Abstract
In vitro time-kill studies and a rabbit model of endocarditis and pyelonephritis were used to define the impact that the order of exposure of Candida albicans to fluconazole (FLC) and amphotericin B (AMB), as sequential and combination therapies, had on the susceptibility of C. albicans to AMB and on the outcome. The contribution of FLC-induced resistance to AMB for C. albicans also was assessed. In vitro, AMB monotherapy rapidly killed each of four C. albicans strains; FLC alone was fungistatic. Preincubation of these fungi with FLC for 18 h prior to exposure to AMB decreased their susceptibilities to AMB for 8 to >40 h. Induced resistance to AMB was transient, but the duration of resistance increased with the length of FLC preincubation. Yeast sequentially incubated with FLC followed by AMB plus FLC (FLC→AMB+FLC) showed fungistatic growth kinetics similar to that of fungi that were exposed to FLC alone. This antagonistic effect persisted for at least 24 h. Simultaneous exposure of C. albicans to AMB and FLC [AMB+FLC(simult)] demonstrated activity similar to that with AMB alone for AMB concentrations of ≥1 μg/ml; antagonism was seen using an AMB concentration of 0.5 μg/ml. The in vitro findings accurately predicted outcomes in our rabbit infection model. In vivo, AMB monotherapy and treatment with AMB for 24 h followed by AMB plus FLC (AMB→AMB+FLC) rapidly sterilized kidneys and cardiac vegetations. AMB+FLC(simult) and FLC→AMB treatments were slower in clearing fungi from infected tissues. FLC monotherapy and FLC→AMB+FLC were both fungistatic and were the least active regimens. No adverse interaction was observed between AMB and FLC for the AMB→FLC regimen. However, FLC→AMB treatment was slower than AMB alone in clearing fungi from tissues. Thus, our in vitro and in vivo studies both demonstrate that preexposure of C. albicans to FLC reduces fungal susceptibility to AMB. The length of FLC preexposure and whether AMB is subsequently used alone or in combination with FLC determine the duration of induced resistance to AMB.
With the increased capacity of medical science to prolong the lives of the immunocompromised host, the incidence of systemic Candida albicans infections is rising (5). Yet despite treatment with fluconazole (FLC) or amphotericin B (AMB) monotherapy, the mortality associated with deep-seated candidal infections remains substantial (2, 22, 23, 25, 30).
In an attempt to improve survival rates, there is much interest in using FLC and AMB in combination. However, the interaction between FLC and AMB remains poorly characterized when these drugs are used concurrently or sequentially. In vitro studies in which FLC and AMB were simultaneously or sequentially introduced to cultures of C. albicans frequently showed this drug combination to be antagonistic (13, 19, 21, 28; P. Banerjee, Q.-F. Liu, A. Louie, M. Shayegani, H. Taber, G. Drusano, and M. Miller, Abstr. 97th Gen. Meet. Am. Soc. Microbiol., abstr. C-252a, p. 164, 1997). Further, using time-kill studies, Vazquez et al. (28) demonstrated that sequential exposure of one C. albicans strain to FLC followed by AMB decreased the susceptibility of the fungus to AMB. This “induced resistance” was transient in that the phenotypic resistance to AMB quickly disappeared after the fungus was transferred to drug-free media. Together these in vitro studies suggest that the interaction between FLC and AMB is antagonistic against C. albicans when this fungus is exposed to these drugs simultaneously and when the fungus is sequentially exposed to FLC followed by either AMB alone or FLC in combination with AMB.
In contrast, in vivo models of systemic candidiasis demonstrate an additive or indifferent interaction between AMB and FLC, regardless of the sequence in which AMB and FLC were added to the treatment regimen (26). However, these in vivo studies were not optimally designed to identify antagonism, if it existed. Thus, the in vivo implications of the in vitro antagonism seen between AMB and FLC remain undefined.
Vazquez et al. induced resistance to AMB for one C. albicans strain (28). In the present study, we expanded on the in vitro studies described by Vazquez et al. to determine whether the induced resistance to AMB was seen for four C. albicans strains that were sequentially exposed to FLC followed by AMB. Also, using the time-kill procedures described by Vazquez et al., we evaluated the interaction between AMB and FLC when the same four C. albicans isolates were simultaneously exposed to FLC and AMB [AMB+FLC(simult)] or sequentially incubated with AMB followed by FLC, FLC followed by AMB, FLC followed by FLC+AMB (FLC→AMB+FLC) and AMB followed by FLC+AMB (AMB→FLC+AMB). Using a rabbit model of C. albicans endocarditis and pyelonephritis, we determined whether the drug interactions between AMB and FLC that were observed in vitro predicted outcomes in vivo. Finally, we determined if the in vitro-induced resistance to AMB described by Vazquez et al. could explain our in vivo findings.
Louie et al. (14) demonstrated that the pharmacodynamic parameter that best predicts the outcome for treatment with FLC is the ratio of the area under the concentration-time curve (AUC) for the drug in serum to the MIC. To maximize the clinical relevance of our in vivo results, we used a dosage of FLC that resulted in a drug AUC for rabbit serum that mimicked the steady-state AUC measured in humans who receive 800 mg of FLC/day (15). For AMB, the pharmacodynamic parameter that defines the efficacy is unknown. Thus, we chose a dose of AMB to use in rabbits that resulted in serum trough and AUC values similar to those seen in humans given 1 mg of this drug/kg of body weight/day (3, 6, 10).
MATERIALS AND METHODS
Fungal isolates.
C. albicans strain B311 was obtained from Vazquez et al. (28). C. albicans ATCC 36082 was obtained from the American Type Culture Collection (Rockville, Md.). C. albicans strains 6 and 95-1939 were selected from our culture collection; each was isolated from the blood of neutropenic patients. Fresh isolates were grown on Sabouraud-dextrose agar (SDA) (Difco, Detroit, Mich.) for 24 h at 35°C before each phase of the investigation.
The MICs for FLC for the fungal strains ranged between 0.125 and 0.5 μg/ml. The MICs for AMB ranged from 0.125 to 0.25 μg/ml. MIC testing was conducted using a macrobroth dilution method specified by NCCLS for yeast (20). The MICs for FLC and AMB were identical when the macrobroth susceptibility testing was conducted in RPMI 1640 and yeast nitrogen broth (YNB) (Difco).
Antifungal agents.
FLC powder was supplied by Pfizer, Inc. (Groton, Conn.), and reagent grade AMB (for the in vitro studies) was purchased from Sigma Inc. (St. Louis, Mo.). For the in vivo studies, AMB-desoxycholate (Adria Laboratories, Columbus, Ohio) was used. FLC solution was prepared in normal saline at a concentration of 3 mg/ml. The FLC solution was prepared prior to each infusion. AMB-desoxycholate was prepared to a concentration of 0.2 mg/ml in 5% dextrose in water. The AMB suspension was stored at 4°C for up to 1 week without a loss of potency.
In vitro time-kill studies for C. albicans B311.
We determined if the transient resistance to AMB reported by Vazquez et al. (28) could be reproduced by our laboratory for the strain of C. albicans used in their investigations. Using the test conditions described by Vazquez et al. (28) with minor modifications, time-kill studies were conducted with C. albicans strain B311. Briefly, a single colony of the fungal strain was incubated in YNB overnight in a shaking water bath at 35°C. The YNB contained 25 μg of FLC per ml of broth. Eighteen hours later, the microorganisms were washed and organisms were inoculated into flasks containing fresh YNB media and either no drug, 25 μg of FLC/ml, 1.5 μg of AMB/ml, or both FLC and AMB at the concentrations indicated. The final concentration of yeast in each flask was 105 CFU/ml. Additional flasks contained yeast cells grown overnight for 18 h in drug-free YNB that were subsequently incubated in fresh YNB broth containing no drug, 25 μg of FLC/ml, 1.5 μg of AMB/ml, or both drugs at the concentrations specified. Controls consisted of yeast incubated overnight in drug-free medium that were then transferred to fresh drug-free medium. (We could not examine the effect of incubating C. albicans with AMB followed by FLC exposure because the fungal densities decreased to undetectable levels within 2 h of AMB exposure). The resulting fungal suspensions were incubated at 35°C. After 0, 2, 4, 6, 8, 12 and 24 h of incubation, a sample was taken from each flask and serially diluted 1:10 in saline. Then, 200 μl of sample from each dilution tube and the original fungal suspensions were plated onto SDA. The agar plates were incubated at 35°C for 48 h before they were read. The relative densities of organisms per milliliter of broth in each group were compared. The lower limit of detection for the quantitative cultures was 50 CFU of C. albicans/ml. Time-kill studies were conducted twice for this C. albicans isolate.
In vitro time-kill studies for three additional C. albicans strains.
The above-described time-kill studies were conducted for three additional C. albicans strains (ATCC 36082, strain 6, and 95-1939) to determine if the induced resistance reported by Vazquez et al. was unique to the single strain he examined (strain B311) or could also be induced in other Candida isolates. Time-kill studies were conducted twice for each fungal strain.
Drug carryover.
Drug carryover was evaluated by inoculating SDA plates with 200 μl of drug-free YNB or YNB supplemented with 1.5 μg of AMB/ml, 25 μg of FLC/ml, or AMB in combination with FLC. The solutions were spread onto the surface of the agar and allowed to be completely absorbed into the medium over 15 min. The plates were inoculated with 200 CFU of C. albicans B311 prepared in 50 μl of YNB. After 48 h of incubation at 35°C, the colony counts for each plate were read and compared. These studies were repeated for each C. albicans strain. These studies demonstrated that our results were not affected by drug carryover (data not shown).
Effect of varying FLC preincubation times on susceptibility of C. albicans to AMB.
The time-kill studies described above were repeated using C. albicans B311. However, the duration of time that the fungus was preincubated with 25 μg of FLC/ml was varied to determine the effect of FLC preincubation times on AMB activity. The FLC preincubation times evaluated were 0, 2, 4, 6, 16, and 40 h. After the preincubation times were reached, the organisms were washed once with normal saline and were then inoculated into flasks containing 100 ml of fresh YNB with no drugs, 1.5 μg of AMB/ml, or 25 μg of FLC/ml. One hundred milliliters of sample was taken for quantitative cultures at 0, 2, 4, 6, 8, 14, and 24 h. The plates were read after 48 h of incubation, and the results were compared. This study was conducted twice.
Time-kill studies to evaluate the impact of preincubation of C. albicans B311 with FLC on the activity of low concentrations of AMB (0.5 μg/ml).
The time-kill studies outlined above were repeated for C. albicans strain B311 using FLC at a concentration of 25 μg/ml and AMB at 0.5 μg/ml. These studies were conducted to determine if the phenotypic resistance to AMB can be induced in Candida organisms that are subsequently exposed to clinically achievable concentrations of AMB. These studies were conducted thrice.
Animals.
Male New Zealand White rabbits weighing 2.0 kg were used. The animals were housed in individual cages and received food and water ad libitum. All animal procedures used were approved by the Institutional Animal Care and Use Committee of Albany Medical College.
Rabbit model of endocarditis and pyelonephritis.
C. albicans B311, a fungal strain that showed induced resistance to fluconazole in vitro, was selected for the in vivo studies. The fungal suspension was prepared as described by Witt and Bayer (31) with minor modifications as described previously (17). C. albicans endocarditis and pyelonephritis were established in animals using the methods of Durack et al. (8) and Witt and Bayer (31), with minor modifications (17). Briefly, under general anesthesia, a sterile vinyl catheter (external diameter, 1.32 mm; Bolab Products, Lake Havasu City, Ariz.) was inserted through an incision in the carotid artery and threaded into the left ventricle. The catheter was left in place for the duration of the study. After 48 h, animals were injected intravenously (i.v.) with 2 × 107 CFU of C. albicans via a marginal ear vein. In untreated rabbits, this fungal inoculum resulted in endocarditis and pyelonephritis in 95 and 100% of animals, respectively.
Therapeutic studies in the rabbit infection model.
Fungal endocarditis and pyelonephritis were established as described above. The infected rabbits were divided into eight groups. Over the course of the study, two to three separate trials were conducted for each treatment regimen. The results from each trial were combined. At the end of the study, there were 5 to 12 animals in each group and time point. Antifungal therapy was initiated 24 h after fungal inoculation. Animals in group I received FLC (42.5 mg/kg) i.v. This total daily dose results in a 24-h AUC for rabbits that mimics the 24-h AUC measured for humans who are given 800 mg of FLC daily (3, 15). Animals in group II received AMB i.v. at 1.0 mg/kg/day. Rabbits in group III received FLC in combination with AMB, at the doses indicated. AMB was given simultaneously with each daily dose of FLC. Treatment with both drugs was begun on the same day. Animals in group IV received FLC and AMB as well. However, the daily dose of AMB was initiated 24 h prior to the first daily dose of FLC. Thereafter, the animals received both drugs each day. Group V also received FLC and AMB. However, in this group, FLC was given alone for 24 h and then combination therapy was initiated. Group VI received FLC as monotherapy for 24 h followed by daily infusions of AMB. Group VII was treated with AMB monotherapy for 24 h followed by FLC monotherapy thereafter. Finally, animals in group VIII served as controls. They received saline in place of FLC and 5% dextrose in water in place of AMB, respectively.
All drugs and saline were given intravenously via a vinyl catheter that was placed into the external jugular vein using the method of Walsh et al. (29). The venous catheter (internal diameter, 1.57 mm; Bolab Products) was inserted at the same time that the aortic valve catheter was placed. Cefazolin (100 mg/kg given intramuscularly) was administered daily for 3 days as a prophylaxis against bacterial infection at the surgical sites.
Treatment was given for 2, 5, 13, or 21 days. Twenty-four hours after the last dose was given, animals from each group were humanely sacrificed with a rapid intravenous infusion of pentobarbital followed by induction of bilateral pneumothoraces. The location of the aortic catheter was confirmed. Aortic valve vegetations and a portion of the right kidney were cultured quantitatively. The samples were homogenized, serially diluted 10-fold, and inoculated onto SDA. The samples were incubated at 35°C for 48 h prior to colony counting. Colony counts were expressed as the number of CFU per gram of each specimen. Comparisons of the relative fungal densities in kidney and cardiac vegetations between treatment groups were made.
To determine the proportion of the fungal population in kidneys and cardiac vegetations that was resistant to AMB, 500 μl of sample from each tube of the dilution series and the undiluted samples were simultaneously plated onto SDA supplemented with 1.5 μg of AMB/ml and onto drug-free agar. After 72 h of incubation, the colonies were enumerated. The numbers of colonies that grew on AMB-supplemented and drug-free media were compared.
Preliminary studies demonstrated that for quantitative cultures of C. albicans prepared in kidneys and cardiac vegetations collected from noninfected rabbits 24 h after the animals received the fourth dose of any of the antifungal drug regimens described above, the culture results were not affected. Thus, our data were not affected by drug carryover.
Impact of length of initial FLC therapy on the activity of AMB in infected rabbits treated with FLC→AMB+FLC and FLC→AMB regimens.
We determined the effect that the length of initial FLC treatment (in FLC→AMB+FLC and FLC→AMB regimens) had on the duration of induced resistance to AMB, in vivo. In these studies, rabbits infected with C. albicans B311 received FLC for either 1 or 5 days before the drug regimen was switched to AMB+FLC or AMB. After 2, 5, 8, or 13 days of treatment with AMB+FLC or AMB, cardiac vegetations and a portion of the right kidney were collected from sacrificed animals and cultured quantitatively. A comparison of outcomes in these studies was possible because FLC had a fungistatic effect, resulting in similar densities of fungi in cardiac vegetations and in kidneys after 1 to 5 days of FLC as monotherapy (data not shown). Thus, differences in densities of fungi in cardiac vegetations and kidneys in the FLC→AMB+FLC and FLC→AMB regimens would be an expression of the effect of FLC on AMB activity. Treatment with FLC was initiated 24 h after rabbits were inoculated with C. albicans B311. Four animals were used in each group at each time point. Separate groups of animals that received FLC alone or AMB alone served as controls. Differences in fungal densities between groups were assessed for time points after AMB or FLC+AMB regimens were initiated.
Pharmacokinetics of AMB and FLC in sera of rabbits.
The pharmacokinetics of FLC and AMB were determined at steady state for infected rabbits. Treatment started 24 h after fungal inoculation. AMB (1 mg/kg/day) was administered to four rabbits for four doses. Another four rabbits received 42.5 mg of FLC/kg per day for 4 days. Prior to administering the last dose of AMB or FLC, a 24-gauge angiocatheter was inserted into the central artery of each animal to obtain blood samples. One milliliter of blood was collected at 0.25, 0.50, 1.0, 2.0, 2.5, 3, 4, 6, 8, 12, and 24 h after drug administration. After the last samples were collected, the animals were sacrificed with pentobarbital given i.v., followed by induction of bilateral pneumothoraces. The serum was separated from the clot and stored at −70°C.
To determine if drug therapy affected the clearance of FLC or AMB, serial creatinine, blood urea nitrogen (BUN), and trough drug levels in serum were assessed in a subset of animals. Four infected animals that were scheduled to receive FLC, AMB, and AMB+FLC(simult) for 13 days had 1.0 ml of blood drawn immediately before the antifungal drug(s) were administered on days 0, 5, and 13 of therapy for measurements of creatinine and BUN levels in serum. Also, sera were collected from these animals at the last two time points for determination of trough antifungal drug levels. In addition, AMB levels were measured in sera collected from three noninfected rabbits 24 h after they were given a single i.v. dose of AMB (1 mg/kg/day); AMB concentrations measured in these animals served as the comparative controls for AMB levels measured in sera of infected animals. FLC trough levels were assessed in the sera of three additional noninfected rabbits 24 h after they received the fourth dose of FLC. These levels were compared with the concentrations of FLC measured in sera of infected animals.
Antifungal drug assays.
The concentrations of FLC in serum were determined using a well diffusion microbiological assay developed by Jorgensen et al. (11) with modifications described by Madu et al. (18). Candida pseudotropicalis (ATCC 46764) was used as the assay organism. Pour plates of the fungus were prepared using synthetic amino acid medium fungal molten agar and were allowed to solidify at room temperature. Four-millimeter-diameter wells were made in the agar. Twenty-microliter aliquots of sera collected from rabbits or standards were dispensed into wells, kept at 4°C for 1 h, and then incubated overnight for 16 h at 30°C. The FLC standards were prepared in normal rabbit serum. The diameters of inhibition for serum samples and standards were measured with a vernier caliper to the nearest 0.1 mm. Antifungal drug concentrations in samples were calculated using the curves derived from FLC standards. The standard curve was linear for concentrations of FLC between 0.5 and 100 μg/ml of serum. For serum samples that resulted in diameters of inhibition that were greater than those associated with the linear portion of the standard curve, the serum samples were diluted 1:4 with saline and retested. Calculation of the concentration of the drug accounted for this. The intraday and interday coefficients of variation of the microbiological assay were 4.9 and 6.8%, respectively.
Concentrations of AMB in serum were determined using a microbiological assay described by Bannatyne et al. (4) and Granich et al. (9) with slight modifications. Paecilomyces variotii (ATCC 22319) was used as the assay organism. The fungus was grown on SDA slants for 5 to 7 days at 35°C. Mature spores from these cultures were harvested with a sterile cotton-tipped applicator and placed into normal saline. The concentration of spores was determined by hemocytometry. Spores were added to molten synthetic amino acid medium fungal agar to a final concentration of 105 spores/ml. Pour plates of the fungal spores were made and allowed to solidify at room temperature. Ten-millimeter-diameter wells were made in the agar, and 100 μl of sample or standards was added to wells. After incubation for 24 h at 35°C, the diameters of zones of inhibition were measured to the nearest 0.1 mm with a vernier caliper. Antifungal drug concentrations in samples were calculated using the curves derived from AMB standards. The standard curve was linear from concentrations of 0.1 to 20 μg/ml. The intraday and interday coefficients of variation of the biological assay were 4.3 and 6.2%, respectively.
The trough levels of AMB and FLC for animals that received 5 or 13 days of treatment with AMB or FLC were measured using the above-described biological assays. For animals that received AMB and FLC in combination, AMB concentrations in serum were measured using a FLC-resistant C. albicans strain (B59630; gift of F. Odds, Janssen Research Foundation, Beerse, Belgium) in the biological assay. Initial studies demonstrated that serum that contained 0.1 to 4.0 μg of AMB/ml together with 1 to 150 μg of FLC/ml yielded the same diameters of zones of growth inhibition as serum containing only AMB. FLC concentrations in sera of animals that received AMB+FLC were determined using a high-performance liquid chromatography (HPLC) method described elsewhere (18). Previously, we demonstrated that FLC concentrations measured using the bioassay and HPLC were equivalent (18). The intraday and interday coefficients of variation of the HPLC at 1 μg/ml were 4.5 and 5.6%, respectively.
Pharmacokinetic analysis.
Pharmacokinetic analysis of the serum samples for FLC and AMB concentration-time relationships were performed with a nonlinear least-squares regression program, RSTRIP II (Micromath Scientific Software, Salt Lake City, Utah). The most appropriate pharmacokinetic models were determined by using model selection criteria based on Akaike's information criterion (1). The Cmax was defined as the highest concentration of a drug measured in serum after the drug was administered. The trough level was defined as the concentration of a drug in serum that was collected just before the next dose of the drug was administered. To determine the AUC in serum, the trapezoidal method was used for the data obtained from time zero to the last time point.
Statistical analysis.
Comparison of colony counts among the different treatment groups for each treatment length was performed by the Kruskal-Wallis test with multiple comparisons followed by Newman-Keuls analysis using the software program True Epistat version 5.3 (Epistat Services, Richardson, Tex.). A P value of <0.05 was considered significant.
In preliminary studies, we reliably detected ≥50 CFU/g of kidneys. For statistical calculations, a value between 0 and 49 CFU/g was randomly assigned by computer to culture-negative specimens. Pooled vegetations from each animal frequently weighed less than 1 g. Thus, culture-negative vegetations were assigned a CFU-per-gram value equal to the inverse of the weight of the sample. For example, a pooled sample weighing 0.05 g was assigned a value of 20 CFU/g, and one weighing 0.1 g was given a value of 10 CFU/g. A P value of < 0.05 was considered significant.
RESULTS
Effect on susceptibility to AMB of preincubating C. albicans strain B311 with 25 μg of FLC/ml prior to exposure to 1.5 μg of AMB/ml.
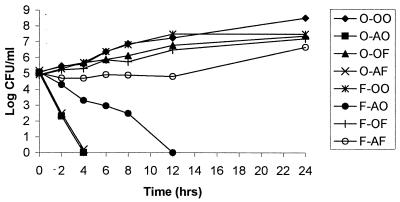
In time-kill studies for C. albicans B311, AMB monotherapy was rapidly fungicidal; the density of yeast in broth was undetectable within 4 h (Fig. 1). Preincubation of this yeast in FLC for 18 h before AMB exposure decreased the rate of killing by AMB (Fig. 1). However, the resistance was transient; after 8 h of incubation with AMB, the number of CFU/ml rapidly fell to undetectable levels.
FIG. 1.
Effect of preincubation of C. albicans strain B311 with 25 μg of FLC/ml for 18 h before the fungus was exposed to 1.5 μg of AMB/ml, 25 μg of FLC/ml, or the combination of FLC and AMB. Transient resistance to AMB was seen in organisms that were preincubated with FLC and subsequently exposed to AMB, but this resistance was persistent for yeast that was subsequently exposed to the combination of FLC and AMB. O-OO, control; O-AO, sequential incubation of yeast in drug-free media followed by AMB; O-OF, preincubation in drug-free media and then FLC; O-AF, sequential incubation of yeast in drug-free media and then in media containing both AMB and FLC; F-OO, preincubation with FLC followed by drug-free media; F-AO, preincubation with FLC followed by AMB; F-OF, sequential incubation of yeast in FLC and then transfer of organisms to fresh media also containing FLC; F-AF, preincubation with FLC followed by treatment with AMB and FLC together.
Incubation of the fungus with both FLC and AMB after the microorganism was preincubated with FLC resulted in a persistence of AMB resistance for the 24-h observation period. Organisms exposed to this sequence of drugs grew at rates that were similar to those for yeast that were incubated with FLC alone (Fig. 1).
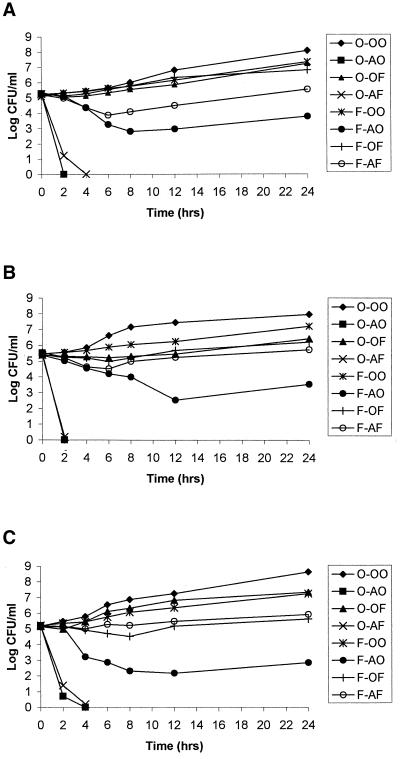
Effect of FLC preincubation on AMB activity for three additional C. albicans strains.
The time-kill results for C. albicans strain B311 were also seen with three other C. albicans strains (Fig. 2). AMB monotherapy was as effective as AMB monotherapy after FLC preincubation. Preincubation of each fungal strain with FLC prior to AMB exposure decreased the activity of AMB. However, in contrast to the transient resistance to AMB seen with C. albicans B311, for C. albicans strains 6, 95-1939, and ATCC 36082, FLC preincubation induced resistance to AMB that lasted for at least 24 h. It is noteworthy that the densities of yeast were always higher when FLC-preincubated yeasts were subsequently exposed to AMB in combination with FLC versus AMB monotherapy. Furthermore, FLC preincubation followed by the combination of FLC plus AMB resulted in fungal densities that were similar to those with FLC monotherapy.
FIG. 2.
Effect of FLC preincubation on the activity of AMB for C. albicans strains 6 (A), ATCC 36082 (B), and 95–1939 (C). The abbreviations are defined in the legend to Fig. 1.
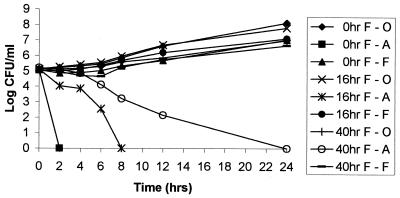
Effect of duration of preincubation with FLC on the duration of induced resistance to AMB.
The duration of induced resistance to AMB seen with C. albicans B311 was dependent on the length of time that the yeast was preincubated with FLC (Fig. 3). Induced resistance to AMB was not seen for organisms that were preincubated with FLC for 2 to 6 h prior to AMB exposure (data not shown). However, 16 h of FLC preincubation resulted in transient resistance that lasted for at least 6 h (Fig. 3). Forty hours of FLC preincubation resulted in resistance to AMB for at least 12 h, and 72 h of azole pretreatment resulted in AMB resistance that lasted for 30 h (data not shown).
FIG. 3.
Impact of the duration of fluconazole preincubation on the activity of AMB for C. albicans B311. Yeast were incubated with 25 μg of FLC/ml for 0, 16, or 40 h and subsequently transferred to media containing 25 μg of FLC (F)/ml, drug-free media, or 1.5 μg of AMB (A)/ml. 0hr F-O, 0hr F-A, and 0hr F-F: yeast incubated in drug-free media (control), AMB, and FLC, respectively, without preincubation in drug-containing media. 16hr F-O, 16hr F-A, 16hr F-F: yeast incubated for 16 h in 25 μg of FLC/ml prior to incubation in drug-free media, AMB, or FLC. 40hr F-O, 40hr F-A, and 40hr F-F: yeast incubated in 25 μg of FLC/ml for 40 h before incubation in drug-free media, AMB, and FLC, respectively.
Preincubation of C. albicans ATCC 36082, strain 6, and 95-1939 with FLC for 16 h resulted in AMB resistance that lasted for at least 42 h (data not shown). Forty hours of FLC pretreatment resulted in AMB resistance that lasted for at least 74 h (data not shown).
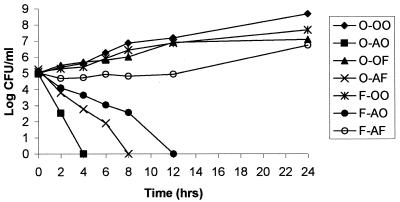
Effect of FLC preincubation on AMB activity in time-kill studies using a lower concentration of AMB.
In the time-kill studies described above, AMB at a concentration of 1.5 μg/ml rapidly killed C. albicans. Thus, we did not observe induced resistance to AMB in C. albicans strains that were exposed simultaneously to 1.5 μg of AMB/ml and 25 μg of FLC/ml. However, when C. albicans B311 was grown in media containing 25 μg of FLC/ml and 0.5 μg of AMB/ml, phenotypic resistance was observed (Fig. 4). Similar results were seen for C. albicans ATCC 36082 (data not shown). However, we could not induce resistance to AMB for C. albicans strains 6 and 95–1939 when these fungal strains were incubated with FLC plus 0.5 μg of AMB/ml (data not shown). Thus, this finding was dependent on the fungal strain studied. These studies suggest that the antagonistic interaction between AMB and FLC may be overcome with higher concentrations of AMB.
FIG. 4.
Effect of FLC preincubation on the activity of low-concentration AMB (0.5 μg/ml) for C. albicans B311. The abbreviations are defined in the legend to Fig. 1.
Pharmacokinetics of FLC and AMB in serum at steady state in infected rabbits.
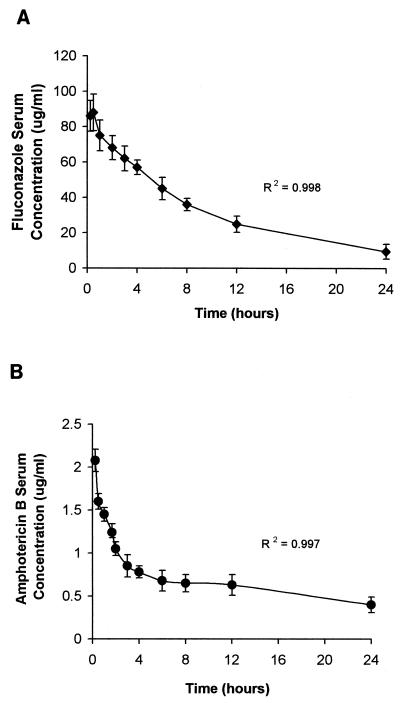
The time-concentration relationship for FLC and AMB at steady state is shown in Fig. 5. The animals were infected with C. albicans. FLC and AMB pharmacokinetics were both best described by a two-compartment model. At steady state, the Cmax and AUC0–24 for FLC in serum were 90.5 ± 10.3 μg/ml and 796.31 ± 26.8 μg · h/ml, respectively. The t1/2β was 8.04 h, and the Cmin (trough) was 11.4 ± 4.2 μg/ml. The Cmax and AUC0–24 for AMB in serum were 2.19 ± 0.36 μg/ml and 16.56 ± 2.79 μg · h/ml, respectively. The t1/2β was 21.4 h, and the Cmin at 24 h was 0.41 ± 0.02 μg/ml.
FIG. 5.
Pharmacokinetics of FLC (42.4 mg/kg per day) (A) and AMB (1.0 mg/kg per day) (B), given i.v., in sera of infected rabbits at steady state.
Serial creatinine and trough AMB and FLC concentrations in sera of rabbits.
Creatinine levels in sera of animals at 0, 5, and 13 days of therapy with AMB, FLC, and AMB+FLC are shown in Table 1. The mean level of creatinine in serum was 0.84 ± 0.05 mg/dl prior to infection. The level of creatinine in serum significantly increased with time only in controls. Creatinine levels in serum tended to increase for rabbits treated with AMB or AMB+FLC by day 13 of therapy (not significant) but were unchanged in animals that received FLC as monotherapy. These results are consistent with those of Chemlal et al. (7), who reported the absence of nephrotoxicity in rabbits treated with AMB (5 mg/kg/day) for 7 days. Similarly, Lee et al. (12) found that the levels of creatinine in serum increased slightly after 13 days of treatment with AMB (1 mg/kg/day). Trough concentrations of FLC and AMB in sera of animals treated with AMB, FLC, and AMB+FLC did not change with time (Table 2).
TABLE 1.
Serial levels of creatinine and BUN in serum measured in infected rabbits treated with various antifungal drug regimens for up to 13 daysa
| Regimen (n)b | Level of creatinine/BUN (mg/dl) in serum
|
||
|---|---|---|---|
| Day 0 | Day 5 | Day 13 | |
| Control (7) | 0.8 ± 0.1/19.4 ± 2.3 | 2.2 ± 0.7/42.5 ± 4.3 | —c |
| FLC (4) | 0.9 ± 0.1/20.3 ± 1.9 | 0.8 ± 0.1/25.4 ± 2.4 | 1.0 ± 0.1/23.4 ± 1.6 |
| AMB (4) | 0.9 ± 0.1/19.1 ± 1.8 | 0.8 ± 0.1/22.7 ± 2.6 | 1.0 ± 0.1/21.2 ± 2.1 |
| AMB+FLC(simult) (4) | 0.8 ± 0.1/19.6 ± 2.3 | 0.9 ± 0.1/24.4 ± 3.0 | 1.0 ± 0.1/23.2 ± 2.3 |
| FLC→AMB+FLC (4) | 0.8 ± 0.1/20.2 ± 3.4 | 0.8 ± 0.1/28.2 ± 2.7 | 0.9 ± 0.1/20.8 ± 1.8 |
| AMB→AMB+FLC (4) | 0.8 ± 0.1/19.6 ± 2.8 | 0.8 ± 0.1/22.1 ± 2.8 | 0.9 ± 0.1/19.3 ± 1.5 |
Results are expressed as means ± 1 standard deviation.
n, number of rabbits in which levels of creatinine and BUN in serum were serially measured over the 13-day study.
—, not done (animals died by day 8 of the study).
TABLE 2.
Trough concentrations of drugs in sera of infected rabbits after 5 and 13 days of treatment with various antifungal drug regimens
| Antifungal regimen | Mean trough AMB/FLC concentrations (μg/ml)a
|
|
|---|---|---|
| Day 5 | Day 13 | |
| FLC | —/11.5 ± 4.3 | —/13.2 ± 5.1 |
| AMB | 0.43 ± 0.04/— | 0.42 ± 0.03/— |
| AMB+FLC(simult) | 0.42 ± 0.02/11.2 ± 4.1 | 0.44 ± 0.02/11.8 ± 4.3 |
| AMB→AMB+FLC | 0.43 ± 0.02/12.7 ± 3.8 | 0.44 ± 0.04/13.0 ± 4.0 |
Values are given as means ± standard deviations. —, not done.
Impact of the sequence of administration of AMB and FLC on the treatment of C. albicans endocarditis.
All untreated animals in the control group died between 6 and 8 days after fungal inoculation. By study design, there were no deaths in any of the groups that received antifungal drug therapy. Death was not a study end point. In our experimental model, differences in fungal densities in various tissue sites over time were used to define interactions between FLC and AMB for regimens in which these drugs were administered concurrently or sequentially.
FLC monotherapy and the FLC→AMB+FLC regimen were fungistatic (Table 3). These regimens demonstrated similar activities at all time points. None of the heart valves were sterilized with these regimens (data not shown). In the latter regimen, FLC completely abrogated the activity of AMB. Although fungal densities associated with FLC+AMB(simult) were similar to those for FLC monotherapy after 5 days of therapy, this regimen sterilized cardiac vegetations in all subjects after 13 days of therapy.
TABLE 3.
Density of C. albicans in cardiac vegetations of rabbits that received 5, 13, and 21 days of antifungal drug therapya
| Antifungal regimen | Density of C. albicansb (no. of animals) for treatment period
|
||
|---|---|---|---|
| 5 days | 13 days | 21 days | |
| Control | 4.63 ± 0.88∗∗ (8) | —c | — |
| FLC | 4.08 ± 0.50∗ (11) | 3.30 ± 0.36∗ (7) | 3.04 ± 0.51∗ (6) |
| FLC→AMB+FLC | 4.28 ± 0.81∗ (8) | 3.52 ± 0.47∗ (7) | 2.56 ± 0.38∗ (5) |
| FLC→AMB | 3.52 ± 0.47∗ (7) | 0# (6) | 0+ (2) |
| FLC+AMB(simult) | 2.91 ± 0.89 (8) | 0# (6) | 0+ (3) |
| AMB→FLC | 2.44 ± 0.63# (7) | 0# (6) | 0+ (2) |
| AMB→AMB+FLC | 0.51 ± 0.37# (8) | 0# (6) | 0+ (2) |
| AMB | 0.43 ± 0.29# (7) | 0# (6) | 0# (5) |
Symbols: ∗, P < 0.02 versus results for AMB; ∗∗, P < 0.008 versus results for AMB; #, P < 0.02 versus results for FLC; +, insufficient number of animals to subject to statistical analysis.
Densities are expressed as log10 CFU ± 1 standard error of the mean.
—, not done.
AMB monotherapy and AMB→AMB+FLC were rapidly fungicidal. After 5 days of treatment, fungal densities in cardiac vegetations were significantly lower for animals that received AMB alone and the AMB→AMB+FLC regimen than for controls (P = 0.008), FLC treatment (P = 0.02), and FLC→AMB+FLC treatment (P = 0.021). At this time point, cardiac vegetations were culture negative in approximately 80% of subjects (data not shown). All valves were culture negative with 13 days of treatment.
The sequential use of AMB followed by FLC (AMB→FLC) rapidly decreased fungal counts early in the treatment course due to the effect of AMB. This was followed by the fungistatic effect of FLC monotherapy. Cardiac vegetations collected after 2 days of therapy were too small to allow fungal densities to be accurately defined by quantitative culture. Therefore, we could not discern whether there was an interaction between AMB and FLC with this drug regimen. However, the sequential use of FLC for 1 day followed by AMB therapy for 4 days (FLC→AMB) clearly decreased the rate of clearance of fungi from cardiac valves. While AMB monotherapy sterilized this site with 5 days of treatment, fungal densities associated with FLC→AMB treatment were similar to those of FLC+AMB(simult) treatment at this time point.
Effect of the sequence of administration of AMB and FLC on the treatment of C. albicans pyelonephritis.
On day 5 of the study, there was no difference in the fungal densities in kidneys of controls and groups that received FLC monotherapy, FLC+AMB(simult), and FLC→AMB+FLC (Table 4). FLC alone and FLC→AMB+FLC demonstrated similar fungistatic activities throughout the study. These regimens sterilize the kidney by day 21 of therapy. In the latter regimen, FLC completely abolished the activity of AMB. FLC+AMB(simult) was as active as FLC alone and FLC→AMB+FLC for the first 5 days of treatment. But this regimen sterilized kidneys by day 13.
TABLE 4.
Density of C. albicans in kidneys of rabbits that received 2, 5, 13, and 21 days of antifungal drug therapya
| Antifungal regimen | Density of C. albicansb (no. of animals) for treatment period
|
|||
|---|---|---|---|---|
| 2 days | 5 days | 13 days | 21 days | |
| Control | 3.97 ± 0.28+ (6) | 4.26 ± 0.26∗∗ (8) | — | — |
| FLC | 3.60 ± 0.34+ (6) | 3.55 ± 0.26∗∗ (12) | 2.34 ± 0.31∗ (7) | 0 (5) |
| FLC→AMB+FLC | 3.52 ± 0.38+ (6) | 3.20 ± 0.17∗∗ (8) | 2.31 ± 0.22∗ (7) | 0 (5) |
| FLC→AMB | 3.55 ± 0.42+ (6) | 0 (6) | 0 (6) | 0 (2) |
| FLC+AMB(simult) | 3.39 ± 0.44+ (6) | 3.29 ± 0.46∗∗ (8) | 0 (6) | 0 (2) |
| AMB→FLC | 2.36 ± 0.41 (6) | 1.86 ± 0.34+ (7) | 0 (6) | 0 (2) |
| AMB→AMB+FLC | 2.14 ± 0.25 (6) | 0 (8) | 0 (6) | 0 (2) |
| AMB | 2.27 ± 0.33 (6) | 0 (8) | 0 (7) | 0 (5) |
Symbols: —, not done; +, P < 0.05 versus results for AMB; ∗, P < 0.001 versus results for AMB; ∗∗, P < 0.0005 versus results for AMB.
Densities are expressed as log10 CFU ± 1 standard error of the mean.
In contrast, 5 days of treatment with AMB, FLC→AMB, and AMB→AMB+FLC rapidly sterilized kidneys [P < 0.0005 versus results with FLC, FLC+AMB(simult), and FLC→AMB+FLC]. FLC+AMB(simult) and AMB→FLC therapies resulted in a slower clearance of organisms from this site.
The fungal densities in kidneys of recipients of AMB→FLC treatment were similar to those for recipients of AMB monotherapy after 2 days of treatment (Table 4). Thus, there was no appreciable interaction between AMB and FLC for the AMB→FLC regimen. This regimen resulted in a rapid decrease in fungal counts with the first 2 days of therapy (due to the effect of AMB), followed by a slower rate of organ sterilization (due to FLC). In contrast, with 2 days of therapy with FLC→AMB (i.e., 1 day of FLC followed by 1 day of AMB), fungal densities in kidneys were 1.2 log10 CFU higher than for AMB→FLC (i.e., 1 day of AMB followed by 1 day of FLC). Fungal counts with the former regimen were similar to those with FLC monotherapy, suggesting an antagonistic effect when FLC was given prior to AMB. However, the antagonistic effect was transient, since kidneys were sterilized after 5 days of therapy. The findings for FLC→AMB were consistent with the induced resistance to AMB that was described in our in vitro time-kill studies.
Decreased susceptibility of Candida to AMB with FLC therapy in vivo: effect of induced resistance to AMB on outcome.
Cardiac vegetations and kidneys collected from animals that received 5 days of the various treatment regimens were quantitatively cultured onto both SDA supplemented with 1.5 μg of AMB/ml and drug-free agar. The numbers of colonies that grew on AMB-supplemented and drug-free agar were compared. For recipients of FLC monotherapy and FLC→AMB+FLC, approximately 33 and 37% of the fungal population, respectively, in both the cardiac vegetations and kidneys demonstrated decreased susceptibility to AMB after 5 days of therapy. After 13 and 21 days of therapy, 14 to 18% of the fungal population demonstrated decreased AMB susceptibility with these regimens. For animals that received FLC→AMB, approximately 27% of colonies in kidneys demonstrated induced resistance to AMB on day 2 of therapy; 17% of organisms isolated from cardiac vegetations demonstrated induced resistance to AMB after 5 days of therapy. For recipients of AMB+FLC(simult), approximately 8% of the fungal population demonstrated decreased AMB susceptibility after 5 days of treatment Fungi cultured from animals treated with AMB as monotherapy, AMB→FLC, AMB→AMB+FLC, and controls remained susceptible to AMB.
Decreased susceptibilities to AMB were detected only when the tissue samples were directly plated onto AMB-containing agar. We could not detect a change in susceptibility to AMB in fungal isolates that were originally plated onto drug-free agar and then recultured onto AMB-supplemented agar. The induced resistance to AMB that was identified in vivo was well predicted by the in vitro time-kill studies.
Effect of varying the duration of initial FLC treatment in FLC→AMB+FLC and FLC→AMB regimens on the duration of induced AMB resistance in C. albicans.
For animals that received only FLC, fungal densities in cardiac vegetations and kidneys were similar at all time points (Table 5). This made it possible to examine the effect that different durations of initial therapy with FLC had on the activity of AMB in regimens in which FLC is subsequently switched to AMB+FLC or AMB alone.
TABLE 5.
Effect of using FLC treatment for 1 or 5 days prior to switching to AMB+FLC or AMB on C. albicans density in cardiac vegetations and kidneys
| Antifungal regimen for: |
C. albicans densitya for no. of days of therapy after switch from FLC treatmentb
|
|||
|---|---|---|---|---|
| 2 days | 5 days | 8 days | 13 days | |
| Cardiac vegetations | ||||
| FLC(1 day)→FLC | 4.43 ± 0.47 | 4.19 ± 0.38 | 3.92 ± 0.35 | |
| FLC(1 day)→AMB+FLC | 4.31 ± 0.21 | 4.22 ± 0.47 | 4.06 ± 0.54 | |
| FLC(5 days)→AMB+FLC | 4.28 ± 0.42 | 4.16 ± 0.33 | 3.83 ± 0.48 | |
| FLC(1 day)→AMB | 2.33 ± 0.52 | 0.84 ± 0.30#+ | 0# | |
| FLC(5 days)→AMB | 3.61 ± 0.44 | 3.15 ± 0.29 | 0# | |
| AMB alone | 0.41 ± 0.36#+ | 0#+ | 0# | |
| Kidneys | ||||
| FLC(1 day)→FLC | 3.84 ± 0.35 | 3.90 ± 0.22 | 3.36 ± 0.39 | 2.73 ± 0.54 |
| FLC(1 day)→AMB+FLC | 3.66 ± 0.47 | 3.41 ± 0.29 | 3.25 ± 0.47 | 2.46 ± 0.38 |
| FLC(5 days)→AMB+FLC | 3.88 ± 0.50 | 3.60 ± 0.38 | 4.16 ± 0.33 | 2.59 ± 0.47 |
| FLC(1 day)→AMB | 3.54 ± 0.33 | 1.06 ± 0.30#+ | 0# | 0# |
| FLC(5 days)→AMB | 3.47 ± 0.43 | 2.88 ± 0.25# | 1.64 ± 0.33# | 0# |
| AMB alone | 1.82 ± 0.27#+ | 0#+ | 0# | 0# |
Densities are expressed as log10 CFU ± 1 standard error of the mean.
For FLC(1 day)→AMB, FLC(5 days)→AMB, FLC(1 day)→AMB+FLC, and FLC(5 days)→AMB+FLC, treatment days were standardized to the number of days that treatment was given after FLC was switched to AMB alone or AMB+FLC. For animals that received only AMB or FLC, treatment days are referenced to when antifungal treatment was begun. Symbols: #, P < 0.05 versus results for FLC; +, P < 0.05 versus results for FLC(5 days)→AMB.
AMB was rapidly fungicidal in both infection sites. Using FLC for 1 day and 5 days prior to starting AMB+FLC resulted in similar outcomes. Both regimens demonstrated fungistatic activities that were similar to those with FLC monotherapy. In animals that received FLC for 1 day prior to changing therapy to AMB [FLC(1 day)→AMB], the rates of clearance of fungi from cardiac vegetations and kidneys were slower than for AMB monotherapy. In animals that received 5 days of FLC therapy [FLC(5 days)→AMB], additional days of AMB therapy were required before these sites were cleared of microorganisms. The prolonged times to clearance of fungi from these cardiac vegetations and kidneys were due to the antagonistic effect of FLC on AMB. These in vivo findings were accurately predicted by our in vitro time-kill studies.
DISCUSSION
A number of in vitro studies report antagonism between FLC and AMB when their interaction was assessed using checkerboard and time-kill methods (13, 21; Banerjee et al., Abstr. 97th Gen. Meet. Am. Soc. Microbiol., 1997). Lewis et al. (13) reported antagonism between FLC and AMB for C. albicans isolates that were sequentially exposed to FLC and then to AMB. Further, Vazquez et al. (28) found that the sequential exposure of C. albicans to FLC followed by AMB caused the fungus to be transiently resistant to AMB. This induced resistance lasted for up to 6 h after FLC was removed from the culture medium (28). These investigators found that preincubation of C. albicans with FLC for as little as 8 h induced resistance to AMB.
Our study confirmed and expanded on the in vitro observations of Vazquez et al. In vitro, we found that phenotypic resistance to AMB was induced in each of four C. albicans strains that were preincubated with FLC for 18 h. The induced resistance to AMB lasted from 8 to >24 h, depending on the Candida isolate evaluated. However, AMB resistance was more profound and persistent in C. albicans isolates that were first incubated with FLC and then exposed to AMB in combination with FLC. Under these conditions, the fungi demonstrated fungistatic growth kinetics similar to that seen with yeast that were incubated with FLC alone. In this regimen, the activity of AMB was completely abolished. Similar to the findings of Vazquez et al., we found that a minimum of 8 h of FLC preincubation was needed to induce resistance to AMB.
Our in vitro observations accurately predicted the outcome in our rabbit model of C. albicans endocarditis and pyelonephritis. In these studies, we evaluated the impact on outcome of the order of initiation of FLC and AMB when these drugs are used sequentially as single agents or as combination therapy. In both cardiac vegetations and kidneys, AMB monotherapy was rapidly fungicidal, while FLC treatment was fungistatic. AMB+FLC(simult) treatment resulted in a kill rate that was between those seen with the individual drugs. AMB→AMB+FLC treatment rapidly sterilized these sites, indicating that the initiation of AMB prior to using this drug in combination with FLC preserved the activity of the most active agent. For FLC→AMB treatment, there was a decrease in the rate of clearance of fungi from cardiac vegetations and kidneys associated with the AMB component of the regimen that was dependent of the duration of FLC that was used as initial therapy. As predicted by our in vitro studies, initiation of FLC treatment prior to using FLC in combination with AMB resulted in fungal densities in tissues that were similar to the fungistatic activity of FLC monotherapy. In this regimen, preceding AMB+FLC with 1 day of FLC treatment was sufficient to abolish the activity of AMB.
In vivo efficacy was predicted by the proportion of the fungal population that demonstrated phenotypic resistance to AMB during therapy. The least effective drug combination, FLC→AMB+FLC, was associated with the highest proportion of AMB resistance in the population. AMB+FLC(simult) and FLC→AMB regimens were associated with smaller populations of AMB-resistant C. albicans and showed greater efficacy than FLC→AMB+FLC. Finally, resistance to AMB was not seen in recipients of AMB monotherapy and AMB→AMB+FLC, the most rapidly fungicidal regimens. Decreased susceptibility to AMB was identified only when tissue specimens were directly plated onto AMB-supplemented agar. If fungi in tissue homogenates were first isolated on drug-free media and then plated onto AMB-supplemented media, decreased susceptibility to AMB was not detected. The mechanism for this observation is uncertain.
The results of our current study are consistent with those of our earlier studies. In a rabbit model of endocarditis using C. albicans strain ATCC 36082, we found that the simultaneous initiation of FLC treatment and AMB treatment reduced the clearance of the fungus from cardiac vegetations compared with the most active regimen, AMB monotherapy (17). Also, combination therapy slowed the clearance of fungi from the kidneys for at least 14 days of treatment, compared with therapy with AMB alone (17). Similarly, in a mouse model of systemic candidiasis, the simultaneous initiation of AMB and FLC as combination therapy decreased the efficacy of AMB, in terms of both fungal loads in kidneys and survival of the infected host (16). Decreased activity of AMB was seen with combination therapy for mice infected with C. albicans strains, with FLC MICs up to 256 μg/ml. The activity of AMB was not affected when combination therapy was given to mice infected with a C. albicans strain that was highly resistant to FLC (FLC MIC, 512 μg/ml).
In contrast to our results, Sugar et al. (26) reported that the interaction between AMB and FLC was additive in their murine model of systemic candidiasis when these drugs were initiated simultaneously. However, in their model the 90 to 100% survival rates observed for animals that received AMB monotherapy placed the survivorship on the top of the dose-response curve. This makes it difficult to observe an antagonistic interaction between AMB and FLC. Also, the results of the quantitative culture of kidneys were below the sensitivity of their assay for all the treatment groups examined. Thus, it is difficult to make any conclusions about the interaction between AMB and FLC from those studies. In another trial (26), these investigators reported a 40% reduction in survival rates, from 62.5% for AMB alone to 37.5% for FLC plus AMB. The difference was not statistically significant, although the number of animals evaluated was small.
Sanati et al. (24) evaluated the interaction between FLC and AMB in a neutropenic murine model of systemic candidiasis. The drugs were started concurrently. Eight days after fungal inoculation, the survival rates were approximately 15, 43, 60, and 72% for mice that received a placebo, FLC, FLC+AMB, and AMB, respectively. The differences in survival rates between groups did not reach statistical significance; however, the possibility of a type II error could not be excluded (24).
In a rabbit model of C. albicans endocarditis, Sanati et al. (24) reported that the interaction between FLC and AMB was indifferent when these drugs were initiated simultaneously. In contrast, using the same fungal strain, Louie et al. (17) noted the combination of FLC+AMB to be antagonistic. Both investigators found the fungal densities in cardiac tissues of FLC+AMB recipients to lie between those found with the two monotherapies. The discrepancy in results may be explained by the fact that Louie et al. observed a larger difference between the fungal densities in the cardiac vegetations of the FLC and AMB monotherapy groups than Sanati (a 5-log difference versus a 2-log difference, respectively). Thus, Louie's model was more sensitive than Sanati's for identifying statistically significant differences between the FLC+AMB treatment group and the other treatment groups. Of note, Louie et al. (17) also reported antagonism between FLC and AMB in the clearance of C. albicans from the kidneys of the same infected rabbits. With 5 and 14 days of therapy, the FLC+AMB regimen was significantly less effective than AMB but more active than FLC. However, by day 21 of therapy, the FLC, AMB, and FLC+AMB regimens all sterilized this site. Thus, antagonism in the kidney was manifested by a delay in the sterilization of this organ.
We believe that it would be inappropriate to dismiss the potential adverse effect of FLC and AMB antagonism on the outcome based on the fact that there were no differences in survival rates between treatment arms in the current study. Importantly, our rabbit infection model was specifically designed to identify differences in fungal densities in tissues that may occur in response to various antifungal drug regimens and not mortality. By design, there were no mortalities in even the least active of the regimens. Thus far, all published in vivo studies that evaluated the activity between FLC and AMB have not documented outcomes to be less than that described for FLC monotherapy. However, in a murine model of systemic candidiasis in which survival rates with AMB and FLC monotherapies were 60 and 0%, respectively, there were no survivors in the group that received AMB and FLC in combination (16). Furthermore, with a mouse model of systemic C. albicans infection, Sugar et al. (27) reported that mortality in mice treated with itraconazole in combination with AMB was 100%, while the mortality rates associated with AMB alone and itraconazole alone were 10 and 80%, respectively. Similar mortality rates were seen regardless of whether AMB and itraconazole were administered concurrently or sequentially.
In summary, we found no negative consequences of switching AMB to FLC during the treatment of deep-seated C. albicans infections. This order of events is commonly seen in the clinic, where AMB empiric therapy is switched to FLC therapy after a deep-seated fungal infection is found to be due to a species that is usually susceptible to FLC. However, our in vitro and in vivo studies and those reported by others suggest that there is no therapeutic advantage in using FLC and AMB, as in AMB→AMB+FLC, FLC→AMB+FLC, or AMB+FLC(simult) regimens, in the treatment of invasive C. albicans infections. Combination therapies using AMB and FLC for Candida infections may result in avoidable toxicity and additional cost without added benefit relative to antifungal drug monotherapy.
ACKNOWLEDGMENT
This project was supported by an educational grant provided by Pfizer, Inc., New York, N.Y.
REFERENCES
- 1.Akaike H. A new look at the statistical model identification. IEEE Trans Automated Control. 1974;19:716–723. [Google Scholar]
- 2.Anaissie E J, Darouiche R O, Abi-Said D, Uzun O, Mera J, Gentry L O, Williams T, Kontoyiannis D P, Karl C L, Bodey G P. Management of invasive candidal infections: results of a prospective, randomized, multicenter study of fluconazole versus amphotericin B and review of the literature. Clin Infect Dis. 1996;23:964–972. doi: 10.1093/clinids/23.5.964. [DOI] [PubMed] [Google Scholar]
- 3.Ayestaran A, Lopez R M, Montoro J B, Estibalez A, Pou L, Julia A, Lopez A, Pascual B. Pharmacokinetics of conventional formulation versus fat emulsion formulation of amphotericin B in a group of patients with neutropenia. Antimicrob Agents Chemother. 1996;40:609–612. doi: 10.1128/aac.40.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bannatyne R M, Cheung R. Discrepant results of amphotericin B assays on fresh versus frozen serum samples. Antimicrob Agents Chemother. 1997;12:550. doi: 10.1128/aac.12.4.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beck-Sague C M, Jarvis W R the National Nosocomial Infections Surveillance System. Secular trends in the epidemiology of nosocomial fungal infections in the United States, 1980-1990. J Infect Dis. 1993;167:1247–1251. doi: 10.1093/infdis/167.5.1247. [DOI] [PubMed] [Google Scholar]
- 6.Chavanet P Y, Garry I, Charlier N, Caillot C, Kisterman J P, D'Athis M, Portier H. Trial of glucose versus fat emulsion preparation of amphotericin for use in HIV infected patients with candidiasis. Br Med J. 1992;305:921–925. doi: 10.1136/bmj.305.6859.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chemlal K, Saint-Julien L, Joly V, Farinotti R, Seta N, Yeni P, Carbon C. Comparison of fluconazole and amphotericin B for treatment of experimental Candida albicans endocarditis in rabbits. Antimicrob Agents Chemother. 1996;40:263–266. doi: 10.1128/aac.40.1.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Durack D T, Beeson P B, Petersdorf R G. Experimental bacterial endocarditis. III. Production and progress of the disease in rabbits. Br J Exp Pathol. 1973;54:142–151. [PMC free article] [PubMed] [Google Scholar]
- 9.Granich G G, Kobayashi G S, Krogstad D J. Sensitive high-pressure liquid chromatographic assay for amphotericin B which incorporates an internal standard. Antimicrob Agents Chemother. 1986;29:584–588. doi: 10.1128/aac.29.4.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heinemann V, Bosse D, Jehn U, Kahny B, Wachholz K, Debus A, Scholz P, Kolb H-J, Wilmanns W. Pharmacokinetics of liposomal amphotericin B (AMBisome) in critically ill patients. Antimicrob Agents Chemother. 1997;41:1275–1280. doi: 10.1128/aac.41.6.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jorgensen J H, Alexander G A, Graybill J R, Drutz D J. Sensitive bioassay for ketoconazole in serum and cerebrospinal fluid. Antimicrob Agents Chemother. 1981;20:59–62. doi: 10.1128/aac.20.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee J W, Amantea M A, Francis P A, Navarro E E, Bacher J, Pizzo P A, Walsh T J. Pharmacokinetics and safety of a unilamellar liposomal formulation of amphotericin B (AMBisome) in rabbits. Antimicrob Agents Chemother. 1994;38:713–718. doi: 10.1128/aac.38.4.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lewis R E, Lund B C, Klepser M E, Ernst E J, Pfaller M A. Assessment of antifungal activities of fluconazole and amphotericin B administered alone and in combination against Candida albicans by using a dynamic in vitro mycotic infection model. Antimicrob Agents Chemother. 1998;42:1382–1386. doi: 10.1128/aac.42.6.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Louie A, Drusano G L, Banerjee P, Liu Q-F, Liu W, Kaw P, Shayegani M, Taber H, Miller M H. Pharmacodynamics of fluconazole in a murine model of systemic candidiasis. Antimicrob Agents Chemother. 1998;42:1105–1109. doi: 10.1128/aac.42.5.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Louie A, Liu Q-F, Drusano G L, Liu W, Mayers M, Anaissie E, Miller M H. Pharmacokinetic studies of fluconazole in rabbits characterizing doses which achieve peak levels in serum and area under the concentration-time curve values which mimic those of high-dose fluconazole in humans. Antimicrob Agents Chemother. 1998;42:1512–1514. doi: 10.1128/aac.42.6.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Louie A, Banerjee P, Drusano G L, Shayegani M, Miller M H. Interaction between fluconazole and amphotericin B in mice with systemic infection due to fluconazole-susceptible and -resistant strains of Candida albicans. Antimicrob Agents Chemother. 1999;43:2841–2847. doi: 10.1128/aac.43.12.2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Louie A, Liu W, Miller D A, Sucke A C, Liu Q-F, Drusano G L, Mayers M, Miller M H. Efficacies of high-dose fluconazole plus amphotericin B and high-dose fluconazole plus 5-fluorocytosine versus amphotericin B, fluconazole, and 5-fluorocytosine monotherapies in treatment of experimental endocarditis, endophthalmitis, and pyelonephritis due to Candida albicans. Animicrob Agents Chemother. 1999;43:2831–2840. doi: 10.1128/aac.43.12.2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Madu A, Cioffe C, Mian U, Burroughs M, Tuomanen E, Mayers M, Schwartz E, Miller M. Pharmacokinetics of fluconazole in cerebrospinal fluid and serum of rabbits: validation of an animal model used to measure drug concentrations in cerebrospinal fluid. Antimicrob Agents Chemother. 1994;38:2111–2115. doi: 10.1128/aac.38.9.2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin E, Maier F, Bhakdi S. Antagonistic effects of fluconazole and 5-fluorocytosine on candidacidal action of amphotericin B in human serum. Antimicrob Agents Chemother. 1994;38:1331–1338. doi: 10.1128/aac.38.6.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing for yeast; tentative standard. M27-T. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1995. [Google Scholar]
- 21.Petrou M A, Rogers T R. Interactions in vitro between polyenes and imidazoles against yeast. J Antimicrob Chemother. 1991;27:491–506. doi: 10.1093/jac/27.4.491. [DOI] [PubMed] [Google Scholar]
- 22.Rentz R M, Halpern M T, Bowden R. The impact of candidemia on length of hospital stay, outcome, and overall cost of illness. Clin Infect Dis. 1998;27:781–789. doi: 10.1086/514955. [DOI] [PubMed] [Google Scholar]
- 23.Rex J H, Bennett J E, Sugar A M, Pappas P G, Van der Horst C M, Edwards J E, Washburn R G, Scheld W M, Karchmer A W, Dine A P, Levenstein M J, Webb C D the Candidemia Study Group; the NIAID Mycoses Study Group. A randomized trial comparing fluconazole with amphotericin B for the treatment of candidemia in patients without neutropenia. N Engl J Med. 1994;331:1325–1330. doi: 10.1056/NEJM199411173312001. [DOI] [PubMed] [Google Scholar]
- 24.Sanati H, Ramos C F, Bayer A S, Ghannoum M A. Combination therapy with amphotericin B and fluconazole against invasive candidiasis in neutropenic-mouse and infective-endocarditis rabbit models. Antimicrob Agents Chemother. 1997;41:1345–1348. doi: 10.1128/aac.41.6.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Solomkin J S, Flohr A M, Simmons R L. Indications for therapy for fungemia in postoperative patients. Arch Surg. 1982;117:1272–1275. doi: 10.1001/archsurg.1982.01380340008003. [DOI] [PubMed] [Google Scholar]
- 26.Sugar A M, Hitchcock C A, Troke P F, Picard M. Combination therapy of murine invasive candidiasis with fluconazole and amphotericin B. Antimicrob Agents Chemother. 1995;39:598–601. doi: 10.1128/AAC.39.3.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sugar A M, Liu X P. Interactions between itraconazole with amphotericin B in the treatment of murine invasive candidiasis. J Infect Dis. 1998;177:1660–1663. doi: 10.1086/515319. [DOI] [PubMed] [Google Scholar]
- 28.Vazquez J A, Argonoza M T, Vaishampayan J K, Akins R A. In vitro interaction between amphotericin B and azoles in Candida albicans. Antimicrob Agents Chemother. 1996;40:2511–2516. doi: 10.1128/aac.40.11.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walsh T J, Bacher J, Pizzo P A. Chronic silastic central venous catheterization for induction, maintenance and support of persistent granulocytopenia in rabbits. Lab Anim Sci. 1988;38:467–471. [PubMed] [Google Scholar]
- 30.Wey S B, Mori M, Pfaller M A, Woolson R F, Wenzel R P. Hospital-acquired candidemia: the attributable mortality and excess length of stay. Arch Intern Med. 1988;148:2642–2645. doi: 10.1001/archinte.148.12.2642. [DOI] [PubMed] [Google Scholar]
- 31.Witt M D, Bayer A S. Comparison of fluconazole and amphotericin B for prevention and treatment of experimental Candida endocarditis. Antimicrob Agents Chemother. 1991;35:2481–2485. doi: 10.1128/aac.35.12.2481. [DOI] [PMC free article] [PubMed] [Google Scholar]