Abstract
Background: Staphylococcus aureus, the most common pathogen in skin and soft tissue infections (SSTI), harbors many well-characterized virulence genes. However, the expression of many of them in SSTIs is unknown. In this study, S. aureus virulence genes expressed in SSTI were investigated. Methods: Fifty-three subjects presenting to the outpatient’s care and emergency departments with a purulent SSTI at two medical centers in Wisconsin, USA, were enrolled in the study. Total mRNA was extracted from the purulent or swab materials, made into cDNA and sequenced on MiSeq platform. The relative cDNA counts to gmk and identifications of the transcripts were carried out with respect to USA300 reference genome and using SAMTOOLS v.1.3 and BWA, respectively. Result: A significantly higher cDNA count was observed for many of the virulence and regulatory gene transcripts in the pus samples compared to the swab samples relative to the cDNA counts for gmk, a housekeeping gene. They were for lukS-PV (18.6 vs. 14.2), isaA (13.4 vs. 8.5), ssaA (4.8 vs. 3.1), hlgC (1.4 vs. 1.33), atl (17.7 vs. 8.33), clfA (3.9 vs. 0.83), eno (6.04 vs. 3.16), fnbA (5.93 vs. 0.33), saeS (6.3 vs. 1.33), saeR (5.4 vs. 3.33) and agrC (5.6 vs. 1.5). Conclusions: A relative increase in the transcripts of several toxins, adhesion and regulatory genes with respect to a gmk in purulent materials suggests their role in situ during SSTIs, perhaps in an orchestrated manner.
Keywords: Staphylococcus aureus, SSTI, Panton–Valentine leukocidin, hemolysins, clumping factors, accessory gene regulators
1. Introduction
Staphylococcus aureus, a Gram-positive bacterium is a major human commensal in the anterior nares and other moist body sites and can be present in approximately 30% of the human population [1,2]. S. aureus commensalism is a major risk factor for future clinical infections when protective barriers of the skin and innate immunity are breached and it is able to penetrate deeper into body tissues. In addition to skin and soft tissue infections (SSTI), it is also capable of causing a variety of other invasive diseases such as pneumonia, osteomyelitis, and bacteremia [3,4,5,6]. Treatment of these infections is a challenge as clinical S. aureus isolates express resistance to most of the anti-staphylococcal antibiotics, including the β-lactam class [7]. S. aureus persists within the host due to its ability to produce a large number of virulence factors and biofilm that enables this bacterium to establish an infection, extract nutrients from the host, and evade immune recognition and clearance [8]. Additionally, recurrence and persistence of S. aureus SSTIs is a common clinical phenomenon and an area of major concern [9].
In the past three decades, there has been continued increase in the incidences of community-acquired methicillin-resistant S. aureus (MRSA) infections [10] and clonally, the majority of these MRSA SSTIs have been due to S. aureus strain, USA300 or USA300 lineage strains [11,12]. Although, these strains harbor genes for several well-described (e.g., Panton–Valentine leukocidin and α-toxin) and other putative virulence factors such as Escherichia coli ampicillin resistance (ear) [13], lipoprotein lipase (lpl10) [14] and some of the staphylococcal superantigen-like proteins [15,16], it is unclear which of these are expressed in the SSTI wounds. To determine the distinctive virulence potential of clinical and carriage S. aureus isolates, including MRSA, many of the earlier studies simply profiled the known and putative virulence genes by annotating the genome or screening specific genes by PCR. For example, besides annotating known virulence genes, Baba et al. also identified 19 putative virulence genes in the CA-MRSA strain, MW2 based on their signature sequence motifs [14]. Similarly, other studies also described the virulence genes profile of S. aureus isolated from carriage and clinical sources to correlate the bacterial genotype with its clinical phenotype [17,18,19,20,21,22,23]. Since the presence and absence of virulence genes in an isolate do not necessarily confirm its expression and true virulence potential, some studies investigated the expression of specific virulence genes using in vitro culture, which again may not correlate to the in vivo conditions. Said-Salim et al. investigated the expression of PVL genes in vitro from several CA-MRSA isolates and noted variability in its expression levels in different strains [24]. The present study aimed to detect the transcript levels of putative toxin genes and virulence genes directly from purulent material following surgical drainage or from wound material using a swab.
The goal of this exploratory study was to identify the known and putative virulence genes that are expressed in situ in S. aureus present in SSTI. The study utilized purulent material or wound swabs collected from acute SSTIs, which were analyzed using a transcriptomic approach to measure gene expression through high-throughput sequencing. In this preliminary characterization, we show several S. aureus genes expressed during SSTI thereby implicating their potential role in SSTI pathogenesis.
2. Materials and Methods
2.1. Subjects and Research Specimens
Patients presenting with a purulent SSTI requiring surgical drainage were enrolled during outpatient visits to the Marshfield Clinic or the emergency department of the University of Wisconsin–Madison. Informed consent was obtained from all participants enrolled in the study. Using a sterile needle and syringe, a pus sample was collected if the SSTI had larger wounds and there was sufficient volume of purulent material. In cases where there was an insufficient volume of pus to collect via syringe (<1 mL), two wound swab samples using a sterile cotton swab (Copan Eswab kit, COPAN Diagnostics, Murrieta, CA, USA) were collected. The size of the wounds ranged from 1 cm × 1 cm to 20 cm × 15 cm. The samples were immediately stored in dry ice followed by storage at −80 °C until processed.
2.2. Screening for Methicillin-Sensitive and Methicillin-Resistant S. aureus
A ten to fifty microliter sample of the pus material was streaked on the blood agar plates (BAP) and subsequently on mannitol salt agar (MSA) plates to isolate S. aureus. Wound swabs were streaked onto BAP followed by streaking the suspected colony on MSA plates. The suspected S. aureus colonies were confirmed by the Staphaurex latex Agglutination Test (Thermo Fischer, Waltham, MA, USA). Identification of MRSA was confirmed by identifying the presence of the mecA gene using polymerase chain reaction (PCR) as previously described [19]. Identification of the seven non-S. aureus isolates was done using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) [25]. The spa typing was performed by the method described in [26] and spa types were determined by using the Ridom database (http://spaserver.ridom.de/, accessed on 1 March 2022).
2.3. RNA Extraction and cDNA Synthesis
Total RNA from the pus or swab samples was extracted using TRIzol reagent. The swab was transferred to a 2 mL centrifuge tube containing 500 μL of Ramel MH broth and 1 mL RNAprotect bacteria reagent. The sample was incubated at room temperature for 5 min and centrifuged at maximum speed for 5 min to pellet the cells. The supernatant was discarded and 1 mL TRIzol was added to the cell pellet. The TRIzol mixture was transferred to a 2 mL lysing matrix tube and homogenized for 20 s. Two hundred microliter of chloroform was added to the tube, and the homogenate was centrifuged at high speed for 10 min to separate into a clear aqueous upper layer containing RNA. RNA from the aqueous layer was precipitated with 500 μL of isopropanol and was purified using Qiagen’s RNeasy kit. DNase treatment was then performed using Ambion’s DNase treatment kit. RNA was converted into cDNA using the Life Technologies high-capacity cDNA reverse transcription kit.
2.4. cDNA Sequencing
Total cDNA samples were purified and concentrated using the QIAquick PCR purification kit. Libraries were prepared for sequencing using the KAPA HyperPlus Library Preparation Kit as described below. Depending on the quantity and quality of cDNA available for each sample, 1–50 ng of starting material was enzymatically fragmented for 2 min at 37 °C. The ends of each fragment were enzymatically repaired, with 5′ phosphorylation and 3′ A-tailing. Illumina (San Diego, CA, USA). TruSeq universal adapters (IDT, Coralville, IA, USA) were duplexed in solution by annealing together at a concentration of 3 µM and ligated to the end-repaired fragments. Ligation reaction products were cleaned up using Agencourt AMPure XP beads (Beckman Coulter, Indianapolis, IN, USA). Libraries were amplified using iTru5 and iTru7 primers, to incorporate dual eight bp indexes. PCR products were cleaned up using size selection beads. The final cDNA libraries were sized using the TapeStation (Agilent Technologies, Santa Clara, CA, USA) and quantitated by qPCR using a KAPA library quantitation kit (KAPA Biosystems, Wilmington, MA, USA). The libraries were pooled in equimolar ratios, diluted to 10 pM, and sequenced on a MiSeq instrument using the V3 150 cycle reagent kit (Illumina, San Diego, CA, USA) [27].
2.5. Data Analysis
Two bioinformatics tools, BWA (0.7.13) and Bowtie (2.2.7) were used to assemble the raw sequences as paired-end fastq files and map the output reads to well-annotated USA300FPR3757 genome to match the cDNA and identify the transcripts. The fraction and counts of reads for the coverage of the coding region of the genes (CDS regions) were processed using the SAMTOOLS v.1.3 with the default settings on the annotated reference S. aureus genome, USA300FPR3757. This process was then applied for each of the samples that generated a usable cDNA count. Summary tables of all the genes for all the samples were generated as per each assembler and the reference genome. Estimation of relative cDNA counts was calculated by dividing the cDNA count of genes of interest by gmk (guanylate kinase), a housekeeping gene [28,29] Welch’s t-test was used to compare the relative cDNA counts between pus and swab samples to determine the significant p values. A p value of ≤0.05 was considered significant.
3. Results and Discussion
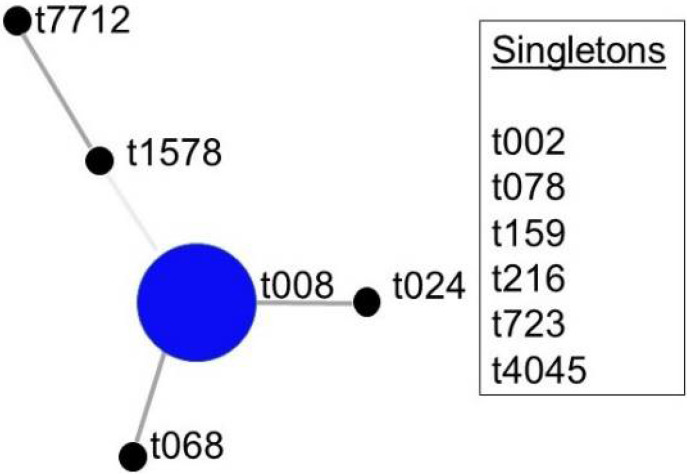
We enrolled 53 patients with SSTI in this study. The average age of the S. aureus-positive subjects was 42.33 years and 17 of the 30 (56.67%) were male. Thirty subjects yielded S. aureus on a BAP from the wound sample. All S. aureus isolates were further confirmed by the latex agglutination test. Seven samples that were negative for S. aureus grew S. caprae, S. capitis, S. epidermidis, S. lugdensis, S. homimis, and Citrobacter braakii as major bacteria confirmed by MALDI-TOF. Twenty of the S. aureus isolates were MRSA as determined by the presence of the mecA gene by PCR and 10 were MSSA. S. aureus isolates from twenty-eight samples were represented by 11 spa types, t008 = 17; t024 = 2; one each of t002, t068, t078, t159, t216, t723, t1578, t4045, t7712, and two isolates were non-typeable (Figure 1). Eight SSTIs were large enough to yield a volume of purulent material while the remainder (n = 20) of the samples were collected by a sterile swab from the surface of the wound. cDNAs could be made only from 23 S. aureus positive samples. Six specimens out of 23 S. aureus positive samples yielded less than 50 total S. aureus cDNA counts and hence they were excluded from further analysis as were the non-S. aureus samples.
Figure 1.
Based upon repeat pattern (BURP) analysis of the 11 spa types, spa-CC t008 was the major clonal complex and there were six singletons.
All cDNA samples yielded sequences that had S. aureus gene transcripts. Total sequencing reads per sample ranged from 3,867,530–7,552,309 (average: 5,432,957) (Table 1). Of these cDNA reads, 43,660 on an average matched with a locus on a S. aureus USA300 genome (Table 1). Expression of as many as 858 S. aureus genes was observed of which 293 genes had at least five cDNA reads. The relative cDNA counts of the genes of interest were measured against the cDNA counts of gmk because of its relatively constant expression during growth phases [28,29].
Table 1.
Number of cDNA reads at different steps of data processing.
| cDNA Reads | Minimum | Maximum | Average |
|---|---|---|---|
| Range of sequencing reads per sample | 3,867,530 | 7,552,309 | 5,432,957 |
| Ranges of cDNA reads that matched to S. aureus USA300 | 255 | 247,463 | 43,660 |
| Ranges of number of sequences that match to an annotated loci | 0 | 77,765 | 11,345 |
| Ranges of the number of loci that have at least one sequence of coverage | 0 | 2372 | 668 |
The virulence factor genes that were prevalent at the site of infection were mainly PVL genes, lukSF-PV, and hemolysin genes, hla, hld, hlgB, and hlgC (Table 2). PVL has a high cytolytic activity to polymorphonuclear leukocytes and macrophages [30,31,32,33,34]. Subunits LukS-PV and LukF-PV assemble and oligomerize on host cells to form β-barrel transmembrane channels that cause osmotic dysregulation and eventually lead to necrosis [35,36,37,38]. These cytotoxic effects are aggravated when they combine with other toxins such as gamma hemolysin [35,37,39].
Table 2.
The average cDNA count of the virulence genes from pus or swab samples and their relative cDNA count to that of gmk.
| Virulence Genes | Gene | Pus (n = 7) | Swab (n = 10) | p Value ** | ||
|---|---|---|---|---|---|---|
| Average cDNA Count | cDNA Count Relative to gmk * | Average cDNA Count | cDNA Count Relative to gmk * | |||
| Panton–Valentine Leukocidin | lukF-PV | 235.4 | 22.6 | 6.8 | 11.3 | 0.06 |
| Panton–Valentine Leukocidin | lukS-PV | 194.4 | 18.6 | 8.5 | 14.2 | ≤0.05 |
| Immunodominant staphylococcal antigen | isaA | 139.3 | 13.4 | 5.1 | 8.5 | ≤0.05 |
| Staphylococcal SecretoryAntigen | ssaA | 49.9 | 4.8 | 1.9 | 3.1 | ≤0.05 |
| Hemolysin D | hld | 39.3 | 3.8 | 2.8 | 4.6 | 0.06 |
| Hemolysin B | hlgB | 26.6 | 2.6 | 0.4 | 0.7 | 0.10 |
| Hemolysin C | hlgC | 15 | 1.4 | 0.8 | 1.33 | ≤0.05 |
| Hemolysin A | hla | 0.7 | 1.3 | 0.8 | 1.33 | 0.09 |
* The average cDNA count of gmk were 10.43 and 0.6 in pus and swab samples, respectively. ** p values are for comparison between pus and swab samples.
Our data showed that PVL is perhaps the most significant toxin produced in SSTI as seen from both the pus and swab samples. Since PVL usually correlates with the severity of skin infections [40], therefore, smaller wounds whose samples could only be collected using a swab showed a lower expression of PVL. Interestingly, Lina et al. (1999) reported that PVL was only expressed in necrotic wounds and was completely absent from superficial folliculitis [41]. Indeed, in a transcriptome study, both PVL and gamma hemolysin expression were found to be several-fold higher in human cutaneous abscess samples (n = 3) when compared to the cultured USA300 strain and mouse infected kidneys in a non-SSTI model [42]. PVL positive strains are often associated with severe persistent and recurrent SSTI [6,10,34,43,44]. Previous studies measuring the transcript levels of virulent genes such as PVL, hlgB, hla, and lukE in cutaneous infections have observed several-fold higher expression levels in them, compared to the same isolates grown in vitro [42,45]. Secretion of these toxins to the cell exterior passing the highly cross-linked peptidoglycan requires enzymes such as IsaA that cleave peptidoglycan. Immunodominant staphylococcal antigen, isaA that was expressed at the site of infection (Table 2) is a lytic transglycosylase, which cleaves peptidoglycan [46]. The immunodominant staphylococcal antigen is highly immunogenic and produces high antibody titers in individuals with S. aureus infection [47]. Treatment with monoclonal antibodies against IsaA protected mice from bacteremia, however, this protection was not explored in SSTI models [48]. Interestingly, most of the virulence genes showed a significantly higher expression in the pus samples (Table 2, Table 3 and Table 4) than in the swab samples. However, hlgB, hld, and hla were high in both pus and swab samples with no special preference to any of these sample types.
Table 3.
The average cDNA count of the adhesion and clumping factor genes from pus or swab samples and their relative cDNA count to that of gmk.
| Adhesion and Clumping Factor Genes | Gene | Pus (n = 7) | Swab (n = 10) | p Values ** | ||
|---|---|---|---|---|---|---|
| Average cDNA Count | cDNA Count Relative to gmk * | Average cDNA Count | cDNA Count Relative to gmk * | |||
| Autolysin | atl | 185.4 | 17.7 | 5 | 8.33 | ≤0.05 |
| Clumping factor | clfB | 110.3 | 10.57 | 7 | 11.6 | ≤0.05 |
| Laminin-binding protein | eno | 63 | 6.04 | 1.9 | 3.16 | ≤0.05 |
| Fibronectin-binding protein | fnbA | 61.9 | 5.93 | 0.2 | 0.33 | ≤0.05 |
| Clumping factor | clfA | 40.9 | 3.9 | 0.5 | 0.83 | ≤0.05 |
| Fibronectin-binding protein | fnbB | 39.1 | 3.75 | 0.4 | 0.66 | ≤0.05 |
| Coagulase | coa | 17.3 | 1.65 | 0 | 0 | ≤0.05 |
| Elastin-binding protein | ebpS | 13.6 | 1.3 | 0.7 | 1.16 | ≤0.05 |
* The average cDNA count of gmk are 10.43 and 0.6 in pus and swab samples, respectively. ** p values are for comparison between pus and swab samples.
Table 4.
The average cDNA count of the regulatory genes from pus or swab samples and their relative cDNA count to that of gmk.
| Regulatory genes | Gene | Pus (n = 7) | Swab (n = 10) | p Value ** | ||
|---|---|---|---|---|---|---|
| Average cDNA Count | cDNA Count Relative to gmk * | Average cDNA Count | cDNA Count Relative to gmk * | |||
| S. aureus exoprotein expression S | saeS | 66.7 | 6.3 | 0.8 | 1.33 | ≤0.05 |
| Accessory gene regulator C | agrC | 58.6 | 5.6 | 0.9 | 1.5 | ≤0.05 |
| S. aureus exoprotein expression R | saeR | 56.3 | 5.4 | 2 | 3.33 | ≤0.05 |
| Accessory gene regulator B | agrB | 25.6 | 2.45 | 0.5 | 0.83 | 0.073 |
* The average cDNA count of gmk were 10.43 and 0.6 in pus and swab samples, respectively. ** p values are for comparison between pus and swab samples.
Transcripts for virulence genes that were not detected in this study were seb, sec, sed, see, seh, sej, and seg2, suggesting their lack of role in SSTI. Although a previous study had detected the presence of enterotoxin gene seb to be more prevalent in S. aureus isolated from SSTI samples and sea to be predominant in S. aureus isolated from non-SSTI samples, their expression levels remain indefinable [49]. Transcripts of seven housekeeping genes of the multilocus sequence typing scheme were also identified, although their cDNA count averages (1.2 to 7) were low.
Adhesion and clumping factor genes that were detected at a higher level were for autolysin, clumping factors B and laminin-binding protein. (Table 3). Autolysin [50,51,52] and clumping factors [53,54,55] are the early proteins involved in adhesion and biofilm formation. Autolysins are the peptidoglycan hydrolases that are suggested to contribute to the appearance of perforating holes throughout the cell wall [56] that may help in the secretion of toxins that would otherwise be contained due to the constraints imposed by the three-dimensional mesh of the cell wall. All of the adhesion and clumping transcripts showed a significant expression in the pus than the swab samples.
Both ClfA and ClfB strongly bind to plasma fibrinogen and cytokeratin promoting bacterial colonization and biofilm formation at the site of a wound during SSTI [57,58,59]. Transcripts for adhesion genes such as eno (laminin-binding protein), fnbA and fnbB (fibronectin-binding proteins A and B), coa (coagulase), and ebps (elastin-binding protein) were also detected (Table 3). Apart from adhesion, they are also involved in blood clumping, platelet activation, and the internalization of S. aureus into host cells [60,61,62]. Surprisingly, we did not detect coagulase transcripts in the swab samples. We also observed transcripts for regulatory genes, accessory gene regulators, agrC and agrB, and S. aureus exoprotein, saeS, and saeR from the purulent samples (Table 4). They all showed significant differences between the pus and the swab samples except for agrB.
Mutations in agr in S. aureus in murine skin infection models have been linked to smaller lesion sizes due to less hemolysin expression and low bacterial burden compared to the wild-type strains [63,64]. Agr (RNAIII) upregulates hla, hlg, hld, lukAB, lukGH, and downregulates cell wall secretary protein IsaA [65,66]. SaeRS is a two-component regulatory system that upregulates coa, hla, hlb, hlg, PVL, and isaA [67,68,69,70]. Both atl and isaA were found to be upregulated in tissues isolated from USA300-positive cutaneous abscess samples [42]. The atl is a major autolysin and plays an important role in cell division and biofilm formation [52,71]. In this study, staphylococcal superantigen such as (ssl) and putative virulence genes such as lipoprotein lipases (lpl10) genes were identified infrequently.
The complexity of the S. aureus pathogenesis is due to the production of different virulence and adhesion proteins at different stages of the disease process. Its microbial surface components recognizing adhesive matrix molecules (MSCRAMMs) help in binding to the host’s extracellular matrix and sometimes more than one molecule bind to the same tissue. Expression of these molecules usually occurs in the colonization phase in contrast to the secreted toxins in the late infection phase [72] In this study, we observed significant differences in gene expression of toxin and adhesion genes between the pus and the swab samples. The higher expression of these genes could be either due to (i) higher density of the S. aureus in the pus, and/or (ii) due to influx of immune cells at the wound site causing higher levels of inflammation. Indeed, in a mouse model study, a higher inoculum of S. aureus increased the inoculum size [73] and direct challenge with purified PVL in a rabbit pneumonia model caused lung damage by cytotoxic granules from the recruited polymorphonuclear at the site of the challenge [74].
It is important to note some limitations of this study. While we detected several important genes for virulence proteins such as PVL, hemolysin, and autolysin, the average cDNA counts of putative genes such as lpl10, seq, sek, etc., were below the average cDNA cut-off except for the ear gene. The overall low cDNA counts matching the S. aureus were likely due to (1) most abscesses contained insufficient purulent material and required sample collection via wound swab, which reduced the amount of S. aureus available for transcript analysis, and (2) a limited number of S. aureus cells present in the wound swab or even the purulent material. These limitations can be controlled by using larger volumes of purulent material to measure transcript levels. This study establishes a foundation for future studies involving larger cohorts with larger volumes of purulent material. In conclusion, interrogation of in situ S. aureus gene expression in SSTI identified a number of known toxin and adhesion/clumping factor genes, suggesting that S. aureus SSTI pathogenesis is likely due to a variety of virulence proteins acting in an orchestrated manner.
Acknowledgments
We would like to thank the anonymous reviewers for their critical feedback and comments to improve this manuscript.
Author Contributions
S.K.S., M.S.P., V.K.S. and M.H., designed the study. J.A. and T.L. performed the experiments. K.G., N.S.E., Z.Y., J.A., T.L., J.P., S.K.S. and M.S.P. analyzed the data. All authors contributed to the writing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The project described was supported by funding from the Marshfield Clinic Research Institute to S.K.S. and M.H. and the Clinical and Translational Science Award (CTSA) program, through the NIH National Center for Advancing Translational Sciences (NCATS), grant UL1TR000427. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Institutional Review Board Statement
The study was approved by the Institutional Review Board of the Marshfield Clinic Research Institute under the protocol # SUL10110.
Informed Consent Statement
All subjects provided informed, signed consent for this study.
Data Availability Statement
All the data is available on request to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kluytmans J., van Belkum A., Verbrugh H. Nasal Carriage of Staphylococcus aureus: Epidemiology, Underlying Mechanisms, and Associated Risks. Clin. Microbiol. Rev. 1997;10:505–520. doi: 10.1128/CMR.10.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wertheim H.F., Melles D.C., Vos M.C., van Leeuwen W., van Belkum A., Verbrugh H.A., Nouwen J.L. The Role of Nasal Carriage in Staphylococcus aureus Infections. Lancet Infect. Dis. 2005;5:751–762. doi: 10.1016/S1473-3099(05)70295-4. [DOI] [PubMed] [Google Scholar]
- 3.Yao D., Yu F., Qin Z., Chen C., He S., Chen Z., Zhang X., Wang L. Molecular Characterization of Staphylococcus aureus Isolates Causing Skin and Soft Tissue Infections (SSTIs) BMC Infect. Dis. 2010;10:133. doi: 10.1186/1471-2334-10-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maina E.K., Kiiyukia C., Wamae C.N., Waiyaki P.G., Kariuki S. Characterization of Methicillin-Resistant Staphylococcus aureus from Skin and Soft Tissue Infections in Patients in Nairobi, Kenya. Int. J. Infect. Dis. 2013;17:e115–e119. doi: 10.1016/j.ijid.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 5.Esposito S., Noviello S., Leone S. Epidemiology and Microbiology of Skin and Soft Tissue Infections. Curr. Opin. Infect. Dis. 2016;29:109–115. doi: 10.1097/QCO.0000000000000239. [DOI] [PubMed] [Google Scholar]
- 6.Harch S.A.J., MacMorran E., Tong S.Y.C., Holt D.C., Wilson J., Athan E., Hewagama S. High Burden of Complicated Skin and Soft Tissue Infections in the Indigenous Population of Central Australia Due to Dominant Panton Valentine Leucocidin Clones ST93-MRSA and CC121-MSSA. BMC Infect. Dis. 2017;17:405. doi: 10.1186/s12879-017-2460-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.David M.Z., Daum R.S. Treatment of Staphylococcus aureus Infections. In: Bagnoli F., Rappuoli R., Grandi G., editors. Staphylococcus Aureus: Microbiology, Pathology, Immunology, Therapy and Prophylaxis. Springer International Publishing; Cham, Switzerland: 2017. pp. 325–383. [DOI] [PubMed] [Google Scholar]
- 8.Yu F., Liu Y., Lv J., Qi X., Lu C., Yu D., Dan L., Liu H., Wang L. Antimicrobial Susceptibility, Virulence Determinant Carriage and Molecular Characteristics of Staphylococcus aureus Isolates Associated with Skin and Soft Tissue Infections. Braz. J. Infect. Dis. 2015;19:614–622. doi: 10.1016/j.bjid.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Creech C.B., Al-Zubeidi D.N., Fritz S.A. Prevention of Recurrent Staphylococcal Skin Infections. Infect. Dis. Clin. North Am. 2015;29:429–464. doi: 10.1016/j.idc.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dukic V.M., Lauderdale D.S., Wilder J., Daum R.S., David M.Z. Epidemics of Community-Associated Methicillin-Resistant Staphylococcus aureus in the United States: A Meta-Analysis. PLoS ONE. 2013;8:e52722. doi: 10.1371/journal.pone.0052722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moran G.J., Krishnadasan A., Gorwitz R.J., Fosheim G.E., McDougal L.K., Carey R.B., Talan D.A. Methicillin-Resistant S. aureus Infections among Patients in the Emergency Department. N. Engl. J. Med. 2006;355:666–674. doi: 10.1056/NEJMoa055356. [DOI] [PubMed] [Google Scholar]
- 12.Kumar N., David M.Z., Boyle-Vavra S., Sieth J., Daum R.S. High Staphylococcus aureus Colonization Prevalence among Patients with Skin and Soft Tissue Infections and Controls in an Urban Emergency Department. J. Clin. Microbiol. 2015;53:810–815. doi: 10.1128/JCM.03221-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh V.K., Ring R.P., Aswani V., Stemper M.E., Kislow J., Ye Z., Shukla S.K. Phylogenetic Distribution and Expression of a Penicillin-Binding Protein Homologue, Ear and Its Significance in Virulence of Staphylococcus aureus. J. Med. Microbiol. 2017;66:1811–1821. doi: 10.1099/jmm.0.000630. [DOI] [PubMed] [Google Scholar]
- 14.Baba T., Takeuchi F., Kuroda M., Yuzawa H., Aoki K., Oguchi A., Nagai Y., Iwama N., Asano K., Naimi T., et al. Genome and Virulence Determinants of High Virulence Community-Acquired MRSA. Lancet. 2002;359:1819–1827. doi: 10.1016/S0140-6736(02)08713-5. [DOI] [PubMed] [Google Scholar]
- 15.Pantrangi M., Singh V.K., Shukla S.K. Regulation of Staphylococcal Superantigen-Like Gene, Ssl8, Expression in Staphylococcus aureus Strain, RN6390. Clin. Med. Res. 2015;13:7–11. doi: 10.3121/cmr.2014.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pantrangi M., Singh V.K., Wolz C., Shukla S.K. Staphylococcal Superantigen-like Genes, Ssl5 and Ssl8, Are Positively Regulated by Sae and Negatively by Agr in the Newman Strain. FEMS Microbiol. Lett. 2010;308:175–184. doi: 10.1111/j.1574-6968.2010.02012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jarraud S., Mougel C., Thioulouse J., Lina G., Meugnier H., Forey F., Nesme X., Etienne J., Vandenesch F. Relationships between Staphylococcus aureus Genetic Background, Virulence Factors, Agr Groups (Alleles), and Human Disease. Infect. Immun. 2002;70:631–641. doi: 10.1128/IAI.70.2.631-641.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naimi T.S., LeDell K.H., Como-Sabetti K., Borchardt S.M., Boxrud D.J., Etienne J., Johnson S.K., Vandenesch F., Fridkin S., O’Boyle C., et al. Comparison of Community- and Health Care–Associated Methicillin-Resistant Staphylococcus aureus Infection. JAMA. 2003;290:2976–2984. doi: 10.1001/jama.290.22.2976. [DOI] [PubMed] [Google Scholar]
- 19.Shukla S.K., Ramaswamy S.V., Conradt J., Stemper M.E., Reich R., Reed K.D., Graviss E.A. Novel Polymorphisms in Mec Genes and a New Mec Complex Type in Methicillin-Resistant Staphylococcus Aureus Isolates Obtained in Rural Wisconsin. Antimicrob Agents Chemother. 2004;48:3080–3085. doi: 10.1128/AAC.48.8.3080-3085.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shukla S.K., Karow M.E., Brady J.M., Stemper M.E., Kislow J., Moore N., Wroblewski K., Chyou P.-H., Warshauer D.M., Reed K.D., et al. Virulence Genes and Genotypic Associations in Nasal Carriage, Community-Associated Methicillin-Susceptible and Methicillin-Resistant USA400 Staphylococcus aureus Isolates. J. Clin. Microbiol. 2010;48:3582–3592. doi: 10.1128/JCM.00657-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shukla S.K., Stemper M.E., Ramaswamy S.V., Conradt J.M., Reich R., Graviss E.A., Reed K.D. Molecular Characteristics of Nosocomial and Native American Community-Associated Methicillin-Resistant Staphylococcus Aureus Clones from Rural Wisconsin. J. Clin. Microbiol. 2004;42:3752–3757. doi: 10.1128/JCM.42.8.3752-3757.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Limbago B., Fosheim G.E., Schoonover V., Crane C.E., Nadle J., Petit S., Heltzel D., Ray S.M., Harrison L.H., Lynfield R., et al. Characterization of Methicillin-Resistant Staphylococcus aureus Isolates Collected in 2005 and 2006 from Patients with Invasive Disease: A Population-Based Analysis. J. Clin. Microbiol. 2009;47:1344–1351. doi: 10.1128/JCM.02264-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Belkum A., Melles D.C., Snijders S.V., van Leeuwen W.B., Wertheim H.F.L., Nouwen J.L., Verbrugh H.A., Etienne J. Clonal Distribution and Differential Occurrence of the Enterotoxin Gene Cluster, Egc, in Carriage- versus Bacteremia-Associated Isolates of Staphylococcus aureus. J. Clin. Microbiol. 2006;44:1555–1557. doi: 10.1128/JCM.44.4.1555-1557.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saïd-Salim B., Mathema B., Braughton K., Davis S., Sinsimer D., Eisner W., Likhoshvay Y., Deleo F.R., Kreiswirth B.N. Differential Distribution and Expression of Panton-Valentine Leucocidin among Community-Acquired Methicillin-Resistant Staphylococcus aureus Strains. J. Clin. Microbiol. 2005;43:3373–3379. doi: 10.1128/JCM.43.7.3373-3379.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang Y., Lin Y., Qiao L. Direct MALDI-TOF MS Identification of Bacterial Mixtures. Anal. Chem. 2018;90:10400–10408. doi: 10.1021/acs.analchem.8b02258. [DOI] [PubMed] [Google Scholar]
- 26.Koreen L., Ramaswamy S.V., Graviss E.A., Naidich S., Musser J.M., Kreiswirth B.N. Spa Typing Method for Discriminating among Staphylococcus aureus Isolates: Implications for Use of a Single Marker to Detect Genetic Micro- and Macrovariation. J. Clin. Microbiol. 2004;42:792–799. doi: 10.1128/JCM.42.2.792-799.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glenn T.C., Nilsen R.A., Kieran T.J., Sanders J.G., Bayona-Vásquez N.J., Finger J.W., Pierson T.W., Bentley K.E., Hoffberg S.L., Louha S., et al. Adapterama I: Universal Stubs and Primers for 384 Unique Dual-Indexed or 147,456 Combinatorially-Indexed Illumina Libraries (ITru & INext) PeerJ. 2019;7:e7755. doi: 10.7717/peerj.7755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eleaume H., Jabbouri S. Comparison of Two Standardisation Methods in Real-Time Quantitative RT-PCR to Follow Staphylococcus aureus Genes Expression during in Vitro Growth. J. Microbiol. Methods. 2004;59:363–370. doi: 10.1016/j.mimet.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 29.Goerke C., Bayer M.G., Wolz C. Quantification of Bacterial Transcripts during Infection Using Competitive Reverse Transcription-PCR (RT-PCR) and LightCycler RT-PCR. Clin. Diagn. Lab. Immunol. 2001;8:279–282. doi: 10.1128/CDLI.8.2.279-282.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alonzo F., III, Torres V.J. The Bicomponent Pore-Forming Leucocidins of Staphylococcus aureus. Microbiol. Mol. Biol. Rev. 2014;78:199–230. doi: 10.1128/MMBR.00055-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aman M.J., Adhikari R.P. Staphylococcal Bicomponent Pore-Forming Toxins: Targets for Prophylaxis and Immunotherapy. Toxins. 2014;6:950–972. doi: 10.3390/toxins6030950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spaan A.N., van Strijp J.A.G., Torres V.J. Leukocidins: Staphylococcal Bi-Component Pore-Forming Toxins Find Their Receptors. Nat. Rev. Microbiol. 2017;15:435–447. doi: 10.1038/nrmicro.2017.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deresinski S. Methicillin-Resistant Staphylococcus Aureus: An Evolutionary, Epidemiologic, and Therapeutic Odyssey. Clin. Infect. Dis. 2005;40:562–573. doi: 10.1086/427701. [DOI] [PubMed] [Google Scholar]
- 34.Melles D.C., van Leeuwen W.B., Boelens H.A.M., Peeters J.K., Verbrugh H.A., van Belkum A. Panton-Valentine Leukocidin Genes in Staphylococcus aureus. Emerg. Infect. Dis. 2006;12:1174–1175. doi: 10.3201/eid1207.050865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Menestrina G., Dalla Serra M., Prévost G. Mode of Action of β-Barrel Pore-Forming Toxins of the Staphylococcal α-Hemolysin Family. Toxicon. 2001;39:1661–1672. doi: 10.1016/S0041-0101(01)00153-2. [DOI] [PubMed] [Google Scholar]
- 36.Guillet V., Roblin P., Werner S., Coraiola M., Menestrina G., Monteil H., Prévost G., Mourey L. Crystal Structure of Leucotoxin S Component: New insight into the staphylococcal β-barrel pore-forming toxins. J. Biol. Chem. 2004;279:41028–41037. doi: 10.1074/jbc.M406904200. [DOI] [PubMed] [Google Scholar]
- 37.Yamashita K., Kawai Y., Tanaka Y., Hirano N., Kaneko J., Tomita N., Ohta M., Kamio Y., Yao M., Tanaka I. Crystal Structure of the Octameric Pore of Staphylococcal γ-Hemolysin Reveals the β-Barrel Pore Formation Mechanism by Two Components. Proc. Natl. Acad. Sci. USA. 2011;108:17314–17319. doi: 10.1073/pnas.1110402108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoong P., Torres V.J. The Effects of Staphylococcus aureus Leukotoxins on the Host: Cell Lysis and Beyond. Curr. Opin. Microbiol. 2013;16:63–69. doi: 10.1016/j.mib.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaneko J., Kamio Y. Bacterial Two-Component and Hetero-Heptameric Pore-Forming Cytolytic Toxins: Structures, Pore-Forming Mechanism, and Organization of the Genes. Biosci. Biotechnol. Biochem. 2004;68:981–1003. doi: 10.1271/bbb.68.981. [DOI] [PubMed] [Google Scholar]
- 40.Yu F., Liu Y., Xu Y., Shang Y., Lou D., Qin Z., Parsons C., Zhou W., Huang X., Li Y., et al. Expression of Panton-Valentine Leukocidin MRNA among Staphylococcus aureus Isolates Associates with Specific Clinical Presentations. PLoS ONE. 2013;8:e83368. doi: 10.1371/journal.pone.0083368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lina G., Piémont Y., Godail-Gamot F., Bes M., Peter M.-O., Gauduchon V., Vandenesch F., Etienne J. Involvement of Panton-Valentine Leukocidin—Producing Staphylococcus aureus in Primary Skin Infections and Pneumonia. Clin. Infect. Dis. 1999;29:1128–1132. doi: 10.1086/313461. [DOI] [PubMed] [Google Scholar]
- 42.Date S.V., Modrusan Z., Lawrence M., Morisaki J.H., Toy K., Shah I.M., Kim J., Park S., Xu M., Basuino L., et al. Global Gene Expression of Methicillin-Resistant Staphylococcus aureus USA300 During Human and Mouse Infection. J. Infect. Dis. 2014;209:1542–1550. doi: 10.1093/infdis/jit668. [DOI] [PubMed] [Google Scholar]
- 43.Boubaker K., Diebold P., Blanc D.S., Vandenesch F., Praz G., Dupuis G., Troillet N. Panton-Valentine Leukocidin and Staphyloccoccal Skin Infections in Schoolchildren. Emerg. Infect. Dis. 2004;10:121–124. doi: 10.3201/eid1001.030144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marazza G., Harbarth S. Serious Staphylococcus aureus Skin Infections That Are Panton-Valentine Leukocidin Positive: A New Challenge. Rev. Med. Suisse. 2007;3:1106–1108. [PubMed] [Google Scholar]
- 45.Loughman J.A., Fritz S.A., Storch G.A., Hunstad D.A. Virulence Gene Expression in Human Community-Acquired Staphylococcus aureus Infection. J. Infect. Dis. 2009;199:294–301. doi: 10.1086/595982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stapleton M.R., Horsburgh M.J., Hayhurst E.J., Wright L., Jonsson I.-M., Tarkowski A., Kokai-Kun J.F., Mond J.J., Foster S.J. Characterization of IsaA and SceD, Two Putative Lytic Transglycosylases of Staphylococcus aureus. J. Bacteriol. 2007;189:7316–7325. doi: 10.1128/JB.00734-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lorenz U., Ohlsen K., Karch H., Hecker M., Thiede A., Hacker J. Human Antibody Response during Sepsis against Targets Expressed by Methicillin Resistant Staphylococcus aureus. FEMS Immunol. Med. Microbiol. 2000;29:145–153. doi: 10.1111/j.1574-695X.2000.tb01517.x. [DOI] [PubMed] [Google Scholar]
- 48.van den Berg S., Bonarius H.P., van Kessel K.P., Elsinga G.S., Kooi N., Westra H., Bosma T., van der Kooi-Pol M.M., Koedijk D.G.A.M., Groen H., et al. A Human Monoclonal Antibody Targeting the Conserved Staphylococcal Antigen IsaA Protects Mice against Staphylococcus aureus Bacteremia. Int. J. Med. Microbiol. 2015;305:55–64. doi: 10.1016/j.ijmm.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 49.Chen K., Lin S., Li P., Song Q., Luo D., Liu T., Zeng L., Zhang W. Characterization of Staphylococcus aureus Isolated from Patients with Burns in a Regional Burn Center, Southeastern China. BMC Infect. Dis. 2018;18:51. doi: 10.1186/s12879-018-2955-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heilmann C., Hartleib J., Hussain M.S., Peters G. The Multifunctional Staphylococcus aureus Autolysin Aaa Mediates Adherence to Immobilized Fibrinogen and Fibronectin. Infect. Immun. 2005;73:4793–4802. doi: 10.1128/IAI.73.8.4793-4802.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hirschhausen N., Schlesier T., Peters G., Heilmann C. Characterization of the Modular Design of the Autolysin/Adhesin Aaa from Staphylococcus aureus. PLoS ONE. 2012;7:e40353. doi: 10.1371/journal.pone.0040353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Porayath C., Suresh M.K., Biswas R., Nair B.G., Mishra N., Pal S. Autolysin Mediated Adherence of Staphylococcus aureus with Fibronectin, Gelatin and Heparin. Int. J. Biol. Macromol. 2018;110:179–184. doi: 10.1016/j.ijbiomac.2018.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fleury O.M., McAleer M.A., Feuillie C., Formosa-Dague C., Sansevere E., Bennett D.E., Towell A.M., McLean W.H.I., Kezic S., Robinson D.A., et al. Clumping Factor B Promotes Adherence of Staphylococcus aureus to Corneocytes in Atopic Dermatitis. Infect. Immun. 2017;85:e00994-16. doi: 10.1128/IAI.00994-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vitry P., Valotteau C., Feuillie C., Bernard S., Alsteens D., Geoghegan J.A., Dufrêne Y.F. Force-Induced Strengthening of the Interaction between Staphylococcus aureus Clumping Factor B and Loricrin. mBio. 2017;8:e01748-17. doi: 10.1128/mBio.01748-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Herman-Bausier P., Labate C., Towell A.M., Derclaye S., Geoghegan J.A., Dufrêne Y.F. Staphylococcus aureus Clumping Factor A Is a Force-Sensitive Molecular Switch That Activates Bacterial Adhesion. Proc. Natl. Acad. Sci. USA. 2018;115:5564–5569. doi: 10.1073/pnas.1718104115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Salamaga B., Kong L., Pasquina-Lemonche L., Lafage L., von Und Zur Muhlen M., Gibson J.F., Grybchuk D., Tooke A.K., Panchal V., Culp E.J., et al. Demonstration of the Role of Cell Wall Homeostasis in Staphylococcus aureus Growth and the Action of Bactericidal Antibiotics. Proc. Natl. Acad. Sci. USA. 2021;118:e2106022118. doi: 10.1073/pnas.2106022118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kintarak S., Whawell S.A., Speight P.M., Packer S., Nair S.P. Internalization of Staphylococcus aureus by Human Keratinocytes. Infect. Immun. 2004;72:5668–5675. doi: 10.1128/IAI.72.10.5668-5675.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Walsh E.J., Miajlovic H., Gorkun O.V., Foster T.J. Identification of the Staphylococcus aureus MSCRAMM Clumping Factor B (ClfB) Binding Site in the AlphaC-Domain of Human Fibrinogen. Microbiology. 2008;154:550–558. doi: 10.1099/mic.0.2007/010868-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Walsh E.J., O’Brien L.M., Liang X., Hook M., Foster T.J. Clumping Factor B, a Fibrinogen-Binding MSCRAMM (Microbial Surface Components Recognizing Adhesive Matrix Molecules) Adhesin of Staphylococcus aureus, Also Binds to the Tail Region of Type I Cytokeratin 10. J. Biol. Chem. 2004;279:50691–50699. doi: 10.1074/jbc.M408713200. [DOI] [PubMed] [Google Scholar]
- 60.Ní Eidhin D., Perkins S., Francois P., Vaudaux P., Höök M., Foster T.J. Clumping Factor B (ClfB), a New Surface-Located Fibrinogen-Binding Adhesin of Staphylococcus aureus. Mol. Microbiol. 1998;30:245–257. doi: 10.1046/j.1365-2958.1998.01050.x. [DOI] [PubMed] [Google Scholar]
- 61.Hauck C.R., Ohlsen K. Sticky Connections: Extracellular Matrix Protein Recognition and Integrin-Mediated Cellular Invasion by Staphylococcus aureus. Curr. Opin. Microbiol. 2006;9:5–11. doi: 10.1016/j.mib.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 62.Shinji H., Yosizawa Y., Tajima A., Iwase T., Sugimoto S., Seki K., Mizunoe Y. Role of Fibronectin-Binding Proteins A and B in in Vitro Cellular Infections and in Vivo Septic Infections by Staphylococcus aureus. Infect. Immun. 2011;79:2215–2223. doi: 10.1128/IAI.00133-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schwan W.R., Langhorne M.H., Ritchie H.D., Stover C.K. Loss of Hemolysin Expression in Staphylococcus aureus Agr Mutants Correlates with Selective Survival during Mixed Infections in Murine Abscesses and Wounds. FEMS Immunol. Med. Microbiol. 2003;38:23–28. doi: 10.1016/S0928-8244(03)00098-1. [DOI] [PubMed] [Google Scholar]
- 64.Montgomery C.P., Boyle-Vavra S., Daum R.S. Importance of the Global Regulators Agr and SaeRS in the Pathogenesis of CA-MRSA USA300 Infection. PLoS ONE. 2010;5:e15177. doi: 10.1371/journal.pone.0015177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Morfeldt E., Taylor D., von Gabain A., Arvidson S. Activation of Alpha-Toxin Translation in Staphylococcus aureus by the Trans-Encoded Antisense RNA, RNAIII. EMBO J. 1995;14:4569–4577. doi: 10.1002/j.1460-2075.1995.tb00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Geisinger E., Adhikari R.P., Jin R., Ross H.F., Novick R.P. Inhibition of Rot Translation by RNAIII, a Key Feature of Agr Function. Mol. Microbiol. 2006;61:1038–1048. doi: 10.1111/j.1365-2958.2006.05292.x. [DOI] [PubMed] [Google Scholar]
- 67.Heyer G., Saba S., Adamo R., Rush W., Soong G., Cheung A., Prince A. Staphylococcus aureus Agr and SarA Functions Are Required for Invasive Infection but Not Inflammatory Responses in the Lung. Infect. Immun. 2002;70:127–133. doi: 10.1128/IAI.70.1.127-133.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Novick R.P., Jiang D. The Staphylococcal SaeRS System Coordinates Environmental Signals with Agr Quorum Sensing. Microbiology. 2003;149:2709–2717. doi: 10.1099/mic.0.26575-0. [DOI] [PubMed] [Google Scholar]
- 69.Steinhuber A., Goerke C., Bayer M.G., Döring G., Wolz C. Molecular Architecture of the Regulatory Locus Sae of Staphylococcus Aureus and Its Impact on Expression of Virulence Factors. J. Bacteriol. 2003;185:6278–6286. doi: 10.1128/JB.185.21.6278-6286.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Flack C.E., Zurek O.W., Meishery D.D., Pallister K.B., Malone C.L., Horswill A.R., Voyich J.M. Differential Regulation of Staphylococcal Virulence by the Sensor Kinase SaeS in Response to Neutrophil-Derived Stimuli. Proc. Natl. Acad. Sci. USA. 2014;111:E2037–E2045. doi: 10.1073/pnas.1322125111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Houston P., Rowe S.E., Pozzi C., Waters E.M., O’Gara J.P. Essential Role for the Major Autolysin in the Fibronectin-Binding Protein-Mediated Staphylococcus aureus Biofilm Phenotype. Infect. Immun. 2011;79:1153–1165. doi: 10.1128/IAI.00364-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gordon R.J., Lowy F.D. Pathogenesis of Methicillin-Resistant Staphylococcus aureus Infection. Clin. Infect. Dis. 2008;46:S350–S359. doi: 10.1086/533591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cho J.S., Zussman J., Donegan N.P., Ramos R.I., Garcia N.C., Uslan D.Z., Iwakura Y., Simon S.I., Cheung A.L., Modlin R.L., et al. Noninvasive In Vivo Imaging to Evaluate Immune Responses and Antimicrobial Therapy against Staphylococcus aureus and USA300 MRSA Skin Infections. J. Invest. Derm. 2011;131:907–915. doi: 10.1038/jid.2010.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Diep B.A., Chan L., Tattevin P., Kajikawa O., Martin T.R., Basuino L., Mai T.T., Marbach H., Braughton K.R., Whitney A.R., et al. Polymorphonuclear Leukocytes Mediate Staphylococcus Aureus Panton-Valentine Leukocidin-Induced Lung Inflammation and Injury. Proc. Natl. Acad. Sci. USA. 2010;107:5587–5592. doi: 10.1073/pnas.0912403107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data is available on request to the corresponding author.