Abstract
Palmitic acid (PA) is a saturated fatty acid (SFA) that can cause an inflammatory response, while docosahexaenoic acid (DHA) is always used as a nutritional modulator due to its anti-inflammatory properties. However, the potential molecular mechanism is still not completely elucidated in fish. Herein, the PA treatment induced an inflammatory response in macrophages of large yellow croaker (Larimichthys crocea). Meanwhile, the mRNA expression of Toll-like receptor (TLR)-related genes, especially tlr22, and the phosphorylation of the mitogen-activated protein kinase (MAPK) pathway were significantly upregulated by PA. Further investigation found that the PA-induced inflammatory response was suppressed by tlr22 knockdown and MAPK inhibitors. Moreover, the results of the peroxisome proliferator-activated receptor γ (PPARγ) agonist and inhibitor treatment proved that PPARγ was involved in the PA-induced inflammation. PA treatment decreased the protein expression of PPARγ, while tlr22 knockdown and MAPK inhibitors recovered the decreased expression. Besides, the PA-induced activation of Nrf2 was regulated by p38 MAPK. Furthermore, DHA-executed anti-inflammatory effects by regulating the phosphorylation of the MAPK pathway and expressions of PPARγ and Nrf2. Overall, the present study revealed that DHA alleviated PA-induced inflammation in macrophages via the TLR22-MAPK-PPARγ/Nrf2 pathway. These results could advance the understanding of the molecular mechanism of the SFA-induced inflammatory response and provide nutritional mitigative strategies.
Keywords: palmitic acid, inflammatory response, Toll-like receptor, docosahexaenoic acid, Larimichthys crocea
1. Introduction
Palmitic acid (PA), one of the most common saturated fatty acids (SFAs), can be transported in cells and converted into phospholipids, diacylglycerol and ceramides [1,2,3]. PA has been reported to induce an inflammatory response of macrophages in mammals, which triggers the activation of various signaling pathways and induces the production of cytokines [4]. Palm oil (PO), enriched with PA, is increasingly used as an alternative to fish oil in aquaculture [5]. Although partially replacing fish oil with PO in diets has no significant effects on the growth performance of cultured fish, the overuse of PO often induces an inflammatory response and suppresses the antioxidant capacity [6,7]. Moreover, PA has been proven to induce an inflammatory response in fish [8]. However, the molecular mechanism of the inflammatory response induced by PA is still not completely understood.
As a family of pattern recognition receptors (PRRs), Toll-like receptors (TLRs) play an essential role in initiating and regulating innate immunity both in mammals and fish [9,10]. The activation of TLRs recruits its adaptor proteins, such as the myeloid differentiation factor 88 (MyD88) and TIR domain containing adaptor-inducing interferon-β (TRIF). Then, downstream signaling cascades are triggered and activated, which could induce the expression of inflammatory genes [11,12]. Mitogen-activated protein kinase (MAPK) and nuclear transcription factor kappa-B (NF-κβ) are the widely studied downstream pathways of TLR. In mammals, PA could act as a strong agonist of TLR2 and TLR4 and activate downstream inflammatory signaling pathways [13,14]. However, the composition of the TLR family in teleost is different from that in mammals, which has some members considered to be fish-specific TLRs. Among all the fish-specific TLRs, TLR22 is widely explored as the typical member in many fish species [15,16,17,18]. Previous studies on large yellow croaker (Larimichthys crocea) have revealed that TLR22 could respond to fatty acids [19,20]. However, the role and downstream regulation mechanism of TLR22 in the PA-induced inflammatory response remain unclear in fish.
N-3 polyunsaturated fatty acids (PUFAs), such as docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), can inhibit inflammation by regulating the activity of inflammatory signaling pathways and influencing the production of lipid mediators [21,22]. PUFAs could inhibit SFA-induced cyclooxygenase-2 expression by mediating a common signaling pathway derived from TLR [23]. Moreover, PUFA supplementation in diets results in a lower incidence of metabolic disease [24]. Previous studies on large yellow croaker have revealed that DHA supplementation in diets significantly improved fish health, and the anti-inflammatory effect of DHA was much stronger than EPA [25,26]. However, the anti-inflammatory molecular mechanism of DHA in fish needs further investigation.
Large yellow croaker is an important mariculture fish in China. In large yellow croaker feed, PO is widely used as a promising alternative to fish oil. However, it is still limited in the mechanism of inflammatory response induced by PA. Nutritional regulation of the immune system provides a potentially powerful strategy for improving fish health and quality. Studies on nutritional regulation in large yellow croaker have been intensively explored [27,28]. Large yellow croaker can be used as a good model animal in nutrition research. Therefore, this study aimed to investigate the molecular mechanism of PA-induced inflammation and its mitigative strategy mediated by DHA in macrophages of large yellow croaker. These results could advance the understanding of the molecular mechanism of the inflammatory response induced by SFAs and provide nutritional strategies against inflammation.
2. Materials and Methods
2.1. Macrophages Culture and Treatment
Large yellow croaker (weight 500 ± 50.26 g) was purchased from a commercial fish farm in Ningbo, China. All experimental procedures performed on fish were in strict accordance with the Management Rule of Laboratory Animals (Chinese Order No. 676 of the State Council, revised 1 March 2017). Macrophages were isolated from fish head kidneys and maintained according to the previous procedure with some modifications [29]. The primary macrophage was cultured in DMEM/F12 medium supplemented with 10% fetal bovine serum (FBS; Gibco, Carlsbad, CA, USA), 100-U/mL penicillin and 100-mg/mL streptomycin at 28 °C. Before stimulation, macrophages were seeded in six-well plates (Corning, Corning, NY, USA) at 2.0 × 106 cells per well. PA (Sigma-Aldrich, St. Louis, MO, USA) was combined with 1% fatty acid-free bovine serum albumin (BSA; Equitech-Bio, Kerrville, TX, USA) to reach a final concentration at 1 mm. Before the fatty acids treatment, cells were starved with DMEM/F12 alone for 1 h. Macrophages were incubated with different concentration of PA at 250 or 500 µM for 12 h. Cells treated with 1% fatty acid-free BSA were considered as the control group.
To confirm the role of the MAPK pathway in PA-induced inflammation, SB203580 (p38 inhibitor; MCE, Jersey City, NJ, USA) and SP600125 (JNK inhibitor; MCE) were used at a final concentration of 10 μm for 2 h before PA treatment. Furthermore, to confirm the role of PPARγ in PA-induced inflammation, troglitazone (PPARγ agonist; MCE) and GW9662 (PPARγ inhibitor; MCE) were incubated at a final concentration of 10 μm for 12 h before PA treatment. The control group was incubated with the same concentration of dimethyl sulfoxide (DMSO; Solarbio, Beijing, China).
To investigate the effect of DHA (Sigma-Aldrich, St. Louis, MO, USA) on PA-induced inflammation, macrophages were incubated with 200-μm DHA for 12 h before PA stimulation. After culturing, the macrophages were harvested for further analysis.
2.2. RNA Isolation and Quantitative Real-Time PCR (RT-qPCR)
Total RNA was extracted from macrophages by RNAiso Plus (Takara, Tokyo, Japan) according to the manufacturer’s instructions. The 1.5% denaturing agarose gel was used to measure the integrity of the RNA. The concentration and quality of extracted total RNA were confirmed by a NanoDrop®2000 spectrophotometer (Thermo Scientific, Waltham, MA, USA). Then, complementary DNA (cDNA) was reverse-transcribed from the extracted RNA with the PrimeScript™ RT reagent kit (Takara, Tokyo, Japan).
The RT-qPCR primers used in this study are shown in Table 1 according to the nucleotide sequences of target genes in large yellow croaker. β-actin was used as the housekeeping gene. RT-qPCR was performed on a CFX96 Touch real-time PCR detection system (Bio-Rad, Hercules, CA, USA) using the SYBR Premix Ex Taq kit (Takara, Tokyo, Japan). The amplification was performed in a total volume of 20 μL, containing 10 μL of SYBR qPCR Master Mix, 6 μL of DEPC water, 2 μL of cDNA and 1 μL of F/R primer. The PCR temperature profile was performed at 95 °C for 2 min and, afterward, 39 cycles of 95 °C for 10 s, 58 °C for 15 s and 72 °C for 10 s. The gene expression levels were calculated via the 2−ΔΔCT method [30].
Table 1.
Primers used for RT-qPCR and the gene accession number.
| Gene | Forward (5′-3′) | Reverse (5′-3′) | Accession Number |
|---|---|---|---|
| β-actin | GACCTGACAGACTACCTCATG | AGTTGAAGGTGGTCTCGTGGA | GU584189 |
| tnf α | ACACCTCTCAGCCACAGGAT | CCGTGTCCCACTCCATAGTT | NM_001303385 |
| il1β | CATAGGGATGGGGACAACGA | AGGGGACGGACACAAGGGTA | XM_010736551 |
| il6 | CGACACACCCACTATTTACAAC | TCCCATTTTCTGAACTGCCTC | XM_010734753 |
| cox2 | CTGGAAAGGCAACACAAGC | CGGTGAGAGTCAGGGACAT | XM_010734489 |
| arg1 | AACCACCCGCAGGATTACG | AAACTCACTGGCATCACCTCA | XM_19269015 |
| il10 | AGTCGGTTACTTTCTGTGGTG | TGTATGACGCAATATGGTCTG | XM_010738826 |
| cd68 | GCAGGGCTTCAATCTGACCAA | AGGATGAGCACCAGCAATGTC | NM_001319937 |
| cd86 | TGTGCGTCTTAGTCTACCTTCT | AAACTCTTCCGTCATCTTGC | XM_010756962 |
| cd209 | GATGGGTGTATTTCAGCGGTAG | TGTTGATAATCACCAGGTCTGC | XM_027278935 |
| tlr1 | TGTGCCACCGTTTGGATA | TTCAGGGCGAACTTGTCG | KF318376 |
| tlr2 | TCTGCTGGTGTCAGAGGTCA | GGTGAATCCGCCATAGGA | XM_027287556 |
| tlr3 | ACTTAGCCCGTTTGTGGAAG | CCAGGCTTAGTTCACGGAGG | XM_019274877 |
| tlr7 | ATGCAATGAGCCAAAGTCT | CATGTGAGTCAATCCCTCC | XM_010743042 |
| tlr13 | CCTCCTGTTTATGGTAGTGTCC | GCTCGTCATGGGTGTTGTAG | XM_010743101 |
| tlr21 | CTTTGCCTACATCACAGGGACT | GAAACACGAGCAGGAGAACATC | KY025428 |
| tlr22 | TATGCGAGCAGGAAGACC | CAGAAACACCAGGATCAGC | GU324977 |
| myd88 | TACGAAGCGACCAATAACCC | ATCAATCAAAGGCCGAAGAT | EU978950 |
| trif | TACAATACTGTTATCCCTCTGCTGC | TCTCTTCTGTTTTCTAATCCTCGCG | MK863372 |
| pparγ | TGTCCGAGCTGGAAGACAAC | TGGGGTCATAGGGCATACCA | XM_010731330 |
| nrf2 | GATGGAAATGGAGGTGATGC | CATGTTCTTTCTGTCGGTGG | XM_010737768 |
| sitlr22 | GCAAGUUUGGUGGUGCUUUTT | AAAGCACCACCAAACUUGCTT | GU324977 |
2.3. Flow Cytometry Analysis
Macrophages treated with PA and BSA (the control group) were harvested and incubated with CD68 or CD209 antibodies at 37 °C for 1 h. CD68 and CD209 antibodies were produced by immunizing rabbits with synthetic recombinant proteins according to sequences from large yellow croaker, and the specificity was verified in previous studies [31]. Alexa Flour 488 goat anti-rabbit IgG (Beyotime Biotechnology, Shanghai, China) was incubated for 45 min at 37 °C to combine with primary antibodies. The percentage of positive cells was detected, and the data was analyzed by a flow cytometer (BD AccuriTM C6, Franklin Lakes, NJ, USA). Gated represent macrophages (R1) were selected. M1 and M2 represented CD68+ and CD209+ populations compared with the control group, respectively.
2.4. Western Blotting
RIPA reagent (Solarbio, Beijing, China) with a supplementation of protease and phosphatase inhibitors (Thermo Fisher Scientific, USA) was used to obtain the proteins of macrophages. Protein concentrations were measured by the BCA Protein Assay Kit (Beyotime Biotechnology, Shanghai, China). All protein concentrations were adjusted to the same level before heating. Western blot experiments were performed as follows: 20 μg of proteins were loaded on 10% SDS-PAGE and then transferred to polyvinylidene difluoride (PVDF) membranes (Merck Millipore, Berlin, Germany). The PVDF membranes were incubated with 5% skim milk for 2 h at room temperature and then incubated with the targeting antibody overnight at 4 °C. The primary antibodies against ERK1/2 (Cat. No. 4695), phospho-ERK1/2 (Cat. No. 4370), JNK1/2 (Cat. No. 9252), phospho-JNK1/2 (Cat. No. 4668), p38 (Cat. No. 8690), phospho-p38 (Cat. No. 9215), IKKβ (Cat. No. 2678), phospho-IKKα/β (Cat. No. 2697) and PPARγ (Cat. No. 2443) were obtained from Cell Signaling Technology (Boston, MA, USA). Anti-GAPDH antibody (Cat. No. TA-08; Golden Bridge Biotechnology, Beijing, China) was used as the reference. Then, the membrane was incubated with the secondary antibody (HRP-labeled Goat Anti-Rabbit IgG (H + L)) for 2 h at room temperature and then was visualized by an electrochemiluminescence kit (Beyotime Biotechnology, Shanghai, China). The target proteins were quantified using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
2.5. RNA Interference
Large yellow croaker TLR22-specific small interfering RNA (siRNA; sense and antisense sequences of siRNA are shown in Table 1; Gene Pharma, Shanghai, China) was transfected into cells using the Xfect™ RNA Transfection Reagent (Takara, Tokyo, Japan) according to the manufacture’s protocol for 36 h to knock down the expression of tlr22 in macrophages. The RNAi negative control (NC) was used as the control group. After transfection for 36 h, cells were treated with PA for another 12 h.
2.6. Statistical Analysis
SPSS 22.0 (IBM, Armonk, NY, USA) was used to perform the statistical analysis, and the results were presented as means ± standard error of the mean (S.E.M.). All data were subjected to independent sample t-tests or one-way analysis of variance (ANOVA) followed by Tukey’s multiple range test. A value of p < 0.05 was considered statistically significant.
3. Results
3.1. PA-Induced Inflammatory Response in Macrophages of Large Yellow Croaker
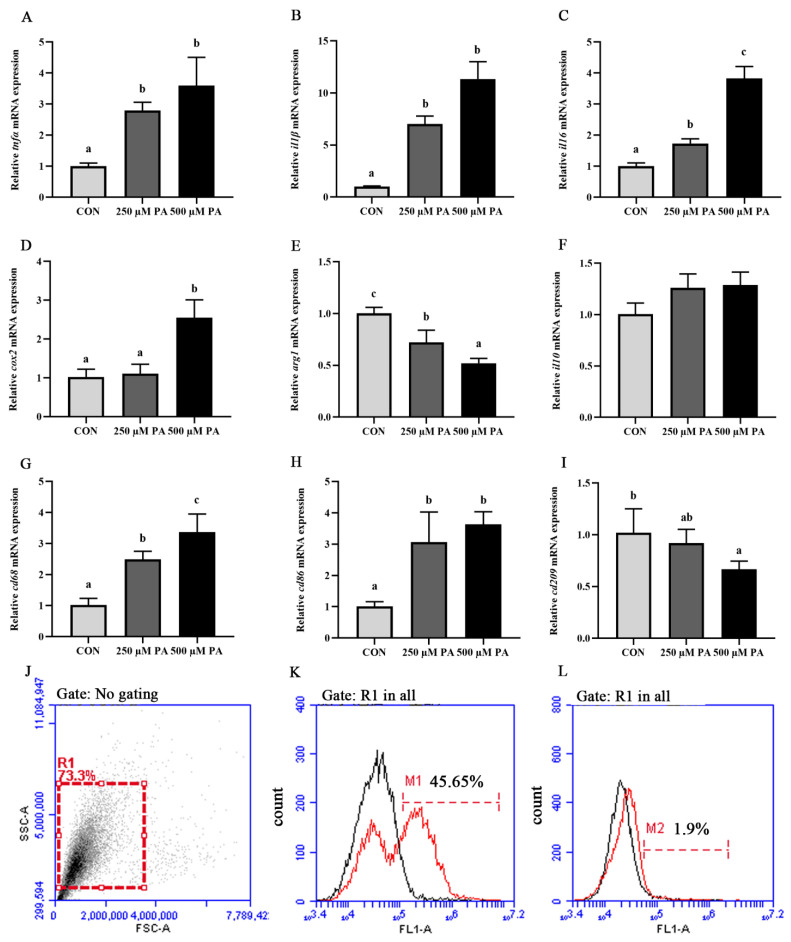
The PA treatment significantly upregulated the mRNA expression levels of proinflammatory genes, including tnfα, il1β, il6 and cox2 (p < 0.05) (Figure 1A–D). The mRNA expression level of anti-inflammatory gene arg1 was significantly decreased in the PA treatment compared with that in the control group (p < 0.05), while the mRNA expression of il10 showed no significant differences (p > 0.05) (Figure 1E,F). Moreover, mRNA expression levels of cd68 and cd86, markers of proinflammatory phenotype macrophages, were significantly increased after PA treatment, whereas the mRNA expression of cd209, a marker of the anti-inflammatory phenotype macrophage, was significantly decreased (p < 0.05) (Figure 1G–I). Gated macrophages (R1) in forward and side scatter (FS-SS) dot plots and combined fluorescence histograms were shown (Figure 1J). The flow cytometry analysis showed that the PA treatment significantly increased the CD68+ population (Figure 1K,L). These results demonstrated that PA treatment induced an inflammatory response in macrophages of large yellow croaker.
Figure 1.
PA induced inflammatory response in macrophages of large yellow croaker. (A–I) mRNA expression levels of inflammatory genes (tnfα, il1β, il6, cox2, arg1 and il10) and macrophage markers (cd68, cd86 and cd209) after PA treatment (n = 6). (J–L) Fluorescence histograms of CD68+ (K) and CD209+ (L) populations after PA treatment (scale of M1). Represent macrophages gated (R1) on a forward scatter (FSC) versus side scatter (SSC) dot plot. Data are presented as the means ± SEM and are analyzed using one-way ANOVA, followed by Tukey’s test. Bars labeled with the same letters are not significantly different (p > 0.05).
3.2. PA Activated the TLR-Related Genes Expression and MAPK Signaling Pathway in Macrophages
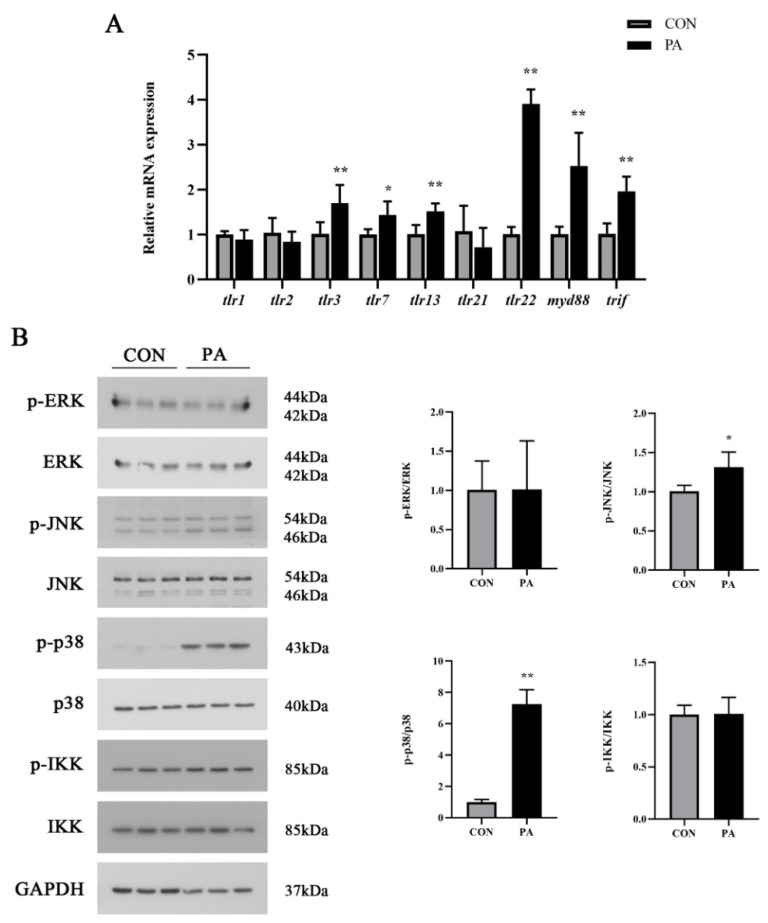
To investigate whether the TLR signaling pathway was activated by PA, TLR-related genes expression and downstream signaling pathways were detected in macrophages after PA treatment. PA treatment significantly upregulated the mRNA expression levels of tlr3, tlr7, tlr13, tlr22, myd88 and trif (p < 0.05), while the mRNA expression levels of tlr1, tlr2 and tlr21 had no significant changes (p > 0.05) (Figure 2A). As a typical specific TLR, the increased expression level of tlr22 was more conspicuous than the others. Moreover, the phosphorylation level of MAPK, including JNK and p38, was significantly increased after the PA treatment (p < 0.05), while the phosphorylation levels of ERK and IKK showed no significant differences (p > 0.05) (Figure 2B). These results revealed that the PA treatment induced TLRs gene expressions, especially tlr22, and activated the MAPK pathway in macrophages.
Figure 2.
PA activated the TLR-related genes expression and MAPK signaling pathway in macrophages. (A) The mRNA expression level of TLR-related genes after PA treatment. (B) Western blot analysis of MAPK signaling activation after PA treatment. The ratios of p-ERK to ERK, p-p38 to p38, p-JNK to JNK and p-IKK to IKK were determined. The data are presented as the means ± SEM (n = 6) and are analyzed using independent t-tests. * p < 0.05 and ** p < 0.01 indicate significant differences compared with the control group.
3.3. Effects of TLR22-MAPK Signaling Pathway on PA-Induced Inflammation in Macrophages
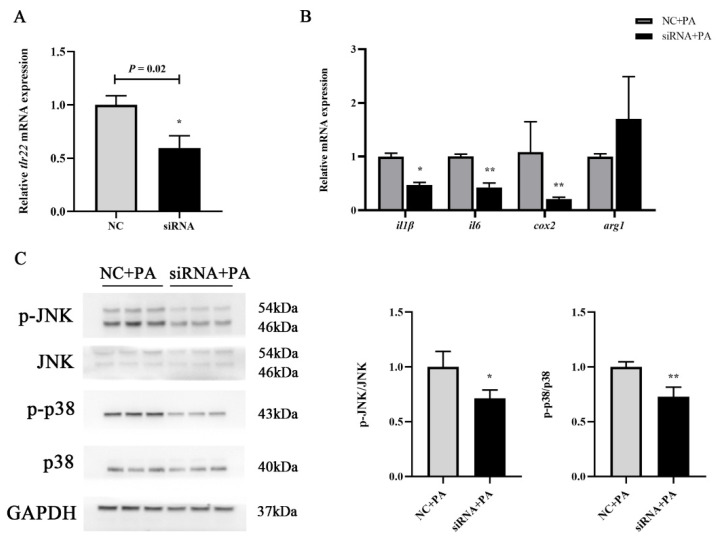
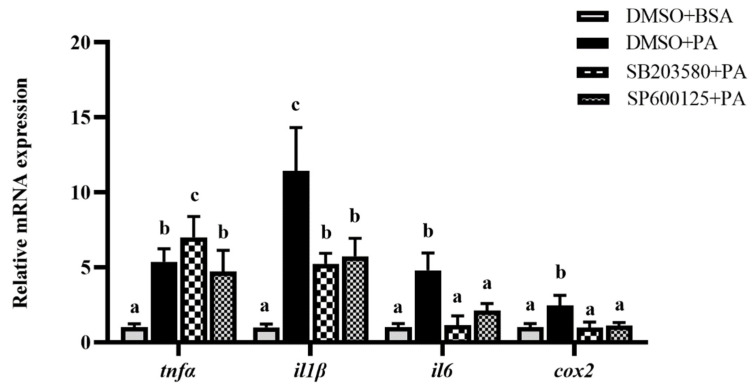
To confirm whether the TLR22-MAPK pathway participated in PA-induced inflammation, TLR22 siRNA was transfected, and MAPK inhibitors were incubated in macrophages. The transfection of siRNA into macrophages significantly inhibited the tlr22 mRNA expression (p < 0.05) (Figure 3A). Meanwhile, tlr22 knockdown significantly suppressed the PA-induced mRNA expression levels of proinflammatory genes, including il1β, il6 and cox2 (p < 0.05), while the mRNA expression level of arg1 showed no significant differences (p > 0.05) (Figure 3B). As the downstream pathway of TLR, the change of MAPK was detected after tlr22 knockdown under PA treatment. The results showed that the PA-induced phosphorylation levels of JNK and p38 were significantly inhibited after tlr22 knockdown (p < 0.05) (Figure 3C). Furthermore, JNK and p38 inhibitor incubation significantly suppressed the PA-induced mRNA expression levels of proinflammatory genes, including il1β, il6 and cox2 (p < 0.05) (Figure 4). These results demonstrated that PA induced an inflammatory response of macrophages via the TLR22-MAPK signaling pathway.
Figure 3.
Effects of tlr22 knockdown on PA-induced inflammation in macrophages. (A) The mRNA expression of tlr22 after tlr22 knockdown. (B) Effects of tlr22 knockdown on the mRNA expression levels of inflammatory genes induced by PA. (C) Effects of tlr22 knockdown on the phosphorylation of the MAPK pathway induced by PA. The ratios of p-p38 to p38 and p-JNK to JNK were determined. The data are presented as the means ± SEM (n = 3) and are analyzed using independent t-tests. * p < 0.05 and ** p < 0.01 indicate significant differences compared with the control group.
Figure 4.
Effects of p38 (SB203580) and JNK (SP600125) inhibitors on the mRNA expression levels of inflammatory genes induced by PA. Data are presented as the means ± SEM (n = 6) and are analyzed using Tukey’s test. Bars labeled with the same letters are not significantly different (p > 0.05).
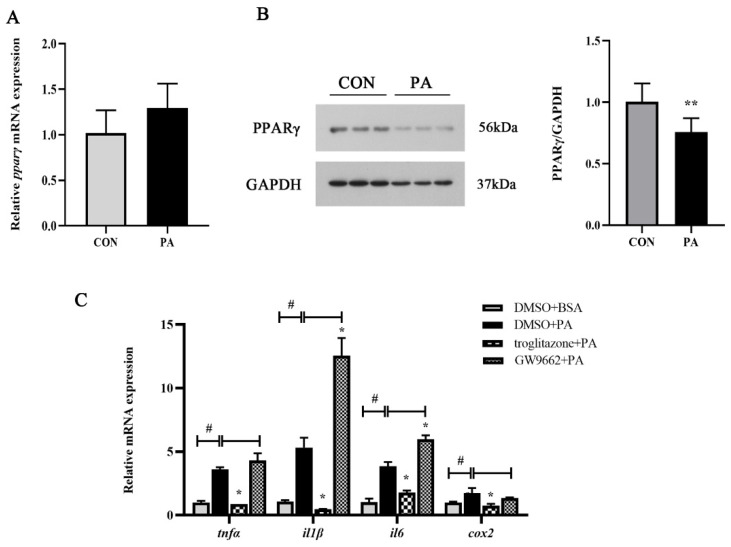
3.4. PPARγ Participated in PA-Induced Inflammation via TLR22-MAPK Pathway
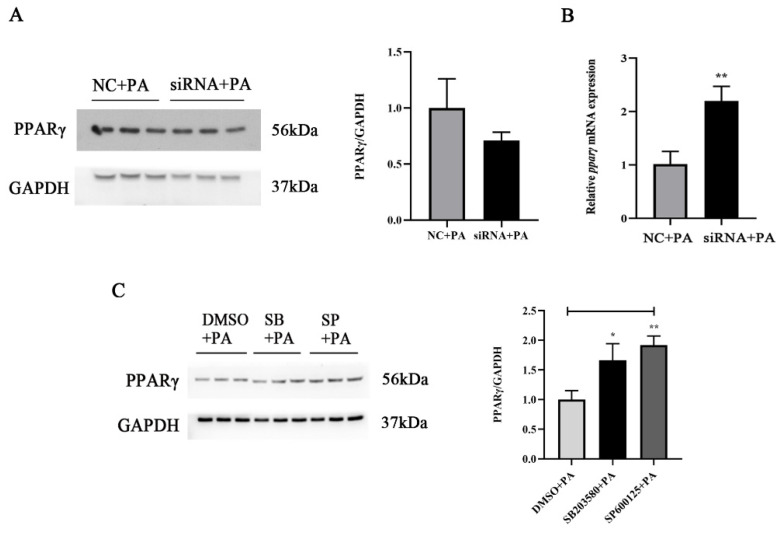
As an integral part of inflammatory responses, we further investigated whether PPARγ was involved in regulating PA-induced inflammation. The PA treatment significantly decreased the protein expression level of PPARγ (p < 0.05), while the mRNA expression level of pparγ showed no significant differences (p > 0.05) (Figure 5A,B). The PPARγ agonist and inhibitor were used to explore the role of PPARγ in PA-induced inflammation. The activation of PPARγ significantly suppressed the PA-induced mRNA expression levels of proinflammatory genes, including tnfα, il1β, il6 and cox2 (p < 0.05) (Figure 5C). Meanwhile, the inhibition of PPARγ significantly enhanced the mRNA expression levels of il1β and il6 (p < 0.05) (Figure 5C). Moreover, tlr22 knockdown significantly enhanced the mRNA expression level of pparγ induced by PA (p < 0.05) but was not significantly different in the protein expression (p > 0.05) (Figure 6A,B). However, p38 and JNK inhibitor incubation significantly enhanced the protein expression level of PPARγ induced by PA (p < 0.05) (Figure 6C). These results revealed that PPARγ participated in PA-induced inflammation via the TLR22-MAPK pathway.
Figure 5.
PPARγ was involved in PA-induced inflammation. (A,B) mRNA and protein expression levels of PPARγ after PA treatment (n = 6). The ratio of PPARγ to GAPDH was determined. (C) Effects of the PPARγ activator (troglitazone) and inhibitor (GW9662) on the mRNA expression levels of the inflammatory genes induced by PA (n = 3). Data are presented as the means ± SEM and analyzed using independent t-tests. # p < 0.05 indicates significant differences compared with the BSA group as the negative control. * p < 0.05 and ** p < 0.01 indicate significant differences compared with the control group.
Figure 6.
PPARγ participated in PA-induced inflammation via the TLR22-MAPK pathway. (A,B) Effects of tlr22 knockdown on the protein and mRNA expression levels of PPARγ induced by PA (n = 3). (C) Effects of the p38 (SB203580) and JNK (SP600125) inhibitors on the protein expression level of PPARγ induced by PA (n = 3). Data are presented as the means ± SEM and analyzed using independent t-tests. * p < 0.05 and ** p < 0.01 indicate significant differences compared with the control group.
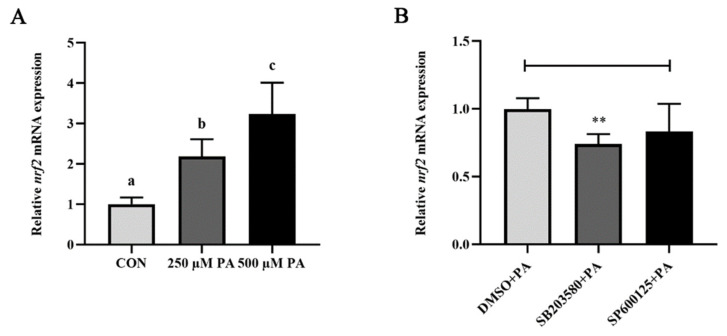
3.5. p38 MAPK Regulated the PA-Induced Activation of Nrf2
Nrf2, which was widely known to be the major regulation of antioxidant processes, also played a role in regulating inflammation. The PA treatment significantly upregulated the mRNA expression level of nrf2 (p < 0.05) (Figure 7A). p38 inhibitor incubation significantly suppressed the PA-induced mRNA expression level of nrf2 (p < 0.05), while the JNK inhibitor had no effects (Figure 7B). These results showed that p38 MAPK regulated the PA-induced activation of Nrf2.
Figure 7.
p38 MAPK regulated the PA-induced activation of Nrf2. (A) The mRNA expression level of nrf2 after PA treatment. (B) Effects of the p38 (SB203580) and JNK (SP600125) inhibitors on the mRNA expression level of nrf2 induced by PA. Data are presented as the means ± SEM (n = 6) and analyzed using one-way ANOVA, followed by Tukey’s test and independent t-tests. Bars labeled with the same letters are not significantly different (p > 0.05 and ** p < 0.01).
3.6. The Protective Effect of DHA against PA-Induced Inflammation
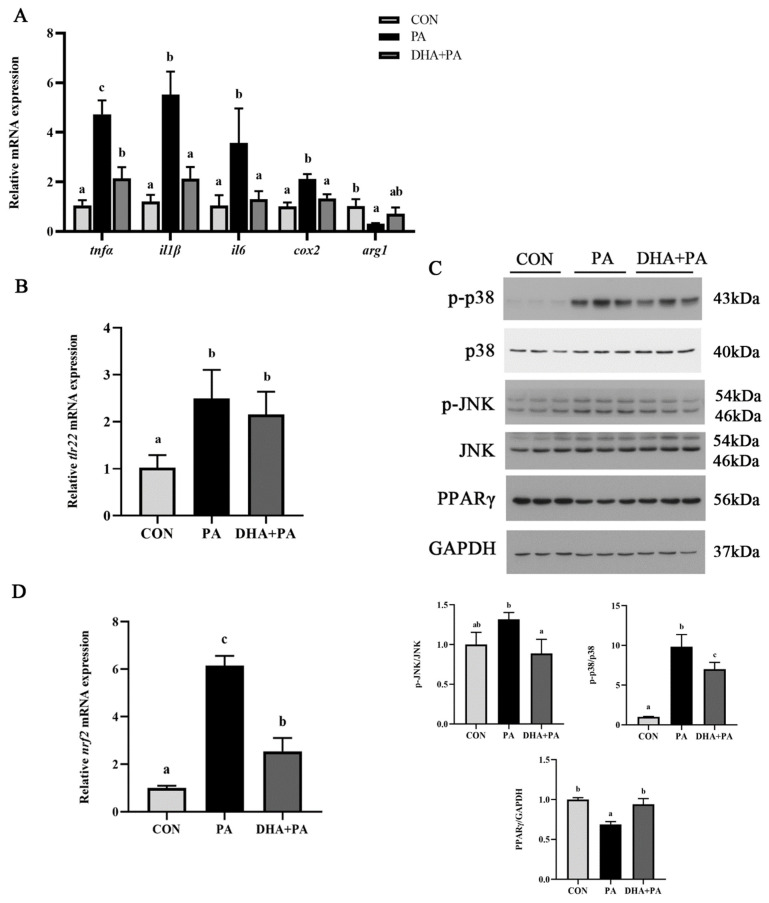
N-3 PUFAs are widely known to exert anti-inflammatory effects. Hence, the protective effect of DHA against PA-induced inflammation in macrophages was investigated. DHA treatment significantly suppressed the PA-induced mRNA expression levels of proinflammatory genes, including tnfα, il1β, il6 and cox2 (p < 0.05), while the mRNA expression of anti-inflammatory gene arg1 was significantly upregulated (p < 0.05) (Figure 8A). However, the mRNA expression level of tlr22 had no significant differences after DHA treatment (p > 0.05) (Figure 8B). Furthermore, the DHA treatment significantly reduced the PA-induced phosphorylation levels of JNK and p38 (p < 0.05) (Figure 8C). Moreover, DHA significantly recovered the decrease of PPARγ protein expression caused by PA (p < 0.05) (Figure 8C). DHA also significantly inhibited the increase of nrf2 mRNA expression induced by PA (p < 0.05) (Figure 8D). These results demonstrated that the protective effect of DHA against the PA-induced inflammatory response could perform via the TLR22-MAPK-PPARγ/Nrf2 signaling pathway.
Figure 8.
The protective effect of DHA against PA-induced inflammation. (A) mRNA expression levels of inflammatory genes after DHA treatment (n = 4). (B) The mRNA expression level of tlr22 after DHA treatment (n = 4). (C) Western blot analysis of MAPK signaling activation and the PPARγ protein level after DHA treatment (n = 3). The ratios of p-p38 to p38, p-JNK to JNK and PPARγ to GAPDH were determined. (D) The mRNA expression level of nrf2 after DHA treatment (n = 4). Data are presented as the means ± SEM and analyzed using one-way ANOVA, followed by Tukey’s test. Bars labeled with the same letters are not significantly different (p > 0.05).
4. Discussion
This study utilized the primary head kidney macrophages, an important immune cell in fish, to investigate the potential molecular mechanism of the PA-induced inflammatory response. In the present study, we found that PA induced proinflammatory gene expressions and promoted proinflammatory macrophage polarization, which was consistent with the observations in mammals [32,33].
Fish rely more on their innate immune system to resist the invasion of bacteria because of their evolutionarily less well-developed adaptive immune system [34]. One of mechanisms by which the innate immune system senses stimulation is through TLR signaling. As the TLR family in teleost was different from that in mammals, the change of TLR-related genes in large yellow croaker was detected according to a previous study [19]. The results showed that PA significantly activated the expressions of TLRs and adaptor proteins. Among them, TLR22 may act as the main response receptor to PA. However, TLR22 orthologs in humans serve as nonfunctional pseudogenes [35]. Furthermore, TLR22 knockdown suppressed PA-induced inflammation in macrophages. The current study, for the first time, revealed the role of TLR22 in PA-induced inflammation in fish. Further investigation found that PA induced the activation of JNK and p38 pathways. Although, a previous study in large yellow croaker showed that PO and PA could activate the NF-κβ pathway in the liver and hepatocytes, the present study found that PA activated MAPK but not NF-κβ in macrophages [36,37]. Meanwhile, the transcriptome analysis in the spleen of large yellow croaker revealed multiple signaling pathways were involved in the antiviral response, including the MAPK, NF-β and JAK pathways [38,39]. Hence, previous studies do not have uniform results on the changes of downstream signaling in response to different stimulations [40,41,42]. These inconsistent results may be due to the fact that different tissues had different regulation mechanisms of TLRs in response to different stimulations. Therefore, the signaling pathways regulated by TLR22 in different stimulations remain to be further explored in fish.
Since PPARγ could be modulated by TLRs and perform an anti-inflammatory effect, we further confirmed whether PPARγ was involved in PA-induced inflammation. The results showed that PA decreased the PPARγ expression at the translation level. The effects of the agonist and inhibitor of PPARγ on inflammatory gene expressions indicated that it presented an anti-inflammatory effect in regulating PA-induced inflammation, which was consistent with previous studies in mammals [43]. Previous studies in large yellow croaker revealed that PPARγ could negatively regulate the expression of inflammatory genes by affecting the promoter activity of genes [44]. Furthermore, the tlr22 knockdown and MAPK inhibitors recovered the decrease of PPARγ expression caused by PA. These results demonstrated that PPARγ participated in PA-induced inflammation via the TLR22-MAPK pathway in macrophages of large yellow croaker.
There is a complex interaction between the inflammatory response and antioxidant systems. Nrf2 is an important transcriptional regulator regulating the expression of varieties of anti-inflammatory and antioxidant genes and plays a central role in the response to stimulation [45]. In the current study, Nrf2 could be activated by PA, and p38 regulated the PA-induced activation of Nrf2. The results in mammals have reported that Nrf2 could participate in the alleviation of chronic inflammation by the MAPK pathway [46,47]. Therefore, the PA-induced activation of Nrf2 in the present study may be to compete and suppress the inflammatory response induced by PA.
N-3 PUFAs perform anti-inflammatory and antioxidant properties through various mechanisms and are always used as nutritional regulation strategies in mammals [48,49]. After understanding the molecular mechanism of PA in regulating inflammation, we further investigated whether DHA could alleviate PA-induced inflammation. The results showed that DHA inhibited the effect of PA on the mRNA expression of proinflammatory genes and the phosphorylation of JNK and p38, which was consistent with the results in mammals [50,51]. However, the mRNA expression of tlr22 showed no differences. Previous studies in mammals suggested that the effect of PUFAs on impacting TLR activation might perform by regulating the lipid and protein compositions of the raft membrane, not directly affecting the TLR mRNA expression [52,53]. The results in our lab also found that DHA might regulate the activation of TLR22 by changing the biophysical properties of the cell membrane (unpublished data). Furthermore, DHA recovered the decrease of PPARγ protein expression induced by PA. DHA may affect PPARγ expression by regulating the signaling pathways or directly activating PPARγ as a ligand [54]. Together, these results revealed that the protective effect of DHA against PA-induced inflammation was executed through the TLR22-MAPK-PPARγ/Nrf2 signaling pathway.
5. Conclusions
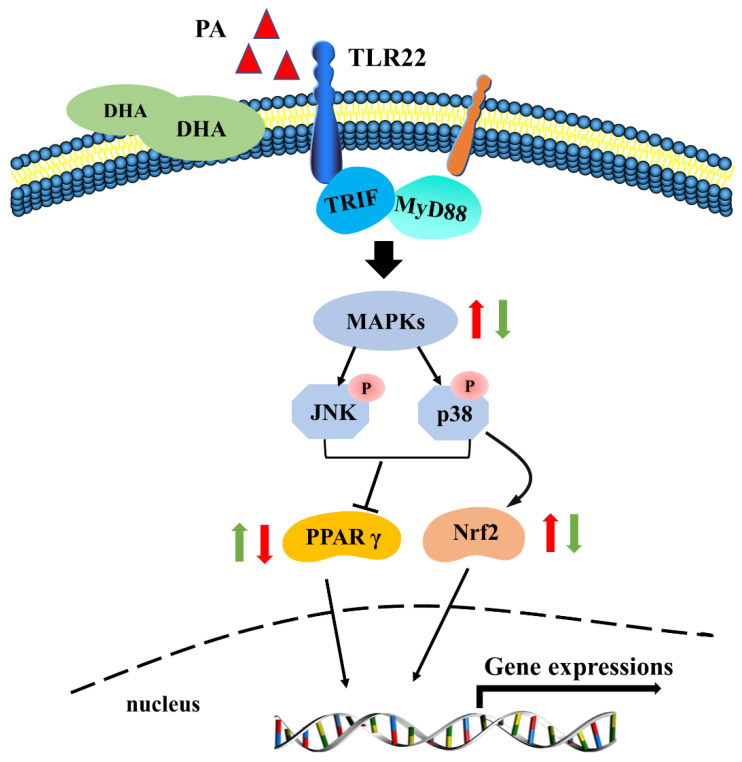
In conclusion, the present study indicated the PA-induced inflammatory response of macrophages via the TLR22-MAPK-PPARγ/Nrf2 signaling pathway in large yellow croaker. Meanwhile, DHA was found to alleviate the PA-induced inflammation, thereby performing anti-inflammatory and antioxidation properties (Figure 9). These results could advance the understanding of the molecular mechanism of the inflammatory response induced by SFAs and provide nutritional strategies against inflammation, thereby improving the utilization rate of PO in aquafeed.
Figure 9.
A working model showed the molecular mechanism of the PA-induced inflammatory response and the protective effect of DHA against the inflammation in macrophages of large yellow croaker.
Author Contributions
D.X.: conceptualization, data curation and writing—review and editing; K.C. and Q.L.: methodology and visualization; S.Z. and J.Z.: software and analyze; S.G. and T.H.: methodology and editing and K.M. and Q.A.: project administration and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Key Program of National Natural Science Foundation of China (Grant No. 31830103), the National Science Fund for Distinguished Young Scholars of China (Grant No. 31525024), the Ten-thousand Talents Program (Grant No. 2018-29), the Scientific and Technological Innovation of Blue Granary (Grant No. 2018YFD0900402) and the Agriculture Research System of China (Grant No. CARS-47-11).
Institutional Review Board Statement
All animal care and handling procedures performed in the present study were approved by the Animal Care Committee of the Ocean University of China (Approval number SPXY2020012).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no competing financial interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chavez J.A., Summers S.A. Characterizing the effects of saturated fatty acids on insulin signaling and ceramide and diacylglycerol accumulation in 3T3-L1 adipocytes and C2C12 myotubes. Arch. Biochem. Biophys. 2003;419:101–109. doi: 10.1016/j.abb.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 2.Peng G., Li L., Liu Y., Pu J., Zhang S., Yu J., Zhao J., Liu P. Oleate blocks palmitate-induced abnormal lipid distribution, endoplasmic reticulum expansion and stress, and insulin resistance in skeletal muscle. Endocrinology. 2011;152:2206–2218. doi: 10.1210/en.2010-1369. [DOI] [PubMed] [Google Scholar]
- 3.Leamy A.K., Egnatchik R.A., Shiota M., Ivanova P.T., Myers D.S., Brown H.A., Young J.D. Enhanced synthesis of saturated phospholipids is associated with ER stress and lipotoxicity in palmitate treated hepatic cells. J. Lipid Res. 2014;55:1478–1488. doi: 10.1194/jlr.M050237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Korbecki J., Bajdak-Rusinek K. The effect of palmitic acid on inflammatory response in macrophages: An overview of molecular mechanisms. Inflamm. Res. 2019;68:915–932. doi: 10.1007/s00011-019-01273-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bell J.G., Henderson R.J., Tocher D.R., McGhee F., Dick J.R., Porter A., Smullen R.P., Sargent J.R. Substituting fish oil with crude palm oil in the diet of Atlantic salmon (Salmo salar) affects muscle fatty acid composition and hepatic fatty acid metabolism. J. Nutr. 2002;132:222–230. doi: 10.1093/jn/132.2.222. [DOI] [PubMed] [Google Scholar]
- 6.Bahurmiz O.M., Ng W.-K. Effects of dietary palm oil source on growth, tissue fatty acid composition and nutrient digestibility of red hybrid tilapia, Oreochromis sp., raised from stocking to marketable size. Aquaculture. 2007;262:382–392. doi: 10.1016/j.aquaculture.2006.11.023. [DOI] [Google Scholar]
- 7.Han Y.Z., Jiang Z.Q., Ren T.J., Koshio S., Gao J., Komilus C.F., Jiang B.Q. Effect of dietary fish oil replacement with palm oil on growth performance, hematology and liver anti-oxidative enzymes of juvenile Japanese flounder Paralichthys olivaceus (Temminck & Schlegel, 1846) J. Appl. Ichthyol. 2015;31:518–524. doi: 10.1111/jai.12776. [DOI] [Google Scholar]
- 8.Zhang J., Liu Q., Pang Y., Xu X., Cui K., Zhang Y., Mai K., Ai Q. Molecular cloning and the involvement of IRE1alpha-XBP1s signaling pathway in palmitic acid induced-Inflammation in primary hepatocytes from large yellow croaker (Larimichthys crocea) Fish Shellfish. Immunol. 2020;98:112–121. doi: 10.1016/j.fsi.2019.12.089. [DOI] [PubMed] [Google Scholar]
- 9.Shi H., Kokoeva M.V., Inouye K., Tzameli I., Yin H., Flier J.S. TLR4 links innate immunity and fatty acid-induced insulin resistance. J. Clin. Investig. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rebl A., Goldammer T., Seyfert H.M. Toll-like receptor signaling in bony fish. Vet. Immunol. Immunopathol. 2010;134:139–150. doi: 10.1016/j.vetimm.2009.09.021. [DOI] [PubMed] [Google Scholar]
- 11.Takeda K., Akira S. TLR signaling pathways. Semin. Immunol. 2004;16:3–9. doi: 10.1016/j.smim.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 12.Akira S., Takeda K. Toll-like receptor signalling. Nat. Rev. Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 13.Rocha D.M., Caldas A.P., Oliveira L.L., Bressan J., Hermsdorff H.H. Saturated fatty acids trigger TLR4-mediated inflammatory response. Atherosclerosis. 2016;244:211–215. doi: 10.1016/j.atherosclerosis.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 14.Huang S., Rutkowsky J.M., Snodgrass R.G., Ono-Moore K.D., Schneider D.A., Newman J.W., Adams S.H., Hwang D.H. Saturated fatty acids activate TLR-mediated proinflammatory signaling pathways. J. Lipid Res. 2012;53:2002–2013. doi: 10.1194/jlr.D029546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Panda R.P., Chakrapani V., Patra S.K., Saha J.N., Jayasankar P., Kar B., Sahoo P.K., Barman H.K. First evidence of comparative responses of Toll-like receptor 22 (TLR22) to relatively resistant and susceptible Indian farmed carps to Argulus siamensis infection. Dev. Comp. Immunol. 2014;47:25–35. doi: 10.1016/j.dci.2014.06.016. [DOI] [PubMed] [Google Scholar]
- 16.Ding X., Lu D.Q., Hou Q.H., Li S.S., Liu X.C., Zhang Y., Lin H.R. Orange-spotted grouper (Epinephelus coioides) toll-like receptor 22: Molecular characterization, expression pattern and pertinent signaling pathways. Fish Shellfish Immunol. 2012;33:494–503. doi: 10.1016/j.fsi.2012.05.034. [DOI] [PubMed] [Google Scholar]
- 17.Li H., Yang G., Ma F., Li T., Yang H., Rombout J.H., An L. Molecular characterization of a fish-specific toll-like receptor 22 (TLR22) gene from common carp (Cyprinus carpio L.): Evolutionary relationship and induced expression upon immune stimulants. Fish Shellfish Immunol. 2017;63:74–86. doi: 10.1016/j.fsi.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 18.Ji J., Ramos-Vicente D., Navas-Perez E., Herrera-Ubeda C., Lizcano J.M., Garcia-Fernandez J., Escriva H., Bayes A., Roher N. Characterization of the TLR family in Branchiostoma lanceolatum and discovery of a novel TLR22-like involved in dsRNA recognition in Amphioxus. Front. Immunol. 2018;9:2525. doi: 10.3389/fimmu.2018.02525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tan P., Dong X., Mai K., Xu W., Ai Q. Vegetable oil induced inflammatory response by altering TLR-NF-kappaB signalling, macrophages infiltration and polarization in adipose tissue of large yellow croaker (Larimichthys crocea) Fish Shellfish Immunol. 2016;59:398–405. doi: 10.1016/j.fsi.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 20.Xiao X., Qin Q., Chen X. Molecular characterization of a Toll-like receptor 22 homologue in large yellow croaker (Pseudosciaena crocea) and promoter activity analysis of its 5’-flanking sequence. Fish Shellfish Immunol. 2011;30:224–233. doi: 10.1016/j.fsi.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 21.Calder P.C. Omega-3 polyunsaturated fatty acids and inflammatory processes: Nutrition or pharmacology? Br. J. Clin. Pharmacol. 2013;75:645–662. doi: 10.1111/j.1365-2125.2012.04374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calder P.C. n−3 Polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am. J. Clin. Nutr. 2006;83:1505S–1519S. doi: 10.1093/ajcn/83.6.1505S. [DOI] [PubMed] [Google Scholar]
- 23.Lee J.Y., Plakidas A., Lee W.H., Heikkinen A., Chanmugam P., Bray G., Hwang D.H. Differential modulation of Toll-like receptors by fatty acids: Preferential inhibition by n-3 polyunsaturated fatty acids. J. Lipid Res. 2003;44:479–486. doi: 10.1194/jlr.M200361-JLR200. [DOI] [PubMed] [Google Scholar]
- 24.Norris P.C., Dennis E.A. Omega-3 fatty acids cause dramatic changes in TLR4 and purinergic eicosanoid signaling. Proc. Natl. Acad. Sci. USA. 2012;109:8517–8522. doi: 10.1073/pnas.1200189109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Q., Cui K., Wu M., Xu D., Mai K., Ai Q. Polyunsaturated fatty acids influence LPS-induced inflammation of fish macrophages through differential modulation of pathogen recognition and p38 MAPK/NF-kappaB signaling. Front. Immunol. 2020;11:559332. doi: 10.3389/fimmu.2020.559332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zuo R., Ai Q., Mai K., Xu W., Wang J., Xu H., Liufu Z., Zhang Y. Effects of dietary n-3 highly unsaturated fatty acids on growth, nonspecific immunity, expression of some immune related genes and disease resistance of large yellow croaker (Larmichthys crocea) following natural infestation of parasites (Cryptocaryon irritans) Fish Shellfish Immunol. 2012;32:249–258. doi: 10.1016/j.fsi.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 27.Xu H., Turchini G.M., Francis D.S., Liang M., Mock T.S., Rombenso A., Ai Q. Are fish what they eat? A fatty acid’s perspective. Prog. Lipid Res. 2020;80:101064. doi: 10.1016/j.plipres.2020.101064. [DOI] [PubMed] [Google Scholar]
- 28.Du J., Xiang X., Li Y., Ji R., Xu H., Mai K., Ai Q. Molecular cloning and characterization of farnesoid X receptor from large yellow croaker (Larimichthys crocea) and the effect of dietary CDCA on the expression of inflammatory genes in intestine and spleen. Comp. Biochem. Physiol. B-Biochem. Mol. Biol. 2018;216:10–17. doi: 10.1016/j.cbpb.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 29.Li Q., Ai Q., Mai K., Xu W., Zheng Y. A comparative study: In vitro effects of EPA and DHA on immune functions of head-kidney macrophages isolated from large yellow croaker (Larmichthys crocea) Fish Shellfish Immunol. 2013;35:933–940. doi: 10.1016/j.fsi.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 30.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 31.Xu D., Li Q., Zhou Y., Shen Y., Lai W., Hao T., Ding Y., Mai K., Ai Q. Functional analysis and regulation mechanism of interferon gamma in macrophages of large yellow croaker (Larimichthys crocea) Int. J. Biol. Macromol. 2022;194:153–162. doi: 10.1016/j.ijbiomac.2021.11.183. [DOI] [PubMed] [Google Scholar]
- 32.Liu S.P., Li X.Y., Li Z., He L.N., Xiao Y., Yan K., Zhou Z.G. Octanoylated Ghrelin Inhibits the Activation of the Palmitic Acid-Induced TLR4/NF-kappaB Signaling Pathway in THP-1 Macrophages. ISRN Endocrinol. 2012;2012:237613. doi: 10.5402/2012/237613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suganami T., Tanimoto-Koyama K., Nishida J., Itoh M., Yuan X., Mizuarai S., Kotani H., Yamaoka S., Miyake K., Aoe S., et al. Role of the Toll-like receptor 4/NF-kappaB pathway in saturated fatty acid-induced inflammatory changes in the interaction between adipocytes and macrophages. Arterioscler. Thromb. Vasc. Biol. 2007;27:84–91. doi: 10.1161/01.ATV.0000251608.09329.9a. [DOI] [PubMed] [Google Scholar]
- 34.Uribe C., Folch H., Enríquez R., Moran G. Innate and adaptive immunity in teleost fish: A review. Vet. Med. 2011;56:486. doi: 10.17221/3294-VETMED. [DOI] [Google Scholar]
- 35.Roach J.C., Glusman G., Rowen L., Kaur A., Purcell M.K., Smith K.D., Hood L.E., Aderem A. The evolution of vertebrate Toll-like receptors. Proc. Natl. Acad. Sci. USA. 2005;102:9577–9582. doi: 10.1073/pnas.0502272102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li X., Ji R., Cui K., Chen Q., Chen Q., Fang W., Mai K., Zhang Y., Xu W., Ai Q. High percentage of dietary palm oil suppressed growth and antioxidant capacity and induced the inflammation by activation of TLR-NF-kappaB signaling pathway in large yellow croaker (Larimichthys crocea) Fish Shellfish Immunol. 2019;87:600–608. doi: 10.1016/j.fsi.2019.01.055. [DOI] [PubMed] [Google Scholar]
- 37.Wang T., Yang B., Ji R., Xu W., Mai K., Ai Q. Omega-3 polyunsaturated fatty acids alleviate hepatic steatosis-induced inflammation through Sirt1-mediated nuclear translocation of NF-kappaB p65 subunit in hepatocytes of large yellow croaker (Larmichthys crocea) Fish Shellfish Immunol. 2017;71:76–82. doi: 10.1016/j.fsi.2017.09.064. [DOI] [PubMed] [Google Scholar]
- 38.Mu Y., Li M., Ding F., Ding Y., Ao J., Hu S., Chen X. De novo characterization of the spleen transcriptome of the large yellow croaker (Pseudosciaena crocea) and analysis of the immune relevant genes and pathways involved in the antiviral response. PLoS ONE. 2014;9:e97471. doi: 10.1371/journal.pone.0097471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mu Y., Ding F., Cui P., Ao J., Hu S., Chen X. Transcriptome and expression profiling analysis revealed changes of multiple signaling pathways involved in immunity in the large yellow croaker during Aeromonas hydrophila infection. BMC Genom. 2010;11:506. doi: 10.1186/1471-2164-11-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ding X., Liang Y., Peng W., Li R., Lin H., Zhang Y., Lu D. Intracellular TLR22 acts as an inflammation equalizer via suppression of NF-κB and selective activation of MAPK pathway in fish. Fish Shellfish Immunol. 2018;72:646–657. doi: 10.1016/j.fsi.2017.11.042. [DOI] [PubMed] [Google Scholar]
- 41.Matsuo A., Oshiumi H., Tsujita T., Mitani H., Kasai H., Yoshimizu M., Matsumoto M., Seya T. Teleost TLR22 recognizes RNA duplex to induce IFN and protect cells from birnaviruses. J. Immunol. 2008;181:3474–3485. doi: 10.4049/jimmunol.181.5.3474. [DOI] [PubMed] [Google Scholar]
- 42.Ji J., Liao Z., Rao Y., Li W., Yang C., Yuan G., Feng H., Xu Z., Shao J., Su J. Thoroughly remold the localization and signaling pathway of TLR22. Front. Immunol. 2020;10:3003. doi: 10.3389/fimmu.2019.03003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li H., Ruan X.Z., Powis S.H., Fernando R., Mon W.Y., Wheeler D.C., Moorhead J.F., Varghese Z. EPA and DHA reduce LPS-induced inflammation responses in HK-2 cells: Evidence for a PPAR-γ–dependent mechanism. Kidney Int. 2005;67:867–874. doi: 10.1111/j.1523-1755.2005.00151.x. [DOI] [PubMed] [Google Scholar]
- 44.Wu M., Li Q., Mai K., Ai Q. Regulation of free fatty acid receptor 4 on inflammatory gene induced by LPS in large yellow croaker (Larimichthys crocea) Front. Immunol. 2021;12:703914. doi: 10.3389/fimmu.2021.703914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaspar J.W., Niture S.K., Jaiswal A.K. Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radic. Biol. Med. 2009;47:1304–1309. doi: 10.1016/j.freeradbiomed.2009.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee M.S., Lee B., Park K.E., Utsuki T., Shin T., Oh C.W., Kim H.R. Dieckol enhances the expression of antioxidant and detoxifying enzymes by the activation of Nrf2-MAPK signalling pathway in HepG2 cells. Food Chem. 2015;174:538–546. doi: 10.1016/j.foodchem.2014.11.090. [DOI] [PubMed] [Google Scholar]
- 47.Yao P., Nussler A., Liu L., Hao L., Song F., Schirmeier A., Nussler N. Quercetin protects human hepatocytes from ethanol-derived oxidative stress by inducing heme oxygenase-1 via the MAPK/Nrf2 pathways. J. Hepatol. 2007;47:253–261. doi: 10.1016/j.jhep.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 48.De Caterina R. n–3 fatty acids in cardiovascular disease. N. Engl. J. Med. 2011;364:2439–2450. doi: 10.1056/NEJMra1008153. [DOI] [PubMed] [Google Scholar]
- 49.Breslow J.L. n−3 Fatty acids and cardiovascular disease. Am. J. Clin. Nutr. 2006;83:1477S–1482S. doi: 10.1093/ajcn/83.6.1477S. [DOI] [PubMed] [Google Scholar]
- 50.Kennedy A., Martinez K., Chuang C.C., LaPoint K., McIntosh M. Saturated fatty acid-mediated inflammation and insulin resistance in adipose tissue: Mechanisms of action and implications. J. Nutr. 2009;139:1–4. doi: 10.3945/jn.108.098269. [DOI] [PubMed] [Google Scholar]
- 51.White P.J., Mitchell P.L., Schwab M., Trottier J., Kang J.X., Barbier O., Marette A. Transgenic omega-3 PUFA enrichment alters morphology and gene expression profile in adipose tissue of obese mice: Potential role for protectins. Metabolism. 2015;64:666–676. doi: 10.1016/j.metabol.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 52.Wong S.W., Kwon M.J., Choi A.M., Kim H.P., Nakahira K., Hwang D.H. Fatty acids modulate Toll-like receptor 4 activation through regulation of receptor dimerization and recruitment into lipid rafts in a reactive oxygen species-dependent manner. J. Biol. Chem. 2009;284:27384–27392. doi: 10.1074/jbc.M109.044065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fessler M.B., Rudel L.L., Brown J.M. Toll-like receptor signaling links dietary fatty acids to the metabolic syndrome. Curr. Opin. Lipidol. 2009;20:379–385. doi: 10.1097/MOL.0b013e32832fa5c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang L., Waltenberger B., Pferschy-Wenzig E.M., Blunder M., Liu X., Malainer C., Blazevic T., Schwaiger S., Rollinger J.M., Heiss E.H., et al. Natural product agonists of peroxisome proliferator-activated receptor gamma (PPARgamma): A review. Biochem. Pharmacol. 2014;92:73–89. doi: 10.1016/j.bcp.2014.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is contained within the article.