Abstract
Context
Clinicians frequently rely on aldosterone thresholds derived from older immunoassays to diagnose primary aldosteronism. Liquid chromatography–tandem mass spectrometry (LC-MS/MS) is increasingly widespread and reported to yield lower aldosterone concentrations.
Objective
Given the health impact of incorrect interpretations of aldosterone levels, we compared measurements using LC-MS/MS and immunoassay across the full range of aldosterone physiology by evaluating distinct regulation by angiotensin II and adrenocorticotropin (ACTH).
Methods
Normotensive volunteers underwent prospective characterization of aldosterone production by immunoassay and LC-MS/MS during 4 conditions (n = 188): oral sodium suppression and restriction (to assess angiotensin II–mediated aldosterone production) and dexamethasone suppression and cosyntropin stimulation (to assess ACTH-mediated aldosterone production).
Results
Serum aldosterone concentrations by LC-MS/MS and immunoassay had a correlation of 0.69 (P < .001), with good agreement (intraclass correlation 0.76; 95% CI 0.52-0.87). Aldosterone was lower by LC-MS/MS than immunoassay (median 10.5 [3.8, 21.9] vs 19.6 [9.5, 28.0] ng/dL; P < .001), with an average difference of 37.2%. The most notable discrepancy was in the clinically discriminatory range <20 ng/dL: 9.9 (7.1, 13.8) ng/dL using immunoassay corresponded to 5.5 (1.4, 8.9) ng/dL by LC-MS/MS (P < .001). Following oral sodium suppression, the aldosterone-to-renin ratio was 4-fold higher using immunoassay (27.2 [19.7, 62.4] vs 6.4 [3.5, 19.1] ng/dL per ng/mL/hour; P < .001).
Conclusion
Aldosterone measurements are substantially lower by LC-MS/MS than immunoassay across the full physiologic range, especially when aldosterone levels were less than 20 ng/dL. These findings highlight the need to recalibrate diagnostic interpretations when measuring aldosterone via LC-MS/MS and provide insights into potential biologic causes of assay differences.
Keywords: aldosterone, primary aldosteronism, hypertension
Mounting evidence identifies primary aldosteronism as an underrecognized and prevalent cause of hypertension [1-7] and excess cardiovascular risk [8-14]. Recent studies have demonstrated a severity spectrum of renin-independent aldosterone production that extends continuously across the “normal range” into primary aldosteronism [1, 15-18] and have shown that circulating aldosterone concentrations in primary aldosteronism can be highly variable [19-21], often lower than conventional diagnostic thresholds [1, 20, 22]. Increasingly, older immunoassay technologies have been supplanted by liquid chromatography–tandem mass spectrometry (LC-MS/MS) for clinical measurement of aldosterone concentration; however, clinical guideline recommendations [22], and most clinical practice patterns, still generally rely on relatively arbitrary thresholds derived from older immunoassay measurements.
With the development and dissemination of the LC-MS/MS approach to aldosterone quantification, studies in clinical patients, largely with hypertension and suspected primary aldosteronism, have reported the performance of LC-MS/MS relative to several immunoassays [23-34]. These studies have demonstrated generally lower aldosterone values by LC-MS/MS, especially in the context of interpreting categorical thresholds for confirmatory testing [26-30], thereby highlighting the importance of assay type in the establishment of diagnostic thresholds for primary aldosteronism and in the potential to confound the interpretation of screening and confirmatory tests. In contrast, prior studies have not compared LC-MS/MS measurements with immunoassay across the entire dynamic range of aldosterone physiology, from levels near the lower limits of detection, as might be seen following aldosterone suppression maneuvers, to those approximating maximally stimulated aldosterone production, as might be seen in states of hyperaldosteronism. Apparently low values may still be clinically relevant, for in some clinical contexts they may still signify states of pathologic aldosterone excess that are erroneously missed. We aimed to compare aldosterone quantification using immunoassay and LC-MS/MS to determine whether differences between assay type were detectable across the full dynamic range of aldosterone production and to quantify the magnitude of these potential differences. To accomplish this goal, we studied aldosterone measurements in participants who underwent 4 controlled physiologic protocols to suppress and to stimulate aldosterone by modifying angiotensin II and adrenocorticotropin (ACTH).
Materials and Methods
Study Participants
Participants were recruited in the Prospective Phenotyping of Autonomous Aldosterone Secretion protocol (NCT03484130). This protocol enrolled “high-risk” normotensive adult volunteers aged 35-70 years with high–normal blood pressure, overweight or obese status, family history of young-onset hypertension, and/or diabetes in order to characterize adrenal function and renin-independent aldosterone production prior to the potential longitudinal development of hypertension. “High-risk” normotensives were selected to enrich the development of hypertension during the course of prospective follow-up. Participants were recruited to have either blood pressures of 120-135/75-85 mmHg with at least 1 or more of the following, or blood pressures of 115-135/70-85 mmHg with at least 2 or more of the following: body mass index (BMI) ≥25 kg/m2, family history of hypertension prior to the age of 60 years, and/or diabetes with glycated hemoglobin <9%. Participants were excluded for known antihypertensive drug use or significant comorbidity including cardiovascular disease, chronic kidney disease, or actively treated cancer. All participants underwent controlled testing of the full range of aldosterone production in response to physiologic modulation of angiotensin II using oral sodium suppression and dietary sodium restriction, and after physiologic modulation of ACTH, using dexamethasone suppression testing and cosyntropin stimulation, as detailed below. Blood samples were prospectively collected and stored in duplicate to facilitate comparison of immunoassay and LC-MS/MS methodologies under all conditions.
Angiotensin II Stimulation and Suppression Protocols
In order to maximally stimulate angiotensin II–mediated aldosterone production, participants received a fixed sodium-restricted diet of 10 mEq sodium (230 mg) and 75 mmol potassium per day for 5 days, prior to presenting to the Clinical Research Center for phlebotomy performed in the morning, while fasting, and after 30 minutes of upright/seated posture. To maximally suppress angiotensin II–mediated aldosterone production, participants then underwent oral sodium suppression with an intake of at least 200 mmol sodium (4600 mg) and 75 mmol potassium for 5 days, again followed by fasting, and upright/seated phlebotomy between 8 am and 9 am. Urine was collected for 24 hours on the final day of both dietary phases; only participants achieving 24-hour urine sodium concentration of ≤50 mEq after sodium restriction and ≥150 mEq after sodium suppression were included in the analyses to ensure that aldosterone measurements represented the ideal stimulated and suppressed states (n = 48 participants and n = 96 paired measurements).
ACTH Stimulation and Suppression Protocols
The range of aldosterone production regulated by ACTH was then evaluated on a separate day on an ad libitum diet. Participants were prescribed a 1 mg oral dose of dexamethasone to be taken between 11 pm and 12 am on the evening before the study visit. On arrival to the Clinical Research Center the following morning at 8 am, they underwent seated phlebotomy while fasting. Participants then received an intravenous bolus of 250 µg of cosyntropin, followed by repeat seated phlebotomy 60 minutes later. Of the 48 subjects with adequate oral sodium suppression and sodium restriction, 46 also successfully completed dexamethasone suppression and cosyntropin stimulation (n = 92 paired measurements).
All blood samples from oral sodium suppression, dietary sodium restriction, dexamethasone suppression, and cosyntropin stimulation protocols (n = 188 paired samples) were collected and frozen at –80°C, with 1 aliquot used for the measurement of serum aldosterone in the Brigham and Women’s Research Assay Core laboratory (Boston, MA, USA) by immunoassay and the other assayed using LC-MS/MS at the University of Michigan (Auchus Lab, Ann Arbor, MI, USA), as described in detail below.
All study participants provided written informed consent, and the study was approved by the Brigham and Women’s Hospital Institutional Review Board.
Laboratory Assays
Measurement of serum aldosterone was performed using 2 analytical techniques: enzyme-linked immunosorbent assay (ELISA) and LC-MS/MS.
The ELISA (IBL-America; Minneapolis, MN, USA; catalog #IB79134, RRID:AB_2813725, https://scicrunch.org/resources/Antibodies/search?q=AB_2813725&l=AB_2813725) has a reported analytical sensitivity of <0.57 ng/dL and a dynamic range of 0.57 ng/dL to 100 ng/dL. The manufacturer’s reported interassay coefficient of variation is 8.6% to 9.9% and the intra-assay coefficient of variation is 3.9% to 9.7%. The study laboratory interassay coefficient of variation is 7.9% to 15.9% (7.9% at a concentration of 51.27 ng/dL and 15.9% at a concentration of 5.58 ng/dL) and the intra-assay coefficient of variation is 4.9% to 13.5% (4.9% at 51.28 ng/dL and 13.5% at 5.67 ng/dL). The immunoassay has reported 17.2% cross-reactivity with tetrahydroaldosterone and otherwise cross-reactivities of <0.02% with prednisolone and <0.003% with cortisol, 11-deoxycortisol, progesterone, testosterone, and androstenedione.
Aldosterone was measured with other steroid analytes using an Agilent 6495 triple quadrupole tandem mass spectrometer with 2-dimensional liquid chromatography (LC) on Agilent 1260 and 1290 systems after supported liquid extraction as previously described [35, 36]. The standard curve was generated as follows: A solution containing all stable isotope–labelled internal standards, including the aldosterone-d8 from Steraloids, was first prepared in acetonitrile. A set of 12 external calibrators (standards) was created from a certified analytical stock solution (1 mg/mL, Cerilliant) via 1:1 serial dilution, using the internal standard solution as solvent and as diluent. The same internal standard solution was added to each sample during processing, so that the amount of internal standard injected after extraction, drying, and reconstitution at 100% recovery would be equal to the amount injected with each standard solution. Standards and reconstituted samples are injected in 40% aqueous methanol, which approximates the LC mobile phase starting conditions, without spiking or extraction. The aldosterone-d8 internal standard corrects for both matrix effects and extraction variability in the samples. Aldosterone and the aldosterone-d8 (Steraloids, Newport, RI) internal standard were eluted from the 150-mm biphenyl resolving column (Phenomenex, Torrance, CA) at 11.9 to 12.2 minutes, with precursor and quantifier (qualifier) product ions of 361.2/315.1 (343.2) and 369.2/351.2 (323.2), respectively. The lower limit of quantitation was estimated from the signal-to-noise ratio of pooled samples, using the Mass Hunter “peak-to-peak” method in a noise region window lacking interfering peaks of 0.25 minutes most often postpeak, and extrapolated to a concentration that yields a signal-to-noise ratio of 5. This theoretical lower limit of quantitation was 1.4 to 2.5 ng/dL, and samples assayed as below the lower limit of quantification were set equal to the lower limit determined for that assay batch. Chromatograms for all samples below quantitation were reviewed and confirmed to have similar signal for the internal standard as quantified samples, which excludes high ion suppression or low recovery as artifacts that precluded quantitation. Only 1 sample was not quantitated (reported as the lower limit) due to a quantifier/qualifier ion ratio outside 80% to 120% of the ratio for the calibrator standard. The intra- and interassay coefficients of variation were <12% for all analytes including aldosterone [35-37].
Statistical Analyses
Variables are shown as mean (SD) or median (interquartile range), unless otherwise noted. Serum aldosterone concentrations are shown as paired values using each of the 2 analytical techniques on blood samples drawn under controlled conditions designed to maximally stimulate and suppress aldosterone production under the regulatory control of angiotensin II and ACTH. The Wilcoxon rank sum test was used to compare median aldosterone concentrations using LC-MS/MS and immunoassay in the full set of 188 samples across the entire range of aldosterone concentrations. The consistency of aldosterone values by immunoassay and LC-MS/MS was evaluated using the Pearson (r) correlation coefficient. Bland–Altman analysis was used to evaluate average bias between aldosterone assays. Absolute agreement between the 2 assays was determined by computing the average intraclass correlation coefficient using a 2-way mixed effects model.
To evaluate the full range of aldosterone physiology, the 2 assays were compared within each of the 4 conditions individually. Aldosterone values from oral sodium suppression and dietary sodium restriction were then combined to evaluate the calibration of the immunoassay relative to LC-MS/MS across the range of aldosterone production under the control of angiotensin II. Serum aldosterone concentrations by immunoassay were divided into 4 clinically relevant and approximately equal-sized categories (<10 ng/dL, 10 to 20 ng/dL, 20 to 30 ng/dL, and ≥30 ng/dL) to facilitate clinically relevant comparisons between median immunoassay and LC-MS/MS values, the median difference between measurements, and the median percent difference. The percent difference was calculated as the aldosterone concentration using immunoassay minus the concentration using LC-MS/MS divided by the immunoassay value. The identical approach was then used to compare immunoassay and LC-MS/MS aldosterone concentrations across the range of aldosterone secretion under the control of ACTH, combining aldosterone values from dexamethasone suppression and cosyntropin stimulation.
After oral sodium suppression only, the aldosterone-to-renin ratio (ARR) was calculated using both LC-MS/MS- and immunoassay-derived aldosterone concentrations divided by the plasma renin activity. The Wilcoxon rank sum test was used to compare ARRs using LC-MS/MS vs immunoassay in the subset of 42 cases with plasma renin activity ≤1.0 ng/mL/hour after sodium suppression.
A P < .05 was considered statistically significant. All analyses were performed using SAS, version 9.4 (Cary, NC), and STATA, version 14 (College Station, TX).
Results
As anticipated on the basis of the study design, the population was obese, with a mean BMI of 30.1 (4.5) kg/m2, and had an untreated blood pressure of 123 (7) over 77 (5) mmHg (Table 1). Total 24-hour urinary sodium excretion confirmed effective sodium balance following loading and restriction protocols, and serum cortisol concentrations confirmed effective dexamethasone suppression and cosyntropin stimulation protocols (Table 1).
Table 1.
Population characteristics of the study population (n = 48)
| Characteristics | All subjects |
|---|---|
| Age, y | 49.3 ± 10.1 |
| Female, n (%) | 22 (45.8) |
| BMI, kg/m2 | 30.1 ± 4.5 |
| Race, n (%) | |
| White | 40 (83.3) |
| Black/African-American | 3 (6.3) |
| Asian | 3 (6.3) |
| American Indian or Alaska Native | 1 (2.1) |
| More than 1 of the above | 1 (2.1) |
| Screening systolic BP, mmHg | 123 ± 7 |
| Screening diastolic BP, mmHg | 77 ± 5 |
| Screening potassium, mEq/L | 4.30 ± 0.35 |
| Screening eGFR, mL/min/1.73 m2 | 89 ± 13 |
| Sodium suppression | |
| 24-hour urine sodium, mmol | 255.0 (193.1, 304.3) |
| Sodium restriction | |
| 24-hour urine sodium, mmol | 13.6 (7.8, 20.4) |
| Dexamethasone suppression | |
| Cortisol, μg/dL | 0.9 (0.7, 1.3) |
| Cosyntropin stimulation | |
| Cortisol, μg/dL | 20.8 (18.7, 24.6) |
Abbreviations: BMI, body mass index; BP, blood pressure; eGFR, estimated glomerular filtration rate.
Overall, aldosterone concentrations using LC-MS/MS and immunoassay were strongly correlated (Pearson r = 0.69, P < .001), and there was good agreement between LC-MS/MS and immunoassay, with an intraclass correlation coefficient of 0.76 (95% CI 0.52-0.87).
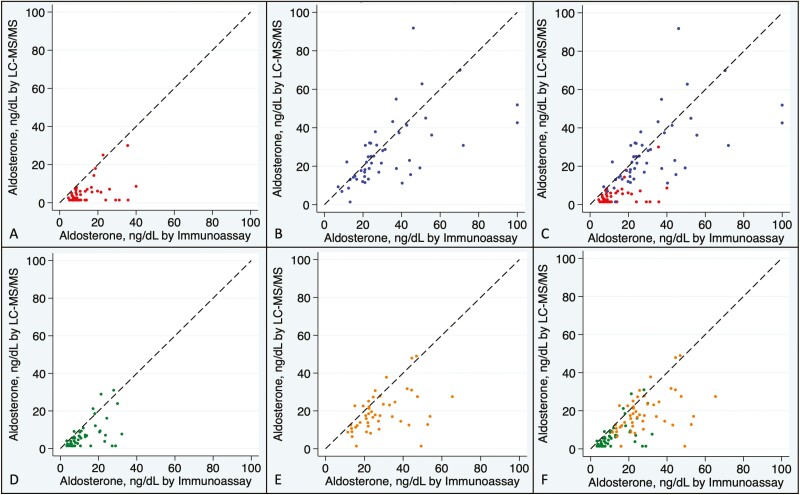
After controlled physiologic suppression of aldosterone with either oral sodium loading (Fig. 1A) or dexamethasone (Fig. 1D), aldosterone concentrations near the lower end of detection were consistently lower using LC-MS/MS than using immunoassay. In the intermediate to higher range of aldosterone values achieved by stimulation with dietary sodium restriction (Fig. 1B) or ACTH stimulation (Fig. 1E), LC-MS/MS again yielded consistently lower aldosterone concentrations than immunoassay. The full range of aldosterone concentrations across angiotensin II and ACTH modulation are shown in Fig. 1C and 1F, respectively.
Figure 1.
Agreement between aldosterone concentration by assay type under conditions of oral sodium suppression (A), dietary sodium restriction (B), combined angiotensin II modulation (C), dexamethasone suppression (D), ACTH stimulation (E), and combined ACTH modulation (F). Samples assayed for serum aldosterone concentration by LC-MS/MS (liquid chromatography–tandem mass spectrometry) and immunoassay are shown from overweight normotensive volunteers after oral sodium suppression (red; A and C), sodium restriction (blue; B and C), dexamethasone suppression (green; D and F), and 250 µg ACTH stimulation (orange; E and F). The dashed line represents identity; ie, the same value of aldosterone returned by both assays.
Aldosterone concentrations were lower using LC-MS/MS in nearly all participants and across the entire dynamic range of aldosterone production. The proportion of aldosterone concentrations that were lower using LC-MS/MS than using immunoassay was 93.8% for values <10 ng/dL, 89.6% for values 10-20 ng/dL, 73.6% for values 20-30 ng/dL, and 82.1% for values >30 ng/dL. This discrepancy was most notable in the clinically relevant ranges of <10 ng/dL and 10 to 20 ng/dL, whereby LC-MS/MS measurements were on average substantially lower than immunoassay (up to 65% lower) (Tables 2 and 3).
Table 2.
Serum aldosterone by immunoassay vs liquid chromatography–tandem mass spectrometry during physiologic maneuvers to modify angiotensin II
| Serum aldosterone by immunoassay | ||||
|---|---|---|---|---|
| <10 ng/dL | 10-20 ng/dL | 20-30 ng/dL | ≥30 ng/dL | |
| N | 26 | 24 | 23 | 23 |
| Aldosterone, ng/dL | 7.7 (6.8, 8.7) |
13.9 (12.1, 16.8) |
23.4 (21.6, 26.1) |
42.7 (35.8, 52.5) |
| Immunoassay | ||||
| LC-MS/MS | 2.5 (1.4, 5.4) |
6.6 (1.4, 13.7) |
21.6 (13.3, 25.3) |
30.8 (18.8, 45.0) |
| Median difference in aldosterone, ng/dL | 4.4 (2.0, 5.9) |
7.4 (2.4, 10.5) |
2.8 (–2.3, 9.5) |
18.5 (1.3, 30.4) |
| Median percent difference in aldosterone, % | 65.8 (24.1, 79.8) |
51.4 (13.5, 86.3) |
13.3 (–10.1, 41.7) |
34.9 (3.3, 61.4) |
| Proportion with aldosterone lower by LC-MS/MS than Immunoassay, n (%) | 25/26 (96.2) |
23/24 (95.8) |
14/23 (60.9) |
19/23 (82.6) |
Results are median with interquartile range. All samples obtained under conditions of oral sodium suppression and sodium restriction to modulate angiotensin II were pooled and categorized based on the aldosterone concentration by immunoassay (n = 96).
Abbreviations: ACTH, adrenocorticotropin; LC-MS/MS, liquid chromatography–tandem mass spectrometry.
Table 3.
Serum aldosterone by immunoassay vs liquid chromatography–tandem mass spectrometry during physiologic maneuvers to modify ACTH
| Serum aldosterone by immunoassay | ||||
|---|---|---|---|---|
| <10 ng/dL | 10-20 ng/dL | 20-30 ng/dL | ≥30 ng/dL | |
| N | 22 | 24 | 30 | 16 |
| Aldosterone, ng/dL | ||||
| Immunoassay | 6.3 (5.4, 7.5) |
13.7 (11.8, 16.1) |
23.7 (22.0, 27.3) |
43.7 (35.1, 48.4) |
| LC-MS/MS | 2.6 (1.4, 4.6) |
9.2 (6.4, 12.4) |
17.1 (9.4, 23.5) |
23.9 (13.7, 31.4) |
| Median difference in aldosterone, ng/dL | 3.1 (1.5, 4.6) |
4.0 (1.3-6.5) |
7.8 (1.1, 12.2) |
18.6 (10.2, 33.7) |
| Median percent difference in aldosterone, % | 53.8 (27.9, 70.0) |
25.9 (11.9, 49.7) |
31.0 (4.9, 57.1) |
46.3 (27.4, 69.6) |
| Proportion with aldosterone lower by LC-MS/MS than immunoassay, n (%) | 20/22 (90.9) |
20/24 (83.3) |
25/30 (83.3) |
13/16 (81.3) |
Results are median with interquartile range. All samples obtained under conditions of dexamethasone suppression and cosyntropin stimulation to modulate ACTH were pooled and categorized based on the aldosterone concentration by immunoassay (n = 92).
Abbreviations: ACTH, adrenocorticotropin; LC-MS/MS, liquid chromatography–tandem mass spectrometry.
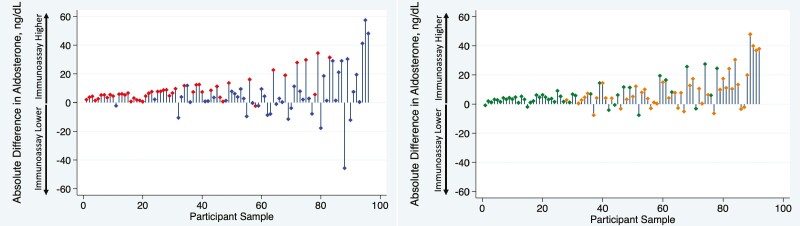
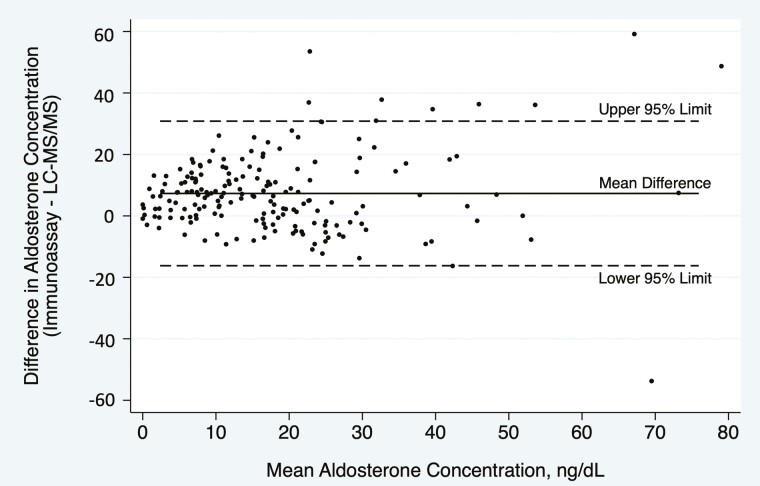
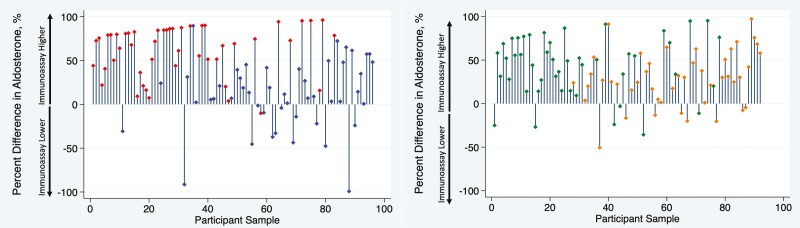
Figure 2 demonstrates that aldosterone concentrations were generally lower using LC-MS/MS than using immunoassay across the full range of aldosterone values obtained by controlled physiologic suppression and stimulation, with the serum aldosterone concentration 37.2% lower using LC-MS/MS than using immunoassay overall (median 10.5 [3.8, 21.9] vs 19.6 [9.5, 28.0] ng/dL; P < .001). The difference in aldosterone concentrations using each assay across the full spectrum of values is shown in a Bland–Altman plot (Fig. 3), which demonstrates a bias of 7.3 ng/dL (SD 12.0 ng/dL) higher aldosterone level using immunoassay. The individual percent differences between LC-MS/MS and immunoassay on a per-sample basis, across the range of values achieved through angiotensin II and ACTH manipulation, are shown in Fig. 4.
Figure 2.
Absolute difference in aldosterone concentration by assay type under conditions of angiotensin II modulation (A) and ACTH modulation (B), ordered from lowest to highest aldosterone concentration. All samples obtained under conditions of oral sodium suppression and dietary sodium restriction to modulate angiotensin II were pooled (A: red, oral sodium suppression; blue; sodium restriction) and all samples obtained under ACTH modulation were pooled (B; green: dexamethasone suppression; orange, ACTH stimulation). Each participant sample is represented, ordered from the lowest to highest aldosterone concentration by immunoassay. The vertical bars represent the absolute difference between immunoassay and LC-MS/MS (liquid chromatography–tandem mass spectrometry), with positive values indicating a higher immunoassay and lower LC-MS/MS result.
Figure 3.
Bland–Altman plot demonstrating agreement between immunoassay and LC-MS/MS. The 2 assays yielded an average difference in aldosterone concentration of 7.3 ng/dL higher (solid line; SD 12.0 ng/dL) by immunoassay than using LC-MS/MS, with 95% limits of agreement from –16.2 to 30.8 ng/dL (dashed lines), comparing all 188 samples from n = 48 subjects across 4 experimental conditions.
Figure 4.
Percent difference in aldosterone concentration by assay type under conditions of angiotensin II modulation (A) and ACTH modulation (B), ordered from lowest to highest aldosterone concentration. All samples obtained under conditions of oral sodium suppression and dietary sodium restriction to modulate angiotensin II were pooled (A: red, oral sodium suppression; blue; sodium restriction) and all samples obtained under ACTH modulation were pooled (B; green: dexamethasone suppression; orange, ACTH stimulation). Each participant sample is represented, ordered from the lowest to highest aldosterone concentration by immunoassay. The vertical bars represent the percent difference between immunoassay and LC-MS/MS (liquid chromatography–tandem mass spectrometry) as a proportion of the immunoassay aldosterone concentration, with positive values indicating a higher immunoassay and lower LC-MS/MS result.
In samples drawn after oral sodium suppression, an ARR of 6.2 [3.4, 14.8] ng/dL per ng/mL/hour using LC-MS/MS corresponded to 23.3 [15.9, 52.8] ng/dL per ng/mL/hour using immunoassay (P < .001). In the subset of 42 subjects with a suppressed plasma renin activity (≤1.0 ng/mL/hour) after oral sodium suppression, the ARR was similarly several fold higher using immunoassay than LC-MS/MS (27.2 [19.7, 62.4] vs 6.4 [3.5, 19.1] ng/dL per ng/mL/hour; P < .001).
Discussion
We have demonstrated that aldosterone concentrations using LC-MS/MS are systematically lower than using immunoassay across the entire physiologic spectrum, ranging from as much as 53.8% to 65.8% lower when maximally suppressed by oral sodium loading or dexamethasone to 34.9% to 46.3% lower under maximal stimulatory conditions of extreme dietary sodium restriction and cosyntropin administration. While clinical guidelines for primary aldosteronism have emphasized the importance of minimizing false positive screening results to limit unnecessary downstream testing [22], our findings highlight that the contemporary clinical concern should be the risk of false negative testing caused by the significantly lower aldosterone concentrations detected using modern LC-MS/MS assay techniques; most clinicians have not yet recalibrated their interpretations to accommodate this finding. Given that primary aldosteronism is highly prevalent [1, 5, 38, 39], yet rarely diagnosed [19, 20, 40-44], it is paramount that clinicians be aware that interpretations of circulating aldosterone and ARR values need to be recalibrated to include much lower values than previously recommended [45].
These data extend the findings of prior studies comparing LC-MS/MS and immunoassay techniques for aldosterone quantification [23-34]. In one of the original papers validating aldosterone measurement using LC-MS/MS in 1997, Fredline et al compared aldosterone concentrations using LC-MS/MS and radioimmunoassay from 78 patients with unclear clinical histories in a mix of supine or upright posture and demonstrated a mean difference of 0.52 ng/dL (SE 0.37 ng/dL) over the range of 0.14 to 47.2 ng/dL [23], with a 6-fold greater difference at lower, compared with higher, concentrations. Multiple subsequent studies comparing a variety of immunoassay and LC-MS/MS techniques in healthy volunteers [25], as well as patients with hypertension and suspected or confirmed primary aldosteronism [27-34] under an array of postural and medication conditions, have demonstrated consistently lower aldosterone values using LC-MS/MS, with immunoassay returning values of plasma aldosterone ranging from 27% to 86% higher than LC-MS/MS, with the greatest difference detected at the lower range of aldosterone concentrations [25-28, 30-34].
While prior studies have demonstrated a consistent discrepancy between assays and have attributed suboptimal and inconsistent accuracy of immunoassay quantification to antibody cross-reactivity and interference [24, 26, 30, 46, 47], these studies have not been designed to interrogate pathophysiologic causes of such a discrepancy. The current study provides insight into potential causes of assay disagreement by modulating aldosterone production in normotensive volunteers not taking antihypertensive medications, under strictly controlled posture, diet, and time of day, and with targeted modulation of each angiotensin II and ACTH to achieve maximal physiologic suppression and stimulation. While angiotensin II predominantly increases zona glomerulosa synthesis of aldosterone, ACTH diffusely stimulates adrenal steroidogenesis; therefore, distinct modulations of each of these regulators can provide clues into the mechanisms underlying assay differences. One putative explanation for the magnified discrepancy between immunoassay and LC-MS/MS at low values is based on the presence of immunoassay antibody cross-reactivity with alternative steroid hormones including aldosterone metabolites such as tetrahydroaldosterone (and possibly also aldosterone-18-glucuronide). Free aldosterone, measured by LC-MS/MS, reflects less than 1% of daily aldosterone metabolite production, whereas tetrahydroaldosterone and aldosterone-18-glucuronide reflect the remainder [48-50]. In the current study, oral sodium and dexamethasone suppression maneuvers to acutely reduce the production of aldosterone, measured exclusively using LC-MS/MS, would be expected to have relatively little impact on longer circulating metabolites concurrently detected by immunoassay. Conversely, with acute stimulation using sodium restriction and cosyntropin, relative increases in aldosterone production would be detected by both LC-MS/MS and immunoassay, without adequate time for substantial equilibration and increases in concentrations of aldosterone metabolites, resulting in a less pronounced difference in reported aldosterone concentration using immunoassay and LC-MS/MS. This is consistent with previous reports among patients with confirmed primary aldosteronism following an oral sodium suppression test, in whom elevated 24-hour urinary aldosterone excretion—measuring the combination of both free aldosterone and aldosterone-18-glucuronide—often corresponded to low concentrations (<10 ng/dL) of circulating free aldosterone measured using LC-MS/MS and poor performance of the ARR [1]. Further, the discrepancies detected between immunoassay and LC-MS/MS were largely consistent, regardless of modulation of aldosterone production via angiotensin II or ACTH, suggesting that cross-reactivity with other zona fasciculata–derived steroids is not the only contributor to assay-based differences. Whether detection of free aldosterone alone, as is done by LC-MS/MS, or detection of total aldosterone, including relevant metabolites, provides better diagnostic accuracy for primary aldosteronism is uncertain, though incorporation of tetrahydroaldosterone into screening has been demonstrated to have high sensitivity and specificity [48] and probably should be re-evaluated in future studies. Routine measurement of tetrahydroaldosterone and aldosterone-18-glucuronide using LC-MS/MS techniques could provide clarification on this question.
The present study demonstrates the need for clinical interpretation of aldosterone values to incorporate explicit awareness of the assay technique used, not only among patients presenting with clinical suspicion of primary aldosteronism, but across the full dynamic range of aldosterone production, especially in the clinically discriminatory range of <20 ng/dL. Recent studies have extended these observations to evaluate the implications of assay-specific diagnostic thresholds for patients with suspected primary aldosteronism undergoing confirmatory testing [27, 28, 31]. Most recently, Eisenhofer et al reported 62 of 240 patients having discordant results of seated saline suppression testing despite method-specific cut-off values, and raised the specter of misdiagnosis in 60% or more of cases labeled with bilateral primary aldosteronism [30].
In the aforementioned studies, the authors emphasized the risk of false positive results, derived from relying on immunoassays, that may lead to unnecessary invasive procedures in the absence of true primary aldosteronism. However, a major limitation in adjudicating which assay is more accurate is the absence of a universal gold standard to define genuine primary aldosteronism, especially in cases of milder forms of primary aldosteronism and those that do not undergo surgical adrenalectomy. Though elevations in aldosterone using LC-MS/MS may be high enough to rule in the diagnosis, several recent studies have also demonstrated the converse—that primary aldosteronism may be erroneously missed based on aldosterone values that are considered “too low.” For example, in a cohort of resistant hypertension patients referred to a hypertension specialty center, 24.5% had a circulating aldosterone concentration below 10 ng/dL using LC-MS/MS despite having confirmed primary aldosteronism defined using oral sodium suppression testing [1, 22]. Since many clinicians consider 10 ng/dL to be an arbitrary exclusionary threshold for primary aldosteronism, the implications are that at least one-quarter of confirmed primary aldosteronism cases within resistant hypertension patients could be erroneously excluded when aldosterone is measured via LC-MS/MS assays. Similarly, in a study of patients with confirmed primary aldosteronism, 30% had a circulating aldosterone concentration below 10 ng/dL on at least 1 occasion when measured using LC-MS/MS [20]. Finally, among 340 patients with confirmed primary aldosteronism who underwent adrenal venous sampling, 26% had an inferior vena cava aldosterone concentration ≤5 ng/dL by LC-MS/MS at the time of AVS [19]. All 3 of these studies underscore the risk of false negative interpretations of aldosterone levels when measured using LC-MS/MS assays. These studies highlight the need for increased clinician awareness of assay characteristics and for updated clinical guidelines to reflect recalibrations adapted to modern diagnostic LC-MS/MS assays that provide more accurate quantification of aldosterone, distinct from its related steroid metabolites [24]. Consequently, recent clinical recommendations have emphasized the need to recalibrate diagnostic thresholds for primary aldosteronism to include aldosterone concentrations as low as 5 or 6 ng/dL, particularly when measured using an LC-MS/MS assay [20, 45, 51]. Similar changes to clinical interpretations of testosterone [52] and cortisol [53, 54] values, due to the use of LC-MS/MS or more modern immunoassays, have already been recognized and recommended in the routine evaluation of hypogonadism and adrenal insufficiency, respectively.
While this study benefits from prospective assessment of adults evaluated under carefully controlled conditions, key limitations must be acknowledged. First, the subjects under study were normotensive volunteers who would not meet current guideline criteria for primary aldosteronism screening in clinical practice. However, this study did not aim to establish diagnostic thresholds to verify the accuracy of LC-MS/MS in diagnosing primary aldosteronism, but rather used established maneuvers to isolate key regulators of aldosterone production—angiotensin II and ACTH—in order to achieve aldosterone concentrations that span the full range over which the comparison of LC-MS/MS with immunoassay is relevant and important. Second, LC-MS/MS was compared with only 1 immunoassay technique despite known discrepancies between immunoassays [47]. Although differences in immunoassay characteristics could influence the precision of the comparison with LC-MS/MS and the strength of the reported interassay correlation, the central findings would not be changed: the interpretation of aldosterone values is highly dependent on assay type, and traditionally “normal” aldosterone concentrations yielded by LC-MS/MS may still represent pathologic aldosterone production. Third, we used only 1 LC-MS/MS platform for all of our measurements. We anticipate that different commercial laboratories could have minor variations in the calibration of their LC-MS/MS measurements; however, when calibrating with the same standard, these differences should be negligible [26].
Conclusions
In summary, by using controlled maneuvers to capture the entire physiologic range of aldosterone production and to isolate distinct, acute regulatory actions of angiotensin II and ACTH, we describe a substantial and consistent discrepancy in aldosterone concentrations between immunoassay and LC-MS/MS, wherein LC-MS/MS measurements are on average approximately 35% lower, and up to 50% to 65% lower in the most clinically relevant range. These differences, especially notable at lower values, mark the need for a recalibration of clinical intuition about what constitutes a circulating aldosterone concentration that is “high enough” for a diagnosis of primary aldosteronism, and raise concern for false negative interpretations that have resulted due to lack of awareness of assay type and calibration. The increased reliance on LC-MS/MS platforms for measuring aldosterone should result in a liberalization of diagnostic interpretations to include lower thresholds [45]. Given the public health burden of hypertension and hypertensive cardiovascular disease, clinician awareness of these systematic differences is critical to ensure accurate interpretation of primary aldosteronism testing so that patients are not deprived of targeted therapies or even cure.
Acknowledgments
We are grateful to the research participants who volunteered for this study.
Glossary
Abbreviations
- ACTH
adrenocorticotropin
- ARR
aldosterone-to-renin ratio; BMI< body mass index
- ELISA
enzyme-linked immunosorbent assay
- LC-MS/MS
liquid chromatography–tandem mass spectrometry
Financial Support
Research reported in this publication was supported by the National Institute of Diabetes and Digestive and Kidney Diseases under award R01 DK115392-04 (A.V.), National Institute of Heart, Lung, and Blood Disorders under award R01 HL153004 (A.V.), by an American Heart Association Career Development Award 852429 (J.M.B.), by a KL2/Catalyst Medical Research Investigator Training (CMeRIT) award from Harvard Catalyst UL1 TR002541 (J.M.B.), and by a Brigham and Women’s Hospital Department of Medicine Innovation Evergreen Fund Award (J.M.B., A.V.).
Disclosures
J.M.L. reports consulting activities with Mineralys Therapeutics, unrelated to the current work. A.V. reports consulting activities with Mineralys Therapeutics, Corecept Therapeutics, and HRA Pharma, all unrelated to the current work. No other authors report anything to disclose.
Data Availability Statement
Data generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Clinical Trial Information
NCT03484130 (registered March 30, 2018).
References
- 1. Brown JM, Siddiqui M, Calhoun DA, et al. . The unrecognized prevalence of primary aldosteronism: a cross-sectional study. Ann Intern Med. 2020;173(1):10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brown JM, Robinson-Cohen C, Luque-Fernandez MA, et al. . The spectrum of subclinical primary aldosteronism and incident hypertension. Ann Intern Med. 2017;167(9):630-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vasan RS, Evans JC, Larson MG, et al. . Serum aldosterone and the incidence of hypertension in nonhypertensive persons. N Engl J Med. 2004;351(1):33-41. [DOI] [PubMed] [Google Scholar]
- 4. Newton-Cheh C, Guo CYY, Gona P, et al. . Clinical and genetic correlates of aldosterone-to-renin ratio and relations to blood pressure in a community sample. Hypertension 2007;49(4):846-856. [DOI] [PubMed] [Google Scholar]
- 5. Rossi GP, Bernini G, Caliumi C, et al. . A prospective study of the prevalence of primary aldosteronism in 1,125 hypertensive patients. J Am Coll Cardiol. 2006;48(11):2293-2300. [DOI] [PubMed] [Google Scholar]
- 6. Stowasser M, Gordon RD, Gunasekera TG, et al. . High rate of detection of primary aldosteronism, including surgically treatable forms, after “non-selective” screening of hypertensive patients. J Hypertens. 2003;21(11):2149-2157. [DOI] [PubMed] [Google Scholar]
- 7. Mulatero P, Stowasser M, Loh KC, et al. . Increased diagnosis of primary aldosteronism, including surgically correctable forms, in centers from five continents. J Clin Endocrinol Metab. 2004;89(3):1045-1050. [DOI] [PubMed] [Google Scholar]
- 8. Hundemer GL, Curhan GC, Yozamp N, Wang M, Vaidya A. Incidence of atrial fibrillation and mineralocorticoid receptor activity in patients with medically and surgically treated primary aldosteronism. JAMA Cardiology. 2018;3(8):768-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Monticone S, D’Ascenzo F, Moretti C, et al. . Cardiovascular events and target organ damage in primary aldosteronism compared with essential hypertension: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2018;6(1):41-50. [DOI] [PubMed] [Google Scholar]
- 10. Rossi GP, Sechi LA, Giacchetti G, Ronconi V, Strazzullo P, Funder JW. Primary aldosteronism: cardiovascular, renal and metabolic implications. Trends Endocrinol Metab. 2008;19(3): 88-90. [DOI] [PubMed] [Google Scholar]
- 11. Reincke M, Fischer E, Gerum S, et al. . Observational study mortality in treated primary aldosteronism: the German Conn’s registry. Hypertension. 2012;60(3):618-624. [DOI] [PubMed] [Google Scholar]
- 12. Mulatero P, Monticone S, Bertello C, et al. . Long-term cardio- and cerebrovascular events in patients with primary aldosteronism. J Clin Endocrinol Metab. 2013;98(12):4826-4833. [DOI] [PubMed] [Google Scholar]
- 13. Milliez P, Girerd X, Plouin PF, Blacher J, Safar ME, Mourad JJ. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol. 2005;45(8):1243-1248. [DOI] [PubMed] [Google Scholar]
- 14. Hundemer GL, Curhan GC, Yozamp N, Wang M, Vaidya A. Cardiometabolic outcomes and mortality in medically treated primary aldosteronism: a retrospective cohort study. Lancet Diabetes Endocrinol. 2018;6(1):51-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Baudrand R, Guarda FJ, Fardella CE, et al. . Continuum of renin-independent aldosteronism in normotension. Hypertension. 2017;69(5):950-956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vaidya A, Mulatero P, Baudrand R, Adler GK. The expanding spectrum of primary aldosteronism: implications for diagnosis, pathogenesis, and treatment. Endocr Rev. 2018;39(6):1057-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vaidya A, Underwood PC, Hopkins PN, et al. . Abnormal aldosterone physiology and cardiometabolic risk factors. Hypertension. 2013;61(4):886-893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brown JM, Underwood PC, Ferri C, et al. . Aldosterone dysregulation with aging predicts renal vascular function and cardiovascular risk. Hypertension. 2014;63(6):1205-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yozamp N, Hundemer GL, Moussa M, et al. . Variability of aldosterone measurements during adrenal venous sampling for primary aldosteronism. Am J Hypertens. 2021;34(1):34-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yozamp N, Hundemer GL, Moussa M, et al. . Intraindividual variability of aldosterone concentrations in primary aldosteronism: implications for case detection. Hypertension. 2021;77(3):891-899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tanabe A, Naruse M, Takagi S, Tsuchiya K, Imaki T, Takano K. Variability in the renin/aldosterone profile under random and standardized sampling conditions in primary aldosteronism. J Clin Endocrinol Metab. 2003;88(6):2489-2494. [DOI] [PubMed] [Google Scholar]
- 22. Funder JW, Carey RM, Mantero F, et al. . The management of primary aldosteronism: case detection, diagnosis, and treatment: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2016;101(5):1889-1916. [DOI] [PubMed] [Google Scholar]
- 23. Fredline VF, Taylor PJ, Dodds HM, Johnson AG. A Reference method for the analysis of aldosterone in blood by high-performance liquid chromatography-atmospheric pressure chemical ionization-tandem mass spectrometry. Anal Biochem. 1997;252(2):308-313. [DOI] [PubMed] [Google Scholar]
- 24. Ray JA, Kushnir MM, Palmer J, Sadjadi S, Rockwood AL, Meikle AW. Enhancement of specificity of aldosterone measurement in human serum and plasma using 2D-LC-MS/MS and comparison with commercial immunoassays. J Chromatogr B Analyt Technol Biomed Life Sci. 2014;970:102-107. [DOI] [PubMed] [Google Scholar]
- 25. le Goff CM, Gonzalez-Antuña A, Peeters SD, et al. . Migration from RIA to LC-MS/MS for aldosterone determination: Implications for clinical practice and determination of plasma and urine reference range intervals in a cohort of healthy Belgian subjects. Clin Mass Spectrom. 2018;9:7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Baron S, Amar L, Faucon AL, et al. . Criteria for diagnosing primary aldosteronism on the basis of liquid chromatography-tandem mass spectrometry determinations of plasma aldosterone concentration. J Hypertens. 2018;36(7):1592-1601. [DOI] [PubMed] [Google Scholar]
- 27. Guo Z, Poglitsch M, McWhinney BC, et al. . Aldosterone LC-MS/MS assay-specific threshold values in screening and confirmatory testing for primary aldosteronism. J Clin Endocrinol Metab. 2018;103(11):3965-3973. [DOI] [PubMed] [Google Scholar]
- 28. Thuzar M, Young K, Ahmed AH. Diagnosis of primary aldosteronism by seated saline suppression test - variability between immunoassay and HPLC-MS/MS. J Clin Endocrinol Metab. 2019;105(3):e477-e483. [DOI] [PubMed] [Google Scholar]
- 29. Fries CM, Bae YJ, Rayes N, et al. . Prospective evaluation of aldosterone LC-MS/ MS-specific cutoffs for the saline infusion test. Eur J Endocrinol. 2020;183(2):191-201. [DOI] [PubMed] [Google Scholar]
- 30. Eisenhofer G, Kurlbaum M, Peitzsch M, et al. . The saline infusion test for primary aldosteronism: implications of immunoassay inaccuracy. J Clin Endocrinol Metab. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fuss CT, Brohm K, Kurlbaum M, et al. . Confirmatory testing of primary aldosteronism with saline infusion test and LC-MS/MS. Eur J Endocrinol. 2021;184(1):167-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nishikawa T, Satoh F, Takashi Y, et al. . Comparison and commutability study between standardized liquid chromatography-mass spectrometry/mass spectrometry (LC-MS/MS) and chemiluminescent enzyme immunoassay for aldosterone measurement in blood. Endocr J. 2022;69(1):45EJ21-45EJ54. [DOI] [PubMed] [Google Scholar]
- 33. Ozeki Y, Tanimura Y, Nagai S. Development of a new chemiluminescent enzyme immunoassay using a two-step sandwich method for measuring aldosterone concentrations. Diagnostics. 2021;11(3):433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yin Y, Ma C, Yu S, et al. . Comparison of three different chemiluminescence assays and a rapid liquid chromatography tandem mass spectrometry method for measuring serum aldosterone. Clin Chem Lab Med. 2019;58(1):95-102. [DOI] [PubMed] [Google Scholar]
- 35. Wright C, O’Day P, Alyamani M, Sharifi N, Auchus RJ. Abiraterone acetate treatment lowers 11-oxygenated androgens. Eur J Endocrinol. 2020;182(4):413-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Turcu AF, Wannachalee T, Tsodikov A, et al. . Comprehensive analysis of steroid biomarkers for guiding primary aldosteronism subtyping. Hypertension. 2020;75(1):183-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nanba AT, Rege J, Ren J, Auchus RJ, Rainey WE, Turcu AF. 11-Oxygenated C19 steroids do not decline with age in women. J Clin Endocrinol Metab. 2019;104(7):2615-2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Monticone S, Burrello J, Tizzani D, et al. . Prevalence and clinical manifestations of primary aldosteronism encountered in primary care practice. J Am Coll Cardiol. 2017;69(14):1811-1820. [DOI] [PubMed] [Google Scholar]
- 39. Omura M, Saito J, Yamaguchi K, Kakuta Y, Nishikawa T. Prospective study on the prevalence of secondary hypertension among hypertensive patients visiting a general outpatient clinic in Japan. Hypertens Res. 2004;27(3):193-202. [DOI] [PubMed] [Google Scholar]
- 40. Jaffe G, Gray Z, Krishnan G, et al. . Screening rates for primary aldosteronism in resistant hypertension: a cohort study. Hypertension 2020;75(3):650-659. [DOI] [PubMed] [Google Scholar]
- 41. Cohen JB, Cohen DL, Herman DS, Leppert JT, Byrd JB, Bhalla V. Testing for primary aldosteronism and mineralocorticoid receptor antagonist use among U.S. veterans. Ann Intern Med. 2021;174(3):289-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hundemer GL, Imsirovic H, Vaidya A, et al. . Screening rates for primary aldosteronism among individuals with hypertension plus hypokalemia: a population-based retrospective cohort study. Hypertension 2022;79(1):178-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sivarajah M, Beninato T, Fahey TJ. Adherence to consensus guidelines for screening of primary aldosteronism in an urban healthcare system. Surgery 2020;167(1):211-215. [DOI] [PubMed] [Google Scholar]
- 44. Ruhle BC, White MG, Alsafran S, Kaplan EL, Angelos P, Grogan RH. Keeping primary aldosteronism in mind: deficiencies in screening at-risk hypertensives. Surgery. 2019;165(1): 221-227. [DOI] [PubMed] [Google Scholar]
- 45. Vaidya A, Carey RM. Evolution of the primary aldosteronism syndrome: updating the approach. J Clin Endocrinol Metab. 2020;105(12):3771-3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Schirpenbach C, Seiler L, Maser-Gluth C, Beuschlein F, Reincke M, Bidlingmaier M. Automated chemiluminescence-immunoassay for aldosterone during dynamic testing: comparison to radioimmunoassays with and without extraction steps. Clin Chem. 2006;52(9):1749-1755. [DOI] [PubMed] [Google Scholar]
- 47. Burrello J, Monticone S, Buffolo F, et al. . Diagnostic accuracy of aldosterone and renin measurement by chemiluminescent immunoassay and radioimmunoassay in primary aldosteronism. J Hypertens. 2016;34(5):920-927. [DOI] [PubMed] [Google Scholar]
- 48. Abdelhamid S, Blomer R, Hommel G, et al. . Urinary tetrahydroaldosterone as a screening method for primary aldosteronism: a comparative study. Am J Hypertens. 2003;16(7): 522-530. [DOI] [PubMed] [Google Scholar]
- 49. Gomez-Sanchez CE, Holland O. Urinary tetrahydroaldosterone and aldosterone-18- glucuronide excretion in white and black normal subjects and hypertensive patients. J Clin Endocrinol Metab. 1981;52(2):214-219. [DOI] [PubMed] [Google Scholar]
- 50. Luetscher JA, Hancock EW, Camargo CA, Dowdy AJ, Nokes GW. Conjugation of 1,2-3 H-aldosterone in human liver and kidneys and renal extraction of aldosterone and labeled conjugates from blood plasma. J Clin Endocrinol Metab. 1965;25:628-638. [DOI] [PubMed] [Google Scholar]
- 51. Funder JW. Primary aldosteronism: three strikes and out. Hypertension. 2021;77(3):900-903. [DOI] [PubMed] [Google Scholar]
- 52. Bhasin S, Pencina M, Jasuja GK, et al. . Reference ranges for testosterone in men generated using liquid chromatography tandem mass spectrometry in a community-based sample of healthy nonobese young men in the Framingham Heart Study and applied to three geographically distinct cohorts. J Clin Endocrinol Metab. 2011;96(8):2430-2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Grassi G, Morelli V, Ceriotti F, et al. . Minding the gap between cortisol levels measured with second-generation assays and current diagnostic thresholds for the diagnosis of adrenal insufficiency: a single-center experience. Hormones. 2000;19(3):425-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kline GA, Buse J, Krause RD. Clinical implications for biochemical diagnostic thresholds of adrenal sufficiency using a highly specific cortisol immunoassay. Clin Biochem. 2017;50(9):475-480. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.