Abstract
Context
The SELECT trial led to the approval of lenvatinib for the treatment of advanced radioiodine-refractory differentiated thyroid carcinomas (DTCs) but also revealed an important adverse event (AE) profile which may limit its use in clinical practice.
Objective
We aim to describe the efficacy and toxicity profiles of lenvatinib in real life.
Methods
We included all patients who received lenvatinib for an advanced DTC at our institution, enrolling 27 patients. We reviewed retrospectively electronic medical records to assess efficacy and AEs.
Results
Among the 24 patients with evaluation of tumor response during treatment, overall response rate (ORR) was 37.0% (95% CI, 19.4%-57.6%), and disease control rate was 85.2% (95% CI, 66.3%-95.8%). The median progression-free survival (PFS) was 12 months (95% CI, 7.5-16.5]. The most prevalent AEs were hypertension (77.8%), fatigue (55.6%), and weight loss (51.9%). At least one grade ≥ 3 AE was experienced by 25/27 patients (92.6%), mostly hypertension (59.3%). Lenvatinib was discontinued due to AEs in 13/27 patients (48.1%). Interestingly, 1 patient experienced a grade 4 posterior reversible encephalopathy syndrome, and another developed a Takotsubo cardiomyopathy.
Conclusion
The safety profile of lenvatinib in our cohort was similar to that reported in the literature, with a predominance of hypertension. Rigorous blood pressure control is therefore essential to avoid discontinuing therapy. We also report 2 severe and rarely described AEs that physicians should watch for. As for efficacy, although less than in the SELECT trial, ORR and PFS were similar to other real-life studies.
Keywords: advanced differentiated thyroid cancer, lenvatinib, efficacy, adverse events, real-life data
Lenvatinib is a multitarget anti-angiogenic tyrosine kinase inhibitor (TKI) [1] that was approved for the treatment of locally recurrent or metastatic, progressive, radioiodine-refractory differentiated thyroid carcinomas (RR-DTC) following the phase 3 randomized controlled trial SELECT [2]. This trial showed an increase of progression-free survival (PFS) by 15 months with lenvatinib compared to placebo, but also an important prevalence of adverse events (AEs), occurring in 97.3% of patients [2].
Although randomized controlled trials are considered the gold standard for determining the efficacy and safety profile of a new medication, they are typically performed in a strictly controlled setting and with a highly selected patient population, which can be quite unlike real-life practice settings [3]. Real-world data on safety and efficacy of lenvatinib would therefore be more applicable to the usual advanced thyroid cancer patient encountered in clinical practice.
To date, few real-life experiences with lenvatinib in RR-DTC have been described [4-9], none of which were in Canadian centers. The objective of this monocentric retrospective cohort study is therefore to describe the efficacy and toxicity profiles of lenvatinib in real life, and to report some unusual adverse events with this treatment.
Methods
Patients
A retrospective analysis of all patients with advanced differentiated thyroid carcinomas (DTC) treated with lenvatinib between May 2016 and June 2021 at our quaternary care academic center in Montreal (Canada), was performed. One patient with concomitant progressive metastatic breast cancer was excluded, leading to a total of 27 patients included in the study.
Radioactive iodine (RAI) refractory disease was defined as per The Martinique Principles [10]. Decision-making regarding treatment initiation was made by a multidisciplinary team of experts at the institutional thyroid tumor board, taking into consideration tumor progression, tumor-related symptoms, size and location of tumors, and patient comorbidities [11]. Of note, in our center, lenvatinib has been used since its Health Canada approval in 2016 as a first-line agent for targeted systemic therapy in patients with progressive RR-DTC and distant metastasis.
Clinical and biochemical follow-up were according to local practice and current guidelines [11-13], comprising a monthly blood and urine work-up, a monthly visit with the medical oncologist, a visit every 3 months with the endocrinologist as well as additional telephone or in-person follow-ups as needed with the Oncology Nurse Practitioner. Twice-daily home blood pressure monitoring was required from each patient, with weekly follow-ups by the Oncology Nurse Practitioner for at least the first 2 months, and if blood pressure remained within target, further follow-ups were as needed.
Treatment
Lenvatinib starting dose was determined by the expert medical oncologist at our center. Usual starting dose in our practice is 24 or 20 milligrams (mg) once daily. In the presence of intolerable side effects, dose reduction occurred in a stepwise manner. Based on AE severity and the judgment of the treating physician, treatment interruptions were also possible. Lenvatinib therapy was continued as long as it was deemed clinically indicated, based on tumor assessment and treatment side effects.
Safety and Efficacy Outcomes
Efficacy was assessed according to the response evaluation criteria in solid tumors (RECIST) v1.1 with computed tomography (CT) scans performed approximately every 3 months, as well as using clinical assessment, and as indicated additional imaging, such as 18Fluorodeoxyglucose positron emission tomography-CT or magnetic resonance imaging (MRI).
Overall response rate (ORR), disease control rate (DCR), median PFS and median overall survival (OS) were analyzed as efficacy outcomes. PFS was defined as the time from initiation of lenvatinib to disease progression, death, or last day of follow-up, whereas OS designates time from initiation of lenvatinib to death or last day of follow-up. The ORR corresponds to the proportion of patients with a complete response (CR) or partial response (PR) as best overall response (BOR). The DCR designates the proportion of patients with a CR, PR, or stable disease as BOR.
Toxicity was monitored at least every month and retrieved retrospectively. Severity was graded using the Common Terminology Criteria for Adverse Events (CTCAE), version 5.0. The frequency, severity, and time to AEs were evaluated for all patients.
Data Source
We obtained study data from electronic medical records. The study was approved by the Centre Hospitalier de l’Université de Montréal’s institutional review board. Confidentiality safeguards were maintained throughout the study period to protect the patients’ best interests.
Statistical Analysis
Statistical analyses were performed using SPSS statistical software (IBM SPSS Statistics for Mac, Version 26.0. Armonk, NY: IBM Corp). Continuous variables are presented using mean, median, and minimum and maximum values. Categorical data were summarized as counts with percentages. The 95% CI for continuous data and proportions were presented where appropriate. Median PFS and median OS were estimated using the Kaplan–Meier method.
Results
Patient Characteristics
Baseline characteristics of the 27 enrolled patients are detailed in Table 1. Mean age upon lenvatinib initiation was 62.8 years (range, 43-81) with a majority of female patients (66.7%), in concordance with thyroid cancer demographics. Most patients (44.4%) had papillary thyroid cancer, while 6 (22.2%), 5 (18.5%), and 4 (14.8%) patients had poorly differentiated, Hürthle cell, and follicular thyroid cancers, respectively. BRAF, RAS, or NTRK mutation status was known for 19 tumors: 12 (44.4%) had no identified mutation, 6 (22.2%) had a BRAF V600E mutation, and 1 had an NTRK fusion that was identified after disease progression on lenvatinib. All patients had an Eastern Cooperative Oncology Groupe (ECOG) performance status ≤ 2 upon therapy initiation, with most patients (74.1%) having an ECOG performance status of 1. At baseline, 63.0% of patients had at least one comorbidity and 25.9% had 2 or more comorbidities. Disease progression within 12 months before lenvatinib initiation was documented in 23/27 patients. The 4 remaining patients had bulky nonoperable primary disease threatening airway integrity and/or causing compressive symptoms. All patients had unresectable distant metastasis, most frequently in the lungs (92.6%), bones (51.9%), and lymph nodes (48.1%). The mean cumulative dose of RAI prior to lenvatinib initiation was 302.8 millicuries (range, 0-825) and all patients with previous RAI treatment were confirmed as RAI-refractory. Six patients (22.2%) could not receive RAI therapy, 5 of them due to inoperable primary tumors and 1 because of patient refusal. Before lenvatinib initiation, local treatment was performed in most patients (81.5%), including radiation therapy in 15 patients (55.6%) and surgery for locoregional metastasis in 7 patients (25.9%). All patients except 1 were receiving lenvatinib as first-line systemic therapy; 1 patient had had a previous anti-VEGF targeted therapy with sorafenib.
Table 1.
Baseline characteristics of patients
| Characteristic | All patients (n = 27) |
|---|---|
| Mean age at lenvatinib initiation, years (range) | 62.8 (43–81) |
| Female, n (%) | 18 (66.7%) |
| Mean BMI at lenvatinib initiation (range), kg/m2 | 28.8 (18.5–42.7) |
| ECOG Performance Status at lenvatinib initiation, n (%) | |
| 0 | 6 (22.2) |
| 1 | 20 (74.1) |
| 2 | 1 (3.7) |
| Comorbidities before lenvatinib initiation, n (%) | |
| Any | 17 (63.0%) |
| Arterial hypertension | 13 |
| Coronary artery disease | 3 |
| Chronic kidney disease (stage ≥ 3) | 1 |
| Asthma/COPD | 2 |
| Diabetes | 4 |
| Multiple sclerosis | 1 |
| Schizophrenia | 1 |
| Tumor histology, n (%) | |
| Papillary | 12 (44.4) |
| Follicular, not Hürthle cell | 4 (14.8) |
| Hürthle cell | 5 (18.5) |
| Poorly differentiated | 6 (22.2) |
| Mutation status, n (%) | |
| BRAF V600E | 6 (22.2) |
| NTRK fusion | 1 (3.7) |
| No NTRK, BRAF, or RAS mutation | 12 (44.4) |
| Unknown | 8 (29.6) |
| Metastatic sites, n (%) | |
| Extra-cervical lymph nodes | 13 (48.1) |
| Lung | 25 (92.6) |
| Bones | 14 (51.9) |
| Brain | 4 (14.8) |
| Liver | 3 (11.1) |
| Right ventricle | 1 (3.7) |
| Kidney | 1 (3.7) |
| Mean cumulative RAI dose (range), mCi | 302.8 (0–825) |
| Previous treatment for recurrent lesions, n (%) | |
| Surgery for locoregional metastasis | 7 (25.9) |
| Surgery for distant metastasis | 7 (25.9) |
| Radiotherapy | 15 (55.6) |
| Stereotactic radiosurgery | 3 (11.1) |
| Intravenous Bisphosphonate | 13 (48.1) |
| None | 5 (18.5) |
| Prior VEGF-targeted therapy, n (%) | 1 (3.7) |
| Median time from diagnosis to lenvatinib initiation (range), months | 73.0 (2–317) |
| Median Lenvatinib starting dose (range), mg | 20 (14–24) |
| Median treatment duration (range), days | 242 (28–1280) |
| Median follow-up after lenvatinib initiation (range), months | 18 (2–60) |
Abbreviations: BMI, body mass index; COPD, chronic obstructive pulmonary disease; ECOG, Eastern Cooperative Oncology Group; RAI, radioactive iodine.
Efficacy
At the cutoff date for analysis, the median follow-up period after lenvatinib initiation was 18 months (range, 2-60) and median treatment duration was 242 days (range, 28-1280). At last follow-up, 20 patients (74.1%) had discontinued lenvatinib therapy, 7 (25.9%) due to disease progression and 13 (48.1%) due to nontolerable AEs. Initial treatment dose was 24, 20, 18, and 14 mg/day in 7 (25.9%), 17 (63.0%), 1 (3.7%), and 2 (7.4%) patients, respectively. The main reasons leading to initiation of treatment with a lower dose were comorbidities, patient age, and physician’s judgment. Median lenvatinib dose at treatment discontinuation was 14 mg per day (range, 10-24).
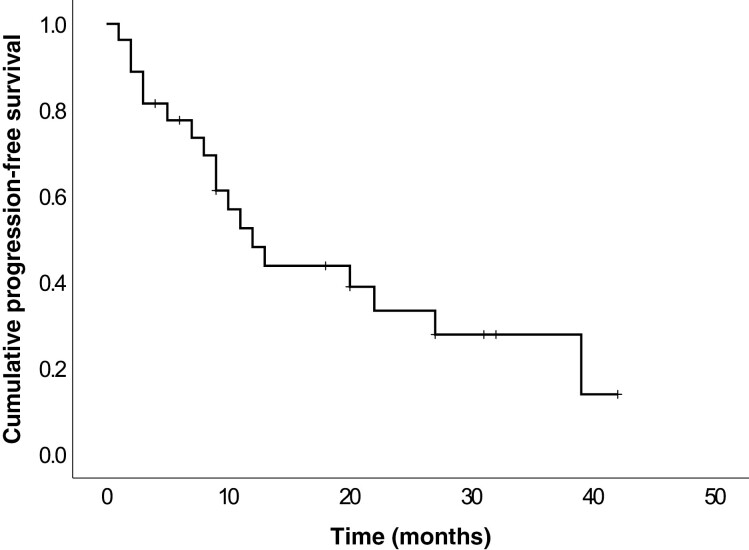
Efficacy outcomes are summarized in Table 2. The BOR was PR in 10 patients (37.0%), stable disease in 13 patients (48.1%), and PD in 1 patient (3.7%). No CR was observed, and response was not evaluable in 3 patients. The ORR was 37% (95% CI, 19.4-57.6) and DCR was 85.2% (95% CI, 66.3-95.8). The median PFS was 12 months (95% CI, 7.5-16.5) (Fig. 1). At the end of the study, 9 patients had died, and all deaths were related to the thyroid neoplasia. As more than half of patients were still alive at the end of the follow-up period, OS could not be assessed. However, the 6-month and 12-month survival rates were 92.6% and 85.2%, respectively.
Table 2.
Efficacy outcomes
| Best overall response, n (%) | |
|---|---|
| NE | 3 (11.1) |
| CR | 0 (0) |
| Stable disease | 13 (48.1) |
| PR | 10 (37.0) |
| PD | 1 (3.7) |
| Overall response ratea, n (%) | 10 (37.0) |
| Disease control rateb, n (%) | 23 (85.2) |
| Median progression-free survival, months (95% CI) | 12.0 (7.5–16.5) |
| Death due to thyroid neoplasia during follow-up, n (%) | 9 (33.3) |
Abbreviations: CR, complete response; NE, not estimable; PD, progressive disease; PR, partial response.
aOverall response rate calculated as CR + PR.
bDisease control rate calculated ad CR + PR + stable disease.
Figure 1.
Kaplan–Meier estimate of progression-free survival from lenvatinib initiation.
Safety and Tolerability
In this real-life cohort, 119 AEs were seen in 26 patients (96.3%), as presented in Table 3. The most frequent AE was hypertension in 77.8% of cases. Other frequent AEs were fatigue (55.6%), weight loss (51.9%), diarrhea (33.3%), proteinuria (33.3%), and anorexia (29.6%). Forty grade ≥ 3 AEs were reported in 25 patients, including hypertension in 16 patients, weight loss in 4 patients, diarrhea in 3 patients, anorexia in 3 patients, and hand-foot syndrome, proteinuria, and hypokalemia in 2 patients each. No grade 5 toxicity was reported. AE-related dose reductions and interruptions were required in 18 (66.7%) and 12 (44.4%) patients respectively. Median time to first AE was 26 days (range, 3-370). Moreover, first dose reduction occurred on day 13 (range, 13-969) while first treatment discontinuation was reported on day 28 (range, 28-1280). At the end of follow-up, 74.1% (20/27) of patients had stopped lenvatinib, 7 due to disease progression and 13 due to treatment toxicity. Table 4 details the reason why toxicity led to treatment discontinuation in each of these patients.
Table 3.
Summary of adverse events
| Adverse Events | All grades, n (%) | Grade ≥ 3, n (%) |
|---|---|---|
| Any adverse event | 26 (96.3) | 25 (92.6) |
| Hypertension | 21 (77.8) | 16 (59.3) |
| Fatigue | 15 (55.6) | 0 (0) |
| Weight loss | 14 (51.9) | 4 (14.8) |
| Diarrhea | 9 (33.3) | 3 (11.1) |
| Proteinuria | 9 (33.3) | 2 (7.4) |
| Anorexia | 8 (29.6) | 3 (11.1) |
| Oral mucositis | 7 (25.9) | 1 (3.7) |
| TSH elevation | 7 (25.9) | n/a |
| Nausea | 6 (22.2) | 1 (3.7) |
| Palmar-plantar erythrodysesthesia syndrome | 6 (22.2) | 2 (7.4) |
| Infections | 5 (18.5) | 1 (3.7) |
| Pulmonary | 1 (3.7) | 1 (3.7) |
| Dental | 1 (3.7) | 0 (0) |
| Urinary tract infection | 2 (7.4) | 0 (0) |
| Pharyngitis | 1 (3.7) | 0 (0) |
| Elevated liver enzymes | 4 (14.8) | 1 (3.7) |
| Anemia | 2 (7.4) | 1 (3.7) |
| Decreased platelet count | 2 (7.4) | 0 (0) |
| Hypokalemia | 2 (7.4) | 2 (7.4) |
| Arthralgia | 1 (3.7) | 1 (3.7) |
| Colitis | 1 (3.7) | 0 (0) |
| Left ventricular systolic dysfunction | 1 (3.7) | 1 (3.7) |
| Maculo-papular rash | 1 (3.7) | 0 (0) |
| Pancreatitis | 1 (3.7) | 1 (3.7) |
| Posterior reversible encephalopathy syndrome | 1 (3.7) | 1 (3.7) |
| Vestibular disorder | 1 (3.7) | 1 (3.7) |
Abbreviation: TSH, thyroid stimulating hormone.
Table 4.
Detail of patients in whom lenvatinib was permanently discontinued due to toxicity
| Patient | Reason for lenvatinib discontinuation |
|---|---|
| #1 | Grade 3 alanine aminotransferase increase (6× ULN) |
| #2 | Grade 2 colitis and fatigue, patient decision not to restart therapy |
| #3 | 30 kg weight loss, severe fatigue, and anorexia despite multiple dose reductions |
| #4 | Important fatigue, weight loss, ECOG-PS 3 despite dose reductions |
| #5 | Grade 2 proteinuria (3 grams/day) despite ARB |
| #6 | Nephrotic syndrome |
| #7 | Grade 3 vestibular disorder |
| #8 | Proteinuria 5 grams/day, recurring after temporary treatment discontinuation and dose reduction |
| #9 | Takotsubo cardiomyopathy with grade 4 left ventricular dysfunction and cardiogenic shock |
| #10 | Grade 3 acute pancreatitis |
| #11 | 20 kg weight loss despite dose reduction |
| #12 | Grade 4 PRES |
| #13 | Grade 3 hand-foot syndrome, 2 grams/day proteinuria despite multiple dose reductions |
Abbreviations: ARB, angiotensin II receptor blocker; ECOG-PS, Eastern Cooperative Oncology Group Performance Status; PRES, posterior reversible encephalopathy syndrome; ULN, upper limit of normal.
Of note, a few severe and rarely reported AEs occurred in our cohort.
One patient experienced Takotsubo cardiomyopathy (TC) with grade 4 left ventricular systolic dysfunction and cardiogenic shock. This 69-year-old female without previously known cardiovascular risk factors had a locally invasive nonoperable papillary thyroid carcinoma with pulmonary metastases, treated with lenvatinib 20 mg daily. Six weeks later, she developed grade 3 hypertension and grade 2 oral mucositis which were managed medically without lenvatinib modification. After a total of 111 days of treatment, she was brought to the emergency room following an episode of presyncope. She was found hypotensive upon arrival, and bedside echocardiography showed regional wall motion abnormalities with a reduced left ventricular ejection fraction (LVEF). Patient was euthyroid with a thyroid stimulating hormone (TSH) level of 1.16 mIU/L. Emergent cardiac catheterization showed no significant coronary atherosclerotic lesion, but ventriculography revealed a typical aspect of TC, with a LVEF of 25% and a severe mitral regurgitation due to a systolic anterior motion of the valve. Patient required a 10-day hospitalization at the cardiac intensive care unit with vasopressor support and transient noninvasive ventilation. On transthoracic echocardiogram (TTE) done on the day of discharge, LVEF had increased to 50%. Follow-up TTE 3 months later showed a LVEF of 65% with normal ventricular contractility. As there was no other apparent cause for the patient’s stress cardiomyopathy, it was attributed to lenvatinib, which was therefore not reinitiated afterwards.
Another patient developed a grade 4 posterior reversible encephalopathy syndrome (PRES). This 77-year-old woman with progressive, metastatic, RAI-refractory Hürthle cell carcinoma was receiving lenvatinib 24 mg daily. She had known hypertension and stable coronary artery disease prior to lenvatinib initiation, with adequate blood pressure control under perindopril 4 mg daily and bisoprolol 5 mg daily. Only 42 days after treatment initiation, she presented with severe headaches and a markedly decreased level of consciousness requiring endotracheal intubation. Ten days prior, she had experienced an increase in her blood pressure to a maximum of 181/108 mmHg with mild grade 1 proteinuria (0.17 g/24 hours), which led to an intensification of her antihypertensive treatment (perindopril was increased to 8 mg daily and amlodipine 5 mg daily was added). Cerebral MRI revealed severe brainstem and cerebellar edema with compression of the fourth ventricle and mild hydrocephalus. Confluent areas of increased signal in the white matter where also present on T2-weighted images. Patient was treated in the intensive care unit with hyperventilation and osmotherapy, as well as a labetalol drip. Lenvatinib was immediately stopped upon admission. A repeat MRI 24 hours later showed marked improvement, and the patient was successfully extubated 4 days after presentation. Having completely recovered, she was discharged home after 2 weeks of hospitalization, and lenvatinib was permanently discontinued.
Finally, a 78-year-old female patient, treated with lenvatinib 20 mg daily for a metastatic poorly differentiated thyroid carcinoma, developed an acute vestibular disorder making her unable to walk and leading to a 2-week long hospitalization. She presented to the emergency room 42 days after lenvatinib initiation with acute-onset dizziness, nausea, axial ataxia, and a gaze-evoked binocular horizontal nystagmus. After a thorough work-up including brain MRI, videonystagmography, and evaluation by neurology and otorhinolaryngology specialists, no cause was identified, which led to the conclusion that these were lenvatinib-related AEs. Throughout her hospital stay, patient’s vestibular symptoms improved with supportive treatment and lenvatinib discontinuation.
Discussion
This retrospective cohort study of lenvatinib in a real-life setting confirms the efficacy and safety profile of this treatment in advanced DTC. Our findings regarding the high frequency of toxicity with lenvatinib were similar to those reported in the literature [2, 4-9], with 96.3% of treatment-related AEs of all grades as compared to 97.3% in the SELECT trial [2]. Moreover, as in previous studies [2, 4-9], the most common AE with lenvatinib therapy in our cohort was hypertension, reinforcing the importance of blood pressure monitoring in patients receiving this treatment. Proteinuria was also common in our cohort, observed in 33.3% of patients (any grade) and leading to treatment discontinuation in 4 patients. In the SELECT trial [2], proteinuria of any grade and grade ≥ 3 occurred in 31% and 10% of patients respectively. However, when looking at real-life data, lenvatinib-associated proteinuria is not consistently described, with some studies reporting no cases of proteinuria [4, 6] while others report an incidence similar to [5], or higher [7, 9] than what was observed in our cohort.
A few less frequent serious adverse events observed in our population are noteworthy.
Although a few cases of TC have been reported with other anti-VEGF agents in patients with progressive renal cancer [14-16], there is to our knowledge only one other case of TC with lenvatinib that has been described in the literature [17]. Across clinical trials in 799 patients with thyroidal and nonthyroidal neoplasms treated with lenvatinib, grade ≥ 3 cardiac dysfunction occurred in 3% of patients, without any specific mention regarding the risk of TC [18]. Although the pathogenesis of TC due to TKI is not well understood, current hypotheses include reduction in nitric oxide levels, impairment of the physiological vascular response to injury and an increased response to catecholamines, all through the downstream effect of VEGF antagonism [15]. Physicians should be aware of this potentially life-threatening complication, which occurred shortly after treatment initiation in our previously healthy patient. Further reports are needed to better understand when patients may be at greatest risk for development of TC after initiation of lenvatinib, and the pathophysiology underlying this AE.
Moreover, TKI-associated PRES, while rare, is increasingly recognized [12]. A few case reports have described the association between PRES and other anti-angiogenic TKIs [17, 19]. Regarding lenvatinib, only one of the 392 patients treated in the SELECT study developed a PRES of grade < 3 [2], and 2 other cases were reported in patients treated for DTC [7] and anaplastic thyroid carcinoma [14]. The pathophysiology of PRES is not entirely clarified but the syndrome has been associated with hypertension, impaired cerebral autoregulation, and endothelial dysfunction [20]. Therefore, it is not surprising that lenvatinib-induced hypertension, through VEGF-receptor antagonism, might lead to PRES. We can further hypothesize that patients with underlying arterial hypertension before lenvatinib initiation might be at an increased risk of TKI-associated PRES, as this was the case for our patient and the patient described by Osawa et al [21], although more data is necessary to confirm this observation.
Finally, to our knowledge, no previous cases of vestibular disorders due to lenvatinib have been reported. Nonetheless, in the SELECT study, 15% of patients developed dizziness of any severity.
The frequency and potential severity of lenvatinib-associated adverse events command careful monitoring and prompt management, in order to limit morbidity and maximize treatment efficacy. In our cohort, through close clinical and biochemical monitoring, various strategies were undertaken to limit adverse events. For instance, blood pressure was rigorously controlled, diarrhea managed with electrolyte replacement and as needed loperamide, and palmar-plantar erythrodysesthesia syndrome was treated with urea-based creams and dermatological evaluation if necessary. However, despite these efforts, drug interruptions for longer than 24 hours due to AEs occurred in 12/27 (44.4%) of patients, which might have contributed to our shorter PFS. In fact, a subanalysis of the SELECT trial [22] showed that patients with shorter treatment interruptions had a greater degree of benefit from lenvatinib.
Multidisciplinary care, easy access to medical providers as well as clearly established management strategies for each potential adverse event have been suggested to minimize dose interruptions and allow optimal management of treatment toxicity [23, 24]. A strategy using outpatient follow-ups by pharmacists in collaboration with oncologists have been shown to improve adverse drug reactions, decrease temporary interruptions and reduce treatment discontinuations [25]. More recently, planned drug holidays have been used in Japan and were shown to prolong PFS as well as time to treatment failure [26].
Regarding efficacy outcomes, the DCR of 85.2% (95% CI, 66.3 to 95.8) and median PFS of 12 months (95% CI, 7.5 to 16.5) in our cohort confirm the efficacy of lenvatinib in advanced DTC. These results are similar to those of most previous real-life studies [4-7], although some studies have reported longer PFS, up to 22 months in an Italian real-life study by De Leo et al [27]. However, this was in a very well selected population with 61.5% of patients having an ECOG performance status of 0 and only 2 patients with bone metastases, which might have biased the PFS. Moreover, the small number of patients in this study (n = 13) may limit its conclusions.
Our efficacy results are also somewhat inferior in comparison to the SELECT trial [2]. This may be accounted for by a few differences in baseline characteristics between patients in the 2 studies. Notably, in our cohort, there were more patients with poorly differentiated thyroid carcinoma (22.2%, as compared to 10.7% in SELECT), and the initial dose of lenvatinib was 24 mg daily in only 7 patients (25.9%). Data from a randomized controlled trial by Brose et al showed better ORR with a starting dose of 24 mg compared to 18 mg [28]. Our population also included 5 patients who had not received any prior radioiodine therapy due to bulky nonoperable primary tumors. This could have contributed to the lower response rate, as baseline tumor size was shown to be an independent factor for predicting PFS on lenvatinib [29].
Thyroid cancer-related mortality in our study was higher than what has been reported. All previously stated factors (lower starting dose, higher prevalence of poorly differentiated disease, higher rates of treatment interruptions, etc.) as well as possibly larger tumor burden upon treatment initiation might explain this observation [12, 29], although our study design does not allow demonstration of this hypothesis.
Our study has some limitations. Data was acquired retrospectively from electronic medical charts, which could have affected its preciseness, especially for AEs, as they might not have been documented as stringently as in a clinical trial. Toxicity was also graded retrospectively through chart review. Moreover, our cohort size may be too small to draw precise conclusions. However, our results are in agreement with those previously reported by other real-life studies, and all patients but one treated with lenvatinib at our center since its approval were included in our analysis, which allows a complete evaluation of lenvatinib safety and efficacy in our local practice.
In conclusion, our data shows that treatment of advanced DTC with lenvatinib is effective, similar to what has been previously reported in other in real-life studies. We also reported some rare but serious AEs that physicians prescribing lenvatinib should be aware of. Nonetheless, AEs are generally manageable with close monitoring, dose reductions or interruptions, and medical treatment, allowing maintenance of lenvatinib as a robust treatment option for advanced DTC. Management of patients receiving lenvatinib by a multidisciplinary team is the best approach to minimize the impact of AEs and help improve overall survival by optimizing treatment efficacy.
Glossary
Abbreviations
- AE
adverse event
- BOR
best overall response
- CR
complete response
- DCR
disease control rate
- DTC
differentiated thyroid cancer
- ECOG
Eastern Cooperative Oncology Group
- LVEF
left ventricular ejection fraction
- MRI
magnetic resonance imaging
- ORR
overall response rate
- OS
overall survival
- PFS
progression-free survival
- PR
partial response
- PRES
posterior reversible encephalopathy syndrome
- RAI
radioactive iodine
- RR-DTC
radioiodine-refractory differentiated thyroid carcinoma
- TC
Takotsubo cardiomyopathy
- TKI
tyrosine kinase inhibitor
- TSH
thyrotropin (thyroid-stimulating hormone)
- TTE
transthoracic echocardiography
Financial Support
No funding was received for this article.
Disclosures
S.H. and R.L. have nothing to disclose. H.M. has received honoraria for advisory boards for Abbott, Bayer, and Eisai as well as speaker honoraria from Eisai. A.B. has received honoraria for advisory boards and as a speaker for Bayer and Eisai, as well as research funding from Eisai, not related to this project. B.L. and G.R. have received honoraria as consultants for Eisai. L.G.S.M. has received honoraria for advisory boards for Amgen, as a speaker for Amgen and AstraZeneca, and as a consultant for Eli Lilly, not related to this project.
Data Availability
All data generated or analyzed during this study are included in this published article.
References
- 1. Cabanillas ME, Ryder M, Jimenez C. Targeted therapy for advanced thyroid cancer: kinase inhibitors and beyond. Endocr Rev. 2019;40(6):1573-1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schlumberger M, Tahara M, Wirth LJ. Lenvatinib in radioiodine-refractory thyroid cancer. N Engl J Med. 2015;372(19):1868. [DOI] [PubMed] [Google Scholar]
- 3. Zuidgeest MGP, Goetz I, Groenwold RHH, Irving E, van Thiel GJMW, Grobbee DE; GetReal Work Package 3. Series: pragmatic trials and real-world evidence: paper 1. Introduction. J Clin Epidemiol. 2017;88:7-13. [DOI] [PubMed] [Google Scholar]
- 4. Berdelou A, Borget I, Godbert Y, et al. Lenvatinib for the treatment of radioiodine-refractory thyroid cancer in real-life practice. Thyroid. 2018;28(1):72-78. [DOI] [PubMed] [Google Scholar]
- 5. Locati LD, Piovesan A, Durante C, et al. Real-world efficacy and safety of lenvatinib: data from a compassionate use in the treatment of radioactive iodine-refractory differentiated thyroid cancer patients in Italy. Eur J Cancer. 2019;118:35-40. [DOI] [PubMed] [Google Scholar]
- 6. Aydemirli MD, Kapiteijn E, Ferrier KRM, et al. Effectiveness and toxicity of lenvatinib in refractory thyroid cancer: Dutch real-life data. Eur J Endocrinol. 2020;182(2):131-138. [DOI] [PubMed] [Google Scholar]
- 7. Masaki C, Sugino K, Saito N, et al. Efficacy and limitations of lenvatinib therapy for radioiodine-refractory differentiated thyroid cancer: real-world experiences. Thyroid. 2020;30(2):214-221. [DOI] [PubMed] [Google Scholar]
- 8. Giani C, Valerio L, Bongiovanni A, et al. Safety and quality-of-life data from an italian expanded access program of lenvatinib for treatment of thyroid cancer. Thyroid. 2021;31(2):224-232. [DOI] [PubMed] [Google Scholar]
- 9. Takahashi S, Tahara M, Ito K, et al. Safety and effectiveness of lenvatinib in 594 patients with unresectable thyroid cancer in an all-case post-marketing observational study in Japan. Adv Ther. 2020;37(9):3850-3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tuttle RM, Ahuja S, Avram AM, et al. Controversies, consensus, and collaboration in the use of 131I Therapy in Differentiated Thyroid Cancer: A Joint Statement from the American Thyroid Association, the European Association of Nuclear Medicine, the Society of Nuclear Medicine and Molecular Imaging, and the European Thyroid Association. Thyroid. 2019;29(4):461-470. [DOI] [PubMed] [Google Scholar]
- 11. Boucher A, Ezzat S, Hotte S, et al. Canadian consensus statement on the management of radioactive iodine-resistant differentiated thyroid cancer. Oral Oncol. 2021;121:105477. [DOI] [PubMed] [Google Scholar]
- 12. Fugazzola L, Elisei R, Fuhrer D, et al. 2019 European Thyroid Association Guidelines for the treatment and follow-up of advanced radioiodine-refractory thyroid cancer. Eur Thyroid J. 2019;8(5):227-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Numico G, Sicuro M, Silvestris N, et al. Takotsubo syndrome in a patient treated with sunitinib for renal cancer. J Clin Oncol. 2012;30(24):e218-e220. [DOI] [PubMed] [Google Scholar]
- 15. White AJ, LaGerche A, Toner GC, Whitbourn RJ. Apical ballooning syndrome during treatment with a vascular endothelial growth factor receptor antagonist. Int J Cardiol. 2009;131(3):e92-e94. [DOI] [PubMed] [Google Scholar]
- 16. Ovadia D, Esquenazi Y, Bucay M, Bachier CR. Association between takotsubo cardiomyopathy and axitinib: case report and review of the literature. J Clin Oncol. 2015;33(1):e1-e3. [DOI] [PubMed] [Google Scholar]
- 17. Chae YK, Chiec L, Adney SK, et al. Posterior reversible encephalopathy syndrome and takotsubo cardiomyopathy associated with lenvatinib therapy for thyroid cancer: a case report and review. Oncotarget. 2018;9(46):28281-28289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. LENVIMA. Highlights of prescribing information. Woodcliff Lake, NJ: Eisai Inc; 2021. [Google Scholar]
- 19. Myint ZW, Sen JM, Watts NL, et al. Reversible posterior leukoencephalopathy syndrome during regorafenib treatment: a case report and literature review of reversible posterior leukoencephalopathy syndrome associated with multikinase inhibitors. Clin Colorectal Cancer. 2014;13(2):127-130. [DOI] [PubMed] [Google Scholar]
- 20. Fugate JE, Rabinstein AA. Posterior reversible encephalopathy syndrome: clinical and radiological manifestations, pathophysiology, and outstanding questions [published correction appears in Lancet Neurol. 2015 Sep;14(9):874]. Lancet Neurol. 2015;14(9):914-925. [DOI] [PubMed] [Google Scholar]
- 21. Osawa Y, Gozawa R, Koyama K, Nakayama T, Sagoh T, Sunaga H. Posterior reversible encephalopathy syndrome after lenvatinib therapy in a patient with anaplastic thyroid carcinoma. Intern Med. 2018;57(7):1015-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tahara M, Brose MS, Wirth LJ, et al. Impact of dose interruption on the efficacy of lenvatinib in a phase 3 study in patients with radioiodine-refractory differentiated thyroid cancer. Eur J Cancer. 2019;106:61-68. [DOI] [PubMed] [Google Scholar]
- 23. Takahashi S, Kiyota N, Tahara M. Optimal use of lenvatinib in the treatment of advanced thyroid cancer. Cancers Head Neck. 2017;2:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tahara M. Management of recurrent or metastatic thyroid cancer. ESMO Open. 2018;3(Suppl 1):e000359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Suzuki S, Horinouchi A, Uozumi S, et al. Impact of outpatient pharmacy interventions on management of thyroid patients receiving lenvatinib. SAGE Open Med. 2020;8:2050312120930906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tahara M, Takami H, Ito Y, et al. Planned drug holiday in a cohort study exploring the effect of lenvatinib on differentiated thyroid cancer. J Clin Oncol. 2021;39(Suppl 15):6070-6070. [Google Scholar]
- 27. De Leo S, Di Stefano M, Persani L, Fugazzola L, Colombo C. Lenvatinib as first-line treatment for advanced thyroid cancer: long progression-free survival. Endocrine. 2021;72(2):462-469. [DOI] [PubMed] [Google Scholar]
- 28. Brose MS, Panaseykin Y, Konda B, et al. A randomized study of lenvatinib 18 mg vs 24 mg in patients with radioiodine-refractory differentiated thyroid cancer. J Clin Endocrinol Metab. 2022;107(3):776-787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Robinson B, Schlumberger M, Wirth L, et al. Characterization of tumor size changes over time from the phase 3 study of lenvatinib in thyroid cancer. J Clin Endocrinol Metab. 2016;101(11):4103-4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.