Abstract
(1) Background: Hypofractionated stereotactic radiotherapy (HSRT) and anti-vascular endothelial growth factor (VEGF) antibodies have been reported to have a promising survival benefit in recent studies. Anlotinib is a new oral VEGF receptor inhibitor. This report describes our experience using HSRT and anlotinib for recurrent glioblastoma (rGBM). (2) Methods: Between December 2019 and June 2020, rGBM patients were retrospectively analysed. Anlotinib was prescribed at 12 mg daily during HSRT. Adjuvant anlotinib was administered d1-14 every 3 weeks. The primary endpoint was the objective response rate (ORR). Secondary endpoints included overall survival (OS), progression-free survival (PFS) after salvage treatment, and toxicity. (3) Results: Five patients were enrolled. The prescribed dose was 25.0 Gy in 5 fractions. The median number of cycles of anlotinib was 21 (14–33). The ORR was 100%. Three (60%) patients had the best outcome of a partial response (PR), and 2 (40%) achieved a complete response (CR). One patient died of tumour progression at the last follow-up. Two patients had grade 2 hand-foot syndrome. (4) Conclusions: Salvage HSRT combined with anlotinib showed a favourable outcome and acceptable toxicity for rGBM. A prospective phase II study (NCT04197492) is ongoing to further investigate the regimen.
Keywords: hypofractionated stereotactic radiotherapy, recurrent high-grade glioma, salvage treatment, anlotinib
1. Introduction
Glioblastoma is the most frequently diagnosed malignant primary brain tumour in adults. Maximum surgical resection with six courses of temozolomide adjuvant chemoradiotherapy is the current standard of care in the first-line management of glioblastoma [1]. However, most patients still suffer from recurrence within eight months after primary treatment, and approximately 90% of recurrences occur within a 2 cm margin of the original tumour resection cavity [2]. The management of recurrent glioblastoma is highly challenging due to resistance to available therapeutic approaches, and treatment outcomes remain uniformly poor.
For recurrent glioblastoma (rGBM), several options have been studied, including surgery, re-irradiation, tumour-treating fields, and systemic therapy. Many second-line targeted agents and chemotherapy regimens have been examined in trials with limited success. The anti-vascular endothelial growth factor (VEGF) antibody bevacizumab has been demonstrated to prolong the progression-free survival (PFS) of glioblastoma (GBM); however, patients still progress after 3–6 months with an OS of 6–9 months after salvage treatments [3]. Anlotinib is a novel tyrosine kinase inhibitor that targets vascular endothelial growth factor receptor (VEGFR) 1/2/3, platelet-derived growth factor receptor (PDGFR), fibroblast growth factor receptors (FGFR) 1/2/3/4, c-Kit, and Ret. It has been reported to have a promising effect on tumour control in an rGBM case report [4]. However, as a salvage treatment, failure ultimately occurs. It is crucial to increase local treatment to reduce the risk of disease progression. The Radiation Therapy Oncology Group (RTOG) 1205 trial reported a prolonged PFS for bevacizumab with hypofractionated stereotactic radiotherapy (HSRT) compared with bevacizumab alone [5]. As the main pattern of failure remains local recurrence, it is crucial to optimize local control to improve survival.
Advances in stereotactic radiation can deliver high doses to tumours while limiting toxicity to normal structures. CyberKnife is a noncoplanar radiosurgery system that allows highly conformal image-guided radiotherapy and shows a promising tumour control effect for central nervous system tumours. A prior retrospective study at our centre showed the efficacy of hypofractionated stereotactic radiotherapy for recurrent high-grade glioma (rHGG) patients with mild toxicity. This study aimed to report the preliminary outcome of HSRT combined with anlotinib. To our knowledge, this is the first cohort of rGBM patients treated with HSRT combined with anlotinib.
2. Materials and Methods
2.1. Eligibility Criteria and Endpoints
This is a retrospective, cross-sectional study that was approved by the local ethics committee. Between December 2019 and June 2020, five rGBM patients received salvage HSRT with anlotinib at Huashan Hospital, Fudan University. All patients received surgery followed by standard chemoradiotherapy before recurrence. Recurrence was confirmed by the Response Assessment in Neuro-Oncology (RANO) criteria. Patients who were able to lie flat to receive radiotherapy and had Karnofsky Performance Status (KPS) scores higher than or equal to 70 were considered eligible for the regimen at our institution. All patients were treated at first recurrence within the radiation field and were not eligible for resection after neurosurgeons’ evaluation. Patients were informed that re-resection, re-irradiation, systemic therapy, and best supportive care were the treatment options and chose to receive the treatment after having fully understood and agreed to the potential harm and benefit.
The outcome endpoint was the objective response rate (ORR). Other endpoints included overall survival (OS) after HSRT, progression-free survival after salvage treatment, the best tumour response defined by the RANO criteria, and toxicity defined by the Common Terminology Criteria for Adverse Events (CTCAE) 5.0.
2.2. Baseline Evaluation and Treatment Delivery
Patients were immobilised with a custom thermoplastic mask and underwent localised 1.25-mm thin-slice computed tomography (CT, GE Light speed Ultra 16 Slice, San Francisco, CA, USA) and 2-mm thin-slice magnetic resonance imaging (MRI) including T1 post-contrast and T2 FLAIR images. CT and MRI scans were then fused using the planning system for contouring. HSRT was delivered by a CyberKnife Radiosurgery System (Accuray, Sunnyvale, CA, USA).
Radiation oncologists, neurosurgeons, and radiation physicists participated in tumour delineation and planning. The prescribed dose was 25.0 Gy in 5 fractions. The gross tumour volume (GTV) was defined as the gadolinium-enhanced tumour on the T1-weighted series. The clinical tumour volume (CTV) was considered equal to the GTV. The planning target volume (PTV) was a uniform 1-mm expansion of the CTV. Multiplan software was used for inverse planning. The prescribed isodose line to the PTV was determined according to the target volume, site, previous irradiation volume, and interval between treatments. Anlotinib (Tai-Tianqing Pharmaceutical Co., Ltd., Jiangsu, China) was prescribed at a dose of 12 mg daily for 14 consecutive days every 3 weeks from the first day of HSRT.
2.3. Assessment and Toxicity
All patients underwent a clinical and radiological follow-up every two months after HSRT. If there was any significant deterioration in the patient’s performance, an MRI was performed immediately. The radiological examination included MRI and other necessary examinations, such as MRI-based spectroscopy, perfusion MRI, and methionine positron emission tomography. The KPS after treatment, adverse event occurrence, and associated clinical outcomes were recorded. Toxicity was assessed using the CTCAE 5.0.
2.4. Statistics
The outcome measures considered were the objective response rate based on the proportion of patients with a best overall response of a confirmed complete response (CR) or partial response (PR). Other measures included overall survival after HSRT, defined as survival from the time of the completion of HSRT to death due to any cause, progression-free survival after salvage treatment, and treatment-related toxicities.
The CTCAE 5.0 was used to assess toxicity. The number of events, number of subjects, and incidence rate are used to describe the measurement. The maximum, minimum, and median values are used to describe the measurements of patient characteristics.
3. Results
3.1. Patient Characteristics
Five glioblastoma patients with clinical and radiographic evidence of recurrence were treated with HSRT between December 2019 and June 2020. All patients were initially treated with a maximum safe resection of gross total resection and adjuvant radiation treatment with a median dose of 60 Gy in 30 fractions with concurrent and maintenance temozolomide. The GTV of adjuvant radiation after surgery was defined by the post-contrast T1 and T2 fluid-attenuated inversion recovery sequences. GTV was expanded 1–2 cm to create CTV. PTV with a 3–5 mm margin was added to the CTV. All patients had information on methyl-guanine-methyltransferase (MGMT), isocitrate dehydrogenase 1 (IDH1), 1p/19q co-deletion, and telomerase reverse transcriptase (TERT) after initial resection. Five patients were TERT- and MGMT-positive, one patient had a 1p/19q co-deletion, and no patient was IDH1-positive. Three patients were male and two were female. The median age was 51 years (range 43–60 years). The KPS score at the time of salvage treatment ranged from 70 to 90. The median time from initial diagnosis to salvage HSRT was 10.4 months, with a range of 7.0 to 14.8 months. The median PTV was 26.9 cm3 (5.5–54.4 cm3). The treatment was delivered daily, and the dose was 25 Gy in five fractions with a median isodose line of 68% (65–70%). Patient characteristics are listed in Table 1.
Table 1.
Patient characteristics and treatment outcomes.
| Case | Age Sex | Interval between Initial Diagnosis and HSRT (Months) | Upfront RT Dose/fx | Upfront Chemotherapy (Cycles) | MGMT | IDH1 | 1p/19q |
|---|---|---|---|---|---|---|---|
| 1 | 60 Male | 12.6 | 60Gy/30 | TMZ (12) | + | - | - |
| 2 | 46 Female | 10.4 | 60Gy/30 | TMZ (6) | + | - | - |
| 3 | 55 Female | 14.8 | 60Gy/30 | TMZ (12) | + | - | + |
| 4 | 51 Male | 10.0 | 60Gy/30 | TMZ (4) | + | - | - |
| 5 | 43 Male | 7.0 | 60Gy/30 | TMZ (4) | + | - | - |
| Case | TERT | Recurrent Lesion | Recurrent PTV (cm3) | KPS at HSRS | Dose (iso-dose line) | Cycles of Anlotinib | F/U Interval from HSRS (months) |
| 1 | + | Left Frontal Lobe | 7.08 | 80 | 68 | 15 | 10 |
| 2 | + | Left Frontal Lobe | 26.94 | 80 | 65 | 14 | 10 |
| 3 | + | Left Occipital Lobe | 54.41 | 70 | 70 | 9 | 6 |
| 4 | + | Right Frontal Lobe | 5.53 | 90 | 70 | 4 | 4 |
| 5 | + | Left and Right Frontal Lobe | 44.33 | 90 | 68 | 8 | 6 |
3.2. Compliance and Toxicities
All patients received the planned radiation dose without interruption. The median number of cycles of anlotinib administered were 21 and ranged from 14 to 33 cycles. No acute clinical morbidity was observed. Grade 2 hand-foot syndrome was observed in two patients during cycles 8 and 10. Anlotinib was discontinued for one week in these two patients. The symptoms were relieved after dermatologic treatment, and the regimen was continued. Details are shown in Table 2. No operations or hospitalisation was required related to acute or delayed toxicity of HSRT and anlotinib.
Table 2.
Best treatment outcomes and adverse events that occurred in rGBM patients.
| Outcomes/AE | Total No. of Patients | No. of Patients | ||
|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | ||
| ORR | 5 (100%) | N/A | ||
| CR | 2 | |||
| PR | 3 | |||
| Haematologic | ||||
| Thrombocytopenia | 1 | 0 | 1 | 0 |
| Nonhaematologic | ||||
| Hand foot syndrome | 2 | 0 | 2 | 0 |
| Rash | 1 | 0 | 1 | 0 |
| Hypertension | 1 | 0 | 1 | 0 |
3.3. Treatment Outcomes
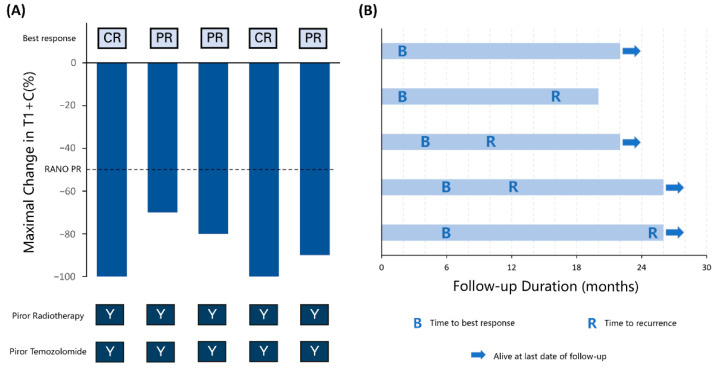
The patients were assessed by the RANO criteria. Three (60%) patients had the best outcome of PR, and two (40%) achieved CR; the ORR was 100% (Figure 1A). The follow-up from the time of HSRT ranged from 20 to 26 months. By the end of the study, four patients had progressive disease (PD) and one patient died of tumour progression (Figure 1B). The overall survival rates following the salvage treatment were 100% and 80% at one and two years, and the PFS rates were 60% and 40%, respectively (see Supplementary Figures S1 and S2).
Figure 1.
(A) Maximal change in the product of the perpendicular diameter in MRI T1 contrast before and after HSRT with anlotinib in each patient. CR, complete response. PR, partial response. RANO, Response Assessment in Neuro-Oncology. Y, yes. (B) Follow-up duration, time to the best response, and time to recurrence in each patient.
4. Discussion
Recurrent glioblastoma has been reported to have a poor prognosis. Due to its therapeutic resistance and aggressiveness, its clinical management is challenging. GBM is a vascularised tumour that produces VEGF. Anti-VEGF treatments have been widely used in recurrent GBM. The mechanism of anti-VEGF treatments may have two aspects. First, inhibiting VEGF and its receptor reduces tumour angiogenesis to produce a hypoxic environment and inhibits tumour growth [6]. Second, the tumour vessel diameter was normalised, and the basement membrane was thin. A reduced volume of tumour microvessels has been reported to be related to longer survival [7].
Bevacizumab is approved for treating recurrent glioblastoma by the US Food and Drug Administration and has become a recommended treatment in the National Comprehensive Cancer Network (NCCN) guidelines, with several phase II and III randomised trials indicating a prolonged PFS compared with chemotherapy alone [8,9,10]. A phase III RCT reported a prolonged median PFS (4.2 vs. 1.5 months) in the bevacizumab and Lomustine groups compared with the Lomustine alone group. However, this trial did not find a difference in OS between the two groups. The grade 3 to 5 toxicity rate in the experimental group was 63.6% [10]. Friedman et al. reported a phase II randomised controlled trial (RCT) in which a higher 6-month PFS rate (50.3% vs. 42.6%) and better ORR (37.8% vs. 28.2%) were observed in the bevacizumab with irinotecan group than in the bevacizumab alone group [9]. Other anti-angiogenic drugs, including sorafenib, pazopanib, sunitinib, etc., were reported in phase I and II trials treating rGBM. The ORR reported for anti-VEGF treatments for rGBM ranged from 6% to 30% (Table 3), and the 6-month PFS ranged from 3% to 63%. The treatment-related toxicity was mild for these anti-VEGF treatments. However, the efficacy seems to be unsatisfactory.
Table 3.
Reported anti-angiogenic treatment for recurrent glioblastoma.
| Author, Year | Treatment | Phase (Sample Size) | Outcome (ORR Rate%) | Median PFS (Months) | Median OS (Months) | 6-Month PFS |
|---|---|---|---|---|---|---|
| Reardon, 2018 [13] | Trebananib | II (11) | 2CR (18) | 0.7 | 11.4 | N/A |
| Reardon, 2005 [14] | Imatinib | II (33) | 3PR (9) | 3.3 | N/A | 27.0% |
| Iwamoto, 2010 [15] | Pazopanib | II (35) | 8PR (22) | 3.0 | 8.1 | 3.0% |
| Pan, 2012 [16] | Sunitinib | II (16) | 0 | N/A | 12.6 | 16.7% |
| Hutterer, 2014 [17] | Sunitinib | II (40) | 0 | 2.0 | 9.2 | 12.5% |
| Hassler, 2014 [18] | Imatinib | II (24) | 2PR (8) | 3.0 | 6.2 | N/A |
| Batchelor, 2010 [19] | Cediranib | II (131) | 1CR, 17PR (14) | 3.0 | 8.0 | 16.0% |
| Gerstner, 2015 [20] | Cediranib | I (45) | 2CR, 2PR (9) | 1.9 | 6.5 | 4.4% |
| Chheda, 2015 [21] | Vandetanib | I (19) | 2PR (11) | 1.9 | 7.2 | 63% |
| McNeill, 2014 [22] | Vandetanib | II (32) | 2PR (6) | 1.7 | 5.6 | N/A |
| Duerinck, 2016 [23] | Axitinib | II (22) | 2CR, 4PR (27) | N/A | 6.7 | 34% |
| Lee, 2012 [24] | Sorafenib | I/II (18) | 2PR (11) | 1.8 | N/A | N/A |
| Groot, 2020 [25] | Aflibercept | II (27) | 8PR (30) | N/A | N/A | N/A |
Anlotinib is an oral novel multi-target tyrosine kinase inhibitor targeting the VEGF1/2/3 receptor, fibroblast growth factor receptor and platelet-derived growth factor receptor. It inhibits more targets than bevacizumab, sunitinib, sorafenib, etc. and has been reported to reduce both tumour proliferation and angiogenesis [3]. Lv et al. published the first case report of the administration of 12 mg anlotinib to an rGBM patient. The patient achieved a PR after 26 days, but the tumour progressed in two months [4]. Wang et al. reported a recurrent GBM patient with an FGFR-TACC3 fusion who was administered anlotinib 12 mg and temozolomide 100 mg/m2. The patient achieved a PR after two months and maintained stable disease for more than 17 months [11].
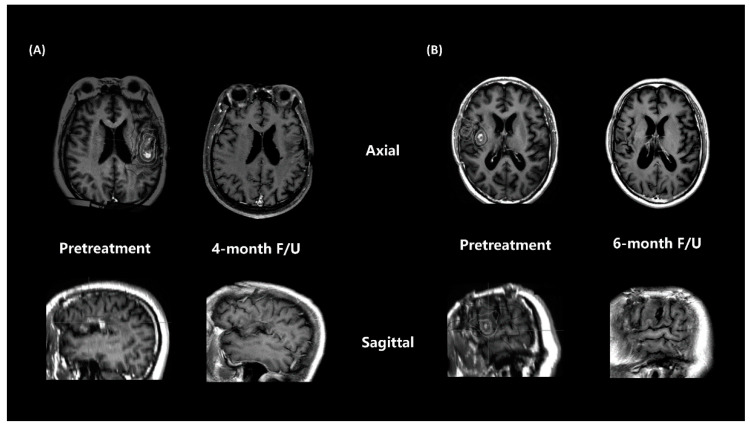
Several reports have suggested that re-irradiation has a reasonable efficacy with acceptable safety profiles in selected patients with recurrent GBM. However, for rGBM, salvage treatment failure ultimately occurs. It is crucial to increase local treatment to reduce recurrence risk. In a meta-analysis, a highly conformal technique with a hypofractionated regimen (e.g., 25 Gy in five fractions or 35 Gy in 10 fractions) is recommended, considering the volume and location of the recurrent tumour. The RTOG 1205 trial reported a prolonged PFS with anti-VEGF treatment with HSRT compared with bevacizumab alone [5]. Philip et al. theorized that additional anti-VEGF treatment sensitised the tumour endothelia to radiotherapy and induced apoptosis [12]. New-generation automated noncoplanar HSRT delivery systems can deliver high-dose treatment by limiting the dose to normal structures and can provide a higher local treatment intensity for recurrent tumours. In this study, the ORR rate of salvage treatment was 100% in two CR and three PR patients. The ORR was higher than other results of anti-VEGF treatments [13,14,15,16,17,18,19,20,21,22,23,24,25], which ranged from 6% to 30% (Table 3). There may be several possible reasons for the promising treatment outcomes. Patient selection may be a reason for good outcomes. All patients had a KPS of 70 or higher, and HSRT was performed after the first recurrence. Moreover, the administration of HSRT increased the local treatment intensity. The preliminary result of RTOG 1205 also reported an increased PFS in the intensified treatment groups. Additionally, patients with a smaller tumour volume may have a better response. The two CR patients (Figure 2A,B) in this study had a relatively smaller PTV (7.08 and 5.53 cm3) than the three PR patients (26.94, 44.33, and 54.41 cm3).
Figure 2.
Contrast-enhanced MRI T1 of responses to HSRT and anlotinib, including (A) patient case 1 and (B) patient case 4, who achieved a complete response.
Salvage HSRT was administered with a full dose of 25 Gy/5 fx for all five patients without any interruption. No radiation necrosis occurred during the follow-up. Grade 2 hand-food syndrome was found in two patients (40%), and rash and hypertension were observed in one patient (20%). These adverse effects were considered to be related to anlotinib. In a phase II randomised trial of non-small-cell lung cancer patients, 28.33% of the subjects had grade 2 hand-foot syndrome, and grade 2 hypertension was observed in 55% of patients [26]. These toxicities were also observed in our study.
The study had some limitations due to its retrospective nature: an inherent patient selection bias was created when the physicians chose eligible patients to receive the regimen. The treatment option was provided for patients with high KPS scores who were not willing to receive standard intravenous bevacizumab treatment. Thus, the cohort was enriched with patients with a better prognosis. Another limitation was that recurrence before salvage treatment was diagnosed by radiological parameters according to the RANO criteria, which is a common practice [27]. However, the lack of biopsy samples limited the information on tumour genomic characterisations. It is crucial to consider whether the previously detected mutation still presents as the dominant clone at the time of recurrence [28]. Further investigation is warranted to explain the potential treatment mechanisms and select good responders to the regimen.
Despite the limitations, this study provides initial evidence of a promising outcome using salvage HSRT with anlotinib in a real-world scenario. Responses were observed in all rGBM patients included in the study. Further investigation is needed to identify patients who can benefit from this regimen. A prospective phase II study HSCK-002 (ClinicalTrials.gov identifier: NCT04197492) is ongoing to further investigate the value of HSRT with anlotinib.
5. Conclusions
Salvage radiosurgery with anlotinib appeared to achieve a clinical benefit with acceptable toxicity for rGBM patients in this preliminary report. A prospective phase II study (NCT04197492) is ongoing to further investigate the value of HSRT with anlotinib in rHGG.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/brainsci12040471/s1. Figure S1: Overall survival (OS) from salvage treatment of all rHGG patients (calculated with the Kaplan–Meier method). Figure S2: Progression-free survival (PFS) from salvage treatment of all rHGG patients (calculated with the Kaplan–Meier method).
Author Contributions
Conceptualization, E.W., X.W., Y.L. and Y.G.; methodology, E.W., X.W. and Y.G.; software, Y.G.; validation, E.W., X.W. and Y.L.; formal analysis, Y.G.; investigation, L.P., J.D. and Y.W.; resources, L.P., E.W. and X.W.; data curation, J.L., X.G., H.Z., C.L., G.M. and X.L.; writing—original draft preparation, Y.G., J.L., X.G.; writing—review and editing, X.W., Y.G. and X.G.; visualization, Y.G.; supervision, E.W.; project administration, X.W.; funding acquisition, X.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China grant number 81727806.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and was approved by the ethics committee of Huashan Hospital.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Attarian F., Taghizadeh-Hesary F., Fanipakdel A., Javadinia S.A., Porouhan P., PeyroShabany B., Fazilat-Panah D. A Systematic Review and Meta-Analysis on the Number of Adjuvant Temozolomide Cycles in Newly Diagnosed Glioblastoma. Front. Oncol. 2021;11:779491. doi: 10.3389/fonc.2021.779491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sneed P.K., Gutin P.H., Larson D.A., Malec M.K., Phillips T.L., Prados M.D., Scharfen C.O., Weaver K.A., Wara W.M. Patterns of Recurrence of Glioblastoma Multiforme after External Irradiation Followed by Implant Boost. Int. J. Radiat. Oncol. Biol. Phys. 1994;29:719–727. doi: 10.1016/0360-3016(94)90559-2. [DOI] [PubMed] [Google Scholar]
- 3.Shen G., Zheng F., Ren D., Du F., Dong Q., Wang Z., Zhao F., Ahmad R., Zhao J. Anlotinib: A Novel Multi-Targeting Tyrosine Kinase Inhibitor in Clinical Development. J. Hematol. Oncol. 2018;11:120. doi: 10.1186/s13045-018-0664-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lv Y., Zhang J., Liu F., Song M., Hou Y., Liang N. Targeted Therapy with Anlotinib for Patient with Recurrent Glioblastoma: A Case Report and Literature Review. Medicine. 2019;98:e15749. doi: 10.1097/MD.0000000000015749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsien C., Pugh S., Dicker A.P., Raizer J.J., Matuszak M.M., Lallana E., Huang J., Algan O., Taylor N., Portelance L. Randomized Phase II Trial of Re-Irradiation and Concurrent Bevacizumab versus Bevacizumab Alone as Treatment for Recurrent Glioblastoma (NRG Oncology/RTOG 1205): Initial Outcomes and RT Plan Quality Report. Int. J. Radiat. Oncol. Biol. Phys. 2019;105:S78. doi: 10.1016/j.ijrobp.2019.06.539. [DOI] [Google Scholar]
- 6.Wong E.T., Gautam S., Malchow C., Lun M., Pan E., Brem S. Bevacizumab for Recurrent Glioblastoma Multiforme: A Meta-Analysis. J. Natl. Compr. Cancer Netw. 2011;9:403–407. doi: 10.6004/jnccn.2011.0037. [DOI] [PubMed] [Google Scholar]
- 7.Sorensen A.G., Batchelor T.T., Zhang W.-T., Chen P.-J., Yeo P., Wang M., Jennings D., Wen P.Y., Lahdenranta J., Ancukiewicz M. A “Vascular Normalization Index” as Potential Mechanistic Biomarker to Predict Survival after a Single Dose of Cediranib in Recurrent Glioblastoma Patients. Cancer Res. 2009;69:5296–5300. doi: 10.1158/0008-5472.CAN-09-0814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vredenburgh J.J., Desjardins A., Herndon J.E., Marcello J., Reardon D.A., Quinn J.A., Rich J.N., Sathornsumetee S., Gururangan S., Sampson J. Bevacizumab plus Irinotecan in Recurrent Glioblastoma Multiforme. J. Clin. Oncol. 2007;25:4722–4729. doi: 10.1200/JCO.2007.12.2440. [DOI] [PubMed] [Google Scholar]
- 9.Friedman H.S., Prados M.D., Wen P.Y., Mikkelsen T., Schiff D., Abrey L.E., Yung W.A., Paleologos N., Nicholas M.K., Jensen R. Bevacizumab Alone and in Combination with Irinotecan in Recurrent Glioblastoma. J. Clin. Oncol. 2009;27:4733–4740. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 10.Wick W., Gorlia T., Bendszus M., Taphoorn M., Sahm F., Harting I., Brandes A.A., Taal W., Domont J., Idbaih A. Lomustine and Bevacizumab in Progressive Glioblastoma. N. Engl. J. Med. 2017;377:1954–1963. doi: 10.1056/NEJMoa1707358. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y., Liang D., Chen J., Chen H., Fan R., Gao Y., Gao Y., Tao R., Zhang H. Targeted Therapy with Anlotinib for a Patient with an Oncogenic FGFR3-TACC3 Fusion and Recurrent Glioblastoma. Oncologist. 2021;26:173–177. doi: 10.1002/onco.13530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gutin P.H., Iwamoto F.M., Beal K., Mohile N.A., Karimi S., Hou B.L., Lymberis S., Yamada Y., Chang J., Abrey L.E. Safety and Efficacy of Bevacizumab with Hypofractionated Stereotactic Irradiation for Recurrent Malignant Gliomas. Int. J. Radiat. Oncol. Biol. Phys. 2009;75:156–163. doi: 10.1016/j.ijrobp.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reardon D.A., Lassman A.B., Schiff D., Yunus S.A., Gerstner E.R., Cloughesy T.F., Lee E.Q., Gaffey S.C., Barrs J., Bruno J. Phase 2 and Biomarker Study of Trebananib, an Angiopoietin-blocking Peptibody, with and without Bevacizumab for Patients with Recurrent Glioblastoma. Cancer. 2018;124:1438–1448. doi: 10.1002/cncr.31172. [DOI] [PubMed] [Google Scholar]
- 14.Reardon D.A., Egorin M.J., Quinn J.A., Rich J.N., Gururangan I., Vredenburgh J.J., Desjardins A., Sathornsumetee S., Provenzale J.M., Herndon J.E. Phase II Study of Imatinib Mesylate plus Hydroxyurea in Adults with Recurrent Glioblastoma Multiforme. J. Clin. Oncol. 2005;23:9359–9368. doi: 10.1200/JCO.2005.03.2185. [DOI] [PubMed] [Google Scholar]
- 15.Iwamoto F.M., Lamborn K.R., Robins H.I., Mehta M.P., Chang S.M., Butowski N.A., DeAngelis L.M., Abrey L.E., Zhang W.-T., Prados M.D. Phase II Trial of Pazopanib (GW786034), an Oral Multi-Targeted Angiogenesis Inhibitor, for Adults with Recurrent Glioblastoma (North American Brain Tumor Consortium Study 06-02) Neuro-Oncology. 2010;12:855–861. doi: 10.1093/neuonc/noq025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pan E., Yu D., Yue B., Potthast L., Chowdhary S., Smith P., Chamberlain M. A Prospective Phase II Single-Institution Trial of Sunitinib for Recurrent Malignant Glioma. J. Neuro-Oncol. 2012;110:111–118. doi: 10.1007/s11060-012-0943-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hutterer M., Nowosielski M., Haybaeck J., Embacher S., Stockhammer F., Gotwald T., Holzner B., Capper D., Preusser M., Marosi C. A Single-Arm Phase II Austrian/German Multicenter Trial on Continuous Daily Sunitinib in Primary Glioblastoma at First Recurrence (SURGE 01-07) Neuro-Oncology. 2014;16:92–102. doi: 10.1093/neuonc/not161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hassler M.R., Vedadinejad M., Flechl B., Haberler C., Preusser M., Hainfellner J.A., Wöhrer A., Dieckmann K.U., Rössler K., Kast R. Response to Imatinib as a Function of Target Kinase Expression in Recurrent Glioblastoma. SpringerPlus. 2014;3:111. doi: 10.1186/2193-1801-3-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Batchelor T.T., Duda D.G., di Tomaso E., Ancukiewicz M., Plotkin S.R., Gerstner E., Eichler A.F., Drappatz J., Hochberg F.H., Benner T. Phase II Study of Cediranib, an Oral Pan–Vascular Endothelial Growth Factor Receptor Tyrosine Kinase Inhibitor, in Patients with Recurrent Glioblastoma. J. Clin. Oncol. 2010;28:2817. doi: 10.1200/JCO.2009.26.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gerstner E.R., Ye X., Duda D.G., Levine M.A., Mikkelsen T., Kaley T.J., Olson J.J., Nabors B.L., Ahluwalia M.S., Wen P.Y. A Phase I Study of Cediranib in Combination with Cilengitide in Patients with Recurrent Glioblastoma. Neuro-Oncology. 2015;17:1386–1392. doi: 10.1093/neuonc/nov085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chheda M.G., Wen P.Y., Hochberg F.H., Chi A.S., Drappatz J., Eichler A.F., Yang D., Beroukhim R., Norden A.D., Gerstner E.R. Vandetanib plus Sirolimus in Adults with Recurrent Glioblastoma: Results of a Phase I and Dose Expansion Cohort Study. J. Neuro-Oncol. 2015;121:627–634. doi: 10.1007/s11060-014-1680-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McNeill K., Iwamoto F., Kreisl T., Sul J., Shih J., Fine H. AT-39A Randomized Phase II Trial of Vandetanib (ZD6474) in Combination with Carboplatin versus Carboplatin Alone in Adults with Recurrent Glioblastoma. Neuro-Oncology. 2014;16((Suppl. S5)):v17. doi: 10.1093/neuonc/nou237.38. [DOI] [Google Scholar]
- 23.Duerinck J., Du Four S., Vandervorst F., D’Haene N., Le Mercier M., Michotte A., Van Binst A.M., Everaert H., Salmon I., Bouttens F. Randomized Phase II Study of Axitinib versus Physicians Best Alternative Choice of Therapy in Patients with Recurrent Glioblastoma. J. Neuro-Oncol. 2016;128:147–155. doi: 10.1007/s11060-016-2092-2. [DOI] [PubMed] [Google Scholar]
- 24.Lee E.Q., Kuhn J., Lamborn K.R., Abrey L., DeAngelis L.M., Lieberman F., Robins H.I., Chang S.M., Yung W.A., Drappatz J. Phase I/II Study of Sorafenib in Combination with Temsirolimus for Recurrent Glioblastoma or Gliosarcoma: North American Brain Tumor Consortium Study 05-02. Neuro-Oncology. 2012;14:1511–1518. doi: 10.1093/neuonc/nos264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Groot J.F., Wen P.Y., Lamborn K., Chang S., Cloughesy T.F., Chen A.P., DeAngelis L.M., Mehta M.P., Gilbert M.R., Yung W.K. Phase II Single Arm Trial of Aflibercept in Patients with Recurrent Temozolomide-Resistant Glioblastoma: NABTC 0601. J. Clin. Oncol. 2008;26((Suppl. S15)):2020. doi: 10.1200/jco.2008.26.15_suppl.2020. [DOI] [Google Scholar]
- 26.Han B., Li K., Zhao Y., Li B., Cheng Y., Zhou J., Lu Y., Shi Y., Wang Z., Jiang L. Anlotinib as a Third-Line Therapy in Patients with Refractory Advanced Non-Small-Cell Lung Cancer: A Multicentre, Randomised Phase II Trial (ALTER0302) Br. J. Cancer. 2018;118:654–661. doi: 10.1038/bjc.2017.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vogelbaum M.A., Jost S., Aghi M.K., Heimberger A.B., Sampson J.H., Wen P.Y., Macdonald D.R., Van den Bent M.J., Chang S.M. Application of Novel Response/Progression Measures for Surgically Delivered Therapies for Gliomas: Response Assessment in Neuro-Oncology (RANO) Working Group. Neurosurgery. 2012;70:234–244. doi: 10.1227/NEU.0b013e318223f5a7. [DOI] [PubMed] [Google Scholar]
- 28.Kaley T., Touat M., Subbiah V., Hollebecque A., Rodon J., Lockhart A.C., Keedy V., Bielle F., Hofheinz R.-D., Joly F. BRAF Inhibition in BRAFV600-Mutant Gliomas: Results from the VE-BASKET Study. J. Clin. Oncol. 2018;36:3477. doi: 10.1200/JCO.2018.78.9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.