Abstract
Actinobacteria of the genus Amycolatopsis are important for antibiotic production and other valuable biotechnological applications such as bioconversion or bioremediation. Despite their importance, tools and methods for their genetic manipulation are less developed than in other actinobacteria such as Streptomyces. We report here the use of the pSAM2 site-specific recombination system to delete antibiotic resistance cassettes used in gene replacement experiments or to create large genomic deletions. For this purpose, we constructed a shuttle vector, replicating in Escherichia coli and Amycolatopsis, expressing the integrase and the excisionase from the Streptomyces integrative and conjugative element pSAM2. These proteins are sufficient for site-specific recombination between the attachment sites attL and attR. We also constructed two plasmids, replicative in E. coli but not in Amycolatopsis, for the integration of the attL and attR sites on each side of a large region targeted for deletion. We exemplified the use of these tools in Amycolatopsis mediterranei by obtaining with high efficiency a marker-free deletion of one single gene in the rifamycin biosynthetic gene cluster or of the entire 90-kb cluster. These robust and simple tools enrich the toolbox for genome engineering in Amycolatopsis.
Keywords: Amycolatopsis, pSAM2 site-specific recombination system, excision tool, large-scale deletion, unmarked mutant, marker recycling
1. Introduction
Actinobacteria of the genus Amycolatopsis are the source of diverse bioactive specialized metabolites [1]. Some species such as Amycolatopsis mediterranei and Amycolatopsis orientalis are for instance used industrially for the production of the medically important antibiotics rifamycins [2] and vancomycin [3,4], respectively. Amycolatopsis spp. are also known for their biotechnological potential as agents of lignin degradation [5] and bioremediation [6] or for the fermentative production of aromatic compounds [7].
Despite the importance of the genus Amycolatopsis, the development of adapted genetic tools has lagged behind that for other actinobacterial genera such as Streptomyces [8,9]. For some time, the introduction of DNA into Amycolatopsis has been a limiting factor, until this problem could be circumvented by the development of a method of direct transformation of Amycolatopsis mycelia [10] or by using intergeneric conjugation from Escherichia coli to introduce single-stranded DNA into the Amycolatopsis host [11,12]. Functional studies often rely on the inactivation/deletion of one or several genes. Recent attempts to use CRISPR-Cas based systems in Amycolatopsis suggested a toxicity linked to dead Cas9 expression [13]. Thus, a genome editing system based on another Cas protein was developed to obtain deletion mutants in Amycolatopsis mediterranei U32 [13], however this implicated the integration of the Cas12a gene and of a hygromycin resistance gene in the chromosome of A. mediterranei U32. To obtain unmarked gene replacement, a more common technique for gene deletion is often used. In this multi-step process, fragments identical to those upstream and downstream of the target gene are cloned contiguously into a suicide vector carrying a selectable marker. This delivery vector is introduced into the actinobacterial strain and its integration by one event of homologous recombination is selected. The transformants are grown for one or several rounds of cultivation under non-selective conditions and single colonies are then screened for the loss of the resistance marker carried by the vector. Sensitive clones, devoid of the vector, are either wild-type (if both homologous recombination events occurred within the same homologous fragment) or deleted of the target gene (if the first and second recombination events occurred within different homologous fragments). Wild-type and mutant colonies can be distinguished by screening, for example using PCR. To facilitate the obtention of mutants by this approach, various methods of counterselecting [14,15,16] the delivery vectors or of visual screening [17,18] have been used in Streptomyces. In Amycolatopsis a chromogenic screening method based on the gusA gene was developed, allowing to distinguish clones still carrying the suicide vector from those who have lost it [7]. A counterselection rpsL-based streptomycin resistance system was also developed for gene deletion in Amycolatopsis [8]. This system, based on the dominance of streptomycin sensitivity, can only be used in a streptomycin resistant mutant.
Other techniques, allowing marker free deletions and marker recycling, based on site-specific recombination (SSR) have been developed to remove the antibiotic marker after knockout mutant selection in some actinobacteria [19]. These techniques are based on the use of an antibiotic resistance gene cassette flanked by SSR sequences, which are recognized by cognate site-specific recombinases. The excision of the antibiotic cassette leaves a short scar (usually smaller than 50 bp) in the chromosome and can be designed to avoid polar effect [19]. Another application of SSR systems for genome engineering is the deletion of complete gene clusters or large genomic regions. Several examples of such application have been reported in Streptomyces, relying on the Cre/loxP SSR system (Herrmann et al. [20] and references therein). This approach allowed the deletion of genomic regions up to 1.5 Mb in Streptomyces avermitilis [21].
An example of well-characterized SSR system is that of pSAM2, an integrative element from Streptomyces ambofaciens [22]. The integrase encoded by the int gene promotes the integration of pSAM2 into the chromosome by intermolecular SSR between the attachment (att) sites, attP carried by pSAM2 and attB located in the bacterial chromosome. After integration, pSAM2 is flanked by the attL (left) and attR (right) sites. These sites can be themselves involved in intramolecular SSR leading to excision of pSAM2, an event requiring both the excisionase (encoded by the xis gene) and the integrase. Detailed studies have been carried out to precisely define the minimal sites required by the pSAM2 SSR system [23,24]. These findings were used for the construction of several excisable antibiotic resistance cassettes in which minimal attL and attR sites flank various antibiotic resistance genes [25]. These selectable cassettes are used in Streptomyces to replace target genes by homologous recombination. After positive selection of the mutant strains, the resistance cassette can be efficiently excised by transiently expressing xis and int carried by an unstable replicative vector. The resulting mutant strains are marker-free and contain a minimal attB site. The size of the scar (33, 34, or 35 bp) is dependent of the excisable cassette used and is chosen in order to maintain the correct reading frame if the deletion is internal to a coding sequence [25].
These cassettes allow marker recycling and could be useful in Amycolatopsis strains, many of which are naturally resistant to several antibiotics [26]. This limits the choice of resistance markers and makes the possibility of recycling them even more attractive. However, if these cassettes can be used for gene replacement in Amycolatopsis, the existing plasmids promoting their excision are unable to replicate in Amycolatopsis. The same is true for plasmids carrying other SSR systems that have been used in Streptomyces, as they all rely on replicons from pIJ101 or pSG5 [10].
Until now, few plasmids have been described in Amycolatopsis species [27], and only four of them (pMEA100, pMEA300, pA387 and pXL100) have been further studied. However, only the replicative vectors derived from the endogenous plasmids pA387 and pXL100 have been shown to replicate into several Amycolatopsis species [12,28].
Here, we report the successful use of the pSAM2 SSR system to construct unmarked deletion mutants in Amycolatopsis, without leaving exogenous sequences within the genome, except for a short scar of 33 bp. For this purpose, we constructed a shuttle vector, replicating in Escherichia coli and Amycolatopsis, which is poorly maintained in Amycolatopsis in the absence of a selection pressure. This plasmid, derived from pRL60 (containing the short pA387 replicon) [29], carries the xis and int genes from pSAM2 and the oriT origin of transfer for interspecific conjugation. Moreover, we used the pSAM2 SSR system for the generation of large deletions. For this purpose, we constructed two plasmids for the integration of the recombination sites attL and attR on each side of a region targeted for deletion. With these newly constructed tools and the previously constructed excisable antibiotic resistance cassettes [25] we demonstrated the efficiency of the pSAM2 SSR system to obtain marker-free in-frame deletion mutants and to generate large-scale deletions in A. mediterranei DSM 40773. These tools enrich the genetic toolbox available for genetic engineering of Amycolatopsis strains.
2. Materials and Methods
2.1. Bacterial Strains, Cultivation Conditions and Strain Manipulation
All strains used in this study are listed in Table 1. E. coli strains were grown at 37 °C in LB. When required, antibiotics were added to E. coli cultures in liquid (or on solid) medium at the following concentrations: ampicillin, 50 µg/mL (or 100 µg/mL in solid); apramycin, 25 µg/mL (or 50 µg/mL); hygromycin, 50 µg/mL (or 150 µg/mL); kanamycin, 25 µg/mL in liquid or solid medium. A. mediterranei strains were grown at 30 °C on GYM agar medium (32) for sporulation before the preparation of spore stocks, in TSB (Tryptic Soy Broth, Becton Dickinson) for DNA extraction and in MP5 (33) for rifamycin production. Conjugations between E. coli ET12567 harboring pUZ8002 (or pUZ8003) and A. mediterranei were carried out according to Kieser et al. (34) using MS medium complemented with 10 mM CaCl2 (35), instead of MgCl2. An overlay (3 mL) of soft nutrient agar (Nutrient Broth (Thermo Scientific, Dardilly, France), with 0.8% agar) containing 25 µg/mL nalidixic acid and the appropriate antibiotics for the selection of exconjugants was added after overnight incubation of the conjugation plates. The plates were incubated for 5–7 days until exconjugants clones became visible. Antibiotic concentration used for A. mediterranei were as follows: apramycin, 50 µg/mL; erythromycin, 75 µg/mL; hygromycin, 75 µg/mL.
Table 1.
Strains used in this study.
| Strain | Description | Reference or Source |
|---|---|---|
| E. coli DH5α | General cloning strain | Promega |
| E. coli ET12567/pUZ8002 | Host strain for conjugation from E. coli to Amycolatopsis | [30,31] |
| E. coli ET12567/pUZ8003 | Host strain for conjugation from E. coli to Amycolatopsis (pUZ8003 is a modified pUZ8002 with aph(3′) gene replaced by bla) | [32] |
| A. mediterranei DSM 40773 | Wild-type (WT) strain | DSMZ |
| DSM 40773 ∆rifK::att1Ωhyg | A. mediterranei rifK deletion mutant with replacement of the rifK gene by the att1Ωhyg hygromycin resistance cassette | This study |
| DSM 40773 ∆rifK::att1 | Unmarked A. mediterranei rifK deletion mutant | This study |
| DSM 40773-pRIF14 | A. mediterranei containing pRIF14 | This study |
| DSM 40773-pRIF12-pRIF14 | A. mediterranei containing both plasmids pRIF14 and pRIF12 | This study |
| DSM 40773 ∆rif::att1 | A. mediterranei with a deletion of the complete rif cluster | This study |
| S. aureus HG003 | Rifamycin sensitive strain used as indicator in bioassay analysis | [33] |
| S. aureus HG003 rpoB (H418Y) | Rifamycin resistant strain used as indicator in bioassay analysis, rpoB mutant of S. aureus HG003 | Marick Esberard and Philippe Bouloc unpublished |
2.2. Plasmids and DNA Manipulations
All plasmids used in this study are listed in Table 2. Details about the plasmids constructed for this study are given in Appendix A. Amplification of DNA fragments for cloning was carried out using the high-fidelity DNA polymerase Q5 (New England BioLabs: NEB, Evry, France). Taq polymerase (Qiagen, Les Ulis, France) was used for verification PCRs. All oligonucleotides used in this study were provided by IDT (Integrated DNA Technologies, Leuven, Belgium) and are listed in Table S1. Restriction and modification (ligase, kinase, etc.) enzymes were purchased from NEB or Thermo Scientific. Plasmid DNA extraction from E. coli was performed using NucleoSpin Plasmid kit from Macherey-Nagel (Hoerdt, France). DNA fragments were purified from agarose gels using the NucleoSpin Gel and PCR Clean-up kit from Macherey-Nagel. Genomic DNA extractions from Amycolatopsis and E. coli transformations were performed according to standard procedures [34,35].
Table 2.
Plasmids used in this study.
| Plasmid | Description a | Reference or Source |
|---|---|---|
| pRL60 | E.coli-Amycolatopsis shuttle plasmid, Ery R, Kan R, amy+, oriT | [29] |
| pOSV236 | E. coli-Streptomyces shuttle plasmid expressing the Xis and Int proteins for site-specific excision of excisable cassettes, Amp R, Pur R, oriT | [36] |
| pOSV504 | Source of the excisable hygromycin cassette (att1Ωhyg), Amp R, Hyg R | [25] |
| pOJ260 | E.coli-Amycolatopsis shuttle plasmid, suicide vector in Amycolatopsis, Apr R, oriT | [37] |
| pOSV805 | E.coli-Amycolatopsis shuttle plasmid, integrative in Amycolatopsis using the ϕBT1 SSR system, Hyg R, oriT | [32] |
| pCR-blunt | E. coli cloning vector, Kan R | Invitrogen |
| pT-atts | pUC derivative containing the attL and attR minimal sites from pSAM2, Amp R, Constructed by Twist Biosciences | This study |
| pRL60∆amy | E.coli-Amycolatopsis shuttle plasmid, derivative of pRL60, amy−, Ery R, Kan R, oriT | This study |
| pEA01 | E.coli-Amycolatopsis shuttle plasmid, pRL60∆amy derivative containing the xis and int genes from pSAM2 under the control of trcp, the pac gene and oriT, Ery R, Kan R, Pur R, oriT | This study |
| pEA02 | E.coli-Amycolatopsis shuttle plasmid, suicide vector in Amycolatopsis; pOJ260 derivative containing the minimal attL sequence and a multi-cloning site, Apr R, oriT | This study |
| pEA03 | E.coli-Amycolatopsis shuttle plasmid, suicide vector in Amycolatopsis; pOSV805 derivative containing the minimal attR sequence and a multi-cloning site, Hyg R, oriT | This study |
| pRIF01 | pCR-blunt derivative containing the downstream homologous region of rifK, KanR | This study |
| pRIF02 | pCR-blunt derivative containing the upstream homologous region of rifK, KanR | This study |
| pRIF05 | pOJ260 derivative containing the excisable hygromycin cassette (att1Ωhyg) flanked by the upstream and downstream homologous region of rifK, for replacement of rifK, Apr R, oriT | This study |
| pRIF09 | pCR-blunt derivative containing the downstream homologous region of the rif cluster, Kan R | This study |
| pRIF10 | pCR-blunt derivative containing the upstream homologous region of the rif cluster, Kan R | This study |
| pRIF12 | E.coli-Amycolatopsis shuttle plasmid, suicide plasmid in Amycolatopsis; pEA02 derivative containing the upstream region of the rif cluster, attL, Apr R, oriT | This study |
| pRIF14 | E.coli-Amycolatopsis shuttle plasmid, suicide plasmid in Amycolatopsis; pEA03 derivative containing the downstream region of the rif cluster, attR, Hyg R, oriT | This study |
a Abbreviations: amy—α-amylase; R—Resistance, Ery—Erythromycin, Kan—Kanamycin, Amp—Ampicillin, Pur—Puromycin, Apr—Apramycin, Hyg—Hygromycin.
2.3. Construction and Verification of A. mediterranei DSM 40773 Mutant Strains
2.3.1. Marker-Free rifK Mutants
Firstly, pRIF05 was transferred to A. mediterranei wild-type by conjugation. Hygromycin resistant exconjugants were selected and then screened for apramycin sensitivity to obtain clones in which the rifK gene was replaced by the att1Ωhyg cassette. The genetic organization of hygromycin-resistant and apramycin-sensitive clones was checked by PCRs targeting the upstream and the downstream junctions formed by the replacement of the rifK gene by the att1Ωhyg cassette. These PCRs were performed using the following couples of primers: LS182/LS161 and LS188/LS189, respectively. For excision of the att1Ωhyg cassette, the plasmid pEA01 was introduced into three independent ∆rifK::att1Ωhyg clones. Erythromycin resistant exconjugants were selected. Hygromycin sensitive clones were then screened and the loss of plasmid pEA01 was finally obtained by successive subculturing on medium without erythromycin. The excision of the cassette and the deletion of the rifK gene was checked by PCR using primers hybridizing upstream and downstream of the rifK gene (LS172 and LS173, respectively). For each mutant clone, the presence of the scar was checked by sequencing the PCR product.
2.3.2. Large-Scale Deletions
Firstly, pRIF14 was transferred to A. mediterranei wild-type by conjugation. Hygromycin resistant exconjugants were selected. The integration of the plasmid pRIF14 was checked by PCR using primers LS190 and LS71 targeting the downstream junction formed by the integration of pRIF14 within the region downstream of the rif cluster. pRIF12 was then introduced into the strain already harboring pRIF14. Apramycin and hygromycin resistant exconjugants were selected. The integration of the plasmid pRIF12 was checked by PCR using primers LS191 and LS70 targeting the upstream junction formed by the integration of pRIF12 within the region upstream of the rif cluster. Finally, the excision of the complete cluster was performed as previously described for the excision of the hygromycin cassette. The deletion of the complete rif cluster was checked by PCR using primers hybridizing upstream and downstream of the rif cluster (LS220 and LS221, respectively) and allowing the amplification of the scar region formed after the excision. For each mutant clone, the presence of the scar was checked by sequencing the PCR product.
2.4. Rifamycin Production
MP5 liquid medium (75 mL of medium in 500 mL baffled Erlenmeyer flasks) was inoculated with 5 × 107 spores of the mutant or the wild-type strains. Cultures were incubated for 10 days at 30 °C under agitation (180 rpm). Cultures were centrifugated and the supernatants used for the assay of the antibacterial activity.
2.5. Antibacterial Activity Assays
Antibacterial activity assays were performed using two Staphylococcus aureus strains as indicator (one strain sensitive to rifamycin and the other resistant). Each S. aureus strain was grown overnight in LB at 37 °C and used to inoculate molten Antibiotic medium 5 (Becton Dickinson, Le Pont de Claix, France). Each indicator plate was loaded with 100 µL of A. mediterranei supernatants and then incubated overnight at 37 °C for growth inhibition analysis. The growth inhibition of strains by rifamycin was verified with 100 µL of MP5 containing or not rifamycin SV (5 mg/mL). The contrast between regions of normal and inhibited growth was enhanced using tetrazolium red (Sigma) as described by Pattee [38]. These assays were performed at least three times for each mutant strain.
3. Results
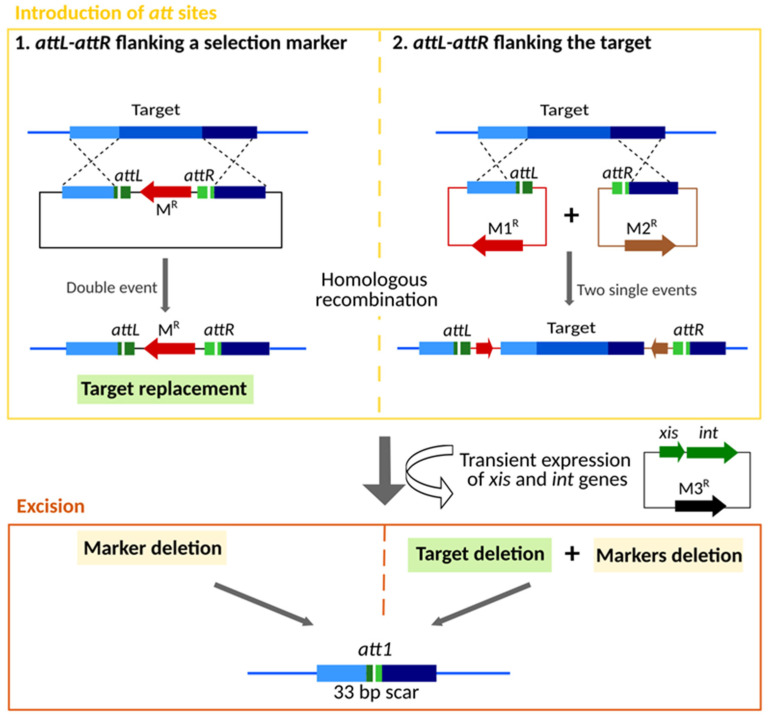
The application of the pSAM2 SSR system requires cis- and trans-acting elements and involves two steps (Figure 1). First, the cis elements, the attL and attR recombination sites, are introduced in the genome via homologous recombination. Then, the trans-acting elements, the xis and int genes, are temporarily expressed to perform SSR and consequently the excision of the region flanked by attL and attR.
Figure 1.
Principle of pSAM2 SSR system and examples of its application (schematic representations not to scale). The SSR system requires cis-acting elements (attL and attR sites) and trans-acting elements (xis and int genes). First, the att sites are introduced in the genome via homologous recombination. The attL and attR sites are integrated in the genome by (1) a double event of homologous recombination in which the target is replaced by an excisable cassette/marker; or (2) two single events of homologous recombination after which the target is flanked by the att sites. Then the xis and int genes are introduced and temporarily expressed to perform the excision of the region flanked by attL-attR sites.
The excision step requires only the transient expression of the int and xis genes. This can be achieved by cloning these genes in a vector self-replicating in Amycolatopsis which is rapidly lost in the absence of a selection pressure. Considering the introduction of attL and attR sites in the genome, two approaches can be used. In the first one, the target gene(s) is replaced by an antibiotic resistance gene flanked by attL and attR. For this purpose, several excisable cassettes, previously constructed [25], are available for cloning in an Amycolatopsis suicide vector, in between sequences identical to the upstream and downstream regions of the target gene. This approach was originally designed to inactivate a single gene by in-frame deletion for functional analysis [39]. It can also be used to delete a few adjacent genes. Even if we used this approach to delete small (<30 kb) biosynthetic gene clusters (BGCs), such as the bicyclomycin BGC [40] and the congocindine BGC [41], the efficiency of the target replacement (double event of recombination) by homologous recombination decreases with the size of the target region. In this context, for large-scale deletions we propose here a second approach in which attL and attR sites are successively introduced at the borders of the target region by homologous recombination (two single events). For this propose attL and attR sites must be cloned in two compatible non-replicative vectors, within the identical sequences required for homologous recombination between the vector and the host genome.
Therefore, in this work we constructed a set of three vectors, one self-replicative in Amycolatopsis for transient expression of the xis and int genes, and two suicide vectors for the integration of the recombination sites attL and attR on each side of target region to be deleted. The application of this tools in Amycolatopsis has then evaluated for single gene or large-scale deletions in A. mediterranei DSM 40773.
3.1. Design of Genetic Tools for pSAM2 SSR System Application
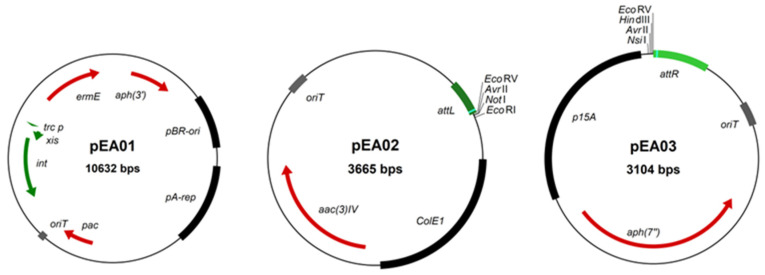
For transient expression of the int and xis genes in Amycolatopsis, we constructed a plasmid relying on the short form of the pA387 replicon contained in pRL60 [29] for replication in Amycolatopsis. If fact, pRL60 has been reported to be unstable in the absence of a selection pressure [42]. The presence of the origin of transfer oriT allows the interspecific transfer of the plasmid from E. coli strain to Amycolatopsis species by conjugation, as widely performed in actinobacteria. In our plasmid, three antibiotic resistance genes are present, conferring kanamycin, erythromycin or puromycin resistance, allowing its selection in various Amycolatopsis genetic backgrounds. The kanamycin marker is used for selection in E. coli strains. The xis and int genes are placed under the control of the promoter trcp, a promoter that efficiently expresses these genes in Streptomyces spp [25]. The resulting plasmid possessing all these features was called pEA01 (Figure 2). This plasmid is available from the Addgene repository (ID 170766).
Figure 2.
Maps of the plasmids constructed in this study. All three plasmids are replicative in E. coli. pEA01 can replicate in Amycolatopsis while pEA02 and pEA03 are suicide vectors in Amycolatopsis. aph(3′): kanamycin resistance gene; pBR-ori: pBR322 origin of replication; pA-rep: short replicon region of pA387; pac: puromycin resistance gene; oriT: origin of transfer; int and xis, integrase and excisionase gene of pSAM2; trcp: trc promoter for the expression of xis and int genes; ermE: erythromycin resistance gene; attL: left attachment site; ColE1: ColE1 origin of replication; aac(3)IV: apramycin resistance gene; aph(7″): hygromycin resistance gene; p15A: p15A origin of replication; attR: right attachment site.
The study of the vector replication, selection and stability was performed in A. mediterranei DSM 40773. After conjugative transfer of pEA01 to A. mediterranei DSM 40773, exconjugants resistant to erythromycin and puromycin were obtained. Of note, the kanamycin marker was not useful for this strain since A. mediterranei DSM 40773 is spontaneously resistant to kanamycin. The instability of pEA01 was assessed in absence of antibiotic selection: After 3 days of growth in media without antibiotic, 99.5% of clones (995 of 1000 analyzed) were sensitive to erythromycin indicating the loss of pEA01 vector. This confirmed that the short pA387 replicon from pRL60 is suitable to generate replicative but unstable vectors in Amycolatopsis, as required for transient expression of genes.
For successive introduction of attL and attR sites at the borders of the target region by homologous recombination, we designed two non-replicative vectors that share minimal sequence identity to avoid recombination between them. As both plasmids are conjugative, they nevertheless share an identical region corresponding to the oriT sequence (≈500 bp). This is the only long stretch of identity as we used different replicons (ColE1 and p15A) for replication in E. coli and different antibiotic resistance genes (apramycin and hygromycin resistance). The two vectors pEA02 and pEA03, contain the attL and attR sites, respectively (Figure 2). They both harbor a multiple cloning site for the easy cloning of the sequences required for homologous recombination. These vectors are suitable for introduction of attL and attR sites at any target borders by a single event of homologous recombination. Detailed instructions to clone homologous regions into these vectors are provided in Figure S1. pEA02 and pEA03 are available from Addgene (ID 172193 and ID 172194, respectively).
The set of three vectors and the efficiency of each approach were thereafter evaluated for single gene or large-scale deletions in Amycolatopsis. We used as target the rifamycin biosynthetic gene cluster of A. mediterranei DSM 40773.
3.2. Cassette Excision
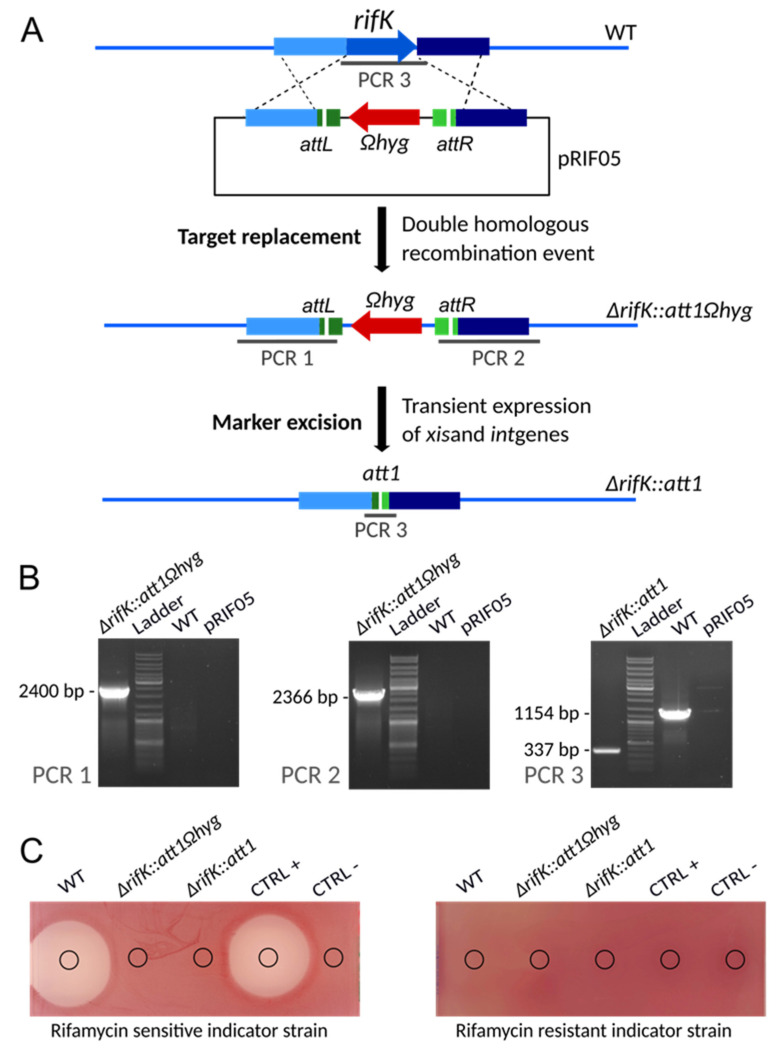
The strategy employed for the obtention of marker-free rifK mutants is summarized in Figure 3A. First rifK was replaced by an excisable hygromycin resistance cassette (att1Ωhyg) following double homologous recombination events between the non-replicative plasmid pRIF05 and the host genome. For that, pRIF05 containing the excisable cassette between the upstream and the downstream homologous regions was introduced into the wild-type strain by conjugation. Hygromycin resistant exconjugants were selected and then tested for sensitivity/resistance to apramycin. Of the 300 hygromycin resistant clones tested, 20 were sensitive to apramycin, indicating that double homologous recombination events occur with a frequency (about 7%) that makes them relatively easy to obtain. These hygromycin-resistant and apramycin-sensitive clones were then checked by PCR on genomic DNA. The verification is based on the amplification of the junctions formed between the upstream or the downstream region of rifK and the att1Ωhyg cassette. As expected only the clone of interest allowed the amplification of DNA fragments with the expected size (PCR 1 and PCR 2 in Figure 3B). The results are shown for one clone, representative of the 20 clones analyzed. Thus, in all the 20 clones, the expected recombination events had occurred. The resulting clones were called A. mediterranei DSM 40773 ∆rifK::att1Ωhyg, and three of them were used in further steps and for phenotypic analysis.
Figure 3.
Construction of an unmarked rifK deletion mutant using an excisable cassette. (A) Schematic representation (not to scale) of the successive steps. First the rifK gene is replaced by the att1Ωhyg cassette via a double homologous recombination event. Then the att1Ωhyg cassette is excised following the introduction of pEA01, resulting in an unmarked deletion mutant containing a 33-bp scar (att1). (B) PCR verification of the strains at different stages of the construction. Verifications are based on three PCRs. The extent of the expected amplicons for each of the PCR is indicated in panel A. The amplicons sizes are indicated on the left side of the pictures. Lanes: ∆rifK::att1Ωhyg- genomic DNA from one of the A. mediterranei DSM43770 ∆rifK::att1Ωhyg clones, representative of all analyzed clones; Ladder-GeneRuler DNA Ladder (SM0331 Thermo Scientific); ∆rifK::att1-genomic DNA from one of the A. mediterranei DSM43770 ∆rifK::att1 clones, representative of all analyzed clones WT-genomic DNA from A. mediterranei DSM43770 wild-type strain; pRIF05-Plasmid pRIF05. (C) Bioassays. The antibacterial activity of the culture supernatants from the wild-type and the mutant strains of A. mediterranei DSM43770 was assayed against S. aureus HG003 (sensitive to rifamycin) and its rpoBH481Y mutant derivative (resistant to rifamycin). MP5 with or without 5 µg/mL of rifamycin SV was used as positive or negative control, respectively.
In the second step, the excision of the att1Ωhyg cassette was performed by introducing the plasmid pEA01 in three independent ∆rifK::att1Ωhyg clones. The erythromycin resistant exconjugants obtained were then screened for resistance/sensitivity to hygromycin to check the loss of the hygromycin cassette. Over the 150 (50 from each parental clone) clones tested 147 were sensitive to hygromycin indicating that the excision occurs with a frequency of 98%. In this case, 18 of these hygromycin sensitive clones (6 from each parental clone) were genetically verified by PCR, targeting the scar region formed by the excision of Ωhyg cassette. As expected, a smaller fragment was amplified with genomic DNA of the sensitive hygromycin clones when compared to the 1154 bp fragment obtained with the wild-type genomic DNA. The results obtained for PCR 3 are also shown in Figure 3B for one clone, representative of the 18 clones tested. The sequence analysis of the 337 bp amplicon, confirmed the excision of the Ωhyg cassette and the formation of a 33 bp scar, corresponding to the att1 sequence [25], as expected. Sanger sequencing results obtained for three independent clones are provided in Supplemental File S1. Moreover, these clones became sensitive to erythromycin, indicating that pEA01 has been lost during cultivation in the absence of erythromycin. The resulting clones were called A. mediterranei DSM 40773 ∆rifK::att1, and three of them (one from each parental clone) were used for phenotypic analyses.
Phenotypic analysis, presented in Figure 3C, was performed using a bacterial growth inhibition assay. The results shown that ∆rifK::att1Ωhyg and ∆rifK::att1 strains did not produce rifamycin contrarily to the wild-type strain, confirming that this gene is essential for antibiotic production.
3.3. Region Excision
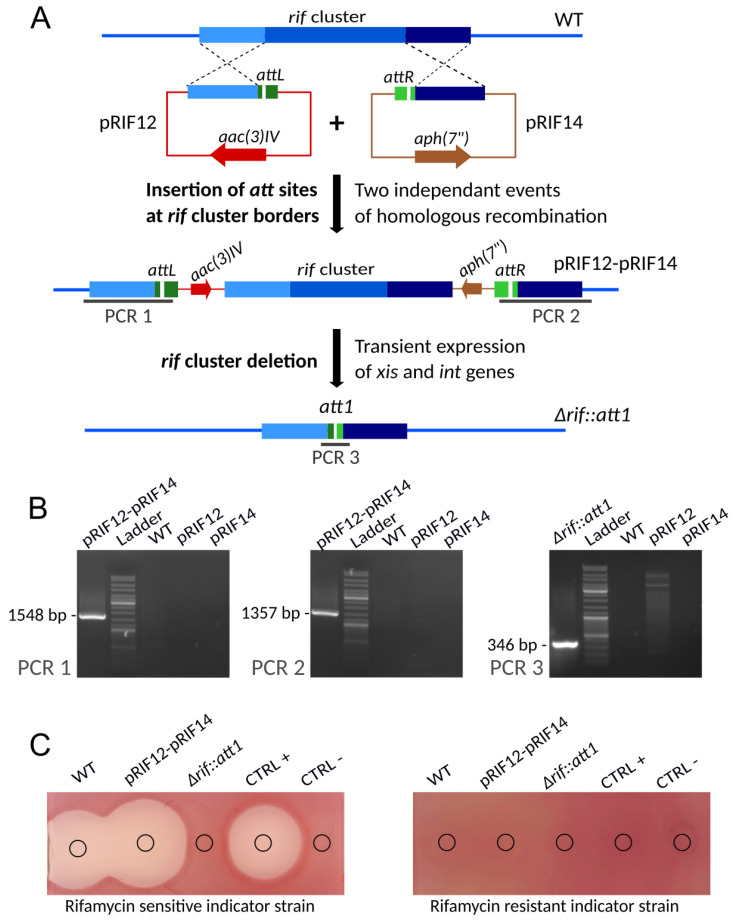
The complete rifamycin gene cluster was targeted to demonstrate the use of the pSAM2 SSR system to generate marker-free large-scale deletions. The rifamycin biosynthetic gene cluster comprises 42 genes covering a region of about 90 kb [43]. The strategy employed to delete this cluster is shown in Figure 4A. First the attL and attR sequences were integrated at the cluster’ extremities via homologous recombination. In a second step, the excision of the region flanked by attL-attR sites and encompassing the rifamycin gene cluster was performed using the pEA01 vector.
Figure 4.
Construction of an unmarked rif cluster deletion mutant. (A) Schematic representation (not to scale) of the successive steps. First the attL and attR sequences are successively integrated upstream and downstream of the rif cluster via two single homologous recombination events. Then the complete region between the attL and attR sites is excised following the introduction of pEA01, resulting in an unmarked rif cluster deletion mutant containing a 33-bp scar (att1). (B) PCR verification of the strains at different stages of the construction. Verifications are based on three PCRs. The extent of the expected amplicons for each of the PCR is indicated in panel A. Lanes: pRIF12-pRIF14-genomic DNA of A. mediterranei DSM43770 harboring pRIF12 and pRIF14, representative of all analyzed exconjugants; Ladder-GeneRuler DNA Ladder (SM0331 Thermo Scientific); WT-genomic DNA of A. mediterranei DSM43770 wild-type strain; pRIF12-Plasmid pRIF12; pRIF14-Plasmid pRIF14; ∆rif::att1-genomic DNA of A. mediterranei with a deletion of the rif cluster. (C) Bioassays. The antibacterial activity of culture supernatants from the wild-type strain A. mediterranei DSM43770, the strain harbouring pRIF12 and pRIF14, and the mutant strain ∆rif::att1 was assayed against S. aureus HG003 (sensitive to rifamycin) and its rpoBH481Y derivative (resistant to rifamycin). The slight difference in inhibition zone size observed for WT and pRIF12-pRIF14 strains is due to the fluctuation of rifamycin production between different flasks. MP5 with or without 5 µg/mL of rifamycin SV was used as positive or negative control, respectively.
For this purpose, fragment identical to the region upstream and downstream of the rifamycin cluster were cloned into pEA02 and pEA03, yielding pRIF12 and pRIF14, respectively. These plasmids were then transferred into the wild-type strain by successive conjugations. After conjugative transfer of pRIF14 into the wild-type strain, clones resistant to hygromycin were selected. These clones were then verified by PCR on genomic DNA to confirm the integration of pRIF14 at the downstream extremity of rifamycin cluster. In a second step, pRIF12 was transferred into the strain already carrying the pRIF14, and clones resistant to apramycin and hygromycin were selected. Then, as previously described for strains carrying the pRIF14, these clones were verified by PCR. The results obtained confirmed the integration of pRIF12 and pRIF14 at the expected chromosomal sites (upstream and downstream to the rifamycin gene cluster) as presented in Figure 4B for PCR 1 and PCR 2. The resulting clones were called A. mediterranei DSM 40773-pRIF12-pRIF14 and were used in further steps and for phenotypic analysis.
The excision of the rifamycin gene cluster was performed in two independent A. mediterranei DSM 40773-pRIF12-pRIF14 clones, using the plasmid pEA01. The erythromycin resistant clones obtained after conjugation of pEA01 were then screened for resistance/sensitivity to hygromycin and/or apramycin to check the loss of hygromycin and/or apramycin cassette. Over the 100 tested exconjugants (50 from each parental clone) that received pEA01 vector, 95 were sensitive to both antibiotics indicating that the excision occurs with a frequency of 95%. In this case, 12 of these sensitive clones (6 from each parental clone) were verified by PCR, targeting the scar region formed after excision (PCR 3 in Figure 4B). As expected, a DNA fragment of 346 bp, containing the att1 sequence [25], was amplified for all the 12 clones tested. Sanger sequencing results obtained for three independent clones are provided in Supplemental File S2. Moreover, these clones became sensitive to erythromycin, indicating that pEA01 has been lost during cultivation in the absence of erythromycin. The resulting clones were called A. mediterranei DSM 40773 ∆rif::att1, and three of them were used for phenotypic analyses.
As previously described for rifK mutants, bacterial growth inhibition assay results confirmed that the ∆rif::att1 strains did not produce rifamycin, as shown in Figure 4C. The integration of pRIF12 and pRIF14 at the border of the cluster does not affect the production of rifamycin.
This experiment validates the functionality of the set of vectors developed in this work and the application of the pSAM2 SSR system to generate large-scale deletions in A. mediterranei DSM 40773.
4. Discussion
The genetic tools that we constructed allow the application of the pSAM2 SSR system in A. mediterranei to generate marker-free small or large deletions. The transient expression of the xis and int genes was achieved by using an unstable replicative vector which is very rapidly lost in the absence of a selection pressure. No additional step of growth in the absence of selection was required to lose the excision plasmid pEA01. It should also be noted that with the delivery suicide vectors that we used (pOJ260, pEA02, pEA03), we did not observed integration by illegitimate recombination as was sometimes the case in Amycolatopsis with other vectors [7,8]. The SSR between attL and attR leading to the excision of the region flanked by these sites is highly efficient (>95%). The efficiency is roughly the same whether these sites are close (98% efficiency with excisable cassettes) or quite distant (e.g., 95% efficiency for sites separated by 90 kb). These tools allow the use of resistance marker for steps where a selection is helpful and the subsequent removal of these markers by SSR to obtain marker-less deletion mutant strains. This allows the recycling of resistance markers for successive rounds of genetic engineering, an advantage for a genus for which the number of usable resistance markers is limited. Moreover, the absence of heterologous antibiotic resistance genes might be important for biotechnological applications. In addition, unmarked in-frame gene deletion prevents polar effects on the expression of downstream genes [25].
The application of pSAM2 SSR system to generate unmarked deletion mutants requires the introduction of several vectors into Amycolatopsis: (1) the introduction of either a suicide vector for gene replacement by a selection marker or two suicide vectors for the introduction of attL and attR at the borders of the target region; (2) the introduction of an unstable self-replicative vector for excision. Thus, this process together with the recently reported CRISPR-Cas12a based method [13], which requires the successive introduction of two constructions, might seem more time-consuming than the ones using double homologous recombination techniques associated to a chromogenic screen [7] or a counterselectable marker [8], which require the introduction of a single vector. In these systems, the integration of a suicide vector by simple crossing over is selected using a resistance marker and then the loss of the vector, after a second event of homologous recombination, can be screen by the colour of the colonies or selected using the counterselectable marker. However, obtaining clones in which a second event of homologous recombination had occurred may require several rounds of sporulation in non-selective media [7], chromogenic screening can be hampered by the natural pigmentation of the colonies in some strains [8] and the use of counterselectable markers often implies to use a mutant strain [8]. With the approach described in this work, the steps based on homologous recombination are always associated with the possibility of selecting or screening the desired events by antibiotic resistance, a simple and robust procedure. The excision step, using the pSAM2 SSR system, results in a very high a very high (>95%) excision frequency, so that selection for the desired mutant is not necessary. After excision, apart a small scar of 33 bp, no exogenous DNA is left in the final strain. The CRISPR-Cas12a genome editing system recently described in A. mediterranei [13] has the advantage of not leaving any scar. However, the CRISPR-Cas12a edited A. mediterranei strains still carries the cas12a and the hygromycin resistance genes integrated in its chromosome [13]. In addition to A. mediterranei, we have been using these tools with success in two other Amycolatopsis species, Amycolatopsis tucumanensis [44] and Amycolatopsis sp. AA4 [45]. Moreover, the pSAM2 SSR system should be functional in many Amycolatopsis strains as no proteins other than Xis and Int are required for SSR, these proteins are expressed from a orthogonal promoter (trcp) which should not be affected by endogenous regulations from the host, and the pSAM2 SSR system, expressed from this same promoter, has previously been shown to be functional in several Streptomyces species (e.g., Streptomyces coelicolor A3(2) [46], Streptomyces lividans [47], Streptomyces venezuelae [48]) and in E. coli [23]. Therefore, the genetic tools developed here enrich the toolbox available for genome engineering in Amycolatopsis spp. They offer a simple, robust, and efficient alternative to other systems using chromogenic screening [7], counterselectable markers [8] or based on CRISPR-Cas technology [13] and should be helpful for the exploration and exploitation of Amycolatopsis spp. biotechnological potential. Moreover, it should be noted that the plasmids pEA02 and pEA03 might be used in Streptomyces spp. in combination with the Streptomyces replicative vectors expressing Xis and Int (pOSV236 [36], pOSV507 or pOSV508 [25]) to obtain large-scale deletions.
Acknowledgments
We thank Rup Lal for the kind gift of the plasmid pRL60 and Marick Esberard and Philippe Bouloc for the kind gift of S. aureus strains. We thank Jorddy Benoist, Anaïs da Costa and Gaëtan Pavard for their technical help and motivation during their internships. We thank Stéphanie Bury-Moné for critical reading of the manuscript and helpful discussions.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/microorganisms10040828/s1, Figure S1: Large-scale marker-free deletions using the pSAM2 SSR system. Table S1: Oligonucleotides used in this study. File S1: Sequence data of marker-free rifK mutants. File S2: Sequence data of marker-free rif cluster mutants.
Appendix A
Plasmid Construction
-
(i)
Construction of the plasmid pEA01 expressing Xis and Int
pRL60 was digested by EcoRI and DNA fragments of 6 kb (aph(3′) gene, pBR-origin and pA-rep) and 1,8 kb (ermE gene) were then purified and ligated, yielding pRL60∆amy. The DNA fragment containing the xis and int genes under control of trcp, the puromycin resistance gene pac and the origin of transfert oriT was isolated from pOSV236 by digestion with NotI and SspI. This 3 kb fragment was then blunt-ended using Klenow enzyme and cloned into pRL60∆amy linearized by EcoRV and dephosphorylated, yielding pEA01. The construction was verified by restriction enzyme digestion and sequencing.
-
(ii)
Construction of the plasmids pEA02 and pEA03 carrying attL and attR
A DNA fragment containing the attL and attR sites surrounded by desired restriction sites was chemically synthesized and cloned into a high copy number plasmid by Twist Bioscience, yielding pT-atts. A BamHI/EcoRI fragment containing the attL sequence flanked by the EcoRV, AvrII and NotI restriction sites was isolated from pT-atts and cloned into pOJ260 linearized by BamHI and EcoRI, yielding pEA02. A PstI/NsiI fragment containing the attR sequence flanked by the EcoRV, HindIII and AvrII restriction sites was isolated from pT-atts and ligated to a 2.8 kb fragment obtained by digestion of pOSV805 by PstI and NsiI, yielding pEA03. It should be noted that the int gene from ϕBT1, present in pOSV805, is no longer present in pEA03. Thus, pEA03 is a suicide vector in Amycolatopsis, as pEA02. Both plasmids were verified by restriction analyzes and partial sequencing.
-
(iii)
Construction of pRIF05 for the inactivation of rifK
The regions upstream and downstream (≈2 kb each) of rifK were amplified by PCR using the following couple of primers: LS151/LS152 and LS153/LS154, respectively. Each PCR product was cloned into pCR-blunt, yielding pRIF02 and pRIF01. Effective cloning of the fragments in pCR-blunt was controlled by restriction analysis and the integrity of the insert was verified by sequencing. The downstream region was then isolated from pRIF01 as a HindIII/PvuII fragment, while the upstream region was isolated from pRIF02 as a BamHI/PvuII fragment. The att1Ωhyg cassette was isolated from pOSV504 by EcoRV. The three DNA fragments were then cloned together into pOJ260 digested by BamHI and HindIII, yielding pRIF05.
-
(iv)
Construction of pRIF12 and pRIF14 for deletion of the rifamycin gene cluster
The regions upstream (1.2 kb) and downstream (1 kb) of the rifamycin (rif) biosynthetic gene cluster were amplified using the pairs of primers: LS155/LS156 and LS157/LS158, respectively. Each PCR product was cloned in pCR-blunt, yielding pRIF10 and pRIF09. Effective cloning of the fragments in pCR-blunt was controlled by restriction analysis and the integrity of the insert was verified by sequencing. The EcoRV/NotI fragment of pRIF10 containing the upstream region of the rif cluster was then cloned into pEA02 digested by the same enzymes yielding pRIF12. In the same way, to construct pRIF14, the downstream region of the rif cluster was isolated from pRIF09 as a NsiI/EcoRV fragment and was then cloned into pEA03 digested by the same enzymes.
Author Contributions
Conceptualization, L.D.F.S., L.C.-P., E.D. and J.-L.P.; funding acquisition, L.D.F.S. and J.-L.P.; investigation, L.D.F.S., L.C.-P. and E.D.; project administration, J.-L.P.; supervision, J.-L.P.; writing—original draft, L.D.F.S.; writing—review and editing, L.D.F.S., E.D. and J.-L.P. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Association Nationale de la Recherche et de la Technologie (CIFRE-2018/0227).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The genetic tools described here are available from Addgene (www.addgene.org, accessed on 15 March 2022), where information (e.g., maps and sequences) can be found.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Song Z., Xu T., Wang J., Hou Y., Liu C., Liu S., Wu S. Secondary metabolites of the genus Amycolatopsis: Structures, bioactivities and biosynthesis. Molecules. 2021;26:1884. doi: 10.3390/molecules26071884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.August P.R., Tang L., Yoon Y.J., Ning S., Müller R., Yu T.W., Taylor M., Hoffmann D., Kim C.G., Zhang X., et al. Biosynthesis of the ansamycin antibiotic rifamycin: Deductions from the molecular analysis of the Rif biosynthetic gene cluster of Amycolatopsis mediterranei S699. Chem. Biol. 1998;5:69–79. doi: 10.1016/S1074-5521(98)90141-7. [DOI] [PubMed] [Google Scholar]
- 3.Van Wageningen A.M.A., Kirkpatrick P.N., Williams D.H., Harris B.R., Kershaw J.K., Lennard N.J., Jones M., Jones S.J.M., Solenberg P.J. Sequencing and analysis of genes involved in the biosynthesis of a vancomycin group antibiotic. Chem. Biol. 1998;5:155–162. doi: 10.1016/S1074-5521(98)90060-6. [DOI] [PubMed] [Google Scholar]
- 4.Zmijewski M.J., Briggs B. Biosynthesis of vancomycin: Identification of tdp-glucose: aglycosyl-vancomycin glucosyltransferase from Amycolatopsis orientalis. FEMS Microbiol. Lett. 1989;59:129–133. doi: 10.1111/j.1574-6968.1989.tb03096.x. [DOI] [Google Scholar]
- 5.Davis J.R., Goodwin L.A., Woyke T., Teshima H., Bruce D., Detter C., Tapia R., Han S., Han J., Pitluck S., et al. Genome sequence of Amycolatopsis sp. strain ATCC 39116, a plant biomass-degrading actinomycete. J. Bacteriol. 2012;194:2396–2397. doi: 10.1128/JB.00186-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Albarracín V.H., Amoroso M.J., Abate C.M. Bioaugmentation of copper polluted soil microcosms with Amycolatopsis tucumanensis to diminish phytoavailable copper for Zea mays plants. Chemosphere. 2010;79:131–137. doi: 10.1016/j.chemosphere.2010.01.038. [DOI] [PubMed] [Google Scholar]
- 7.Fleige C., Meyer F., Steinbüchel A. Metabolic engineering of the actinomycete Amycolatopsis sp. strain ATCC 39116 towards enhanced production of natural vanillin. Appl. Environ. Microbiol. 2016;82:3410–3419. doi: 10.1128/AEM.00802-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meyer F., Pupkes H., Steinbüchel A. development of an improved system for the generation of knockout mutants of Amycolatopsis sp. strain ATCC 39116. Appl. Environ. Microbiol. 2017;83:e02660-16. doi: 10.1128/AEM.02660-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao H., Murugesan B., Hoßbach J., Evans S.K., Stark W.M., Smith M.C.M. integrating vectors for genetic studies in the rare actinomycete Amycolatopsis marina. BMC Biotechnol. 2019;19:32. doi: 10.1186/s12896-019-0521-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Madoń J., Hütter R. Transformation system for Amycolatopsis (Nocardia) mediterranei: Direct transformation of mycelium with plasmid DNA. J. Bacteriol. 1991;173:6325–6331. doi: 10.1128/jb.173.20.6325-6331.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stegmann E., Pelzer S., Wilken K., Wohlleben W. development of three different gene cloning systems for genetic investigation of the new species Amycolatopsis japonicum MG417-CF17, the ethylenediaminedisuccinic acid producer. J. Biotechnol. 2001;92:195–204. doi: 10.1016/S0168-1656(01)00360-1. [DOI] [PubMed] [Google Scholar]
- 12.Malhotra S., Majumdar S., Kumar M., Bhasin V.K., Gartemann K.H., Lal R. Nucleotide sequence of plasmid pA387 of Amycolatopsis benzoatilytica and construction of a conjugative shuttle Vector. J. Basic Microbiol. 2008;48:177–185. doi: 10.1002/jobm.200700326. [DOI] [PubMed] [Google Scholar]
- 13.Zhou Y., Liu X., Wu J., Zhao G., Wang J. CRISPR-Cas12a-assisted genome editing in Amycolatopsis mediterranei. Front. Bioeng. Biotechnol. 2020;8:1–9. doi: 10.3389/fbioe.2020.00698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hosted T.J., Baltz R.H. Use of RpsL for dominance selection and gene replacement in Streptomyces roseosporus. J. Bacteriol. 1997;179:180–186. doi: 10.1128/jb.179.1.180-186.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dubeau M.P., Ghinet M.G., Jacques P.É., Clermont N., Beaulieu C., Brzezinski R. Cytosine deaminase as a negative selection marker for gene disruption and replacement in the genus Streptomyces and other actinobacteria. Appl. Environ. Microbiol. 2009;75:1211–1214. doi: 10.1128/AEM.02139-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Potúčková L., Kelemen G.H., Findlay K.C., Lonetto M.A., Buttner M.J., Kormanec J. A New RNA polymerase sigma factor, σf is required for the late stages of morphological differentiation in Streptomyces spp. Mol. Microbiol. 1995;17:37–48. doi: 10.1111/j.1365-2958.1995.mmi_17010037.x. [DOI] [PubMed] [Google Scholar]
- 17.Knirschova R., Novakova R., Mingyar E., Bekeova C., Homerova D., Kormanec J. Utilization of a reporter system based on the blue pigment indigoidine biosynthetic gene bpsA for detection of promoter activity and deletion of genes in Streptomyces. J. Microbiol. Methods. 2015;113:1–3. doi: 10.1016/j.mimet.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 18.Rezuchova B., Homerova D., Sevcikova B., Núñez L.E., Novakova R., Feckova L., Skultety L., Cortés J., Kormanec J. An efficient blue-white screening system for markerless deletions and stable integrations in Streptomyces chromosomes based on the blue pigment indigoidine biosynthetic gene bpsA. Appl. Microbiol. Biotechnol. 2018;102:10231–10244. doi: 10.1007/s00253-018-9393-7. [DOI] [PubMed] [Google Scholar]
- 19.Siegl T., Luzhetskyy A. Actinomycetes genome engineering approaches. Antonie van Leeuwenhoek, Int. J. Gen. Mol. Microbiol. 2012;102:503–516. doi: 10.1007/s10482-012-9795-y. [DOI] [PubMed] [Google Scholar]
- 20.Herrmann S., Siegl T., Luzhetska M., Jilg L.P., Welle E., Erb A., Leadlay P.F., Bechthold A., Luzhetskyy A. Site-specific recombination strategies for engineering actinomycete. Appl. Environ. Microbiol. 2012;78:1804–1812. doi: 10.1128/AEM.06054-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Komatsu M., Uchiyama T., Omura S., Cane D.E., Ikeda H. Genome-minimized Streptomyces host for the heterologous expression of secondary metabolism. Proc. Natl. Acad. Sci. USA. 2010;107:2646–2651. doi: 10.1073/pnas.0914833107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boccard F., Smokvina T., Pernodet J.L., Friedmann A., Guérineau M. The integrated conjugative plasmid pSAM2 of Streptomyces ambofaciens is related to temperate bacteriophages. EMBO J. 1989;8:973–980. doi: 10.1002/j.1460-2075.1989.tb03460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raynal A., Tuphile K., Gerbaud C., Luther T., Guérineau M., Pernodet J.L. Structure of the chromosomal insertion site for pSAM2: Functional analysis in Escherichia coli. Mol. Microbiol. 1998;28:333–342. doi: 10.1046/j.1365-2958.1998.00799.x. [DOI] [PubMed] [Google Scholar]
- 24.Raynal A., Friedmann A., Tuphile K., Guérineau M., Pernodet J.L. Characterization of the attP site of the integrative element pSAM2 from Streptomyces ambofaciens. Microbiology. 2002;148:61–67. doi: 10.1099/00221287-148-1-61. [DOI] [PubMed] [Google Scholar]
- 25.Raynal A., Karray F., Tuphile K., Darbon-Rongère E., Pernodet J.-L. Excisable cassettes: New tools for functional analysis of Streptomyces genomes. Appl. Environ. Microbiol. 2006;72:4839–4844. doi: 10.1128/AEM.00167-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khanna M., Dua M., Lal R. Selection of suitable marker genes for the development of cloning vectors and electroporation in different strains of Amycolatopsis mediterranei. Microbiol. Res. 1998;153:205–211. doi: 10.1016/S0944-5013(98)80002-5. [DOI] [PubMed] [Google Scholar]
- 27.Malhotra S., Lal R. The genus Amycolatopsis: Indigenous plasmids, cloning vectors and gene transfer systems. Indian J. Microbiol. 2007;47:3–14. doi: 10.1007/s12088-007-0003-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu L., Li Y., Zhu L., Zhao W., Chen D., Huang W., Yang S. Characterization of plasmid pXL100 from Amycolatopsis orientalis HCCB10007 and construction of a shuttle vector. J. Basic Microbiol. 2015;55:247–254. doi: 10.1002/jobm.201400210. [DOI] [PubMed] [Google Scholar]
- 29.Lal R., Khanna R., Dhingra N., Khanna M., Lal S. Development of an improved cloning vector and transformation system in Amycolatopsis mediterranei (Nocardia mediterranei) J. Antibiot. (Tokyo) 1998;51:161–169. doi: 10.7164/antibiotics.51.161. [DOI] [PubMed] [Google Scholar]
- 30.Paget M.S., Chamberlin L., Atrih A., Foster S.J., Buttner M.J. Evidence that the extracytoplasmic function sigma factor sigmae is required for normal cell wall structure in Streptomyces coelicolor A3(2) J. Bacteriol. 1999;181:204–211. doi: 10.1128/JB.181.1.204-211.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MacNeil D.J., Gewain K.M., Ruby C.L., Dezeny G., Gibbons P.H., MacNeil T. Analysis of Streptomyces avermitilis genes required for avermectin biosynthesis utilizing a novel integration vector. Gene. 1992;111:61–68. doi: 10.1016/0378-1119(92)90603-M. [DOI] [PubMed] [Google Scholar]
- 32.Aubry C., Pernodet J.-L., Lautru S. Modular and integrative vectors for synthetic biology applications in Streptomyces spp. Appl. Environ. Microbiol. 2019;85:e00485-19. doi: 10.1128/AEM.00485-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herbert S., Ziebandt A.-K., Ohlsen K., Schäfer T., Hecker M., Albrecht D., Novick R., Götz F. Repair of global regulators in Staphylococcus aureus 8325 and comparative analysis with other clinical isolates. Infect. Immun. 2010;78:2877–2889. doi: 10.1128/IAI.00088-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kieser T., Chater K.F., Bibb M.J., Buttner M.J., Hopwood D.A. Pratical Streptomyces Genetics. John Innes Foundation; Norwich: 2000. [Google Scholar]
- 35.Sambrook J., Russell D.W. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY, USA: 2001. [Google Scholar]
- 36.Nguyen H.C., Karray F., Lautru S., Gagnat J., Lebrihi A., Huynh T.D.H., Pernodet J.L. Glycosylation steps during spiramycin biosynthesis in Streptomyces ambofaciens: Involvement of three glycosyltransferases and their interplay with two auxiliary proteins. Antimicrob. Agents Chemother. 2010;54:2830–2839. doi: 10.1128/AAC.01602-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bierman M., Logan R., O’Brien K., Seno E.T., Nagaraja Rao R., Schoner B.E. Plasmid cloning vectors for the conjugal transfer of DNA from Escherichia coli to Streptomyces spp. Gene. 1992;116:43–49. doi: 10.1016/0378-1119(92)90627-2. [DOI] [PubMed] [Google Scholar]
- 38.Pattee P.A. Use of tetrazolium for improved resolution of bacteriophage plaques. J. Bacteriol. 1966;92:787–788. doi: 10.1128/jb.92.3.787-788.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nguyen H.C., Darbon E., Thai R., Pernodet J.L., Lautru S. Post-PKS tailoring steps of the spiramycin macrolactone ring in Streptomyces ambofaciens. Antimicrob. Agents Chemother. 2013;57:3836–3842. doi: 10.1128/AAC.00512-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Witwinowski J., Moutiez M., Coupet M., Correia I., Belin P., Ruzzini A., Saulnier C., Caraty L., Favry E., Seguin J., et al. Study of bicyclomycin biosynthesis in Streptomyces cinnamoneus by genetic and biochemical approaches. Sci. Rep. 2019;9:20226. doi: 10.1038/s41598-019-56747-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Juguet M., Lautru S., Francou F.X., Nezbedová Š., Leblond P., Gondry M., Pernodet J.L. An iterative nonribosomal peptide synthetase assembles the pyrrole-amide antibiotic congocidine in Streptomyces ambofaciens. Chem. Biol. 2009;16:421–431. doi: 10.1016/j.chembiol.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 42.Tuteja D., Dua M., Khanna R., Dhingra N., Khanna M., Kaur H., Saxena D.M., Lal R. The importance of homologous recombination in the generation of large deletions in hybrid plasmids in Amycolatopsis mediterranei. Plasmid. 2000;43:1–11. doi: 10.1006/plas.1999.1426. [DOI] [PubMed] [Google Scholar]
- 43.Li C., Liu X., Lei C., Yan H., Shao Z., Wang Y., Zhao G., Wang J., Ding X. RifZ (AMED_0655) is a pathway-specific regulator for rifamycin biosynthesis in Amycolatopsis mediterranei. Appl. Environ. Microbiol. 2017;83:1–10. doi: 10.1128/AEM.03201-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Santos L.D.F. Ph.D. Thesis. University Paris-Saclay; Gif-sur-Yvette, France: 2022. Metabolic Engineering of Actinobacteria for the Production of Flavor. [Google Scholar]
- 45.Caraty-Philippe L., da Silva A., Pernodet J.L., Darbon E. (Université Paris-Saclay, CEA, CNRS, Institute for Integrative Biology of the Cell (I2BC), 91198 Gif-sur-Yvette, France). Characterization of diketopiperazine biosynthetic gene clusters in Amycolatopsis sp. AA4. Personal communication. 2022. manuscript in preparation .
- 46.Boubakri H., Seghezzi N., Duchateau M., Gominet M., Kofroňová O., Benada O., Mazodier P., Pernodet J.L. The absence of pupylation (prokaryotic ubiquitin-like protein modification) affects morphological and physiological differentiation in Streptomyces coelicolor. J. Bacteriol. 2015;197:3388–3399. doi: 10.1128/JB.00591-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shikura N., Darbon E., Esnault C., Deniset-Besseau A., Xu D., Lejeune C., Jacquet E., Nhiri N., Sago L., Cornu D., et al. The phosin PptA plays a negative role in the regulation of antibiotic production in Streptomyces lividans. Antibiotics. 2021;10:325. doi: 10.3390/antibiotics10030325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Witwinowski J. Ph.D. Thesis. Université Paris-Saclay; Gif-sur-Yvette, France: 2017. Caractérisation de Voies de Biosynthèse de Dicétopipérazines Chez les Actinobactéries. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The genetic tools described here are available from Addgene (www.addgene.org, accessed on 15 March 2022), where information (e.g., maps and sequences) can be found.