Abstract
Background:
Increased physical activity (PA) may protect against asthma but PA can trigger asthma symptoms.
Objective:
To investigate relationships between moderate to vigorous PA (MVPA) assessed during routine care visits and incident asthma.
Methods:
Retrospective cohort of 542,486 children, 2–17 years from 2010–2017 with MVPA assessed (exercise vital sign) during routine care visits. Association of MVPA and asthma was analyzed using Cox proportional hazards (HR) regression models as a function of age with MVPA and body mass index (BMI) as time-varying factors, adjusted for race/ethnicity, socioeconomic status, air pollution.
Results:
Mean MVPA was 5.4 (SD 4.4) h/wk. Crude asthma incidence density rate (IDR) was highest in children with < 1 h/wk of MVPA (IDR 9.07, 95% CI 8.79, 9.36) and lowest in children engaging in 4–7 h/wk of MVPA (IDR 6.55, 95% CI 6.33, 6.77). In adjusted models, an increase in MVPA was associated with lower asthma risk in children reporting 0 h/wk of MVPA (HR 0.981, 95% CI 0.973, 0.990). In children with ≥8 h/wk of MVPA, an increase in MVPA was associated with higher asthma risk (1.005,95% CI 1.002, 1.009). There was no significant BMI by MVPA interaction.
Conclusion:
Increasing MVPA in children with low activity levels is associated with lower asthma risk, children reporting high levels of activity may experience greater asthma risk as their activity levels increase further. Understanding the role of PA in the development of asthma and assessing MVPA during routine care visits in children may help to develop targeted interventions and guide asthma management.
Keywords: Physical activity, obesity, asthma, children
INTRODUCTION
Asthma, one of the most common chronic clinical syndromes in children, affects approximately 5.5 million children in the United States, and has significant impacts on families and society including school absenteeism, emergency room visits, and healthcare related costs.1–3 While the underlying etiology of asthma development is multifactorial, unhealthy lifestyle factors, including decreased physical activity, increased sedentary time, and poor nutrition, likely play a role.4 Further, it is now well established that obesity, which is associated with a sedentary lifestyle and reduced participation in physical activity,5 is a significant risk factor for both asthma development and increased morbidity.6
Physical activity, a modifiable risk factor, plays an essential role in healthy growth and development in children and adolescents. However, over the past decades, significant reductions in physical activity have led to decreased aerobic fitness and coincided with dramatically increasing rates of asthma and obesity.7, 8 Exercise is a common trigger for bronchoconstriction in children with asthma, but poor asthma control, asthma severity, exercise-induced laryngeal dysfunction, or air pollution exposure could be contributing factors.9 Increased particle deposition in the lower airways from air pollution during exercise may put children in urban environments at higher risk for asthma.10, 11 Some studies have shown that increased physical activity is protective against the development of asthma in contrast to other studies where higher levels of physical activity increased the risk of asthma symptoms.5, 12–14 Quantifying physical activity, particularly in large population-based studies is challenging and the various physical activity tools/questionnaires as well as categorization of physical activity can contribute to these mixed results. In children, only a handful of prospective studies examining weight status and the risk of asthma have accounted for physical activity levels.15 Lastly, the majority of studies examining relationships between physical activity, weight status and asthma have been cross-sectional with only a few longitudinal cohorts.16–18
The objective of our study was to characterize the association of physical activity, obesity and asthma risk in a population-based cohort of children enrolled in a large comprehensive health care system in which physical activity is assessed as “exercise vital sign” regularly during routine care.19 The Kaiser Permanente (KP) “exercise vital sign” is a simple screening tool using 2-questions to assess moderate to vigorous physical activity (MVPA). Leveraging data collected from this tool allows us to examine the effect of MVPA over time. We hypothesized that increased physical activity would decrease the risk of asthma among normal weight and overweight/obese children.
PATIENTS AND METHODS
Study Design and Setting
A retrospective cohort study between Jan 01, 2010 to Dec 31, 2017 was conducted among health plan members of Kaiser Permanente Southern California (KPSC), a large, prepaid, integrated managed healthcare system that served approximately 4.7 million members throughout Southern California as of March 2020 (~19.5% of the Southern California population). Members received their care in medical offices and hospitals owned by KPSC throughout the seven-county region. The membership is diverse and similar in socioeconomic characteristics to the region’s census demographics.20 The Kaiser Permanente Southern California Institutional Review Board approved the study and granted a waiver for informed consent.
Study population
Routine assessment of physical activity during most medical visits in KPSC medical offices including pediatric clinics was implemented in 2010. The population for the present analysis consisted of children and adolescents between the ages of 2 and 17 who were actively enrolled in a KPSC health plan between Jan 01, 2010 to Dec 31, 2017.21 Eligible youth (n=953,388) had a baseline visit with MVPA assessment (exercise vital sign) during the study period, at least 6 months of health care coverage prior to their baseline visit to rule out pre-existing asthma, at least 12 months of follow-up, were not pregnant, and had no prior indication of asthma, wheezing, allergic rhinitis, or complex care conditions (Figure 1).22, 23 We then excluded youth without plausible weight and height measurements defined as above or below 3 SD from the mean as well as BMI below the 0.5th (11.02 kg/m2) and above the 99.5th percentile (39.98 kg/m2) and those without physical activity assessment.24 The final analytical cohort was comprised of 542,486 youth.
Figure 1.
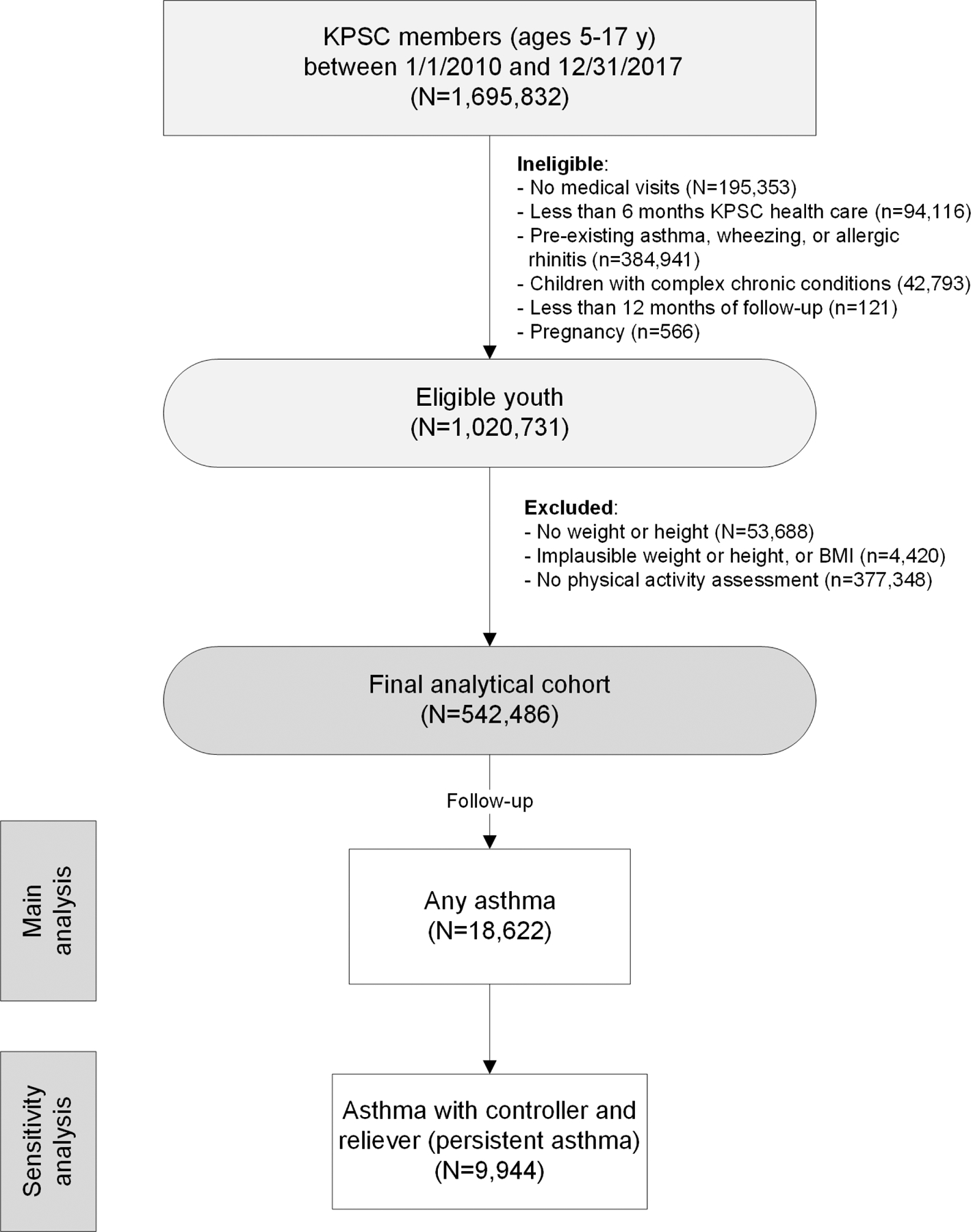
Study flow chart.
Physical activity and body mass index
The exercise vital sign is a modification of the Behavioral Risk Factor Surveillance System (BRFSS) and Youth Risk Behavior Surveillance (YRBS) physical activity questions.25, 26 In adults, the measure has shown reliable test/retest responses and acceptable results for time trends.27–29 At KPSC, the exercise vital sign was first implemented in 2009 in routine adult care and in 2010 for pediatric care to identify patients not meeting recommended physical activity levels.25 Medical assistants and nurses collect exercise vital sign responses during the outpatient visit before the provider interacts with the patient. The exercise vital sign can be administered in less than 30 seconds. For children, the exercise vital sign is assessed during many pediatric outpatient visits but limited to a maximum of one assessment every 3 months if more medical visits occur. In adults, the exercise vital sign showed good discriminant validity and seemed to result in more conservative estimates of physical activity behavior when compared with national surveys.19 The exercise vital sign consists of two brief questions directed to the parent or guardian of the pediatric patient until the age of 12 years and to the patient directly for adolescents 13 years and older: 1) “On average, how many days per week does your child (do you) engage in moderate-to-vigorous physical activity (like a brisk walk)?” and 2) “On average, how many minutes does your child (do you) engage in physical activity at this level?” These responses are multiplied to give minutes per week of reported MVPA. The questions are asked by a medical assistant or nurse when they are recording traditional vital signs and are triggered by the EHR system. MVPA assessments were available in 63% of pediatric members with annual assessments of ~39.5% in all years.
Body weight and height were obtained from the electronic medical records. They are routinely measured by trained staff on calibrated scales at almost every medical office visit.30 Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters. Definitions of weight class in children and adolescents were based on the sex-specific BMI-for-age growth charts developed by the Centers for Disease Control and Prevention (CDC).31 For descriptive purposes, youth were categorized at baseline as underweight (BMI-for-age <5th percentile), normal weight (BMI-for-age ≥5th to <85th percentile), overweight (BMI-for-age ≥85th to <95th percentile), moderately obese (BMI-for age ≥95th to <97th percentile), and severely obese (BMI-for age ≥97th percentile).
Follow-up for asthma incidence
Children were followed for incident asthma until their last follow-up visit, death, or the end of the study on September 30, 2018. Asthma was defined as 1) physician-diagnosed asthma (International Classification of Diseases, 9th Revision, Clinical Modification codes ICD-9-CM 493.xx, ICD-10 J45.xx) and at least 2 prescriptions specific to asthma rescue or controller medication (inhaled corticosteroids, oral corticosteroids, leukotriene modifiers, long-acting beta-agonists, mast-cell stabilizers, other oral bronchodilators, and short-acting beta-agonists) within 24 months after diagnosis, or 2) physician-diagnosed wheezing (ICD-9-CM 786.07, ICD-10 R06.2) and at least 2 prescriptions specific to asthma rescue or controller medication within 12 months after diagnosis.
To address concerns that the association between physical activity and asthma is driven by possible exercise-induced asthma, persistent asthma defined as diagnosis of asthma and prescription of both a rescue and controller medication was used as secondary outcome.32
Covariates
We obtained self-reported race and ethnicity information from health plan administrative records and birth records. We categorized self-reported race/ethnicity as non-Hispanic White, Hispanic (regardless of race), African American, Asian or Pacific Islander, and other or unknown race/ethnicity. We used insurance through government health care assistance programs such as Medicaid, as a proxy for low annual income. To control for the exposure to air pollutants, we used ozone concentrations and emission of fine particles using CalEnvironScreen 3.0.32, 33 Ozone concentration, measured in parts per million (ppm), is calculated as the maximum amount of ozone in an 8-hour period per day that is over the 8-hour state standard of 0.07 ppm. The daily excess concentrations are then averaged over three years (2009–2011) to create one community ozone value for every census tract. Fine particle emission is measure as annual means in micrograms per cubic meter (μg/m3) in 2009–2011. Data for both PM2.5 and ozone were estimated at the center of the census tract.32, 33
Statistical Analysis
We compared clinical and demographic characteristics by physical activity group (≤1.0 h, 1.0–4.0 h, 4.1–7.9 h, ≥8 h) and by BMI group (<85th, 85-<95th, 95-<97th, ≥97th percentile of BMI-for-age). Incidence rates of asthma were estimated by dividing the number of asthma cases by the total person-years of follow-up, overall and within each sub-group. Confidence intervals (CIs) were estimated assuming that the occurrence of asthma follows Poisson distribution. We analyzed the association of physical activity, body mass index and asthma using Cox proportional hazards regression models with age as time-scale.34, 35 Baseline factors, low annual income and self-reported race/ethnicity were treated as fixed factors, physical activity (MVPA hours/week) and BMI (kg/m2) were modeled as time-varying continuous factors.36–38 We assessed the potential non-linear relationship between MVPA and asthma by including quadratic MVPA2 terms in the model. Based on the lowest Akaike information criterion (AIC), the models that best fit our data included both a linear MVPA term and a quadratic MVPA2 term. All BMI and/or MVPA measurements available were included in the analysis. Missing values for physical activity and BMI were imputed by using the average of non-missing values from the closest date prior to or after the date of the missing measure.39 For some youth, only one physical activity (n=115,989) or one BMI assessment (n=45,003) was conducted and treated as constant. We tested effect modification by adding interaction terms between MVPA and other variables in the model. We did not find any significant interactions beyond those reported. We performed several sensitivity analyses: 1) To address concerns that the association between physical activity and asthma is driven by possible exercise-induced asthma, we repeated our final models restricted to persistent asthma as the outcome, defined as diagnosis of asthma and prescription of both rescue and controller medication; 2) To address concerns of bias by pre-existing but undiagnosed asthma, we excluded incident asthma which occurred during the first 6 months of follow-up; 3) To address concerns of MVPA reporting accuracy in children ages 2–4 years, we repeated the analysis excluding MVPA measures and cases that occurred before the age of 5 years. All statistical analyses were conducted using SAS version 9.4 (SAS Institute, Inc., Cary, NC, USA).
RESULTS
Study population characteristics
The study population was 48.2% male, 53.6% Hispanic, and 22.1% receiving government health care assistance (Table 1). Parents/guardians or children reported a mean of 5.4 (SD 4.4) h/week of MVPA with 17.7% of children with ≤1 h/week of MVPA. Overall, 32.1% of children were overweight or obese. MVPA decreased with increasing body weight class (Table E1).
Table 1.
Cohort characteristics by physical activity level
| Total1 | Moderate to vigorous physical activity (h/week) | ||||
|---|---|---|---|---|---|
| ≤1 | 1–4 | 4.1–7.9 | 8+ | ||
| N, % | 542,486 (100) | 96,057 (17.7) | 116,318 (21.4) | 117,395 (21.6) | 212,716 (39.2) |
| Sex | |||||
| Male | 261372 (48.2) | 42117 (43.8) | 52635 (45.3) | 55878 (47.6) | 110742 (52.1) |
| Female | 281114 (51.8) | 53940 (56.2) | 63683 (54.7) | 61517 (52.4) | 101974 (47.9) |
| Age (years) | |||||
| 2–5 | 163711 (30.2) | 37954 (39.5) | 23206 (20.0) | 23296 (19.8) | 79255 (37.3) |
| 6–11 | 165814 (30.6) | 19085 (19.9) | 42135 (36.2) | 39545 (33.7) | 65049 (30.6) |
| 12–14 | 99501 (18.3) | 12542 (13.1) | 24670 (21.2) | 30159 (25.7) | 32130 (15.1) |
| 15–17 | 113460 (20.9) | 26476 (27.6) | 26307 (22.6) | 24395 (20.8) | 36282 (17.1) |
| Race/Ethnicity (self-report) | |||||
| Non-Hispanic White | 138703 (25.6) | 22609 (23.5) | 27364 (23.5) | 28563 (24.3) | 60167 (28.3) |
| Hispanic | 290582 (53.6) | 54667 (56.9) | 64999 (55.9) | 64403 (54.9) | 106513 (50.1) |
| African American | 38971 (7.2) | 6359 (6.6) | 7846 (6.7) | 8686 (7.4) | 16080 (7.6) |
| Asian/Pacific Islander | 39626 (7.3) | 6769 (7.0) | 8617 (7.4) | 8006 (6.8) | 16234 (7.6) |
| Other/Unknown | 34604 (6.4) | 5653 (5.9) | 7492 (6.4) | 7737 (6.6) | 13722 (6.5) |
| Body weight class | |||||
| Under/Normal weight | 368597 (67.9) | 63756 (66.4) | 74133 (63.7) | 77142 (65.7) | 153566 (72.2) |
| Overweight | 86832 (16.0) | 15038 (15.7) | 19829 (17) | 19983 (17) | 31982 (15.0) |
| Moderately obese | 27347 (5.0) | 4982 (5.2) | 6862 (5.9) | 6474 (5.5) | 9029 (4.2) |
| Severely obese | 59710 (11.1) | 12281 (12.8) | 15494 (13.3) | 13796 (11.8) | 18139 (8.5) |
| Government health care assistance | |||||
| No | 422767 (77.9) | 74199 (77.2) | 89713 (77.1) | 91003 (77.5) | 167852 (78.9) |
| Yes | 119719 (22.1) | 21858 (22.8) | 26605 (22.9) | 26392 (22.5) | 44864 (21.1) |
Asthma incidence rates
In 2,401,653 person-years and a mean follow-up time of 4.43 (SD 2.52) years, we identified 18,622 incident asthma cases. Of these, 9,944 cases were classified as persistent asthma, defined as diagnosis of asthma with a prescription of both controller and rescue medication and 8,050 cases of intermittent asthma with a prescription of rescue medication only. Another 628 children with asthma had a controller prescription only. Overall, the crude incident rate of asthma was 7.754 (95% CI 7.643, 7.866) per 1,000 person years (Table 2). Incidence of asthma was highest in children 2–5 years of age, African American children, and those receiving government health care assistance. The incidence of asthma increased with BMI-for-age class with an incidence of 8.810 (95% CI 8.463, 9.171) per 1000 person years in children with severe obesity compared to 7.650 (95% CI 7.517, 7.786) per 1000 person years in children who were underweight or normal weight. The crude incidence of asthma varied by MVPA categories, with the incidence of asthma highest in those reporting ≤1 h/week (9.070/1000 person years) and lowest in those reporting 4.1–7.9 h/week of MVPA (6.549 per 1000 person years).
Table 2:
Crude asthma incidence per 1,000 person-years
| Total | Cases | Person years | Incident rate (95% CI) per 1,000 person-years | |
|---|---|---|---|---|
| Overall | 542,486 | 18,622 | 2,401,653 | 7.754 (7.643, 7.866) |
| Age (years) | ||||
| 2–5 | 163,711 | 9,449 | 639,570 | 14.774 (14.479, 15.075) |
| 6–11 | 165,814 | 5,257 | 763,971 | 6.881 (6.698, 7.070) |
| 12–14 | 99,501 | 2,296 | 474,846 | 4.835 (4.641, 5.037) |
| 15–17 | 113,460 | 1,620 | 523,266 | 2.943 (2.949, 3.250) |
| Sex | ||||
| Male | 261,372 | 9,080 | 1,150,803 | 7.890 (7.730, 8.054 |
| Female | 281,114 | 9,542 | 1,250,851 | 7.628 (7.477, 7.783) |
| Race/Ethnicity | ||||
| Non-Hispanic/White | 138,703 | 4,610 | 596,783 | 7.725 (7.505, 7.951) |
| Hispanic | 290,582 | 10,159 | 1,324,531 | 7.670 (7.522, 7.820) |
| African American | 38,971 | 1,782 | 183,171 | 9.729 (9.287, 10.191) |
| Asian/Pacific Islander | 39,626 | 1,284 | 175,591 | 7.312 (6.923, 7.724) |
| Other/Unknown | 34,604 | 787 | 121,577 | 6.473 (6.036, 6.942) |
| Government health care assistance | ||||
| No | 422,767 | 13,689 | 1,898,499 | 7.210 (7.091, 7.332) |
| Yes | 119,719 | 4,933 | 503,155 | 9.804 (9.534, 10.082) |
| Body weight class | ||||
| Underweight/Normal | 368,597 | 12,356 | 1,615,118 | 7.650 (7.517, 7.786) |
| Overweight | 86,832 | 2,948 | 391,585 | 7.528 (7.261, 7.805) |
| Moderate Obese | 27,347 | 941 | 125,143 | 7.519 (7.054, 8.016) |
| Severe Obese | 59,710 | 2,377 | 269,808 | 8.810 (8.463, 9.171) |
| Moderate to vigorous physical activity (h/week) | ||||
| 0–1 | 96,057 | 3,980 | 438,820 | 9.070 (8.792, 9.356) |
| 1–4 | 116,318 | 3,893 | 563,108 | 6.913 (6.700, 7.134) |
| 4.1–7.9 | 117,395 | 3,396 | 518,532 | 6.549 (6.333, 6.773) |
| ≥8 | 212,716 | 7,353 | 881,194 | 8.344 (8.156, 8.537) |
Effect of MVPA and BMI
In models adjusted for sex, self-reported race/ethnicity, and annual income, MVPA was associated with the risk of asthma (Table 3). We found a significant quadratic time-varying MVPA term, indicating that the effect of increasing MVPA on asthma risk changes depending on the current MVPA level. An increase of MVPA was associated with a lower asthma risk at lower levels (0–4 h/week) of MVPA. For sedentary children with 0 hours of MVPA, an increase in 1 h/wk of MVPA decreased their asthma risk (HR 0.981, 95% CI 0.973, 0.990, Figure 2A). In contrast, for high-active children with ≥8 hours MVPA, the risk of asthma increased for each additional 1 h/wk of MVPA (HR 1.005, 95% CI 1.002, 1.009). These relationships remained significant even after adjusting for air pollution exposure.
Table 3:
Adjusted Hazard ratios for incident asthma risk by current moderate to vigorous physical activity (MVPA) level and increase of MVPA by 1 h/week
| HR (95% CI)* | |||
|---|---|---|---|
| Asthma type: | All asthma | Persistent asthma | |
| Model: | Model 1 | Model 1 + air pollution | Model 1 |
| N | 542,486 | 542,486 | 542,486 |
| Cases (n) | 18,622 | 18,622 | 8,050 |
| Current MVPA-level (h/wk) | (Figure 2A) | (Figure 2B) | |
| 0 | 0.981 (0.973, 0.990) | 0.981 (0.971, 0.990) | 0.978 (0.966, 0.989) |
| 2 | 0.987 (0.981, 0.994) | 0.987 (0.980, 0.994) | 0.985 (0.976, 0.994) |
| 4 | 0.993 (0.988, 0.998) | 0.993 (0.988, 0.999) | 0.992 (0.985, 0.999) |
| 6 | 0.999 (0.996, 1.003) | 1.000 (0.996, 1.004) | 0.999 (0.994, 1.004) |
| 8 | 1.005 (1.002, 1.009) | 1.006 (1.003, 1.010) | 1.007 (1.002, 1.011) |
| 10 | 1.011 (1.007, 1.016) | 1.013 (1.008, 1.018) | 1.014 (1.008, 1.020) |
| 12 | 1.017 (1.011, 1.023) | 1.020 (1.013, 1.026) | 1.022 (1.013, 1.030) |
| 14 | 1.023 (1.015, 1.031) | 1.026 (1.018, 1.035) | 1.029 (1.018, 1.040) |
| 16 | 1.029 (1.019, 1.040) | 1.033 (1.022, 1.044) | 1.037 (1.023, 1.050) |
| 18 | 1.036 (1.023, 1.048) | 1.040 (1.026, 1.053) | 1.044 (1.028, 1.061) |
| 20 | 1.042 (1.027, 1.057) | 1.046 (1.031, 1.062) | 1.052 (1.032, 1.072) |
Hazard ratios (HR) < 1.0 indicate that increasing MVPA by 1 h/wk is associated with a lower asthma risk at a given current level of MVPA. In contrast, HR >1.0 indicated that increasing MVPA by 1 h/wk is associated with a higher asthma risk at a given current level of MVPA. Model 1: Adjusted for sex, race/ethnicity, government health care assistance and stratified by baseline year. Missing values for BMI or MVPA were imputed.
Figure 2.
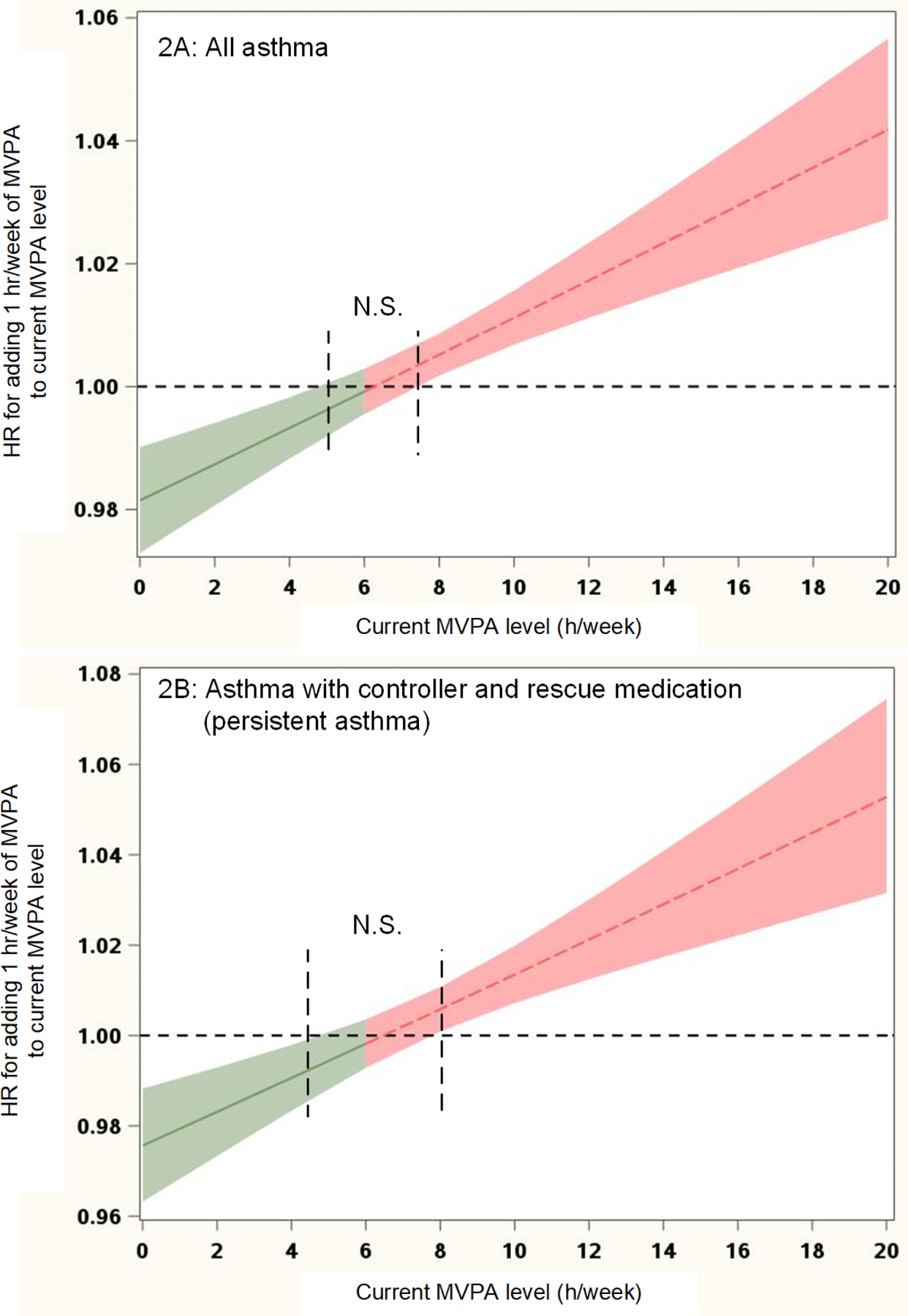
Effect of MVPA on the risk of any asthma and persistent asthma (prescription of rescue and controller medication)
*Adjusted for sex, race/ethnicity, government health care assistance, and air pollution (ozone levels); stratified by baseline year. Missing values for BMI or MVPA were imputed (equivalent to Model 1 in Table 3).
Green indicates a decrease and red an increase in asthma risk
BMI was positively associated with asthma risk (HR 1.042 for each kg/m2, 95% CI 1.039, 1.046). However, there was no significant interaction between BMI and MVPA.
Sensitivity analyses
Demographics of the eligible (n=953,388) and analytical cohort (n= 542,486) were essentially the same (Table E2).
Limiting incident asthma to persistent asthma slightly strengthened the effects compared to all incident asthma cases as outcomes (Figure 2B, Table 3).
Excluding incident cases of asthma occurring in the first 6 months of follow up (excluded cases n=3,488) did not essentially alter the results of the analysis (Table E3). Results remained unaltered when we excluded cases and MVPA measures before the age of 5 years (excluded cases n=5,040).
DISCUSSION
In this large retrospective population-based cohort of 542,486 Southern California children, MVPA was associated with decreased asthma risk in children reporting low levels of physical activity but increased asthma risk in children reporting higher levels of physical activity. To our knowledge, this is the first longitudinal analysis evaluating physical activity and weight status on asthma risk in children in the United States.
Physical activity plays a key role in healthy child growth and development but is usually, not regularly assessed, monitored or prescribed during routine pediatric care.40 To fill this gap, a self-reported exercise vital sign has been incorporated into electronic health records in health systems including Intermountain Healthcare and Kaiser Permanente (KP) which is embedded into a decision support tool alerting medical providers of inadequate physical activity to prompt counseling or support referral.19, 41–43 In adults, the KP exercise vital sign was a valid proxy measure of physical activity in COPD, for example, patients who reported higher physical activity had less airway obstruction, used less supplemental oxygen and higher quality of life compared to inactive and less active patients.44
Studies investigating the relationship between physical activity and asthma development have been contradictory.8, 45 The lack of standardized, validated approaches to measure PA can contribute to these mixed results. Objective measures of physical activity using accelerometer have been used in only a few primarily cross-sectional studies.46 Two systematic reviews found that physical activity played a protective role for asthma in children and adults.45, 46 In a birth cohort, Byberg et al found low levels of PA between 3–6 and 6–10 years of age were positively associated with asthma by 10.8 years.18 Vogelberg et al studied 2910 adolescents and found that increasing physical activity was associated with decreased prevalence of wheeze in unadjusted analyses, however, the association was no longer significant after adjusting for smoking.14
In contrast to above studies, other studies have found no relationship between physical activity and asthma outcomes.46 Eijkemans et al studied 1838 children from a birth cohort, measured physical activity, sedentary behavior and screen time by questionnaire and accelerometers (in a subset) at age 4 or 5 years and assessed asthma development between age 6 and 10.16 The authors found that levels of physical activity at early school age, assessed by questionnaire or accelerometer, was not associated with asthma development in childhood. A population based study of 4983 children found no longitudinal associations between physical activity and asthma in either direction.17 However, the frequency of assessments occurred only every 2 years and their definition of current asthma was based on any asthma medication or wheezing related illness in the past 12 months in contrast to our definition of asthma based on at least 2 prescriptions of asthma medications as well as continuous assessments of physical activity and asthma at medical visits.
Exercise is a common trigger for bronchoconstriction in children with asthma, but poor asthma control, air pollution exposure, or fitness could be contributing factors.9 Asthma prevalence is increased in athletes and there have been a few cross-sectional studies reporting increased risk of asthma associated with high levels of physical activity in children and adults.13, 47 While we do not know if the children in this cohort had exercise-induced asthma, which may cause children to limit their levels of physical activity, we restricted our analysis on those with persistent asthma (both a prescription of controller and rescue medications); this restriction slightly strengthened the findings. Another possible explanation relevant to our study population in Southern California is the impact of environmental exposures and air pollution on asthma risk in children who spend a lot of their time active outdoors,33 as exercise can increase air pollution exposure through increased ventilation and lower nasal responses. However, we did try to control for air pollution exposure.
Our findings that the association between physical activity and asthma risk differs by the child’s level of physical activity suggests that there may be a healthy range of physical activity levels. Del Giacco and colleagues hypothesized that there is a U-shaped dose-response relationship between physical activity levels and asthma with both inactivity and strenuous exercise associated with higher asthma risk.48 Studies in animals and humans suggest that aerobic exercise may have an anti-inflammatory effect through a reduction in pro-inflammatory cytokine production, decreased airway smooth muscle hypertrophy and hyperplasia, and enhanced regulatory T cell responses. Additionally, improving cardiorespiratory fitness can improve asthma symptoms, control and quality of life in asthmatics.8, 49 In elite athletes, asthma risk is increased and may be secondary to increased exposure to environmental agents through increased ventilation, ongoing damage and reduced repair to the respiratory epithelium, and increase airways inflammation.48 Alternatively, the likelihood that athletes have increased numbers of exercise bouts, each of which can lead to exercise induced bronchoconstriction (EIB), suggests that the general anti-inflammatory effect of physical fitness is mitigated by the pro-inflammatory response of each acute bout of exercise. Exercise-induced laryngeal dysfunction is also common in highly active children and can mimic EIB.50
One of the major strengths of our study include medical record data from a large, community-based population with asthma outcomes based on a diagnosis by a health care providers as well as prescription of asthma-specific medications. Previous studies have relied on parental report of asthma diagnosis, which may be over-reported and not supported by a physician diagnosis. BMI was based on measured weight and height instead of self-report. The study population is generally reflective of Southern California and includes a high proportion of children born to low income families.21 The cohort study design reduced the chance of possible bias inherent in case-control and hospital-based studies. The longitudinal design over 8 years of follow-up where exercise vital sign was collected at most clinic visits is based on a large, multi-ethnic pediatric population which allowed us to investigate the effect of physical activity and other covariates such as BMI over time instead of relying on baseline PA and BMI. The large sample size enabled evaluation of the physical activity and BMI with higher precision than previous studies and allowed us to examine interactions.
Limitations include the possibility of residual confounding inherent to the observational design, including the possibility of differential distribution of unmeasured or incompletely measured confounders. To address concerns of potential confounding, we considered a wide range of possible confounders including environmental exposure. Another limitation is the use of exercise vital sign based on parental report of physical activity and not an objective measure of physical activity such as accelerometers. Moreover, data on reliability and validity of an exercise vital sign in children is almost completely lacking.50 Quantifying physical activity in younger children is challenging, however sensitivity analyses excluding children 2–4 years of age were unchanged. Because physical activity was not assessed at every visit and imputed, we also performed sensitivity analyses without imputing physical activity with comparable results. Last, our ability to identify exercise-induced bronchoconstriction was limited, but we attempted to restrict the analyses to those with persistent asthma.
Conclusions.
Asthma risk differed by levels of reported levels of moderate to vigorous physical activity., Children reporting relatively low levels of physical activity had lower asthma risk as activity levels increased in contrast to children reporting higher levels of activity who manifested greater asthma risk as activity levels increased. Highly active children may have increased exercise-induced bronchoconstriction or air pollution exposure. Asthma risk also increased with BMI. Understanding the role of physical activity and BMI, both modifiable factors, as well as the role of routine screening for exercise as a vital sign in the development of asthma in children will help to develop targeted interventions and guide asthma management.
Supplementary Material
Highlights:
What is already known on this topic? In general, physical activity is important for healthy growth and development for children but is a common trigger for asthma symptoms.
What does this article add to our knowledge? This study shows that different levels of physical activity are associated with asthma risk in children and adolescents.
How does this study impact current management guidelines? These findings emphasize the need to assess physical activity levels in children when considering asthma risk.
Acknowledgments
This work was supported by Kaiser Permanente Direct Community Benefit Funds and the National Center for Advancing Translational Sciences (grant KL2 TR0001416 and UO1 TR002004).
Statement of Financial Disclosure
The authors report no specific funding in relation to this research and no conflicts of interest to disclose. Dr. Lu was an employee of the University of California, Irvine during the concept, data collection and development of this publication. Dr. Lu is currently an employee of Novartis Pharma.
Funding Sources:
The present study was supported by Kaiser Permanente Direct Community Benefit Funds, NCATS KL2 TR0001416, and NCATS U01 TR002004
Abbreviations
- BMI
body mass index
- CI
confidence intervals
- HR
Cox proportional hazards
- IDR
incidence density rate
- KPSC
Kaiser Permanente Southern California
- MVPA
moderate to vigorous physical activity
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: The authors report no conflicts of interest to disclose. Dr. Lu was an employee of the University of California, Irvine during the concept, data collection and development of this publication. Dr. Lu is currently an employee of Novartis Pharma.
References
- 1.Centers of Disease Control and Prevention (CDC). Asthma - Most Recent Asthma Data - Current Asthma Prevalence 2016. . https://www.cdc.gov/asthma/most_recent_data.htm 2016.
- 2.Skinner AC, Ravanbakht SN, Skelton JA, Perrin EM, Armstrong SC. Prevalence of Obesity and Severe Obesity in US Children, 1999–2016. Pediatrics 2018; 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of Obesity Among Adults and Youth: United States, 2015–2016. NCHS Data Brief 2017:1–8. [PubMed] [Google Scholar]
- 4.Lu KD, Forno E, Radom-Aizik S, Cooper DM. Low fitness and increased sedentary time are associated with worse asthma-The National Youth Fitness Survey. Pediatr Pulmonol 2020; 55:1116–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ford ES. The epidemiology of obesity and asthma. J Allergy Clin Immunol 2005; 115:897–909; quiz 10. [DOI] [PubMed] [Google Scholar]
- 6.Lucas SR, Platts-Mills TA. Physical activity and exercise in asthma: relevance to etiology and treatment. J Allergy Clin Immunol 2005; 115:928–34. [DOI] [PubMed] [Google Scholar]
- 7.Lochte L, Nielsen KG, Petersen PE, Platts-Mills TA. Childhood asthma and physical activity: a systematic review with meta-analysis and Graphic Appraisal Tool for Epidemiology assessment. BMC Pediatr 2016; 16:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lang JE. The impact of exercise on asthma. Curr Opin Allergy Clin Immunol 2019; 19:118–25. [DOI] [PubMed] [Google Scholar]
- 9.Oravisjarvi K, Pietikainen M, Ruuskanen J, Rautio A, Voutilainen A, Keiski RL. Effects of physical activity on the deposition of traffic-related particles into the human lungs in silico. Sci Total Environ 2011; 409:4511–8. [DOI] [PubMed] [Google Scholar]
- 10.Lovinsky-Desir S, Jung KH, Rundle AG, Hoepner LA, Bautista JB, Perera FP, et al. Physical activity, black carbon exposure and airway inflammation in an urban adolescent cohort. Environ Res 2016; 151:756–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vlaski E, Stavric K, Seckova L, Kimovska M, Isjanovska R. Influence of physical activity and television-watching time on asthma and allergic rhinitis among young adolescents: preventive or aggravating? Allergol Immunopathol (Madr) 2008; 36:247–53. [DOI] [PubMed] [Google Scholar]
- 12.Lu KD, Billimek J, Bar-Yoseph R, Radom-Aizik S, Cooper DM, Anton-Culver H. Sex Differences in the Relationship between Fitness and Obesity on Risk for Asthma in Adolescents. J Pediatr 2016; 176:36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitchell EA, Beasley R, Bjorksten B, Crane J, Garcia-Marcos L, Keil U, et al. The association between BMI, vigorous physical activity and television viewing and the risk of symptoms of asthma, rhinoconjunctivitis and eczema in children and adolescents: ISAAC Phase Three. Clin Exp Allergy 2013; 43:73–84. [DOI] [PubMed] [Google Scholar]
- 14.Vogelberg C, Hirsch T, Radon K, Dressel H, Windstetter D, Weinmayr G, et al. Leisure time activity and new onset of wheezing during adolescence. Eur Respir J 2007; 30:672–6. [DOI] [PubMed] [Google Scholar]
- 15.Visness CM, London SJ, Daniels JL, Kaufman JS, Yeatts KB, Siega-Riz AM, et al. Association of childhood obesity with atopic and nonatopic asthma: results from the National Health and Nutrition Examination Survey 1999–2006. J Asthma 2010; 47:822–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eijkemans M, Mommers M, Remmers T, Draaisma JMT, Prins MH, Thijs C. Physical activity and asthma development in childhood: Prospective birth cohort study. Pediatr Pulmonol 2020; 55:76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cassim R, Milanzi E, Koplin JJ, Dharmage SC, Russell MA. Physical activity and asthma: cause or consequence? A bidirectional longitudinal analysis. J Epidemiol Community Health 2018; 72:770–5. [DOI] [PubMed] [Google Scholar]
- 18.Byberg KK, Eide GE, Forman MR, Juliusson PB, Oymar K. Body mass index and physical activity in early childhood are associated with atopic sensitization, atopic dermatitis and asthma in later childhood. Clin Transl Allergy 2016; 6:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Golightly YM, Allen KD, Ambrose KR, Stiller JL, Evenson KR, Voisin C, et al. Physical Activity as a Vital Sign: A Systematic Review. Prev Chronic Dis 2017; 14:E123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koebnick C, Coleman KJ, Black MH, Smith N, Der-Sarkissian JK, Jacobsen SJ, et al. Cohort profile: the KPSC Children’s Health Study, a population-based study of 920 000 children and adolescents in southern California. Int J Epidemiol 2012; 41:627–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koebnick C, Langer-Gould AM, Gould MK, Chao CR, Iyer RL, Smith N, et al. Sociodemographic characteristics of members of a large, integrated health care system: comparison with US Census Bureau data. Perm J 2012; 16:37–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feinstein JA, Russell S, DeWitt PE, Feudtner C, Dai D, Bennett TD. R Package for Pediatric Complex Chronic Condition Classification. JAMA Pediatr 2018; 172:596–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr 2014; 14:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith N, Coleman KJ, Lawrence JM, Quinn VP, Getahun D, Reynolds K, et al. Body weight and height data in electronic medical records of children. Int J Pediatr Obes 2010; 5:237–42. [DOI] [PubMed] [Google Scholar]
- 25.Coleman KJ, Ngor E, Reynolds K, Quinn VP, Koebnick C, Young DR, et al. Initial validation of an exercise “vital sign” in electronic medical records. Med Sci Sports Exerc 2012; 44:2071–6. [DOI] [PubMed] [Google Scholar]
- 26.Kann L, McManus T, Harris WA, Shanklin SL, Flint KH, Queen B, et al. Youth Risk Behavior Surveillance - United States, 2017. MMWR Surveill Summ 2018; 67:1–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mucci LA, Wood PA, Cohen B, Clements KM, Brawarsky P, Brooks DR. Validity of self-reported health plan information in a population-based health survey. J Public Health Manag Pract 2006; 12:570–7. [DOI] [PubMed] [Google Scholar]
- 28.Evenson KR, McGinn AP. Test-retest reliability of adult surveillance measures for physical activity and inactivity. Am J Prev Med 2005; 28:470–8. [DOI] [PubMed] [Google Scholar]
- 29.Brown WJ, Trost SG, Bauman A, Mummery K, Owen N. Test-retest reliability of four physical activity measures used in population surveys. J Sci Med Sport 2004; 7:205–15. [DOI] [PubMed] [Google Scholar]
- 30.Koebnick C, Mohan YD, Li X, Young DR. Secular Trends of Overweight and Obesity in Young Southern Californians 2008–2013. J Pediatr 2015; 167:1264–71 e2. [DOI] [PubMed] [Google Scholar]
- 31.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat 11 2002; 11:1–190. [PubMed] [Google Scholar]
- 32.Schatz M, Zeiger RS, Yang SJ, Chen W, Crawford WW, Sajjan SG, et al. Persistent asthma defined using HEDIS versus survey criteria. Am J Manag Care 2010; 16:e281–8. [PubMed] [Google Scholar]
- 33.Delfino RJ, Wu J, Tjoa T, Gullesserian SK, Nickerson B, Gillen DL. Asthma morbidity and ambient air pollution: effect modification by residential traffic-related air pollution. Epidemiology 2014; 25:48–57. [DOI] [PubMed] [Google Scholar]
- 34.Thiebaut AC, Benichou J. Choice of time-scale in Cox’s model analysis of epidemiologic cohort data: a simulation study. Stat Med 2004; 23:3803–20. [DOI] [PubMed] [Google Scholar]
- 35.Korn EL, Graubard BI, Midthune D. Time-to-event analysis of longitudinal follow-up of a survey: choice of the time-scale. Am J Epidemiol 1997; 145:72–80. [DOI] [PubMed] [Google Scholar]
- 36.coxme: Mixed Effects Cox Models. R package version 2.2–5. 2015.] Available from https://cran.r-project.org/web/packages/coxme/index.html.
- 37.Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model. New York, NY: Springer New York; 2000. [Google Scholar]
- 38.Zhang Z, Reinikainen J, Adeleke KA, Pieterse ME, Groothuis-Oudshoorn CGM. Time-varying covariates and coefficients in Cox regression models. Ann Transl Med 2018; 6:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Z Missing data imputation: focusing on single imputation. Ann Transl Med 2016; 4:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lobelo F, Muth ND, Hanson S, Nemeth BA, Council On Sports M, Fitness, et al. Physical Activity Assessment and Counseling in Pediatric Clinical Settings. Pediatrics 2020; 145. [DOI] [PubMed] [Google Scholar]
- 41.Kuntz JL, Young DR, Saelens BE, Frank LD, Meenan RT, Dickerson JF, et al. Validity of the Exercise Vital Sign Tool to Assess Physical Activity. Am J Prev Med 2021; 60:866–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Quiles NN, McCullough AK, Piao L. Validity and Reliability of the Exercise Vital Sign Questionnaire in an Ethnically Diverse Group: A Pilot Study. J Prim Care Community Health 2019; 10:2150132719844062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bowen PG, Mankowski RT, Harper SA, Buford TW. Exercise is Medicine as a Vital Sign: Challenges and Opportunities. Transl J Am Coll Sports Med 2019; 4:1–7. [PMC free article] [PubMed] [Google Scholar]
- 44.Liu I, Moy M, Estrada E, Rippberger E, Nguyen HQ. An “Exercise Vital Sign” Is a Valid Proxy Measure of Physical Activity in COPD in Routine Clinical Care. Translat J Am Coll Sports Medicine 2017; 2:148. [Google Scholar]
- 45.Cassim R, Dharmage SC, Koplin JJ, Milanzi E, Paro FM, Russell MA. Does physical activity strengthen lungs and protect against asthma in childhood? A systematic review. Pediatr Allergy Immunol 2019; 30:739–51. [DOI] [PubMed] [Google Scholar]
- 46.Eijkemans M, Mommers M, Draaisma JM, Thijs C, Prins MH. Physical activity and asthma: a systematic review and meta-analysis. PLoS One 2012; 7:e50775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jerning C, Martinander E, Bjerg A, Ekerljung L, Franklin KA, Jarvholm B, et al. Asthma and physical activity--a population based study results from the Swedish GA(2)LEN survey. Respir Med 2013; 107:1651–8. [DOI] [PubMed] [Google Scholar]
- 48.Del Giacco SR, Firinu D, Bjermer L, Carlsen KH. Exercise and asthma: an overview. Eur Clin Respir J 2015; 2:27984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lu KD, Cooper DM, Haddad F, Radom-Aizik S. Four Months of a School-Based Exercise Program Improved Aerobic Fitness and Clinical Outcomes in a Low-SES Population of Normal Weight and Overweight/Obese Children With Asthma. Front Pediatr 2018; 6:380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nelson VR, Masocol RV, Asif IM. Associations Between the Physical Activity Vital Sign and Cardiometabolic Risk Factors in High-Risk Youth and Adolescents. Sports Health 2020; 12:23–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


