Abstract
Gemifloxacin mesylate (SB-265805-S, LB-20304a) is a potent, novel fluoroquinolone agent with a broad spectrum of antibacterial activity. The pharmacokinetics and tolerability of oral gemifloxacin were characterized in two parallel group studies in healthy male volunteers after doses of 160, 320, 480, and 640 mg once daily for 7 days. Multiple serum or plasma and urine samples were collected on days 1 and 7 and were analyzed for gemifloxacin by high-performance liquid chromatography (HPLC)–fluorescence (study 1) or HPLC-mass spectrometry (study 2). Safety assessments included vital signs, 12-lead electrocardiogram (ECG) readings, hematology, clinical chemistry, urinalysis, and adverse experience monitoring. Gemifloxacin was rapidly absorbed, with a time to maximum concentration of approximately 1 h after dosing followed by a biexponential decline in concentration. Generally, maximum concentration and area under the concentration-time curve (AUC) increased linearly with dose after either single or repeat doses. Mean ± standard deviation values of AUC0–τ on day 7 were 4.92 ± 1.08, 9.06 ± 2.20, 12.2 ± 3.69, and 20.1 ± 3.67 μg·h/ml following 160-, 320-, 480-, and 640-mg doses, respectively. The terminal-phase half-life was approximately 7 to 8 h, independent of dose, and was similar following single and repeated administrations. There was minimal accumulation of gemifloxacin after multiple dosing. Approximately 20 to 30% of the administered dose was excreted unchanged in the urine. The renal clearance was 160 ml/min on average after single and multiple doses, which was slightly greater than the accepted glomerular filtration rate (approximately 120 ml/min). These data show that the pharmacokinetics of gemifloxacin are linear and independent of dose. Gemifloxacin was generally well tolerated, although one subject was withdrawn from the study after 6 days at 640 mg for mild, transient elevations of alanine aminotransferase and aspartate aminotransferase not associated with any clinical signs or symptoms. There were no other significant changes in clinical chemistry, hematology or urinalysis parameters, vital signs, or ECG readings. In conclusion, the results of these studies, combined with the antibacterial spectrum and potency, support the further investigation of once-daily administration of gemifloxacin for indications such as respiratory tract and urinary tract infections.
Gemifloxacin, (R,S)-7-(3-aminomethyl-4-syn-methoxyimino-1-pyrrolidinyl)-1-cyclopropyl-6-fluoro-1,4-dihydro-4- oxo-1,8-naphthyridine-3-carboxylic acid methanesulfonate (SB-265805; LB-20304), is a new fluoroquinolone antibacterial agent with a broad spectrum of activity (2, 3, 5). It has shown potent antibacterial activity against clinical isolates and reference strains in both in vitro studies and experimental infections in animals (4; V. Berry, R. Page, J. Satterfield, R. Straub, and G. Woodnutt, Abstr. 21st Int. Congress Chemother., p. 146, 1999; V. Berry, R. Page, J. Satterfield, C. Singley, R. Straub, and G. Woodnutt, Abstr. 21st Int. Congress Chemother., p. 148, 1999; M. E. Erwin, R. N. Jones, and the Quality Control Study Group, Letter, J. Clin. Microbiol. 37:279–280, 1999). Activity against gram-positive organisms is particularly enhanced, with gemifloxacin displaying activity fourfold higher than that of moxifloxacin against Streptococcus pneumoniae (MIC at which 90% of isolates are inhibited [MIC90], 0.06 μg/ml) in vitro (4). Gemifloxacin is also highly potent against penicillin-resistant strains of S. pneumoniae (MIC90, 0.03 μg/ml) (D. Hardy, D. Amsterdam, L. Mandell, and C. Rotstein, Abstr. 21st Int. Congress Chemother., p. 146, 1999). In addition, gemifloxacin has high activity against the other major pathogens involved in respiratory tract infections, Haemophilus influenzae and Moraxella catarrhalis (MIC90s, 0.015 and 0.03 μg/ml, respectively), and the atypical organisms Legionella pneumophila (MIC90, 0.008 μg/ml), Chlamydia spp. (MIC range, 0.06 to 0.12 μg/ml), and Mycoplasma spp. (MIC range, 0.001 to 0.1 μg/ml) (D. Felmingham, M. Robbins, C. Dencer, H. Salman, I. Mathias, and G. Ridgway, Abstr. 21st Int. Congress Chemother., p. 131, 1999; P. Hannan and G. Woodnutt, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 101, p. 257, 1998). Gemifloxacin has also been shown to be highly active against many organisms isolated from urinary tract infections (MIC90 for members of the family Enterobacteriaceae, 0.25 μg/ml) (2; K. G. Naber, K. Hollauer, D. Kirchbauer, and W. Witte, Abstr. 9th Eur. Congress Clin. Microbiol. Infect. Dis., p. 144, 1999). Two single-dose pharmacokinetic studies in healthy male subjects showed that gemifloxacin was well-tolerated at doses up to 800 mg (A. Allen, E. Bygate, M. Teillol-Foo, S. Oliver, M. Johnson, and C. Ward, Abstr. 21st Int. Congress Chemother., p. 137, 1999). The long terminal-phase half-life (8 h) in humans suggests the possibility of once-daily dosing. Plasma protein binding of gemifloxacin is low (approximately 60%; SmithKline Beecham, unpublished data.) The present studies were designed to evaluate the pharmacokinetics and tolerability of gemifloxacin following multiple once-daily oral dosing to healthy male subjects in double-blind, placebo-controlled trials.
(Preliminary pharmacokinetics from these studies have been presented previously [A. Allen, E. Bygate, M. Teillol-Foo, S. Oliver, M. Johnson, and C. Ward, Abstr. 21st Int. Congress Chemother., p. 137, 1999].)
MATERIALS AND METHODS
Subjects.
Forty (16 in study 1; 24 in study 2) healthy adult male Caucasian volunteers participated in two studies. The age ranged from 21 to 43 years (means, 31.9 ± 7.1 years and 29.3 ± 6.1 years for studies 1 and 2, respectively), and the average weights were 72.2 ± 8.7 kg and 77.0 ± 9.4 kg for studies 1 and 2, respectively. Before the study all subjects were assessed by a complete medical history and physical examination and a 12-lead resting electrocardiogram (ECG). Blood samples were collected for clinical and hematology studies. Urine was collected for urinalysis and drug screening. Subjects did not use any medication for at least 7 days before dosing. All subjects fully satisfied the inclusion and exclusion criteria. All participants gave written informed consent before any study procedures were performed. The study protocol and consent forms were reviewed and approved by the Besselaar Covance Independent Ethics Committee.
Study design.
Two double-blind, randomized, placebo-controlled, sequential-group, multiple-dose studies were conducted. In study 1, two treatment groups of eight subjects received once-daily multiple oral doses of gemifloxacin or placebo (randomized 3:1). Subjects received oral doses of 160 mg (n = 6) or 320 mg (n = 6) of gemifloxacin or placebo (n = 4) in the fasting state. In study 2, two groups of 12 subjects (randomized 2:1) received once-daily multiple oral doses of 480 mg (n = 8) or 640 mg (n = 8) of gemifloxacin, or placebo (n = 8), in the fasting state. Gemifloxacin or placebo was administered after an overnight fast with 240 ml of water. Subjects were required to avoid lying down, eating, or drinking beverages other than water until 2 or 4 h (studies 1 and 2, respectively) after dosing, after which a standard meal was served. Subjects were excluded from the study if they had used any prescription drug within 14 days, any over-the-counter drug within 7 days, or any investigational drug within 4 months before participation in the study. Also excluded were subjects who were smokers or had smoked in the 6 months prior to dosing and subjects with a known drug or alcohol dependence or drug allergy. Subjects abstained from alcohol- and caffeine-containing foods and beverages until 48 h after each dose and from sunbathing or using sun beds or sun lamps from 7 days prior to the first dose until after the poststudy assessment.
Safety.
All subjects were under close, continuous observation in the study unit throughout each 7-day investigation period. Subjects were asked a nonleading probe question (“How have you been feeling since you were last asked?”) at intervals after dosing in order to elicit reports of adverse events (AEs). In addition, all spontaneously reported AEs were recorded throughout the study. The likelihood that the events were due to gemifloxacin was assessed by a physician who was blinded to treatment, according to a five-point scale in study 1 (none, improbable, possible, probable, definite) and according to a similar four-point scale in study 2 (unrelated, unlikely, suspected, probable). In addition to AE monitoring, safety assessments throughout the study day included vital signs, hematology, clinical chemistry, and urinalysis, including N-acetylglucosamine and β2-microglobulin measurement. Urine was filtered through warmed filters at 37°C immediately after voiding, and the residue was examined microscopically for the presence of urinary drug crystals. The filtrate and unfiltered urine were also examined immediately and 1 to 2 h after voiding. Twelve-lead ECG recordings were made at 1 h postdose (the approximate time of maximal concentration in plasma) on days 1 and 7 for all subjects and at 12 h postdose in study 2, as well as predose and at the end of the dosing period.
Sampling.
Blood samples (10 ml) were taken by venipuncture of the antecubital veins (or via an indwelling catheter) predose and at 0.25, 0.50, 0.75, 1, 1.5, 2, 3, 4, 6, 8, 12, 18, and 24 h on days 1 and 7 (plus 36 and 48 h after dosing on day 7 for study 1 only). Blood samples were also collected prior to dosing on days 3, 4, 5, and 6. For study 1, the blood samples were allowed to clot at room temperature and in the dark for at least 30 min, and for study 2, blood samples were collected in tubes containing the anticoagulant EDTA. Blood samples were centrifuged for 15 min at 2,000 × g and 4°C, and separated serum or plasma was frozen at approximately −20°C. The urine samples were collected prior to dosing (−12 to 0 h) and over the intervals 0 to 6, 6 to 12, and 12 to 24 h on days 1 and 7 (plus 24 to 48 h postdose on day 7 for study 1 only). A 10-ml aliquot of each sample was transferred into polystyrene tubes and immediately frozen at approximately −20°C.
HPLC-fluorescence assay (study 1).
The concentrations of gemifloxacin in serum and urine from study 1 were determined by a reversed-phase high-performance liquid chromatography (HPLC) method with fluorescence detection (SmithKline Beecham, unpublished data). Following liquid/liquid extraction into chloroform and back extraction into phosphate buffer, chromatographic separation was carried out using a PLRP-S column (Polymer Laboratories, Church Stretton, Shropshire, United Kingdom) and a trifluoroacetic acid-acetonitrile mobile phase. Gemifloxacin and an internal standard (a structural analogue of gemifloxacin) were detected by fluorescence with excitation and emission wavelengths of 337 and 460 nm, respectively. The calibration curve of the serum method was linear over a range of concentrations in serum of 0.01 to 5 μg/ml (correlation coefficient, 0.99085). Intra- and interassay coefficients of variation over the range 0.02 to 5 μg/ml were less than 3% and 12%, respectively. At 0.01 μg/ml, the limit of quantitation, intra- and interassay coefficients of variation were less than 3% and 20%, respectively. The calibration curve of the urine method was linear over a range of concentrations in urine of 1 to 100 μg/ml (correlation coefficient, 0.98132), and the intra- and interassay coefficients of variation over this range were less than 11% and 14%, respectively. The lower limit of quantification for gemifloxacin was 0.01 μg/ml in serum and 1.0 μg/ml in urine using a 1-ml aliquot.
HPLC-MS/MS assay (study 2).
The concentrations of gemifloxacin in plasma and urine from study 2 were determined by a reversed-phase HPLC method with mass spectroscopy (MS) detection (SmithKline Beecham, unpublished data). Protein precipitation, using acetonitrile containing the internal standard (a structural analogue of gemifloxacin), was used to extract gemifloxacin from plasma, and an aliquot of the supernatant was injected onto the HPLC equipment. Urine was diluted with mobile phase containing the internal standard prior to injection onto the HPLC equipment. Chromatographic separation was carried out using a PLRP-S column and an ammonium acetate-acetonitrile mobile phase. Gemifloxacin and an internal standard (a structural analogue of gemifloxacin) were detected by positive-ion MS/MS employing a Turbo IonSpray interface. The calibration curve of the plasma method was linear over a range of concentrations in plasma of 0.01 to 5 μg/ml (correlation coefficient, 0.9990), and the intra- and interassay coefficients of variation over this range were less than 6%. The calibration curve of the urine method was linear over a range of concentrations in urine of 0.01 to 5 μg/ml (correlation coefficient, 0.9992), and the intra- and interassay coefficients of variation over this range were less than 9%. The lower limit of quantification for gemifloxacin was 0.01 μg/ml in plasma and urine using a 0.05-ml aliquot.
Pharmacokinetic analysis.
Serum and plasma gemifloxacin concentration-time data were analyzed by noncompartmental methods using an in-house program written and validated for SAS version 6.12. The apparent terminal elimination rate constant (λz) was derived from the log linear disposition phase of the concentration-time curve using linear least-squares regression with visual inspection of the data to determine the appropriate number of terminal points to calculate λz. The corresponding terminal-phase elimination half-life was calculated as ln(2)/λz. Area under the serum or plasma concentration-time curve from time zero to the last quantifiable concentration in serum or plasma (AUC0–t) was determined using the linear trapezoidal rule for each incremental trapezoid and the log trapezoidal rule for each trapezoid after the first occurrence of maximal concentration in plasma (Cmax). The area under the serum or plasma concentration-time curve extrapolated to infinity (AUC0–∞) was calculated as the sum of the AUC0–t and C(t)/λz, where C(t) is the predicted concentration from the log linear regression analysis at the last quantifiable time point and λz is the elimination rate constant. Cmax and the time to Cmax (Tmax) were obtained directly from the serum and plasma concentration-time data. Renal clearance was calculated as the ratio of the amount of gemifloxacin excreted in the urine divided by AUC. The extent of accumulation was calculated as the ratio of AUC0–τ(day 7) to AUC0–τ(day 1). The predicted accumulation ratio was calculated as the ratio of AUC0–∞(day 1) to AUC0–τ(day 1). The linearity ratio was calculated as the ratio of AUC0–τ(day 7) to AUC0–∞(day 1).
RESULTS
Pharmacokinetics of gemifloxacin.
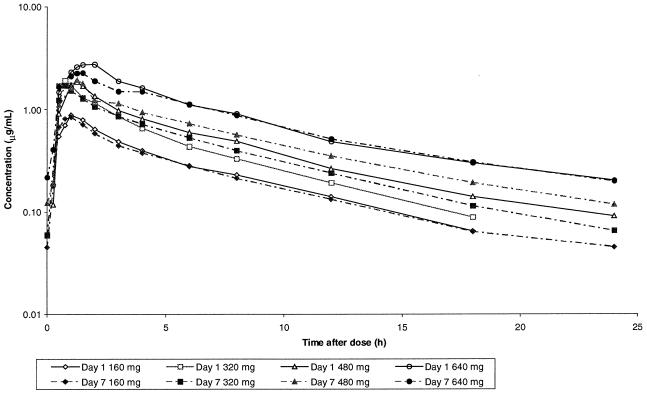
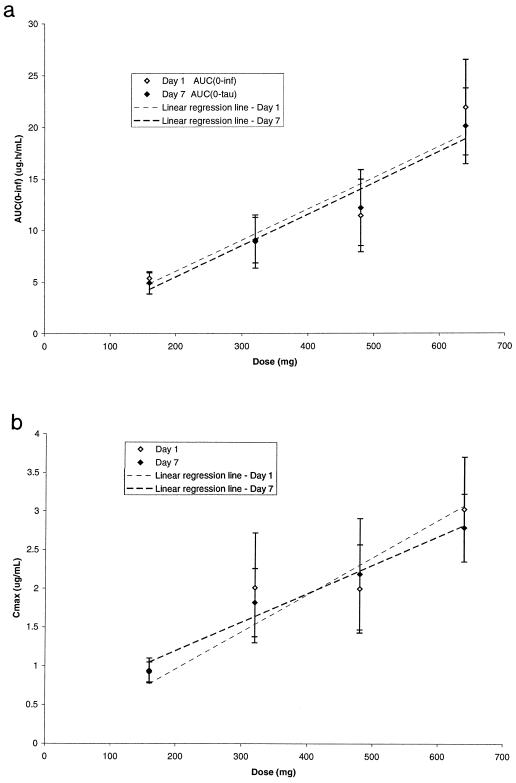
Gemifloxacin was rapidly absorbed, with Tmax being approximately 1 h after dosing followed by an apparent biexponential decline in concentration based on visual inspection of the log-concentration-time plots (Fig. 1). Mean trough concentrations in serum of subjects receiving 160 mg were 0.046, 0.049, 0.051, 0.050, and 0.045 μg/ml and in those receiving 320 mg were 0.070, 0.062, 0.071, 0.068 and 0.060 μg/ml on days 3, 4, 5, 6, and 7, respectively. Mean trough concentrations in plasma of subjects receiving 480 mg were 0.115, 0.117, and 0.122 μg/ml and in those receiving 640 mg were 0.230, 0.234, and 0.218 μg/ml on days 5, 6, and 7, respectively. Visual inspection of the trough gemifloxacin concentrations indicated that steady state had been attained by at most the fourth dose. The pharmacokinetic parameters for gemifloxacin in healthy male subjects following single and repeated oral doses are given in Table 1. Generally, Cmax and AUC increased linearly with dose after either single or repeat doses (Table 1; Fig. 2). Mean ± standard deviation values of AUC0–τ on day 7 were 4.92 ± 1.08, 9.06 ± 2.20, 12.2 ± 3.69, and 20.1 ± 3.67 μg·h/ml following 160-, 320-, 480-, and 640-mg doses, respectively. There was minimal accumulation of gemifloxacin after multiple dosing. The mean observed accumulation ratios were 0.99, 1.10, 1.20, and 0.99 for doses of 160, 320, 480, and 640 mg, respectively. These values were generally consistent with the minimal accumulation predicted from the single-dose data at 160, 320, 480, and 640 mg of 1.07, 1.05, 1.10, and 1.14, respectively.
FIG. 1.
Mean concentrations of gemifloxacin in serum and plasma following single and repeat oral administration in healthy male volunteers.
TABLE 1.
Pharmacokinetic parameters of gemifloxacin following single oral administration and once-daily oral administration for 7 days to healthy male subjectsa
| Gemifloxacin dose | Cmax (μg/ml) | Tmax (h) | AUC0–τ (μg · h/ml) | AUC0–∞ (μg · h/ml) | t1/2 (h) | Cmax (μg/ml) | Ae (%) | CLR (ml/min) |
|---|---|---|---|---|---|---|---|---|
| 160 mg (n = 6) | ||||||||
| Day 1 | 0.92 (0.13) | 1.1 (0.5–1.5) | 5.03 (0.46) | 5.36 (0.53) | 6.09 (0.70) | ND | 24.4 (2.50) | 130 (14.4) |
| Day 7 | 0.94 (0.16) | 0.9 (0.5–1.0) | 4.92 (1.08) | ND | 6.86 (0.51) | 0.045 (0.020) | 21.5 (7.15) | 119 (38.7) |
| 320 mg (n = 6) | ||||||||
| Day 1 | 2.01 (0.71) | 0.8 (0.5–1.0) | 8.49 (2.53) | 8.92 (2.59) | 5.87 (0.56) | ND | 22.2 (5.12) | 146 (37.6) |
| Day 7 | 1.82 (0.44) | 0.8 (0.5–1.5) | 9.06 (2.20) | ND | 6.16 (0.36) | 0.060 (0.015) | 22.0 (6.01) | 134 (37.7) |
| 480 mg (n = 8) | ||||||||
| Day 1 | 2.00 (0.57) | 1.00 (0.75–1.50) | 10.4 (3.11) | 11.4 (3.51) | 7.72 (1.25) | ND | 29.6 (5.21) | 242 (92.8) |
| Day 7 | 2.19 (0.72) | 1.00 (0.75–1.00) | 12.2 (3.69) | ND | 7.65 (0.80) | 0.122 (0.042) | 29.3 (4.21) | 196 (57.9) |
| 640 mg (n = 8) | ||||||||
| Day 1 | 3.03 (0.68) | 1.53 (0.50–2.00) | 19.2 (3.93) | 21.9 (4.61) | 9.29 (1.40) | ND | 30.1 (2.26) | 167 (38.8) |
| Day 7 | 2.79 (0.44) | 1.50 (0.75–1.50) | 20.1 (3.67) | ND | 8.60 (1.52) | 0.218 (0.061) | 27.3 (3.06) | 141 (20.3) |
Values for Tmax are medians (ranges); all others are arithmetic means (standard deviations). t1/2, terminal-phase elimination half life; Cmin, minimum concentration in plasma; Ae, amount excreted unchanged in urine; CLR, renal clearance; ND, not determined.
FIG. 2.
(a) AUC0–∞ versus dose; (b) Cmax versus dose. All values are means ± standard deviations.
The terminal-phase half-life was approximately 7 to 8 h, independent of dose, and was similar following single or repeated administration. The linearity ratios were 0.92, 1.04, 1.07, and 0.87 for doses of 160, 320, 480, and 640 mg, respectively.
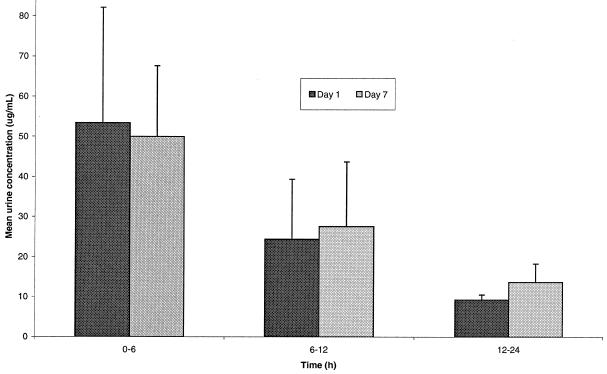
On average, approximately 20 to 30% of the administered gemifloxacin dose was excreted unchanged in the urine over 24 h following both single and repeat dosing, the vast majority (>80% of total) being excreted after the first 12 h (Fig. 3). The renal clearance in these healthy volunteers with normal renal function was, on average, 160 ml/min after single and multiple doses, which was slightly greater than the accepted glomerular filtration rate (approximately 120 ml/min), suggestive of active renal secretion. These data show that the pharmacokinetics of gemifloxacin are linear and independent of dose.
FIG. 3.
Mean urinary concentrations of gemifloxacin in healthy volunteers following single and repeat oral administration of 320 mg.
Tolerability.
All doses of gemifloxacin from 160 to 640 mg were well tolerated. There were no deaths or serious AEs during the studies. Similar numbers of subjects reported a similar number of AEs across all treatment groups; therefore, there was no evidence of a drug- or dose-related increase in the incidence of AEs across both studies. The most common AEs over both studies were headache (17 events), nausea (12 events), and abdominal pain (6 events), with no other AEs reported more than twice in both studies. Of these AEs the following were considered possibly, probably, or definitely related to study medication: six headaches (two events reported while the subject was on placebo), four experiences of nausea, and three experiences of abdominal pain. Other AEs considered possibly related to medication were rash, sweating, eye pain, myalgia, constipation, dizziness, and taste perversion. Most AEs were mild, and all resolved spontaneously. Two cases of rash were reported: one subject in the 640-mg group experienced a widespread, pruritic, mobilliform rash which had its onset on day 8, after discharge from the unit. It was considered consistent with a drug eruption and was treated with antihistamines. A second subject in the 160-mg group had a mild maculopapular rash confined to the forearms, which began on day 3 and resolved spontaneously after 4 days, despite continued dosing with gemifloxacin.
Mild, clinically asymptomatic, reversible increases in alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were seen in three of the eight subjects at the 640-mg dose level. One subject was withdrawn from the study after 6 days at 640 mg, as the increases were greater than twice the upper limit of the reference range (maximum values were 179 IU/liter for ALT and 103 IU/liter for AST on day 7). Two other subjects had rises in AST and ALT above the upper limit of the reference range which reached a peak on days 7 to 8. Maximum values were 51 and 55 IU/liter for AST (reference range, 10 to 41 IU/liter) and 70 and 94 IU/liter for ALT (reference range, 10 to 49 IU/liter). In all three cases, the transaminases returned quickly towards baseline within a few days of cessation of the drug. There were no changes in transaminase levels in the placebo or other gemifloxacin dose groups.
No drug crystals were seen in any of the urine samples from study 1. Brown, amorphous crystals were seen on the filters from 4 of 528 freshly voided urine samples in study 2, and in three of these, there was only a trace on the filter. Evidence of possible in vivo urinary crystal formation was seen in a single urine sample from one subject at the 640-mg dose level. The crystals were seen on the filter on day 1, in the sample with the highest urinary gemifloxacin concentrations for this individual, although no crystals were seen in subsequent urine samples. Crystals were also observed in seven other urine samples but not on the filter (two subjects on placebo, one on 480 mg, and two on 640 mg) and hence were considered to have formed ex vivo as the urine cooled. There were no changes in plasma creatinine or urea and no hematuria or casts in the urine sediment, and N-acetylglucosamine and β2-microglobulin levels were normal in all subjects. There were no other clinically relevant changes in results of laboratory investigations or vital signs at the doses investigated. There were no clinically significant changes in QT interval corrected for heart rate or other ECG parameters.
DISCUSSION
Consistent with the findings in previous single-dose studies, gemifloxacin was rapidly absorbed and AUC and Cmax increased linearly with dose. Mean AUC0-∞ and Cmax values did not deviate notably from linearity over the range of doses studied (160 to 640 mg). It should be noted that this overview summarizes data from two studies, neither of which was of a crossover design, and thus, pharmacokinetic parameters were not strictly comparable between the different dosing groups. Following once-daily oral doses of 160, 320, 480, or 640 mg of gemifloxacin, steady-state concentrations in serum and plasma were attained by, at most, the fourth daily dose. The mean accumulation ratios of 0.99 to 1.20 were consistent with those of a drug with a terminal-phase half-life of, on average, 8 h. The minimal accumulation predicted from single-dose data for once-daily dosing was consistent with the observed mean values. In addition, there appeared to be no change in elimination half-life following repeat dosing, and the linearity ratio was approximately unity, indicating that the pharmacokinetics of gemifloxacin were linear throughout the once-daily multiple dosing regimens. Following once-daily dosing at 320 mg, accumulation has been shown to be minimal while trough concentrations in serum and plasma were still at measurable levels. The relatively high concentrations in serum and plasma observed over 24 h, compared to MICs for the relevant pathogens, and the good activity against gram-positive bacteria (MIC90 for S. pneumoniae, 0.06 μg/ml [4]; MIC90 for penicillin-resistant strains of S. pneumoniae, 0.03 μg/ml [Hardy et al., 21st ICC]; MIC90 for H. influenzae, 0.015 μg/ml; and MIC90 for M. catarrhalis, 0.03 μg/ml [Felmingham et al., 21st ICC; Hannan and Woodnutt, 38th ICAAC]) suggest that gemifloxacin is likely to be useful for the treatment of respiratory infections.
Approximately 20 to 30% of the administered dose was excreted unchanged in the urine. The renal clearance was 160 ml/min on average after single and multiple doses, indicating that once-daily repeat dosing did not affect renal clearance appreciably at any dose. Renal clearance was slightly greater than the accepted glomerular filtration rate (approximately 120 ml/min), suggesting involvement of active secretion in the renal elimination of gemifloxacin. Concentrations in urine (mean range, 10 to 50 μg/ml) of gemifloxacin over 0 to 24 h (Fig. 3) for the 320-mg dose are sufficient to kill most gram-negative bacteria responsible for urinary tract infections (MIC90 for the family Enterobacteriaceae, 0.25 μg/ml) (2; Naber et al., 9th ECCMID). This observation suggests that gemifloxacin is also likely to be useful in the treatment of a wide range of urinary tract infections.
Gemifloxacin was generally well tolerated following oral administration of doses of 160 to 640 mg once daily for 7 days. One subject was withdrawn from the study after 6 days at 640 mg for mild, transient elevations of ALT and AST not associated with any clinical signs or symptoms. These changes in hepatic enzymes with gemifloxacin were not considered to be of clinical concern, and hepatic changes have been reported with other fluoroquinolones (1). Fluoroquinolones as a class are known to produce crystalluria in the alkaline urine of a number of experimental animals (6). As human urine is normally acidic, problems are not routinely expected, but crystalluria has occasionally been observed in humans after the administration of other fluoroquinolones, such as ciprofloxacin (8) or norfloxacin (7). Crystals possibly formed in vivo were observed in the urine of one subject in the highest dose group, and these were assumed to be drug crystals, although they were not analyzed for confirmation. Crystals observed in the urine on standing or in the filtrate but not in the filter were considered to be the result of ex vivo crystal formation as the samples cooled. There were no clinical signs or symptoms of renal damage in any of the subjects. There were no other significant changes in clinical chemistry, hematology or urinalysis parameters, vital signs, or ECG readings.
In conclusion, the results of these studies, combined with the antibacterial spectrum and potency of gemifloxacin, support the further investigation of once-daily administration of gemifloxacin for indications such as respiratory tract and urinary tract infections.
REFERENCES
- 1.Ball P, Tillotson G. Tolerability of fluoroquinolone antibiotics. Drug Safety. 1995;13:343–358. doi: 10.2165/00002018-199513060-00004. [DOI] [PubMed] [Google Scholar]
- 2.Cormican M G, Jones R N. Antimicrobial activity and spectrum of LB 20304, a novel fluoronaphthyridone. Antimicrob Agents Chemother. 1997;41:204–211. doi: 10.1128/aac.41.1.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hohl A F, Frei R, Pünter V, von Graevenitz A, Knapp C, Washington J, Johnson D, Jones R N. International multicenter investigation of LB20304, a new fluoronaphthyridone. Clin Microbiol Infect. 1998;4:280–284. doi: 10.1111/j.1469-0691.1998.tb00057.x. [DOI] [PubMed] [Google Scholar]
- 4.Johnson D M, Jones R N, Erwin M E. Anti-streptococcal activity of SB-265805 (LB20304), a novel fluoronaphthyridone, compared with five other compounds, including quality control guidelines. Diagn Microbiol Infect Dis. 1999;33:87–91. doi: 10.1016/s0732-8893(98)00104-7. [DOI] [PubMed] [Google Scholar]
- 5.Oh J-I, Paek K-S, Ahn M-J, Kim M-Y, Hong C Y, Kim I-C, Kwak J-H. In vitro and in vivo evaluations of LB20304, a new fluoronaphthyridone. Antimicrob Agents Chemother. 1996;40:1564–1568. doi: 10.1128/aac.40.6.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schluter G. Ciprofloxacin: review of potential toxicologic effects. Am J Med. 1987;82(Suppl. 4A):91–93. [Google Scholar]
- 7.Swanson B N, Boppana V K, Vlasses P H, et al. Norfloxacin disposition after sequentially increasing oral doses. Antimicrob Agents Chemother. 1983;23:284–288. doi: 10.1128/aac.23.2.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thorsteinsson S B, Bergan T, Oddsdottir S, et al. Crystalluria and ciprofloxacin, influence of urinary pH and hydration. Chemotherapy. 1986;32:408–417. doi: 10.1159/000238444. [DOI] [PubMed] [Google Scholar]