Abstract
Cystic fibrosis (CF) is a life-threatening, inherited, multi-organ disease that renders patients susceptible throughout their lives to chronic and ultimately deteriorating protracted pulmonary infections. Those infections are dominated in adulthood by the opportunistic pathogen, Pseudomonas aeruginosa (Pa). As with other advancing respiratory illnesses, people with CF (pwCF) also frequently suffer from gastroesophageal reflux disease (GERD), including bile aspiration into the lung. GERD is a major co-morbidity factor in pwCF, with a reported prevalence of 35–81% in affected individuals. Bile is associated with the early acquisition of Pa in CF patients and in vitro studies show that it causes Pa to adopt a chronic lifestyle. We hypothesized that Pa is chemoattracted to bile in the lung environment. To evaluate, we developed a novel chemotaxis experimental system mimicking the lung environment using CF-derived bronchial epithelial (CFBE) cells which allowed for the evaluation of Pa (strain PAO1) chemotaxis in a physiological scenario superior to the standard in vitro systems. We performed qualitative and quantitative chemotaxis tests using this new experimental system, and microcapillary assays to demonstrate that bovine bile is a chemoattractant for Pa and is positively correlated with bile concentration. These results further buttress the hypothesis that bile likely contributes to the colonization and pathogenesis of Pa in the lung, particularly in pwCF.
Keywords: chemotaxis, bile, Pseudomonas aeruginosa, cystic fibrosis, bronchial epithelial cells
1. Introduction
Bacterial chemotaxis is the directional navigation of an organism toward or away from certain chemical gradients, which may enhance their access to favorable substances or avoid hostile niches, respectively [1,2,3]. Chemotaxis signaling pathways are broadly distributed among bacterial pathogens and the phenotype is linked to their pathogenicity, playing an essential role in initial localization, subsequent colonization, and infection of the host [4]. For decades, the vast majority of bacterial chemotaxis studies have been performed using in vitro capillary assay methods, initially developed by Adler (1966) [1] for the quantification of Escherichia coli chemotaxis, or with some further modifications for other bacterial pathogens [5]. To better mimic the in situ bacterial chemoattractions during pathogenesis, we have developed a model that builds on the principles of Adler’s classic assay system. Our new chemotaxis experimental system utilizes a human cystic fibrosis (CF) bronchial epithelial (CFBE) cell environment that mimics CF airway epithelia [6,7,8]. Here, we present the application of this new assay centering on a scenario where Pseudomonas aeruginosa (Pa) encounters bile.
CF is a life-threatening, inherited, multi-organ disease, characterized by a universal thickening of a person’s mucosal layers, derived from mutations in the CF transmembrane conductance regulator (CFTR) gene [9,10]. Throughout their lives, people with CF (pwCF) are susceptible to chronic and ultimately deteriorating, persistent, polymicrobial lung infections, which account for over 90% of the morbidity and mortality associated with the disease [11]. Pa is arguably the most important bacterial pathogen among pwCF [12,13]. Once a pwCF is colonized with Pa, it is tough to eradicate, leading to adaptation and chronic infections in the majority of older children and adults [12,14]. Insights into how Pa finds its initial niches during early infection in pwCF could drive new clinical strategies to control or defeat this opportunistic pathogen.
One of the drivers of CF pulmonary infections is hypothesized to be aspirated bile, a consequence of gastroesophageal reflux disease (GERD) [15,16]. First described in 1975 [17], GERD is a major co-morbidity factor in pwCF, ranging between 35–81% with elevated prevalence since childhood (there is a lacking specific guideline for GERD in CF, bringing the underestimation into context without considering the asymptomatic/silent GERD cases) [15,16]. GERD-derived bile aspiration is associated with the early airway tract acquisition of Pa in CF patients [18]. Indeed, despite the inherent bactericidal nature of bile [19,20], Pa can tolerate it and even replicate in bile [21,22]. Additionally, bile stimulates Pa virulence, facilitates colonization, and further enhances infection through stimulating biofilm formation, Type VI Secretion, efflux pump expression, quorum sensing, and antibiotic tolerance, all of which are linked to the switch from an acute lifestyle to a persistent phase of infection [20,21,23,24,25]. To date, there is still a lack of knowledge on any chemotaxis behavior of Pa as a major CF pathogen toward GERD-derived bile, since most studies on bile chemotaxis have been limited to enteropathogenic bacteria, including Vibrio cholerae, Campylobacter jejuni, and Salmonella spp. [4,19,23,26]. We hypothesized that Pa would be attracted to bile and thus we used our new chemotaxis experimental system, which consists of human CFBE cells that mimic CF airway epithelia [6,7], as well as microcapillary assays to test the hypothesis.
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
Biological replicates of P. aeruginosa PAO1 [27] were cultured in a minimal salts medium (MSB) supplemented with 27.5 mM of glucose, 0.5% (w/v) casamino acids (CAS) (obtained from Amresco at the highest purity commercially available), and 3% (w/v) bovine bile and incubated overnight at 37 °C. Secondary cultures were inoculated for 2 h at 37 °C in MSB with 3% bovine bile (the chemoattractant). Bile supplementation of overnight and secondary cultures was applied to provide Pa with time for metabolic adaptations. These growth conditions were used in all the experiments.
2.2. CFBE-Cell Preparation
CFBE cells (CFBE41o-), originally collected from a CF patient, are homozygous for the ΔF508-CFTR mutation, [6]. The CFBE cell line used in this study was a gift from Dr. J. P. Clancy, [28] as described previously [29]. The cells were maintained in Eagle’s minimal essential medium (MEM), supplemented with fetal bovine serum (FBS; 10% v/v), L-glutamine (2 mM), penicillin (50 U/mL), plasmocin (5 mg/mL), puromycin (2 mg/mL), and streptomycin (50 mg/mL), and placed in a 5% CO2/95% air incubator at 37 °C [7,29]. CFBE cells were grown at a seeding density of 2 × 106 in glass-bottom Petri dishes (MatTek Corporation) for 7–9 days to establish confluent monolayers [29]. The MEM cell growth medium was switched to a modified one, known as “imaging medium” [29], on the day the experiment was carried out.
2.3. Chemotaxis Assays
2.3.1. Chemoattractant Preparation
The chemoattractant (bovine bile) was prepared in chemotaxis buffer (CB; sodium phosphate buffer (50 mM; pH 7.0), disodium ethylenediaminetetraacetic acid (EDTA) (10 µM), and glycerol (0.05% (w/v)) [30]. To obtain physiology relevant concentrations [24,31], bile was diluted in CB in a series of 10-fold dilutions from 3% to 0.0003% (w/v). All studies were performed using bovine bile since human and bovine biles have similar compositions, but with different proportions of bile acids [32,33]. All bile solutions were mixed with 2% (w/v) low-melting-temperature-agarose (NuSieve GTG, Lonza) and were drawn into a 1 μL microcapillary tube in preparation for chemotaxis experiments.
2.3.2. Qualitative Capillary Assay
The assay was performed as previously described by Parales and Harwood (2002) [34]. Briefly, a mid-exponential PAO1 pellet was harvested, washed once with CB and resuspended in aerated CB to a final OD600 = 0.1, and then located in a chamber made by a coverslip and a glass U-tube with prior verification of bacterial cells’ motility by microscopy. Microcapillaries, filled with each tested concentration of bile in a low-melting-temperature gel in CB, were inserted into the pool of bacteria. CAS and CB were included as positive or negative controls, respectively. The chemotactic response at the mouth of the capillaries was visualized at time points 0 and 5 min using dark-field microscopy at 4x magnification (an Olympus IX73 inverted microscope plus an Olympus TH4-100 halogen illuminator) and recorded using an Olympus DP73 CCD camera with the Olympus cellSens standard version 1.8 software. The dark-field illumination was generated by a Ph2 ring in the long-working distance condenser NA 0.55 using a UPlanFL N 4x NA 0.13 objective. Photographs were processed for contrast and brightness and centered using Adobe Photoshop Lightroom.
2.3.3. Quantitative Capillary Assay
Quantitative capillary assays were performed as described previously [1,5]. Briefly, the capillary tubes were 1 μL disposable micropipettes sealed on one end using flame and filled with the chemoattractant bile resuspended in CB. P. aeruginosa PAO1 cultures were prepared as described above. PAO1 cells were harvested in the mid-exponential phase of growth (OD660 = 0.4–0.6) by centrifugation at 4600 revolutions per min (rpm) for 5 min, washed once with CB, and then bacterial cells were resuspended in CB to an OD660 of 0.1. PAO1 was pipetted into each U-tube, and 1 µL microcapillary tubes containing different bile concentrations (0.0003–3% w/v) were added to each U-tube. The assay was run for 30 min at room temperature, after which the microcapillaries were removed and their contents were serially diluted and plated to quantify colony forming units (CFU)s in capillaries. In all experiments, CB was included as a negative control and used for the normalization of the results.
2.3.4. CFBE Cell-Bacteria Chemotaxis Assay
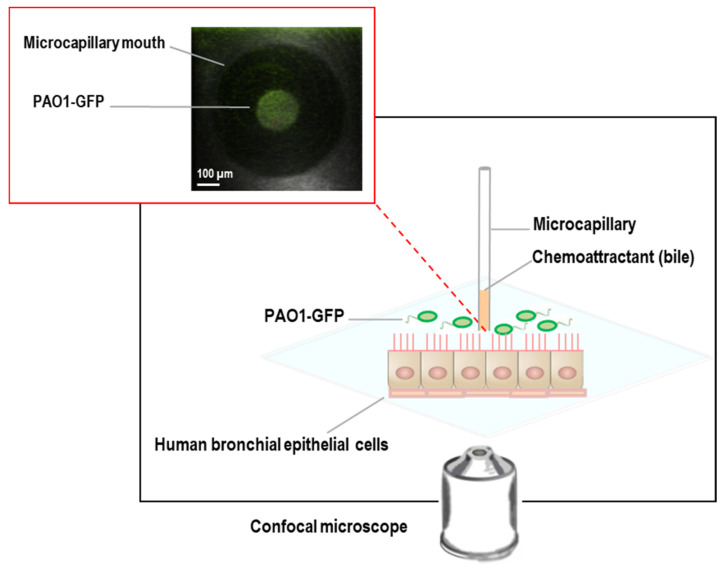
In our experimental system (Figure 1), a monolayer culture of CFBE cells is grown on the bottom of a glass Petri dish above which sits a microcapillary tube containing bovine bile as the potential chemoattractant with physiologically relevant concentrations between 0.03% and 3% (w/v). Green fluorescent protein (GFP)-labeled P. aeruginosa PAO1 (PAO1-GFP) [35] cells were prepared, as described previously in Section 2.3.3, were introduced, and their movement was tracked over 10 min using confocal imaging. The capillary containing the bile component in a gel of 2% low-melting-temperature agarose was lowered into a suspension of motile PAO1-GFP and CFBE cells in a glass-bottom Petri dish closed with a lid in which a hole allowed the introduction of the capillary tubes, just to put the mouth in contact with the bacteria. GFP-expressing PAO1 motility was confirmed prior to the introduction using 0.3% (w/v) soft agar plates. The health and viability of CFBE cells in the presence of bile were confirmed using the LIVE/DEAD kit (Invitrogen; data not shown). In addition, to investigate the impact of any damage to the CFBE cells, we exposed the cells to −80 °C for 30 min and then 37 °C for 15 min as described in Text S1. The results confirmed that damage to the CFBE cells did not influence the migration of Pa, noting there was no difference between intact cells as well as damaged cells (Supplementary Text S1 and Figure S1).
Figure 1.
Human bronchial epithelial cell-bacteria chemotaxis system. The inset illustrates chemoattraction of green fluorescent protein (GFP)-labeled P. aeruginosa PAO1 (PAO1-GFP) cells towards bile.
2.3.5. Replicates
Data are presented as the mean ± SE of at least five independent experiments, with three technical replicates each.
2.4. Microscopy
Confocal microscopy imaging was conducted on a Nikon TE2000 Live-Scan Swept Field Confocal microscope equipped with a QuantEM:512SCEMCCD camera (Photometrics, Tucson, AZ, USA), located in the Live Cell Imaging Core at the Dartmouth Lung Biology Center. In qualitative assays, confocal microscopy images were taken by the objective 10x during a time course between 0 and 10 min (time intervals of 2 min) in a system where a microcapillary containing the bile component in a gel of 2% low-melting-temperature agarose was lowered into a suspension of motile, PAO1-GFP and CFBE cells, as described before.
3. Results and Discussion
3.1. P. aeruginosa PAO1 Is Attracted toward Bovine Bile and Correlates with Bile Concentration
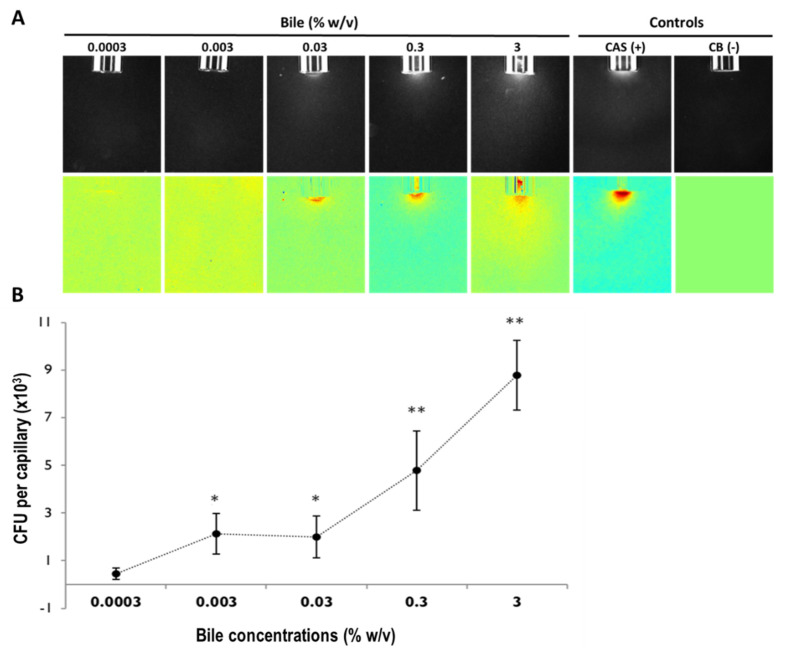
First, we evaluated and confirmed Pa chemotaxis toward bile using the traditional qualitative (Figure 2A) and quantitative (Figure 2B, Supplementary Figure S2) microcapillary chemotaxis assays using a range of physiologically relevant bovine bile concentrations (0.0003–3% w/v) [24,31]. Casamino acids (CAS) and the chemotaxis buffer (CB) were used as the positive and negative controls, respectively. Figure 2 illustrates the PAO1 chemoattraction response toward bovine bile in a concentration-related manner.
Figure 2.
Chemotaxis of P. aeruginosa PAO1 toward bovine bile in the absence of cystic fibrosis -derived bronchial epithelial (CFBE) cells using qualitative (A) and quantitative (B) microcapillary assays. (A) PAO1 chemoattraction towards bile (0.0003–3% w/v). Top panel: Dark-field images of bacterial cells gathered at the mouth of microcapillaries containing attractants at time 5 min. Below panel: Color map “jet” (MATLAB R2013b version 8.2; www.Mathworks.com, accessed on 5 September 2013) representing a normalization with the time 0 min for each treatment. The positive control (casamino acids, (0.2%, w/v) (CAS)) and the negative control (chemotaxis buffer (CB)) are shown for contrast. (B) Quantitative assay illustrating the concentration-response curve of P. aeruginosa PAO1 to bovine bile (0.0003–0.3% w/v) diluted in chemotaxis buffer. Results are averaged of at least 18 capillaries from 7 independent experiments. The results have been normalized with CB. (Results with no normalization have been illustrated in Supplementary Figure S2). Error bars indicate standard error. A comparison between the five concentrations of bile tested and the negative control is indicated by the ANOVA test (* 0.01 < p ≤ 0.05; ** p ≤ 0.01).
3.2. PAO1 Is Attracted to Bovine Bile in the Novel CFBE Experimental System
The CFBE cell line was originally isolated from a pwCF (generated by Dr. Gruenert, UCSF) and subsequently was immortalized [6]. This cell line model recapitulates several aspects of CF lung disease, including the capability to form electrically-tight cell layers with functional cell-to-cell contacts, and has been used for gathering information about CF at the cellular level [7,36]. It was also used to elucidate mechanisms associated with Pa colonization, biofilm formation, and antimicrobial-agent efficacy evaluation [29,37].
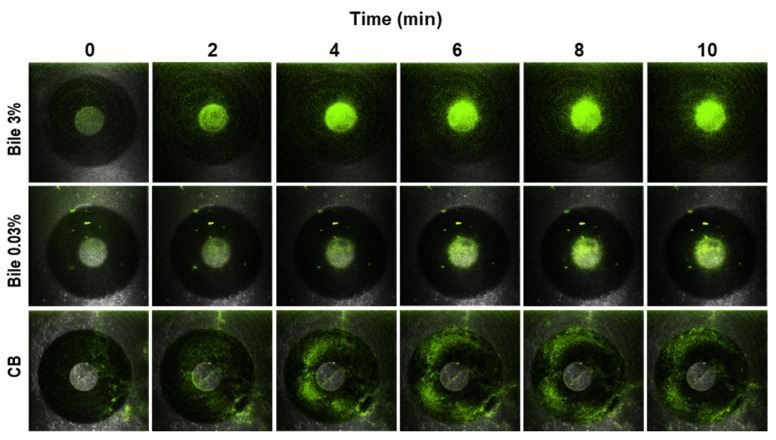
First, to investigate whether the expression of GFP affected the chemotaxis response of PAO1, we tested the chemoattraction response of both the wild-type strain (no GFP) and GFP-labeled PAO1 toward 3% (w/v) bile in quantitative microcapillary assays as described before. Both strains exhibited the same chemoattraction, which was approximately fifty times higher in the presence of bile than the CB control (data not shown). We then evaluated the chemotaxis response of PAO1-GFP in the new chemotaxis assay. In a time-lapse experiment, PAO1-GFP can be seen (Figure 3) concentrating around the microcapillary mouth containing bovine bile, in a concentration- and time-dependent manner. By contrast, PAO1-GFP did not aggregate around the capillary mouth.
Figure 3.
Chemotaxis of green fluorescent protein (GFP)-labeled P. aeruginosa PAO1 toward bovine bile using cystic fibrosis-derived bronchial epithelial (CFBE) cells. Confocal microscopy images (10×) of the chemoattraction of GFP-labeled PAO1 toward 0.03% and 3% (w/v) bovine bile on CFBE cells at times 0–10 min (two top panels). Chemotaxis buffer (CB) with no response is shown for contrast (below panel). PAO1 is attracted to bovine bile in an intact respiratory CFBE cell model, as illustrated in Figure 1.
4. Conclusions
This experimental system demonstrates a new use for the CFBE cell culture system. Here, we show how Pa colonization in the CF lung may be preceded or enhanced by chemotactic responses to bile. We suggest that this model can facilitate studying Pa, and other motile CF opportunistic bacterial pathogens (e.g., Burkholderia cepacia complex and Stenotrophomonas maltophilia), chemotaxis, initial binding, as well as colonization at the same time [23,24,25,29,37]. In addition, this model could be useful for studies with eukaryotic cells, such as neutrophils, in the context of CF lung. Using this CF-derived model, which is more biologically relevant than the traditional standard assay systems, provides a closer insight into the chemoattraction of Pa toward bile in a biological condition that models the CF lung environment. This is a promising start and additional adjustments to this novel approach can be developed to closely represent the CF lung niche.
Here, our studies reveal that bile could be implicated in the accumulation of critical masses of Pa in the lung. Subsequent studies to assess the Pa chemoreceptor(s) involved in the PAO1 response, particularly toward individual constituents of bile [20,38], as well as an assessment of whether other Pa strains also exhibit chemoattraction, might cast additional light on Pa pathogenesis in the CF lung.
Acknowledgments
P. aeruginosa PAO1 came from our lab collection, while the PAO1-GFP strain was kindly provided by George O’Toole’s lab at Dartmouth College. We are grateful to Valentina Sedlacek and Rachel Dokko for their technical assistance. S.H. was supported by the Women in Science Program at Dartmouth College.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/microorganisms10040716/s1, Text S1, Damaged cystic fibrosis-derived bronchial epithelial (CFBE41o-, here CFBE) cells do not influence the chemotaxis of P. aeruginosa PAO1 toward bovine bile, Figure S1: Quantitative chemotaxis assay of P. aeruginosa PAO1 toward bile (3% w/v) in intact and damaged human cystic fibrosis bronchial epithelial (CFBE) cells, Figure S2: Quantitative assay illustrating non-normalized concentration-response of P. aeruginosa PAO1 to bovine bile (0.0003–0.3% w/v) diluted in chemotaxis buffer (CB). References [1,30] are cited in the Supplementary Materials.
Author Contributions
Conceptualization, I.S., B.A.S. and J.E.H.; methodology, I.S., S.H. and Q.Y.; data analysis, I.S. and J.E.H.; writing—original draft preparation, S.B.; writing—review and editing, S.B., J.E.H., I.S. and B.D.; funding acquisition, J.E.H. and B.A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the US Cystic Fibrosis Foundation, HILL17P0 for J.E.H., and the NIH and CF Foundation, STANTO19R0. R01 HL151385-01A for B.A.S.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are presented in the paper as well as the supporting information. Original data files will be available upon request from the authors.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Adler J. Chemotaxis in bacteria. Science. 1966;153:708–716. doi: 10.1126/science.153.3737.708. [DOI] [PubMed] [Google Scholar]
- 2.Webre D.J., Wolanin P.M., Stock J.B. Bacterial chemotaxis. Cur. Biol. 2003;13:R47–R49. doi: 10.1016/S0960-9822(02)01424-0. [DOI] [PubMed] [Google Scholar]
- 3.Wadhams G.H., Armitage J.P. Making sense of it all: Bacterial chemotaxis. Nat. Rev. Mol. Cell. Biol. 2004;5:1024–1237. doi: 10.1038/nrm1524. [DOI] [PubMed] [Google Scholar]
- 4.Matilla M.A., Krell T. The effect of bacterial chemotaxis on host infection and pathogenicity. FEMS Microbiol. Rev. 2018;42:fux052. doi: 10.1093/femsre/fux052. [DOI] [PubMed] [Google Scholar]
- 5.Sampedro I., Parales R.E., Krell T., Hill J.E. Pseudomonas chemotaxis. FEMS Microbiol. Rev. 2015;39:17–46. doi: 10.1111/1574-6976.12081. [DOI] [PubMed] [Google Scholar]
- 6.Goncz K.K., Kunzelmann K., Xu Z., Gruenert D.C. Targeted replacement of normal and mutant CFTR sequences in human airway epithelial cells using DNA fragments. Hum. Mol. Genet. 1998;7:1913–1919. doi: 10.1093/hmg/7.12.1913. [DOI] [PubMed] [Google Scholar]
- 7.Ehrhardt C., Collnot E.M., Baldes C., Becker U., Laue M., Kim K.J., Lehr C.M. Towards an in vitro model of cystic fibrosis small airway epithelium: Characterisation of the human bronchial epithelial cell line CFBE41o- Cell Tissue Res. 2006;323:405–415. doi: 10.1007/s00441-005-0062-7. [DOI] [PubMed] [Google Scholar]
- 8.Gottschalk L.B., Vecchio-Pagan B., Sharma N., Han S.T., Franca A., Wohler E.S., Batista D.A., Goff L.A., Cutting G.R. Creation and characterization of an airway epithelial cell line for stable expression of CFTR variants. J. Cyst. Fibros. 2016;15:285–294. doi: 10.1016/j.jcf.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ratjen F., Bell S.C., Rowe S.M., Goss C.H., Quittner A.L., Bush A. Cystic fibrosis. Nat. Rev. Dis. Primers. 2015;1:15010. doi: 10.1038/nrdp.2015.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shteinberg M., Haq I.J., Polineni D., Davies J.C. Cystic fibrosis. Lancet. 2021;397:2195–2211. doi: 10.1016/S0140-6736(20)32542-3. [DOI] [PubMed] [Google Scholar]
- 11.Goetz D., Ren C.L. Review of Cystic Fibrosis. Pediatr. Ann. 2019;48:e154–e161. doi: 10.3928/19382359-20190327-01. [DOI] [PubMed] [Google Scholar]
- 12.Rossi E., La Rosa R., Bartell J.A., Marvig R.L., Haagensen J.A.J., Sommer L.M., Molin S., Johansen H.K. Pseudomonas aeruginosa adaptation and evolution in patients with cystic fibrosis. Nat. Rev. Microbiol. 2021;19:331–342. doi: 10.1038/s41579-020-00477-5. [DOI] [PubMed] [Google Scholar]
- 13.Moradali M.F., Ghods S., Rehm B.H. Pseudomonas aeruginosa lifestyle: A paradigm for adaptation, survival, and persistence. Front. Cell Infect. Microbiol. 2017;7:39. doi: 10.3389/fcimb.2017.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faure E., Kwong K., Nguyen D. Pseudomonas aeruginosa in Chronic Lung Infections: How to Adapt within the Host? Front. Immunol. 2018;9:2416. doi: 10.3389/fimmu.2018.02416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bongiovanni A., Manti S., Parisi G.F., Papale M., Mulè E., Rotolo N., Leonardi S. Focus on gastroesophageal reflux disease in patients with cystic fibrosis. World J. Gastroenterol. 2020;26:6322–6334. doi: 10.3748/wjg.v26.i41.6322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robinson N.B., DiMango E. Prevalence of gastroesophageal reflux in cystic fibrosis and implications for lung disease. Ann. Am. Thorac. Soc. 2014;11:964–968. doi: 10.1513/AnnalsATS.201401-044FR. [DOI] [PubMed] [Google Scholar]
- 17.Feigelson J., Sauvegrain J. Refux gastro-oesophagien dans la mucoviscidose [Letter: Gastro-esophageal reflux in mucoviscidosis] Nouv Presse Med. 1975;4:2729–2730. (In French) [PubMed] [Google Scholar]
- 18.Reen F.J., Woods D.F., Mooij M.J., Chróinín M.N., Mullane D., Zhou L., Quille J., Fitzpatrick D., Glennon J.D., McGlacken G.P., et al. Aspirated bile: A major host trigger modulating respiratory pathogen colonisation in cystic fibrosis patients. Eur. J. Clin. Microbiol. Infect. Dis. 2014;33:1763–1771. doi: 10.1007/s10096-014-2133-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hay A.J., Zhu J. In Sickness and in Health: The Relationships between Bacteria and Bile in the Human Gut. Adv. Appl. Microbiol. 2016;96:43–64. doi: 10.1016/bs.aambs.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 20.Begley M., Gahan C.G., Hill C. The interaction between bacteria and bile. FEMS Microbiol. Rev. 2005;29:625–651. doi: 10.1016/j.femsre.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 21.Gipson K.S., Nickerson K.P., Drenkard E., Llanos-Chea A., Dogiparthi S.K., Lanter B.B., Hibbler R.M., Yonker L.M., Hurley B.P., Faherty C.S. The Great ESKAPE: Exploring the Crossroads of Bile and Antibiotic Resistance in Bacterial Pathogens. Infect. Immun. 2020;88:e00865-19. doi: 10.1128/IAI.00865-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bachta K.E.R., Allen J.P., Cheung B.H., Chiu C.H., Hauser A.R. Systemic infection facilitates transmission of Pseudomonas aeruginosa in mice. Nat. Commun. 2020;11:543. doi: 10.1038/s41467-020-14363-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sistrunk J.R., Nickerson K.P., Chanin R.B., Rasko D.A., Faherty C.S. Survival of the Fittest: How Bacterial Pathogens Utilize Bile to Enhance Infection. Clin. Microbiol. Rev. 2016;29:819–836. doi: 10.1128/CMR.00031-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reen F.J., Flynn S., Woods D., Dunphy N., Chróinín M.N., Mullane D., Stick S., Adams C., O’Gara F. Bile signalling promotes chronic respiratory infections and antibiotic tolerance. Sci. Rep. 2016;6:29768. doi: 10.1038/srep29768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flynn S., Reen F.J., O’Gara F. Exposure to Bile Leads to the Emergence of Adaptive Signaling Variants in the Opportunistic Pathogen Pseudomonas aeruginosa. Front. Microbiol. 2019;10:2013. doi: 10.3389/fmicb.2019.02013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hugdahl M.B., Beery J.T., Doyle M.P. Chemotactic behavior of Campylobacter jejuni. Infect. Immun. 1988;56:1560–1566. doi: 10.1128/iai.56.6.1560-1566.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holloway B.W. Genetics of Pseudomonas. Bacteriol. Rev. 1969;33:419–443. doi: 10.1128/br.33.3.419-443.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hentchel-Franks K., Lozano D., Eubanks-Tarn V., Cobb B., Fan L., Oster R., Sorscher E., Clancy J.P. Activation of airway cl- secretion in human subjects by adenosine. Am. J. Respir. Cell Mol. Biol. 2004;31:140–146. doi: 10.1165/rcmb.2004-0012OC. [DOI] [PubMed] [Google Scholar]
- 29.Yu Q., Griffin E.F., Moreau-Marquis S., Schwartzman J.D., Stanton B.A., O’Toole G.A. In vitro evaluation of tobramycin and aztreonam versus Pseudomonas aeruginosa biofilms on cystic fibrosis-derived human airway epithelial cells. J. Antimicrob. Chemother. 2012;67:2673–2681. doi: 10.1093/jac/dks296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sampedro I., Kato J., Hill J.E. Elastin degradation product isodesmosine is a chemoattractant for Pseudomonas aeruginosa. Microbiology. 2015;161:1496–1503. doi: 10.1099/mic.0.000090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reen F.J., Woods D.F., Mooij M.J., Adams C., O’Gara F. Respiratory pathogens adopt a chronic lifestyle in response to bile. PLoS ONE. 2012;7:e45978. doi: 10.1371/journal.pone.0045978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tagliacozzi D., Mozzi A.F., Casetta B., Bertucci P., Bernardini S., Di Ilio C., Urbani A., Federici G. Quantitative analysis of bile acids in human plasma by liquid chromatography-electrospray tandem mass spectrometry: A simple and rapid one-step method. Clin. Chem. Lab. Med. 2003;41:1633–1641. doi: 10.1515/CCLM.2003.247. [DOI] [PubMed] [Google Scholar]
- 33.Washizu T., Tomoda I., Kaneko J.J. Serum bile acid composition of the dog, cow, horse and human. J. Vet. Med. Sci. 1991;53:81–86. doi: 10.1292/jvms.53.81. [DOI] [PubMed] [Google Scholar]
- 34.Parales R.E., Harwood C.S. Bacterial chemotaxis to pollutants and plant-derived aromatic molecules. Curr. Opin. Microbiol. 2002;5:266–273. doi: 10.1016/S1369-5274(02)00320-X. [DOI] [PubMed] [Google Scholar]
- 35.Bloemberg G.V., O’Toole G.A., Lugtenberg B.J., Kolter R. Green fluorescent protein as a marker for Pseudomonas spp. Appl. Environ. Microbiol. 1997;63:4543–4551. doi: 10.1128/aem.63.11.4543-4551.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olivares E., Badel-Berchoux S., Provot C., Jaulhac B., Prévost G., Bernardi T., Jehl F. Tobramycin and Amikacin Delay Adhesion and Microcolony Formation in Pseudomonas aeruginosa Cystic Fibrosis Isolates. Front. Microbiol. 2017;8:1289. doi: 10.3389/fmicb.2017.01289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwarzer C., Fischer H., Machen T.E. Chemotaxis and Binding of Pseudomonas aeruginosa to Scratch-Wounded Human Cystic Fibrosis Airway Epithelial Cells. PLoS ONE. 2016;11:e0150109. doi: 10.1371/journal.pone.0150109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Farina A., Dumonceau J.M., Lescuyer P. Proteomic analysis of human bile and potential applications for cancer diagnosis. Expert. Rev. Proteom. 2009;6:285–301. doi: 10.1586/epr.09.12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are presented in the paper as well as the supporting information. Original data files will be available upon request from the authors.