Abstract
The present study compared the abilities of different lipid carriers of amphotericin B (AMB) to activate murine peritoneal macrophages, as assessed by their capacities to produce nitric oxide (NO) and tumor necrosis factor alpha (TNF-α). Although AMB alone did not induce NO production, synergy was observed with gamma interferon but not with lipopolysaccharide. This synergy could not be explained by the mobilization of the nuclear activation factor NF-κB by AMB. On the other hand, AMB induced TNF-α production without a costimulator and no synergy was observed. Anti-TNF-α antibodies did not influence NO production, and an inhibitor of NO synthase did not affect TNF-α production, indicating that the production of one of these effector molecules was independent of that of the other. The incorporation of AMB into lipid carriers reduced NO and TNF-α production with all formulations but more so with liposomes than with lipid complexes. NO production was correlated with the induction of NO synthase II, revealed by Western blotting. The extent of association of AMB with macrophages depended on the formulation, especially on the AMB/lipids ratio: the higher the ratio was, the greater the AMB association with macrophages. However, there was no clear correlation between AMB association with macrophages, whether internalized or bound to the membrane, and immunostimulating effects. These results may explain the reduced toxicities of lipid-based formulations of AMB.
Amphotericin B (AMB) is considered to be the antifungal agent of choice for the treatment of many disseminated infections in immunocompromised patients (AIDS patients, cancer patients, or transplant recipients), despite its toxicity. Many hypotheses concerning the mechanisms of activity and/or toxicity of AMB have been put forward. In addition to its well-known property of complexing membrane sterols, AMB might also activate the innate immune defense functions of macrophages.
The immunomodulating effects of AMB may be exerted on polynuclear neutrophils, macrophages, natural killer cells, T cells, B cells, and lymph node cells. In particular, stimulation of macrophage-mediated defense mechanisms with AMB has been proposed as an interesting therapeutic strategy to supplement the direct antifungal activity of the drug. These immunostimulating effects may be mediated by the production of cytokines as well as by low-molecular-weight effector molecules (53).
Increased production of tumor necrosis factor alpha (TNF-α) (9, 42, 48), interleukin-1 (IL-1) (8, 11, 34, 42), IL-6 (17, 32), and macrophage colony-stimulating factor (M-CSF) (16) has been shown for macrophages treated with AMB, as has the production of prostaglandin E-2 (18). This cytokine and prostaglandin production may be responsible for some of the toxic effects of AMB, such as chills and fever (8, 18, 34). AMB has also been shown to trigger the respiratory burst (33, 51) leading to the generation of active oxygen metabolites, e.g., H2O2 and O2− (50), in macrophages and polymorphonuclear leukocytes. These products have been shown to reinforce the antimicrobial activity of AMB by their cytotoxic effects (41, 50, 51). On the other hand, AMB has been found to induce nitric oxide (NO) production as a result of NO synthase (NOS) induction only in the presence of a costimulator such as gamma interferon (IFN-γ), lipopolysaccharide (LPS), and M-CSF (16, 21, 37). This NO production may also increase the antimicrobial activity of AMB (29, 49). AMB has also been shown to modify some other functions of phagocytic cells: chemotaxis, adherence, and phagocytosis (39, 54).
Recent advances in the clinical application of AMB have focused on the improvement of its therapeutic index through reduction of toxicity as a result of its incorporation into lipid carriers. Several colloidal AMB preparations (Table 1) have appeared on the market or are undergoing clinical trials (5, 26). These preparations are either liposomes, such as AmBisome and ampholiposomes, or lipid complexes, such as Amphotec and Abelcet. It has already been observed that some of the immunostimulating effects mentioned above may be modified when AMB is associated with lipid carriers (3, 31, 33, 43). However, no systematic study comparing all the different formulations has been made.
TABLE 1.
AMB lipid formulations
| AMB formulation | Composition (molar ratio)a | AMB/lipids ratio (mol%) | Charge | Shape | Size (μm) |
|---|---|---|---|---|---|
| Liposomes | |||||
| AmBisome | HSPC/CHOL/DSPG/AMB (2:1:0.8:0.4) | 10 | Negative | Small unilamellar vesicles | 0.08 |
| Ampholiposomes | EPC/CHOL/SA/AMB (4:3:1:0.4) | 5 | Positive | Oligolamellar vesicles | 0.2–0.3 |
| Complexes | |||||
| Amphotec | CS/AMB (1:1) | 50 | Negative | Colloidal dispersion discs | 0.12 |
| Abelcet | DMPC/DMPG/AMB (7:3:5) | 33 | Negative | Lipid complexes (sheets) | 1–5 |
| LC-AMB | DMPC/DMPG/AMB (7:3:5) | 33 | Negative | Lipid complexes | 0.25 |
HSPC, hydrogenated soya phosphatidylcholine; CHOL, cholesterol; DSPG, distearoyl phosphatidylglycerol; EPC, egg yolk phosphatidylcholine; SA, stearylamine; CS, cholesteryl sulfate.
In order to evaluate the role of AMB lipid preparations in macrophage immunostimulation and the implication of this immunostimulation for antimicrobial therapy, the present study compares the abilities of free AMB and AMB associated with different lipid carriers, both commercial and prepared in our laboratory, to activate murine peritoneal macrophages in vitro. Among the possible effector molecules synthesized by macrophages, NO and TNF-α were chosen for detailed study because of their known cytotoxicities in vivo. Furthermore, the relationship between this activation and the uptake of AMB in different lipid carriers by the macrophages was investigated in an attempt to determine the mechanism of immunostimulation.
MATERIALS AND METHODS
Chemicals.
AMB was purchased from Sigma (Paris, France) and dissolved at 10 mg/ml in dimethyl sulfoxide before being diluted in culture medium. The commercial formulation Fungizone (mixed micelles with deoxycholate) was obtained from Squibb (Neuilly, France). The different lipid formulations are described in Table 1. AmBisome and ampholiposomes were kind gifts, respectively, from Nexstar Pharmaceutical (now Gilead Sciences, Foster City, Calif.) and the Pharmacie Centrale des Hôpitaux de Paris. Amphotec was obtained from Liposome Technology, Inc. (Menlo Park, Calif.), and Abelcet was obtained from the Liposome Company Ltd. (London, United Kingdom). Dimyristoyl phosphatidylcholine (DMPC) and dimyristoyl phosphatidylglycerol (DMPG) were purchased from Avanti Polar Lipids, Inc. (Alabaster, Ala.). Solvents and other reagents were obtained from Carlo Erba Reagenti (Val de Reuil, France).
Tissue culture products were obtained from Gibco (Eragny, France), and LPS was obtained from Salmonellae enteritis isolated by the Westphal method from Difco (distributed by Tebu, Le-Perray-en-Yvelines, France). Tissue culture flasks and 24-well plates from Dominique Dutscher (Brumath, France) and 96-well plates (Nunc) were obtained from ATGC (Noisy-le-Grand, France). Recombinant mouse IFN-γ, TNF-α, and anti-murine TNF-α antibody were obtained from Genzyme (Paris, France); the neutralizing dose for the last was 0.2 to 0.4 μg/ml according to the manufacturer. Actinomycin D, Triton X-100, sulfanilamide, N-(1-naphthyl)-ethylenediamine, sodium lauryl sulfate, dimethyl formamide, dimethylthiazol diphenyltetrazolium bromide (MTT), actinomycin D, and cytochalasin B were supplied by Sigma (I'Isle Abeau, France). All reagents and media for tissue culture experiments were tested for LPS content by a colorimetric Limulus amebocyte lysate assay (detection limit, 11 pg/ml; Whittaker M. A. Bioproducts, Walkersville, Md.). Polymyxin B sulfate was obtained from Fluka (Mulhouse, France), and l-N-monomethyl arginine (l-NMMA) was obtained from CalBiochem (Meudon, France).
Preparation of LC-AMB.
A colloidal dispersion of AMB (lipid complex of AMB [LC-AMB]) was prepared using the process solvent displacement methods described by Stainmesse et al. (45). Briefly, AMB (3.5 mg) was dissolved in methanol (15 ml) together with DMPC (3.5 mg) and DMPG (1.5 mg), and this organic solution was added to 15 ml of pure water. Methanol was removed, and the preparation was concentrated to a volume of 5 ml by low-pressure evaporation. The mean particle diameter, measured by laser light scattering on a typical preparation (Nanosizer N4; Coultronics, Margency, France), was 250 ± 50 nm (mean ± standard deviation for three runs), with a polydispersity index of 0.12.
Cell culture.
Elicited mouse peritoneal macrophages were harvested from 20- to 25-g female CD1 mice (Charles River Ltd., Saint Aubin les Elbeuf, France), 5 days after intraperitoneal injection of 1.5 ml of thioglycolate broth, by irrigating the peritoneal cavity with 6 ml of ice-cold medium (RPMI 1640 Glutamax-I) supplemented with fetal calf serum (FCS) (10% [vol/vol]). Five milliliters of medium containing cells was recovered. The cell suspensions were pooled and washed twice with complete medium (RPMI 1640 medium, supplemented with 10% FCS, 100 IU of penicillin per ml, and 100 IU of streptomycin per ml). After viable cells were counted in the presence of trypan blue, the macrophage suspension was diluted to the desired concentration, 106 cells/ml, in complete medium and plated into flat-bottomed 96-well plates at 100 μl/well. The cells were allowed to adhere for 3 h at 37°C in a humidified 95% air–5% CO2 atmosphere.
RAW 264.7 (ECACC catalogue number 91062702) cells were maintained in RPMI 1640 Glutamax-I medium, containing less than 5 pg of endotoxin per ml, supplemented with 5% decomplemented FCS guaranteed to have less than 20 pg of endotoxin and antibiotics per ml.
Macrophage activation.
After adherence, the medium in 96-well plates containing mouse peritoneal macrophages was removed and replaced by medium containing the different formulations of AMB at various concentrations in the presence of a costimulator where stated below. The costimulator was either IFN-γ at 20 IU/ml or LPS at 5 μg/ml (in both cases at suboptimal doses). After an activation phase (from 4 to 72 h), the medium was removed and kept for assay of TNF-α or nitrite, the latter being measured as an indication of NOS induction. In order to elucidate the role of TNF-α as a costimulant for NOS induction, similar experiments were performed in the presence of 10 μg of anti-TNF-α antibody per ml. Similarly, to determine the influence of NO on TNF-α secretion, a competitive inhibitor of NOS, l-NMMA (1 mM) was added during this phase in some experiments. To check that the formulations did not contain nitrite, which could be taken as indicating NO production, and did not have direct effects on L929 cells, similar manipulations were carried out on wells without macrophages.
Finally, to ensure that the immunomodulating effect of the AMB formulations were not due to contamination with traces of LPS, we performed experiments in which the formulations were preincubated with polymyxin B (2 μg/ml) for 15 min at 37°C before being added to the cells.
Measurement of nitrite.
Briefly, 100 μl of the culture medium was incubated with 200 μl of Griess reagent (19) (1% sulfanilamide–0.1% naphthylethylene diamine dihydrochloride) at room temperature for 30 min in the dark. The absorbance was measured at 540 nm using a Labsystem microplate reader. A calibration curve was prepared by using sodium nitrite in culture medium. The lower detection limit of this method was 1 μM nitrite. Medium containing a range of dilutions of the different AMB formulations incubated at 37°C between 6 and 170 h in the absence of macrophages did not contain nitrite or other material reacting with the Griess reagent and did not affect the growth of L929 cells (data not shown).
Measurement of TNF-α activity.
TNF-α activity was measured by the cytotoxicity assay on L929 mouse fibroblasts (ECACC catalogue number 85011425) in the presence of 2 μg of actinomycin D per ml as described by Flick and Gifford (15). Mouse peritoneal macrophages were incubated for 5 h with an immunomodulator as described above. Subconfluent L929 cells were suspended at 4 × 105 cells/ml in RPMI 1640 containing 10% serum. Aliquots (100 μl) of this cell suspension were dispensed into individual wells of microtitration plates. After adherence (4 h), the medium was removed and 100 μl of conditioned medium from macrophages was added. A calibration curve with recombinant mouse TNF-α was performed in each assay. The plates were incubated overnight at 37°C in a humidified 5% CO2 incubator. Cell viability was determined by a colorimetric assay using the tetrazolium salt MTT (12). We verified that L929 viability was restored when anti-TNF-α antibody was added.
Measurement of cell viability.
The protein contents of the macrophage monolayers were determined at the end of each experiment after the monolayers were washed twice in warm phosphate-buffered saline (PBS) and then lysed in 0.1% (wt/vol) Triton X-100, using the Bio-Rad (Ivry-sur-Seine, France) detergent-compatible assay (Lowry method) and bovine serum albumin as a standard. The MTT conversion method (12) was also used.
Association of AMB with macrophages.
AMB was measured by high-performance liquid chromatography in a Waters-Millipore (Saint-Quentin-en-Yvelines, France) system consisting of a model 501 pump, a Kromasil C18 column (150 by 3 mm, 5-μm particle diameter (AIT Chromato, Saint-Nom-La-Breteche, France), a WISP 712 automatic injector, and a model 484 UV detector with its wavelength set at 405 nm. The mobile phase was composed of acetic acid, acetonitrile, and water (41/43/16 [vol/vol/vol]), at a flow rate of 0.4 ml/min, with pressure at 1,200 lb/in2. A calibration curve (10 to 50,000 ng/ml) was prepared from a stock solution of AMB in dimethyl sulfoxide, which was further diluted in methanol–Triton X-100 (3/1); the limit of detection was 1 ng/ml.
After an activation period (5 or 18 h), the medium was removed and the cells were washed twice with PBS. The macrophages were lysed with 100 μl of Triton X-100 (1%), AMB was extracted with 300 μl of methanol, and 50 μl of the supernatant after centrifugation (15,000 × g, 30 min, 4°C) was assayed. Each experiment was carried out three times using 2 wells of a 24-well plate (106 cells/well), with three measurements of the contents of each well being taken.
For experimentation with cytochalasin B, cells were incubated with cytochalasin B (5 μg/ml) for 15 min before addition of AMB formulations.
Preparation of cellular protein extracts.
Peritoneal macrophages were plated in 24-well plates at a density of 106 cells/well. After 4 h of adherence, immunomodulators were added in culture medium containing 5% serum as described above. As a positive control, two wells were treated with IFN-γ (100 IU/ml) and LPS (100 ng/liter) and other samples were treated with LPS (100 ng/liter) alone or with IFN-γ (20 IU/ml) alone. After 18 h, the medium was removed and the cell monolayers were washed twice with ice-cold PBS. The monolayers were treated with 60 μl of lysis buffer (10 mM Tris buffer [pH 7.5], 50 mM NaCl, 5 mM EDTA, 1% NP-40, 1 mM phenylmethylsulfonyl fluoride, 5 μg of pepstatin per ml, 5 μg of aprotinin per ml, 5 μg of leupeptin per ml, 2 mM benzamidine) for 20 min on ice and then scraped and collected. The tubes were centrifuged at 4°C to remove cell debris, and the protein content of the supernatant was determined using the Bio-Rad detergent-compatible assay. Lysates were stored aliquoted at 20°C until electrophoresis.
Western blot analysis for NOSII.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis was performed under reducing conditions on a 7.5% gel. Equal amounts of protein (50 μg) from each lysate were loaded. Proteins were then transferred to a nitrocellulose membrane (Optitran BA-S 83; Schleicher and Schuell Inc., Dassell, Germany) at 30 V and stored overnight in 25 mM Tris–192 mM glycine (pH 8.3)–20% methanol. Nonspecific binding sites were blocked by incubation with 5% skimmed milk in Tris-buffered saline (TBS; 25 mM Tris [pH 7.5], 150 mM NaCl) containing 0.2% Tween 20 for 1 h at 37°C. The membrane was incubated with anti-NOS II murine antibody (Santa Cruz Biotechnology Inc., supplied by Tebu, Le-Perray-en-Yvelines, France) at 0.5 μg/ml in TBS for 2 h at room temperature. After five washes with TBS–Tween 20–0.5% skimmed milk, the secondary antiserum (goat-anti-rabbit immunoglobulin G conjugated with horseradish peroxidase; Sigma Immunochemicals, St.-Quentin-Fallavier, France), diluted 1/10,000 in TBS buffer, was added for 2 h at room temperature. The above-described washing procedure was repeated. The immunoreactive bands were revealed by the enhanced chemiluminescence (ECL) system (Amersham, Orsay, France) and detected with photographic film (Hyperfilm ECL; Amersham). The film was scanned, converted into a bitmap, and reproduced using Microsoft PowerPoint. Nitrite was measured in 100-μl samples of conditioned culture medium as described above.
Investigation of the role of NF-κB.
In order to determine whether AMB with or without costimulators could mobilize the nuclear factor NF-κB, the murine macrophage cell line RAW 264.7 was temporarily transfected with the plasmid 3enh-κb-LUC, which contains the luciferase gene under the control of three synthetic copies of the κB consensus sequence of the immunoglobulin κ-chain promoter, kindly supplied by Nicole Israel, Institut Pasteur, Paris, France. Prior to the experiment, it was confirmed that NOS activity could be induced in these cells by combinations of IFN-γ and LPS. RAW cells (4 × 106 per ml) were treated with 100 ng of plasmid per μg in the presence of a cationic lipid preparation. They were then plated at 3 × 106 cells/well. After 15 min the LPS, IFN-γ, TNF-α, and AMB in various combinations were added for 5 h. Thereafter, cell lysates were prepared before determination of luciferase activity by a chemiluminescence technique.
RESULTS
AMB. (i) NO production.
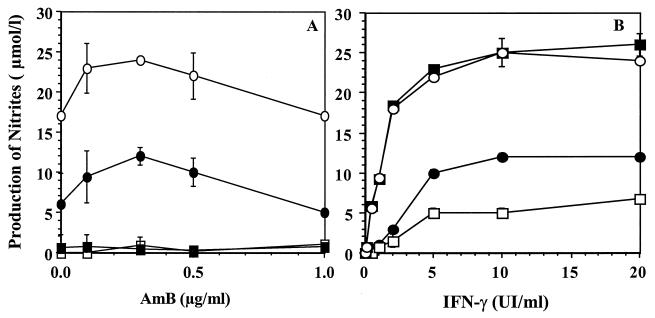
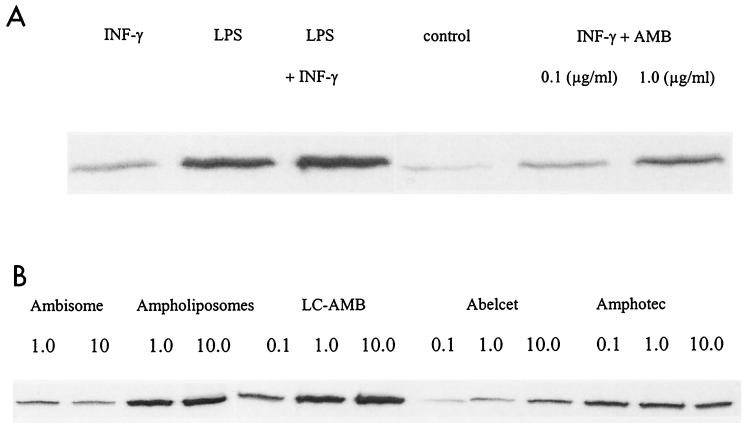
NO production by macrophages incubated with free AMB was observed only in the presence of IFN-γ (Fig. 1A). This production seemed to be dose dependent until the concentration of AMB reached 1 μg/ml, which is the threshold of toxicity as judged by the MTT test and protein quantification. The highest amounts of nitrite were found between 5 and 10 IU of IFN-γ per ml (Fig. 1B), which represent a synergistic association (although this association produced less NO than the combination IFN-γ–LPS). In all cases, no synergy was observed between AMB and LPS, although LPS could increase the response to IFN-γ alone (Fig. 1B). The induction of NOS II by the combination of AMB and IFN-γ was confirmed by Western blotting (Fig. 2A).
FIG. 1.
Immunostimulating effect of AMB on NO production. Macrophages (105 cells per well) were cultured with various combinations of immunostimulants. After an activation phase of 24 h, nitrite production was determined as described in Materials and Methods. (A) Nitrite production as a function of AMB concentration. ■, AMB alone; ●, AMB with IFN-γ (5 IU/ml); □, AMB with LPS (5 μg/ml); ○, AMB with IFN-γ (5 IU/ml) and LPS (5 μg/ml). (B) Nitrite production as a function of IFN-γ concentration. □, IFN-γ alone; ●, IFN-γ with AMB (0.3 μg/ml); ○, IFN-γ with LPS (5 μg/ml); ■, IFN-γ with AMB (0.3 μg/ml) and LPS (5 μg/ml).
FIG. 2.
(A) NOS II (134 kDa) expression in macrophages revealed by Western blotting after treatment with AMB, IFN-γ (20 IU/ml), and LPS (10 μg/ml) as described in Materials and Methods. (B) NOS II (134 kDa) expression in macrophages revealed by Western blotting after treatment with different lipid formulations of AMB (concentrations are noted in micrograms per milliliter) with IFN-γ (20 IU/ml), as described in Materials and Methods.
(ii) TNF-α production.
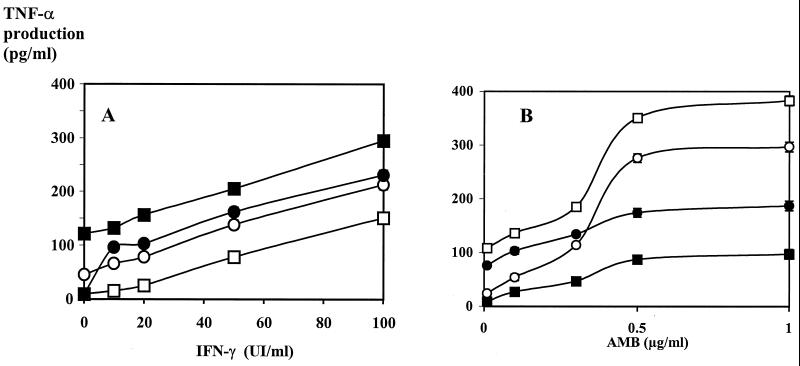
AMB induced TNF-α production in a dose-dependent manner without any costimulator (Fig. 3B). As regards NO production, a steep increase was observed between AMB concentrations of 0.3 and 0.5 μg/ml. When AMB was combined with IFN-γ (or LPS), no significant synergy for TNF-α production and only additions of the effect of AMB to that of the costimulator were observed (Fig. 3A). No significant differences were observed between Fungizone and free AMB (dissolved in dimethyl sulfoxide or prepared like LC-AMB), as far as the immunomodulatory effects were concerned. The production of NO and TNF-α induced by AMB formulations did not diminish on preincubation with polymyxin B (2 μg/ml).
FIG. 3.
Immunostimulating effect of AMB on TNF-α production. Macrophages (105 cells per well) were cultured with various combinations of immunostimulants after an activation phase of 5 h. TNF-α production was determined as described in Materials and Methods. (A) TNF-α production as a function of IFN-γ concentration. □, IFN-γ alone; ○, IFN-γ with AMB (0.3 μg/ml); ●, IFN-γ with LPS (5 μg/ml); ■, IFN-γ with AMB (0.3 μg/ml) and LPS (5 μg/ml). (B) TNF-α production as a function of AMB concentration. ■, AMB alone; ●, AMB with LPS (5 μg/ml); ○, AMB with IFN-γ (5 IU/ml); □, AMB with IFN-γ (5 IU/ml) and LPS (5 μg/ml).
(iii) Relationship between NO and TNF-α production.
It was possible that TNF-α acted as a costimulator for NO production. However, when macrophages were activated in the presence of a blocking antibody to TNF-α, no change in NO production was observed, whatever the experimental conditions were (dose of AMB, costimulator, formulation, and time of activation). Similarly, the production of TNF-α induced by the different combinations of immunostimulators was not altered in the presence of an NOS inhibitor, l-NMMA, at a concentration (1 mM) sufficient to completely block NO production (data not shown). Thus, the production of NO and that of TNF-α were induced directly and independently by AMB.
(iv) Determination of the role of NF-κB.
In order to understand the mechanism by which AMB was able to stimulate NO and TNF-α production in macrophages, we investigated whether the nuclear transcription factor NF-κB might be involved. It is known that NF-κB is necessary for NOS II gene transcription but that IFN-γ alone cannot mobilize this factor, whereas LPS can (52). The synergy observed between AMB and IFN-γ but not between AMB and LPS in our present results is consistent with the hypothesis that AMB might mobilize NF-κB and thus replace LPS in the two-signal mechanism of macrophage activation. Furthermore, NF-κB is also implicated in the induction of TNF-α production.
In an attempt to detect the mobilization of NF-κB by AMB, a plasmid carrying a luciferase reporter gene under the control of a strongly NF-κB-dependent promoter was introduced into the macrophage-like cell line RAW 264.7. These cells were treated with various combinations of immunostimulants. It had previously been established that this cell line responded to AMB, IFN-γ, and LPS in a way similar to that of primary cultures of murine peritoneal macrophages. Furthermore, measurement of nitrite in culture medium from the experimental groups yielded results comparable with those shown in Fig. 1A, that is, synergy between IFN-γ and AMB for the induction of NOS II activity.
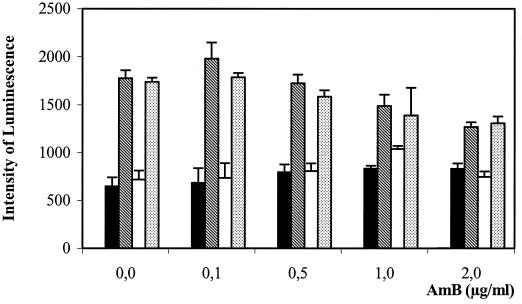
Figure 4 shows a threefold increase in luciferase activity after treatment with LPS (10 ng/ml) in the absence of AMB, showing that transfection was efficient. As expected, IFN-γ (20 IU/ml) did not induce luciferase expression alone and did not increase the expression induced by LPS. However, the treatment with AMB alone (up to 2 μg/ml) did not increase the intensity of chemiluminescence. Furthermore, when AMB was added in the presence of LPS, luciferase expression was not reinforced; rather, it was slightly decreased. These results indicate that AMB does not mobilize NF-κB in macrophages and that its effects on NOSII induction and TNF-α production must be mediated by some other intracellular signaling pathway(s).
FIG. 4.
Response of an NF-κB-controlled luciferase gene transfected into RAW 264.7 cells to AMB with or without other costimulants after 5 h of activation. Filled bars, AMB alone; cross-hatched bars, AMB with 10 ng of LPS per ml; open bars, AMB with 20 IU of IFN-γ per ml; dotted bars, AMB with 20 IU of IFN-γ per ml and 10 μg of LPS per ml.
Lipid formulations of AMB. (i) NO and TNF-α production.
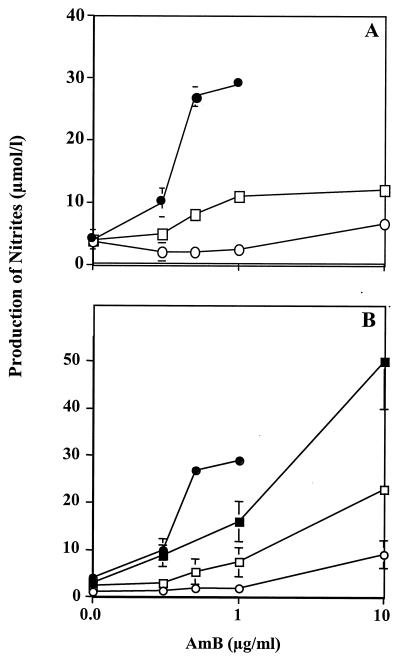
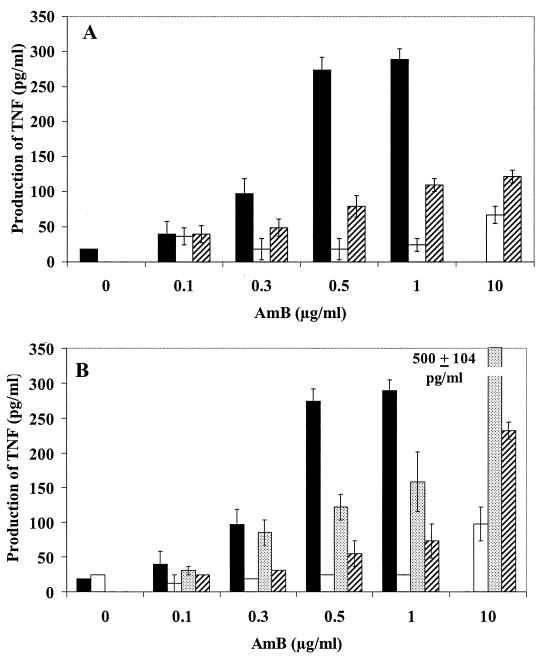
In the presence of IFN-γ, AMB that was associated with lipid carriers stimulated significantly less NO production (Fig. 5) than free AMB up to the subtoxic dose of 1 μg/ml. The levels of expression of NOS observed by Western blot analysis (Fig. 2B) were roughly correlated with nitrite production, showing that this difference was at the level of NOS induction rather than the result of a posttranscriptional modification of its activity. TNF-α production was also reduced when AMB was presented associated with lipid formulations. A dose-response curve shaped similarly to that of free AMB was observed (Fig. 6).
FIG. 5.
Effects of lipid formulations of AMB on NO production. Macrophages (105 per well) were cultured with IFN-γ (20 IU/ml, suboptimum dose) and various concentrations and formulations of AMB. After an activation phase of 18 h, nitrite production was determined as described in Materials and Methods. (A) Liposomes. ●, AMB; ○, AmBisome; □, ampholiposomes. (B) Complexes. ●, AMB; ○, Abelcet; □, Amphotec; ■, LC-AMB.
FIG. 6.
Effects of lipid formulations of AMB on TNF-α production. Macrophages (105 per well) were cultured with IFN-γ (20 IU/ml) and various concentrations and formulations of AMB. After an activation phase of 5 h, TNF-α production was determined as described in Materials and Methods. (A) Liposomes. Filled bars, AMB; open bars, AmBisome, cross-hatched bars, ampholiposomes. (B) Complexes. Filled bars, AMB; open bars, Abelcet; dotted bars, LC-AMB; cross-hatched bars, Amphotec.
At 10 μg of AMB per ml, a concentration at which free AMB was toxic, the lipid formulations were well tolerated as determined by the MTT conversion test and by measurement of the protein content of the monolayers (data not shown). At this concentration, all the formulations except LC-AMB and Amphotec induced lower NO and TNF-α production than did free AMB at 1 μg/ml. The two small-sized complexes, Amphotec and LC-AMB, showed the smallest reductions in NO and TNF-α production compared with the levels produced by AMB, whereas the large Abelcet complexes and the two liposomal formulations induced lower levels of TNF-α and NO. The small unilamellar AmBisome and the Abelcet ribbons of several microns yielded similar results. Thus, there did not seem to be any correlation between size or form and immunostimulating effects. Furthermore, the lipid composition did not seem to be directly related to activity, since the commercially available Abelcet and its modified form had the same lipid composition and AMB/lipids ratio (DMPC/DMPG/AMB, 7/3/5) but induced very different amounts of NO and TNF-α.
In an attempt to determine why different formulations of AMB led to such different results in terms of NO and TNF-α production, we then investigated the association of AMB presented in different forms with macrophages.
(ii) Uptake of AMB presented to macrophages in different formulations.
Association of all the formulations was dose dependent, but considerable differences between them were noted (Table 2). The amount of AMB that associated after presentation as a liposome formulation (AmBisome and ampholiposomes) was similar to or not much greater than that with control AMB. On the other hand, AMB presented as a complex, especially in Abelcet, was associated to a much greater extent than control AMB. The level of association of LC-AMB was intermediate between those of liposomes and the commercial complexes. Although the association increased with increasing AMB concentration in the medium, this trend was not linear, suggesting a saturable component, but a plateau was not reached, even with Abelcet. On the other hand, the association reached equilibrium quickly, since it was only slightly higher after 18 h than after 3 h, except with LC-AMB.
TABLE 2.
Influence of lipid formulations on the amount of AMB associated with macrophages
| AMB formulation | Amt of AMB (ng/μg of protein)c associated with macrophages at the following AMB concn (μg/ml):
|
|||||
|---|---|---|---|---|---|---|
| 0.1
|
1
|
10
|
||||
| 3 h | 18 h | 3 h | 18 h | 3 h | 18 h | |
| AMB | 92 ± 14 | 123 ± 33 | 190 ± 14 | 254 ± 24b | —d | — |
| AmBisome | 118 ± 8a | 159 ± 11ab | 198 ± 30 | 202 ± 27a | 329 ± 9 | 550 ± 36b |
| Ampholiposomes | 91 ± 5 | 181 ± 8ab | 228 ± 5a | 291 ± 29ab | 1,174 ± 198 | 1,384 ± 79b |
| Abelcet | 1,293 ± 47a | 1,450 ± 256a | 2,990 ± 152a | 2,520 ± 294ab | 13,698 ± 302 | 15,268 ± 344b |
| LC-AMB | 182 ± 19a | 279 ± 15ab | 527 ± 51a | 706 ± 17ab | 2,069 ± 72 | 3,561 ± 123b |
| Amphotec | 462 ± 21a | 538 ± 32ab | 1,899 ± 190a | 1,947 ± 67a | 8,154 ± 226 | 8,335 ± 247 |
Significant difference from results with AMB by Student's t test (P > 0.05).
Significant difference from level of association at 3 h by Student's t test (P > 0.05).
Values are means ± standard deviations of six measurements.
—, amount was toxic.
Whatever the formulation, association was considerably reduced at 4°C compared with that at 37°C (data not shown) except with the positively charged ampholiposomes at a high concentration. The last result may indicate adsorption onto the negatively charged cell membrane by electrostatic interaction. On the other hand, the results obtained in the presence of cytochalasin B (data not shown) were more variable, with the degree of inhibition depending on the formulation and the dose.
(iii) Relationship between AMB association and immunostimulating effect.
Whatever the mechanism of association of AMB with macrophages, it is clear that the production of NO and that of TNF-α (Fig. 4 and 5, respectively) cannot be directly related to the total amount of AMB associated. In fact, the complex which showed the highest association (Abelcet) induced the smallest NO and TNF-α production. On other hand, the liposomes, which showed a level of association similar to that of AMB, differed in their immunostimulating effects (ampholiposomes activated much more than AmBisome), although both were less efficient than AMB. The LC-AMB preparation, which showed intermediate association, induced the highest NO and TNF-α production among the formulations.
DISCUSSION
A number of pathogens can survive and multiply within cells of the mononuclear phagocyte system because they have developed mechanisms to protect themselves from host defense systems. This ability to survive and multiply provides a reservoir of microorganisms, which can provoke recurrent infection. Colloidal formulations can be advantageous in treating these types of infection because they themselves are concentrated within phagocyte cells. Thus, microorganisms within the phagocytes may be directly reached, and furthermore, antibiotic-loaded macrophages may migrate to sites of infection. For example, the use of liposomes has been shown to increase the amount of AMB at the sites of Aspergillus infection (36). Colloidal systems have also been used to deliver immunostimulating molecules to macrophages to stimulate their innate antimicrobial activity.
There is growing evidence that AMB has immunostimulating activity and that this may be responsible for part of AMB's antimicrobial activity. Several authors have noted differences in the in vitro and in vivo sensitivities to AMB of various microorganisms, namely, Listeria monocytogenes (6, 35, 46), Schistosoma mansoni (38), Toxoplasma gondii (22), and Trypanosoma cruzi (23), suggesting that indirect mechanisms are involved. The fact that AMB therapy has sometimes been found to be less effective in severely immunocompromised patients (14) also suggests that immunostimulation plays a role in the action of the antibiotic against pathogenic agents. Among the different effector molecules which may be produced by AMB-stimulated macrophages, NO and TNF-α are often cited. Several studies have demonstrated the implication of NO in the antimicrobial action of AMB. Tohyama et al. (47) showed that the NOS inhibitor l-MMNA reduced the anticryptococcal activity of AMB in vitro. However, in another study, NO was found to be cytostatic rather than cytotoxic (49). On the other hand, several authors have shown that TNF-α can increase the capacity of macrophages and polymorphonuclear leukocytes to phagocytose and kill Candida albicans (17) and improve the survival of infected mice. Furthermore, the autocrine secretion of TNF has been shown to contribute to NO production and killing of intracellular parasites, such as Leishmania major (20).
Therefore, the production of these two molecules was used to compare the effects of free and lipid-associated AMB in peritoneal macrophages. Our results indicating that AMB-induced NO production only in the presence of IFN-γ as a costimulator are in accord with those obtained by several other authors in vitro (21, 31, 37) as well as in vivo (25). The dose of IFN-γ necessary for costimulation of mouse peritoneal macrophages (20 IU ml) (Fig. 1B) was inferior to that needed for the J774A1 cell line (21, 37). M-CSF (16) and OK432 (48) have also been found to act as costimulants for NO production with AMB. In our experiments, however, LPS could not replace IFN-γ as a costimulant, although LPS and IFN-γ acted in synergy to induce NO in mouse peritoneal macrophages and reached a higher level of activity than the combination AMB–IFN-γ. The production of NO and TNF-α induced by AMB formulations did not diminish on preincubation with polymyxin B, indicating that traces of LPS were not responsible.
The induction of the NOSII protein by the different immunostimulant combinations was confirmed by Western blotting. Furthermore, the intensity of the NOS II band was roughly proportional to the AMB dose (Fig. 2). Unlike NO production, TNF-α secretion was stimulated by AMB alone and the results of combinations with IFN-γ and LPS were merely additive, as described by Louie et al. in vitro with thioglycollate-stimulated macrophages (31) and in vivo (32). The use of anti-TNF-α antibody confirmed that the effect of conditioned medium on L929 cells was due to TNF-α and not to AMB itself or another secreted mediator. It was possible that the TNF-α induced by AMB might have acted as a cofactor for NOS II induction, as was observed by Drapier (13). However, the fact that NO production was maintained in the presence of anti-TNF-α antibody eliminates this possibility. On the other hand, TNF-α production has been reported to be down-regulated by NO (24, 44). However, in our experiments complete inhibition of NO production had no effect on TNF-α production, in accordance with the observations of Boss et al. (4).
Since the nuclear activation factor κB is implicated in both TNF-α production and NOS II induction, it was interesting to check whether AMB could mobilize this factor. This mechanism would be consistent with the interactions observed between different immunostimulants: IFN-γ does not activate NF-κB and must be combined with a costimulant which does (e.g., LPS) for NOS II induction (52). We thus speculated that AMB might replace LPS as the NF-κB activator. This hypothesis was tested in a macrophage-like cell line (RAW 264.7) temporarily transfected with a reporter gene under the control of a strongly NF-κB-dependent promoter. The results (Fig. 4) clearly show that AMB did not activate NF-κB or increase its activation by LPS. Therefore, some other mechanism, such as protein kinase C activation (40), must explain the action of AMB on NO and TNF-α production.
In contrast to the situation with free AMB, the immunostimulating properties of lipid-based formulations have not been thoroughly evaluated previously. Our results clearly show that, at equivalent concentrations, the production of NO and TNF-α induced in mouse peritoneal macrophages by the lipid formulations was always less than that induced by free AMB but remained dose dependent. The different formulations reduced the immunostimulating activity of AMB to different extents. Similar results concerning TNF-α production were obtained with AmBisome in vitro (31) and in vivo (3). On the other hand, Sculier and Body (43) observed increased TNF-α production in cancer patients treated with ampholiposomes, but no comparison with free AMB was made.
Several explanations of the reduced immunostimulating effects of lipid formulations of AMB are possible. The presence of some types of liposome has been observed to interfere with the processes of NO and TNF-α production. Negatively charged unloaded liposomes (containing phosphatidylserine or phosphatidic acid), but not neutral ones, have been observed to inhibit NO production by macrophages as a result of suppression of NOS induction (2) and also to inhibit TNF production by interfering with tyrosine phosphorylation of a 41-kDa protein (1). However, the molar proportion of negatively charged lipid was very high (33 to 50%) and the concentration of lipid which gave significant inhibition (about 350 μM) was much greater than that associated with 1μg of AMB per ml in our formulations (1 to 20 μM). Phosphatidylglycerol was not tested. On the other hand, negatively charged unilamellar liposomes containing 5% phosphatidylserine were observed to inhibit TNF-α by a posttranslational mechanism (7). Again, the concentration of lipid which gave significant inhibition (6 mM) was higher than that used in our experiments. No toxicity was observed by the MTT test or by measurement of the protein content of the cells; thus, an effect on cell viability can be excluded. Furthermore, the production of NO and TNF-α was dose dependent and continued to increase when the AMB concentration was increased from 1 to 10 μg/ml (with a corresponding 10-fold increase in lipid concentration). This type of dose-response curve would not be expected if the lipids had a direct effect on the ability of the macrophages to become activated. Furthermore, the effects on NO and on TNF-α production were correlated even though their production was independent.
In our experiments, we tested the effects of the lipids used to prepare LC-AMB (DMPC/DMPG, 7/3) processed by the same solvent displacement technique without the antibiotic. The resulting colloid alone induced moderate NO and TNF-α production (Table 3), and more importantly, when it was presented at the same time as a solution of AMB, it did not reduce the levels of production of NO and TNF-α compared with the levels stimulated by AMB alone. Thus, the slight reductions in levels of NO and TNF-α produced that were observed when AMB was presented as LC-AMB and the greater reductions observed with Abelcet (which has the same lipid composition) could not be explained by an inhibitory effect of the lipids. It would have been interesting to test the effect of the lipids used in the other formulations, but since these were gifts from the manufacturers, the corresponding “blank” formulations were not available, and even if we had tried to reproduce these formulations in the laboratory, they would not necessarily have been representative since the preparation procedures for the commercial preparations have not been disclosed. Furthermore, in the cases of the complexes with high AMB/lipids ratios, the morphology of the objects would not be the same in the absence of AMB, since the antibiotic influences the lipid organization. In fact, the particles prepared by nanoprecipation from the DMPC-DMPG lipid mixture, as a control for Abelcet and LC-AMB, were polydisperse and unlike either of the two AMB complexes. The results obtained with them (Table 3) led us to think that it was not the lipids per se but the AMB-lipid interactions which were important in modulating macrophage activation.
TABLE 3.
Influence of the lipid on the activation of murine peritoneal macrophagesa
| AMB formulation | AMB concn (μg/ml) | NO2− concn (μmol/liter) | TNF-α concn (pg/ml) |
|---|---|---|---|
| AMBc | 1.0 | 25 ± 4 | 297 ± 12 |
| 10.0 | —d | — | |
| Abelcet | 1.0 | 2 ± 0.2b | 23 ± 3b |
| 10.0 | 9 ± 0.4 | 86 ± 6 | |
| LC-AMBc | 1.0 | 16 ± 4 | 178 ± 36b |
| 10.0 | 50 ± 7 | 467 ± 56 | |
| Lipids (DMPC/DMPG, 7/3) | 1.0 | 2 ± 0.3b | 18 ± 3b |
| LC-AMB lipid equivalentc | 10.0 | 10 ± 0.6 | 73 ± 4 |
| Mixed lipids (DMPC/DMPG, 7/3) | 1.0 | 24 ± 6 | 281 ± 19 |
| LC-AMB lipid equivalentc + AMBc | 10.0 | — | — |
Macrophages (105 per well) were cultured with various concentrations of AMB with IFN-γ (20 IU/ml). After an activation phase of 24 h, levels of nitrite and TNF-α production were determined as described in Materials and Methods. Results are means ± standard deviations of three measurements.
Significant difference from results with AMB by Student's t test (P > 0.05).
Prepared by a solvent displacement method.
—, amount was toxic.
As far as NO production was concerned, the intensities of the bands attributed to NOS II on Western blotting were approximately proportional to the extent of NO production, indicating modifications at the level of enzyme induction rather than a posttranslational modification or inhibition of enzyme activity. This and the fact that the same tendencies were observed for NO and TNF-α suggest that the formulations affect the process of immunostimulation by AMB at an early stage, perhaps at the level of the availability of AMB.
Table 2 shows that the immunostimulating activity of the formulations cannot be correlated with their total association with macrophages. However, the mode of association with the cells may be important. AMB may be associated with macrophages either by phagocytosis of intact particles or by diffusion into the cell membrane after cell-particle contact or after release of AMB from the formulations. Work by Legrand et al. (28) has shown that the larger the particle, the higher the proportion of phagocytosis. Thus, free AMB or AMB from Fungizone was associated mainly by diffusion, while the large Abelcet particles were principally taken up by phagocytosis. However, in our study there was no correlation between particle size and NO and TNF-α production. Another important factor could be the amount of AMB released into the medium after dissociation from the formulation. A study of the release of AMB from liposomes by monitoring changes in its spectral properties showed that this process was much more rapid from ampholiposomes than from AmBisome (27). This result may be explained by the strength of binding of AMB to the lipids. The higher AMB/lipids ratio in the complexes (Table 1) have been shown to lead to a more rapid release (P. Legrand, unpublished results). This leads to hypotheses that the immunostimulating effects are due to free AMB in the culture medium, which interacts with the cell membrane in a particular way (30, 54), and that AMB remaining anchored within the formulations is ineffective. However, the contrasting results obtained with LC-AMB and Abelcet indicate that the AMB/lipids ratio is not the only important factor. Here, the difference in morphology may play a role. The large Abelcet ribbons are rapidly removed from the medium by phagocytosis, especially because of sedimentation in this unstirred culture system, and AMB within phagolysosomes may be ineffective in terms of stimulating NO and TNF-α production. Therefore, our results indicate that two factors are important in determining the immunostimulating properties of AMB within lipid formulations: the rate of release of AMB from the formulations and the kinetics of association with cells. These two processes combine to explain the high NO and TNF-α production observed for LC-AMB at 10 μg/ml.
The results presented in this paper clearly demonstrate that the immunostimulating properties of AMB depend on the formulation in which it is presented to macrophages. However, these results cannot be extrapolated directly to the in vivo situation, since other factors, such as the biodistribution of the carriers and their effects on other types of immunocompetent cells, have to be taken into account. The question remains as to the consequences of these differences for their toxicity and efficacy. All the formulations studied here reduced the toxicity of AMB in vitro and in vivo. However, the new formulation, LC-AMB, which shows the highest NO and TNF production, was found to have a 50% lethal dose similar to that of AmBisome (unpublished data), the least toxic of the commercial formulations and that which most reduces NO and TNF production. The production of proinflammatory cytokines such as IL-1 (8, 34), IL-6 (17, 32), and TNF-α has been proposed as a cause of some side effects of AMB, while treatments which reduce this production, namely, the use of antibodies (32) or dexamethasone (9) and pentoxilline (11), can reduce this toxicity. In particular, TNF-α production induced by AMB and its derivatives in human immunodeficiency virus-infected macrophages can stimulate virus replication (10). Therefore, AMB formulations which minimize TNF-α production would be advantageous for therapy of infections in AIDS patients.
On the other hand, the reduced immunostimulating capacity of lipid formulations of AMB may reduce their efficacy against intracellular infections and may explain why some authors have observed poor antifungal activity in immunocompromised patients. It would be interesting to test the impact of reduced NO and TNF-α production on the activity of AMB on macrophages infected by pathogens. Therefore, a study of the immunostimulating and antiparasitic activities of these formulations in infected macrophages will be the subject of a forthcoming publication.
ACKNOWLEDGMENTS
This work was supported in part by a grant from the BQR98 of Paris XI University and a personal grant, Louis Forest et Georges Canat, from la Chancellerie des Universités de Paris to the first-listed author.
We thank Beatrice Wolferslerger for technical help with transfection experiments and the technicians of the Faculty's Central Animal House for the maintenance of the mice. AmBisome and ampholiposomes were kind gifts, respectively, from Nexstar Pharmaceutical (now Gilead Sciences) and from the Pharmacie Centrale des Hôpitaux de Paris. Amphotec was generously provided by Liposome Technology Inc., and Abelcet was provided by the Liposome Company Ltd. (London, United Kingdom).
REFERENCES
- 1.Aramaki Y, Matsuno R, Nitta F, Arima H, Tsuchiya S. Negatively charged liposomes inhibit tyrosine phosphorylation of 41-kDa protein in murine macrophages stimulated with LPS. Biochem Biophys Res Commun. 1997;231:827–830. doi: 10.1006/bbrc.1996.5999. [DOI] [PubMed] [Google Scholar]
- 2.Aramaki Y, Nitta F, Matsuno R, Morimura Y, Tsuchiya S. Inhibitory effects of negatively charged liposomes on nitric oxide production from macrophages stimulated by LPS. Biochem Biophys Res Commun. 1996;220:1–6. doi: 10.1006/bbrc.1996.0346. [DOI] [PubMed] [Google Scholar]
- 3.Arning M, Kliche K O, Heer-Sonderhoff A H, Wehmeier A. Infusion-related toxicity of three different amphotericin B formulations and its relation to cytokine plasma levels. Mycoses. 1995;38:459–465. doi: 10.1111/j.1439-0507.1995.tb00020.x. [DOI] [PubMed] [Google Scholar]
- 4.Boss J M, Laster S M, Gooding L R. Sensitivity to tumour necrosis factor-mediated cytolysis is unrelated to manganous superoxide dismutase messenger RNA levels among transformed mouse fibroblasts. Immunology. 1991;73:309–315. [PMC free article] [PubMed] [Google Scholar]
- 5.Brajtburg J, Bolard J. Carrier effects on biological activity of amphotericin B. Clin Microbiol Rev. 1996;9:512–531. doi: 10.1128/cmr.9.4.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brajtburg J, Elberg S, Kobayashi G S, Medoff G. Toxicity and induction of resistance to Listeria monocytogenes infection by amphotericin B in inbred strains of mice. Infect Immun. 1986;54:303–307. doi: 10.1128/iai.54.2.303-307.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brisseau G F, Kresta A, Schouten D, Bohnen J M, Shek P N, Fok E, Rotstein O D. Unilamellar liposomes modulate secretion of tumor necrosis factor by lipopolysaccharide-stimulated macrophages. Antimicrob Agents Chemother. 1994;38:2671–2675. doi: 10.1128/aac.38.11.2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chia J K, McManus E J. In vitro tumor necrosis factor induction assay for analysis of febrile toxicity associated with amphotericin B preparations. Antimicrob Agents Chemother. 1990;34:906–908. doi: 10.1128/aac.34.5.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chia J K, Pollack M. Amphotericin B induces tumor necrosis factor production by murine macrophages. J Infect Dis. 1989;159:113–116. doi: 10.1093/infdis/159.1.113. [DOI] [PubMed] [Google Scholar]
- 10.Clayette P, Martin M, Beringue V, Dereuddre-Bosquet N, Adjou K T, Seman M, Dormont D. Effects of MS-8209, an amphotericin B derivative, on tumor necrosis factor alpha synthesis and human immunodeficiency virus replication in macrophages. Antimicrob Agents Chemother. 2000;44:405–407. doi: 10.1128/aac.44.2.405-407.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cleary J D, Chapman S W, Nolan R L. Pharmacologic modulation of interleukin-1 expression by amphotericin B-stimulated human mononuclear cells. Antimicrob Agents Chemother. 1992;36:977–981. doi: 10.1128/aac.36.5.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Denizot F, Lang R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods. 1986;89:271–277. doi: 10.1016/0022-1759(86)90368-6. [DOI] [PubMed] [Google Scholar]
- 13.Drapier J C. Nitric oxide and macrophages. Pathol Biol. 1997;45:110–114. . (In French.) [PubMed] [Google Scholar]
- 14.Dromer F, Charreire J. Improved amphotericin B activity by a monoclonal anti-Cryptococcus neoformans antibody: study during murine cryptococcosis and mechanisms of action. J Infect Dis. 1991;163:1114–1120. doi: 10.1093/infdis/163.5.1114. [DOI] [PubMed] [Google Scholar]
- 15.Flick D A, Gifford G E. Production of tumor necrosis factor in unprimed mice: mechanism of endotoxin-mediated tumor necrosis. Immunobiology. 1986;171:320–328. doi: 10.1016/S0171-2985(86)80064-X. [DOI] [PubMed] [Google Scholar]
- 16.Fujita H, Masuda H, Nakajima T, Yada K, Watanabe M, Kagitani Y. Protective effect of human macrophage colony-stimulating factor on fungal infection (2). In vitro effect of human macrophage colony-stimulating factor on systemic aspergillosis and in vitro effect on the activities of macrophage. Kansenshogaku Zasshi. 1995;69:582–589. doi: 10.11150/kansenshogakuzasshi1970.69.582. . (In Japanese) [DOI] [PubMed] [Google Scholar]
- 17.Ghezzi M C, Raponi G, Filadoro F, Mancini C. The release of TNF-alpha and IL-6 from human monocytes stimulated by filtrates of Candida albicans after treatment with amphotericin B. J Antimicrob Chemother. 1994;33:1039–1043. doi: 10.1093/jac/33.5.1039. [DOI] [PubMed] [Google Scholar]
- 18.Gigliotti F, Shenep J L, Lott L, Thornton D. Induction of prostaglandin synthesis as the mechanism responsible for the chills and fever produced by infusing amphotericin B. J Infect Dis. 1987;156:784–789. doi: 10.1093/infdis/156.5.784. [DOI] [PubMed] [Google Scholar]
- 19.Green B N, Warguer D A, Glowgoski J, Skipper P L, Wishnok J S, Tannenbaum J R. Analysis of nitrate, nitrite and (15)N nitrate in biological fluids. Anal Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 20.Green S J, Crawford R M, Hockmeyer J T, Meltzer M S, Nacy C A. Leishmania major amastigotes initiate the l-arginine-dependent killing mechanism in IFN-gamma-stimulated macrophages by induction of tumor necrosis factor-alpha. J Immunol. 1990;145:4290–4297. [PubMed] [Google Scholar]
- 21.Herrmann J L, Dubois N, Fourgeaud M, Basset D, Lagrange P H. Synergic inhibitory activity of amphotericin-B and gamma interferon against intracellular Cryptococcus neoformans in murine macrophages. J Antimicrob Chemother. 1994;34:1051–1058. doi: 10.1093/jac/34.6.1051. [DOI] [PubMed] [Google Scholar]
- 22.Hisaeda H, Sakai T, Nagasawa H, Ishikawa H, Yasutomo K, Maekawa Y, Himeno K. Contribution of extrathymic gamma delta T cells to the expression of heat-shock protein and to protective immunity in mice infected with Toxoplasma gondii. Immunology. 1996;88:551–557. doi: 10.1046/j.1365-2567.1996.d01-694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horvath A E, Zierdt C H. The effect of amphotericin B on Trypanosoma cruzi in vitro and in vivo. J Trop Med Hyg. 1974;77:144–149. [PubMed] [Google Scholar]
- 24.Iuvone T, Van Osselaer N, D'Acquisto F, Carnuccio R, Herman A G. Differential effect of l-NAME and S-methyl-isothiourea on leukocyte emigration in carrageenin-soaked sponge implants in rat. Br J Pharmacol. 1997;121:1637–1644. doi: 10.1038/sj.bjp.0701317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joly V, Saint-Julien L, Carbon C, Yeni P. In vivo activity of interferon-gamma in combination with amphotericin B in the treatment of experimental cryptococcosis. J Infect Dis. 1994;170:1331–1334. doi: 10.1093/infdis/170.5.1331. [DOI] [PubMed] [Google Scholar]
- 26.Lasic D D, Papahadjopoulos D, editors. Medical applications of liposomes. Amsterdam, The Netherlands: Elsevier; 1998. [Google Scholar]
- 27.Legrand P, Cheron M, Leroy L, Bolard J. Release of amphotericin B from delivery systems and its action against fungal and mammalian cells. J Drug Target. 1997;4:311–319. doi: 10.3109/10611869708995847. [DOI] [PubMed] [Google Scholar]
- 28.Legrand P, Vertut-Doi A, Bolard J. Comparative internalization and recycling of different amphotericin B formulations by a macrophage-like cell line. J Antimicrob Chemother. 1996;37:519–533. doi: 10.1093/jac/37.3.519. [DOI] [PubMed] [Google Scholar]
- 29.Liew F Y, Li Y, Millott S. Tumour necrosis factor (TNF-alpha) in leishmaniasis. II. TNF-alpha-induced macrophage leishmanicidal activity is mediated by nitric oxide from l-arginine. Immunology. 1990;71:556–559. [PMC free article] [PubMed] [Google Scholar]
- 30.Lingaas E, Midtvedt T. Dissociated effect of amphotericin B and deoxycholate on phagocytosis of Escherichia coli by human polymorphonuclear neutrophils. Drugs Exp Clin Res. 1985;11:747–754. [PubMed] [Google Scholar]
- 31.Louie A, Baltch A L, Franke M A, Smith R P, Gordon M A. Comparative capacity of four antifungal agents to stimulate murine macrophages to produce tumour necrosis factor alpha: an effect that is attenuated by pentoxifylline, liposomal vesicles, and dexamethasone. J Antimicrob Chemother. 1994;34:975–987. doi: 10.1093/jac/34.6.975. [DOI] [PubMed] [Google Scholar]
- 32.Louie A, Baltch A L, Smith R P, Franke M A, Ritz W J, Singh J K, Gordon M A. Fluconazole and amphotericin B antifungal therapies do not negate the protective effect of endogenous tumor necrosis factor in a murine model of fatal disseminated candidiasis. J Infect Dis. 1995;171:406–415. doi: 10.1093/infdis/171.2.406. [DOI] [PubMed] [Google Scholar]
- 33.Marzzullo L, Souza L C, Campa A. Effect of amphotericin B associated with a lipid emulsion on the oxidative burst of human polymorphonuclear leukocytes. Gen Pharmacol. 1997;28:203–207. doi: 10.1016/s0306-3623(96)00220-0. [DOI] [PubMed] [Google Scholar]
- 34.Matsumoto T, Kaku M, Furuya N, Usui T, Kohno S, Tomono K, Tateda K, Hirakata Y, Yamaguchi K. Amphotericin B-induced resistance to Pseudomonas aeruginosa infection in mice. J Antibiot (Tokyo) 1993;46:777–784. doi: 10.7164/antibiotics.46.777. [DOI] [PubMed] [Google Scholar]
- 35.Medoff G, Kwan C N, Schlessinger D, Kobayashi G S. Permeability control in animal cells by polyenes: a possibility. Antimicrob Agents Chemother. 1973;3:441–443. doi: 10.1128/aac.3.3.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mehta R T, McQueen T J, Keyhani A, Lopez-Berestein G. Phagocyte transport as mechanism for enhanced therapeutic activity of liposomal amphotericin B. Chemotherapy. 1994;40:256–264. doi: 10.1159/000239202. [DOI] [PubMed] [Google Scholar]
- 37.Mozaffarian N, Berman J W, Casadevall A. Enhancement of nitric oxide synthesis by macrophages represents an additional mechanism of action for amphotericin B. Antimicrob Agents Chemother. 1997;41:1825–1829. doi: 10.1128/aac.41.8.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olds G R, Stewart S J, Ellner J J. Amphotericin B-induced resistance to Schistosoma mansoni. J Immunol. 1981;126:1667–1670. [PubMed] [Google Scholar]
- 39.Pallister C J, Johnson E M, Warnock D W, Elliot P J, Reeves D F. In-vitro effects of liposome-encapsulated amphotericin B (AmBisome) and amphotericin B-deoxycholate (Fungizone) on the phagocytic and candidacidal function of human polymorphonuclear leucocytes. J Antimicrob Chemother. 1992;30:313–320. doi: 10.1093/jac/30.3.313. [DOI] [PubMed] [Google Scholar]
- 40.Paul A, Doherty K, Plevin R. Differential regulation by protein kinase C isoforms of nitric oxide synthase induction in RAW 264.7 macrophages and rat aortic smooth muscle cells. Br J Pharmacol. 1997;120:940–946. doi: 10.1038/sj.bjp.0700976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perfect J R, Granger D L, Durack D T. Effects of antifungal agents and gamma interferon on macrophage cytotoxicity for fungi and tumor cells. J Infect Dis. 1987;156:316–323. doi: 10.1093/infdis/156.2.316. [DOI] [PubMed] [Google Scholar]
- 42.Rogers P D, Jenkins J K, Chapman S W, Ndebele K, Chapman B A, Cleary J D. Amphotericin B activation of human genes encoding for cytokines. J Infect Dis. 1998;178:1726–1733. doi: 10.1086/314495. [DOI] [PubMed] [Google Scholar]
- 43.Sculier J P, Body J J. Intravenous administration of amphotericin B entrapped in liposomes: induction of high serum levels of TNF alpha. Ann Oncol. 1991;2:141–144. doi: 10.1093/oxfordjournals.annonc.a057878. [DOI] [PubMed] [Google Scholar]
- 44.Seyler I, Appel M, Devissaguet J P, Legrand P, Barratt G. Relationship between NO-synthase activity and TNF-alpha secretion in mouse macrophage lines stimulated by a muramyl peptide entrapped in nanocapsules. Int J Immunopharmacol. 1996;18:385–392. doi: 10.1016/s0192-0561(96)00041-0. [DOI] [PubMed] [Google Scholar]
- 45.Stainmesse, S., H. Fessi, J. P. Devissaguet, and F. Puisieux. 1989. Procede de preparation de systeme colloidaux dispersibles de lipides amphiphiles sous forme de liposomes submicroniques. European patent 894018571.
- 46.Thomas M Z, Medoff G, Kobayashi G S. Changes in murine resistance to Listeria monocytogenes infection induced by amphotericin B. J Infect Dis. 1973;127:373–377. doi: 10.1093/infdis/127.4.373. [DOI] [PubMed] [Google Scholar]
- 47.Tohyama M, Kawakami K, Saito A. Anticryptococcal effect of amphotericin B is mediated through macrophage production of nitric oxide. Antimicrob Agents Chemother. 1996;40:1919–1923. doi: 10.1128/aac.40.8.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tokuda Y, Tsuji M, Yamazaki M, Kimura S, Abe S, Yamaguchi H. Augmentation of murine tumor necrosis factor production by amphotericin B in vitro and in vivo. Antimicrob Agents Chemother. 1993;37:2228–2230. doi: 10.1128/aac.37.10.2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vazquez-Torres A, Jones-Carson J, Balish E. Nitric oxide production does not directly increase macrophage candidacidal activity. Infect Immun. 1995;63:1142–1144. doi: 10.1128/iai.63.3.1142-1144.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wilson E, Thorson L, Speert D P. Enhancement of macrophage superoxide anion production by amphotericin B. Antimicrob Agents Chemother. 1991;35:796–800. doi: 10.1128/aac.35.5.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wolf J E, Massof S E. In vivo activation of macrophage oxidative burst activity by cytokines and amphotericin B. Infect Immun. 1990;58:1296–1300. doi: 10.1128/iai.58.5.1296-1300.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xie Q, Nathan C. The high-output nitric oxide pathway: role and regulation. J Leukoc Biol. 1994;56:576–582. doi: 10.1002/jlb.56.5.576. [DOI] [PubMed] [Google Scholar]
- 53.Yamaguchi H, Abe S, Tokuda Y. Immunomodulating activity of antifungal drugs. Ann NY Acad Sci. 1993;685:447–457. doi: 10.1111/j.1749-6632.1993.tb35905.x. [DOI] [PubMed] [Google Scholar]
- 54.Yasui K, Masuda M, Matsuoka T, Yamazaki M, Komiyama A, Akabane T, Murata K. Miconazole and amphotericin B alter polymorphonuclear leukocyte functions and membrane fluidity in similar fashions. Antimicrob Agents Chemother. 1988;32:1864–1868. doi: 10.1128/aac.32.12.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]