Abstract
Local, national, and international health agencies have advocated multi-pronged public health strategies to limit infections and prevent deaths. The availability of safe and effective vaccines is critical in the control of a pandemic. Several adverse events have been reported globally following reception of different vaccines, with limited or no data from Africa. This cross-sectional epidemiological study investigated adverse events following COVID-19 vaccination in Africans from April–June, 2021 using a structured online questionnaire. Out of 1200 participants recruited, a total of 80.8% (n = 969) respondents from 35 countries, including 22 African countries and 13 countries where Africans live in the diaspora, reported adverse events. Over half of the vaccinees were male (53.0%) and frontline healthcare workers (55.7%), respectively. A total of 15.6% (n = 151) reported previous exposure to SARS-CoV-2, while about one-fourth, 24.8% (n = 240), reported different underlying health conditions prior to vaccination. Fatal cases were 5.1% (n = 49), while other significant heterogenous events were reported in three categories: very common, common, and uncommon, with the latter including enlarged lymph nodes 2.4% (n = 23), menstrual disorder 0.5% (n = 5), and increased libido 0.2% (n = 2). The study provided useful data for concerned authorities and institutions to prepare plans that will address issues related to COVID-19 vaccines.
Keywords: vaccine adverse events, SARS-CoV-2, COVID-19 vaccine, public health, vaccination, adverse events following immunisation (AEFI)
1. Introduction
As the COVID-19 pandemic progresses, multilateral agencies under the coordination of the World Health Organisation (WHO) are working round the clock to stem the tide of continuing infection and transmission, not only by supplying medical kits and equipment to various healthcare systems, but also through coordinated distribution of safe and effective vaccines. Vaccination and immunisation remain the best options for the prevention and control of diseases worldwide [1]. To date, 15 November 2021, more than 7.5 billion doses of COVID-19 vaccines have been administered among 52.1% of the global population, with an approximate of 31.2 million new doses administered daily. However, only 4.5% of the population in low-income countries have been vaccinated, with approximately 300 million doses administered for full or partial vaccination in Africa (https://ourworldindata.org/covid-vaccinations?country=OWID_WRL (accessed on 7 November 2021)). It is instructive to understand that Africa has an estimated population of 1.4 billion (amounting to only 21.4% of Africans vaccinated).
The COVID-19 Vaccines Global Access (COVAX) facility was launched on the 24 February 2021 to improve COVID-19 vaccine deliveries to Africa [2]. Ghana and Cote D’Ivoire were the first two countries to receive the WHO-approved COVISHIELD vaccine on 24 and 26 February 2021, respectively. These deliveries were followed by those for Nigeria, Angola, Democratic Republic of Congo, and Gambia on the 2 March 2021. These were followed by Rwanda on the 3 March, the same day as Kenya, Sudan, and Malawi, with Rwanda being the first country to receive both the AstraZeneca/Oxford vaccine and the Pfizer–BioNTech mRNA vaccine from the COVAX facility [2]. In addition, Benin received 144,000 COVISHIELD vaccines on the 11th March 2021.
The Oxford–AstraZeneca vaccine was the most widely used because of its ease of storage at a temperature of +2 to +8 °C and associated logistics, but it is not without its adverse events, as is observed for other COVID-19 vaccines. Generally, vaccines may produce adverse reactions due to idiosyncrasies or because the body’s immunological system is set to always recognise foreign and infectious agents, and there is the possibility of it responding physiologically through the cellular and/or humoral pathways. According to the CDC in 2021, any local or systemic health problem or side effect that occurs after vaccination or immunisation is referred to as an adverse event (ADE) following vaccination or immunisation (AEFI) [3]. Global adverse events following COVID-19 vaccination vary based on the type of vaccine, but the most common symptoms reported include fatigue, headache, muscle and joint pain, allergic skin reaction, and chills, while the most prevalent events include low-grade fever and pain or redness at the site of injection, often felt a few days after vaccination. Severe adverse events are possible, but the chances are low [4,5,6,7,8].
Nevertheless, despite the identification of serious and fatal adverse events following COVID-19 vaccination, a causal relationship has not been really established [9,10,11]. Severe adverse events following vaccination have been identified by the United States of America (USA) Vaccine Adverse Event Reporting System (VAERS). According to the report of May 2021, 2 to 5 people per million vaccinated in the USA developed a severe and rare anaphylactic reaction that occurred 30 min after vaccination. VAERS also filed 32 confirmed reports of people who developed thrombosis with thrombocytopenia syndrome (TTS) after getting the Johnson & Johnson (J&J) vaccine.
Moreover, other reports identified the occurrence of myocarditis and pericarditis in vaccinees who were vaccinated with the Pfizer–BioNTech and Moderna COVID-19 vaccines, mostly in adolescents and young adults. In addition, VAERS reported 9810 human deaths from 442 million doses of COVID-19 vaccines administered in the USA from 14 December 2020, through 15 November 2021 [12]. Although a causal link of death to COVID-19 vaccines is still being examined, important reports attributed the death that occurred to a rare, serious adverse event involving blood clots with low platelets following the J&J vaccine [12]. On the other hand, thrombotic and thromboembolic adverse events and related causes of death have been reported across European countries following AstraZeneca/COVISHIELD vaccine administration [13], including but not limited to the United Kingdom [14].
Until 14 November 2021, there are a total of 15,287 (0.07%) AEFI reported following 22,749,817 doses of COVID-19 vaccines administered in Ontario, with an overall reporting rate of 67.2 per 100,000 doses and specific rates of 56.9 per 100,000 doses administered for the Pfizer–BioNTech vaccine, 81.6 per 100,000 doses for the Moderna vaccine, and 141.9 per 100,000 doses for the AstraZeneca/COVISHIELD vaccine. Furthermore, 29 reports of deaths plausibly associated with receipt of the COVID-19 vaccine have been filed, and a causal relationship to any vaccine is still being investigated [7].
Despite the outstanding achievements in the development and rapid approval of the different vaccines, Africa is still at high risk of a likely long period of COVID-19 community spread and hospitalisation, mainly due to unwillingness of Africans to receive the vaccines [15] combined with the scarcity of vaccines, lack of infrastructure for vaccine production on the continent, and paucity of funds. Furthermore, there is a dearth of information and lack of reporting or surveillance data on adverse events following COVID-19 vaccination in Africa. Furthermore, there is need for active surveillance and public health research communication in order to provide media supports for public health researchers and improve health literacy for the populace, especially among the youths, via social media to counter fake news campaigns on adverse events following COVID-19 vaccination [16].
Hence, this public health surveillance was designed to investigate adverse events associated with COVID-19 vaccination in the African population and the management options being employed by all individuals reporting adverse events.
2. Materials and Methods
2.1. Study Design and Participants
A descriptive, cross-sectional, continent-based study was carried out to monitor adverse events following COVID-19 vaccination in Africans living in Africa and diaspora from April–June 2021. An online survey instrument was deployed for data collection from consenting participants recruited using a convenience sampling method. Inclusion criteria were being an African regardless of location, 18 years of age and above, and having received any of the COVID-19 vaccines. Those who have not received the vaccines were excluded from the survey. Participants who met the inclusion criteria were only eligible if they had access to an electronic medium with Internet capability. Physical and interviewer-based questionnaires were avoided to reduce the risk of contracting and spreading SARS-CoV-2/COVID-19. An initial probable target of ≥50–≤100 respondents per country was planned to be recruited from North, Southern, West, and East Africa. This strategy was selected because it was difficult to determine the exact number of persons among the target population per country who met all the inclusion criteria for online survey participation. Since the survey was also conducted on a willing participant basis, the number of final recruits was difficult to determine. Therefore, our data should be carefully interpreted, as it does not epitomise the entire African population.
2.2. Questionnaire
A structured, multiple-choice pretested questionnaire was designed in English and translated into French and Arabic by native language experts to capture the specific and logical respondents’ quantitative data in Arabic- and French-speaking countries, respectively. The questionnaire was designed based on the WHO, FDA, and UK-NHS classification of common and uncommon COVID-19 vaccination reactions, to elicit information about respondents’ socio-economic and demographic characteristics, health status, past medical history, adverse events following vaccination, and how those events were managed. The questionnaire was uploaded to Google Forms (https://forms.gle/k9KYGp7wC4JNjZeL6, Alpha Inc., Turlock, CA, USA) (Appendix A) for distribution and a one-time data collection process through various online social media platforms including WhatsApp, Facebook, Instagram, Telegram, Twitter, LinkedIn, and emails. The questionnaire was first pretested among 30 respondents in different countries before its administration by research collaborators in various countries including Egypt, Ghana, Kenya, Morocco, Namibia, Nigeria, Rwanda, South Africa, Somalia, and Sudan, among others. Continuous sharing was done on social media, with sponsored adverts on Facebook deployed as reminders to encourage participation.
2.3. Ethics Approval and Consent
Ethical approval for the study was obtained from various institutional review committees of Nigeria (University of Ilorin), Egypt (Ahram Canadian University, Faculty of Oral and Dental Medicine) and Kenya (Kenyatta University). Guidelines and code of ethics for human research were observed in line with the Declaration of Helsinki—Ethical Principles for Medical Research involving Human Subjects (64th World Medical Association General Assembly, Fortaleza, Brazil, October 2013). Participation in the study was voluntary, allowing any participant to quit at any stage without submitting the online form. All participants consented to the study by initially selecting an option to ‘voluntarily agree or disagree to participate in the current study’, leading to the study questionnaire or ‘finished’ page as the case may be. Data collection was anonymous, and respondents’ information was kept highly confidential.
2.4. Data Analyses and Statistics
The collated public health surveillance data on adverse vaccine events were retrieved from the Google form in Excel format for sorting and coding. Categorical variables were presented as frequencies and proportions using descriptive statistics. Chi-square test and Fisher’s exact test for 2 × 2 tables were used to test for statistical significance between variables and the demographic (independent) values. Variables considered included demographic characteristics such as age (categories), gender, educational attainment, community type (rural, urban, semi-urban), questionnaire items concerning adverse events, and subsequent reactions following vaccination. All the vaccinated participants were included in the adverse events’ data analyses irrespective of the number of COVID-19 vaccine doses or type received. The proportion of vaccinees who reported side events post-vaccination were calculated. Differences in severity between post-vaccination adverse events were compared to investigate the variability in different categories and between variables. The event of previous exposure to SARS-CoV-2/COVID-19 and the consequence of adverse reaction was compared to the naive population.
No adjustments were made for missing data, and all analyses used complete case analysis. p-values were two-sided, and analyses were carried out at 95% confidence interval using Statistical Package for the Social Sciences (SPSS) software v.22 (SPSS Inc., Chicago, IL, USA) and GraphPad Prism 9.0.0 (121) (GraphPad Software Inc., San Diego, CA, USA).
3. Results
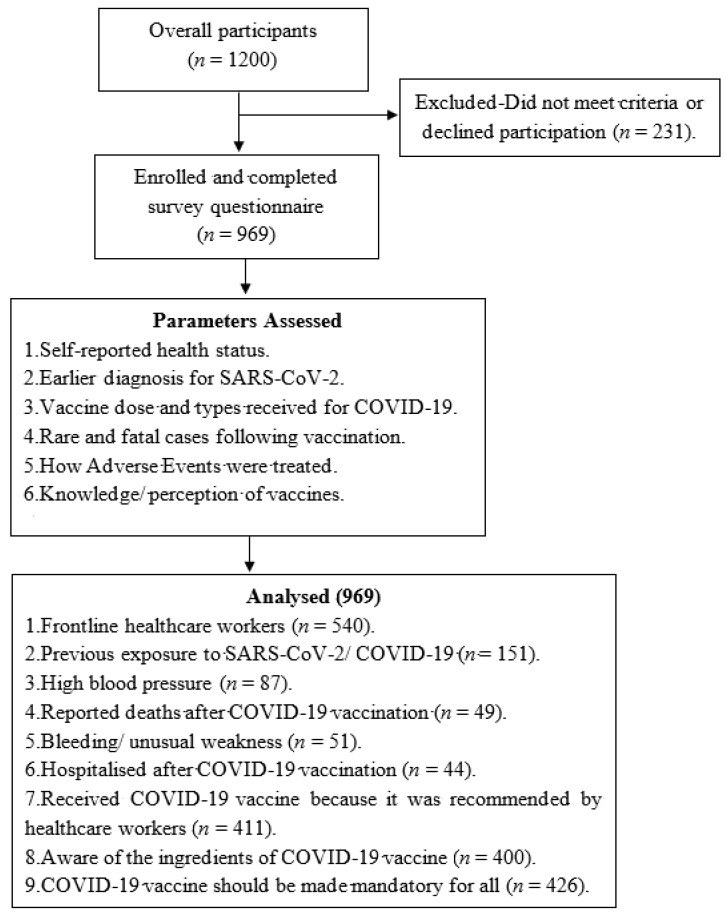
This study detailed the recruitment of participants, past exposure to SARS-CoV-2, exclusion criteria, hospitalisation following vaccination, the observed adverse events, and their treatment by Africans, including recommendation for COVID-19 vaccination (Figure 1).
Figure 1.
Flow chart of participants experiencing adverse events following COVID-19 Vaccination in Africa.
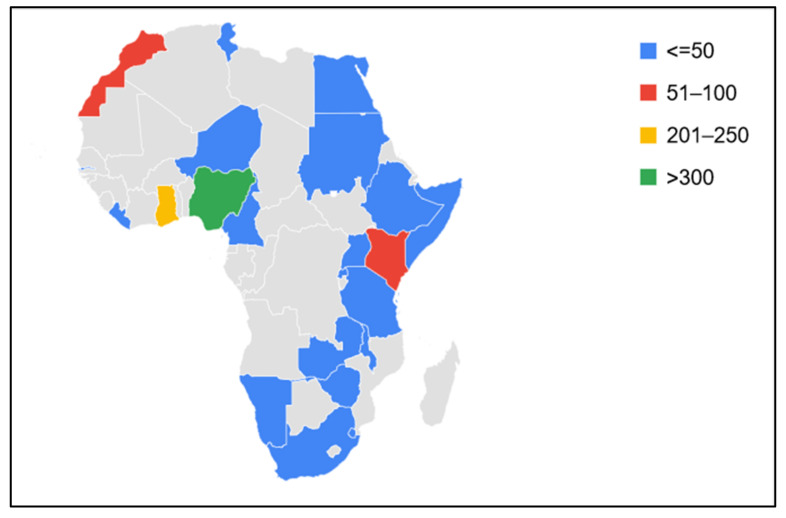
Out of the overall participants (n = 1200), a total of 19.2% (n = 231) were excluded, as they did not meet the study inclusion criteria or declined participation by not giving their consent, while 80.8% (n = 969) reported different adverse events following COVID-19 vaccination from 35 countries, including 22 African countries: Cameroon, Egypt, Ethiopia, Gambia, Ghana, Liberia, Kenya, Malawi, Morocco, Namibia, Niger, Nigeria, Rwanda, Somalia, South Africa, Tanzania, Tunisia, Uganda, Swaziland, Sudan, Zambia, and Zimbabwe (Figure 2).
Figure 2.
Distribution of respondents to questions on adverse events following COVID-19 vaccination in African countries.
Nigeria recorded the highest number of participants (33.7%), followed by Ghana (23.3%) and Kenya (9.7%), respectively, while the fewest responses were documented from participants in Somalia, Sudan, and Rwanda, recording proportions of 5.1%, 4.1%, and 2.8%, respectively (Table 1).
Table 1.
Distribution in some African countries based on the number of willing participants.
| Country | Frequency | Proportion (%) |
|---|---|---|
| Nigeria | 327 | 33.7 |
| Ghana | 226 | 23.3 |
| Kenya | 94 | 9.7 |
| Diaspora | 83 | 8.6 |
| Morocco | 52 | 5.4 |
| Egypt | 50 | 5.2 |
| Somalia | 49 | 5.1 |
| Sudan | 40 | 4.1 |
| Rwanda | 27 | 2.8 |
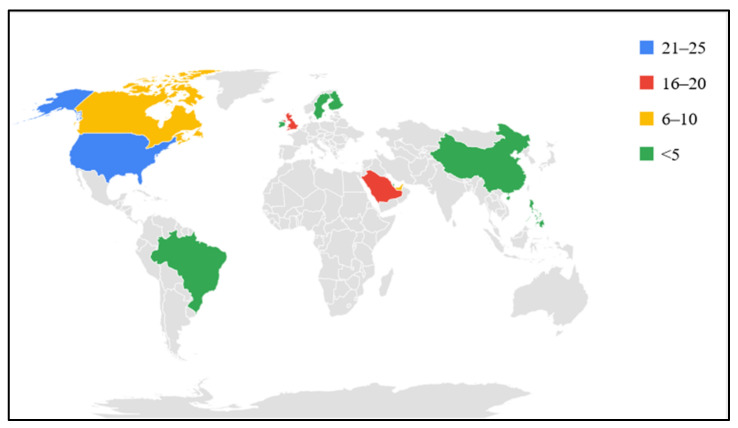
Participants from 13 countries (USA, UK, Saudi Arabia, Canada, UAE, Bahrain, Qatar, Brazil, China, Finland, Ireland, Philippines, and Sweden) represent Africans who live in the diaspora (Figure 3).
Figure 3.
Distribution of some Africans vaccinated against COVID-19 living in the diaspora.
Different demographic profiles of Africans vaccinated against COVID-19 were investigated, with the highest proportion, 40.7%, in the young (25–34 years) population and a decrease in the vaccinated population as the age increases except for the age category 18–24 years (Table 2).
Table 2.
Demographic distribution of Africans vaccinated for COVID-19.
| Category | Frequency | Percentage |
|---|---|---|
| Age | ||
| 18–24 | 84 | 8.7 |
| 25–34 | 394 | 40.7 |
| 35–44 | 268 | 27.7 |
| 45–54 | 123 | 12.7 |
| 55–64 | 69 | 7.1 |
| >65 | 31 | 3.2 |
| Gender | ||
| Male | 514 | 53.0 |
| Female | 455 | 47.0 |
| Education | ||
| Tertiary | 804 | 83.0 |
| Secondary | 49 | 5.1 |
| Primary | 10 | 1.0 |
| Others | 89 | 9.2 |
| None | 17 | 1.8 |
| Occupation | ||
| Frontline Healthcare Workers | 540 | 55.7 |
| Frontline Non-healthcare Workers | 127 | 13.1 |
| Others | 302 | 31.2 |
| Community | ||
| Urban | 727 | 75.0 |
| Semi-urban | 149 | 15.4 |
| Rural | 93 | 9.6 |
A total of 15.6% (n = 151) reported previous exposure to SARS-CoV-2/COVID-19. Earlier investigators opined that prior exposure may alter the response of participants to COVID-19 vaccination. About a quarter, 240/969 (24.8%), of vaccinated Africans reported different underlying conditions prior to COVID-19 vaccination. Cardiovascular diseases such as high blood pressure and heart diseases accounted for more than one-third (38.8%), followed by asthma (17.9%) and diabetes (12.9%), while glaucoma and arthritis at 0.2% each were the least reported (Table 3).
Table 3.
African respondents earlier diagnosed positive for SARS-CoV-2 and underlying conditions before COVID-19 vaccination.
| Frequency (n = 969) | Proportion (%) | |
|---|---|---|
| Earlier diagnosed positive | ||
| No | 799 | 82.5 |
| Yes | 151 | 15.6 |
| Missing | 19 | 2.0 |
| Underlying conditions | ||
| No | 729 | 75.2 |
| Yes | 240 | 24.8 |
| CVD | 93 | 38.8 |
| Asthma | 43 | 17.9 |
| Diabetes | 31 | 12.9 |
| Obesity | 26 | 10.8 |
| Infectious diseases | 14 | 5.8 |
| GIT Diseases | 10 | 4.2 |
| Haematological disorder | 9 | 3.8 |
| Cancer | 5 | 2.1 |
| Arthritis | 2 | 0.8 |
| Glaucoma | 2 | 0.8 |
| Others | 5 | 2.1 |
The study showed a high distribution and administration of adenovector vaccines in Africa compared to other vaccines, constituting 79% of the total, with mRNA, inactivated whole virus, and live attenuated vaccines representing 10.8%, 6.6%, and 0.2%, respectively. Oxford–AstraZeneca remains the most (77.8%) administered COVID-19 vaccine across different countries in Africa (Table 4).
Table 4.
Vaccine dose and types administered for COVID-19 in Africa.
| Frequency n = 969 |
Proportion % |
|
|---|---|---|
| Vaccine dose | ||
| First dose | 811 | 83.7 |
| Complete dose | 158 | 16.3 |
| Vaccine type | ||
| Adenovector | 766 | 79 |
| mRNA | 105 | 10.8 |
| Inactivated whole virus | 64 | 6.6 |
| Live attenuated vaccine | 2 | 0.2 |
| Others | 32 | 3.3 |
| Vaccine Brand | ||
| Oxford–AstraZeneca | 754 | 77.8 |
| Johnson & Johnson | 5 | 0.5 |
| Covaxin | 9 | 0.9 |
| Sinopharm-BBIBP | 44 | 4.5 |
| Moderna | 17 | 1.8 |
| Pfizer–BioNTech | 88 | 9.1 |
| CoronaVac | 6 | 0.6 |
| Sputnik V | 7 | 0.7 |
| Sinopharm-WIBP | 5 | 0.5 |
| Covi Vac | 2 | 0.2 |
| Others | 32 | 3.3 |
This study also compared adverse events in adenoviral vaccines vs. others for COVID-19 vaccination in Africa based on the different types of vaccine production technologies of adenovector (Oxford–AstraZeneca, Johnson & Johnson, Sputnik V), mRNA (Moderna, Pfizer–BioNTech, and CureVac), inactivated vaccine (Sinopharm, SinoVac/CoronaVac, and Covaxin), and live attenuated vaccine (Covi Vac). For bleeding, the highest incidence of adverse events following vaccination was reported in participants who took inactivated vaccine, at 6/57 (10.5%), followed by mRNA of 5/98 (5.1%) while adenovector accounted for the lowest, at 37/733 (5.0%). Generally, adenovector vaccines were reported to account for the lowest incidence of adverse events compared to other classes of vaccines reported on in this study (Table 5).
Table 5.
Comparison of adverse events in adenoviral vaccines vs. others for COVID-19 in Africa.
| Bleeding | n = 918 | AEFV | % | p-Value |
|---|---|---|---|---|
| Adenovector | 733 | 37 | 5 | 0.33 |
| mRNA | 98 | 5 | 5.1 | |
| Inactivated vaccine | 57 | 6 | 10.5 | |
| Live attenuated | 2 | 0 | 0 | |
| Others | 28 | 3 | 10.7 | |
| Seizure | ||||
| Adenovector | 728 | 31 | 4.3 | 0.10 |
| mRNA | 102 | 3 | 2.9 | |
| Inactivated vaccine | 60 | 4 | 6.7 | |
| Live attenuated | 2 | 0 | 0 | |
| Others | 28 | 4 | 14.3 | |
| Breathing difficulty | ||||
| Adenovector | 750 | 19 | 2.5 | 0.90 |
| mRNA | 100 | 3 | 3 | |
| Inactivated vaccine | 61 | 2 | 3.3 | |
| Live attenuated | 2 | 0 | 0 | |
| Others | 30 | 5 | 16.7 | |
| Hearing/Vision | ||||
| Adenovector | 757 | 11 | 1.5 | 0.09 |
| mRNA | 101 | 3 | 2.9 | |
| Inactivated vaccine | 60 | 3 | 5 | |
| Live attenuated | 2 | 0 | 0 | |
| Others | 28 | 4 | 14.3 | |
| Severe allergic reaction | ||||
| Adenovector | 752 | 16 | 2.1 | 0.35 |
| mRNA | 102 | 3 | 2.9 | |
| Inactivated vaccine | 60 | 3 | 5 | |
| Live attenuated | 2 | 0 | 0 | |
| Others | 28 | 4 | 14.3 | |
These data showcase incidents of serious, rare, and fatal cases following COVID-19 vaccination, including bleeding/unusual weakness at 5.3% (n = 51), reported number of deaths at 5.1% (n = 49), convulsion at 4.3% (n = 42), breathing difficulty at 2.7% (n = 26), and hearing/vision problems at 2.2% (n = 21). It is probable that these may have been missed in clinical trials despite the willingness of Africans 35.8% (n = 347) to partake in clinical trials (Table 6).
Table 6.
Rare and fatal cases following COVID-19 vaccination among Africans.
| Frequency (n = 969) |
Percent (%) |
p-Value | |
|---|---|---|---|
| Bleeding/Unusual weakness | |||
| No | 918 | 94.7 | <0.0001 |
| Yes | 51 | 5.3 | |
| Died after vaccination | |||
| No | 920 | 94.9 | <0.0001 |
| * Yes | 49 | 5.1 | |
| Seizure (convulsion) or high fever after hours or a few days | |||
| No | 927 | 95.7 | <0.0001 |
| Yes | 42 | 4.3 | |
| Breathing difficulty | |||
| No | 943 | 97.3 | <0.0001 |
| Yes | 26 | 2.7 | |
| Hearing/Vision problem | |||
| No | 948 | 97.8 | <0.0001 |
| Yes | 21 | 2.2 | |
| Clinical trial | |||
| No | 622 | 64.2 | <0.0001 |
| Yes | 347 | 35.8 |
* The deaths were accounted for by the healthcare workers that attended to the vaccinees with adverse events leading to deaths or the family members of the dead persons.
We have determined some heterogenous adverse events following COVID-19 vaccines’ administration in Africa. These heterogenous adverse events among Africans were statistically significant and can be reported in three categories: uncommon, common, and very common signs. Reported uncommon signs include feeling dizzy at 11.9% (n = 116), abdominal pain at 3.3% (n = 32), itchy skin or rash at 2.9% (n = 28), enlarged lymph nodes at 2.4% (n = 23), and menstrual disorder at 0.5% (n = 5). Out of the common adverse events, fever at 33% (n = 320), injection site swelling, redness, or lump at 18.2% (n = 176), and influenza-like symptoms at 12.1% (n = 117) were the most reported, while fatigue at 40% (n = 388), tenderness at 39.2% (n = 380), and headache at 37.5% (n = 363) represent the topmost very common signs (Table 7).
Table 7.
Adverse events following COVID-19 vaccination among Africans.
| Frequency (n = 969) |
Percentage | p-Value | |
|---|---|---|---|
| I experienced uncommon signs including: | <0.0001 | ||
| None | 651 | 67.1 | |
| Feeling dizzy | 116 | 11.9 | |
| Decreased appetite | 62 | 6.4 | |
| Excessive sweating | 41 | 4.2 | |
| Abdominal pain | 32 | 3.3 | |
| Itchy skin or rash | 28 | 2.9 | |
| Enlarged lymph nodes | 23 | 2.4 | |
| Menstrual disorder | 5 | 0.5 | |
| Hunger | 4 | 0.4 | |
| Increased libido | 2 | 0.2 | |
| I experienced common signs including: | |||
| None | 508 | 52.4 | <0.0001 |
| Fever | 320 | 33.0 | |
| Swelling, redness or a lump at the injection site | 176 | 18.2 | |
| Flu-like symptoms such as high temperature, sore throat, runny nose, cough and chills | 117 | 12.1 | |
| Being sick (vomiting) | 44 | 4.5 | |
| Diarrhoea | 20 | 2.1 | |
| Heaviness of the head | 2 | 0.2 | |
| Bone ache | 1 | 0.1 | |
| Lymph node enlargement | 1 | 0.1 | |
| I experienced very common signs including: | <0.0001 | ||
| None | 220 | 22.7 | |
| Feeling tired/fatigued | 388 | 40.0 | |
| Tenderness, pain, warmth, itching or bruising where the injection was given | 380 | 39.2 | |
| Headache | 363 | 37.5 | |
| Generally feeling unwell | 339 | 34.9 | |
| Chills or feeling feverish | 293 | 30.2 | |
| Joint pain/muscle ache | 269 | 27.8 | |
| Feeling sick/nausea | 115 | 11.9 | |
| Deep sleep | 5 | 0.5 | |
| Lymph in armpits | 3 | 0.3 | |
| Mouth sores | 1 | 0.1 | |
| Boil | 1 | 0.1 | |
| Experienced lower sex drive | 1 | 0.1 | |
| Diarrhoea | 1 | 0.1 | |
| Ear pain | 1 | 0.1 | |
| Chest pain | 1 | 0.1 | |
| Vomiting | 1 | 0.1 | |
| Blood (red) spot on left eye | 1 | 0.1 | |
| Tender swollen tongue, loss of taste and appetite | 1 | 0.1 | |
| Insomnia | 1 | 0.1 | |
| Dry cough | 1 | 0.1 | |
| Rhinitis | 1 | 0.1 | |
| Numbness at neck and hand after 2nd dose for one night | 1 | 0.1 |
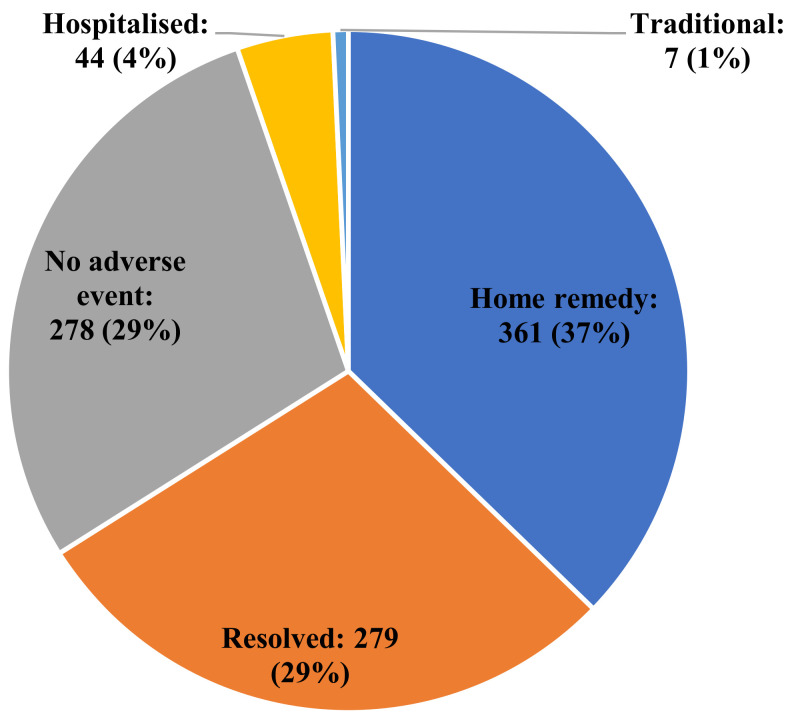
A large proportion of Africans with adverse events following COVID-19 vaccination, 37% (n = 361), managed and treated the reactions at home without any visit to the hospital, while 1% (n = 7) sought the option of traditional remedy (Figure 4).
Figure 4.
How adverse events of COVID-19 vaccination were treated among Africans.
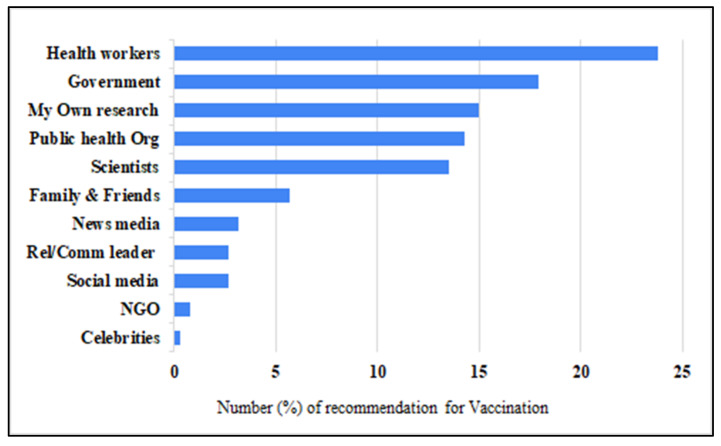
Study participants were vaccinated for COVID-19 significantly based on the recommendations given by healthcare workers (23.8%), various levels of governments (17.9%), personal research/investigation (15.0%), public health organisations/institutions (14.3%), and scientists (13.5%). These sources are about 5–10 times greater enablers of COVID-19 vaccination compared to the recommendations received from religious and community leaders (2.7%), social media (2.7%), not-for-profit organisations (NGOs) (0.8%), and celebrities (0.3%) (Figure 5).
Figure 5.
Distribution of recommendations that enhanced COVID-19 vaccination among Africans.
4. Discussion
This is a report of the first pan-African findings on rare and fatal cases following COVID-19 vaccination among Africans, using the common vaccines administered in the continent. It also detailed past exposure to SARS-CoV-2 and hospitalisation following vaccination, how adverse events were treated by Africans, and how they were recommended for COVID-19 vaccination (Figure 1). The major strengths of this research include the fact that it is a population-based and geographically diverse study over time [17]. Surveillance for adverse events following vaccination is crucial to ensure safety, maintain trust, and guide policy-makers. This is further corroborated by several studies reported elsewhere, including in Asia [18,19,20], America [12,16], and Europe [21,22].
In this study, respondents from Nigeria, Ghana, and Kenya contributed more to the survey (Table 1); whether this was due to willingness of the respondents to complete the questionnaire, the intensity of administration of the questionnaire, ability to access the questionnaires, or the effect of population distributions is unclear. However, the reasons for low participation in online surveys in Africa are obvious and multifaceted, including but not limited to the lack of power supply, poor access to or irregular Internet and electronic gadgets, lack of motivation, perception of questionnaires as too burdensome or intrusive, unexplained context of the objectives, or ethics-related issues.
Different demographic profiles of Africans vaccinated against COVID-19 were investigated, including the young population, who are at higher risk of blood clots [23] and other adverse events. We observed a decrease in the vaccinated population as age increases except for the age category 18–24 years. This trend agreed with previous reports that youths are more willing to partake in vaccination compared to the older population [19,24]. Furthermore, previous work reported the largest number of doses (5,124,940) in vaccinees aged 18 to 49 years [17]. It should, however, be noted that online questionnaire administration may also facilitate more inclusion of the youth group.
More than half of the vaccinated population are male (53.0%) (Table 2), and this observation is similar to previous findings, though no significant statistical difference was observed in AEFI reported based on gender, implying no correlation between gender and reactogenicity [20]. The proportion of frontline healthcare workers that participated in the study was high (55.7%), an unsurprising observation. Is it important to prioritise vaccination and proper surveillance of AEFI among healthcare workers in order to reduce occupational hazards and the burden of hospitalisation associated with SARS-CoV-2/COVID-19 infection.
In this study, a total of 15.6% (n = 151) reported previous exposure to SARS-CoV-2/COVID-19, and earlier investigators opined that prior exposure may alter the response of participants to COVID-19 vaccination. Further studies are needed to unravel the effect and mechanism of immunological response of past exposure to SARS-CoV-2/COVID-19 and vaccination, especially among the African populace. Although there are burgeoning data on the mystery and mechanistic roles surrounding the interference of these underlying health conditions in the general population, including among Africans [25], they remain potential predictors of various adverse events following COVID-19 vaccination.
The data reported here showcase incidents of serious rare and fatal cases following COVID-19 vaccination. It is probable that some of these may have been missed in clinical trials; only 35.8% (n = 347) of Africans showed willingness to partake in clinical trials (Table 6). The serious, rare, and fatal cases reported amongst Africans agree with the adverse events and deaths reported by the European Medicines Agency [21], including the fatal cases, along with the blood, ear, eye, and respiratory disorders following COVID-19 vaccination.
Although the cause of death was reported as COVID-19 vaccination complication, as stated and confirmed by the deceased’s loved ones, families, friends, and healthcare workers/caregivers, coupled with the fact that a high proportion of the vaccinees reported had underlying/co-morbid conditions, this does not sufficiently prove that the vaccines caused the adverse events. Even though this study classified adverse events following COVID-19 vaccination as serious in accordance with the WHO definition that an adverse events following vaccination is considered serious if it is life-threatening, results in hospitalisation or disability/incapacity, or leads to fatality, there is need for further investigation using the WHO causality assessment algorithm to classify adverse events following immunisation/vaccination as ‘consistent causal association’, ‘inconsistent causal association’, ‘indeterminate’, or ‘not-classifiable’ in relation to COVID-19 vaccination among Africans [26,27]. The WHO causality assessment algorithm will further provide information on vaccine safety based on the causal link between vaccines and serious adverse events following COVID-19 vaccination.
Although some of the adverse events were reported to resolve within a few days after vaccination, in actual fact, they may be the reactions of the immune system shortly after vaccination (also known as reactogenicity) [22,28]. The CDC recommends that individuals having severe allergic reactions immediately (within 4 h) or some days after administration of the vaccine should refrain from getting a second shot of the type of vaccine that produced the event [29]. Generally, the WHO, CDC, major international organisations, and experts in vaccinology agree that the efficacy of the current COVID-19 vaccines at reducing the spread of SARS-CoV-2, disease complications, and deaths far outweighs the likely risk of adverse events.
We have determined some heterogenous adverse events to have followed COVID-19 vaccine’ administration in Africa. These heterogenous adverse events among Africans were statistically significant and can be reported in three categories: uncommon, common, and very common, as reported in the results section. These adverse events among Africans reported here align with similar ones reported from other continents, including dizziness, fever, and tenderness at the injection site, while abdominal pain and menstrual disorder were less reported in other populations [19,21,22].
Nonetheless, transient local inflammation signalling neutrophils and antigen-presenting cells to the site of injection is expected after intramuscular administration of lipid-nanoparticle-formulated mRNA vaccines. According to the CDC, following the administration of the first dose of the COVID-19 vaccine, if an itch or swollen or painful rash is observed in a person, said person(s) should be treated with antihistamine or acetaminophen. If fatigue or pain is observed, treatment is equally recommended before such candidates proceed for the second shot of the vaccine based on availability to affirm complete protection [29]. In all cases, it becomes necessary to critically evaluate patients’ previous medical histories and vaccine-associated allergies in detail; it is also important to monitor vaccinated persons for at least 30 min following COVID-19 vaccine administration to ensure that no immediate untoward events are observed.
In this study, adverse events were managed and treated at home without any visit to the hospital (37%; n = 361) or with the use of traditional remedy (1%; n = 7). These observations agree with the previous review [22] that only a limited proportion of patients who experienced serious adverse events elsewhere considered treatment. This may be a reflection of the following: (1) the under-resourced situation of most healthcare facilities in Africa; (2) the lack of healthcare service delivery where it is most-needed, particularly, among the poor, and (3) the costs associated with seeking healthcare in Africa, including the lack of health insurance for the majority of the populations. In addition, previous experiences of people seeking hospitalisation including wrong or unfavourable evaluations and diagnoses by physicians or healthcare organisations, the low perceived need to seek medical care due to views that the illnesses or symptoms will improve over time, and time constraints on visiting hospitals may also be major constraints [30].
The study participants were vaccinated for COVID-19, based on various recommendations or knowledge channels (Figure 5), with the five top sources being 5–10 times greater enablers of COVID-19 vaccination compared to the least used sources (religious and community leaders (2.7%), social media (2.7%), not-for-profit organisations (NGOs) (0.8%), and celebrities (0.3%)); it is recommended that for society to benefit optimally from COVID-19 vaccine coverage, the earlier proposal of Anjorin et al. [15] on the use of a multi-channel vaccination campaign strategy is necessary for excellent vaccine uptakes.
In the new world, a reporting system known as the Vaccine Adverse Event Reporting System (VAERS) has been introduced by the CDC and US Food and Drug Administration (FDA) to record any adverse events known to occur in the future after vaccination. The system also proffers invaluable information to vaccinologists to guarantee safety [31]. Whether such technologies will reach wide adoption in Africa is doubtful. However, select African countries are utilising similar technologies. This includes, for instance, the Med Safety App, originally developed by the WEB-Recognising Adverse Drug Reactions (WEB-RADR) project, which was aimed to enable self-reporting suspected adverse events following vaccines or drugs for proper monitoring. In Nigeria, for instance, such monitoring is conducted by the National Agency for Food and Drug Administration and Control (NAFDAC) (https://www.nafdac.gov.ng/wp-content/uploads/Publications/Others/Events_PDF/how-to-download-the-med-safety-app-1-1.pdf (accessed on 30 November 2021)), and in South Africa, the tool is equally applied (Med Safety App—SAHPRA (https://medsafety.sahpra.org.za (accessed on 30 November 2021))). Therefore, there is a need to promote the utilisation of easy-to-access and report tools such as the above and encourage other African countries that are yet to voluntarily adopt and utilise such available reporting systems to join the league of adoptees for proper documentation and future informed decision-making.
While COVID-19 vaccines are undoubtedly beneficial, the safety concerns, including those adverse events identified in this work, hospitalisation, fatalities, and excess economic spending on health are relevant and should not be ignored [32]. Nevertheless, the benefits of being vaccinated against COVID-19 for perceived disease risks outweigh these identified demerits, and a decision on accepting the vaccine should be strengthened on this basis [33]. Given that it is difficult to determine what degree of protection against COVID-19 disease is attributable to the vaccine’s effects in the body, the risk perceptions of COVID-19 are often measured as the perceived likelihood of contracting the disease and the perceived severity of the symptoms. These perceptions also have an emotional dimension, including fear and worry components [34]. In Catalonia, based on a previous model, the benefit/cost ratio of COVID-19 vaccination was estimated at 3.4 from a social perspective and 1.4 from a health system perspective, and such benefits are associated with the monetisation of the reduction in mortality and cases with sequelae, intervention, and reduction in the use of resources [35].
We have evaluated the adverse events following the use of COVID-19 vaccines in Africa and report our findings. In this study, a number of proposed pathogenic mechanisms may have been responsible, through which the vaccines may have produced the observed adverse events, including the following: Firstly, the vaccine-induced immune thrombotic thrombocytopenia, which trigger platelet activation. Through the vaccine adenoviral non-replicating vectors (AVV), the COVID-19 vaccines may (i) spread rapidly into the blood stream, (ii) promote the early production of high levels of IL-6, (iii) interact with erythrocytes, platelets, mast cells, and endothelia, (iv) favour the presence of extracellular DNA at the site of injection, and (v) activate platelets and mast cells to release PF4 and heparin [36]. Secondly, the mechanism of anaphylaxis may also be IgE-mediated, with polyethylene glycol as the inciting antigen. However, other unknown complement-mediated mechanisms may be possible in individuals without a previous history of allergy [37].
However, our study is subjected to some limitations. For one, since the study is based on a willingness to participate, it became difficult to obtain proportional representation per country or to have all the countries enrolled based on density of vaccination per country or other logical considerations. This may have affected the statistically empirical determination of expected number of participants per country, thus skewing the analyses, including for the outcomes that are less frequent. It should be noted that some variables and predictors of interest were not captured in this preliminary report, perhaps due to the intrusive nature of the associated questions, which may discourage the participants from cooperating with filling the questionnaires.
5. Conclusions
We hereby report adverse events following COVID-19 vaccination from a total of 80.8% (n = 969) Africans in 35 different countries. Previous exposure to SARS-CoV-2/COVID-19 was reported by 15.6% (n = 151) participants with various underlying diseases. Oxford–AstraZeneca remains the most (77.8%) administered COVID-19 vaccine across African countries. The most worrisome, rare, and fatal cases include bleeding/unusual weakness at 5.3% (n = 51), reported number of deaths at 5.1% (n = 49), convulsion at 4.3% (n = 42), breathing difficulty at 2.7% (n = 26), and hearing/vision problems at 2.2% (n = 21) while heterogenous adverse events were reported in three categories: very common, common, and uncommon, with the latter including abdominal pain 3.3% (n = 32), enlarged lymph nodes 2.4% (n = 23), and menstrual disorder 0.5% (n = 5). Perhaps an advanced, government-based, more robust real-time online data-capturing system for reporting continuous sentinel AEFI surveillance, instituted in different African countries, from which future data may be mined for analysis, may be necessary. Such a database may be managed by the Africa Union or its agency, the Africa Centres for Disease Control and Prevention, on behalf of the member states, similar to databases that exist in other continents.
Acknowledgments
We hereby acknowledge all the participants that gave their consent to be part of this study and colleagues that offered useful suggestions to improve the quality of the questionnaire and the entire manuscript.
Appendix A. Study Questionnaire
-
Consent Statement: Do you agree to participate in the study?
I disagree with participating in the current study (0), end survey.
I voluntarily agree to participate in the current study (1), Continue……..
Adverse Events Following COVID-19 Vaccination in Africa
Biodata
-
Have you been vaccinated against COVID-19?
Yes (1), proceed, or No (0), end of survey.
Age………………….18–24 (1); 25–34 (2); 35–44 (3); 45–54 (4); 55–64 (5); >65 (6)
Gender (M/F). Male (1) Female (2) Other.
Highest Level of Education Completed: None (0) Primary (1) Secondary (2) Tertiary (3) Other (4).
Country of residence…………… A drop down of all countries.…………….
Community setting (Urban/semi-urban/rural) Rural (1) Semi-urban (2) Urban (3). Dropdown menu to choose state.
Occupation…………………. Frontline medical/healthcare worker (1) Frontline non-healthcare worker (2) Other (3).
Self-reported Health Status
I have been diagnosed positive to SARS-CoV-2/COVID-19 before? Yes/No
I have received the COVID-19 vaccine………… First dose (0) Complete dose (1).
Date of first dose……………
Date of Second dose…………………
I know the brand name of the COVID-19 vaccine I was administered. Dropdown menu to choose vaccine brand name
-
I experienced very common signs including:
Tenderness, pain, warmth, itching or bruising where the injection was given.
generally feeling unwell.
feeling tired/fatigue.
chills or feeling feverish.
Headache.
feeling sick/nausea.
joint pain/muscle ache.
Dropdown menu to choose multiple common signs
-
I experienced common signs including:
swelling, redness or a lump at the injection site
fever
being sick (vomiting) or diarrhoea.
flu-like symptoms, such as high temperature, sore throat, runny nose, cough and chills.
Dropdown menu to choose multiple common signs.
-
I experienced uncommon signs including:
Feeling dizzy
decreased appetite
abdominal pain
enlarged lymph nodes
excessive sweating, itchy skin or rash.
Dropdown menu to choose multiple common signs.
Multiple choice questions (Yes/No):
I experienced unknown severe allergic reaction (anaphylaxis).
I experienced fever in 5–7 days following vaccination. Yes (1) No (0).
I had pain at the site of COVID-19 vaccine injection. Yes (1) No (0).
I experienced joint or muscle pain. Yes (1) No (0).
I experienced general rash 7 and 10 days after vaccination Yes (1) No (0).
I had seizure (black-out or convulsions) or high fever (after few hours or a few days) Yes (1) No (0).
I had problems with hearing or vision, Hives (other itching or irritation) Yes (1) No (0).
I experienced extreme drowsiness, and fainting. Yes (1) No (0).
I experienced bleeding, or unusual weakness. Yes (1) No (0).
I experienced difficulty in breathing or swallowing. Yes (1) No (0).
Please state any other adverse event/sign or symptom you experienced following COVID-19 vaccination (if not already captured above). Yes (1) No (0).
How was the adverse event treated? ……………….. At home (1) Hospital (2) Traditional remedy (3) I did not treat it, it resolved spontaneously (4) I did not have any adverse effect (5). Other (6).
Have you ever had severe allergic reaction (anaphylaxis) following any other vaccination Yes (1) No (0).
I know somebody who died following COVID-19 vaccination complication? Yes (1) No (0).
Do you have any underlying disease(s)/health challenge(s)? Yes (1) No (0).
Please state the underlying disease(s)/health challenge(s) you had before taking the COVID-19 vaccine (Select all that apply).
Please state any other underlying disease(s)/health challenge(s) you had before taking the COVID-19 vaccine (if not captured above).
Knowledge/Perception of Vaccines and Acceptance of COVID-19 Vaccine
I understand how vaccines work. No (0), Yes (1).
I would be willing to participate in a clinical trial for a coronavirus vaccine. No (0), Yes (1).
Are you aware of the ingredients of COVID-19 vaccine? No (0), Yes (1).
Are you aware of the side effects of the COVID-19 vaccine? No (0), Yes (1).
I received the COVID-19 vaccine because it was recommended by religious leaders (1), community elders (2), NGO (3) Government (4) Healthcare workers (5), scientists (6), news media (7), social media (8), celebrities (9), schools (10), public health organizations (NCDC) (11), my own research (12), friends (13), family (14), Not applicable (15).
I got the COVID-19 vaccine jab because of the ease of my house/office to the vaccination center. No (0), Yes (1).
To reach my nearest vaccination center, it took <15 min (1), <30 min (2), 1 h (3), <2 h (4), >2 h (5).
The COVID-19 vaccine should be made mandatory for all. No (0), Yes (1), Maybe (2).
-
For future cohort study, can we contact you through your email?
Yes (1), email: ……………………., No (0), end survey.
Author Contributions
A.A.A.: Conceptualisation and original draft; J.B.N., K.S.A., J.E., H.E.: Part of manuscript draft; A.A.A., J.E., K.S.A.: Methodology; A.A.A., I.A.O., J.B.N., H.E., J.E., A.I.A., G.G., A.M.M., M.F.Y.M., Z.E.M., T.A., L.N., M.S., B.L.S., Y.R., N.E., K.O.W., Z.E.M., R.M.: Questionnaire design and data collection; A.A.A., I.A.O., A.I.A., H.E., J.B.N., A.M.M., Y.R., Z.E.M., K.O.W., B.L.S., R.M.: Data merging, and coding; A.A.A., I.A.O.: Statistical analyses; A.A.A., F.O.F., I.A.O., N.E., K.O.W.: Results interpretation; A.A.A., K.O.W., I.A.O., N.E., K.S.A., F.O.F.: Writing, review, and editing; A.A.A., H.E., J.E., K.S.A., J.B.N., R.M.: Reference collation. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the institutional review boards (ethics committees) from Nigeria (University of Ilorin, approval code: FVM/uerc/em/001), Egypt (Ahram Canadian University, Faculty of Oral and Dental Medicine, approval code: IRB 00012891 #7), and Kenya (Kenyatta University, approval code: PKU/2267/11411).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent was obtained from all participants.
Data Availability Statement
Relevant data have been included in this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO Vaccines and Immunization. [(accessed on 28 November 2021)]. Available online: https://www.who.int/health-topics/vaccines-and-immunization#tab=tab_1.
- 2.WHO First COVID-19 COVAX Vaccine Doses Administered in Africa. [(accessed on 28 November 2021)]. Available online: https://www.who.int/news/item/01-03-2021-first-covid-19-covax-vaccine-doses-administered-in-africa.
- 3.CDC . Understanding Adverse Events and Side Effects. CDC; Atlanta, GA, USA: 2021. [Google Scholar]
- 4.Konu Y.R., Gbeasor-Komlanvi F.A., Yerima M., Sadio A.J., Tchankoni M.K., Zida-Compaore W.I.C., Nayo-Apetsianyi J., Afanvi K.A., Agoro S., Salou M. Prevalence of severe adverse events among health professionals after receiving the first dose of the ChAdOx1 nCoV-19 coronavirus vaccine (Covishield) in Togo, March 2021. Arch. Public Health. 2021;79:207. doi: 10.1186/s13690-021-00741-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li X., Ostropolets A., Makadia R., Shoaibi A., Rao G., Sena A.G., Martinez-Hernandez E., Delmestri A., Verhamme K., Rijnbeek P.R., et al. Characterising the background incidence rates of adverse events of special interest for covid-19 vaccines in eight countries: Multinational network cohort study. BMJ. 2021;373:n1435. doi: 10.1136/bmj.n1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Menni C., Klaser K., May A., Polidori L., Capdevila J., Louca P., Sudre C.H., Nguyen L.H., Drew D.A., Merino J. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: A prospective observational study. Lancet Infect. Dis. 2021;21:939–949. doi: 10.1016/S1473-3099(21)00224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Public-Health-Ontario Adverse Events Following Immunization (AEFIs) for COVID-19 in Ontario. [(accessed on 23 January 2022)]. Available online: https://www.publichealthontario.ca/-/media/documents/ncov/epi/covid-19-aefi-report.pdf?sc_lang=en.
- 8.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Pérez Marc G., Moreira E.D., Zerbini C., et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edmonds C.E., Zuckerman S.P., Conant E.F. Management of unilateral axillary lymphadenopathy detected on breast MRI in the era of coronavirus disease (COVID-19) vaccination. Am. J. Roentgenol. 2021;217:831–834. doi: 10.2214/AJR.21.25604. [DOI] [PubMed] [Google Scholar]
- 10.Klimek L., Novak N., Hamelmann E., Werfel T., Wagenmann M., Taube C., Bauer A., Merk H., Rabe U., Jung K., et al. Severe allergic reactions after COVID-19 vaccination with the Pfizer/BioNTech vaccine in Great Britain and USA. Allergo J. Int. 2021;30:51–55. doi: 10.1007/s40629-020-00160-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Renoud L., Khouri C., Revol B., Lepelley M., Perez J., Roustit M., Cracowski J.-L. Association of Facial Paralysis With mRNA COVID-19 Vaccines: A Disproportionality Analysis Using the World Health Organization Pharmacovigilance Database. JAMA Intern. Med. 2021;181:1243–1245. doi: 10.1001/jamainternmed.2021.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.CDC Selected Adverse Events Reported after COVID-19 Vaccination. [(accessed on 28 November 2021)]; Available online: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/adverse-events.html.
- 13.ECDC Suspected Adverse Reactions to COVID19 Vaccination and the Safety of Substances of Human Origin—3 June 2021. [(accessed on 13 January 2022)]. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/Suspected-adverse-reactions-to-COVID-19-vaccination-and-safety-of-SoHO.pdf.
- 14.MHRA . Coronavirus Vaccine—Weekly Summary of Yellow Card Reporting. Medicines & Healthcare Products Regulatory Agency; London, UK: 2022. [(accessed on 23 March 2022)]. Available online: https://www.gov.uk/government/publications/coronavirus-covid-19-vaccine-adverse-reactions/coronavirus-vaccine-summary-of-yellow-card-reporting. [Google Scholar]
- 15.Anjorin A., Odetokun I., Abioye A., Elnadi H., Umoren M., Damaris B., Eyedo J., Umar H., Nyandwi J., Abdalla M., et al. Will Africans Take COVID-19 Vaccination? PLoS ONE. 2021;16:e0260575. doi: 10.1371/journal.pone.0260575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bianchi F.P., Tafuri S. A public health perspective on the responsibility of mass media for the outcome of the anti-COVID-19 vaccination campaign: The AstraZeneca case. Ann. Ig. 2022 doi: 10.7416/ai.2022.2499. [DOI] [PubMed] [Google Scholar]
- 17.Klein N.P., Lewis N., Goddard K., Fireman B., Zerbo O., Hanson K.E., Donahue J.G., Kharbanda E.O., Naleway A., Nelson J.C. Surveillance for adverse events after COVID-19 mRNA vaccination. JAMA. 2021;326:1390–1399. doi: 10.1001/jama.2021.15072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Esba L.C.A., Al Jeraisy M. Reported Adverse Effects following COVID-19 Vaccination at a Tertiary Care Hospital, Focus on Cerebral Venous Sinus Thrombosis (CVST) Expert Rev. Vaccines. 2021;20:1037–1042. doi: 10.1080/14760584.2021.1940145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jeon M., Kim J., Oh C.E., Lee J.-Y. Adverse events following immunization associated with coronavirus disease 2019 vaccination reported in the mobile vaccine adverse events reporting system. J. Korean Med. Sci. 2021;36:e114. doi: 10.3346/jkms.2021.36.e114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sakinah E.N., Nugraha M.Y., Qodar T.S., Mulyono B.W., Tohari A.I. COVID-19 Vaccines Programs: Adverse events following immunization (AEFI) among medical Clerkship Student in Jember, Indonesia. BMC Pharmacol. Toxicol. 2021;22:58. doi: 10.1186/s40360-021-00528-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.EMA, European Medicines Agency Committee for Medicinal Products for Human Use (CHMP). Assessment Report—Comirnaty (Pfizer/BioNTech). EMA/707383/2020. 2021. [(accessed on 19 February 2021)]. Available online: https://www.ema.europa.eu/en/documents/assessment-report/comirnaty-epar-public-assessment-report_en.pdf.
- 22.Hernández A.F., Calina D., Poulas K., Docea A.O., Tsatsakis A.M. Safety of COVID-19 vaccines administered in the EU: Should we be concerned? Toxicol. Rep. 2021;8:871–879. doi: 10.1016/j.toxrep.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhatia M., Putcha V.R., Dwivedi L.K., Singh D. Serious adverse events and fatal outcomes following COVID-19 vaccination in the UK: Lessons for other countries. Int. J. Community Soc. Dev. 2021;3:396–402. doi: 10.1177/25166026211053485. [DOI] [Google Scholar]
- 24.Zhang Y., Zeng G., Pan H., Li C., Hu Y., Chu K., Han W., Chen Z., Tang R., Yin W. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: A randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect. Dis. 2021;21:181–192. doi: 10.1016/S1473-3099(20)30843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anjorin A., Abioye A., Asowata O., Soipe A., Kazeem M., Adesanya I., Raji M., Adesanya M., Oke F., Lawal F. Comorbidities and the COVID-19 pandemic dynamics in Africa. Trop. Med. Int. Health. 2021;26:2–13. doi: 10.1111/tmi.13504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.WHO Causality Assessment of an Adverse Event Following Immunization (AEFI). User Manual for the Revised WHO Classification, January 2018. [(accessed on 23 March 2022)]. Available online: https://apps.who.int/iris/bitstream/handle/10665/259959/9789241513654-eng.pdf?sequence=1&isAllowed=y.
- 27.Stefanizzi P., Stella P., Ancona D., Malcangi K.N., Bianchi F.P., De Nitto S., Ferorelli D., Germinario C.A., Tafuri S. Adverse Events Following Measles-Mumps-Rubella-Varicella Vaccination and the Case of Seizures: A Post Marketing Active Surveillance in Puglia Italian Region, 2017–2018. Vaccines. 2019;7:140. doi: 10.3390/vaccines7040140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Forni G., Mantovani A. COVID-19 vaccines: Where we stand and challenges ahead. Cell Death Differ. 2021;28:626–639. doi: 10.1038/s41418-020-00720-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.CDC What to Do if You Had an Allergic Reaction after Getting a COVID-19 Vaccine. [(accessed on 26 January 2022)]; Available online: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/allergic-reaction.html.
- 30.Taber J.M., Leyva B., Persoskie A. Why do people avoid medical care? A qualitative study using national data. J. Gen. Intern. Med. 2015;30:290–297. doi: 10.1007/s11606-014-3089-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.CDC Vaccine Adverse Event Reporting System (VAERS) [(accessed on 28 November 2021)]; Available online: https://www.cdc.gov/vaccinesafety/ensuringsafety/monitoring/vaers/index.html.
- 32.Shaker M., Abrams E.M., Greenhawt M. A Cost-Effectiveness Evaluation of Hospitalizations, Fatalities, and Economic Outcomes Associated with Universal Versus Anaphylaxis Risk-Stratified COVID-19 Vaccination Strategies. J. Allergy Clin. Immunol. Pract. 2021;9:2658–2668.e3. doi: 10.1016/j.jaip.2021.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karlsson C.K., Soveri A., Lewandowsky S., Karlsson L., Karlsson H., Nolvi S., Karukivi M., Lindfelt M., Antfolk J. Fearing the disease or the vaccine: The case of COVID-19. Personal. Individ. Differ. 2021;172:110590. doi: 10.1016/j.paid.2020.110590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Loewenstein G.F., Weber E.U., Hsee C.K., Welch N. Risk as feelings. Psychol. Bull. 2001;127:267–286. doi: 10.1037/0033-2909.127.2.267. [DOI] [PubMed] [Google Scholar]
- 35.López F., Català M., Prats C., Estrada O., Oliva I., Prat N., Isnard M., Vallès R., Vilar M., Clotet B., et al. A Cost–Benefit Analysis of COVID-19 Vaccination in Catalonia. Vaccines. 2022;10:59. doi: 10.3390/vaccines10010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Azzarone B., Veneziani I., Moretta L., Maggi E. Pathogenic Mechanisms of Vaccine-Induced Immune Thrombotic Thrombocytopenia in People Receiving Anti-COVID-19 Adenoviral-Based Vaccines: A Proposal. Front. Immunol. 2021;12:728513. doi: 10.3389/fimmu.2021.728513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Edwards K.M., Orenstein W.A. COVID-19: Vaccines. [(accessed on 5 March 2022)]. Available online: https://www.uptodate.com/contents/covid-19-vaccines.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Relevant data have been included in this manuscript.