Abstract
Chlorpyrifos (CPF) is one of the widely used organophosphorus pesticides in agriculture activities and its presence in the aquatic environment has been broadly recorded. In the present study, we investigated the effect of CPF exposure on oxidative stress, innate immunity, sexual hormones, and DNA integrity of female African catfish, Clarias gariepinus, in addition to the potential use of dietary supplementation of papaya, Carica papaya (CP), extract against CPF toxicity. Apparent healthy female catfish (300 ± 10 g) were divided into four groups with three replicates each. The first group served as the negative control (fed on a basal diet) and the other groups exposed to CPF (8.75 µg/L) with or without CP extract (250 mg/kg body weight) for six weeks. The results revealed that CPF exposure exhibited marked elevations in stress markers (glucose and cortisol), serum aspartate aminotransferase, alanine aminotransferase activities, testosterone, and luteinizing hormone level. Moreover, CPF increased the percentage of hepatic DNA damage. In addition, catfish exposed to CPF experienced significant decline in serum total protein, albumin, follicles stimulating hormone, estradiol hormone levels, AChE, immunoglobulin, and lysozyme activity. CPF induced significantly oxidative stress in hepatic and renal tissues. The dietary supplementation with CP extract at a level of 250 mg/kg body weight succeeded to alleviate the negative effects of CPF on the physiological, immunological, and antioxidant status of female catfish. In addition, CP extract alleviated the endocrine disruption and hepatic DNA damage and counteracted the subchronic CPF toxicity in female African catfish. Finally, the CP extract may be used as a feed additive in the aquatic diet.
Keywords: Clarias gariepinus, chlorpyrifos, Carica papaya, oxidative stress, DNA fragmentation, Reproductive hormones, immunity
1. Introduction
Chlorpyrifos (CPF; O, O-diethyl O-(3,5,6-trichloro-2-pyridyl-phosphorothioate)) is a broad-spectrum organophosphorus pesticide used extensively for the management of domestic and agricultural pests [1]. Its release into the aquatic environment results in toxicity to non-target aquatic species [2]. Therefore, researchers have focused on the toxicity of the CPF on aquatic organisms and their potential adverse effects on the aquatic community [3,4,5]. CPF causes hepatic, neurobehavioral, reproductive, and endocrine dysfunction in fish [6,7,8,9]. It can also lead to oxidative stress [10,11] and genotoxicity in fish [12,13]. In addition, Jiao et al. [14] and Zhang et al. [11] found that common carp exposed to CPF impaired immune functions and caused respiratory disfunction. Xing et al. [3] reported a reduction in acetylcholinesterase and carboxylesterase activities in the brain and muscle of common carp due to CPF exposure.
In the past few years, much more interest has been paid to the use of medicinal plants as feed supplements worldwide to reduce the toxicity induced by different chemicals, heavy metals, drugs, and pesticides [15,16,17,18,19,20,21,22]. Carica papaya (family: Caricaceae) is popularly known as pawpaw and papaya [23]. Papaya is cultivated widely all over the world, particularly in tropical countries such as Malaysia, the West Indies, and Africa [24,25]. C. papaya is known to have antioxidant and immune-modulating activities and is traditionally utilized for medicinal purposes [26]. The papaya fruit has a delicious taste and is a good source of carotene, vitamin C, vitamin B, flavonoids, folate, pantothenic acid, potassium, and magnesium [27,28]. Additionally, Li et al. [29] reported that the active constituents of papaya including chymopapain and papain are utilized to treat joint pain and digestive issues. Extracts of ripe fruits are additionally utilized for an assortment of medicinal purposes, for example, medications for treating ringworm, intestinal sickness, and hypertension. Furthermore, papaya includes a range of phytochemicals such as natural phenols [30] and polyphenols which prevent biomolecules oxidation through their free radical scavenging capabilities [31] or by increasing the activity of endogenous antioxidant enzymes [32].
African catfish, Clarias gariepinus, is a cosmopolitan ‘big’ Clarias species with wide distribution in Africa and several temperate regions around the world. Its high demand in aquaculture derives not only in growing to a big size but also from being hardy to withstand stress [33]. Thus, C. gariepinus is considered a suitable model for toxicological studies [34,35,36,37]. To the best of our knowledge, most of the toxicity studies have used male catfish as experimental models except few studies which revealed degenerative changes in the ovary including ooplasm vacuolization, follicular lining damage, and deshaped oocytes [38]. In addition, lead and mercury exposure induced development impairments in the ovaries and degenerative vitologenic oocytes [39] and significantly increased the percentage of germinal vesicles breakdown [40]. Therefore, the present study was designed to evaluate the toxicity of CPF on sexual hormones level, redux status, physiological and immunological status, and DNA integrity of female catfish, C. gariepinus. In addition, determining the palliative effect of C. papaya extract against the toxicity of CPF was investigated.
2. Materials and Methods
2.1. Plant Authentication, Aqueous Extraction, and Bioactive Components of the Extract
Mature fresh papaya, C. papaya (CP), fruit was obtained during the winter of 2020 from the Ministry of Agriculture and Land Reclamation, Cairo, Egypt. The fruits were identified in the Horticulture Department, Faculty of Agriculture, Ain Shams University, and the voucher was kept in the herbarium of the same department. The fruit was peeled and the cream-hued seeds were discarded. The aqueous extract was obtained as follows: a total of 1000 g of the fruit was mixed well with 1000 mL of physiological saline (sodium chloride 0.9%) and incubated at room temperature for 72 h [41,42]. The extract was then sieved into a clean container and stored in a refrigerator until use. The used concentration of CP aqueous extract was 250 mg/kg body weight as dietary supplementation according to Abouzed et al. [43]. The identification of bioactive components of the CP extract was conducted by gas chromatography-mass spectrometry (GC-MS). The most abundant identified phenolic compounds in CP extract were 16.0% caffeic acid and 13.0% flavonoid quercetin-3-O-rutinoside. In addition, several phytochemicals were recorded such as 1.6% ferulic acid, 5.0% rutin, 0.8% gallic, and 0.6% protocatechuic acids conjugates. The other phytocomponents found in CP in terms of their relative abundance were tetradecanoic acid, octadecanoic acid, hexadecanoic acid, and methyl ester.
2.2. Fish Maintenance
A total of 350 aberrant healthy female catfish, C. gariepinus (average weight 300 ± 10 g, length 40 ± 3 cm), were obtained from Abassa fish farm, Sharqia governorate, Egypt. Fish were transferred to the laboratory in 100 L well-aerated fiberglass tanks. Fish were treated with (0.5% w/v) potassium permanganate solution for 1 min. to remove dermal adherents. The fish were acclimatized for 2 weeks in identical glass aquaria measuring 80 × 60 × 40 cm having 85 L of dechlorinated aerated tap water under laboratory conditions. The daily water exchange rate was 30%. The water quality parameters were as follows: pH was 7.5 ± 0.3, temperature was 28 ± 1 °C, dissolved oxygen was 6.5 ± 0.4 mg/L, alkalinity was 122 mg/L, and hardness was 152 mg/L CaCO3. Fish were fed on a commercial diet (Aller-Aqua, Giza Governorate, Egypt) at a rate of 3% of body weight per day. The proximate chemical composition of the used diet was 32.00% crude protein, 8.00% crude lipid, 4.5% fiber, and 8.5% ash.
All procedures and handling methods used in the current study had been accepted by the Research Ethical Committee (R.E.C.) of Faculty of Women for Arts, Science & Education, Ain shams University, Cairo, Egypt.
2.3. Experimental Design
2.3.1. Determination 96 h LC50 of Chloropyrofis
The half-lethal concentration (LC50) of Chloropyrofis (CPF; National Company for Fertilizers and Chemicals, Cairo, Egypt) was determined with the definitive test by the static renewal bioassay method of Litchfield and Wilcoxon [44]. Briefly, five groups each of eight fish were exposed to various concentrations of CPF (0, 25, 50, 100, 200, or 400 µg/L.). The half-lethal concentration (LC50) of chlorpyrifos was determined according to the following formula:
| LC50 = the highest concentration − ∑a × b/n | (1) |
where a: constant factor of difference between groups; b: mean value of dead fish between every two successive groups; and n: number of fish in each group.
2.3.2. Sublethal Exposure
After the acclimatization period, catfish were distributed into four groups in triplicates containing 12 fish and exposed to the following treatments for six weeks: Fish in the 1st group was fed the basal diet and reserved as the control group. Fish in 2nd group was exposed to 10% of the LC50 of CPF (8.75 µg/L). Fish in 3rd group was dietary administered CP extract (250 mg/kg body weight). Fish in 4th group was exposed to CPF (8.75 µg/L) and administered CP extract (250 mg/kg body weight). In the CPF exposed groups, with each water exchange, the new water was dosed with the same CPF concentration to maintain the same exposure level throughout the experiment.
2.3.3. Blood Sampling
Six fish from each treatment were collected at the end of the experiment and anaesthetized with 0.02% benzocaine solution. Blood samples were taken from the caudal arteries and allowed to clot at room temperature in clean dry centrifuge tubes before being centrifuged at 800× g for 15 min at 4 °C to determine biochemical and immunologic characteristics.
2.3.4. Biochemical Assays
All biochemical assays were conducted using commercial kits (Bio-Diagnostic Co., Cairo, Egypt). Serum glucose and cortisol levels were assessed using the methods described by Foster and Dunn [45], respectively. Serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were detected by [46]. Serum total protein and albumin were measured using the methods described by Henry [47] and Doumas et al. [48], respectively. Serum globulin was determined by subtracting the albumin value from the total protein value of the same sample. The AChE activity was determined according to [49].
2.3.5. Determination of Tissue Lipid Peroxidation and Antioxidant Enzyme Activities
Samples from hepatopancreas and renal tissues were homogenized in cold phosphate buffer saline (0.1 M, pH 7.4) using a Potter-Elvejhem glass and Teflon homogenizer. The homogenate was centrifuged at 1600× g at 4 °C for 10 min. Supernatants were stored at −20 °C until analysis. Lipid peroxidation (LPO) levels were determined according to Uchiyama and Mihara [50]. Superoxide dismutase (SOD) activity was determined using the method described by Nishikimi et al. [51]. Catalase (CAT) was measured according to the method of Aebi [52]. Total antioxidant capacity (TAC) and reduced glutathione (GSH) levels were estimated using the method of Koracevic et al. [53] and Beutler [54], respectively, using commercial kit (Gamma Trade Co., Cairo, Egypt).
2.3.6. Immunological Assays
According to Ellis (1990), serum lysozyme (LYZ) was measured using a turbidimetric technique using Micrococcus luteus as the target in phosphate buffer (pH = 6.2). (1990). According to Secombes [55], the respiratory burst (RB) activity of the whole blood sample was assessed using Nitroblue Tetrazolium dye. The IgM was precipitated in polyethylene glycol, the initial and final total proteins were subtracted, and immunoglobulin (IgM) was quantified [56].
2.3.7. Reproductive Hormones Examination
Serum 17-β Estradiol (E2) and follicle-stimulating hormone (FSH) levels were assessed by ELISA according to Abraham [57] and Knobil [58], respectively. Luteinizing hormone (LH) and testosterone levels were detected according to the method of Tietz et al. [59], respectively, using commercial kit (Gamma Trade Co., Cairo, Egypt).
2.3.8. Hepatic DNA Fragmentation Measurement
The DNA fragmentation of hepatic specimens of female catfish was determined according to the method described by Kurita-Ochiai et al. [60] using a spectrophotometer (Micro lab 200 Vital Scientific Dieren, The Netherlands) at 575 or 600 nm against reagent blank. The percentage of fragmented DNA was assessed by the following formula:
| % of fragmented DNA = fragmented DNA/(fragmented DNA + intact DNA) × 100 | (2) |
2.4. Statistical Analyses
The results were presented as means ± standard error. Data were statistically analyzed using analysis of variance, one-way ANOVA, to evaluate effects of CPF toxicity and dietary PC extract. Differences among means were tested at the 5% probability level using Duncan’s multi-comparison test. All the statistical analyses were done using SPSS (version 20; SPSS, Richmond, VA, USA).
3. Results
3.1. Determination of the 96 h LC50 of Chlorpyrifos
The 96 h LC50 value of CPF was determined as 87.5 µg/L (Table 1) and a sublethal concentration of CPF equal to 10% of the LC50 (8.75 µg CPF/L) was used in the current investigation as chronic stressors.
Table 1.
Half-lethal concentration (LC50) of Chlorpyrifos (CPF) in female African catfish, Clarias gariepinus.
| CPF Concentration (µg/L) | No. of Fish/Concentration | No. of Alive Fish |
No. of Dead Fish |
a | b | a × b |
|---|---|---|---|---|---|---|
| 0.00 | 8 | 8 | 0 | 0.0 | 0.0 | 0.00 |
| 25.00 | 8 | 6 | 2 | 25 | 1.00 | 25 |
| 50.00 | 8 | 4 | 4 | 25 | 3.00 | 75 |
| 100.00 | 8 | 2 | 6 | 50 | 5.00 | 250 |
| 200.00 | 8 | 1 | 7 | 100 | 6.50 | 650 |
| 400.00 | 8 | 0 | 8 | 200 | 7.50 | 1500 |
| ∑ a × b = | 2500 | |||||
Half-lethal concentration (LC50) of chlorpyrifos = the highest concentration − ∑a × b/n; a: constant factor of difference between groups; b: mean value of dead fish between every two successive groups; and n: number of fish in each group.
3.2. Biochemical Analysis
Female African catfish exposed to a sublethal level of CPF showed marked elevations in levels of serum liver enzyme activities (AST, ALT), glucose, and cortisol levels compared to the control fish (Table 2). In comparison to the control tilapia, there was a considerable decrease in serum AchE, total protein, albumin, and globulin levels (Table 2). On the contrary, CP extract (250 mg/kg bw) into the diet along with CPF-exposed catfish normalized levels of all the tested parameters to near the levels of the control group (Table 2). Fish of the control group (1st group) and fish treated with CP extract (2nd group) had nearly the same values of the tested biochemical parameters.
Table 2.
Blood biochemical parameters in female African catfish exposed to chlorpyrifos (8.75 µg/L.) and/or co-administrated with papaya, Carica papaya, aqueous extract (250 mg/kg body weight) for 6 weeks.
| Items | Control | Chlorpyrifos | Papaya | Chlorpyrifos + Papaya |
|---|---|---|---|---|
| Aspartate aminotransferase (µ/L) | 48.15 ± 0.47 b | 62.14 ± 0.51 a | 46.21 ± 0.84 b | 48.29 ± 0.13 b |
| Alanine aminotransferase (µ/L) | 18.05 ±0.37 a | 10.21 ± 0.04 b | 17.92 ± 0.34 a | 17.41 ± 0.087 a |
| Glucose (mg/dL) | 86.87 ± 0.34 b | 120.31 ± 1.31 a | 86.57 ± 0.41 b | 88.05 ± 0.36 b |
| Cortisol (µg/dL) | 11.66 ± 0.28 b | 21.21 ± 0.09 a | 11.57 ± 0.19 c | 13.51 ± 0.16 b |
| Acetylcholinestrase (µ/L) | 545.23 ± 1.16 a | 381.27 ± 2.27 c | 545.17 ± 2.18 a | 550.35 ± 1.35 b |
| Total proteins (g/dL) | 6.53 ± 0.12 a | 3.14 ± 0.26 b | 6.64 ± 0.17 a | 6.35 ± 0.01 a |
| Albumin (g/dL) | 3.87 ± 0.12 a | 1.95 ± 0.21 b | 3.75 ± 0.11 a | 3.89 ± 0.09 a |
| Globulin (g/dL) | 2.66 ± 0.11 a | 1.19 ± 0.01 b | 2.89 ± 0.31 a | 2.46 ± 0.14 a |
Means with different superscript letters in the same row for each parameter are significantly different (p < 0.05).
3.3. Lipid Peroxidation and Antioxidant Enzyme Activities
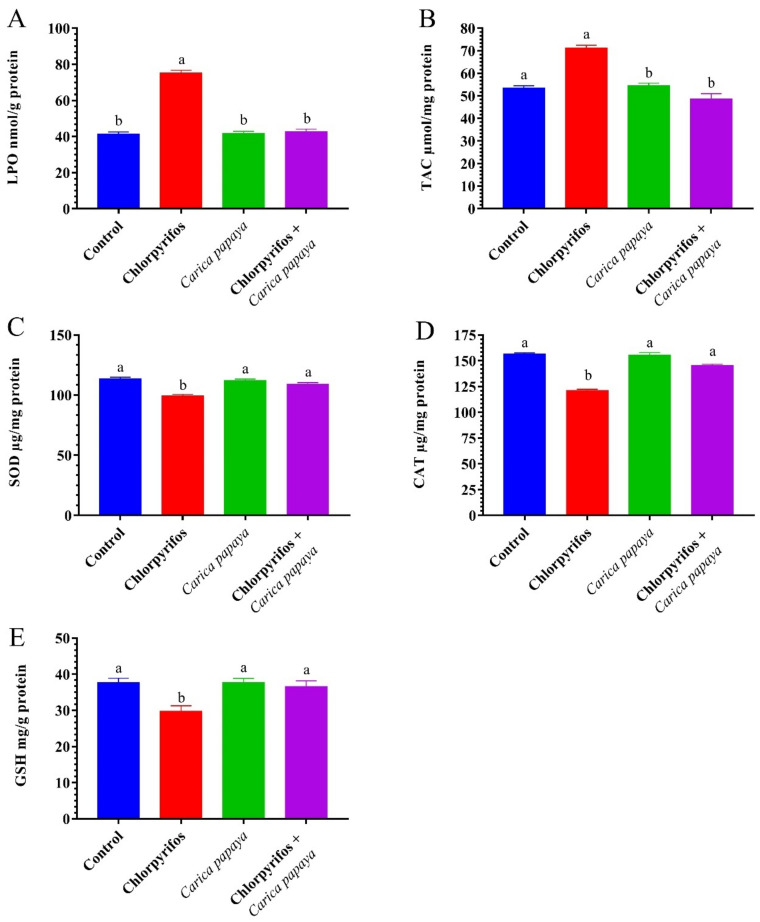
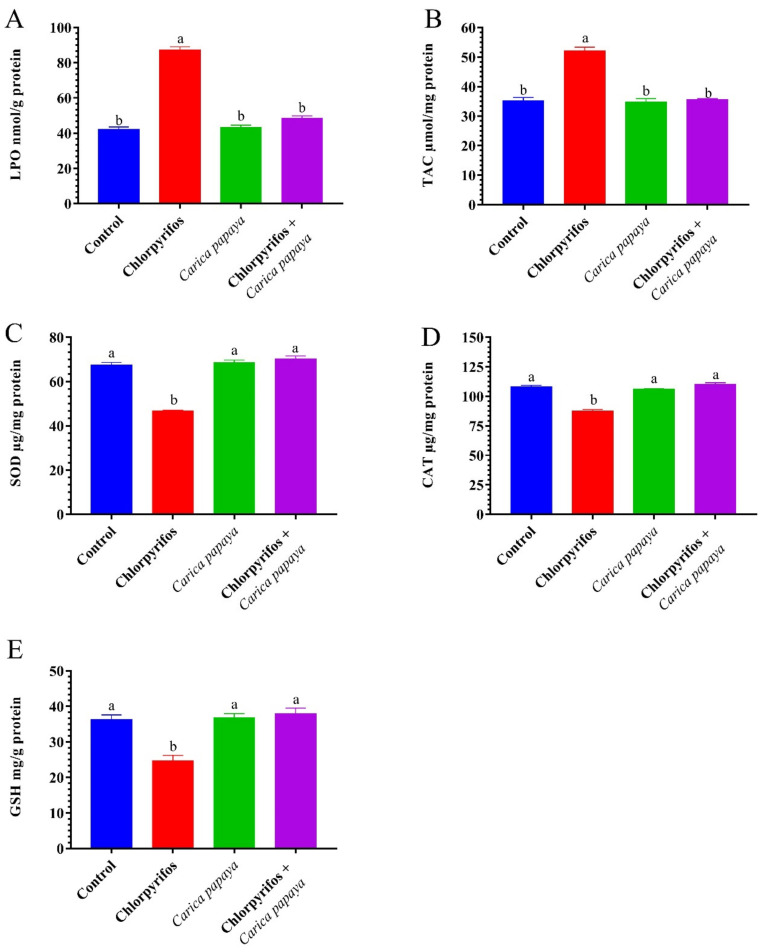
The exposure of female catfish to CPF caused a marked (p < 0.05) increase in hepatorenal LPO and TAC levels as presented in Figure 1 and Figure 2. The CAT, SOD, and GSH activities significantly decreased in the liver and kidney tissues compared to the control group (Figure 1 and Figure 2). The administration with CP extract 250 mg/kg bw of showed no significant difference in hepatorenal antioxidant enzyme activities compared to the control group. Treatment of CPF-exposed fish with CP extract resulted in a significant decrease in LPO and TAC levels and a marked an improvement in hepatorenal oxidative stress enzymes.
Figure 1.
Lipid peroxidation (A), total antioxidant capacity (B), superoxide dismutase (C), total antioxidant capacity (D), catalase (D), and reduced glutathione (E) of hepatopancreas tissue in female African catfish, Clarias gariepinus, exposed to chlorpyrifos (8.75 µg/L.) and co-administrated with papaya, Carica papaya, aqueous extract (250 mg/kg body weight) for 6 weeks. The column bearing with different letters are significantly different (p < 0.05).
Figure 2.
Lipid peroxidation (A), total antioxidant capacity (B), superoxide dismutase (C), total antioxidant capacity (D), catalase (D), and reduced glutathione (E) of renal tissue in female African catfish, Clarias gariepinus, exposed to chlorpyrifos (8.75 µg/L) and co-administrated with papaya, Carica papaya, aqueous extract (250 mg/kg body weight) for 6 weeks. The column bearing with different letters are significantly different (p < 0.05).
3.4. Innate Immune Response
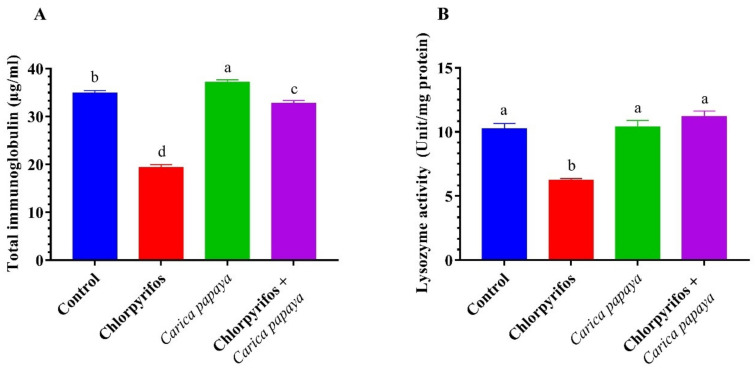
Catfish exposed to (8.75 µg/L.) CPF for 6 weeks exhibited a significant reduction in the lysozyme activity and immunoglobulins levels (Figure 3). The combined treatment of CPF and CP extract resulted in a marked enhancement in the lysozyme activity and immunoglobulins levels.
Figure 3.
Total immunoglobulin (A) and lysozyme (B) of female African catfish, Clarias gariepinus, exposed to chlorpyrifos (8.75 µg/L.) and co-administrated with papaya, Carica papaya aqueous extract (250 mg/kg body weight) for 6 weeks. The column bearing with different letters are significantly different (p < 0.05).
3.5. Reproductive Hormones Analysis
The concentrations of 17-β E2 and FSH in female catfish were significantly reduced during CPF exposure (Table 3). On the other hand, levels of LH and testosterone were markedly elevated (p < 0.05) in the group exposed to CPF (8.75 µg/L.) for 6 weeks (Table 3). Co-administration of CP extract (250 mg/kg bw) with CPF exposure caused a significant increase in serum FSH and 17-β E2 levels and restored the concentrations of LH and testosterone to the normal values in comparison with CPF-exposed fish (Table 3).
Table 3.
Reproductive hormones in female African catfish, Clarias gariepinus, exposed to chlorpyrifos (8.75 µg/L.) and/or co-administrated with papaya, Carica papaya, aqueous extract (250 mg/kg body weight) for 6 weeks.
| Items | Control | Chlorpyrifos | Papaya | Chlorpyrifos + Papaya |
|---|---|---|---|---|
| Follicle-stimulating hormone (µ/L) | 0.68 ± 0.05 a | 0.26 ± 0.06 c | 0.72 ± 0.04 a | 0.67 ± 0.03 b |
| Luteinizing hormone (µ/L) | 0.33 ± 0.01 b | 0.78 ± 0.09 a | 0.31 ± 0.04 b | 0.32 ± 0.05 b |
| Estradiol (17-β E2; g/mL) | 349.31 ± 0.41 a | 253.51 ± 0.15 c | 350.21 ± 0.23 a | 339.24 ± 0.13 b |
| Testosterone (g/mL) | 54.15 ± 0.34 b | 80.52 ± 0.25 a | 55.20 ± 0.41 b | 50.34 ± 0.31 c |
Means with different superscript letters in the same column for each parameter are significantly different (p < 0.05).
3.6. Liver DNA Fragmentation
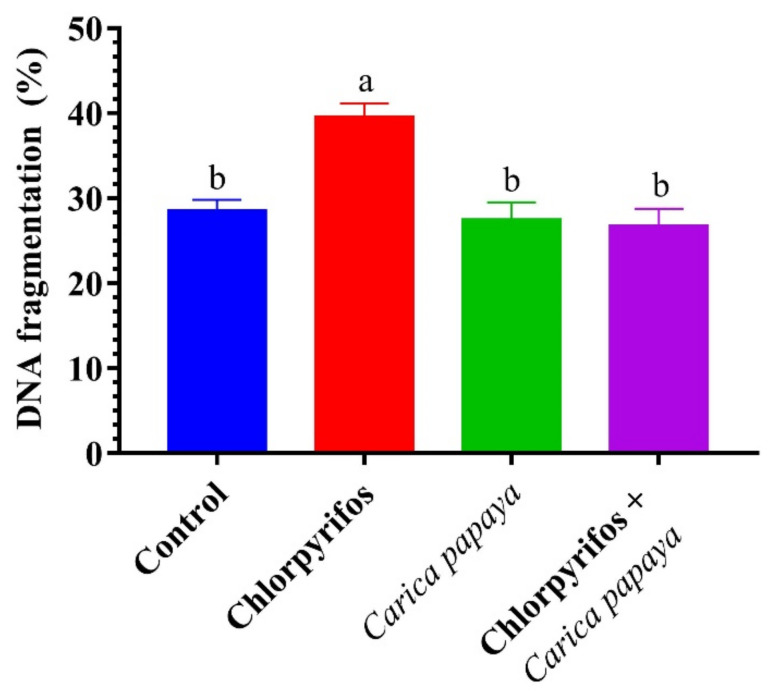
CPF-exposed fish showed a significant (p < 0.05) increase in the percentage of hepatic DNA fragmentation when compared to the control catfish, as seen in Figure 4. In contrast, combined treatment with CPF and CP extract showed a significant decrement in the percentage of hepatic DNA fragmentation compared to the CPF-intoxicated group (Figure 4).
Figure 4.
DNA fragmentation (%) of female African catfish, Clarias gariepinus, exposed to chlorpyrifos (8.75 µg/L.) and co-administrated with papaya, Carica papaya aqueous extract (250 mg/kg body weight) for 6 weeks. The column bearing with different letters are significantly different (p < 0.05).
4. Discussion
The 96 h LC50 value of CPF in female African catfish was found to be 87.5 µg/L. In similar studies, the 96 h LC50 of CPF in O. mossambicus with a mean body weight 3.0 g was 25.97 µg/L [61]. Meanwhile, the 96 h LC50 values of technical grade (94% a.i.) and commercial CPF (20% EC) on Nile tilapia with average body weight 2.64 ± 0.16 g were 109 µg/L and 47 µg/L, respectively [5]. They attributed the difference in CPF toxicity on fish to the type of CPF where they suggested that the technical grade (active ingredient) of CPF was more toxic to fish than its emulsified concentration. In addition, Paracampo et al. [62] found that the 96 h LC50 of CPF was 105 µg/L for Cnesterodon decemmaculatus with a maximum length of 25 and 45 mm for males and females, respectively. The 96 h LC50 value of CPF for Cyprinus carpio with mean body weight (47.51 ± 3.24 g.) was 0.160 mg/L [63]. Meanwhile, Zhang et al. [11] found that the 96 h LC50 of CPF in C. carpio (average body weight of 15.00 ± 5.0 g) was 149.0 μg/L. The sensitivity of fish to CPF toxicity could be related to many different reasons such as age, fish species, exposure period, fish size, and ecological circumstances.
In the present study, serum AST and ALT exhibited high values in CPF-exposed catfish without feeding on CP extract. The results showed that the hepatic tissue of catfish was severely impaired by CPF exposure while CP extract represented a functional remedy for fish. In accordance with the present findings, African catfish exposed to CPF or other insecticides had higher blood levels of AST and ALT [34,64,65], although other research found a significant drop in liver enzyme levels when feed additives were added to fish diets [17,64]. In addition, the CP extract had more hepatoprotection against CCl4 toxicity than vitamin E. Hence, the results indicated that CP extract possesses hepatoprotective properties [66].
In the same way, glucose and cortisol displayed high levels in fish exposed to CPF [36,67]. This hyperglycemia seems to be a sign of stress in fish exposed to toxicity and is also associated with an elevation in cortisol [17,68] to cope with the toxicity effects as an attempt to cover the energy demands [69]. The results also showed a marked reduction in AChE of catfish exposed to CPF for 6 weeks. Salbego et al. [70] described the inhibition of AChE to the oxidative stress generated by pesticide exposure which provides a convenient non-destructive means of monitoring exposure to pesticides [71]. Many researchers have focused on the ability of CPF to cause hyperglycemia where exposure to CPF decreased serum insulin levels and increased serum glucose levels (hyperglycemia) [72,73,74]. Degeneration and necrosis of glandular acini and beta cells of the pancreas with a proliferation of interlobular ducts in broilers after exposure to CPF supported the alterations in the level of insulin and glucose [75]. Pancreatic beta cells contain muscarinic receptors which play a vital role in insulin production and secretion [76]. By inhibiting AChE activity, CPF increases the accumulation of acetylcholine leading to more stimulation of its receptors and subsequently down-regulates these receptors [77], causing a reduction in insulin synthesis [78]. Furthermore, prolonged stimulation by AChE may reduce beta cell sensitivity to glucose [79]. Hence, inhibition of AChE activity plays a role to some extent in CPF-induced hyperglycemia [80]. This is consistent with the findings of Narra et al. [1] and Hamed and El-Sayed [15] in which the crab Barytelphusa guerini was treated with CPF and Oreochromis niloticus exposed to pendimethalin. In the present study, the feeding of CPF-exposed catfish on the supplemented diet with CP extract reduced glucose and cortisol levels followed by an increment in AChE activity. These findings could be attributed to the bioactive compounds of CP which stimulates glucose uptake exhibiting hypoglycemia. Similar results were documented in Nile tilapia fed diets containing green tea leaves [20] and Moringa oleifera leaves [15] and CPF also diminished the levels of total protein, albumin, and globulin in catfish. The vascular leakage and high proteolysis rate are to account for the lower total protein level [81,82,83], while the decreased albumin level can be attributed to the high rate of renal excretion and failure of protein synthesis due to liver malfunction in the fish exposed to CPF [84,85]. Meanwhile, feeding with CP extract restored the levels of total protein, albumin, and globulin in female catfish exposed to CPF for six weeks. The enhanced serum total protein and its derivatives in CPF-exposed catfish co-administrated with dietary CP extract suggest immune-stimulatory effects of CP in African catfish. In addition, the co-treatment of cypermethrin-intoxicated Nile tilapia with guava leaves extract enhanced serum total protein, albumin, and globulin levels [17].
Environmental contaminants or their metabolites produce oxidative damage in fish [18,69,86,87,88]. In the present study, the antioxidant profile in catfish exposed to CPF exhibited a marked rise in hepatorenal LPO and TAC and declines in the levels of TAC, CAT, SOD, and GSH. The LPO or oxidation of polyunsaturated fatty acids has been observed and used to monitor the effect of pollutants [89,90,91,92]. In the present study, the LPO level increased significantly in the hepatic and renal tissues of female C. gariepinus after exposure to CPF for six weeks. Increased LPO levels could be attributed to the inequality state between the production and removal of reactive oxygen species (ROSs) in the cell due to oxidative stress which allows ROSs to attack the lipids, proteins, and DNA of the cell leading to mitochondrial dysfunction and DNA fragmentation which finally causes cell death [93]. CP extract inclusion in catfish feeds markedly decreased the LPO levels due to its constituents of antioxidant phytochemicals such as lycopene, vitamin C, vitamin E, beta-carotene, quercetin, flavonoids, and phenolic compounds which scavenge free radicals generated after CPF exposure [26,94,95].
In all living beings, ROSs are transformed into bland metabolites by antioxidant enzymes (SOD and CAT), which may decrease or increase under chemical exposure [96]. In the present study, the SOD and CAT activities decreased in the hepatic and renal tissues of catfish exposed to 8.75 µg CPF/L. The inhibitory effect may be a result of the overproduction of ROSs induced by CPF exposure, whereas the SOD-CAT system is considered the first line of defense against oxidative damage. The SOD catalyzes the conversion of superoxide anions (O2−) into H2O2 and CAT converts H2O2 into H2O and O2 [17,97]. In a similar study, Kadry et al. [98] reported that antioxidant enzyme activities were reduced in the liver of female C. gariepinus exposed to atrazine for six weeks. In addition, Abdel-Daim et al. [99] found a marked decline in CAT and SOD activities of tilapia fish exposed to CPF. Dietary supplementation of CP extract in the catfish diet resulted in enhancement of the action of the antioxidant enzymes CAT and SOD activities and TAC level through the removal of excess ROSs to combat oxidative stress. These positive effects could be due to the PC extract content of antioxidant phytochemicals.
The observed reduction in GSH values in liver and kidney tissues of CPF exposed catfish is considered a primary concern of CPF-induced oxidative stress. The reduction in GSH could be due to its depletion to oppose oxidative destruction in response to pollutant exposure [36]. In addition, Shaban et al. [100] explained the decrease in GSH level due to the high demand and consumption of the tripeptide for lipid hydroperoxide metabolism. Similar studies supported this result [101]. The co-administration of CP aqueous extract restored the GSH levels in hepatorenal tissues of catfish after CPF exposure compared with the control fish, where CP acted as an antioxidant and subsequently reduced the consumption of GSH.
In the present study, the total immunoglobulin and lysozyme activity experienced low values in CPF-intoxicated catfish suggesting an immune suppression effect of CPF. Several insecticides could induce immune suppression effects in the fish by altering the transcription of important mediators of the fish’s immune system [17,102]. Feeding catfish a CP-enriched diet during exposure to CPF for six weeks substantially restored the innate immunity parameters, indicating an enhancement of the non-specific innate immunity defense in fish. The results could be attributed to the phytochemical compounds of CP aqueous extract such as lycopene, vitamin C, vitamin E, beta-carotene, and quercetin [26,94,95] as well as the proteolytic enzymes in CP aqueous extract (papain and chymopapain) which could enhance feed utilization and improve the general animal performance [103]. Accordingly, the CP extract revealed a potential immune stimulant property on fish and could alleviate the immunotoxicity effects of CPF. In similar study, the dietary guava leaf powder exhibited immune-stimulatory effects against cypermethrin toxicity in Nile tilapia [17]. In addition, [104] found that dietary pomegranate peel powder ameliorated the immune-toxicological effects of silver nanoparticles on Nile tilapia.
Endocrine disruption is another adverse effect of environmental pollutants. The pituitary hormones (LH and FSH) play an important role in the reproductive process [105,106]. The results of the current work showed significant reductions in concentrations of FSH and 17- β E2 hormones and noticeable elevations in LH and testosterone levels after exposure of female catfish to CPF for 6 weeks. The increase in testosterone in female catfish after exposure to CPF could be attributed to increasing the activity of 17 alpha-hydroxylase enzymes involved in testosterone synthesis. Similar studies [71] recorded that testosterone levels increased in female catfish exposed to bisphenol-A. Additionally, the LH hormone of juvenile African catfish was significantly increased after exposure to nonyl phenol for 2 weeks [107]. Our results are also in accordance with da Silva et al. [108] who found that estradiol concentrations decreased in rats intoxicated with bisphenol-S during pregnancy and lactation stages. The reduction in the estradiol level could be attributed to the hypothalamus-pituitary-ovarian axis which was damaged by CPF. The hormonal changes in gonads of CPF-intoxicated female catfish reflect their sensitivity to CPF, suggesting that CPF has androgenic effects. Co-administration with CP aqueous extract (250 mg/kg bw) caused significant improvements in reproductive hormone concentrations of CPF-exposed fish. This improvement may be because of flavonoids, polyphenols, and antioxidant components in the CP aqueous extract [109]. Likewise, the quercetin that was reported in CP extract protected against deltamethrin- and cypermethrin-induced reproductive system toxicity and oxidative damage in rats [95]. This demonstrated that CP aqueous extract has a hormonal recovery effect against CPF.
The current investigation exhibited that exposure of catfish to CPF at a level of 8.75 µg/L resulted in a marked increase in the percentage of liver DNA fragmentation. CPF has the potential to generate mutagenic impacts by generating oxidative DNA damage [25,109]. These genotoxic effects of CPF could be attributed to the generation of free radicals and the imbalance in calcium homeostasis due to the damage in the endoplasmic reticulum membrane [64]. Dietary treatment of female catfish with CP extract at a level of 250 mg/kg body weight reduced the percentage in DNA damage of hepatic tissues after exposure to CPF via oxidation by H2O2. This study suggests that feeding catfish on CP extract had a powerful antioxidant effect that succeed to reduce the adverse effects of free radicals and inhibit the oxidation of molecules such as lipids and DNA. Hence, CP induced a significant depletion in the elevation level of DNA fragmentation in liver tissues of CPF-intoxicated catfish.
5. Conclusions
According to the present findings, it could be concluded that the exposure of female African catfish Clarias gariepinus to chlorpyrifos (CPF) caused variations in serum biochemical, immunological assays, and oxidative damage in different tissues. These parameters could also be considered good indicators for monitoring pollution in the aquatic ecosystem including fish. On the other hand, dietary supplementation with papaya Carica papaya (CP) extract could improve the physiological and immunological status and alleviated hormonal disruption of catfish exposed to with CPF. Consequently, feeding fish with a CP-extract-enriched diet could minimized the negative impacts of CPF toxicity.
Acknowledgments
The authors would like to acknowledge the Deanship of Scientific Research, Vice Presidency for Graduate Studies and Scientific Research, King Faisal University, Saudi Arabia [Project No. GRANT463].
Author Contributions
Conceptualization, H.S.H. and W.F.M.; methodology, H.S.H. and W.F.M.; software; validation, A.T.M., H.S.H. and W.F.M.; formal analysis, A.T.M., H.S.H. and W.F.M.; investigation, H.S.H. and W.F.M.; resources, A.T.M. and H.S.E.-B.; data curation, A.T.M., H.S.H. and W.F.M.; writing—original draft preparation, A.T.M., H.S.H. and W.F.M.; writing—review and editing A.T.M. and H.S.H.; visualization, A.T.M. and H.S.E.-B.; supervision, H.S.H. and W.F.M.; project administration, A.T.M. and H.S.H.; funding acquisition, A.T.M. and H.S.E.-B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Deanship of Scientific Research, Vice Presidency for Graduate Studies and Scientific Research, King Faisal University, Saudi Arabia [Project No. GRANT463].
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Research Ethical Committee of Faculty of Women for Arts, Science & Education, Ain shams University, Cairo, Egypt.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the authors upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Narra M.R., Rajender K., Reddy R.R., Rao J.V., Begum G. The role of vitamin C as antioxidant in protection of biochemical and haematological stress induced by chlorpyrifos in freshwater fish Clarias batrachus. Chemosphere. 2015;132:172–178. doi: 10.1016/j.chemosphere.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 2.Palma P., Palma V., Fernandes R., Soares A., Barbosa I. Acute toxicity of atrazine, endosulfan sulphate and chlorpyrifos to Vibrio fischeri, Thamnocephalus platyurus and Daphnia magna, relative to their concentrations in surface waters from the Alentejo region of Portugal. Bull. Environ. Contam. Toxicol. 2008;81:485–489. doi: 10.1007/s00128-008-9517-3. [DOI] [PubMed] [Google Scholar]
- 3.Xing H., Han Y., Li S., Wang J., Wang X., Xu S. Alterations in mRNA expression of acetylcholinesterase in brain and muscle of common carp exposed to atrazine and chlorpyrifos. Ecotoxicol. Environ. Saf. 2010;73:1666–1670. doi: 10.1016/j.ecoenv.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 4.John E.M., Shaike J.M. Chlorpyrifos: Pollution and remediation. Environ. Chem. Lett. 2015;13:269–291. doi: 10.1007/s10311-015-0513-7. [DOI] [Google Scholar]
- 5.Majumder R., Kaviraj A. Acute and sublethal effects of organophosphate insecticide chlorpyrifos on freshwater fish Oreochromis niloticus. Drug Chem. Toxicol. 2019;42:487–495. doi: 10.1080/01480545.2018.1425425. [DOI] [PubMed] [Google Scholar]
- 6.Ahmed N.S., Mohamed A.S., Abdel-Wahhab M.A. Chlorpyrifos-induced oxidative stress and histological changes in retinas and kidney in rats: Protective role of ascorbic acid and alpha tocopherol. Pestic. Biochem. Physiol. 2010;98:33–38. doi: 10.1016/j.pestbp.2010.04.006. [DOI] [Google Scholar]
- 7.Oruç E.Ö. Oxidative stress, steroid hormone concentrations and acetylcholinesterase activity in Oreochromis niloticus exposed to chlorpyrifos. Pestic. Biochem. Physiol. 2010;96:160–166. doi: 10.1016/j.pestbp.2009.11.005. [DOI] [Google Scholar]
- 8.Richendrfer H., Pelkowski S.D., Colwill R.M., Créton R. Developmental sub-chronic exposure to chlorpyrifos reduces anxiety-related behavior in zebrafish larvae. Neurotoxicol. Teratol. 2012;34:458–465. doi: 10.1016/j.ntt.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hussein R.M., Mohamed W.R., Omar H.A. A neuroprotective role of kaempferol against chlorpyrifos-induced oxidative stress and memory deficits in rats via GSK3β-Nrf2 signaling pathway. Pestic. Biochem. Physiol. 2018;152:29–37. doi: 10.1016/j.pestbp.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 10.Xing H., Wang X., Sun G., Gao X., Xu S., Wang X. Effects of atrazine and chlorpyrifos on activity and transcription of glutathione S-transferase in common carp (Cyprinus carpio L.) Environ. Toxicol. Pharmacol. 2012;33:233–244. doi: 10.1016/j.etap.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Z., Liu Q., Cai J., Yang J., Shen Q., Xu S. Chlorpyrifos exposure in common carp (Cyprinus carpio L.) leads to oxidative stress and immune responses. Fish Shellfish Immunol. 2017;67:604–611. doi: 10.1016/j.fsi.2017.06.048. [DOI] [PubMed] [Google Scholar]
- 12.Xing H., Li S., Wang X., Gao X., Xu S., Wang X. Effects of atrazine and chlorpyrifos on the mRNA levels of HSP70 and HSC70 in the liver, brain, kidney and gill of common carp (Cyprinus carpio L.) Chemosphere. 2013;90:910–916. doi: 10.1016/j.chemosphere.2012.06.028. [DOI] [PubMed] [Google Scholar]
- 13.Wang J., Wang J., Zhu L., Xie H., Shao B., Hou X. The enzyme toxicity and genotoxicity of chlorpyrifos and its toxic metabolite TCP to zebrafish Danio rerio. Ecotoxicology. 2014;23:1858–1869. doi: 10.1007/s10646-014-1321-8. [DOI] [PubMed] [Google Scholar]
- 14.Jiao W., Han Q., Xu Y., Jiang H., Xing H., Teng X. Impaired immune function and structural integrity in the gills of common carp (Cyprinus carpio L.) caused by chlorpyrifos exposure: Through oxidative stress and apoptosis. Fish Shellfish Immunol. 2019;86:239–245. doi: 10.1016/j.fsi.2018.08.060. [DOI] [PubMed] [Google Scholar]
- 15.Hamed H.S., El-Sayed Y.S. Antioxidant activities of Moringa oleifera leaf extract against pendimethalin-induced oxidative stress and genotoxicity in Nile tilapia, Oreochromis niloticus (L.) Fish Physiol. Biochem. 2019;45:71–82. doi: 10.1007/s10695-018-0535-8. [DOI] [PubMed] [Google Scholar]
- 16.Van Doan H., Hoseinifar S.H., Sringarm K., Jaturasitha S., Yuangsoi B., Dawood M.A., Esteban M.Á., Ringø E., Faggio C. Effects of Assam tea extract on growth, skin mucus, serum immunity and disease resistance of Nile tilapia (Oreochromis niloticus) against Streptococcus agalactiae. Fish Shellfish Immunol. 2019;93:428–435. doi: 10.1016/j.fsi.2019.07.077. [DOI] [PubMed] [Google Scholar]
- 17.Abdel-Tawwab M., Hamed H.S. Antagonistic effects of dietary guava (Psidium guajava) leaves extract on growth, hemato-biochemical, and immunity response of cypermethrin-intoxicated Nile tilapia, Oreochromis niloticus, fingerlings. Aquaculture. 2020;529:735668. doi: 10.1016/j.aquaculture.2020.735668. [DOI] [Google Scholar]
- 18.García-Beltrán J.M., Mansour A.T., Alsaqufi A.S., Ali H.M., Esteban M.Á. Effects of aqueous and ethanolic leaf extracts from drumstick tree (Moringa oleifera) on gilthead seabream (Sparus aurata L.) leucocytes, and their cytotoxic, antitumor, bactericidal and antioxidant activities. Fish Shellfish Immunol. 2020;106:44–55. doi: 10.1016/j.fsi.2020.06.054. [DOI] [PubMed] [Google Scholar]
- 19.Hamed H.S., Ismal S.M., Faggio C. Effect of allicin on antioxidant defense system, and immune response after carbofuran exposure in Nile tilapia, Oreochromis niloticus. Comp. Biochem. Physiol. C Toxicol. 2021;240:108919. doi: 10.1016/j.cbpc.2020.108919. [DOI] [PubMed] [Google Scholar]
- 20.Sinha R., Jindal R., Faggio C. Nephroprotective effect of Emblica officinalis fruit extract against malachite green toxicity in piscine model: Ultrastructure and oxidative stress study. Microsc. Res. Tech. 2021;84:1911–1919. doi: 10.1002/jemt.23747. [DOI] [PubMed] [Google Scholar]
- 21.Sinha R., Jindal R., Faggio C. Protective effect of Emblica officinalis in Cyprinus carpio against hepatotoxicity induced by malachite green: Ultrastructural and molecular analysis. Appl. Sci. 2021;11:3507. doi: 10.3390/app11083507. [DOI] [Google Scholar]
- 22.Rashidian G., Boldaji J.T., Rainis S., Prokić M.D., Faggio C. Oregano (Origanum vulgare) extract enhances zebrafish (Danio rerio) growth performance, serum and mucus innate immune responses and resistance against Aeromonas hydrophila challenge. Animals. 2021;11:299. doi: 10.3390/ani11020299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Madinah N., Nozmo M., Ezekiel I. The protective effects of aqueous extract of Carica papaya seeds in paracetamol induced nephrotoxicity in male wistar rats. Afr. Health Sci. 2015;15:598–605. doi: 10.4314/ahs.v15i2.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sengupta S., Das B., Prasad M., Acharyya P., Ghose T.K. A comparative survey of genetic diversity among a set of Caricaceae accessions using microsatellite markers. SpringerPlus. 2013;2:345. doi: 10.1186/2193-1801-2-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou L., David A.C., Robert E.P. Defoliation and fruit removal effects on papaya fruit production, sugar accumulation, and sucrose metabolism. J. Am. Soc. Hortic. Sci. 2000;125:644–652. doi: 10.21273/JASHS.125.5.644. [DOI] [Google Scholar]
- 26.Sadek K.M. Antioxidant and immunostimulant effect of Carica papaya Linn. aqueous extract in acrylamide intoxicated rats. Acta Inform. Med. 2012;20:180–185. doi: 10.5455/aim.2012.20.180-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Otsuki N., Dang N.H., Kumagai E., Kondo A., Iwata S., Morimoto C. Aqueous extract of Carica papaya leaves exhibits anti-tumor activity and immunomodulatory effects. J. Ethnopharmacol. 2010;127:760–767. doi: 10.1016/j.jep.2009.11.024. [DOI] [PubMed] [Google Scholar]
- 28.Mannaa F.A., Abdel-Wahhab K.G., Abdel-Wahhab M.A. Prevention of cardiotoxicity of aflatoxin B1 via dietary supplementation of papaya fruit extracts in rats. Cytotechnology. 2014;66:327–334. doi: 10.1007/s10616-013-9579-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li M., Su E., You P., Gong X., Sun M., Xu D., Wei D. Purification and in situ immobilization of papain with aqueous two-phase system. PLoS ONE. 2010;5:e15168. doi: 10.1371/journal.pone.0015168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khan J., Yadav J., Srivastava Y., Pal P. In vitro evaluation of antimicrobial properties of Carica papaya. Int. J. Biol. Pharm. Allied Sci. 2012;1:933–945. [Google Scholar]
- 31.Urquiaga I., Leighton F. Plant polyphenol antioxidants and oxidative stress. Biol. Res. 2000;33:55–64. doi: 10.4067/S0716-97602000000200004. [DOI] [PubMed] [Google Scholar]
- 32.La Marca M., Beffy P., Della Croce C., Gervasi P., Iori R., Puccinelli E., Longo V. Structural influence of isothiocyanates on expression of cytochrome P450, phase II enzymes, and activation of Nrf2 in primary rat hepatocytes. Food Chem. Toxicol. 2012;50:2822–2830. doi: 10.1016/j.fct.2012.05.044. [DOI] [PubMed] [Google Scholar]
- 33.Oluah N.S., Mgbenka B.O., Nwani C.D., Aguzie I.O., Ngene I.C., Oluah C. Tissue-specific changes in Ca2+-ATPase and Na+/K+-ATPase activities in freshwater African catfish Clarias gariepinus juvenile exposed to oxadiazon. J. Basic Appl. Zool. 2020;81:51. doi: 10.1186/s41936-020-00186-8. [DOI] [Google Scholar]
- 34.Hamed H.S. Ameliorative effects of Spirulina platensis on deltamethrin-induced biochemical alterations and oxidative stress in the African catfish; Clarias gariepinus. Open J. Mar. Sci. 2016;6:62112. doi: 10.4236/ojms.2016.61001. [DOI] [Google Scholar]
- 35.Rocha S., Streit D.P., Jr., Marques L.S., Varela A.S., Jr., Corcini C.D., Hoshiba M.A. Toxic effects of mercury chloride on silver catfish (Rhamdia quelen) spermatozoa. Aquacult. Res. 2018;49:963–968. doi: 10.1111/are.13543. [DOI] [Google Scholar]
- 36.Hamed H.S., Osman A.G. Modulatory effect of lycopene against carbofuran toxicity in African catfish, Clarias gariepinus. Fish Physiol. Biochem. 2017;43:1721–1731. doi: 10.1007/s10695-017-0404-x. [DOI] [PubMed] [Google Scholar]
- 37.Harikrishnan R., Devi G., Balasundaram C., Van Doan H., Jaturasitha S., Ringø E., Faggio C. Effect of chrysophanic acid on immune response and immune genes transcriptomic profile in Catla catla against Aeromonas hydrophila. Sci. Rep. 2021;11:612. doi: 10.1038/s41598-020-79629-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dhanulkar M., Tirpude J., Pradhan N., Mohite A., Khapekar H., Gahukar P. Assessment of toxicity of 2-methyltetrahydrofuran on the ovarian tissue of african catfish Clarias gariepinus (burchell, 1822)-a histopathological study. Int. J. Res. Biosci. Agric. Technol. 2021;18:179–187. [Google Scholar]
- 39.Ali A.M., Mohamed N.A., Bakhoum S.A., Abdel-Kader H.H., Ahmed M.A. Positive Effects of Meso-2, 3-Dimercaptosuccinic Acid against Oxidative Stress of Lead and Mercury in the Catfish Clarias gariepinus of Lake Maryout, Egypt. Egypt. J. Aquat. Biol. Fish. 2020;24:391–407. doi: 10.21608/ejabf.2020.100258. [DOI] [Google Scholar]
- 40.Gautam G.J., Chaube R. Differential effects of heavy metals (cadmium, cobalt, lead and mercury) on oocyte maturation and ovulation of the catfish Heteropneustes fossilis: An in vitro study. Turkish J. Fish. Aquat. Sci. 2018;18:1205–1214. [Google Scholar]
- 41.Adeniyi F., Ogunyemi E. Toxicity studies on an unripe Carica papaya aqueous extract: Biochemical and haematological effects in wistar albino rats. J. Med. Plant Res. 2007;1:1–4. [Google Scholar]
- 42.El-Nekeety A.A., Abdel-Wahhab K.G., Abdel-Aziem S.H., Mannaa F.A., Hassan N.S., Abdel-Wahhab M.A. Papaya fruits extracts enhance the antioxidant capacity and modulate the genotoxicity and oxidative stress in the kidney of rats fed ochratoxin A-contaminated diet. J. Appl. Pharm. Sci. 2017;7:111–121. [Google Scholar]
- 43.Abouzed T.K., Sadek K.M., Ayoub M.M., Saleh E.A., Nasr S.M., El-Sayed Y.S., Shoukry M. Papaya extract upregulates the immune and antioxidants-related genes, and proteins expression in milk somatic cells of Friesian dairy cows. J. Anim. Physiol. Anim. Nutr. 2019;103:407–415. doi: 10.1111/jpn.13032. [DOI] [PubMed] [Google Scholar]
- 44.Litchfield J.J., Wilcoxon F. A simplified method of evaluating dose-effect experiments. J. Pharmacol. Exp. Ther. 1949;96:99–113. [PubMed] [Google Scholar]
- 45.Foster L.B., Dunn R.T. Single-antibody technique for radioimmunoassay of cortisol in unextracted serum or plasma. Clin. Chem. 1974;20:365–368. doi: 10.1093/clinchem/20.3.365. [DOI] [PubMed] [Google Scholar]
- 46.Reitman S., Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol. 1957;28:56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 47.Henry R. Clinical Chemistry. Harper and Row Publisher; New York, NY, USA: 1964. Colorimetric determination of total protein; p. 181. [Google Scholar]
- 48.Doumas B.T., Watson W.A., Biggs H.G. Albumin standards and the measurement of serum albumin with bromcresol green. Clin. Chim. Acta. 1971;31:87–96. doi: 10.1016/0009-8981(71)90365-2. [DOI] [PubMed] [Google Scholar]
- 49.Knedel M., Böttger R. Eine kinetische Methode zur Bestimmung der Aktivität der Pseudocholinesterase (Acylcholin-acylhydrolase 3.1. 1.8.) Klin. Wochenschr. 1967;45:325–327. doi: 10.1007/BF01747115. [DOI] [PubMed] [Google Scholar]
- 50.Uchiyama M., Mihara M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem. 1978;86:271–278. doi: 10.1016/0003-2697(78)90342-1. [DOI] [PubMed] [Google Scholar]
- 51.Nishikimi M., Rao N.A., Yagi K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 1972;46:849–854. doi: 10.1016/S0006-291X(72)80218-3. [DOI] [PubMed] [Google Scholar]
- 52.Aebi H. Methods Enzymol. Volume 105. Elsevier; Amsterdam, The Netherlands: 1984. Catalase in vitro; pp. 121–126. [DOI] [PubMed] [Google Scholar]
- 53.Koracevic D., Koracevic G., Djordjevic V., Andrejevic S., Cosic V. Method for the measurement of antioxidant activity in human fluids. J. Clin. Pathol. 2001;54:356–361. doi: 10.1136/jcp.54.5.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Beutler E. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 1963;61:882–888. [PubMed] [Google Scholar]
- 55.Secombes C. Isolation of salmonid macrophages and analysis of their killing activity. Tech. Fish Immunol. 1990;1:137–154. [Google Scholar]
- 56.Siwicki A.I., Anderson D.P. Nonspecific defense mechanisms assay in fish. II. Potential killing activity of neutrophils and macrophages, lysozyme activity in serum and organs and total immunoglobulin (Ig) level in serum. In: Siwicki A.I., Anderson D.P., Waluga J., editors. Fish Diseases Diagnosis and Preventions Methods. Wydawnictwo Instytutu Rybactwa Strodladowego; Olsztyn, Poland: 1993. pp. 105–111. [Google Scholar]
- 57.Abraham G. The application of natural steroid radioimmunoassay to gynecologic endocrinology. In: Abraham G., editor. Radioassay System in Clinical Endocrinology. Marcel Dekker; New York, NY, USA: 1981. pp. 475–529. [Google Scholar]
- 58.Knobil E. The Neuroendocrine Control of the Menstrual Cycle. In: Greep R.O., editor. Proceedings of the 1979 Laurentian Hormone Conference. Volume 36. Academic Press; Boston, MA, USA: 1980. pp. 53–88. [DOI] [PubMed] [Google Scholar]
- 59.Tietz N.W., Finley P.R., Pruden E. Clinical Guide to Laboratory Tests. 3rd ed. Volume 624 WB Saunders Company; Philadelphia, PA, USA: 1995. [Google Scholar]
- 60.Kurita-Ochiai T., Fukushima K., Ochiai K. Lipopolysaccharide stimulates butyric acid-induced apoptosis in human peripheral blood mononuclear cells. Infect. Immun. 1999;67:22–29. doi: 10.1128/IAI.67.1.22-29.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rao J.V., Rani C.S., Kavitha P., Rao R.N., Madhavendra S. Toxicity of chlorpyrifos to the fish Oreochromis mossambicus. Bull. Environ. Contam. Toxicol. 2003;70:985–992. doi: 10.1007/s00128-003-0079-0. [DOI] [PubMed] [Google Scholar]
- 62.Paracampo A., Solis M., Bonetto C., Mugni H. Acute toxicity of chlorpyrifos to the non-target organism Cnesterodon decemmaculatus. Int. J. Environ. Health Res. 2015;25:96–103. doi: 10.1080/09603123.2014.903903. [DOI] [PubMed] [Google Scholar]
- 63.Yonar M.E. Chlorpyrifos-induced biochemical changes in Cyprinus carpio: Ameliorative effect of curcumin. Ecotoxicol. Environ. Saf. 2018;151:49–54. doi: 10.1016/j.ecoenv.2017.12.065. [DOI] [PubMed] [Google Scholar]
- 64.Abdel-Wahhab M.A., Aljawish A., Kenawy A.M., El-Nekeety A.A., Hamed H.S., Abdel-Aziem S.H. Grafting of gallic acid onto chitosan nano particles enhances antioxidant activities in vitro and protects against ochratoxin A toxicity in catfish (Clarias gariepinus) Environ. Toxicol. Pharmacol. 2016;41:279–288. doi: 10.1016/j.etap.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 65.Hamed H.S., Abdel-Tawwab M. Ameliorative effect of propolis supplementation on alleviating bisphenol-A toxicity: Growth performance, biochemical variables, and oxidative stress biomarkers of Nile tilapia, Oreochromis niloticus (L.) fingerlings. Comp. Biochem. Physiol. C Toxicol. 2017;202:63–69. doi: 10.1016/j.cbpc.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 66.Sadeque M.Z., Begum Z.A. Protective effect of dried fruits of Carica papaya on hepatotoxicity in rat. Bangladesh J. Pharmacol. 2010;5:48–50. doi: 10.3329/bjp.v5i1.5305. [DOI] [Google Scholar]
- 67.Adel M., Dadar M., Khajavi S.H., Pourgholam R., Karimí B., Velisek J. Hematological, biochemical and histopathological changes in Caspian brown trout (Salmo trutta caspius Kessler, 1877) following exposure to sublethal concentrations of chlorpyrifos. Toxin Rev. 2017;36:73–79. doi: 10.1080/15569543.2016.1230631. [DOI] [Google Scholar]
- 68.Barton B.A. Stress in fishes: A diversity of responses with particular reference to changes in circulating corticosteroids. Integr. Comp. Biol. 2002;42:517–525. doi: 10.1093/icb/42.3.517. [DOI] [PubMed] [Google Scholar]
- 69.Mansour A.T., Espinosa C., García-Beltrán J.M., Miao L., Francisco D.C.C., Alsaqufi A.S., Esteban M.Á. Dietary supplementation of drumstick tree, Moringa oleifera, improves mucosal immune response in skin and gills of seabream, Sparus aurata, and attenuates the effect of hydrogen peroxide exposure. Fish. Physiol. Biochem. 2020;46:981–996. doi: 10.1007/s10695-020-00763-2. [DOI] [PubMed] [Google Scholar]
- 70.Salbego J., Pretto A., Gioda C.R., de Menezes C.C., Lazzari R., Radünz Neto J., Baldisserotto B., Loro V.L. Herbicide formulation with glyphosate affects growth, acetylcholinesterase activity, and metabolic and hematological parameters in piava (Leporinus obtusidens) Arch. Environ. Contam. Toxicol. 2010;58:740–745. doi: 10.1007/s00244-009-9464-y. [DOI] [PubMed] [Google Scholar]
- 71.Hamed H.S., Ali R.M., Shaheen A.A., Hussein N.M. Chitosan nanoparticles alleviated endocrine disruption, oxidative damage, and genotoxicity of Bisphenol-A-intoxicated female African catfish. Comp. Biochem. Physiol. C Toxicol. 2021;248:109104. doi: 10.1016/j.cbpc.2021.109104. [DOI] [PubMed] [Google Scholar]
- 72.Kalender Y., Kaya S., Durak D., Uzun F.G., Demir F. Protective effects of catechin and quercetin on antioxidant status, lipid peroxidation and testis-histoarchitecture induced by chlorpyrifos in male rats. Environ. Toxicol. Pharmacol. 2012;33:141–148. doi: 10.1016/j.etap.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 73.Acker C.I., Nogueira C.W. Chlorpyrifos acute exposure induces hyperglycemia and hyperlipidemia in rats. Chemosphere. 2012;89:602–608. doi: 10.1016/j.chemosphere.2012.05.059. [DOI] [PubMed] [Google Scholar]
- 74.Hamza R.Z.M.M. Hyperglycemic effect of chlorpyrifos, profenofos and possible ameliorative role of propolis and ginseng. Sci. Agric. 2014;5:9–14. [Google Scholar]
- 75.Kammon A., Brar R., Sodhi S., Banga H., Singh J., Nagra N. Chlorpyrifos chronic toxicity in broilers and effect of vitamin C. Open Vet. J. 2011;1:21–27. [PMC free article] [PubMed] [Google Scholar]
- 76.Duttaroy A., Zimliki C.L., Gautam D., Cui Y., Mears D., Wess J. Muscarinic stimulation of pancreatic insulin and glucagon release is abolished in m3 muscarinic acetylcholine receptor-deficient mice. Diabetes. 2004;53:1714–1720. doi: 10.2337/diabetes.53.7.1714. [DOI] [PubMed] [Google Scholar]
- 77.van Koppen C.J., Kaiser B. Regulation of muscarinic acetylcholine receptor signaling. Pharmacol. Ther. 2003;98:197–220. doi: 10.1016/S0163-7258(03)00032-9. [DOI] [PubMed] [Google Scholar]
- 78.Montgomery M., Kamel F., Saldana T., Alavanja M., Sandler D. Incident diabetes and pesticide exposure among licensed pesticide applicators: Agricultural Health Study, 1993–2003. Am. J. Epidemiol. 2008;167:1235–1246. doi: 10.1093/aje/kwn028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gilon P., Henquin J.-C. Mechanisms and physiological significance of the cholinergic control of pancreatic β-cell function. Endocr. Rev. 2001;22:565–604. doi: 10.1210/edrv.22.5.0440. [DOI] [PubMed] [Google Scholar]
- 80.Joshi A.K.R., Rajini P. Hyperglycemic and stressogenic effects of monocrotophos in rats: Evidence for the involvement of acetylcholinesterase inhibition. Exp. Toxicol. Pathol. 2012;64:115–120. doi: 10.1016/j.etp.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 81.Ellis A., Hastings T., Munro A. The role of Aeromonas salmonicida extracellular products in the pathology of furunculosis. J. Fish Dis. 1981;4:41–51. doi: 10.1111/j.1365-2761.1981.tb01108.x. [DOI] [Google Scholar]
- 82.Mansour A.T., Miao L., Espinosa C., García-Beltrán J.M., Francisco D.C.C., Esteban M.Á. Effects of dietary inclusion of Moringa oleifera leaves on growth and some systemic and mucosal immune parameters of seabream. Fish Physiol. Biochem. 2018;44:1223–1240. doi: 10.1007/s10695-018-0515-z. [DOI] [PubMed] [Google Scholar]
- 83.Sallam A.E., Mansour A.T., Srour T.M., Goda A.M.A. Effects of different carotenoid supplementation sources with or without sodium taurocholate on growth, feed utilization, carotenoid content and antioxidant status in fry of the European seabass, Dicentrarchus Labrax. Aquacultuture Res. 2017;48:848–3858. doi: 10.1111/are.13212. [DOI] [Google Scholar]
- 84.Kori-Siakpere O. Some alterations in haematological parameters in Clarias, isheriensis (Sydenham) exposed to sublethal concentration of water-born lead. BioScience Res. Commun. 1995;8:93–98. [Google Scholar]
- 85.Jaffer N.S., Rabee A.M., Al-Chalabi S.M. Biochemical and hematological parameters and histological alterations in fish Cyprinus carpio L. as biomarkers for water pollution with chlorpyrifos. Hum. Ecol. Risk Assess. Int. J. 2017;23:605–616. doi: 10.1080/10807039.2016.1261626. [DOI] [Google Scholar]
- 86.Velisek J., Stara A., Kolarova J., Svobodova Z. Biochemical, physiological and morfological responses in common carp (Cyprinus carpio L.) after long-term exposure to terbutryn in real environmental concentration. Pestic. Biochem. Physiol. 2011;100:305–313. doi: 10.1016/j.pestbp.2011.05.004. [DOI] [Google Scholar]
- 87.Forouhar Vajargah M., Imanpoor M.R., Shabani A., Hedayati A., Faggio C. Effect of long-term exposure of silver nanoparticles on growth indices, hematological and biochemical parameters and gonad histology of male goldfish (Carassius auratus gibelio) Microsc. Res. Tech. 2019;82:1224–1230. doi: 10.1002/jemt.23271. [DOI] [PubMed] [Google Scholar]
- 88.Mansour W.A., Abdelsalam N.R., Tanekhy M., Khaled A.A., Mansour A.T. Toxicity, inflammatory and antioxidant genes expression, and physiological changes of green synthesis silver nanoparticles on Nile tilapia (Oreochromis niloticus) fingerlings. Comp. Biochem. Physiol. C Toxicol. 2021;247:109068. doi: 10.1016/j.cbpc.2021.109068. [DOI] [PubMed] [Google Scholar]
- 89.Khalil H.S., Mansour A.T., Goda A.M.A., Omar E.A. Effect of selenium yeast supplementation on growth performance, feed utilization, lipid profile, liver and intestine histological changes, and economic benefit in meagre, Argyrosomus regius, fingerlings. Aquaculture. 2019;501:135–143. doi: 10.1016/j.aquaculture.2018.11.018. [DOI] [Google Scholar]
- 90.Stegeman J., Brouwer M., Richard T., Förlin L., Fowler B., Sanders B., Van Veld P. Molecular responses to environmental contamination: Enzyme and protein systems as indicators of chemical exposure and effect. In: Huggett R.J., Klmerle R.A., Mehrle P.M., Bergman H.L., editors. Biomarkers: Biochemical, Physiological and Histological Markers of Anthropogenic Stress. 1st ed. Lewis Publishers; Chelsea, MI, USA: 1992. pp. 235–335. [Google Scholar]
- 91.Ahmadifar E., Sheikhzadeh N., Roshanaei K., Dargahi N., Faggio C. Can dietary ginger (Zingiber officinale) alter biochemical and immunological parameters and gene expression related to growth, immunity and antioxidant system in zebrafish (Danio rerio)? Aquaculture. 2019;507:341–348. doi: 10.1016/j.aquaculture.2019.04.049. [DOI] [Google Scholar]
- 92.Sula E., Aliko V., Marku E., Nuro A., Faggio C. Evaluation of kidney histopathological alterations in crucian carp, Carassius carassius, from a pesticide and PCB-contaminated freshwater ecosystem, using light microscopy and organ index mathematical model. Int. J. Aquat. Biol. 2020;8:154–165. [Google Scholar]
- 93.Upasani C., Balaraman R. Protective effect of Spirulina on lead induced deleterious changes in the lipid peroxidation and endogenous antioxidants in rats. Phytother. Res. 2003;17:330–334. doi: 10.1002/ptr.1135. [DOI] [PubMed] [Google Scholar]
- 94.Boeira S.P., Borges Filho C., Del’Fabbro L., Roman S.S., Royes L.F.F., Fighera M.R., Jessé C.R., Oliveira M.S., Furian A.F. Lycopene treatment prevents hematological, reproductive and histopathological damage induced by acute zearalenone administration in male Swiss mice. Exp. Toxicol. Pathol. 2014;66:179–185. doi: 10.1016/j.etp.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 95.Sharma P., Khan I.A., Singh R. Curcumin and quercetin ameliorated cypermethrin and deltamethrin-induced reproductive system impairment in male wistar rats by upregulating the activity of pituitary-gonadal hormones and steroidogenic enzymes. Int. J. Fertil. Steril. 2018;12:72. doi: 10.22074/ijfs.2018.5160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Clasen B., Leitemperger J., Murussi C., Pretto A., Menezes C., Dalabona F., Marchezan E., Adaime M.B., Zanella R., Loro V.L. Carbofuran promotes biochemical changes in carp exposed to rice field and laboratory conditions. Ecotoxicol. Environ. Saf. 2014;101:77–82. doi: 10.1016/j.ecoenv.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 97.Aliko V., Qirjo M., Sula E., Morina V., Faggio C. Antioxidant defense system, immune response and erythron profile modulation in gold fish, Carassius auratus, after acute manganese treatment. Fish Shellfish Immunol. 2018;76:101–109. doi: 10.1016/j.fsi.2018.02.042. [DOI] [PubMed] [Google Scholar]
- 98.Kadry S., Amer A., Marzouk M., Hanna M., Azmy A., Hamed H. Vitamin E as antioxidant in female african catfish (Clarias gariepinus) exposed to chronic toxicity of atrazine. Egypt. J. Aquat. Biol. Fish. 2012;16:83–98. doi: 10.21608/ejabf.2012.2127. [DOI] [Google Scholar]
- 99.Abdel-Daim M.M., Dawood M.A., Elbadawy M., Aleya L., Alkahtani S. Spirulina platensis reduced oxidative damage induced by chlorpyrifos toxicity in Nile tilapia (Oreochromis niloticus) Animals. 2020;10:473. doi: 10.3390/ani10030473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shaban N.Z., El-Kersh M.A., El-Rashidy F.H., Habashy N.H. Protective role of Punica granatum (pomegranate) peel and seed oil extracts on diethylnitrosamine and phenobarbital-induced hepatic injury in male rats. Food Chem. 2013;141:1587–1596. doi: 10.1016/j.foodchem.2013.04.134. [DOI] [PubMed] [Google Scholar]
- 101.Xie C., Ge M., Jin J., Xu H., Mao L., Geng S., Wu J., Zhu J., Li X., Zhong C. Mechanism investigation on bisphenol S-induced oxidative stress and inflammation in murine RAW264. 7 cells: The role of NLRP3 inflammasome, TLR4, Nrf2 and MAPK. J. Hazard. Mater. 2020;394:122549. doi: 10.1016/j.jhazmat.2020.122549. [DOI] [PubMed] [Google Scholar]
- 102.Sharma D.K., Ansari B.A. Effects of Deltamethrin on CAT, LPO and GSH in Tissues of Zebrafish Danio rerio. Res. J. Environ. Toxicol. 2013;7:38. doi: 10.3923/rjet.2013.38.46. [DOI] [Google Scholar]
- 103.Mohr T., Desser L. Plant proteolytic enzyme papain abrogates angiogenic activation of human umbilical vein endothelial cells (HUVEC) in vitro. BMC Complement. Altern. Med. 2013;13:231. doi: 10.1186/1472-6882-13-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hamed H.S., Abdel-Tawwab M. Dietary pomegranate (Punica granatum) peel mitigated the adverse effects of silver nanoparticles on the performance, haemato-biochemical, antioxidant, and immune responses of Nile tilapia fingerlings. Aquaculture. 2021;540:736742. doi: 10.1016/j.aquaculture.2021.736742. [DOI] [PubMed] [Google Scholar]
- 105.Yaron Z., Sivan B. Reproduction. In: Evans D., Claibourne J., editors. The Physiology of Fishes. 3rd ed. CRC, Taylor and Francis; Boca Raton, FL, USA: 2006. pp. 343–386. [Google Scholar]
- 106.El-Gamal M.M., Othman S.I., Abdel-Rahim M.M., Mansour A.T., Alsaqufi A.S., El Atafy M.M., Mona M.H., Allam A.A. Palaemon and artemia supplemented diet enhances sea bass, Dicentrarchus labrax, broodstock reproductive performance and egg quality. Aquacult. Rep. 2020;16:100290. doi: 10.1016/j.aqrep.2020.100290. [DOI] [Google Scholar]
- 107.Van Baal J., Hassing G., Goos H., Schulz R. Modulatory effects of 4-nonyphenol on LH production in the African catfish pituitary; Proceedings of the 6th International Symposium on the Reproductive Physiology of Fish; Bergen, Norway. 4–6 June 1999; p. 373. [Google Scholar]
- 108.da Silva B.S., Pietrobon C.B., Bertasso I.M., Lopes B.P., Carvalho J.C., Peixoto-Silva N., Santos T.R., Claudio-Neto S., Manhães A.C., Oliveira E. Short and long-term effects of bisphenol S (BPS) exposure during pregnancy and lactation on plasma lipids, hormones, and behavior in rats. Environ. Pollut. 2019;250:312–322. doi: 10.1016/j.envpol.2019.03.100. [DOI] [PubMed] [Google Scholar]
- 109.Mogahed H.E., El-Rhman A., Afifi N., Barakat H. Protective effects of cactus and/or papaya juices against hepatic and testicular toxicity induced by chlorpyrifos in Albino rats. World J. Pharm. Res. 2019;8:142–161. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the authors upon reasonable request.