Abstract
Endometriosis is a chronic gynecological disease that causes numerous severe symptoms in affected women. Revealing alterations of the molecular processes in ectopic endometrial tissue is the current policy for understanding the pathomechanisms and discovering potential novel therapeutic targets. Examining molecular processes of eutopic endometrium is likely to be a convenient method to compare it with the molecular alterations observed in ectopic tissues. The aim of the present study was to determine what proportion of the surgically resected eutopic endometrial samples is suitable for further experiments so that these can be comparable with endometriosis. Final hospital reports and histopathology reports of a 3-year-long period (1162 cases) were analysed. The application of a retrospective screening method promoted the categorization of these cases, and quantification of the categorized cases was accomplished. In addition, results obtained from cultured endometrium samples were also detailed. Only a small number of the harvested endometrial samples was suitable for further molecular analysis, while preoperative screening protocol could enlarge this fraction. Applying clinical and histopathological selection and exclusion criteria for tissue screening and histopathological examination of samples could ensure the comparability of healthy endometrium with endometriosis. The present study could be useful for researchers who intend to perform molecular experiments to compare endometriosis with the physiological processes of the endometrium.
Keywords: endometrium, curettage, hysteroscopy, endometrial sampling, in vitro endometrial culturing
1. Introduction
Endometriosis is a gynaecological disease described as the formation of endometrial-like tissue outside the uterine cavity [1]. Although the definite origin and pathogenesis of the disease are still unknown [2], several theories exist describing the development of the lesions [3]. In addition to the widely accepted retrograde menstruation theory, embryonic rest, lympho-vascular metastasis, and coelomic metaplasia hypotheses have also been settled in the last decade [4]. In recent studies, stem cell-originated endometriosis has also been suggested [5]: ectopic tissue occurs from endogenous stem cells in the endometrium or from bone-marrow-derived stem cells that differentiate into endometrial cells [6]. Various molecular alterations have been described in endometriosis that may affect several emerging downstream pathways such as transcriptional regulation, cell cycle regulation, and cell adhesion [7,8].
The endometrium is a multicellular tissue that forms the inner layer of the uterus. Different processes that lead to the development of endometriosis can result from physiological alterations observed in the endometrium [8]. Hence, it may be hypothesized that eutopic endometrium can be an appropriate control to detect the different molecular changes observed in the ectopic tissue. However, due to many reasons, it is not easy to harvest healthy human endometrium, and careful consideration is needed to reveal the different molecular processes of this tissue [9]. Therefore, the aim of this present study is to assess the suitability of the clinically extracted endometrial samples for further experimental research. To answer this question, more than 1000 cases of a 3-year-long period were examined, where endometrial harvesting happened. In addition, with the help of eight cultured samples, examples of possible general pitfalls during the selection of suitable samples are shown.
2. Materials and Methods
2.1. Collection of Data
Lists from a medical database of patients provided by the Medical Record Office (University of Debrecen) were studied (Research ethics committee approval number: H.0180-2020). Cases based on this list were collected from the period between January 2017 and March 2020, when samples of endometrial biopsies and curettages were extracted by the surgeons of the Obstetrics and Gynaecology Clinic. These tissues were submitted with an ICD (international classification of diseases) code of irregular menstrual bleeding to the Pathology Department for histopathological analysis. In total, the final hospital reports and histopathology reports of 1162 cases were analysed. The following entries were collected: the age of the patient, the appropriate surgical indication, the type of surgery, and finally, the diagnosis in the histopathology report.
2.2. Grouping of Histopathological Diagnoses
In total, 21 different diagnoses were described in the histopathology reports. Firstly, all cases were separated into these 21 groups. To make it easier to handle the different cases, all diagnoses were categorized into 10 groups based on their similarities. These were the following: proliferative phase endometrium, secretory phase endometrium, menstrual phase endometrium, group for effects of exogenous hormones, menopausal endometrium, samples inadequate for analysis, endometrial polyp, endometrial hyperplasia, malignant tumors, and endometritis (Table 1).
Table 1.
21 different histopathological diagnoses categorized into 10 groups.
| Groups | Histopathological Diagnoses |
|---|---|
| Proliferative phase endometrium | - Proliferative phase endometrium |
| Secretory phase endometrium | - Secretory phase endometrium |
| Menstrual phase endometrium | - Menstrual phase endometrium |
| Exogenous hormones | - Pseudo-decidualization - Effects of gestagens |
| Menopausal endometrium | - Pseudo-menopausal endometrium - Endometrial atrophy |
| Inadequate for analysis | - Desquamated endometrium - Inadequate sample - Submucosal uterine leiomyoma |
| Endometrial polyp | - Endometrial polyp |
| Endometrial hyperplasia | - Simple endometrial hyperplasia - Simple glandular endometrial hyperplasia - Complex endometrial hyperplasia - Adenomatous endometrial hyperplasia - Simple glandular atypical endometrial hyperplasia - Complex atypical endometrial hyperplasia |
| Malignant tumors | - Endometrioid adenocarcinoma - Uterine carcinosarcoma |
| Endometritis | - Acute endometritis - Chronic endometritis |
2.3. Using Clinical Exclusion Criteria
In freshly extracted samples, the diagnosis included in the histopathology report was unknown at the time the tissue was submitted to a laboratory for in vitro tissue culturing. Therefore, all samples suggesting underlying pathological processes of the uterus must be eliminated. Every case suitable for further molecular analysis was selected. The following aspects constituted the basis of exclusion: 1. every sample was excluded in which the patient was older than 45 years; 2. surgery was performed because of an established pathological process of the uterus or the type of operation was not only aimed at the extraction of the endometrium; 3. endometriosis or pathological conditions can be revealed in the anamnesis of the patient. The following cases of surgical indication were excluded: postmenopausal bleeding, perimenopausal bleeding, cervical atypia, IUD removal, pathological findings on ultrasound, and preoperative curettage before excision of an intrauterine tumor (Table 2).
Table 2.
Figure illustrating the applied selection and exclusion of cases with different examples.
| Clinical Selection | Type of Operation | Indication of Surgery | Age | Histopathological Diagnosis | Endometriosis/Adenomyosis |
|---|---|---|---|---|---|
| curettage | heavy menstrual bleeding (HMB) | 46 | proliferative phase endometrium | no | |
| LEEP + curettage | cervical cytologic atypia | 28 | CIN-II, proliferative phase endometrium | no | |
| curettage | heavy menstrual bleeding (HMB) | 39 | proliferative phase endometrium | no | |
| curettage | heavy menstrual bleeding (HMB) | 44 | disordered proliferative endometrium | no | |
| hysteroscopy (HSC) | pathological findings on ultrasound | 44 | proliferative phase endometrium | no | |
| hysteroscopy (HSC) | heavy menstrual bleeding (HMB) | 26 | proliferative phase endometrium | yes | |
| hysteroscopy (HSC) | heavy menstrual bleeding (HMB) | 31 | proliferative phase endometrium | no | |
| transcervical resection of polyp (TCRP) | pathological findings on ultrasound | 28 | disordered proliferative endometrium | no | |
| hysteroscopy (HSC) | pathological findings on ultrasound | 39 | proliferative phase endometrium | no | |
| curettage | pathological findings on ultrasound | 48 | proliferative phase endometrium | no | |
| selected | |||||
| excluded | |||||
| histopathologic exclusion | |||||
| reason of exclusion | |||||
2.4. Using Clinical Selection Criteria
Considering the exclusion criteria, every case was selected where the patient was younger than 45 years, where surgery was performed because of heavy menstrual bleeding (menorrhagia, abnormal menstruation, irregular periods), and finally, where an operation was aimed at the extraction of the eutopic endometrium. The following types of operation were selected: D&C (dilation and curettage) scraping procedures, such as curettage, fractional curettage (F&C), abrasion and fractional abrasion, in addition to hysteroscopy with endometrial biopsy (HSC) and transcervical resection of endometrium (TCRE). Scraping (D&C) and hysteroscopy with endometrial biopsy (HSC) procedures were also examined separately from each other (Table 2).
2.5. Using Histopathological Selection and Exclusion Criteria
Knowing the diagnoses of the histopathology report, only proliferative and secretory phase endometria were accepted as appropriate control samples for experimental research. Considering these diagnoses only, every case was excluded where the histopathology report described a condition that could affect the signalling pathways of the physiological endometrium: disordered proliferative endometrium, adenomyosis, and cervical intraepithelial neoplasia (CIN) (Table 2).
2.6. Tissue Culturing
Endometrial tissues harvested by biopsy during HSC procedures were obtained from the Department of Gynaecology. Tissues were processed for histopathological analysis with ICD codes different from irregular menstrual bleeding. Evaluation of the uterine cavity was performed as part of diagnostic hysteroscopy. No other uterine pathologies could be detected preoperatively. Every patient was younger than 45 years. These samples were minced into 60 × 15 mm cell culture dishes (Eppendorf, North America, Inc., New York, NY, USA) and were fixed to a 15 µL matrigel drop (Cultrex® BME, Type 2). Dishes were filled with 4.5 g/L Glucose DMEM (Lonza, Bend, OR, USA), and the medium was changed every day. Tissues were divided into control and hormone-treated groups. Knowing the first day of the last menstruation (LMP), samples were maintained until the 24th day of the menstrual cycle. During the whole culturing period, protocol against tissue contamination was kept.
2.7. Hormone Application
For hormone administration, 17β-estradiol (E2) and progesterone (P4) solutions (Sigma-Aldrich, St. Louis, MO, USA) were applied to the medium of the hormone-treated groups. Given the first day of the LMP, whenever the endometrial sample was submitted to the laboratory, the relevant day of the menstrual cycle could be calculated. Four different concentrations of E2 and P4 solutions were available. This way, the final concentrations of hormones in the medium were the mean serum levels observed in the early follicular, follicular, ovulatory, and luteal phases of the ovarian cycle. Until the last day of hormone administration, hormone concentrations were changed when the sample reached another phase of the ovarian cycle (Table 3). The aim of this procedure was to imitate the in vivo hormonal changes of the menstrual cycle.
Table 3.
Hormone administration mimicking the hormonal changes of a 24 day long menstrual cycle. Mean serum levels of E2 and P4 were used for treatment.
| Menstruation | Proliferative Phase | Secretory Phase | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 |
|
Early Follicaular Phase E2 = 8.7–75 ng/L → 42 ng/L |
|||||||||||||||||||||||
|
Follicular Phase E2 = 12.5–166 ng/L → 89 ng/L P4 = 0.06–0.9 µg/L → 0.5 µg/L |
|||||||||||||||||||||||
|
Ovulation E2 = 85.8–498 ng/L → 292 ng/L P4 = 0.1–12 µg/L → 6.1 µg/L |
|||||||||||||||||||||||
|
Luteal Phase E2 = 43.8–211 ng/L → 112.7 ng/L P4 = 1.8–24 µg/L → 12.9 µg/L |
|||||||||||||||||||||||
2.8. H&E Staining
After the last day of the treatment period, tissues were fixed in 4% paraformaldehyde (PFA) solution for 6 h at least. On the day of sample submission, one part of the removed tissue (‘Fresh’) was fixed directly after the operation. Following paraffinization, histological slides were sectioned to StarFrost® microscope slides (Knittel Glass, Brunswick, Germany). After deparaffinization, slides were stained with haematoxylin (VWR International, Radnor, PA, USA) and eosin (Amresco, Fountain Parkway Solon, OH, USA) histological staining method. Photomicrographs with 10×, 20× and 40× magnifications were taken from the stained slides with a light microscope (BX-53 Microscope, Olympus Microscopes).
2.9. Mathematical Analysis
After counting the number of cases in different histopathological diagnostic groups during the retrospective analysis, data were converted to percentages to make the results more transparent.
2.10. Ethical Approval
Research ethics committee approval numbers of the present study are the followings: H.0180-2020 and 28966-2/2018/EKU.
3. Results
3.1. Histopathological Findings of the Samples
In the histopathology reports, 17.64 and 10.67% of the samples were diagnosed as proliferative and secretory phase endometria. The remaining tissues were verified as menstrual phase endometria (1.46%), menopausal endometria (4.56%), and pathological endometria: endometrial polyp (13.51%), endometrial hyperplasia (24.01%), malignant tumors (4.04%), and endometritis (1.72%). Overall, 16.18% of the samples seemed inadequate for further analysis, and 6.20% of the endometria showed signs of exogenous hormonal effects (Table 4). All histopathological diagnoses of these mentioned 10 groups are detailed in Table 1.
Table 4.
Histopathological findings of 1162 cases.
| Group | Case Number | Percentage (%) |
|---|---|---|
| Proliferative phase endometrium | 205 | 17.64 |
| Secretory phase endometrium | 124 | 10.67 |
| Menstrual phase endometrium | 17 | 1.46 |
| Exogenous hormones | 72 | 6.20 |
| Menopausal endometrium | 53 | 4.56 |
| Inadequate for analysis | 188 | 16.18 |
| Endometrial polyp | 157 | 13.51 |
| Endometrial hyperplasia | 279 | 24.01 |
| Malignant tumors | 47 | 4.04 |
| Endometritis | 20 | 1.72 |
| Total | 1162 | 100 |
3.2. Histopathological Findings of Endometrial Scraping and Biopsy Procedures
Of the total 1162 cases aiming at the extraction of the endometrium, 949 tissues were removed by only scraping and hysteroscopy with endometrial biopsy surgical procedures. The resection of the remaining 213 samples was an additional surgery or another surgical procedure. Knowing the histopathology reports of these 949 cases, 15.07% and 10.01% of the samples were diagnosed as proliferative and secretory phase endometria. The remaining tissues were verified as menstrual phase endometria (1.79%), menopausal endometria (4.32%), and pathological endometria: endometrial polyp (12.33%), endometrial hyperplasia (26.66%), malignant tumors (4.43%), and endometritis (1.05%). Overall, 19.07% of the samples seemed inadequate for further analysis, and 5.27% of the endometria showed signs of exogenous hormonal effects (Table 5). All histopathological diagnoses of these mentioned 10 groups are detailed in Table 1.
Table 5.
Histopathological findings of cases where operation aimed at the extraction of endometrium.
| Group | Case Number | Percentage (%) |
|---|---|---|
| Proliferative phase endometrium | 143 | 15.07 |
| Secretory phase endometrium | 95 | 10.01 |
| Menstrual phase endometrium | 17 | 1.79 |
| Exogenous hormones | 50 | 5.27 |
| Menopausal endometrium | 41 | 4.32 |
| Inadequate for analysis | 181 | 19.07 |
| Endometrial polyp | 117 | 12.33 |
| Endometrial hyperplasia | 253 | 26.66 |
| Malignant tumors | 42 | 4.43 |
| Endometritis | 10 | 1.05 |
| Total | 949 | 100 |
3.3. Histopathological Findings of Endometrial Scraping Procedures (D&C)
From the previously mentioned 949 cases, 833 endometria were extracted by scraping surgical procedures. In the histopathology reports of these 833 cases, 14.17 and 9.84% of the samples were diagnosed as proliferative and secretory phase endometria. The remaining tissues were verified as menstrual phase endometria (1.80%), menopausal endometria (4.44%) and pathological endometria: endometrial polyp (12.24%), endometrial hyperplasia (27.25%), malignant tumors (4.32%), and endometritis (1.20%). Overall, 19.69% of the samples seemed inadequate for further analysis, and 5.04% of the endometria showed signs of exogenous hormonal effects (Table 6). All histopathological diagnoses of these mentioned 10 groups are detailed in Table 1.
Table 6.
Histopathological findings of samples extracted by scraping procedure.
| Group | Case Number | Percentage (%) |
|---|---|---|
| Proliferative phase endometrium | 118 | 14.17 |
| Secretory phase endometrium | 82 | 9.84 |
| Menstrual phase endometrium | 15 | 1.80 |
| Exogenous hormones | 42 | 5.04 |
| Menopausal endometrium | 37 | 4.44 |
| Inadequate for analysis | 164 | 19.69 |
| Endometrial polyp | 102 | 12.24 |
| Endometrial hyperplasia | 227 | 27.25 |
| Malignant tumors | 36 | 4.32 |
| Endometritis | 10 | 1.20 |
| Total | 883 | 100 |
3.4. Histopathological Findings of Hysteroscopy with Endometrial Biopsy Procedures (HSC)
From the previously mentioned 949 cases, 116 endometria were extracted by hysteroscopy with endometrial biopsy procedures. In the histopathology reports of these 116 cases, 21.55 and 11.21% of the samples were diagnosed as proliferative and secretory phase endometria. The remaining tissues were verified as menstrual phase endometria (1.72%), menopausal endometria (3.45%), and pathological endometria: endometrial polyp (12.93%), endometrial hyperplasia (22.41%) and malignant tumors (5.17%). There were no histopathological findings for endometritis. Overall, 14.66% of the samples seemed inadequate for further analysis, and 6.90% of the endometria showed signs of exogenous hormonal effects (Table 7). All histopathological diagnoses of these mentioned 10 groups are detailed in Table 1.
Table 7.
Histopathological findings of samples extracted by hysteroscopy with endometrial biopsy procedure.
| Group | Case Number | Percentage (%) |
|---|---|---|
| Proliferative phase endometrium | 25 | 21.55 |
| Secretory phase endometrium | 13 | 11.21 |
| Menstrual phase endometrium | 2 | 1.72 |
| Exogenous hormones | 8 | 6.90 |
| Menopausal endometrium | 4 | 3.45 |
| Inadequate for analysis | 17 | 14.66 |
| Endometrial polyp | 15 | 12.93 |
| Endometrial hyperplasia | 26 | 22.41 |
| Malignant tumors | 6 | 5.17 |
| Endometritis | 0 | 0.00 |
| Total | 116 | 100 |
3.5. Clinically Suitable Samples for In Vitro Experimental Research Extracted by Scraping and Hysteroscopy with Endometrial Biopsy Procedures
Without knowing the histopathological findings of the samples, only using the previously described clinical exclusion and selection criteria, altogether 145 endometria (15.28%) seemed suitable for further experimental analysis from the total 949 cases (Table 8). By retrospective analysis, it was proved that from these 145 tissues, 22.07% and 15.86% of the samples were diagnosed as proliferative and secretory phase endometria. The remaining tissues were verified as menstrual phase endometria (3.45%), menopausal endometria (1.38%), and pathological endometria: endometrial polyp (8.97%), endometrial hyperplasia (31.03%), malignant tumors (0.69%), and endometritis (0.69%). Overall, 10.34% of the samples seemed inadequate for further analysis, and 5.52% of the endometria showed signs of exogenous hormonal effects (Table 9). All histopathological diagnoses of these mentioned 10 groups are detailed in Table 1.
Table 8.
Cases that were suitable by applying clinical selection and exclusion criteria.
| Operation | Total Case Number | Clinically Suitable (Case Number) | Clinically Suitable (%) |
|---|---|---|---|
| D&C + HSC | 949 | 145 | 15.28 |
| D&C | 833 | 116 | 13.93 |
| HSC | 116 | 29 | 25.00 |
Table 9.
Histopathological findings of clinically suitable samples.
| Group | Case Number | Percentage (%) |
|---|---|---|
| Proliferative phase endometrium | 32 | 22.07 |
| Secretory phase endometrium | 23 | 15.86 |
| Menstrual phase endometrium | 5 | 3.45 |
| Exogenous hormones | 8 | 5.52 |
| Menopausal endometrium | 2 | 1.38 |
| Inadequate for analysis | 15 | 10.34 |
| Endometrial polyp | 13 | 8.97 |
| Endometrial hyperplasia | 45 | 31.03 |
| Malignant tumors | 1 | 0.69 |
| Endometritis | 1 | 0.69 |
| Total | 145 | 100 |
3.6. Clinically Suitable Samples for In Vitro Experimental Research Extracted by Scraping Procedures (D&C)
From the previously mentioned 145 cases, 116 endometria were extracted by scraping surgical procedures (Table 8). By retrospective analysis, only regarding these cases, it was proven that 19.83% and 17.24% of the samples were diagnosed as proliferative and secretory phase endometria. The remaining tissues were verified as menstrual phase endometria (3.45%), menopausal endometria (1.72%), and pathological endometria: endometrial polyp (9.48%), endometrial hyperplasia (31.90%), and endometritis (0.86%). There were no histopathological findings for malignant tumors. Overall, 10.34% of the samples were inadequate for further analysis, and 5.17% of the endometria showed signs of exogenous hormonal effects (Table 10). All histopathological diagnoses of these mentioned 10 groups are detailed in Table 1.
Table 10.
Histopathological findings of clinically suitable samples extracted by scraping procedure.
| Group | Case Number | Percentage (%) |
|---|---|---|
| Proliferative phase endometrium | 23 | 19.83 |
| Secretory phase endometrium | 20 | 17.24 |
| Menstrual phase endometrium | 4 | 3.45 |
| Exogenous hormones | 6 | 5.17 |
| Menopausal endometrium | 2 | 1.72 |
| Inadequate for analysis | 12 | 10.34 |
| Endometrial polyp | 11 | 9.48 |
| Endometrial hyperplasia | 37 | 31.90 |
| Malignant tumors | 0 | 0.00 |
| Endometritis | 1 | 0.86 |
| Total | 116 | 100 |
3.7. Clinically Suitable Samples for In Vitro Experimental Research Extracted by Hysteroscopy with Endometrial Biopsy Procedures (HSC)
From the previously mentioned 145 cases, 29 endometria were extracted by hysteroscopy with endometrial biopsy procedures (Table 8). By retrospective analysis, only regarding these cases, it was proven that 31.03 and 10.34% of the samples were diagnosed as proliferative and secretory phase endometria. The remaining tissues were verified as menstrual phase endometria (3.45%) and pathological endometria: endometrial polyp (6.90%), endometrial hyperplasia (27.59%), and malignant tumors (3.45%). There were no histopathological findings for endometritis or menopausal endometria. Overall, 10.34% of the samples were inadequate for further analysis, and 6.90% of the endometria showed signs of exogenous hormonal effects (Table 11). All histopathological diagnoses of these mentioned 10 groups are detailed in Table 1.
Table 11.
Histopathological findings of clinically suitable samples extracted by hysteroscopy with endometrial biopsy procedure.
| Group | Case Number | Percentage (%) |
|---|---|---|
| Proliferative phase endometrium | 9 | 31.03 |
| Secretory phase endometrium | 3 | 10.34 |
| Menstrual phase endometrium | 1 | 3.45 |
| Exogenous hormones | 2 | 6.90 |
| Menopausal endometrium | 0 | 0.00 |
| Inadequate for analysis | 3 | 10.34 |
| Endometrial polyp | 2 | 6.90 |
| Endometrial hyperplasia | 8 | 27.59 |
| Malignant tumors | 1 | 3.45 |
| Endometritis | 0 | 0.00 |
| Total | 29 | 100 |
3.8. Suitable Samples for Further In Vitro Experimental Research Studies
For the reasons detailed below, only proliferative and secretory phase endometria are suitable for experimental research. Of the total number of examined samples (949 cases), 145 (15.3%) were suitable after using only clinical selection and exclusion criteria. From these, 116 were harvested by scraping and 29 by hysteroscopy with endometrial biopsy procedures. The number of samples suitable for molecular experiments (suitability detailed in Section 2.3, Section 2.4 and Section 2.5) was revealed with the help of retrospective analysis. Altogether, 32 samples were suitable by using histopathological selection and exclusion criteria. This is 3.37% of the 949 cases and 22.07% of the 145 cases. Regarding the scraping procedures, 28 endometria were suitable for further in vitro experimental analysis. This is 3.36% of the 833 and 24.14% of the 116 cases. Regarding the hysteroscopy with endometrial biopsy procedures, four endometria were suitable for further in vitro experimental analysis. This is 3.45% of the 116 and 13.79% of the 29 cases (Table 12).
Table 12.
Suitable samples.
| D&C + HSC | D&C | HSC | ||||
|---|---|---|---|---|---|---|
| Total Case Number | Clinically Suitable | Total Case Number | Clinically Suitable | Total Case Number | Clinically Suitable | |
| 949 | 145 | 833 | 116 | 116 | 29 | |
| Proliferative phase endometrium | ||||||
| Suitable (case number) |
13 | 11 | 2 | |||
| Suitable (%) | 1.37 | 8.97 | 1.32 | 9.48 | 1.72 | 6.90 |
| Secretory phase endometrium | ||||||
| Suitable (case number) |
19 | 17 | 2 | |||
| Suitable (%) | 2.00 | 13.10 | 2.04 | 14.66 | 1.72 | 6.90 |
| Proliferative and secretory phase endometria | ||||||
| Suitable (case number) |
32 | 28 | 4 | |||
| Suitable (%) | 3.37 | 22.07 | 3.36 | 24.14 | 3.45 | 13.79 |
3.9. Experiences Obtained from the Submitted and Cultured Eutopic Endometrial Tissues
Every sample was analysed with histopathological assessment. One piece of submitted tissue was fixed with 4% PFA on the day of endometrium removal to verify the histopathological diagnosis of the harvested sample, determine the actual histological status and be able to compare the submitted tissue with the cultured tissues. After the treatment period (following the 24th day of the menstrual cycle), the control and the hormone-administered samples were also assessed with histopathological analysis. Results are demonstrated with the example of different cases.
Case 1 endometrium (Supplementary Figure S1) contained a thick layer of submucosal myometrium and was composed of secretory phase glands and stroma. However, the whole thickness of the secretory phase endometrium could not be observed since the layer close to the uterine cavity was missing. Furthermore, histopathological analysis of another sample harvested from the same uterus verified polypoid adenomyoma. In addition, this kind of thick myometrium layer in a sample always raises the possibility of the presence of submucosal uterine leiomyoma. Hence, this endometrium would be excluded from further molecular examinations. Moreover, cultured tissues underwent necrosis during the treatment period.
Case 2 endometrium (Supplementary Figure S2) comprised secretory phase tissue. Nevertheless, in ‘Fresh’ samples, interstitial bleeding was observed, suggesting injury of the tissue. In this case, the culture also underwent necrosis.
In Case 3 endometrium (Supplementary Figure S3) glands showed the signs of artificial injury. In addition, histopathological analysis of another removed piece from the same endometrium verified endometrial polyp. Hence, this endometrium would be excluded from further molecular examinations. Cultured tissues showed signs of necrosis, and in control and hormone-treated groups, severe necrosis of stroma was observed. Moreover, one minced portion contained squamous metaplasia of the endocervix.
Case 4 endometrium (Supplementary Figure S4) showed the signs of another type of artificial injury. Gland cells separated from the stromal cells and disintegrated from each other.
Cultured tissues of Case 5 endometrium (Supplementary Figure S5) underwent necrosis during the treatment period. Histopathological analysis of the ‘Fresh’ sample verified that the tissue was disordered proliferative endometrium as proliferative and secretory phase glands could be found alongside each other.
In Case 6 endometrium (Supplementary Figure S6), another type of disordered proliferative endometrium was confirmed. In this sample, the appearance of proliferative phase stroma was observed along with secretory phase glands.
Histopathological analysis of Case 7 endometrium (Supplementary Figure S7) confirmed healthy, secretory phase endometrium. Cultured tissues could be maintained until the last day of the treatment period. In control groups, secretory phase glands produced mucus. The same condition was observed in hormone-administered groups, where very mild necrosis was observed around the intact endometrial glands.
Case 8 endometrium (Supplementary Figure S8) was similar to Case 7 endometrium. In hormone-treated samples, the secretory phase of endometrium appeared more progressed compared to the control group.
4. Discussion
The formation of endometriosis can be the result of the involvement of stem cell populations that can be found in the human endometrium [10]. The issue regarding the differences in gene expression between the eutopic and ectopic tissues has been raised in some studies [11,12]. Considering these, it seems logical to conclude that eutopic endometrium is a suitable candidate for comparison with endometriosis in experimental research. This way, changes detected in the ectopic tissue can be confirmed with the results obtained from a healthy endometrium. It could be a question of interest why it is inevitable to examine human endometrium in an in vitro experimental research instead of using endometrial cell lines or animal models.
Endometrial cells become terminally differentiated during each menstrual cycle [13], while endometrial stem cells (ESC) are responsible for the monthly regeneration of the endometrium [14]. These could be endometrial mesenchymal stem cells, endometrial epithelial progenitors, side population cells, and bone marrow-derived stem cells that support the proliferation of stromal and epithelial compartments and are located in both layers of the endometrium [15,16]. Basal and functional layers contain endometrial glands embedded in a multicellular stroma consisting of fibroblasts, immune cells and vascular components [17]. Regarding all these, a diverse population of cells can be found in the endometrium; they affect each other and form an endometrial milieu responsible for the complexity of the menstrual cycle and renewing the endometrial tissue. Nevertheless, endometrial cell lines used in different research are derived from pathological endometria or isolated from menstrual blood and generally comprise only one cell type (e.g., mesenchymal stem cells) of the endometrium [18,19,20,21]. Furthermore, these can also be immortalized cell lines [22]. Both the cell lines trying to model the processes of endometriosis [23] as well as the endometrial cell lines demonstrate one or several aspects of the physiology of the endometrium but are not applicable for understanding the entire complexity of the tissue. In addition, there is an absence of cell-cell interactions in these models, which are characteristic of the complex microenvironment of the endometrium [20].
One of the main problems with using animal models to investigate the processes of the endometrium is that most laboratory animals have an oestrus cycle instead of a menstrual cycle, except for menstruating primates [24,25]. The latter animals are expensive to house, and the use of them for routine screenings is unethical. [26]. Another controversy regarding the use of animals is that endometriosis only develops spontaneously in humans and menstruating primates [26]. Although several models have been used to examine the pathophysiology of the disease [27,28,29,30], they have limitations and can not mimic or reproduce all aspects of endometriosis [29,30].
As endometrial cell lines and animal models are not capable of fully reflecting the physiological characteristics of the endometrium [20], examining the human endometrium itself can be a convenient decision to reveal the physiological or pathological molecular processes of this tissue as they consist of the same cells. Histopathological diagnosis of endometriosis consists of identifying two or more of the following cell types: endometrial gland cells, endometrial epithelial cells, endometrial stromal cells and hemosiderin-laden macrophages [1]. The endometrial complexity and extensive capability of the tissue to transform are hallmarked by the diversity of cell types in the tissue, including epithelial, stromal, vascular (endothelium, pericytes and vascular smooth muscle), and immune cells [17,31,32]. Histologically, the endometrium is divided into a basal and a functional layer [33]. Because of the hormonal changes (different blood levels of oestrogen and progesterone), the endometrium undergoes cyclic episodes of proliferation, differentiation, and, in the absence of embryo implantation, the shedding of the functional layer [17]. Regeneration of endometrium after menstruation is provided by endometrial stem cells [34]. It has been found that these stem cells, found in both layers of the endometrium, can be distributed by retrograde menstrual efflux, and may contribute to the establishment of ectopic endometrium [35]. In recent studies, stem cell-originated endometriosis has also been suggested [5]: ectopic tissue occurs from endogenous stem cells in the endometrium or from bone-marrow-derived stem cells that differentiate into endometrial cells [6].
Harvesting healthy endometrium is a challenging process that also requires ethical consideration. Extraction of this sample from the uterine cavity is only possible by appropriate surgical indication. Moreover, operations are generally performed due to a presupposed underlying pathological process in the uterus. Three main surgical procedures provide the extraction of the endometrium: scraping (curettage), hysteroscopy, and hysterectomy procedures [9,36].
The aim of our present retrospective analysis was to determine what proportion of the surgically removed human eutopic samples are suitable as appropriate controls to reveal molecular alterations observed in ectopic tissues. This study covers the cases of a 3-year-long period and intends to reveal what the chances are that a healthy, surgically removed endometrial sample submitted to a laboratory is eligible to be processed for further in vitro experimental research. A list of 1162 final hospital reports and histopathology reports was examined where endometria have been submitted to histopathological analysis with an ICD code of irregular menstrual bleeding. This is one of the most commonly used codes submitted to the Pathology Departments together with endometrial samples. Altogether 949 operations were aimed at endometrial harvesting only. The remaining 213 cases were excluded, these being hysterectomy procedures or operations where endometrial harvesting was only a complementary part of another surgical procedure. In most cases, a hysterectomy is performed because of a confirmed pathological condition [9]. Hence, in the present research, only endometria extracted by scraping and biopsy procedures were examined. It has been described that in pathological endometria, numerous molecular alterations can be observed (e.g., in endometrial hyperplasia and tumors) [37,38,39,40]. For this reason, only healthy endometria can be an appropriate choice to examine molecular processes in a control experiment. In addition, it has been described that perimenopausal endometrium has altered gene expression compared to the premenopausal tissue [41]. Thus, as early menopause occurs at or before the age of 45 [42], every sample was excluded where the patient was older than 45 years.
At the time the endometrial tissue is submitted to an experimental research laboratory directly after the surgical procedure, histopathological diagnosis is unknown. In this case, based on our research, the chance of examining pathological endometrium is more than 96%. Hence, it should be considered to use preliminary clinical exclusion and selection criteria to decrease the probability of getting pathological tissue. With the help of these, it was shown that the chance of examining a healthy endometrium is more than 22%.
The reason for examining the D&C and HSC cases separately is that endometrial samples harvested with these procedures are examined in different types of molecular research. Tissues removed with scraping procedures mainly contain the functional layer and are suitable for fertility and perimenopausal research, while endometria harvested by hysteroscopy with endometrial biopsy also contain the basalis layer, and are suitable for stem cell examinations [9]. At the time of sample submission, the chance of examining physiological endometrium is a little more than 3% in the case of scraping and hysteroscopy with endometrial biopsy procedures also. With the help of using clinical exclusion and selection criteria, the chance of examining healthy endometrium is more than 24% regarding the scraping procedure and more than 13% for hysteroscopy with endometrial biopsy procedure. The proportion of endometrial samples with pathological findings was also revealed, although it was not the aim of the present research, contrary to previous studies [36,43].
To examine molecular processes of a healthy endometrium, the use of samples extracted with surgical indications other than menstrual disorders should be considered. Endometrium harvested during TCRS (transcervical resection of the uterine septum) or diagnostic hysteroscopy could be good candidate methods; nevertheless, histopathological findings observed in these kinds of samples have not been described yet. Removing the uterus of brain-dead women who are candidates for organ donation would also be a possibility to extract endometrium; however, it is supposed that these uteri would be used to treat absolute uterine factor infertility patients in the future [44]. The main limitation of the present research was that only samples submitted with an ICD code of irregular menstrual bleeding were examined, and endometria submitted with different ICD codes were not.
Regarding cultured samples, it was tested whether it is possible to maintain a minced piece of endometrium under laboratory circumstances. Hormonal changes in the menstrual cycle were mimicked with estradiol and progesterone administration. As a normal menstrual cycle lasts between 25 and 35 days [45], the length of the treatment period was 24 days and samples were fixed in formalin solution on the 25th day. Analysis of eight cases revealed the problems and difficulties that a molecular researcher faces while doing experiments with eutopic endometrium. First of all, submitted samples can be injured artificially at the time of removal. Harvested tissue can sustain mechanical injuries and interstitial bleedings during the operation. It is unlikely that these injuries are dependent on individual surgeons. Moreover, except for the layers of the endometrium, samples can be composed of other cell types, such as smooth muscle cells of submucosal myometrium or epithelial cells of the endocervix. These common pitfalls have also been described in previous studies [9]. Furthermore, in spite of applying clinical selection and exclusion criteria, during histopathological analysis, pathological conditions can be revealed, such as disordered proliferative endometrium or endometrial polyp. Regarding our results, healthy endometria outlive the culturing procedures and can be used for further analysis.
5. Conclusions
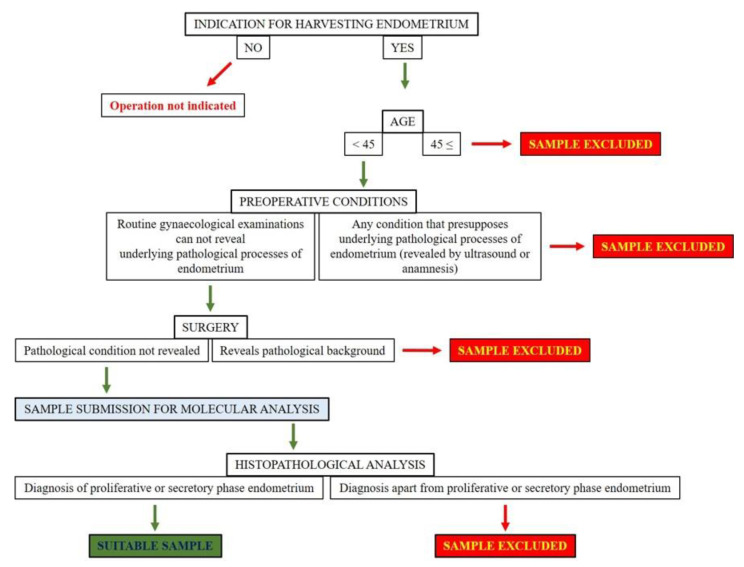
In summary, since the possibility of harvesting pathological tissue is very high, it is a major challenge for experimental researchers to achieve reliable results from human eutopic endometrium while examining molecular processes. Using clinical exclusion and selection criteria for screening pathological samples increases the chance of examining healthy endometrium. Retrospective histopathological analysis is indispensable to ensure that results were obtained from physiological tissue (Figure 1). Samples where the pathological condition was confirmed are recommended to be excluded from experimental research. This way, the results from eutopic endometrium can be compared with the molecular processes observed in endometriosis to reveal whether there are differences between the two tissues.
Figure 1.
Recommended protocol for the involvement of eutopic endometrium samples to experimental research studies.
Acknowledgments
Special thanks to our laboratory assistant, Krisztina Bíróné Barna for her honourable work. We thank Lili Schwieters for proofreading the manuscript.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/diagnostics12040970/s1, Figure S1: Case 1 endometrium; Figure S2: Case 2 endometrium; Figure S3: Case 3 endometrium; Figure S4: Case 4 endometrium; Figure S5: Case 5 endometrium; Figure S6: Case 6 endometrium; Figure S7: Case 7 endometrium; Figure S8: Case 8 endometrium.
Author Contributions
Conceptualization, D.R., A.J. and T.J.; Data curation, V.S., L.F. and M.K.; Formal analysis, B.O.; Methodology, V.S., L.F., M.K., P.T., B.O. and T.J.; Writing—original draft, V.S.; Writing—review & editing, D.R., P.T., A.S.L., A.J. and T.J. All authors have read and agreed to the published version of the manuscript.
Funding
Research was supported by the ÚNKP-17-2 New National Excellence Program of the Ministry for Innovation and Technology, by the ÚNKP-19-3 New National Excellence Program of the Ministry for Innovation and Technology, by the ÚNKP-20-3-II New National Excellence Program of the Ministry for Innovation and Technology from the source of the National Research, Development and Innovation Fund. The research was supported by the EFOP-3.6.3-VEKOP-16-2017-00009 project co-financed by EU and the European Social Fund. The research was supported by NKFIHK139396 and by MTA-TKI 14016.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of University of Debrecen: research ethics committee approval numbers of the present study are the followings: H.0180-2020 and 28966-2/2018/EKU.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that there are no conflicts of interest that could be perceived as prejudicing the impartiality of the research reported.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hsu A.L., Khachikyan I., Stratton P. Invasive and noninvasive methods for the diagnosis of endometriosis. Clin. Obstet. Gynecol. 2010;53:413–419. doi: 10.1097/GRF.0b013e3181db7ce8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rolla E. Endometriosis: Advances and controversies in classification, pathogenesis, diagnosis, and treatment. F1000Res. 2019;8:529. doi: 10.12688/f1000research.14817.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lagana A.S., Vitale S.G., Salmeri F.M., Triolo O., Ban Frangez H., Vrtacnik-Bokal E., Stojanovska L., Apostolopoulos V., Granese R., Sofo V. Unus pro omnibus, omnes pro uno: A novel, evidence-based, unifying theory for the pathogenesis of endometriosis. Med. Hypotheses. 2017;103:10–20. doi: 10.1016/j.mehy.2017.03.032. [DOI] [PubMed] [Google Scholar]
- 4.Chang J.H., Au H.K., Lee W.C., Chi C.C., Ling T.Y., Wang L.M., Kao S.H., Huang Y.H., Tzeng C.R. Expression of the pluripotent transcription factor OCT4 promotes cell migration in endometriosis. Fertil. Steril. 2013;99:1332–1339.e5. doi: 10.1016/j.fertnstert.2012.11.033. [DOI] [PubMed] [Google Scholar]
- 5.Lagana A.S., Salmeri F.M., Vitale S.G., Triolo O., Gotte M. Stem cell trafficking during endometriosis: May epigenetics play a pivotal role? Reprod. Sci. 2018;25:978–979. doi: 10.1177/1933719116687661. [DOI] [PubMed] [Google Scholar]
- 6.Macer M.L., Taylor H.S. Endometriosis and infertility: A review of the pathogenesis and treatment of endometriosis-associated infertility. Obstet. Gynecol. Clin. N. Am. 2012;39:535–549. doi: 10.1016/j.ogc.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bouquet De Joliniere J., Ayoubi J.M., Gianaroli L., Dubuisson J.B., Gogusev J., Feki A. Endometriosis: A new cellular and molecular genetic approach for understanding the pathogenesis and evolutivity. Front. Surg. 2014;1:16. doi: 10.3389/fsurg.2014.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lagana A.S., Garzon S., Gotte M., Vigano P., Franchi M., Ghezzi F., Martin D.C. The pathogenesis of endometriosis: Molecular and cell biology insights. Int. J. Mol. Sci. 2019;20:5615. doi: 10.3390/ijms20225615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maclean A., Kamal A., Adishesh M., Alnafakh R., Tempest N., Hapangama D.K. Human uterine biopsy: Research value and common pitfalls. Int. J. Reprod. Med. 2020;2020:9275360. doi: 10.1155/2020/9275360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sourial S., Tempest N., Hapangama D.K. Theories on the pathogenesis of endometriosis. Int. J. Reprod. Med. 2014;2014:179515. doi: 10.1155/2014/179515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borghese B., Mondon F., Noel J.C., Fayt I., Mignot T.M., Vaiman D., Chapron C. Gene expression profile for ectopic versus eutopic endometrium provides new insights into endometriosis oncogenic potential. Mol. Endocrinol. 2008;22:2557–2562. doi: 10.1210/me.2008-0322. [DOI] [PubMed] [Google Scholar]
- 12.Gabriel M., Fey V., Heinosalo T., Adhikari P., Rytkonen K., Komulainen T., Huhtinen K., Laajala T.D., Siitari H., Virkki A., et al. A relational database to identify differentially expressed genes in the endometrium and endometriosis lesions. Sci. Data. 2020;7:284. doi: 10.1038/s41597-020-00623-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evans J., Salamonsen L.A., Winship A., Menkhorst E., Nie G., Gargett C.E., Dimitriadis E. Fertile ground: Human endometrial programming and lessons in health and disease. Nat. Rev. Endocrinol. 2016;12:654–667. doi: 10.1038/nrendo.2016.116. [DOI] [PubMed] [Google Scholar]
- 14.Gargett C.E., Schwab K.E., Zillwood R.M., Nguyen H.P., Wu D. Isolation and culture of epithelial progenitors and mesenchymal stem cells from human endometrium. Biol. Reprod. 2009;80:1136–1145. doi: 10.1095/biolreprod.108.075226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cousins F.L., Dorien F.O., Gargett C.E. Endometrial stem/progenitor cells and their role in the pathogenesis of endometriosis. Best Pract. Res. Clin. Obstet. Gynaecol. 2018;50:27–38. doi: 10.1016/j.bpobgyn.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 16.Maruyama T. Endometrial stem/progenitor cells. J. Obstet. Gynaecol. Res. 2014;40:2015–2022. doi: 10.1111/jog.12501. [DOI] [PubMed] [Google Scholar]
- 17.Gibson D.A., Simitsidellis I., Collins F., Saunders P.T.K. Endometrial intracrinology: Oestrogens, androgens and endometrial disorders. Int. J. Mol. Sci. 2018;19:3276. doi: 10.3390/ijms19103276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Du X., Yuan Q., Qu Y., Zhou Y., Bei J. Endometrial mesenchymal stem cells isolated from menstrual blood by adherence. Stem Cells Int. 2016;2016:3573846. doi: 10.1155/2016/3573846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Musina R.A., Belyavski A.V., Tarusova O.V., Solovyova E.V., Sukhikh G.T. Endometrial mesenchymal stem cells isolated from the menstrual blood. Bull. Exp. Biol. Med. 2008;145:539–543. doi: 10.1007/s10517-008-0136-0. [DOI] [PubMed] [Google Scholar]
- 20.Nikolakopoulou K., Turco M.Y. Investigation of infertility using endometrial organoids. Reproduction. 2021;161:R113–R127. doi: 10.1530/REP-20-0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noumoff J., Haydock S.W., Sachdeva R., Heyner S., Pritchard M.L. Characteristics of cell lines derived from normal and malignant endometrial tissue. Gynecol. Oncol. 1987;27:141–149. doi: 10.1016/0090-8258(87)90286-1. [DOI] [PubMed] [Google Scholar]
- 22.Holdsworth-Carson S.J., Colgrave E.M., Donoghue J.F., Fung J.N., Churchill M.L., Mortlock S., Paiva P., Healey M., Montgomery G.W., Girling J.E., et al. Generation of immortalized human endometrial stromal cell lines with different endometriosis risk genotypes. Mol. Hum. Reprod. 2019;25:194–205. doi: 10.1093/molehr/gaz006. [DOI] [PubMed] [Google Scholar]
- 23.Fan H. In-vitro models of human endometriosis. Exp. Ther. Med. 2020;19:1617–1625. doi: 10.3892/etm.2019.8363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ajayi A.F., Akhigbe R.E. Staging of the estrous cycle and induction of estrus in experimental rodents: An update. Fertil. Res. Pract. 2020;6:5. doi: 10.1186/s40738-020-00074-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sato J., Nasu M., Tsuchitani M. Comparative histopathology of the estrous or menstrual cycle in laboratory animals. J. Toxicol. Pathol. 2016;29:155–162. doi: 10.1293/tox.2016-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simitsidellis I., Gibson D.A., Saunders P.T.K. Animal models of endometriosis: Replicating the aetiology and symptoms of the human disorder. Best Pract. Res. Clin. Endocrinol. Metab. 2018;32:257–269. doi: 10.1016/j.beem.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 27.Grummer R. Animal models in endometriosis research. Hum. Reprod. Update. 2006;12:641–649. doi: 10.1093/humupd/dml026. [DOI] [PubMed] [Google Scholar]
- 28.Story L., Kennedy S. Animal studies in endometriosis: A review. ILAR J. 2004;45:132–138. doi: 10.1093/ilar.45.2.132. [DOI] [PubMed] [Google Scholar]
- 29.Taniguchi F., Wibisono H., Mon Khine Y., Harada T. Animal models for research on endometriosis. Front. Biosci. 2021;13:37–53. doi: 10.2741/871. [DOI] [PubMed] [Google Scholar]
- 30.Tirado-Gonzalez I., Barrientos G., Tariverdian N., Arck P.C., Garcia M.G., Klapp B.F., Blois S.M. Endometriosis research: Animal models for the study of a complex disease. J. Reprod. Immunol. 2010;86:141–147. doi: 10.1016/j.jri.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 31.Critchley H.O.D., Maybin J.A., Armstrong G.M., Williams A.R.W. Physiology of the endometrium and regulation of menstruation. Physiol. Rev. 2020;100:1149–1179. doi: 10.1152/physrev.00031.2019. [DOI] [PubMed] [Google Scholar]
- 32.Simitsidellis I., Saunders P.T.K., Gibson D.A. Androgens and endometrium: New insights and new targets. Mol. Cell Endocrinol. 2018;465:48–60. doi: 10.1016/j.mce.2017.09.022. [DOI] [PubMed] [Google Scholar]
- 33.Gibson D.A., Simitsidellis I., Collins F., Saunders P.T.K. Androgens, oestrogens and endometrium: A fine balance between perfection and pathology. J. Endocrinol. 2020;246:R75–R93. doi: 10.1530/JOE-20-0106. [DOI] [PubMed] [Google Scholar]
- 34.Tempest N., Jansen M., Baker A.M., Hill C.J., Hale M., Magee D., Treanor D., Wright N.A., Hapangama D.K. Histological 3D reconstruction and in vivo lineage tracing of the human endometrium. J. Pathol. 2020;251:440–451. doi: 10.1002/path.5478. [DOI] [PubMed] [Google Scholar]
- 35.Proestling K., Birner P., Balendran S., Nirtl N., Marton E., Yerlikaya G., Kuessel L., Reischer T., Wenzl R., Streubel B., et al. Enhanced expression of the stemness-related factors OCT4, SOX15 and TWIST1 in ectopic endometrium of endometriosis patients. Reprod. Biol. Endocrinol. 2016;14:81. doi: 10.1186/s12958-016-0215-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCluggage W.G. My approach to the interpretation of endometrial biopsies and curettings. J. Clin. Pathol. 2006;59:801–812. doi: 10.1136/jcp.2005.029702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lax S.F. Molecular genetic changes in epithelial, stromal and mixed neoplasms of the endometrium. Pathology. 2007;39:46–54. doi: 10.1080/00313020601146822. [DOI] [PubMed] [Google Scholar]
- 38.Llobet D., Pallares J., Yeramian A., Santacana M., Eritja N., Velasco A., Dolcet X., Matias-Guiu X. Molecular pathology of endometrial carcinoma: Practical aspects from the diagnostic and therapeutic viewpoints. J. Clin. Pathol. 2009;62:777–785. doi: 10.1136/jcp.2008.056101. [DOI] [PubMed] [Google Scholar]
- 39.Yeramian A., Moreno-Bueno G., Dolcet X., Catasus L., Abal M., Colas E., Reventos J., Palacios J., Prat J., Matias-Guiu X. Endometrial carcinoma: Molecular alterations involved in tumor development and progression. Oncogene. 2013;32:403–413. doi: 10.1038/onc.2012.76. [DOI] [PubMed] [Google Scholar]
- 40.Zauber P., Denehy T.R., Taylor R.R., Ongcapin E.H., Marotta S., Sabbath-Solitare M. Strong correlation between molecular changes in endometrial carcinomas and concomitant hyperplasia. Int. J. Gynecol. Cancer. 2015;25:863–868. doi: 10.1097/IGC.0000000000000421. [DOI] [PubMed] [Google Scholar]
- 41.Erikson D.W., Barragan F., Piltonen T.T., Chen J.C., Balayan S., Irwin J.C., Giudice L.C. Stromal fibroblasts from perimenopausal endometrium exhibit a different transcriptome than those from the premenopausal endometrium. Biol. Reprod. 2017;97:387–399. doi: 10.1093/biolre/iox092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shuster L.T., Rhodes D.J., Gostout B.S., Grossardt B.R., Rocca W.A. Premature menopause or early menopause: Long-term health consequences. Maturitas. 2010;65:161–166. doi: 10.1016/j.maturitas.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Inal Z.O., Inal H.A., Kucukosmanoglu I., Kucukkendirci H. Assessment of endometrial sampling and histopathological results: Analysis of 4247 cases. Eurasian J. Med. 2017;49:44–47. doi: 10.5152/eurasianjmed.2017.16269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brannstrom M. Uterus transplantation and beyond. J. Mater. Sci. Mater. Med. 2017;28:70. doi: 10.1007/s10856-017-5872-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bull J.R., Rowland S.P., Scherwitzl E.B., Scherwitzl R., Danielsson K.G., Harper J. Real-world menstrual cycle characteristics of more than 600,000 menstrual cycles. NPJ Digit. Med. 2019;2:83. doi: 10.1038/s41746-019-0152-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.