Summary
Background
Studies have shown increased mortality among women living with HIV diagnosed with breast cancer compared with HIV-negative women with breast cancer. We aimed to examine how this HIV differential varies by patient or breast tumour characteristics.
Methods
The African Breast Cancer–Disparities in Outcomes (ABC-DO) study is a prospective cohort of women (aged ≥18 years) with incident breast cancer recruited consecutively at diagnosis (2014–17) from hospitals in Namibia, Nigeria, South Africa, Uganda, and Zambia. Detailed clinical and epidemiological data, including self-reported or tested HIV status, were collected at baseline. Participants were actively followed up via telephone calls every 3 months. The primary outcome was all-cause mortality, assessed in all women who had at least one updated vital status after baseline interview. Using Cox regression, we examined differences in overall survival by HIV status in the cohort, and across country and patient subgroups, adjusted for age, tumour grade, and tumour stage at cancer diagnosis.
Findings
Between Sept 8, 2014, and Dec 31, 2017, we recruited 2154 women with primary breast cancer, 519 of whom were excluded due to their countries having small numbers of women with HIV for comparison. Among the remaining 1635 women, 313 (19%) were living with HIV, 1184 (72%) were HIV negative, and 138 (9%) had unknown HIV status. At breast cancer diagnosis, women with HIV were younger and had lower body-mass index (BMI) than their HIV-negative counterparts, but had similar tumour stage, grade, and receptor subtypes. At the end of the follow-up (Jan 1, 2019), a higher proportion of women with HIV (137 [44%] of 313) had died than had HIV-negative women (432 [37%] of 1184). Crude 3-year survival was 9% lower for women with HIV (46% [95% CI 40–53]) than for HIV-negative women (55% [52–59]; hazard ratio (HR) 1·41 [1·15–1·74]). The HIV survival differential did not differ by age, BMI, tumour subtype, or tumour grade, but was stronger in women with non-metastatic disease (3-year survival 52% HIV-positive vs 63% HIV-negative women, adjusted HR 1·65 [1·30–2·10]), whereas women with metastatic cancer had low survival, regardless of HIV status.
Interpretation
The larger survival deficit among women with HIV with non-metastatic breast cancer calls for a better understanding of the reasons underlying this differential (eg, biological mechanisms, health behaviours, detrimental HIV–breast cancer treatment interactions, or higher HIV background mortality) to inform strategies for reducing mortality among this patient group.
Funding
Susan G Komen, International Agency for Research on Cancer, National Cancer Institute, and UK-Commonwealth Scholarships.
Introduction
Breast cancer is the most common female cancer worldwide.1 Although it is not an HIV-associated cancer, breast cancer is common in women living with HIV.2 In 2012, an estimated 6325 new breast cancer cases were diagnosed among 16·0 million women with HIV globally.2 The breast cancer burden in this population is expected to increase, partly due to improved survival outcomes in women with HIV as a result of the rollout of antiretroviral therapy (ART) for HIV,3 meaning that women with HIV now live to ages when breast cancer incidence is highest.
Globally, most women with HIV reside in low-resource and high HIV-prevalence settings, such as sub-Saharan Africa. Breast cancer survival in sub-Saharan Africa is low (<50% at 5 years in most sub-Saharan Africa countries4) compared with 85–90% in high-income countries,5 and is lower among women with HIV. Previous studies (appendix p 2) indicate increased all-cause mortality after breast cancer diagnosis among women with HIV compared with setting-matched HIV-negative women. This is not surprising as women with HIV have, in general, higher mortality rates than the general population.6 In sub-Saharan Africa, estimates of the excess mortality seen among women with HIV (vs HIV-negative women) diagnosed with breast cancer ranged from an increase of 40–50% in South Africa and Mozambique to 80–100% in Botswana and Uganda.7–10 Similarly, studies in the USA11–15 showed an adjusted 50% and 80% increased risk for all-cause and breast cancer-specific mortality, respectively (appendix p 2). A meta-analysis reported a pooled-adjusted 90% increased risk,16 albeit with marked between-study heterogeneity as most studies were either based on small numbers of women with HIV and, specifically in sub-Saharan Africa, had suboptimal follow-up time or losses to follow-up of greater than 40% (appendix p 2).8,9
The reasons for increased mortality in women with HIV compared with HIV-negative women with breast cancer remain unclear. This disparity might solely reflect the increased background mortality among women with HIV versus the general population. Alternatively, survival gaps might occur in women with HIV if their compromised immune system facilitates tumour progression, if they have poorer prognostic factors for breast cancer, if treatment regimens and intensities differ by HIV status, or if there are ART–chemotherapy interferences.8,17,18 Women with HIV are diagnosed with breast cancer at younger ages than are HIV-negative women because women with HIV are typically younger than the general population. However, there is little or unclear evidence that stage at cancer diagnosis differs by HIV status. Most studies in the USA have shown that women with HIV are more likely to present with advanced-stage breast cancer, whilst most studies in sub-Saharan Africa have indicated either no such differences or only a modest excess in the prevalence of advanced-stage at diagnosis of breast cancer among women with HIV.16 Excess weight is associated with poorer overall survival after a breast cancer diagnosis.19 Being underweight or overweight also complicates HIV management and could contribute to increased cardiovascular disease risk or mortality (or both).20 Finally, the survival deficits in women with HIV with breast cancer can vary by tumour hormone receptor status. ART affects breast tissue, with about 5% of men on ART experiencing breast enlargement (including gynaecomastia21) suggesting a potential role of ART on circulating sex hormone concentrations. Therefore, the difference in survival after a breast cancer diagnosis between women with and without HIV (hereafter referred to as the HIV differential) might reflect, at least in part, an ART effect that could differ according to the tumour hormone receptor status. To date, no study has examined whether the HIV differential varies across patient subgroups defined by patient and tumour characteristics at diagnosis.
The African Breast Cancer–Disparities in Outcomes (ABC-DO) study is a multi-country prospective study of women newly diagnosed with breast cancer in five sub-Saharan Africa countries.22 In a previous ABC-DO analysis,23 women with HIV experienced a 48% (95% CI 22–81) increase in all-cause mortality after adjusting for age and tumour stage at diagnosis. In this analysis, we aimed to examine whether prognostic factors (such as age, stage, receptor status, and body-mass index [BMI]) explain the lower survival outcomes in women with HIV than in HIV-negative women and explore how the HIV differential varies across different sub-Saharan Africa settings and patient subgroups.
Methods
Study design and setting
The ABC-DO study recruited women with incident primary breast cancer in five countries in sub-Saharan Africa: Namibia, South Africa, and Zambia in southern Africa where HIV prevalence is high, Uganda in east Africa where HIV prevalence is intermediate, and Nigeria in west Africa where HIV prevalence is low.
Participants with a diagnosis of breast cancer were recruited from participating hospitals. The characteristics of participating hospitals and the study protocol have been described in detail previously.22 The study was approved by all institutional ethics committees (appendix p 1). All participants provided informed written or thumbprint consent before recruitment.
Procedures
Between Sept 8, 2014, and Dec 31, 2017, women (aged ≥18 years) with cytological, histological (>80% of all participants23), or clinical diagnosis of primary breast cancer were recruited into the study. Participants answered a face-to-face baseline interview, and consented to study access to their medical records, to provide tumour tissue specimens, and to be actively followed up via telephone interviews every 3 months. The baseline questionnaire included detailed socio-demographic data, such as age, formal education attained, access to nine household amenities used to generate site-specific tertiles of socioeconomic position,23,24 knowledge (before diagnosis) that breast cancer is curable if detected early, cohabitation status, residential status (urban vs rural), and presence of other non-HIV comorbidities. BMI was derived from height and weight measured at breast cancer diagnosis. HIV status was also captured at breast cancer diagnosis. In South Africa, all participants who did not report being HIV-positive were tested for HIV.18 Those who refused were considered to be of unknown HIV status. For all other sites, HIV status was self-reported on the baseline questionnaire and also on the presenting symptoms questionnaire. For this analysis, a woman was considered HIV-positive if responses were positive on either questionnaire.
Data on current ART use, CD4 count, and HIV RNA (HIV-1 vs HIV-2) were also collected. Standard pro formas were used to extract clinical, pathological, and treatment information from hospital records on tumour TNM stage25 (assessed clinically using ultrasound, x-ray, or surgical information); cytology or histology; grade; and oestrogen, progesterone, and human epidermal growth factor 2 (HER2) receptor status. Oestrogen-positivity and progesterone-positivity were defined as at least 1% immunohistochemistry staining for the respective receptor, and HER2-positivity as an immunohistochemistry score of 3 by immunohistochemistry or a positive fluorescence in-situ hybridisation result.
Follow-up was done actively via telephone calls every 3 months to the woman or her next-of-kin. At each contact, the date, vital status, and contact details were updated. The ABC-DO protocol was implemented using a specifically designed mHealth application that prompted real-time to-call lists, resulting in much lower loss to follow-up (5% at 3 years) and timely study notification of deaths (median 9·1 weeks [IQR 3·9–14·0]), most of which were reported by the next-of-kin (92%).26
Outcomes
The primary outcome was all-cause mortality, analysed for all women who had at least one updated vital status after baseline interview. We analysed overall survival up to 3 years by HIV status, on a time-since-diagnosis scale (ie, date of breast cancer diagnosis was time zero). Follow-up commenced on the latest date of either breast cancer diagnosis or—effectively incorporating left-censoring—ABC-DO recruitment date and continued until the earliest of: date of death from any cause, date last known alive (among those lost to follow-up), or administrative censoring on the earliest of 3 years post-diagnosis or Jan 1, 2019.
Statistical analysis
As per the published protocol,22 the ABC-DO study invited all eligible women to participate over a period of at least 2 years in each country in order to recruit at least 300 women in each participating hospital. For an exposure prevalence of 30% and 50%, respectively, and over 3-year follow-up, ABC-DO would have 80% power to detect hazard ratios (HRs) of 1·7 and 2·6 for 100 deaths in smaller sites, and 1·5 and 2·1 for 160 deaths in larger sites, assuming 0·85 and 0·5 survival probabilities for stage I or II and stage III or IV, respectively. We tested associations between patient or tumour characteristics and HIV status using χ2 tests. We generated Kaplan-Meier curves to estimate crude 3-year survival by HIV status. A multivariable Cox proportional hazards model, stratified by country (due to varying HIV prevalence, patient profiles, catchment populations, and health systems), was used to estimate HR for HIV. We first looked at a simpler model with HIV status as the only exposure. Then, each factor (ie, age, tumour stage, tumour grade, education, socioeconomic position, and tumour receptor subtype) was added to the model to examine the change in the HR for HIV status on survival associated with each factor. Kaplan-Meier curves showed that the HIV differential was stronger after 18 months since breast cancer diagnosis than before. Therefore, as a post-hoc analysis, we performed a Lexis expansion on follow-up time up to 18 months and beyond 18 months to evaluate the HIV–time interaction and then estimated HRs for HIV based on these follow-up times respectively. We also tested the interactions between HIV status and each one of the factors (age, tumour stage, tumour grade, education, socioeconomic position, and tumour receptor subtypes), including BMI and ART use. For factors where there was evidence for interaction, we built models comparing women with HIV in each subgroup to the HIV-negative women in the comparison subgroup, to pinpoint HIV-specific differences that were independent of the effect of the characteristic itself.
In sensitivity analyses, we re-classified women with unknown HIV status as being either HIV-positive or HIV-negative (as two separate analyses), to examine whether any potential misclassification of HIV status might affect the findings. We checked all models for the validity of the proportional hazards assumption for HIV status using Schoenfeld’s residuals. All analyses were done in Stata (version 16; StataCorp, College Station, TX, USA).
Role of the funding source
The funders of the study had no role in the study design, data collection, data analysis and interpretation, writing of the report, or in the decision to submit it for publication.
Results
Between Sept 8, 2014, and Dec 31, 2017, we recruited 2154 women with primary breast cancer, of whom 332 (15%) were HIV positive, 1608 (75%) were HIV negative, and 214 (10%) had an unknown HIV status. Due to small numbers of women with HIV in Nigeria (12 [3%] vs 374 HIV negative or unknown) and in non-Black groups in Namibia (three [3%] vs 94) and South Africa (four [11%] vs 32), these patient groups (519 women) were excluded from the analysis. We enrolled 1635 Black women from Namibia (n=384), South Africa (n=632), Uganda (n=421), and Zambia (n=198).
Of the 169 women considered to be positive for HIV from ABC-DO study sites where HIV status was self-reported, 130 (77%) had concordant HIV self-report in both questionnaires (median 31 days apart [IQR 6–113]), 20 (12%) had a HIV-positive self-report in the presenting symptoms questionnaire only, and 19 (11%) in the baseline interview only. Data on CD4 count and HIV RNA were largely missing, except for women in South Africa. Data on receptor status were largely missing in Uganda and Zambia due to either unavailability of immunohistochemistry testing or patients being unable to cover the costs. Thus, analyses on receptor status were restricted to Namibia and South Africa.
Among women included in the present analysis, the overall HIV prevalence was 19% (313 of 1635) but ranged from 14% (57 of 421) in Uganda to 26% (163 of 632) in South Africa (table 1; ie, representing the countries where HIV prevalence in Black African women was over 10%). Age at breast cancer diagnosis, socioeconomic position, educational level attained, BMI, cohabitation status, and presence of other comorbidities were all associated with HIV status. Women with HIV were younger at breast cancer diagnosis than their HIV-negative counterparts (table 1). The percentage of women with HIV who were overweight or obese was lower than among HIV-negative women. Most women with HIV were on ART. Cancer stage, grade, and receptor subtypes at diagnosis were not associated with HIV status (table 1). The proportion of late-stage (TNM III and IV) breast cancer at diagnosis was high, but was similar among women with HIV and HIV-negative women for all countries combined and for each country individually (table 1). The proportion of women with triple-negative breast tumours was also similar between women with and without HIV in Namibia and South Africa, where immunohistochemistry testing was routinely available.
Table 1.
Patient and tumour characteristics in the ABC-DO cohort, by HIV status and country
| Namibia (n=384) | South Africa (n=632) | Uganda (n=421) | Zambia (n=198) | All sites (n=1635) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HIV+ | HIV− | HIV+ | HIV− | HIV+ | HIV− | HIV+ | HIV− | HIV+ | HIV− | |
| Characteristic | N (%†) | N (%†) | N (%†) | N (%†) | N (%†) | N (%†) | N (%†) | N (%†) | N (%) | N (%†) |
| Total (row % η ) | 57 (14·8) | 309 (80.5) | 163 (25·8) | 446 (70.6) | 57 (13·5) | 315 (74.8) | 36 (18·2) | 114 (57.6) | 313 (19·1) | 1184 (72.4) |
| Age at BC diagnosis (years) | ||||||||||
| 18–29 | 1 (1·7) | 14 (4.5) | 3 (1·8) | 6 (1.3) | 4 (7·0) | 20 (6.4) | 3 (8·3) | 9 (7·9) | 11 (3·5) | 49 (4·1) |
| 30–39 | 14 (24·6) | 50 (16.1) | 30 (18·4) | 42 (9.4) | 7 (12·3) | 73 (23·2) | 10 (27·8) | 22 (19·3) | 61 (19·5) | 187 (15·8) |
| 40–49 | 23 (40·4) | 74 (24.0) | 73 (44·8) | 89 (20.0) | 27 (47·4) | 86 (27·3) | 13 (36·1) | 22 (19·3) | 136 (43·5) | 271 (22.9) |
| 50–59 | 13 (22·8) | 79 (25.6) | 34 (20·9) | 108 (24.2) | 13 (22·8) | 69 (21·9) | 10 (27.8) | 30 (26·3) | 70 (22·4) | 286 (24·2) |
| 60–69 | 5 (8·8) | 45 (14.6) | 18 (11·0) | 102 (22.9) | 6 (10·5) | 40 (12·7 | 0 | 14 (12·3) | 29 (9.2) | 201 (17·0) |
| 70+ | 1 (1·7) | 47 (15.2) | 5 (3·1) | 99 (22.2) | 0 | 27 (8·5) | 0 | 17 (14·9) | 6 (1·9) | 190 (16·0) |
| P‡= 0·008 | P < 0·001 | P= 0·01 | P= 0·02 | P < 0·001 | ||||||
| Education attained | ||||||||||
| None/primary | 26 (45·6) | 164 (53.1) | 23 (14·1) | 114 (25.6) | 32 (56·1) | 183 (58.1) | 15 (41·7) | 61 (53.5) | 96 (30·7) | 522 (44.1) |
| Secondary/above | 31 (54·4) | 145 (46.9) | 139 (85·3) | 327 (74.3) | 25 (43·9) | 132 (41.9) | 21 (58·3)) | 53 (46.5) | 216 (69·0) | 657 (55.5) |
| Missing | ·· | ·· | 1 (0·6) | 5 (1·1) | ·· | ·· | ·· | ·· | 1 (0·3) | 5 (0·4) |
| P= 0·3 | P= 0·002 | P= 0·8 | P= 0·2 | P < 0·001 | ||||||
| Socio-economic position β | ||||||||||
| Low | 33 (57·9) | 129 (41.7) | 109 (67·9) | 189 (42.3) | 28 (49·1) | 188 (59.7) | 13 (36·1) | 43 (37.7) | 183 (58·5) | 549 (46.4) |
| Medium | 18 (31·6) | 108 (35·0) | 38 (23·3) | 176 (39.5) | 15 (26·3) | 68 (21.6) | 13 (36·1) | 37 (32.5) | 84 (26·8) | 389 (32·9) |
| High | 6 (10·5) | 72 (23·3) | 15 (9·2) | 77 (17.3) | 14 (24·6) | 59 (18.7) | 10 (27·8) | 34 (29.8) | 45 (14·4) | 242 (20.4) |
| Missing | 1 (0·6) | 4 (0·9) | 1 (0·3) | 4 (0·3) | ||||||
| P= 0·04 | P < 0·001 | P= 0·3 | P= 0·9 | P= 0·001 | ||||||
| Area of residence | ||||||||||
| Urban | 32 (56·1) | 176 (57.0) | ·· | ·· | 15 (26·3) | 88 (27.9) | 25 (69·4) | 70 (61.4) | 72 (48·0) | 334 (45.3) |
| Rural | 25 (43·9) | 133 (43·0) | ·· | ·· | 42 (73·7) | 227 (72.1) | 11 (30·6) | 44 (38.6) | 78 (52·0) | 404 (54.7) |
| P= 0·9 | ·· | P= 0·8 | P= 0·4 | P= 0·5 | ||||||
| Body Mass Index (Kg/m2) | ||||||||||
| <18·5 | 7 (12·3) | 29 (9·4) | 9 (5·5) | 4 (0·9) | 0 | 14 (4·4) | 4 (11·0) | 3 (2·6) | 20 (6·4) | 50 (4·2) |
| 18·5–<25 | 24 (42·1) | 98 (31·7) | 57 (34·9) | 54 (12·1) | 27 (47·4) | 141 (44·8) | 17 (47·2) | 43 (37·7) | 125 (39·9) | 336 (28·4) |
| 25–<30 | 17 (29·8) | 81 (26·2) | 34 (20·9) | 91 (20·4) | 17 (29·8) | 106 (33·7) | 10 (27·8) | 30 (26·3) | 78 (24·9) | 308 (26·0) |
| 30+ | 8 (14·0) | 87 (28·2) | 51 (31·3) | 264 (59·2) | 11 (19·3) | 48 (15·2) | 2 (5·7) | 31 (27·2) | 72 (23·0) | 430 (36·3) |
| Missing | 1 (1·8) | 14 (4·5) | 12 (7·4) | 33 (7·4) | 2 (3·5) | 6 (1·9) | 3 (8·3) | 7 (6·2) | 18 (5.8) | 60 (5.1) |
| P= 0·1 | P < 0·001 | P= 0·3 | P= 0·01 | P < 0·001 | ||||||
| Cohabiting | ||||||||||
| No | 44 (77·2) | 211 (68·3) | 124 (76·1) | 314 (70·4) | 39 (68·4) | 167 (53·0) | 19 (52·8) | 46 (40·4) | 226 (72·2) | 738 (62·3) |
| Yes | 13 (22·8) | 98 (31·7) | 39 (23·9) | 132 (29·6) | 18 (31·6) | 148 (47·0) | 17 (47·2) | 68 (59·6) | 87 (27·8) | 446 (37·7) |
| P= 0·4 | P= 0·3 | P= 0·03 | P= 0·4 | P= 0·001 | ||||||
| Comorbidities α | ||||||||||
| No | 35 (61·4) | 147 (47·6) | 94 (57·7) | 214 (48.0) | 47 (82·5) | 214 (67·9) | 29 (80·6) | 69 (60·5) | 205 (65·5) | 644 (54·4) |
| Yes | 22 (38·6) | 162 (52·4) | 69 (42·3) | 232 (52.0) | 10 (17·5) | 101 (32·1) | 7 (19·4) | 45 (39·5) | 108 (34·5) | 540 (45·6) |
| P= 0·06 | P= 0·03 | P= 0·03 | P= 0·03 | P < 0·001 | ||||||
| Knowledge BC is curable ζ | ||||||||||
| Yes | 39 (68·4) | 238 (77·0) | ·· | ·· | 23 (40·4) | 126 (40·0) | 21 (58·3) | 80 (70·2) | 83 (55·3) | 444 (60·2) |
| No/NK | 18 (31·6) | 71 (23·0) | ·· | ·· | 34 (59·6) | 189 (60·0) | 15 (41·7) | 34 (29·8) | 67 (44·7) | 294 (39·8) |
| P= 0·2 | ·· | P= 0·9 | P= 0·2 | P= 0·3 | ||||||
| Stage at BC diagnosis | ||||||||||
| I/II | 21 (36·8) | 112 (36·3) | 76 (46·6) | 214 (48·0) | 13 (22·8) | 105 (33·4) | 10 (27·8) | 43 (37·7) | 120 (38·3) | 474 (40·0) |
| III | 30 (52·7) | 145 (46·9) | 65 (39·9) | 171 (38·3) | 28 (49·2) | 140 (44·4) | 15 (41·7) | 44 (38·6) | 138 (44·1) | 500 (42·2) |
| IV | 6 (10·5) | 52 (16·8) | 22 (13·5) | 60 (13·5) | 8 (14·0) | 52 (16·5) | 3 (8·3) | 7 (6·2) | 39 (12·5) | 171 (14·5) |
| Unknown | ·· | ·· | 0 (0) | 1 (0·2) | 8 (14·0) | 18 (5·7) | 8 (22·2) | 20 (17·5) | 16 (5·1) | 39 (3·3) |
| P= 0·5 | P= 0·9 | P= 0·08 | P= 0·7 | P= 0·3 | ||||||
| Tumour grade | ||||||||||
| 1 | 8 (14·0) | 48 (15·5) | 12 (7·4) | 24 (5·4) | 7 (12·3) | 62 (19·7) | 1 (2·8) | 11 (9·7) | 28 (9·0) | 145 (12·3) |
| 2 | 18 (31·6) | 114 (36·9) | 85 (52·1) | 220 (49·3) | 10 (17·5) | 51 (16·2) | 11 (30·6) | 29 (25·4) | 124 (39·6) | 414 (35·0) |
| 3 | 17 (29·8) | 67 (21·7) | 57 (35·0) | 182 (40·8) | 14 (24·6) | 68 (21·6) | 7 (19·4) | 22 (19·3) | 95 (30·4) | 339 (28·6) |
| Not specified | 14 (24·6) | 80 (25·9) | 9 (5·5) | 20 (4·5) | 26 (45·6) | 134 (42·5) | 17 (47·2) | 52 (45·6) | 66 (21·0) | 286 (24·1) |
| P= 0·6 | P= 0·5 | P= 0·6 | P= 0·6 | P= 0·2 | ||||||
| Receptor subtype § | ||||||||||
| HR+γ, HER2- | 25 (43·9) | 150 (48·5) | 74 (45·4) | 236 (52·9) | ·· | ·· | ·· | ·· | 99 (45·0) | 386 (51·1) |
| HR+, HER2+ | 17 (29·8) | 65 (21·0) | 41 (25·1) | 101 (22·7) | ·· | ·· | ·· | ·· | 58 (26·4) | 166 (22·0) |
| HR-ω, HER2+ | 2 (3·5) | 30 (9·7) | 9 (5·5) | 22 (4·9) | ·· | ·· | ·· | ·· | 11 (5·0) | 52 (6·9) |
| HR-, HER2- | 12 (21·0) | 49 (15·9) | 33 (20·3) | 69 (15·5) | ·· | ·· | ·· | ·· | 45 (20·4) | 118 (15·6) |
| Unknown | 1 (1·8) | 15 (4·9) | 6 (3·7) | 18 (4·0) | 7 (3·2) | 33 (4·4) | ||||
| P= 0·2 | P= 0·3 | ·· | ·· | P= 0·1 | ||||||
| ART¥ use | ||||||||||
| No | 2 (3·5) | · | 44 (27·0) | · | 4 (7·0) | ·· | 2 (5·6) | ·· | 52 (16·6) | ·· |
| Yes | 53 (93·0) | · | 118 (72·4) | · | 40 (70·2) | ·· | 32 (88·9) | ·· | 243 (77·6) | ·· |
| Unknown | 2 (3·5) | 1 (0·6) | 13 (22·8) | 2 (5·5) | 18 (5·8) | |||||
NK= not known. BC= breast cancer
Column percentage including missing values where applicable
row percentage by HIV status including small number of women whose HIV status was unknown; 18 in Namibia, 23 in South Africa, 49 in Uganda and 48 in Zambia
All P-values in this table are chi-squared test P-values comparing HIV+ and HIV− women only
Socio-economic position (SEP) was derived from a score of combined self-reported access to amenities including home ownership, indoor water, flush toilet, electricity, vehicle, refrigerator, landline, gar or electric stove and a bed. SEP categories (low, middle or high) were constructed based on country specific distribution of the SEP score tertiles.
Comorbidities include: Tuberculosis, Hepatitis, Hypertension, Heart disease, Diabetes, Anaemia, COPD, Asthma, other cancer, other infections and other diseases
Data on this variable was not available for South Africa because a slightly different questionnaire was used at this site
Information on tumour molecular subtype was available for Namibia and South Africa only
ΗR+ = hormone receptor positive (ER+/PR+)
ΗR− = hormone receptor negative (ER−/PR−)
Antiretroviral therapy, restricted to HIV-positive women only
Of the 1635 women who were followed up for up to 3 years, 632 (39%) died, 44% (137 of 313) of whom had HIV and 37% (432 of 1184) did not, 855 (52%) were alive at 3 years, and 148 (9%) were censored early (statistical loss to follow-up; table 2). The crude overall survival at 3 years for all sites combined was 46% (95% CI 40–53) in women with HIV versus 55% (52–59) in HIV-negative women. The crude HR for the difference in all-cause mortality between women with and without HIV was 1·35 (95% CI 1·11–1·63) for all ABC-DO sites combined, but this value varied across sites (table 3). Absolute differences in 3-year overall survival estimates between women with and without HIV of at least 15% were seen in South Africa and Zambia, compared with 9% in Namibia and less than 1% in Uganda. In Uganda, women with HIV experienced lower mortality rates within the first 6 months of diagnosis than did HIV-negative women, but higher mortality rates thereafter (figure 1). Relative to the other ABC-DO sites, the finding that survival did not differ by HIV status in Uganda does not necessarily mean a better survival experience in women with HIV in this country, but rather that women with and without HIV experienced particularly low survival (appendix p 5). Lower survival was also seen in the small proportion of women with unknown HIV status compared with HIV-negative women (table 2).
Table 2.
ABC-DO study follow up, deaths and crude 3-year overall survival estimates by HIV status and country
| All sites (n=1635) | Namibia (n=384) | South Africa (n=632) | Uganda (n=421) | Zambia (n=198) | ||
|---|---|---|---|---|---|---|
| Statistic | HIV status | |||||
| No· women followed up | HIV− | 1,184 | 309 | 446 | 315 | 114 |
| HIV+ | 313 | 57 | 163 | 57 | 36 | |
| HIV unknown | 138 | 18 | 23 | 49 | 48 | |
| Median age at BC diagnosis (IQR) | HIV− | 52·4 (42·0–63·6) | 51·7 (41·6–63·6) | 58·0 (47·0–67·0) | 47·0 (38·5–57·3) | 50·5 (39·2–61·4) |
| HIV+ | 45·2 (40·0–52·9) | 45·2 (39·8–51·6) | 45·0 (41·0–54·0) | 45·3 (42·3–51·8) | 44·9 (38·1–53·6) | |
| HIV unknown | 50·9 (41·3–65·0) | 57·9 (46·4–69·0) | 58·0 (39·0–74·0) | 48·5 (40·1–55·4) | 48·0 (41·2–66·1) | |
| No· deaths at 3 years | HIV− | 432 | 118 | 125 | 157 | 32 |
| HIV+ | 137 | 25 | 62 | 29 | 21 | |
| HIV unknown | 63 | 9 | 9 | 25 | 20 | |
| Median follow-up, years (IQR) | HIV− | 2·1 (1·3–2·9) | 2·8 (1·8–3·0) | 1·9 (1·3–2·5) | 2·3 (1·1–3·0) | 1·7 (1·0–2·2) |
| HIV+ | 1·9 (1·1–2·7) | 2·8 (1·7–3·0) | 1·7 (1·1–2·4) | 2·2 (1·2–2·9) | 1·8 (0·5–2·2) | |
| HIV unknown | 1·7 (0·9–2·7) | 2·4 (1·1–3·0) | 1·2 (0·6–1·7) | 2·4 (1·0–2·4) | 1·5 (0·9–2·2) | |
| Cumulative loss to follow-up at 3 years, n (row %) | HIV− | 106 (9·0) | 8 (2·6) | 60 (13·5) | 13 (4·1) | 25 (21·9) |
| HIV+ | 24 (7·7) | 0 | 17 (10·4) | 3 (5·3) | 4 (11·1) | |
| HIV unknown | 18 (13·0) | 0 | 5 (21·7) | 4 (8·2) | 9 (18·8) | |
| Crude 3-year survival % (95% CI) | HIV− | 55·4 (52·0–58·6) | 57·6 (51·4–63·3) | 63·4 (57·0–69·1) | 44·6 (38·6–50·4) | 65·5 (54·6–74·4) |
| HIV+ | 46·3 (39·5–52·8) | 54·6 (40·6–66·6) | 46·3 (34·0–57·8) | 44·4 (30·5–57·4) | 26·5 (11·3–44·6) | |
| HIV unknown | 42·4 (32.3–52·2) | 42·9 (19·0–65·0) | 48·1 (20·0–71·8) | 43·7 (28·9–57·5) | ·· | |
| Crude 3-year survival % (95% CI) conditional on being alive at 18 months | HIV− | 73·9 (70·0–77·3) | 76·5 (70·3–81·6) | 78·2 (70·8–84·0) | 67·5 (60·1–73·7) | 89·3 (77·4–95·1) |
| HIV+ | 66·2 (57·4–73·6) | 73·5 (57·3–84·5) | 61·4 (44·7–74·5) | 71·1 (51·3–84·0) | 48·6 (20·1–72·3) | |
| HIV unknown | 68·6 (53·7–79·6) | 85·7 (33·4–97·9) | 70·0 (22·5–91·8) | 64·4 (44·7–78·6) | ·· | |
| Crude 3-year survival % (95% CI) among women with non-metastatic disease at diagnosis | HIV− | 63·2 (59·4–66·7) | 67·1 (60·3–72·9) | 70·5 (63·5–76·5) | 53·3 (46·4–59·8) | 63·3 (50·4–73·6) |
| HIV+ | 52·1 (44·4–59·2) | 59·7 (44·7–71·8) | 51·1 (37·4–63·2) | 56·0 (38·3–70·5) | 25·6 (8·4–47·3) | |
|
Crude 3-year survival % (95% CI) among
women with metastatic disease at diagnosis |
HIV− | 12·7 (7·8–18·8) | 15·0 (6·9–26·0) | 16·8 (6·7–30·7) | 6·5 (2·1–14·4) | ·· |
| HIV+ | 16·5 (5·5–32·7) | 16·0 (5·4–9·5) | 18·5 (1·6–50·4) | 10·4 (0·5–37·8) | ·· |
BC: breast cancer; CI: confidence interval; IQR: Inter-quartile range; N/A: not applicable
··No observations
Table 3.
Adjustedξ Hazard ratios (stratified by country) for the association between HIV status and 3-year survival, 18-months survival and 3-year survival conditional on surviving the first 18 months for all ABC-DO sites
| Namibia | South Africa | Uganda | Zambia | All sites | All sites - FU up to 18 months | All sites - if alive at 18 months |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model | HIV status | Deaths | HR (95% CI) | Deaths | HR (95% CI) | Deaths | HR (95% CI) | Deaths | HR (95% CI) | Deaths | HR (95% CI) | Deaths | HR (95% CI) | Deaths | HR (95% CI) |
| Crude | HIV− | 118 | 1·00 | 125 | 1·00 | 157 | 1·00 | 32 | 1·00 | 432 | 1·00 | 269 | 1·00 | 163 | 1·00 |
| HIV+ | 25 | 1·14 | 62 | 1·47 | 29 | 1·09 | 21 | 2·23 | 137 | 1·35 | 87 | 0·95 | 50 | 1·4 | |
| (0·74–1·76) | (1·09–2·00) | (0·73–1·61) | (1·29–3·87) | (1·11–1·63) | (0·74–1·22) | (1·02–1·93) | |||||||||
| + Age | HIV− | 118 | 1·00 | 125 | 1·00 | 157 | 1·00 | 32 | 1·00 | 432 | 1·00 | 269 | 1·00 | 163 | 1·00 |
| HIV+ | 25 | 1·23 | 62 | 1·67 | 29 | 1·14 | 21 | 2·87 | 137 | 1·46 | 87 | 0·99 | 50 | 1·48 | |
| (0·79–1·92) | (1·20–2·31) | (0·76–1·70) | (1·57–5·25) | (1·19–1·78) | (0·77–1·28) | (1·06–2·06) | |||||||||
| + Stage | HIV− | 118 | 1·00 | 125 | 1·00 | 157 | 1·00 | 32 | 1·00 | 432 | 1·00 | 269 | 1·00 | 163 | 1·00 |
| HIV+ | 25 | 1·39 | 62 | 1·58 | 29 | 1·10 | 21 | 3·12 | 137 | 1·46 | 87 | 0·97 | 50 | 1·53 | |
| (0·88–2·19) | (1·14–2·20) | (0·73–1·66) | (1·66–5·87) | (1·19–1·79) | (0·75–1·27) | (1·09–2·15) | |||||||||
| + Other factors γ | HIV− | 118 | 1·00 | 123 | 1·00 | 157 | 1·00 | 32 | 1·00 | 430 | 1·00 | 266 | 1·00 | 163 | 1·00 |
| HIV+ | 25 | 1·26 | 61 | 1·61 | 29 | 1·09 | 21 | 3·10 | 136 | 1·41 | 85 | 0·98 | 50 | 1·48 | |
| (0·79–2·03) | (1·14–2·28) | (0·72–1·65) | (1·64–5·88) | (1·15–1·74) | (0·75–1·29) | (1·06–2·08) | |||||||||
| + Receptor subtypes | HIV− | 112 | 1·00 | 120 | 1·00 | ·· | ·· | ·· | ·· | 232 | 1·00 | 141 | 1·00 | 91 | 1·00 |
| HIV+ | 25 | 1·36 | 59 | 1·54 | ·· | ·· | ·· | ·· | 84 | 1·43 | 50 | 1·22 | 34 | 1·56 | |
| (0·84–2·20) | (1·08–2·20) | ·· | ·· | ·· | ·· | (1·08–1·88) | (0·83–1·79) | (1·01–2·40) | |||||||
To a simple model with HIV status as the only exposure, each of the other factors were added sequentially to the model one at a time i.e. age, then stage etc.
Other factors include tumour grade, educational level and socio economic position
Figure 1.
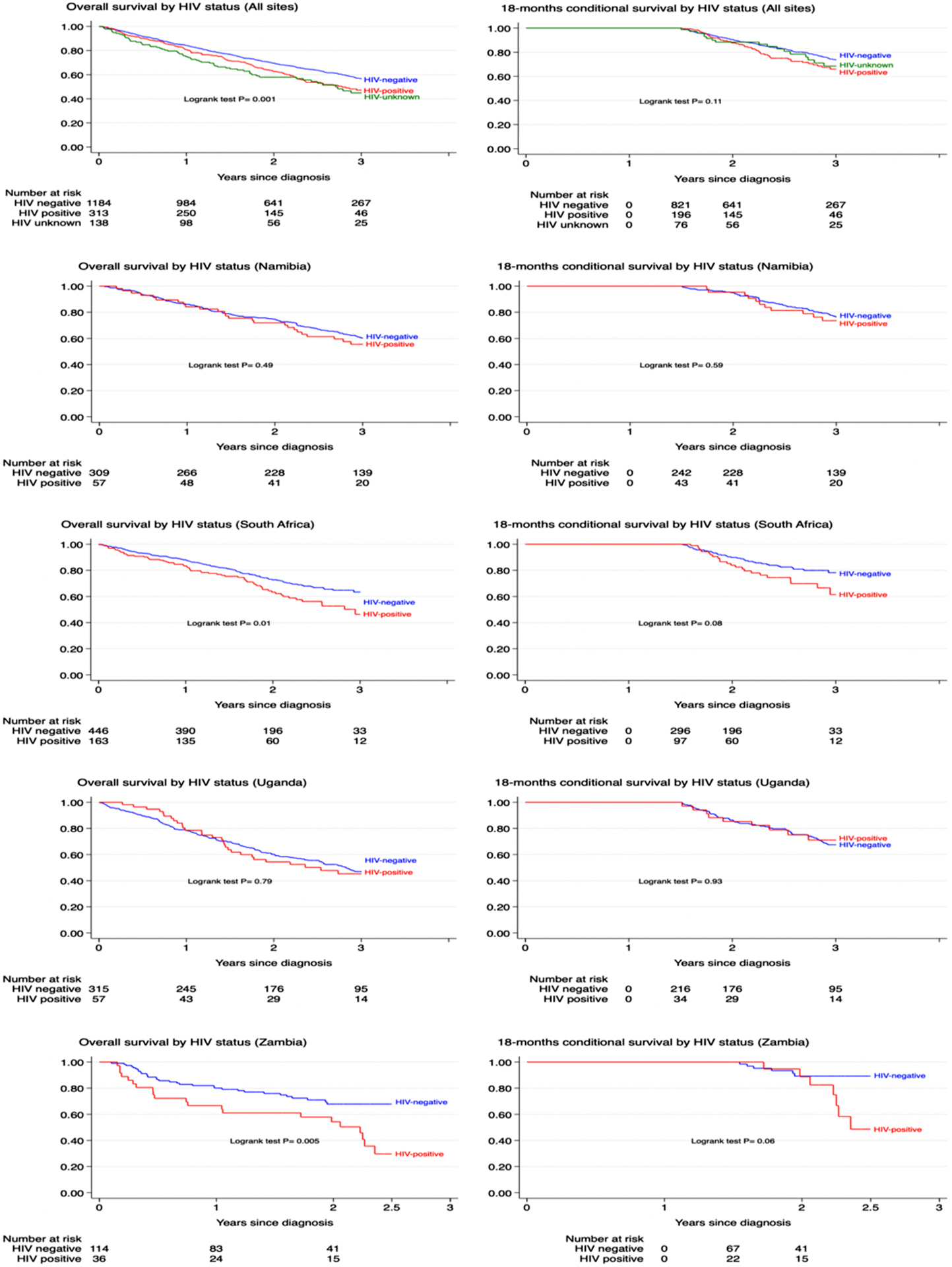
Kaplan Meier curves for crude 3-year overall survival following breast cancer diagnosis by HIV status in the ABC-DO cohort, for all sites and by country: (a) 3-year survival; (b) 3-year survival conditional on being alive at 18-months
The differences by HIV status became marginally stronger when adjusted for relevant patient and tumour characteristics (adjusted HR [aHR] 1·41, 95% CI 1·15–1·74), including tumour receptor subtypes which were available in Namibia and South Africa only (aHR 1·43, 1·08–1·88 vs corresponding crude HR 1·35, 1·05–1·72 for these two countries). Based on observed steeper survival curves in women with HIV beyond 18 months (figure 1), follow-up time was split at 18 months. Absolute survival differences by HIV status were modest within the first 18 months of follow-up (figure 1, table 2), but became more marked beyond 18 months (table 2). The relative HIV differentials were stronger after 18 months of follow-up (crude HR 1·40, 95% CI 1·02–1·93) than within the first 18 months of follow-up (0·95, 0·74–1·22; pinteraction=0·06). When restricted to women with non-metastatic disease at diagnosis (stages I–III), the magnitude of the HIV differential for overall 3-year all-cause mortality (adjusted for age, stage, and grade) increased from 1·52 (1·24–1·87) to 1·64 (1·29–2·09; appendix pp 5, 8). Among women with non-metastatic disease, the HIV differential was stronger for individuals who survived beyond the first 18 months (adjusted HR 1·58, 1·09–2·28; appendix p 5) than for those alive within the first 18 months (1·05, 0·75–1·46). There were no differences by HIV status among women with metastatic disease (adjusted HR 1·10, 0·70–1·73) even when restricted to those alive beyond 18 months (1·14, 0·42–3·11; appendix p 5). However, the absolute difference in survival percentage between women with and without HIV was consistent at all stages (table 2). Therefore, the observed HIV–time interaction was not due to early deaths among women with metastatic disease, but rather points to a real strengthening of the HIV differential over time.
Figure 2 shows survival differences by HIV status according to patient and tumour characteristics. The increased all-cause mortality among women with HIV differed by tumour stage at diagnosis (pinteraction=0·06) and ART (p=0·0001) only. Relative to their HIV-negative counterparts of the same stage, women with HIV with tumour stages I or II and tumour stage III had, respectively, increases of 58% (aHR 1·58, 95% CI 0·98–2·57) and 73% (aHR 1·73, 1·31–2·28) in all-cause mortality, whereas women with HIV with stage IV disease had similar all-cause mortality as HIV-negative women (aHR 1·10, 0·70–1·73). Overall, among women with non-metastatic breast cancer, those with HIV had an increase of 65% (aHR 1·65, 1·30–2·10) in all-cause mortality compared with HIV-negative women of the same stage.
Figure 2.
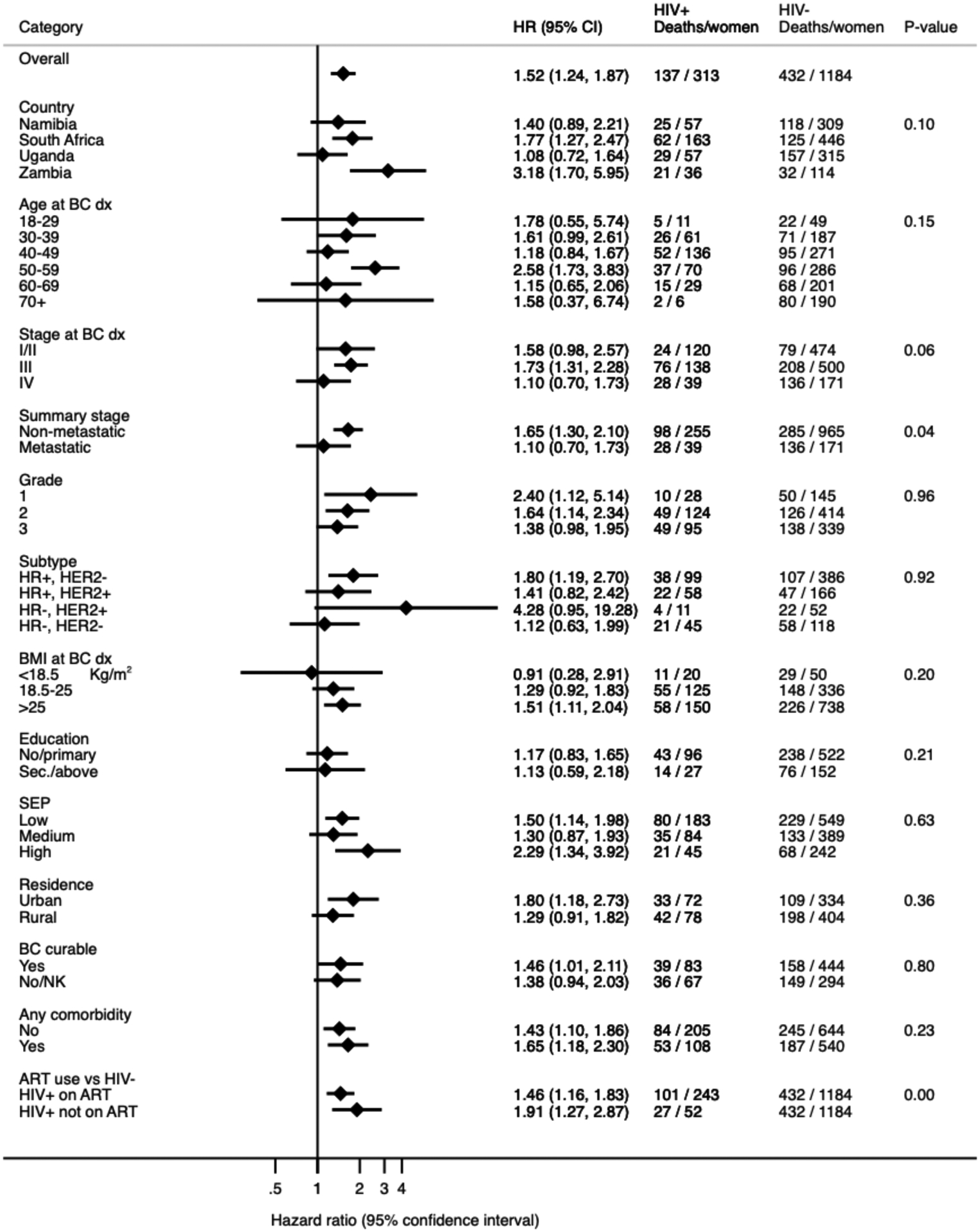
Hazard ratios for 3-year all-cause mortality in HIV-positive compared to HIV-negative breast cancer women, by patient and tumour characteristics in the ABC-DO cohort (all sites combined)
ART: anti-retroviral therapy; BC: breast cancer; BMI: body mass index; CI: confidence interval; dx: diagnosis; BC curable: woman’s knowledge of whether breast cancer is curable if detected early
HR: hazard ratio; NK: not known; Sec: secondary education; SEP: socio-economic position (see Table 1).
HR adjusted for age, tumour stage and tumour grade at breast cancer diagnosis.
P-value for interaction between HIV status and each patient and tumour variable listed.
Women with HIV had increased all-cause mortality compared with their HIV-negative counterparts of the same tumour stage (appendix p 9). The magnitude of this increase corresponded to a single shift in stage (eg, women with HIV with stage IIB breast cancer had mortality rates almost reaching those for HIV-negative women with stage IIIA breast cancer), with the exception of women with stage IV (ie, metastatic disease). Relative to HIV-negative women, women with HIV not on ART had poorer survival (aHR 1·91, 95% CI 1·27–2·87) than did women with HIV on ART (1·46, 1·16–1·83; figure 2). There was no clear evidence that the HIV differential was modified by BMI or tumour receptor subtypes, although women with HIV who were overweight or obese (aHR 1·51, 1·11–2·04), and those with hormone receptor-positive or HER2-negative tumours (1·80, 1·19–2·70), had increased all-cause mortality, but estimates were based on small numbers. There were small differences in the adjusted HR for HIV among women with non-metastatic disease for the different assumptions on HIV unknowns: 1·55 (1·26–1·91) if assumed HIV positive; 1·60 (1·26–2·03) if HIV negative; and 1·63 (1·29–2·09) if dropped from the analysis (appendix p 6).
Discussion
In this large, prospective, multi-country ABC-DO study in sub-Saharan Africa, women living with HIV who were diagnosed with breast cancer, particularly those with non-metastatic disease, experienced increased all-cause mortality relative to their HIV-negative counterparts, with the effect persisting upon adjustment for other relevant prognostic factors. However, differences by HIV status were more pronounced in some settings than others. In Zambia, women with HIV had higher risk of mortality than HIV-negative women. In Uganda, 3-year overall survival was very low, regardless of HIV status, and most women presented with late-stage disease.
Similar to other studies in the region7,10,27,28 and elsewhere2,12,14 women with HIV in the ABC-DO cohort were diagnosed with breast cancer at younger ages than were HIV-negative women, given that women with HIV are younger than the general population. Similar to other studies,10,28 albeit not all,7,17,29 our study found that the prevalence of late-stage breast cancer at diagnosis did not differ by HIV status. However, women with HIV had increased risk of mortality than did HIV-negative women for the same tumour stage, with the relative HIV differential being stronger among those with non-metastatic disease at diagnosis (65% increased mortality risk). A study in the USA reported comparable overall survival by HIV status between women with stage I–III tumours only and all women (stages I–IV), but the proportion of women with metastatic disease was small.17 Although on this scale of analysis the relative increase in mortality was stronger in women with non-metastatic disease than in those with metastatic disease, it remains possible that the absolute difference in mortality rates between women with and without HIV is constant at all stages and this constant difference would represent a smaller relative increase (more difficult to detect) in women with metastatic disease. Excess bodyweight is associated with poor survival from breast cancer.19 We found no evidence that the HIV differential was modified by BMI. However, the difference in all-cause mortality between women with and without HIV was stronger among overweight or obese women. These findings are consistent with reports of increased risk of obesity and increased risk of, and mortality from, cardiovascular diseases among women with HIV on ART.20 Although women with HIV on ART had poor survival outcomes compared with HIV-negative women, they had better survival outcomes than did women with HIV who were not on ART, emphasising the importance of ART adherence on cancer survival.11
Studies in the USA have shown that both overall and breast cancer-specific survival is poor in women with HIV (vs HIV-negative women) even after adjusting for stage at diagnosis and type of cancer treatment received.12,14,17 To date, no such studies have been conducted in sub-Saharan Africa. Our finding that the HIV differential was stronger in women with non-metastatic disease and manifested only beyond 18 months follow-up suggests that the excess deaths in women with HIV were not due to immediate treatment-related toxicity but rather point to a longer term treatment pathway or differential, highlighting the importance of early diagnosis and timely access to effective breast cancer and HIV treatments among women with HIV. More consideration should be given to the feasibility and effectiveness of integrating breast cancer care into existing functional HIV or cervical cancer services through the promotion of breast cancer awareness among women with HIV and their health-care workers, coupled with implementation of efficient patient navigation systems to minimise delays in cancer diagnosis and initiation of treatment, and minimise disruptions and abandonment of cancer or HIV treatments. Our findings underscore the need for standard clinical guidelines on how best to simultaneously manage the two conditions. Previously, we reported that women with HIV were less likely (vs HIV-negative women) to receive curative treatment for breast cancer within the first 12 months of diagnosis,30 either due to scarcity of oncologists or expertise on how to co-manage both diseases, particularly in Zambia and Uganda. Furthermore, the absence of universal free health care and increased out-of-pocket costs of cancer care might have resulted in long delays to cancer treatment initiation and to interruptions and abandonment of HIV or cancer treatments.
The strengths of this study include its large size, the considerable proportion (19%) of women with HIV, the prospective multi-country design, the collection of detailed clinical and epidemiological data, and the use of an mHealth application to minimise losses to follow-up.
This study has several limitations. First, self-reported HIV status might have led to non-differential exposure misclassification and underestimation of the effect of HIV status on survival. However, the high percentage (>50%) of women with HIV tested in South Africa, high level of within-woman agreement on HIV self-reports taken at two distinct timepoints, and consistency of the findings across the different settings indicate that misclassification of self-reported HIV status was probably minimal. Sensitivity analyses also showed that any potential misclassification of HIV status would have had little effect on the findings. Second, the high proportion of missing data for CD4 count and viral load and the absence of data on HIV stage and management history prevented examination of whether severity of HIV/AIDS contributed to the poorer survival outcomes seen among women with HIV. We could not examine whether survival differences vary by HIV-1 versus HIV-2 types due to the absence of such data and the exclusion of women from Nigeria, west Africa (where HIV-2 is most prevalent) due to low HIV prevalence (<4%). Third, the absence of data on cause of death precluded examination of cause-specific survival differential by HIV status. Lastly, the hospital-based nature of ABC-DO might limit the generalisation of study findings.
In summary, this ABC-DO study showed that women with HIV had lower survival outcomes at 3 years post-diagnosis for breast cancer than their HIV-negative counterparts, even after taking into account other prognostic factors assessed at diagnosis. To prevent deaths in this patient group a better understanding is needed of the reasons for and the extent to which survival differentials by HIV status in sub-Saharan Africa reflect underlying biological mechanisms, differential access to (or compliance with) appropriate treatment regimens, detrimental HIV–breast cancer treatment interactions, or simply increased background mortality associated with HIV.
Supplementary Material
Figure 3.
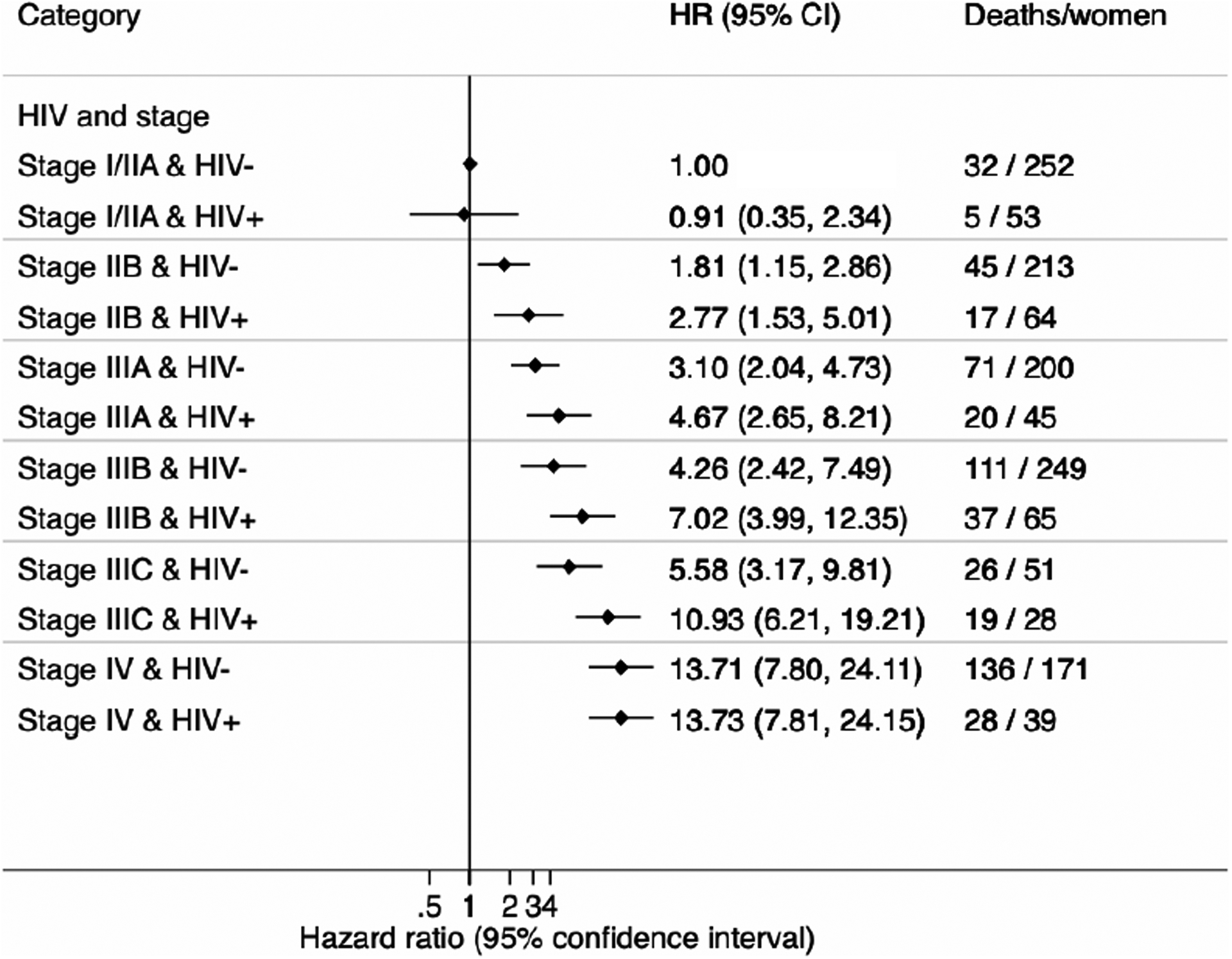
Hazard ratios for 3-year all-cause mortality stratified by HIV- status and tumour stage at breast cancer diagnosis in the ABC-DO cohort (all sites combined)
CI: confidence interval; HR: hazard ratio
HRs adjusted for age and tumour grade at breast cancer diagnosis
Research in context.
Evidence before this study
We identified a recent systematic review of studies published up to Jan 1, 2020, and updated it to Jan 1, 2021, by searching the Embase and PubMed (Ovid) databases using the search terms “breast cancer”, “HIV”, and “survival”, with no language restrictions. The systematic review, comprising seven distinct study populations, showed that women living with HIV diagnosed with breast cancer had increased all-cause mortality compared with their HIV-negative counterparts with breast cancer, both in North America and sub-Saharan Africa. However, the review also highlighted marked between-study heterogeneity in the magnitude of the differences in mortality between women with and without HIV (increases of 1·6–4·6 times); studies with a small number of women with HIV; and, specifically in sub-Saharan Africa, studies with suboptimal follow-up owing to high losses to follow-up. Our updated search identified only one additional publication, from our African Breast Cancer–Disparities in Outcomes (ABC-DO) study, which showed a 1·5-times increase in all-cause mortality among women with HIV diagnosed with breast cancer in sub-Saharan Africa. None of these studies examined whether differences in overall survival vary by patient or tumour characteristics between women with and without HIV diagnosed with breast cancer.
Added value of this study
The ABC-DO study is a multi-country, hospital-based, prospective study that recruited women with incident breast cancer who were diagnosed in 2014–17 in five sub-Saharan Africa countries (Namibia, Nigeria, South Africa, Uganda, and Zambia) with varying HIV prevalence and different health-care systems. Detailed clinical and epidemiological data were collected at baseline, including self-reported or tested HIV status, and the cohort underwent active follow-up by mobile phone every 3 months. The ABC-DO study has the largest number of women with HIV diagnosed with breast cancer and lowest loss-to-follow-up ever reported in sub-Saharan Africa. We examined how the previously reported association between HIV status and survival after a diagnosis of breast cancer in the ABC-DO cohort might be modified by other factors. 3-year overall survival was lower for women with HIV than for HIV-negative women.
Implications of all the available evidence
The findings of the present study show that women with HIV diagnosed with breast cancer were at higher risk of dying within 3 years of their cancer diagnosis than their HIV-negative counterparts. Identifying the reasons for the higher all-cause mortality among women with HIV (eg, underlying biological mechanisms, poor access to [or compliance with] appropriate treatment regimens, detrimental HIV–breast cancer treatment interactions, or simply increased background mortality associated with HIV) is crucial if early deaths among this patient group are to be prevented.
Acknowledgments
The ABC-DO study was sponsored by Susan G Komen (grants IIR13264158, GSP18IARC001, and GSP19IARC001), the National Cancer Institute (grants R01CA244559 and R01CA250012), and the International Agency for Research on Cancer Branch of Environmental and Lifestyle Epidemiology. The work reported in this paper was undertaken as part of PhD research of SC supported by the UK-Commonwealth Scholarships. Where authors are identified as personnel of the International Agency for Research on Cancer (IARC) or WHO, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy, or views of IARC or WHO. We thank the participants, their families, and the study interviewers for taking part in the study. This paper is dedicated to the memory of our colleague, Prof Charles A Adisa, who was the founder of the ABC-DO study in Abia, Nigeria.
Footnotes
Declaration of interests
We declare no competing interests.
Data sharing
ABC-DO study data, including on individual participants, are available and can be accessed at the International Agency for Research on Cancer through collaborative work. Requests should be made to env@iarc.fr.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136: E359–86. [DOI] [PubMed] [Google Scholar]
- 2.McCormack VA, Febvey-Combes O, Ginsburg O, Dos-Santos-Silva I. Breast cancer in women living with HIV: a first global estimate. Int J Cancer 2018; 143: 2732–40. [DOI] [PubMed] [Google Scholar]
- 3.Shiels MS, Pfeiffer RM, Gail MH, et al. Cancer burden in the HIV-infected population in the United States. J Natl Cancer Inst 2011; 103: 753–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Joko-Fru WY, Miranda-Filho A, Soerjomataram I, et al. Breast cancer survival in sub-Saharan Africa by age, stage at diagnosis and human development index: a population-based registry study. Int J Cancer 2020; 146: 1208–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018; 391: 1023–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brinkhof MWG, Boulle A, Weigel R, et al. Mortality of HIV-infected patients starting antiretroviral therapy in sub-Saharan Africa: comparison with HIV-unrelated mortality. PLoS Med 2009; 6: e1000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brandão MDRA, Guisseve A, Bata G, et al. HIV infection in breast cancer patients from Mozambique: a prospective cohort study. Ann Oncol 2019; 30: iii69. [Google Scholar]
- 8.Coghill AE, Newcomb PA, Madeleine MM, et al. Contribution of HIV infection to mortality among cancer patients in Uganda. AIDS 2013; 27: 2933–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cubasch H, Dickens C, Joffe M, et al. Breast cancer survival in Soweto, Johannesburg, South Africa: a receptor-defined cohort of women diagnosed from 2009 to 11. Cancer Epidemiol 2018; 52: 120–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sadigh KHR, Tapela N. HIV is associated with decreased breast cancer survival: a prospective cohort study. Conference on Retroviruses and Opportunistic Infections; March 4–7, 2019 (abstr 16). [Google Scholar]
- 11.Biggar RJ, Engels EA, Ly S, et al. Survival after cancer diagnosis in persons with AIDS. J Acquir Immune Defic Syndr 2005; 39: 293–99. [DOI] [PubMed] [Google Scholar]
- 12.Chhatre S, Schapira M, Metzger DS, Jayadevappa R. Association between HIV infection and outcomes of care among Medicare enrollees with breast cancer. EClinicalMedicine 2019; 17: 100205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coghill AE, Pfeiffer RM, Shiels MS, Engels EA. Excess mortality among HIV-Infected Individuals with cancer in the United States. Cancer Epidemiol Biomarkers Prev 2017; 26: 1027–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coghill AE, Shiels MS, Suneja G, Engels EA. Elevated cancer-specific mortality among HIV-infected patients in the United States. J Clin Oncol 2015; 33: 2376–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coghill AE, Suneja G, Rositch AF, Shiels MS, Engels EA. HIV infection, cancer treatment regimens, and cancer outcomes among elderly adults in the United States. JAMA Oncol 2019; 5: e191742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brandão M, Bruzzone M, Franzoi MA, et al. Impact of HIV infection on baseline characteristics and survival of women with breast cancer: a systematic review and meta-analysis. AIDS 2021; 35: 605–18. [DOI] [PubMed] [Google Scholar]
- 17.Coghill AE, Han X, Suneja G, Lin CC, Jemal A, Shiels MS. Advanced stage at diagnosis and elevated mortality among US patients with cancer infected with HIV in the National Cancer Data Base. Cancer 2019; 125: 2868–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cubasch H, Ruff P, Joffe M, et al. South African breast cancer and HIV outcomes study: methods and baseline assessment. J Glob Oncol 2017; 3: 114–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan DSM, Vieira AR, Aune D, et al. Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol 2014; 25: 1901–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Biggs C, Spooner E. Obesity and HIV: a compounding problem. South Afr J Clin Nutr 2018; 31: 78–83. [Google Scholar]
- 21.Shawarira-Bote S, Shamu T, Chimbetete C. Gynecomastia in HIV-positive adult men receiving efavirenz-based antiretroviral therapy at Newlands clinic, Harare, Zimbabwe. BMC Infect Dis 2019; 19: 715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKenzie F, Zietsman A, Galukande M, et al. African Breast Cancer-Disparities in Outcomes (ABC-DO): protocol of a multicountry mobile health prospective study of breast cancer survival in sub-Saharan Africa. BMJ Open 2016; 6: e011390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCormack V, McKenzie F, Foerster M, et al. Breast cancer survival and survival gap apportionment in sub-Saharan Africa (ABC-DO): a prospective cohort study. Lancet Glob Health 2020; 8: e1203–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McKenzie F, Zietsman A, Galukande M, et al. Drivers of advanced stage at breast cancer diagnosis in the multicountry African breast cancer - disparities in outcomes (ABC-DO) study. Int J Cancer 2018; 142: 1568–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010; 17: 1471–74. [DOI] [PubMed] [Google Scholar]
- 26.Foerster M, Anele A, Adisa C, et al. Few losses to follow-up in a sub-Saharan African cancer cohort via active mobile health follow-up. Am J Epidemiol 2020; 189: 1185–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cubasch H, Joffe M, Hanisch R, et al. Breast cancer characteristics and HIV among 1,092 women in Soweto, South Africa. Breast Cancer Res Treat 2013; 140: 177–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Langenhoven L, Barnardt P, Neugut AI, Jacobson JS. Phenotype and treatment of breast cancer in hiv-positive and -negative women in Cape Town, South Africa. J Glob Oncol 2016; 2: 284–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Phakathi B, Cubasch H, Nietz S, et al. Clinico-pathological characteristics among South African women with breast cancer receiving anti-retroviral therapy for HIV. Breast 2019; 43: 123–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Foerster M, Anderson BO, McKenzie F, et al. Inequities in breast cancer treatment in sub-Saharan Africa: findings from a prospective multi-country observational study. Breast Cancer Res 2019; 21: 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
ABC-DO study data, including on individual participants, are available and can be accessed at the International Agency for Research on Cancer through collaborative work. Requests should be made to env@iarc.fr.


