Abstract
Background:
Prenatal phthalate exposure has been associated with lower birth weight but also higher weight in childhood. Few studies have examined weight or adiposity from birth to childhood and thus cannot assess growth trajectories associated with exposure.
Objective:
We assessed associations between maternal phthalate exposures in pregnancy and child weight and adiposity measured prenatally through childhood (3–6 years of age).
Methods:
Within The Infant Development and the Environment Study (TIDES), a prospective pregnancy cohort, we analyzed a panel of phthalate metabolites in urine collected at two visits from early and late gestation (). We estimated average phthalate metabolite associations with child weight -scores from gestation (estimated by ultrasound), birth, and 1, 3, 4, and 6 years of age using linear mixed-effects (LME) models. We also modeled associations with adiposity -scores from birth (weight for length) and 1, 3, 4, and 6 years of age [body mass index (BMI)] using LME models.
Results:
For weight, we observed inverse associations between several phthalate metabolites and birth weight -scores, but no associations were observed with postnatal weight -scores in LME models. Regarding adiposity, we observed inverse associations between phthalate metabolites and weight-for-length -scores at birth, but positive associations were observed with BMI -scores at 3–4 years of age in LME models. For example, mono-ethyl phthalate was associated with a 0.17-unit decrease in birth weight-for-length -score [95% confidence interval (CI): , ] and a 0.18-unit increase in 4-years-of-age BMI -score (95% CI: 0.04, 0.32).
Discussion:
We observed associations between prenatal exposure to phthalates and lower weight at birth but not at childhood follow-up visits. However, for adiposity, we observed an interesting pattern of association with low adiposity at delivery as well as high adiposity at 3–4 years of age. Although it is not clear from our results whether these associations occur within the same children, such a pattern of adiposity in early life has been linked to cardiometabolic disease in adulthood and deserves special attention as an outcome in the study of prenatal exposures in the developmental origins of health and disease. https://doi.org/10.1289/EHP10077
Introduction
Phthalates are found ubiquitously in the environment in personal care products, food and beverage packaging materials, and vinyl products. Prenatal exposure to some phthalates has been associated with lower birth weight or reduced fetal growth in human populations.1 Conversely, some studies have observed a positive association between the same prenatal phthalate exposures and childhood weight and body mass index (BMI).2–4 Few studies, however, have examined prenatal phthalate exposure in relation to weight or adiposity measured longitudinally from the prenatal period to childhood.
Early work by Barker et al. demonstrated that low birth weight is associated with cardiovascular and metabolic disease in adulthood.5 However, this research has evolved to an understanding that the trajectory of growth in early life is a stronger predictor of subsequent health than is any cross-sectional measurement.6 Repeatedly, human studies have identified associations between a pattern of low birth weight with rapid weight gain in the first months or years of life and later cardiometabolic disease.6–8 Thus, an adverse trajectory of early life growth, characterized by smallness in the prenatal period and largeness in childhood, is arguably a more important end point than size at any individual time point.
The Developmental Origins of Health and Disease (DOHaD) framework suggests fetal programming in utero in response to maternal exposures has the capacity to affect developmental trajectories throughout the offspring’s life course.9 Phthalates, among other endocrine disruptors, are hypothesized to elicit the phenotype of low adiposity at birth and excess adiposity in childhood and later in life.10 Therefore, in the present study, we examined associations between prenatal exposure to phthalates and weight and adiposity in the prenatal period through early childhood (3–6 years of age) and explored associations with longitudinal patterns of weight and adiposity using group-based trajectory models.
Methods
Study Population
We conducted our analysis within The Infant Development and the Environment Study (TIDES), a prospective pregnancy cohort in which women were recruited early in gestation (at , based on early pregnancy ultrasound or last menstrual period) between 2010 and 2012 at four U.S. sites [University of California, San Francisco (UCSF), University of Minnesota (UMN), University of Rochester Medical Center (URMC), University of Washington (UW)].11 Participants provided informed consent, spoke English, were free of medical complications that would preclude participation in the study, and planned to deliver at one of the study site hospitals. During pregnancy, women were asked to participate in three study visits [ (9–12), 20 (10–22), and 32 (30–35), respectively] to provide biological specimens (urine, blood) and to respond to questionnaires. Self-administered questionnaires from the first study visit, which were primarily completed electronically at home through a secure portal, were used to collect information on the covariates used in the present study, including maternal age, race/ethnicity, education level, and prepregnancy BMI.11 Regarding race, participants were asked to select one of the following categories: Black or African American; Asian; American Indian or Alaska Native; Native Hawaiian or Pacific Islander; White; More than one race; Other; or Unknown. Ethnicity (Hispanic or Latino vs. not Hispanic or Latino) was assessed in a separate question. These categories were combined into White (non-Hispanic white), Black (non-Hispanic Black or African American), and Other (all other categories) for the present analyses because of small sample sizes. Current smoking (yes/no) was also self-reported at each study visit and combined to indicate ever smoking in pregnancy for the present analysis. A birth exam with anthropometrics was completed in the entire cohort, and follow-up to 1 year of age (boys only) was part of the original study design. At 3 years of age, all participants were invited to provide information via mail about child height and weight. Finally, all mother–child pairs were invited to participate in study visits at 4 and 6 years of age. Figure 1 displays the timeline for sample collection and outcome data collection. Study activities were approved by the institutional review boards (IRBs) at all study sites. The present analysis was deemed Not Human Subjects Research by the IRB at the National Institute of Environmental Health Sciences (NIEHS).
Figure 1.
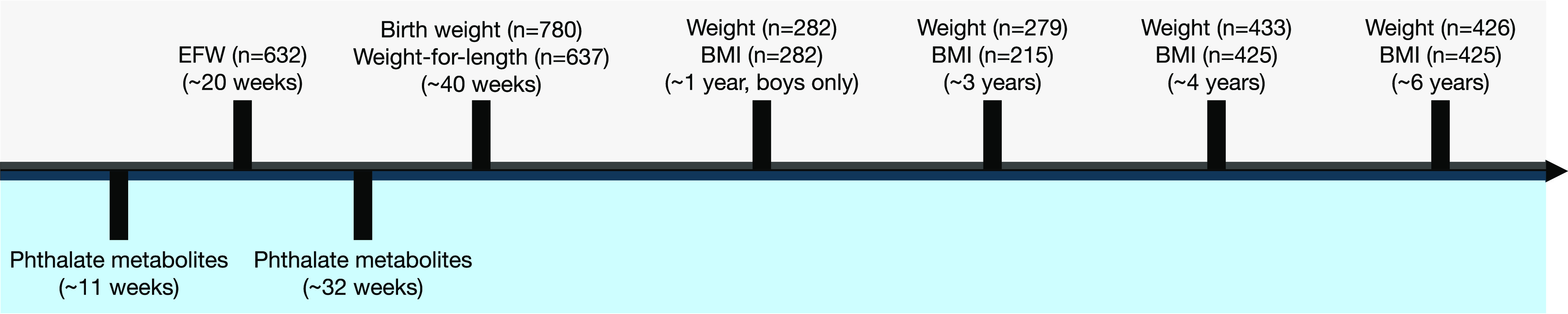
Timeline of urinary phthalate metabolite measurements and weight and BMI outcome measurements from The Infant Development and the Environment Study included in the present analyses. Visit 1 occurred at gestation and visit 3 occurred at 32 wk gestation. Note: BMI, body mass index; EFW, estimated fetal weight.
Phthalate Exposure in Pregnancy
We used phthalate metabolite concentrations measured in samples from visits 1 and 3 for analyses in the present study. A panel of phthalate and phthalate alternative metabolites was measured via high-performance liquid chromatography at the National Center for Environmental Health, Centers for Disease Control and Prevention (CDC), or UW, as described in detail elsewhere.12 Briefly, for visit 1, all samples from mothers carrying a male fetus and a quarter of samples from mothers carrying a female fetus were analyzed at the CDC. The remainder of samples from mothers carrying a female fetus were analyzed at UW. For visit 3, all samples were analyzed at the CDC. A subset of samples were also analyzed for phthalate metabolites at visit 2 (); however, we did not include these measurements in our analyses given that the sample size was small and measurements were only performed on samples from mothers carrying a male fetus.12
For the present analysis, we focused on the 11 phthalate metabolites that were measured in the majority of participants and had of concentrations above the limit of detection (LOD), as shown in Table S1 and reported elsewhere,12 including mono-ethyl phthalate (MEP), mono--butyl phthalate (MBP), monobenzyl phthalate (MBzP), mono-isobutyl phthalate (MiBP), mono (3-carboxypropyl) phthalate (MCPP), monocarboxyoctyl phthalate (MCOP), monocarboxy-isononyl phthalate (MCNP), and four (DEHP) metabolites [mono () phthalate (MEHP), mono () phthalate (MEHHP), mono () phthalate (MEOHP), and mono () phthalate (MECPP)]. Concentrations below the LOD were replaced with the LOD divided by the square root of 2.
Urine samples were analyzed for specific gravity at the time of sample collection.13 Phthalate metabolites were corrected for specific gravity using the formula of Boeniger14 and measurements from visit 1 and visit 3 were averaged to obtain a more stable estimate of each participant’s exposure across the course of pregnancy. For most metabolites, averages were based on two measurements for 707 (91%) participants. If only one measurement was available, the single value was used in place of the average [73 (9%) participants]. For MCOP and MCNP, which were analyzed in later batches only, only one measurement was available for 430 (56%) and 339 (44%) participants, respectively. Finally, the DEHP metabolite averages (MEHP, MEHHP, MEOHP, and MECPP) were combined in a molar sum (hereafter presented as ).15
Child Weight and BMI -Scores
Our primary outcome measures were child weight and BMI -scores, the latter being an acceptable proxy for adiposity.16 Child weight and BMI measurements were collected and standardized to -scores, as described below, for 780 participants who had at least one phthalate measurement available during gestation, a singleton pregnancy, and birth weight measured at delivery. The number of observations for weight or BMI varied across each time point (as shown in Table 1), and none of these missing outcome measurements were imputed.
Table 1.
Distributions of child age, weight, and body mass index (BMI) measures (medians and 25th and 75th percentiles) by study visit in the TIDES study population ().
| Study visit | Age (y)a | Weight (kg)b | BMI ()c | Weight -score | BMI -scorec | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median | 25th P | 75th P | Median | 25th P | 75th P | Median | 25th P | 75th P | Median | 25th P | 75th P | Median | 25th P | 75th P | ||||||
| 20 Wk | 632 | 19.7 | 19.0 | 20.3 | 632 | 313 | 277 | 364 | — | — | — | — | 632 | 0.91 | 0.21 | 1.96 | — | — | — | — |
| Birth | 780 | 39.6 | 38.7 | 40.6 | 780 | 3.35 | 3.05 | 3.68 | 637 | 6.80 | 63.4 | 72.6 | 780 | 0.30 | 0.97 | 637 | 0.29 | 0.93 | ||
| Age (y) | ||||||||||||||||||||
| 1d | 282 | 1.04 | 1.00 | 1.13 | 282 | 10.1 | 9.47 | 10.9 | 282 | 17.1 | 16.2 | 17.9 | 282 | 0.20 | 0.85 | 282 | 0.28 | 0.90 | ||
| 3 | 279 | 2.92 | 2.42 | 3.25 | 279 | 14.5 | 12.9 | 15.9 | 215 | 16.2 | 15.4 | 17.2 | 279 | 0.35 | 0.87 | 215 | 0.16 | 0.95 | ||
| 4 | 433 | 4.51 | 4.24 | 4.69 | 433 | 18.2 | 16.5 | 19.5 | 425 | 15.7 | 14.6 | 16.6 | 433 | 0.44 | 0.93 | 425 | 0.24 | 0.82 | ||
| 6 | 426 | 6.21 | 6.08 | 6.48 | 426 | 22.5 | 20.1 | 24.5 | 425 | 15.9 | 14.8 | 16.8 | 426 | 0.29 | 0.89 | 425 | 0.33 | 0.87 | ||
Note: —, not applicable; P, percentile; TIDES, The Infant Development and the Environment Study.
Age at 20 wk and at birth is in weeks gestation.
Weight for 20-wk ultrasound measurements (estimated fetal weight) is in grams.
For the birth exam, weight-to-length ratio is presented instead of BMI, and weight-to-length ratio -score is presented instead of BMI -score. Weight-to-length ratio is reported in kilograms per meter.
No data collected for females at 1 year of age.
Mid-Pregnancy Ultrasound Measures
Ultrasound measures of estimated fetal weight (EFW) collected between 18 and 23 wk of gestation, that is, the approximate window for routine anatomy ultrasounds, were abstracted from medical records. If EFW was not reported, we calculated EFW using the formula of Hadlock from measures of abdominal circumference, head circumference, and femur length.17 If participants had multiple ultrasounds available within the 18–23 wk window, the EFW measurement occurring closest to 20 wk gestation was retained for analysis.
We calculated EFW -scores using the World Health Organization (WHO) fetal growth charts as a standard population because, as opposed to other standards, sex-specific charts are available and references are available for fetuses prior to 22 wk of gestation.18 We created percentiles for each measurement as recommended by Kiserud et al.19 and subsequently assigned each percentile a corresponding -score, as has been done with birth weight data.20 The final number of observations for EFW -scores was 632 (Table 1).
Birth Measurements
Beginning at birth, we created weight -scores, as well as adiposity -scores (based on weight-for-length or BMI). Birth weight (in grams) was collected from medical records for all newborns. Sex-specific -scores for birth weight for gestational age were calculated from the International Fetal and Newborn Growth Consortium for the 21st Century (INTERGROWTH-21st) standards.21 Neonatal length was assessed at an exam performed shortly after birth.22 We only used length measurements that were recorded within 1 wk () of birth. Combining these with the birth weight measures above, we calculated weight-for-length -scores, also using the INTERGROWTH-21st standards.21 Weight-for-length is preferred to BMI or ponderal index as a measure of adiposity in this age range because it is a better predictor of infant free fat mass.23 The final numbers of observations for birth weight -scores and weight-for-length -scores were 780 and 637, respectively (Table 1).
One-Year-of-Age Measurements
One-year-of-age weight and length were assessed at an in-person visit in boys only (per funding; range in years: 0.8–1.7). Weight was measured using the Seca Infant Scale Model #334 to the nearest 10 g, and length was measured with the Seca Infantometer Model #416 to the nearest 0.1 cm. Sex- and age-specific -scores for weight and BMI were calculated using WHO growth charts that are optimized for children under 2 years of age24. The final number of observations for 1 year of age weight and BMI -scores was 282 (Table 1).
Approximately Three-Years-of Age Measurements
Early preschool period weight and length measurements in boys and girls were assessed once via maternal report in a mailed questionnaire. The range in ages at this time point was wider than others (, ); thus, data for this period were restricted to measurements collected at - and -of-age visits. Sex- and age-specific -scores for weight and BMI were calculated using CDC growth charts [25]. The final numbers of observations for 3-years-of-age weight and BMI -scores were 279 and 215, respectively (Table 1).
Four- and 6-Years of-Age Measurements
Weight and height at 4 and 6 years of age in boys and girls were measured at an in-person visit by study staff who received in-person training for a standardized protocol. The range in years for the 4-years-of-age visit was 4–5.8 y, and for the 6-years-of-age visit, 5.8–8 y. Weight was measured on a digital scale after the child removed any footwear or heavy clothing. Height was measured after the child removed any footwear using a Shorr board. Both height and weight measurements were taken twice and averaged for analyses, and a third measure was taken if the first two had too much variability. Sex- and age-specific -scores for weight and BMI were also calculated using CDC growth charts and implausible -scores were identified in the same manner.25 For 4 years of age, the final numbers of observations for weight and BMI -scores were 433 and 425, respectively. For 6 years of age, there were 426 and 425 observations, respectively (Table 1).
Statistical Analysis
All statistical analyses were performed using R (version 3.6.3; R Development Core Team)26 and SAS (version 9.4; SAS Institute). Demographic characteristics of the study sample were summarized. In addition, the 25th, 50th, and 75th percentiles of the average prenatal phthalate concentrations, as well as the child age, weight, and BMI measurements at each study visit, were calculated.
Linear mixed-effects models.
In our primary analysis of weight and BMI -scores, we used linear mixed-effects (LME) models to estimate the unadjusted and adjusted mean difference and 95% confidence intervals (CIs) in repeated growth measures for an interquartile range (IQR)-increase in maternal average urinary phthalate measurements. Specifically, outcomes for child weight models included an EFW -score from ultrasounds taken at of gestation, birth weight -scores measured at delivery, and child weight -scores collected at 1, 3, 4, and 6 years of age. For child BMI models, outcomes included weight-for-length -scores measured at delivery and child BMI -scores collected at 1, 3, 4, and 6 years of age. We included an interaction term between exposure and study visit for all models because preliminary analyses showed that the exposure–outcome associations differed based on the timing of outcome assessment. The -value for a likelihood ratio test comparing the full model without phthalate metabolite vs. a full model with phthalate metabolite was used to identify significant () overall associations. All models were adjusted for the following covariates, which were selected a priori based on a directed acyclic graph: maternal age (y, continuous), maternal race (White/Black/Other), education level as a proxy for socioeconomic status (High school or less/Any technical school or college/Graduate work), prepregnancy BMI (in kilograms per meter squared, continuous), and Study site (UCSF/UMN/URMC/UW). We adjusted for race as a confounder because phthalate exposure levels and patterns of birth weight and childhood weight gain are both associated with race owing to social factors. Missing covariate data was minimal, with the exception of smoking (10%; Table 2), and was imputed 10 times (with 10 iterations) using multiple imputation chained equations (MICE). Missing exposure and outcome data were not imputed. LME models were constructed using the lme4 package27 and imputation was performed using the mice package in R.28 As part of the model-building process, we explored nonlinearity using models fit with natural cubic splines. Models fit with spline terms were compared with models including only a simple linear term using likelihood ratio tests. The -values from these comparisons were all , indicating little evidence of a nonlinear association. Therefore, in our modeling approach, we used simple linear terms to represent phthalate measures.
Table 2.
Demographic characteristics of the TIDES study population ().
| Demographic characteristics | (%) or mean (SD) |
|---|---|
| Study center | |
| University of California, San Francisco | 187 (24) |
| University of Minnesota | 209 (27) |
| University of Rochester Medical Center | 221 (28) |
| University of Washington | 163 (21) |
| Maternal age (y) | 30.5 (5.56) |
| Missing | 2 |
| Maternal race | |
| White | 532 (68) |
| Black | 101 (13) |
| Othera | 145 (19) |
| Missing | 2 |
| Maternal education level | |
| High school or less | 109 (14) |
| Any technical school/college | 335 (43) |
| Graduate work | 327 (42) |
| Missing | 9 |
| Prepregnancy BMI (kg/m2) | 25.7 (6.37) |
| Missing | 11 |
| Ever smoked during pregnancy | |
| No | 643 (92) |
| Yes | 57 (8) |
| Missing | 80 |
| Child sex | |
| Female | 398 (51) |
| Male | 382 (49) |
Note: BMI, body mass index; TIDES, The Infant Development and the Environment Study; SD, standard deviation.
Other includes Asian (), American Indian or Alaska Native (), Native Hawaiian or Pacific Islander (), multiracial (), Other (), and Unknown ().
Several sensitivity analyses were performed to ensure the reported results were robust. First, the potential effect of smoking in pregnancy, although uncommon in this study population, was examined by reconstructing models with additional adjustment for self-reported smoking status. Second, the potential for confounding by child sex or for sex-specific associations between phthalates and growth were examined. To investigate this possibility, we reconstructed our models with adjustment for child sex. In addition, we created models that were stratified according to child sex, and tested for interactions by sex with likelihood ratio tests that compared models with a two-way interaction () vs. a three-way interaction (), with used to identify significant differences in associations by sex. Third, because some of the urine samples used for phthalate metabolite measurement were collected after the time of the ultrasound for EFW, we examined associations with phthalate metabolite concentrations at visits 1 and 3 separately and provided a Spearman correlation matrix between the repeated measures to contextualize those results. Last, to examine the influence of missingness on our results, we examined demographic characteristics among individuals in the overall sample compared with those who contributed data to the 6-years-of-age visit only and additionally recreated our LME models with inverse probability weights (IPWs) to account for missing growth outcomes. IPWs were constructed using logistic regression models with predictors of missingness for each outcome. Significant (i.e., ) predictors of outcome missingness were initially identified using univariate models. We examined covariates included in our primary models (listed in Table 2) as well as additional variables that we expected might be related to missingness, including alcohol consumption, income, parity, marital status, and previous diagnosis of diabetes. Predictors that were significantly associated with missingness in univariate models were included in subsequent multivariate models, and only the predictors that maintained statistical significance were retained in final models for parsimony. Final multivariate models for IPW included the following predictors of missingness: maternal race and alcohol consumption for weight outcomes; and study center, maternal race, and maternal age for BMI outcomes. Weights were stabilized prior to incorporation into statistical analyses.
Group-Based Trajectory Models.
As a secondary approach, we used group-based trajectory modeling to identify subgroups of individuals sharing similar patterns of weight or BMI change over time.29 Based on previous work using this method,30 we explored two- to five-group solutions for both our weight and BMI trajectory analyses, with a default cubic polynomial to allow flexibility in trajectory shape. Following identification of the optimal number of groups, nonsignificant polynomial terms were removed from the trajectory model in favor of parsimony.31 We evaluated the optimal number of groups using substantive knowledge and statistical criteria.31 We selected the highest number of groups that met the following criteria: log Bayes factor, that is, , required to be ; average posterior probabilities of class membership required to be ; and smallest sample size in each group required to be at least 5% of the study sample.31 Participants were subsequently assigned to the group trajectory corresponding to their highest posterior inclusion probability. Weight and BMI trajectories were visualized by graphically displaying the predicted mean -score and 95% CIs for each group.
Following group assignment, we tabulated the distribution of demographic variables according to weight- or BMI-trajectory group assignments. In addition, we calculated the 25th, 50th, and 75th percentiles of phthalate metabolite concentrations according to group membership. Crude differences in metabolite concentrations between weight or BMI trajectory groups were examined using Kruskal-Wallis tests. Subsequently, we used linear regression models to estimate the association between trajectory group membership (independent variable) and urinary phthalate concentrations (dependent variable). Specifically, we report the population marginal mean phthalate metabolite concentrations within each weight- and BMI-trajectory group. To ensure comparability across analyses, these models included the same covariates as our LME models. Group-based trajectory modeling was performed using PROC TRAJ,29 and population marginal mean phthalate concentrations were calculated using the LSMEANS statement in SAS. Missing data were imputed (as described above) and results were averaged across all data sets using MIANALYZE in SAS.
Results
Of the 969 participants enrolled in the study, we included 780 for the present analysis who had a singleton pregnancy, at least one phthalate metabolite measurement from pregnancy, as well as birth weight at delivery. Mothers from our study population were primarily White (68%) and well educated (Table 2). As part of our study design, we had complete information on birth weight. Availability of weight and adiposity measures by study visit are displayed in Table 1, and by sex in Table S2. Ultrasound estimates of fetal weight were available between 18 and 22 wk of gestation on 632 participants (81%). At 1 year of age, 74% of boys from this sample participated in the in-person follow-up, and girls were not examined at this time point. Participation in the 3-years-of-age questionnaire was low overall (36% for weight, 28% for BMI). However, attendance at the 4- and 6-years-of-age in-person visits was higher; 62% of participants participated in at least one of the two visits. At 4 years of age, 56% of participants had a weight measurement and 54% had a BMI measurement, and availability at 6 years of age was similar. Total number of visits attended and the last observed visit are displayed in Table S3.
Distributions of pregnancy-averaged phthalate metabolite concentrations, corrected for urinary specific gravity, are presented in Table 3. All associations for subsequent analyses are presented as the change per IQR difference.
Table 3.
Distributions of average phthalate metabolite concentrations in maternal urine (ng/mL) (as medians and 25th and 75th percentiles).
| Phthalate metabolite | Median | 25th P | 75th P | IQR | |
|---|---|---|---|---|---|
| MEP | 780 | 37.3 | 16.1 | 88.1 | 72.0 |
| MBP | 780 | 8.99 | 5.60 | 14.5 | 8.91 |
| MBzP | 780 | 4.49 | 2.25 | 8.85 | 6.60 |
| MiBP | 780 | 5.97 | 3.67 | 9.94 | 6.26 |
| MCPP | 780 | 2.03 | 1.19 | 4.41 | 3.21 |
| MCOP | 769 | 15.7 | 7.51 | 34.7 | 27.2 |
| MCNP | 769 | 2.45 | 1.58 | 4.19 | 2.60 |
| ΣDEHPa | 780 | 0.09 | 0.06 | 0.13 | 0.07 |
Note: Phthalate metabolite concentrations were corrected for urinary specific gravity prior to averaging. IQR, interquartile range; MBP, mono--butyl phthalate; MBzP, monobenzyl phthalate; MCNP, monocarboxy-isononyl phthalate; MCOP, monocarboxyoctyl phthalate; MCPP, mono(3-carboxypropyl) phthalate; MEP, mono-ethyl phthalate; MiBP, mono-isobutyl phthalate; P, percentile; , summed di-2-ethylhexyl phthalate.
Units are in nanomoles per milliliter.
LME Models
Results from LME models for weight showed a significant overall association for MEP, MBzP, and MiBP. Associations (95% CIs) outputted from each study visit are displayed in Figure 2 and Table S4. MEP, MBzP, MiBP, and MBP were all inversely associated with weight at birth, but generally not with weight measurements at other study visits. For example, an IQR difference in MEP was associated with a 0.14 decrease in birth weight -score (95% CI: , ), which corresponds to at birth; however, associations at other study visits were largely null [ (95% CI: , 0.08); (95% CI: , 0.18); (95% CI: , 0.15); (95% CI: , 0.17); .06 (95% CI: , 0.19)].
Figure 2.
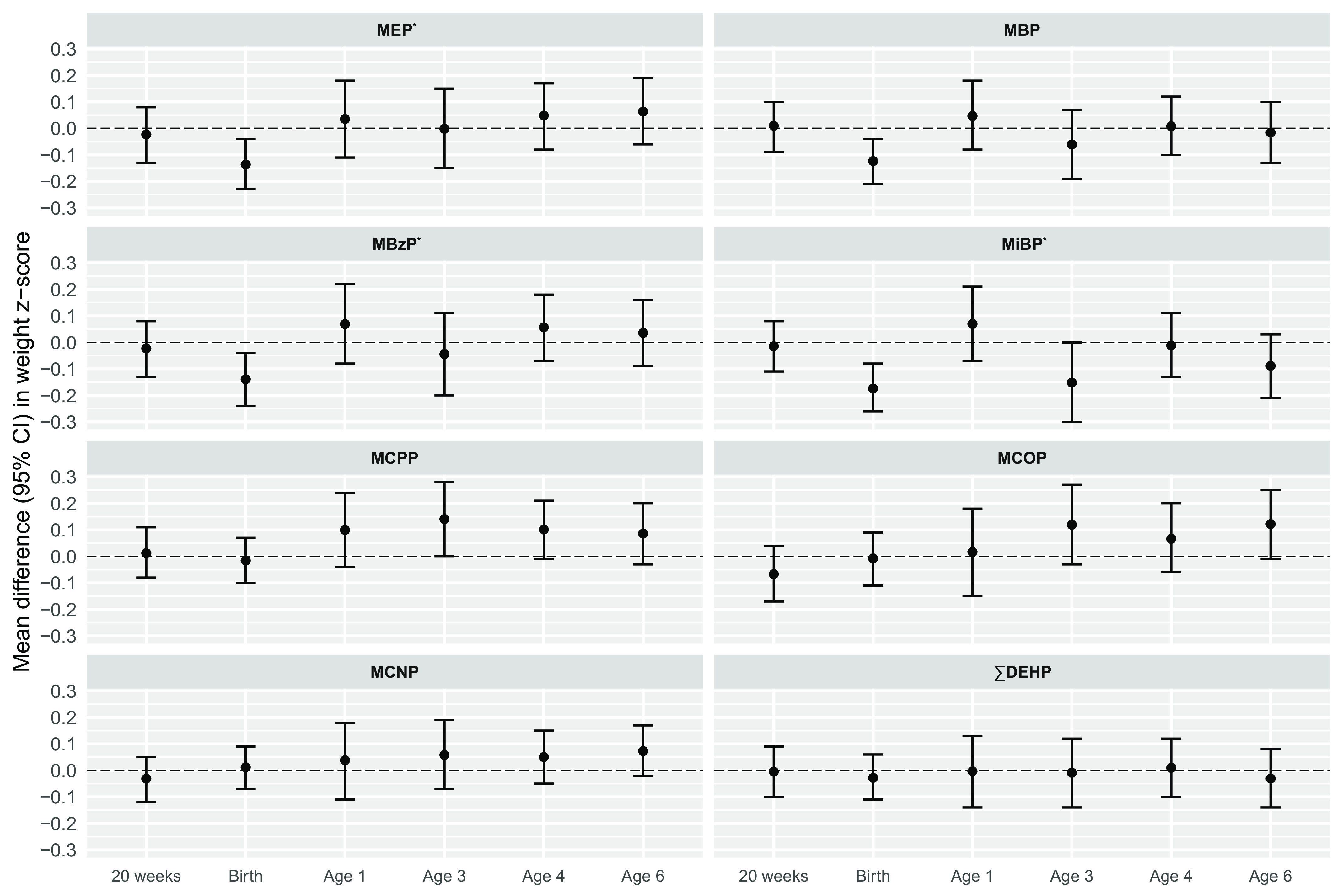
Adjusted difference in weight -scores (95% CIs) per IQR increase in pregnancy average of individual phthalate metabolite biomarkers (). Effect estimates were calculated using linear mixed-effects models adjusted for maternal age (y), race (White/Black/Other), education level (High school or less/Any technical school or college/Graduate work), prepregnancy BMI (), and study site (UCSF/UMN/URMC/UW). For models of MCOP and MCNP, . No data was collected for females at 1 year of age. *, for overall association of phthalate metabolite on outcome based on likelihood ratio tests. Corresponding effect estimates are presented in Table S4. Note: BMI, body mass index; CI, confidence interval; IQR, interquartile range; MBP, mono--butyl phthalate; MBzP, monobenzyl phthalate; MCNP, monocarboxy-isononyl phthalate; MCOP, monocarboxyoctyl phthalate; MCPP, mono(3-carboxypropyl) phthalate; MEP, mono-ethyl phthalate; MiBP, mono-isobutyl phthalate; , summed di-2-ethylhexyl phthalate; UCSF, University of California, San Francisco; UMN, University of Minnesota; URMC, University of Rochester Medical Center; UW, University of Washington.
LME models for BMI showed significant overall associations for all phthalate metabolites except for MCNP and . Results from individual study visits showed inverse associations between MEP, MBP, MBzP, and MiBP and weight for length at birth, and positive associations between MEP, MBP, MBzP, MCPP, and MCOP at 3 years of age, 4 years of age, or both. (Figure 3; Table S5). For example, MEP was associated with a 0.17 decrease in birth weight-for-length -score (95% CI: , −0.05), and a 0.18-unit increase in 4-years-of-age BMI -score (95% CI: 0.04, 0.32). These associations roughly correspond to 0.12 and , respectively.
Figure 3.
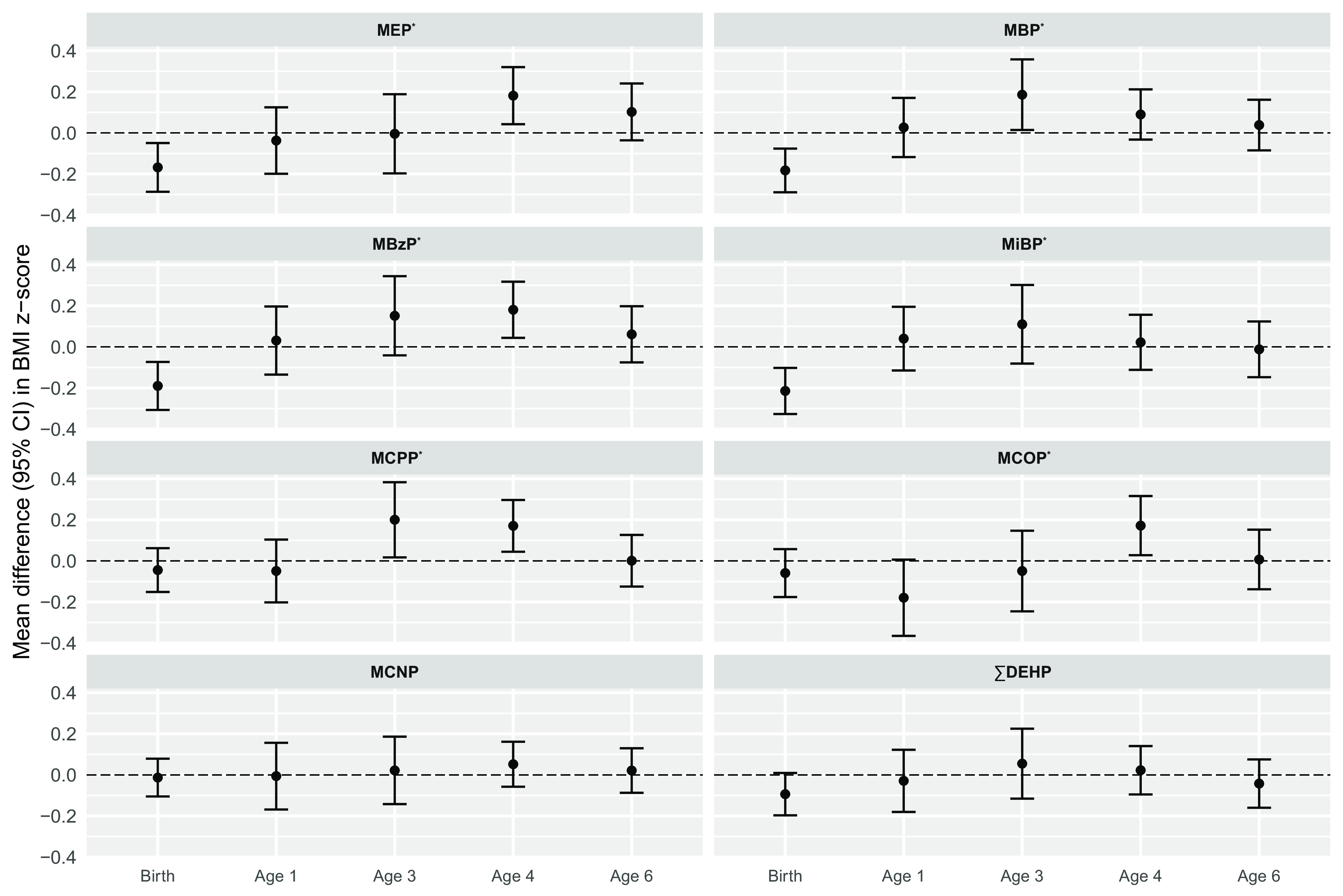
Adjusted difference in BMI -scores (95% CIs) per IQR increase in pregnancy average of individual phthalate metabolite biomarkers (). Effect estimates were calculated using linear mixed-effects models adjusted for maternal age (y), race (White, Black, other), education level (high school or less/any technical school or college/graduate work), prepregnancy BMI (), and study site (UCSF, UMN, URMC, UW). For the birth exam, weight-to-length ratio -scores are presented instead of BMI -scores. For models of MCOP and MCNP, . No data was collected for females at 1 year of age. *, for overall association of phthalate metabolite on outcome based on likelihood ratio tests. Corresponding effect estimates are presented in Table S5. Note: BMI, body mass index; CI, confidence interval; IQR, interquartile ratio; MBP, mono--butyl phthalate; MBzP, monobenzyl phthalate; MCNP, monocarboxy-isononyl phthalate; MCOP, monocarboxyoctyl phthalate; MCPP, mono(3-carboxypropyl) phthalate; MEP, mono-ethyl phthalate; MiBP, mono-isobutyl phthalate; , summed di-2-ethylhexyl phthalate; UCSF, University of California, San Francisco; UMN, University of Minnesota; URMC, University of Rochester Medical Center; UW, University of Washington.
Sensitivity analyses for our LME model results showed general robustness to model misspecification. First, our primary associations were only slightly attenuated compared with those from models without adjustment for covariates (Tables S6 and S7 for weight and BMI, respectively). Second, adjustment for maternal smoking had almost no impact on associations (Tables S8 and S9). Adjustment for child sex similarly had little effect (Tables S10 and S11). However, likelihood ratio tests showed that differences by sex for models of weight -scores, but not BMI -scores, were statistically significant for all phthalate metabolites (all ; Table S12). Models of weight -scores that were stratified by sex showed the same direction of effects in girls and boys, but with slightly greater associations (i.e., more negative associations between MEP, MBzP, and MiBP) in boys (Table S13). Models of BMI -scores stratified by sex showed similar associations between girls and boys at birth, but at 4 years of age the associations were greater in magnitude (i.e., more positive) for boys (Table S14). Third, we observed low-to-moderate correlations between phthalate metabolite concentrations from visits 1 and 3 (Figure S1). Models where phthalate metabolites from visits 1 and 3 were modeled separately showed that associations were similar, although attenuated as expected owing to measurement error, for measurements from either time point (Tables S15 and S16 for weight and BMI, respectively). Finally, demographic characteristics were similar in individuals from the overall sample compared with the those who contributed data to the 6-years-of-age visit (Table S17), and models with IPW showed that results were similar after accounting for missing growth outcomes (Tables S18 and S19 for weight and BMI, respectively).
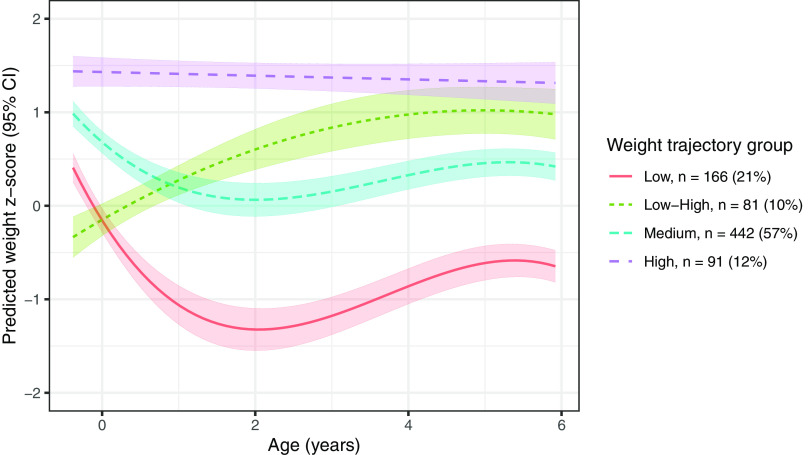
Group-Based Trajectory Models
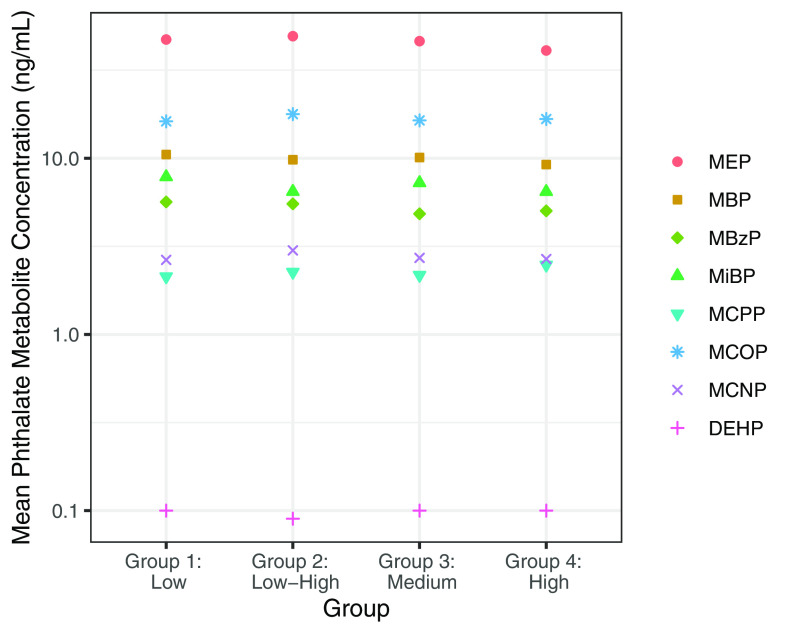
In our secondary approach, we identified four groups of individuals with unique weight trajectories (Figure 4). The model fit with four groups had a log Bayes factor , had sufficient average posterior probabilities of inclusion (78%), and had sufficient sample size within each group (Table S20). The four groups can be roughly characterized as “low” (lower weight -scores from 20 wk gestation to 6 years of age), “medium” (intermediate weight -scores), “high” (consistently highest weight -scores), and “low–high” (lower weight -scores at 20 wk gestation and birth, but higher weight -scores at 4 and 6 years of age). The low–high group comprised individuals who were more often from the URMC study site, self-identified as Black, had a lower education level, and smoked during pregnancy (Table S21). We hypothesized that individuals in the low–high group would also have the highest exposure to phthalates. In general, however, pregnancy-averaged phthalate metabolite concentrations did not differ across groups, either when crudely estimated (Table S22) or when estimated from adjusted least square models that accounted for maternal age, race, education level, prepregnancy BMI, and study site (Figure 5, Table S23).
Figure 4.
Predicted mean (95% CI) trajectories for weight -scores from 20 wk to 6 years of age and estimated using group-based trajectory modeling. Note: CI, confidence interval.
Figure 5.
Adjusted least square mean phthalate metabolite concentrations by weight -score growth trajectory groupings. Effect estimates were calculated using linear models adjusted for maternal age (y), race (White/Black/Other), education level (High school or less/Any technical school or college/Graduate work), prepregnancy BMI (), and study site (UCSF/UMN/URMC/UW). Least square mean values are presented numerically in Table S23. Note: BMI, body mass index; MBP, mono--butyl phthalate; MBzP, monobenzyl phthalate; MCNP, monocarboxy-isononyl phthalate; MCOP, monocarboxyoctyl phthalate; MCPP, mono(3-carboxypropyl) phthalate; MEP, mono-ethyl phthalate; MiBP, mono-isobutyl phthalate; DEHP, di-2-ethylhexyl phthalate; UCSF, University of California, San Francisco; UMN, University of Minnesota; URMC, University of Rochester Medical Center; UW, University of Washington.
We also investigated BMI trajectories using group-based trajectory modeling; however, we did not identify a low–high group as we expected. The optimal number of groups was three (Table S24). These groups could roughly be characterized as low, medium, and high for BMI (or weight-for-length) -scores from birth to 6 years of age (Figure S2). The fewer, less-informative groups (as compared with groups for weight) could be due to fewer observations per participant ( as opposed to for models of weight), as well as to fewer study visits (i.e., no fetal measure for BMI). BMI groups did not appear to be strongly linked to participants’ demographic characteristics (Table S25) nor to pregnancy-averaged phthalate metabolite concentrations from adjusted models (Table S26).
Discussion
Within a cohort of pregnant women and their children from four U.S. sites, we observed associations between prenatal exposure to some phthalates and low adiposity at delivery (i.e., weight-for-length -scores), as well as high adiposity at 3–6 years of age (i.e., BMI -scores). This was most evident for MEP, MBzP, and MBP. Fewer associations were observed between phthalate metabolites and weight measurements, although several metabolites (MEP, MBP, MBzP, and MiBP) were inversely associated with weight at birth.
Our findings point toward an association between prenatal phthalate exposure and adiposity, rather than weight in other compartments. In the prenatal period, phthalate metabolites were not associated with EFW at gestation. During this period, fetal mass is predominantly composed of skeleton and organs, whereas adipose accumulates late in pregnancy.32,33 At birth, after adipose deposition has occurred,32,33 we observed inverse associations between several phthalate metabolites and weight and similar—if not stronger—associations with weight-for-length. Finally, at study visits in early childhood (3–6 years of age), we observed few associations between exposure and weight, but positive associations with BMI.
An interesting animal study observed a pattern of the effects of DEHP on adiposity that is similar to what we observed in the TIDES study population, although our findings were for different phthalates. Strakovsky et al. found that rats exposed prenatally to DEHP had reduced adiposity, but not weight, at birth.34 Furthermore, the exposed rats experienced a catch-up in adipocyte maturation and lipid accumulation that resulted in overweight, and exacerbated the effects of a high-fat diet in adulthood (i.e., resulted in greater weight gain than was otherwise observed with the high-fat diet).34 Although, to our knowledge, similar studies have not been performed with the phthalates that were associated with adiposity in our study, other phthalate monoesters are known to activate peroxisome proliferator-activated receptors in the same fashion as DEHP metabolites.35,36 Given that these receptors are known to regulate maturation of adipocytes, our findings are biologically plausible.37,38
Despite our observed dimorphic associations (i.e., low adiposity at birth and high adiposity in childhood), our statistical approach does not elucidate whether this pattern occurs among the same individuals. It is also possible that our results are showing associations with two groups—one where exposure is associated with low birth weight, but no associations in childhood, and one where exposure is associated with no changes at birth but higher adipose in childhood. However, the pattern of low adiposity at birth and rapid catch-up growth is of great importance for later-life cardiometabolic health.6,9 We attempted to investigate associations with this pattern directly but employing group-based trajectory models for weight and adipose -scores from the prenatal period to 6 years of age. These models relax the assumption that all individuals have the same growth curve, and allows for investigation of subgroups that follow unique trajectories.39 These models identified unique phenotypes of weight, including a low–high group that may be problematic for later-life cardiovascular and metabolic health.6 However, a similar trajectory for BMI was not identified within our data set, as we anticipated. Given the results for weight from our LME models, it was not surprising that phthalate metabolites were not associated with the weight trajectories in our study. However, our approach may be useful for future investigation of these and other environmental contaminants in populations where such a trajectory of low adiposity at birth and high adiposity in childhood exists.
Our findings add to a large body of literature on the associations between prenatal phthalate exposure and birth weight, as well as childhood weight and BMI. The literature on prenatal phthalate exposure and size at birth is quite mixed, although many report an inverse association with low molecular weight phthalates, including MEP and MBP.1,40 Notably, very few of these studies report on measures of adiposity, such as weight for length or ponderal index at birth.41,42 The data on prenatal phthalate exposure and childhood weight or BMI is similarly mixed.2–4,43–48 In our analysis, we observed positive associations between prenatal measures of MEP, MBP, MBzP, MCPP, and MCOP with BMI -scores from 3 to 6 years of age, but not childhood weight. The heterogeneity in findings could be attributable to numerous factors, including study population characteristics (e.g., prevalence of childhood obesity), differences in exposure assessment approaches, timing of follow-up, and availability of covariate data. Strong conclusions about the obesogenic effects of phthalate exposure in utero must wait until larger studies, such as the Environmental influences on Child Health Outcomes (ECHO) program, can fully assess the impacts of these factors.49
Our study is relatively unique in combining data from the neonatal period through childhood in the same analysis, and among existing studies has the largest sample size. Previously, Botton et al. observed associations between MEP and early childhood growth velocity (weight and height) as well as BMI at 5 years of age.2 Heggeseth et al. also observed positive associations between prenatal MEP and BMI between 2 and 14 years of age using multiple longitudinal approaches.50 Finally, Yang et al. created BMI trajectories from birth to 14 years of age with somewhat mixed findings for associations with prenatal phthalate metabolite tertiles.51 All studies used different statistical approaches. Our primary analysis used LME models, which have improved power for testing overall associations (as compared with multiple cross-sectional models). These models also enable testing of differences in associations based on timing of outcome measurement and can easily handle missingness. However, as above, this approach cannot provide information about the pattern of associations. The group-based trajectory approach, which may prove useful in this regard, has been used frequently in the study of childhood BMI52 but less so in investigating the environmental origins of adverse weight gain trajectories.53 Future studies with additional study visits and observations, or that include populations who are at higher risk for adverse weight gain patterns (e.g., individuals residing in food deserts who have restricted access to fresh foods54), may be able to observe a low–high BMI group that would be more relevant for associations with prenatal phthalate exposure.
The primary limitation to our study was loss to follow-up from birth to 6 years of age, which is not uncommon in birth cohort studies but could limit the generalizability of our findings. In the pooled study of prenatal phthalate exposure and childhood BMI, Buckley et al. examined the impact of loss to follow-up and observed that children with follow-up were more likely to have a higher BMI.3 Associations were similar when they accounted for missingness in their model, which is consistent with what we observed in our sensitivity analysis. A second limitation is lack of information on maternal or childhood diet. Exposure to some phthalates occurs through consumption of contaminated foods, particularly those that are high in fat and may be linked to childhood obesity.55 However, our primary findings were for the primary metabolite of di-ethyl phthalate (DEP), which is found at very low levels across food groups.55 Third, the generalizability of our findings may also be limited by the fact that our population was primarily White and highly educated. Fourth, we did not have information available on childhood phthalate exposure for our analyses. Although correlations between prenatal and childhood exposure levels are known to be low,56 there is a possibility that exposures during the childhood period could partially explain our findings. Fifth, our estimation of phthalate metabolite concentrations for individuals in each trajectory group did not account for the uncertainty in trajectory assignment. However, it is expected that this would bias any differences observed toward the null.57 Sixth, although we averaged two urinary measurements of phthalate metabolites in pregnancy to create a more stable estimate of exposure, more measurements would have been better, given that phthalates are variable across the course of gestation.58 Seventh, the data on height and weight from the 3-years-of-age visit were self-reported. Because error in reporting is unlikely to be related to phthalate exposure levels, this may have biased results from this visit toward the null. Eighth, although our data had four U.S. sites, these data were from populations in urban or urban-adjacent locations and thus our findings may not be generalizable to populations in rural locations. Finally, we know that prenatal phthalate metabolites are correlated with one another, including in this study population (Spearman ).59 We created single-pollutant models to best compare our findings to previous studies and did not create copollutant models or models of cumulative associations so that we could pay more attention to approaches we used to model outcome. However, future studies should also consider using one of the many mixtures approaches available to address these questions.
The strengths of our study included its prospective design from the prenatal period through childhood and the use of a large cohort with four U.S. sites. Furthermore, we fully used the longitudinal data with LME models, as well as group-based trajectory models, the latter of which have seldom been applied in the study of environmental exposures and early life weight and adipose gain and may have greater clinical utility.
Our findings coalesce the knowledge from the cross-sectional literature on the impact of prenatal phthalate exposure on weight and adipose of the baby at birth through childhood. We observed associations between prenatal phthalate metabolites and decreased adiposity at birth and higher adiposity in childhood from 3 to 6 years of age. Given the high rates of childhood obesity in the United States,60 continued investigation of the role of environmental exposures such as phthalates is warranted.
Supplementary Material
Acknowledgments
We thank K. Christenbury and E. Rowley for their assistance with data management and statistical analyses, and A. Calafat (Centers for Disease Control and Prevention), as well as the laboratory at the University of Washington for urinary phthalate metabolite analyses. Finally, we gratefully acknowledge the study coordinators, G. Alcedo, S. Caveglia, S. Grover, A. Cordeiro, and S. Moe, as well as the study’s medical record abstractors: S. Bedell, A. Carter, S. Caveglia, A. Hart, S. King, E. Laschansky, A. Santilli, and S. Singh.
This research was supported in part by the Intramural Research Program of the National Institute of Environmental Health Sciences (NIEHS), National Institutes of Health (NIH; ZIAES103313 to K.K.F.); by the following NIEHS/NIH grants: R01ES016863 (S.H.S.), R01ES025169 (S.H.S.), P30ES023515, R01ES016863-02S4, and P30 ES005022; and by the NIH Office of the Director (UG3/UH3 OD023305 to L.T.).
References
- 1.Kamai EM, McElrath TF, Ferguson KK. 2019. Fetal growth in environmental epidemiology: mechanisms, limitations, and a review of associations with biomarkers of non-persistent chemical exposures during pregnancy. Environ Health 18(1):43, PMID: , 10.1186/s12940-019-0480-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Botton J, Philippat C, Calafat AM, Carles S, Charles MA, Slama R, et al. 2016. Phthalate pregnancy exposure and male offspring growth from the intra-uterine period to five years of age. Environ Res 151:601–609, PMID: , 10.1016/j.envres.2016.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buckley JP, Engel SM, Braun JM, Whyatt RM, Daniels JL, Mendez MA, et al. 2016. Prenatal phthalate exposures and body mass index among 4- to 7-year-old children: a pooled analysis. Epidemiology 27(3):449–458, PMID: , 10.1097/EDE.0000000000000436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harley KG, Berger K, Rauch S, Kogut K, Claus Henn B, Calafat AM, et al. 2017. Association of prenatal urinary phthalate metabolite concentrations and childhood BMI and obesity. Pediatr Res 82(3):405–415, PMID: , 10.1038/pr.2017.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barker DJP, Gluckman PD, Godfrey KM, Harding JE, Owens JA, Robinson JS, et al. 1993. Fetal nutrition and cardiovascular disease in adult life. Lancet 341(8850):938–941, PMID: , 10.1016/0140-6736(93)91224-A. [DOI] [PubMed] [Google Scholar]
- 6.Leunissen RWJ, Kerkhof GF, Stijnen T, Hokken-Koelega A. 2009. Timing and tempo of first-year rapid growth in relation to cardiovascular and metabolic risk profile in early adulthood. JAMA 301(21):2234–2242, PMID: , 10.1001/jama.2009.761. [DOI] [PubMed] [Google Scholar]
- 7.Chomtho S, Wells JC, Williams JE, Davies PS, Lucas A, Fewtrell MS, et al. 2008. Infant growth and later body composition: evidence from the 4-component model. Am J Clin Nutr 87(6):1776–1784, PMID: , 10.1093/ajcn/87.6.1776. [DOI] [PubMed] [Google Scholar]
- 8.Ekelund U, Ong KK, Linné Y, Neovius M, Brage S, Dunger DB, et al. 2007. Association of weight gain in infancy and early childhood with metabolic risk in young adults. J Clin Endocrinol Metab 92(1):98–103, PMID: , 10.1210/jc.2006-1071. [DOI] [PubMed] [Google Scholar]
- 9.McMillen IC, Rattanatray L, Duffield JA, Morrison JL, MacLaughlin SM, Gentili S, et al. 2009. The early origins of later obesity: pathways and mechanisms. Adv Exp Med Biol 646:71–81, PMID: , 10.1007/978-1-4020-9173-5_8. [DOI] [PubMed] [Google Scholar]
- 10.Casals-Casas C, Desvergne B. 2011. Endocrine disruptors: from endocrine to metabolic disruption. Annu Rev Physiol 73:135–162, PMID: , 10.1146/annurev-physiol-012110-142200. [DOI] [PubMed] [Google Scholar]
- 11.Barrett ES, Sathyanarayana S, Janssen S, Redmon JB, Nguyen RHN, Kobrosly R, et al. 2014. Environmental health attitudes and behaviors: findings from a large pregnancy cohort study. European J Obstet Gynecol Reprod Biol 176:119–125, 10.1016/j.ejogrb.2014.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferguson KK, Rosen EM, Barrett ES, Nguyen RHN, Bush N, McElrath TF, et al. 2019. Joint impact of phthalate exposure and stressful life events in pregnancy on preterm birth. Environ Int 133(pt B):105254, PMID: , 10.1016/j.envint.2019.105254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swan SH, Sathyanarayana S, Barrett ES, Janssen S, Liu F, Nguyen RHN, et al. 2015. First trimester phthalate exposure and anogenital distance in newborns. Hum Reprod 30(4):963–972, PMID: , 10.1093/humrep/deu363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boeniger MF, Lowry LK, Rosenberg J. 1993. Interpretation of urine results used to assess chemical exposure with emphasis on creatinine adjustments: a review. Am Ind Hyg Assoc J 54(10):615–627, PMID: , 10.1080/15298669391355134. [DOI] [PubMed] [Google Scholar]
- 15.Wolff MS, Engel SM, Berkowitz GS, Ye X, Silva MJ, Zhu C, et al. 2008. Prenatal phenol and phthalate exposures and birth outcomes. Environ Health Perspect 116(8):1092–1097, PMID: , 10.1289/ehp.11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kakinami L, Henderson M, Chiolero A, Cole TJ, Paradis G. 2014. Identifying the best body mass index metric to assess adiposity change in children. Arch Dis Child 99(11):1020–1024, PMID: , 10.1136/archdischild-2013-305163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hadlock FP, Harrist RB, Sharman RS, Deter RL, Park SK. 1985. Estimation of fetal weight with the use of head, body, and femur measurements—a prospective study. Am J Obstet Gynecol 151(3):333–337, PMID: , 10.1016/0002-9378(85)90298-4. [DOI] [PubMed] [Google Scholar]
- 18.Kiserud T, Piaggio G, Carroli G, Widmer M, Carvalho J, Neerup Jensen L, et al. 2017. The World Health Organization fetal growth charts: a multinational longitudinal study of ultrasound biometric measurements and estimated fetal weight. PLoS Med 14(1):e1002220, PMID: , 10.1371/journal.pmed.1002220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kiserud T, Benachi A, Hecher K, Perez RG, Carvalho J, Piaggio G, et al. 2018. The World Health Organization fetal growth charts: concept, findings, interpretation, and application. Am J Obstet Gynecol 218(suppl 2):S619–S629, PMID: , 10.1016/j.ajog.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 20.Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. 2003. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr 3:6, PMID: , 10.1186/1471-2431-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Villar J, Ismail LC, Victora CG, Ohuma EO, Bertino E, Altman DG, et al. 2014. International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st project. Lancet 384(9946):857–868, PMID: , 10.1016/S0140-6736(14)60932-6. [DOI] [PubMed] [Google Scholar]
- 22.Sathyanarayana S, Grady R, Redmon JB, Ivicek K, Barrett E, Janssen S, et al. 2015. Anogenital distance and penile width measurements in The Infant Development and the Environment Study (TIDES): methods and predictors. J Pediatr Urol 11(2):76.e1–e6, PMID: , 10.1016/j.jpurol.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Villar J, Puglia FA, Fenton TR, Cheikh Ismail L, Staines-Urias E, Giuliani F, et al. 2017. Body composition at birth and its relationship with neonatal anthropometric ratios: the newborn body composition study of the INTERGROWTH-21st project. Pediatr Res 82(2):305–316, PMID: , 10.1038/pr.2017.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.CDC (Centers for Disease Control and Prevention). 2016. A SAS program for the WHO growth charts (ages 0 to < 2 years). https://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas-who.htm [accessed 21 January 2020].
- 25.CDC. 2020. A SAS Program for the 2000 CDC Growth Charts (ages 0 to < 20 years). https://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm [accessed 21 January 2020].
- 26.Rebuli ME, Patisaul HB. 2016. Assessment of sex specific endocrine disrupting effects in the prenatal and pre-pubertal rodent brain. J Steroid Biochem Mol Biol 160:148–159, PMID: , 10.1016/j.jsbmb.2015.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bates D, Mächler M, Bolker B, Walker S. et al. 2014. Fitting linear mixed-effects models using lme4. arXiv. Preprint posted online June 23, 2014, 10.48550/arXiv.1406.5823. [DOI] [Google Scholar]
- 28.van Buuren S, Groothuis-Oudshoorn K. 2011. mice: multivariate imputation by chained equations in R. J Stat Soft 45(3):1–68, 10.18637/jss.v045.i03. [DOI] [Google Scholar]
- 29.Jones BL, Nagin DS, Roeder K. 2001. A SAS procedure based on mixture models for estimating developmental trajectories. Sociol Methods Res 29(3):374–393, 10.1177/0049124101029003005. [DOI] [Google Scholar]
- 30.Arogbokun O, Rosen E, Keil AP, Milne GL, Barrett E, Nguyen R, et al. 2021. Maternal oxidative stress biomarkers in pregnancy and child growth from birth to age 6. J Clin Endocrinol Metab 106(5):1427–1436, PMID: , 10.1210/clinem/dgab018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andruff H, Carraro N, Thompson A, Gaudreau P, Louvet B. 2009. Latent class growth modelling: a tutorial. Tutor Quant Methods Psychol 5(1):11–24, 10.20982/tqmp.05.1.p011. [DOI] [Google Scholar]
- 32.Orsso CE, Colin-Ramirez E, Field CJ, Madsen KL, Prado CM, Haqq AM. 2020. Adipose tissue development and expansion from the womb to adolescence: an overview. Nutrients 12(9):2735, PMID: , 10.3390/nu12092735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Toro-Ramos T, Paley C, Pi-Sunyer FX, Gallagher D. 2015. Body composition during fetal development and infancy through the age of 5 years. Eur J Clin Nutr 69(12):1279–1289, PMID: , 10.1038/ejcn.2015.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strakovsky RS, Lezmi S, Shkoda I, Flaws JA, Helferich WG, Pan YX. 2015. In utero growth restriction and catch-up adipogenesis after developmental di (2-ethylhexyl) phthalate exposure cause glucose intolerance in adult male rats following a high-fat dietary challenge. J Nutr Biochem 26(11):1208–1220, PMID: , 10.1016/j.jnutbio.2015.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bility MT, Thompson JT, McKee RH, David RM, Butala JH, Vanden Heuvel JP, et al. 2004. Activation of mouse and human peroxisome proliferator-activated receptors (PPARs) by phthalate monoesters. Toxicol Sci 82(1):170–182, PMID: , 10.1093/toxsci/kfh253. [DOI] [PubMed] [Google Scholar]
- 36.Hurst CH, Waxman DJ. 2003. Activation of PPARα and PPARγ by environmental phthalate monoesters. Toxicol Sci 74(2):297–308, PMID: , 10.1093/toxsci/kfg145. [DOI] [PubMed] [Google Scholar]
- 37.Desvergne B, Feige JN, Casals-Casas C. 2009. PPAR-mediated activity of phthalates: a link to the obesity epidemic? Mol Cell Endocrinol 304(1–2):43–48, PMID: , 10.1016/j.mce.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 38.Tontonoz P, Hu E, Spiegelman BM. 1995. Regulation of adipocyte gene expression and differentiation by peroxisome proliferator activated receptor γ. Curr Opin Genet Dev 5(5):571–576, PMID: , 10.1016/0959-437X(95)80025-5. [DOI] [PubMed] [Google Scholar]
- 39.Ram N, Grimm KJ. 2009. Methods and measures: a method for identifying differences in longitudinal change among unobserved groups. Int J Behav Dev 33(6):565–576, PMID: , 10.1177/0165025409343765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Santos S, Sol CM, van Zwol-Janssens C, Philips EM, Asimakopoulos AG, Martinez-Moral MP, et al. 2021. Maternal phthalate urine concentrations, fetal growth and adverse birth outcomes. A population-based prospective cohort study. Environ Int 151:106443, PMID: , 10.1016/j.envint.2021.106443. [DOI] [PubMed] [Google Scholar]
- 41.Kim JH, Park H, Lee J, Cho G, Choi S, Choi G, et al. 2016. Association of diethylhexyl phthalate with obesity-related markers and body mass change from birth to 3 months of age. J Epidemiol Community Health 70(5):466–472, PMID: , 10.1136/jech-2015-206315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu Y, Wan Y, Zhang B, Zhou A, Huo W, Wu C, et al. 2018. Relationship between maternal phthalate exposure and offspring size at birth. Sci Total Environ 612:1072–1078, PMID: , 10.1016/j.scitotenv.2017.08.207. [DOI] [PubMed] [Google Scholar]
- 43.Buckley JP, Engel SM, Mendez MA, Richardson DB, Daniels JL, Calafat AM, et al. 2016. Prenatal phthalate exposures and childhood fat mass in a New York City cohort. Environ Health Perspect 124(4):507–513, PMID: , 10.1289/ehp.1509788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maresca MM, Hoepner LA, Hassoun A, Oberfield SE, Mooney SJ, Calafat AM, et al. 2016. Prenatal exposure to phthalates and childhood body size in an urban cohort. Environ Health Perspect 124(4):514–520, PMID: , 10.1289/ehp.1408750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vafeiadi M, Myridakis A, Roumeliotaki T, Margetaki K, Chalkiadaki G, Dermitzaki E, et al. 2018. Association of early life exposure to phthalates with obesity and cardiometabolic traits in childhood: sex specific associations. Front Public Health 6:327, PMID: , 10.3389/fpubh.2018.00327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang TC, Peterson KE, Meeker JD, Sánchez BN, Zhang Z, Cantoral A, et al. 2017. Bisphenol A and phthalates in utero and in childhood: association with child BMI z-score and adiposity. Environ Res 156:326–333, PMID: , 10.1016/j.envres.2017.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shoaff J, Papandonatos GD, Calafat AM, Ye X, Chen A, Lanphear BP, et al. 2017. Early-life phthalate exposure and adiposity at 8 years of age. Environ Health Perspect 125(9):097008, PMID: , 10.1289/EHP1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Valvi D, Casas M, Romaguera D, Monfort N, Ventura R, Martinez D, et al. 2015. Prenatal phthalate exposure and childhood growth and blood pressure: evidence from the Spanish INMA-Sabadell birth cohort study. Environ Health Perspect 123(10):1022–1029, PMID: , 10.1289/ehp.1408887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Buckley JP, Barrett ES, Beamer PI, Bennett DH, Bloom MS, Fennell TR, et al. 2020. Opportunities for evaluating chemical exposures and child health in the United States: the Environmental influences on Child Health Outcomes (ECHO) program. J Expo Sci Environ Epidemiol 30(3):397–419, PMID: , 10.1038/s41370-020-0211-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heggeseth BC, Holland N, Eskenazi B, Kogut K, Harley KG. 2019. Heterogeneity in childhood body mass trajectories in relation to prenatal phthalate exposure. Environ Res 175:22–33, PMID: , 10.1016/j.envres.2019.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang TC, Peterson KE, Meeker JD, Sánchez BN, Zhang Z, Cantoral A, et al. 2018. Exposure to bisphenol A and phthalates metabolites in the third trimester of pregnancy and BMI trajectories. Pediatr Obes 13(9):550–557, PMID: , 10.1111/ijpo.12279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mattsson M, Maher GM, Boland F, Fitzgerald AP, Murray DM, Biesma R, et al. 2019. Group‐based trajectory modelling for BMI trajectories in childhood: a systematic review. Obes Rev 20(7):998–1015, PMID: , 10.1111/obr.12842. [DOI] [PubMed] [Google Scholar]
- 53.Carter MA, Dubois L, Tremblay MS, Taljaard M, Jones BL. 2012. Trajectories of childhood weight gain: the relative importance of local environment versus individual social and early life factors. PLoS One 7(10):e47065, PMID: , 10.1371/journal.pone.0047065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Howlett E, Davis C, Burton S. 2016. From food desert to food oasis: the potential influence of food retailers on childhood obesity rates. J Bus Ethics 139(2):215–224, 10.1007/s10551-015-2605-5. [DOI] [Google Scholar]
- 55.Serrano SE, Braun J, Trasande L, Dills R, Sathyanarayana S. 2014. Phthalates and diet: a review of the food monitoring and epidemiology data. Environ Health 13(1):43, PMID: , 10.1186/1476-069X-13-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lewis RC, Meeker JD, Peterson KE, Lee JM, Pace GG, Cantoral A, et al. 2013. Predictors of urinary bisphenol A and phthalate metabolite concentrations in Mexican children. Chemosphere 93(10):2390–2398, PMID: , 10.1016/j.chemosphere.2013.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van de Schoot R, Sijbrandij M, Winter SD, Depaoli S, Vermunt JK. 2017. The GRoLTS-checklist: Guidelines for Reporting on Latent Trajectory Studies. Struct Equ Modeling 24(3):451–467, 10.1080/10705511.2016.1247646. [DOI] [Google Scholar]
- 58.Casas M, Basagaña X, Sakhi AK, Haug LS, Philippat C, Granum B, et al. 2018. Variability of urinary concentrations of non-persistent chemicals in pregnant women and school-aged children. Environ Int 121(pt 1):561–573, PMID: , 10.1016/j.envint.2018.09.046. [DOI] [PubMed] [Google Scholar]
- 59.van ’t Erve TJ, Rosen EM, Barrett ES, Nguyen RHN, Sathyanarayana S, Milne GL, et al. 2019. Phthalates and phthalate alternatives have diverse associations with oxidative stress and inflammation in pregnant women. Environ Sci Technol 53(6):3258–3267, PMID: , 10.1021/acs.est.8b05729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ogden CL, Carroll MD, Kit BK, Flegal KM. 2014. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA 311(8):806–814, PMID: , 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.