Abstract
Among other factors, food intolerance is cardinal in triggering irritable bowel syndrome (IBS) symptoms in a significant percentage of patients. As a result, specific dietary patterns are the first-line therapeutic approach. The low-FODMAP diet (LFD) is gaining ground as the most well-documented diet intervention that significantly reduces IBS symptoms. Though the LFD improves symptoms, the diet’s impact on intestinal low-grade inflammation, one of the cardinal mechanisms contributing to symptom development, remains doubtful. On the other hand, the Mediterranean diet (MedDiet) is recommended for chronic low-grade inflammation-related diseases because of its anti-inflammatory properties, derived predominantly from olive oil and phenolic compounds. Thus far, the role of a modified LFD, enriched with the MedDiet’s anti-inflammatory components, has not been evaluated in IBS patients. This review aims to examine the hypothesis of a potential combination of the immunomodulatory effects of the MedDiet with the LFD to improve IBS symptoms.
Keywords: irritable bowel syndrome, low-FODMAP diet, inflammatory bowel disease, Mediterranean diet, inflammation, gut microbiota
1. Introduction
Irritable bowel syndrome (IBS) is a highly prevalent chronic functional gastrointestinal disorder. The cardinal symptoms include abdominal pain, flatulence, bloating, and changes in bowel habits related to stool frequency and consistency, in the absence of detectable structural and biochemical abnormalities [1,2].
IBS is divided into four subtypes, depending on the predominant bowel habit alteration, according to the Rome IV criteria: IBS with constipation (IBS-C), IBS with diarrhea (IBS-D), IBS with defecation alterations in between constipation and diarrhea (IBS-M), and unclassified IBS (IBS-U). IBS symptoms may appear after an infectious disease, e.g., enteritis from viral (Norwalk), parasitic (Cryptosporidium spp. or Giardia (Giardia duodenalis or Giardia lamblia)), or bacterial (Campylobacter jejuni and Salmonella) infection (postinfectious IBS) [3,4,5]. The underlying mechanisms of postinfectious IBS (PI-IBS) pathogenesis, though not clearly understood, are potentially caused by residual inflammation or by alterations in mucosal immunocytes (B and T cells), mast cells, enterochromaffin-like cells, and the intestinal flora [4,6], as well as altered intestinal permeability (decreased expression of the tight junction-associated proteins Zonula occludens-1 (ZO-1) and α-cathenin) [7].
Gut commensal bacteria number a few trillion, with the predominant bacterial phyla Firmicutes (e.g., Lactobacillus), Actinobacteria (e.g., Bifidobacteria), Bacteroidetes (e.g., B. fragilis), and Proteobacteria (e.g., E. coli, Yersinia, Salmonella) living in a balanced relationship with viruses, fungi, archaea, protozoa, and the host. The beneficial bacteria Lactobacilli and Bifidobacteria have anti-inflammatory properties, protecting the barrier function, among other things [1]. In IBS, there is an imbalance between “good” and “bad” flora called dysbiosis, which has been associated with disruption of the mucosal enteric barrier, loss of immunotolerance, and low-grade inflammation limited to the intestine [6,8,9,10].
Low-grade mucosal inflammation represented by chronic inflammatory cells, such as mucosal enteroendocrine cells and T lymphocytes, that have been found in rectal mucosal biopsy specimens and visceral hypersensitivity are present in all IBS subtypes (including PI-IBS), providing evidence to the contemporary theory that IBS is not a functional disorder [11,12,13].
Up to 89% of IBS patients associate their intestinal distention and pain with food consumption. Additionally, almost 90% of them choose to eat less food to lessen postprandial discomfort; therefore, specific dietary patterns are used as a first-line therapeutic approach, showing beneficial effects [1,3]. Studies have shown that poorly absorbed dietary components are fermented by the intestinal bacteria, causing osmotic effects in the colon with gas production, pain, and diarrhea [14,15]. The acronym FODMAPs (fermentable oligosaccharides, disaccharides, monosaccharides, and polyols) is used by Monash University researchers to describe a family of poorly absorbed, osmotically active short-chain carbohydrates (SCCs) rapidly fermented by the gut bacteria. A low-FODMAP (LFD) diet significantly reduces IBS symptoms compared to a habitual Australian diet [16]; however, although the LFD improves IBS symptoms, its efficacy regarding the reduction in inflammation is not proven [17]. On the contrary, the well-known anti-inflammatory benefits of the Mediterranean diet (MedDiet) may occur due to olive oil as the principal source of fat, which contains at least 30 phenolic compounds [17,18]. Polyphenols have antioxidant, anti-inflammatory, and antimicrobial effects [17]. Consequently, the MedDiet is an effective dietary model for chronic low-grade inflammation-related diseases such as cardiovascular disease, atherosclerosis, metabolic syndrome, and obesity [19,20,21].
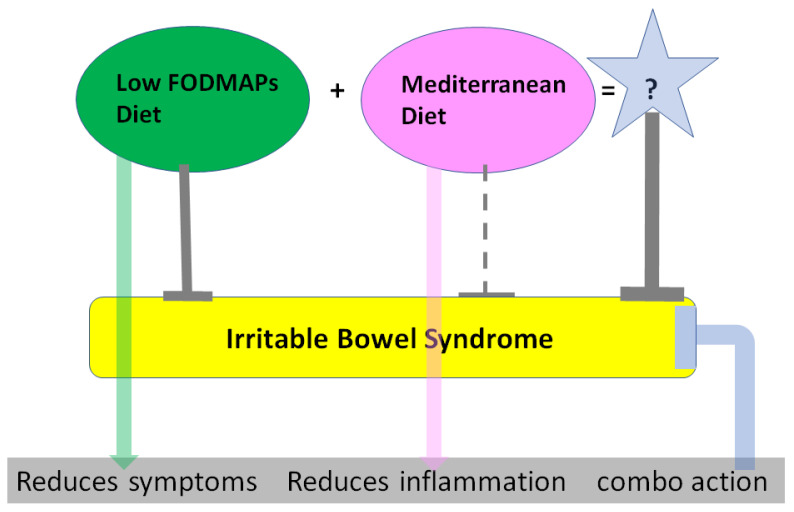
Considering the value of the LFD in improving symptoms in IBS patients, and the proven anti-inflammatory properties of the Mediterranean diet in many clinical conditions, we aimed to assess the immunomodulatory effect of the MedDiet combined with the LFD in managing IBS (Figure 1).
Figure 1.
Schematic presentation of the hypothesis of combining the Mediterranean diet with the low-FODMAP diet for the management of irritable bowel syndrome.
2. Materials and Methods
We performed a literature search in the PubMed and Cochrane databases for articles written in the English language. We used evidence from original articles written in the English language, excluding reviews, abstracts, conference presentations, pediatric studies, editorials, and study protocols. Given the well-known efficacy of the low-FODMAP diet in alleviating IBS symptoms, we used the terms “Mediterranean Diet”, “irritable bowel disease”, and “inflammation”, in order to discuss a potential extrapolation of the results of this literature search on the treatment of IBS with the combination of the two diets.
3. Results
The literature searches revealed no results. Thus, in the absence of data exploring the immunomodulatory effects of the MedDiet on IBS symptoms, we review the immunomodulatory effects of the MedDiet below, as well as the pathophysiologic correctness of combining the two diets for the management of this functional disorder.
3.1. The Effect of LFD on IBS Subtypes, Gut Microbiota, and the Immune System
The LFD focuses on limiting the intake of carbohydrates found in wheat-based products, onion, garlic, legumes, dairy, and many fruits and vegetables. These carbohydrates are incompletely absorbed in the small intestine and cause gas and water retention. Fruits and vegetables high in FODMAPs are often reduced but not replaced with low-FODMAP alternatives [22]. As a result, long-term use of the LFD, which decreases prebiotic intake, will probably have unpleasant effects on the gut microbiota [23]. Moreover, constipation is associated with low fiber intake, and the LFD further decreases fiber consumption [24]. Hence, treatment for IBS-C is targeted towards increasing fiber consumption and fiber supplements, in order to alleviate constipation, as recommended (Level II Evidence, Grade B) [1,25]. While the LFD relieves IBS-C patients’ symptoms to a lesser extent compared to other subtypes, it has been postulated that the LFD is a beneficial treatment regardless of the IBS classification. Marsh et al. indicated that the LFD clinically and significantly improved all symptoms, with IBS-C patients benefiting less [24]. Additionally, lower fiber intake correlated with decreased symptoms among IBS-D subjects, but this did not apply to the other IBS subtypes. In a recent meta-analysis, van Lanen et al. showed the efficacy of the LFD among all IBS subtypes. Patients with constipation as the predominant symptom and with a mixed stool pattern were similarly relieved and the symptoms subsided, but the results were not consistent among the studies. Furthermore, they underlined that the subgroups were small, and they concluded that more studies are needed to determine if the efficacy of the LFD is consistent among IBS subtypes [26].
Venter et al. highlighted the bidirectional relationship between diet and the immune system underlying the protective role of specific nutrients. Nutritional status may also have an effect on the gut microbiota and the intestinal mucous membrane [27]. Many reports have linked dysbiosis with IBS, referring to a significant reduction in the genera Lactobacillus and Bifidobacterium compared to healthy people [6,8,9,10]. The LFD reduces dietary prebiotics (fructans and GOS) that stimulate immunomodulatory bacteria (Bifidobacteria and Faecalibacterium prausnitzii) and interact with PRRs, protecting the intestinal barrier and epithelial cells [28] (Table 1). This effect is a limitation of the LFD given the barrier dysfunction in IBS-D and PI-IBS [29].
Table 1.
| Effect of the LFD on Gut Bacteria | |
|---|---|
| Bifidobacterium (Actinobacteria) |
|
| Bacteroides |
|
| Lactobacillus |
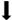
|
| Clostridium cluster XIVa |
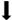
|
| Clostridium cluster IV |
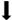
|
| Faecalibacterium prausnitzii |
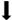
|
| Mycoplasma hominis |
|
| Mycoplasma hominis |
|
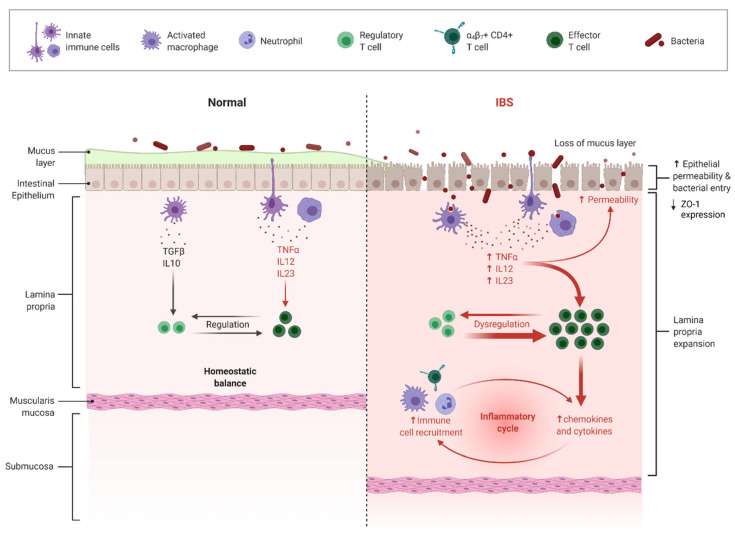
The restrictive phase of the LFD (4 weeks) led to dysbiosis in 42% of IBS patients, while 46% of them did not experience any dysbiosis [8]. A systematic review showed that increased intestinal permeability was present in 37–62% of IBS-D and 16–50% of PI-IBS patients and correlated with barrier dysfunction and diarrhea severity [31]. Intestinal permeability results in visceral hypersensitivity and reduces ZO-1 expression [32]. Endotoxins such as LPS are components of the outer membrane of Gram-negative bacteria which enter the lamina propria, inducing inflammation and increased cytokine expression. Inflammation leads to further elevation in blood endotoxins and circulating cytokines due to intestinal permeability as a result of mucosal damage [33,34] (Figure 2).
Figure 2.
Intestinal epithelium in healthy and IBS patients. Figure 2 was created using BioRender, Toronto, Canada, Subscription agreement number: TU237PZA2O for Microorganisms.
Until now, we believed that the LFD reduces the beneficial bacteria only in IBS patients with altered gut microbiota and dysbiosis. The same findings were reported in healthy volunteers, showing that the LFD decreases bacterial amounts overall and more specifically the abundance of Actinobacteria—mainly Bifidobacteria [30]. Investigators [35] suggest probiotic administration in parallel with the LFD to restore the loss of beneficial bacteria. The role of probiotics in bowel function and symptom relief in IBS patients is supported by more than 80 trials and approximately 10,000 patients [33].
Restricting fermentable substrates for saccharolytic gut bacteria reduces SCFA production. While SCFAs inhibit intestinal colonization by pathogenic bacteria, their reduction leads to a lower amount of substrate for colonocytes and an increase in microbes [25,29]. Long-term LFD consumption may induce adverse effects beyond colonocyte metabolism and micronutrient deficiencies [15,25]. Although there is not enough evidence, we may speculate that long-term LFD consumption leads to deficiencies similar to those from exclusion diets. Consumption of a gluten-free diet (GFD), for instance, excludes cereals (wheat, barley, rye) like the LFD and exposes patients at high risk for iron, folate, and zinc inadequacies. Fiber consumption in the LFD is restricted (including legumes and some fruits and vegetables). Thus, imbalances in nutritional status and symptom exacerbation are expected, particularly in IBS-C. Recently, a double-blind crossover trial was conducted in 26 patients with IBS, which were randomly assigned to one of three low-FODMAP diets differing only in the total fiber content (control 23 g/d; minimally fermented sugarcane bagasse 33 g/d; and the combination of sugarcane bagasse with resistant starch ∼45 g/d). The results showed that supplementation of fibers during the initiation of a low-FODMAP diet did not alter the symptomatic response in patients with IBS but augmented stool bulk and normalized low stool water content and slow transit, indicating the beneficial role of the combination of dietary fiber and a low-FODMAP diet in IBS symptom management [36]. Moreover, restriction of lactose-containing dairy products limits calcium intake and depletes vitamin D stores. Finally, the LFD contains low amounts of phenolic compounds, anthocyanins, and antioxidants—flavonoids, carotenoids, and vitamin C—which are components of fruits and vegetables (e.g., onions, garlic, cherries, blackberries) [37].
3.2. The Effect of MedDiet on the Immune System
The MedDiet’s characteristics include olive oil consumption, fruits, vegetables, cereals (mainly whole grain), legumes, and nuts. Fish, white meat, eggs, and fermented dairy products (cheese and yogurt) are also included but in moderate amounts. Small amounts of red meat and foods rich in sugars are permitted. Wine—preferably red—with meals is recommended in moderate quantity and frequency. Fat intake comprises 40–50% of daily calories with emphasis on monounsaturated fatty acids (MUFAs), mainly from olive oil (15–25% of calories), in contrast to saturated fatty acids (SFAs), which comprises less than 8% of the consumed daily fat. There is high intake of omega-3 fatty acids from fish and other seafood and plant sources, and the ratio of omega-6/omega-3 is low (2:1–1:1 compared to 14:1 in other European countries). In addition, high consumption of dietary fiber and antioxidant compounds may act together to produce favorable anti-inflammatory effects [19]. The MedDiet is characterized by an abundance of antioxidant/anti-inflammatory components, although their detailed descriptions are beyond the scope of this review. The predominant anti-inflammatory ingredients include omega-3 fatty acids, olive oil and phenolic compounds, and fibers.
3.2.1. Omega-3 Fatty Acids
The polyunsaturated fatty acids (PUFAs) are omega-3 (α-linolenic acid (α-LA), eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA)) and omega-6 PUFAs (linoleic acid (LA), arachidonic acid (AA)) [38]. Western-type diets are rich in omega-6 PUFAs in comparison with omega-3 PUFAs, with the consumption ratio reaching 20–30:1. Omega-3 PUFAs and their bioactive metabolites compete with omega-6 PUFAs to promote the resolution of inflammation [39,40]. EPA and DHA inhibit the activation of the transcription factor NF-κB (Nuclear Factor–B) in macrophages. They can impede leukocyte chemotaxis, adhesion molecule expression, and leukocyte–endothelial adhesive interactions. Eventually, they prevent eicosanoid production, inflammatory cytokine secretion (Tumor Necrosis Factor-alpha (TNF-a) and interleukin (IL)-6), T-cell reactivity, and phagocytosis [41,42].
3.2.2. Olive Oil and Phenolic Compounds
Virgin olive oil (VOO) daily intake, the primary source of fat in the MedDiet, ranges between 25 mL and 50 mL. It is high in MUFAs, with the dominant lipid, oleic acid, found in proportions up to 83% [43]. The Western diet has been associated with high CRP, IL-6, vascular cell adhesion molecule-1 (VCAM-1), and Intercellular Adhesion Molecule-1 (ICAM-1) levels. Endothelial cells are activated in response to the inflammatory process to produce cytokines and adhesion molecules. Many studies have shown that VOO decreases plasma concentrations of IL-6 and CRP and suppresses the expression of VCAM-1 and ICAM-1. The ATTICA study showed that adherence to the MedDiet was associated with a reduction of 20% in CRP and 17% in IL-6 levels. Similar results were reached by the Nurses’ Health Study, indicating that high adherence to the MedDiet was associated with lower concentrations of inflammatory biomarkers (CRP, IL6) [19]. The VOO benefits are related to the components in the unsaponifiable fraction (about 2%), including phenolic compounds (absent in oils derived from seeds or fruits), phytosterols, and tocopherols. Phenolic concentrations in extra-VOO (EVOO) consist of various components (phenolic acids, lignans, flavones, flavone glycosides, phenolic alcohols, and secoiridoids) [44]. Over 90% of phenolic compounds constitute secoiridoids (oleuropein, oleocanthal, and ligstroside aglycone). Their alcohol derivatives, hydroxytyrosol (HT) and tyrosol, and flavonoids and lignans are considered potential agents against inflammation and exhibit inhibitory action on NF-κB [45]. EVOO interferes with arachidonic acid that represents the beginning of the inflammatory response. The first step is prostaglandin production mediated by cyclooxygenase (COX). Simultaneously, inflammatory cytokines induce NF-κB expression and NLRP3 inflammasome assembly. EVOO decreases COX concentrations and inflammatory cytokines and impairs the NF-κB pathway [43,46]. Molecular mechanisms of polyphenol anti-inflammatory properties further include inhibition of COX-2, Lipoxygenase (LOX), inducible Nitric oxide synthase (iNOS), and the activating protein-1 (AP-1), and activation of phase II antioxidant enzymes, mitogen-activated protein kinase (MAPK), protein kinase-C (PKC), and nuclear factor erythroid 2-related factor (NRF2) [47]. Furthermore, HT inhibits the activation of the TLR-4 and NK-kB pathways, which are associated with intestinal permeability [44]. Aromatic plants, spices, seeds, and nuts rich in polyphenols are abundant in the MedDiet. Polyphenols are divided into two subgroups, flavonoids and non-flavonoids [44]. Flavonoids have a protective role against cancer, gastrointestinal disorders, and other diseases [48]. In the group of phenolic compounds, there are, beyond HT, two main components: resveratrol (RSV) and quercetin (QUE) [44,49]. QUE inhibits the activation of the NF-κB pathway induced by IL-1β. In addition, it impedes TNF-induced IFN-γ-inducible protein ten and macrophage inflammatory protein two gene expression in the murine small intestinal epithelial cell line Mode-K [48]. RSV is a natural antioxidant with anti-inflammatory potency. RSV and its metabolites and conjugated products inhibit NF-κB and eicosanoid production, may block TLR4, proinflammatory genes, and IL-17, and activate NRF2. The effects of RSV on the gut microbiota have been studied in mouse models. RSV supplements decreased the Firmicutes/Bacteroidetes ratio, avoiding Enterococcus faecalis growth and achieving Lactobacillus and Bifidobacterium proliferation [44].
3.2.3. Fiber
Fiber and carbohydrates are the energy source for gut bacteria, and their fermentation produces short-chain fatty acids (SCFAs). SCFAs lead to an increase in Bifidobacteria, Bacteroidetes, and Akkermansia muciniphila, with positive effects on barrier integrity, motility, and alleviation of inflammation [40]. Fiber intake in the MedDiet is two times higher than in a usual Western diet [40]. Fiber is divided into soluble and insoluble forms. Insoluble fiber captures water in the stool and increases gut peristalsis and stool bulk. It reduces the time of colonic fermentation, contributes to microbial diversity, and prevents inflammation. Soluble fibers are prebiotics with anti-inflammatory potential (fructooligosaccharides (FOS), galactooligosaccharides (GOS)) [44]. β-Glucans are the principal soluble fibers found in abundance in oat grain (free of FODMAPs) and barley or wheat. Numerous studies have shown that β-glucans can significantly stimulate several types of immune responses [44]. Recently, Zyla et al. showed that β-glucans reduce the gene expression of proinflammatory cytokines in the colon in rat models with trinitrobenzene sulfonic acid (TNBS)-induced CD [50].
4. Conclusions
To the best of our knowledge, there is no study that has examined the effects of the MedDiet—full of fruits, vegetables, and legumes, and therefore rich in FODMAPs—on IBS patients, thus far. IBS is characterized by low-grade inflammation restricted to the bowels, and the LFD alleviates IBS symptoms, but it is ineffective in inflammatory response management. On the contrary, the MedDiet has anti-inflammatory and immunomodulating properties and increases the abundance of bacterial species in the intestine that are beneficial for the host. Thus, the MedDiet is not only considered a prudent dietary pattern, but also a scientifically proven method that is able to provide benefits for the management of several diseases and an overall improvement in health, while the LFD might be considered a possibility to identify, if present, some intolerances. Recently, the beneficial effect of supplementing the LFD with either dietary fiber or probiotics has been reported, limiting constipation and dysbiosis, respectively; thus, similar effects might be detected with adequate consumption of allowed fruits and vegetables rich in prebiotics, abundant in the MedDiet (Table 2).
Table 2.
Common foods in the MedDiet, free of FODMAPs according to the Monash University application.
| Food | Components | Action |
|---|---|---|
| Oat | β-Glucans (fibers) | prebiotic action [51] |
| Olives/olive oil | Hydroxy-tyrosol (phenolic compound) |
anti-inflammatory action [44] |
| Walnuts | ω-3 PUFAs | prebiotic action [52] |
| Fish | ω-3 PUFAs | anti-inflammatory action [53] |
| Wine | Resveratrol (phenolic compound) |
anti-inflammatory action [54] |
| Orange | Quercetin (phenolic compound) |
prebiotic action [55] anti-inflammatory action [56] |
| Mandarin (Imperial, clementine) |
Quercetin (phenolic compound) |
anti-inflammatory action [57] |
| Tomatoes | Quercetin (phenolic compound) |
prebiotic action [58] |
| Oregano, rosemary, thyme, and cumin | Phenolic compounds | anti-inflammatory action [59] |
The novelty of this review is the concept of a new dietary pattern with the synergistic action of the LFD and MedDiet as a dietary treatment for IBS. We speculate that enriching the LFD with the MedDiet’s components with anti-inflammatory and prebiotic actions may potently optimize the effects of the LFD and eliminate its drawbacks.
Prebiotics promote the growth of beneficial bacteria and the development of intestinal epithelial cells while reshaping the gut microbiota. Intake of VOO, fish, and specific fruits and vegetables and moderate wine consumption according to the MedDiet pyramid, as well as limited intake of red meat, proceed foods (i.e., bacon, sausages), and refined oils, in all phases of the LFD could be crucial to reduce the inflammatory status.
The limitation of this new concept is the lack of evidence from clinical trials to support it. However, there is evidence that the MedDiet is beneficial for the symptom management of patients with quiescent (absence of laboratory and endoscopic findings of inflammation) IBD—the so-called IBS-like syndrome [60]. Moreover, the concept of a new dietary pattern with the synergistic action of the LFD and MedDiet as a dietary intervention for IBS is currently under evaluation in the ClinicalTrials.gov Identifier: NCT03997708 study, where we compare the efficacy of a combination of the MedDiet and the LFD and the nutritional recommendations of NICE in managing IBS. Additionally, the collected fecal samples will be used to assess the effect of each intervention on the gut microbiota, which might shed light on the underlying mechanisms of the additive or synergistic effect of the two dietary interventions.
Abbreviations
| 5-HT | 5-Hydroxytryptamine |
| α-LA | α-Linolenic Acid |
| AA | Arachidonic Acid |
| AP-1 | Activating Protein-1 |
| CD | Crohn Disease |
| COX | Cyclooxygenase |
| CRH | Corticotropin-Releasing Hormone |
| CRP | C-Reactive Protein |
| DHA | Docosahexaenoic Acid |
| EFSA | European Food Safety Authority |
| EPA | Eicosapentaenoic Acid |
| EVOO | Extra Virgin Olive Oil |
| FCP | Fecal Calprotectin |
| FOS | Fructooligosaccharides |
| GFD | Gluten-Free Diet |
| GI | Gastrointestinal |
| GLUT-2 | Glucose Transporter 2 |
| GLUT-5 | Glucose Transporter 5 |
| GOS | Galactooligosaccharides |
| HPA | Hypothalamic–Pituitary–Adrenal |
| HT | Hydroxytyrosol |
| IBD | Inflammatory Bowel Disease |
| IBS | Irritable Bowel Syndrome |
| IBDQ | Inflammatory Bowel Disease Questionnaire |
| ICAM-1 | Intercellular Adhesion Molecule-1 |
| IL-6 | Interleukin 6 |
| iNOS | Inducible Nitric Oxide Synthase |
| LA | Linolenic Acid |
| LFD | Low-FODMAP Diet |
| LOX | Lipoxygenase |
| MAPK | Mitogen-Activated Protein Kinase |
| MedDiet | Mediterranean Diet |
| MUFAs | Monounsaturated Fatty Acids |
| NF-κΒ | Nuclear Factor–B |
| NRF2 | Nuclear Factor Erythroid 2-Related Factor |
| PI–IBS | Postinfectious Irritable Bowel Syndrome |
| PKC | Protein Kinase-C |
| PPRs | Pattern Recognition Receptors |
| PUFAs | Polyunsaturated Fatty Acids |
| QoL | Quality of Life |
| QUE | Quercetin |
| RCTs | Randomized Controlled Trials |
| RSV | Resveratrol |
| SCFAs | Short-Chain Fatty Acids |
| SFAs | Saturated Fatty Acids |
| SIBDQ | Short IBD Quality of Life Questionnaire |
| SIBO | Small Intestine Bacterial Overgrowth |
| TLRs | Toll-Like Receptors |
| TNBS | 2,4,6-Trinitrobenzene Sulfonic Acid |
| TNF-a | Tumor Necrosis Factor-Alpha |
| UC | Ulcerative Colitis |
| VCAM-1 | Vascular Cell Adhesion Molecule-1 |
| VOO | Virgin Olive Oil |
| ZO-1 | Zonula Occludens-1 |
Author Contributions
A.K. conceived the presented idea and wrote the manuscript. S.L. and K.P. equally contributed to the search strategy. K.K., E.H. and I.S.P. contributed to the analysis and interpretation of the results. A.K. and M.N. designed the figures. K.T. supervised the work. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hadjivasilis A., Tsioutis C., Michalinos A., Ntourakis D., Christodoulou D.K., Agouridis A.P. New Insights into Irritable Bowel Syndrome: From Pathophysiology to Treatment. Ann. Gastroenterol. 2019;32:554–564. doi: 10.20524/aog.2019.0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saha L. Irritable bowel syndrome: Pathogenesis, diagnosis, treatment, and evidence-based medicine. World J. Gastroenterol. 2014;20:6759–6773. doi: 10.3748/wjg.v20.i22.6759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adriani A., Ribaldone D.G., Astegiano M., Durazzo M., Saracco G.M., Pellicano R. Irritable bowel syndrome: The clinical approach. Panminerva Med. 2018;60:213–222. doi: 10.23736/S0031-0808.18.03541-3. [DOI] [PubMed] [Google Scholar]
- 4.Radovanovic-Dinic B., Tesic-Rajkovic S., Grgov S., Petrovic G., Zivković V. Irritable bowel syndrome—From etiopathogenesis to therapy. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2018;162:1–9. doi: 10.5507/bp.2017.057. [DOI] [PubMed] [Google Scholar]
- 5.Lacy B.E., Patel N.K. Rome criteria and a diagnostic approach to irritable bowel syndrome. [(accessed on 20 February 2022)];J. Clin. Med. 2017 6:99. doi: 10.3390/jcm6110099. Available online: http://www.ncbi.nlm.nih.gov/pubmed/29072609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang L., Alammar N., Singh R., Nanavati J., Song Y., Chaudhary R. Gut Microbial Dysbiosis in the Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis of Case-Control Studies. J. Acad. Nutr. Diet. 2020;120:565–586. doi: 10.1016/j.jand.2019.05.015. [DOI] [PubMed] [Google Scholar]
- 7.Vandeputte D., Joossens M. Effects of Low and High FODMAP Diets on Human Gastrointestinal Microbiota Composition in Adults with Intestinal Diseases: A Systematic Review. Microorganisms. 2020;8:1638. doi: 10.3390/microorganisms8111638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yan R., Andrew L., Marlow E., Kunaratnam K., Devine A., Dunican I., Christophersen C. Dietary Fibre Intervention for Gut Microbiota, Sleep, and Mental Health in Adults with Irritable Bowel Syndrome: A Scoping Review. Nutrients. 2021;13:2159. doi: 10.3390/nu13072159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rajilić-Stojanović M., Jonkers D.M., Salonen A., Hanevik K., Raes J., Jalanka J., de Vos W.M., Manichanh C., Golic N., Enck P., et al. Intestinal Microbiota and Diet in IBS: Causes, Consequences, or Epiphenomena? Am. J. Gastroenterol. 2015;110:278–287. doi: 10.1038/ajg.2014.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chumpitazi B.P., Hollister E.B., Oezguen N., Tsai C.M., McMeans A.R., Luna R.A., Savidge T.C., Versalovic J., Shulman R.J. Gut microbiota influences low fermentable substrate diet efficacy in children with irritable bowel syndrome. Gut Microbes. 2014;5:165–175. doi: 10.4161/gmic.27923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spiller R.C., Jenkins D., Thornley J.P., Hebden J.M., Wright T., Skinner M., Neal K.R. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut. 2000;47:804–811. doi: 10.1136/gut.47.6.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gwee K.A., Leong Y.L., Graham C., McKendrick M.W., Collins S.M., Walters S.J. The role of psychological and biological factors in postinfective gut dysfunction. Gut. 1999;44:400–406. doi: 10.1136/gut.44.3.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vannucchi M.G., Evangelista S. Experimental Models of Irritable Bowel Syndrome and the Role of the Enteric Neurotransmission. J. Clin. Med. 2018;3:4. doi: 10.3390/jcm7010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barrett J.S., Gearry R.B., Muir J.G., Irving P.M., Rose R., Rosella O. Dietary poorly absorbed, short-chain carbohydrates increase delivery of water and fermentable substrates to the proximal colon. Aliment. Pharmacol. Ther. 2010;31:874–882. doi: 10.1111/j.1365-2036.2010.04237.x. [DOI] [PubMed] [Google Scholar]
- 15.Altobelli E., Del Negro V., Angeletti P.M., Latella G. Low-FODMAP Diet Improves Irritable Bowel Syndrome Symptoms: A Meta-Analysis. Nutrients. 2017;9:940. doi: 10.3390/nu9090940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Basnayake C. Treatment of irritable bowel syndrome. Aust. Prescr. 2018;41:145–149. doi: 10.18773/austprescr.2018.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weber A.T., Shah N.D., Sauk J., Limketkai B.N. Popular Diet Trends for Inflammatory Bowel Diseases: Claims and Evidence. Curr. Treat. Options Gastroenterol. 2019;17:564–576. doi: 10.1007/s11938-019-00248-z. [DOI] [PubMed] [Google Scholar]
- 18.Tuck K.L., Hayball P.J. Major phenolic compounds in olive oil: Metabolism and health effects. J. Nutr. Biochem. 2002;13:636–644. doi: 10.1016/S0955-2863(02)00229-2. [DOI] [PubMed] [Google Scholar]
- 19.Casas R., Sacanella E., Estruch R. The immune protective effect of the Mediterranean diet against chronic low-grade inflammatory diseases. Endocr. Metab. Immune. Disord. Drug Targets. 2014;14:245–254. doi: 10.2174/1871530314666140922153350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Minelli P., Montinari M.R. The Mediterranean Diet and Cardioprotection: Historical Overview and Current Research. J. Multidiscip. Healthc. 2019;12:805–815. doi: 10.2147/JMDH.S219875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nani A., Murtaza B., Khan A.S., Khan N., Hichami A. Antioxidant and Anti-Inflammatory Potential of Polyphenols Contained in Mediterranean Diet in Obesity: Molecular Mechanisms. Molecules. 2021;26:985. doi: 10.3390/molecules26040985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bellini M., Tonarelli S., Nagy A.G., Pancetti A., Costa F., Ricchiuti A., de Bortoli N., Mosca M., Marchi S., Rossi A. Low FODMAP Diet: Evidence, Doubts, and Hopes. Nutrients. 2020;12:148. doi: 10.3390/nu12010148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nybacka S., Störsrud S., Lindqvist H.M., Törnblom H., Simrén M., Winkvist A. Habitual FODMAP Intake in Relation to Symptom Severity and Pattern in Patients with Irritable Bowel Syndrome. Nutrients. 2020;13:27. doi: 10.3390/nu13010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marsh A., Eslick E.M., Eslick G.D. Does a diet low in FODMAPs reduce symptoms associated with functional gastrointestinal disorders? A comprehensive systematic review and meta-analysis. Eur. J. Nutr. 2016;55:897–906. doi: 10.1007/s00394-015-0922-1. [DOI] [PubMed] [Google Scholar]
- 25.Rao S.S.C., Yu S., Fedewa A. Systematic review: Dietary fibre and FODMAP-restricted diet in the management of constipation and irritable bowel syndrome. Aliment. Pharmacol. Ther. 2015;41:1256–1270. doi: 10.1111/apt.13167. [DOI] [PubMed] [Google Scholar]
- 26.Van Lanen A.-S., de Bree A., Greyling A. Efficacy of a low-FODMAP diet in adult irritable bowel syndrome: A systematic review and meta-analysis. Eur. J. Nutr. 2021;60:3505–3522. doi: 10.1007/s00394-021-02620-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Venter C., Eyerich S., Sarin T., Klatt K.C. Nutrition and the Immune System: A Complicated Tango. Nutrients. 2020;12:818. doi: 10.3390/nu12030818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cox S.R., Prince A.C., Myers C.E., Irving P.M., Lindsay J.O., Lomer M.C. Fermentable Carbohydrates [FODMAPs] Exacerbate Functional Gastrointestinal Symptoms in Patients with Inflammatory Bowel Disease: A Randomised, Double-blind, Placebo-controlled, Cross-over, Re-challenge Trial. J. Crohn’s Colitis. 2017;11:1420–1429. doi: 10.1093/ecco-jcc/jjx073. [DOI] [PubMed] [Google Scholar]
- 29.Wilson B., Cox S.R., Whelan K. Challenges of the low FODMAP diet for managing irritable bowel syndrome and approaches to their minimisation and mitigation. Proc. Nutr. Soc. 2021;80:19–28. doi: 10.1017/S0029665120006990. [DOI] [PubMed] [Google Scholar]
- 30.Sloan T.J., Jalanka J., Major G.A.D., Krishnasamy S., Pritchard S., Abdelrazig S. A low FODMAP diet is associated with changes in the microbiota and reduction in breath hydrogen but not colonic volume in healthy subjects. PLoS ONE. 2018;13:e0201410. doi: 10.1371/journal.pone.0201410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hanning N., Edwinson A.L., Ceuleers H., Peters S.A., De Man J.G., Hassett L.C., De Winter B.Y., Grover M. Intestinal barrier dysfunction in irritable bowel syndrome: A systematic review. Ther. Adv. Gastroenterol. 2021;14:1756284821993586. doi: 10.1177/1756284821993586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scuderi S.A., Casili G., Lanza M., Filippone A., Paterniti I., Esposito E., Campolo M. Modulation of NLRP3 Inflammasome Attenuated Inflammatory Response Associated to Diarrhea-Predominant Irritable Bowel Syndrome. Biomedicines. 2020;8:519. doi: 10.3390/biomedicines8110519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raskov H., Burcharth J., Pommergaard H.-C., Rosenberg J. Irritable bowel syndrome, the microbiota and the gut-brain axis. Gut Microbes. 2016;7:365–383. doi: 10.1080/19490976.2016.1218585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou S.-Y., Gillilland M., Wu X., Leelasinjaroen P., Zhang G., Zhou H., Ye B., Lu Y., Owyang C. FODMAP diet modulates visceral nociception by lipopolysaccharide-mediated intestinal inflammation and barrier dysfunction. J. Clin. Investig. 2017;128:267–280. doi: 10.1172/JCI92390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Staudacher H.M., Scholz M., Lomer M.C., Ralph F.S., Irving P.M., Lindsay J.O., Fava F., Tuohy K., Whelan K. Gut microbiota associations with diet in irritable bowel syndrome and the effect of low FODMAP diet and probiotics. Clin. Nutr. 2021;40:1861–1870. doi: 10.1016/j.clnu.2020.10.013. [DOI] [PubMed] [Google Scholar]
- 36.So D., Yao C.K., Ardalan Z.S., Thwaites P.A., Kalantar-Zadeh K., Gibson P.R., Muir J.G. Supplementing Dietary Fibers with a Low-FODMAP Diet in Irritable Bowel Syndrome: A Randomized Controlled Crossover Trial. [(accessed on 20 February 2022)];Clin. Gastroenterol. Hepatol. 2021 doi: 10.1016/j.cgh.2021.12.016. Available online: http://www.ncbi.nlm.nih.gov/pubmed/34929392. [DOI] [PubMed] [Google Scholar]
- 37.Catassi G., Lionetti E., Gatti S., Catassi C. The Low FODMAP Diet: Many Question Marks for a Catchy Acronym. Nutrients. 2017;9:292. doi: 10.3390/nu9030292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mori T.A. Marine OMEGA-3 fatty acids in the prevention of cardiovascular disease. Fitoterapia. 2017;123:51–58. doi: 10.1016/j.fitote.2017.09.015. [DOI] [PubMed] [Google Scholar]
- 39.Li X., Bi X., Wang S., Zhang Z., Li F., Zhao A.Z. Therapeutic Potential of ω-3 Polyunsaturated Fatty Acids in Human Autoimmune Diseases. Front. Immunol. 2019;10:2241. doi: 10.3389/fimmu.2019.02241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barrea L., Caprio M., Tuccinardi D., Moriconi E., Di Renzo L., Muscogiuri G. Could ketogenic diet “starve” cancer? Emerging evidence. Crit. Rev. Food Sci. Nutr. 2022;62:1800–1821. doi: 10.1080/10408398.2020.1847030. [DOI] [PubMed] [Google Scholar]
- 41.Calder P.C. n-3 fatty acids, inflammation and immunity: New mechanisms to explain old actions. Proc. Nutr. Soc. 2013;72:326–336. doi: 10.1017/S0029665113001031. [DOI] [PubMed] [Google Scholar]
- 42.Gutiérrez S., Svahn S.L., Johansson M.E. Effects of Omega-3 Fatty Acids on Immune Cells. Int. J. Mol. Sci. 2019;20:5028. doi: 10.3390/ijms20205028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cariello M., Contursi A., Gadaleta R.M., Piccinin E., De Santis S., Piglionica M., Spaziante A.F., Sabbà C., Villani G., Moschetta A. Extra-Virgin Olive Oil from Apulian Cultivars and Intestinal Inflammation. Nutrients. 2020;12:1084. doi: 10.3390/nu12041084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.García-Montero C., Fraile-Martínez O., Gómez-Lahoz A.M., Pekarek L., Castellanos A.J., Noguerales-Fraguas F. Nutritional Components in Western Diet Versus Mediterranean Diet at the Gut Microbiota-Immune System Interplay. Implications for Health and Disease. Nutrients. 2021;13:699. doi: 10.3390/nu13020699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lucas L., Russell A., Keast R. Molecular Mechanisms of Inflammation. Anti-Inflammatory Benefits of Virgin Olive Oil and the Phenolic Compound Oleocanthal. Curr. Pharm. Des. 2011;17:754–768. doi: 10.2174/138161211795428911. [DOI] [PubMed] [Google Scholar]
- 46.Taticchi A., Urbani S., Albi E., Servili M., Codini M., Traina G. In Vitro Anti-Inflammatory Effects of Phenolic Compounds from Moraiolo Virgin Olive Oil (MVOO) in Brain Cells via Regulating the TLR4/NLRP3 Axis. Molecules. 2019;24:4523. doi: 10.3390/molecules24244523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hussain T., Tan B., Yin Y., Blachier F., Tossou M.C., Rahu N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell. Longev. 2016;2016:7432797. doi: 10.1155/2016/7432797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.González-Gallego J., García-Mediavilla M.V., Sánchez-Campos S., Tuñón M.J. Fruit polyphenols, immunity and inflammation. Br. J. Nutr. 2010;104((Suppl. S104)):S15–S27. doi: 10.1017/S0007114510003910. [DOI] [PubMed] [Google Scholar]
- 49.Nanayakkara W.S., Skidmore P.M., O’Brien L., Wilkinson T.J., Gearry R.B. Efficacy of the low FODMAP diet for treating irritable bowel syndrome: The evidence to date. Clin. Exp. Gastroenterol. 2016;9:131–142. doi: 10.2147/CEG.S86798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Żyła E., Dziendzikowska K., Kamola D., Wilczak J., Sapierzyński R., Harasym J. Anti-Inflammatory Activity of Oat Beta-Glucans in a Crohn’s Disease Model: Time- and Molar Mass-Dependent Effects. Int. J. Mol. Sci. 2021;22:4485. doi: 10.3390/ijms22094485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kristek A., Wiese M., Heuer P., Kosik O., Schär M.Y., Soycan G. Oat bran, but not its isolated bioactive β-glucans or polyphenols, have a bifidogenic effect in an in vitro fermentation model of the gut microbiota. Br. J. Nutr. 2019;121:549–559. doi: 10.1017/S0007114518003501. [DOI] [PubMed] [Google Scholar]
- 52.Bamberger C., Rossmeier A., Lechner K., Wu L., Waldmann E., Fischer S., Stark R.G., Altenhofer J., Henze K., Parhofer K.G. A Walnut-Enriched Diet Affects Gut Microbiome in Healthy Caucasian Subjects: A Randomized, Controlled Trial. Nutrients. 2018;10:244. doi: 10.3390/nu10020244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wall R., Ross R.P., Fitzgerald G.F., Stanton C. Fatty acids from fish: The anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr. Rev. 2010;68:280–289. doi: 10.1111/j.1753-4887.2010.00287.x. [DOI] [PubMed] [Google Scholar]
- 54.Chalons P., Amor S., Courtaut F., Cantos-Villar E., Richard T., Auger C., Chabert P., Schni-Kerth V., Aires V., Delmas D. Study of Potential Anti-Inflammatory Effects of Red Wine Extract and Resveratrol through a Modulation of Interleukin-1-Beta in Macrophages. Nutrients. 2018;10:1856. doi: 10.3390/nu10121856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Costabile A., Walton G.E., Tzortzis G., Vulevic J., Charalampopoulos D., Gibson G.R. Effects of Orange Juice Formulation on Prebiotic Functionality Using an In Vitro Colonic Model System. PLoS ONE. 2015;10:e0121955. doi: 10.1371/journal.pone.0121955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Coelho R.C.L.A., Hermsdorff H.H.M., Bressan J. Anti-inflammatory Properties of Orange Juice: Possible Favorable Molecular and Metabolic Effects. Mater. Veg. 2013;68:1–10. doi: 10.1007/s11130-013-0343-3. [DOI] [PubMed] [Google Scholar]
- 57.Lv X., Zhao S., Ning Z., Zeng H., Shu Y., Tao O., Xiao C., Lu C., Liu Y. Citrus fruits as a treasure trove of active natural metabolites that potentially provide benefits for human health. Chem. Cent. J. 2015;9:1–14. doi: 10.1186/s13065-015-0145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.García-Alonso F.J., González-Barrio R., Martín-Pozuelo G., Hidalgo N., Navarro-González I., Masuero D., Soini E., Vrhovsek U., Periago M.J. A study of the prebiotic-like effects of tomato juice consumption in rats with diet-induced non-alcoholic fatty liver disease (NAFLD) Food Funct. 2017;8:3542–3552. doi: 10.1039/C7FO00393E. [DOI] [PubMed] [Google Scholar]
- 59.Gutiérrez-Grijalva E.P., Picos-Salas M.A., Leyva-López N., Criollo-Mendoza M.S., Vazquez-Olivo G., Heredia J.B. Flavonoids and Phenolic Acids from Oregano: Occurrence, Biological Activity and Health Benefits. Plants. 2017;7:2. doi: 10.3390/plants7010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Van Lingen E. A Mediterranean Diet-Based Lifestyle Intervention in Inflammatory Bowel Disease Patients with Quiescent Disease; A Pilot Study. Am. J. Biomed. Sci. Res. 2021;13:47–53. doi: 10.34297/AJBSR.2021.13.001830. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.