Abstract
The ability of trovafloxacin and ciprofloxacin to select efflux mutants in vivo was studied in a model of acute Pseudomonas aeruginosa pneumonia in rats. Twelve hours after intratracheal inoculation of 106 CFU of P. aeruginosa strain PAO1 enmeshed in agar beads, two groups of 12 rats were treated by three intraperitoneal injections of each antibiotic given every 5 h. Dosing regimens were chosen to obtain a comparable area under the concentration-time curve from 0 to infinity/MIC ratio of 27.9 min for trovafloxacin (75 mg/kg of body weight) and of 32.6 min for ciprofloxacin (12.5 mg/kg). Twelve rats were left untreated and served as controls. Rats were sacrificed 12 h after the last injection (34 h after infection) for lung bacteriological studies. Selection of resistant bacteria was determined by plating lung homogenates on Trypticase soy agar plates containing antibiotic. In untreated animals, the frequency of resistant colonies was 10-fold higher than in agar beads. Compared to controls, both treatment regimens resulted in a 2-log reduction of lung bacterial load. The frequency of resistant colonies was 10-fold less with trovafloxacin than with ciprofloxacin at twice the MIC (7.4 × 10−5 versus 8.4 × 10−4, respectively) (P < 0.05) and at four times the MIC (6.2 × 10−4 versus 5.0 × 10−5, respectively) (P < 0.05). A multidrug resistance phenotype typical of efflux mutants was observed in all 41 randomly tested colonies obtained from treated and untreated rats. In agreement with in vitro results, trovafloxacin and ciprofloxacin preferentially selected MexCD-OprJ and MexEF-OprN overproducers, respectively. These results demonstrate the differential ability of trovafloxacin and ciprofloxacin to select efflux mutants in vivo and highlight the rapid emergence of those mutants, even without treatment.
Pseudomonas aeruginosa is an opportunistic pathogen characterized by an intrinsic resistance to various antibiotics. This property mainly results from the operation of broad-spectrum drug efflux pumps, which limit the intracellular accumulation of antibiotics in addition to the low membrane permeability of the bacteria (19). Four different efflux systems have been described so far. They share a similar genetic and structural organization but differ in substrate specificity and regulation. The constitutively expressed MexAB-OprM system (15, 24) has the broadest substrate spectrum of all bacterial efflux pumps described so far, including quinolones, tetracycline, chloramphenicol (14), trimethoprim (8), β-lactam antibiotics (13), β-lactamase inhibitors (17), and detergents and solvent molecules (16). A second efflux system, MexCD-OprJ (25), is responsible for efflux of quinolones, erythromycin (21), and cephems (5, 20). The third efflux pump, MexEF-OprN (10), transports chloramphenicol as well as quinolones and trimethoprim. The recently discovered MexXY (23) system is responsible for the active efflux of quinolones, tetracycline, and erythromycin and is involved in the intrinsic resistance of P. aeruginosa to aminoglycosides (27). Quinolones are particularly prone to select multidrug-resistant (MDR) mutants overexpressing efflux systems in vitro (9, 12, 22). In a previous work, Köhler et al. demonstrated that quinolones vary in their capacity to select efflux systems in vitro (9). Of interest, the frequency of resistant colonies was 10- to 100-fold less with trovafloxacin than with ciprofloxacin and older fluoroquinolones. Furthermore, trovafloxacin almost exclusively selected MexCD-OprJ-overproducing mutants, while ciprofloxacin mainly selected MexEF-OprN overproducers.
Although MDR strains overexpressing efflux pumps have been isolated from patients (30) who have received antibiotic therapy, the frequency of emergence of such mutants and the direct relationship between antibiotic treatment and selection of a particular resistance mechanism remain unclear. Furthermore, the selection of antibiotic-resistant bacteria in vivo may be influenced by many factors, including host defenses, pharmacokinetics, diffusion of the antibiotic into the infected site, and heterogeneity of the infection. Indeed, previous studies have suggested that efflux pumps may play a physiological role in vivo, such as resistance to diverse hydrophobic agents in Neisseria gonorrhoeae (6) and Escherichia coli (18, 29), allowing the bacteria to adapt to a hostile environment (bile salts or toxic fatty acids). The aim of our study was therefore to compare, in an experimental model of acute P. aeruginosa pneumonia in rats, the qualitative and quantitative abilities of ciprofloxacin and of trovafloxacin to select antibiotic-resistant mutants.
(This work was presented at the 39th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, Calif., 1999 [abstr. no. 673].)
MATERIALS AND METHODS
Bacterial strains and media.
The antibiotic-susceptible P. aeruginosa laboratory strain PAO1 that was previously used for in vitro selection experiments (9) was also used in this study. MICs of ciprofloxacin (Bayer AG) and trovafloxacin (Pfizer) against PAO1 were 0.12 μg/ml and 1 μg/ml, respectively. Strains were grown at 37°C in Trypticase soy (TS) broth.
Experimental model. (i) Preparation of the inoculum.
The model of acute P. aeruginosa pneumonia has been formerly developed by researchers F. Faurisson, M. Brun-Pascaud, and M. Zhong (Abstr. 30th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 529, 1990), and O. Join-Lambert, F. Faurisson, and C. Carbon (Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. B-51, 1998). This model is derived from the model of chronic P. aeruginosa pneumonia described by Cash et al. (3). PAO1 was grown overnight in TS broth and washed and resuspended in phosphate-buffered saline (PBS, pH 7.0). An aliquot of this suspension was added to 2% melted agar at 50°C to obtain a final bacterial concentration of approximately 108 CFU/ml. Thirty milliliters of sterile heavy mineral oil (Sigma) warmed to 50°C was added to 10 ml of melted agar containing bacteria and mixed vigorously during 1 min. The oil-agar mixture was then cooled rapidly by placing crushed ice around the vessel while stirring continuously for 5 min. During this time, agar droplets solidified into beads. Agar beads were then separated from oil by a low-speed centrifugation (200 × g, 5 min, 4°C). After removal of the supernatant oil, beads were washed once with 10 ml of 0.5% sodium deoxycholic acid, once with 10 ml of 0.25% sodium deochycholic acid, and three times in PBS (1,000 × g, 20 min, 4°C). Beads were then resuspended in an equal volume of PBS and kept at 4°C during the inoculation time. The final bacterial concentration was 107 CFU/ml.
(ii) Infection of animals.
Male Sprague-Dawley rats (Charles River, Saint Aubin Les Elbeufs, France) weighing 200 to 250 g were anesthetized by an intraperitoneal (i.p.) injection of 20 mg of ketamine per kg of body weight. A cervical incision was performed under ether inhalation for complete analgesia. Then 0.1 ml of the agar bead suspension containing 106 CFU was instilled by the transtracheal route after exposure of the trachea. Following inoculation, animals were maintained at 37°C until they were awake.
(iii) Natural outcome and histopathology of lung infection with PAO1.
The mortality rate, lung weight, histology, and bacterial counts at death were studied in 32 untreated rats that received an inoculum of 106 CFU of PAO1.
(iv) Pharmacokinetics.
Plasma areas under the concentration-time curve (AUCs) were determined in infected rats in order to obtain antibiotic concentrations comparable to those observed in humans and a similar AUC from 0 to infinity (AUC0–∞)/MIC of about 30 min for both antibiotics. Ciprofloxacin and trovafloxacin were dissolved in sterile water and were heated at 60°C, 2 min before each injection. Drug concentrations were measured in plasma and in lung homogenates by high-performance liquid chromatography as previously described (1, 28) after an i.p. injection of ciprofloxacin (25 and 12.5 mg/kg) or trovafloxacin (25 and 75 mg/kg). Blood sampling (three animals per point) was performed by intracardiac puncture 1, 3, 5, and 12 h after antibiotic injection. Lung antibiotic concentrations were measured 1 and 5 h after a 25-mg/kg injection of antibiotics. The AUC0–∞ of both antibiotics was determined by using a two-compartment open model, by trapezoidal rule with correction for the final phase with the 1.1 version of Siphar/Win software (Simed, Créteil, France).
(v) Treatments.
Twelve hours after bacterial challenge, two groups of 12 rats were treated by three i.p. injections of ciprofloxacin (12.5 mg/kg) or trovafloxacin (75 mg/kg) given every 5 h. A third group of 12 animals was left untreated and served as control. All rats were killed 12 h after the last dose of antibiotic (i.e., 34 h after the bacterial challenge for all three groups) by an i.p. injection of phenobarbital. Rats which died before the sacrifice were excluded from the study. Lungs were removed and homogenized in 1 ml of physiological saline. Bacterial counts were measured by plating serial dilutions of lung homogenates on TS agar plates incubated at 37°C during 48 h.
(vi) Analysis of in vivo-selected resistant mutants.
Antibiotic-resistant colonies were obtained by plating 100 μl of homogenized lung tissue on TS agar plates containing ciprofloxacin or trovafloxacin at two and four times their MIC against PAO1. Spontaneously resistant colonies emerging in untreated animals were selected by the same method on ciprofloxacin- or trovafloxacin-containing agar plates. As controls, 100-μl aliquots of the overnight bacterial suspension used to prepare the agar beads and 100 μl of the agar bead suspension were also plated on TS agar containing 0.25 μg of ciprofloxacin/ml or 2 μg of trovafloxacin/ml. Two or three randomly chosen colonies per plate were sampled, independently of their size, at two and four times the MIC. Their MIC and antibiotic resistance phenotype were determined by susceptibility testing using Luria-Bertani agar gradient plates containing ciprofloxacin, trovafloxacin, cefpirome, aztreonam, chloramphenicol, or imipenem. The resistance mechanism was deduced as described previously (9). Briefly, mutants which displayed (compared to the PAO1 wild-type strain) increased resistance to quinolones, aztreonam, and cefpirome but not to imipenem were designated MexAB-OprM overproducers. Mutants with increased MICs for quinolones and cefpirome but unchanged or decreased MICs for aztreonam were designated MexCD-OprJ-overexpressing strains. Finally, mutants displaying increased MICs for quinolones, chloramphenicol, and imipenem but unchanged MICs for the other antibiotics tested were designated MexEF-OprN overproducers. If an identical phenotype of resistance was observed in colonies sampled from the same plate, implying that two colonies of the same clone were sampled, only one colony was retained for the study.
(vii) Statistics.
Bacterial counts are presented as mean ± standard deviation (SD) log CFU/gram. The frequency of resistant strains emerging under treatment and in controls was calculated as follows: resistant bacterial count observed on antibiotic plates/CFU obtained on TS plates. Because of the logarithmic distribution of frequencies, mean frequencies of resistant strains (expressed as mean ± SD) were calculated by using the logarithmic transformed value of each individual frequency. Comparisons of mean frequencies between groups were calculated with logarithmic data. For better legibility of results, mean frequencies are presented as the geometric mean and 95% confidence interval of the mean, calculated as follows: geometric mean ± 1.96 × SD/(number of animals)1/2. Differences between groups were studied by a variance analysis, which when significant, was followed by a Bonferroni-Dunn test. Differences in efflux phenotype distributions betwee n treated groups were studied with the χ2 test. For all statistical tests, a P value lower than 0.05 was considered significant.
RESULTS
Natural outcome and histopathology of lung infection in experimental model.
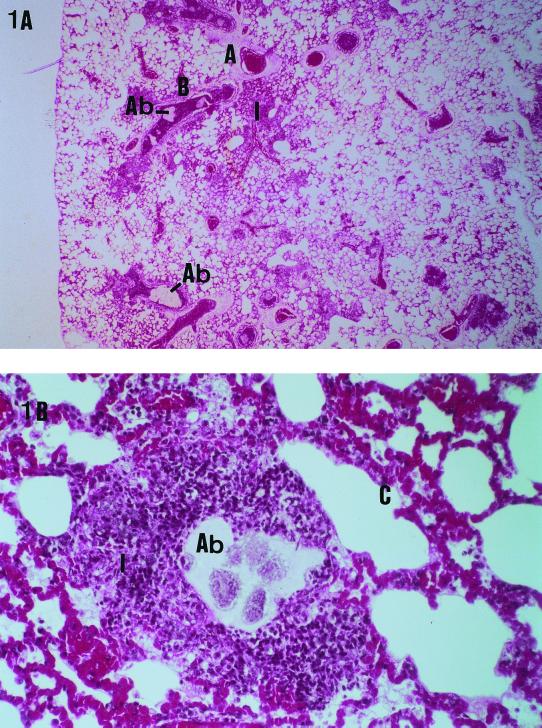
The cumulative mortality rate of 32 rats infected by an inoculum of 106 CFU of PAO1 was 72% 3 days after bacterial challenge. Macroscopic examination of lungs in dead animals showed an aspect of bilateral heterogeneous pneumonia with a threefold increase in lung weight (4.76 ± 0.56 g versus 1.4 ± 0.2 g in uninfected rats) and a lung bacterial load of 8.5 ± 0.8 log CFU/g. Histological examination of these lungs confirmed the presence of a multifocal heterogeneous bronchopneumonia with agar beads trapped in distal bronchioles, surrounded by polymorphonuclear infiltrates and exudates (Fig. 1).
FIG. 1.
Histopathologic aspects of P. aeruginosa acute pneumonia in rats. (A) Histological examination of lungs of animals at death (mean lung bacterial load, 8.5 ± 0.8 log CFU/g; hematoxylin and eosin safran staining), showing the presence of a multifocal heterogeneous bronchopneumonia with agar beads trapped in distal bronchioles, surrounded by polymorphonuclear infiltrates and exudates. Original magnification, approximately ×25. (B) Surrounding interalveolar septa are thickened, due to the presence of congestive capillaries. Original magnification, approximately ×400. Abbreviations: A, pulmonary artery; B, bronchus; Ab, agar bead; C, congestive interalveolar septa; I, polymorphonuclear infiltrate.
Dosing regimens.
The pharmacokinetics of ciprofloxacin and trovafloxacin were similar in infected rats after a single i.p. dose of 25 mg/kg (Table 1). One hour after a single injection of 25 mg of antibiotic/kg, the concentrations measured in plasma and lung were respectively 2.2 ± 0.3 μg/ml and 1.5 ± 0.3 μg/g for trovafloxacin versus 2.1 ± 0.3 μg/ml and 1.8 ± 0.3 μg/g for ciprofloxacin, thus showing a rapid equilibration of both antibiotics in plasma and lung. With these dosing regimens, the AUC0–∞/MIC ratio was higher for ciprofloxacin than for trovafloxacin because of the lower MIC of ciprofloxacin (0.12 μg/ml) than for trovafloxacin (1 μg/ml). Therefore, the dosing regimens were adjusted to 12.5 mg of ciprofloxacin/kg and 75 mg of trovafloxacin/kg to obtain comparable AUC0–∞/MIC ratios of 32.6 and 27.9 min, respectively. These dosing regimens were used in all subsequent experiments.
TABLE 1.
Pharmacokinetics of antibiotics in plasma and in lung (mean ± SD) in infected rats after a single i.p. injectiona
| Drug used (mg/kg) | Antibiotic concn for plasma (μg/ml)/lung (μg/g)
|
AUC0–∞ (μg · min/ml) | AUC0–∞/MICb (min) | |
|---|---|---|---|---|
| Peak (Cmax, 1 h)e | Trough (5 h) | |||
| Ciprofloxacin (25) | 2.1 ± 0.3/1.8 ± 0.3 | 0.3 ± 0.1/0.4 ± 0.02 | 8.1 | 64.4 |
| Ciprofloxacin (12.5)c | 1.5 ± 0.2/NDd | 0.2 ± 0.1/ND | 3.9 | 32.6 |
| Trovafloxacin (25) | 2.2 ± 0.3/1.5 ± 0.3 | 0.5 ± 0.1/0.4 ± 0.08 | 11.6 | 11.6 |
| Trovafloxacin (75)c | 7.0 ± 0.4/ND | 2.6 ± 0.1/ND | 27.9 | 27.9 |
Each value is the mean of measurements made in at least three animals.
Ciprofloxacin and trovafloxacin MICs for PAO1 were 0.12 and 1 μg/ml, respectively.
Regimen was used in in vivo experiments.
ND, not done.
Cmax, maximum concentration of drug in serum.
Antibiotic treatments and isolation of resistant mutants.
Ciprofloxacin (12.5 mg/kg) or trovafloxacin (75 mg/kg) dosing regimens allowed us to obtain a comparable 2-log decrease of the lung bacterial load in comparison with controls (Table 2). Two animals in the trovafloxacin treatment group and one in the ciprofloxacin treatment group died before the end of experiments and were excluded from the study.
TABLE 2.
Resistant colonies isolated from lungs of fluoroquinolone-treated (3 i.p. injections at 5-h intervals) and of untreated control animals (spontaneous mutants)
| Regimen or control category | Lung bacterial counts (log CFU/g) | Bacterial counts in log CFU/g (no. of animals with resistant colonies/no. of animals)
|
|
|---|---|---|---|
| at 2× MIC | at 4× MIC | ||
| Ciprofloxacin (12.5 mg/kg) | 6.0 ± 0.5ab | 3.1 ± 1.1bc (7/11bc) | 3.1 ± 1.2bd (5/11c) |
| Trovafloxacin (75 mg/kg) | 6.4 ± 0.5a | 2.3 ± 1.0c (9/10c) | 2.2 ± 0.7c (6/10c) |
| Control rats | 8.0 ± 0.5 | ||
| Colonies on ciprofloxacin plates | 2.5 ± 0.6 (9/12) | 2.1 ± 0.5 (8/12e) | |
| Colonies on trovafloxacin plates | 2.4 ± 0.7 (4/12) | 1.9 ± 0.3 (3/12) | |
P < 0.001 versus controls.
No significant difference versus trovafloxacin at 75 mg/kg.
No significant difference versus spontaneous efflux mutants to the corresponding antibiotic in controls.
P = 0.03 versus the count of spontaneous ciprofloxacin mutants in controls.
P = 0.04 versus the percentage of animals with trovafloxacin mutants at four times the MIC in controls.
In treated rats, the number of animals yielding resistant colonies was comparable after ciprofloxacin or trovafloxacin therapy (7 of 11 versus 9 of 10 rats at twice the MIC; 5 of 11 versus 6 of 10 rats at four times the MIC, respectively), and no significant difference was observed between the number of resistant colonies per gram of lung tissue isolated from ciprofloxacin- or trovafloxacin-treated rats (Table 2). The same is true for untreated rats, where the counts of resistant colonies emerging either on ciprofloxacin- or on trovafloxacin-containing agar plates were comparable (Table 2).
However, notable differences between the two treatments were observed in the frequency of resistant colonies. For instance, the frequency of resistant mutants obtained after ciprofloxacin exposure (8.4 × 10−4 at twice the MIC) was significantly higher (P < 0.05) (Table 3) than after trovafloxacin exposure (7.4 × 10−5). At twice the MIC these frequencies are 300-fold (ciprofloxacin) and 100-fold (trovafloxacin) higher than those of spontaneous mutants emerging in untreated control rats. Interestingly, the frequencies of resistant colonies isolated from untreated control rats were higher than in the agar bead suspension or in the overnight culture (Table 3).
TABLE 3.
Frequency of resistant colonies (geometric mean, 95% confidence interval) emerging in treated and control rats, in agar beads, and in the overnight culture used to prepare agar beads
| Source of studied colonies | Frequency of resistant colonies [95% confidence interval] (no. of animals)
|
|
|---|---|---|
| at 2× MIC | at 4× MIC | |
| Ciprofloxacin-resistant colonies | ||
| Ciprofloxacin-treated rats (dosage, 12.5 mg/kg) | 8.4 × 10−4 [1.3 × 10−4 to 5.3 × 10−3a,b] (7) | 6.2 × 10−4 [5.6 × 10−5 to 6.9 × 10−3ab] (5) |
| Control ratsd | 2.6 × 10−6 [1.6 × 10−6 to 4.3 × 10−6c] (9) | 8.3 × 10−7 [4.9 × 10−7 to 1.4 × 10−6c] (8) |
| Agar beads | 2.2 × 10−7 | <1.8 × 10−7 |
| Overnight culture | 3.6 × 10−7 | 1.8 × 10−8 |
| Trovafloxacin-resistant colonies | ||
| Trovafloxacin-treated rats (dosage, 75 mg/kg) | 7.4 × 10−5 [2.1 × 10−5 to 2.7 × 10−4a] (9) | 5.0 × 10−5 [1.7 × 10−5 to 1.4 × 10−4a] (6) |
| Control ratsd | 7.2 × 10−7 [2.6 × 10−7 to 2.0 × 10−6] (4) | 1.6 × 10−7 [6.6 × 10−8 to 3.7 × 10−7] (3) |
| Agar beads | <1.8 × 10−7 | NDe |
| Overnight culture | 2.2 × 10−8 | ND |
P < 0.05 versus corresponding controls.
P < 0.05 versus trovafloxacin at 75 mg/kg.
No significant difference versus spontaneous efflux mutants isolated on trovafloxacin agar plates in controls.
Spontaneous efflux mutants obtained from untreated rats and grown on agar plates containing the corresponding antibiotic.
ND, not determined.
Analysis of resistant colonies.
The analysis of the resistance phenotype of 41 randomly selected colonies from treated and untreated rats revealed only MDR mutants exhibiting a typical efflux pump phenotype with cross-resistance to nonquinolone agents. The type of efflux pump mutants selected was treatment dependent. At twice the MIC, ciprofloxacin treatment generated mainly MexEF-OprN-overproducing mutants (87 versus 20% in untreated controls), while trovafloxacin predominantly selected MexCD-OprJ overproducers (62.5 versus 8% in untreated controls) (P < 0.05) (Table 4). At four times the MIC, the most susceptible colonies obtained at twice the MIC were eradicated (especially for trovafloxacin, where only MexCD-OprJ overproducers were obtained), but no shift toward MexCD-OprJ overproducers was observed on agar plates containing four times the MIC of ciprofloxacin (data not shown). By contrast, MexAB-OprM mutants represented a high percentage of spontaneously emerging mutants isolated from untreated control rats (40 and 54% on ciprofloxacin- and trovafloxacin-containing plates, respectively). When the distribution of efflux mutants isolated on ciprofloxacin-containing plates was compared for the overnight culture and the agar bead suspension, a significant increase of MexAB-OprM overproducers (from 0 to 70%) was observed (Table 4), suggesting that conditions used during the bead preparation might have favored this type of efflux mutant.
TABLE 4.
Efflux pump phenotype of selected resistant strains obtained after treatment with ciprofloxacin or trovafloxacin in untreated rats, in agar beads, and in the overnight culture used to prepare agar beadsa
| Source of studied strains | No. of strains studied | % Efflux phenotype of studied strains [95% confidence interval]
|
||
|---|---|---|---|---|
| MexAB-OprM | MexEF-OprN | MexCD-OprJ | ||
| Ciprofloxacin efflux mutants | ||||
| Ciprofloxacin-treated rats (12.5 mg/kg)bc | 15 | 13 [−5 to 31] | 87 [69 to 105] | 0 |
| Control rats | 5 | 40 [−3 to 83] | 20 [15 to 55] | 40 [−3 to 83] |
| Agar beads | 10 | 70 | 30 | 0 |
| Overnight culture | 20 | 0 | 38 | 62 |
| Trovafloxacin efflux mutants | ||||
| Trovafloxacin-treated rats (75 mg/kg)b | 8 | 12.5 [11 to 36] | 25 [−4 to 54] | 62.5 [29 to 96] |
| Control rats | 13 | 54 [27 to 81] | 38 [20 to 56] | 8 [−14 to 30] |
| Agar beadsd | 0 | ND | ND | ND |
| Overnight culture | 20 | 0 | 0 | 100 |
Examined on TS agar plates containing the corresponding antibiotic at twice the MIC.
P < 0.05 versus controls.
P < 0.05 versus trovafloxacin (75 mg/kg).
No mutants were obtained after plating agar beads on trovafloxacin agar. ND, not done.
In summary, the results clearly demonstrate that exposure of P. aeruginosa to quinolones in the animal selects preferentially efflux mutants. A more unexpected result is that even in untreated control rats, the frequency of efflux mutants was increased compared to in vitro conditions, which could suggest a selective advantage of this type of mutant in vivo.
DISCUSSION
Despite progress in antimicrobial therapy, P. aeruginosa lung infection remains a dreadful disease associated with a high level of mortality. Such severity relies partly on host-related factors, since P. aeruginosa lung infections usually occur in mechanically ventilated or cystic fibrosis patients (4, 11), and partly on the high ability of the bacterium to persist in the airways and to develop resistance under antimicrobial therapy. Understanding the resistance mechanisms developed by the bacteria in vivo should help to develop new treatments against P. aeruginosa, including new antibiotic strategies but also new approaches to decrease bacterial virulence (7).
In the present study we analyzed the emergence of resistant mutants both in the absence and presence of antibiotic treatment in an experimental model of P. aeruginosa acute pneumonia. The in vivo model has the advantage of taking into account factors such as pharmacokinetics, postantibiotic effect, and antimicrobial diffusion into the infected site. In the particular setting of P. aeruginosa lung infection, these factors are of critical importance because of the heterogeneity of lung infection, which involves both the terminal bronchioles and the surrounding lung (2, 26). Furthermore, the additional pressure exerted by the host on the bacteria, which have to adapt to their new environment, may influence the selection of antibiotic resistance mechanisms in vivo. In this respect, our model allowed us to study the emergence of resistance in P. aeruginosa either in the absence of treatment during the acute phase of infection or in the presence of antibiotics.
In this study, ciprofloxacin or trovafloxacin was used under comparable pharmacokinetic conditions, i.e., comparable AUC0−∞/MIC ratios. These treatments resulted in a similar 100-fold reduction of the lung bacterial load compared to that in untreated rats. This rather poor reduction is certainly due to suboptimal fluoroquinolone dosing regimens (AUC0−∞/MIC of 30 min), which in contrast probably favored the selection of resistant mutants. Besides, the low solubility of trovafloxacin precluded the possibility of studying the selection of a resistance mechanism at higher dosing regimens.
Compared to untreated rats, the frequency of in vivo-selected resistant bacteria increased about 300-fold and 100-fold after treatment with ciprofloxacin and trovafloxacin, respectively. This increase was mainly due to the killing of susceptible bacteria under treatment, since the counts of mutants per gram of lung tissue were not statistically different in treated and untreated rats. Mutant frequencies were 10-fold higher after treatment with ciprofloxacin than after treatment with trovafloxacin. This is in agreement with previous in vitro data (9) reporting a 10- to 100-fold-lower frequency of mutants emerging after exposure to trovafloxacin than after ciprofloxacin. Therefore, at the dosing regimens used in this study, a weak but significant quantitative difference existed between the two antibiotics in their relative ability to select resistant mutants in vivo.
More importantly, we found that all the resistant colonies tested from both untreated and treated animals presented an efflux phenotype. Different efflux mutant phenotypes were obtained according to the pressure selection exerted, demonstrating that plating lung homogenates in antibiotic-containing agar plates was an accurate method to isolate preselected mutants. In treated animals, the appearance of efflux mutants may be explained by the low antibiotic concentrations achieved in the lung and the short exposure time (about 15 h), which both probably favor the selection of efflux mutants. Our data clearly demonstrate that in the present model, efflux is the predominant resistance mechanism developed by P. aeruginosa after fluoroquinolone exposure. None of the clones analyzed presented changes in quinolone MICs only. Therefore, if target mutations (gyrase and topoisomerase IV) have occurred, they emerged simultaneously with the efflux mutation.
The phenotypic analysis of randomly selected resistant clones from lung homogenates demonstrated that trovafloxacin selected mainly MexCD-OprJ overproducers (62.5 versus 8% in controls). These data are in agreement with formerly published in vitro results (9). Ciprofloxacin predominantly selected mutants overexpressing the MexEF-OprN system (87% of strains versus 20% in controls). In vitro, these mutants were also predominant at two times the MIC, whereas MexCD-OprJ overproducers were predominant at higher ciprofloxacin concentrations (four times the MIC) (9). The fact that no MexCD-OprJ mutants were obtained in ciprofloxacin-treated rats despite peak plasma levels well above the MIC of ciprofloxacin may be due to the variations of antibiotic concentrations obtained in vivo, which is not the case in vitro. If we consider only the minimum antibiotic concentration obtained at trough in vivo, we observe that for both antibiotics these concentrations correspond to two times the MIC for the wild-type PAO1. At these concentrations, the predominant phenotype of mutants obtained in vitro is MexEF-OprN for ciprofloxacin and MexCD-OprJ for trovafloxacin (9), which is in agreement with in vivo data. This phenomenon may suggest that a continuous selection pressure needs to be exerted in order to select mutants overexpressing a particular efflux system. Finally, the low prevalence of MexAB-OprM mutants in treated rats may be explained by the lower MIC increase conferred by this efflux system than those conferred by the MexCD-OprJ and MexEF-OprN systems (9).
More unexpectedly, a significant population of efflux mutants spontaneously emerged in untreated rats at frequencies which were about 10-fold higher than in agar beads or in the overnight culture. This result suggests that efflux pump overexpression may confer a selective advantage to the bacteria in vivo by providing a defense mechanism against potentially harmful agents in the lung environment.
In conclusion, these results show that trovafloxacin and ciprofloxacin qualitatively and quantitatively differ in their ability to select efflux mutants and suggest that pharmacokinetics and host-bacterial interactions may influence the emergence of efflux mutants in vivo. Also, the spontaneous emergence of efflux mutants in untreated rats is an important observation, since it may explain the failure to eradicate P. aeruginosa when suboptimal antibiotic dosing regimens are used.
ACKNOWLEDGMENT
This work was partly supported by a grant from Pfizer Inc.
REFERENCES
- 1.Awni W M, Clarkson J, Gray D R P. Determination of ciprofloxacin and its 7-ethylenediamine metabolite in human serum and urine by high performance liquid chromatography. J Chromatogr. 1987;419:414–420. doi: 10.1016/0378-4347(87)80309-2. [DOI] [PubMed] [Google Scholar]
- 2.Baltimore R S, Christie C D, Smith G J. Immunohistopathologic localization of Pseudomonas aeruginosa in lungs from patients with cystic fibrosis. Implication for the pathogenesis of progressive lung deterioration. Am Rev Respir Dis. 1989;140:1650–1661. doi: 10.1164/ajrccm/140.6.1650. [DOI] [PubMed] [Google Scholar]
- 3.Cash H A, Woods D E, McCullough B, Johanson W G, Jr, Bass J A. A rat model of chronic respiratory infection with Pseudomonas aeruginosa. Am Rev Respir Dis. 1979;119:453–459. doi: 10.1164/arrd.1979.119.3.453. [DOI] [PubMed] [Google Scholar]
- 4.Fagon J Y, Chastre J, Hance A J, Montravers P, Novara A, Gibert C. Nosocomial pneumonia in ventilated patients: a cohort study evaluating attributable mortality and hospital stay. Am J Med. 1993;94:281–288. doi: 10.1016/0002-9343(93)90060-3. [DOI] [PubMed] [Google Scholar]
- 5.Gotoh N, Tsujimoto H, Tsuda M, Okamoto K, Nomura A, Wada T, Nakahashi M, Nishino T. Characterization of the MexC-MexD-OprJ multidrug efflux system in ΔmexA-mexB-oprM mutants of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1998;42:1938–1943. doi: 10.1128/aac.42.8.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hagman K E, Pan W, Spratt B G, Balthazard J T, Judd C, Shafer W M. Resistance of Neisseria gonorrhoeae to antimicrobial hydrophobic agents is modulated by the mtrCDE efflux system. Microbiology. 1995;141:611–622. doi: 10.1099/13500872-141-3-611. [DOI] [PubMed] [Google Scholar]
- 7.Hartman G, Wise R. Quorum sensing: potential means of treating gram-negative infections? Lancet. 1998;351:848–849. doi: 10.1016/S0140-6736(05)70282-8. [DOI] [PubMed] [Google Scholar]
- 8.Köhler T, Kok M, Michea-Hamzehpour M, Plesiat P, Gotoh N, Nishino T, Curty L K, Pechere J C. Multidrug efflux in intrinsic resistance to trimethoprim and sulfamethoxazole in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1996;40:2288–2290. doi: 10.1128/aac.40.10.2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Köhler T, Michéa-Hamzehpour M, Plésiat P, Kahr A, Pechère J C. Differential selection of multidrug efflux systems by quinolones in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1997;41:2540–2543. doi: 10.1128/aac.41.11.2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Köhler T, Michea-Hamzehpour M, Henze U, Gotoh N, Curty L K, Pechère J C. Characterization of MexE-MexF-OprN, a positively regulated multidrug efflux system of Pseudomonas aeruginosa. Mol Microbiol. 1997;23:345–354. doi: 10.1046/j.1365-2958.1997.2281594.x. [DOI] [PubMed] [Google Scholar]
- 11.Kumczycki L, Murphy T M, Bellanti J A. Pseudomonas colonization in cystic fibrosis: a study of 160 patients. JAMA. 1978;240:30–34. [PubMed] [Google Scholar]
- 12.Legakis N J, Tzouvelekis L S, Makris A, Kotsifaki H. Outer membrane alterations in multiresistant mutants of Pseudomonas aeruginosa selected by ciprofloxacin. Antimicrob Agents Chemother. 1989;33:124–127. doi: 10.1128/aac.33.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li X-Z, Ma D, Livermore D M, Nikaido H. Role of efflux pump(s) in intrinsic resistance of Pseudomonas aeruginosa: active efflux as contributing factor to β-lactam resistance. Antimicrob Agents Chemother. 1994;38:1742–1752. doi: 10.1128/aac.38.8.1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li X-Z, Livermore D M, Nikaido H. Role of efflux pump(s) in intrinsic resistance of Pseudomonas aeruginosa: resistance to tetracycline, chloramphenicol and norfloxacin. Antimicrob Agents Chemother. 1994;38:1732–1741. doi: 10.1128/aac.38.8.1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li X-Z, Nikaido H, Poole K. Role of MexA-MexB-OprM in antibiotic efflux in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1995;39:1948–1953. doi: 10.1128/aac.39.9.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li X-Z, Zhang L, Poole K. Role of the multidrug efflux systems of Pseudomonas aeruginosa in organic solvent tolerance. J Bacteriol. 1998;180:2987–2991. doi: 10.1128/jb.180.11.2987-2991.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li X-Z, Zhang L, Srikumar R, Poole K. β-Lactamase inhibitors are substrates for the multidrug efflux pumps of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1998;42:399–403. doi: 10.1128/aac.42.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma D, Cook D N, Atserti M, Pon N G, Nikaido H, Hearst J E. Genes acrA and AcrB encode a stress-induced system of Escherichia coli. Mol Microbiol. 1995;16:45–55. doi: 10.1111/j.1365-2958.1995.tb02390.x. [DOI] [PubMed] [Google Scholar]
- 19.Ma D, Cook D N, Hearst J E, Nikaido H. Efflux pumps and drug resistance in Gram negative bacteria. Trends Microbiol. 1994;2:489–493. doi: 10.1016/0966-842x(94)90654-8. [DOI] [PubMed] [Google Scholar]
- 20.Masuda N, Gotoh N, Ohya S, Nishino T. Quantitative correlation between susceptibility and OprJ production in NfxB mutants of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1996;40:909–913. doi: 10.1128/aac.40.4.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Michéa-Hamzehpour M, Pechère J-C, Plésiat P, Köhler T. OprK and OprM define two genetically distinct multidrug efflux systems in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1995;39:2392–2396. doi: 10.1128/aac.39.11.2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michea-Hamzehpour M, Lucain M C, Pechere J-C. Resistance to pefloxacin in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1991;35:512–518. doi: 10.1128/aac.35.3.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mine T, Morita Y, Kataoka A, Mizushima T, Tsuchiya T. Expression in Escherichia coli of a new multidrug efflux pump, MexXY, from Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1999;43:415–417. doi: 10.1128/aac.43.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poole K, Krebes K, McNally C, Neshat S. Multiple antibiotic resistance in Pseudomonas aeruginosa: evidence for involvement of an efflux operon. J Bacteriol. 1993;175:7363–7372. doi: 10.1128/jb.175.22.7363-7372.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poole K, Gotoh N, Tsujimoto H, Zhao Q, Wada A, Yamasaki T, Neshat S, Yamagishi J, Li X Z, Nishino T. Overexpression of the mexC-mexD-oprJ efflux operon in nfxB-type multidrug-resistant strains of Pseudomonas aeruginosa. Mol Microbiol. 1996;21:713–724. doi: 10.1046/j.1365-2958.1996.281397.x. [DOI] [PubMed] [Google Scholar]
- 26.Potts S B, Roggli V L, Spock A. Immunohistologic quantification of Pseudomonas aeruginosa in the tracheobronchial tree from patients with cystic fibrosis. Pediatr Pathol Lab Med. 1995;15:707–721. doi: 10.3109/15513819509027007. [DOI] [PubMed] [Google Scholar]
- 27.Ramos-Aires J, Köhler T, Nikaido H, Plésiat P. Involvement of an active efflux system in the natural resistance of Pseudomonas aeruginosa to aminoglycosides. Antimicrob Agents Chemother. 1999;43:2624–2628. doi: 10.1128/aac.43.11.2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Teng R, Girard D, Gootz T D, Foulds G, Liston T E. Pharmacokinetics of trovafloxacin (CP-99, 219), a new quinolone, in rats, dogs, and monkeys. Antimicrob Agents Chemother. 1996;40:561–566. doi: 10.1128/aac.40.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thanassi D G, Cheng L W, Nikaido H. Active efflux of bile salts in Escherichia coli. J Bacteriol. 1997;179:2512–2518. doi: 10.1128/jb.179.8.2512-2518.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ziha-Zarifi I, Llanes C, Köhler T, Pechère J-C, Plésiat P. In vivo emergence of multidrug resistant mutants of Pseudomonas aeruginosa overexpressing the active efflux system MexA-MexB-OprM. Antimicrob Agents Chemother. 1999;43:287–291. doi: 10.1128/aac.43.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]