Abstract
The objective of this study was to determine if there is a pharmacokinetic interaction when amprenavir is given with rifabutin or rifampin and to determine the effects of these drugs on the erythromycin breath test (ERMBT). Twenty-four healthy male subjects were randomized to one of two cohorts. All subjects received amprenavir (1,200 mg twice a day) for 4 days, followed by a 7-day washout period, followed by either rifabutin (300 mg once a day [QD]) (cohort 1) or rifampin (600 mg QD) (cohort 2) for 14 days. Cohort 1 then received amprenavir plus rifabutin for 10 days, and cohort 2 received amprenavir plus rifampin for 4 days. Serial plasma and urine samples for measurement of amprenavir, rifabutin, and rifampin and their 25-O-desacetyl metabolites, were measured by high-performance liquid chromatography. Rifabutin did not significantly affect amprenavir's pharmacokinetics. Amprenavir significantly increased the area under the curve at steady state (AUCss) of rifabutin by 2.93-fold and the AUCss of 25-O-desacetylrifabutin by 13.3-fold. Rifampin significantly decreased the AUCss of amprenavir by 82%, but amprenavir had no effect on rifampin pharmacokinetics. Amprenavir decreased the results of the ERMBT by 83%. The results of the ERMBT after 2 weeks of rifabutin and rifampin therapy were increased 187 and 156%, respectively. Amprenavir plus rifampin was well tolerated. Amprenavir plus rifabutin was poorly tolerated, and 5 of 11 subjects discontinued therapy. Rifampin markedly increases the metabolic clearance of amprenavir, and coadministration is contraindicated. Amprenavir significantly decreases clearance of rifabutin and 25-O-desacetylrifabutin, and the combination is poorly tolerated. Amprenavir inhibits the ERMBT, and rifampin and rifabutin are equipotent inducers of the ERMBT.
Amprenavir (USAN approved, VX-478, 141W94; Glaxo Wellcome Inc., Research Triangle Park, N.C.) is a new human immunodeficiency virus type 1 (HIV-1) protease inhibitor which has potent in vitro and in vivo activity for HIV (2). In vitro data indicate that amprenavir is extensively metabolized by the microsomal P450 enzyme, CYP3A4, to oxidized products (8, 28, 38). In humans, less than 2% of an oral dose of amprenavir appears in urine as unchanged drug (38).
The CYP3A4 isoenzyme is the major route of metabolism for many drugs, including all of the currently available protease inhibitors (9, 10, 19, 24). The rifamycin antibiotics rifampin and rifabutin are well-known inducers of this isoenzyme (16, 18, 23, 24, 31; J. A. Smith, T. C. Hardin, T. F. Patterson, M. G. Rinaldi, and J. R. Graybill, Abstr. 2nd Natl. Conf. Hum. Retrovir. Relat. Infect., abstr. 126, p. 77, 1995), although rifabutin is believed to cause substantially less isoenzyme induction than rifampin (16, 23). In addition, the multidrug transporter, P-glycoprotein contributes to elimination of the protease inhibitors, and rifampin increases the activity of this transporter (17, 20, 35, 37). The potential for an interaction between amprenavir and these rifamycin antibiotics is clinically important because HIV-infected patients may receive rifampin or rifabutin for the prevention and treatment of opportunistic infections caused by mycobacteria (4, 6). The prevalence of infection caused by Mycobacterium tuberculosis, including multi drug-resistant tuberculosis, has increased with the emergence of AIDS (6). This has made treatment difficult, because rifampin induces metabolism of the available HIV-1 protease inhibitors and reduces the mean area under the curve (AUC) for individual agents by 35 to 92% (4, 6). Guidelines from the Centers for Disease Control and Prevention recommend that rifampin not be used with most protease inhibitors, and if rifabutin is used in place of rifampin, that it be given at a reduced dose (6). This study was undertaken to determine if a pharmacokinetic interaction exists when amprenavir and rifabutin or rifampin are coadministered.
The erythromycin breath test (ERMBT) is a measure of hepatic CYP3A4 activity (14, 36; ERMBT assay product information, Metabolic Solutions Inc., Nashua, N.H.). The inclusion of the ERMBT in this study was intended to evaluate the following questions. (i) Is amprenavir an inhibitor of hepatic CYP3A4 in vivo? (ii) What are the effects of rifabutin and rifampin on CYP3A4 activity as measured by the ERMBT? (iii) Do the results of the ERMBT help explain the pharmacokinetics of amprenavir when administered alone and in combination with rifabutin and rifampin?
MATERIALS AND METHODS
Subjects.
Nonsmoking men, aged 18 to 55 years, were eligible for this study, which was approved by the Committee on the Conduct of Human Research at Virginia Commonwealth University (VCU). Each subject gave written informed consent. A complete medical history; physical examination that included vital signs; and routine laboratory tests that included a 13-test chemistry screen, complete blood count with differential, urinalysis (dipstick), urine drug screen for illicit controlled substances, test for HIV antibiodies, and electrocardiogram were completed for each subject. Subjects were ineligible if they had a clinically significant abnormality at the screening evaluation, had donated blood within the past month, were taking concomitant medication(s) which could not be withheld for the duration of the study, or had a prior adverse reaction to rifabutin, rifampin, or another rifamycin antibiotic. Subjects were instructed to use a barrier method of contraception (condoms) while enrolled in the study and for a minimum of 1 month after administration of their last dose of study drugs. Additionally, subjects abstained from taking concomitant medications and from consuming alcohol from 48 h before each treatment day until discharge from the study center. The same restrictions were placed on consumption of grapefruit and grapefruit juice. Tea, coffee, chocolate, and other beverages and foods containing methyl xanthines were prohibited on each pharmacokinetic sampling day.
Experimental design and procedures.
This was an open-label, parallel-group, three-period study conducted at the School of Pharmacy Center for Drug Studies, VCU/Medical College of Virginia Campus. Following the screening evaluation, the study consisted of a treatment phase of up to 5 weeks' duration and a follow-up evaluation comprising up to four separate visits over a 3-month period. The screening evaluation was scheduled within 14 days before administration of the first dose of study drug. Subjects eligible after screening were randomized to two dosing cohorts (dosing cohort 1 [DC1] and DC2) and received the following treatments.
(i) DC1.
Subjects in DC1 were treated as follows: dosing days 1 to 4 (treatment 1), amprenavir (1,200 mg twice daily) for 3½ days, followed by a 7-day washout period; dosing days 5 to 18 (treatment 2), rifabutin (300 mg every morning) for 14 days; dosing days 19 to 28 (treatment 3), amprenavir (1,200 mg twice daily) plus rifabutin (300 mg every morning) for 10 days.
(ii) DC2.
Subjects in DC2 were treated as follows: dosing days 1 to 4 (treatment 1), amprenavir (1,200 mg twice daily) for 3½ days, followed by a 7-day washout period; dosing days 5 to 18 (treatment 4), rifampin (600 mg every morning) for 14 days; dosing days 19 to 22 (treatment 5), amprenavir (1,200 mg twice daily) plus rifampin (600 mg every morning) for 4 days.
Subjects received the first dose of amprenavir (treatment 1, DC1 and DC2) under the supervision of the study center staff. Subjects then left the center with sufficient drug for the next four doses and were instructed to complete a diary card to record the exact time they took their doses, the number of capsules taken, and any missed doses. The evening (6:00 p.m.) before dosing day 4, subjects returned to the study center, where a breath alcohol test was performed, in addition to a review of concomitant medications and other restricted substances since their last visit. Diary cards and drug containers were inspected and deviations from the dosing schedule (e.g., missed doses) were recorded. Subjects remained overnight in the study center.
On dosing day 4, drug was administered at 8:00 a.m. with 240 ml of tap water after an overnight fast (from at least 12:00 midnight). Water was prohibited for 4 h predosing until 4 h postdosing. Standard, balanced meals were served 4 and 10 h postdosing. Before dosing on day 4, blood samples for hematology and serum chemistry and urine for dipstick analyses were obtained. Serial blood samples for pharmacokinetic evaluation of amprenavir were drawn 5 min before dosing and up to 12 h postdosing (see schedule below). Urine for pharmacokinetic evaluation of amprenavir was collected 15 min before dosing and then at intervals up to 12 h postdosing (see below for exact timing of samples). Subjects were discharged from the study center after completion of all 12-h-postdosing procedures.
After a washout period of 7 days, subjects returned to the study center. The first dose of either rifabutin (treatment 2, DC1) or rifampin (treatment 4, DC2) was administered to each subject under staff supervision, after which subjects were given enough drug for the next 5 days. Subjects then left the study center. During this period, subjects self-administered either rifabutin or rifampin as directed and were asked to complete a drug diary as before. Subjects returned to the study center the morning of dosing day 11 for clinical laboratory evaluations as described before and an ERMBT (below). Diary cards and drug containers were inspected for compliance monitoring. After subjects were determined to be well, they were given enough rifabutin or rifampin sufficient for the next 6 days and were allowed to leave the study center.
The evening before dosing day 18, subjects returned to the study center and remained there for 2 nights, until completion of all 24-h-postdosing procedures. On arrival, diary cards and drug containers were again inspected for compliance and a breath alcohol test was performed. Subjects fasted after midnight. On the morning of dosing day 18, subjects received their last dose of rifabutin or rifampin. Clinical and laboratory evaluations were conducted as before. The only difference was that urine and serial blood samples for measurement of rifabutin and rifampin and their respective 25-O-desacetyl metabolites were collected up to 24 h postdosing. Once all 24-h-postdosing procedures were completed, if the subjects were well, they were allowed to leave the study center. Before leaving, the first dose of the combination amprenavir and rifabutin (treatment 3, DC1) or amprenavir and rifampin (treatment 5, DC2) was administered to subjects under staff supervision. Subjects were then given enough drug to self-administer over the next 2 days (DC2) or 8 days (DC1). Subjects completed a diary card as previously described.
Subjects were instructed to return to the study center the evening before dosing day 22 (DC2) or 28 (DC1), where they were to remain for 2 nights, until completion of all 24-h-postdosing procedures. On arrival, diary cards and drug containers were again inspected for compliance and a breath alcohol test was performed. Subjects fasted after midnight. On the morning of dosing day 22 (or 28), subjects received their last doses of amprenavir and rifampin (or amprenavir and rifabutin). Clinical and laboratory evaluations were conducted as before, with serial blood samples collected for up to 12 h postdosing and urine collected up to 12 h (for amprenavir) and up to 24 h postdosing for rifabutin, rifampin, and their respective metabolites.
The first follow-up evaluation occurred within 7 to 10 days after completion of the final treatment period. This comprised a physical examination, vital signs, electrocardiogram, clinical laboratory evaluations, and an ERMBT. If a subject's liver function tests (LFTs) were not clinically significant, then subsequent visits for follow-up LFTs were scheduled 1, 2, and 3 months after completion of the treatment phase. If a subject's LFTs were significantly elevated, additional follow-up visits were scheduled at weekly intervals until resolved.
Administration and analysis of the ERMBT.
The ERMBT (Metabolic Solutions, Inc.) was administered within 1 week before treatment period 1 (to establish baseline), at 2 h after the final dose of amprenavir (treatment 1), at 2 h after the morning dose of either rifampin or rifabutin at 7 and 14 days, at 2 h after the final dose of combined treatment with amprenavir and rifampin or rifabutin, and at the follow-up evaluation. For each ERMBT, subjects received an intravenous injection containing 3 μCi of [N-methyl-14C] erythromycin as a 1-min bolus infusion according to the manufacturer's directions. Twenty minutes later, subjects exhaled through a straw into a vial containing 20 ml of a 50:50 hyamine-ethanol solution containing thymolphthaline until there was a color change, from blue to clear, indicating the trapping of 2 mmol of CO2.
All ERMBT samples were assayed at the VCU School of Pharmacy Biopharmaceutical Analysis Laboratory. Liquid scintillation counting was used to measure exhaled 14CO2. Ten milliliters of Insta-Gel XF scintillation cocktail (Packard Instrument Co.) was added to decolorize samples in the scintillation vials; samples were mixed well and left in the dark at room temperature for at least 16 h. Scintillation in the samples was counted on a Packard Model Tricarb 4530 for 14C using terminators of 1% standard deviation or 10 min, whichever came first. Generally, scintillation in the samples was counted for 10 min. Counts per minute were converted to disintegrations per minute using a quench curve. Results of the ERMBT are expressed as percent erythromycin dose metabolized during the 1st h postinjection and are calculated from disintegrations per minute as previously described (34). The change in isoenzyme activity due to the study drug(s) was described using the equation 1 − (treatment period value/baseline value). The intra-assay precision (coefficient of variation [CV]) ranged from 3.4 to 3.8%.
Sample procurement and assay of plasma and urine samples for amprenavir, rifampin, and rifabutin and their respective metabolites.
On dosing days 4 and 28 (DC1) or 22 (DC2), blood was obtained for determination of amprenavir concentrations in plasma on 15 separate occasions during the respective treatment periods: 0 (taken 5 min before dosing), 0.25, 0.5, 0.75, 1, 1.5, 2, 2.5, 3, 4, 5, 6, 8, 10, and 12 h after drug administration. On dosing days 18 and 28 (DC1) or 22 (DC2), blood samples to determine concentrations of rifabutin, rifampin, and their 25-O-desacetyl metabolites in plasma were drawn at the same interval as amprenavir blood samples, but with additional samples for amprenavir, rifampin, rifabutin, and metabolites at 16 and 24 h postdosing. Blood samples were collected by venipuncture or peripheral venous catheter. Each blood sample for amprenavir analysis was collected in a prelabeled 4-ml lavender-stoppered Vacutainer tube (containing freeze-dried K2EDTA). Blood for analysis of the concentrations of rifabutin, rifampin, and their metabolites in plasma was drawn in 5-ml green-stoppered tubes containing freeze-dried sodium heparin. Ascorbic acid was added to prevent oxidation of rifampin and rifabutin. The exact time that each blood sample was drawn was recorded. Once separated, the plasma was stored in a polypropylene tube in an upright position at −20°C until shipment for analysis.
Urine was collected from each subject for the determination of concentrations of amprenavir, rifabutin, rifampin, and their 25-desacetyl metabolite. On dosing day 4, urine was collected just prior to dosing and over the collection intervals: 0 to 4, 4 to 8, and 8 to 12 h postdosing. On dosing days 18 and 28 (DC1) and 22 (DC2) urine was collected at the same times for dosing day 4 with an additional collection 12 to 24 h postdosing. Samples were stabilized with ascorbic acid. Urine was stored in a refrigerator during each collection interval. At the end of each collection period, the urine was weighed and two 10-ml aliquots were transferred to 13-ml tubes and stored in an upright position at −20°C until shipment for analysis.
Concentrations of amprenavir were determined at Glaxo Wellcome Research and Development with a semiautomated solid-phase extraction method. A 0.5-ml portion of plasma was combined with 0.5 ml of internal standard solution (VB 11599; 5.0 μg/ml). Solid-phase extraction was performed with a Waters MilliLab Workstation and C18 Sep Pak cartridge. Extraction cartridges were primed with methanol followed by water. The sample plus internal standard was loaded on the cartridge, and the cartridge was washed with water and methanol (65:35, vol/vol). The compound was eluted from the cartridge with 2.5 ml of acetonitrile (100%) and blown to dryness under gentle nitrogen gas at 50°C in a Turbo Vap. The sample was reconstituted with acetonitrile and water (45:55, vol/vol) and vortexed to mix, and 50 μl was injected. Amprenavir was detected by fluorescence (excitation λ = 245 nm; emission λ = 340 nm). The analytical column was a Waters Symmetry C18 column (3.9 by 150 mm) maintained at 40°C. The mobile phase was also acetonitrile and water (45:55, vol/vol), set at a flow rate of 0.8 ml/min. The amprenavir calibration standard concentrations ranged from 10 to 1,000 ng/ml, and the amprenavir plasma control concentrations were 30, 400, and 800 ng/ml. The limit of quantitation was 10 ng/ml. The interassay precision (CV) ranged from 1.8 to 4.7%.
Analysis of rifampin and 25-desacetylrifampin in human urine and plasma was performed by PPD-Development, Middleton, Wis. using validated methodologies. Aliquots of plasma and urine were stabilized with ascorbic acid solution. Internal standard (rifamycin) was added, and the analytes were isolated by liquid-liquid extraction into an organic phase (chloroform–methyl-t-butyl ether). The sample was evaporated, and the residues were reconstituted in a methanol solution. Concentrations were determined by high-performance liquid chromatography with UV detection. The data was acquired and interpolated against a calibration curve. The range of the assay was 0.10 to 10.0 μg/ml for rifampin in plasma, 0.05 to 5.0 μg/ml for 25-desacetylrifampin in plasma, 0.50 to 200 ng/ml for rifampin in urine, and 0.25 to 100 μg/ml for 25-desacetylrifampin in urine. The specificity, accuracy, precision, limits of quantification of the method, and recovery of both rifampin and the 25-desacetyl metabolites were evaluated with analytical standards, calibration standards, and spiked plasma standards to validate the assays. Accuracy, expressed as mean percent difference from the theoretical value, was demonstrated to be <10% for both rifampin and 25-desacetylrifampin in plasma and urine. Precision, expressed as a maximum CV, was demonstrated to be <10% for both rifampin and 25-desacetylrifampin in plasma and urine.
Analysis of rifabutin and 25-desacetylrifabutin in human urine and plasma was performed by BAS Analytics, West Lafayette, Ind., using validated methodologies. Aliquots of plasma and urine were combined with ethanol and an internal standard (N-propylrifabutin). The analytes were isolated by liquid-liquid extraction into an organic phase (n-butylchloride). The supernatant was removed and evaporated to dryness. The residues were reconstituted in trifluoroacetic acid-acetonitrile-water (0.2:32:68, vol/vol/vol). Further extraction of impurities was performed by liquid-liquid extraction into hexane. Concentrations were determined by high-performance liquid chromatography with UV detection from the resultant aqueous phase. The data were acquired and interpolated against a calibration curve. The range of the assay was 5 to 500 ng/ml for rifabutin in plasma, 3.7 to 300 ng/ml for 25-desacetylrifabutin in plasma, 50 to 5,000 ng/ml for rifabutin in urine, and 36.8 to 2,940 ng/ml for 25-desacetylrifabutin in urine. The specificity, accuracy, precision, limits of quantification of the method, and recovery of both rifabutin and the 25-desacetyl metabolite were evaluated with analytical standards, calibration standards, and spiked plasma standards to validate the assays. Accuracy, expressed as mean percent difference from the theoretical value, was demonstrated to be <15% for both rifabutin and 25-desacetylrifabutin in plasma and urine. Precision, expressed as a maximum CV, was demonstrated to be <10% for both rifabutin and 25-desacetylrifabutin in plasma and urine.
Pharmacokinetic analysis.
The observed peak concentrations in plasma at steady state (Cmax,ss) of amprenavir, rifabutin, and rifampin and the time to Cmax,ss (Tmax,ss) were obtained by inspection of the individual plasma concentration-time data. Individual estimates of the apparent terminal elimination rate constant (k) for each drug were obtained by log-linear regression of the terminal portions of the plasma concentration-time curves. Half-lives were calculated as 0.693/k. The steady-state AUC (AUCss) from time zero to the last quantifiable sample at 24 for rifampin and rifabutin or at 12 h for amprenavir was calculated by the linear trapezoidal method. The AUC from 0 h to infinity was calculated by adding Clast/k to AUCss. The apparent total clearance from plasma at steady state (CL/F) was calculated as dose/AUC. Similar formulae were used to determine the pharmacokinetic parameters for the metabolites (with the exception of CL/F). For each metabolite, the ratio of the metabolite AUC to that of the parent drug was also calculated based upon the AUC.
Urine pharmacokinetic parameters were determined for rifabutin, rifampin, and their 25-O-desacetyl metabolites. Renal clearance (CLR) was calculated as Aess/AUCss, where Aess is the amount of drug excreted in the urine over the steady-state dosing interval. The percentages of rifabutin, rifampin, and their 25-O-desacetyl metabolites eliminated in the urine were calculated based on parent compound equivalent weights.
Statistical analysis.
Data are presented as mean values ± standard deviations. Pharmacokinetic parameters other than Tmax, were log transformed, and analysis of variance with treatment as the fixed effect and subject as the random effect was performed on the calculated parameters. The geometric least-squares (LS) mean ratio and its 90% CI were calculated for each pharmacokinetic parameter and for the results of the ERMBT. Two one-sided t tests (90% CI) were performed on each dosing cohort to compare treatments. Pearson's correlation coefficient was calculated to assess the relationship of ERMBT to pharmacokinetic parameters of amprenavir, rifabutin, and rifampin. Differences and associations were interpreted as statistically significant when P was ≤0.05.
RESULTS
Subject characteristics.
Twenty-four healthy, HIV-seronegative, male subjects were enrolled in the study (19 Caucasian, 4 African-American, 1 Asian), with 12 subjects in each dosing cohort. The subjects' ages ranged from 18 to 49 years (mean, 27.3 years), and their weights ranged from 58.8 to 100 kg (mean, 75.9 kg). Demographic characteristics were similar between the two dosing cohorts.
Tolerability of study medications.
All 24 subjects completed treatment period 1 (amprenavir alone). The most common adverse events that occurred during treatment with amprenavir alone in both cohorts were nausea, oral numbness, dizziness, diarrhea, and headache.
In DC1, one subject was removed after failing to return to the study center on the evening of dosing day 17. The combination treatment with amprenavir and rifabutin was poorly tolerated. Five of the remaining eleven subjects were withdrawn from the study between day 1 and day 9 of combination therapy due to adverse events. The adverse events consisted of chiefly flu-like symptoms (e.g., headache, nausea, fever, myalgia, tiredness, vomiting, diarrhea, chills, weakness) and laboratory abnormalities (predominantly leukopenia). There was a clear decline in white blood cell (WBC) and neutrophil counts associated with therapy. At screening, DC1 had a mean WBC count of 5.98 × 103/mm3 (60% granulocytes). Following completion of amprenavir-alone, rifabutin-alone, and combination therapy, the mean WBC counts were 6.22 × 103/mm3 (53% granulocytes), 3.88 × 103/mm3 (46% granulocytes), and 3.24 × 103/mm3 (47% granulocytes). Seven of 11 subjects starting rifabutin and amprenavir had WBC counts of less than 3,000 cells/ml compared with none of the subjects in the rifampin arm.
In DC2, 11 of 12 subjects completed the study. One subject was removed following treatment period 1 (amprenavir alone) due to development of a maculopapular rash. The combination of amprenavir and rifampin was otherwise well tolerated. There were no hematological adverse effects associated with combination therapy. The most common adverse effect seen during rifampin therapy, alone or in combination with amprenavir, was a discoloration of the urine consistent with the known effects of rifampin.
Pharmacokinetics.
Twenty-four subjects were included in the pharmacokinetic analysis of amprenavir alone, 11 subjects for rifabutin alone and 11 for rifampin alone. There were 11 subjects included in the pharmacokinetic analysis of amprenavir and rifampin when given in combination, but there were only 6 subjects included in the pharmacokinetic analysis when amprenavir was given in combination with rifabutin.
Amprenavir.
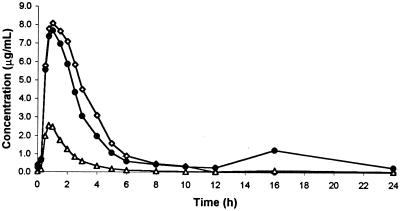
Table 1 provides the summary pharmacokinetic parameters, geometric LS mean ratio, and the 90% CI estimates for amprenavir pharmacokinetic parameters, alone and in combination with rifabutin and rifampin. There were no statistically significant differences in the pharmacokinetics of amprenavir when coadministered with rifabutin, but only 6 subjects were available for a full pharmacokinetic profile. In contrast, rifampin resulted in statistically significant decreases in AUCss, Cmax,ss and minimum concentration in plasma (Cmin,ss) (82, 70, and 92%, respectively) of amprenavir and a 5.45-fold increase in amprenavir CL/F. Figure 1 illustrates the effects of rifampin and rifabutin on the mean concentration-time profile of amprenavir.
TABLE 1.
Summary of results of geometric LS means for amprenavir pharmacokinetic parametersa
| Treatment no. or ratio | Descriptionb | AUCss (h · μg/ml) | Cmax,ss (μg/ml) | Cmin,ss (μg/ml) | Tmax,ss (h)c | CL/F (ml/min) |
|---|---|---|---|---|---|---|
| 1 (n = 24) | APV 1,200 mg BID | 27.32 (24.01, 31.08) | 9.20 (7.89, 10.74) | 0.32 (0.25, 0.39) | 1.00 (0.87, 1.38) | 732 (644, 833) |
| 3d | APV 1,200 mg BID + RFB 300 mg QD | 29.12 (17.05, 28.69) | 8.56 (7.17, 10.23) | 0.25 (0.14, 0.42) | 1.12 (0.73, 1.50) | 904 (697, 1,173) |
| 5 (n = 11)d | 4.99 (3.96, 6.28) | 2.78 (2.07, 3.72) | 0.02 (0.02, 0.03) | 0.75 (0.62, 1.00) | 4,010 (3,187, 5,046) | |
| Ratio 3:1 (90% CI) | 0.85 (0.72–1.00) | 0.93 (0.75–1.10) | 0.85 (0.62–1.17) | 0.00 (−0.64–0.38) | 1.18 (1.00–1.38) | |
| Ratio 5:1 (90% CI) (n = 11) | APV 1,200 mg BID + RFP 600 mg QD | 0.18 (0.16–0.22) | 0.30 (0.24–0.38) | 0.08 (0.05–0.11) | −0.25 (−0.76–0.00) | 5.45 (4.64–6.39) |
Unless otherwise indicated data in parentheses indicate 95% CI.
Abbreviations: APV, amprenavir; RFB, rifabutin; RFP, rifampin; BID, twice daily; QD, once daily.
Median and median difference.
Based upon 6 subjects only.
FIG. 1.
Mean amprenavir concentrations in plasma following 1,200 mg administered alone (open diamond), following 2 weeks of rifampin (600 mg once daily) (open triangle), and following 2 weeks of rifabutin (300 mg once daily) (closed circle).
Rifabutin.
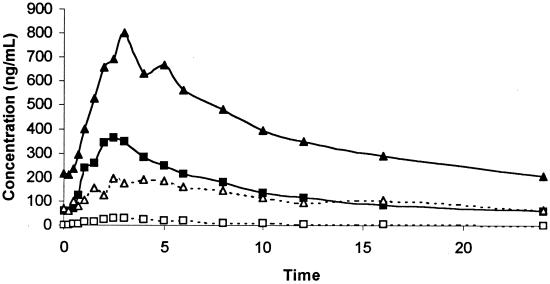
Table 2 provides the summary pharmacokinetic data for rifabutin alone and when administered with amprenavir. There were statistically significant 2.93-, 2.19-, and 3.71-fold increases in rifabutin AUCss, Cmax,ss and Cmin,ss when coadministered with amprenavir. There was a 66% decrease in rifabutin CL/F. The median Tmax,ss following administration of the combined treatment was 1.0 h longer than after administration of rifabutin alone. There was a 2.51-fold increase in the amount of rifabutin excreted in the urine as unchanged parent drug when it was administered with amprenavir. Figure 2 illustrates the effect of amprenavir on the mean concentration-time profile of rifabutin.
TABLE 2.
Summary of results of geometric LS means (95% CI) for rifabutin and 25-O-desacetylrifabutin pharmacokinetic parameters
| Treatment no. or ratio | Descriptiona | AUCss (h · μg/ml) | Cmax,ss (μg/ml) | Cmin,ss (μg/ml) | CL/F (ml/min) | Tmax,ss (h)b | CLR (ml/min) | % Dose |
|---|---|---|---|---|---|---|---|---|
| 2 | RFB 300 mg QD (n = 11) | 3.39 (2.84, 4.03) | 0.38 (0.30, 0.48) | 0.06 (0.04, 0.07) | 1,477 (1,241, 1,758) | 2.01 (1.00, 3.00) | 58.4 (48.6, 70.1) | 4.06 (3.17, 4.95) |
| 3 | APV 1,200 mg BID + RFB 300 mg QD (n = 6) | 9.92 (8.07, 12.20) | 0.84 (0.64, 1.10) | 0.20 (0.15, 0.28) | 504 (410, 619) | 3.74 (2.48, 5.00) | 53.02 (53.02, 64.33) | 10.18 (9.11, 11.35) |
| Ratio 3:2 (90% CI) (n = 6) | 2.93 (2.56–3.35) | 2.19 (1.82–2.64) | 3.71 (2.71–5.09) | 0.34 (0.30–0.39) | 1.00 (0.24–2.50) | 0.91 (0.84–0.98) | 2.51 (2.33–2.69) | |
| 2 | 25-RFB 300 mg QD (n = 11) | 0.23 (0.19, 0.28) | 0.031 (0.26, 0.36) | 0.002 (−0.01, 0.01) | 0.070 (0.046, 0.095) | 2.50 (2.00, 5.00) | 123.6 (109.8, 138.2) | 0.61 (0.37, 0.86) |
| 3 | APV 1,200 mg BID + 25-RFB 300 mg QD (n = 6) | 3.06 (2.35, 3.98) | 0.226 (0.178, 0.286) | 0.066 (0.0053, 0.079) | 0.301 (0.268, 0.333) | 2.74 (1.48, 6.0) | 94.8 (82.9, 108.3) | 5.85 (5.53, 6.17) |
| 4 | Ratio 3:2 (90% CI) (n = 6) | 13.35 (10.93–16.30) | 7.39 (5.87–9.29) | 32.92 (26.6–39.2) | 4.27 (3.90–4.64) | 0.24 (−0.75–1.24) | 0.77 (0.71–0.82) | 9.51 (9.09–9.93) |
25-RFB, 25-O-desacetylrifabutin. See footnote b of Table 1 for other abbreviations.
Median and median difference.
FIG. 2.
Mean rifabutin and 25-O-desacetylrifabutin concentrations in plasma following doses 300 mg once daily given for 2 weeks (closed squares and open squares, respectively) and following coadministration with amprenavir (1,200 mg) (closed triangles and open triangles, respectively).
25-O-Desacetylrifabutin.
Table 2 provides the summary pharmacokinetic data for 25-O-desacetylrifabutin, when rifabutin was administered alone and in combination with amprenavir. When coadministered, the AUCss, Cmax,ss and Cmin,ss of 25-O-desacetylrifabutin were increased by 13.35-, 7.39-, and 32.9-fold, respectively, compared to rifabutin alone. There was a 4.27-fold increase in the AUC ratio (AUC of 25-O-desacetylrifabutin to that of rifabutin). There was a 23% decrease in CLR when rifabutin was administered with amprenavir. The percent dose of rifabutin excreted in the urine as 25-O-desacetylrifabutin was 9.5-fold greater following the administration of rifabutin with amprenavir. Figure 2 illustrates the effect of amprenavir on the mean concentration-time profile of 25-O-desacetylrifabutin.
Rifampin.
There were no significant differences in AUCss, Cmax,ss and CL/F when rifampin was given alone and in combination with amprenavir (Table 3). There was a 22% decrease in CLR and an 18% decrease in the amount of rifampin excreted in the urine as parent drug following coadministration with amprenavir.
TABLE 3.
Summary of results of geometric LS means (95% CI) for rifampin pharmacokinetic parameters
| Treatment no. or ratio | Descriptionb | AUCss (h · μg/ml) | Cmax,ss (μg/ml) | CL/F (ml/min) | Tmax,ss (h)a | CLR (ml/min) | % Dose |
|---|---|---|---|---|---|---|---|
| 4 | 600 mg RFP QD | 28.08 (22.37, 35.26) | 8.50 (6.55, 11.03) | 356 (284, 447) | 1.48 (1.00, 2.00) | 18.33 (15.39, 21.83) | 5.49 (4.29, 6.69) |
| 5 | 600 mg RFP QD + 1,200 mg APV BID | 28.30 (22.54, 35.53) | 8.41 (6.48, 10.92) | 353 (281, 444) | 1.50 (1.24, 2.00) | 14.38 (12.07, 17.13) | 4.28 (3.07, 5.48) |
| Ratio 5:4 (90% CI) | 1.01 (0.90, 1.13) | 0.99 (0.87, 1.12) | 0.99 (0.88, 1.11) | 1.01 (0.49, 0.51) | 0.78 (0.68, 0.90) | 0.78 (0.69, 0.87) |
Median and median difference.
See footnote b of Table 1 for abbreviations.
25-O-Desacetylrifampin.
There were no statistically significant differences in 25-O-desacetylrifampin pharmacokinetics when rifampin was administered alone or in combination with amprenavir (data not shown).
Erythromycin breath test.
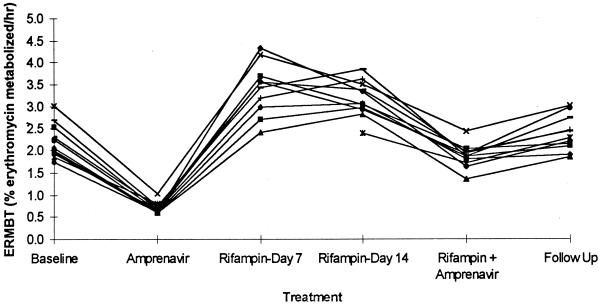
Amprenavir treatment reduced the LS mean ratio for the ERMBT to 17% of baseline for both dosing cohorts, and both rifampin and rifabutin significantly increased the ERMBT at 1 and 2 weeks of treatment. At follow-up, the ERMBT mean ratios had returned to baseline for both dosing cohorts. Figure 3 illustrates the ERMBT results for the rifampin group. Results for the rifabutin group were similar (data not shown), except for the combination treatment regimen of rifabutin plus amprenavir, in which the ERMBT result was significantly lower.
FIG. 3.
Individual ERMBT results at baseline; following administration of amprenavir (1,200 mg twice daily), administration of rifampin (600 mg once daily) for 7 and 14 days, and administration of the combination of amprenavir and rifampin; and at follow-up.
There was no significant linear correlation between baseline ERMBT and the AUCss of amprenavir (r2 = 0.00; P > 0.05) or the AUCss of rifabutin (r2 = 0.05; P > 0.05), nor was there a significant correlation between the magnitude of percent increase in the ERMBT result following 14 days of rifampin and the magnitude of percent decrease in the AUCss for amprenavir after 14 days of rifampin (r2 = 0.11; P < 0.05).
DISCUSSION
The pharmacokinetics of amprenavir, rifabutin, and rifampin when administered alone are in agreement with those reported in previous studies (1, 2, 5, 29). The effects of rifabutin and rifampin on amprenavir pharmacokinetics are consistent with observations for other protease inhibitors, indicating that amprenavir is a substrate for CYP3A4 (8, 28), rifabutin is a less potent inducer of metabolic clearance for protease inhibitors than is rifampin (4, 6, 10, 16, 23, 24), and rifabutin and its 25-O-desacetyl metabolite are metabolized by CYP3A4 (15, 32).
Rifabutin and amprenavir.
Amprenavir significantly decreased clearance of rifabutin and 25-O-desacetylrifabutin. These results are similar to the effects of ritonavir(5) and other protease inhibitors (4, 6, 10, 24). on the pharmacokinetics of rifabutin. An assessment of the effect of rifabutin on the pharmacokinetics of amprenavir is confounded by the poor tolerability of the combination and the relatively few subjects who completed combination therapy. Although there is a 15% mean reduction in amprenavir AUCss, only six subjects were able to provide full pharmacokinetic data. However, even a true difference of this magnitude is unlikely to be clinically important.
The effects of amprenavir on the pharmacokinetics of rifabutin are clinically significant, and 5 of 11 subjects were unable to complete treatment due to adverse drug events. These flu-like symptoms and neutropenia have been previously reported when high doses of rifabutin are given alone (22, 27) or coadministered with CYP3A4 inhibitors such as HIV-1 protease inhibitors (E. Sun, M. Heath-Chiozzi, D. W. Cameron, A. Hsu, R. G. Granneman, and C. J. Maurath, Proc. Abstr. XI Int. Conf. AIDS, 1996), fluconazole (33), and clarithromycin (12, 13). These adverse effects are likely caused by increased concentrations of rifabutin and/or 25-O-desacetylrifabutin, such as those observed in this study. It is therefore recommended that the dose of rifabutin be decreased by at least 50% if medically indicated for concomitant use with amprenavir. In addition, patients should be observed for uveitis and flu-like symptoms and monitored for leukopenia.
Rifampin and amprenavir.
The coadministration of amprenavir and rifampin resulted in significant changes in the pharmacokinetics of amprenavir, including an 82% decrease in the AUCss and an increase of greater than fivefold in amprenavir CL/F. This most likely reflects induction of hepatic and intestinal CYP3A4 by rifampin, and possibly enhancement of P-glycoprotein transport, resulting in an increase in clearance of amprenavir. Decreases in amprenavir concentrations in plasma of the magnitude observed in this study are of probable clinical relevance as trough concentrations are below the 95% inhibitory concentration for HIV isolates (2). Of the steady-state pharmacokinetic parameters for the HIV-1 protease inhibitors, Cmin,ss has been shown to be the best predictor of antiviral response, as well as being associated with the development of resistance (7, 21, 30). Because rifampin markedly increases amprenavir's metabolism, coadministration of these drugs is contraindicated (4, 6).
The administration of amprenavir with rifampin had no effect on the pharmacokinetics of rifampin or its metabolite 25-O-desacetylrifampin. This is consistent with statements that rifampin is not a substrate for CYP3A4 (4). There was a 22% decrease in rifampin and a 12% decrease in 25-O-desacetylrifampin CLR. Amprenavir may slightly inhibit the CLR of rifampin, but the effect is not clinically significant.
ERMBT results.
Amprenavir reduced hepatic CYP3A4 activity, as measured by the ERMBT, to 17% of baseline. Similar results were observed in our other studies with amprenavir (3, 25). Compared with the baseline, the mean ERMBT increased at 7 and 14 days of rifabutin and rifampin treatment by 181 and 187% and 164 and 156%, respectively. It appears that induction is not greater following 2 weeks of rifamycin therapy than following 1 week. These results are similar to the findings of a prior investigation with rifampin that revealed a mean 186% increase in the ERMBT (11). The effects of rifabutin on the ERMBT have not been previously reported.
ACKNOWLEDGMENTS
This work was supported by a grant from Glaxo Wellcome, Inc.
Appreciation is expressed to Cindy Rawls (Glaxo Wellcome, Inc.), who performed the amprenavir assays; to Clark March (School of Pharmacy, VCU), who performed the ERMBT scintillation counts; and to the staff of the VCU School of Pharmacy Center for Drug Studies.
REFERENCES
- 1.Acocella G. Clinical pharmacokinetics of rifampicin. Clin Pharmacokinet. 1978;3:108–127. doi: 10.2165/00003088-197803020-00002. [DOI] [PubMed] [Google Scholar]
- 2.Adkins J C, Faulds D. Amprenavir. Drugs. 1998;55:837–842. doi: 10.2165/00003495-199855060-00015. [DOI] [PubMed] [Google Scholar]
- 3.Brophy D F, Israel D S, Pastor A, Gillotin C, Chittick G E, Symonds W T, Lou Y, Sadler B M, Polk R E. Pharmacokinetic interaction between amprenavir and clarithromycin in healthy male volunteers. Antimicrob Agents Chemother. 2000;44:978–984. doi: 10.1128/aac.44.4.978-984.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burman W J, Gallicano K, Peloquin C. Therapeutic implications of drug interactions in the treatment of human immunodeficiency virus-related tuberculosis. Clin Infect Dis. 1999;28:419–430. doi: 10.1086/515174. [DOI] [PubMed] [Google Scholar]
- 5.Cato A, III, Cavanaugh J, Shi H, Hsu A, Leonard J, Granneman R. The effect of multiple doses of ritonavir on the pharmacokinetics of rifabutin. Clin Pharmacol Ther. 1998;63:414–421. doi: 10.1016/S0009-9236(98)90036-4. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Updated guidelines for the use of rifabutin or rifampin for the treatment and prevention of tuberculosis among HIV-infected patients taking protease inhibitors or nonnucleoside reverse transcriptase inhibitors. Morb Mortal Wkly Rep. 2000;48:185–189. [PubMed] [Google Scholar]
- 7.Danner S, Carr A, Leonard J M, Lehman L M, Gudiol F, Gonzales J, Raventos A, Rubio R, Bouza E, Pintado V. A short-term study of the safety, pharmacokinetics, and efficacy of ritonavir, an inhibitor of HIV-1 protease. N Engl J Med. 1995;7:1528–1533. doi: 10.1056/NEJM199512073332303. [DOI] [PubMed] [Google Scholar]
- 8.Decker C J, Laitinen L M, Bridson G W, Raybuck S A, Tung R D, Chaturvedi P R. Metabolism of amprenavir in liver microsomes: role of CYP3A4 inhibition for drug interactions. J Pharm Sci. 1998;87:803–807. doi: 10.1021/js980029p. [DOI] [PubMed] [Google Scholar]
- 9.Fitzsimmons M E, Collins J M. Selective biotransformation of the human immunodeficiency virus protease inhibitor saquinavir by human small-intestinal cytochrome P4503A4: potential contribution to high first-pass metabolism. Drug Metab Dispos. 1997;25:256–266. [PubMed] [Google Scholar]
- 10.Flexner C. HIV-protease inhibitors. N Engl J Med. 1998;338:1281–1292. doi: 10.1056/NEJM199804303381808. [DOI] [PubMed] [Google Scholar]
- 11.Garaibeh M N, Gillen L P, Osborne B, Schwartz J I, Waldman S A. Effect of multiple doses of rifampin on the [14C N-methyl] erythromycin breath test in healthy male volunteers. J Clin Pharmacol. 1998;38:492–495. doi: 10.1002/j.1552-4604.1998.tb05785.x. [DOI] [PubMed] [Google Scholar]
- 12.Griffith D E, Brown D A, Girard W M, Wallace R J. Adverse events associated with high-dose rifabutin in macrolide-containing regimens for the treatment of Mycobacterium avium complex lung disease. Clin Infect Dis. 1995;21:594–598. doi: 10.1093/clinids/21.3.594. [DOI] [PubMed] [Google Scholar]
- 13.Hafner R, Bethel J, Power M, Landry B, Banach M, Mole L, Standiford H C, Follansbee S, Kumar P, Raasch R, Drusano G. Tolerance and pharmacokinetic interactions of rifabutin and clarithromycin in human immunodeficiency virus-infected volunteers. Antimicrob Agents Chemother. 1998;42:631–639. doi: 10.1128/aac.42.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hall S D, Thummel K E, Watkins P B, Lown K S, Benet L Z, Paine M F, Mayo R R, Turgeon D K, Bailey D G, Fontana R J, Wrighton S A. Molecular and physical mechanisms of first-pass extraction. Drug Metab Dispos. 1999;27:161–16. [PubMed] [Google Scholar]
- 15.Iatsimirskaia E, Tulebaev S, Storozhuk E, Utkin I, Smith D, Gerber N, Koudriakova T. Metabolism of rifabutin in human enterocyte and liver microsomes: kinetic parameters, identification of enzyme systems, and drug interactions with macrolides and antifungal agents. Clin Pharmacol Ther. 1997;61:554–562. doi: 10.1016/S0009-9236(97)90135-1. [DOI] [PubMed] [Google Scholar]
- 16.Jamis-Dow C A, Katki A G, Collins J M, Klecker R W. Rifampin and rifabutin and their metabolism by human liver esterases. Xenobiotica. 1997;27:1015–1024. doi: 10.1080/004982597239994. [DOI] [PubMed] [Google Scholar]
- 17.Kim R B, Fromm M F, Wandel C, Leake B, Wood A J J, Roden D M, Wilkinson G R. The drug transporter P-glycoprotein limits oral absorption and brain entry of HIV-1 protease inhibitors. J Clin Investig. 1998;101:289–294. doi: 10.1172/JCI1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kohlars J C, Schmeidlin-Ren P, Schuetz J D, Fang C, Watkins P B. Identification of rifampin-inducible P450IIIA4 (CYP3A4) in human small bowel enterocytes. J Clin Investig. 1992;90:1871–1878. doi: 10.1172/JCI116064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koudriakova T, Iatsimirskaia E, Utkin I, Gangl E, Vouros P, Storozhuk E, Ozra D, Marinina J, Gerber N. Metabolism of the human immunodeficiency virus protease inhibitors indinavir and ritonavir by human intestinal microsomes and expressed cytochrome P450 3A4/3A5: mechanism-based inactivation of cytochrome P450 3A by ritonavir. Drug Metab Dispos. 1998;26:552–561. [PubMed] [Google Scholar]
- 20.Lee C G L, Gottesman M M, Cardarelli C O, Ramachandra M, Jeang K T, Ambudkar S V, Pastan I, Dey S. HIV-1 protease inhibitors are substrates for the MDR1 multidrug transporter. Biochemistry. 1998;37:3594–3601. doi: 10.1021/bi972709x. [DOI] [PubMed] [Google Scholar]
- 21.Lorenzi P, Yerly S, Abderrakim K, Fathi M, Rutschmann O T, von Overback J, Ledue D, Perrin L, Hirschel B. Toxicity, efficacy, plasma drug concentrations and protease mutations in patients with advanced HIV infection treated with ritonavir plus saquinavir. AIDS. 1997;11:F95–99. doi: 10.1097/00002030-199712000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Maddix D S, Tallian K B, Mead P S. Rifabutin: a review with emphasis on its role in the prevention and treatment of disseminated Mycobacterium avium complex infection. Ann Pharmacother. 1994;28:1250–1254. doi: 10.1177/106002809402801108. [DOI] [PubMed] [Google Scholar]
- 23.Perucca E, Grimaldi R, Frigo G M, Sardi A, Monig H, Ohnhaus E E. Comparative effects of rifabutin and rifampicin on hepatic microsomal enzyme activity in normal subjects. Eur J Clin Pharmacol. 1988;34:595–599. doi: 10.1007/BF00615223. [DOI] [PubMed] [Google Scholar]
- 24.Piscitelli S C, Flexner C, Minor J R, Polis M A, Masur H. Drug interactions in patients infected with human immunodeficiency virus. Clin Infect Dis. 1996;23:685–693. doi: 10.1093/clinids/23.4.685. [DOI] [PubMed] [Google Scholar]
- 25.Polk R E, Crouch M A, Israel D, Pastor A, Sadler B M, Chittick G E, Symonds W T, Gouldin W, Lou Y. Pharmacokinetic interaction between ketoconazole and amprenavir after single doses in healthy men. Pharmacotherapy. 1999;19:1378–1384. doi: 10.1592/phco.19.18.1378.30905. [DOI] [PubMed] [Google Scholar]
- 26.Salphati L, Benet L Z. Modulation of P-glycoprotein expression by cytochrome P450 3A inducers in male and female rat livers. Biochem Pharmacol. 1998;55:387–395. doi: 10.1016/s0006-2952(97)00436-x. [DOI] [PubMed] [Google Scholar]
- 27.Siegal F P, Eilbott D, Burger H, Gehan K, Davidson B, Kaell A T, Weiser B. Dose-limiting toxicity of rifabutin in AIDS-related complex: syndromes of arthralgia/arthritis. AIDS. 1990;4:433–441. doi: 10.1097/00002030-199005000-00009. [DOI] [PubMed] [Google Scholar]
- 28.Singh R, Taylor L C, Chang S Y. In vitro metabolism of a potent HIV-protease inhibitor (141W94) using rat, monkey and human liver S9. Rapid Commun Mass Spectrom. 1996;10:1019–1026. doi: 10.1002/(SICI)1097-0231(19960715)10:9<1019::AID-RCM618>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 29.Skinner M H, Blaschke T F. Clinical pharmacokinetics of rifabutin. Clin Pharmacokinet. 1995;28:115–125. doi: 10.2165/00003088-199528020-00003. [DOI] [PubMed] [Google Scholar]
- 30.Stein D S, Fish D G, Bilello J A, Preston S L, Martineau G L, Drusano G L. A 24-week open-label phase I/II evaluation of the HIV protease inhibitor MK-639 (indinavir) AIDS. 1996;10:485–492. doi: 10.1097/00002030-199605000-00006. [DOI] [PubMed] [Google Scholar]
- 31.Strayhorn V A, Baciewicz A M, Self T H. Update on rifampin drug interactions, III. Arch Intern Med. 1997;157:2453–2458. [PubMed] [Google Scholar]
- 32.Trapnell C B, Jamis-Dow C, Klecker R W, Collins J M. Metabolism of rifabutin and its 25-desacetyl metabolite, LM 565, by human liver microsomes and recombinant human cytochrome P-450 3A4: relevance to clinical interaction with fluconazole. Antimicrob Agents Chemother. 1997;41:924–926. doi: 10.1128/aac.41.5.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trapnell C B, Narang P K, Li R, Lavelle J P. Increased plasma rifabutin levels with concomitant fluconazole therapy in HIV-infected patients. Ann Intern Med. 1996;124:573–576. doi: 10.7326/0003-4819-124-6-199603150-00006. [DOI] [PubMed] [Google Scholar]
- 34.Wagner D. CYP3A4 and the erythromycin breath test. Clin Pharmacol Ther. 1998;64:129. doi: 10.1016/S0009-9236(98)90031-5. [DOI] [PubMed] [Google Scholar]
- 35.Washington C B, Duran G E, Man M C, Sikic B I, Blaschke T. Interaction of anti-HIV protease inhibitors with the multidrug transporter P-glycoprotein (P-gp) in human cultured cells. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;19:203–209. doi: 10.1097/00042560-199811010-00001. [DOI] [PubMed] [Google Scholar]
- 36.Watkins P B. Non-invasive tests of CYP3A enzymes. Pharmacogenetics. 1994;4:171–184. doi: 10.1097/00008571-199408000-00001. [DOI] [PubMed] [Google Scholar]
- 37.Wille R T, Lown K S, Huszczo M F, Schmiedlen-Ren P, Watkins P B. Short term effects of medications on CYP3A4 and P-glycoprotein expression in human intestinal mucosa. Gastroenterology. 1997;112:A419. [Google Scholar]
- 38.Woolley J, Studenberg S, Sadler B, et al. The disposition of [14−C]-amprenavir in human volunteers. Pharm Sci. 1998;1(Suppl.):2044. . (Abstract.) [Google Scholar]