.1080/08830185.2020.1756284. [DOI] [PubMed] [Google Scholar]
- 79.Reiter R.J., Tan D.X., Mayo J.C., Sainz R.M., Leon J., Czarnocki Z. Melatonin as an antioxidant: Biochemical mechanisms and pathophysiological implications in humans. Acta Biochim. Pol. 2003;50:1129–1146. doi: 10.18388/abp.2003_3637. [DOI] [PubMed] [Google Scholar]
- 80.Suzen S. Melatonin and synthetic analogs as antioxidants. Curr. Drug Deliv. 2013;10:71–75. doi: 10.2174/1567201811310010013. [DOI] [PubMed] [Google Scholar]
- 81.Sugden D. Psychopharmacological effects of melatonin in mouse and rat. J. Pharmacol. Exp. Ther. 1983;227:587–591. [PubMed] [Google Scholar]
- 82.Benavides M.A., Bosland M.C., da Silva C.P., Sares C.T.G., de Oliveira A.M.C., Kemp R., Reis R., Martins V.R., Sampaio S.V., Bland K.I., et al. L-Methionine inhibits growth of human pancreatic cancer cells. Anti-Cancer Drugs. 2014;25:200–203. doi: 10.1097/CAD.0000000000000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Catanesi M., Brandolini L., D’Angelo M., Benedetti E., Tupone M.G., Alfonsetti M., Cabri E., Iaconis D., Fratelli M., Cimini A., et al. L-methionine protects against oxidative stress and mitochondrial dysfunction in an in vitro model of Parkinson’s disease. Antioxidants. 2021;10:1467. doi: 10.3390/antiox10091467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Garlick P.J. Toxicity of methionine in humans. J. Nutr. 2006;136:1722S–1725S. doi: 10.1093/jn/136.6.1722S. [DOI] [PubMed] [Google Scholar]
- 85.Tapia-Rojas C., Lindsay C.B., Montecinos-Oliva C., Arrazola M.S., Retamales R.M., Bunout D., Hirsch S., Inestrosa N.C. Is L-methionine a trigger factor for Alzheimer’s-like neurodegeneration?: Changes in Aβ oligomers, tau phosphorylation, synaptic proteins, Wnt signaling and behavioral impairment in wild-type mice. Mol. Neurodegener. 2015;10:62. doi: 10.1186/s13024-015-0057-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kamat J.P., Devasagayam T.P. Nicotinamide (vitamin B3) as an effective antioxidant against oxidative damage in rat brain mitochondria. Redox Rep. 1999;4:179–184. doi: 10.1179/135100099101534882. [DOI] [PubMed] [Google Scholar]
- 87.Kreth S., Ledderose C., Luchting B., Weis F., Thiel M. Immunomodulatory properties of pentoxifylline are mediated via adenosine-dependent pathways. Shock. 2010;34:10–16. doi: 10.1097/SHK.0b013e3181cdc3e2. [DOI] [PubMed] [Google Scholar]
- 88.Seirafianpour F., Mozafarpoor S., Fattahi N., Sadeghzadeh-Bazargan A., Hanifiha M., Goodarzi A. Treatment of COVID-19 with pentoxifylline: Could it be a potential adjuvant therapy? Dermatol. Ther. 2020;33:e13733. doi: 10.1111/dth.13733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zein C.O., Lopez R., Fu X., Kirwan J.P., Yerian L.M., McCullough A.J., Hazen S.L., Feldstein A.E. Pentoxifylline decreases oxidized lipid products in nonalcoholic steatohepatitis: New evidence on the potential therapeutic mechanism. Hepatology. 2012;56:1291–1299. doi: 10.1002/hep.25778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lewis R.J., Tatken R.L. Registry of Toxic Effects of Chemical Substances. NIOSH, US Government Printing; Washington, DC, USA: 1978. [Google Scholar]
- 91.Tikhaze A.K., Lankin B.Z., Konovalova G.G., Shumaev K.B., Kaminnyi A.I., Kozachenko A.I., Gurevich S.M., Nagler L.G., Zaitseva T.M., Kukharchuk V.V. Antioxidant probucol as an effective scavenger of lipid radicals in low density lipoproteinsin vivo and in vitro. Bull. Exp. Biol. Med. 1999;128:818–821. doi: 10.1007/BF02433824. [DOI] [PubMed] [Google Scholar]
- 92.Lebeau J.E. Toxicologie animale du probucol [Animal toxicity studies of probucol (author’s translation)] Nouv. Presse Med. 1980;9:3001–3004. [PubMed] [Google Scholar]
- 93.Chen X., Lu X.-Z., Gao Y., Shi X.-C., Yu W.-G. Molecular mechanisms of antioxidant effects of propylene glycol mannate sulfate. Yao Xue Xue Bao. 2004;39:13–16. [PubMed] [Google Scholar]
- 94.Sax N.I. Final report on the safety assessment of propylene glycol and polypropylene glycols. J. Am. Coll. Toxicol. 1994;13:437–491. [Google Scholar]
- 95.Melo-Cavalcante A.A.D.C., Sousa J.M.C., Alencar M.V.O.B., Santos J.V.D.O., da Mata A.M.O., Paz M.F.C.J., de Carvalho R.M., Nunes N.M.F., Islam M., Mendes A.N., et al. Retinol palmitate and ascorbic acid: Role in oncological prevention and therapy. Biomed. Pharmacother. 2019;109:1394–1405. doi: 10.1016/j.biopha.2018.10.115. [DOI] [PubMed] [Google Scholar]
- 96.Midha I.K., Kumar N., Kumar A., Madan T. Mega doses of retinol: A possible immunomodulation in COVID-19 illness in resource-limited settings. Rev. Med. Virol. 2021;31:1–14. doi: 10.1002/rmv.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Moussa Z., Judeh Z.M.A., Ahmed S.A. Nonenzymatic exogenous and endogenous antioxidants. In: Das K., Das S., Biradar M.S., Bobbarala V., Tata S.S., editors. Free Radical Medicine and Biology. IntechOpen; London, UK: 2020. [DOI] [Google Scholar]
- 98.Kamm J.J. Toxicology, carcinogenicity, and teratogenicity of some orally administered retinoids. J. Am. Acad. Dermatol. 1982;6:652–659. doi: 10.1016/S0190-9622(82)70054-4. [DOI] [PubMed] [Google Scholar]
- 99.Penniston K.L., Tanumihardjo S.A. The acute and chronic toxic effects of vitamin A. Am. J. Clin. Nutr. 2006;83:191–201. doi: 10.1093/ajcn/83.2.191. [DOI] [PubMed] [Google Scholar]
- 100.Ebert P.S., Malinin G.I. Induction of erythroid differentiation in friend murine erythroleukemic cells by inorganic selenium compounds. Biochem. Biophys. Res. Commun. 1979;86:340–349. doi: 10.1016/0006-291X(79)90871-4. [DOI] [PubMed] [Google Scholar]
- 101.Nuttall K.L. Evaluating selenium poisoning. Ann. Clin. Lab. Sci. 2006;36:409–420. [PubMed] [Google Scholar]
- 102.Khan S.L., Siddiqui F.A. Beta-sitosterol: As immunostimulant, antioxidant and inhibitor of SARS-CoV-2 spike glycoprotein. Arch. Pharmacol. Ther. 2020;2:12–16. [Google Scholar]
- 103.Baskar A.A., Al Numair K.S., Paulraj M.G., Alsaif M.A., Al Muamar M., Ignacimuthu S. β-sitosterol prevents lipid peroxidation and improves antioxidant status and histoarchitecture in rats with 1,2-dimethylhydrazine-induced colon cancer. J. Med. Food. 2012;15:335–343. doi: 10.1089/jmf.2011.1780. [DOI] [PubMed] [Google Scholar]
- 104.Rieger M.M. Kirk-Othmer Encyclopedia of Chemical Technology. 3rd ed. Wiley-Interscience; New York, NY, USA: 1978. [DOI] [Google Scholar]
- 105.Armstrong C., Husbands M., Scott G. Mechanisms of antioxidant action: Antioxidant-active products formed from the dialkyl thiodipropionate esters. Eur. Polym. J. 1979;15:241–248. doi: 10.1016/0014-3057(79)90170-8. [DOI] [Google Scholar]
- 106.Das Gupta S., Suh N. Tocopherols in cancer: An update. Mol. Nutr. Food Res. 2016;60:1354–1363. doi: 10.1002/mnfr.201500847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chew E.Y., Clemons T.E., Agrón E., Sperduto R.D., SanGiovanni J.P., Kurinij N., Davis M.D. Long-term effects of vitamins C and E, β-carotene, and zinc on age-related macular degeneration: AREDS report no. 35. Ophthalmology. 2013;120:1604–1611.e4. doi: 10.1016/j.ophtha.2013.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shults C.W., Oakes D., Kieburtz K., Beal M.F., Haas R., Plumb S., Juncos J.L., Nutt J., Shoulson I., Carter J., et al. Effects of coenzyme Q10 in early Parkinson disease: Evidence of slowing of the functional decline. Arch. Neurol. 2002;59:1541–1550. doi: 10.1001/archneur.59.10.1541. [DOI] [PubMed] [Google Scholar]
- 109.Ernster L., Dallner G. Biochemical, physiological and medical aspects of ubiquinone function. Biochim. Biophys. Acta. 1995;1271:195–204. doi: 10.1016/0925-4439(95)00028-3. [DOI] [PubMed] [Google Scholar]
- 110.Sangsefidi Z.S., Yaghoubi F., Hajiahmadi S., Hosseinzadeh M. The effect of coenzyme Q10 supplementation on oxidative stress: A systematic review and meta-analysis of randomized controlled clinical trials. Food Sci. Nutr. 2020;8:1766–1776. doi: 10.1002/fsn3.1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hatakeyama S., Kawase S., Yoshimura I. Comparative oral toxicity of coenzyme Q10 and its (2Z)-isomer in rats: Single and four-week repeated dose toxicity studies. J. Nutr. Sci. Vitaminol. 2006;52:9–20. doi: 10.3177/jnsv.52.9. [DOI] [PubMed] [Google Scholar]
- 112.Prasad A.S. Zinc is an antioxidant and anti-inflammatory agent: Its role in human health. Front. Nutr. 2014;1:14. doi: 10.3389/fnut.2014.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cingolani V. Hypothesis of zinc ascorbate as best zinc ionophore for raising anti-viral resistance against COVID-19. J. Med. Virol. 2021;93:5205–5208. doi: 10.1002/jmv.26989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lee S.R. Critical role of zinc as either an antioxidant or a prooxidant in cellular systems. Oxid. Med. Cell. Longev. 2018:9156285. doi: 10.1155/2018/9156285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Forman H.J., Davies K.J., Ursini F. How do nutritional antioxidants really work: Nucleophilic tone and para-hormesis versus free radical scavenging in vivo. Free Radic. Biol. Med. 2014;66:24–35. doi: 10.1016/j.freeradbiomed.2013.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gueven N., Ravishankar P., Eri R., Rybalka E. Idebenone: When an antioxidant is not an antioxidant. Redox Biol. 2021;38:101812. doi: 10.1016/j.redox.2020.101812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Khansari N., Shakiba Y., Mahmoudi M. Chronic inflammation and oxidative stress as a major cause of age-related diseases and cancer. Recent Pat. Inflamm. Allergy Drug Discov. 2009;3:73–80. doi: 10.2174/187221309787158371. [DOI] [PubMed] [Google Scholar]
- 118.Medini H., Zirman A., Mishmar D. Immune system cells from COVID-19 patients display compromised mitochondrial-nuclear expression co-regulation and rewiring toward glycolysis. iScience. 2021;24:103471. doi: 10.1016/j.isci.2021.103471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cappanera S., Palumbo M., Kwan S., Priante G., Martella L., Saraca L., Sicari F., Vernelli C., Di Giuli C., Andreani P., et al. When does the cytokine storm begin in COVID-19 patients? A quick score to recognize it. J. Clin. Med. 2021;10:297. doi: 10.3390/jcm10020297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yang L., Xie X., Tu Z., Fu J., Xu D., Zhou Y. The signal pathways and treatment of cytokine storm in COVID-19. Signal Transduct. Target. Ther. 2021;6:255. doi: 10.1038/s41392-021-00679-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.De Flora S., Balansky R., LA Maestra S. Antioxidants and COVID-19. J. Prev. Med. Hyg. 2021;62:E34–E45. doi: 10.15167/2421-4248/jpmh2021.62.1S3.1895. (In Italian) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jorge-Aarón R.-M., Rosa-Ester M.-P. N-acetylcysteine as a potential treatment for COVID-19. Future Microbiol. 2020;15:959–962. doi: 10.2217/fmb-2020-0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Peter A.E., Sandeep B.V., Rao B.G., Kalpana V.L. Calming the storm: Natural immunosuppressants as adjuvants to target the cytokine storm in COVID-19. Front. Pharmacol. 2021;11:583777. doi: 10.3389/fphar.2020.583777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.LaForge M., Elbim C., Frère C., Hémadi M., Massaad C., Nuss P., Benoliel J.-J., Becker C. Tissue damage from neutrophil-induced oxidative stress in COVID-19. Nat. Rev. Immunol. 2020;20:515–516. doi: 10.1038/s41577-020-0407-1. Correction in Nat. Rev. Immunol. 2020, 20, 579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ramos E., López-Muñoz F., Gil-Martín E., Egea J., Álvarez-Merz I., Painuli S., Semwal P., Martins N., Hernández-Guijo J., Romero A. The Coronavirus disease 2019 (COVID-19): Key emphasis on melatonin safety and therapeutic efficacy. Antioxidants. 2021;10:1152. doi: 10.3390/antiox10071152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sies H., Parnham M.J. Potential therapeutic use of ebselen for COVID-19 and other respiratory viral infections. Free Radic. Biol. Med. 2020;156:107–112. doi: 10.1016/j.freeradbiomed.2020.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Santi C., Scimmi C., Sancineto L. Ebselen and analogues: Pharmacological properties and synthetic strategies for their preparation. Molecules. 2021;26:4230. doi: 10.3390/molecules26144230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Liu F., Zhu Y., Zhang J., Li Y., Peng Z. Intravenous high-dose vitamin C for the treatment of severe COVID-19: Study protocol for a multicentre randomised controlled trial. BMJ Open. 2020;10:e039519. doi: 10.1136/bmjopen-2020-039519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gönen M.S., Alaylıoğlu M., Durcan E., Özdemir Y., Şahin S., Konukoğlu D., Nohut O.K., Ürkmez S., Küçükece B., Balkan I.I., et al. Rapid and effective vitamin D supplementation may present better clinical outcomes in COVID-19 (SARS-CoV-2) patients by altering serum INOS1, IL1B, IFNg, cathelicidin-LL37, and ICAM1. Nutrients. 2021;13:4047. doi: 10.3390/nu13114047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Khurana R.K., Jain A., Jain A., Sharma T., Singh B., Kesharwani P. Administration of antioxidants in cancer: Debate of the decade. Drug Discov. Today. 2018;23:763–770. doi: 10.1016/j.drudis.2018.01.021. [DOI] [PubMed] [Google Scholar]
- 131.Brion L.P., Bell E.F., Raghuveer T.S. Vitamin E supplementation for prevention of morbidity and mortality in preterm infants. Cochrane Database Syst. Rev. 2003;2010:CD003665. doi: 10.1002/14651858.CD003665. [DOI] [PubMed] [Google Scholar]
- 132.Poljsak B., Šuput D., Milisav I. Achieving the balance between ROS and antioxidants: When to use the synthetic antioxidants. Oxid. Med. Cell. Longev. 2013;2013:956792. doi: 10.1155/2013/956792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Stroehlein J.K., Wallqvist J., Iannizzi C., Mikolajewska A., Metzendorf M.-I., Benstoem C., Meybohm P., Becker M., Skoetz N., Stegemann M., et al. Vitamin D supplementation for the treatment of COVID-19: A living systematic review. Cochrane Database Syst. Rev. 2021:CD015043. doi: 10.1002/14651858.cd015043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Milan S.J., Hart A., Wilkinson M. Vitamin C for asthma and exercise-induced bronchoconstriction. Cochrane Database Syst. Rev. 2013:CD010391. doi: 10.1002/14651858.CD010391.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Evans J.R., Lawrenson J.G. Antioxidant vitamin and mineral supplements for slowing the progression of age-related macular degeneration. Cochrane Database Syst. Rev. 2017;2017:CD000254. doi: 10.1002/14651858.CD000254.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rutjes A.W.S., Denton A.D., Di Nisio M., Chong L.-Y., Abraham R.P., Al-Assaf A.S., Anderson J.L., Malik A.M., Vernooij R., Martínez G., et al. Vitamin and mineral supplementation for maintaining cognitive function in cognitively healthy people in mid and late life. Cochrane Database Syst. Rev. 2018;2019:CD011906. doi: 10.1002/14651858.CD011906.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Scholz S.S., Borgstedt R., Ebeling N., Menzel L.C., Jansen G., Rehberg S. Mortality in septic patients treated with vitamin C: A systematic meta-analysis. Crit. Care. 2021;25:17. doi: 10.1186/s13054-020-03438-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhang Y., Fang F., Tang J., Jia L., Feng Y., Xu P., Faramand A. Association between vitamin D supplementation and mortality: Systematic review and meta-analysis. BMJ. 2019;366:l4673. doi: 10.1136/bmj.l4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Curtis A.J., Bullen M., Piccenna L., McNeil J.J. Vitamin E supplementation and mortality in healthy people: A meta-analysis of randomised controlled trials. Cardiovasc. Drugs Ther. 2014;28:563–573. doi: 10.1007/s10557-014-6560-7. [DOI] [PubMed] [Google Scholar]
- 140.Rouhani P., Kelishadi M.R., Saneei P. Effect of zinc supplementation on mortality in under 5-year children: A systematic review and meta-analysis of randomized clinical trials. Eur. J. Nutr. 2022;61:37–54. doi: 10.1007/s00394-021-02604-1. [DOI] [PubMed] [Google Scholar]
- 141.Keum N., Lee D.H., Greenwood D.C., Manson J.E., Giovannucci E. Vitamin D supplementation and total cancer incidence and mortality: A meta-analysis of randomized controlled trials. Ann. Oncol. 2019;30:733–743. doi: 10.1093/annonc/mdz059. Erratum in BMJ 2020, 22, m2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Passerieux E., Hayot M., Gilles C., Pincemail J., Mercier J., Chenivesse D. Oxidative stress and dystrophy Facioscapulohumeral: Effects of vitamin C, vitamin E, zinc gluconate and selenomethionine supplementation. Free Radic. Biol. Med. 2014;75:S14. doi: 10.1016/j.freeradbiomed.2014.10.587. [DOI] [PubMed] [Google Scholar]
- 143.Yamashita S., Arai H., Bujo H., Masuda D., Ohama T., Ishibashi T., Yanagi K., Doi Y., Nakagawa S., Yamashiro K., et al. Probucol trial for secondary prevention of atherosclerotic events in patients with coronary heart disease (PROSPECTIVE) J. Atheroscler. Thromb. 2021;28:103–123. doi: 10.5551/jat.55327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Villarruz-Sulit M.V., Forster R., Dans A.L., Tan F.N., Sulit D.V. Chelation therapy for atherosclerotic cardiovascular disease. Cochrane Database Syst. Rev. 2020;5:CD002785. doi: 10.1002/14651858.cd002785.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mohammadi S., Shokri J., Ranjkesh M., Hamed S.A., Monajjemzadeh F. Comparative physicochemical stability and clinical anti-wrinkle efficacy of transdermal emulgel preparations of 5% sodium ascorbyl phosphate and or ascorbic acid on human volunteers. J. Cosmet. Dermatol. 2021;20:174–180. doi: 10.1111/jocd.13471. [DOI] [PubMed] [Google Scholar]
- 146.Rattanawiwatpong P., Wanitphakdeedecha R., Bumrungpert A., Maiprasert M. Anti-aging and brightening effects of a topical treatment containing vitamin C, vitamin E, and raspberry leaf cell culture extract: A split-face, randomized controlled trial. J. Cosmet. Dermatol. 2020;19:671–676. doi: 10.1111/jocd.13305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rusanova I., Martínez-Ruiz L., Florido J., Rodríguez-Santana C., Guerra-Librero A., Acuña-Castroviejo D., Escames G. Protective effects of melatonin on the skin: Future perspectives. Int. J. Mol. Sci. 2019;20:4948. doi: 10.3390/ijms20194948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hatem S., Nasr M., Moftah N., Ragai M.H., Geneidi A.S., Elkheshen S.A. Clinical cosmeceutical repurposing of melatonin in androgenic alopecia using nanostructured lipid carriers prepared with antioxidant oils. Expert Opin. Drug Deliv. 2018;15:927–935. doi: 10.1080/17425247.2018.1517740. [DOI] [PubMed] [Google Scholar]
- 149.Schillaci C., Nepravishta R., Bellomaria A. Antioxidants in food and pharmaceutical research. Alb. J. Pharm. Sci. 2014;1:15–25. [Google Scholar]
- 150.Carocho M., Morales P., Ferreira I.C. Antioxidants: Reviewing the chemistry, food applications, legislation and role as preservatives. Trends Food Sci. Technol. 2018;71:107–120. doi: 10.1016/j.tifs.2017.11.008. [DOI] [Google Scholar]
- 151.Chaumont M., Van De Borne P., Bernard A., Van Muylem A., Deprez G., Ullmo J., Starczewska E., Briki R., De Hemptinne Q., Zaher W., et al. Fourth generation e-cigarette vaping induces transient lung inflammation and gas exchange disturbances: Results from two randomized clinical trials. Am. J. Physiol. Lung Cell. Mol. Physiol. 2019;316:L705–L719. doi: 10.1152/ajplung.00492.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Semba R.D. The discovery of the vitamins. Int. J. Vitam. Nutr. Res. 2012;82:310–315. doi: 10.1024/0300-9831/a000124. [DOI] [PubMed] [Google Scholar]
- 153.Bowden M.E., Crow A.B., Sullivan T. Pharmaceutical Achievers: The Human Face of Pharmaceutical Research. Chemical Heritage Foundation; Philadelphia, PA, USA: 2003. pp. 32–41. [Google Scholar]
- 154.Delanghe T., Huyghe J., Lee S., Priem D., Van Coillie S., Gilbert B., Choi S.M., Vandenabeele P., Degterev A., Cuny G.D., et al. Antioxidant and food additive BHA prevents TNF cytotoxicity by acting as a direct RIPK1 inhibitor. Cell Death Dis. 2021;12:699. doi: 10.1038/s41419-021-03994-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Murakami Y., Kawata A., Katayama T., Fujisawa S. Anti-inflammatory activity of the artificial antioxidants 2-tert-butyl-4-methoxyphenol (BHA), 2,6-di-tert-butyl-4-methylphenol (BHT) and 2,4,6-tri-tert-butylphenol (TBP), and their various combinations. In Vivo. 2015;29:197–206. [PubMed] [Google Scholar]
- 156.Vo Q.V., Van Gon T., Van Bay M., Mechler A. Antioxidant activities of monosubstituted indolinonic hydroxylamines: A thermodynamic and kinetic study. J. Phys. Chem. B. 2019;123:10672–10679. doi: 10.1021/acs.jpcb.9b08912. [DOI] [PubMed] [Google Scholar]
- 157.Hrizi A., Cailler M., Romdhani-Younes M., Carcenac Y., Thibonnet J. Synthesis of new highly functionalized 1H-indole-2-carbonitriles via cross-coupling reactions. Molecules. 2021;26:5287. doi: 10.3390/molecules26175287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kovacikova L., Prnova M., Majekova M., Bohac A., Karasu C., Stefek M. Development of novel indole-based bifunctional aldose reductase inhibitors/antioxidants as promising drugs for the treatment of diabetic complications. Molecules. 2021;26:2867. doi: 10.3390/molecules26102867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Reisman S.A., Gahir S.S., Lee C.-Y.I., Proksch J.W., Sakamoto M., Ward K.W. Pharmacokinetics and pharmacodynamics of the novel Nrf2 activator omaveloxolone in primates. Drug Des. Dev. Ther. 2019;13:1259–1270. doi: 10.2147/DDDT.S193889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Forman H.J., Zhang H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021;20:689–709. doi: 10.1038/s41573-021-00233-1. Erratum in Nat. Rev. Drug. Discov. 2021, 20, 652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Carlström K.E., Ewing E., Granqvist M., Gyllenberg A., Aeinehband S., Enoksson S.L., Checa A., Badam T.V.S., Huang J., Gomez-Cabrero D., et al. Therapeutic efficacy of dimethyl fumarate in relapsing-remitting multiple sclerosis associates with ROS pathway in monocytes. Nat. Commun. 2019;10:3081. doi: 10.1038/s41467-019-11139-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wang Y., Bhargava P. Diroximel fumarate to treat multiple sclerosis. Drugs Today. 2020;56:431. doi: 10.1358/dot.2020.56.7.3151521. [DOI] [PubMed] [Google Scholar]
- 163.Mills E.A., Ogrodnik M.A., Plave A., Mao-Draayer Y. Emerging understanding of the mechanism of action for dimethyl fumarate in the treatment of multiple sclerosis. Front. Neurol. 2018;9:5. doi: 10.3389/fneur.2018.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wundes A., Wray S., Gold R., Singer B.A., Jasinska E., Ziemssen T., de Seze J., Repovic P., Chen H., Hanna J., et al. Improved gastrointestinal profile with diroximel fumarate is associated with a positive impact on quality of life compared with dimethyl fumarate: Results from the randomized, double-blind, phase III EVOLVE-MS-2 study. Ther. Adv. Neurol. Disord. 2021;14:1756286421993999. doi: 10.1177/1756286421993999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Li S., Li H., Xu X., Saw P.E., Zhang L. Nanocarrier-mediated antioxidant delivery for liver diseases. Theranostics. 2020;10:1262–1280. doi: 10.7150/thno.38834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lushchak O., Zayachkivska A., Vaiserman A. Metallic nanoantioxidants as potential therapeutics for type 2 diabetes: A hypothetical background and translational perspectives. Oxid. Med. Cell. Longev. 2018;2018:3407375. doi: 10.1155/2018/3407375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zhang C., Wang X., Du J., Gu Z., Zhao Y. Reactive oxygen species-regulating strategies based on nanomaterials for disease treatment. Adv. Sci. 2020;8:2002797. doi: 10.1002/advs.202002797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Mazzotta E., Orlando C., Muzzalupo R. New nanomaterials with intrinsic antioxidant activity by surface functionalization of niosomes with natural phenolic acids. Pharmaceutics. 2021;13:766. doi: 10.3390/pharmaceutics13060766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Khalil I., Yehye W.A., Etxeberria A.E., Alhadi A.A., Dezfooli S.M., Julkapli N.B.M., Basirun W.J., Seyfoddin A. Nanoantioxidants: Recent trends in antioxidant delivery applications. Antioxidants. 2020;9:24. doi: 10.3390/antiox9010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Pastor-Maldonado C.J., Suárez-Rivero J.M., Povea-Cabello S., Álvarez-Córdoba M., Villalón-García I., Munuera-Cabeza M., Suárez-Carrillo A., Talaverón-Rey M., Sánchez-Alcázar J.A. Coenzyme Q10: Novel formulations and medical trends. Int. J. Mol. Sci. 2020;21:8432. doi: 10.3390/ijms21228432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Piazzini V., D’Ambrosio M., Luceri C., Cinci L., Landucci E., Bilia A.R., Bergonzi M.C. Formulation of nanomicelles to improve the solubility and the oral absorption of silymarin. Molecules. 2019;24:1688. doi: 10.3390/molecules24091688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Stamatoski A., Fidoski J., Vasilev A., Todorovska L., Petlichkovski A. New nanoantioxidant approach to improve healing of oral surgery wounds: A randomised, pilot placebo-controlled, double-blind clinical trial. Int. J. Oral Maxillofac. Surg. 2017;46:249. doi: 10.1016/j.ijom.2017.02.841. [DOI] [Google Scholar]