Abstract
Porcine enteric coronaviruses have caused immense economic losses to the global pig industry, and pose a potential risk for cross-species transmission. The clinical symptoms of the porcine enteric coronaviruses (CoVs) are similar, making it difficult to distinguish between the specific pathogens by symptoms alone. Here, a multiplex nucleic acid detection platform based on CRISPR/Cas12a and multiplex reverse transcriptase loop-mediated isothermal amplification (RT-LAMP) was developed for the detection of four diarrhea CoVs: porcine epidemic diarrhea virus (PEDV), transmissible gastroenteritis virus (TGEV), porcine deltacoronavirus (PDCoV), and swine acute diarrhea syndrome coronavirus (SADS-CoV). With this strategy, we realized a visual colorimetric readout visible to the naked eye without specialized instrumentation by using a ROX-labeled single-stranded DNA-fluorescence-quenched (ssDNA-FQ) reporter. Our method achieved single-copy sensitivity with no cross-reactivity in the identification and detection of the target viruses. In addition, we successfully detected these four enteric CoVs from RNA of clinical samples. Thus, we established a rapid, sensitive, and on-site multiplex molecular differential diagnosis technology for porcine enteric CoVs.
Keywords: porcine enteric coronaviruses, CRISPR/Cas12a, multiplex loop-mediated isothermal amplification, single-strand DNA-FQ reporter
1. Introduction
Coronaviruses (CoVs) are a class of viruses that cause respiratory tract infections in mammals and birds. The porcine enteric CoV has spread on a large scale worldwide, severely affecting the efficiency of pork production and the animal husbandry economy [1,2,3]. It is currently known that four porcine CoVs can cause porcine diarrhea, including three α-CoVs (transmissible gastroenteritis virus (TGEV), porcine epidemic diarrhea virus (PEDV), and swine acute diarrhea syndrome coronavirus (SADS-CoV), as well as one δ-CoV [Porcine Deltacoronavirus (PDCoV)] [4,5,6]. Among them, TGEV has been circulating in pigs for decades, whereas PEDV, PDCoV, and SADS-CoV are considered as emerging CoVs [6]. Moreover, the latest research has shown that SADS-CoV replicates effectively in human cells, suggesting that the virus is a potential threat to humans [7]. However, these four porcine enteric CoVs cannot be distinguished by their clinical symptoms [8]. Therefore, the development of low-cost, innovative point-of-care (POC) tests for detection and diagnosis of porcine CoVs has become a priority in biosensors and bioassays.
A variety of porcine CoVs detection methods are currently applied, including immunofluorescence (IF) or immunohistochemistry (IHC) tests, in situ hybridization, nucleic acid amplification-based methods, etc. Among them, Reverse Transcription-Polymerase Chain Reaction/Real-time quantitative (RT–PCR/qPCR) is the main laboratory diagnosis method for detecting porcine CoVs, mainly targeting conservative genes, such as M, N, or ORF3 genes [9,10,11,12,13]. The multiplex PCR detection method for viruses is fast and efficient and has been developed in the nucleic acid detection field [12]. This assay for the detection of major diarrheal viruses in pig herds has greatly improved the efficiency of detection [12,14,15]. However, there are still many limitations in PCR-based diagnoses, such as expensive equipment, a lack of well-trained operators, and limited laboratory environments, which pose many challenges in conducting diagnoses outside the laboratory [16,17]. Therefore, it is urgent to develop a POC diagnostic platform for rapidly detecting multiple porcine diarrhea CoVs in the field, which can be quickly applied to diagnosis in resource-poor or remote small pig farms. Especially for the simultaneous on-site measurement of different CoVs from a single sample, multiplexed POC testing is necessary.
Recently, clustered regularly interspaced short palindromic repeat (CRISPR)- and CRISPR-associated protein (Cas)-based biosensing platforms have been developed for their high specificity, simplicity, and ease of field deployment. CRISPR-associated endoribonucleases, such as Cas13a, Cas12a, Cas12b, and Cas14, perform indiscriminate collateral cleavage activities when the Cas/small guide RNA (sgRNA)/target ternary complex is formed. By combining these Cas proteins with a preamplification step, such as PCR, recombinase polymerase amplification (RPA) or loop-mediated isothermal amplification (LAMP) and single-stranded DNA-fluorescence-quenched (ssDNA-FQ) reporter, researchers have developed various biosensing platforms for nucleic acid detection: SHERLOCK [18], DETECTR [19], HOLMES [20], CDetection [21] and Cas14-DETECTR [22]. These provide attomolar sensitivity and single-base specificity for the one-site detection of pathogenic viruses [23,24,25], such as severe acute respiratory syndrome (SARS)-CoV-2 [26,27] and African swine fever virus (ASFV) [28,29]. However, these CRISPR-based nucleic acid detection technologies still require blue or UV light transilluminators for fluorescence readout and expensive commercial lateral flow strips for visual readout.
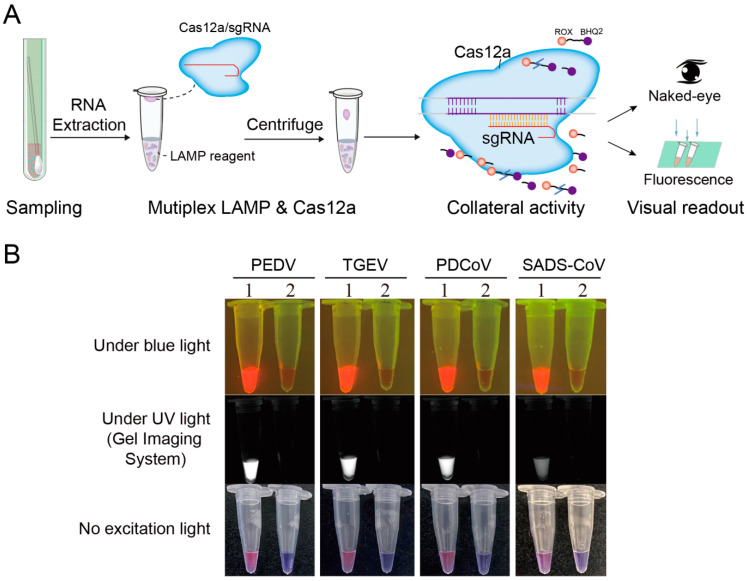
In this study, we developed a CRISPR-/Cas12a-based nucleic acid colorimetric detection system combined with multiplex LAMP (multiplex LAMP-Cas12a), which allows the detection of PEDV, TGEV, PDCoV, and SADS-CoV with the naked eye. We also evaluated its application in RNA of clinical samples collected from infected farm pigs. Thus, our technique can be used to rapidly and efficiently detect the four pathogenic swine enteric CoVs.
2. Materials and Methods
2.1. Preparation of Nucleic Acid Template
The target ORF3 gene of virulent field PEDV strain (KJ642645.1), the N gene of TGEV (GQ374566.1), the N gene of PDCoV (KY586149.1), and the N gene of SADS-CoV (MG775253.1) and their corresponding partial fragments were synthesized (TSINGKE, Beijing, China) according to the highly conserved region of the gene sequences in GenBank (https://www.ncbi.nlm.nih.gov/genbank/, accessed on 20 February 2022) and cloned into the pMD18T vector (Supplementary Table S1).
The RNAs of clinical samples of PEDV, TGEV, and PDCoV were extracted from the supernatant of virus-infected cells or tissues samples. SADS-CoV RNA was transcribed in vitro. Specifically, the SADS-CoV-N plasmid was amplified using a forward primer containing a T7 promoter and a reverse primer (Supplementary Table S2). The volume of the PCR assay was 20 μL, composed of 10 μL of Extaq Mix, the prime mixture (10 pmol forward primer and 10 pmol reverse primer), and 5 ng of the DNA template. The PCR product was purified using a PCR Purification Kit (Beijing, China, Tianmo Sci&Tech Development). The amplified purified PCR product then served as the DNA template for the in vitro transcription reactions using the HiScribe™ T7 High Yield RNA Synthesis Kit (E2040S, NEB, Ipswich, MA, USA) following the manufacturer’s instructions. The in vitro transcribed RNA was purified by acid-phenol-chloroform (NEB) extraction and resuspended in RNase-free water following isopropanol precipitation. The concentration of the RNA was quantified by a NanoDrop One™ spectrophotometer (Software version 2.7, Thermo Fisher Scientific, Waltham, MA, USA).
2.2. Preparation of sgRNAs
SgRNAs targeting conserved genes were designed with CRISPR-offinder software [30]. The designed sgRNA was complemented to the target sites with a “5′TTTN” protospacer adjacent motif (PAM) in the DNA strand opposite the target sequences (Supplementary Table S2). The sgRNA templates were amplified from a pUC57-T7-sgRNA (Supplementary Table S1) using a forward primer containing a T7 promoter and a reverse primer containing different nucleotide target sequences. Then, sgRNAs were transcribed and purified in vitro.
2.3. CRISPR/Cas12a Combined with PCR Assay
Primers were designed by Primer Premier 5.0 (PREMIER Biosoft) based on the highly conserved sequences of the ORF3 gene targeting PEDV, which were synthesized and cloned into the pMD18T vector (Supplementary Table S1). PCR assay was performed in a thermal cycler PCR system with an amplification reaction as follows: 30 cycles of denaturation at 94 °C for 30 s, annealing at 55 °C for 30 s, extension at 72 °C for 16 s, and a final extension at 72 °C for 5 min. Upon completion, 3 μL of each sample was isolated for agarose gel electrophoresis analysis and the Cas12a cleavage assay. The primers used for PCR are listed in Supplementary Table S1. All primers were synthesized by TSINGKE (Beijing, China).
2.4. Optimize the CRISPR/Cas12a Detection System Using ROX or JOE-Dye ssDNA-FQ Reporters
The CRISPR/Cas12a detection assay mainly contains the Cas12a protein, sgRNA, an ssDNA-FQ reporter, and buffer. The ssDNA-FQ reporter is digested by the Cas12a protein after the formation of the ternary complex (Cas12a protein, sgRNA and target DNA), releasing a fluorescent signal. The volume of the Cas12a cleavage assay was 20 μL, which contained 250 nM Cas12a, 500 nM sgRNA, 300 nM ssDNA-FQ reporter (JOE-N12-BHQ1 or ROX-N12-BHQ2) [31], and 2 μL NEBuffer 2.1. The Cas12a reactions were incubated at 37 °C for 20 min and inactivated at 98 °C for 2 min.
2.5. Sensitivity of CRISPR/Cas12a Combined with PCR Assay
The copy number of the PEDV-ORF3 plasmid containing a 320 bp sequence was calculated based on the plasmid, and the insert molecular weight was as follows: number of copies = (amount × 6.022 × 1023)/(length × 1 × 109 × 660) (https://toptipbio.com/dna-copy-number-qpcr/, accessed on 20 February 2022). The plasmid DNA was serially diluted from 8 × 107 to 1 × 100 copies/μL. PCR was performed on the dilution series, and the 3 μL products were analyzed by gel electrophoresis. Parallel samples were then analyzed using the Cas12a cleavage reaction, incubated at 37 °C for 15 min and inactivated at 98 °C for 2 min, and the fluorescence was read using a blue or UV Light Transilluminator or a microplate reader.
2.6. Optimization of the CRISPR/Cas12a Combined with LAMP Assay (LAMP-Cas12a)
Several sets of LAMP primer pairs were designed based on the highly active sgRNA targeting the PEDV ORF3 gene (Supplementary Table S1), and the PEDV-ORF3 plasmid DNA (10 ng) was then amplified by the LAMP assay. The LAMP amplification assay contained 0.16 U of Bst 3.0 DNA polymerase (NEB, Ipswich, MA, USA), 2.5 μL of 10 × isothermal amplification buffer, 6 mM MgSO4, 1.4 mM each of dNTP Mix (Cwbio, CW0941S, CoWin BioSciences, Cambridge, MA, USA), and 2.5 μL of primer mix (10 × primer: 1.6 μM of PEDV-ORF3-FIP/BIP; 0.2 μM of PEDV-ORF3-F3/B3, 0.4 μM of PEDV-ORF3-LF/LB). LAMP reactions were incubated at 65 °C for 40 min and then inactivated at 85 °C for 5 min. The 3 μL products were analyzed by gel electrophoresis or by the Cas12a cleavage reaction. The best primer pairs were then determined based on their fluorescence intensity combined with gel electrophoresis bands to determine the strategy for the LAMP-Cas12a assay.
The range of reaction temperatures (T) or times (t) for the LAMP amplification assay are broad; the temperature range is 50–72 °C, and the time range is greater than or equal to 5 or 10 min. Therefore, different temperatures or times were designed according to the reaction range to determine the reaction conditions for this experiment. First, to determine the reaction temperature, a total of eight temperature (°C) gradients—53, 55, 57.5, 60, 62.5, 65, 67.5, 71—were designed, and the reaction temperature of the negative control was 65 °C. Then, to determine the reaction time, a total of eight reaction times (min)—5, 15, 20, 25, 30, 35, 40, 45—were designed, and the reaction time of the negative control was 45 min. Finally, the analysis of the LAMP amplification products was consistent with the above.
2.7. Sensitivity of the CRISPR/Cas12a Combined with LAMP Assay
The copy number of the plasmid DNA was calculated based on the plasmid and insert molecular weight. The plasmid DNA was serially diluted from 1 × 104 to 1 × 10−1 copies/μL. LAMP assay was performed on the dilution series, and the 3 μL products were analyzed by gel electrophoresis. Parallel samples were then analyzed using the Cas12a cleavage reaction, incubated at 37 °C for 10 min and inactivated at 98 °C for 2 min, and the fluorescence was read using a blue or UV Light Transilluminator or a microplate reader.
2.8. Specificity of the CRISPR/Cas12a Combined with LAMP Assay
First, to evaluate the specificity of the LAMP primer pairs, the four plasmid DNA samples (10 ng) of the diarrhea CoVs were amplified with the following pairs of primers: PEDV-LAMP-2, TGEV-LAMP-1, PDCoV-LAMP-1, and SADS-CoV-LAMP-1 (Supplementary Table S2). LAMP reactions were incubated at 65 °C for 20 min and inactivated at 85 °C for 5 min, and the products were verified by agarose gel electrophoresis. Then, to further determine the specificity of the sgRNA of the CRISPR/Cas12a cleavage assay, four amplicons were assayed using the Cas12a detection method with the specific sgRNA (Supplementary Table S2).
2.9. CRISPR/Cas12a Combined with RT-LAMP Assay
To determine whether the RT-LAMP-Cas12a assay can be used for the detection of RNA viruses, PEDV RNA was amplified using the RT-LAMP assay, and the products were cleaved by Cas12a. To further increase the accuracy of Cas12a, the two systems, RT-LAMP amplification and Cas12a cleaved assay, were integrated into one system. The 25 μL reagent was administered into the bottom of the tube containing the RT-LAMP reaction solution and 12.5 μM ROX-dye ssDNA-FQ reporter. The pipe cap reagent contained 1000 nM of sgRNA, 500 nM of the Cas12a protein, and 2 μL of NEBuffer 2.1. In addition, paraffin oil was added to the liquid surface to prevent evaporation and to control temperature. Reactions were incubated at 65 °C for 40 min and incubated at 37 °C for 15 min after centrifugation to observe the colorimetric/fluorescence readouts.
2.10. Detection of PDEV, TGEV, PDCoV, and SADS-CoV by a Multiplex LAMP-Cas12a Assay
To address a coinfection scenario in actual production, a plasmid DNA mixture of PEDV, TGEV, PDCoV, and SADS-CoV (1:1:1:1) was amplified using multiplex LAMP with an optimized primer ratio. The multiplex LAMP reactions were incubated at 65 °C for 20 min and inactivated at 85 °C for 5 min. In addition, paraffin oil was added to the liquid surface to prevent evaporation and to control the temperature. The pipe cap reagent contained 1000 nM of sgRNA, 500 nM of the Cas12a protein, and 2 μL of NEBuffer 2.1. After the multiplex LAMP reaction, the CRISPR/Cas12a solution was centrifuged to observe the colorimetric/fluorescence readout. The amplicons were analyzed using the Cas12a cleavage reaction, incubated at 37 °C for 20 min, and inactivated at 98 °C for 2 min. The co-infected viruses were distinguished by different CRISPR/Cas12a assays.
3. Results
3.1. Development and Optimization of PEDV, TGEV, PDCoV, and SADS-CoV Detection Based on CRISPR/Cas12a Cleavage Assay
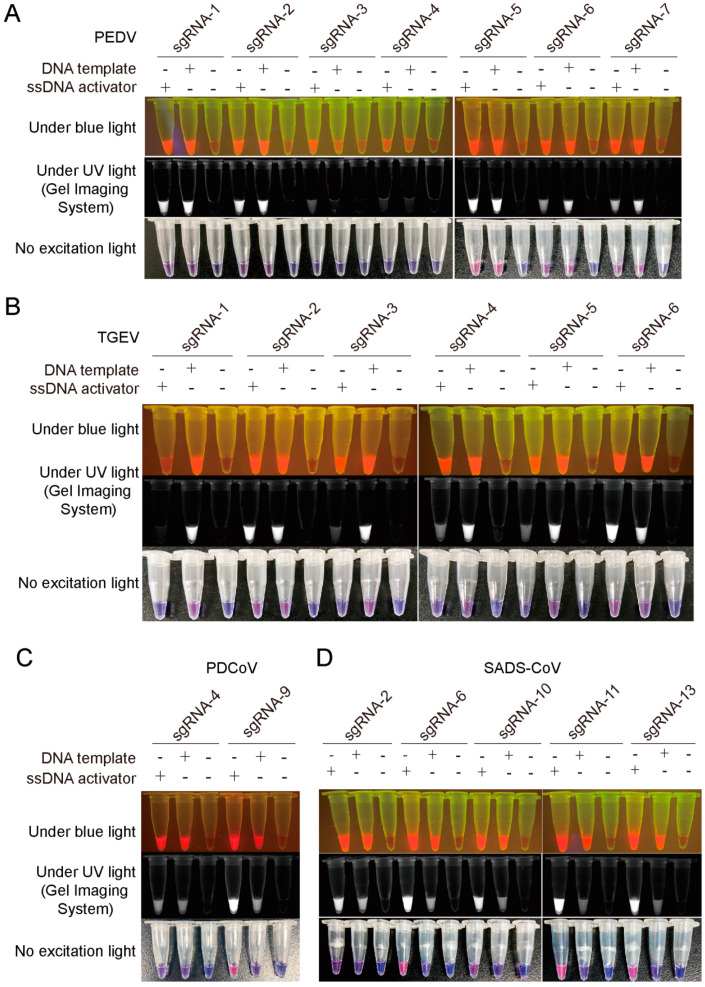
To establish CRISPR-/Cas12a-based nucleic acid detection technology for PEDV, TGEV, PDCoV, and SADS-CoV, we first identified the most active sgRNA to detect each CoVs. We designed seven sgRNAs targeting the PEDV ORF3 gene (Figure S1A), and used the PEDV-ORF3 plasmid as a template for amplification (Figure S1B). We then collected the PCR purified products as the DNA template and the complementary ssDNA activator targeted by the sgRNA sequence to test the activity of the seven sgRNAs. The results showed that an sgRNA named sgRNA5 showed the highest activity (Figure 1A and Figure S1C,D). We then identified the sgRNAs with the highest activity against the TGEV N gene, the PDCoV N gene, and the SADS-CoV N gene, which were sgRNA2, sgRNA9, and sgRNA6, respectively (Figure S2 and Figure 1B–D).
Figure 1.
Screening of highly active sgRNAs for detection of PEDV, TGEV, PDCoV, and SADS-CoV. Identification of the highly active sgRNA targeting PEDV ORF3 gene (A), TGEV N gene (B), PDCoV N gene (C), and SADS-CoV N gene (D) by CRISPR/Cas12a cleavage assay; DNA template represents the amplification fragment of ORF3 or N gene as the template; ssDNA activator represents the complementary single-stranded DNA of the sgRNA. No excitation light stands for white light. PEDV, porcine epidemic diarrhea virus; TGEV, transmissible gastroenteritis virus; PDCoV, porcine deltacoronavirus; SADS-CoV, swine acute diarrhea syndrome coronavirus.
In addition, the colorimetric signals of CRISPR/Cas12a cleavage assay using JOE-N12-BHQ1 and ROX-N12-BHQ2 reporters were compared. We found that the reaction of using ROX-N12-BHQ2 as a reporter could be clearly observed with directly naked eye (Figure S1C,D). The solution changed visibly as the concentration of the ssDNA-FQ reporter increased (Figure S1E,F), indicating that the selection of ROX-N12-BHQ2 as ssDNA-FQ reporter facilitates CRISPR/Cas12a nucleic acid visual detection, and the optimal concentration was 12.5 µM (Figure S1F). To further improve the naked-eye detectability of the colorimetric signal of CRISPR/Cas12a nucleic acid detection, we optimized the concentrations of the sgRNA and the Cas12a enzyme, and the results showed that the optimal concentrations were 1 µM and 0.5 µM, respectively (Figure S1G,H).
3.2. Establishment of the CRISPR/Cas12a Combined with LAMP Assay for the Detection of PEDV, TGEV, PDCoV, and SADS-CoV
Next, a CRISPR/Cas12a combined with LAMP assay was established for the detection of four pathogenic swine enteric CoVs. We designed four sets of LAMP primers based on sgRNA5 targeting the PEDV ORF3 gene, and selected the best primer pairs by gel electrophoresis and the CRISPR/Cas12a cleavage assay. As shown in Figure S3A,B, the LAMP-2 primer pairs for PEDV had the highest amplification efficiency. The temperature range for LAMP amplification was between 57.5 °C and 67.5 °C, and 65 °C was used as the optimal reaction temperature in our study (Figure S3C,E). When the amplification time (t) was ≥15 min (Lane 2), the LAMP products at 65 °C using the LAMP-2 primer pairs could be clearly detected in the CRISPR/Cas12a cleavage assay with the naked eye (Figure S3F). However, the most appropriate amplification time was 20 min by agarose gel electrophoresis (Figure S3D).
For TGEV, the results showed that the primer pairs LAMP-2, LAMP3, and LAMP-4 caused false-positive readouts after visual detection by CRISPR/Cas12a cleavage assay in the no-template controls (Figure S4B). Thus, the best LAMP amplification primer pair for the TGEV N gene was LAMP-1 (Figure S4A–C). There was no significant difference in the amplification efficiency of PDCoV. In subsequent experiments, LAMP-1 was selected as the target primer pair for the PDCoV N gene (Figure S5A,B). Similarly, LAMP-1 was chosen as the best primer pair for the SADS-CoV N gene (Figure S6A–C).
3.3. Sensitivity Comparison between CRISPR/Cas12a Combined with PCR and LAMP Assays for the Detection of PEDV, TGEV, PDCoV, and SADS-CoV
We evaluated the sensitivity of the PCR or LAMP-Cas12a assay for the detection of four pathogenic swine enteric CoVs. The PEDV-ORF3 plasmid DNA was diluted from 8 × 107 to 8 × 100 copies/μL for PCR reaction. As shown in Figure S7A, the limit of detection (LoD) for the PCR assay was 8 × 102 copies/μL according to the concentration gradient test (Lane 6). Subsequently, the PCR amplification products of ORF3 were further digested by Cas12a cleavage assay and then detected by the blue or UV Light Transilluminator. The results showed that the sensitivity of the PCR-Cas12a assay for PEDV could reach 8 × 102 copies/μL using the plasmid containing ORF3 as template (tube 6) (Figure S7B), but the LoD with the naked eye detection of PEDV by PCR-Cas12a was 8 × 103 copies/μL (Lane 5) (Figure S7B). Furthermore, we collected the fluorescence signal values of the colorimetric/fluorescence Cas12a cleavage assay through a multifunctional microplate reader, and the result was the same as those of the blue or UV light assay (Figure S7C).
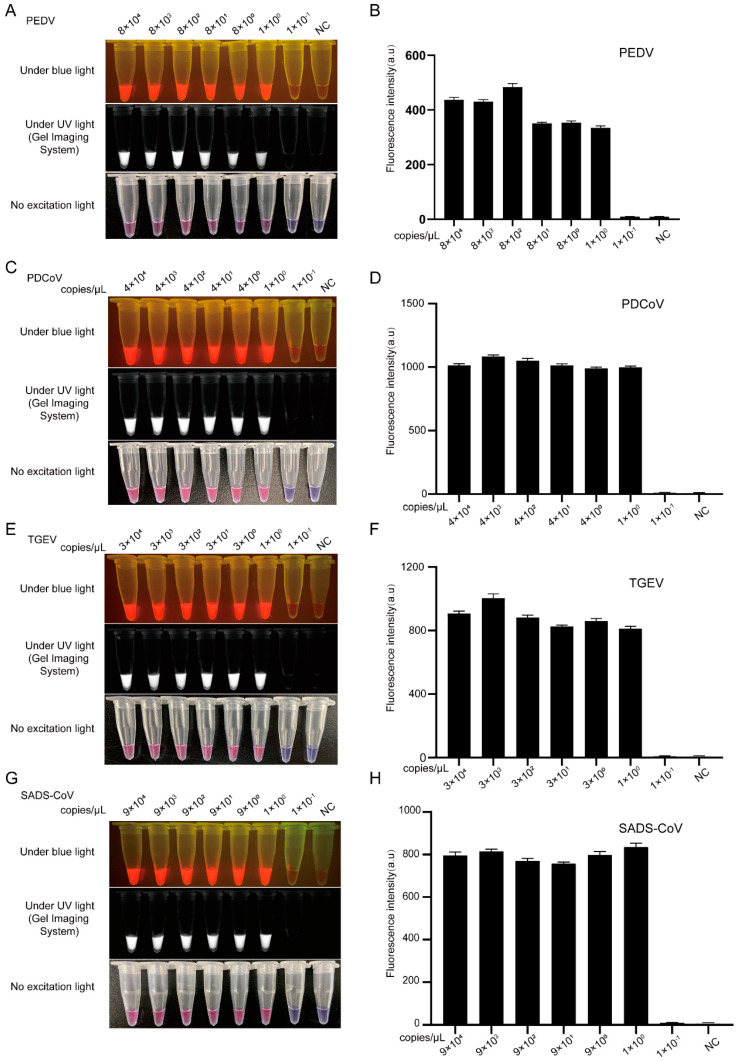
To further evaluate the sensitivity of the LAMP-Cas12a assay, we diluted the plasmid DNAs of PEDV-ORF3, TGEV-N, PDCoV-N, and SADS-CoV-N from 1 × 104 to 1 × 10−1 copies/μL. As shown in Figure 2A,C,E,G, the LoD for LAMP-Cas12a with the naked eye almost reached a single copy (tube 6), corresponding with the LoD of the multifunctional microplate reader (Figure 2B,D,F,H), which had a sensitivity a thousand times higher than that of the PCR-Cas12a assay (Figure S7).
Figure 2.
Sensitivity of CRISPR/Cas12a combined with LAMP assay for portable detection of PEDV, PDCoV, TGEV, and SADS-CoV. (A,C,E,G) Colorimetric/fluorescence signals from a series of 10-fold dilutions of plasmid DNA by CRISPR/Cas12a coupled with LAMP assay. (B,D,F,H) Sensitivity of the CRISPR/Cas12a coupled with LAMP assay quantified by a multi-functional microplate reader (n = 3). No excitation light stands for white light; PEDV, porcine epidemic diarrhea virus; TGEV, transmissible gastroenteritis virus; PDCoV, porcine deltacoronavirus; SADS-CoV, swine acute diarrhea syndrome coronavirus; NC, negative control. Data are represented as mean ± SEM.
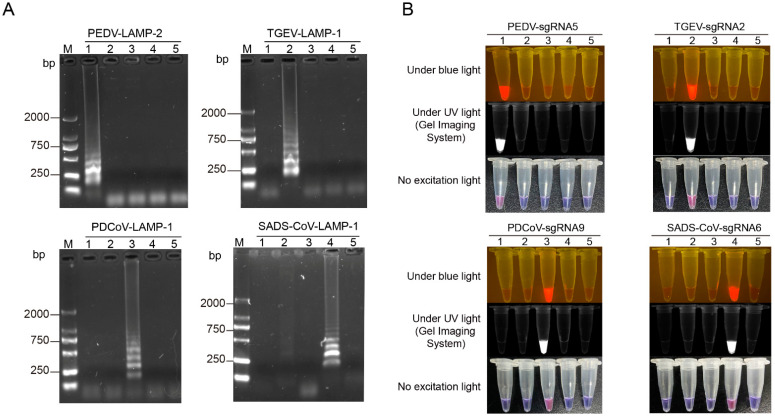
3.4. Specificity Analysis of the LAMP-Cas12a Assay for the Detection of Four Porcine Coronaviruses
To confirm the specificity of the LAMP-Cas12a assay, we evaluated the specificity of the LAMP primer pairs and the sgRNA. First, LAMP primer pairs targeting the PEDV ORF3 gene, the TGEV N gene, the PDCoV N gene, or the SADS-CoV N gene were used to amplify the four viral plasmids above. The results showed that each set of primer pairs could only amplify the unique band of the plasmid containing the target gene, indicating that the LAMP primer pairs designed for each virus in this study were specific (Figure 3A). Subsequently, to verify the specificity of the sgRNA, the highly active sgRNAs of the four CoVs were analyzed by the colorimetric/fluorescence Cas12a cleavage assay. As shown in Figure 3B, only the tube containing the corresponding target fragment had colorimetric/fluorescence signals, while the other tubes had no signal readout, Thus, the sgRNA identified in this study is specific.
Figure 3.
Specificity of LAMP-Cas12a assay for detection of four porcine coronaviruses. (A) The agarose gel electrophoresis for detection of specific LAMP amplicons; Lane M: 2000 DNA Ladder; bp: base pairs; Lane 1: PEDV-ORF3 plasmid as DNA template; Lane 2: TGEV-N plasmid as DNA template; Lane 3: PDCoV-N plasmid as DNA template; Lane 4: SADS-CoV-N plasmid as DNA template; Lane 5: non-template control (NTC). (B) The colorimetric/fluorescence signal detection under the blue (470 nM) and UV light; LAMP-amplified products of PEDV-ORF3 plasmid (1), TGEV-N plasmid (2), PDCoV-N plasmid (3), SADS-CoV-N plasmid (4), non-template control (5). No excitation light stands for white light; PEDV, porcine epidemic diarrhea virus; TGEV, transmissible gastroenteritis virus; PDCoV, porcine deltacoronavirus; SADS-CoV, swine acute diarrhea syndrome coronavirus.
3.5. Detection of Clinical RNA Samples of the Four Porcine Coronaviruses with an RT-LAMP-Cas12a Assay
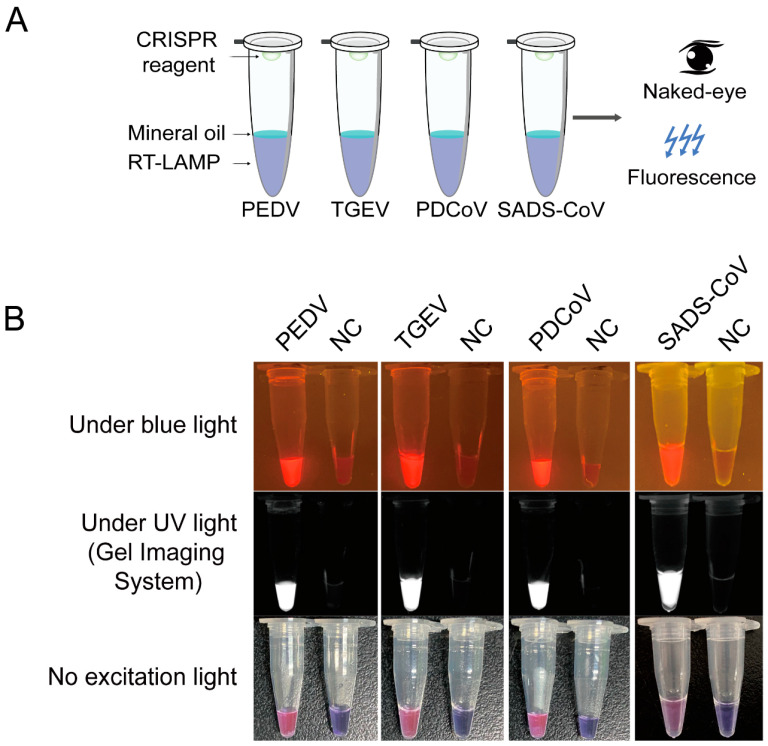
To apply this newly developed method to field samples, we attempted to detect viral RNA with RT-LAMP-Cas12a directly in a one-tube assay (Figure 4A). As shown in Figure 4B, strong visual colorimetric/fluorescence signals appeared when samples had the corresponding virus. In the no-template control, there was no visual colorimetric/fluorescence signal. Therefore, the method based on CRISPR/Cas12a combined with RT-LAMP assay realized the detection of RNA in clinical samples. In addition, the one-tube operation process has the potential to reduce the aerosol pollution caused by opening the cover, and can also save time. Therefore, one-tube detection will become a useful method for detecting viral infections.
Figure 4.
One-tube detection of four porcine coronaviruses in clinical samples based on CRISPR/Cas12a combined with RT-LAMP assay. (A) Diagram of principle of the contamination-free one-tube CRISPR/Cas12a combined with RT-LAMP assay detection of clinical samples. (B) Colorimetric/fluorescence readout of four porcine CoVs by one-tube multiplex RT-LAMP-Cas12a assay. CRISPR, clustered regularly interspaced short palindromic repeats; RT-LAMP, reverse transcription-loop-mediated isothermal amplification; No excitation light stands for white light; PEDV, porcine epidemic diarrhea virus; TGEV, transmissible gastroenteritis virus; PDCoV, porcine deltacoronavirus; SADS-CoV, swine acute diarrhea syndrome coronavirus; NC, negative control.
3.6. Detection of the Four Porcine Coronaviruses by a Multiplex LAMP-Cas12a Assay
In clinical cases, the symptoms of pig diarrhea are generally caused by cross-infection. Multiplex amplification is able to amplify multiple target genes simultaneously in one reaction, which can greatly improve detection efficiency. In the process of multiplex RT-LAMP amplification, primer pairing and competitive amplification could cause many problems, such as dimerizations, nonspecific amplification, and low amplification efficiency. Therefore, it is necessary to optimize the concentration ratio of the primers for each target sequence. First, we explored the concentration ratio of LAMP primers for amplifying the PEDV ORF3 gene and the TGEV N gene, and established a double-LAMP amplification combined with the CRISPR/Cas12a cleavage assay. The results showed that the PEDV ORF3 gene and the TGEV N gene could be amplified simultaneously in one-tube reaction assay (Figure S8A), and lower concentrations (1/2 or 1/3 of the original concentration) of LAMP amplification primers targeting the PEDV-ORF3 gene were beneficial to CRISPR/Cas12a visual detection (Figure S8B). This suggested that appropriately reducing the primer concentration could prevent the interaction between primer sequences to a certain extent.
Subsequently, triple-LAMP amplification was established to identify PEDV, TGEV, and PDCoV simultaneously. The results showed that the LAMP amplification primer pairs targeting the TGEV N gene had a strong amplification ability, and the primer concentration had little effect on it (Figure S8C). However, the LAMP amplification efficiencies of PEDV and PDCoV depended on the primer concentration (Figure S8D). Based on the above results, we established quadruple-LAMP amplification (Figure S8E,F). The results showed that the concentration of LAMP primer pairs targeting the TGEV N gene had no significant effect on its amplification efficiency, while the amplification efficiencies of the PEDV ORF3 gene, the PDCoV N gene and the SADS-CoV N gene were positively correlated with primer concentration (Figure S8F). It was suggested that the primer concentrations for PEDV, PDCoV, and SADS-CoV could be appropriately increased, and the primer concentration for TGEV could be reduced to achieve better detection efficiency with multiplex LAMP amplification.
To balance the amplification efficiency of multiplex LAMP and enhance the colorimetric/fluorescence readout of the CRISPR/Cas12a cleavage assay, we adjusted the LAMP primer concentrations (2/5, 6/25, 3/5, and 3/5 of the original concentrations for PEDV, TGEV, PDCoV, and SADS-CoV, respectively). In addition, to avoid aerosol pollution, we established a one-tube multiplex LAMP combined with the CRISPR/Cas12a system (Figure 5A), which was successfully used for the plasmid DNA of the four porcine CoVs (Figure 5B).
Figure 5.
Establishment of one-tube multiplex LAMP-Cas12a assay for detection of four porcine diarrhea coronaviruses. (A) Schematic diagram experimental flow and principle of contamination-free one-tube multiplex LAMP-Cas12a assay. (B) Colorimetric/fluorescence readout of four porcine coronaviruses by one-tube multiplex LAMP-Cas12a assay. 1: plasmid DNA mixture of PEDV, TGEV, PDCoV, and SADS-CoV (1:1:1:1) as templates; 2: non-template control (NTC). sgRNA, small guide RNA; LAMP, loop-mediated isothermal amplification; ROX-BHQ2, ROX-labeled ssDNA-FQ reporter; No excitation light stands for white light; PEDV, porcine epidemic diarrhea virus; TGEV, transmissible gastroenteritis virus; PDCoV, porcine deltacoronavirus; SADS-CoV, swine acute diarrhea syndrome coronavirus.
4. Discussion
To simplify the detection and on-site diagnosis, we developed a multiplex nucleic acid detection method based on CRISPR/Cas12a and multiplex RT-LAMP for the detection of four porcine diarrhea CoVs. Several studies have reported gel-based PCR and qPCR for the simultaneous detection of porcine diarrheal viruses [32]. In comparison, multiplex LAMP-Cas12a assay has the following advantages: when combined with RT-LAMP, complete reverse transcription and amplification can be completed at constant temperature. We found that the LoD for LAMP-Cas12a with the naked eye reached a single copy, which indicated a sensitivity a thousand times higher than that of PCR-Cas12a (from 8 × 102 to 8 × 103 copies/μL) visual detection of the four CoVs. The possible reason is that the amplification and detection efficiency in combination with LAMP is higher than that in PCR. Thus, the multiplex LAMP-Cas12a assay had potentially achieved the sensitivity of a single copy, but further validation was required, while the use of the CRISPR/Cas12a system guaranteed a higher detection specificity, based on Cas12a nuclease and sgRNA detection and typing of target DNA. In addition, as the ORF3 gene of PEDV has been used in several reports to differentiate between field-and vaccine-derived isolates, it was chosen in this study for the detection of classical PEDV strains [33].
To achieve naked-eye visual detection of viruses, a variety of ssDNA-FQ reporters have been developed [31]. The ROX-labeled ssDNA-FQ reporter allows a direct interpretation of the assay results by observation of visual colorimetric changes with the naked eye [31]. This ssDNA-FQ reporter could be synthesized stably from biological companies in large quantities, in contrast to the expensive gold nanoparticles used in Cas12a/Cas13a-AuNP visual assays [34,35,36,37,38]. The operation process of the one-tube method does not require the transfer of pre-amplified products, which avoids false-positive readouts due to aerosol contamination by pre-amplified product formation [39,40] and saves detection time. For example, the total detection time of the multiplex LAMP-Cas12a assay is only about 25 min.
Compared with other previously reported nucleic acid detection platforms based on CRISPR, which could only detect one pathogen per test [18,19,41], our multiplex LAMP-Cas12a assay could amplify four pathogens with only one-tube reaction, greatly improving diagnosis efficiency. The SHERLOCK V2 platform performed four-channel multiplex detection using different orthogonal cas effectors, and the CARMEN method was able to simultaneously differentiate 169 pathogens in a microwell array [42,43]. However, they both require specialized instruments and expensive experimental reagents, making them difficult to apply to on-site diagnostics. A multiplex RT-RPA combined with separate LbCas12a/AuNP visual assay, reported to simultaneously detect five viruses in apples, has application prospects for on-site deployment in the field [44]. Compared with preamplification by multiplex RT-RPA, our LAMP-Cas12a assay combined with multiplex isothermal amplification assays is cost-effective and more sensitive (almost up to single copy). In fact, it is very difficult to simultaneously detect four CoVs in a single tube, so while our method using only multiplex RT-LAMP is performed simultaneously, it requires different CRISPR/Cas12a assays to distinguish co-infected viruses. In the future, we plan to continue to improve the multiplex LAMP-Cas12a assay in a single tube.
5. Conclusions
Taken together, we successfully established a multiplex, field-deployable, and visual naked-eye detection method for the detection of four porcine diarrhea epidemic CoVs in one test. This newly developed viral RNA diagnostic method can help diagnose the status and prevalence of four porcine enteric CoVs.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/v14040833/s1, Figure S1: Screening of high-activity sgRNAs for detection of PEDV ORF3 gene and optimum reaction conditions; Figure S2. Screening of high-activity sgRNAs for detection of PDCoV N and SADS-CoV N gene; Figure S3. Optimization of the reaction conditions in LAMP for PEDV detection; Figure S4. Screening of LAMP primer pairs for detection of TGEV N gene; Figure S5. Screening of LAMP primer pairs for detection of PDCoV N gene; Figure S6. Screening of LAMP primer pairs for detection of SADS-CoV N gene; Figure S7. Sensitivity of the PCR-Cas12a assay for detection of ORF3 gene using serially diluted ORF3-plasmid (8×107 to 8×100 copies/μL); Figure S8. Establishment of multiple LAMP-Cas12a assay for porcine diarrhea coronavirus; Table S1. Synthetic insert cloned into pUC57 and pMD18T vectors; Table S2. Primers and oligos used in this study.
Author Contributions
Most of the experimental work was co-conducted by J.L., X.C., and D.T., with minor contributions from L.S., L.Z., B.X., H.L., S.Z., and X.L. (Xinyun Li) and X.L. (Xiangdong Liu). L.N. and S.X. conceived the project and designed the experiments. J.L. and D.T. wrote the manuscript. S.X. and L.N. helped in revising the manuscript. All authors contributed to manuscript revision. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Science Foundation of China (No. 32072685), the Sichuan Science and Technology Support Program (No. SCSZTD-2021-09), the earmarked fund for China Agriculture Research System (No. CARS-36-05B).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.de Wilde A.H., Snijder E.J., Kikkert M., van Hemert M.J. Host Factors in Coronavirus Replication. Curr. Top. Microbiol. Immunol. 2018;419:1–42. doi: 10.1007/82_2017_25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haake C., Cook S., Pusterla N., Murphy B. Coronavirus Infections in Companion Animals: Virology, Epidemiology, Clinical and Pathologic Features. Viruses. 2020;12:1023. doi: 10.3390/v12091023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang Q., Yoo D. Immune evasion of porcine enteric coronaviruses and viral modulation of antiviral innate signaling. Virus Res. 2016;226:128–141. doi: 10.1016/j.virusres.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woo P.C., Lau S.K., Lam C.S., Lau C.C., Tsang A.K., Lau J.H., Bai R., Teng J.L., Tsang C.C., Wang M., et al. Discovery of seven novel Mammalian and avian coronaviruses in the genus deltacoronavirus supports bat coronaviruses as the gene source of alphacoronavirus and betacoronavirus and avian coronaviruses as the gene source of gammacoronavirus and deltacoronavirus. J. Virol. 2012;86:3995–4008. doi: 10.1128/JVI.06540-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Q., Wang H.Y. Porcine enteric coronaviruses: An updated overview of the pathogenesis, prevalence, and diagnosis. Vet. Res. Commun. 2021;45:75–86. doi: 10.1007/s11259-021-09808-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Q., Vlasova A.N., Kenney S.P., Saif L.J. Emerging and re-emerging coronaviruses in pigs. Curr. Opin. Virol. 2019;34:39–49. doi: 10.1016/j.coviro.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edwards C.E., Yount B.L., Graham R.L., Leist S.R., Hou Y.J., Dinnon K.H., Sims A.C., Swanstrom J., Gully K., Scobey T.D., et al. Swine acute diarrhea syndrome coronavirus replication in primary human cells reveals potential susceptibility to infection. Proc. Natl. Acad. Sci. USA. 2020;117:26915–26925. doi: 10.1073/pnas.2001046117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jung K., Saif L.J., Wang Q. Porcine epidemic diarrhea virus (PEDV): An update on etiology, transmission, pathogenesis, and prevention and control. Virus Res. 2020;286:198045. doi: 10.1016/j.virusres.2020.198045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bigault L., Brown P., Bernard C., Blanchard Y., Grasland B. Porcine epidemic diarrhea virus: Viral RNA detection and quantification using a validated one-step real time RT-PCR. J. Virol. Methods. 2020;283:113906. doi: 10.1016/j.jviromet.2020.113906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou L., Sun Y., Wu J.L., Mai K.J., Chen G.H., Wu Z.X., Bai Y., Li D., Zhou Z.H., Cheng J., et al. Development of a TaqMan-based real-time RT-PCR assay for the detection of SADS-CoV associated with severe diarrhea disease in pigs. J. Virol. Methods. 2018;255:66–70. doi: 10.1016/j.jviromet.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song D., Zhou X., Peng Q., Chen Y., Zhang F., Huang T., Zhang T., Li A., Huang D., Wu Q., et al. Newly Emerged Porcine Deltacoronavirus Associated With Diarrhoea in Swine in China: Identification, Prevalence and Full-Length Genome Sequence Analysis. Transbound. Emerg. Dis. 2015;62:575–580. doi: 10.1111/tbed.12399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang X., Chen J., Yao G., Guo Q., Wang J., Liu G. A TaqMan-probe-based multiplex real-time RT-qPCR for simultaneous detection of porcine enteric coronaviruses. Appl. Microbiol. Biotechnol. 2019;103:4943–4952. doi: 10.1007/s00253-019-09835-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang J. Porcine deltacoronavirus: Overview of infection dynamics, diagnostic methods, prevalence and genetic evolution. Virus Res. 2016;226:71–84. doi: 10.1016/j.virusres.2016.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ding G., Fu Y., Li B., Chen J., Wang J., Yin B., Sha W., Liu G. Development of a multiplex RT-PCR for the detection of major diarrhoeal viruses in pig herds in China. Transbound. Emerg. Dis. 2020;67:678–685. doi: 10.1111/tbed.13385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang J., Tsai Y.L., Lee P.Y., Chen Q., Zhang Y., Chiang C.J., Shen Y.H., Li F.C., Chang H.F., Gauger P.C., et al. Evaluation of two singleplex reverse transcription-Insulated isothermal PCR tests and a duplex real-time RT-PCR test for the detection of porcine epidemic diarrhea virus and porcine deltacoronavirus. J. Virol. Methods. 2016;234:34–42. doi: 10.1016/j.jviromet.2016.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schrader C., Schielke A., Ellerbroek L., Johne R. PCR inhibitors—Occurrence, properties and removal. J. Appl. Microbiol. 2012;113:1014–1026. doi: 10.1111/j.1365-2672.2012.05384.x. [DOI] [PubMed] [Google Scholar]
- 17.Hedman J., Rådström P. Overcoming inhibition in real-time diagnostic PCR. Methods Mol. Biol. 2013;943:17–48. doi: 10.1007/978-1-60327-353-4_2. [DOI] [PubMed] [Google Scholar]
- 18.Gootenberg J.S., Abudayyeh O.O., Lee J.W., Essletzbichler P., Dy A.J., Joung J., Verdine V., Donghia N., Daringer N.M., Freije C.A., et al. Nucleic acid detection with CRISPR-Cas13a/C2c2. Science. 2017;356:438–442. doi: 10.1126/science.aam9321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen J.S., Ma E., Harrington L.B., Da Costa M., Tian X., Palefsky J.M., Doudna J.A. CRISPR-Cas12a target binding unleashes indiscriminate single-stranded DNase activity. Science. 2018;360:436–439. doi: 10.1126/science.aar6245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li S.Y., Cheng Q.X., Wang J.M., Li X.Y., Zhang Z.L., Gao S., Cao R.B., Zhao G.P., Wang J. CRISPR-Cas12a-assisted nucleic acid detection. Cell Discov. 2018;4:20. doi: 10.1038/s41421-018-0028-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Teng F., Guo L., Cui T., Wang X.G., Xu K., Gao Q., Zhou Q., Li W. CDetection: CRISPR-Cas12b-based DNA detection with sub-attomolar sensitivity and single-base specificity. Genome Biol. 2019;20:132. doi: 10.1186/s13059-019-1742-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harrington L.B., Burstein D., Chen J.S., Paez-Espino D., Ma E., Witte I.P., Cofsky J.C., Kyrpides N.C., Banfield J.F., Doudna J.A. Programmed DNA destruction by miniature CRISPR-Cas14 enzymes. Science. 2018;362:839–842. doi: 10.1126/science.aav4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang B., Wang R., Wang D., Wu J., Li J., Wang J., Liu H., Wang Y. Cas12aVDet: A CRISPR/Cas12a-Based Platform for Rapid and Visual Nucleic Acid Detection. Anal. Chem. 2019;91:12156–12161. doi: 10.1021/acs.analchem.9b01526. [DOI] [PubMed] [Google Scholar]
- 24.Ding X., Yin K., Li Z., Lalla R.V., Ballesteros E., Sfeir M.M., Liu C. Ultrasensitive and visual detection of SARS-CoV-2 using all-in-one dual CRISPR-Cas12a assay. Nat. Commun. 2020;11:4711. doi: 10.1038/s41467-020-18575-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He Q., Yu D., Bao M., Korensky G., Chen J., Shin M., Kim J., Park M., Qin P., Du K. High-throughput and all-solution phase African Swine Fever Virus (ASFV) detection using CRISPR-Cas12a and fluorescence based point-of-care system. Biosens. Bioelectron. 2020;154:112068. doi: 10.1016/j.bios.2020.112068. [DOI] [PubMed] [Google Scholar]
- 26.Broughton J.P., Deng X., Yu G., Fasching C.L., Servellita V., Singh J., Miao X., Streithorst J.A., Granados A., Sotomayor-Gonzalez A., et al. CRISPR-Cas12-based detection of SARS-CoV-2. Nat. Biotechnol. 2020;38:870–874. doi: 10.1038/s41587-020-0513-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang R., Qian C., Pang Y., Li M., Yang Y., Ma H., Zhao M., Qian F., Yu H., Liu Z., et al. opvCRISPR: One-pot visual RT-LAMP-CRISPR platform for SARS-cov-2 detection. Biosens. Bioelectron. 2021;172:112766. doi: 10.1016/j.bios.2020.112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tao D., Liu J., Nie X., Xu B., Tran-Thi T.N., Niu L., Liu X., Ruan J., Lan X., Peng G., et al. Application of CRISPR-Cas12a Enhanced Fluorescence Assay Coupled with Nucleic Acid Amplification for the Sensitive Detection of African Swine Fever Virus. ACS Synth. Biol. 2020;9:2339–2350. doi: 10.1021/acssynbio.0c00057. [DOI] [PubMed] [Google Scholar]
- 29.Wang X., Ji P., Fan H., Dang L., Wan W., Liu S., Li Y., Yu W., Li X., Ma X., et al. CRISPR/Cas12a technology combined with immunochromatographic strips for portable detection of African swine fever virus. Commun. Biol. 2020;3:62. doi: 10.1038/s42003-020-0796-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao C., Zheng X., Qu W., Li G., Li X., Miao Y.L., Han X., Liu X., Li Z., Ma Y., et al. CRISPR-offinder: A CRISPR guide RNA design and off-target searching tool for user-defined protospacer adjacent motif. Int. J. Biol. Sci. 2017;13:1470–1478. doi: 10.7150/ijbs.21312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xie S., Tao D., Fu Y., Xu B., Tang Y., Steinaa L., Hemmink J.D., Pan W., Huang X., Nie X., et al. Rapid Visual CRISPR Assay: A Naked-Eye Colorimetric Detection Method for Nucleic Acids Based on CRISPR/Cas12a and a Convolutional Neural Network. ACS Synth. Biol. 2022;11:383–396. doi: 10.1021/acssynbio.1c00474. [DOI] [PubMed] [Google Scholar]
- 32.Yang Y.L., Yu J.Q., Huang Y.W. Swine enteric alphacoronavirus (swine acute diarrhea syndrome coronavirus): An update three years after its discovery. Virus Res. 2020;285:198024. doi: 10.1016/j.virusres.2020.198024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang K., Liang Y., Li Y., Liu Q., Zhang W., Yin D., Song X., Shao Y., Tu J., Qi K. Reverse transcription-enzymatic recombinase amplification coupled with CRISPR-Cas12a for rapid detection and differentiation of PEDV wild-type strains and attenuated vaccine strains. Anal. Bioanal. Chem. 2021;413:7521–7529. doi: 10.1007/s00216-021-03716-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Y., Mansour H., Wang T., Poojari S., Li F. Naked-Eye Detection of Grapevine Red-Blotch Viral Infection Using a Plasmonic CRISPR Cas12a Assay. Anal. Chem. 2019;91:11510–11513. doi: 10.1021/acs.analchem.9b03545. [DOI] [PubMed] [Google Scholar]
- 35.Yuan C., Tian T., Sun J., Hu M., Wang X., Xiong E., Cheng M., Bao Y., Lin W., Jiang J., et al. Universal and Naked-Eye Gene Detection Platform Based on the Clustered Regularly Interspaced Short Palindromic Repeats/Cas12a/13a System. Anal. Chem. 2020;92:4029–4037. doi: 10.1021/acs.analchem.9b05597. [DOI] [PubMed] [Google Scholar]
- 36.Hu M., Yuan C., Tian T., Wang X., Sun J., Xiong E., Zhou X. Single-Step, Salt-Aging-Free, and Thiol-Free Freezing Construction of AuNP-Based Bioprobes for Advancing CRISPR-Based Diagnostics. J. Am. Chem. Soc. 2020;142:7506–7513. doi: 10.1021/jacs.0c00217. [DOI] [PubMed] [Google Scholar]
- 37.Choi J.H., Lim J., Shin M., Paek S.H., Choi J.W. CRISPR-Cas12a-Based Nucleic Acid Amplification-Free DNA Biosensor via Au Nanoparticle-Assisted Metal-Enhanced Fluorescence and Colorimetric Analysis. Nano Lett. 2021;21:693–699. doi: 10.1021/acs.nanolett.0c04303. [DOI] [PubMed] [Google Scholar]
- 38.Liu J., Chen J., Wu D., Huang M., Chen J., Pan R., Wu Y., Li G. CRISPR-/Cas12a-Mediated Liposome-Amplified Strategy for the Surface-Enhanced Raman Scattering and Naked-Eye Detection of Nucleic Acid and Application to Food Authenticity Screening. Anal. Chem. 2021;93:10167–10174. doi: 10.1021/acs.analchem.1c01163. [DOI] [PubMed] [Google Scholar]
- 39.Chen Y., Shi Y., Chen Y., Yang Z., Wu H., Zhou Z., Li J., Ping J., He L., Shen H., et al. Contamination-free visual detection of SARS-CoV-2 with CRISPR/Cas12a: A promising method in the point-of-care detection. Biosens. Bioelectron. 2020;169:112642. doi: 10.1016/j.bios.2020.112642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pang B., Xu J., Liu Y., Peng H., Feng W., Cao Y., Wu J., Xiao H., Pabbaraju K., Tipples G., et al. Isothermal Amplification and Ambient Visualization in a Single Tube for the Detection of SARS-CoV-2 Using Loop-Mediated Amplification and CRISPR Technology. Anal. Chem. 2020;92:16204–16212. doi: 10.1021/acs.analchem.0c04047. [DOI] [PubMed] [Google Scholar]
- 41.Myhrvold C., Freije C.A., Gootenberg J.S., Abudayyeh O.O., Metsky H.C., Durbin A.F., Kellner M.J., Tan A.L., Paul L.M., Parham L.A., et al. Field-deployable viral diagnostics using CRISPR-Cas13. Science. 2018;360:444–448. doi: 10.1126/science.aas8836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gootenberg J.S., Abudayyeh O.O., Kellner M.J., Joung J., Collins J.J., Zhang F. Multiplexed and portable nucleic acid detection platform with Cas13, Cas12a, and Csm6. Science. 2018;360:439–444. doi: 10.1126/science.aaq0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ackerman C.M., Myhrvold C., Thakku S.G., Freije C.A., Metsky H.C., Yang D.K., Ye S.H., Boehm C.K., Kosoko-Thoroddsen T.F., Kehe J., et al. Massively multiplexed nucleic acid detection with Cas13. Nature. 2020;582:277–282. doi: 10.1038/s41586-020-2279-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jiao J., Kong K., Han J., Song S., Bai T., Song C., Wang M., Yan Z., Zhang H., Zhang R., et al. Field detection of multiple RNA viruses/viroids in apple using a CRISPR/Cas12a-based visual assay. Plant Biotechnol. J. 2021;19:394–405. doi: 10.1111/pbi.13474. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available on request from the corresponding author.