Abstract
Swine influenza (SI) is a major respiratory disease of swine; SI is due to the influenza A virus of swine (IAV-S), a highly contagious virus with zoonotic potential. The intensity of IAV-S surveillance varies among countries because it is not a reportable disease and causes limited mortality in swine. Although Asia accounts for half of all pig production worldwide, SI is not well managed in those countries. Rigorously managing SI on pig farms could markedly reduce the economic losses, the likelihood of novel reassortants among IAV-S, and the zoonotic IAV-S infections in humans. Vaccination of pigs is a key control measure for SI, but its efficacy relies on the optimal antigenic matching of vaccine strains with the viral strains circulating in the field. Here, we phylogenetically reviewed the genetic diversity of the hemagglutinin gene among IAVs-S that have circulated in Asia during the last decade. This analysis revealed the existence of country-specific clades in both the H1 and H3 subtypes and cross-border transmission of IAVs-S. Our findings underscore the importance of choosing vaccine antigens for each geographic region according to both genetic and antigenic analyses of the circulating IAV-S to effectively manage SI in Asia.
Keywords: pig, respiratory disease, phylogeny, vaccine
1. Introduction
Swine influenza (SI) is a respiratory disease caused by the influenza A virus of swine (IAV-S), which belongs to the genus Alphainfluenza virus in the family Orthomyxoviridae; IAV-S carries eight negative-strand segments of RNAs as its genome [1]. IAV-S is highly contagious and a major pathogen of the porcine respiratory disease complex (PRDC): morbidity can reach as high as 100% within a herd. SI causes flu-like symptoms in pigs similar to those of human influenza, including high fever, coughing, and depression; in addition, SI results in stunting and delayed weight-gain in fattening and finishing pigs [2,3,4,5]. Coinfection with Mycoplasma spp., porcine reproductive and respiratory syndrome virus (PRRS virus), porcine circovirus 2 (PCV-2), or other respiratory pathogens can exacerbate the symptoms of SI [2,6]. The pig industry in the United Kingdom loses approximately GBP 65 million per year due to SI [7]. In addition, asymptomatic infection with IAV-S has been widely recognized recently [8,9,10]. Overall economic losses due to IAV-S in the pig industry are very substantial [4,11,12].
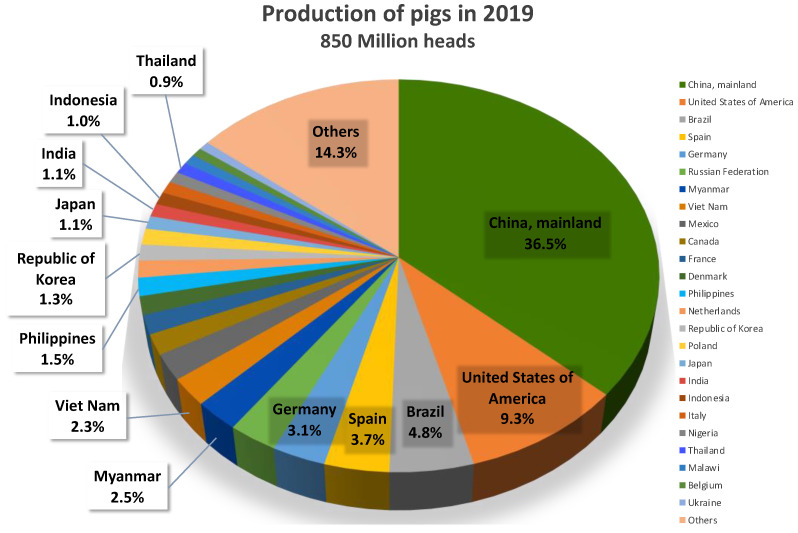
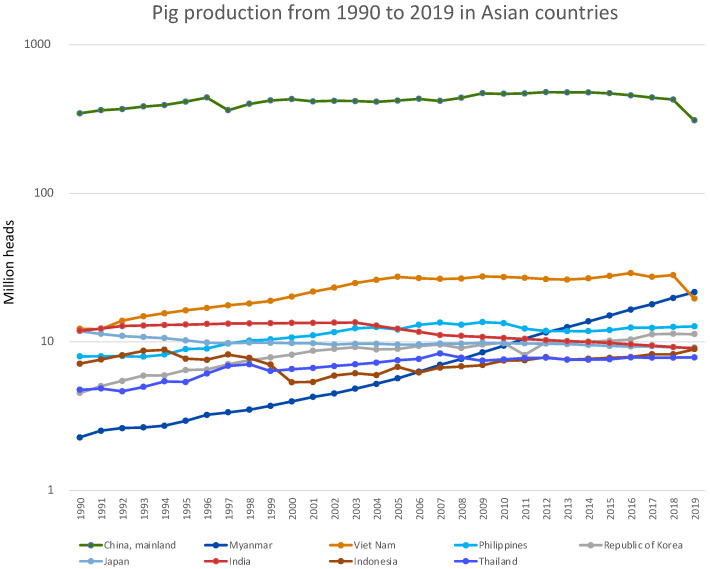
Half of all pigs worldwide are produced in Asian countries, with China as the top pig-producing country, accounting for 36.5% of worldwide pig production in 2019 [13]. In addition, Myanmar and Vietnam each accounted for more than 2% of overall pig production, followed by the Philippines, Korea, Japan, India, Indonesia, and Thailand among the Asian countries (Figure 1). In particular, the pig production industry in Myanmar has increased steadily over the last three decades, with a current market share that is 10 times what it was initially (Figure 2). However, the intensity of IAV-S surveillance varies among Asian countries [14,15,16], because SI is not a reportable disease and because SI has far less of an effect on the pig industry than other enzootic diseases in Asia, such as classical swine fever, foot-and-mouth disease, PRRS, and the recently emerged African swine fever.
Figure 1.
The top five pig-producing countries worldwide and the top nine pig-producing Asian countries. Data was obtained from FAOSTAT (http://www.fao.org/faostat/en/#data/QCL, accessed on 9 July 2021).
Figure 2.
Trends in pig production from 1990 through 2019 for the nine Asian countries in Figure 1.
Combined with robust hygiene practices, vaccination is one of the most effective control measures to reduce SI-associated economic loss at the farm level [4]. However, due to the error-prone nature of the viral RNA polymerase [17], non-synonymous substitutions in the hemagglutinin (HA) genes accumulate over time [14,18,19,20,21]. In particular, the accumulation of substitutions in the antigenic sites of the HA protein, a primary target of host neutralizing antibodies, can lead to antigenic drift from the ancestral strain [22,23,24,25]. The effectiveness of SI vaccines rests heavily on correct antigenic matching between viral strains circulating in the field and the vaccine strain. Although other factors including antigen content and type of adjuvant may influence vaccine efficiency [26,27,28], antigenic match is the leading determinant in vaccine efficacy [29,30,31]. Therefore, maintaining the efficacy of commercially available SI vaccines requires continued monitoring of the antigenicity of circulating strains through hemagglutination-inhibition or neutralizing tests—followed by challenge studies, if necessary—and subsequent updating of vaccine composition [15,32,33]. However, in many countries, updating the composition of a vaccine involves a cumbersome registration process that is almost equal in effort to registering a new vaccine. Unlike that for human seasonal influenza vaccines, the process for updating SI vaccine composition hinders a timely update [33]. An exception to this drawback is the autogenous vaccines available in the United States; these herd-specific vaccines are designed specifically against an IAV-S isolate from a herd within the pig production system where the vaccine is used [3,34,35].
IAVs-S of the H1N1, H1N2, and H3N2 subtypes are currently circulating worldwide. The established clade classification system for the HA gene of H1 subtype IAVs-S recognizes three major lineages—1A, 1B, and 1C—and their sub-lineages [14]. Clade 1A used to be called ‘classical IAVs-S’, which share a common ancestor with Spanish Flu [36]; genes derived from human seasonal IAVs since 2009 (i.e., H1N1pdm) are recognized as sub-clade 1A.3.3.2. Clade 1B consists of genes that originated from human seasonal H1 IAVs before H1N1pdm, including the so-called Russian Flu circulating from 1977 to 2009 in humans. Viruses in clade 1C originated from the Eurasian avian (EA) influenza virus lineage, which has been endemic in the European pig population since the late 1970s [37]. In contrast, although reverse-zoonotic (i.e., human to pig) transmission of human seasonal H3 IAVs-S occasionally has occurred in different parts of world [18,21,24,38,39,40,41,42], no definitive H3 HA clade classification system has yet been established.
Neither the nucleotide nor amino acid sequence of the HA gene of IAVs-S can be used alone to infer the antigenic similarity [32] or efficacy of vaccines against the IAVs-S circulating in a country [27]. However, understanding the genetic diversity and evolution of IAVs-S provides pivotal information for designing effective SI vaccines for Asian countries, where SI vaccines are less recognized as a tool for controlling SI. For example, IAV-S vaccine coverage in Japan is estimated at <10% [18]. Despite the many studies regarding the genetic diversity of the HA genes of IAVs-S in individual countries, including China [20,43,44,45,46,47,48], Japan [18,23,25,49,50,51,52,53], Korea [5,54,55,56,57], Thailand [19,21,22,58,59], and Vietnam [42,60,61,62], the evolutionary relationship among Asian IAV-S isolates has not been examined in detail. Therefore, we phylogenetically reviewed HA sequence data obtained from the GISAID EpiFlu Database to clarify the cross-regional and national characteristics of the genetic diversity of the HA genes of IAVs-S in Asian countries, with particular focus on the IAV-S sequences generated during the last decade (i.e., 2010 through 2020).
2. H1 Subtype IAVs-S
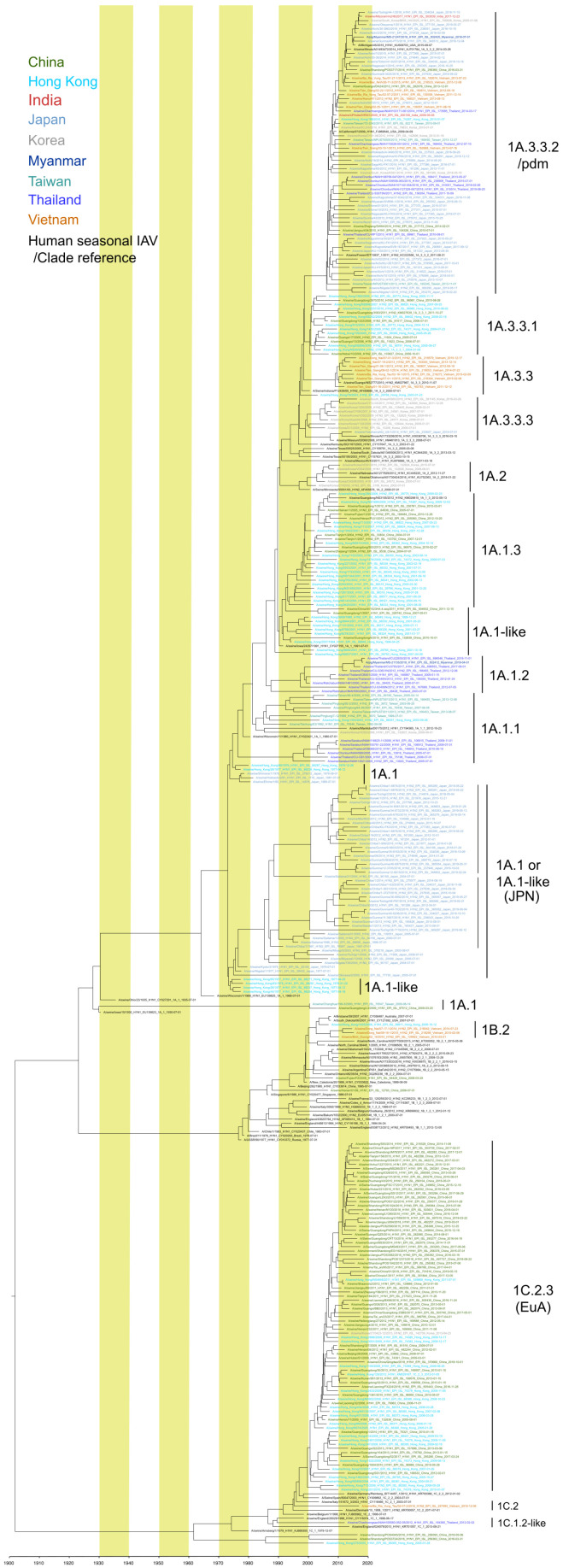
Several subclades of H1 IAVs-S have cocirculated in Asian countries since 2010 (Table 1). Similar to the situation in other parts of the world [63,64,65,66,67], reverse-zoonotic transmission of H1N1pdm clade 1A.3.3.2, has occurred in China, Hong Kong, India, Japan, Korea, Myanmar, Taiwan, Thailand, and Vietnam; some of these 1A.3.3.2 viruses reassorted with the IAVs-S strains previously circulating in those countries and regions [18,19,20,22,43,44,49,52,58,60,68]. The precise geographic location where H1N1pdm (clade 1A.3.3.2) originated in swine remains unknown. However, the phylogenetic analysis of H1N1pdm strains isolated from Asia suggests that several incidents of the reverse-zoonotic transmission of H1N1pdm might have occurred in various countries (Figure 3). However, a lineage originating from any of these transmissions has not become the predominant IAV-S in any of these countries or regions. Although, clade 1A.3.3.2 is common throughout Asia, the prevalence of the other clades differs substantially from one country or region to another.
Table 1.
Prevalence of each clade of IAV-S H1 isolates in each Asian country since 2010.
| Country or Region | Total No. of Sequences in GISAID a | Total No. of Sequences after Cd-Hit (99.5% Identity) b | Proportion (%) of Each Clade among H1 IAV-S H1 Since 2010 (%) c | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1A.1/1A.1-like | 1A.1.1 | 1A.1.2 | 1A.1.3 | 1A.2 | 1A.3.3 | 1A.3.3.1 | 1A.3.3.2 | 1A.3.3.3 | 1B.2 | 1C.1-2-like | 1C.2 | 1C.2.3 | |||
| China | 317 | 134 | 1.4 | 3.0 | 1.5 | 11.9 | 82.1 | ||||||||
| Hong Kong | 3 | 3 | 33.3 | 66.7 | |||||||||||
| India | 1 | 1 | 100.0 | ||||||||||||
| Japan | 473 | 119 | 56.3 | 42.9 | 0.8 | ||||||||||
| Korea | 16 | 8 | 12.5 | 50.0 | 25.0 | 12.5 | |||||||||
| Myanmar | 4 | 2 | 50.0 | 50.0 | |||||||||||
| Taiwan | 9 | 8 | 37.5 | 62.5 | |||||||||||
| Thailand | 138 | 29 | 3.4 | 24.1 | 69.0 | 3.4 | |||||||||
| Vietnam | 199 | 28 | 32.1 | 42.9 | 21.4 | 3.6 | |||||||||
a Sequences of isolates that were isolated after 2010 and contained the entire HA coding region were counted. b Sequences more than 99.5% identical were clustered by using cd-hit [69]. To avoid overestimation of a particular clade due to the prevalence derived from a single farm or outbreak, a single representative sequence for each cluster underwent prevalence analysis because inclusion of all the sequences available in the database may result in overestimation. The 99.5% identity threshold was set empirically. Sequences remaining after using cd-hit were classified according to clade; the number of sequences in each clade was divided by the total number of sequences remaining after cd-hit clustering to calculate each clade’s percentage. c Clade classification was done by using the Swine H1Clade Classification Tool at Influenza Research Database (IRD: https://www.fludb.org/brc/h1CladeClassifier.spg?method=ShowCleanInputPage&decorator=influenza, accessed on 30 July 2021).
Figure 3.
Maximum clade credibility (MCC) phylogenetic tree of the HA genes of Asian H1 IAVs-S. All available H1 HA gene sequences with complete HA coding regions from IAVs-S isolated in the nine Asian countries in Figure 2 were downloaded from GISAID on 8 July 2021. Sequence data from the Philippines were unavailable at the time of the download. Sequence data for each country or region underwent cd-hit clustering [69] (threshold, 98.5% identity) to reduce the number of sequences and increase visualization. ‘China’ refers to mainland China, and Hong Kong and Taiwan were considered as separate regions because they differ in pig trade and other livestock-related practices that might affect the prevalence of IAV-S. For the calculation, when only the year was available as the isolation date from GISAID, 1 July was used as the tentative isolation date; when only the year and month were available, the 15th was used as the day. This tree was constructed to illustrate the overall evolutionary pathway of Asian IAVs-S, not for precise calculation of the divergence time. Various the human seasonal IAVs and IAVs-S from other countries have been included as clade indicators.
In China and Hong Kong, viruses belonging to clades 1A.3.3.1 and 1A.1.3 were isolated in the early 2000s [43,70]. Recent intensive surveillance in mainland China and Hong Kong has revealed the predominance of the 1C.2.3 lineage, which derived from EA-like IAVs-S, during 2011 to 2018 [43,45,46] followed by 1A.3.3.2. Within the 1C.2.3, antigenic drift was apparent between isolates in the early 2010s and the late 2010s in mainland China. They showed low cross reactivity in the hemagglutination-inhibition test to the antiserum raised against the human H1N1pdm strain isolated in 2015 [46]. Clearly, antigenicity differs between the 1C.2.3 isolates from 2011 to 2013 and those from 2016 to 2018.
In Vietnam, clades 1A.3.2.2, 1A.3.3 and 1B.2 appeared to be codominant [61]. During 2010 through 2015, 1A.3.3 strains circulated in southern Vietnam, whereas 1B.2 viruses were isolated in both the north and south. Although the 1C.2 clade was not well established throughout Vietnam, 1C.2 viruses—represented by A/swine/Ba Ria Vung Tau/02-07-2/2016—were isolated in southern Vietnam [62]. Transborder introduction of 1C.2 viruses into Vietnam from Europe was suggested, given that the farm where the 1C.2 viruses were isolated had purchased the pigs from another farm that imported pigs from Europe [62].
In Korea, viruses belonging to clade 1A.3.3.3 appeared as early as in 2004, were considered to originate from gamma-lineage strain(s) that circulated in the United States [5], and circulated throughout Korea until 2016. Furthermore, the 1A.2 viruses that circulated in the United States and Mexico were reported in Korea, however, the most recent strain of this clade was reported in 2010. IAVs-S of clade 1C.2.3 closely related to those circulating in Hong Kong were reported in Korea in 2013 but no later [57]. Pig importation has been suggested as the mechanism through which multiple IAVs-S from abroad have been introduced into Korea.
In Thailand, viruses belonging to 1A.1.2 and 1A.1.1 cocirculated in the 2000s [21,71,72], but only members of 1A.1.2 were reported during the 2010s [19,22]. Because sequence data regarding the 1A.1.2. viruses in the 2010s were obtained mainly through the longitudinal surveillance and the studies that focused on only a few farms, whether this clade became dominant throughout the Thai pig population is unknown. It is noteworthy that a 1A.1.2 isolate from Myanmar—A/pig/Myanmar/MS-21135-HA/2018 [68]—is closely related to A/swine/Thailand/CU22630/2018, indicating the transborder movement of IAVs-S between the neighboring countries. EA-like IAV-S belonging to 1C.1.2 was isolated from a farm in Thailand in 2012 [73], but no further reports of this clade have been made.
In Japan, the 1A.1-like clade has become established as a unique clade consisting exclusively of Japanese isolates since the 1980s [18,49,74]. Except for 1A.3.3.2 viruses originating from H1N1pdm strains, the IAVs-S in Japan has evolved as a single major clade since its first recognition in 1977 [50]. This single clade diverged into two sub-clades in the mid-1980s, both of which are circulating in Japan currently [18]. Whether this genetic divergence is indicative of the antigenic divergence has not yet been studied. In addition, although a virus belonging to 1A.3.3.3—A/swine/YokohamaAQ(US)/1/2014—was isolated from an imported pig under quarantine at a national Animal Quarantine Station in Japan, further dissemination of this clade in Japan has not been recognized.
3. H3 Subtype IAVs-S
Several clades of IAVs-S harboring the HA gene of human seasonal H3 HA lineages have been observed in Asian countries during the last decade. A/Bin Duong/03_06/2010 (BD)-like strains have been isolated in Vietnam [42,61], China, and Hong Kong [43]. The BD-like strains obtained in Vietnam originated from a human seasonal H3N2 strain that circulated between 2004 and 2005 [42]. An antigenic comparison of A/Bin Duong/03_06/2010 with a human H3N2 strain isolated in 2004 (a possible ancestor of A/Bin Duong/03_06/2010) and a human strain isolated in 2009 revealed considerable antigenic differences among these viruses [23], thus demonstrating independent antigenic drift in the pig population among viruses with a common origin. Although not directly related to BD-like strains, Japanese IAVs-S—represented by A/swine/Osaka-C/12-20/2008—similarly were shown to originate from a human seasonal H3N2 strains circulating around 2004 [23]. However, a descendant of these strains has not become the predominant strain in Japan.
In Japan, viruses carrying the HA gene of human seasonal H3N2 strains circulating during the 1999–2000 season—represented by A/swine/Kagoshima/37-7201/2019—have become established during the last decade [18,23,53,74]. These H3 subtype IAVs-S have been isolated in various regions of the country since 2000, suggesting dissemination of this clade of IAVs-S throughout Japan. Although IAVs-S with an HA of similar origin—represented by A/swine/Korea/CAS05/2004—have been isolated in Korea [5], they have not become established there.
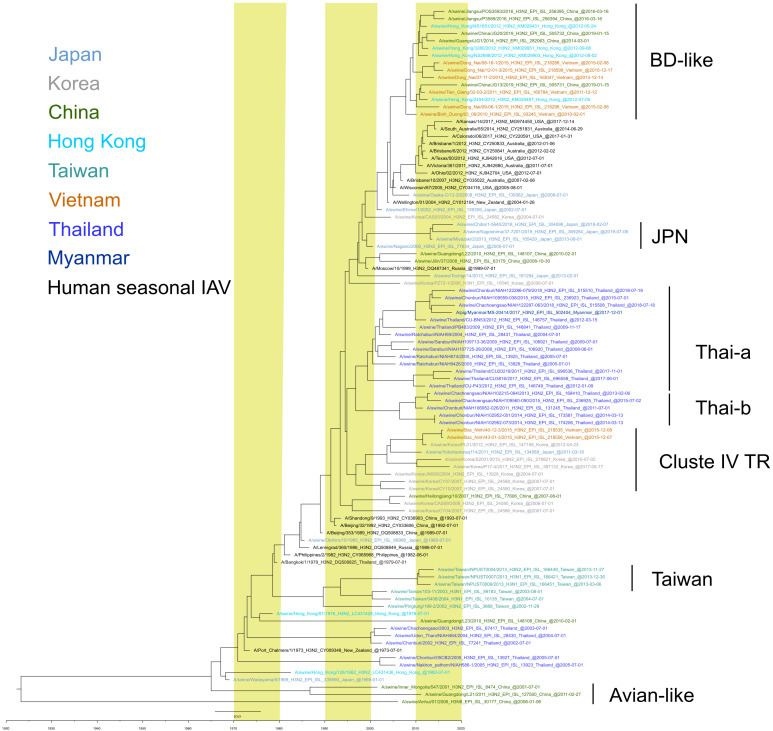
In Thailand, viruses with the HA gene of the human seasonal H3N2 virus that circulated during 1996–1997 [21,22] diverged into at least two sub-clades, Thai-a and Thai-b (Figure 4) [22], which have become dominant in Thailand since 2010. Thai-a and Thai-b have replaced the two older human-like lineages—represented, respectively, by A/swine/Udon Thani/NIAH464/2004 and A/swine/Nakhon pathom/NIAH586-1/2005—[19,21,71] which harbored the human seasonal H3 HA that circulated during the early 1970s. Although Thai-a and Thai-b viruses isolated from 2015 through 2017 did not differ antigenically from each other, they differed in antigenicity from the human seasonal H3 viruses isolated during the same period [22]. Descendants of the Thai-b lineage—represented by A/pig/Myanmar/MS-20414/2017—have been reported in Myanmar, suggesting a transborder dissemination of IAVs-S in the region [68].
Figure 4.
Maximum clade credibility (MCC) phylogenetic tree of the HA genes of H3 subtype Asian IAVs-S. All available H3 HA gene sequences with complete HA coding regions from IAVs-S isolated in the nine Asian countries in Figure 2 were downloaded from GISAID on 8 July 2021. Sequence data from the Philippines and Indonesia were unavailable at the time of the download. Sequence data for each country or region underwent cd-hit clustering [69] (threshold, 98.5% identity) to reduce the number of sequences and increase visualization. Countries and regions are defined as in Figure 3. For the calculation, when only the year was available as the isolation date from GISAID, 1 July was used as the tentative isolation date; when only the year and month were available, the 15th was used as the day. This tree was constructed to illustrate the overall evolutionary pathway of Asian IAVs-S, not for precise calculation of the divergence time. Various human seasonal IAVs and IAVs-S from other countries have been included as clade indicators.
IAVs-S with the HA gene of cluster IV triple-reassortant (TR) IAVs-S, whose HA gene originated from a human seasonal H3N2 virus that circulated around 1998 in North America [41,75], were isolated thereafter in Korea [55], Vietnam [61], and at an Animal Quarantine Station in Japan [49]. The strain A/swine/Yokohama/aq114/2011 was isolated from the specimens taken from a pig imported from Canada, indicating that the virus entered Japan through pig transportation and that it had not been introduced into the Japanese pig population. In contrast, Korean and Vietnamese IAVs-S in this clade have been isolated on farms in Korea since 2004 and in Vietnam since 2015, thus indicating their establishment in the pig populations of these two countries.
In Taiwan, IAVs-S carrying an HA gene that originated from a human seasonal H3N2 strain isolated around 1980 became established for a decade, from 2002 through 2013. These H3 HA viruses had either N1 or N2 NA genes, indicating reassortments with other IAVs. Given that an ancestral human strain of these Taiwanese IAVs is estimated to have circulated during the late 1970s, the H3HA IAVs-S appeared to have been introduced just once into the Taiwanese pig population. However, whether these or similar strains are still circulating is unknown.
Several strains with an H3 HA from avian influenza viruses or an equine influenza virus have been isolated from pigs in China [47,76,77]. However, these IAVs-S appeared to have resulted from a sporadic incident and have not become established in the pig population of China.
4. H5 Subtype IAVs-S
Since 2004, highly pathogenic avian influenza viruses (HPAIVs) carrying an H5 HA gene originated from A/goose/Guangdong/1/96—the so-called Goose/Guangdong (Gs/Gd) lineage—have disseminated throughout the Asian poultry population (Table 2). Concurrently, H5 HPAIVs of the Gs/Gd lineage have sporadically been isolated from pigs in both China [78,79] and Indonesia [80]. In China since 2003, both the H5N1 and H5N6 subtypes were reported [78]. Chinese IAVs-S of the H5N1 subtype belonged to clades 0, 2.3.2.1c, 2.3.4, 5, 7.2, and 9 [78], whereas those of the H5N6 subtype belonged to clade 2.3.4.4 [79]. In Indonesia, H5N1 IAVs-S belonging to clades 2.1.1, 2.1.3 and 2.1.3.3 were isolated from 2005 through 2007. In Korea, an H5N2 IAV-S whose HA gene did not derive from the Gs/Gd lineage but instead belonged to the EA lineage was isolated in 2004 [81].
Table 2.
H5 IAVs-S isolated in Asian countries.
| Representative Strain from Each Cluster a | Isolation Date b | Subtype | Country | Clade c | GISAID Isolate ID | Reference |
|---|---|---|---|---|---|---|
| A/swine/Shandong/2/03 | 2003 | H5N1 | China | 0 | EPI_ISL_4138 | none available |
| A/swine/Banten/UT2071/2005 | 2005 | H5N1 | Indonesia | 2.1.1 | EPI_ISL_76964 | [80] |
| A/swine/Tabanan/061/2006 | 30 September 2006 | H5N1 | Indonesia | 2.1.3 | EPI_ISL_190517 | none available |
| A/swine/Banten/UT6008/2007 | 2007 | H5N1 | Indonesia | 2.1.3 | EPI_ISL_76972 | [80] |
| A/swine/East_Java/UT6010/2007 | 2007 | H5N1 | Indonesia | 2.1.3.3 | EPI_ISL_76973 | [80] |
| A/swine/Korea/C12/2008 | 2008 | H5N2 | Korea | EA-nonGsGD | EPI_ISL_28756 | [81] |
| A/swine/Jiangsu/1/2008 | December 2008 | H5N1 | China | 7.2 | EPI_ISL_144531 | [78] |
| A/swine/Jiangsu/2/2009 | January 2009 | H5N1 | China | 2.3.4 | EPI_ISL_144532 | [78] |
| A/swine/Guangdong/2/2014 | 18 June 2014 | H5N6 | China | 2.3.4.4 | EPI_ISL_196101 | [79] |
| A/swine/Shandong/SD1/2014 | 14 October 2014 | H5N1 | China | 5 | EPI_ISL_226073 | none available |
| A/swine/Shandong/SD2/2014 | 3 November 2014 | H5N1 | China | 9 | EPI_ISL_226074 | none available |
| A/swine/Zhejiang/SW57/2015 | January 2015 | H5N1 | China | 2.3.2.1c | EPI_ISL_393329 | none available |
| A/swine/Guangdong/G3/2015 | 26 March 2015 | H5N6 | China | 2.3.4.4 | EPI_ISL_266596 | none available |
a Sequences found in GISAID, which contains the entire open reading frame of the HA protein of isolates from each country, separately underwent cd-hit clustering (threshold, 98.5% homology); the most recent isolate in each cluster is listed as the representative strain. b Isolation date is given as Day Month Year, unless the month or date (or both) was unavailable. c Clade classification was performed at https://www.fludb.org/brc/h5n1Classifier.spg?method=ShowCleanInputPage&decorator=influenza on 11 August 2021.
5. H9N2 Subtype IAVs-S
IAVs of H9N2 subtype have been endemic in poultry in Asian countries since the late 1990s [82]. HA genes of this subtype have diversified as the endemic intensified. H9 HA genes have been classified into four major lineages—h9.1, h9.2, h9.3, and h9.4 [83]. The h9.1 and h9.2 lineages are known as the “North American” lineages; the clades h9.3 and h9.4 are further divided into sub-clades indicated with four-digit numerals (e.g., h9.3.3.1, h9.4.1.1). Sub-clades h9.4.1 and h9.4.2 are the so-called the G1- and Y280/G9-lineages, respectively, and h9.3.3 is Y439 or Korean lineage. Sporadic transmission from poultry to swine occurred in China, Hong Kong, and Korea from the late 1990s until 2010. H9N2 IAVs-S from poultry in China and Hong Kong belonged to the h9.4 lineage [83,84,85,86,87,88,89], whereas those in Korea fall into the h9.3, particularly h9.3.3.1 (Table 3). In addition, sequence of Chinese IAV-S belonging to h9.1—A/swine/Yantai/16/2012 (Table 3)—has been reported, suggesting that direct transmission of an AIV directly from a wild bird into Chinese pig population might have occurred.
Table 3.
H9 IAV-S isolated in Asian countries.
| Representative Strain from Each Cluster a | Isolation Date b | Subtype | Country | Clade c | GISAID Isolate ID | Reference |
|---|---|---|---|---|---|---|
| A/swine/Hong Kong/10/1998 | 1998 | H9N2 | Hong Kong | h9.4.2.3 | EPI_ISL_379214 | [90] |
| A/swine/ShanDong/1/2003 | 2003 | H9N2 | China | h9.4.2.3 | EPI_ISL_3465 | [87] |
| A/swine/Guangxi/58/2005 | 2005 | H9N2 | China | h9.4.2.3 | EPI_ISL_12580 | [84] |
| A/swine/Jiangxi/1/2004 | 2004 | H9N2 | China | h9.4.2.4 | EPI_ISL_15583 | [84] |
| A/swine/Korea/S452/2004 | 2004 | H9N2 | Korea | h9.3.3.1 | EPI_ISL_4617 | none available |
| A/swine/Guangxi/7/2007 | 1 February 2007 | H9N2 | China | h9.4.2.4 | EPI_ISL_81608 | [84] |
| A/swine/Henan/Y1/2009 | 2 July 2009 | H9N2 | China | h9.4.2.5 | EPI_ISL_139172 | [88] |
| A/swine/Shanghai/Y1/2009 | 13 October 2009 | H9N2 | China | h9.4.2.5 | EPI_ISL_139173 | none available |
| A/swine/Guangdong/L1/2010 | 30 January 2010 | H9N2 | China | h9.4.2.1 | EPI_ISL_179451 | [89] |
| A/swine/Yantai/16/2012 | 21 September 2012 | H9N2 | China | h9.1 | EPI_ISL_229212 | none available |
| A/swine/China/SPF_embryonated_chicken_eggs/2015 | 18 May 2015 | H9N2 | China | h9.4.2.5 | EPI_ISL_381335 | none available |
| A/swine/Shandong/TA009/2019 | April 2019 | H9N2 | China | h9.4.2.5 | EPI_ISL_503942 | none available |
a Sequences found in GISAID, which contains the entire open reading frame of the HA protein of isolates from each country, separately underwent cd-hit clustering [69] (threshold, 98.5% homology); the most recent isolate in each cluster was listed as the representative strain. b Isolation date is as Day Month Year, unless the month or date (or both) was unavailable. c Clade classification follows Jiang, et al., 2012.
6. IAVs-S of Subtypes Other Than H1, H3, H5, and H9
Owing to intensive surveillance, AIVs have been isolated from pigs in China and Korea (Table 4). Viruses of the subtype H4N1 [91], H4N8 [92], H6N6 [93], H7N9, and H10N5 [94] have been isolated in China and of subtypes H7N2 [95] and H11N6 in Korea. Except for those isolated from specimens collected at slaughterhouses, these viruses were isolated from specimens collected from pigs with respiratory symptoms, including coughing and nasal discharge. These findings indicated that, although viruses of these AIV subtypes caused diseases in infected pigs, they rarely became established in the pig population.
Table 4.
IAVs-S of subtypes other than H1, H3, H5, and H9 subtypes isolated in Asian countries.
| Strain a | Isolation Date b | Subtype | Country | GISAID Isolate ID | Reference |
|---|---|---|---|---|---|
| A/swine/HuBei/06/2009 | 24 May 2009 | H4N1 | China | EPI_ISL_130351 | [91] |
| A/swine/Guangdong/K4/2011 | 21 October 2011 | H4N8 | China | EPI_ISL_127501 | [92] |
| A/swine/Yangzhou/080/2009 | January 2009 | H6N6 | China | EPI_ISL_139117 | none available |
| A/swine/Guangdong/K6/2010 | 17 January 2010 | H6N6 | China | EPI_ISL_89164 | [93] |
| A/swine/KU/16/2001 | 2001 | H7N2 | Korea | EPI_ISL_85566 | [95] |
| A/swine/eastern_China/005/2017 | 20 August 2017 | H7N9 | China | EPI_ISL_505059 | none available |
| A/swine/eastern_China/HH24/2017 | 7 August 2017 | H7N9 | China | EPI_ISL_505060 | none available |
| A/swine/Hubei/10/2008 | 30 April 2008 | H10N5 | China | EPI_ISL_129071 | [94] |
| A/swine/KU/2/2001 | 2001 | H11N6 | Korea | EPI_ISL_80217 | none available |
a IAVs-S from Asian countries found in GISAID, which contains the entire open reading frame of each HA protein. b Isolation date is given as Day Month Year, unless the month or date (or both) was unavailable.
7. Discussion
Our current phylogenetic comparison across the IAVs-S isolated from different countries and regions in Asia revealed country- or region-specific evolution in the HA of both the H1 and H3 subtypes of IAVs-S. Among H1 HA subtypes, although clade 1A.3.3.2 viruses derived from human pandemic strains circulated in all of the Asian countries that we compared, co-circulation of other clade(s) of H1 IAVs-S also was evident in most of these countries (Table 1). Co-circulating clades and the relative proportions of each within the H1 subtype vary from one country or region to another. Regarding the H3 HA isolates, although most of them originated from human seasonal H3 IAVs, the clade that predominated differed between Asian countries and regions.
Combining rigorous hygiene practices with a robust IAV-S vaccination program effectively controls IAV-S outbreaks and reduces their economic impact on swine farms. For SI vaccines to be maximally efficacious, the viral strain in the vaccine must be antigenically matched to those circulating on swine farms. In this regard, genetic characterization of IAV-S coupled with their antigenic characterization and followed by timely updates of the vaccine components are essential for each country or region. Differences in antigenicity between IAV-S clades co-circulating in Japanese swine populations have been demonstrated [23]. For example, antiserum produced against the 1A-like virus A/swine/Tochigi/2/2011 (H1N2) reacted eightfold less strongly to A/swine/Yamagata/11/2010 (H1N1) which belongs to clade 1A.3.3.2. Antigenic change among co-circulating clades of a subtype within a country or region or among countries could be accelerated over time, if IAVs-S evolve independently. Should that evolution reaches a point where a vaccine component directed toward one clade no longer offers cross-protection against the other, multiple antigenic components for such subtype need to be included in the vaccine, similar to some vaccines commercially available in North America and Europe [4].
Antigenic change between viruses belonging to the same clade is known to occur within a country or region as well as between them [32]. For example, according to calculation of the mean pairwise antigenic distance (MPD), viruses isolated from Japan and Hong Kong, both belonging to clade 1A as classified by the current criteria, differed by 5.6 antigenic units (AU) [32]. Our current phylogenetic comparison demonstrated that the 1A1.1/1A.1-like isolates from Japan and Hong Kong diverged during the mid-1970s. When such genetic differentiation between countries or regions also increases the antigenic distance, a region- or country-specific SI vaccine is needed.
Human infection by IAVs-S or their reassortants is a threat to public health, as realized by the emergence of H1N1pdm due to reassortment among IAVs-S [96,97,98]. Control of the IAVs-S in pig populations is important not only for preventing the disease burden on pigs but also to prevent the human infection. Incidental human infections with IAVs-S have occurred in North America, Europe, and Australia [99,100,101,102,103,104,105]. Although no large outbreaks in humans have erupted recently in Asian countries, several incidents have been reported in China [46,106,107], Thailand [108], and Vietnam [61]. IAVs-S of the H1 and H3 subtypes are mostly antigenically different from the human seasonal influenza viruses, such that most of the human population lacks protective antibodies against these viruses [109,110,111,112], except for those originating from human H1N1pdm strains. Human infection by IAVs-S has the potential to cause an outbreak and lead to a pandemic, as we have experienced in 2009. Caretakers at farms in Asia are at high risk to IAV-S infection [107,113] and high likelihood of passing that infection to the rest of the country’s population. Effective IAV-S vaccination programs on pig farms could reduce the exposure of humans to IAVs-S and thus the potential risk of a human pandemic [114].
In conclusion, our review of genetic information regarding the HA genes of Asian IAVs-S reveals sparse sequence data from countries where the pig industry is thriving, including Myanmar, the Philippines, India, and Indonesia. Due to a lack of knowledge and the unavailability of effective vaccines, SI is not recognized as a major swine disease by pig producers in these countries. Therefore, most Asian countries neither monitor circulating IAVs-S nor vaccinate against IAV-S routinely. Furthermore, our findings reveal geographical segregation of the genetic evolution of the HA genes within the subtypes. This genetic segregation points to the need to develop region- or country-specific IAV-S vaccines for their maximum efficacy. However, genetic segregation is not always directly correlated with antigenic segregation. Therefore, the antigenic diversity of IAVs-S needs to be evaluated concurrently with available genetic diversity data to develop vaccines that are well matched to—and thus highly protective against—the IAVs-S circulating in the swine populations in each country or region in Asia. Whether such an approach is feasible remains to be seen.
Acknowledgments
We thank the contributing laboratories of GISAID for depositing the hemagglutinin sequences of IAVs-S into the database.
Author Contributions
Conceptualization, T.S. and B.N.H.; Construction of phylogenetic trees, T.S. and S.S.; writing—original draft preparation, T.S.; writing—review and editing, J.M., Y.U. and B.N.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by a collaboration research agreement between the National Institute of Animal Health, NARO, and MSD-Animal Health.
Conflicts of Interest
The authors of this manuscript have the following competing interests: B.N.H. is employed at MSD Animal Health, a commercial company.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pleschka S. Overview of influenza viruses. Curr. Top. Microbiol. Immunol. 2013;370:1–20. doi: 10.1007/82_2012_272. [DOI] [PubMed] [Google Scholar]
- 2.Deblanc C., Robert F., Pinard T., Gorin S., Quéguiner S., Gautier-Bouchardon A.V., Ferré S., Garraud J.M., Cariolet R., Brack M., et al. Pre-infection of pigs with Mycoplasma hyopneumoniae induces oxidative stress that influences outcomes of a subsequent infection with a swine influenza virus of H1N1 subtype. Vet. Microbiol. 2013;162:643–651. doi: 10.1016/j.vetmic.2012.11.028. [DOI] [PubMed] [Google Scholar]
- 3.Draayer H. Autogenous vaccines: Determining product need and antigen selection, production and testing considerations. Dev. Biol. 2004;117:43–47. [PubMed] [Google Scholar]
- 4.Gracia J.C.M., Pearce D.S., Masic A., Balasch M. Influenza A Virus in Swine: Epidemiology, Challenges and Vaccination Strategies. Front. Vet. Sci. 2020;7:647. doi: 10.3389/fvets.2020.00647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pascua P.N.Q., Song M.S., Lee J.H., Choi H.W., Han J.H., Kim J.H., Yoo G.J., Kim C.J., Choi Y.K. Seroprevalence and genetic evolutions of swine influenza viruses under vaccination pressure in Korean swine herds. Virus Res. 2008;138:43–49. doi: 10.1016/j.virusres.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 6.Choi Y.K., Goyal S.M., Joo H.S. Retrospective analysis of etiologic agents associated with respiratory diseases in pigs. Can. Vet. J. 2003;44:735–737. [PMC free article] [PubMed] [Google Scholar]
- 7.Fao U.N. The human influenza due to a novel subtype H1N1. Empres Watch. 2009:1–3. [Google Scholar]
- 8.Takemae N., Shobugawa Y., Nguyen P.T., Nguyen T., Nguyen T.N., To T.L., Thai P.D., Nguyen T.D., Nguyen D.T., Nguyen D.K., et al. Effect of herd size on subclinical infection of swine in Vietnam with influenza A viruses. BMC Vet. Res. 2016;12:227. doi: 10.1186/s12917-016-0844-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bowman A., Nolting J., Nelson S., Slemons R. Subclinical influenza virus A infections in pigs exhibited at agricultural fairs, Ohio, USA, 2009–2011. Emerg. Infect. Dis. 2012;18:1945–1950. doi: 10.3201/eid1812.121116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hause B.M., Duff J.W., Scheidt A. Virus detection using metagenomic sequencing of swine nasal and rectal swabs. J. Swine Health Prod. 2016;24:304–308. [Google Scholar]
- 11.Díaz J.A.C., Fitzgerald R.M., Shalloo L., da Costa M.R., Niemi J., Leonard F.C., Kyriazakis I., Manzanilla E.G. Financial Analysis of Herd Status and Vaccination Practices for Porcine Reproductive and Respiratory Syndrome Virus, Swine Influenza Virus, and Mycoplasma hyopneumoniae in Farrow-to-Finish Pig Farms Using a Bio-Economic Simulation Model. Front. Vet. Sci. 2020;7:556674. doi: 10.3389/fvets.2020.556674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bennett R., IJpelaar J. Updated estimates of the costs associated with thirty four endemic livestock diseases in Great Britain: A note. J. Agric. Econ. 2005;56:135–144. doi: 10.1111/j.1477-9552.2005.tb00126.x. [DOI] [Google Scholar]
- 13.FAO U. FAOSTAT. [(accessed on 9 July 2021)]. Available online: http://www.fao.org/faostat/en/#data/QCL.
- 14.Anderson T.K., Macken C.A., Lewis N.S., Scheuermann R.H., Van Reeth K., Brown I.H., Swenson S.L., Simon G., Saito T., Berhane Y., et al. A Phylogeny-Based Global Nomenclature System and Automated Annotation Tool for H1 Hemagglutinin Genes from Swine Influenza A Viruses. mSphere. 2016;1:e00275-16. doi: 10.1128/mSphere.00275-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vincent A., Awada L., Brown I., Chen H., Claes F., Dauphin G., Donis R., Culhane M., Hamilton K., Lewis N., et al. Review of influenza A virus in swine worldwide: A call for increased surveillance and research. Zoonoses Public Health. 2014;61:4–17. doi: 10.1111/zph.12049. [DOI] [PubMed] [Google Scholar]
- 16.Chauhan R.P., Gordon M.L. A systematic review analyzing the prevalence and circulation of influenza viruses in swine population worldwide. Pathogens. 2020;9:355. doi: 10.3390/pathogens9050355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palese P., Shaw M. Orthomyxovirdae: The Virus and THEIR Replication. 5th ed. Lippincott Williams & WIlkins; Philadelphia, PA, USA: 2007. pp. 1647–1689. [Google Scholar]
- 18.Mine J., Uchida Y., Takemae N., Saito T. Genetic Characterization of Influenza A Viruses in Japanese Swine in 2015 to 2019. J. Virol. 2020;94:e02169-19. doi: 10.1128/JVI.02169-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nonthabenjawan N., Chanvatik S., Chaiyawong S., Jairak W., Boonyapisusopha S., Tuanudom R., Thontiravong A., Bunpapong N., Amonsin A. Genetic diversity of swine influenza viruses in Thai swine farms, 2011–2014. Virus Genes. 2015;50:221–230. doi: 10.1007/s11262-014-1153-x. [DOI] [PubMed] [Google Scholar]
- 20.Zhao Y., Sun F., Li L., Chen T., Cao S., Ding G., Cong F., Liu J., Qin L., Liu S., et al. Evolution and pathogenicity of the H1 and H3 subtypes of swine influenza virus in mice between 2016 and 2019 in China. Viruses. 2020;12:298. doi: 10.3390/v12030298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takemae N., Parchariyanon S., Damrongwatanapokin S., Uchida Y., Ruttanapumma R., Watanabe C., Yamaguchi S., Saito T. Genetic diversity of swine influenza viruses isolated from pigs during 2000 to 2005 in Thailand. Influenza Other Respir. Viruses. 2008;2:181–189. doi: 10.1111/j.1750-2659.2008.00062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mine J., Abe H., Parchariyanon S., Boonpornprasert P., Ubonyaem N., Nuansrichay B., Takemae N., Tanikawa T., Tsunekuni R., Uchida Y., et al. Genetic and antigenic dynamics of influenza A viruses of swine on pig farms in Thailand. Arch. Virol. 2019;164:457–472. doi: 10.1007/s00705-018-4091-4. [DOI] [PubMed] [Google Scholar]
- 23.Takemae N., Nguyen T., Ngo L.T., Hiromoto Y., Uchida Y., Pham V.P., Kageyama T., Kasuo S., Shimada S., Yamashita Y., et al. Antigenic variation of H1N1, H1N2 and H3N2 swine influenza viruses in Japan and Vietnam. Arch. Virol. 2013;158:859–876. doi: 10.1007/s00705-013-1616-8. [DOI] [PubMed] [Google Scholar]
- 24.de Jong J., Smith D., Lapedes A., Donatelli I., Campitelli L., Barigazzi G., Van Reeth K., Jones T., Rimmelzwaan G., Osterhaus A., et al. Antigenic and genetic evolution of swine influenza A (H3N2) viruses in Europe. J. Virol. 2007;81:4315–4322. doi: 10.1128/JVI.02458-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Katsuda K., Sato S., Shirahata T., Lindstrom S., Nerome R., Ishida M., Nerome K., Goto H. Antigenic and genetic characteristics of H1N1 human influenza virus isolated from pigs in Japan. J. Gen. Virol. 1995;76:1247–1249. doi: 10.1099/0022-1317-76-5-1247. [DOI] [PubMed] [Google Scholar]
- 26.Kyriakis C.S., Gramer M.R., Barbé F., Van Doorsselaere J., Van Reeth K. Efficacy of commercial swine influenza vaccines against challenge with a recent European H1N1 field isolate. Vet. Microbiol. 2010;144:67–74. doi: 10.1016/j.vetmic.2009.12.039. [DOI] [PubMed] [Google Scholar]
- 27.Van Reeth K., Labarque G., De Clercq S., Pensaert M. Efficacy of vaccination of pigs with different H1N1 swine influenza viruses using a recent challenge strain and different parameters of protection. Vaccine. 2001;19:4479–4486. doi: 10.1016/S0264-410X(01)00206-7. [DOI] [PubMed] [Google Scholar]
- 28.Van Reeth K., Gregory V., Hay A., Pensaert M. Protection against a European H1N2 swine influenza virus in pigs previously infected with H1N1 and/or H3N2 subtypes. Vaccine. 2003;21:1375–1381. doi: 10.1016/S0264-410X(02)00688-6. [DOI] [PubMed] [Google Scholar]
- 29.De Vleeschauwer A., Qiu Y., Van Reeth K. Vaccination-challenge studies with a Port Chalmers/73 (H3N2)-based swine influenza virus vaccine: Reflections on vaccine strain updates and on the vaccine potency test. Vaccine. 2015;33:2360–2366. doi: 10.1016/j.vaccine.2015.03.031. [DOI] [PubMed] [Google Scholar]
- 30.Van Reeth K., Van Gucht S., Pensaert M. Investigations of the efficacy of European H1N1- and H3N2-based swine influenza vaccines against the novel H1N2 subtype. Vet. Rec. 2003;153:9–13. doi: 10.1136/vr.153.1.9. [DOI] [PubMed] [Google Scholar]
- 31.Everett H.E., Aramouni M., Coward V., Ramsay A., Kelly M., Morgan S., Tchilian E., Canini L., Woolhouse M.E.J., Gilbert S., et al. Vaccine-mediated protection of pigs against infection with pandemic H1N1 2009 swine influenza A virus requires a close antigenic match between the vaccine antigen and challenge virus. Vaccine. 2019;37:2288–2293. doi: 10.1016/j.vaccine.2019.02.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lewis N.S., Russell C.A., Langat P., Anderson T.K., Berger K., Bielejec F., Burke D.F., Dudas G., Fonville J.M., Fouchier R.A., et al. The global antigenic diversity of swine influenza A viruses. eLife. 2016;5:e12217. doi: 10.7554/eLife.12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Reeth K., Ma W. Swine influenza virus vaccines: To change or not to change-that’s the question. Curr. Top. Microbiol. Immunol. 2013;370:173–200. doi: 10.1007/82_2012_266. [DOI] [PubMed] [Google Scholar]
- 34.Vincent A., Ma W., Lager K., Janke B., Richt J. Swine influenza viruses a North American perspective. Adv. Virus Res. 2008;72:127–154. doi: 10.1016/S0065-3527(08)00403-X. [DOI] [PubMed] [Google Scholar]
- 35.Sandbulte M., Spickler A., Zaabel P., Roth J. Optimal Use of Vaccines for Control of Influenza A Virus in Swine. Vaccines. 2015;3:22–73. doi: 10.3390/vaccines3010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schultz-Cherry S., Olsen C.W., Easterday B.C. History of Swine influenza. Curr. Top. Microbiol. Immunol. 2013;370:21–28. doi: 10.1007/82_2011_197. [DOI] [PubMed] [Google Scholar]
- 37.Pensaert M., Ottis K., Vandeputte J., Kaplan M.M., Bachmann P.A. Evidence for the natural transmission of influenza A virus from wild ducts to swine and its potential importance for man. Bull. World Health Organ. 1981;59:75–78. [PMC free article] [PubMed] [Google Scholar]
- 38.Cappuccio J., Pena L., Dibárbora M., Rimondi A., Piñeyro P., Insarralde L., Quiroga M., Machuca M., Craig M., Olivera V., et al. Outbreak of swine influenza in Argentina reveals a non-contemporary human H3N2 virus highly transmissible among pigs. J. Gen. Virol. 2011;92:2871–2878. doi: 10.1099/vir.0.036590-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bolton M.J., Abente E.J., Venkatesh D., Stratton J.A., Zeller M., Anderson T.K., Lewis N.S., Vincent A.L. Antigenic evolution of H3N2 influenza A viruses in swine in the United States from 2012 to 2016. Influenza Other Respir. Viruses. 2019;13:83–90. doi: 10.1111/irv.12610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou N.N., Senne D.A., Landgraf J.S., Swenson S.L., Erickson G., Rossow K., Liu L., Yoon K.-J., Krauss S., Webster R.G. Genetic Reassortment of Avian, Swine, and Human Influenza A Viruses in American Pigs. J. Virol. 1999;73:8851–8856. doi: 10.1128/JVI.73.10.8851-8856.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Webby R., Swenson S., Krauss S., Gerrish P., Goyal S., Webster R. Evolution of swine H3N2 influenza viruses in the United States. J. Virol. 2000;74:8243–8251. doi: 10.1128/JVI.74.18.8243-8251.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ngo L.T., Hiromoto Y., Pham V.P., Hong H.T., Nguyen L.H.T., Le V.T., Takemae N., Saito T. Isolation of novel triple-reassortant swine H3N2 influenza viruses possessing the hemagglutinin and neuraminidase genes of a seasonal influenza virus in Vietnam in 2010. Influenza Other Respir. Viruses. 2012;6:6–10. doi: 10.1111/j.1750-2659.2011.00267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liang H., Lam T.T.Y., Fan X., Chen X., Zeng Y., Zhou J., Duan L., Tse M., Chan C.H., Li L., et al. Expansion of Genotypic Diversity and Establishment of 2009 H1N1 Pandemic-Origin Internal Genes in Pigs in China. J. Virol. 2014;88:10864–10874. doi: 10.1128/JVI.01327-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.He P., Wang G., Mo Y., Yu Q., Xiao X., Yang W., Zhao W., Guo X., Chen Q., He J., et al. Novel triple-reassortant influenza viruses in pigs, Guangxi, China. Emerg. Microbes Infect. 2018;7:1–9. doi: 10.1038/s41426-018-0088-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun Y.-F., Wang X.-H., Li X.-L., Zhang L., Li H.-H., Lu C., Yang C.-L., Feng J., Han W., Ren W.-K., et al. Novel triple-reassortant H1N1 swine influenza viruses in pigs in Tianjin, Northern China. Vet. Microbiol. 2016;183:85–91. doi: 10.1016/j.vetmic.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 46.Sun H., Xiao Y., Liu J., Wang D., Li F., Wang C., Li C., Zhu J., Song J., Sun H., et al. Prevalent Eurasian avian-like H1N1 swine influenza virus with 2009 pandemic viral genes facilitating human infection. Proc. Natl. Acad. Sci. USA. 2020;117:17204–17210. doi: 10.1073/pnas.1921186117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Su S., Chen J.-D., Qi H.-T., Zhu W.-J., Xie J.-X., Huang Z., Tan L.-K., Qi W.-B., Zhang G.-H. Complete Genome Sequence of a Novel Avian-Like H3N2 Swine Influenza Virus Discovered in Southern China. J. Virol. 2012;86:9533. doi: 10.1128/JVI.01315-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu H., Zhang P.-C., Zhou Y.-J., Li G.-X., Pan J., Yan L.-P., Shi X.-X., Liu H.-L., Tong G.-Z. Isolation and genetic characterization of avian-like H1N1 and novel ressortant H1N2 influenza viruses from pigs in China. Biochem. Biophys. Res. Commun. 2009;386:278–283. doi: 10.1016/j.bbrc.2009.05.056. [DOI] [PubMed] [Google Scholar]
- 49.Matsuu A., Uchida Y., Takemae N., Mawatari T., Kasai S., Yoneyama T., Nakamura K.R., Eto M., Saito T. Genetic characterization of swine influenza viruses isolated in Japan between 2009 and 2012. Microbiol. Immunol. 2012;56:792–803. doi: 10.1111/j.1348-0421.2012.00501.x. [DOI] [PubMed] [Google Scholar]
- 50.Nerome K., Ishida M., Oya A., Oda K. The Possible Origin of H1N1(Hsw1N1) Virus in the Swine Population of Japan and Antigenic Analysis of the Isolates. J. Gen. Virol. 1982;62:171–175. doi: 10.1099/0022-1317-62-1-171. [DOI] [PubMed] [Google Scholar]
- 51.Ito T., Kawaoka Y., Vines A., Ishikawa H., Asai T., Kida H. Continued circulation of reassortant H1N2 influenza viruses in pigs in Japan. Arch. Virol. 1998;143:1173–1182. doi: 10.1007/s007050050415. [DOI] [PubMed] [Google Scholar]
- 52.Kirisawa R., Ogasawara Y., Yoshitake H., Koda A., Furuya T. Genomic reassortants of pandemic A (H1N1) 2009 virus and endemic porcine H1 and H3 viruses in swine in Japan. J. Vet. Med. Sci. 2014;76:1457–1470. doi: 10.1292/jvms.14-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ozawa M., Matsuu A., Yonezawa K., Igarashi M., Okuya K., Kawabata T., Ito K., Tsukiyama-Kohara K., Taneno A., Deguchi E. Efficient isolation of Swine influenza viruses by age-targeted specimen collection. J. Clin. Microbiol. 2015;53:1331–1338. doi: 10.1128/JCM.02941-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim J.I., Lee I., Park S., Lee S., Hwang M.W., Bae J.Y., Heo J., Kim D., Jang S.I., Kim K., et al. Phylogenetic analysis of a swine influenza a(H3N2) virus isolated in Korea in 2012. PLoS ONE. 2014;9:e88782. doi: 10.1371/journal.pone.0088782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Noh J.Y., Lo V.T., Kim Y.J., Yoon S.-W., Jeong D.G., Na W., Song D., Kim H.K. Complete Coding Sequence of a Swine Influenza A Variant (H3N2) Virus Isolated in the Republic of Korea in 2017. Microbiol. Resour. Announc. 2020;9:e01355-19. doi: 10.1128/MRA.01355-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pascua P.N.Q., Lim G.J., Kwon H.I., Park S.J., Kim E.H., Song M.S., Kim C.J., Choi Y.K. Emergence of H3N2pM-like and novel reassortant H3N1 swine viruses possessing segments derived from the A (H1N1)pdm09 influenza virus, Korea. Influenza Other Respir. Viruses. 2013;7:1283–1291. doi: 10.1111/irv.12154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pascua P.N.Q., Lim G.J., Kwon H.I., Kim Y., Kim E.H., Park S.J., Kim S.M., Decano A.G., Choi E.J., Jeon H.Y., et al. Complete genome sequences of novel reassortant H1N2 swine influenza viruses isolated from pigs in the Republic of Korea. Genome Announc. 2013;1:e00552-13. doi: 10.1128/genomeA.00552-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hiromoto Y., Parchariyanon S., Ketusing N., Netrabukkana P., Hayashi T., Kobayashi T., Takemae N., Saito T. Isolation of the Pandemic (H1N1) 2009 virus and its reassortant with an H3N2 swine influenza virus from healthy weaning pigs in Thailand in 2011. Virus Res. 2012;169:175–181. doi: 10.1016/j.virusres.2012.07.025. [DOI] [PubMed] [Google Scholar]
- 59.Nasamran C., Janetanakit T., Chiyawong S., Boonyapisitsopa S., Bunpapong N., Prakairungnamthip D., Thontiravong A., Amonsin A. Persistence of pdm2009-H1N1 internal genes of swine influenza in pigs, Thailand. Sci. Rep. 2020;10:19847. doi: 10.1038/s41598-020-76771-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baudon E., Poon L.L., Dao T.D., Pham N.T., Cowling B.J., Peyre M., Nguyen K.V., Peiris M. Detection of Novel Reassortant Influenza A (H3N2) and H1N1 2009 Pandemic Viruses in Swine in Hanoi, Vietnam. Zoonoses Public Health. 2015;62:429–434. doi: 10.1111/zph.12164. [DOI] [PubMed] [Google Scholar]
- 61.Takemae N., Harada M., Nguyen P.T., Nguyen T., Nguyen T.N., To T.L., Nguyen T.D., Pham V.P., Le V.T., Do H.T., et al. Influenza A Viruses of Swine (IAV-S) in Vietnam from 2010 to 2015: Multiple Introductions of A(H1N1)pdm09 Viruses into the Pig Population and Diversifying Genetic Constellations of Enzootic IAV-S. J. Virol. 2017;91:e01490-16. doi: 10.1128/JVI.01490-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Takemae N., Nguyen P.T., Le V.T., Nguyen T.N., To T.L., Nguyen T.D., Pham V.P., Vo H.V., Le Q.V.T., Do H.T., et al. Appearance of reassortant European avian-origin H1 influenza A viruses of swine in Vietnam. Transbound. Emerg. Dis. 2018;65:1110–1116. doi: 10.1111/tbed.12849. [DOI] [PubMed] [Google Scholar]
- 63.Njabo K., Fuller T., Chasar A., Pollinger J., Cattoli G., Terregino C., Monne I., Reynes J., Njouom R., Smith T. Pandemic A/H1N1/2009 influenza virus in swine, Cameroon, 2010. Vet. Microbiol. 2012;156:189–192. doi: 10.1016/j.vetmic.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Howden K., Brockhoff E., Caya F., McLeod L., Lavoie M., Ing J., Bystrom J., Alexandersen S., Pasick J., Berhane Y., et al. An investigation into human pandemic influenza virus (H1N1) 2009 on an Alberta swine farm. Can. Vet. J. 2009;50:1153–1161. [PMC free article] [PubMed] [Google Scholar]
- 65.Hofshagen M., Gjerset B., Er C., Tarpai A., Brun E., Dannevig B., Bruheim T., Fostad I., Iversen B., Hungnes O., et al. Pandemic influenza A(H1N1)v: Human to pig transmission in Norway? Eurosurveillance. 2009;14:19406. doi: 10.2807/ese.14.45.19406-en. [DOI] [PubMed] [Google Scholar]
- 66.Moreno A., Di Trani L., Alborali L., Vaccari G., Barbieri I., Falcone E., Sozzi E., Puzelli S., Ferri G., Cordioli P. First Pandemic H1N1 Outbreak from a Pig Farm in Italy. Open Virol. J. 2010;4:52–56. doi: 10.2174/1874357901004010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Welsh M., Baird P., Guelbenzu-Gonzalo M., Hanna A., Reid S., Essen S., Russell C., Thomas S., Barrass L., McNeilly F., et al. Initial incursion of pandemic (H1N1) 2009 influenza A virus into European pigs. Vet. Rec. 2010;166:642–645. doi: 10.1136/vr.4851. [DOI] [PubMed] [Google Scholar]
- 68.Mon P.P., Thurain K., Janetanakit T., Nasamran C., Bunpapong N., Aye A.M., San Y.Y., Tun T.N., Amonsin A. Swine influenza viruses and pandemic H1N1-2009 infection in pigs, Myanmar. Transbound. Emerg. Dis. 2020;67:2653–2666. doi: 10.1111/tbed.13616. [DOI] [PubMed] [Google Scholar]
- 69.Fu L., Niu B., Zhu Z., Wu S., Li W. CD-HIT: Accelerated for clustering the next-generation sequencing data. Bioinformatics. 2012;28:3150–3152. doi: 10.1093/bioinformatics/bts565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lam T., Zhu H., Wang J., Smith D., Holmes E., Webster R., Webby R., Peiris J., Guan Y. Reassortment events among swine influenza A viruses in China: Implications for the origin of the 2009 influenza pandemic. J. Virol. 2011;85:10279–10285. doi: 10.1128/JVI.05262-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chutinimitku L.S., Thippamom N., Damrongwatanapokin S., Payungporn S., Thanawongnuwech R., Amonsin A., Boonsuk P., Sreta D., Bunpong N., Tantilertcharoen R., et al. Genetic characterization of H1N1, H1N2 and H3N2 swine influenza virus in Thailand. Arch. Virol. 2008;153:1049–1056. doi: 10.1007/s00705-008-0097-7. [DOI] [PubMed] [Google Scholar]
- 72.Kitikoon P., Sreta D., Tuanudom R., Amonsin A., Suradhat S., Oraveerakul K., Poovorawan Y., Thanawongnuwech R. Serological evidence of pig-to-human influenza virus transmission on Thai swine farms. Vet. Microbiol. 2011;148:413–418. doi: 10.1016/j.vetmic.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 73.Abe H., Mine J., Parchariyanon S., Takemae N., Boonpornprasert P., Ubonyaem N., Patcharasinghawut P., Nuansrichay B., Tanikawa T., Tsunekuni R., et al. Co-infection of influenza A viruses of swine contributes to effective shuffling of gene segments in a naturally reared pig. Virology. 2015;484:203–212. doi: 10.1016/j.virol.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 74.Kanehira K., Takemae N., Uchida Y., Hikono H., Saito T. Reassortant swine influenza viruses isolated in Japan contain genes from pandemic A(H1N1) 2009. Microbiol. Immunol. 2014;58:327–341. doi: 10.1111/1348-0421.12152. [DOI] [PubMed] [Google Scholar]
- 75.Olsen C., Karasin A., Carman S., Li Y., Bastien N., Ojkic D., Alves D., Charbonneau G., Henning B., Low D., et al. Triple reassortant H3N2 influenza A viruses, Canada, 2005. Emerg. Infect. Dis. 2006;12:113–1135. doi: 10.3201/eid1207.060268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tu J., Zhou H., Jiang T., Li C., Zhang A., Guo X., Zou W., Chen H., Jin M. Isolation and molecular characterization of equine H3N8 influenza viruses from pigs in China. Arch. Virol. 2009;154:887–890. doi: 10.1007/s00705-009-0381-1. [DOI] [PubMed] [Google Scholar]
- 77.Tian Z., Zhou G., Zheng B., Qiu H., Ni J., Yang H., Yin X., Hu S., Tong G. A recombinant pseudorabies virus encoding the HA gene from H3N2 subtype swine influenza virus protects mice from virulent challenge. Vet. Immunol. Immunopathol. 2006;111:211–218. doi: 10.1016/j.vetimm.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 78.He L., Zhao G., Zhong L., Liu Q., Duan Z., Gu M., Wang X., Liu X., Liu X. Isolation and characterization of two H5N1 influenza viruses from swine in Jiangsu Province of China. Arch. Virol. 2013;158:2531–2541. doi: 10.1007/s00705-013-1771-y. [DOI] [PubMed] [Google Scholar]
- 79.Li X., Fu Y., Yang J., Guo J., He J., Guo J., Weng S., Jia Y., Liu B., Li X., et al. Genetic and biological characterization of two novel reassortant H5N6 swine influenza viruses in mice and chickens. Infect. Genet. Evol. 2015;36:462–466. doi: 10.1016/j.meegid.2015.08.017. [DOI] [PubMed] [Google Scholar]
- 80.Nidom C.A., Takano R., Yamada S., Sakai-Tagawa Y., Daulay S., Aswadi D., Suzuki T., Suzuki Y., Shinya K., Iwatsuki-Horimoto K., et al. Influenza A (H5N1) viruses from pigs, Indonesia. Emerg. Infect. Dis. 2010;16:1515–1523. doi: 10.3201/eid1610.100508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee J.H., Pascua P.N.Q., Song M.-S., Baek Y.H., Kim C.-J., Choi H.-W., Sung M.-H., Webby R.J., Webster R.G., Poo H., et al. Isolation and Genetic Characterization of H5N2 Influenza Viruses from Pigs in Korea. J. Virol. 2009;83:4205–4215. doi: 10.1128/JVI.02403-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Carnaccini S., Perez D.R. H9 influenza viruses: An emerging challenge. Cold Spring Harb. Perspect. Med. 2020;10:a038588. doi: 10.1101/cshperspect.a038588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jiang W., Liu S., Hou G., Li J., Zhuang Q., Wang S., Zhang P., Chen J. Chinese and Global Distribution of H9 Subtype Avian Influenza Viruses. PLoS ONE. 2012;7:e52671. doi: 10.1371/journal.pone.0052671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yu H., Hua R.H., Wei T.C., Zhou Y.J., Tian Z.J., Li G.X., Liu T.Q., Tong G.Z. Isolation and genetic characterization of avian origin H9N2 influenza viruses from pigs in China. Vet. Microbiol. 2008;131:82–92. doi: 10.1016/j.vetmic.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 85.Yu H., Zhou Y.-J., Li G.-X., Ma J.-H., Yan L.-P., Wang B., Yang F.-R., Huang M., Tong G.-Z. Genetic diversity of H9N2 influenza viruses from pigs in China: A potential threat to human health? Vet. Microbiol. 2011;149:254–261. doi: 10.1016/j.vetmic.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 86.Cong Y.L., Pu J., Liu Q.F., Wang S., Zhang G.Z., Zhang X.L., Fan W.X., Brown E.G., Liu J.H. Antigenic and genetic characterization of H9N2 swine influenza viruses in China. J. Gen. Virol. 2007;88:2035–2041. doi: 10.1099/vir.0.82783-0. [DOI] [PubMed] [Google Scholar]
- 87.Xu C., Fan W., Wei R., Zhao H. Isolation and identification of swine influenza recombinant A/Swine/Shandong/1/2003(H9N2) virus. Microbes Infect. 2004;6:919–925. doi: 10.1016/j.micinf.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 88.Ge F., Li X., Ju H., Yang D., Liu J., Qi X., Wang J., Yang X., Qiu Y., Liu P., et al. Genotypic evolution and antigenicity of H9N2 influenza viruses in Shanghai, China. Arch. Virol. 2016;161:1437–1445. doi: 10.1007/s00705-016-2767-1. [DOI] [PubMed] [Google Scholar]
- 89.Kong W., Huang L., Zhang G. Isolation and phylogenetic analysis of H9N2 swine influenza virus from sick pigs in Southern China in 2010. J. Anim. Vet. Adv. 2011;10:2331–2342. doi: 10.1007/s13337-011-0035-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Saito T., Lim W., Suzuki T., Suzuki Y., Kida H., Nishimura S.-I., Tashiro M. Characterization of a human H9N2 influenza virus isolated in Hong Kong. Vaccine. 2001;20:125–133. doi: 10.1016/S0264-410X(01)00279-1. [DOI] [PubMed] [Google Scholar]
- 91.Hu Y., Liu X., Li S., Guo X., Yang Y., Jin M. Complete Genome Sequence of a Novel H4N1 Influenza Virus Isolated from a Pig in Central China. J. Virol. 2012;86:13879. doi: 10.1128/JVI.02726-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Su S., Qi W., Chen J., Cao N., Zhu W., Yuan L., Wang H., Zhang G. Complete genome sequence of an avian-like H4N8 swine influenza virus discovered in southern China. J. Virol. 2012;86:9542. doi: 10.1128/JVI.01475-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang G., Wenbao W.K., Ping Q.L., Zongxi L., Liangzong C., Haitao H., Nan Q., Wenhua C., Furong W., Zhangyong Z., et al. Identification of an H6N6 swine influenza virus in southern China. Infect. Genet. Evol. 2011;11:1174–1177. doi: 10.1016/j.meegid.2011.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang N., Zou W., Yang Y., Guo X., Hua Y., Zhang Q., Zhao Z., Jin M. Complete Genome Sequence of an H10N5 Avian Influenza Virus Isolated from Pigs in Central China. J. Virol. 2012;86:13865–13866. doi: 10.1128/JVI.02687-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kwon T.Y., Lee S.S., Kim C.Y., Shin J.Y., Sunwoo S.Y., Lyoo Y.S. Genetic characterization of H7N2 influenza virus isolated from pigs. Vet. Microbiol. 2011;153:393–397. doi: 10.1016/j.vetmic.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 96.Centers for Disease Control and Prevention Swine influenza A (H1N1) infection in two children--Southern California, March-April 2009. MMWR. Morb. Mortal. Wkly. Rep. 2009;58:400–402. [PubMed] [Google Scholar]
- 97.Neumann G., Noda T., Kawaoka Y. Emergence and pandemic potential of swine-origin H1N1 influenza virus. Nature. 2009;459:931–939. doi: 10.1038/nature08157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Smith G., Vijaykrishna D., Bahl J., Lycett S., Worobey M., Pybus O., Ma S., Cheung C., Raghwani J., Bhatt S., et al. Origins and evolutionary genomics of the 2009 swine-origin H1N1 influenza A epidemic. Nature. 2009;459:1122–1125. doi: 10.1038/nature08182. [DOI] [PubMed] [Google Scholar]
- 99.Centers for Disease Control and Prevention Swine-origin influenza A (H3N2) virus infection in two children--Indiana and Pennsylvania, July-August 2011. MMWR. Morb. Mortal. Wkly. Rep. 2011;60:1213–1215. [PubMed] [Google Scholar]
- 100.Cox C., Neises D., Garten R., Bryant B., Hesse R., Anderson G., Trevino-Garrison I., Shu B., Lindstrom S., Klimov A., et al. Swine influenza virus A (H3N2) infection in human, Kansas, USA, 2009. Emerg. Infect. Dis. 2011;17:1143–1144. doi: 10.3201/eid1706.101488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Epperson S., Jhung M., Richards S., Quinlisk P., Ball L., Moll M., Boulton R., Haddy L., Biggerstaff M., Brammer L., et al. Human infections with influenza A(H3N2) variant virus in the United States, 2011–2012. Clin. Infect. Dis. 2013;57((Suppl. 1)):S4–S11. doi: 10.1093/cid/cit272. [DOI] [PubMed] [Google Scholar]
- 102.Jhung M., Epperson S., Biggerstaff M., Allen D., Balish A., Barnes N., Beaudoin A., Berman L., Bidol S., Blanton L., et al. Outbreak of variant influenza A(H3N2) virus in the United States. Clin. Infect. Dis. 2013;57:1703–1712. doi: 10.1093/cid/cit649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Deng Y., Wong F., Spirason N., Kaye M., Beazley R., Grau M., Shan S., Stevens V., Subbarao K., Sullivan S., et al. Locally Acquired Human Infection with Swine-Origin Influenza A(H3N2) Variant Virus, Australia, 2018. Emerg. Infect. Dis. 2020;26:143–147. doi: 10.3201/eid2601.191144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Parys A., Vandoorn E., King J., Graaf A., Pohlmann A., Beer M., Harder T., Van Reeth K. Human Infection with Eurasian Avian-Like Swine Influenza A(H1N1) Virus, the Netherlands, September 2019. Emerg. Infect. Dis. 2021;27:939–943. doi: 10.3201/eid2703.201863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.de Jong J., Paccaud M., de Ronde-Verloop F., Huffels N., Verwei C., Weijers T., Bangma P., van Kregten E., Kerckhaert J., Wicki F. Isolation of swine-like influenza A(H1N1) viruses from man in Switzerland and The Netherlands. Ann. I’Institut Pasteur. Virol. 1988;139:429–437. doi: 10.1016/S0769-2617(88)80078-9. [DOI] [PubMed] [Google Scholar]
- 106.Li X., Guo L., Liu C., Cheng Y., Kong M., Yang L., Zhuang Z., Liu J., Zou M., Dong X., et al. Human infection with a novel reassortant Eurasian-avian lineage swine H1N1 virus in northern China. Emerg. Microbes Infect. 2019;8:1535–1545. doi: 10.1080/22221751.2019.1679611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zu R., Dong L., Qi X., Wang D., Zou S., Bai T., Li M., Li X., Zhao X., Xu C., et al. Virological and serological study of human infection with swine influenza A H1N1 virus in China. Virology. 2013;446:49–55. doi: 10.1016/j.virol.2013.07.022. [DOI] [PubMed] [Google Scholar]
- 108.Komadina N., Roque V., Thawatsupha P., Rimando-Magalong J., Waicharoen S., Bomasang E., Sawanpanyalert P., Rivera M., Iannello P., Hurt A., et al. Genetic analysis of two influenza A (H1) swine viruses isolated from humans in Thailand and the Philippines. Virus Genes. 2007;35:161–165. doi: 10.1007/s11262-007-0097-9. [DOI] [PubMed] [Google Scholar]
- 109.Hoa L.N.M., Bryant J.E., Choisy M., Nguyet L.A., Bao N.T., Trang N.H., Chuc N.T.K., Toan T.K., Saito T., Takemae N., et al. Population susceptibility to a variant swine-origin influenza virus A(H3N2) in Vietnam, 2011–2012. Epidemiol. Infect. 2015;143:2959–2964. doi: 10.1017/S0950268815000187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lorbach J., Fitzgerald T., Nolan C., Nolting J., Treanor J., Topham D., Bowman A. Gaps in Serologic Immunity against Contemporary Swine-Origin Influenza A Viruses among Healthy Individuals in the United States. Viruses. 2021;13:127. doi: 10.3390/v13010127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Vandoorn E., Leroux-Roels I., Leroux-Roels G., Parys A., Vincent A., Van Reeth K. Detection of H1 Swine Influenza A Virus Antibodies in Human Serum Samples by Age Group 1. Emerg. Infect. Dis. 2020;26:2118–2128. doi: 10.3201/eid2609.191796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Qiu Y., Muller C., Van Reeth K. Lower seroreactivity to European than to North American H3N2 swine influenza viruses in humans, Luxembourg, 2010. Euro Surveill. Bull. Eur. Sur Les Mal. Transm. Eur. Commun. Dis. Bull. 2015;20:25–33. doi: 10.2807/1560-7917.ES2015.20.13.21078. [DOI] [PubMed] [Google Scholar]
- 113.Borkenhagen L., Wang G., Simmons R., Bi Z., Lu B., Wang X., Wang C., Chen S., Song S., Li M., et al. High Risk of Influenza Virus Infection Among Swine Workers: Examining a Dynamic Cohort in China. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2020;71:622–629. doi: 10.1093/cid/ciz865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lorbach J.N., Nelson S.W., Lauterbach S.E., Nolting J.M., Kenah E., McBride D.S., Culhane M.R., Goodell C., Bowman A.S. Influenza Vaccination of Swine Reduces Public Health Risk at the Swine-Human Interface. mSphere. 2021;6:e01170-20. doi: 10.1128/mSphere.01170-20. [DOI] [PMC free article] [PubMed] [Google Scholar]