Abstract
The main objectives of the present study were to investigate anti-Vibrio spp., antibiofilms, and anti-quorum-sensing (anti-QS) properties of caraway essential oil in relation to their phytochemical composition. The results obtained show the identification of twelve compounds, with carvone (58.2%) and limonene (38.5%) being the main ones. The obtained essential oil (EO) is particularly active against all Vibrio spp. species, with bacteriostatic action against all tested strains (MBC/MIC ratio ≥ 4) and with inhibition zones with high diameters of growth, ranging from 8.66 ± 0.58 mm for V. furnisii ATCC 35016 to 37.33 ± 0.58 mm for V. alginolyticus ATCC 17749. Caraway essential oil (Carvone/limonene chemotype) exhibits antioxidant activities by using four tests (DPPH = 15 ± 0.23 mg/mL; reducing power = 7.8 ± 0.01 mg/mL; β-carotene = 3.9 ± 0.025 mg/mL; chelating power = 6.8 ± 0.05 mg/mL). This oil is particularly able to prevent cell-to-cell communication by inhibiting swarming motility, production of elastase and protease in Pseudomonas aeruginosa PAO1, and violacein production in C. violaceum in a concentration-dependent manner. A molecular docking approach shows good interaction of the identified bioactive molecules in caraway EO, with known target enzymes involved in antioxidant, antibacterial, and anti-QS activities having high binding energy. Overall, the obtained results highlight the possible use of caraway essential oil against pathogenic Vibrio species and to attenuate the secretion of virulence-related factors controlled by QS systems in Gram-negative bacteria. Therefore, this oil can be used by food industries to prevent biofilm formation on abiotic surfaces by Vibrio strains.
Keywords: volatile oil, Carum carvi L., antioxidant, Vibrio spp., biofilm, anti-quorum sensing, pharmacokinetics, molecular docking
1. Introduction
Bacteria belonging to the genus Vibrio are natural hosts of the marine environment [1,2,3]. The ubiquity of these bacteria in the marine environment and the potential seriousness of these infections, especially for sensitive people, have drawn attention to these microorganisms [4,5]. Ultra-recent advances in understandings of bacterial behavior have shown the existence of a cell-to-cell communication mechanism called quorum sensing, which is involved in the regulation of social behavior and expression of virulence factors involved in their pathogenicity [6]. Plant-derived molecules, due to their wide spectrum of biological activities and especially due to their antimicrobial and antioxidant properties, have long been well recognized in medicine and pharmacy [7], such as antioxidant [8,9,10,11,12,13], antimicrobial [10,11,12,13], cytotoxicity [12,13], anti-acetylcholinesterase [12,13], and antidiabetic [13] properties as well as anti-inflammatory [9] and preventive effects on the cardiac remodeling process [14]. Thus, essential oils (EOs) from many plants have been well-used for hundreds of years to fight an infinite number of pathogens, including bacteria, viruses, and fungi [14,15,16]. Recently, great attention has been paid to the efficacy and safety of nanomedicine based on natural products in preclinical models of various diseases as well as for other applications [17,18,19,20,21]. EO also has anti-QS activity and targets the QS system via three pathways, either by the breakdown of the synthesis of signal molecules or their degradation or by blocking their receptors. Biofilm formation is controlled by QS mechanisms, which makes biofilm formation and QS two central and interconnected features of the social life of bacteria. Indeed, the fight against biofilms can be defined according to two main axes: preventing the formation of biofilms and destroying them when they are already present [22].
Carum carvi L., or caraway, belongs to the Apiaceae family, which is a biennial plant species with fine and grooved stems. The leaves are oblong, and its white flowers, sometimes pink, appear in umbels. Its seeds are 5 mm in measurement. Its size does not exceed 60/75 cm in height at most. Carvi is made up of D-carvone-rich essential oil, together with fatty oils and polysaccharides [23]. The fruits are used in traditional medicine as a carminative, and it is added to young children’s dishes to help digestion and is added to food products in rye bread for its taste and flavor [24]. Caraway possesses several pharmacological properties, including antispasmodic, emmenagogue, expectorant, galactagogue, stimulant, stomachic, and tonic properties [24]. Moreover, carvi EO has been proved for its cancer chemopreventive effects against colon premalignant injuries induced by dimethylhydrazine [25]. The antimicrobial, antioxidant, anti-acetylcholinesterase, and antidiabetic activities of caraway essential oil have also been reported in our laboratory [16].
Hence, the aim of this work was to study the chemical composition of caraway seeds commonly used in the Saudi kitchen to prepare fish and shellfish dishes. Antibacterial activity has been tested against several pathogenic Vibrio spp. strains. The ability of the obtained volatile oil to scavenge reactive oxygen species in vitro using different assays has been assessed. An in silico approach was performed in order to elucidate its physicochemical properties, pharmacokinetic properties, drug-likeness, and toxicity prediction of the identified bioactive compounds in caraway essential oil.
2. Results
2.1. Chemical Profile of C. carvi EO
The yield of extraction was about 3.52% (v/w) based on dry weight. Twelve phytoconstituents representing 98.7% of the total oil composition were identified (Table 1).
Table 1.
Chemical composition of Carum carvi L. (seeds) EO. l.r.i.: Linear Retention Index.
| Code | Components | l.r.i. | Percentage | MW (g·mol−1) | Chemical Formula |
|---|---|---|---|---|---|
| Monoterpene hydrocarbons (39%) | |||||
| 1 | Myrcene | 993 | 0.4 | 136.238 | C10H16 |
| 2 | D-3-carene | 1013 | 0.1 | 136.238 | C10H16 |
| 3 | Limonene | 1032 | 38.5 | 136.238 | C10H16 |
| Oxygenated monoterpenes (59.6%) | |||||
| 4 | trans-p-mentha-2.8-dien-1-ol | 1126 | 0.1 | 152.237 | C10H16O |
| 5 | cis-limonene oxide | 1136 | 0.1 | 152.230 | C10H16O |
| 6 | cis-p-mentha-2.8-dien-1-ol | 1139 | 0.1 | 152.230 | C10H16O |
| 7 | cis-dihydrocarvone | 1195 | 0.7 | 152.230 | C10H16O |
| 8 | trans-dihydrocarvone | 1202 | 0.2 | 152.230 | C10H16O |
| 9 | trans-carveol | 1219 | 0.1 | 152.230 | C10H16O |
| 10 | cis-carveol | 1231 | 0.1 | 152.230 | C10H16O |
| 11 | Carvone | 1242 | 58.2 | 150.220 | C10H14O |
| Phenylpropanoids (0.1%) | |||||
| 12 | Eugenol | 1358 | 0.1 | 164.200 | C10H12O2 |
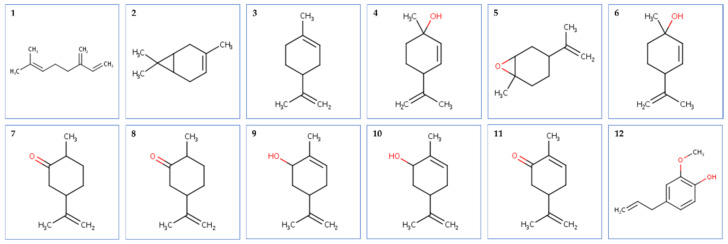
The tested oil was dominated by oxygenated monoterpenes (59.6%), followed by monoterpene hydrocarbons (39%) and phenylpropanoids (0.1%). Carvone (58.2%) and limonene (38.5%) were the main compounds identified. The chemical structures of the identified compounds are represented in Figure 1.
Figure 1.
Structure of phytocompounds identified by GC-MS technique from caraway essential oil. Legend: (1): Myrcene; (2): D-3-carene; (3): Limonene; (4): trans-p-mentha-2.8-dien-1-ol; (5): cis-limonene oxide; (6): cis-p-mentha-2.8-dien-1-ol; (7): cis-dihydrocarvone; (8): trans-dihydrocarvone; (9): trans-carveol; (10): cis-carveol; (11): Carvone; (12): Eugenol.
2.2. Antioxidant Activities Screening
The antioxidant activity of caraway essential oil examined using four different assays (DPPH, reducing power, β-carotene, and chelating power) is outlined in Table 2. According to the results of the DPPH free radical scavenging assay, caraway essential oil exhibits potent antioxidant effects with an IC50 value of 15 ± 0.23 mg/mL, which is significantly (p < 0.05) lower than that of BHT and ascorbic acid, used as standards.
Table 2.
Antioxidant activities of caraway essential oil compared to ascorbic acid and BHT. BHT: Butylated hydroxytoluene. The letters (a–c) indicate a significant difference between the different antioxidant methods according to the Duncan test (p < 0.05).
| DPPH IC50 (mg/mL) |
Reducing Power EC50 (mg/mL) |
β-Carotene IC50 (mg/mL) |
Chelating Power IC50 (mg/mL) |
|
|---|---|---|---|---|
| C. carvi EO | 15 ± 0.23 a | 7.8 ± 0.01 c | 3.9 ± 0.025 a | 6.8 ± 0.05 b |
| Ascorbic acid | 12 ± 0.01 b | 25 ± 0.01 a | - | - |
| BHT | 11.5 ± 0.62 b | 23.00 ± 1.0 b | 4.60 ± 1.60 a | - |
| EDTA | - | - | - | 32.50 ± 1.32 a |
Interestingly, the results show that caraway essential oil displays a significantly higher redox property (2.95–3.2 times) towards the reducing power test when compared to the commercial standards, BHT and ascorbic acid. Moreover, the chelating power of caraway essential oil is significantly (p < 0.05) higher (4.7 times) than that of EDTA (IC50 = 32.50 ± 1.32 mg/mL), used as a positive control. A comparison of the capacity of caraway essential oil to inhibit linoleic acid oxidation to that of BHT points out very similar results (IC50 = 3.9 ± 0.025 vs. 4.60 ± 1.60) with no significant difference (p > 0.05).
2.3. Antimicrobial Activity
2.3.1. Vibriocidal Activities
The antimicrobial activity of caraway essential oil was qualitatively and quantitatively assessed by the presence or absence of an inhibition zone, MIC, and MBC values (Table 3). The results obtained from the disc diffusion method indicate that caraway chemotype (Carvone/limonene) tested at 10 mg/disc exhibited antimicrobial activity against all Vibrio spp. with various degrees of antimicrobial activity depending on the strain tested.
Table 3.
Antimicrobial activity of the tested Carum essential oil evaluated by disc diffusion and microdilution assays as compared to five antibiotics. GIZ: Mean of Growth Inhibition Zone. The letters (a–o) indicate a significant difference between the different means of GIZ according to the Duncan test (p < 0.05). C: chloramphenicol 30 µg; AM: ampicillin 10 µg; E: erythromycin 10 µg; TE: tetracycline 5 µg; G: gentamycin 10 µg.
| Microorganisms Tested | Disc Diffusion Assay | Microdilution Assay | |||||||
|---|---|---|---|---|---|---|---|---|---|
| (GIZ ± SD) | C | AM | E | TE | G | MIC | MBC | MBC/MIC Ratio | |
| V. cholerae ATCC 9459 | 23.33 ± 0.58 l | 27 | 8 | 6 | 20 | 18 | 0.022 | 5.781 | >4; Bacteriostatic |
| V. cholerae | 11.67 ± 0.58 cd | 8 | 10 | 6 | 21 | 20 | 0.022 | 5.781 | >4; Bacteriostatic |
| V. vulnificus ATCC 27962 | 30.33 ± 0.58 n | 17 | 7 | 26 | 24 | 16 | 0.022 | 23.125 | >4; Bacteriostatic |
| V. vulnificus S 5 | 11.33 ± 0.58 c | 18 | 7 | 17 | 13 | 19 | 0.022 | 5.781 | >4; Bacteriostatic |
| V. vulnificus V 30 | 13.33 ± 0.58 e | 30 | 6 | 6 | 25 | 20 | 0.022 | 11.562 | >4; Bacteriostatic |
| V. parahaemolyticus ATCC 17802 | 16.00 ± 0.00 g | 8 | 7 | 7 | 17 | 17 | 0.022 | 11.562 | >4; Bacteriostatic |
| V. parahaemolyticus ATCC 43996 | 22.66 ± 0.58 l | 22 | 7 | 14 | 17 | 18 | 0.022 | 2.890 | >4; Bacteriostatic |
| V. parahaemolyticus I12 | 16.66 ± 0.58 gh | 7 | 7 | 14 | 20 | 16 | 0.022 | 11.562 | >4; Bacteriostatic |
| V. parahaemolyticus I22 | 25.33 ± 0.58 m | 19 | 10 | 14 | 15 | 20 | 0.022 | 11.562 | >4; Bacteriostatic |
| V. parahaemolyticus | 12.66 ± 0.58 de | 24 | 7 | 12 | 14 | 18 | 0.022 | 5.781 | >4; Bacteriostatic |
| V. parahaemolyticus S 949 | 11.33 ± 0.58 fg | 13 | 7 | 7 | 13 | 20 | 0.022 | 5.781 | >4; Bacteriostatic |
| V. parahaemolyticus S 950 | 11.67 ± 0.58 cd | 13 | 10 | 7 | 15 | 17 | 0.022 | 11.562 | >4; Bacteriostatic |
| V. alginolyticus ATCC 33787 | 17.66 ± 0.58 h | 22 | 6 | 22 | 17 | 17 | 0.022 | 11.562 | >4; Bacteriostatic |
| V. alginolyticus ATCC 17749 | 37.33 ± 0.58 o | 25 | 7 | 7 | 16 | 12 | 0.045 | 2.890 | >4; Bacteriostatic |
| V. alginolyticus | 24.66 ± 0.58 m | 20 | 6 | 20 | 15 | 10 | 0.022 | 11.562 | >4; Bacteriostatic |
| V. alginolyticus S 6 | 11.67 ± 0.58 cd | 13 | 6 | 7 | 11 | 12 | 0.022 | 2.890 | >4; Bacteriostatic |
| V. alginolyticus S 7 | 12.67 ± 0.58 de | 29 | 15 | 14 | 7 | 23 | 0.090 | 23.125 | >4; Bacteriostatic |
| V. alginolyticus S 5 | 22.66 ± 0.58 l | 18 | 8 | 15 | 13 | 20 | 0.022 | 2.891 | >4; Bacteriostatic |
| V. furnisii ATCC 35016 | 8.66 ± 0.58 a | 33 | 7 | 30 | 20 | 13 | 0.022 | 23.125 | >4; Bacteriostatic |
| V. cincinnatiensis ATCC 35912 | 10.33 ± 058 b | 24 | 7 | 30 | 18 | 15 | 0.022 | 23.125 | >4; Bacteriostatic |
| V. proteolyticus ATCC 15338 | 20.33 ± 0.58 j | 24 | 8 | 10 | 16 | 16 | 0.022 | 11.562 | >4; Bacteriostatic |
| V. natrigens ATCC 14048 | 21.67 ± 0.58 k | 18 | 13 | 25 | 16 | 14 | 0.022 | 5.781 | >4; Bacteriostatic |
| V. mimicus ATCC 33653 | 21.33 ± 0.58 k | 18 | 10 | 12 | 15 | 14 | 0.022 | 11.562 | >4; Bacteriostatic |
| V. fluvialis ATCC 33809 | 14.66 ± 0.58 f | 11 | 7 | 7 | 17 | 18 | 0.022 | 11.562 | >4; Bacteriostatic |
| V. anguillarum | 23.66 ± 0.58 l | 24 | 6 | 19 | 14 | 20 | 0.045 | 5.781 | >4; Bacteriostatic |
| V. carhiaccae ATCC 35084 | 14.33 ± 0.58 f | 6 | 10 | 7 | 15 | 17 | 0.022 | 5.781 | >4; Bacteriostatic |
| V. harveyi ATCC 18293 | 17.66 ± 0.58 h | 27 | 7 | 12 | 16 | 20 | 0.022 | 5.781 | >4; Bacteriostatic |
| V. diazotrophicus ATCC 33466 | 12.00 ± 0.00 cd | 22 | 8 | 30 | 19 | 18 | 0.022 | 11.562 | >4; Bacteriostatic |
| V. tapetis CECT 4600T | 20.00 ± 1.00 ij | 10 | 19 | 6 | 34 | 15 | 0.022 | 11.562 | >4; Bacteriostatic |
| V. splendidus ATCC 33125 | 19.33 ± 0.58 i | 22 | 6 | 22 | 24 | 16 | 0.022 | 11.562 | >4; Bacteriostatic |
| A. hydrophila ATCC 7966T | 16.66 ± 0.58 gh | 23 | 8 | 7 | 19 | 15 | 0.022 | 11.562 | >4; Bacteriostatic |
In fact, the obtained EO from caraway seeds was tested against a large collection of Vibrio spp. strains, including pathogenic ones isolated from diseased reared fish (Dicentrarchus labrax and Sparus aurata), from Mytilus edulis, and from seawater. At 10 mg/disc, carvone-limonene rich oil was able to inhibit the growth of all Vibrio ssp. strains with different degrees. Indeed, V. alginolyticus strains were the most sensitive ones with growth inhibition zones (GIZs) ranging from 11.67 ± 0.58 mm to 37.33 ± 0.58 mm. Similarly, V. parahaemolyticus strains were also sensitive to the tested oil with GIZs ranging from 11.67 ± 0.58 mm for V. parahaemolyticus isolated from mussels to 25.33 ± 0.58 mm for V. parahaemolyticus isolated from seawater. In addition, a low concentration of caraway EO was needed to kill almost all tested strains with MBC values ranging from 5.781 mg/mL to 23.125 mg/mL. Using the MBC/MIC ratio, the tested oil exhibited bacteriostatic activity against all tested bacteria (MBC/MIC ratio > 4). All these data are summarized in Table 3.
2.3.2. Biofilm Inhibition and Eradication
Vibrionaceae members are mainly aquatic bacteria present in different forms: those that are free planktonic in oceans or estuaries, those that are associated with biotic or abiotic surfaces in biofilms, and finally those that colonize marine animals. Caraway volatile oil was tested for its ability to prevent and eradicate the biofilms formed by four Vibrio species, including V. cholerae, V. vulnificus, V. parahaemolyticus, and V. alginolyticus by using the XTT technique.
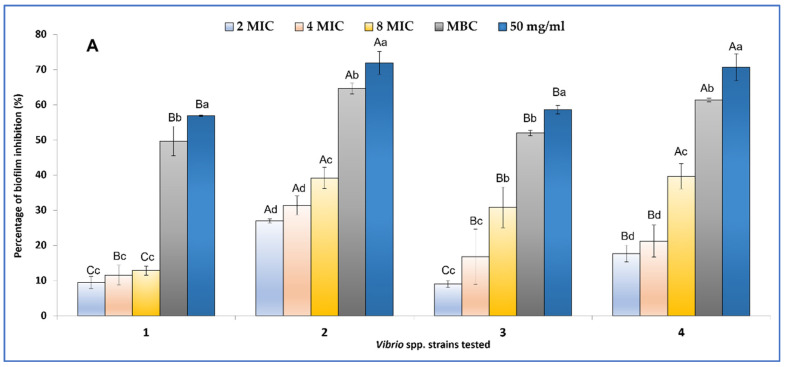
Results obtained show that biofilm formation (Figure 2A) was inhibited by 9.46 ± 1.67% for V. alginolyticus ATCC 33787, by 26.95 ± 0.65% for V. parahaemolyticus ATCC 17802, by 9.02 ± 1.03% for V. vulnificus ATCC 27962, and by 17.66 ± 2.39% for V. cholerae ATCC 9459 at 0.044 mg/mL (2MIC value). At sub-MIC concentrations (50 mg/mL), the formation of biofilms on 96-well plates was inhibited by 71.90 ± 3.26% for V. parahaemolyticus ATCC 17802 and by 70.66 ± 3.84% for V. cholerae ATCC 9459.
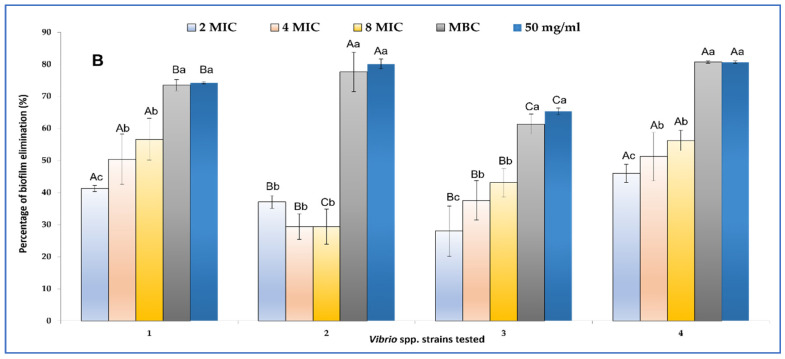
Figure 2.
Effects of different concentrations of C. carvi EO on biofilm formation (A) and eradication (B) expressed as percentages of inhibition evaluated by the XTT technique: V. alginolyticus ATCC 33787 (1, MIC value = 0.022 mg/mL), V. parahaemolyticus ATCC 17802 (2, MIC value = 0.022 mg/mL), V. vulnificus ATCC 27962 (3, MIC value = 0.022 mg/mL), and V. cholerae ATCC 9459 (4). Errors bars represent standard deviation. Values are the average of at least three independent determinations. Means followed by the same letters are not significantly different at p < 0.05 based on Duncan’s multiple range test. Small letters are used to compare different concentrations within the same strain, and capital letters are used to compare means between the same concentrations between strains.
Interestingly, the tested caraway essential oil was able to eradicate more than 50% of preformed V. alginolyticus ATCC 3378 and V. cholerae ATCC 9459 biofilms at 0.088 mg/mL. MBC values are needed to eradicate 50% of biofilms formed by V. parahaemolyticus ATCC 17,802 and V. vulnificus ATCC 27,962 (Figure 2B).
2.3.3. Anti-QS Activity
Caraway essential oil was also tested against virulence-related properties controlled by the QS system in C. violaceum (violacein production) and P. aeruginosa PAO1 (swarming, elastase, and protease production). In fact, caraway essential oil and its main compound (Carvone) were able to inhibit the swarming activity of P. aeruginosa PAO1 starter strain on LB-0.5% in a concentration-dependent manner (Table 4). The motility of this bacterium was reduced by 67.90% at 0.05 mg/mL for caraway essential oil and by 79.01% at 2.5 mg/mL. The main compound, carvone, was able to inhibit the motility of P. aeruginosa PAO1 by 71% at 0.05 mg/mL and by more than 79.62% at 2.5 mg/mL of the same essential oil.
Table 4.
Effect of caraway essential oil and its major compound (carvone) on the swarming activity of P. aeruginosa PAO1 strain expressed as mean diameter of growth on LB-0.5% agar (mm).
| Control (PAO1 Strain) |
Concentrations Tested (mg/mL) | |||||
|---|---|---|---|---|---|---|
| 2.5 | 1.25 | 0.625 | 0.5 | 0.05 | ||
| C. carvi EO | 54.00 ± 0.00 | 11.33 ± 0.57 | 13.33 ± 0.57 | 15.66 ± 0.57 | 16.00 ± 0.00 | 17.33 ± 1.15 |
| Carvone | 54.00 ± 0.00 | 11.00 ± 0.00 | 11.66 ± 0.57 | 13.00 ± 0.00 | 14.33 ± 0.57 | 15.66 ± 0.57 |
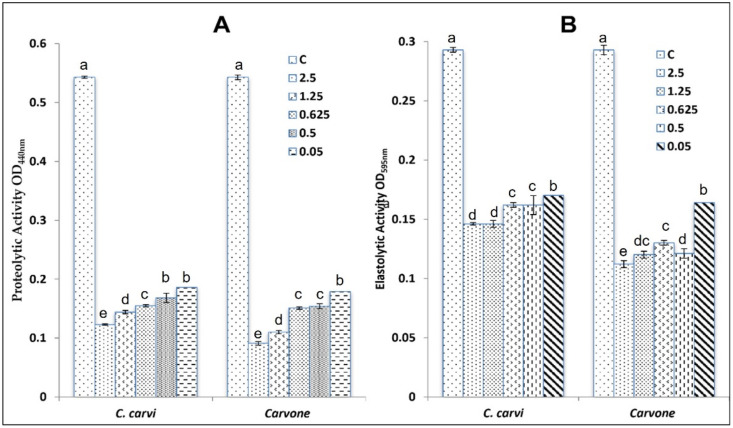
Production of elastase and protease by the PAO1 strain was also inhibited by caraway essential oil, as shown in Figure 3. In fact, proteolytic activity was reduced by 65.74% at 0.05 mg/mL for caraway essential oil and by 67.03% for carvone. At high concentrations (2.5 mg/mL), this oil inhibited the production of protease enzymes in the PAO1 strain by 77.34% and by 83.24% for carvone. Elastase production was also affected by caraway essential oil and carvone in a concentration manner. The highest inhibition was recorded at 2.5 mg/mL and ranged from 50.17% for the oil to 61.77% for its main compound (carvone).
Figure 3.
Effect of caraway essential oil and its main compound (Carvone) tested at different concentrations on P. aeruginosa PAO1 (virulence-related properties controlled by quorum-sensing system: (A) proteolytic activity; (B) elastolytic activity). Values are the average of at least three independent determinations. Means followed by the same letters are not significantly different at p < 0.05 based on Duncan’s multiple range test.
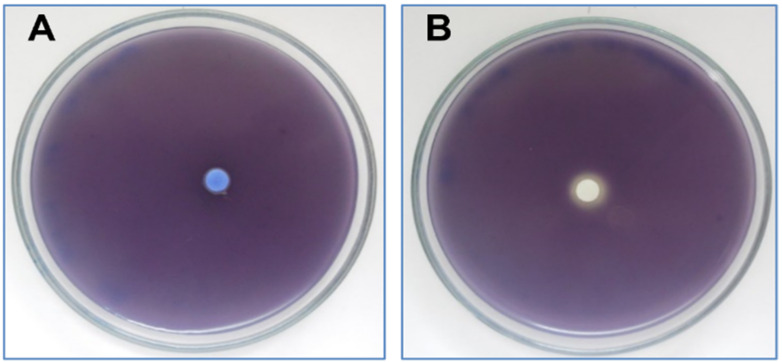
This oil has the ability to inhibit the production of violacein by two different techniques: on LB agar Petri dishes using the mutant strain CV026 and on microtitre plates by using C. violaceum-type strains (ATCC 12472). The results obtained show that the tested oil (Figure 4A) and its major compound (carvone) were unable to inhibit the production of violacein at 2 mg/disc, whereas limonene at 2 mg/disc (Figure 4B) slightly inhibited violacein production (Inhibition zone = 2 mm).
Figure 4.
(A) Effect of caraway essential oil on violacein production by C. violaceum CV026 tested on Lauria–Bertani agar as compared to limonene at 2 mg/disc (B).
As depicted in Table 5, caraway essential oil inhibited the production of violacein by C. violaceum wild type (ATCC 12472) in a concentration-dependent manner. In fact, at an MIC value of 10 mg/mL, the percentage of violacein production in 96-well plates was about 47.57 ± 3.7%. At low MIC values (MIC/32 = 0.312 mg/mL), the production of this pigment was inhibited by 25.28 ± 4.3%. All these data are summarized in Table 5.
Table 5.
Inhibition of violacein production by C. violaceum ATCC 12472 by caraway essential oil at different MIC values. The letters (a,b) indicate significant difference according to the Duncan test (p < 0.05).
| Concentration Tested | % of Violacein Inhibition |
|---|---|
| MIC; (10 mg/mL) | 47.57 ± 3.7 a |
| MIC/2; (5 mg/mL) | 32.26 ± 2.2 b |
| MIC/4; (2.5 mg/mL) | 29.17 ± 1.3 b |
| MIC/8; (1.25 mg/mL) | 28.21 ± 6.1 b |
| MIC/16; (0.625 mg/mL) | 27.89 ± 6.0 b |
| MIC/32; (0.312 mg/mL) | 25.28 ± 4.3 b |
2.4. Molecular Docking Analysis
2.4.1. Molecular Docking Antimicrobial Receptor Proteins
DNA gyrase is an enzyme belonging to a member of bacterial topoisomerase, which, by introducing transient breaks to both DNA strands, can control the topology of DNA during transcription, replication, and recombination. Therefore, this enzyme is essential for bacterial survival and can mainly be exploited as an antibacterial drug target. Here, we attempted to investigate the binding pattern of the most relevant phytocompounds. Molecular docking of trans-dihydrocarvone, eugenol, and trans-carveol, the top three compounds that have the best binding affinity, were performed to identify their binding sites on the structures of S. aureus tyrosyl-tRNA synthetase (PDB ID, 1JIJ) and topoisomerase II DNA gyrase (PDB ID, 2XCT) proteins. In fact, trans-Dihydrocarvone, trans-carveol, and eugenol were found to be the most stable complexes with tyrosyl-tRNA synthetase possessing a binding energy of −6.3 kcal/mol, −6.4 kcal/mol, and −6.3 kcal/mol, respectively.
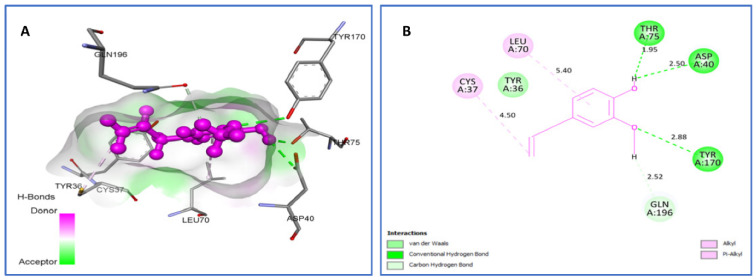
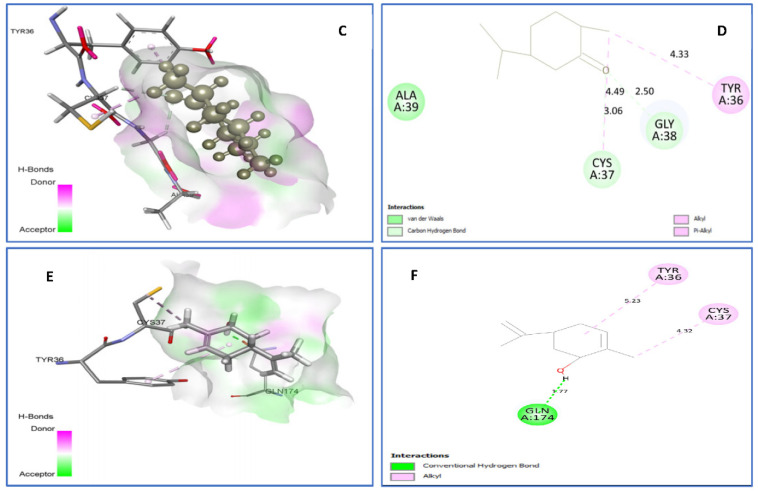
As shown in Figure 5, trans-Dihydrocarvone forms van der Waals interactions with Ala39; C-H bonds with Cys37(3.06) and Gly38 (2.50); and Alkyl/Pi-Alkyl with Tyr36 (4.33) and Cys37 (4.49) amino acids. Trans-Carveol binds to active sites via hydrophobic interactions with Cys37, Ala39, and Tyr36 residues; however, eugenol establishes van der Waals bonding with Tyr36; H bonds with Asp40 (2.50), Thr75 (1.95), and Tyr170 (2.88); C-H bonds with Gln196 (2.52); and Alkyl/Pi-Alkyl with Cys37 (4.50), Leu70 (5.40).
Figure 5.
Two-dimensional (2D) and three-dimensional (3D) residual interactions network of eugenol (A,B), trans-dihydrocarvone (C,D), and trans-carveol (E,F) with the active site of tyrosyl-tRNA synthetase (PDB Id: 1JIJ) enzyme.
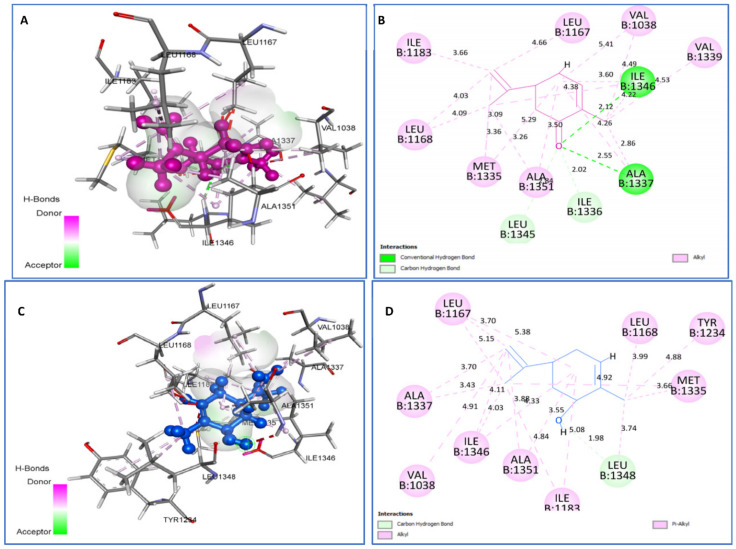
The best-docked natural ligands for topoisomerase II DNA gyrase were found to be cis-carveol and carvone, with the same binding energies of −5.3 kcal/mol. Cis-Carveol forms C-H bonds with Leu1348 and Alkyl/Pi-Alkyl with Ala1351, Ile1346, Leu1168, Met1335, Leu1348, Leu1167, Ile1183, Ile1346, Val1038, and Tyr1234. However, carvone binds to 2XCT by two H bonds, Ala1337 (2.55) and Ile1346 (2.12); one C-H bond with Ile1336 and Leu1345; and Alkyl interactions with Val1038, Ala1337, Met1335, Ile1346, Leu1168, Val1339, and Leu1167 residues (Figure 6).
Figure 6.
Two-dimensional (2D) and three-dimensional (3D) residual interactions network of carvone (A,B) and cis-carveol (C,D) with the active site of topoisomerase II DNA gyrase (PDB Id: 2XCT) enzyme.
2.4.2. Molecular Docking against Antioxidant Receptor Proteins
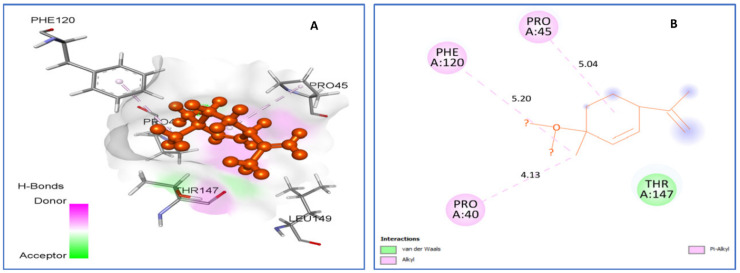
Human peroxiredoxin 5, located mainly in mitochondria, peroxisomes, and cytosol, is implicated in antioxidant protective mechanisms as well as in signal transduction in cells, and thus it is a potential target for evaluation of antioxidant activity. Trans-p-mentha-2.8-dien-1-ol (Figure 7) was found to be best-docked to human peroxiredoxin 5 with a binding energy of −5.2 kcal/mol. It formed hydrophobic interactions with residues Thr147, Pro40 (4.13), Pro45 (5.04), and Phe120 (5.20).
Figure 7.
Two-dimensional (2D) and three-dimensional (3D) residual interactions network of trans-p-mentha-2.8-dien-1-ol (A,B) with the active site of Human peroxiredoxin 5 (PDB ID: 1HD2) enzyme.
2.4.3. Molecular Docking against QS Receptor Proteins
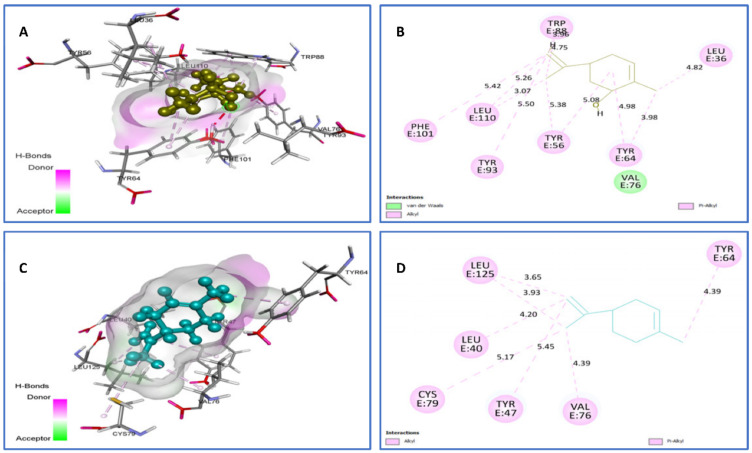
To obtain insight of the possibility of binding interactions between identified phytocompounds and QS receptors, docking studies were performed. The crystal structures of QS signal receptors LasR (PDB ID, 2UV0) and (PDB ID: 3IX3), from P. aeruginosa as a key regulator of P. aeruginosa pathogenesis, and CviR (PDB ID, 3QP1), from C. violaceum ATCC 12472, were used for docking analysis. Limonene and trans-carveol were selected as the top natural ligands against LasR (PDB ID 2UV0, Figure 8) because of their good binding energies within the active site, which are −7.4 kcal/mol and −7.5 kcal/mol, respectively. Their contacts suggest that limonene forms van der Waals interactions with Val76 and Alkyl/Pi-Alkyl with Tyr93 (5.50), Tyr56 (5.08) (5.38), Tyr64 (3.98) (4.98), Leu36 (4.82), Leu110 (3.07) (5.26) (5.42), and Trp88 (3.96) (4.75), all of which are reported in the literature based on X-ray structural observations (Figure 8). In addition, the trans-carveol-2UV0 complex involves the following interactions: Alkyl/Pi-Alkyl with Cys79 (5.17), Tyr47 (5.45), Val76 4.39, Leu40 (4.20), Leu125 (3.65) (3.93), and Tyr64 (4.93).
Figure 8.
Two-dimensional (2D) and three-dimensional (3D) residual interactions network of trans-carveol (A,B) and limonene (C,D) with the active site of LasR enzyme (PDB ID, 2UV0).
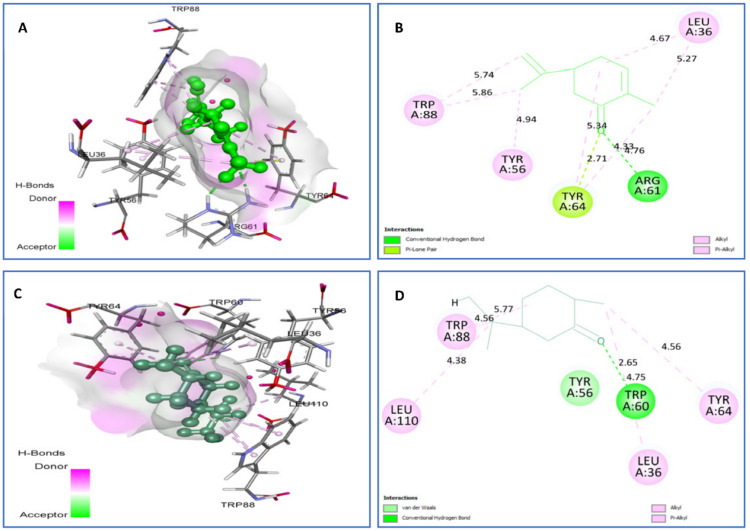
Towards LasR (PDB ID: 3IX3; Figure 9), carvone and trans-dihydrocarvone were also selected as the top natural ligands. Carvone was found to interact with the following 3IX3 residues: H-bonds with Arg61 (2.71), P-Lone Pair with Tyr64 (2.71), and Alkyl/Pi-Alkyl with Leu36 (4.67) (5.27), Tyr56 (4.94), Tyr64 (4.33) (4.76), and Trp88 (5.74) (5.86). However, trans-dihydrocarvone was able to establish H bonds with Trp60 (2.65), van der Waals contacts with Tyr56, and Alkyl/Pi-Alkyl with Leu36 (4.75), Tyr64 (4.56), Trp88 (4.56) (5.71), and Leu110 (4.38).
Figure 9.
Two-dimensional (2D) and three-dimensional (3D) residual interactions network of carvone (A,B) and trans-dihydrocarvone (C,D) with the active site of LasR enzyme (PDB ID, 3IX3).
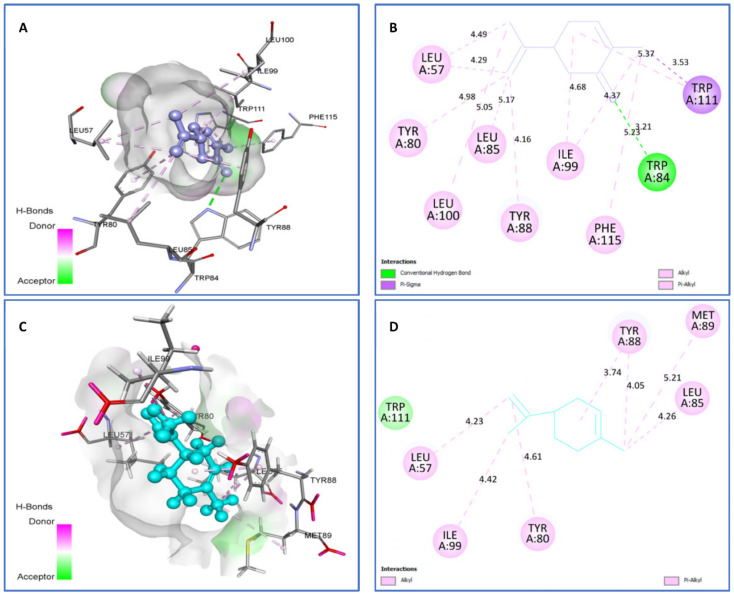
On the other hand, towards the CviR protein (Figure 10), limonene (−7.4 kcal/mol) and carvone (−7.5 kcal/mol) were highly bound ligands, with limonene showing van der Waals interactions with Trp111 and Alkyl/Pi-Alkyl with Ile99 (4.42), Tyr80 (4.61), Leu57 (4.23), Leu85 (4.26), Met89 (5.21), and Tyr88 (3.74) (5.21). However, carvone displayed one hydrogen bonding interaction with Trp84 (3.21), one Pi-Sigma with Trp111 (3.53), and several Alkyl/Pi-Alkyl with Leu100 (5.05), Tyr80 (4.98), Leu57 (4.29) (4.49), Leu85 (5.17), Tyr88 (4.16), Ile99 (4.37) (4.68), Phe115 (5.23), and Trp111 (5.37) amino acid residues.
Figure 10.
Two-dimensional (2D) and three-dimensional (3D) residual interactions network of carvone (A,B), and limonene (C,D) with the active site of CviR enzyme (PDB ID, 3QP1).
Table 6 summarizes the best-identified bioactive compounds in caraway essential oil with all target proteins, their interacting residues, and binding energies (Kcal/mol).
Table 6.
Best phytoconstituents with the lowest binding energies and their interaction residues with selected target proteins.
| Compounds | Interacting Residues Receptor vs. Targets |
Binding Energy (kcal/mol) |
|---|---|---|
| trans-p-mentha-2.8-dien-1-ol vs. 1HD2 | van der Waals: Thr147. Alkyl/Pi-Alkyl: Pro40 (4.13), Pro45 (5.04), Phe120 (5.20). | −5.2 |
| trans-dihydrocarvone vs. IJIJ | van der Waals: Ala39. C-H bond: Cys37(3.06), Gly38 (2.50), Alkyl/Pi-Alkyl: Tyr36 (4.33), Cys37 (4.49). | −6.3 |
| trans-carveol vs. 1JIJ | Alkyl/Pi-Alkyl: Cys37 (), Ala39 (), Tyr36 (). | −6.4 |
| Eugenol vs. IJIJ | van der Waals: Tyr36. H bond: Asp40 (2.50), Thr75 (1.95), Tyr170 (2.88). C-H bond: Gln196 (2.52). Alkyl/Pi-Alkyl: Cys37(4.50), Leu70 (5.40). | −6.3 |
| cis-carveol vs. 2XCT | C-H bond: Leu1348; Alkyl/Pi-Alkyl: Ala1351, Ile1346, Leu1168, Met1335, Leu1348, Leu1167, Ile1183, Ile1346, Val1038, Tyr1234. | −5.3 |
| Carvone vs. 2XCT | H bond: Ala1337 (2.55), Ile1346 (2.12). C-H bond: Ile1336, Leu1345. Alkyl: Val1038, Ala1337, Met1335, Ile1346, Leu1168, Val1339, Leu1167. | −5.3 |
| Limonene vs. 2UV0 | van der Waals: Val76, Alkyl/Pi-Alkyl: Tyr93 (5.50), Tyr56 (5.08) (5.38), Tyr64 (3.98) (4.98), Leu36 (4.82), Leu110 (3.07) (5.26) (5.42), Trp88 (3.96) (4.75). | −7.4 |
| trans-carveol vs. 2UV0 | Alkyl/Pi-Alkyl: Cys79 (5.17), Tyr47 (5.45), Val76 4.39, Leu40 (4.20), Leu125 (3.65) (3.93), Tyr64 (4.93). | −7.5 |
| Carvone vs. 3IX3 | H bond: Arg61 (2.71). Alkyl/Pi-Alkyl: Leu36 (4.67) (5.27), Tyr56 (4.94), Tyr64 (4.33) (4.76), Trp88 (5.74) (5.86). P-Lone Pair: Tyr64 (2.71) | −7.5 |
| trans-dihydrocarvone vs. 3IX3 | van der Waals: Tyr56. H bond: Trp60 (2.65), Alkyl/Pi-Alkyl: Leu36 (4.75), Tyr64 (4.56), Trp88 (4.56) (5.71), Leu110 (4.38). | −7.5 |
| Limonene vs. 3QP1 | van der Waals: Trp111, Alkyl/Pi-Alkyl: Ile99 (4.42), Tyr80 (4.61), Leu57 (4.23), Leu85 (4.26), Met89 (5.21), Tyr88 (3.74) (5.21). | −7.4 |
| Carvone vs. 3QP1 | H bond: Trp84 (3.21). Pi-Sigma: Trp111 (3.53). Alkyl/Pi-Alkyl: Leu100 (5.05), Tyr80 (4.98), Leu57 (4.29) (4.49), Leu85 (5.17), Tyr88 (4.16), Ile99 (4.37) (4.68), Phe115 (5.23), Trp111(5.37). | −7.5 |
2.5. ADMET Analysis
The study of the disposition of a drug molecule within an organism is an indispensable part of drug discovery based on the assessment of its pharmacokinetic properties, named Absorption, Distribution, Metabolism, Excretion, and Toxicity (ADMET) [23,24,25]. The ADMET properties, as derived from the admetSAR tool, reveal that all identified compounds have a good Human Intestinal Absorption (HIA) score, meaning that they are well-absorbed in the intestinal tract upon oral administration. They were found to easily penetrate to Caco-2 and are promoted to be potential substrates and inhibitors for P-glycoprotein (P-gp), which effluxes drugs and various compounds to undergo further metabolism and clearance, and they are thought to cross the blood–brain barrier (BBB) easily. In terms of metabolism, we found that all analogues are non-substrates and non-inhibitors of any CYP450 isoenzymes (with some exception for compound 5), suggesting that they do not obstruct the biotransformation of drugs metabolized by the CYP450 enzyme. The toxicity profile was predicted through different parameters; the two compounds were identified as non-inhibitors of hERG (human-Ether-a-go-go-related), which is a gene that encodes the protein channel that potassium uses to contribute to heart rate activity, meaning that they do not disrupt the heart. Based on the Ames test, all compounds do not show any mutagenic effects. Moreover, they are not carcinogenic (except myrcene), and they are without any hepatotoxic effect. The acute toxicity test revealed that they are unlikely to present acute hazards (Table 7).
Table 7.
ADMET properties of the identified compounds from caraway essential oil. Compounds 1–12 are the same as those listed in Table 1.
| ADMET Predicted Profile | 1 | 2 | 3 | 4 | ||||
|---|---|---|---|---|---|---|---|---|
| Results | Probability | Results | Probability | Results | Probability | Results | Probability | |
| Absorption | ||||||||
| Human Intestinal Absorption | + | 0.9698 | + | 0.9819 | + | 0.9692 | + | 0.9795 |
| Caco-2 Permeability | + | 0.7783 | + | 0.7793 | + | 0.7994 | + | 0.6792 |
| Blood–Brain Barrier | + | 0.9967 | + | 0.9911 | + | 0.9962 | + | 0.9564 |
| Human oral bioavailability | - | 0.5286 | + | 0.8000 | + | 0.6143 | - | 0.6286 |
| Subcellular localization | Nucleus | 0.5972 | Lysosomes | 0.7499 | Lysosomes | 0.6471 | Lysosomes | 0.5661 |
| P-glycoprotein inhibitor | - | 0.9810 | - | 0.9663 | - | 0.9834 | - | 0.9846 |
| P-glycoprotein substrate | - | 0.9692 | - | 0.9250 | - | 0.9317 | - | 0.8969 |
| Distribution and Metabolism | ||||||||
| CYP3A4 substrate | - | 0.6665 | - | 0.5787 | - | 0.6282 | - | 0.5108 |
| CYP2C9 substrate | - | 0.8209 | - | 0.7890 | - | 0.8110 | - | 0.7759 |
| CYP2D6 substrate | - | 0.7550 | - | 0.7441 | - | 0.7415 | - | 0.7764 |
| CYP3A4 inhibition | - | 0.9747 | - | 0.8365 | - | 0.9257 | - | 0.7643 |
| CYP2C9 inhibition | - | 0.9178 | - | 0.7654 | - | 0.9308 | - | 0.8692 |
| CYP2C19 inhibition | - | 0.8849 | - | 0.7419 | - | 0.8906 | - | 0.8058 |
| CYP2D6 inhibition | - | 0.9341 | - | 0.9171 | - | 0.9398 | - | 0.9189 |
| CYP1A2 inhibition | - | 0.7790 | - | 0.7877 | - | 0.7497 | - | 0.8169 |
| CYP inhibitory promiscuity | - | 0.7215 | - | 0.7928 | - | 0.7657 | - | 0.9195 |
| Excretion and Toxicity | ||||||||
| Carcinogenicity | + | 0.5429 | - | 0.7571 | - | 0.4589 | - | 0.7857 |
| Ames mutagenesis | - | 0.9200 | - | 0.8700 | - | 1.0000 | - | 0.8600 |
| Human ether-a-go-go inhibition | - | 0.6620 | - | 0.4935 | - | 0.5586 | - | 0.5665 |
| Micronuclear | - | 0.9900 | - | 0.9200 | - | 1.0000 | - | 0.9700 |
| Hepatotoxicity | - | 0.8250 | - | 0.8250 | - | 0.8250 | - | 0.8000 |
| Acute Oral Toxicity | III | 0.8030 | III | 0.8186 | III | 0.9069 | III | 0.8083 |
| Biodegradation | + | 0.9000 | + | 0.5500 | + | 0.9500 | + | 0.8000 |
| ADMET Predicted Profile (Regression) | ||||||||
| Water solubility | −3.443 | −4.227 | −3.937 | −1.609 | ||||
| Plasma protein binding | 0.433 | 0.837 | 0.373 | 0.555 | ||||
| Acute Oral Toxicity | 1.66 | 1.5 | 1.856 | 3.839 | ||||
| Tetrahymena pyriformis | 1.193 | 0.252 | 0.113 | −0.445 | ||||
| ADMET Predicted Profile | 5 | 6 | 7 | 8 | ||||
| Results | Probability | Results | Probability | Results | Probability | Results | Probability | |
| Absorption | ||||||||
| Human Intestinal Absorption | + | 0.9840 | + | 0.9795 | + | 0.9873 | + | 0.9873 |
| Caco-2 Permeability | + | 0.7132 | + | 0.6792 | + | 0.7226 | + | 0.7226 |
| Blood–Brain Barrier | + | 0.9743 | + | 0.9564 | + | 0.9778 | + | 0.9778 |
| Human oral bioavailability | + | 0.7000 | - | 0.6286 | + | 0.6286 | + | 0.6286 |
| Subcellular localization | Lysosomes | 0.5804 | Lysosomes | 0.5661 | Mitochondria | 0.5743 | Mitochondria | 0.5743 |
| P-glycoprotein inhibitor | - | 0.9840 | - | 0.9846 | - | 0.9772 | - | 0.9772 |
| P-glycoprotein substrate | - | 0.9223 | - | 0.8969 | - | 0.9097 | - | 0.9097 |
| Distribution and Metabolism | ||||||||
| CYP3A4 substrate | + | 0.5411 | - | 0.5108 | - | 0.6066 | - | 0.6066 |
| CYP2C9 substrate | - | 1.0000 | - | 0.7759 | - | 0.8012 | - | 0.8012 |
| CYP2D6 substrate | - | 0.7083 | - | 0.7764 | - | 0.7855 | - | 0.7855 |
| CYP3A4 inhibition | - | 0.8418 | - | 0.7643 | - | 0.9166 | - | 0.9166 |
| CYP2C9 inhibition | - | 0.5512 | - | 0.8692 | - | 0.9518 | - | 0.9518 |
| CYP2C19 inhibition | + | 0.5571 | - | 0.8058 | - | 0.8753 | - | 0.8753 |
| CYP2D6 inhibition | - | 0.9316 | - | 0.9189 | - | 0.9395 | - | 0.9395 |
| CYP1A2 inhibition | + | 0.7089 | - | 0.8169 | - | 0.7044 | - | 0.7044 |
| CYP inhibitory promiscuity | - | 0.8628 | - | 0.9195 | - | 0.8829 | - | 0.8829 |
| Excretion and Toxicity | ||||||||
| Carcinogenicity | - | 0.8857 | - | 0.7857 | - | 0.7857 | - | 0.7857 |
| Ames mutagenesis | - | 0.9000 | - | 0.8600 | - | 0.8200 | - | 0.8200 |
| Human ether-a-go-go inhibition | - | 0.5581 | - | 0.5665 | - | 0.7524 | - | 0.7524 |
| Micronuclear | - | 0.8100 | - | 0.9700 | - | 1.0000 | - | 1.0000 |
| Hepatotoxicity | - | 0.7500 | - | 0.8000 | - | 0.8500 | - | 0.8500 |
| Acute Oral Toxicity | III | 0.8102 | III | 0.8083 | III | 0.8245 | III | 0.8245 |
| Biodegradation | + | 0.6000 | + | 0.8000 | + | 0.8500 | + | 0.8500 |
| ADMET Predicted Profile (Regression) | ||||||||
| Water solubility | −2.724 | −1.609 | −2.117 | −2.117 | ||||
| Plasma protein binding | 0.589 | 0.555 | 0.394 | 0.394 | ||||
| Acute Oral Toxicity | 1.438 | 3.839 | 2.061 | 2.061 | ||||
| Tetrahymena pyriformis | 0.762 | −0.445 | 0.236 | 0.236 | ||||
| ADMET Predicted Profile | 9 | 10 | 11 | 12 | ||||
| Results | Probability | Results | Probability | Results | Probability | Results | Probability | |
| Absorption | ||||||||
| Human Intestinal Absorption | + | 0.9859 | + | 0.9859 | + | 0.9956 | + | 0.9767 |
| Caco-2 Permeability | + | 0.7041 | + | 0.7041 | + | 0.7047 | + | 0.6118 |
| Blood–Brain Barrier | + | 0.9406 | + | 0.9406 | + | 0.9791 | + | 0.9260 |
| Human oral bioavailability | + | 0.5714 | + | 0.5714 | + | 0.5714 | - | 0.5857 |
| Subcellular localization | Mitochondria | 0.4869 | Mitochondria | 0.4869 | Mitochondria | 0.6420 | Mitochondria | 0.7362 |
| P-glycoprotein inhibitor | - | 0.9772 | - | 0.9772 | - | 0.9716 | - | 0.9765 |
| P-glycoprotein substrate | - | 0.8702 | - | 0.8702 | - | 0.9360 | - | 0.8741 |
| Distribution and Metabolism | ||||||||
| CYP3A4 substrate | - | 0.6433 | - | 0.6433 | - | 0.6171 | + | 0.5312 |
| CYP2C9 substrate | - | 0.8090 | - | 0.8090 | - | 0.8078 | - | 1.0000 |
| CYP2D6 substrate | - | 0.7021 | - | 0.7021 | - | 0.8631 | - | 0.6817 |
| CYP3A4 inhibition | - | 0.8309 | - | 0.8309 | - | 0.8964 | - | 0.9404 |
| CYP2C9 inhibition | - | 0.9206 | - | 0.9206 | - | 0.9425 | - | 0.9581 |
| CYP2C19 inhibition | - | 0.7038 | - | 0.7038 | - | 0.6994 | - | 0.8321 |
| CYP2D6 inhibition | - | 0.8987 | - | 0.8987 | - | 0.9069 | - | 0.9267 |
| CYP1A2 inhibition | - | 0.8306 | - | 0.8306 | - | 0.8146 | - | 0.8382 |
| CYP inhibitory promiscuity | - | 0.8346 | - | 0.8346 | - | 0.8246 | - | 0.9270 |
| Excretion and Toxicity | ||||||||
| Carcinogenicity | - | 0.8571 | - | 0.8571 | - | 0.6768 | - | 0.8429 |
| Ames mutagenesis | - | 0.9500 | - | 0.9500 | - | 0.9600 | - | 0.6300 |
| Human ether-a-go-go inhibition | - | 0.5592 | - | 0.5592 | - | 0.5429 | - | 0.5685 |
| Micronuclear | - | 0.8900 | - | 0.8900 | - | 0.8100 | - | 0.9700 |
| Hepatotoxicity | - | 0.8000 | - | 0.8000 | - | 0.6750 | - | 0.8750 |
| Acute Oral Toxicity | III | 0.8021 | III | 0.8021 | III | 0.8144 | III | 0.7376 |
| Biodegradation | + | 0.7750 | + | 0.7750 | + | 0.6500 | - | 0.5250 |
| ADMET Predicted Profile (Regression) | ||||||||
| Water solubility | −2.258 | −2.258 | −1.998 | −2.874 | ||||
| Plasma protein binding | 0.546 | 0.546 | 0.654 | 0.895 | ||||
| Acute Oral Toxicity | 1.896 | 1.896 | 1.845 | 3.394 | ||||
| Tetrahymena pyriformis | 0.162 | 0.162 | 0.6 | 0.012 | ||||
3. Discussion
Spices such as caraway (C. carvi L.) are widely used in Saudi Arabia to spice and aromatize fish and shellfish dishes. These plant species can be promising sources of phytochemical compounds active against Vibrio spp. pathogenic strains associated with seafood products. Therefore, the main objectives of the present study were to study the anti-Vibrio spp. activity of its EO in relation with its phytochemical composition. Our study revealed the identification of carvone (58.2%) and limonene (38.5%) as its main compounds. These percentages are in accordance with those reported by the European Pharmacopoeia, which defines that caraway essential oil must contain (50.0–65.0%) of carvone (30.0–45.0%) of limonene, (0.1–1.0%) b-myrcene, maximum (2.5%) trans-dihydrocarvone and a maximum of 2.5% for trans-carveol [26]. Additionally, it has been well documented that these two compounds are the main chemotype constituents of caraway essential oil from Tunisia, France, Greece, Ukraine, Moldova, Austria, and Norway, ranging from 38.4% to 45.9% [27].
The tested carvone/limonene chemotype of caraway essential oil is active against all Vibrio spp. with high growth inhibition zones and low MIC and MBC values. In fact, we reported the effectiveness of many plants species against the same strains tested, including Mentha longifolia, M. pulegium, Eugenia caryophyllata, Thymus vulgaris and Rosmarinus officinalis Cuminum cyminum L., M. spicata [28], Elettaria cardamomum [29], Petroselinum crispum and Ocimum basilicum [30], and Allium roseum var. odoratissimum [31].
In addition, thymol and carvacrol-rich Lippia berlandieri Schauer EO from Mexico (48% thymol/23% carvacrol and 25% thymol/40% carvacrol, respectively) are able to inhibit the growth of V. alginolyticus, V. parahaemolyticus, and V. vulnificus in shrimps [32]. In 2017, Partovi and colleagues [33] reported that essential oil extracted from Artemisia absinthium, Zataria multiflora Boiss., Pulicaria gnaphalodes, Trachyspermum ammi, and C. cyminum are highly active against pathogenic V. parahaemolyticus strains as compared to non-pathogenic ones with high diameters of growth inhibition zones, ranging from 23 ± 2.47 mm to 31 ± 1.41 mm for V. parahaemolyticus ATCC 43996 and from 15 ± 0.00 mm to 24 ± 1.41 mm for V. parahaemolyticus ATCC 17802. They also reported that low concentrations of these EOs are needed to inhibit the growth of these two V. parahaemolyticus ATCC strains (MIC values range from 0.025 to >3%, v/v), and MBC values range from 0.05 to >3% (v/v) [33].
All these differences in the effectiveness of Vibrio spp. for EO can be mainly attributed to the main compositions of the tested oils, the strains used, and the techniques applied. In fact, we mention the contributions of minor compounds, especially dihydrocarvone, eugenol, and trans-carveol as predicted by molecular docking, in our study. Dihydrocarvone as a monoterpene ketone has been illustrated for its antimicrobial activity as a potential growth inhibitor of yeast fungi such as Saccharomyces cerevisiae, C. albicans, and Cryptococcus neoformans [34].
The tested caraway essential oil also exhibited good antioxidant activity using four different assays (DPPH, reducing power, β-carotene, and chelating power) as compared to the standard molecules used (BHT, ascorbic acid, and EDTA). In fact, previous reports outline the antioxidant properties of caraway harvested from different ecotypes [16]. Additionally, it is appropriate to highlight here that the mechanism of EO action or its individual chemical constituents mainly depend on their chemical nature or chemical composition. Since antioxidant compounds can mitigate oxidative stress because of their scavenging capacity and/or their reducing power, research into powerful new antioxidants from plants has gained momentum in recent times. As has been shown, the antioxidant activity of C. carvi EO is likely due to the highly oxygenated mono-terpene content for which carvone is a major compound (58.2%). In addition, carvone (2-methyl-5-(1-methylethenyl)-2-cyclohexen-1-one, C10H14O), with its two enantiomeric forms, (−)-carvone and (+)-carvone, is an oxygenated monoterpene possessing a greater capacity to capture free radicals and to reduce power, and it has wide applicability in the food, beverage, and cosmetic industries. Our results corroborate very well with those of Hajlaoui et al. [16].
Our results show that the tested caraway essential oil (chemotype carvone/limonene) is able to inhibit/eradicate Vibrio spp. biofilm and to interfere with quorum-sensing systems in both P. aeruginosa PAO1 and C. violaceum starter strains. In fact, concentrations as low as 2MIC values (about 0.044 mg/mL) of this EO are able to inhibit biofilm formation by four Vibrio species, including V. alginolyticus ATCC 33787, V. parahaemolyticus ATCC 17802, V. vulnificus ATCC 27962, and V. cholerae ATCC 9459, with a percentage of biofilm inhibition ranging from 9.46 ± 1.67% to 26.95 ± 0.65%. In addition, the tested oil exhibits the ability to eradicate the biofilms formed by these Vibrio by 50% at 0.088 mg/mL. Similar results were reported by our team and other researchers who demonstrated the use of EO as a good alternative to Vibrio spp. biofilm formation and eradication [30,35,36]. In fact, EOs from P. crispum (Chemotype 1,3,8-p-menthatriene/β-phellandrene) and O. basilicum (Chemotype linalool/(E)-methylcinnamate) are able to inhibit and eradicate mature biofilms formed by the same strains at low concentrations. Similarly, M. spicata EO (Chemotype carvone/limonene) is able to inhibit biofilm formation by 11.5% and 11.6% for V. alginolyticus ATCC 33787, and by 28% and 40% for V. vulnificus ATCC 27562 at 0.046 and 0.092 mg/mL, respectively. The same EOs are able to eradicate more than 50% of preformed V. cholerae ATCC 9459 and V. alginolyticus ATCC 3378 biofilms at 0.092 mg/mL. In 2019, Mendes and colleagues [37] reported that Protium heptaphyllum EO (chemotype β-phellandrene/p-cymene) is able to inhibit biofilm formation by V. parahaemolyticus at 4 mg/mL due to increases in cell permeability causing the leakage of intracellular components and electrolytes. More recently, Mizan and colleagues [36] reported that clove, thyme, and garlic EOs are able to decrease colony count in biofilms formed on stainless-steel coupons by 0.48, 1.18, and 1.18 log CFU/cm2 at 1xMIC and by 3.60, 4.20, and 2.60 log CFU/cm2 at 8xMIC, respectively.
It has been demonstrated that many EOs can interfere with cell-to-cell communication by regulating virulence factors in many bacteria, including P. aeruginosa PAO1 and C. violaceum [38,39,40,41]. Our results show that caraway essential oil is able to inhibit the motility of P. aeruginosa PAO1 and to decrease the production of elastase and proteases at low concentrations. At MIC values, this EO and its main compound (carvone) are able to inhibit production of violacein by 25.28 ± 4.3%. By direct contact on Lauria–Bertani agar plates, no inhibition was recorded for both caraway essential oil and carvone. In fact, many EOs and phytochemical compounds are described for their abilities to decreases the production of virulence-related properties controlled by the quorum-sensing system in Gram-negative bacteria in a concentration-dependent manner [42,43,44,45]. These activities can be explicated by the effect of carvone (main compound identified in caraway essential oil) as a natural monoterpenoid with a high ability to control biofilm formation elimination by 80% at 60 to 70 µg/mL and violacein production in C. violaceum ATCC 12472 at the same concentration [46]. Similarly, it has been demonstrated that limonene is able to inhibit biofilm formation by P. aeruginosa ATCC 27853 and P. aeruginosa HT5 with different magnitudes and to decrease production of elastase enzymes at concentrations ranging from 0.1 to 4 mg/mL and ranging from (75% to 52%) to (80% to 66%), respectively [47]. Additionally, Luciardi and colleagues [48] reported that the attenuation of swarming, pyocyanin, and elastase production in the P. aeruginosa strain by limonene pure compounds is enhanced by compounds identified in Citrus limon oil. This result points out that biological activities of essential oils can be explicated by the synergism between single compounds identified [49].
Regarding docking, these findings corroborate well with our previous work, in which viridiflorol, methyleugenol, isocembrol, eugenol, α-selinene, and β-caryophyllene oxide [50], as well as 1,3-di-O-caffeoyquinic acid, p-coumaric acid, trans-ferulic acid, naringin, rosmarinic acid, rutin, salviolinic acid, 4,5-di-O-caffeoyquinic acid, apegen-in-7-o-glucoside, quercetrin (quercetin-3-o-rhamonoside), and cirsiliol [51], share the same amino acids when interacting with human peroxiredoxin 5 (1HD2). Moreover, Cys47, Thr44, Gly46, Thr147, Pro40, Pro45, Phe120, Arg127, and Leu149 are the main contributors in the stabilizing of the complex ascorbic acid-1HD2. Moreover, by analyzing the crystal structure of human peroxiredoxin 5 (1HD2), our results corroborate also with those of Noumi et al. [52], in which they docked the identified phytocompounds from methanolic T. polium extract to determine their binding modes from one side and from another site of the active residues of human peroxiredoxin 5. Recently, with the same therapeutic target, it was reported that substituted pyrazolone and dipyrazolotriazine derivatives are linked to the same active side residues of 1HD2 as those that were found in this work [53]. De Clercq et al. [54] showed that one side of the active site pocket contains several hydrophobic residues, including Leu116, Ile119, and Phe120, whose side chains are located near the benzoate aromatic ring, which can act as a hydroxyl radical scavenger (via its benzoate ion).
Our docking results between S. aureus tyrosyl-tRNA synthetase (PDB ID, 1JIJ) and trans-dihydrocarvone, trans-carveol, and eugenol are highly consistent with those involved in the ternary structure of S. aureus TyrRS, showing Cys37, Gly38, Ala39, Asp40, His47, Gly49, His50, Leu70, Thr75, Gln174, Asp177, Gln190, Gly192, Asp195, and Pro222 [55]. Moreover, these interactions are in full accordance with our previous work on phytocompounds from Piper cubeba L. EO [55] as well as with the results obtained from docking the identified compounds from C. gigantea flower extract [55]. In addition, the docking study of fused pyridine derivatives and new imidazo [4,5-b]pyridine-5-thione analogues has been reported to bind to several common amino acids obtained in our work [53,56]. Our docking data for the LasR binding domain corroborate with those obtained by Eswaramoorthy et al. [57], who docked isolated carbazole alkaloids and coumarins from roots of Clausena anisate. The authors found that their compounds possess common residues such as the commercial anti-QS ciprofloxacin. The same trend has been observed with the docking results of cladodionen, which has been proved for its high potential QS inhibitory effect against P. aeruginosa PAO1 [58].
4. Materials and Methods
4.1. Plant Material Sampling and Extraction of EO
Caraway seeds were purchased from a local market in August 2021 (Mahdia, Tunisia). Botanical identification was performed by Dr. Zouhair Noumi, University of Sfax, Tunisia (Voucher No: AN-0004). Essential oil from C. carvi L. seeds (100 g) was extracted by hydrodistillation using a Clevenger apparatus for 4 h with distilled water (500 mL). The EO was dried over anhydrous sodium sulphate and was stored in sealed glass vials in a refrigerator at 4 °C until analysis. The yield of extraction was about 4% (4 mL/100 g of dry seeds).
4.2. Analysis of the Volatile Compounds
GC-MS analysis was performed with a Varian CP-3800 GC equipped with an HP-5 capillary column (30 m × 0.25 mm; coating thickness 0.25 μm) and a Varian Saturn 2000 ion trap mass detector. Analytical conditions were the following: injector and transfer line temperatures were 220 and 240 °C, respectively; oven temperature was programmed from 60 to 240 °C at 3 °C/min; carrier gas helium was 1 mL/min; 0.2 μL 10% hexane solution was injected; and the split ratio was 1:30. Identification of the constituents was based on comparisons of the retention times with those of authentic standards, comparing their Linear Retention Indices relative to the series of n-hydrocarbons, and it was conducted by computer matching against commercial libraries (NIST 98 and ADAMS 95) and a home-made library of mass spectra built up from pure substances and components of known essential oils and MS literature data. Linear retention indices were calculated using the n-alkanes series (C8–C23) using the Van den Dool and Kratz formula. Moreover, the molecular weights of all identified substances were confirmed by chromatography chemical ionization mass spectrometry (GC-MS), using MeOH as a CI-ionizing gas [42,59].
4.3. Biological Activities of Caraway Essential Oil
4.3.1. Evaluation of Anti-Vibrio spp. Activities
The antimicrobial activity of caraway essential oil was tested against a large collection of Vibrio spp. strains, including 17 type strains, 13 Vibrio spp. strains isolated from seawater, fish, and shellfish products, and 1 Aeromonas hydrophila ATCC 7966T strain. These microorganisms were previously isolated from diseased Sparus aurata, Dicentrarchus labrax, and Mytilus edulis in Tunisia [28], and the type strains were kindly provided by Professor Stefania Zanetti from the Department of Biomedical Sciences (University of Sassari, Sassari, Italy), Professor Jesús López Romalde from the Department of Microbiology and Parasitology (CIBUS-Facultad de Biologia, Universidad de Santiago, Santiago de Compostela, Spain), Professor Donatela Ottaviani from the Italian Reference Center for Microbiological and Chemical Control on Shellfish-State Veterinary Institute for Umbria and the Marches (IZSUM, Ancona, Italy), Professor Miguel Angel Morinigo from the Department of Microbiology (Facultad de ciencia de Malaga, Campus de Teatinos, Spain), and Professor Bruno Gomez Gil (Mazatlán Unit for Aquaculture, Sinaloa, Mexico).
Two techniques were used: (i) disk diffusion assay for the determination of the diameters of growth of inhibition zones estimated on Mueller–Hinton agar medium, and (ii) microdilution assay for the determination of the minimal inhibitory concentrations (MICs) and the minimal bactericidal concentrations (MBCs) [35,60]. Vibrio strains were grown on a Mueller–Hinton agar medium supplemented with 1% NaCl from culture stock, and pure colonies were used to prepare 0.5 McFarland turbidity. A cotton swab was used to inoculate fresh Petri dishes. Sterile filter paper disks (6 mm in diameter, Biolife, Italy) were impregnated with 10 mg of caraway EO (10.81 µL/disc) and then placed on the cultured plates. The treated Petri dishes were kept for 1 h at 4 °C and then incubated overnight at 37 °C. The diameters of growth of inhibition zones around the disks were estimated using a 1 cm flat ruler. Five antibiotics (C: chloramphenicol 30 µg; AM: ampicillin 10 µg; E: erythromycin 10 µg; TE: tetracycline 5 µg; and G: gentamycin 10 µg) were used as standard drugs against the tested Vibrio strains.
For the microdilution method, twofold serial dilution of the EO in DMSO-5% was prepared in 96-well plates, starting from 25 µL/mL (23.125 mg/mL) in Mueller–Hinton broth medium with 1% NaCl. A total of 5 µL of microbial inoculum was added to each well containing 100 µL of the serially diluted caraway essential oil. All microtiter plates were incubated overnight at 37 °C. MICs were defined as the lowest concentrations that are able to inhibit the growth of a specific microorganism. To determine MBC values, 3 µL from all the wells with no visible growth were point-inoculated on a Mueller–Hinton agar medium (1% NaCl). After 24 h of incubation, the concentration at which the Vibrio spp. strain with no growth was recorded as the MBC value.
4.3.2. Evaluation of Antioxidant Activities
Antioxidant activity experiments were carried out by using four different assays: DPPH tests as described by Mseddi et al. (2020) [22], β-Carotene bleaching tests, and reducing/chelating power by using the protocols previously described [61,62,63].
For the DPPH assay, 0.25 mL of a 0.2 mM DPPH• methanolic solution was mixed with 1 mL of essential oil at different concentrations (5, 10, 15, and 20 mg/mL) or with 1 mL of control sample. The mixture was left for 30 min at room temperature in the dark. The absorbance was measured at 515 nm, and the scavenging activity (SA%) against DPPH radicals was calculated using the following Equation (1):
| SA% = [(Ac − As)/Ac] × 100 | (1) |
where Ac is the absorbance of the control at 30 min and As is the absorbance of the sample at 30 min. IC50 values represent the essential oil scavenging 50% of DPPH radicals. All samples were analyzed in triplicate.
For ferrous ion chelating activity, different concentrations of essential oil (1, 5, and 15 mg/mL) were added to 0.05 mL of 2 mM FeCl2·4H2O solution and were left for incubation at room temperature for 5 min. Afterwards, the reaction was initiated by adding 0.1 mL of 5 mM ferrozine, and the mixture was adjusted to 3 mL with deionized water, shaken vigorously, and left standing at room temperature for 10 min. The absorbance of the solution was then measured at 562 nm. The percentage of inhibition of ferrozine–Fe2+ complex formation was calculated using the following Equation (2):
| Metal chelating activity (%) = [(Ac − As)/Ac] × 100 | (2) |
where Ac is the absorbance of the control and As is the absorbance of the sample. Results are expressed as IC50. The IC50 values are the concentrations required to chelate 50% of ferrous ions present in the system. Analyses were run in triplicate.
For the reducing power assay, 1 mL of caraway essential oil was (1, 5, 10 mg/mL) mixed with 2.5 mL of phosphate buffer (0.2 M, pH 6.6) and 2.5 mL of K3Fe(CN)6 solution (1 g/100 mL). The mixture was incubated at 50 °C for 25 min, 2.5 mL of a trichloroacetic acid solution (10 g/100 mL) was added, and the mixture was centrifuged for 10 min at 650× g. Finally, 2.5 mL of the upper layer was mixed with 2.5 mL of distilled water and 0.5 mL of FeCl3 aqueous solution (0.1 g/100 mL). The absorbance of the mixture was measured at 700 nm.
The EC50 value (mg/mL) is the effective concentration at which the absorbance was 0.5 for the reducing power. Ascorbic acid was used as a positive control.
For the linoleic acid system, 0.2 mg of β-carotene was dissolved in 2 mL of chloroform and was added to 20 mg of linoleic acid and 200 mg of Tween 40. After removing CHCl3 under a vacuum, oxygenated water (100 mL) was added, and the flask was vigorously shaken until all material dissolved. The emulsion obtained was freshly prepared before each experiment. An aliquot of 150 μL of emulsion was distributed into each of the wells of 96-well microtiter plates, and 10 mg of essential oil or BHA standard solution was added. An equal amount of emulsion was used for the blank sample. The microtiter plate was incubated at 45 °C, and the absorbance was measured at 490 nm using a visible/UV microplate kinetics reader (EL × 808, Bio-Tek instruments, Winooski, VT, USA). Readings of all samples were performed immediately (t = 0 min) and after 120 min of incubation. The antioxidant activity (AA) of the essential oil was evaluated in terms of β-carotene blanching using the following Equation (3):
| AA% = [(A0 − At)/A0] × 100 | (3) |
where A0 is the absorbance of the control at 0 min and At is the absorbance of the sample (essential oil or BHA) at 120 min. The results are expressed as IC50 values (mg/mL).
4.4. Screening for Anti-Quorum Sensing Activities
4.4.1. Effect on Violacein Production
Chromobacterium violaceum strain ATCC 12472, C. violaceum CV026, and P. aeruginosa PAO1 biosensor strains were selected to study the effects of caraway essential oil against some virulence traits controlled by the Quorum-Sensing System in both Gram-negative bacteria [40,42]. In fact, C. violaceum ATCC 12472 was used in qualitative screening by using the protocol previously described by Noumi et al. [64].
For the inhibition of violacein pigment production on agar media, caraway essential oil and its main compound (carvone) were loaded at 2 mg/disc on the surface of CV026-inoculated Lauria–Bertani agar plates supplemented with C6-HSL (50 µL of 1 mg/mL stock). The zone of violacein inhibition was detected by the presence of colourless but viable cells around the disks, and the zone of growth inhibition was also recorded by clear zones around the disks.
For violacein inhibition using a microtiter plate assay, 10 µL of C. violaceum ATCC 12472 was added into wells of sterile microtiter plates containing Lauria–Bertani broth. It was then incubated at 30 °C for 18 h in the presence and absence of various concentrations of caraway essential oil, ranging from 0.3125 mg/mL to 10 mg/mL at 30 °C for 18 h, and it was observed for inhibition of violacein production. For quantification of violacein, the contents of the wells were aspirated into Eppendorf tubes and centrifuged (8000 rpm 6 min) to collect cells. Violacein was extracted from the cells using water-saturated n-butanol. The extracted violacein was separated from the cell debris by centrifugation and quantified by recording OD585 readings spectrophotometrically. Percentage inhibition of violacein by the essential oil was calculated with respect to the control, and 50% inhibition concentration (IC50) was recorded.
4.4.2. Effect on QS-Controlled Virulence Factor Production in P. aeruginosa PAO1
The effects of caraway essential oil on some factors were regulated by the QS system, including swarming motility and proteolytic and elastolytic activities, by using the protocol previously described [42].
In the swarming motility test, overnight cultures of a P. aeruginosa PAO1 strain were point inoculated at the center of semi-solid agar media (1% peptone, 0.5% NaCl, 0.5% agar, and 0.5% of filter-sterilized D-glucose) containing different concentrations of caraway essential oil (0.05, 0.5, 0.625, 1.25, and 2.5 mg/mL). The plate without the essential oil was used as a control. Swarming migration was recorded by following swarm fronts of bacterial cells and is expressed in mm.
For its effect on elastolytic activity, P. aeruginosa PAO1 was cultivated at 37 °C for 16 h in Lauria–Bertani broth media supplemented with different concentrations of caraway essential oil (0.05, 0.5, 0.625, 1.25, and 2.5 mg/mL). For the experiment, 100 μL of each concentration was mixed with 900 μL of elastin Congo red (ECR) buffer (100 mM Tris, 1 mM CaCl2, pH 7.5) containing 20 mg of ECR (Sigma) and was incubated for 3 h at 37 °C. After centrifugation, 200 μL of the supernatant was transferred to sterile 96-well plates, and the optical density was estimated at 495 nm.
To estimate the effects of caraway essential oil on proteolytic activity in a PAO1 starter strain, 100 μL of the bacterial culture was mixed with 900 μL of a buffer containing 3 mg of azocasein (Sigma). Eppendorf tubes were than incubated for 30 min at 37 °C.
100 μL of trichloroacetic acid (TCA, 10%) were added to each tube, and reactions were kept for 30 min. After centrifugation, the optical density of 200 μL of the supernatant was estimated at 440 nm.
4.5. In Silico Approach
4.5.1. Ligand Preparation
Three-dimensional structures of the ligands were retrieved through PubChem (https://pubchem.ncbi.nlm.nih.gov (accessed on 15 December 2021)) chemical information resources [65]. All the ligands were energy minimized using Avogadro, an advanced molecule editor and visualizer [66]. All the minimized ligands were converted to pdbqt before the docking procedure.
4.5.2. Protein Preparation
The receptor proteins (PDB ID: 1HD2, 1JIJ, 2UV0, 2XCT, 2QP1, 3IX3, 3QPR, and 3HIR) were selected from the RSCB protein data bank (http://www.rcsb.org/ (accessed on 15 December 2021)). Water molecules and co-crystal ligands were removed from each of the proteins. The proteins were assigned polar hydrogens, Kollman charges, solvation parameters, and fragmental volumes via the Graphical User Interface program AutoDock Tools (ADT) to prepare pdbqt files. The grid around each protein was created around the binding pocket using ADT [67,68]. AutoGrid was used to create a grid map using a grid box. The grid size and grid dimensions were set for each protein according to the binding pockets, as shown in Table 8. AutoDock Vina was used to dock proteins and ligands utilizing the grid box attributes defined in the configuration file. Proteins were set as rigid throughout the docking operation, and ligands were labeled flexible. Findings with a positional root-mean-square deviation (RMSD) of less than 1.0 were grouped together and represented by the result with the lowest binding free energy. For intra-molecular interaction analysis, the pose with the lowest binding energy or affinity was selected and aligned with the receptor structure [69]. Interaction analysis was conducted by the ‘View Interaction’ protocol from the Discovery Studio (DS) program [Dassault Systems, BIOVIA Corp., San Diego, CA, USA, v 20.1].
Table 8.
Grid size and dimensions for the receptors.
| Protein (PDB ID) | Grid Size (x, y, z Points) |
Grid Dimension Center (x, y, z Coordinates) |
Grid Spacing in Å |
|---|---|---|---|
| 1HD2 | 40 × 40 × 40 | 7.089, 41.659, 34.385 | 0.375 |
| 1JIJ | 40 × 40 × 40 | −11.273, 13.817, 86.080 | 0.375 |
| 2UV0 | 40 × 40 × 40 | 23.998, 16.050, 80.315 | 0.375 |
| 2XCT | 52 × 52 × 52 | 30.098, 32.836, 89.243 | 0.375 |
| 3IX3 | 52 × 52 × 52 | 14.630, −1.973, 9.690 | 0.375 |
| 3QP1 | 38 × 40 × 40 | 20.546, 12.912, 49.410 | 0.375 |
| 3QPR | 126 × 126 × 126 | 54.057, 84.102, 205.034 | 0.375 |
| 3HIR | 60 × 60 × 60 | −26.388, 13.803, −15.845 | 0.375 |
4.6. ADMET Predicted Properties
The ADMET profiles of the top major identified compounds were predicted using the admetSAR online server (http://lmmd.ecust.edu.cn:8000/ (accessed on 15 December 2021)). The admetSAR server provides a user-friendly interface to easily search for chemical profiles, by CASRN, and common names and similarity searches with more than 40 predictive models were implemented in admetSAR for new chemical ADMET properties for in silico filtering.
4.7. Statistical Analysis
Average values of three replicates were calculated using the SPSS 25.0 (SPSS Inc., Chicago, IL, USA) statistical package for Windows. Differences in means were calculated using Duncan’s multiple-range test for means with a 95% confidence interval (p ≤ 0.05).
5. Conclusions
Overall, we report in this paper the identification of carvone and limonene as main compounds in C. carvi essential oil. This EO exhibits potent activity against several pathogenic and non-pathogenic Vibrio strains frequently isolated from fish and shellfish products with high diameters of growth inhibition zones and low minimal inhibitory concentrations for almost all tested strains. Caraway was particularly able to regulate the production of several virulence-related factors in P. aeruginosa PAO1 and C. violaceum biosensor strains. In fact, this EO was able to inhibit and eradicate biofilms formed by V. alginolyticus, V. parahaemolyticus, V. vulnificus, and V. cholerae species at sub-MIC concentrations. At 2.5 mg/mL, the production of elastase and protease by P. aeruginosa PAO1 was reduced by 50.17%, and 77.34%, respectively. Our computational study reveals potent ADME properties and maximal binding affinities against the tested proteins. The obtained results highlight the potential use of C. carvi essential oil to control pathogenic bacteria belonging to the Vibrio genus.
Author Contributions
Conceptualization M.S., K.A., S.G. and A.K.; methodology, M.S., K.A., S.G. and A.K.; formal analysis, M.S., K.A., S.G. and A.K.; software, M.S. and A.K.; investigation, M.S., K.A., S.G. and A.K.; writing—original draft preparation, M.S., K.A., S.G. and A.K.; writing—review and editing, M.S., K.A., S.G. and A.K.; supervision, A.K. and M.S; funding acquisition, K.A.; project administration, K.A. All authors have read and agreed to the published version of the manuscript.
Funding
The author(s) gratefully acknowledge Qassim University, represented by the Deanship of Scientific Research, for its financial support of this research under the number (10215-cos-2020-1-3-I) during the academic year 1442 AH/2020 AD.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data generated and analyzed during this study are included in this article.
Conflicts of Interest
The authors declare that there are no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Snoussi M., Chaieb K., Mahmoud R., Bakhrouf A. Quantitative study, identification and antibiotics sensitivity of some Vibrionaceae associated to a marine fish hatchery. Ann. Microbiol. 2006;56:289–293. doi: 10.1007/BF03175020. [DOI] [Google Scholar]
- 2.Baker-Austin C., Oliver J.D., Alam M., Ali A., Waldor M.K., Qadri F., Martinez-Urtaza J. Vibrio spp. infections. Nat. Rev. Dis. Primers. 2018;4:1–19. doi: 10.1038/s41572-018-0005-8. [DOI] [PubMed] [Google Scholar]
- 3.Dutta D., Kaushik A., Kumar D., Bag S. Foodborne Pathogenic Vibrios: Antimicrobial Resistance. Front. Microbiol. 2021;12:638331. doi: 10.3389/fmicb.2021.638331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ali A., Parisi A., Conversano M., Iannacci A., D’Emilio F., Mercurio V., Normano G. Food-Borne Bacteria Associated with Seafoods: A Brief Review. J. Food Qual. Hazards Control. 2020;7:4–10. doi: 10.18502/jfqhc.7.1.2446. [DOI] [Google Scholar]
- 5.Dumen E., Ekici G., Ergin S., Bayrakal G.M. Presence of Foodborne Pathogens in Seafood and Risk Ranking for Pathogens. Foodborne. Pathog. Dis. 2020;17:541–546. doi: 10.1089/fpd.2019.2753. [DOI] [PubMed] [Google Scholar]
- 6.Sheyda A., Alexander D.K., Marvin W., Stephen P.D. Bacterial Quorum Sensing during Infection. Annu. Rev. Microbiol. 2020;74:201–219. doi: 10.1146/annurev-micro-032020-093845. [DOI] [PubMed] [Google Scholar]
- 7.Aye M.M., Aung H.T., Sein M.M., Armijos C. A Review on the phytochemistry, medicinal properties and pharmacological activities of 15 selected Myanmar medicinal plants. Molecules. 2019;24:293. doi: 10.3390/molecules24020293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Felhi S., Hajlaoui H., Ncir M., Bakari S., Ktari N., Saoudi M., Gharsallah N., Kadri A. Nutritional, phytochemical and antioxidant evaluation and FT-IR analysis of freeze-dried extracts of Ecballium elaterium fruit juice from three localities. Food Sci. Technol. 2016;36:646–655. doi: 10.1590/1678-457x.12916. [DOI] [Google Scholar]
- 9.Felhi S., Saoudi M., Daoud A., Hajlaoui H., Ncir M., Chaabane R., El Feki A., Gharsallah N., Kadri A. Investigation of phytochemical contents, in vitro antioxidant and antibacterial behavior and in vivo anti-inflammatory potential of Ecballium elaterium methanol fruits extract. Food Sci. Technol. 2017;37:558–563. doi: 10.1590/1678-457x.26316. [DOI] [Google Scholar]
- 10.Bakari S., Daoud A., Felhi S., Smaoui S., Gharsallah N., Kadri A. Proximate analysis, mineral composition, phytochemical contents antioxidant and antimicrobial activities and GC-MS investigation of various solvent extracts of Cactus cladode. Food Sci. Technol. 2017;27:286–293. doi: 10.1590/1678-457x.20116. [DOI] [Google Scholar]
- 11.Bakari S., Hajlaoui H., Daoud A., Mighri H., Ross-Garcia J.M., Gharsallah N., Kadri A. Phytochemicals, antioxidant and antimicrobial potentials and LC-MS analysis of hydroalcoholic extracts of leaves and flowers of Erodium glaucophyllum collected from Tunisian Sahara. Food Sci. Technol. 2018;38:310–317. doi: 10.1590/fst.04517. [DOI] [Google Scholar]
- 12.Hajlaoui H., Mighri H., Aouni M., Gharsallah N., Kadri A. Chemical composition and in vitro evaluation of antioxidant antimicrobial, cytotoxicity and anti-acetylcholinesterase properties of Tunisian Origanum majorana L. essential oil. Microb. Pathog. 2016;95:86–94. doi: 10.1016/j.micpath.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 13.Hajlaoui H., Arraouadi S., Noumi E., Aouadi K., Adnan M., Khan M.A., Kadri A., Snoussi M. antimicrobial, antioxidant, anti-acetylcholinesterase, antidiabetic, and pharmacokinetic properties of Carum carvi L. and Coriandrum sativum L. essential oils alone and in combination. Molecules. 2021;26:3625. doi: 10.3390/molecules26123625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daoud A., Ben Mefteh F., Mnafgui K., Turki M., Jmal S., Ben Amar R., Ayadi F., ElFeki A., Abid L., Rateb M.E., et al. Cardiopreventive effect of ethanolic extract of date palm pollen against isoproterenol induced myocardial infarction in rats through the inhibition of the angiotensin-converting enzyme. Exp. Toxicol. Pathol. 2017;69:656–665. doi: 10.1016/j.etp.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 15.Ben Mefteh F., Daoud A., Chenari Bouket A., Thissera B., Kadri Y., Cherif-Silini H., Eshelli M., Alenezi F.N., Vallat A., Oszako T., et al. Date palm trees root-derived endophytes as fungal cell factories for diverse bioactive metabolites. Int. J. Mol. Sci. 2018;19:1986. doi: 10.3390/ijms19071986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bakari S., Ncir M., Felhi S., Hajlaoui H., Saoudi M., Gharsallah N., Kadri A. Chemical composition and in vitro evaluation of total phenolic, flavonoid, and antioxidant properties of essential oil and solvent extract from the aerial parts of Teucrium polium grown in Tunisia. Food Sci. Biotechnol. 2015;24:1943–1949. doi: 10.1007/s10068-015-0256-z. [DOI] [Google Scholar]
- 17.Baig B., Abdel Halim S., Farrukh A., Greish Y., Amin A. Current status of nanomaterial-based treatment for hepatocellular carcinoma. Biomed. Pharmacother. 2019;116:108852. doi: 10.1016/j.biopha.2019.108852. [DOI] [PubMed] [Google Scholar]
- 18.Benassi E., Fan K., Sun Q., Dukenbayev K., Wang Q., Shaimoldina A., Tassanbiyeva A., Nurtay L., Nurkesh A., Kutzhanova A., et al. Generation of particle assemblies mimicking enzymatic activity by processing of herbal food: The case of rhizoma polygonati and other natural ingredients in traditional Chinese medicine. Nanoscale Adv. 2021;3:2222. doi: 10.1039/D0NA00958J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xie Y., Mu C., Kazybay B., Sun Q., Kutzhanova A., Nazarbek G., Xu N., Nurtay L., Wang Q., Amin A., et al. Network pharmacology and experimental investigation of Rhizoma polygonati extract targeted kinase with herbzyme activity for potent drug delivery. Drug Deliv. 2021;28:2187–2197. doi: 10.1080/10717544.2021.1977422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El-Kharrag R., Amin A., Hisaindee S., Greish Y., Karam S.M. Development of a therapeutic model of precancerous liver using crocin-coated magnetite nanoparticles. Int. J. Oncol. 2017;50:212–222. doi: 10.3892/ijo.2016.3769. [DOI] [PubMed] [Google Scholar]
- 21.Kadri A., Zarai Z., Chobba I.B., Gharsallah N., Damak M., Békir A. Chemical composition and in vitro antioxidant activities of Thymelaea hirsuta L: Essential oil from Tunisia. Afr. J. Biotechnol. 2011;10:2930–2935. [Google Scholar]
- 22.Mseddi K., Alimi F., Noumi E., Veettil V.N., Deshpande S., Adnan M., Hamdi A., Elkahoui S., Alghamdi A., Kadri A., et al. Thymus musilii Velen. as a promising source of potent bioactive compounds with its pharmacological properties: In vitro and in silico analysis. Arab. J. Chem. 2020;13:6782–6801. doi: 10.1016/j.arabjc.2020.06.032. [DOI] [Google Scholar]
- 23.Goyal M., Gupta V.K., Singh N., Mrinal Carum carvi— An Updated Review. Indian J. Pharm. Biol. Res. 2018;6:14–24. [Google Scholar]
- 24.Rasooli I., Allameh A. Caraway (Carum carvi L.) essential oils. In: Preedy V.R., editor. Essential Oils in Food Preservation, Flavor and Safety. Academic Press; San Diego, CA, USA: 2016. pp. 287–293. Chapter 32. [Google Scholar]
- 25.Dadkhah A., Allameh A., Khalafi H., Ashrafihelan J. Inhibitory effects of dietary caraway essential oils on 1,2-dimethylhydrazine-induced colon carcinogenesis is mediated by liver xenobiotic metabolizing enzymes. Nutr. Cancer. 2010;63:46–54. doi: 10.1080/01635581.2010.516473. [DOI] [PubMed] [Google Scholar]
- 26.European Pharmacopoeia. 7th ed. Vol. 1 Council of Europe; Strasbourg, France: 2010. [Google Scholar]
- 27.Raal A., Arak E., Orav A. The content and composition of the essential oil found in Carum carvi L. commercial fruits obtained from different countries. J. Essent. Oil Res. 2012;24:53–59. doi: 10.1080/10412905.2012.646016. [DOI] [Google Scholar]
- 28.Snoussi M., Hajlaoui H., Noumi E., Usai D., Sechi L.A., Zanetti S., Bakhrouf A. In-vitro anti-Vibrio spp. activity and chemical composition of some Tunisian aromatic plants. World J. Microbiol. Biotechnol. 2008;24:3071–3076. doi: 10.1007/s11274-008-9811-6. [DOI] [Google Scholar]
- 29.Snoussi M., Noumi E., Dehmani A., Flamini G., Aouni M., Alsieni M., Al-sieni A. Chemical composition and antimicrobial activities of Elettaria cardamomum L. (Manton) essential oil: A high activity against a wide range of food borne and medically important bacteria and fungi. J. Chem. Biol. Phys. Sci. 2015;6:248–259. [Google Scholar]
- 30.Snoussi M., Dehmeni A., Noumi E., Flamini G., Papetti A. Chemical composition and Antibiofilm activity of Petroselinum crispum and Ocimum basilicum essential oils against Vibrio spp. strains. Microb. Pathog. 2016;90:13–21. doi: 10.1016/j.micpath.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 31.Snoussi M., Trabelsi N., Dehmeni A., Benzekri R., Bouslama L., Hajlaoui B., Al-sieni A., Papetti A. Phytochemical analysis, antimicrobial and antioxidant activities of Allium roseum var. odoratissimum (Desf.) Coss extracts. Ind. Crops Products. 2016;89:533–542. doi: 10.1016/j.indcrop.2016.05.048. [DOI] [Google Scholar]
- 32.Gracia-Valenzuela M.H., Vergara-Jiménez M.J., Baez-Flores M.E., Cabrera-Chavez F. Antimicrobial effect of dietary oregano essential oil against vibrio bacteria in shrimps. Arch. Biol. Sci. 2014;66:1367–1370. doi: 10.2298/ABS1404367G. [DOI] [Google Scholar]
- 33.Partovi R., Khanjari A., Abbaszadeh S., Sharifzadeh A. Chemical composition and antimicrobial effect of five essential oils on pathogenic and non -pathogenic Vibrio parahaemolyticus. Nut. Food Sci. Res. 2017;4:43–52. doi: 10.18869/acadpub.nfsr.4.2.6. [DOI] [Google Scholar]
- 34.Porto C., Stüker C.Z., Mallmann A.S., Simionatto E., Flach A., Canto-Dorow T., Silva U.F., Dalcol I.I., Morel A.F. (R)-(−)-carvone and (1R, 4R)-trans-(+)-dihydrocarvone from Poiretia latifolia vogel. J. Braz. Chem. Soc. 2010;21:782–786. doi: 10.1590/S0103-50532010000500003. [DOI] [Google Scholar]
- 35.Snoussi M., Noumi E., Trabelsi N., Flamini G., Papetti A., De Feo V. Mentha spicata essential oil: Chemical composition, antioxidant and antibacterial activities against planktonic and biofilm cultures of Vibrio spp. strains. Molecules. 2015;20:14402–14424. doi: 10.3390/molecules200814402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mizan M.F.R., Ashrafudoulla M., Hossain M.I., Cho H.R., Ha S.D. Effect of essential oils on pathogenic and biofilm-forming Vibrio parahaemolyticus strains. Biofouling. 2020;36:467–478. doi: 10.1080/08927014.2020.1772243. [DOI] [PubMed] [Google Scholar]
- 37.Mendes J.L., de Araújo T.F., Geraldo de Carvalho M., Catunda A., Júnior F.E. Chemical composition and mechanism of vibriocidal action of essential oil from resin of Protium heptaphyllum. Sci. J. 2019;2019:9563213. doi: 10.1155/2019/9563213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nazzaro F., Fratianni F., De Martino L., Coppola R., De Feo V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals. 2013;6:1451–1474. doi: 10.3390/ph6121451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bai A.J., Vittal R.R. Quorum sensing inhibitory and anti-biofilm activity of essential oils and their in vivo efficacy in food systems. Food Biotechnol. 2014;28:269–292. doi: 10.1080/08905436.2014.932287. [DOI] [Google Scholar]
- 40.Noumi E., Merghni A., Alreshidi, M.M., Haddad O., Akmadar G., De Martino L., Mastouri M., Ceylan O., Snoussi M., Al-sieni A., et al. Chromobacterium violaceum and Pseudomonas aeruginosa PAO1: Models for evaluating anti-quorum sensing activity of Melaleuca alternifolia essential oil and its main component terpinen-4-ol. Molecules. 2018;23:2672. doi: 10.3390/molecules23102672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Camele I., Elshafie H.S., Caputo L., De Feo V. Anti-quorum sensing and antimicrobial effect of Mediterranean plant essential oils against phytopathogenic bacteria. Front. Microbiol. 2019;10:2619. doi: 10.3389/fmicb.2019.02619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Snoussi M., Noumi E., Punchappady-Devasya R., Trabelsi N., Kanekar S. Antioxidant properties and anti-quorum sensing potential of Carum copticum essential oil and phenolics against Chromobacterium violaceum. J. Food Sci. Technol. 2018;55:2824–2832. doi: 10.1007/s13197-018-3219-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cáceres M., Hidalgo W., Stashenko E., Torres R., Ortiz C. Essential oils of aromatic plants with antibacterial, anti-biofilm and anti-quorum sensing activities against pathogenic bacteria. Antibiotics. 2020;9:147. doi: 10.3390/antibiotics9040147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alreshidi M., Noumi E., Bouslama L., Ceylan O., Veettil V.N., Adnan M., Danciu C., Elkahoui S., Badraoui R., Al-Motair K.A., et al. Phytochemical screening, antibacterial, antifungal, antiviral, cytotoxic, and anti-quorum-sensing properties of Teucrium polium L. aerial parts methanolic extract. Plants. 2020;9:1418. doi: 10.3390/plants9111418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vasavi H.S., Arun A.B., Rekha P.D. Anti-quorum sensing potential of Adenanthera pavonina. Pharmacogn. Res. 2015;7:105–109. doi: 10.4103/0974-8490.147220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kanekar S., Fathima F., Rekha P.D. Carvone—A quorum sensing inhibitor blocks biofilm formation in Chromobacterium violaceum. Nat. Prod. Res. 2021:1–6. doi: 10.1080/14786419.2021.1993214. [DOI] [PubMed] [Google Scholar]
- 47.Luciardi M.C., Blázquez M.A., Alberto M.R., Cartagena E., Arena M.E. Grapefruit essential oils inhibit quorum sensing of Pseudomonas aeruginosa. Food Sci. Technol. Int. 2020;26:231–241. doi: 10.1177/1082013219883465. [DOI] [PubMed] [Google Scholar]
- 48.Luciardi M.C., Blázquez M.A., Alberto M.R., Cartagena E., Arena M.E. Lemon oils attenuate the pathogenicity of Pseudomonas aeruginosa by quorum sensing inhibition. Molecules. 2021;26:2863. doi: 10.3390/molecules26102863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nazzaro F., Fratianni F., Coppola R., Feo V. Essential Oils and Antifungal Activity. Pharmaceuticals. 2017;10:86. doi: 10.3390/ph10040086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alminderej F., Bakari S., Almundarij T.I., Snoussi M., Aouadi K., Kadri A. Antioxidant activities of a new chemotype of Piper cubeba L. fruit essential oil (Methyleugenol/Eugenol): In Silico molecular docking and ADMET studies. Plants. 2020;9:1534. doi: 10.3390/plants9111534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aouadi K., Hajlaoui H., Arraouadi S., Ghannay S., Snoussi M., Kadri A. HPLC/MS phytochemical profiling with antioxidant activities of Echium humile Desf. extracts: ADMET prediction and computational study targeting human peroxiredoxin 5 receptor. Agronomy. 2021;11:2165. doi: 10.3390/agronomy11112165. [DOI] [Google Scholar]
- 52.Noumi E., Snoussi M., Anouar E.H., Alreshidi M., Veettil V.N., Elkahoui S., Adnan M., Patel M., Kadri A., Aouadi K., et al. HR-LCMS-Based Metabolite Profiling, Antioxidant, and Anticancer Properties of Teucrium polium L. Methanolic Extract: Computational and In Vitro Study. Antioxidants. 2020;9:1089. doi: 10.3390/antiox9111089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Othman I.M.M., Gad-Elkareem M.A.M., Anouar E.H., Aouadi K., Snoussi M., Kadri A. New substituted pyrazolones and dipyrazolotriazines as promising tyrosyl-tRNA synthetase and peroxiredoxin-5 inhibitors: Design, synthesis, molecular docking and structure-activity relationship (SAR) analysis. Bioorg. Chem. 2021;109:104704. doi: 10.1016/j.bioorg.2021.104704. [DOI] [PubMed] [Google Scholar]
- 54.De Clercq E., Naesens L., De Bolle L., Schols D., Zhang Y., Neyts J. Antiviral agents active against human herpesviruses HHV-6, HHV-7 and HHV-8. Rev. Med. Virol. 2001;11:381–395. doi: 10.1002/rmv.336. [DOI] [PubMed] [Google Scholar]
- 55.Alminderej F., Bakari S., Almundarij T.I., Snoussi M., Aouadi K., Kadri A. Antimicrobial and wound healing potential of a new chemotype from Piper cubeba L. essential oil and in silico study on S. aureus tyrosyl-tRNA synthetase protein. Plants. 2021;10:205. doi: 10.3390/plants10020205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Othman I.M.M., Gad-Elkareem M.A.M., Anouar E.H., Snoussi M., Aouadi K., Kadri A. Novel fused pyridine derivatives containing pyrimidine moiety as prospective tyrosyl-tRNA synthetase inhibitors: Design, synthesis, pharmacokinetics and molecular docking studies. J. Mol. Struct. 2020;1219:128651. doi: 10.1016/j.molstruc.2020.128651. [DOI] [Google Scholar]
- 57.Eswaramoorthy R., Hailekiros H., Kedir F., Endale M. In silico molecular docking, dft analysis and admet studies of carbazole alkaloid and coumarins from roots of Clausena anisata: A potent inhibitor for quorum sensing. Adv. Appl. Bioinform. Chem. 2021;5:13–24. doi: 10.2147/AABC.S290912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang M., Zhao L., Wu H., Zhao C., Gong Q., Yu W. Cladodionen Is a Potential Quorum Sensing Inhibitor Against Pseudomonas aeruginosa. Mar. Drugs. 2020;18:205. doi: 10.3390/md18040205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hajlaoui H., Snoussi M., Ben Jannet H., Elmighri Z., Bakhrouf A. Chemical composition and antimicrobial activities of Tunisian essential oil of Mentha longifolia L. ssp. used in the folkloric medicine. Ann. Microbiol. 2008;58:513–520. doi: 10.1007/BF03175551. [DOI] [Google Scholar]
- 60.Ben Salha G., Herrera Díaz R., Lengliz O., Abderrabba M., Labidi J. Effect of the chemical composition of free-terpene hydrocarbons essential oils on antifungal activity. Molecules. 2019;24:3532. doi: 10.3390/molecules24193532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Koleva I.I., van Beek T.A., Linssen J.P., de Groot A., Evstatieva L.N. Screening of plant extracts for antioxidant activity: A comparative study on three testing methods. Phytochem. Anal. 2002;13:8–17. doi: 10.1002/pca.611. [DOI] [PubMed] [Google Scholar]
- 62.Hajlaoui H., Mighri H., Noumi E., Snoussi M., Trabelsi N., Ksouri R., Bakhrouf A. Chemical composition and biological activities of Tunisian Cuminum cyminum L. essential oil: A high effectiveness against Vibrio spp. strains. Food Chem. Toxicol. 2010;48:2186–2192. doi: 10.1016/j.fct.2010.05.044. [DOI] [PubMed] [Google Scholar]
- 63.Oyaizu M. Studies on product of browning reaction prepared from glucose amine. Jpn. J. Nut. 1986;44:307. doi: 10.5264/eiyogakuzashi.44.307. [DOI] [Google Scholar]
- 64.Noumi E., Snoussi M., Alreshidi M.M., Rekha P.D., Saptami K., Caputo L., De Martino L., Souza L.F., Msaada K., Mancini E., et al. Chemical and biological evaluation of essential oils from Cardamom species. Molecules. 2018;23:2818. doi: 10.3390/molecules23112818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim S., Chen J., Cheng T., Gindulyte A., He J., He S., Li Q., Shoemaker B.A., Thiessen P.A., Yu B., et al. PubChem in 2021: New data content and improved web interfaces. Nucleic Acids Res. 2021;49:D1388–D1395. doi: 10.1093/nar/gkaa971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hanwell M.D., Curtis D.E., Lonie D.C., Vandermeersch T., Zurek E., Hutchison G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 2012;4:17. doi: 10.1186/1758-2946-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tasleem M., Ishrat R., Islam A., Ahmad F., Hassan I. Structural Characterization, Homology Modeling and Docking Studies of ARG674 Mutation in MyH8 Gene Associated with Trismus-Pseudocamptodactyly Syndrome. Lett. Drug Des. Discov. 2014;11:1177–1187. doi: 10.2174/1570180811666140717190217. [DOI] [Google Scholar]
- 68.Trott O., Olson A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010;31:455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Azam S.S., Abbasi S.W. Molecular docking studies for the identification of novel melatoninergic inhibitors for acetylserotonin-O-methyltransferase using different docking routines. Theor Biol Med. Model. 2013;10:63. doi: 10.1186/1742-4682-10-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data generated and analyzed during this study are included in this article.