Abstract
Embraced with apolipoproteins (Apo) B and Apo E, triglyceride-enriched very-low-density lipoprotein (VLDL) is secreted by the liver into circulation, mainly during post-meal hours. Here, we present a brief review of the physiological role of VLDL and a systemic review of the emerging evidence supporting its pathological roles. VLDL promotes atherosclerosis in metabolic syndrome (MetS). VLDL isolated from subjects with MetS exhibits cytotoxicity to atrial myocytes, induces atrial myopathy, and promotes vulnerability to atrial fibrillation. VLDL levels are affected by a number of endocrinological disorders and can be increased by therapeutic supplementation with cortisol, growth hormone, progesterone, and estrogen. VLDL promotes aldosterone secretion, which contributes to hypertension. VLDL induces neuroinflammation, leading to cognitive dysfunction. VLDL levels are also correlated with chronic kidney disease, autoimmune disorders, and some dermatological diseases. The extra-hepatic secretion of VLDL derived from intestinal dysbiosis is suggested to be harmful. Emerging evidence suggests disturbed VLDL metabolism in sleep disorders and in cancer development and progression. In addition to VLDL, the VLDL receptor (VLDLR) may affect both VLDL metabolism and carcinogenesis. Overall, emerging evidence supports the pathological roles of VLDL in multi-organ diseases. To better understand the fundamental mechanisms of how VLDL promotes disease development, elucidation of the quality control of VLDL and of the regulation and signaling of VLDLR should be indispensable. With this, successful VLDL-targeted therapies can be discovered in the future.
Keywords: very-low-density lipoprotein (VLDL), VLDL receptor (VLDLR), metabolic syndrome (MetS), insulin resistance, metabolic associated fatty liver disease (MAFLD), endocrinological disorders, cardiovascular disorders, cognitive dysfunction, cancer, atrial myopathy
1. Introduction of Very-Low-Density Lipoprotein (VLDL)
1.1. Structure Characteristics of VLDL
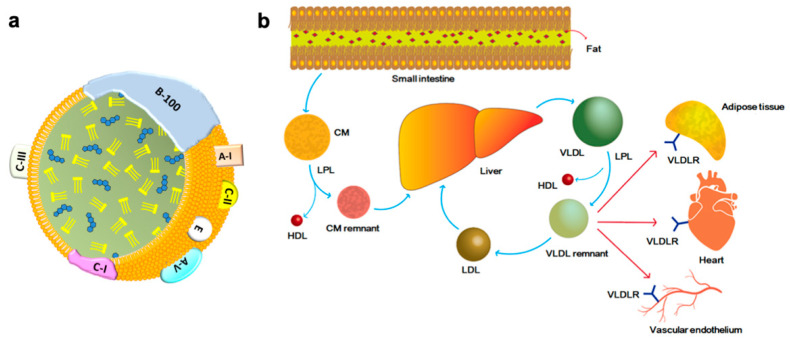
The major lipid content transported by the VLDL is triglyceride (TG) which consists of 50 to 70% particle mass, the remaining 10 to 25% mass consisting of cholesterol ester (CE), and less than 10% of fatty acid [1,2,3,4]. The surface proteins of VLDL include apolipoprotein (Apo) B-100, Apo C-I, Apo C-II, Apo C-III, and Apo E (Figure 1a). Among these, Apo B-100 is the core structural protein and is produced by the liver [5,6]. Apolipoproteins function not only as structural components but also as ligands for cell-surface receptors, and as cofactors for various enzymes such as lipoprotein lipase (LPL) [4,5]. Physiological function, metabolism, and the involvement of disease mechanisms in VLDL are largely regulated and affected by apolipoproteins.
Figure 1.
Structure (a) and metabolism (b) of very-low-density lipoprotein (VLDL). VLDL is a triglyceride (TG)-rich lipoprotein composed of TG, cholesterol ester (CE), phospholipid membrane, apolipoprotein (Apo) A, B-100, and C. The TG consists of 50–70% total mass, and CE consists 10–25%. The remainings are proteins and fatty acids. After fat-content meal intake, chylomicron (CM) is secreted by the intestine into blood circulation, and later transformed to CM remnant by lipoprotein lipase (LPL) and ultimately internalized into the liver where VLDL is produced. In circulation, the hepatic secreted VLDL is transformed to VLDL remnant by the activity of LPL. Both VLDL and VLDL remnant bind VLDL receptor (VLDLR), which is expressed in adipocyte, cardiomyocyte, vascular endothelium in various tissues.
1.2. Hepatic Secretion of VLDL
The production and metabolic pathways of VLDL are shown in the Figure 1b. After fat content meal intake, the intestine secretes chylomicron (CM), which reacts with the LPL and transforms into CM remnants. CM remnants are internalized into the liver and are a major resource of triglycerides (TG) and CE. In hepatocytes, TG and CE are transferred to Apo B-100 in the endoplasmic reticulum. The size of VLDL is enlarged with increased TG production in the liver, and the availability of Apo B-100 also affects the lipid content of VLDL [5,7,8,9,10]. After secretion into the blood circulation, VLDL interacts with LPL on the capillary endothelium in tissues (such as adipose, cardiac, and skeletal muscles) where TG is removed from VLDL for storage or utilization [5,10,11,12,13,14]. One study reported that Apo B-48 containing VLDL can be secreted by the intestinal epithelium in animal experiments. However, the physiological role of this alternative VLDL secretion pathway remains unclear [15]. Abnormal VLDL secretion can be related to an imbalance in the intestinal microbiota, and impaired intestinal bacterial flora may also act as a factor in postprandial dyslipidemia [15,16,17,18].
1.3. Metabolism of VLDL
VLDL can either be hydrolyzed by LPL or taken up by the VLDL receptor (see below). After hydrolysis by LPL, VLDL is transformed into VLDL remnant and intermediate-density lipoprotein (IDL). Apo C-II is transferred to high-density lipoprotein (HDL), which also exchanges TG and phospholipids with CE via the cholesterol ester transfer protein (CEPT). In contrast, VLDL remnants and IDL receive Apo E from HDL [5,14,19,20,21,22]. Approximately half of the IDL can be recognized by the liver using Apo B-100. The rest of the IDL loses Apo E and TG, and becomes low-density lipoprotein (LDL), which is ultimately taken up by the liver via the LDL receptor. Some lipid contents, such as sphingolipids and subclasses of Apo A and C, can affect the metabolism of VLDL [23,24]. For instance, the possession of Apo A-II, A-V, C-II, and C-III makes VLDL associated with insulin resistance [19,25,26,27,28,29]. Further details are provided in the following sections.
1.4. Tissue Expression and Function of VLDL Receptor (VLDLR)
VLDL can be taken up by VLDLR, which is expressed abundantly in adipocytes, cardiomyocytes, and the endothelium [13,30,31]. VLDLR also binds the postprandial remnant-like protein (RLP) in the peripheral tissue. Apo E serves as the ligand for VLDLR; therefore, VLDLR recognizes Apo E-containing lipoproteins, including VLDL, VLDL remnant, and IDL. VLDLR can also bind to molecules such as reelin [13], clusterin [32], and tissue factor inhibitors [33]. VLDLR also interacts with LPL and modulates LDL-mediated TG hydrolysis [34,35,36]. In addition to its important regulatory role in lipid metabolism, VLDLR is found associated with insulin resistance [33,37] and is involved in multiple diseases such as diabetic retinopathy [38], atrial fibrillation [31,39], hypertensive cardiomyopathy [40], and Alzheimer’s disease [13].
1.5. Physiological Function of VLDL
VLDL production is affected by intake and the secretion is increased during the postprandial state. Excessive nutrition and a high-fat diet (HFD) lead to higher VLDL secretion [41,42], and this phenomenon is more dominant in subjects with hypertriglyceridemia. The postprandial secretion peak of VLDL has been reported with different findings ranging from 30 min to 6 h post-meals. Although there is inconsistency in the timing of peak postprandial VLDL levels, these studies consistently found higher and longer plateaus of postprandial VLDL levels in subjects with metabolic syndrome (MetS) and insulin resistance than in normal subjects [11,39,41,42,43,44,45,46,47]. Other factors that influence VLDL secretion include body status, sex, and race. The mean plasma concentration of VLDL was higher in the obese subjects. Compared with men, women have lower VLDL concentrations and stronger VLDL clearance [48,49]. African Americans secret lesser VLDL than Caucasians [48,50,51]. The cause of the sex difference in VLDL secretion remains unclear, although some studies have shown that sex hormones do not affect VLDL formation and metabolism [52,53]. Except for lipid transportation and metabolism, VLDL can promote thrombin generation and inhibit fibrinolysis [54,55,56], and can bind coagulation factors VII and X [57,58]. Patients lacking Apo B lipoproteins have reduced platelet activation [59]. This clinical phenotype of abetalipoproteinaemia supports the hypothesis that Apo B intervenes in thrombosis regulation.
2. Proposed Pathological Roles for VLDL
2.1. Metabolic Associated Fatty Liver Disease (MAFLD) and Hepatitis
The MAFLD subjects have a higher VLDL secretion rate due to increased hydrolysis of intrahepatic TG, and they present with loss of acute reduction in VLDL secretion, but without a difference in Apo B-100 secretion rate [60,61]. The insulin-suppression effect on VLDL is impaired in men with MAFLD regarding the secretion, oxidation, concentration, and reduction of particle size oxidation [62]. In subjects with greater body weight and insulin resistance, Apo C-III of VLDL is increased, which enhances the uptake of VLDL by hepatocytes [20,63,64]. However, in MAFLD patients with severe hepatic fibrosis, plasma TG level, TG ratio of VLDL and circulating total VLDL mass are all reduced [65].
VLDL contributes to sex differences in MAFLD. Men are more likely than women to have MAFLD [66]. For postmenopausal women, the risk for MAFLD is rising due to a decline in estrogen [67,68,69]. A recent animal study showed that estrogen-related receptor (ERR)-α deficiency leads to decreased VLDL secretion, resulting in hepatic lipid accumulation and the development of MAFLD. ERR-α is a nuclear hormone receptor that is involved in multiple metabolic processes [66]. In addition to postmenopausal status, treatment with selective estrogen receptor modulators, such as tamoxifen, suppresses hepatic ERR-α activity and impairs VLDL secretion and promotes hepatic lipid accumulation [66,70]. Therefore, reduced VLDL secretion may be a major contributor to MAFLD in postmenopausal women. Interestingly, the reduced VLDL secretion capability is inheritable as the western diet feeding mother introduces deoxyribonucleic acid (DNA) hypermethylation of hepatic Apo B to male offspring, along with an increased risk of insulin resistance and MAFLD [71,72].
Decreased adiponectin levels, which are commonly observed in subjects with MetS and MAFLD, result in increased circulating total VLDL mass and particle amounts [65]. Other molecules involved in MAFLD and VLDL expression include AMP-activated protein kinase (AMPK) and mammalian target of rapamycin (mTOR) [73,74,75]. Ceramide, a type of lipid content in VLDL, is correlated with the severity of MAFLD [76,77]. Plasma dihydroceramides, which are also carried by VLDL, are correlated with MAFLD severity in type 2 diabetes [77,78].
Hepatitis C virus (HCV) is capable of binding TG-rich lipoproteins, including VLDL, and forming the lipo-viro-particles (LVPs). For instance, HCV glycoprotein E2 is present in LVPs [79,80], and it protects HCV from antibody-mediated immunoreaction, and promotes virus uptake by lipoprotein receptors in hepatocytes [81]. HCV viral load is negatively correlated with LPL activity, and positively correlated with the Apo C-III content of VLDL [82]. On the other hand, lipid homoeostasis is disturbed by HCV through interaction with the host lipid metabolism via several mechanisms such as increased lipogenesis, reduction of fatty acid oxidation, and reduced TG content in the secreted VLDL [83]. Direct antiviral agents that lead to efficient HCV eradication have been shown to restore the abnormal TG to cholesterol ratios in VLDL, and LDL as well as favorable lipoprotein metabolism [84].
Oxidative stress promotes the development of MAFLD in animal study [75]. Nuclear factor erythroid 2-related factor 2 (Nrf2), a transcription factor sensitive to antioxidant responses and activated under oxidative stress, is related to VLDL secretion. Fumarate, an intermediate product of the tricarboxylic acid cycle, is a source of Nrf2 activation. Mice fed an imbalanced diet (high-fat diet or methionine choline-deficient diet) showed decreased Nrf2 expression along with oxidative stress and lipotoxicity of hepatocytes. When the translocation of Nrf2 is suppressed, VLDL maturation is impaired, and lipid accumulation occurs, ultimately leading to hepatic steatosis and MAFLD development [75]. Oxidative stress-induced Nrf2 signaling has also been demonstrated in another animal model of alcoholic hepatitis with elevated VLDLR expression [85].
Upregulation of VLDLR expression in the liver, which occurs after prolonged exposure to endoplasmic reticulum stress, occurs via activation of transcription factor 4 (ATF4) signaling and induces hepatic steatosis [86]. Fibroblast growth factor 21 also promotes endoplasmic reticulum stress-related VLDLR overexpression in hepatic steatosis [87]. In addition, peroxisome proliferator-activated receptor (PPAR)-α activation by fenofibrate treatment, which reduces plasma TG levels, is correlated with the modulation of VLDLR expression [88].
2.2. Insulin Resistance and Metabolic Syndrome
Insulin can suppress VLDL production, and there is increased production and reduced clearance of VLDL in insulin resistance, which is often associated with hypertriglyceridemia [62,89,90,91,92]. Glucagon also reduces hepatic VLDL production [93]. Subjects with insulin resistance have a reduced ability to store lipids in adipose tissue, and are therefore prone to hyperlipidemia and ectopic lipid accumulation [42,94]. In subjects with type 2 diabetes, the ability of insulin to suppress VLDL production is impaired [89,95], and their postprandial VLDL concentration is elevated, although the clearance rate of postprandial VLDL is similar to that in non-diabetic subjects [46]. In studies using ex-labeled VLDL1 and VLDL2 particles to investigate VLDL kinetics during hyperinsulinemia, the size (TG/Apo B ratio) and Apo B-100 concentration were significantly reduced in healthy men but were unaltered in men with type 2 diabetes due to hyperinsulinemia, suggesting that the fatty acid oxidation rate was substantially suppressed in diabetic subjects [89,96].
ATP-binding cassette transporter A1 (ABCA1) is an integral cell membrane protein that acts as a mediator of HDL biogenesis. Pancreatic ABCA1 is involved in insulin secretion by β-cells [97]. The ABCA1 of skeletal muscle enhances glucose uptake by enhancing Akt phosphorylation and glucose transporter type 4 transfer to the plasma membrane. Hepatic ABCA1 is involved in the regulation of VLDL production by affecting Apo B trafficking [98]. Patients with complete ABCA1 deficiency had <5% of normal plasma HDL levels and elevated VLDL and TG levels. Genetic variations in ABCA1 are associated with the MetS phenotype, and a broad range of diseases such as coronary heart disease, type 2 diabetes, neurological disorders, and age-related macular degeneration [99].
Emerging evidence has shown that in MetS, VLDL becomes cytotoxic, pro-inflammatory, and atherogenic, and is involved in the pathogenesis of various diseases [31,39,100,101,102,103]. Increased Apo C-III, Apo E-III, and E-IV of VLDL in MetS exhibit reduced LPL activity and function [8,27,104,105]. VLDL in MetS exhibits the ability to induce apoptosis by promoting reactive oxygen species production, especially in endothelial cells and sub-endothelial macrophages [59,104]. Furthermore, macrophages stimulated by VLDL lipolysis products exhibit proinflammatory effects [33,106]. The size of VLDL is thought to be related to PPAR-α regulation in the clearance of plasma fatty acids after VLDL hydrolysis [107]. LPL activity affects the diameter of VLDL particles, and large VLDL particles are more abundantly in MetS and insulin resistance [108].
2.3. Other Endocrinological Disorders
Here we describe several endocrinological disorders and hormones that are correlated with VLDL/TG metabolism. A summary of this section is provided in Table 1.
Table 1.
Summary of endocrinological effects on very-low-density lipoprotein (VLDL).
| Hormone/Diseases | Effects Related to VLDL | Reference |
|---|---|---|
| Cushing syndrome | Increased secretion | [110] |
| Exogenous cortisol | Declining degradation and increased adipose lipolysis | [111] |
| Aldosterone | Activation of aldosterone production | [114,115,116] |
| Growth hormone deficiency | Increased production and decreased clearance | [110,118] |
| Growth hormone treatment | Increased adipose lipolysis and increased clearance | [110,118,119] |
| Hypothyroidism | Reduced degradation with increased secretion | [122,123] |
| Androgen | Androgen-deprivation therapy: increased level | [128] |
| Transgender males with testosterone therapy: increased level | [129,130,131] | |
| Polycystic ovary syndrome | Increased level | [132,133] |
| Estrogen/progesterone therapy | Increased level | [110,134,135] |
| Prolactinoma | Unclear | [110,143,144,145,146] |
In Cushing syndrome, increased circulating VLDL and LDL are present as dyslipidemia with elevated plasma CE and TG [109]. Increased VLDL is caused by greater secretion of VLDL than in normal subjects without alteration in VLDL clearance [110]. Exogenous cortisol stimulation with glucocorticoid treatment reduces the degradation of Apo B and increases adipose tissue lipolysis, resulting in elevated circulatory VLDL as well [111]. Increased risk of cardiovascular diseases, and dyslipidemia in Cushing syndrome and in glucocorticoid treatment are all suggested to be correlated with elevated VLDL/TG levels [112,113].
The adrenal gland hormone aldosterone is also associated with VLDL. Aldosterone production is promoted by VLDL stimulation and is mediated by the PLC/IP3/PKC signaling pathway [114,115,116]. This mechanism can partially explain how the most commonly used lipid-lowering drug statin is associated with reduced aldosterone levels in hypertensive and diabetic subjects [117].
Growth hormone deficiency is related to elevated VLDL production, decreased VLDL clearance, and elevated TG levels. Growth hormone replacement therapy promotes VLDL clearance, but simultaneously increases VLDL secretion from adipose tissue by facilitating lipolysis [110,118]. Therefore, although growth hormones promote fatty acid oxidation, they do not reduce VLDL secretion, but instead can further increase plasma VLDL and TG levels [119]. This phenomenon can explain why subjects with hypopituitarism are prone to cardiovascular and cerebrovascular diseases [120]. The other disease acromegaly with excessive growth hormone has impaired LPL function and increased non-esterified fatty acid in plasma, as well as hepatic overproduction of VLDL [110,121].
Thyroid hormones affect LPL activity, cholesterol, and lipoprotein metabolism. In hypothyroidism, LPL function is reduced and the hepatic VLDL secretion rate increases [122,123]. Severe hypothyroidism with thyroid-stimulating hormone (TSH) levels >10 mIU/L is associated with cardiovascular disease and with dyslipidemia, such as elevated total cholesterol, LDL, TG, and lower HDL. Nevertheless, the meta-analysis did not show a significant difference in VLDL, Apo A-I, or Apo B levels [124]. Thyromimetics (thyroid replacement therapy) potentially accelerates energy expenditure and exerts hypolipidemic effects with improved lipid profile [110,125].
Regarding sex hormones, testosterone does not affect VLDL metabolism [53,126,127]. While androgen deprivation therapy may elevate VLDL levels [128], testosterone treatment in patients with androgen deficiency has limited effects on VLDL levels [110]. In transgender males, however, increased VLDL/TG is observed after testosterone therapy, probably due to combined suppressed levels of estrogen [129,130,131].
In subjects with polycystic ovary syndrome (PCOS), elevated VLDL levels are commonly observed and can improve after effective treatment for PCOS. There is no influence on other lipid profiles [132,133]. The effects of hormone replacement therapy on VLDL metabolism are still controversial. Some studies have shown that estrogen supplementation increases VLDL and body fat mass [134], and similar findings have been observed with progesterone therapy [110,135]. For women of reproductive age, elevated cardiovascular risk is only observed with high-dose estrogen combined with oral contraceptives [136]. Elevated VLDL levels in hormone replacement therapy have also been reported to be associated with increased cardiovascular risk [137]. The impact of sex-affirming hormone therapy on lipoprotein metabolism parameters is largely undetermined. Observational studies have shown the effects of 1 year administration of estrogen or testosterone in transgender persons. The results showed unfavorable changes in the lipid profile in transmen and favorable changes in transwomen [129,131].
In pregnant women, VLDL levels increase owing to decreased activities of LPL and hepatic lipase [138,139]. During pregnancy, the production of VLDL is also facilitated by hormone-sensitive lipase in adipose tissue [140]. Fatty acids and cholesterol from VLDL serve as the energy source for the mother and placenta [141]. In pregnant women with gestational diabetes and preeclampsia, VLDL levels also increase significantly due to increased insulin resistance [140,142].
Prolactinoma is associated with higher LDL levels; however, the effect of prolactin on VLDL/TG remains unclear [110,143,144,145,146]. The standard therapy for prolactinomas, dopamine agonist therapy, improves insulin sensitivity, decreases LDL-cholesterol levels, and improves the body mass index [124]. The impact of circadian rhythm on disturbing lipid metabolism has been suggested by several animal studies revealing the change in intestinal microbiota as a potential mechanism [147,148,149].
2.4. Cardiovascular Disorders
The impact of VLDL on cardiovascular diseases has been observed in the correlation between atherosclerosis and coronary events [150]. VLDL is also associated with carotid intima-media thickness and vascular stiffness [151]. Recent studies showed that plasma VLDL level is positively related with major adverse limb events for peripheral arterial occlusive diseases [152,153]. Accumulation of TG-rich lipoproteins contributes to the rupture of atherosclerotic plaques [57]. A large Chinese cohort study showed an association between elevated VLDL concentration and coronary heart disease [154]. CE rather than TG in VLDL particles is correlated with an increased risk of acute coronary syndromes [36]. VLDL levels are also correlated with increased mortality in patients with cardiovascular diseases [155]. Elevated VLDL concentrations increase microvascular events in type 2 diabetes by increasing blood viscosity [156]. Other underlying mechanisms include pro-atherogenic effects, [27,33,37,41,47,157] and promotion of thromboembolism with hypercoagulability [54,56,158].
The composition of Apo affects the atherogenic effects of VLDL. Clinical observations have shown that cardiovascular risk is associated with higher Apo B levels and lower Apo C-III of VLDL [3,104,159]. Apo B is suggested to be a major driver in the development of atherosclerosis [8]. In contrast, loss-of-function Apo C-III mutations are associated with low cardiovascular risk [160]. Nevertheless, the effect of Apo C-III on VLDL is complex and it inhibits the interaction between VLDL and its receptors [5]. In addition to Apo B and Apo C, Apo E can also induce atherosclerosis by serving as a ligand for receptors on macrophages, promoting foam cell formation and inflammatory process [161,162,163]. The Apo E of VLDL remnants activates metalloprotease expression and therefore also promotes atherosclerotic plaque rupture [164]. The other pathogenic mechanism of VLDL to atherosclerosis that has been reported by our colleagues is the cytotoxicity exerted by its electronegative charge. The electronegative VLDL has altered lipid content and induces significant apoptosis and senescence of endothelial cells [101,165,166].
Beyond atherosclerosis, our team has conducted a series of studies with animal experiments and clinical observations to reveal the pathogenic role of VLDL in atrial remodeling, which is the preclinical stage of atrial fibrillation. VLDL that is isolated from subjects with MetS, rather than VLDL from healthy subjects, induces cytotoxicity and excess lipid accumulation in atrial tissue, disturbs calcium signaling and resulting sarcomere protein derangement, and affects gap junction of cardiac conduction system, leading to atrial remodeling and vulnerability to atrial fibrillation [31,39,100,167]. The electronegatively charged VLDL, particularly postprandial VLDL, may be a significant and independent predictor of atrial remodeling in MetS [39].
2.5. Neurological Disorders
VLDLR is abundantly expressed in the peripheral nervous system, the cerebellum, and the cerebral cortex. The involvement of VLDLR in Alzheimer’s disease has been observed in clinical observations [168,169,170]. In addition, VLDLR has been detected in senile plaques in the brain [171]. Recent studies have revealed the ability of VLDLR to interact with multiple ligands and molecules such as reelin and clusterin, which are correlated with Alzheimer’s disease [13]. Reelin depletion is regarded as an early phenomenon in Alzheimer’s disease [172,173], whereas clusterin promotes amyloid beta clearance [32,174,175]. Currently, it is suggested that VLDLR is able to interact in the onset and progression of Alzheimer’s disease without the interaction of VLDL.
There is some evidence suggesting a role for VLDL in psychological disorders. In patients with schizophrenia, data revealed a link to insulin resistance, elevated VLDL levels [176], and increased medium and large VLDL levels [177,178]. Elevated TG/VLDL ratio is also correlated with an increased risk of suicide [179] and impaired cognitive function [180]. In autism spectrum disorder, decreased VLDL levels and Apo B are observed along with increased free fatty acids in the circulation [181]. Sleep quality may affect the VLDL metabolism. An observational study showed that sleep disturbance, sleep medication use, and daytime dysfunction due to poor sleep quality are all associated with elevated serum VLDL levels [182]. This may partially explain the association between poor sleep quality and cardiovascular risk [183].
Our colleagues found that VLDL isolated from subjects with MetS was able to induce neuroinflammation and cognitive dysfunction [184]. Typically, VLDL does not pass through the blood-brain barrier (BBB). Studies have suggested that VLDL is enabled by metabolic stress that weakens the BBB and electronegative VLDL can trigger neuroinflammation by activating microglia [102,184]. In addition, increased uptake of VLDL by microglia via the Apo E receptor is associated with impaired LPL function, which affects cognitive function and promotes Alzheimer’s disease [185,186]. Accumulation of VLDL in the medio-basal hypothalamus is also associated with neuroinflammation, regardless of the condition of the BBB [187]. These findings together support the pathogenic role of VLDL in neuroinflammation via microglia and suggest that VLDL is related to an increased risk of cognitive dysfunction in MetS.
2.6. Kidney Diseases
Hypertriglyceridemia is the major dyslipidemia in patients with chronic kidney disease (CKD) [188], and the primary cause is the impairment of VLDL clearance [189]. In CKD, VLDL hydrolysis is impaired, and HDL concentration is reduced [26]. Elevated VLDL levels cause oxidative stress in CKD [190]. Moreover, plasma Apo C-III is significantly higher in the CKD group than in normal subjects, which further contributes to insulin resistance and hyperglycemia [191].
VLDL clearance is also reduced in nephrotic syndrome. The mechanism is suggested to be the suppression of VLDLR in an animal study [192], and impaired LPL function due to upregulation of angiopoietin-like protein 4 (ANGPTL4) level [193,194]. In addition, VLDL can be taken up by mesangial cells to exert direct cytotoxicity and cause progression of nephrotic syndrome. Other proposed mechanisms include elevated proprotein convertase subtilisin kexin type 9 (PCSK9), which contributes to elevated LDL and immature HDL levels [195].
2.7. Inflammation, Autoimmune Disorders and Miscellanies
Generally, MetS is considered a chronic inflammatory state, and VLDL is positively related to microinflammation in endothelial cells, and to activation of monocytes and expression of cytokines in the extrahepatic tissue [47,100,101,106,196,197]. In insulin resistance and MetS, the expression of ceramides is increased on the plasma membrane [198,199], and the proinflammatory function of macrophages is promoted by the over-absorption of VLDL lipolysis products [77,91,198,200,201,202]. Intracellular ceramides are upregulated in macrophages after incubation with VLDL and exhibit a proinflammatory response [106]. In contrast, multiple cytokines can elevate VLDL levels, such as interleukin (IL)-1, IL-2, and IL-6 [203]. VLDL concentration increases within 2 h after exposure to lipopolysaccharide, and this effect can be sustained for more than 24 h [204]. In an animal study, the proinflammatory effects were more dominant when exposed to postprandial VLDL, which further increased cytokine and integrin activation [45]. Furthermore, a recent molecular study suggested the therapeutic effects of insulin-inducible gene-1 (Insig-1) and gene-2 (insig-2) on excess VLDL biosynthesis and hyperlipidemia. The upregulation of Insig-1 and 2 suppresses the activation of sterol regulatory element-binding protein (SREBP), which is a transcription factor that promotes lipogenesis and is able to inhibit the inflammation induced by VLDL [205].
VLDL levels have also been observed in multiple autoimmune disorders. Elevated VLDL levels are observed in patients with systemic lupus erythematosus (SLE) [206,207]. Young women with SLE are at high risk of cardiovascular disease related to dyslipidemia and abnormal VLDL expression [208]. In subjects with primary antiphospholipid syndrome, upregulation of Apo C-III activity leads to decreased VLDL clearance and elevated circulating VLDL levels [209]. In patients with MetS, VLDL levels are positively correlated with disease severity in rheumatoid arthritis [210]. The most commonly used therapy for patients with autoimmune diseases is steroids, which have been proven to increase plasma VLDL concentrations [111,147,148,211]. As VLDL can induce activation of inflammation, monitoring of the lipid profile, including VLDL concentration, is suggested to be important during the treatment and follow-up of patients with autoimmune diseases.
The effects of VLDL on dermatological diseases have also been demonstrated. In vitiligo, which manifests as pigmentation impairment caused by oxidative stress in melanocytes, elevated TG levels commonly coexisted [212]. In psoriasis, MetS is a poor prognostic factor, and VLDL levels are higher in psoriasis patients with MetS than in those without MetS [213]. In patients with lichen planus, a chronic inflammatory disease affecting the mucosa and skin, significantly elevated VLDL levels and associated cardiovascular risk have also been observed [214]. Whether VLDL is pathogenic in the mechanism of the aforementioned dermatological diseases remains unknown. Nevertheless, elevated VLDL levels can be an important biomarker of cardiovascular risk in these diseased populations.
In the aging population, sarcopenia has been recognized as an important adverse outcome marker and is associated with dyslipidemia, especially VLDL [215]. This observation suggests that skeletal muscle affects the metabolism of VLDL, and vice versa. However, the underlying mechanism remains unclear.
3. Association of VLDL in Cancers
Dyslipidemia is associated with the growth and progression of some malignancies [216]. Similar to ordinary cells, lipids regulate cellular and intracellular signaling, modify the fluidity and lipid rafts of membranes, and directly affect lipid-derived mediators in cancer cells. These mechanisms further affect tumor biology, such as immune escape and cellular invasion [216,217,218]. For instance, high-fat intake has been shown to activate the vascular endothelial growth factor and promote angiogenesis, thereby increasing the proliferation of malignant cells [217]. In highly progressive cancer cells, lipid biosynthesis and uptake were also increased [219,220]. A recent study revealed that CD36, a well-known fatty acid translocase, is correlated with the metastatic ability of cancers and is involved in hepatic VLDL secretion [221]. Upregulation of CD36 is also associated with a higher ability to metastasize, especially for epithelial cancer, as well as promoting VLDL secretion [222]. CD36 is involved in signaling cascades that take up extracellular lipids, and further provides energy for the cell. CD36+ cancer cells are thought to gain the energy for anchoring and surviving at sites distant from the tumor origin [223]. Therefore, increased circulating VLDL levels may be associated with progression of CD36+ malignancies. The interaction between VLDL and malignancies is a novel topic, and in the following section, we summarize recent studies that focus on the relationship between VLDL and the oncogenesis and/or the outcome of cancers.
3.1. Breast Cancer
Breast cancer has the most basic and clinical studies focused on the impact of VLDL, and VLDL was found to promote tumor proliferation and progression by promoting angiogenesis, cell migration, and invasion. In a rodent model, breast cancer MDA-MB-231 cells were pre-incubated with charged-defined subfractions of LDL (L1 and L5) and VLDL, and then injected into animals via the tail vein. The results showed that cancer cells incubated with either VLDL, L5, or L1 promoted aggressiveness, but only VLDL incubation exhibited anchorage-independence and caused more lung metastasis. This finding suggests that VLDL promotes lung metastasis in vivo [224]. VLDL uptake brings lipids and offers a sustainable source of energy for cancer cells [225]. The effects of different lipoproteins were compared in human epidermal growth factor receptor 2 (HER2)-overexpressing breast cancer cells and it has been shown that VLDL causes cancer cell growth and morphological changes, and increases cell viability as well [226].
Other studies have investigated the role of miRNAs in breast cancer and their relationship to VLDLR expression. The tumor suppressor miRNA-1297 was positively correlated with VLDLR expression. In contrast, oncogenic miRNA-4465 was negatively correlated with VLDLR expression. Reduced miRNA-1297 was associated with decreased VLDLR expression in highly progressive breast cancer. Furthermore, the expression of VLDLR is also negatively correlated with the abundance of Ki-67, a marker of proliferation [227,228]. It is likely that VLDLR expression affects cancer behavior through undetermined mechanisms. In addition, VLDL levels were inversely associated with the development of breast cancer in postmenopausal women [229] and negatively correlated with high mammographic breast density, a risk factor for breast cancer [230]. This paradoxical correlation between VLDL and breast cancer outcomes may be due to the opposing effects of estrogen on VLDL and breast cancer. However, further studies are required to determine the underlying mechanisms.
3.2. Hepatocellular Carcinoma
Lipid accumulation due to MAFLD and/or genetic predisposition is a risk factor for hepatic cell carcinoma (HCC) [231]. Similar to breast cancer, VLDL also affects HCC development and progression. Recent animal study found that mutation of transmembrane 6 superfamily member 2 (TM6SF2) gene causes worse fibrosis and promotes carcinogenesis of MAFLD [232,233]. TM6SF2 is localized to the membrane of the endoplasmic reticulum, and is essential for the lipidation of Apo B during VLDL synthesis. Impaired TM6SF2 leads to decreased VLDL secretion, which consequently causes hepatic steatosis (MAFLD) and worsens fibrosis in the liver [232]. In hepatoma cells, hypoxia-inducible factor-1 (HIF-1) is upregulated and it can enhance VLDLR expression and VLDL uptake by cells. This mechanism promotes cancer progression [234].
3.3. Other Cancers
VLDL levels were elevated in lung cancer patients compared to non-cancer subjects [235]. However, in the lung cancer population, HDL level was the only prognostic factor [236]. Nevertheless, the role of VLDL in lung cancer remains unclear.
Two Indian clinical studies revealed an inverse correlation between serum VLDL levels and leukoplakia, which is a precursor lesion of oral cancer [237,238]. The major risk factors for oral cancer are smoking, alcohol consumption, and betel nut consumption. These factors increase free-radical production and further damage the cell membrane. Decreased VLDL levels are suggested to be a consequence of increased consumption due to cell membrane repair and oxidative stress [239]. Serum VLDL level has also been suggested as an indicator of the severity of oral cancer and pre-cancer lesions.
While studies focusing on VLDL and other cancers are still sparse, numerous studies have shown evidence of a correlation between elevated TG levels and carcinogenesis risk for small cell lung cancer, breast cancer, pancreatic cancer, and ovarian cancer [217,240]. However, the underlying mechanisms remain largely unknown.
4. Conclusions
VLDL is produced in the liver and is involved in MAFLD, MetS, atherosclerotic diseases, cognitive dysfunction, autoimmune disorders, HCC and breast cancers. Moreover, VLDL inherits cytotoxicity from MetS with undetermined mechanisms and may cause atrial myopathy in the pre-clinical stage of atrial fibrillation. VLDL levels are affected by multiple endocrine systems and VLDL promotes aldosterone secretion. The proposed pathological roles of VLDL awaiting determination include the extrahepatic secretion of VLDL, sleep disorders, neurodegenerative diseases, and a large spectrum of cancers. Overall, there is emerging evidence supporting the pathological roles of VLDL in various diseases across multiple systems. Elucidation of the quality control and metabolism of VLDL, instead of the sole correlation of the VLDL level, may enhance our understanding of its contribution to health and disease mechanisms. Ultimately, the potential VLDL-targeted therapies cab be discovered and be successful.
Acknowledgments
The first author would like to acknowledge the technical support and help from Lee’s laboratory staff including Yu-Hsun Lin, Hua-Shiung Liu, Wun-Jyun Jhuang, and Yi-Hsiung Lin.
Abbreviations
The following abbreviations are used in this manuscript:
| VLDL | Very-low-density lipoprotein |
| VLDLR | VLDL receptor |
| Apo | Apolipoprotein |
| MetS | Metabolic syndrome |
| TG | Triglyceride |
| CE | Cholesterol ester |
| LPL | Lipoprotein lipase |
| CM | Chylomicron |
| IDL | Intermediate-density lipoprotein |
| HDL | High-density lipoprotein |
| LDL | Low-density lipoprotein |
| CEPT | Cholesterol ester transfer protein |
| RLP | Remnant like protein |
| HFD | High-fat diet |
| MAFLD | Metabolic associated fatty liver disease |
| ERR-α | Estrogen-related receptor |
| DNA | Deoxyribonucleic acid |
| AMPK | AMP-activated protein kinase |
| mTOR | Mammalian target of rapamycin |
| HCV | Hepatitis C virus |
| LVP | Lipo-viro-particle |
| Nrf2 | Nuclear factor erythroid 2-related factor |
| PPAR | Peroxisome proliferator-activated receptor |
| ATF4 | Activation of transcription factor 4 |
| ABCA1 | ATP-binding cassette transporter A1 |
| TSH | Thyroid stimulating hormone |
| PCOS | Polycystic ovary syndrome |
| BBB | Blood-brain barrier |
| CKD | Chronic kidney disease |
| ANGPTL4 | Angiopoietin-like protein 4 |
| PCSK9 | Proprotein convertase subtilisin kexin type 9 |
| IL | Interleukin |
| Insig | Insulin-inducible gene |
| SREBP | Sterol regulatory elements-binding protein |
| SLE | Systemic lupus erythematosus |
| HER2 | Human epidermal growth factor receptor 2 |
| HCC | Hepatocellular carcinoma |
| TM6SF2 | Transmembrane 6 superfamily member 2 |
| HIF-1 | Hypoxia-inducible factor-1 |
Author Contributions
Literature searching and writing—original draft preparation, J.-K.H.; writing—review and editing, project administration, and funding acquisition H.-C.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Kaohsiung Medical University Hospital (KMUH97-7G31, KMUH103-3T03, KMUH104-4T04, KMUH107-7R11, and KMUH108-8R10), Kaohsiung Medical University (KMU-TP104D04), Taiwan Ministry of Science and Technology grants (MOST 10314-B-037-080-MY3), the NSYSU-KMU Joint Research Project (#NSYSUKMU 104-P027), and the KMU Global Networking Talent Plan (110KMUOR01).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Klein S. The case of visceral fat: Argument for the defense. J. Clin. Investig. 2004;113:1530–1532. doi: 10.1172/JCI200422028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nielsen S., Guo Z., Johnson C.M., Hensrud D.D., Jensen M.D. Splanchnic lipolysis in human obesity. J. Clin. Investig. 2004;113:1582–1588. doi: 10.1172/JCI21047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ginsberg H.N., Packard C.J., Chapman M.J., Borén J., Aguilar-Salinas C.A., Averna M., Catapano A.L. Triglyceride-rich lipoproteins and their remnants: Metabolic insights, role in atherosclerotic cardiovascular disease, and emerging therapeutic strategies—A consensus statement from the European Atherosclerosis Society. Eur. Heart J. 2021;42:4791–4806. doi: 10.1093/eurheartj/ehab551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ding W.Y., Protty M.B., Davies I.G., Lip G.Y. Relationship between lipoproteins, thrombosis, and atrial fibrillation. Cardiovasc. Res. 2021;118:716–731. doi: 10.1093/cvr/cvab017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feingold K.R., Grunfeld C. Introduction to Lipids and Lipoproteins. MDText.com, Inc.; South Dartmouth, MA, USA: 2015. [Google Scholar]
- 6.Fisher E.A. The degradation of apolipoprotein B100: Multiple opportunities to regulate VLDL triglyceride production by different proteolytic pathways. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids. 2012;1821:778–781. doi: 10.1016/j.bbalip.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Packard C., Demant T., Stewart J., Bedford D., Caslake M., Schwertfeger G., Bedynek A., Shepherd J., Seidel D. Apolipoprotein B metabolism and the distribution of VLDL and LDL subfractions. J. Lipid Res. 2000;41:305–317. doi: 10.1016/S0022-2275(20)32065-4. [DOI] [PubMed] [Google Scholar]
- 8.Marston N.A., Giugliano R.P., Melloni G.E., Park J.G., Morrill V., Blazing M.A., Ference B., Stein E., Stroes E.S., Braunwald E., et al. Association of Apolipoprotein B–Containing Lipoproteins and Risk of Myocardial Infarction in Individuals With and Without Atherosclerosis: Distinguishing Between Particle Concentration, Type, and Content. JAMA Cardiol. 2021;7:250–256. doi: 10.1001/jamacardio.2021.5083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sniderman A.D., Thanassoulis G., Glavinovic T., Navar A.M., Pencina M., Catapano A., Ference B.A. Apolipoprotein B particles and cardiovascular disease: A narrative review. JAMA Cardiol. 2019;4:1287–1295. doi: 10.1001/jamacardio.2019.3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tiwari S., Siddiqi S.A. Intracellular trafficking and secretion of VLDL. Arterioscler. Thromb. Vasc. Biol. 2012;32:1079–1086. doi: 10.1161/ATVBAHA.111.241471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakajima K., Tokita Y., Tanaka A., Takahashi S. The VLDL receptor plays a key role in the metabolism of postprandial remnant lipoproteins. Clin. Chim. Acta. 2019;495:382–393. doi: 10.1016/j.cca.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 12.Péterfy M. Lipase maturation factor 1: A lipase chaperone involved in lipid metabolism. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids. 2012;1821:790–794. doi: 10.1016/j.bbalip.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dlugosz P., Nimpf J. The reelin receptors apolipoprotein E receptor 2 (ApoER2) and VLDL receptor. Int. J. Mol. Sci. 2018;19:3090. doi: 10.3390/ijms19103090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kindel T., Lee D.M., Tso P. The mechanism of the formation and secretion of chylomicrons. Atheroscler. Suppl. 2010;11:11–16. doi: 10.1016/j.atherosclerosissup.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 15.Ko C.-W., Qu J., Black D.D., Tso P. Regulation of intestinal lipid metabolism: Current concepts and relevance to disease. Nat. Rev. Gastroenterol. Hepatol. 2020;17:169–183. doi: 10.1038/s41575-019-0250-7. [DOI] [PubMed] [Google Scholar]
- 16.Le Roy T., Lécuyer E., Chassaing B., Rhimi M., Lhomme M., Boudebbouze S., Ichou F., Haro Barceló J., Huby T., Guerin M., et al. The intestinal microbiota regulates host cholesterol homeostasis. BMC Biol. 2019;17:94. doi: 10.1186/s12915-019-0715-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kvit K.B., Kharchenko N.V., Kharchenko V.V., Chornenka O.I., Chornovus R.I., Dorofeeva U.S., Draganchuk O.B., Slaba O.M. The role of small intestinal bacterial overgrowth in the pathogenesis of hyperlipidemia. Wiadomości Lek. 2019;72:645–649. doi: 10.36740/WLek201904127. [DOI] [PubMed] [Google Scholar]
- 18.Yu Y., Raka F., Adeli K. The role of the gut microbiota in lipid and lipoprotein metabolism. J. Clin. Med. 2019;8:2227. doi: 10.3390/jcm8122227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jayaraman S., Pérez A., Miñambres I., Sánchez-Quesada J.L., Gursky O. Heparin binding triggers human VLDL remodeling by circulating lipoprotein lipase: Relevance to VLDL functionality in health and disease. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids. 2022;1867:159064. doi: 10.1016/j.bbalip.2021.159064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi S.H., Ginsberg H.N. Increased very low density lipoprotein (VLDL) secretion, hepatic steatosis, and insulin resistance. Trends Endocrinol. Metab. 2011;22:353–363. doi: 10.1016/j.tem.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodríguez-Mortera R., Caccavello R., Garay-Sevilla M.E., Gugliucci A. Higher ANGPTL3, apoC-III, and apoB48 dyslipidemia, and lower lipoprotein lipase concentrations are associated with dysfunctional visceral fat in adolescents with obesity. Clin. Chim. Acta. 2020;508:61–68. doi: 10.1016/j.cca.2020.05.014. [DOI] [PubMed] [Google Scholar]
- 22.Wieczorek E., Ćwiklińska A., Kuchta A., Kortas-Stempak B., Gliwińska A., Jankowski M. The Differential Effects of HDL Subpopulations on Lipoprotein Lipase (LPL)-Mediated VLDL Catabolism. Biomedicines. 2021;9:1839. doi: 10.3390/biomedicines9121839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ng T.W.K., Ooi E.M.M., Watts G.F., Chan D.C., Meikle P.J., Barrett P.H. Association of plasma ceramides and sphingomyelin with VLDL apoB-100 fractional catabolic rate before and after rosuvastatin treatment. J. Clin. Endocrinol. Metab. 2015;100:2497–2501. doi: 10.1210/jc.2014-4348. [DOI] [PubMed] [Google Scholar]
- 24.Yamanashi Y., Takada T., Yamamoto H., Suzuki H. NPC1L1 facilitates sphingomyelin absorption and regulates diet-induced production of VLDL/LDL-associated S1P. Nutrients. 2020;12:2641. doi: 10.3390/nu12092641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schaap F.G., Rensen P.C., Voshol P.J., Vrins C., van der Vliet H.N., Chamuleau R.A., Havekes L.M., Groen A.K., van Dijk K.W. ApoAV reduces plasma triglycerides by inhibiting very low density lipoprotein-triglyceride (VLDL-TG) production and stimulating lipoprotein lipase-mediated VLDL-TG hydrolysis. J. Biol. Chem. 2004;279:27941–27947. doi: 10.1074/jbc.M403240200. [DOI] [PubMed] [Google Scholar]
- 26.Ćwiklińska A., Cackowska M., Wieczorek E., Król E., Kowalski R., Kuchta A., Kortas-Stempak B., Gliwińska A., Dąbkowski K., Zielińska L., et al. Progression of chronic kidney disease affects HDL impact on lipoprotein lipase (LPL)-mediated VLDL lipolysis efficiency. Kidney Blood Press. Res. 2018;43:970–978. doi: 10.1159/000490686. [DOI] [PubMed] [Google Scholar]
- 27.Silbernagel G., Scharnagl H., Kleber M.E., Hoffmann M.M., Delgado G., Stojakovic T., Gary T., Zeng L., Ritsch A., Zewinger S., et al. Common APOC3 variants are associated with circulating ApoC-III and VLDL cholesterol but not with total apolipoprotein B and coronary artery disease. Atherosclerosis. 2020;311:84–90. doi: 10.1016/j.atherosclerosis.2020.08.017. [DOI] [PubMed] [Google Scholar]
- 28.Zhang J., Rocha N.D.A., McCullough A.P.A. Contribution of ApoCIII to diabetic dyslipidemia and treatment with volanesorsen. Rev. Cardiovasc. Med. 2018;19:13–19. doi: 10.31083/j.rcm.2018.01.890. [DOI] [PubMed] [Google Scholar]
- 29.Olkkonen V.M., Sinisalo J., Jauhiainen M. New medications targeting triglyceride-rich lipoproteins: Can inhibition of ANGPTL3 or apoC-III reduce the residual cardiovascular risk? Atherosclerosis. 2018;272:27–32. doi: 10.1016/j.atherosclerosis.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 30.Mineo C. Lipoprotein receptor signalling in atherosclerosis. Cardiovasc. Res. 2020;116:1254–1274. doi: 10.1093/cvr/cvz338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee M.H., Smyser C.D., Shimony J.S. Very-low-density lipoprotein of metabolic syndrome modulates gap junctions and slows cardiac conduction. Sci. Rep. 2017;7:12050. doi: 10.1038/s41598-017-11416-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leeb C., Eresheim C., Nimpf J. Clusterin is a ligand for apolipoprotein E receptor 2 (ApoER2) and very low density lipoprotein receptor (VLDLR) and signals via the Reelin-signaling pathway. J. Biol. Chem. 2014;289:4161–4172. doi: 10.1074/jbc.M113.529271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takahashi S. Triglyceride rich lipoprotein-LPL-VLDL receptor and Lp (a)-VLDL receptor pathways for macrophage foam cell formation. J. Atheroscler. Thromb. 2017;24:552–559. doi: 10.5551/jat.RV17004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goudriaan J.R., Santo S.M.E., Voshol P.J., Teusink B., van Dijk K.W., van Vlijmen B.J., Romijn J.A., Havekes L.M., Rensen P.C. The VLDL receptor plays a major role in chylomicron metabolism by enhancing LPL-mediated triglyceride hydrolysis. J. Lipid Res. 2004;45:1475–1481. doi: 10.1194/jlr.M400009-JLR200. [DOI] [PubMed] [Google Scholar]
- 35.Kozarsky K.E., Jooss K., Donahee M., Strauss J.F., Wilson J. Effective treatment of familial hypercholesterolaemia in the mouse model using adenovirus–mediated transfer of the VLDL receptor gene. Nat. Genet. 1996;13:54–62. doi: 10.1038/ng0596-54. [DOI] [PubMed] [Google Scholar]
- 36.Kobayashi K., Oka K., Forte T., Ishida B., Teng B., Ishimura-Oka K., Nakamuta M., Chan L. Reversal of Hypercholesterolemia in Low Density Lipoprotein Receptor Knockout Mice by Adenovirus-mediated Gene Transfer of the Very Low Density Lipoprotein Receptor. J. Biol. Chem. 1996;271:6852–6860. doi: 10.1074/jbc.271.12.6852. [DOI] [PubMed] [Google Scholar]
- 37.Balling M., Afzal S., Varbo A., Langsted A., Davey Smith G., Nordestgaard B.G. VLDL cholesterol accounts for one-half of the risk of myocardial infarction associated with apoB-containing lipoproteins. J. Am. Coll. Cardiol. 2020;76:2725–2735. doi: 10.1016/j.jacc.2020.09.610. [DOI] [PubMed] [Google Scholar]
- 38.Chen Y., Hu Y., Lu K., Flannery J., Ma J.-X. Very low density lipoprotein receptor, a negative regulator of the wnt signaling pathway and choroidal neovascularization. J. Biol. Chem. 2007;282:34420–34428. doi: 10.1074/jbc.M611289200. [DOI] [PubMed] [Google Scholar]
- 39.Lee H.-C., Shin S.-J., Huang J.-K., Lin M.-Y., Lin Y.-H., Ke L.-Y., Jiang H.-J., Tsai W.-C., Chao M.-F., Lin Y.-H. The role of postprandial very-low-density lipoprotein in the development of atrial remodeling in metabolic syndrome. Lipids Health Dis. 2020;19:210. doi: 10.1186/s12944-020-01386-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nusse R., Clevers H. Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell. 2017;169:985–999. doi: 10.1016/j.cell.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 41.Samson C.E., Galia A.L.B., Llave K.I.C., Zacarias M.B., Mercado-Asis L.B. Postprandial peaking and plateauing of triglycerides and VLDL in patients with underlying cardiovascular diseases despite treatment. Int. J. Endocrinol. Metab. 2012;10:587. doi: 10.5812/ijem.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lambert J.E., Parks E.J. Postprandial metabolism of meal triglyceride in humans. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids. 2012;1821:721–726. doi: 10.1016/j.bbalip.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakajima K., Nakano T., Tokita Y., Nagamine T., Inazu A., Kobayashi J., Mabuchi H., Stanhope K.L., Havel P.J., Okazaki M., et al. Postprandial lipoprotein metabolism: VLDL vs chylomicrons. Clin. Chim. Acta. 2011;412:1306–1318. doi: 10.1016/j.cca.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakajima K., Nakajima K. Remnant Lipoproteins: A Subfraction of Plasma Triglyceride-Rich Lipoproteins Associated with Postprandial Hyperlipidemia. Clin. Exp. Thromb. Hemost. 2014;1:45–53. doi: 10.14345/ceth.14013. [DOI] [Google Scholar]
- 45.Hartigh L.J.D., Altman R., Norman J.E., Rutledge J.C. Postprandial VLDL lipolysis products increase monocyte adhesion and lipid droplet formation via activation of ERK2 and NFκB. Am. J. Physiol. Heart Circ. Physiol. 2014;306:H109–H120. doi: 10.1152/ajpheart.00137.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Søndergaard E., Johansen R., Jensen M.D., Nielsen S. Postprandial VLDL-TG metabolism in type 2 diabetes. Metabolism. 2017;75:25–35. doi: 10.1016/j.metabol.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 47.Nakajima K., Tanaka A. Atherogenic postprandial remnant lipoproteins; VLDL remnants as a causal factor in atherosclerosis. Clin. Chim. Acta. 2018;478:200–215. doi: 10.1016/j.cca.2017.12.039. [DOI] [PubMed] [Google Scholar]
- 48.Mittendorfer B., Yoshino M., Patterson B.W., Klein S. VLDL triglyceride kinetics in lean, overweight, and obese men and women. J. Clin. Endocrinol. Metab. 2016;101:4151–4160. doi: 10.1210/jc.2016-1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Palmisano B.T., Zhu L., Eckel R.H., Stafford J.M. Sex differences in lipid and lipoprotein metabolism. Mol. Metab. 2018;15:45–55. doi: 10.1016/j.molmet.2018.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sumner A.E., Furtado J.D., Courville A.B., Ricks M., Younger-Coleman N., Tulloch-Reid M.K., Sacks F.M. ApoC-III and visceral adipose tissue contribute to paradoxically normal triglyceride levels in insulin-resistant African-American women. Nutr. Metab. 2013;10:73. doi: 10.1186/1743-7075-10-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miller B.V., Patterson B.W., Okunade A., Klein S. Fatty acid and very low density lipoprotein metabolism in obese African American and Caucasian women with type 2 diabetes. J. Lipid Res. 2012;53:2767–2772. doi: 10.1194/jlr.P030593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gormsen L.C., Høst C., Hjerrild B.E., Gravholt C.H., Nielsen S. Acute estrogen exposure does not affect basal very low-density lipoprotein–triglyceride production or oxidation in postmenopausal women. Eur. J. Endocrinol. 2010;163:421–426. doi: 10.1530/EJE-10-0551. [DOI] [PubMed] [Google Scholar]
- 53.Wang X., Smith G.I., Patterson B.W., Reeds D.N., Kampelman J., Magkos F., Mittendorfer B. Testosterone increases the muscle protein synthesis rate but does not affect very-low-density lipoprotein metabolism in obese premenopausal women. Am. J. Physiol. Endocrinol. Metab. 2012;302:E740–E746. doi: 10.1152/ajpendo.00533.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Olufadi R., Byrne C.D. Effects of VLDL and remnant particles on platelets. Pathophysiol. Haemost. Thromb. 2006;35:281–291. doi: 10.1159/000093221. [DOI] [PubMed] [Google Scholar]
- 55.Klein S., Spannagl M., Engelmann B. Phosphatidylethanolamine participates in the stimulation of the contact system of coagulation by very-low-density lipoproteins. Arterioscler. Thromb. Vasc. Biol. 2001;21:1695–1700. doi: 10.1161/atvb.21.10.1695. [DOI] [PubMed] [Google Scholar]
- 56.Ouweneel A.B., Van Eck M. Lipoproteins as modulators of atherothrombosis: From endothelial function to primary and secondary coagulation. Vasc. Pharmacol. 2016;82:1–10. doi: 10.1016/j.vph.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 57.Doi H., Kugiyama K., Oka H., Sugiyama S., Ogata N., Koide S.-I., Nakamura S.-I., Yasue H. Remnant lipoproteins induce proatherothrombogenic molecules in endothelial cells through a redox-sensitive mechanism. Circulation. 2000;102:670–676. doi: 10.1161/01.CIR.102.6.670. [DOI] [PubMed] [Google Scholar]
- 58.De Sousa J.C., Soria C., Ayrault-Jarrier M., Pastier D., Bruckert E., Amiral J., Bereziat G., Caen J.P. Association between coagulation factors VII and X with triglyceride rich lipoproteins. J. Clin. Pathol. 1988;41:940–944. doi: 10.1136/jcp.41.9.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Surya I., Mommersteeg M., Gorter G., Erkelens D.W., Akkerman J.-W.N. Abnormal platelet functions in a patient with abetalipoproteinemia. Thromb. Haemost. 1991;65:306–311. doi: 10.1055/s-0038-1648140. [DOI] [PubMed] [Google Scholar]
- 60.Fabbrini E., Mohammed B.S., Magkos F., Korenblat K.M., Patterson B.W., Klein S. Alterations in adipose tissue and hepatic lipid kinetics in obese men and women with nonalcoholic fatty liver disease. Gastroenterology. 2008;134:424–431. doi: 10.1053/j.gastro.2007.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sparks J.D., Sparks C.E., Adeli K. Selective hepatic insulin resistance, VLDL overproduction, and hypertriglyceridemia. Arterioscler. Thromb. Vasc. Biol. 2012;32:2104–2112. doi: 10.1161/ATVBAHA.111.241463. [DOI] [PubMed] [Google Scholar]
- 62.Poulsen M.K., Nellemann B., Stødkilde-Jørgensen H., Pedersen S.B., Grønbæk H., Nielsen S. Impaired insulin suppression of VLDL-triglyceride kinetics in nonalcoholic fatty liver disease. J. Clin. Endocrinol. Metab. 2016;101:1637–1646. doi: 10.1210/jc.2015-3476. [DOI] [PubMed] [Google Scholar]
- 63.Cohn J.S., Patterson B.W., Uffelman K.D., Davignon J., Steiner G. Rate of production of plasma and very-low-density lipoprotein (VLDL) apolipoprotein C-III is strongly related to the concentration and level of production of VLDL triglyceride in male subjects with different body weights and levels of insulin sensitivity. J. Clin. Endocrinol. Metab. 2004;89:3949–3955. doi: 10.1210/jc.2003-032056. [DOI] [PubMed] [Google Scholar]
- 64.Rodriguez V., Newman J., Schwartzbard A.Z. Towards more specific treatment for diabetic dyslipidemia. Curr. Opin. Lipidol. 2018;29:307. doi: 10.1097/MOL.0000000000000528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lucero D., Miksztowicz V., Gualano G., Longo C., Landeira G., Álvarez E., Zago V., Brites F., Berg G., Fassio E., et al. Nonalcoholic fatty liver disease associated with metabolic syndrome: Influence of liver fibrosis stages on characteristics of very low-density lipoproteins. Clin. Chim. Acta. 2017;473:1–8. doi: 10.1016/j.cca.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 66.Yang M., Liu Q., Huang T., Tan W., Qu L., Chen T., Guan M. Dysfunction of estrogen-related receptor alpha-dependent hepatic VLDL secretion contributes to sex disparity in NAFLD/NASH development. Theranostics. 2020;10:10874. doi: 10.7150/thno.47037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang J.D., Abdelmalek M.F., Guy C.D., Gill R.M., Lavine J.E., Yates K., Wilson L. Patient sex, reproductive status, and synthetic hormone use associate with histologic severity of nonalcoholic steatohepatitis. Clin. Gastroenterol. Hepatol. 2017;15:127–131.e2. doi: 10.1016/j.cgh.2016.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Garcia-Carretero R., Vigil-Medina L., Barquero-Perez O., Ramos-Lopez J. Relevant features in nonalcoholic steatohepatitis determined using machine learning for feature selection. Metab. Syndr. Relat. Disord. 2019;17:444–451. doi: 10.1089/met.2019.0052. [DOI] [PubMed] [Google Scholar]
- 69.Ballestri S., Nascimbeni F., Baldelli E., Marrazzo A., Romagnoli D., Lonardo A. NAFLD as a sexual dimorphic disease: Role of gender and reproductive status in the development and progression of nonalcoholic fatty liver disease and inherent cardiovascular risk. Adv. Ther. 2017;34:1291–1326. doi: 10.1007/s12325-017-0556-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Birzniece V., Barrett P.H.R., Ho K.K.Y. Tamoxifen reduces hepatic VLDL production and GH secretion in women: A possible mechanism for steatosis development. Eur. J. Endocrinol. 2017;177:137–143. doi: 10.1530/EJE-17-0151. [DOI] [PubMed] [Google Scholar]
- 71.Pruis M.G.M., Lendvai A., Bloks V.W., Zwier M.V., Baller J.F.W., de Bruin A., Groen A.K., Plösch T. Maternal western diet primes non-alcoholic fatty liver disease in adult mouse offspring. Acta Physiol. 2014;210:215–227. doi: 10.1111/apha.12197. [DOI] [PubMed] [Google Scholar]
- 72.Chen H.C., Chen Y.Z., Wang C.H., Lin F.J. The nonalcoholic fatty liver disease-like phenotype and lowered serum VLDL are associated with decreased expression and DNA hypermethylation of hepatic ApoB in male offspring of ApoE deficient mothers fed a with Western diet. J. Nutr. Biochem. 2020;77:108319. doi: 10.1016/j.jnutbio.2019.108319. [DOI] [PubMed] [Google Scholar]
- 73.Shen B., Zhao C., Wang Y., Peng Y., Cheng J., Li Z., Feng H. Aucubin inhibited lipid accumulation and oxidative stress via Nrf2/HO-1 and AMPK signalling pathways. J. Cell. Mol. Med. 2019;23:4063–4075. doi: 10.1111/jcmm.14293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shen B., Feng H., Cheng J., Li Z., Jin M., Zhao L., Liu G. Geniposide alleviates non-alcohol fatty liver disease via regulating Nrf2/AMPK/mTOR signalling pathways. J. Cell. Mol. Med. 2020;24:5097–5108. doi: 10.1111/jcmm.15139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sano A., Kakazu E., Hamada S., Inoue J., Ninomiya M., Iwata T., Tsuruoka M., Sato K., Masamune A. Steatotic Hepatocytes Release Mature VLDL Through Methionine and Tyrosine Metabolism in a Keap1-Nrf2–Dependent Manner. Hepatology. 2021;74:1271–1286. doi: 10.1002/hep.31808. [DOI] [PubMed] [Google Scholar]
- 76.Nikolova-Karakashian M. Alcoholic and non-alcoholic fatty liver disease: Focus on ceramide. Adv. Biol. Regul. 2018;70:40–50. doi: 10.1016/j.jbior.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 77.Carlier A., Phan F., Szpigel A., Hajduch E., Salem J.-E., Gautheron J., Le Goff W., Guérin M., Lachkar F., Ratziu V., et al. Dihydroceramides in Triglyceride-Enriched VLDL Are Associated with Nonalcoholic Fatty Liver Disease Severity in Type 2 Diabetes. Cell Rep. Med. 2020;1:100154. doi: 10.1016/j.xcrm.2020.100154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Al-Mrabeh A., Zhyzhneuskaya S.V., Peters C., Barnes A.C., Melhem S., Jesuthasan A., Aribisala B., Hollingsworth K.G., Lietz G., Mathers J.C., et al. Hepatic lipoprotein export and remission of human type 2 diabetes after weight loss. Cell Metab. 2020;31:233–249.e4. doi: 10.1016/j.cmet.2019.11.018. [DOI] [PubMed] [Google Scholar]
- 79.André P., Komurian-Pradel F., Deforges S., Perret M., Berland J.L., Sodoyer M., Pol S., Bréchot C., Paranhos-Baccalà G., Lotteau V. Characterization of low-and very-low-density hepatitis C virus RNA-containing particles. J. Virol. 2002;76:6919–6928. doi: 10.1128/JVI.76.14.6919-6928.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vercauteren K., Mesalam A.A., Leroux-Roels G., Meuleman P. Impact of lipids and lipoproteins on hepatitis C virus infection and virus neutralization. World J. Gastroenterol. WJG. 2014;20:15975. doi: 10.3748/wjg.v20.i43.15975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nielsen S.U., Bassendine M.F., Burt A.D., Martin C., Pumeechockchai W., Toms G.L. Association between hepatitis C virus and very-low-density lipoprotein (VLDL)/LDL analyzed in iodixanol density gradients. J. Virol. 2006;80:2418–2428. doi: 10.1128/JVI.80.5.2418-2428.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sun H.-Y., Lin C.-C., Lee J.-C., Wang S.-W., Cheng P.-N., Wu I.-C., Chang T.-T., Lai M.-D., Shieh D.-B., Young K.-C. Very low-density lipoprotein/lipo-viro particles reverse lipoprotein lipase-mediated inhibition of hepatitis C virus infection via apolipoprotein C-III. Gut. 2013;62:1193–1203. doi: 10.1136/gutjnl-2011-301798. [DOI] [PubMed] [Google Scholar]
- 83.Serfaty L. Metabolic Manifestations of Hepatitis C Virus: Diabetes Mellitus, Dyslipidemia. Clin. Liver Dis. 2017;21:475–486. doi: 10.1016/j.cld.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 84.Sun H.-Y., Cheng P.-N., Tseng C.-Y., Tsai W.-J., Chiu Y.-C., Young K.-C. Favouring modulation of circulating lipoproteins and lipid loading capacity by direct antiviral agents grazoprevir/elbasvir or ledipasvir/sofosbuvir treatment against chronic HCV infection. Gut. 2018;67:1342–1350. doi: 10.1136/gutjnl-2017-313832. [DOI] [PubMed] [Google Scholar]
- 85.Wang Z., Dou X., Li S., Zhang X., Sun X., Zhou Z., Song Z. Nuclear factor (erythroid-derived 2)-like 2 activation-induced hepatic very-low-density lipoprotein receptor overexpression in response to oxidative stress contributes to alcoholic liver disease in mice. Hepatology. 2014;59:1381–1392. doi: 10.1002/hep.26912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jo H., Choe S.S., Shin K.C., Jang H., Lee J.H., Seong J.K., Back S.H., Kim J.B. Endoplasmic reticulum stress induces hepatic steatosis via increased expression of the hepatic very low-density lipoprotein receptor. Hepatology. 2013;57:1366–1377. doi: 10.1002/hep.26126. [DOI] [PubMed] [Google Scholar]
- 87.Zarei M. Hepatic regulation of VLDL receptor by PPARβ/δ and FGF21 modulates non-alcoholic fatty liver disease. Mol. Metab. 2018;8:117–131. doi: 10.1016/j.molmet.2017.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gao Y., Shen W., Lu B., Zhang Q., Hu Y., Chen Y. Upregulation of hepatic VLDLR via PPARα is required for the triglyceride-lowering effect of fenofibrate. J. Lipid Res. 2014;55:1622–1633. doi: 10.1194/jlr.M041988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sørensen L.P., Andersen I.R., Søndergaard E., Gormsen L.C., Schmitz O., Christiansen J.S., Nielsen S. Basal and insulin mediated VLDL-triglyceride kinetics in type 2 diabetic men. Diabetes. 2011;60:88–96. doi: 10.2337/db10-0564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tricò D., Natali A., Mari A., Ferrannini E., Santoro N., Caprio S. Triglyceride-rich very low-density lipoproteins (VLDL) are independently associated with insulin secretion in a multiethnic cohort of adolescents. Diabetes Obes. Metab. 2018;20:2905–2910. doi: 10.1111/dom.13467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mucinski J.M., Manrique-Acevedo C., Kasumov T., Garrett T.J., Gaballah A., Parks E.J. Relationships between Very Low-Density Lipoproteins–Ceramides,− Diacylglycerols, and–Triacylglycerols in Insulin-Resistant Men. Lipids. 2020;55:387–393. doi: 10.1002/lipd.12244. [DOI] [PubMed] [Google Scholar]
- 92.Nielsen S., Karpe F. Determinants of VLDL-triglycerides production. Curr. Opin. Lipidol. 2012;23:321–326. doi: 10.1097/MOL.0b013e3283544956. [DOI] [PubMed] [Google Scholar]
- 93.Xiao C., Pavlic M., Szeto L., Patterson B.W., Lewis G.F. Effects of acute hyperglucagonemia on hepatic and intestinal lipoprotein production and clearance in healthy humans. Diabetes. 2011;60:383–390. doi: 10.2337/db10-0763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shulman G.I. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N. Engl. J. Med. 2014;371:1131–1141. doi: 10.1056/NEJMra1011035. [DOI] [PubMed] [Google Scholar]
- 95.Gormsen L.C., Nellemann B., Sørensen L.P., Jensen M.D., Christiansen J.S., Nielsen S. Impact of body composition on very-low-density lipoprotein-triglycerides kinetics. Am. J. Physiol. Endocrinol. Metab. 2009;296:E165–E173. doi: 10.1152/ajpendo.90675.2008. [DOI] [PubMed] [Google Scholar]
- 96.Johansen R.F., Søndergaard E., Sørensen L.P., Jurik A.G., Christiansen J.S., Nielsen S. Basal and insulin-regulated VLDL1 and VLDL2 kinetics in men with type 2 diabetes. Diabetologia. 2016;59:833–843. doi: 10.1007/s00125-015-3856-5. [DOI] [PubMed] [Google Scholar]
- 97.Brunham L.R., Kruit J., Pape T.D., Timmins J.M., Maijer-Reuwer A., Vasanji Z., Marsh B.J., Rodrigues B., Johnson J.D., Parks J.S., et al. β-cell ABCA1 influences insulin secretion, glucose homeostasis and response to thiazolidinedione treatment. Nat. Med. 2007;13:340–347. doi: 10.1038/nm1546. [DOI] [PubMed] [Google Scholar]
- 98.Babashamsi M.M., Koukhaloo S.Z., Halalkhor S., Salimi A. ABCA1 and metabolic syndrome; a review of the ABCA1 role in HDL-VLDL production, insulin-glucose homeostasis, inflammation and obesity. Diabetes Metab. Syndr. Clin. Res. Rev. 2019;13:1529–1534. doi: 10.1016/j.dsx.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 99.Jacobo-Albavera L., Domínguez-Pérez M., Medina-Leyte D., González-Garrido A., Villarreal-Molina T. The Role of the ATP-Binding Cassette A1 (ABCA1) in Human Disease. Int. J. Mol. Sci. 2021;22:1593. doi: 10.3390/ijms22041593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lee H.-C., Lin H.-T., Ke L.-Y., Wei C., Hsiao Y.-L., Chu C.-S., Lai W.-T., Shin S.-J., Chen C.-H., Sheu S.-H., et al. VLDL from metabolic syndrome individuals enhanced lipid accumulation in atria with association of susceptibility to atrial fibrillation. Int. J. Mol. Sci. 2016;17:134. doi: 10.3390/ijms17010134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chen C.-H., Lu J., Chen S.-H., Huang R.Y., Yilmaz H.R., Dong J., Elayda M.A., Dixon R.A., Yang C.-Y. Effects of electronegative VLDL on endothelium damage in metabolic syndrome. Diabetes Care. 2012;35:648–653. doi: 10.2337/dc11-1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li C.-L., Chu C.-H., Lee H.-C., Chou M.-C., Liu C.-K., Chen C.-H., Ke L.-Y., Chen S.-L. Immunoregulatory effects of very low density lipoprotein from healthy individuals and metabolic syndrome patients on glial cells. Immunobiology. 2019;224:632–637. doi: 10.1016/j.imbio.2019.07.005. [DOI] [PubMed] [Google Scholar]
- 103.Kornetova E.G., Kornetov A.N., Mednova I.A., Dubrovskaya V.V., Boiko A.S., Bokhan N.A., Loonen A.J.M., Ivanova S.A. Changes in body fat and related biochemical parameters associated with atypical antipsychotic drug treatment in schizophrenia patients with or without metabolic syndrome. Front. Psychiatry. 2019;10:803. doi: 10.3389/fpsyt.2019.00803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mendivil C.O., Rimm E.B., Furtado J., Sacks F.M. Apolipoprotein E in VLDL and LDL with apolipoprotein C-III is associated with a lower risk of coronary heart disease. J. Am. Heart Assoc. 2013;2:e000130. doi: 10.1161/JAHA.113.000130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Whitacre B.E., Howles P., Street S., Morris J., Swertfeger D., Davidson W.S. Apolipoprotein E content of VLDL limits LPL-mediated triglyceride hydrolysis. J. Lipid Res. 2022;63:100157. doi: 10.1016/j.jlr.2021.100157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shin K.C., Hwang I., Choe S.S., Park J., Jianhong C., Kim J.I., Lee G.Y., Choi S.H., Ching J., Kovalik J.-P., et al. Macrophage VLDLR mediates obesity-induced insulin resistance with adipose tissue inflammation. Nat. Commun. 2017;8:1087. doi: 10.1038/s41467-017-01232-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ruby M., Goldenson B., Orasanu G., Johnston T.P., Plutzky J., Krauss R.M. VLDL hydrolysis by LPL activates PPAR-α through generation of unbound fatty acids. J. Lipid Res. 2010;51:2275–2281. doi: 10.1194/jlr.M005561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wood A.C., Glasser S., Garvey W.T., Kabagambe E.K., Borecki I.B., Tiwari H.K., Tsai M.Y., Hopkins P.N., Ordovas J.M., Arnett D.K. Lipoprotein lipase S447X variant associated with VLDL, LDL and HDL diameter clustering in the MetS. Lipids Health Dis. 2011;10:143. doi: 10.1186/1476-511X-10-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Giordano R., Picu A., Marinazzo E., D’Angelo V., Berardelli R., Karamouzis I., Forno D., Zinnà D., Maccario M., Ghigo E., et al. Metabolic and cardiovascular outcomes in patients with Cushing’s syndrome of different aetiologies during active disease and 1 year after remission. Clin. Endocrinol. 2011;75:354–360. doi: 10.1111/j.1365-2265.2011.04055.x. [DOI] [PubMed] [Google Scholar]
- 110.Feingold K.R., Brinton E.A., Grunfeld C. The effect of endocrine disorders on lipids and lipoproteins. [(accessed on 25 March 2022)];Endotext. 2020 Available online: https://www.ncbi.nlm.nih.gov/books/NBK409608/ [Google Scholar]
- 111.Guia R.M.d., Herzig S. Glucocorticoid Signaling. Springer; Berlin/Heidelberg, Germany: 2015. How do glucocorticoids regulate lipid metabolism? pp. 127–144. [DOI] [PubMed] [Google Scholar]
- 112.Barbot M., Zilio M., Scaroni C. Cushing’s syndrome: Overview of clinical presentation, diagnostic tools and complications. Best Pract. Res. Clin. Endocrinol. Metab. 2020;34:101380. doi: 10.1016/j.beem.2020.101380. [DOI] [PubMed] [Google Scholar]
- 113.Hakami O.A., Ahmed S., Karavitaki N. Epidemiology and mortality of Cushing’s syndrome. Best Pract. Res. Clin. Endocrinol. Metab. 2021;35:101521. doi: 10.1016/j.beem.2021.101521. [DOI] [PubMed] [Google Scholar]
- 114.Tsai Y.-Y., Rainey W.E., Johnson M.H., Bollag W.B. VLDL-activated cell signaling pathways that stimulate adrenal cell aldosterone production. Mol. Cell. Endocrinol. 2016;433:138–146. doi: 10.1016/j.mce.2016.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tsai Y.-Y., E Rainey W., Bollag W.B. Very low-density lipoprotein (VLDL)-induced signals mediating aldosterone production. J. Endocrinol. 2017;232:R115. doi: 10.1530/JOE-16-0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bollag W.B. Role of phospholipases in adrenal steroidogenesis. J. Endocrinol. 2016;229:R29–R41. doi: 10.1530/JOE-16-0007. [DOI] [PubMed] [Google Scholar]
- 117.Baudrand R., Pojoga L.H., Vaidya A., Garza A.E., A Vöhringer P., Jeunemaitre X., Hopkins P.N., Yao T.M., Williams J., Adler G.K., et al. Statin use and adrenal aldosterone production in hypertensive and diabetic subjects. Circulation. 2015;132:1825–1833. doi: 10.1161/CIRCULATIONAHA.115.016759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Moller N., Vendelbo M.H., Kampmann U., Christensen B., Madsen M., Norrelund H., Jorgensen J.O. Growth hormone and protein metabolism. Clin. Nutr. 2009;28:597–603. doi: 10.1016/j.clnu.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 119.Moller N., Jørgensen J.O.L. Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Endocr. Rev. 2009;30:152–177. doi: 10.1210/er.2008-0027. [DOI] [PubMed] [Google Scholar]
- 120.Stochholm K., Gravholt C.H., Laursen T., Laurberg P., Andersen M., Kristensen L., Feldt-Rasmussen U., Christiansen J.S., Frydenberg M., Green A. Mortality and GH deficiency: A nationwide study. Eur. J. Endocrinol. 2007;157:9–18. doi: 10.1530/EJE-07-0013. [DOI] [PubMed] [Google Scholar]
- 121.Twickler T.B., Dallinga-Thie G., Zelissen P.M.J., Koppeschaar H.P.F., Erkelens D.W. The atherogenic plasma remnant-like particle cholesterol concentration is increased in the fasting and postprandial state in active acromegalic patients. Clin. Endocrinol. 2001;55:69–75. doi: 10.1046/j.1365-2265.2001.01326.x. [DOI] [PubMed] [Google Scholar]
- 122.Fabbrini E., Magkos F., Patterson B.W., Mittendorfer B., Klein S. Subclinical hypothyroidism and hyperthyroidism have opposite effects on hepatic very-low-density lipoprotein-triglyceride kinetics. J. Clin. Endocrinol. Metab. 2012;97:E414–E418. doi: 10.1210/jc.2011-2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yang L., Yin R., Wang Z., Wang X., Zhang Y., Zhao D. Circulating Angptl3 and Angptl8 are increased in patients with hypothyroidism. BioMed Res. Int. 2019;2019:3814687. doi: 10.1155/2019/3814687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Treister-Goltzman Y., Yarza S., Peleg R. Lipid profile in mild subclinical hypothyroidism: Systematic review and meta-analysis. Minerva Endocrinol (Torino) 2021;46:428–440. doi: 10.23736/S2724-6507.20.03197-1. [DOI] [PubMed] [Google Scholar]
- 125.Duntas L.H., Brenta G. The effect of thyroid disorders on lipid levels and metabolism. Med. Clin. 2012;96:269–281. doi: 10.1016/j.mcna.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 126.Høst C., Gormsen L.C., Christensen B., Jessen N., Hougaard D.M., Christiansen J.S., Pedersen S.B., Jensen M.D., Nielsen S., Gravholt C.H. Independent effects of testosterone on lipid oxidation and VLDL-TG production: A randomized, double-blind, placebo-controlled, crossover study. Diabetes. 2013;62:1409–1416. doi: 10.2337/db12-0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Corona G., Rastrelli G., Di Pasquale G., Sforza A., Mannucci E., Maggi M. Testosterone and cardiovascular risk: Meta-analysis of interventional studies. J. Sex. Med. 2018;15:820–838. doi: 10.1016/j.jsxm.2018.04.641. [DOI] [PubMed] [Google Scholar]
- 128.Salvador C., Planas J., Agreda F., Placer J., Trilla E., López M., Morote J. Analysis of the lipid profile and atherogenic risk during androgen deprivation therapy in prostate cancer patients. Urol. Int. 2013;90:41–44. doi: 10.1159/000342814. [DOI] [PubMed] [Google Scholar]
- 129.Maraka S., Singh Ospina N., Rodriguez-Gutierrez R., Davidge-Pitts C.J., Nippoldt T.B., Prokop L.J., Murad M.H. Sex steroids and cardiovascular outcomes in transgender individuals: A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2017;102:3914–3923. doi: 10.1210/jc.2017-01643. [DOI] [PubMed] [Google Scholar]
- 130.Gava G., Mancini I., Cerpolini S., Baldassarre M., Seracchioli R., Meriggiola M.C. Testosterone undecanoate and testosterone enanthate injections are both effective and safe in transmen over 5 years of administration. Clin. Endocrinol. 2018;89:878–886. doi: 10.1111/cen.13821. [DOI] [PubMed] [Google Scholar]
- 131.Van Velzen D.M., Paldino A., Klaver M., Nota N.M., Defreyne J., Hovingh G.K., Thijs A., Simsek S., T’Sjoen G., Heijer M.D. Cardiometabolic effects of testosterone in transmen and estrogen plus cyproterone acetate in transwomen. J. Clin. Endocrinol. Metab. 2019;104:1937–1947. doi: 10.1210/jc.2018-02138. [DOI] [PubMed] [Google Scholar]
- 132.Alves A.C., Valcarcel B., Mäkinen V.-P., Morin-Papunen L., Sebert S., Kangas A.J., Soininen P., Das S., De Iorio M., Coin L., et al. Metabolic profiling of polycystic ovary syndrome reveals interactions with abdominal obesity. Int. J. Obes. 2017;41:1331–1340. doi: 10.1038/ijo.2017.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Samimi M., Dadkhah A., Kashani H.H., Tajabadi-Ebrahimi M., Hosseini E.S., Asemi Z. The effects of synbiotic supplementation on metabolic status in women with polycystic ovary syndrome: A randomized double-blind clinical trial. Probiotics Antimicrob. Proteins. 2019;11:1355–1361. doi: 10.1007/s12602-018-9405-z. [DOI] [PubMed] [Google Scholar]
- 134.Marchand G.B., Carreau A.-M., Weisnagel S.J., Bergeron J., Labrie F., Lemieux S., Tchernof A. Increased body fat mass explains the positive association between circulating estradiol and insulin resistance in postmenopausal women. Am. J. Physiol. Endocrinol. Metab. 2018;314:E448–E456. doi: 10.1152/ajpendo.00293.2017. [DOI] [PubMed] [Google Scholar]
- 135.Lamon-Fava S., Diffenderfer M.R., Barrett P.H.R., Wan W.Y., Postfai B., Nartsupha C., Dolnikowski G.G., Schaefer E.J. Differential effects of estrogen and progestin on apolipoprotein B100 and B48 kinetics in postmenopausal women. Lipids. 2018;53:167–175. doi: 10.1002/lipd.12011. [DOI] [PubMed] [Google Scholar]
- 136.Roach R.E., Helmerhorst F.M., Lijfering W.M., Stijnen T., Algra A., Dekkers O. Combined oral contraceptives: The risk of myocardial infarction and ischemic stroke. Cochrane Database Syst. Rev. 2015;2015:CD011054. doi: 10.1002/14651858.CD011054.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kuller L.H. Hormone replacement therapy and risk of cardiovascular disease: Implications of the results of the Women’s Health Initiative. Arterioscler. Thromb. Vasc. Biol. 2003;23:11–16. doi: 10.1161/01.ATV.0000046033.32478.6D. [DOI] [PubMed] [Google Scholar]
- 138.Basaran A. Pregnancy-induced hyperlipoproteinemia: Review of the literature. Reprod. Sci. 2009;16:431–437. doi: 10.1177/1933719108330569. [DOI] [PubMed] [Google Scholar]
- 139.Alvarez J.J., Montelongo A., Iglesias A., Lasunción M.A., Herrera E. Longitudinal study on lipoprotein profile, high density lipoprotein subclass, and postheparin lipases during gestation in women. J. Lipid Res. 1996;37:299–308. doi: 10.1016/S0022-2275(20)37617-3. [DOI] [PubMed] [Google Scholar]
- 140.Cibickova L., Schovanek J., Karasek D. Changes in serum lipid levels during pregnancy in women with gestational diabetes. A narrative review. Biomed. Pap. Med. Fac. Palacky Univ. Olomouc. 2021;165:8–12. doi: 10.5507/bp.2021.009. [DOI] [PubMed] [Google Scholar]
- 141.Toescu V., Nuttall S.L., Martin U., Nightingale P., Kendall M.J., Brydon P., Dunne F. Changes in plasma lipids and markers of oxidative stress in normal pregnancy and pregnancies complicated by diabetes. Clin. Sci. 2004;106:93–98. doi: 10.1042/CS20030175. [DOI] [PubMed] [Google Scholar]
- 142.Yadav S., Agrawal M., Hariharan C., Dewani D., Vadera K., Krishna N. A comparative study of serum lipid profile of women with preeclampsia and normotensive pregnancy. J. Datta Meghe Inst. Med. Sci. Univ. 2018;13:83. doi: 10.4103/jdmimsu.jdmimsu_70_17. [DOI] [Google Scholar]
- 143.Silva C.M.D.S., Barbosa F.R., Lima G.A., Warszawski L., Fontes R., Domingues R.C., Gadelha M.R. BMI and metabolic profile in patients with prolactinoma before and after treatment with dopamine agonists. Obesity. 2011;19:800–805. doi: 10.1038/oby.2010.150. [DOI] [PubMed] [Google Scholar]
- 144.Inancli S.S., Usluogullari A., Ustu Y., Caner S., Tam A.A., Ersoy R., Cakir B. Effect of cabergoline on insulin sensitivity, inflammation, and carotid intima media thickness in patients with prolactinoma. Endocrine. 2013;44:193–199. doi: 10.1007/s12020-012-9857-y. [DOI] [PubMed] [Google Scholar]
- 145.Schwetz V., Librizzi R., Trummer C., Theiler G., Stiegler C., Pieber T.R., Obermayer-Pietsch B., Pilz S. Treatment of hyperprolactinaemia reduces total cholesterol and LDL in patients with prolactinomas. Metab. Brain Dis. 2017;32:155–161. doi: 10.1007/s11011-016-9882-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Byberg S., Futtrup J., Andreassen M., Krogh J. Metabolic effects of dopamine agonists in patients with prolactinomas: A systematic review and meta-analysis. Endocr. Connect. 2019;8:1395–1404. doi: 10.1530/EC-19-0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bursać B., Djordjevic A., Veličković N., Milutinović D.V., Petrović S., Teofilović A., Gligorovska L., Preitner F., Tappy L., Matić G. Involvement of glucocorticoid prereceptor metabolism and signaling in rat visceral adipose tissue lipid metabolism after chronic stress combined with high-fructose diet. Mol. Cell. Endocrinol. 2018;476:110–118. doi: 10.1016/j.mce.2018.04.015. [DOI] [PubMed] [Google Scholar]
- 148.Wu T., Yang L., Jiang J., Ni Y., Zhu J., Zheng X., Wang Q., Lu X., Fu Z. Chronic glucocorticoid treatment induced circadian clock disorder leads to lipid metabolism and gut microbiota alterations in rats. Life Sci. 2018;192:173–182. doi: 10.1016/j.lfs.2017.11.049. [DOI] [PubMed] [Google Scholar]
- 149.Li Y., Ma J., Yao K., Su W., Tan B., Wu X., Huang X., Li T., Yin Y., Tosini G., et al. Circadian rhythms and obesity: Timekeeping governs lipid metabolism. J. Pineal Res. 2020;69:e12682. doi: 10.1111/jpi.12682. [DOI] [PubMed] [Google Scholar]
- 150.Sacks F.M., Alaupovic P., Moye L.A., Cole T.G., Sussex B., Stampfer M.J., Pfeffer M.A., Braunwald E. VLDL, apolipoproteins B, CIII, and E, and risk of recurrent coronary events in the Cholesterol and Recurrent Events (CARE) trial. Circulation. 2000;102:1886–1892. doi: 10.1161/01.CIR.102.16.1886. [DOI] [PubMed] [Google Scholar]
- 151.Gentile M., Iannuzzi A., Giallauria F., D’Andrea A., Venturini E., Pacileo M., Covetti G., Panico C., Mattiello A., Vitale G., et al. Association between Very Low-Density Lipoprotein Cholesterol (VLDL-C) and Carotid Intima-Media Thickness in Postmenopausal Women Without Overt Cardiovascular Disease and on LDL-C Target Levels. J. Clin. Med. 2020;9:1422. doi: 10.3390/jcm9051422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Heidemann B.E., Koopal C., Bots M.L., Asselbergs F., Westerink J., Visseren F.L. The relation between VLDL-cholesterol and risk of cardiovascular events in patients with manifest cardiovascular disease. Int. J. Cardiol. 2021;322:251–257. doi: 10.1016/j.ijcard.2020.08.030. [DOI] [PubMed] [Google Scholar]
- 153.Duran E.K., Aday A.W., Cook N.R., Buring J.E., Ridker P.M., Pradhan A.D. Triglyceride-rich lipoprotein cholesterol, small dense LDL cholesterol, and incident cardiovascular disease. J. Am. Coll. Cardiol. 2020;75:2122–2135. doi: 10.1016/j.jacc.2020.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ren J., Grundy S.M., Liu J., Wang W., Wang M., Sun J., Liu J., Li Y., Wu Z., Zhao D. Long-term coronary heart disease risk associated with very-low-density lipoprotein cholesterol in Chinese: The results of a 15-Year Chinese Multi-Provincial Cohort Study (CMCS) Atherosclerosis. 2010;211:327–332. doi: 10.1016/j.atherosclerosis.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 155.Varbo A., Freiberg J.J., Nordestgaard B.G. Extreme nonfasting remnant cholesterol vs extreme LDL cholesterol as contributors to cardiovascular disease and all-cause mortality in 90000 individuals from the general population. Clin. Chem. 2015;61:533–543. doi: 10.1373/clinchem.2014.234146. [DOI] [PubMed] [Google Scholar]
- 156.Rosenson R.S., Lee M.L., Chen Q. Association of total VLDL particle concentrations with elevated blood viscosity in patients with type 2 diabetes. Diabetes Res. Clin. Pract. 2022;183:109180. doi: 10.1016/j.diabres.2021.109180. [DOI] [PubMed] [Google Scholar]
- 157.Hollstein T., Vogt A., Grenkowitz T., Stojakovic T., März W., Laufs U., Bölükbasi B., Steinhagen-Thiessen E., Scharnagl H., Kassner U. Treatment with PCSK9 inhibitors reduces atherogenic VLDL remnants in a real-world study. Vasc. Pharmacol. 2019;116:8–15. doi: 10.1016/j.vph.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 158.Boulet M.M., Cheillan D., Di Filippo M., Buisson C., Michalski M.-C., Moulin P., Calzada C. Large triglyceride-rich lipoproteins from fasting patients with type 2 diabetes activate platelets. Diabetes Metab. 2020;46:54–60. doi: 10.1016/j.diabet.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 159.Kawakami A., Aikawa M., Libby P., Alcaide P., Luscinskas F.W., Sacks F.M. Apolipoprotein CIII in apolipoprotein B lipoproteins enhances the adhesion of human monocytic cells to endothelial cells. Circulation. 2006;113:691–700. doi: 10.1161/CIRCULATIONAHA.105.591743. [DOI] [PubMed] [Google Scholar]
- 160.Wulff A.B., Nordestgaard B.G., Tybjærg-Hansen A. APOC3 loss-of-function mutations, remnant cholesterol, low-density lipoprotein cholesterol, and cardiovascular risk: Mediation-and meta-analyses of 137 895 individuals. Arterioscler. Thromb. Vasc. Biol. 2018;38:660–668. doi: 10.1161/ATVBAHA.117.310473. [DOI] [PubMed] [Google Scholar]
- 161.Nordestgaard B.G. Triglyceride-rich lipoproteins and atherosclerotic cardiovascular disease: New insights from epidemiology, genetics, and biology. Circ. Res. 2016;118:547–563. doi: 10.1161/CIRCRESAHA.115.306249. [DOI] [PubMed] [Google Scholar]
- 162.Pourcet B., Staels B. Alternative macrophages in atherosclerosis: Not always protective! J. Clin. Investig. 2018;128:910–912. doi: 10.1172/JCI120123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Barrett T.J. Macrophages in atherosclerosis regression. Arterioscler. Thromb. Vasc. Biol. 2020;40:20–33. doi: 10.1161/ATVBAHA.119.312802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Liberale L., Dallegri F., Carbone F., Montecucco F. Pathophysiological relevance of macrophage subsets in atherogenesis. Thromb. Haemost. 2017;26:7–18. doi: 10.1160/TH16-08-0593. [DOI] [PubMed] [Google Scholar]
- 165.Shen M.-Y., Hsu J.-F., Chen F.-Y., Lu J., Chang C.-M., Madjid M., Dean J., Dixon R.A.F., Shayani S., Chou T.-C., et al. Combined LDL and VLDL electronegativity correlates with coronary heart disease risk in asymptomatic individuals. J. Clin. Med. 2019;8:1193. doi: 10.3390/jcm8081193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wang Y.-C., Lee A.-S., Lu L.-S., Ke L.-Y., Chen W.-Y., Dong J.-W., Lu J., Chen Z., Chu C.-S., Chan H.-C., et al. Human electronegative LDL induces mitochondrial dysfunction and premature senescence of vascular cells in vivo. Aging Cell. 2018;17:e12792. doi: 10.1111/acel.12792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lee H.-C., Lin Y.-H. The pathogenic role of very low density lipoprotein on atrial remodeling in the metabolic syndrome. Int. J. Mol. Sci. 2020;21:891. doi: 10.3390/ijms21030891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Okuizumi K., Onodera O., Namba Y., Ikeda K., Yamamoto T., Seki K., Ueki A., Nanko S., Tanaka H., Takahashi H., et al. Genetic association of the very low density lipoprotein (VLDL) receptor gene with sporadic Alzheimer’s disease. Nat. Genet. 1995;11:207–209. doi: 10.1038/ng1095-207. [DOI] [PubMed] [Google Scholar]
- 169.Pritchard M., Saunders A., Gaskell P., Small G., Conneally P., Rosi B., Yamaoka L., Roses A., Haines J., Pericak-Vance M. No association between very low density lipoprotein receptor (VLDL-R) and Alzheimer disease in American Caucasians. Neurosci. Lett. 1996;209:105–108. doi: 10.1016/0304-3940(96)12630-6. [DOI] [PubMed] [Google Scholar]
- 170.Christie R.H., Chung H., Rebeck G.W., Strickland D., Hyman B.T. Expression of the very low-density lipoprotein receptor (VLDL-r), an apolipoprotein-E receptor, in the central nervous system and in Alzheimer’s disease. J. Neuropathol. Exp. Neurol. 1996;55:491–498. doi: 10.1097/00005072-199604000-00012. [DOI] [PubMed] [Google Scholar]
- 171.Nakamura Y., Yamamoto M., Kumamaru E. Significance of the variant and full-length forms of the very low density lipoprotein receptor in brain. Brain Res. 2001;922:209–215. doi: 10.1016/S0006-8993(01)03170-5. [DOI] [PubMed] [Google Scholar]
- 172.Herring A., Donath A., Steiner K.M., Widera M.P., Hamzehian S., Kanakis D., Kölble K., ElAli A., Hermann D.M., Paulus W., et al. Reelin depletion is an early phenomenon of Alzheimer’s pathology. J. Alzheimer’s Dis. 2012;30:963–979. doi: 10.3233/JAD-2012-112069. [DOI] [PubMed] [Google Scholar]
- 173.Rahimi-Balaei M., Jiao X., Dalvand A., Shabanipour S., Chung S.H., Amiri S., Kong J., Marzban H. Mutations in Reelin pathway are associated with abnormal expression of microglial IgG FC receptors in the cerebellar cortex. Mol. Biol. Rep. 2020;47:5323–5331. doi: 10.1007/s11033-020-05614-0. [DOI] [PubMed] [Google Scholar]
- 174.Nelson A.R., Sagare A.P., Zlokovic B.V. Role of clusterin in the brain vascular clearance of amyloid-β. Proc. Natl. Acad. Sci. USA. 2017;114:8681–8682. doi: 10.1073/pnas.1711357114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wojtas A.M., Kang S.S., Olley B.M., Gatherer M., Shinohara M., Lozano P.A., Liu C.-C., Kurti A., Baker K.E., Dickson D.W., et al. Loss of clusterin shifts amyloid deposition to the cerebrovasculature via disruption of perivascular drainage pathways. Proc. Natl. Acad. Sci. USA. 2017;114:E6962–E6971. doi: 10.1073/pnas.1701137114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Anjum S., Bathla M., Panchal S., Singh G.P., Singh M. Metabolic syndrome in drug naïve schizophrenic patients. Diabetes Metab. Syndr. Clin. Res. Rev. 2018;12:135–140. doi: 10.1016/j.dsx.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 177.Solberg D.K., Bentsen H., Refsum H., Andreassen O.A. Lipid profiles in schizophrenia associated with clinical traits: A five year follow-up study. BMC Psychiatry. 2016;16:299. doi: 10.1186/s12888-016-1006-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lin B.D., Alkema A., Peters T., Zinkstok J., Libuda L., Hebebrand J., Antel J., Hinney A., Cahn W., Adan R., et al. Assessing causal links between metabolic traits, inflammation and schizophrenia: A univariable and multivariable, bidirectional Mendelian-randomization study. Int. J. Epidemiol. 2019;48:1505–1514. doi: 10.1093/ije/dyz176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Tatar Z.B. The relationship between serum lipid levels and lifetime suicide attempts in patients with schizophrenia. Med. Sci. 2018;7:826–830. doi: 10.5455/medscience.2018.07.8849. [DOI] [Google Scholar]
- 180.Nandeesha H., Keshri N., Rajappa M., Menon V. Association of hyperglycaemia and hyperlipidaemia with cognitive dysfunction in schizophrenia spectrum disorder. Arch. Physiol. Biochem. 2020:1–8. doi: 10.1080/13813455.2020.1839500. [DOI] [PubMed] [Google Scholar]
- 181.Usui N., Iwata K., Miyachi T., Takagai S., Wakusawa K., Nara T., Tsuchiya K.J., Matsumoto K., Kurita D., Kameno Y., et al. VLDL-specific increases of fatty acids in autism spectrum disorder correlate with social interaction. EBioMedicine. 2020;58:102917. doi: 10.1016/j.ebiom.2020.102917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Geovanini G.R., Lorenzi-Filho G., de Paula L.K., Oliveira C.M., Alvim R.D.O., Beijamini F., Negrão A.B., von Schantz M., Knutson K., Krieger J.E., et al. Poor sleep quality and lipid profile in a rural cohort (The Baependi Heart Study) Sleep Med. 2019;57:30–35. doi: 10.1016/j.sleep.2018.12.028. [DOI] [PubMed] [Google Scholar]
- 183.Depner C.M., Stothard E.R., Wright K.P., Jr. Metabolic consequences of sleep and circadian disorders. Curr. Diabetes Rep. 2014;14:507. doi: 10.1007/s11892-014-0507-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Lin Y.-S., Liu C.-K., Lee H.-C., Chou M.-C., Ke L.-Y., Chen C.-H., Chen S.-L. Electronegative very-low-density lipoprotein induces brain inflammation and cognitive dysfunction in mice. Sci. Rep. 2021;11:6013. doi: 10.1038/s41598-021-85502-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Loving B.A., Bruce K.D. Lipid and lipoprotein metabolism in microglia. Front. Physiol. 2020;11:393. doi: 10.3389/fphys.2020.00393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ren L., Ren X. Meta-analyses of four polymorphisms of lipoprotein lipase associated with the risk of Alzheimer’s disease. Neurosci. Lett. 2016;619:73–78. doi: 10.1016/j.neulet.2016.03.021. [DOI] [PubMed] [Google Scholar]
- 187.Folick A., Koliwad S.K., Valdearcos M. Microglial lipid biology in the hypothalamic regulation of metabolic homeostasis. Front. Endocrinol. 2021;12:591. doi: 10.3389/fendo.2021.668396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Navaneethan S.D., Schold J.D., Arrigain S., Thomas G., Jolly S.E., Poggio E.D., Schreiber M.J., Sarnak M.J., Nally J.V. Serum triglycerides and risk for death in Stage 3 and Stage 4 chronic kidney disease. Nephrol. Dial. Transplant. 2012;27:3228–3234. doi: 10.1093/ndt/gfs058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Björnson E., Adiels M., Taskinen M.-R., Borén J. Kinetics of plasma triglycerides in abdominal obesity. Curr. Opin. Lipidol. 2017;28:11–18. doi: 10.1097/MOL.0000000000000375. [DOI] [PubMed] [Google Scholar]
- 190.Miljkovic M., Stefanovic A., Simic-Ogrizovic S., Vekic J., Bogavac-Stanojevic N., Cerne D., Kocbek P., Marc J., Jelic-Ivanovic Z., Spasojevic-Kalimanovska V., et al. Association of dyslipidemia, oxidative stress, and inflammation with redox status in VLDL, LDL, and HDL lipoproteins in patients with renal disease. Angiology. 2018;69:861–870. doi: 10.1177/0003319718780041. [DOI] [PubMed] [Google Scholar]
- 191.Chan D.T., Dogra G.K., Irish A.B., Ooi E.M., Barrett P.H., Chan D.C., Watts G.F. Chronic kidney disease delays VLDL-apoB-100 particle catabolism: Potential role of apolipoprotein C-III. J. Lipid Res. 2009;50:2524–2531. doi: 10.1194/jlr.P900003-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Liang K., Vaziri N.D. Vaziri, Acquired VLDL receptor deficiency in experimental nephrosis. Kidney Int. 1997;51:1761–1765. doi: 10.1038/ki.1997.242. [DOI] [PubMed] [Google Scholar]
- 193.Clement L.C., Macé C., Avila-Casado C., Joles J.A., Kersten S., Chugh S.S. Circulating angiopoietin-like 4 links proteinuria with hypertriglyceridemia in nephrotic syndrome. Nat. Med. 2014;20:37–46. doi: 10.1038/nm.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Macé C., Chugh S.S. Nephrotic syndrome: Components, connections, and angiopoietin-like 4–related therapeutics. J. Am. Soc. Nephrol. 2014;25:2393–2398. doi: 10.1681/ASN.2014030267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Agrawal S., Zaritsky J.J., Fornoni A., Smoyer W.E. Dyslipidaemia in nephrotic syndrome: Mechanisms and treatment. Nat. Rev. Nephrol. 2018;14:57–70. doi: 10.1038/nrneph.2017.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Hirao Y., Nakajima K., Machida T., Murakami M., Ito Y. Development of a novel homogeneous assay for remnant lipoprotein particle cholesterol. J. Appl. Lab. Med. 2018;3:26–36. doi: 10.1373/jalm.2017.024919. [DOI] [PubMed] [Google Scholar]
- 197.Iqbal J., Al Qarni A., Hawwari A., Alghanem A.F., Ahmed G. Metabolic syndrome, dyslipidemia and regulation of lipoprotein metabolism. Curr. Diabetes Rev. 2018;14:427–433. doi: 10.2174/1573399813666170705161039. [DOI] [PubMed] [Google Scholar]
- 198.Chaurasia B., Summers S.A. Ceramides–lipotoxic inducers of metabolic disorders. Trends Endocrinol. Metab. 2015;26:538–550. doi: 10.1016/j.tem.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 199.Xia J.Y., Holland W.L., Kusminski C.M., Sun K., Sharma A., Pearson M.J., Sifuentes A.J., McDonald J.G., Gordillo R., Scherer P.E. Targeted induction of ceramide degradation leads to improved systemic metabolism and reduced hepatic steatosis. Cell Metab. 2015;22:266–278. doi: 10.1016/j.cmet.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Raichur S., Wang S.T., Chan P.W., Li Y., Ching J., Chaurasia B., Dogra S., Öhman M.K., Takeda K., Sugii S., et al. CerS2 haploinsufficiency inhibits β-oxidation and confers susceptibility to diet-induced steatohepatitis and insulin resistance. Cell Metab. 2014;20:687–695. doi: 10.1016/j.cmet.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 201.Turpin S.M., Nicholls H.T., Willmes D.M., Mourier A., Brodesser S., Wunderlich C.M., Brüning J.C. Obesity-induced CerS6-dependent C16: 0 ceramide production promotes weight gain and glucose intolerance. Cell Metab. 2014;20:678–686. doi: 10.1016/j.cmet.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 202.Gosejacob D., Jäger P.S., Dorp K.V., Frejno M., Carstensen A.C., Köhnke M., Degen J., Dörmann P., Hoch M. Ceramide synthase 5 is essential to maintain C16: 0-ceramide pools and contributes to the development of diet-induced obesity. J. Biol. Chem. 2016;291:6989–7003. doi: 10.1074/jbc.M115.691212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Khovidhunkit W., Kim M.S., Memon R.A., Shigenaga J.K., Moser A.H., Feingold K.R., Grunfeld C. Effects of infection and inflammation on lipid and lipoprotein metabolism: Mechanisms and consequences to the host. J. Lipid Res. 2004;45:1169–1196. doi: 10.1194/jlr.R300019-JLR200. [DOI] [PubMed] [Google Scholar]
- 204.Feingold K.R., Grunfeld C. The effect of inflammation and infection on lipids and lipoproteins. [(accessed on 25 March 2022)];Endotext. 2019 Available online: https://www.ncbi.nlm.nih.gov/sites/books/NBK326741/ [Google Scholar]
- 205.Khound R., Shen J., Song Y., Santra D., Su Q. Phytoceuticals in fenugreek ameliorate VLDL overproduction and insulin resistance via the insig signaling pathway. Mol. Nutr. Food Res. 2018;62:1700541. doi: 10.1002/mnfr.201700541. [DOI] [PubMed] [Google Scholar]
- 206.Garrido J.A.D.J.B., Alba H.A.R., Núñez É.H., Olán F. Dyslipidaemia and atherogenic risk in patients with systemic lupus erythematosus. Med. Clínica. 2016;147:63–66. doi: 10.1016/j.medcli.2016.03.030. [DOI] [PubMed] [Google Scholar]
- 207.Pocovi-Gerardino G., Correa-Rodríguez M., Rubio J.-L.C., Fernández R.R., Amada M.M., Caparros M.-G.C., Rueda-Medina B., Ortego-Centeno N. The Relationships of High-Sensitivity C-Reactive Protein and Homocysteine Levels With Disease Activity, Damage Accrual, and Cardiovascular Risk in Systemic Lupus Erythematosus. Biol. Res. Nurs. 2020;22:169–177. doi: 10.1177/1099800419889192. [DOI] [PubMed] [Google Scholar]
- 208.Zhou B., Xia Y., She J. Dysregulated serum lipid profile and its correlation to disease activity in young female adults diagnosed with systemic lupus erythematosus: A cross-sectional study. Lipids Health Dis. 2020;19:40. doi: 10.1186/s12944-020-01232-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Araújo D.M., Rodrigues C.E.M., Gonçalves N.G.G., Rabelo-Júnior C.N., Lobo M.D.P., Moreira R.D.A., Monteiro-Moreira A.C.D.O. Proteins Involved in the Induction of Procoagulant Activity and Autoimmune Response in Patients With Primary Antiphospholipid Syndrome. Clin. Appl. Thromb./Hemost. 2020;26:1076029620905338. doi: 10.1177/1076029620905338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.García-Chagollán M., Hernández-Martínez S.E., Rojas-Romero A.E., Muñoz-Valle J.F., Sigala-Arellano R., Cerpa-Cruz S., Hernández-Bello J. Metabolic syndrome in rheumatoid arthritis patients: Relationship among its clinical components. J. Clin. Lab. Anal. 2021;35:e23666. doi: 10.1002/jcla.23666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Robinson G., Pineda-Torra I., Ciurtin C., Jury E.C. Lipid metabolism in autoimmune rheumatic disease: Implications for modern and conventional therapies. J. Clin. Investig. 2022;132:e148552. doi: 10.1172/JCI148552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Sinha P., Nigam P., Swain J. Association of Metabolic Syndrome with Vitiligo--A Case Control Study. J. Evol. Med. Dent. Sci. 2019;8:2783–2787. [Google Scholar]
- 213.Teklu M., Zhou W., Kapoor P., Patel N., Dey A.K., Sorokin A.V., Mehta N.N. Metabolic syndrome and its factors are associated with noncalcified coronary burden in psoriasis: An observational cohort study. J. Am. Acad. Dermatol. 2021;84:1329–1338. doi: 10.1016/j.jaad.2020.12.044. [DOI] [PubMed] [Google Scholar]
- 214.Singla R., Ashwini P., Jayadev B. Lichen planus and metabolic syndrome: Is there a relation? Indian Dermatol. Online J. 2019;10:555. doi: 10.4103/idoj.IDOJ_499_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Gong H., Liu Y., Lyu X., Dong L., Zhang X. Lipoprotein subfractions in patients with sarcopenia and their relevance to skeletal muscle mass and function. Exp. Gerontol. 2022;159:111668. doi: 10.1016/j.exger.2021.111668. [DOI] [PubMed] [Google Scholar]
- 216.Butler L.M., Perone Y., Dehairs J., Lupien L.E., de Laat V., Talebi A., Loda M., Kinlaw W.B., Swinnen J.V. Lipids and cancer: Emerging roles in pathogenesis, diagnosis and therapeutic intervention. Adv. Drug Deliv. Rev. 2020;159:245–293. doi: 10.1016/j.addr.2020.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Long J., Zhang C.J., Zhu N., Du K., Yin Y.F., Tan X., Qin L. Lipid metabolism and carcinogenesis, cancer development. Am. J. Cancer Res. 2018;8:778. [PMC free article] [PubMed] [Google Scholar]
- 218.Paoli P., Giannoni E., Chiarugi P. Anoikis molecular pathways and its role in cancer progression. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2013;1833:3481–3498. doi: 10.1016/j.bbamcr.2013.06.026. [DOI] [PubMed] [Google Scholar]
- 219.Menendez J.A., Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat. Rev. Cancer. 2007;7:763–777. doi: 10.1038/nrc2222. [DOI] [PubMed] [Google Scholar]
- 220.Nomura D.K., Long J.Z., Niessen S., Hoover H.S., Ng S.-W., Cravatt B.F. Monoacylglycerol lipase regulates a fatty acid network that promotes cancer pathogenesis. Cell. 2010;140:49–61. doi: 10.1016/j.cell.2009.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Love-Gregory L., Sherva R., Schappe T., Qi J.-S., McCrea J., Klein S., Connelly M.A., Abumrad N.A. Common CD36 SNPs reduce protein expression and may contribute to a protective atherogenic profile. Hum. Mol. Genet. 2011;20:193–201. doi: 10.1093/hmg/ddq449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Enciu A.M., Radu E., Popescu I.D., Hinescu M.E., Ceafalan L.C. Targeting CD36 as biomarker for metastasis prognostic: How far from translation into clinical practice? BioMed Res. Int. 2018;2018:7801202. doi: 10.1155/2018/7801202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Pascual G., Avgustinova A., Mejetta S., Martín M., Castellanos A., Attolini C.S.-O., Berenguer A., Prats N., Toll A., Hueto J.A., et al. Targeting metastasis-initiating cells through the fatty acid receptor CD36. Nature. 2017;541:41–45. doi: 10.1038/nature20791. [DOI] [PubMed] [Google Scholar]
- 224.Lu C.W., Lo Y.H., Chen C.H., Lin C.Y., Tsai C.H., Chen P.J., Yuan S.S.F. VLDL and LDL, but not HDL, promote breast cancer cell proliferation, metastasis and angiogenesis. Cancer Lett. 2017;388:130–138. doi: 10.1016/j.canlet.2016.11.033. [DOI] [PubMed] [Google Scholar]
- 225.Lupien L.E., Bloch K., Dehairs J., Traphagen N.A., Feng W.W., Davis W.L., Kinlaw W.B. Endocytosis of very low-density lipoproteins: An unexpected mechanism for lipid acquisition by breast cancer cells. J. Lipid Res. 2020;61:205–218. doi: 10.1194/jlr.RA119000327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Siti Z., Seoparjoo A., Shahrul H. Lipoproteins modulate growth and P-glycoprotein expression in drug-resistant HER2-overexpressed breast cancer cells. Heliyon. 2019;5:e01573. doi: 10.1016/j.heliyon.2019.e01573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Mosapour A., Tehrani F.S.K., Atri M. Expression level of VLDL receptor and VLDL-c levels in the malignant and benign breast tumors: The correlation with miRNA-4465 and miRNA-1297. Mol. Cell. Probes. 2020;53:101624. doi: 10.1016/j.mcp.2020.101624. [DOI] [PubMed] [Google Scholar]
- 228.Alizadeh-Fanalou S., Khosravi M., Alian F., Rokhsartalb-Azar S., Nazarizadeh A., Karimi-Dehkordi M., Mohammadi F. Dual role of microRNA-1297 in the suppression and progression of human malignancies. Biomed. Pharmacother. 2021;141:111863. doi: 10.1016/j.biopha.2021.111863. [DOI] [PubMed] [Google Scholar]
- 229.Debik J., Schaefer H., Andreassen T., Wang F., Fang F., Cannet C., Giskeodegard G.F. Lipoprotein and metabolite associations to breast cancer risk in the HUNT2 study. medRxiv. 2021 doi: 10.1101/2021.10.08.21264729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Bendinelli B., Vignoli A., Palli D., Assedi M., Ambrogetti D., Luchinat C., Caini S., Saieva C., Turano P., Masala G. Prediagnostic circulating metabolites in female breast cancer cases with low and high mammographic breast density. Sci. Rep. 2021;11:13025. doi: 10.1038/s41598-021-92508-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Bianco C., Jamialahmadi O., Pelusi S., Baselli G., Dongiovanni P., Zanoni I., Santoro L., Maier S., Liguori A., Meroni M., et al. Non-invasive stratification of hepatocellular carcinoma risk in non-alcoholic fatty liver using polygenic risk scores. J. Hepatol. 2021;74:775–782. doi: 10.1016/j.jhep.2020.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Jiang X., Qian H., Ding W. New Glance at the Role of TM6SF2 in Lipid Metabolism and Liver Cancer. Wiley Online Library; Hoboken, NJ, USA: 2021. pp. 1141–1144. [DOI] [PubMed] [Google Scholar]
- 233.Newberry E.P., Hall Z., Xie Y., Molitor E.A., Bayguinov P.O., Strout G.W., Davidson N.O. Liver-Specific Deletion of Mouse Tm6sf2 Promotes Steatosis, Fibrosis, and Hepatocellular Cancer. Hepatology. 2021;74:1203–1219. doi: 10.1002/hep.31771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Shen G.-M., Zhao Y.-Z., Chen M.-T., Zhang F.-L., Liu X.-L., Wang Y., Liu C.-Z., Yu J., Zhang J.-W. Hypoxia-inducible factor-1 (HIF-1) promotes LDL and VLDL uptake through inducing VLDLR under hypoxia. Biochem. J. 2012;441:675–683. doi: 10.1042/BJ20111377. [DOI] [PubMed] [Google Scholar]
- 235.Şahin F., Aslan A.F. Relationship between inflammatory and biological markers and lung cancer. J. Clin. Med. 2018;7:160. doi: 10.3390/jcm7070160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Chi P.-D., Liu W., Chen H., Zhang J.-P., Lin Y., Zheng X., Liu W., Dai S. High-density lipoprotein cholesterol is a favorable prognostic factor and negatively correlated with C-reactive protein level in non-small cell lung carcinoma. PLoS ONE. 2014;9:e91080. doi: 10.1371/journal.pone.0091080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Ramaswamy P., Kiran C.S., Raju B.M., Swathi M., Anusha A., Sharanya G.E. Serum lipid profile in patients with oral potentially malignant disorders. J. Indian Acad. Oral Med. Radiol. 2019;31:323. [Google Scholar]
- 238.Goel P., Garg R., Raghavan V. Lipid profile in oral potentially malignant disorders. J. Indian Acad. Oral Med. Radiol. 2014;26:374. doi: 10.4103/0972-1363.155633. [DOI] [Google Scholar]
- 239.Garg D., Sunil M.K., Singh P.P., Singla N., Rani S.A., Kaur B. Serum lipid profile in oral precancer and cancer: A diagnostic or prognostic marker? J. Int. Oral Health JIOH. 2014;6:33. [PMC free article] [PubMed] [Google Scholar]
- 240.Wei L.J., Zhang C., Zhang H., Wei X., Li S.X., Liu J.T., Ren X.B. A case-control study on the association between serum lipid level and the risk of breast cancer. Chin. J. Prev. Med. 2016;50:1091–1095. doi: 10.3760/cma.j.issn.0253-9624.2016.12.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.