Abstract
Physical inactivity and sedentary behavior (SB) have attracted growing attention globally since they relate to noninfectious chronic diseases (NCDs) and could further result in the loss of life. This systematic literature review aimed to identify existing evidence on the efficacy of mobile health (mHealth) technology in inducing physical activity and reducing sedentary behavior for physically inactive people. Studies were included if they used a smartphone app in an intervention to improve physical activity and/or sedentary behavior for physically inactive individuals. Interventions could be stand-alone interventions or multi-component interventions, including an app as one of several intervention components. A total of nine studies were included, and all were randomized controlled trials. Two studies involved interventions delivered solely via a mobile application (stand-alone intervention) and seven studies involved interventions that used apps and other intervention strategies (multi-component intervention). Methodological quality was assessed, and the overall quality of the studies was ensured. The pooled data favored intervention in improving physical activity and reducing sedentary behavior. This review provided evidence that mobile health intervention improved physical activity and reduced sedentary behavior among inactive individuals. More beneficial effects can be guaranteed when interventions include multiple components. Further studies that maintain the effectiveness of such interventions are required to maximize user engagement and intervention efficacy.
Keywords: physically inactive people, meta-analysis, mobile health, physical activity, sedentary behavior
1. Introduction
Regular physical activity (PA) could promote individual physical and mental health [1]. The World Health Organization (WHO) and the American College of Sports Medicine (ACSM) advocate that “adults aged 18–64 should do at least 150 min of moderate-intensity aerobic PA or do at least 75 min of vigorous-intensity aerobic PA per week or an equivalent combination of both” [2]. However, nearly 58% of individuals have not achieved the recommended amount of activity (i.e., 2.5 h per week) [3], and thus, they are considered physically inactive. In fact, physical inactivity has been deemed as the fourth driver of global mortality and the chief cause of non-communicable diseases (NCDs) [4]. Meanwhile, meta-analyses show that even those who are meeting these recommendations may still be sitting for a long while, which could lead to potential health risks associated with sedentary behavior (SB) [5,6]. Therefore, the two growingly interrelated themes, “sedentary and inactive”, have become a global health challenge faced by humans [7].
Health interventions based on the behavior analysis present the potentials to increase daily physical activity levels [8,9]. Therefore, it is critical to explore preventive interventions that the general population could easily follow. However, traditional face-to-face interventions in public health may not achieve such a purpose [10,11,12,13,14]. This is because people may have limited access due to internal/personal (e.g., lack of time or motivation) and external obstacles (e.g., commuting conditions and the expense of activities) [15,16]. With the development of digital technologies, the concept of mobile health (mHealth) interventions has emerged, which refers to medical and public health practice supported by mobile phones, patient-monitoring devices, Personal Digit Assistant (PDA), and other wireless devices [17].
As reported, mobile health applications have been broadly employed to gather and analyze health-related data and accordingly to design interventions that could help facilitate positive behavior changes for healthy purposes [18]. Particularly, mHealth could target intervening in physical inactivity and sedentary behaviors that require durative persistence since mHealth can offer interactive technologies (e.g., activity reminders and peer support) capable of increasing users’ adherence to the interventions [19,20]. Meanwhile, the mHealth interventions, whose designs tend to be user-friendly [9], can be easily delivered anywhere and anytime, benefiting from the broad popularity of mobile devices [21]. Thus, mHealth are expected to promote the public health care universally.
The domain of mHealth has attracted research interests from multiple disciplines such as sociology and psychology. Though several systematic reviews on mHealth interventions indicate that it is efficacious for average people in promoting behavioral conversion and improving well-being [22,23], there are still some limitations or weaknesses. On the one hand, most of the systemic reviews in the field of mHealth interventions are organized by just focusing on the mHealth application [22,23,24,25] or smartphones [26,27], mHealth interventions based on PDA or other wireless devices tend to be ignored by the former studies. On the other hand, some positive impacts of the mHealth interventions on the PA and the SB are reported [23,24,25,26,27], while the specialized discussion as well as the systematic reviews targeting the representative population—the inactive group—in the trend of the global lack of PA and the popularity of SB is thus far insufficient. These identified research problems motivate this study to target the overall formats of mHealth interventions and the specific inactive population.
Hence, this study aims to systematically review and investigate to what extent mHealth interventions can help to improve physical activity and mitigate sedentary behavior among the inactive group. It synthesizes the outcomes reported in the included randomized controlled trials (RCTs) based on the comprehension of the problems that existed in the related systematic reviews and meta-analyses on health management and support by means of the mHealth. Through the meta-analysis, this paper explores the change in the duration of PA and SB brought by mHealth interventions among the inactive group and thus proposes measures such as selecting the proper exercise plans in mHealth applications to promote individual physical activity and reduce sedentary behavior.
This paper could add value to present knowledge. Our research indicates that the inactive group tends to exhibit its unique and non-linear nature in terms of the duration of PA and SB after the intervention. In other words, there is a sharp response at the initial stage and a successively irregular declination afterward. The focus on inactive individuals could enhance the specificity of current studies on mHealth interventions. As a result, more reliable suggestions for the improvement in mHealth interventions particularly targeting the inactive group could be proposed. We could thus expect to observe some beneficial changes such as being less sedentary and more physically active among inactive individuals by following our research results and arousing their cognition of seeking effective support from mHealth interventions.
2. Method
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Guidelines and Cochrane Handbook for Systematic Reviews of Interventions were used as methodological templates for this review [28].
2.1. Information Sources and Search Strategy
Literature searches were conducted in March 2022. Five databases were searched, including Web of Science, CINAHL, Scopus, PsychInfo, and PubMed. The sources were limited to peer-reviewed journal articles published between 2006 and 2022. It was considered unlikely that mobile health interventions were included before 2006 when smartphones were introduced. To organize and construct the search string(s), we follow the PICO approach [29]: population, intervention, comparison, and outcomes of interest (see Table 1). Two concepts were selected to develop the search query (Table 2). Searched digital libraries and the corresponding search string(s) used are shown in Table 3. Searches followed the PRISMA statement targeting the following keywords in the title or abstract.
Table 1.
PICO for research questions.
| Concept | Description of Detail |
|---|---|
| Population | Inactive Population |
| Intervention | Mobile health intervention |
| Comparison | Control group without the mobile health intervention |
| Outcomes | Effects of mobile health intervention on physical activity and sedentary behavior |
Table 2.
Major search terms.
| Concept | Description of Detail |
|---|---|
| Intervention | Application OR App OR Smartphone OR Smart Phone |
| Outcomes | Sedentary OR sedentary behavior OR sedentary behavior OR sitting OR screen time OR inactive OR inactivity |
Table 3.
List of search strings in different digital library.
| Digital Library. | Query String | Scope | Limitation |
|---|---|---|---|
| Web of science | AB = (application OR app OR smartphone OR smartphone OR tablet) AND AB = (sedentary OR sedentary behavior OR sedentary behavior OR sitting OR screen time OR inactive OR inactivity) | Abstract | Limited to journals, English, 2006–2022 |
| CINAHL | AB = (application OR app OR smartphone OR smartphone OR tablet) AND AB = (sedentary OR sedentary behavior OR sedentary behavior OR sitting OR screen time OR inactive OR inactivity) | Abstract | Limited to journals, English, 2006–2022 |
| Scopus | (TITLE-ABS-KEY (application OR app OR smartphone OR smart AND phone OR tablet) AND TITLE-ABS-KEY (sedentary OR sedentary AND behavior OR sedentary AND behavior OR sitting OR screen AND time OR inactive OR inactivity)) AND PUBYEAR > 2006 AND (LIMIT-TO (PUBSTAGE, “final”)) AND (LIMIT-TO (DOCTYPE, “ar”) OR LIMIT-TO (DOCTYPE, “cp”)) AND (LIMIT-TO (LANGUAGE, “English”)) | Title, Abstract and keywords | Limited to journals, English, publication stage: final, 2006–2022 |
| PsychInfo | (application OR app OR smartphone OR smartphone OR tablet).tw. AND (sedentary OR sedentary behavior OR sedentary behavior OR sitting OR screen time OR inactive OR inactivity).tw. | Title and Abstract | Limited to journals, English, human, 2006–2022 |
| PubMed | (application [Title/Abstract] OR app [Title/Abstract] OR smartphone [Title/Abstract] OR smartphone [Title/Abstract] OR tablet [Title/Abstract]) AND (sedentary [Title/Abstract] OR sedentary behavior [Title/Abstract] OR sedentary behavior [Title/Abstract] OR sitting [Title/Abstract] OR screen time [Title/Abstract] OR inactive [Title/Abstract] OR inactivity [Title/Abstract]) | Title and Abstract | Limited to journals, English, human, 2006–2022 |
The search results were imported into Mendeley bibliographic software. Duplicate studies were removed. The titles and abstracts of all identified studies were screened to identify potentially relevant papers. Studies that did not meet the inclusion criteria and those whose titles/abstracts were obviously not related to the topic of interest were excluded from this review. To ensure that there was no potential for missing primary research, we also searched for those in the automatic search. Backward snowballing was conducted after screening the abstract and title. Then, full-text papers of potentially relevant studies were retrieved and assessed for eligibility by two reviewers. Where uncertainties arose regarding study inclusion, a consensus was achieved through discussion with the third reviewer.
2.2. Inclusion and Exclusion Criteria
Identified studies were screened for eligibility if they met the following inclusion criteria: (1) inactive population, which is the general population without disease that cannot meet the recommended standard of PA or tends to be sedentary daily; (2) published RCTs with mobile health intervention influencing at least one of the following lifestyle behaviors: PA, SB; (3) the mobile health intervention could be a stand-alone intervention or a multi-component intervention; and (4) the report of a study written by the researchers who actually performed the study.
Exclusion criteria leading to studies being classified as ineligible were: (1) age <18 years and age >64; (2) non-experimental study designs; (3) mobile technology was used to provide information versus used for self-management; and (4) clinically diagnosed populations with the exception of those who are overweight or obese.
2.3. Data Extraction and Risk of Bias of Included Studies
The following data were independently extracted from each paper using a standardized form: author, study design, duration, participant characteristics, intervention description, dependent variables, outcome measures, and comparison groups—similar to those used in other systematic reviews [22,30,31]. Two reviewers independently extracted data from each included study. Both reviewers one and two agreed on the data extraction in over 70% of the studies. Disagreement was easily resolved by discussion and consensus with a third reviewer.
The risk of bias assessment of the included studies was conducted by two reviewers using the Cochrane Collaboration’s risk of bias tool [32]. It considers bias originating from the following domains: (i) Random sequence generation (selection bias); (ii) allocation concealment (selection bias); (iii) blinding of participants and personal (performance bias); (iv) blinding of outcome assessment (detection bias); (v) incomplete outcome data (attrition data); (vi) selective reporting (reporting bias); and (vii) other bias. Two reviewers independently marked risk of bias level for each domain. Where inconsistency arose regarding the risk of bias, a consensus was achieved through discussion with the third reviewer.
Studies were considered low-risk of bias for blinding of the outcome assessment (domain (iv)) if the objective measures of PA and SB were used for data collection. Instead, the risk of bias in the study outcome assessment was considered high if the data collection on the outcome is based on subjective measures. For the selective reporting (domain (vi)), studies were judged to be low-risk if there were published protocol papers and the study followed the plan. In the absence of publicly available protocol papers, studies reporting all outcomes mentioned in the methodology are considered low-risk bias of selective reporting.
2.4. Study Quality Assessment
Twenty-five-point criteria adapted from the CONSORT checklists, which are applicable to control trail and other study designs, were used to assess the quality of the included studies [30]. This approach has been used in other reviews [22,30]. Each criterion from the CONSORT checklists was scored as 1 (fulfilled), 0.5 (not all sub-items making up the criterion were fulfilled), 0 (not fulfilled or unclear), or not applicable to the study design. A percentage of fulfilled criteria can be calculated by dividing the obtained study quality score by the highest attainable score that has been used in other reviews [22,30]. Not applicable criteria were discounted from the attainable study quality score. The rate of the included studies was categorized into high (>66.7%), mid (50–66.6%) or low (<50%) study quality. Reviewers independently scored a random half of RCTs. Then, reviewers cross-assessed 4 of 9 RCTs (44%) and reached consensus on disagreements. Reviewers reassessed the remaining studies by applying the consensus criteria.
2.5. Strategy for Data Synthesis and Analysis
Eight studies [21,33,34,35,36,37,38] had continuous outcomes for measures of PA across the same scale, allowing meta-analysis of mean differences (MD). The units of the PA data reported in the study were mostly minutes per day or week of varying intensity. If the study provided data on the amount of time spent on physical activity per week, these were translated into minutes per day (e.g., 420 min/week = 60 min/day), which was also used in other reviews [31,39]. Studies in which the information was unavailable or reported units could not be converted to the scale of min/day were not included in the meta-analyses [40]. If there is more than one measure of PA, objective data takes precedence over subjective data. If there is more than one objective measure of PA, preference will be given to the measure that best represents overall PA [31]. If the focus of a study is to increase vigorous PA, then vigorous PA data will be prioritized and used in the analysis. The other two meta-analyses performed for interventions reported follow-up PA and SB outcome measures. Given the small sample size, subgroup analyses were not performed.
The imported data were assessed for statistical heterogeneity. A random effects model would be adopted when the value was moderate (30% to 60%) to substantial (50% to 90%). Otherwise, a fixed effects model would be adopted. All results of the intervention group and control group were summarized by means and standard deviations.
3. Results
3.1. Search Results
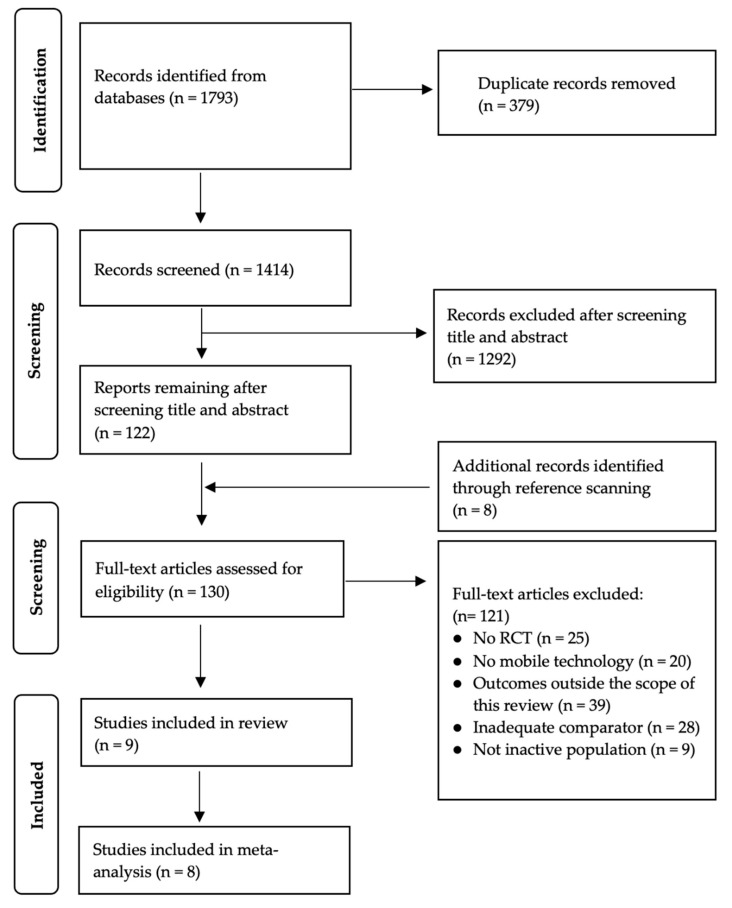
The PRISMA flow diagram of the study selection process is presented in Figure 1. A total of 1793 studies were included in the review after employing the search strategy. The number reduced to 1414 after removing 379 duplicates, which were consequently assessed against the title and abstract. A total of 1292 articles were excluded after screening the title and abstract. References of eligible studies were manually scanned to identify any additional studies and a further eight papers from our backward snowballing search. Further filtering was conducted by screening the full text of the study. Among these, 121 articles were removed since they were not considered highly related to our focus area, e.g., not RCT (n = 25), not using mobile technology for the intervention (n = 20), outcomes outside the scope of this review (n = 39), inadequate comparator (n = 28), and targeting population not inactive (n = 9). This resulted in the inclusion of nine articles.
Figure 1.
PRISMA flow diagram.
3.2. Study Characteristics
Characteristics of the app intervention studies included in this review are presented in Table A1 in Appendix A. Five studies were conducted in North America [35,36,37,38,40], one study in Australia [34], one study in Europe [33], one study in Turkey [21] and another one in Spain [41]. Nine studies were randomized controlled trials (n = 9) with 2-group [21,33,34,35,36,37,40,41] or 3-group [38] study designs.
Of the nine included studies (n = 1495 participants), 1188 participants (79.5%) were female. Seven studies were carried out in mixed gender populations [21,33,34,35,36,40,41]. Two studies were carried out among female participants only [37,38]. One study targeted pregnant women between 10 and 20 weeks of gestation [37].
Nine mobile health interventions were designed to increase overall daily PA among the inactive group. Six studies were physical activity interventions alone [21,33,34,36,38,40], and both PA and SB were targeted in two studies [35,37,41]. One study targeted PA, quality of life, self-efficacy, and exercise motivation for the inactive group [21].
Two studies involved interventions delivered solely via a mobile application (stand-alone intervention) [36,40] and seven studies [21,33,34,35,37,38,41] involved interventions that used both mobile apps and other intervention strategies (multi-component intervention), such as physical education sessions, counseling sessions, information pamphlet, motivational emails, online community and pedometer.
The duration and intensity of the intervention in included studies varied. The intervention time ranged from 3 weeks [33] to 6 months [38]. The type of control groups also varied. One study used a wait-list control [34], two studies used a non-intervention control group [21,35] and three studies provided their control group with basic health information and instructions [33,36,41]. One study compared the intervention group providing the physical promotion app and diet app with the control group providing a diet app alone [40]. Two studies provided their control group with the accelerometer compared with accelerometer and app for the intervention group [37,38].
A variety of PA measurement tools were used. One study used more than one measurement tool [40]. Eight studies used objective measures including: accelerometer [35,38,40], pedometer [34], smart band [33,37,41] and smartphone [21,36]. Subjective questionnaires were used in three studies [21,34,37,41].
3.3. Risk of Bias
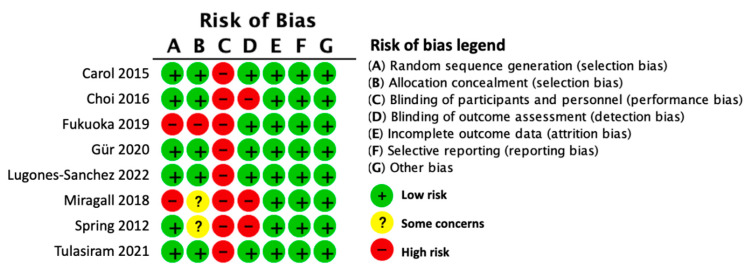
The assessment for each risk of bias item across all included studies is presented in Figure 2. All studies carried a high risk of in participants’ personal blinding owing to the nature of the interventions [21,33,34,35,36,37,38,40,41]. For the selection bias, two studies [33,38] lacked an explanation for how they generated the random sequences. Allocation concealment bias was noted in three studies [33,35,38]. Two of them did not mention the allocation process, which implies some concerns [33,35]. Three studies were considered to have a high risk of bias in blinding the outcome assessment [33,35,37].
Figure 2.
Risk of bias summary.
3.4. Effect of Intervention
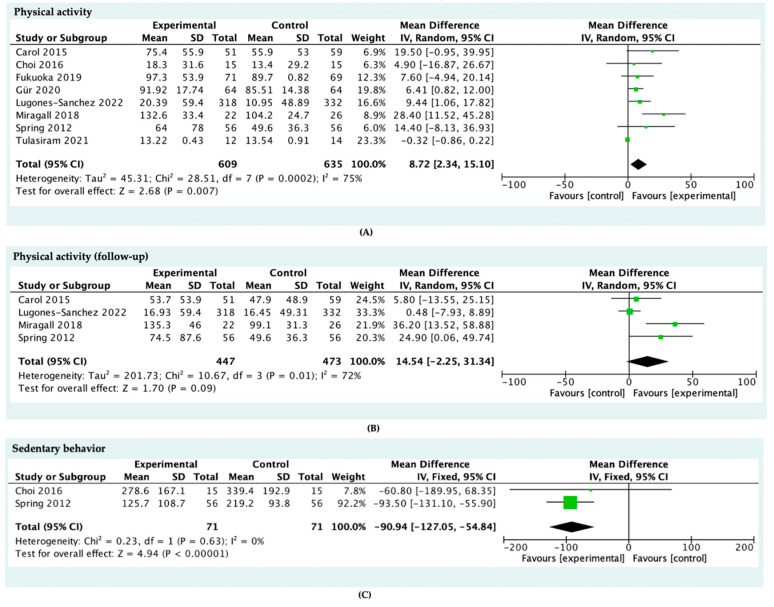
Nine studies [21,33,34,35,36,37,38,40,41] examined the effects of mHealth intervention on PA; One study was excluded because the required information was not available [40]. Data were pooled from eight interventions for meta-analysis. The result of the meta-analysis was statistically significant and favored mHealth interventions (MD = 8.72, 95% CI = 2.34 to 15.10). Four studies [33,34,35,41] reported the follow-up effects of mHealth intervention on physical activity; the meta-analysis result of the four pooled studies was statistically significant and favored mHealth interventions (MD = 14.54, 95% CI = −2.25 to 31.34).
Two studies [35,37] examined the effects of mHealth intervention on SB; all studies were included in the meta-analysis (see Figure 3). Data were pooled from two interventions for meta-analysis. The result of the meta-analysis was statistically significant and favored mHealth interventions (MD = −90.94, 95% CI = −121.05 to −54.84).
Figure 3.
Meta-analysis of the effect of mobile health applications among the inactive population on (A) physical activity, (B) physical activity follow-up, and (C) sedentary behavior.
3.5. Study Quality
The quality assessment result of the included studies can be found in Table A2 in Appendix B. The quality of included studies ranged from low (n = 1) [33] to mid (n = 1) [35] and high (n = 7) [21,34,36,37,38,40,41]. Most included studies fulfill the CONSORT criteria to provide a strong scientific rationale and described their participant eligibility, statistical methods and interventions clearly. Few studies reported sample size calculations [21,36,38,40,41] (n = 5) and included blinding procedures in their study design (n = 2) [34,40].
4. Discussion
The systematic review and meta-analysis were conducted to quantify the reliable evidence about the impact of mHealth interventions on the PA promotion and behavior modification among inactive individuals. The reviewed studies delivered interventions by means of mobile applications. The duration of the intervention varied from 3 weeks to 6 months. Our results revealed that interventions using mHealth could strongly increase the PA level and reduce the SB among the inactive participants.
In general, some studies [35,36,40,41] concluded that mHealth is effective in promoting exercise among inactive people, the results of the meta-analysis reported in this article also confirmed the observable utility of mHealth intervention. Furthermore, the reliability of our conclusions is enhanced by the results of our study quality assessment (Table A2 in Appendix B). As shown in Figure 3, the PA time of the intervention group increased on average by 8.72 min of PA per day. In other words, this meant an inactive individual could obtain an increase of 61.04 min on PA each week, which accounted for nearly 40 percent of the recommendation level [2]. Additionally, four studies reported long-term follow-up measures of PA [33,34,35,41]. The magnitude of the increase reached 14.54 min per day, which demonstrated the beneficial effect could be nicely sustained in the long run. This contradicted the result from a relevant study that argued people can hardly keep a modified healthy lifestyle for more than six months [42]. One possible explanation is that the use of multiple components in the reviewed studies led to better outcomes [43].
The increase in PA time appeared to be more prominent post-intervention according to this meta-analysis. It must be noted that a small proportion of studies in our paper reported follow-up measures for enhancing PA, highlighting the lack of evidence for a long-term increase in PA. Therefore, it is not possible to evaluate the long-term effectiveness of mHealth intervention. This lack of evidence in examining the effectiveness of long-term follow-up is seen in other reviews [26,44,45].
Given clinical recommendations suggest ongoing behavioral support is necessary for lifestyle changes to be sustained [6,7,8,9], continuous use of mHealth apps could make this feasible and cost-effective. In terms of the generalizability of these results, participants included in reported studies were male and female adults, with a BMI indicating over-weight or obesity, predominantly from the U.S. or other developed countries and occasionally with a diagnosed disease, such as diabetes. Results could therefore be generalized to clinical populations such as diabetes mellitus or osteoarthritis patients [46,47], and more research would be required in developing countries. In addition, the limited sample size could influence the reliability of our conclusion. Given the fact that prolonged behavior change could bring out health benefits [31] and improve other dimensions of physical fitness such as BMI and weight, the behavior change therefore requires a relatively long-term observation [43,48,49]. Hence, future research that studies long-period health behavior change is highlighted.
There is a study [12] suggesting that increased PA does not necessarily lead to improved SB when using traditional face-to-face intervention methods, while the results of the meta-analysis in this paper show synergistic benefits of mHealth for the increase in PA and the decrease in SB. It was shown that interventions targeting ease of SB present a mean reduction of 90.94 min in SB time. The result was inspiring since the evidence indicated that just 30 min of SB reallocated to light PA could deliver clinically considerable health outcomes [5]. Compared with another meta-analysis focusing on an ordinary group [31], the result of inactive people within this review showed an additional reduction of 45.94 min per day in SB time. We speculated that the daily sedentary feature of the inactive group led to this extra change in the SB ease effects. Thus, the inactive group deserves specific analysis when conducting studies at a large scale involving a huge number of ordinary people on physical activity and sedentary behavior in order to avoid its misleading impacts on the results. In addition, different inclusion criteria and the sample size could also be considered as one of the reasons. Based on the above discussions, we propose the following suggestions. First, future research could further evaluate the relationship between PA and SB, such as building up accurate quantitative models. Additionally, further exploration is required to gather data from long-term interventions on SB to assess the potentially retentive ability of mHealth in the reduction effect on the SB.
The present findings may present practical implications for mHealth intervention in the future. Long-term user engagement and solid theoretical foundation for mHealth intervention are required for achieving the improved PA. However, the effectiveness of different behavior change theories could not be examined in the present review since limited intervention theory information was given in the included studies. It is likely that intuitive app-use can have a better influence on enhancing the exercise level of inactive individuals. Together with the findings, we hereby propose recommendations for better practical effects of mHealth interventions. First, mHealth providers can develop more functions to improve the app adherence. For example, social participation can be included when designing an mHealth solution. Second, some behavior intervention techniques could be implemented to the mHealth solutions. Examples include “social support”, “hints”, and “goal setting”. In this study, there was a lack of clear and consistent reporting on what behavior change techniques were used in the intervention. The reporting of intervention content should be improved for assessing the effectiveness of the behavior intervention techniques.
5. Limitation
This study exhibits some limitations and therefore calls for future studies. First, this systematic review was not registered on PROSPERO, which is considered a major limitation of our study. However, the search strategy, study selection, and quality assessment were carried out in accordance with established guidelines. Multiple researchers participated in this review to ensure the accuracy of the data and the credibility of the results. We also conducted a meta-analysis of all studies, along with a summary of the risk of bias for all studies. However, there is still a risk that bias could be diluted in the discussion and conclusions of this review. This risk can be reduced by assessing the quality of evidence for each outcome, for example, using the GRADE system. Second, the samples of this study were dominated by females. The gender differences cannot be ignored in the analysis since the characteristics of males and females vary, which could result in diverse behavior habit, schedule flexibility, and long-term mental status. Future research should control variables and make the male–female ratio more balanced to ensure the results reflect the impact of the intervention on general individuals. Plus, multi-group analysis for males and females is appreciated as well. Third, when appraising the same outcome categorized in our study, over one indicator was adopted. Thus, we faced the difficulty of selecting and making a consensus on the best-suited measurement. A further limitation is mainly relevant to the duration—most of the included studies lasted less than eight weeks. As a result, the long-term impact cannot be observed. Finally, only one study referred to the related theoretical frameworks, and thus, we might not identify its concrete role in the context of this study.
6. Conclusions
In conclusion, the interventions using mHealth could well accommodate the requirements of increasing PA and reducing SB among a specific population—inactive individuals. Future studies could follow our research approach to explore the long-term effect on mental health and the benefits of homogeneous methods on the pretext of mHealth in the light of the overall health of the inactive. In practice, our results could provide suggestions for the functions of mHealth to better improve physical activity and mitigate sedentary behavior.
Acknowledgments
The authors would like to thank Sherah Kurnia from The University of Melbourne and Alemayehu Molla from RMIT University for the initial review of this study.
Appendix A
Table A1.
Synthesis of main variables collected from RCTs (n = 9).
| Study | Study Design | Duration | Participant Characteristic | Setting | Intervention | Dependent Variables | Outcome Measure | Comparison Group |
|---|---|---|---|---|---|---|---|---|
| [36] | RCT | Intervention exposure: 4 weeks | N = 26 Age 18~25 years Male (7) and Female (19) USA |
Home | The SmPh app allows step tacking | App impact on cardiorespiratory fitness of college-going adults | Outcome: (i) Aerobic capacity; (ii) Ventilatory equivalent of carbondioxide (iii) Anaerobic threshold (iv) Treadmill distance and time; (v)Heart rate |
Traditional walking prescription |
| [40] | RCT | Intervention exposure: 8 weeks | N = 95 Age >45 years Male (26) and Female (69) USA |
Home | Three mobile apps shape the user towards more physical activity and fewer daily sedentary time | App impact on daily activity and sedentary time | Outcome: (i) Physical activity (i.e., accelerometer-derived moderate-to-vigorous physical activity) and (ii) Sedentary behavior (i.e., accelerometer-derived sedentary time, EMA-derived sitting time) |
A diet-tracking control app |
| [35] | 2-group RCT |
Intervention exposure: 3 week, 20 week follow-up | N = 204 Age 21~60 years Male (48) and Female (156) USA |
Home | (i) Behavior treatment; (ii) Handheld tool to record and self-regulate their targeted behaviors |
The effect of Remote coaching supported by mobile tech- nology and financial incentives to improve diet and activity. | Outcome: (i) Fat and fruit/vegetable consumption; (ii) the saturated fat goal: the Harris–Benedict equation; (iii) Minutes of physical and sedentary activity |
No control group |
| [33] | 2-group RCT |
Intervention exposure: 3-week intervention and a 3 months follow-up | N = 76 Age 18~40 years Female (65), Male (11) UK |
Community, workplace home | (i) Fitbit One to measure steps and provides motivational messages; (ii) Internet-based motivational intervention |
The effect of an Internet-based motivational intervention supported by pedometers on physical activity | Outcome: (i) Physical activity; (ii) Stages of Change Questionnaire for exercise; (iii) Decision Balance Questionnaire for exercise; (iv) Self-Efficacy Questionnaire; (v) Processes of Change Questionnaire |
Without Fitbit one and internet-based motivational intervention |
| [34] | 2-group RCT |
Intervention exposure: 50-day, 20 week follow-up | N = 76 Age 18~65 years Female (82), Male (26) Australia |
Home | (i) Active Team app to encourage friendly rivalry within friendship group; (ii)A pedometer to measure steps |
The effect of an online social networking physical activity intervention with pedometers delivered via Facebook app | Outcome: Physical activities (Active Australia Survey, Assessment of Quality of Life-6D (AQoL-6D) scale, 36-item Short Form Health Survey) |
Teams allocated to the control condition were placed on a waiting list to receive access to the intervention (app and pedometer) |
| [37] | 2-group RCT |
Intervention exposure:12 weeks | N = 30 Age 30~36 years pregnant women between 10 and 20 weeks of gestation USA |
Home | (i) Initial Brief In-Person Session; (ii) Mobile phone app plus Fitbit |
The effect of mobile health intervention in promoting physical activity in Pregnant women | Outcome: Physical activities (The Stanford Brief Physical Activity Survey) Other measures: (i) The Self-Efficacy for Physical Activity; (ii) survey, quiz, scale and checklist |
Fitbit Ultra only (accelerometer) |
| [38] | 3-group RCT |
Intervention exposure:3 months | N = 210 Age 40~60 years Female (210) USA |
Home | Use the app and accelerometer for 9 months | The effects of APP on levels of physical activity | Outcome: Physical activity Other measures: (i) Survey, quiz, scale |
Control group: use accelerometer for 9 months |
| [21] | 2-group RCT |
Intervention exposure: 8 weeks | N = 128 Age 19–26 years Female (112), Male (16) Turkey |
Home | (i) ERVE smartphone app; (ii) An educational video each week under the exercise education component of the ap; (iii) Researchers sent a message once a week to increase motivation. |
The effects of a smartphone app on physical activity, quality of life, self-efficacy, and exercise motivation for inactive people | Primary outcome: self-efficacy, health-related quality of life, and motivational orientation for exercise Secondary outcome: BMI and levels of physical activity |
No intervention |
| [41] | 2-group RCT | Intervention exposure: 3 months | N = 650 Age 20–65 years Female (445) Male (205) Spain |
Primary care center | (i) 5 min of lifestyle counseling before randomization; (ii) low-intensity intervention consisting of a smartphone with the EVIDENT 3 app and a smart band for 3 months; |
The effects of a smartphone app combined with a smart band on weight loss, physical activity, and caloric intake in a population with overweight and obesity | (i) Body weight; (ii) Physical activity: International Physical Activity Questionnaire–Short Form; (iii) Caloric intake (kcal/day) and dietary habits: semiquantitative Food Frequency Questionnaire. |
A brief counseling only |
Appendix B
Table A2.
Synthesis of quality assessment of included RCTs (n = 9).
| [36] | [40] | [35] | [33] | [34] | [37] | [38] | [21] | [41] | |
| Title and abstract | |||||||||
| (a) identification as randomized trial in title; (b) structured summary | 1 | 1 | 1 | 0.5 | 1 | 1 | 1 | 1 | 1 |
| Introduction | |||||||||
| (a) scientific background/rationale; (b) specific objectives/hypotheses | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Methods | |||||||||
| Trial design: (a) description of trial design; (b) changes in methods after trial commencement | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Participants | |||||||||
| (a) eligibility criteria; (b) settings and locations of data collection | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Interventions | |||||||||
| Descriptions of sufficient details to allow replication | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Outcomes | |||||||||
| (a) pre-specified primary and secondary outcomes; (b) changes to outcomes after trial commencement | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Sample size | |||||||||
| (a) how sample size was determined; (b) if applicable, interim analysis/stopping guidelines | 0.5 | 0.5 | 0 | 0 | 0 | 0 | 0.5 | 0.5 | 0.5 |
| Randomization—sequence generation | |||||||||
| (a) method used; (b) type of randomization including any type of restriction | 0.5 | 1 | 1 | 0 | 0.5 | 0.5 | 0 | 0.5 | 0.5 |
| Allocation concealment mechanism | |||||||||
| Implementation of random allocation sequence, including concealment | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 |
| Implementation | |||||||||
| Who generated random allocation sequence, who enrolled participants, who assigned participants | 0 | 1 | 0 | 0 | 0.5 | 1 | 0 | 1 | 1 |
| Blinding | |||||||||
| (a) if done, who was blinded and how; (b) if relevant, similarity of interventions | 0 | 0.5 | 0 | 0 | 0.5 | NA | NA | NA | NA |
| Statistical methods | |||||||||
| Statistical methods used (a) for primary outcomes; (b) additional analyses | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Results | |||||||||
| Participant flow (a) number of participants randomized, receiving treatment, and analyzed; (b) losses and exclusions, with reasons |
1 | 1 | 1 | 0.5 | 1 | 1 | 1 | 1 | 1 |
| Recruitment | |||||||||
| (a) dates of recruitment and follow-up; (b) why the trial ended | 0.5, NA | 0.5, NA | 0.5, NA | 0.5,NA | 0.5,NA | 0.5,NA | 0.5,NA | 0.5,NA | 0.5,NA |
| Baseline data | |||||||||
| A table with baseline demographic and clinical characteristics for each group | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 |
| Numbers analyzed | |||||||||
| For each group, number of participants included in each analyses | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Outcomes and Estimation | |||||||||
| (a) results for each group, and the estimated effect size and its precision; (b) absolute and relative effect sizes for binary outcomes | 0.5, NA | 0.5, NA | 0, NA | 0, NA | 0.5, NA | 0.5, NA | 0.5, NA | 0.5, NA | 0.5, NA |
| Ancillary analyses | |||||||||
| Results of any other analyses performed, distinguishing pre-specified from exploratory | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Harms | |||||||||
| Harms or unintended effects in each group | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 |
| Discussion | |||||||||
| Limitations Trial limitations/bias/multiplicity of analyses | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Generalisability | |||||||||
| Generalisability (external validity/applicability) of findings | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| Interpretation | |||||||||
| Consistent with results and balanced | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Other information | |||||||||
| RegistrationRegistration number and name of registry | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 1 |
| Protocol | |||||||||
| Where full trial protocol can be accessed | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 |
| Funding | |||||||||
| Sources of funding/ role of funders | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 |
| Study quality score attainable | 24 | 24 | 24 | 24 | 24 | 23 | 23 | 23 | 23 |
| Study quality score | 16 | 21.5 | 15.5 | 11.5 | 19 | 17 | 17.5 | 16 | 18 |
| Study quality percentage | 66.7 | 87.8 | 63.2 | 46.9 | 77.6 | 70.8 | 76.1 | 69.6 | 78.2 |
| Study quality rating | High | high | mid | low | high | High | High | High | High |
Author Contributions
Conceptualization, M.Z. and W.W.; methodology, W.W. and M.L.; software, W.W.; formal analysis, M.Z. and W.W.; validation, M.Z., W.W. and M.L.; investigation, M.Z. and W.W.; data curation, H.S.; writing—original draft preparation, M.Z. and W.W.; writing—review and editing, M.L.; visualization, W.W.; supervision, Y.Z.; project administration, M.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data generated during this study is provided in the main manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Breda J., Jakovljevic J., Rathmes G., Mendes R., Fontaine O., Hollmann S., Rütten A., Gelius P., Kahlmeier S., Galea G. Promoting health-enhancing physical activity in Europe: Current state of surveillance, policy development and implementation. Health Policy. 2018;122:519–527. doi: 10.1016/j.healthpol.2018.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Waxman A. WHO global strategy on diet, physical activity and health. Food Nutr. Bull. 2004;25:292–302. doi: 10.1177/156482650402500310. [DOI] [PubMed] [Google Scholar]
- 3.Committee PAGA, Physical Activity Guidelines Advisory Committee Physical activity guidelines advisory committee report, 2008: To the Secretary of Health and Human Services. Nutr. Rev. 2009;67:114–120. doi: 10.1111/j.1753-4887.2008.00136.x. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization . Noncommunicable Diseases Progress Monitor 2015. WHO; Geneva, Switzerland: 2015. p. 232. [Google Scholar]
- 5.Chastin S.F.M., Granat M.H. Methods for objective measure, quantification and analysis of sedentary behaviour and inactivity. Gait Posture. 2010;31:82–86. doi: 10.1016/j.gaitpost.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 6.Patterson R., McNamara E., Tainio M., de Sá T.H., Smith A.D., Sharp S.J., Edwards P., Woodcock J., Brage S., Wijndaele K. Sedentary behaviour and risk of all-cause, cardiovascular and cancer mortality, and incident type 2 diabetes: A systematic review and dose response meta-analysis. Eur. J. Epidemiol. 2018;33:811–829. doi: 10.1007/s10654-018-0380-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization . Global Action Plan on Physical Activity 2018-2030: More Active People for a Healthier World. Volume 28 World Health Organization; Geneva, Switzerland: 2018. [Google Scholar]
- 8.Dounavi K., Tsoumani O. Mobile Health Applications in Weight Management: A Systematic Literature Review. Am. J. Prev. Med. 2019;56:894–903. doi: 10.1016/j.amepre.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 9.Cooper J.O., Heron T.E., Heward W.L. Improving and assessing the quality of behavioral measurement. Appl. Behav. Anal. 2007;3:102–124. [Google Scholar]
- 10.Parrish A.M., Chong K.H., Moriarty A.L., Batterham M., Ridgers N.D. Interventions to Change School Recess Activity Levels in Children and Adolescents: A Systematic Review and Meta-Analysis. Sports Med. 2020;50:2145–2173. doi: 10.1007/s40279-020-01347-z. [DOI] [PubMed] [Google Scholar]
- 11.Das P., Horton R. Physical activity—time to take it seriously and regularly. Lancet. 2016;388:1254–1255. doi: 10.1016/S0140-6736(16)31070-4. [DOI] [PubMed] [Google Scholar]
- 12.Gardner B., Smith L., Lorencatto F., Hamer M., Biddle S.J.H. How to reduce sitting time? A review of behaviour change strategies used in sedentary behaviour reduction interventions among adults. Health Psychol. Rev. 2016;10:89–112. doi: 10.1080/17437199.2015.1082146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hobbs N., Godfrey A., Lara J., Errington L., Meyer T.D., Rochester L., White M., Mathers J.C., Sniehotta F.F. Are behavioral interventions effective in increasing physical activity at 12 to 36 months in adults aged 55 to 70 years? A systematic review and meta-analysis. BMC Med. 2013;11:75. doi: 10.1186/1741-7015-11-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin A., Fitzsimons C., Jepson R., Saunders D.H., Van Der Ploeg H.P., Teixeira P.J., Gray C.M., Mutrie N. Interventions with potential to reduce sedentary time in adults: Systematic review and meta-analysis. Br. J. Sports Med. 2015;49:1056–1063. doi: 10.1136/bjsports-2014-094524. [DOI] [PubMed] [Google Scholar]
- 15.Pate R.R., Saunders R.P., O’Neill J.R., Dowda M. Overcoming barriers to physical activity: Helping youth be more active. ACSM’s Heal. Fit. J. 2011;15:7–12. doi: 10.1249/FIT.0b013e318201c8ee. [DOI] [Google Scholar]
- 16.Ebben W., Brudzynski L. Motivations and barriers to exercise among college students. J. Exerc. Physiol. Online. 2008;11:1–11. [Google Scholar]
- 17.Ryu S. Book Review: mHealth: New Horizons for Health through Mobile Technologies: Based on the Findings of the Second Global Survey on eHealth (Global Observatory for eHealth Series, Volume 3) Healthc. Inform. Res. 2012;18:231. doi: 10.4258/hir.2012.18.3.231. [DOI] [Google Scholar]
- 18.Tate E.B., Spruijt-Metz D., O’Reilly G., Jordan-Marsh M., Gotsis M., Pentz M.A., Dunton G.F. mHealth approaches to child obesity prevention: Successes, unique challenges, and next directions. Transl. Behav. Med. 2013;3:406–415. doi: 10.1007/s13142-013-0222-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu F., Kong X., Cao J., Chen S., Li C., Huang J., Gu D., Kelly T.N. Mobile phone intervention and weight loss among overweight and obese adults: A meta-analysis of randomized controlled trials. Am. J. Epidemiol. 2015;181:337–348. doi: 10.1093/aje/kwu260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Logan A.G. Transforming hypertension management using mobile health technology for telemonitoring and self-care support. Can. J. Cardiol. 2013;29:579–585. doi: 10.1016/j.cjca.2013.02.024. [DOI] [PubMed] [Google Scholar]
- 21.Gür F., Gür G.C., Ayan V. The Effect of the ERVE Smartphone App on Physical Activity, Quality of Life, Self-Efficacy, and Exercise Motivation for Inactive People: A Randomized Controlled Trial. Eur. J. Integr. Med. 2020;39:101198. doi: 10.1016/j.eujim.2020.101198. [DOI] [Google Scholar]
- 22.Lee M., Lee H., Kim Y., Kim J., Cho M., Jang J., Jang H. Mobile app-based health promotion programs: A systematic review of the literature. Int. J. Environ. Res. Public Health. 2018;15:2838. doi: 10.3390/ijerph15122838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schoeppe S., Alley S., Van Lippevelde W., Bray N.A., Williams S.L., Duncan M.J., Vandelanotte C. Efficacy of interventions that use apps to improve diet, physical activity and sedentary behaviour: A systematic review. Int. J. Behav. Nutr. Phys. Act. 2016;13:127. doi: 10.1186/s12966-016-0454-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laranjo L., Ding D., Heleno B., Kocaballi B., Quiroz J.C., Tong H.L., Chahwan B., Neves A.L., Gabarron E., Dao K.P., et al. Do smartphone applications and activity trackers increase physical activity in adults? Systematic review, meta-analysis and metaregression. Br. J. Sports Med. 2021;55:422–432. doi: 10.1136/bjsports-2020-102892. [DOI] [PubMed] [Google Scholar]
- 25.Romeo A., Edney S., Plotnikoff R., Curtis R., Ryan J., Sanders I., Crozier A., Maher C. Can smartphone apps increase physical activity? systematic review and meta-analysis. J. Med. Internet Res. 2019;21:e12053. doi: 10.2196/12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feter N., dos Santos T.S., Caputo E.L., da Silva M.C. What is the role of smartphones on physical activity promotion? A systematic review and meta-analysis. Int. J. Public Health. 2019;64:679–690. doi: 10.1007/s00038-019-01210-7. [DOI] [PubMed] [Google Scholar]
- 27.Stephens J., Allen J. Mobile phone interventions to increase physical activity and reduce weight: A systematic review. J. Cardiovasc. Nurs. 2013;28:320–329. doi: 10.1097/JCN.0b013e318250a3e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J. Clin. Epidemiol. 2009;62:1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 29.McCulloch M. Systematic reviews and meta-analyses: An illustrated step-by-step guide. Natl. Med. J. India. 2004;17:86–95. [PubMed] [Google Scholar]
- 30.Davies C.A., Spence J.C., Vandelanotte C., Caperchione C.M., Mummery W.K. Meta-analysis of internet-delivered interventions to increase physical activity levels. Int. J. Behav. Nutr. Phys. Act. 2012;9:52. doi: 10.1186/1479-5868-9-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stephenson A., McDonough S.M., Murphy M.H., Nugent C.D., Mair J.L. Using computer, mobile and wearable technology enhanced interventions to reduce sedentary behaviour: A systematic review and meta-analysis. Int. J. Behav. Nutr. Phys. Act. 2017;14:105. doi: 10.1186/s12966-017-0561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Higgins J.P.T., Thomas J., Chandler J., Cumpston M., Li T., Page M.J., Welch V.A. Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons; New York, NY, USA: 2019. [Google Scholar]
- 33.Miragall M., Domínguez-Rodríguez A., Navarro J., Cebolla A., Baños R.M. Increasing physical activity through an Internet-based motivational intervention supported by pedometers in a sample of sedentary students: A randomised controlled trial. Psychol. Health. 2018;33:465–482. doi: 10.1080/08870446.2017.1368511. [DOI] [PubMed] [Google Scholar]
- 34.Maher C., Ferguson M., Vandelanotte C., Plotnikoff R., De Bourdeaudhuij I., Thomas S., Nelson-Field K., Olds T. A web-based, social networking physical activity intervention for insufficiently active adults delivered via Facebook app: Randomized controlled trial. J. Med. Internet Res. 2015;17:e4086. doi: 10.2196/jmir.4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spring B., Schneider K., McFadden H.G., Vaughn J., Kozak A.T., Smith M., Moller A.C., Epstein L.H., DeMott A., Hedeker D., et al. Multiple behavior changes in diet and activity: A randomized controlled trial using mobile technology. Arch. Intern. Med. 2012;172:789–796. doi: 10.1001/archinternmed.2012.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tulasiram B., Chandrasekaran B. Are smartphones better in guiding physical activity among sedentary young adults? A randomised controlled trial. Muscles Ligaments Tendons J. 2021;11:83–91. doi: 10.32098/mltj.01.2021.10. [DOI] [Google Scholar]
- 37.Choi J.W., Lee J.H., Vittinghoff E., Fukuoka Y. mHealth Physical Activity Intervention: A Randomized Pilot Study in Physically Inactive Pregnant Women. Matern. Child Health J. 2016;20:1091–1101. doi: 10.1007/s10995-015-1895-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fukuoka Y., Haskell W., Lin F., Vittinghoff E. Short- And long-term effects of a mobile phone app in conjunction with brief in-person counseling on physical activity among Physically InactiveWomen the mPED Randomized Clinical Trial. JAMA Netw. Open. 2019;2:e194281. doi: 10.1001/jamanetworkopen.2019.4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Master H., Bley J.A., Coronado R.A., Robinette P.E., White D.K., Pennings J.S., Archer K.R. Effects of physical activity interventions using wearables to improve objectively-measured and patient-reported outcomes in adults following orthopaedic surgical procedures: A systematic review. PLoS ONE. 2022;17:e0263562. doi: 10.1371/journal.pone.0263562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.King A.C., Hekler E.B., Grieco L.A., Winter S.J., Sheats J.L., Buman M.P., Banerjee B., Robinson T.N., Cirimele J. Effects of three motivationally targeted mobile device applications on initial physical activity and sedentary behavior change in midlife and older adults: A randomized trial. PLoS ONE. 2016;11:e0156370. doi: 10.1371/journal.pone.0156370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lugones-Sanchez C., Recio-Rodriguez J.I., Agudo-Conde C., Repiso-Gento I., Adalia E.G., Ramirez-Manent J.I., Sanchez-Calavera M.A., Rodriguez-Sanchez E., Gomez-Marcos M.A., Garcia-Ortiz L. Long-term Effectiveness of a Smartphone App Combined With a Smart Band on Weight Loss, Physical Activity, and Caloric Intake in a Population With Overweight and Obesity (Evident 3 Study): Randomized Controlled Trial. J. Med. Internet Res. 2022;24:e30416. doi: 10.2196/30416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prochaska J.O., Velicer W.F. The transtheoretical model of health behavior change. Am. J. Health Promot. 1997;12:38–48. doi: 10.4278/0890-1171-12.1.38. [DOI] [PubMed] [Google Scholar]
- 43.Shin Y.H., Kim S.K., Lee M. Mobile phone interventions to improve adolescents’ physical health: A systematic review and meta-analysis. Public Health Nurs. 2019;36:787–799. doi: 10.1111/phn.12655. [DOI] [PubMed] [Google Scholar]
- 44.Pradal-Cano L., Lozano-Ruiz C., Pereyra-Rodríguez J.J., Saigí-Rubió F., Bach-Faig A., Esquius L., Xavier Medina F., Aguilar-Martínez A. Using mobile applications to increase physical activity: A systematic review. Int. J. Environ. Res. Public Health. 2020;17:8238. doi: 10.3390/ijerph17218238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gal R., May A.M., van Overmeeren E.J., Simons M., Monninkhof E.M. The Effect of Physical Activity Interventions Comprising Wearables and Smartphone Applications on Physical Activity: A Systematic Review and Meta-analysis. Sports Med.-Open. 2018;4:42. doi: 10.1186/s40798-018-0157-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rodriguez-León C., Villalonga C., Munoz-Torres M., Ruiz J.R., Banos O. Mobile and wearable technology for the monitoring of diabetes-related parameters: Systematic review. JMIR mHealth uHealth. 2021;9:e25138. doi: 10.2196/25138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cudejko T., Button K., Willott J., Al-Amri M. Applications of wearable technology in a real-life setting in people with knee osteoarthritis: A systematic scoping review. J. Clin. Med. 2021;10:5645. doi: 10.3390/jcm10235645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bogart L.M., Elliott M.N., Cowgill B.O., Klein D.J., Hawes-Dawson J., Uyeda K., Schuster M.A. Two-Year BMI outcomes from a school-based intervention for nutrition and exercise: A randomized trial. Pediatrics. 2016;137:e20152493. doi: 10.1542/peds.2015-2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Quelly S.B., Norris A.E., Dipietro J.L. Impact of mobile apps to combat obesity in children and adolescents: A systematic literature review. J. Spec. Pediatr. Nurs. 2016;21:5–17. doi: 10.1111/jspn.12134. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data generated during this study is provided in the main manuscript.