Abstract
This work aims to explore the differences in phytochemical composition and biological properties of five strawberry hybrids (Fragaria × ananassa Duch.), and highlights the non-edible part (byproduct) as a source of self-remedy natural herb along with fruits. HPLC/DAD/HRESIMS technique was used in the dereplication of ten ethanolic extracts of five strawberry cultivars leaves and fruits (Festival, Red Merlin, Suzana, Tamar and Winter Dawn). Total phenolic and total flavonoid contents were established using Folin–Ciocalteu and aluminum chloride colorimetric assays, respectively. Ethanolic extracts of leaves and fruits from Festival and Red Merlin cultivars were selected to investigate their anti-hyperglycemic activity using streptozotocin-induced diabetic rats. Oxidative stress markers, lipid profile and kidney and liver function tests were assessed. The results revealed different chemical profiles of ten samples with the identification of 37 metabolites, represented mainly as flavonoids and phenolic acid derivatives. Phytochemical investigation resulted in the isolation of seven known phenolic compounds; quercetin, kaempferol, p-coumaric acid, p-tyrosol, methyl gallate, trans-tiliroside and eutigoside A. Suzana cultivar was the richest cultivar with flavonoids and total phenolics except for the total flavonoid content in leaves referred to Festival cultivar. Ethanolic extract of leaves, especially Festival cultivar was the most bioactive one. The results established the role of strawberry leaves along with fruits as an antioxidant and hypoglycemic natural remedy.
This work aims to explore the differences in phytochemical composition and biological properties of five strawberry hybrids (Fragaria × ananassa Duch.), and highlights the non-edible part (byproduct) as a source of self-remedy natural herb along with fruits.
1. Introduction
Berry fruits consumption improves human health due to the high content of nutrients and polyphenolics.1 Strawberry is a kind of berries and considered a global commercial crop, which belongs to genus Fragaria in the Rosaceae family. Its closest relatives are Duchesnea and Potentilla.1,2 Strawberry fruits contain a high level of nutritive components as minerals (Fe, P, K, Mg, Mn, Cu, and I2),3 vitamins (A, E, K, B1, B2, B3, B5, B6, B12, and especially vitamin C (60 mg per 100 g FW)),3 small essential molecules as choline, betaine, folate, fatty acids, and dietary fiber.1,3,4 Strawberry fruits also contain non-nutritive polyphenolic compounds such as anthocyanins, proanthocyanidins, and ellagitannins, flavonols.4,5
Strawberries consumption plays a good protective role against chronic diseases such as Alzheimer's dementia,6 cancer, and cardiovascular diseases,1 along with other health benefits as an anti-inflammatory and wound healing activities,7 antiplatelet aggregation,8 anti-obesity and anti-melanogenesis,9 anti-nephropathy10 and antidiabetic.11
Interestingly, the strawberry leaves also contain a wide range of phenolic compound classes which have not been exploited,12–14 and not fully known, which may hold more interest.15 Strawberry leaves contain an enormous amount of kaempferol and quercetin derivatives, chlorogenic and caffeic acid derivatives, ellagitannins, and octadecatrienoic acid derivatives.16 This may explain why leaves of (black and red raspberries, and strawberry) have higher total phenolic content and antioxidant capacity than their fruits.17
Besides, strawberry fruits were reported for their high antioxidant capacity in comparison to 12 studied fruits even in fresh or dry weight, referring to the antioxidant power not only to vitamin C but to other components.18 Decoctions of wild strawberry leaves were used as antihypertensive treatment.16,19
Diabetes mellitus (DM) is the disease of 347 million people worldwide. It will be the 7th leading cause of death in 2030.20 Currently, the prevalence of type 2 diabetes (T2DM) in Egypt is around 15.6% of all adults aged 20 to 79 which about 8 222 600 case.21 It is heavy burden on our health and economic systems. Diabetic patients develop different types of complications, such as renal disease, retinopathy, cardiovascular disease, diabetic-foot22 but the most threatening one is the development of malignancies and chronic diseases which affect the life quality of the patients if it is not well controlled and the complications are not precisely managed.
Both synthetic antidiabetic drugs and synthetic protective agents exhibited several side effects so, broadcast for new natural sources, that have attracted attention as good and safe alternatives.23–25 Strawberry leaf extract was reported to elevate serum insulin level, improve renal functions and free thyroid hormones in vivo.26
According to Zhu et al.,9in vitro study of different organs from strawberry plant suggested that each part contain different phenolics which may vary in polarity or numbers of functional groups or antioxidant power. The study also found a positive correlation between the antioxidant power of tested ethanolic extracts and their total phenolic content. Herein, we are interested in discovering the nature of five strawberry (Fragaria × ananassa Duch.) cultivars cultivated in Egypt, focusing on leaves as a promising source for many health-related applications.14 This study aimed to illustrate how the cultivar type and breeding process affect phytochemical contents of the strawberry plant by the assessment of total flavonoid and phenolic contents, establishing metabolite profiling, and assessing the potential antidiabetic effect of the most consumed cultivars in Egypt cv. Festival and cv. Red Merlin. It was worth mentioned it is the first comparison of these five cultivars.
2. Materials and methods
2.1. Chemicals and reagents
Acetonitrile, methanol, and formic acid (HPLC grade) were purchased from Merck (Darmstadt, Germany) and distilled water was purified using a Milli-Q system (Millipore, MA, USA). n-Hexane, dichloromethane, ethyl acetate and butanol, sodium carbonate, potassium acetate, aluminum chloride were purchased from El-Gomhorya Co. (Egypt). Quercetin, kaempferol, rutin, gallic acid, Folin–Ciocalteu, MeOH-d4, DMSO-d4, silica gel mesh 60, Sephadex LH 20 were purchased from Sigma Co. (USA). All chemical reagents and solvents (analytical grade, BDH).
STZ (streptozotocin) was purchased from (Sigma Co., USA). Gliclazide tablets were purchased from Servier Egypt Industries Ltd. (Giza, Egypt). In serum, glucose GOD-PAP enzymatic colorimetric kit was purchased from Spinreact S.A. (Sant Esteve de Bas, Spain). Hemoglobin A1C chromatographic–spectrophotometric ion-exchange Kit was purchased from Biosystems Reagents and Instruments Inc. (Barcelona, Spain). Serum aspartate aminotransferase (AST), alanine aminotransferase, (ALT) and alkaline phosphatase (ALP), total bilirubin (T.B), and total cholesterol (T.C) were assayed by Randox kits (Antrim, United Kingdom). Triglycerides (T.G) was assayed by Cayman kit (Ann Arbor, USA). Blood urea, creatinine and uric acid were determined by an enzymatic colorimetric kit purchased from Diamond Diagnostics (Egypt). Total antioxidant capacity, reduced glutathione, and lipid peroxide (malondialdehyde) colorimetric method kits were purchased from Biodiagnostic Inc. (River Falls, WI).
2.2. Plant material
Leaves and fruits from five cultivars of strawberry (Fragaria × ananassa Duch.) cvs. Festival, Red Merlin, Suzana, Tamar and Winter Dawn were collected from Pico farms, Om-Saber region, El-Beheira Governorate, Delta region, Egypt, which belongs to PICO Modern Agriculture Company (MACO). Taxonomical identity was kindly verified by the dean of the farm. The cultivars were authenticated and the genetic similarities were confirmed by DNA fingerprinting. The leaves were dried in the shade and the fruits were used fresh.
2.3. Chemical profiling using HPLC/DAD/HRMS
HPLC analysis was performed using the Thermo Accela HPLC system (Thermo Fisher Scientific, San Joes, CA, USA) coupled to Thermo Instruments MS system (Finnigan LTQ/LTQ Orbitrap). Gradient elution was applied using a Poroshell EC-C18 RP analytical HPLC column (2.7 μm, 2.1 × 100 mm, Agilent, USA), with a mobile phase of 0–100% MeCN over 25 min followed by 100% MeCN over 5 min at a flow rate of 0.5 mL min−1. The capillary temperature and voltage were kept at 260 °C and 45 V, respectively. Auxiliary gas flow rate 10–20 arbitrary units, sheath gas flow rate 40–50 arbitrary units and spray voltage 4.5 kV. The full scan mass ranges from 100–2000 amu (maximum resolution 60 000). All injected samples were 20 μL. The analytes were identified based on the following criteria: retention times, the exact mass of monoisotopic ion and comparison of the experimental and calculated isotopic pattern (Relative Isotopic Abundances RIA errors less than 15%) using a database containing the protonated exact monoisotopic masses list and built on the extracted ion chromatograms (EIC) of the expected [M + H]+ ions of each compound, and the database was created by analyzing pure standard solutions.
2.4. Extraction and isolation
2.4.1. Sample preparation for chemical profiling and in vivo antidiabetic studies
Ethanolic extracts of leaves and fruits from five strawberry cultivars were prepared by macerating 250 g of air-dried powdered leaves, and 500 g of fresh fruits separately in successive portions of 70% ethanol till exhaustion. The ethanolic extract in each sample was filtered and evaporated on a rotary evaporator under reduced pressure at a temperature not exceeding 45 °C to obtain a semisolid residue. Each 100 g dry weight (DW) leaves yielded 38 g dry extract, and 100 g fresh weight (FW) fruits produced 10.5 g dry extract.
2.4.2. Sample preparation for phenolic compounds isolation
The air-dried leaves of strawberry (Fragaria × ananassa Duch.) cv. Festival (1.3 kg) were extracted with successive portions of 70% ethanol till exhaustion. The collected portions were concentrated on Rotary evaporator under reduced pressure at a temperature not exceeding 45 °C to yield (500 g) crude extract. This crude extract was suspended in distilled water (1 L), and then successively partitioned with n-hexane, dichloromethane, ethyl acetate, and n-butanol to give n-hexane (10.5 g), dichloromethane (17.5 g), ethyl acetate (30 g) and n-butanol (50 g) fractions. These fractions were screened for flavonoids and phenolic acid detection by TLC.
Ethyl acetate fraction (30 g) was a promising to be chromatographed on silica gel 60 in a liquid chromatography column (LC) (70 × 5 cm), eluting with 100% dichloromethane (DCM) and increasing the polarity by 10% stepwise with methanol till 100% methanol. The similar eluates were collected according to their TLC profiles. A promising subfraction (6.1 g) was chromatographed on (250 g) silica gel (mesh 60) liquid column chromatography (70 × 3 cm). Eluting with dichloromethane 100% (DCM), then a gradual increase of methanol (MeOH) (2% methanol till 100%) was applied. The major promising spots were detected in the subfractions 3, 4 and 5 which named fraction I (1.2 g), fraction II (1.2 g) and fraction III (2.3 g), respectively. These subfractions deserved to be re-chromatographed on silica gel in liquid chromatography and subsequently purified on Sephadex LH 20, to yield seven compounds 1–7.
2.4.3. Sample preparation for colorimetric assays
Homogenized fruits (25 g FW) and powdered leaves (1 g DW) of five cultivars were separately extracted with 25 mL 80% methanol and left overnight at room temperature, then centrifuged and the supernatant transferred to 25 mL measuring flask. The volume was completed to 25 mL (stock solution), to final concentration (0.2 g mL−1 for fruit) and (0.04 g mL−1 for leaves).27,28
2.5. Determination of total phenolic, and flavonoid contents using colorimetric assays
Total phenolic content (TPC) of samples was determined using the Folin–Ciocalteu colorimetric assay.29 Briefly, an aliquot of each extract (100 μL) was mixed with 7 mL of deionized water and 0.5 mL of Folin–Ciocalteu reagent in 10 mL volumetric flask and then incubated for 3 min at room temperature in the dark, then 1.5 mL of sodium carbonate (Na2CO3) (20% w/v) was added, and the volume completed to 10 mL with deionized water and incubated for 2 hours at room temperature under dark conditions. The intensity of the developed color was measured at 725 nm against a prepared blank [gallic acid was used as standard (1 mg mL−1)] using a UV/vis spectrophotometer (Jenway, England). Total phenolic content was calculated from the regression equation of the standard plot [Y = 111.84x + 1.1697, R2 = 0.9953]. The data expressed as μg of gallic acid equivalents (GAE) per mg dry extract of leaves (DW basis), and per mg dry extract of fruits (FW basis).
The total flavonoid contents of leaves and fruits were determined using the aluminum chloride colorimetric assay according to ref. 30 with some modifications. Briefly, an aliquot (1 mL) of extract solution was added to a 10 mL volumetric flask containing (3 mL) of methanol. To the flask, 0.2 mL of 10% aluminum chloride, 0.2 mL of 1 M potassium acetate and 5.6 mL of distilled water were added, and then remained at room temperature for 30 minutes in the dark. The intensity of the developed color was measured at 420 nm immediately against a prepared blank reagent [rutin was used as standard (1 mg mL−1)] using a UV/vis spectrophotometer (Jenway, England). The total flavonoid contents were calculated from the regression equation of the standard plot [Y = 2996x − 16.54, R2 = 0.995] and all values were expressed. The data expressed as μg of rutin equivalents (GAE) per mg dry extract of leaves (DW basis), and per mg dry extract of fruits (FW basis). The samples were analyzed in triplicate.
2.6. In vivo antidiabetic study
2.6.1. Experimental animals
Forty two female albino rats (Sprague-Dawley strain) weighing 150–170 g purchased from the animal house of the NODCAR, Egypt. Animals were kept under laboratory standard conditions of relative humidity, temperature, and light/night cycles. They were watered and fed ad libitum on high fat diet and allowed to adapt for a two weeks former to the experiment. All animal procedures were performed in accordance with the Guidelines for Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication no. 85-23, revised 1996), and experiments were approved by the Ethics Committee for Animal Experimentation, Faculty of pharmacy, Nahda university, Beni-Suef, Egypt (approval sheet number NUB-022-020).
2.6.2. Acute oral toxicity LD50
The median lethal dose LD50 of the ethanolic extracts from leaves and fruits of strawberry cv. Festival and cv. Red Merlin, were determined according to the method previously described.31
2.6.3. Induction of experimental type II diabetes mellitus
Type 2 diabetes mellitus was induced in female albino rats according to the previous study.32 In the beginning, rats were fed on a high fat diet containing 50% kcal fat for 14 days, and then injected intraperitoneal (i.p.) by a single dose of freshly prepared streptozotocin (STZ, 50 mg per kg b. wt, in 0.1 M citrate buffer pH 4.5). The rats were given a 10% glucose solution after 6 hours of STZ administration for the next 24 hours to overcome drug-induced hypoglycemia. After one week, the rats were screened for fasting blood glucose levels and, rats with blood glucose levels of 250–300 mg dL−1 were taken for study and served as diabetic.33
2.6.4. Experimental design
A total of 42 rats were randomly divided into 7 groups comprising 6 animals in each group:
Group 1: normal rats injected with 1 mL citrate buffer (0.1 M, pH 4.5) and served as a negative control for 4 weeks.
Group 2: STZ-induced diabetic rats served as a positive control for 4 weeks.
Group 3 and 4: diabetic rats treated with once a day with ethanolic extracts of leaves from cv. Festival and cv. Red Merlin at a fixed dose of 250 mg per kg b. wt for 4 weeks.
Group 5 and 6: diabetic rats treated with once a day with ethanolic extracts of fruits from cv. Festival and cv. Red Merlin at a fixed dose of 250 mg per kg b. wt for 4 weeks.
Group 7: diabetic rats treated with gliclazide at a dose of 7.2 mg per kg b. wt for 4 weeks which equivalent to 80 mg human recommended dose.
2.6.5. Samples collection
Treatment with four ethanolic extracts of leaves and fruits of strawberry as the tested compounds and gliclazide as standard drug, were started after 48 hours of STZ injection for 28 days. These treatments were applied once every day for 28 days after STZ injection (induction of diabetes).
At the end of the experimental period, blood samples were collected from the retro-orbital venous plexus through the eye canthus of anesthetized rats after an overnight fast. Sera were separated by centrifugation at 2000 rpm for 15 min and stored at −80 °C for further biochemical analysis.
2.6.6. Biochemical analysis
Assessment of blood glucose level was accomplished by enzymatic colorimetric method according to ref. 34, glycated hemoglobin (HbA1C%) level was determined according to the method of ref. 35. Serum lipoproteins (HDLc, LDLc, VLDLc) assessment was established according to the method of ref. 36, but serum total glycerides (T.G) evaluated following the method of ref. 37. Serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), total bilirubin (T.B), and total cholesterol (T.C) were assayed by following the manufacturer's guidelines of Randox kits (Antrim, United Kingdom). Assessment of renal functions was established by evaluating serum levels creatinine according to the method of ref. 38, uric acid39 and urea.40 Antioxidative stress was evaluated by assessment serum levels of reduced glutathione (GSH),41 malondialdehyde (MDA)42 and total antioxidant capacity (TAC).43
2.6.7. Statistical analysis
All data were expressed as mean ± SE for six readings. The data were analyzed by one-way analysis of variance (ANOVA) followed by Duncan's multiple range test.44 Statistical analysis was performed using the Statistical Package for Social Science (SPSS) computer program, Version 22 produced by IBM Software, Inc. Chicago, USA. Differences were considered significant at P < 0.05.
3. Results
3.1. Chemical profiling of strawberry extracts
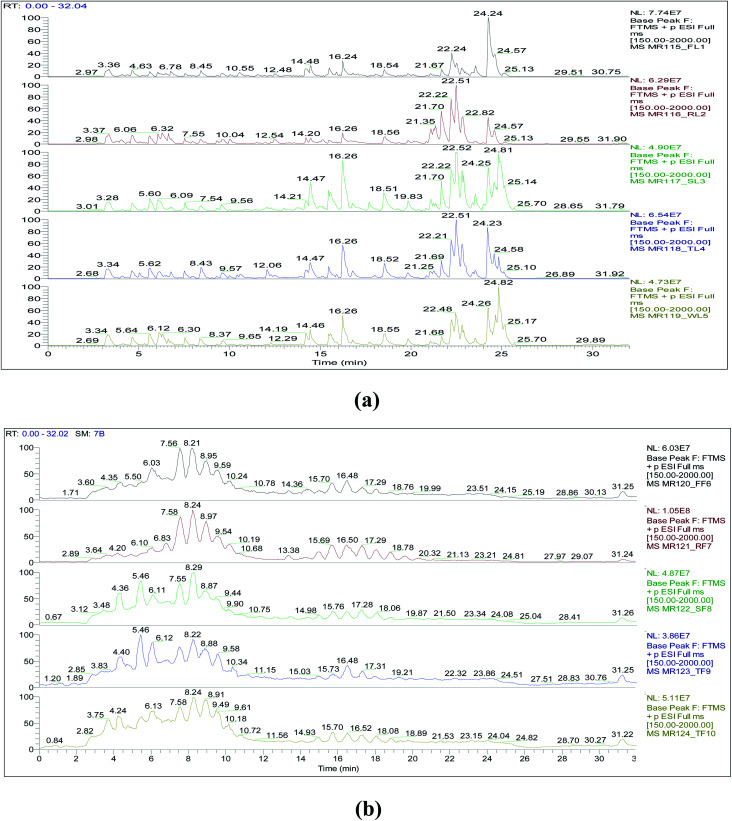
The chemical profiles of ethanolic extracts of five strawberry fruits and their leaves (Fig. 1), with their retention times and m/z of the 37 identified compounds were listed in Table 1. The 37 metabolites were putatively identified using HPLC/DAD/HRESIMS depending on their retention times, mass data, software library and previously reported data. The structural classes include 23 polyphenolic compounds, two aldehydes, one ionone glucoside, three sterols, one saponin, and seven terpenoids.
Fig. 1. LC-HRMS full scan (100–2000 m/z) profiles in ESI positive ion mode of strawberry ethanolic extracts, obtained from five cultivars: (a) leaves; (b) fruits. Where, FL: Festival leaf; RL: Red Merlin leaf; SL: Suzana leaf; TL: Tamar leaf; WL: Winter Dawn leaf; FF: Festival fruit; RF: Red Merlin fruit; SF: Suzana fruit; TF: Tamar fruit; WF: Winter Dawn fruit (for peak identification, please refer to Table 1).
Features (i.e. compounds) annotated from ethanolic extracts of leaves and fruits from five cvs. (Fragaria × ananassa Duch.) by HPLC/DAD/ESI-HRMS (see Fig. 1)a.
| Peak no. | R t | Mass | ESI-HRMS (m/z) (M + H) | Molecular formula | Annotation | Strawberry cultivar & organ |
|---|---|---|---|---|---|---|
| 1 | 4.63 | 106 | 107.0498 | C7H6O | Benzaldehyde | All |
| 2 | 5.60 | 136 | 137.0599 | C8H8O2 | 4-Methoxy-benzaldehyde | FL, RL, SL, TL, WL, SF, TF, WF |
| 3 | 5.68 | 1569 | 1570.178 | C68H48O44 | Sanguiin H-10 | FL, RL, SL, TL, WL, Rf, SF, WF |
| 4 | 6.07 | 164 | 165.0544 | C9H8O3 | p-Coumaric acid | FL, RL, SL, TL, WL, FF, RF |
| 5 | 6.09 | 1871 | 1872.262 | C82H54O52 | Agrimoniin/sanguiin H-6 | FL, RL, SL, TL, WL, SF, WF |
| 6 | 6.11 | 152 | 153.0548 | C8H8O3 | Methyl salicylate | FF, RF, SF, TF, WF (fruit) |
| 7 | 6.12 | 302 | 303.0137 | C14H6O8 | Ellagic acid | FL, RL, SL, WL, FF, RF, SF, TF, WF |
| 8 | 6.30 | 340 | 341.1237 | C16H20O8 | Methyl glucosyl-p-coumaroate | All |
| 9 | 6.34 | 476 | 477.1942 | C24H28O10 | Grayanoside A | FL, RL, SL, TL, RF, WF |
| 10 | 6.49 | 310 | 311.1120 | C15H18O7 | O-trans-Cinnamoyl-β-d-glucopyranose | FL, RL, SL, TL, WL (leaf) |
| 11 | 6.78 | 372 | 373.1491 | C17H24O9 | Lysidiside A | FL, RL, SL, TL, WL (leaf) |
| 12 | 7.55 | 286 | 287.0555 | C15H10O6 | Kaempferol | All |
| 13 | 7.61 | 290 | 291.0869 | C15H14O6 | Catechin | FL, RL, SL, TL, WL, FF, RF, SF, WF |
| 14 | 8.43 | 254 | 255.0655 | C15H10O4 | 5,7-Dihydroxy-2-phenyl-chromen-4-one (chrysin) | FL, RL, SL, TL, WL (leaf) |
| 15 | 9.56 | 304 | 305.0650 | C15H12O7 | Taxifolin | All |
| 16 | 10.04 | 578 | 579.1499 | C30H26O12 | Procyanidin B3 | RL, TL, FF, RF, SF, TF, WF |
| 17 | 10.43 | 594 | 595.1449 | C30H26O13 | trans-Tiliroside | FL, RL, TL, FF, RF, SF, TF, WF |
| 18 | 10.55 | 436 | 437.1070 | C20H20O11 | Taxifolin 3-O-α-l-arabinofuranoside | FL, TL, TF |
| 19 | 12.06 | 384 | 385.1076 | C24H16O5 | 7-O-Cinnamoylchrysin | TL, WL, FF, RF, SF |
| 20 | 12.50 | 705 | 706.1895 | C36H33O15 | Afzelechin(4α → 8)pelargonidin 3-O-β-d-glucopyranoside | FF, RF, SF, TF, WF (fruit) |
| 21 | 14.20 | 721 | 722.1848 | C36H33O16 | Catechin(4α → 8)pelargonidin 3-O-β-d-glucopyranoside | FF, RF, SF, TF, WF (fruit) |
| 22 | 14.47 | 468 | 469.0890 | C21H21O10Cl | Pelargonidin-3-glucoside chloride (callistephinchlorid) | FF, RF, SF, TF, WF (fruit) |
| 23 | 14.93 | 136 | 137.1327 | C10H16 | p-Mentha-1,8-diene (limonene) | FF, RF, SF, TF, WF (fruit) |
| 24 | 15.14 | 506 | 507.3889 | C26H34O10 | Aviculin/icariside E4 | FL, RL, SL, TL (leaf) |
| 25 | 15.69 | 436 | 437.1443 | C21H24O10 | Phloridzin | FF, RF, SF, TF, WF (fruit) |
| 26 | 15.76 | 449 | 449.1073 | C21H21O11+ | Cyanidin-3-glucoside | FF, RF, SF, TF, WF (fruit) |
| 27 | 16.26 | 680 | 681.3849 | C36H56O12 | Suavissimoside R1 | All |
| 28 | 17.29 | 386 | 387.2011 | C19H30O8 | (6S,9R)-Roseoside | FF, RF, SF, TF, WF (fruit) |
| 29 | 18.51 | 504 | 505.3520 | C30H48O6 | Sericic acid | FL, RL, SL, TL, WL (leaf) |
| 30 | 18.72 | 386 | 387.2018 | C19H30O8 | Citroside A | FF, RF, SF (fruit) |
| 31 | 21.70 | 456 | 457.3679 | C30H48O3 | Ursolic acid | FL, RL, SL, TL, WL (leaf) |
| 32 | 22.22 | 472 | 473.3622 | C30H48O4 | Polnolic acid | FL, RL, SL, TL, WL (leaf) |
| 33 | 22.52 | 488 | 489.3579 | C30H48O5 | Tormentic acid | FL, RL, SL, TL, WL (leaf) |
| 34 | 22.82 | 484 | 485.3260 | C30H44O5 | Fupenzic acid | FL, RL, SL, TL, WL (leaf) |
| 35 | 24.23 | 400 | 401.3770 | C28H48O | Campesterol | FL, RL, SL, TL, WL (leaf) |
| 36 | 24.58 | 414 | 415.3939 | C29H50O | β-Sitosterol | FL, RL, SL, TL, WL (leaf) |
| 37 | 24.81 | 412 | 413.3778 | C29H48O | Stigmasterol | SL, TL, WL (leaf) |
Where, FL: Festival leaf; RL: Red Merlin leaf; SL: Suzana leaf; TL: Tamar leaf; WL: Winter Dawn leaf; FF: Festival fruit; RF: Red Merlin fruit; SF: Suzana fruit; TF: Tamar fruit; WF: Winter Dawn fruit.
Analysis of LC-HRMS data of leaves indicated a very close metabolite pattern which only differs in the presence and absence of some metabolites in each cultivar as shown in Fig. 1 and Table 1. As the leaves, analysis of corresponding fruits indicated a close metabolite pattern with the same differences. Some metabolites were identified only in leaves as (O-trans-cinnamoyl-β-d-glucopyranose, lysidiside A, chrysin, aviculin, sericic acid, ursolic acid, polnolic acid, tormentic acid, fupenzic acid, campesterol, β-sitosterol, and stigmasterol) as shown in Table 1. On the other hand, some metabolites were identified only in the fruits as (methyl salicylate, all anthocyanins, condensed anthocyanins, limonene, phloridzin, citroside A and roseoside).
Most of the terpenes were present in leaves, while very few in fruits. On the other hand, flavonoids and phenolic metabolites were presented in higher numbers. Kaempferol, chrysin, taxifolin, ellagic acid, and 4-methoxy benzaldehyde were the most abundant compounds identified in the fruits. In contrast, tormentic acid, β-sitosterol, stigmasterol, polnolic acid, ursolic acid, sericic acid, and suavissimoside R1 were the most abundant compounds identified in the leaves as shown in Table 1.
3.2. Isolation of phenolic compounds from strawberry leaves cv. Festival
The ethanolic extract of dried strawberry leaves cv. Festival was fractionated sequentially by different solvents to give a promising ethyl acetate fraction, according to TLC for flavonoids and phenolics detection. This fraction several chromatographed to yield the major promising spots in subfractions I, II, III. The three subfractions were sequentially purified on Sephadex LH 20 columns separately to provide seven pure compounds. These compounds were identified by their analysis of 1H-NMR and DEPT-Q as kaempferol (1),45p-coumaric acid (2),46 quercetin (3),45p-tyrosol (4),47 methyl gallate (5),48 eutigoside A (6)12,49 and trans-tiliroside (7).50
3.3. Total phenolic, and flavonoid contents using colorimetric assays
The total phenolic and flavonoid contents of fruits and leaves are represented in Table 2, where TPC (total phenolic content) was varied from (42.63–72.63 μg gallic per mg extract DW) for leaves, and (20–35.24 μg gallic per mg extract FW) for fruits. So, there was a significant difference in the total phenolic content between leaves and fruits, and within each cultivar (cultivar dependent). Suzana leaves and fruits had the highest values of total phenolics, but Tamar leaves were the lowest among the studied leaves, and Festival fruits were the lowest among fruits. The total flavonoid content was varied from (52.89–67.1 μg rutin per mg extract DW) for leaves and from (8.57–20.95 μg rutin per mg extract FW) for fruits. So, there was a significant difference in the total flavonoid content between the leaves and fruits and within each cultivar. Suzana fruit had the highest content of flavonoid among studied fruits, but Festival leaf had the highest content among the leaves. Tamar cultivar had the lowest value among both leaves and fruits.
Amounts of total phenolics and flavonoids in methanolic extracts of leaves and fruits from five cvs. (Fragaria × ananassa Duch.).
| Strawberry cultivars | Total phenolic content concentrationa, μg GAE per mg extract | Total flavonoid content concentrationb, μg RE mg−1 |
|---|---|---|
| FL | 62.37 ± 0.1 | 67.1 ± 0.4 |
| RL | 72.1 ± 0.1 | 65.79 ± 0.1 |
| SL | 72.63 ± 0.1 | 55.26 ± 0.1 |
| TL | 42.63 ± 0.2 | 54.21 ± 0.1 |
| WL | 66.58 ± 0.2 | 52.89 ± 0.1 |
| FF | 20 ± 0.1 | 9.52 ± 0.2 |
| RF | 33.33 ± 0.1 | 17.14 ± 0.2 |
| SF | 35.24 ± 0.3 | 20.95 ± 0.1 |
| TF | 21.1 ± 0.5 | 16.19 ± 0.3 |
| WF | 31.42 ± 0.1 | 8.57 ± 0.1 |
Calculated as gallic acid equivalent (GAE). Values are presented as the mean of triplicates ± SE.
Calculated as rutin equivalent (RE). Where, FL: Festival leaf; RL: Red Merlin leaf; SL: Suzana leaf; TL: Tamar leaf; WL: Winter Dawn leaf; FF: Festival fruit; RF: Red Merlin fruit; SF: Suzana fruit; TF: Tamar fruit; WF: Winter Dawn fruit.
3.4. In vivo antidiabetic study
3.4.1. Acute oral toxicity study (LD50)
There was no mortality in animals at all doses of different studied treatments up to 5 g per kg b. wt, so the oral LD50 of the treatments is more than 5 g kg−1. The biological potential of the treatments evaluated using one fixed daily dose of one-twenty (1/20) of the maximum dosage (250 mg per kg b. wt), according to the Organization for Economic Co-operation and Development (OECD)-423 guidelines.51
3.4.2. Effect of strawberry extracts on blood glucose level and glycated hemoglobin
In STZ-diabetic rats, blood glucose level and glycated hemoglobin were significantly increased (P < 0.05) comparing to normal rats. Blood glucose level and glycosylated hemoglobin were significantly decreased (P < 0.05) in all treated diabetic rats compared with those in STZ-diabetic rats. Ethanolic extracts of strawberry were significantly decreased the blood glucose level and glycosylated hemoglobin. Ethanolic extract of leaves was more potent than fruits from both cultivars. Leaf extract from cv. Festival showed the most potent activity where reversed the blood glucose level to half in comparison to STZ-diabetic rats from (276.6 to 142.9 mg dL−1), followed by cv. Red Merlin (151.0 mg dL−1), then extracts of fruits. Ethanolic extract from cv. Festival decreased glycated hemoglobin HbA1C% from (9.617 to 7.215) as illustrated in Table 3, followed by leaf extract of cv. Red Merlin, then fruit extract from cv. Festival and finally fruit extract of cv. Red Merlin. Glycated hemoglobin level (HbA1C%) has been largely used as a biomarker for glycemic control in type 2 diabetes, and a reduction in HbA1C% is used to display the efficacy of diabetes treatments.52
Effect of different treatments on serum level of glucose and glycated hemoglobina.
| Groups | Serum glucose (mg dL−1) | HbA1C% |
|---|---|---|
| Normal control | 78.4 ± 1.64a | 5.413 ± 0.158a |
| STZ-diabetic rats | 276.6 ± 6.32f | 9.617 ± 0.205e |
| Diabetic rats treated with ethanolic extracts of leaves of | ||
| cv. Festival | 142.9 ± 3.30b | 7.215 ± 0.154b |
| cv. Red Merlin | 151.0 ± 4.40bc | 7.405 ± 0.146b |
| Diabetic rats treated with ethanolic extracts of fruits of | ||
| cv. Festival | 157.7 ± 4.64cd | 7.790 ± 0.112bc |
| cv. Red Merlin | 165.8 ± 4.26de | 7.995 ± 0.066cd |
| Diabetic rats treated with gliclazide | 82.9 ± 3.13a | 5.947 ± 0.133a |
Each value represents the mean of 6 rats ± S.E. (standard error). Values within a column followed by different letters are significantly different (P < 0.05) by SPSS Duncan. Where STZ: streptozotocin; HbA1C: glycated hemoglobin.
These results agreed with the previous study.10 Generally, leaves were more potent than fruits and cv. Festival was better than cv. Red Merlin. We can conclude that strawberry leaves and fruits have anti-hyperglycemic activity.
3.4.3. Effect of strawberry extracts on liver function biomarkers in diabetic rats
All liver functions significantly elevated (P < 0.05) in STZ-diabetic rats compared to normal rats. Serum levels of AST, ALT, ALP and total bilirubin were significantly decreased (P < 0.05) in all diabetic treated rats in comparison to the STZ-diabetic rats Table 4. Ethanolic leaf extract from cv. Festival was the most potent one, followed by leaf extract of cv. Red Merlin, then fruit extract of cv. Festival and finally fruit extract of cv. Red Merlin. ALT level remarkably decreased in all diabetic treated rats in comparison to normal and STZ-diabetic rats, and all extracts were better than the standard treatment. ALT level was decreased from (147.17 ± 5.63 to 50.82 ± 2.25 U mL−1) upon administration of ethanolic leaves extract of cv. Festival. Generally, leaves were more potent than fruits, and cv. Festival was more effective than cv. Red Merlin.
Effect of different treatments on serum level of ALT, AST, ALP, and total bilirubina.
| Groups | Serum AST activity (U mL−1) | Serum ALT activity (U mL−1) | Serum ALP activity (U L−1) | Total bilirubin (mg dL−1) |
|---|---|---|---|---|
| Normal control | 23.33 ± 0.89a | 53.83 ± 1.35abc | 110.17 ± 5.25a | 0.77 ± 0.01a |
| STZ-diabetic rats | 58.66 ± 1.801f | 147.17 ± 5.63f | 232.77 ± 10.48f | 1.43 ± 0.04d |
| Diabetic rats treated with ethanolic extracts of leaves of | ||||
| cv. Festival | 35.2 ± 1.08b | 50.82 ± 2.25a | 140.67 ± 7.31cd | 0.911 ± 0.01b |
| cv. Red Merlin | 36.37 ± 1.13bc | 51.32 ± 1.94ab | 144.35 ± 5.33cd | 0.92 ± 0.01b |
| Diabetic rats treated with ethanolic extracts of fruits of | ||||
| cv. Festival | 39.55 ± 0.801cd | 55.7 ± 1.68abc | 162.97 ± 7.33de | 1.001 ± 0.03c |
| cv. Red Merlin | 41.17 ± 1.29de | 56.87 ± 2.33abcd | 163 ± 6.13de | 1.015 ± 0.03c |
| Diabetic rats treated with gliclazide | 26.85 ± 1.02a | 64.6 ± 1.62de | 130.6 ± 4.82b | 0.871 ± 0.02b |
Each value represents the mean of 6 rats ± S.E. (standard error). Values within a column followed by different letters are significantly different (P < 0.05) by SPSS Duncan. Where STZ: streptozotocin; AST: aspartate transaminase; ALT: alanine transaminase; ALP: alkaline phosphatase.
3.4.4. Effect of strawberry extracts on lipid profile in diabetic rats
In STZ-diabetic rats, T.G, T.C, LDLc, VLDLc were increased significantly (P < 0.05), and HDLc decreased significantly (P < 0.05) in comparison to normal rats. All lipid profile markers were ameliorated significantly (P < 0.05) in all treated diabetic rats in comparison to STZ-diabetic rats as shown in Tables 5–7. Ethanolic leaf extract from cv. Festival was the most potent one where decreased T.G from (123.7 ± 4.83 to 84.4 ± 2.16 mg dL−1) and showed a remarkable decrease in serum lipoproteins (LDLc and VLDL), and noteworthy increase in (HDLc) in comparison to STZ-diabetic rats.
Effect of different treatments on serum level of triglycerides and total cholesterola.
| Groups | T.G (mg dL−1) | T.C (mg dL−1) |
|---|---|---|
| Normal control | 51.8 ± 0.79a | 78.75 ± 0.66a |
| STZ-diabetic rats | 123.7 ± 4.83f | 155.4 ± 2.55f |
| Diabetic rats treated with ethanolic extracts of leaves of | ||
| cv. Festival | 84.4 ± 2.16bc | 87.8 ± 0.88ab |
| cv. Red Merlin | 89.13 ± 4.83bc | 90.86 ± 1.91bc |
| Diabetic rats treated with ethanolic extracts of fruits of | ||
| cv. Festival | 92.8 ± 3.62cd | 98.61 ± 2.18cd |
| cv. Red Merlin | 94.95 ± 3.4cde | 103.05 ± 3.69de |
| Diabetic rats treated with gliclazide | 79.4 ± 1.29b | 82.4 ± 1.01ab |
Each value represents the mean of 6 rats ± S.E. (standard error). Values within a column followed by different letters are significantly different (P < 0.05) by SPSS Duncan. Where STZ: streptozotocin; T.G: triglycerides; T.C: total cholesterol.
Effect of different treatments on serum level of HDL-cholesterol, LDL-cholesterol, and VLDL-cholesterola.
| Groups | HDLC (mg dL−1) | LDLC (mg dL−1) | VLDLC (mg dL−1) |
|---|---|---|---|
| Normal control | 44.8 ± 0.821e | 23.6 ± 1.15a | 10.35 ± 0.16a |
| STZ-diabetic rats | 32.06 ± 1.65a | 98.6 ± 2.35f | 24.75 ± 0.96f |
| Diabetic rats treated with ethanolic extracts of leaves of | |||
| cv. Festival | 42.26 ± 1.07bc | 28.66 ± 0.93ab | 16.86 ± 0.43bc |
| cv. Red Merlin | 41.83 ± 1.46b | 31.2 ± 2.04ab | 17.81 ± 0.95bc |
| Diabetic rats treated with ethanolic extracts of fruits of | |||
| cv. Festival | 41.58 ± 1.34b | 38.46 ± 1.94bc | 18.55 ± 0.73cd |
| cv. Red Merlin | 41.2 ± 1.4b | 42.86 ± 2.47cd | 18.98 ± 0.68cde |
| Diabetic rats treated with gliclazide | 43.71 ± 1.09d | 66.55 ± 0.95e | 15.88 ± 0.25b |
Each value represents the mean of 6 rats ± S.E. (standard error). Values within a column followed by different letters are significantly different (P < 0.05) by SPSS Duncan. Where HDLc: high-density lipoprotein cholesterol; LDLc: low-density lipoprotein cholesterol; VLDL: very low-density lipoprotein cholesterol.
Effect of different treatments on serum level of risk ratiosa.
| Groups | T.C/HDLc | LDL/HDLc |
|---|---|---|
| Normal control | 0.52 ± 0.03a | 1.76 ± 0.04a |
| STZ-diabetic rats | 3.12 ± 0.21f | 4.9 ± 0.23d |
| Diabetic rats treated with ethanolic extracts of leaves of | ||
| cv. Festival | 0.68 ± 0.03ab | 2.08 ± 0.04ab |
| cv. Red Merlin | 0.75 ± 0.07ab | 2.18 ± 0.09ab |
| Diabetic rats treated with ethanolic extracts of fruits of | ||
| cv. Festival | 0.93 ± 0.06abc | 2.38 ± 0.09bc |
| cv. Red Merlin | 1.03 ± 0.04bcd | 2.5 ± 0.05bc |
| Diabetic rats treated with gliclazide | 1.52 ± 0.02e | 1.88 ± 0.03a |
Each value represents the mean of 6 rats ± S.E. (standard error). Values within a column followed by different letters are significantly different (P < 0.05) by SPSS Duncan. Where STZ: streptozotocin; T.C/HDLc: risk ratio 1 of total cholesterol to high-density lipoprotein cholesterol; LDLc/HDLc: risk ratio 2 of low-density lipoprotein cholesterol to high-density lipoprotein cholesterol.
In the STZ-diabetic rats risk 1 ratio (T.C/HDLc) and risk 2 ratio (LDLc/HDLc) were increased significantly (P < 0.05) in comparison to normal rats. Both risk ratios were decreased significantly (P < 0.05) in contrast to STZ-diabetic rats by all tested treatments as shown in Table 7. Risk 1 ratio (T.C/HDLc) was decreased significantly (P < 0.05) in diabetic rats treated with ethanolic extracts of both cultivars and organs in comparison to STZ-diabetic rats and standard control. All tested ethanolic extracts were decreased the all readings in risk 1 ratio to more than half, but in risk 2 ratio were decreased nearly to the half in comparison to the STZ-diabetic rats. Finally, we accomplish that both organs of strawberry can manage diabetic hyperlipidemia, but leaves were more effective than fruits, and cv. Festival more effective than cv. Red Merlin. This agreed with the study of ref. 53, where the freeze-dried strawberry fruits were found effective in controlling diabetic hyperlipidemia in alloxan diabetic rats, referring to the high antioxidant polyphenolics content.
3.4.5. Effect of strawberry extracts on renal functions in diabetic rats
All kidney functions were significantly (P < 0.05) increased in STZ-diabetic rats compared to normal rats. Serum (urea, creatinine and uric acid) were significantly (P < 0.05) decreased in all treated diabetic rats in comparison to STZ-diabetic rats. For all measured activities in this work, Festival cultivar is preferred except for renal functions where cv. Red Merlin ameliorated all activities more than cv. Festival. Besides, ethanolic extract from leaves of cv. Red Merlin lowered all renal parameters nearly equal to the standard treatment (gliclazide) as shown in Table 8. Ethanolic leaf extracts from both cultivars decreased serum urea with about 35% compared to STZ-diabetic rats.
Effect of different treatments on serum urea, creatinine, and uric acida.
| Groups | Serum urea (mg dL−1) | Serum creatinine (mg dL−1) | Serum uric acid (mg dL−1) |
|---|---|---|---|
| Normal control | 40.48 ± 1.31a | 0.74 ± 0.02a | 2.46 ± 0.08a |
| STZ-diabetic rats | 78.55 ± 1.43f | 1.62 ± 0.02e | 4.24 ± 0.15e |
| Diabetic rats treated with ethanolic extracts of leaves of | |||
| cv. Festival | 51.83 ± 0.94c | 0.895 ± 0.01c | 3.1 ± 0.11bc |
| cv. Red Merlin | 48.71 ± 0.88bc | 0.85 ± 0.01bc | 2.99 ± 0.1b |
| Diabetic rats treated with ethanolic extracts of fruits of | |||
| cv. Festival | 55.78 ± 1.02e | 1.024 ± 0.01d | 3.48 ± 0.12cd |
| cv. Red Merlin | 53.43 ± 0.98de | 0.976 ± 0.01d | 3.39 ± 0.12cd |
| Diabetic rats treated with gliclazide | 45.71 ± 2.06b | 0.827 ± 0.01b | 2.86 ± 0.09b |
Each value represents the mean of 6 rats ± S.E. (standard error). Values within a column followed by different letters are significantly different (P < 0.05) by SPSS Duncan; where STZ: streptozotocin.
Generally, leaves were more potent than fruits, and cv. Red Merlin was more effective than cv. Festival which agreed with study,10 where the aqueous extract from strawberry leaf had anti-inflammatory, anti-apoptosis, antioxidant and antidiabetic in diabetic nephropathy. Finally, these results may provide a basis for the prevention of diabetes-associated nephrotoxicity with urate-lowering agents.
3.4.6. Effect of strawberry extracts on oxidative stress in diabetic rats
Oxidative stress markers significantly (P < 0.05) increased in STZ-diabetic rats compared to normal rats. Total antioxidant capacity (TAC) and reduced glutathione (GSH) levels were significantly (P < 0.05) increased in all treated diabetic rats comparing to STZ-diabetic rats. Malondialdehyde (MDA) level was significantly (P < 0.05) decreased in all treated diabetic rats comparing to STZ-diabetic rats Table 9. Total antioxidant capacity (TAC) has increased dramatically in diabetic rats treated with alcoholic extracts of both cultivars and organs. TAC level was increased from (0.26 to either 0.48 or 0.45 mmol L−1) by ethanolic leaf extracts from cv. Festival and cv. Red Merlin, respectively.
Effect of different treatments on serum levels of TAC, GSH, and MDAa.
| Groups | TAC (mmol L−1) | GSH (mg dL−1) | MDA (nmol L−1) |
|---|---|---|---|
| Normal control | 0.51 ± 0.009f | 50.28 ± 0.907cd | 13.05 ± 0.28a |
| STZ-diabetic rats | 0.26 ± 0.006a | 25.96 ± 0.84a | 26.43 ± 0.93f |
| Diabetic rats treated with ethanolic extracts of leaves of | |||
| cv. Festival | 0.48 ± 0.011e | 51.66 ± 1.66d | 15.86 ± 0.57bc |
| cv. Red Merlin | 0.45 ± 0.01d | 47.98 ± 1.53cd | 16.38 ± 0.58bc |
| Diabetic rats treated with ethanolic extracts of fruits of | |||
| cv. Festival | 0.43 ± 0.01c | 46.75 ± 1.516bc | 17.18 ± 0.61cd |
| cv. Red Merlin | 0.41 ± 0.009b | 43.63 ± 1.41b | 18.76 ± 0.66de |
| Diabetic rats treated with gliclazide | 0.59 ± 0.013g | 57.05 ± 1.27e | 14.36 ± 0.31ab |
Each value represents the mean of 6 rats ± S.E. (standard error). Values within a column followed by different letters are significantly different (P < 0.05) by SPSS Duncan. Where STZ: streptozotocin; TAC: total antioxidant capacity in (mmol L−1); GSH: glutathione in (mg dL−1); MDA: malondialdehyde in (nmol L−1).
Reduced glutathione level (GSH) was duplicated in diabetic rats treated with ethanolic leaf extract from cv. Festival from (25.96 to 51.66 mg dL−1), and showed a notable increase in comparison to normal rats. On the other hand, the malondialdehyde (MDA) level significantly (P < 0.05) decreased in diabetic rats treated with ethanolic leaf extracts from both cultivars followed by their corresponding fruits in comparison to STZ-diabetic rats. Generally, strawberry leaves showed antioxidative stress more than fruits and cv. Festival more potent than cv. Red Merlin. This was in agreement with the study of ref. 54, who found the byproducts of strawberry (leaves & stems) had strong antioxidant power using the DPPH method, and the ethanolic extract of leaves was better than of stem and fruits.
4. Discussion
HPLC/DAD/HRESIMS revealed closely similar chemical profiles of ethanolic extracts of five strawberry cultivars (leaves and fruits), but differed in the presence of some compounds and absence of others as shown in Fig. 1 and Table 1. This analysis revealed the identification of 37 different compounds in both leaves and fruits, and six of them illustrated as taxifolin 3-O-α-l-arabinofuranoside, polnolic acid, fupenzic acid, sericic acid, suavissimoside R1 and lysidiside A, where firstly reported in strawberry leaves. Besides, isolation of seven phenolic compounds from cv. Festival leaves. All strawberry phytochemicals showed a synergistic action on health improvement and afford a complementary strategy in therapeutic management of hyperglycemia along with its complications via consumption.11 Total phenolic content (TPC) of strawberry plant varied depending on many factors, as the cultivar genotype, organ type, ripening stage, environmental conditions as well as the methods of extraction and analysis.17,55 Genotype is a major reason for qualitative and quantitative differences in the phenolic contents,56 as stated by our results. Study of Kårlund et al.57 proved a significant difference in total phenolic contents between the three studied cultivars (fruits), ranged from 155–199 mg per 100 g FW GAE. In the study of ref. 58, the total phenolic content of strawberry fruits ranged from 2234.62 mg per kg FW (cv. Oso grande) to 1743.47 mg per kg FW (cv. Camino real). Sometimes the difference in TPC about 1.6 fold between studied cultivars such as the study of Wang et al.17 Also, we found that the total phenolic content significantly differs by organ type in the same plant, where phenolic content of strawberry leaves was much more than the fruits in fresh form or extracts as stated in the study of Zhu et al.9 So, consumption of fruits of different cultivars might be more useful.27
cv. Suzana fruits were the richest in total flavonoid and phenolic contents, followed by cv. Red Merlin fruits. Leaves of cv. Festival were the richest in total flavonoids followed by leaves of cv. Red Merlin which occupied the second degree after cv. Suzana. In all readings, cv. Suzana showed the highest total phenolic content, followed by cv. Red Merlin, but other cultivars showed a fluctuation results. According to the classification of Peterson and Dwyer59 strawberry fruits and leaves contain high amount of flavonoids, thus signifying the probable role of leaves as nutraceutical source, and ingredient of functional food products.60
Lifestyle, diet regimen of diabetic patients along with traditional herbal remedies may be valuable strategies in the development of alternative therapeutics that may be more effective than oral antidiabetic drugs.52,61 Consumption of rich polyphenolic diet as strawberry fruits9 has been recommended to be unique supplemental and nutraceutical treatments to regulate carbohydrate metabolism and hyperglycemia in diabetes mellitus, through inhibition of α-amylase and α-glucosidase enzymes.62 As suggested, the ingestion of strawberry fruit after meal controls the rapid increase of postprandial blood glucose level.63 Strawberries rich in flavonoids mainly anthocyanins, were reported to cause an ATP-dependent enhancement of glucose-stimulated insulin secretion from isolated islets and associated with lower peripheral insulin resistance.8,61 In addition, dietary anthocyanins and their metabolites suppress pro-inflammatory cytokine production.7,64 All biological activity of strawberry leaves and fruits may be attributed to many major compounds, previously identified and isolated from the strawberry plant. Quercetin was reported to exhibit anti-inflammatory, anti-hyperuricemic and anti-dyslipidemia effects.65 Kaempferol possessed the most anti-inflammatory activity in between eight studied compounds,66 also the two previous flavonols and their derivatives were the dominant flavonol groups in strawberry leaves.14 Methyl gallate is effective in preventing H2O2-induced oxidative stress and DNA damage in Madin-Darby canine kidney (MDCK) cells,67p-coumaric acid revealed promising anti-inflammatory activity,68p-tyrosol attenuated the hepatic lipid peroxidation caused by high fat diet, and restored the redox equilibrium of the antioxidant glutathione GSH, besides inhibited palmitic acid induced oxidative stress in hepatocytes,69 and also it decreased hyperglycemia by its antioxidant effect and through regulating the key enzymes of carbohydrates metabolism.70 Thus may contribute to the potential antidiabetic effect of strawberry leaves and their fruits, and explain the management role of different studied treatments against diabetic-associated abnormalities as (hyperglycemia, hyperlipidemia, oxidative stress, liver dysfunction, renal dysfunction).
5. Conclusion
Results acquired in this study established the impact of genetic background on health-promoting activity of the strawberry plant, where all result variations are cultivar dependent and also organ dependent. The leaves as a byproduct of plant gave surprising superior results more than the fruits. So, we suggest manipulating these leaves (byproduct) as a promising herbal tea product even alone or in admixture with freeze-dried strawberry fruits, exhibiting anti-hyperglycemic and antioxidant potential. Also, we can manipulate the freeze-dried powder of fruits or their ethanolic extract in a capsulated dosage form as a food supplement and controlling obesity, as the same ethanolic extract of leaves. Finally, there are multiple byproduct parts of strawberry plant need for further more investigations as calyx, crown, stolon and root.
Conflicts of interest
We declare that authors have no conflict of interest.
Supplementary Material
References
- Miller K. Feucht W. Schmid M. Bioactive compounds of strawberry and blueberry and their potential health effects based on human intervention studies: A brief overview. Nutrients. 2019;11:1–12. doi: 10.3390/nu11071510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock J. F., Sjulin T. M. and Lobos G. A., Strawberries, in Temp. Fruit Crop Breed., Springer, 2008, pp. 393–437 [Google Scholar]
- Giampieri F. Tulipani S. Alvarez-Suarez J. M. Quiles J. L. Mezzetti B. Battino M. The strawberry: Composition, nutritional quality, and impact on human health. Nutrition. 2012;28:9–19. doi: 10.1016/j.nut.2011.08.009. doi: 10.1016/j.nut.2011.08.009. [DOI] [PubMed] [Google Scholar]
- Sun J. Liu X. Yang T. Slovin J. Chena P. Profiling polyphenols of two diploid strawberry (Fragaria vesca) inbred lines using UHPLC-HRMS. Food Chem. 2014;146:289–298. doi: 10.1016/j.foodchem.2013.08.089.Profiling. doi: 10.1016/j.foodchem.2013.08.089.Profiling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhu A. K. Miller M. G. Thangthaeng N. Scott T. M. Shukitt-Hale B. Edirisinghe I. Burton-Freeman B. Metabolic fate of strawberry polyphenols after chronic intake in healthy older adults. Food Funct. 2018;9:96–106. doi: 10.1039/c7fo01843f. doi: 10.1039/c7fo01843f. [DOI] [PubMed] [Google Scholar]
- Agarwal P. Wang Y. Holland T. Bennett D. Morris M. Strawberry Consumption Associated with Reduced Alzheimer's Dementia Risk (FS05-06-19) Curr. Dev. Nutr. 2019;3:1268. doi: 10.1093/cdn/nzz052.fs05-06-19. [DOI] [Google Scholar]
- Van de Velde F. Esposito D. Grace M. H. Pirovani M. E. Lila M. A. Anti-inflammatory and wound healing properties of polyphenolic extracts from strawberry and blackberry fruits. Food Res. Int. 2019;121:453–462. doi: 10.1016/j.foodres.2018.11.059. doi: 10.1016/j.foodres.2018.11.059. [DOI] [PubMed] [Google Scholar]
- Parra-Palma C. Moya-León M. A. Ramos P. Fuentes E. Palomo I. Torres C. A. Linking the platelet antiaggregation effect of different strawberries species with antioxidants: Metabolomic and transcript profiling of polyphenols. Bol. Latinoam. Caribe Plant. Med. Aromat. 2018;17:36–52. [Google Scholar]
- Zhu Q. Nakagawa T. Kishikawa A. Ohnuki K. Shimizu K. In vitro bioactivities and phytochemical profile of various parts of the strawberry (Fragaria × ananassa var. Amaou) J. Funct. Foods. 2015;13:38–49. doi: 10.1016/j.jff.2014.12.026. doi: 10.1016/j.jff.2014.12.026. [DOI] [Google Scholar]
- Ibrahim D. S. El-maksoud M. A. E. A. Effect of strawberry (Fragaria × ananassa) leaf extract on diabetic nephropathy in rats. Int. J. Exp. Pathol. 2015;96:1–7. doi: 10.1111/iep.12116. doi: 10.1111/iep.12116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheik Abdulazeez S. Effects of freeze-dried Fragaria × ananassa powder on alloxan-induced diabetic complications in Wistar rats. J. Taibah Univ. Med. Sci. 2014;9:268–273. doi: 10.1016/j.jtumed.2014.03.007. [DOI] [Google Scholar]
- Hanhineva K. Soininen P. Anttonen M. J. Kokko H. Rogachev I. Aharoni A. Laatikainena R. Kärenlampi S. NMR and UPLC-qTOF-MS/MS characterisation of novel phenylethanol derivatives of phenylpropanoid glucosides from the leaves of strawberry (Fragaria × ananassa cv. Jonsok) Phytochem. Anal. 2009;20:353–364. doi: 10.1002/pca.1133. doi: 10.1002/pca.1133. [DOI] [PubMed] [Google Scholar]
- Battino M. Beekwilder J. Denoyes-Rothan B. Laimer M. McDougall G. J. Mezzetti B. Bioactive compounds in berries relevant to human health. Nutr. Rev. 2009;67:145–150. doi: 10.1111/j.1753-4887.2009.00178.x. [DOI] [PubMed] [Google Scholar]
- Kårlund A. Hanhineva K. Lehtonen M. McDougall G. J. Stewartc D. Karjalainena R. O. Non-targeted metabolite profiling highlights the potential of strawberry leaves as a resource for specific bioactive compounds. J. Sci. Food Agric. 2017;97:2182–2190. doi: 10.1002/jsfa.8027. doi: 10.1002/jsfa.8027. [DOI] [PubMed] [Google Scholar]
- Oszmiański J. Wojdyło A. Gorzelany J. Kapusta I. Identification and characterization of low molecular weight polyphenols in berry leaf extracts by HPLC-DAD and LC-ESI/MS. J. Agric. Food Chem. 2011;59:12830–12835. doi: 10.1021/jf203052j. doi: 10.1021/jf203052j. [DOI] [PubMed] [Google Scholar]
- Haugeneder A. Trinkl J. Härtl K. Hoffmann T. Allwood J. W. Schwab W. Answering biological questions by analysis of the strawberry metabolome. Metabolomics. 2018;14:1–10. doi: 10.1007/s11306-018-1441-x. doi: 10.1007/s11306-018-1441-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. Y. Lin H. S. Antioxidant activity in fruits and leaves of blackberry, raspberry, and strawberry varies with cultivar and developmental stage. J. Agric. Food Chem. 2000;48:140–146. doi: 10.1021/jf9908345. doi: 10.1021/jf9908345. [DOI] [PubMed] [Google Scholar]
- Wang H. Cao G. Prior R. L. Total antioxidant capacity of fruits. J. Agric. Food Chem. 1996;44:701–705. doi: 10.1021/jf950579y. doi: 10.1021/jf950579y. [DOI] [Google Scholar]
- D'Urso G. Pizza C. Piacente S. Montoro P. Combination of LC-MS based metabolomics and antioxidant activity for evaluation of bioactive compounds in Fragaria vesca leaves from Italy. J. Pharm. Biomed. Anal. 2018;150:233–240. doi: 10.1016/j.jpba.2017.12.005. doi: 10.1016/j.jpba.2017.12.005. [DOI] [PubMed] [Google Scholar]
- Shaw J. E. Sicree R. A. Zimmet P. Z. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Hegazi R. El-Gamal M. Abdel-Hady N. Hamdy O. Epidemiology of and risk factors for type 2 diabetes in Egypt. Ann. Glob. Heal. 2015;81:814–820. doi: 10.1016/j.aogh.2015.12.011. [DOI] [PubMed] [Google Scholar];
- Daniels M. A. Kan C. Willmes D. M. Ismail K. Pistrosch F. Hopkins D. Mingrone G. Bornstein S. R. Birkenfeld A. L. Pharmacogenomics in type 2 diabetes: oral antidiabetic drugs. Pharmacogenomics J. 2016;16:399–410. doi: 10.1038/tpj.2016.54. [DOI] [PubMed] [Google Scholar]
- Othman E. M. Hintzsche H. Stopper H. Signaling steps in the induction of genomic damage by insulin in colon and kidney cells. Free Radical Biol. Med. 2014;68:247–257. doi: 10.1016/j.freeradbiomed.2013.12.010. [DOI] [PubMed] [Google Scholar]
- Othman E. M. Naseem M. Awad E. Dandekar T. Stopper H. The Plant Hormone Cytokinin Confers Protection against Oxidative Stress in Mammalian Cells. PLoS One. 2016;11:e0168386. doi: 10.1371/journal.pone.0168386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadvand H. Tavafi M. Khosrowbeygi A. Amelioration of altered antioxidant enzymes activity and glomerulosclerosis by coenzyme Q10 in alloxan-induced diabetic rats. J. Diabetes Complications. 2012;26:476–482. doi: 10.1016/j.jdiacomp.2012.06.004. [DOI] [PubMed] [Google Scholar]
- Mohamed N. E. Ashour S. E. Influence of ethanolic extract of strawberry leaves for abrogating bromate hazards in male rats. J. Basic Appl. Zool. 2019;80:19. doi: 10.1186/s41936-019-0088-0. [DOI] [Google Scholar]
- Cordenunsi B. R. Genovese M. I. Oliveira Do Nascimento J. R. Aymoto Hassimotto N. M. José Dos Santos R. Lajolo F. M. Effects of temperature on the chemical composition and antioxidant activity of three strawberry cultivars. Food Chem. 2005;91:113–121. doi: 10.1016/j.foodchem.2004.05.054. doi: 10.1016/j.foodchem.2004.05.054. [DOI] [Google Scholar]
- Mechikova G. Y. Stepanova T. A. Zaguzova E. V. Quantitative determination of total phenols in strawberry leaves. Pharm. Chem. J. 2007;41:97–100. doi: 10.1007/s11094-007-0021-6. doi: 10.1007/s11094-007-0021-6. [DOI] [Google Scholar]
- Singleton V. L. Rossi J. A. Colorimetry of total phenolics with phosphomolybdic–phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965;16:144–158. [Google Scholar]
- Chang C.-C. Yang M.-H. Wen H.-M. Chern J.-C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. Yaowu Shipin Fenxi. 2002;10:178–182. [Google Scholar]
- Lorke D. A new approach to practical acute toxicity testing. Arch. Toxicol. 1983;54:275–287. doi: 10.1007/BF01234480. [DOI] [PubMed] [Google Scholar]
- Manautou J. E. Nowicki M. T. Aleksunes L. M. Sawant S. P. V Dnyanmote A. Mehendale H. M. Renal and hepatic transporter expression in type 2 diabetic rats. Drug Metab. Lett. 2008;2:11–17. doi: 10.2174/187231208783478425. [DOI] [PubMed] [Google Scholar]
- Srinivasan S. Pari L. Ameliorative effect of diosmin, a citrus flavonoid against streptozotocin-nicotinamide generated oxidative stress induced diabetic rats. Chem.-Biol. Interact. 2012;195:43–51. doi: 10.1016/j.cbi.2011.10.003. [DOI] [PubMed] [Google Scholar]
- Milardović S. Kruhak I. Iveković D. Rumenjak V. Tkalčec M. Grabarić B. S. Glucose determination in blood samples using flow injection analysis and an amperometric biosensor based on glucose oxidase immobilized on hexacyanoferrate modified nickel electrode. Anal. Chim. Acta. 1997;350:91–96. doi: 10.1016/S0003-2670(97)00308-5. [DOI] [Google Scholar]
- Bisse E. Abraham E. C. New less temperature-sensitive microchromatographic method for the separation and quantitation of glycosylated hemoglobins using a non-cyanide buffer system. J. Chromatogr. B: Biomed. Sci. Appl. 1985;344:81–91. doi: 10.1016/S0378-4347(00)82009-5. [DOI] [PubMed] [Google Scholar]
- Lopes-Virella M. F. Stone P. Ellis S. Colwell J. A. Cholesterol determination in high-density lipoproteins separated by three different methods. Clin. Chem. 1977;23:882–884. doi: 10.1093/clinchem/23.5.882. [DOI] [PubMed] [Google Scholar]
- Fossati P. Prencipe L. Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clin. Chem. 1982;28:2077–2080. doi: 10.1093/clinchem/28.10.2077. [DOI] [PubMed] [Google Scholar]
- Fossati P. Prencipe L. Berti G. Enzymic creatinine assay: a new colorimetric method based on hydrogen peroxide measurement. Clin. Chem. 1983;29:1494–1496. doi: 10.1093/clinchem/29.8.1494. [DOI] [PubMed] [Google Scholar]
- Fossati P. Prencipe L. Berti G. Use of 3,5-dichloro-2-hydroxybenzenesulfonic acid/4-aminophenazone chromogenic system in direct enzymic assay of uric acid in serum and urine. Clin. Chem. 1980;26:227–231. doi: 10.1093/clinchem/26.2.227. [DOI] [PubMed] [Google Scholar]
- Tietz N. W., Clinical Guide to Laboratory Tests (ELISA), W. B. Saunders, Co., Philadelphia, 3rd edn, 1995 [Google Scholar]
- Srivastava S. K. Use of hydroperoxides in the studies of glutathione metabolism in rat lens. Exp. Eye Res. 1976;22:577–585. doi: 10.1016/0014-4835(76)90002-6. [DOI] [PubMed] [Google Scholar]
- Uchiyama M. and Mihara M., Determination of malondialdehyde, 1978 [Google Scholar]
- Koracevic D. Koracevic G. Djordjevic V. Andrejevic S. Cosic V. Method for the measurement of antioxidant activity in human fluids. J. Clin. Pathol. 2001;54:356–361. doi: 10.1136/jcp.54.5.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan D. B. Multiple range tests for correlated and heteroscedastic means. Biometrics. 1957;13:164–176. doi: 10.2307/2527799. [DOI] [Google Scholar]
- Tien V. N. Duc L. V. Thanh T. B. Isolated Compounds and Cardiotonic Effect on the Isolated Rabbit Heart of Methanolic Flower Extract of Nerium oleander L. Res. J. Phytochem. 2016;10:21–29. doi: 10.3923/rjphyto.2016.21.29. doi: 10.3923/rjphyto.2016.21.29. [DOI] [Google Scholar]
- Forino M. Tartaglione L. Aversano C. D. Ciminiello P. NMR-based identification of the phenolic profile of fruits of Lycium barbarum (goji berries). Isolation and structural determination of a novel N-feruloyl tyramine dimer as the most abundant antioxidant polyphenol of goji berries. Food Chem. 2016;194:1254–1259. doi: 10.1016/j.foodchem.2015.08.129. doi: 10.1016/j.foodchem.2015.08.129. [DOI] [PubMed] [Google Scholar]
- Pande G. Akoh C. C. Enzymatic Synthesis of Tyrosol-Based Phenolipids: Characterization and Effect of Alkyl Chain Unsaturation on the Antioxidant Activities in Bulk Oil and Oil-in-Water Emulsion, JAOCS. J. Am. Oil Chem. Soc. 2016;93:329–337. doi: 10.1007/s11746-015-2775-4. doi: 10.1007/s11746-015-2775-4. [DOI] [Google Scholar]
- Hisham D. M. N. Lip J. M. Noh J. M. Normah A. Identification and isolation of methyl gallate as a polar chemical marker for Labisia pumila Benth. J. Trop. Agric. Food Sci. 2011;39:279–284. [Google Scholar]
- Su Y.-Q. Shen Y.-H. Tang J. Zhang W.-D. Chemical Constituents Of Incarvillea mairei VAR. grandiflora. Chem. Nat. Compd. 2010;46:94–95. doi: 10.1007/s10600-010-9540-6. [DOI] [Google Scholar]
- Liao C.-R. Kuo Y.-H. Ho Y.-L. Wang C.-Y. Yang C.-S. Lin C.-W. Chang Y.-S. Studies on cytotoxic constituents from the leaves of Elaeagnus oldhamii Maxim. in non-small cell lung cancer A549 cells. Molecules. 2014;19:9515–9534. doi: 10.3390/molecules19079515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OECD (Organisation for Economic Co-operation and Development), Guidelines for the testing of chemicals/Section 4: Health Effects. Test No. 425, Acute Oral Toxicity: Up-and-Down Procedure OECD, OECD Publishing, 2001 [Google Scholar]
- Bonadonna R. C. Borghi C. Consoli A. Volpe M. Novel antidiabetic drugs and cardiovascular risk: primum non nocere. Nutr., Metab. Cardiovasc. Dis. 2016;26:759–766. doi: 10.1016/j.numecd.2016.05.007. [DOI] [PubMed] [Google Scholar]
- Abdulazeez S. S., Freeze dried strawberry powder ameliorates alloxan induced hyperlipidemia in diabetic rats, 2015 [Google Scholar]
- Fidrianny I. Syifa F. Insanu M. In vitro Antioxidant Profiles and Phytochemical Content of Different Organs of Strawberry (Fragaria × ananassa Duchesne) Asian J. Sci. Res. 2019;12:241–248. doi: 10.3923/ajsr.2019.241.248. doi: 10.3923/ajsr.2019.241.248. [DOI] [Google Scholar]
- Olsson M. E. Ekvall J. Gustavsson K. E. Nilsson J. Pillai D. Sjöholm I. Svensson U. Åkesson B. Nyman M. G. L. Antioxidants, Low Molecular Weight Carbohydrates, and Total Antioxidant Capacity in Strawberries (Fragaria × ananassa): Effects of Cultivar, Ripening, and Storage. J. Agric. Food Chem. 2004;52:2490–2498. doi: 10.1021/jf030461e. doi: 10.1021/jf030461e. [DOI] [PubMed] [Google Scholar]
- Mandave P. C. Pawar P. K. Ranjekar P. K. Mantri N. Kuvalekar A. A. Comprehensive evaluation of in vitro antioxidant activity, total phenols and chemical profiles of two commercially important strawberry varieties. Sci. Hortic. 2014;172:124–134. doi: 10.1016/j.scienta.2014.03.002. doi: 10.1016/j.scienta.2014.03.002. [DOI] [Google Scholar]
- Kårlund A. Hanhineva K. Lehtonen M. Karjalainen R. O. Sandell M. Nontargeted metabolite profiles and sensory properties of strawberry cultivars grown both organically and conventionally. J. Agric. Food Chem. 2015;63:1010–1019. doi: 10.1021/jf505183j. doi: 10.1021/jf505183j. [DOI] [PubMed] [Google Scholar]
- Pineli L. de L. d. O. Moretti C. L. dos Santos M. S. Campos A. B. Brasileiro A. V. Córdova A. C. Chiarello M. D. Antioxidants and other chemical and physical characteristics of two strawberry cultivars at different ripeness stages. J. Food Compos. Anal. 2011;24:11–16. doi: 10.1016/j.jfca.2010.05.004. doi: 10.1016/j.jfca.2010.05.004. [DOI] [Google Scholar]
- Peterson J. Dwyer J. Taxonomic classification helps identify flavonoid-containing foods on a semiquantitative food frequency questionnaire. J. Am. Diet. Assoc. 1998;98:677–685. doi: 10.1016/S0002-8223(98)00153-9. [DOI] [PubMed] [Google Scholar]
- Raudoniute I. Rovira J. Venskutonis P. R. Damašius J. Rivero-Pérez M. D. González-Sanjosé M. L. Antioxidant properties of garden strawberry leaf extract and its effect on fish oil oxidation. Int. J. Food Sci. Technol. 2011;46:935–943. doi: 10.1111/j.1365-2621.2011.02582.x. doi: 10.1111/j.1365-2621.2011.02582.x. [DOI] [Google Scholar]
- Marles R. J. Farnsworth N. R. Antidiabetic plants and their active constituents. Phytomedicine. 1995;2:137–189. doi: 10.1016/S0944-7113(11)80059-0. [DOI] [PubMed] [Google Scholar]
- Rasouli H. Hosseini-Ghazvini S. M.-B. Adibi H. Khodarahmi R. Differential α-amylase/α-glucosidase inhibitory activities of plant-derived phenolic compounds: a virtual screening perspective for the treatment of obesity and diabetes. Food Funct. 2017;8:1942–1954. doi: 10.1039/C7FO00220C. [DOI] [PubMed] [Google Scholar]
- Yang D. Xie H. Jiang Y. Wei X. Phenolics from strawberry cv. Falandi and their antioxidant and α -glucosidase inhibitory activities. Food Chem. 2016;194:857–863. doi: 10.1016/j.foodchem.2015.08.091. doi: 10.1016/j.foodchem.2015.08.091. [DOI] [PubMed] [Google Scholar]
- Jennings A. Welch A. A. Spector T. Macgregor A. Cassidy A. Intakes of Anthocyanins and Flavones Are Associated with Biomarkers of Insulin Resistance and Inflammation in Women. J. Nutr. 2014;144:202–208. doi: 10.3945/jn.113.184358.The. doi: 10.3945/jn.113.184358.The. [DOI] [PubMed] [Google Scholar]
- Wang C. Pan Y. Zhang Q. Wang F. Kong L. Quercetin and Allopurinol Ameliorate Kidney Injury in STZ-Treated Rats with Regulation of Renal NLRP3 Inflammasome Activation and Lipid Accumulation. PLoS One. 2012;7:1–14. doi: 10.1371/journal.pone.0038285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe K. I. Kwon J. H. Park K. H. Oh M. H. Kim M. H. Kim H. H. Cho S. H. Chung E. K. Ha S. Y. Lee M. W. The antioxidant and anti-inflammatory effects of phenolic compounds isolated from the root of Rhodiola sachalinensis A. BOR. Molecules. 2012;17:11484–11494. doi: 10.3390/molecules171011484. doi: 10.3390/molecules171011484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh T.-J. Liu T.-Z. Chia Y.-C. Chern C.-L. Lu F.-J. Chuang M. Mau S.-Y. Chen S.-H. Syu Y.-H. Chen C.-H. Protective effect of methyl gallate from Toona sinensis (Meliaceae) against hydrogen peroxide-induced oxidative stress and DNA damage in MDCK cells. Food Chem. Toxicol. 2004;42:843–850. doi: 10.1016/j.fct.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Nile S. H. Ko E. Y. Kim D. H. Keum Y.-S. Screening of ferulic acid related compounds as inhibitors of xanthine oxidase and cyclooxygenase-2 with anti-inflammatory activity. Rev. Bras. Farmacogn. 2016;26:50–55. doi: 10.1016/j.bjp.2015.08.013. [DOI] [Google Scholar]
- Sarna L. K. Sid V. Wang P. Siow Y. L. House J. D. Karmin O. Tyrosol attenuates high fat diet-induced hepatic oxidative stress: Potential involvement of cystathionine β-synthase and cystathionine γ-lyase. Lipids. 2016;51:583–590. doi: 10.1007/s11745-015-4084-y. [DOI] [PubMed] [Google Scholar]
- Chandramohan R. Pari L. Rathinam A. Sheikh B. A. Tyrosol, a phenolic compound, ameliorates hyperglycemia by regulating key enzymes of carbohydrate metabolism in streptozotocin induced diabetic rats. Chem.-Biol. Interact. 2015;229:44–54. doi: 10.1016/j.cbi.2015.01.026. [DOI] [PubMed] [Google Scholar]