Abstract
Abiotic stresses, such as those induced by climatic factors or contaminants, and biotic stresses prompted by phytopathogens and pests inflict tremendous losses in agriculture and are major threats to worldwide food security. In addition, climate changes will exacerbate these factors as well as their negative impact on crops. Drought, salinity, heavy metals, pesticides, and drugs are major environmental problems that need deep attention, and effective and sustainable strategies to mitigate their effects on the environment need to be developed. Besides, sustainable solutions for agrocontrol must be developed as alternatives to conventional agrochemicals. In this sense, nanotechnology offers promising solutions to mitigate environmental stress effects on plants, increasing plant tolerance to the stressor, for the remediation of environmental contaminants, and to protect plants against pathogens. In this review, nano-sized TiO2 (nTiO2) and ZnO (nZnO) are scrutinized, and their potential to ameliorate drought, salinity, and xenobiotics effects in plants are emphasized, in addition to their antimicrobial potential for plant disease management. Understanding the level of stress alleviation in plants by these nanomaterials (NM) and relating them with the application conditions/methods is imperative to define the most sustainable and effective approaches to be adopted. Although broad-spectrum reviews exist, this article provides focused information on nTiO2 and nZnO for improving our understanding of the ameliorative potential that these NM show, addressing the gaps in the literature.
Keywords: drought, metal stress, phytotoxicity, phytopathogens, salinity, stress mitigation
1. Introduction
Nanotechnology is gaining prominence in the agro-food system as consequence of the positive reports released in the last half decade highlighting the promising applications that nanomaterials may have in plant fortification, enhancing crop tolerance to abiotic stresses, and improving plant defence against pathogens [1,2,3,4,5,6,7,8,9,10,11,12]. Besides, nanotechnology offers a route to make agriculture more sustainable and precise, as it can contribute to the reduction of the amount of agrochemicals used in farming and their consequent accumulation in the environment, decrease the production cost of conventional fertilizers, smartly deliver active molecules to enhance crop performance and improve plant disease prevention and control, and mitigate the effects of environmental pollutants, pesticide degradation, micronutrients for efficient use, etc. [13]. Thanks to the reduced size of nanomaterials (NM), they show an increased surface-to-volume ratio, reactivity, and frequently distinct properties from their bulk or ionic counterparts [14]. Their particular physicochemical properties enable them to increase the efficiency of agrochemicals at the same time as decreasing the number of active compounds and raw materials needed to produce them and to be used in agriculture. These features will decrease the environmental impact of agrochemicals and contribute to the development of new strategies to overcome the challenges of modern agriculture, including climate changes, crop diseases, water limitations, and contaminated/poor soils, which altogether result in crop yield decline and food insecurity [15].
The general impact of NM in plants has been reviewed recently [16,17,18,19,20], as well as the use of nanocarriers to deliver active compounds to plants [21,22,23], the broad applications of NM to improve plant growth and plant stress tolerance [24,25,26,27,28,29,30,31], and the potential of NM for plant disease management [32]. Here, we focus on TiO2 (nTiO2) and ZnO (nZnO) nano-formulations as plant protective and ameliorative agents against (a)biotic stress based on the reports released in the last five years of research, highlighting their potential to cope with drought and salinity stresses, as well as their potential in metal stress mitigation and plant protection. This approach will allow readers to gain comprehensive knowledge about the potential applications of these materials as stress mitigators, as nano-fertilizers, and antimicrobial solutions for agrocontrol, in seed science, in growth promotion, metal remediation, etc.
2. Metal-Based Nanomaterials and Their Impact on Plants
Collectively, NM hold great promise for boosting crop production, as well as offering a safer alternative to synthetic counterparts for the environment when adopting green-synthesis to produce them. Metal-based NM show desirable characteristics that can be further explored and used in multiple agricultural proposes: higher effectiveness against plant pathogens, either due to biocide traits or to the modulation of plant defense mechanisms and of the proteome/metabolome [33,34,35]; stimulatory effects, improving plant physiological attributes and regulating plant metabolism [3,4,36]; prophylactic activity against adverse climatic/environmental conditions by promoting nutrient uptake/accumulation, modulating the antioxidant response, etc. [5,6,37]; smart delivery to plants of fertilizers, biostimulants, and pesticides as a result of controlled release of bioactive compounds [7]; and binding phases for both organic and inorganic contaminants, making them promising strategies for the remediation of polluted soils/waters [8,9,10,38].
After entering the plants by leaves and roots, nTiO2 and nZnO interact with plants at different levels, leading to several molecular, biochemical, and physiological changes [11]. The effects, positive or negative, depend on several factors, such as nanoparticles’ chemical nature, reactivity, size, and concentration [12]. Despite the large amount of information available on the effects of NM on the expression level of many genes and cellular mechanisms, much more needs to be done to unravel the mechanisms of action triggered by these nanoparticles (NP) [12].
One of the main processes improved by nTiO2 and nZnO is photosynthesis. In the case of the nTiO2, this may result from the photocatalytic properties of these NP, increasing the water hydrolyzation induced by light into oxygen, electrons, and protons. The electrons and protons produced enter the electron transport chain during the light reaction phase of photosynthesis, leading to a general increment of the photosynthetic process [39]. Besides that, biochemical and molecular studies also pinpoint an enhancement of the content and expression of LHCII b genes in the thylakoid membrane by nTiO2, promoting light absorption in chloroplasts [40]. In addition, ribulose-1,5-biphosphate carboxylase/oxygenase (RuBisCO) is also very sensitive to nTiO2, and increases in the rate of photosynthesis by nTiO2 can be attributed to RuBisCO activase acceleration facilitating the carboxylation [26]. Thus, higher photosynthesis can result in an increased supply of photoassimilates in leaves and better growth. Additionally, the stimulatory effect of nTiO2 in nutrient uptake and use efficiency can also be related to the increase of pigment levels and photosynthesis. nTiO2 improves nitrate reductase activity, increasing nitrogen assimilation and therefore increasing amino acids and protein production and growth [41]. Concerning the effects of nTiO2 on plant oxidative stress and antioxidant responses, it is described that these NM can act as pro-oxidants and antioxidants in modulation ROS signaling [42]. The changes in ROS level induced by nTiO2 are mostly associated with alterations in chloroplast function, one of the sites of major ROS production centers in plants [42]. In turn, it was also demonstrated that nTiO2 induces the activation of the biosynthesis of antioxidants, such as vitamin E [43].
The beneficial effects of nZnO in several plant processes, such as photosynthesis and the antioxidant system, are related to the putative increase of Zn availability and/or to the molecular effects of these NM [44,45]. Zn is an essential element that acts as a cofactor of a large number of key enzymes (e.g., SOD, carbonic anhydrase, and glutathione dehydrogenase), and it is involved in the metabolism of carbohydrates and proteins [46]. nZnO acts at the pigment synthesis level, promoting carotene and chlorophyll biosynthesis [47]. Moreover, these NPs can strengthen the plant vascular system, particularly the metaxylem tissues, improving the nutritional status [44]. Additionally, nZnO is described to modulate many genes and transcription factors (e.g., ARP, MPK4, MKK2, SKRD2, MYC, bHLH, EREB, HsfA1a, R2R3MYB, and WRKY1) associated with physiological, hormonal, and developmental responses, and abiotic stress tolerance [44,48]. Moreover, a recent study highlights that nZnO can induce epigenetic modifications, downregulating the histone deacetylases gene (HDA3) [44]. Furthermore, nZnO also modulates the transcription of genes of the antioxidant system, leading to a protective response by increasing the activity of several antioxidant enzymes [49].
Despite the evident advantage, and the potential to revolutionize the actual agricultural system, metal-based NM also have some ecotoxicological implications, as they may show a certain level of toxicity for plants [18,50,51,52], fungi, algae, and microorganisms [7,53]. Concerning the phytotoxicity of nTiO2 [54,55,56,57] and nZnO [58,59,60,61], it is frequently associated with higher doses; nevertheless, other factors such as plant species [62,63,64], exposure period [65,66,67], and crystalline phase in the case of nTiO2 [63,68] also play a role.
3. The Potential of nTiO2 and nZnO in Increasing Abiotic Stress Tolerance to Plants
Abiotic stresses are environmental factors that can limit plant growth, development, and productivity, and global climate change scenarios have contributed substantially to the intensification of these factors [69]. Therefore, strategies that reduce their adverse impact on plants need to be implemented to increase the resilience of plants to stress conditions. Among the several strategies adopted to mitigate the negative effects of abiotic stresses in plants, nanotechnology–particularly the use of NM, mostly NP—is one of the most promising [11].
Several metal-based NM, such as nTiO2 and nZnO, have been extensively studied in the last years due to their environmentally favorable use in agriculture, particularly in the promotion of plant growth and their protective role under stress conditions [39]. These studies have been mostly conducted in plants exposed to drought and salinity, since these abiotic stresses are the most common and produce a stronger impact on plant productivity [70,71,72]. The beneficial effects of the application of nTiO2 and nZnO in plants exposed to other abiotic stresses, such as high and low temperature [11,73], were less studied. For instance, under cold stress, nTiO2 foliar application (5 mg L−1) in Cicer arietinum increases the antioxidant enzymes activity, RuBisCO and phosphoenolpyruvate carboxylase, and the levels of pigments [74,75]. Moreover, these NPs reduce H2O2 content and membrane damage. The same NM applied in Lycopersicon esculentum (nano-anatase with 16 nm; 0.05, 0.1 and 0.2 g L−1) exposed to heat stress enhanced photosynthesis and promoted stomatal opening [76]. In wheat plants under heat conditions, foliar application of nZnO (10 ppm, size 80 nm) increased the antioxidant enzymes activity (e.g., SOD, CAT, GST, and peroxidase) and reduced the levels of lipid peroxidation [77].
3.1. Drought and Salinity
Climate changes have contributed to the increase of global drought, changing the precipitation patterns and increasing the periods without or with low precipitation [69]. Drought is therefore considered one of the most natural hazards, with important consequences in the agriculture sector and food security [70]. For instance, the occurrence of drought events in the European Union (particularly in southern and western parts) resulted in annual agriculture economic losses of around 10% [71]. Furthermore, global soil salinization is increasing due to climate change [72]. Intensive farming together with low-quality irrigation water and poor drainage have strongly contributed to soil salinization [78]. Currently, around 62 million hectares of the world’s irrigated area suffer from salinity, and this situation will be aggravated, particularly in the arid and semi-arid regions [79]. Therefore, drought and salinity are major global concerns and key factors that decline plant performance, yield, and productivity [78,80].
A common feature of these abiotic stresses is that they affect one of the most important key processes in plants—photosynthesis—reducing photosynthetic reactions and pigments levels but increasing the production of reactive oxygen species (ROS) leading to oxidative stress [72]. To control the levels of ROS and oxidative damages, plants can activate the antioxidant system, composed of enzymatic (e.g., superoxide dismutase (SOD), catalase (CAT), peroxidase (POD), and ascorbate peroxidase (APX)), and non-enzymatic (e.g., glutathione, ascorbate (AsA), and flavonoids) antioxidants [51]. In addition, plants can develop stress tolerance mechanisms to avoid negative effects of stress; nevertheless, they vary between species and depend on the intensity and duration of the stress event [72].
The use of stress mitigation agents to alleviate the negative impact of these abiotic stresses can also be an affordable strategy to maintain plant growth and productivity. Therefore, the search for new stress mitigation strategies that ensure food and nutritional security under the rising global population has increased. Several metal-based NM have been investigated and their potential to mitigate drought and salt stress adverse effects demonstrated in several works in different species (e.g., [28,39]). NP application in drought and salt-stressed plants has been reported to increase the availability of water in leaves and to promote growth (above and below-ground), biomass production, nutrient uptake, carbohydrate accumulation, and photosynthesis (Table 1 and Table 2). NM can also modulate phytohormone and osmolyte levels under drought and salinity conditions and contribute to the reduction of oxidative stress by the upregulation of the antioxidant battery (Table 1 and Table 2). Additionally, under salt stress conditions, NP can help to regulate ion balance, reducing Na+ toxicity and increasing the uptake of K+ in plants [28].
Table 1.
Effects of nTiO2 on plants under drought conditions.
| Stress Conditions | nTiO2 Crystalline Phase; Concentrations; Primary (PS) or Hydrodynamic Size (HS) | Plant Species | Application Method | Ameliorative Effects | Ref. |
|---|---|---|---|---|---|
| After seed-filling maintained at 50% FC | Anatase; 10, 100, and 500 mg L−1; PS 10–25 nm. |
Linum usitatissimum L. | Three foliar applications at the initial seed-filling | Increased leaf carotenoids and seed protein | [88] |
| PEG-6000 solutions at −0.4 and −0.8 MPa | Not stated; 500 and 2000 mg L−1 PS 10–25 nm. |
Triticum aestivum L. | Seed priming for 7 days | Increased shoot and root length, as well as fresh weight | [83] |
| 75% and 50% FC for 6 weeks | Not stated; 500, 1000 and 2000 mg kg−1 soil; PS 10–25 nm. |
Triticum aestivum L. | Soil amended | Increased leaf RWC, chlorophylls and carotenoids levels, and antioxidant enzymes (APX, CAT). Decreased lipid peroxidation and H2O2 production | [82] |
| 45% FC for 15 days | Not stated; 20 and 40 mg kg−1 soil; PS 347–447 nm. |
Triticum aestivum L. | Soil amended | Increased root length, hormone level (IAA and GA), proline and sugars. Improvement of the antioxidant enzymes (SOD, POD and CAT) and nutrient uptake (K and P) | [81] |
| 105 and 140 mm evaporation from the class A evaporation pan (mm day−1) | Anatase; 50 and 100 mg L−1 PS 10–25 nm. |
Zea mays L. | Foliar spray twice with an interval of 2 weeks (at the 4–6 leaf stage and 2 weeks after that) | Increased leaf relative water content, Fv/Fm, carotenoids, chlorophylls, proline, soluble protein and grain yield. Improvement of the activity of the antioxidant enzymes (SOD, APX and CAT) | [84] |
Table 2.
Effects of nTiO2 on plants under salinity conditions.
| Stress Conditions | nTiO2 Crystalline Phase; Concentrations; Primary (PS) or Hydrodynamic Size (HS) | Plant Species | Application Method | Ameliorative Effects | Ref. |
|---|---|---|---|---|---|
| 180 mM NaCl for 28 days | Not stated; 160, 320 and 480 mg L−1; PS 20–78 nm. |
Vicia faba L. | Two foliar applications, 3 and 10 days after NaCl treatments | Increased root and shoot length and dry weight. Improvement of the levels of proline, sugars, chlorophylls and carotenoids. Increased antioxidant enzyme activities (SOD, POD, APX and CAT). Decreased lipid peroxidation and H2O2 production |
[42] |
| 100 and 200 mM NaCl for 40 days | Anatase; 500, 1000 and 2000 mg kg−1; PS 10–25 nm. |
Hordeum vulgare L. | Soil amended | Increased root length, leaf RWC, net CO2 assimilation rate, stomatal conductance, transpiration rate, chlorophyll and proline. Improvement of the antioxidant enzyme activities (APX and CAT). Decreased lipid peroxidation |
[87] |
| 50 and 100 mM NaCl for 2 weeks | Anatase; 100 and 200 mg L−1; PS 25 nm. |
Stevia rebaudiana Bertoni. | Foliar spray for 3 times (during the growth period) | Increased root and shoot height, fresh and dry weight, leaf RWC, chlorophylls, carotenoids contents. Improvement of the net CO2 assimilation rate and Fv/Fm. Increased antioxidant enzyme activities (SOD, POD, APX and CAT). Decreased lipid peroxidation and H2O2 production |
[86] |
3.1.1. Ameliorative Effects of nTiO2 in Plants Grown under Drought or Salinity Stress
nTiO2 is one of the most studied NPs, with applications in several areas, such as pharmaceutical, medicinal, industrial, and agricultural fields [39]. Most of the benefits of the application of these NM in plants, particularly at the photosynthesis level under both optimal and abiotic stress conditions, are related to the photocatalytic properties of nTiO2 [81]. Within the three crystalline structures of nTiO2 (anatase, rutile, and brookite), anatase exhibits the highest catalytic activity. The several advantages of nTiO2 application in plant species exposed to drought and salt stress conditions are summarized in Table 1 and Table 2.
Concerning drought, several studies have been conducted with wheat plants treated with nTiO2. Faraji and Sepehri [82,83] reported several positive effects in a controlled experiment using different nTiO2 concentrations for seed priming or soil amendment, respectively, before water deficit treatments (Table 1). These authors described that seed priming with nTiO2 promoted wheat shoot and root length and fresh weight, and soil amendment increased leaf water availability (relative water content—RWC) despite the higher stomatal conductance and transpiration and photosynthetic pigment levels (chlorophylls and carotenoids). Furthermore, in the same species and adding nTiO2 in pot soil, Mustafa et al. [81] demonstrated that they modulated the levels of hormones (increase of IAA and GA), proline, and carbohydrates under drought stress conditions. The wheat root length and nutrient uptake (K and P) were also improved by this NP under stress [81]. In maize plants, Karvar et al. [84] reported that nTiO2 foliar application increased the leaf RWC, Fv/Fm, carotenoids, chlorophylls, proline, soluble protein, and grain yield when plants were under drought. Besides photosynthesis, the production of secondary metabolites is also improved by the application of nTiO2 under drought conditions. Moreover, the enzymatic antioxidant system is also activated by nTiO2 in response to drought [39]. The activity of CAT, APX, and POD increased in basil, wheat, and maize plants treated with nTiO2 [81,82,84,85], reducing the levels of oxidative stress by decreasing the production of H2O2 and lipid peroxidation [82].
In the case of salt stress, the benefits of nTiO2 application are very similar to drought. In a study conducted by Sheikhalipour et al. [86], the nTiO2 foliar treatment of stevia plants exposed to different levels of salinity (50 mM and 100 mM NaCl) improved leaf availability (RWC), chlorophylls and carotenoids levels, and photosynthesis (net CO2 assimilation rate and Fv/Fm), leading to higher plant height and weight. At increased levels of salinity (180 mM), faba bean plants also respond to foliar nTiO2 treatment, increasing the levels of photosynthetic pigments, sugars, and proline, resulting in an improvement of growth [42]. nTiO2 application also ameliorated oxidative stress, activating several antioxidant enzymes (SOD, CAT, APX, and CAT) and reducing the levels of lipid peroxidation and H2O2 production [42,86]. In barley plants exposed to high levels of salinity (100 and 200 mM), nTiO2 application in soil improved photosynthesis (net CO2 assimilation rate, stomatal conductance, and transpiration rate) and chlorophyll and proline levels [87]. Moreover, barley leaf relative water content (RWC) and root length increased, as well as the activities of CAT and SOD, which contributed to the decrease of lipid peroxidation under salinity [87].
3.1.2. Ameliorative Effects of nZnO in Plants Grown under Drought or Salinity Stress
nZnO has been widely used in several areas (e.g., cosmetic and medicine), but its higher popularity arises from its use in fertilizers and pesticides manufacture [89]. Zn is an important cofactor of several essential enzymes, and the benefits of nZnO application in plants are in part based on the increased availability of this nutrient to the plant, which leads to improvements on several metabolic pathways [45]. Several advantages of nZnO application in plant species have been reported under both drought and salt stress conditions (Table 3 and Table 4).
Table 3.
Effects of nZnO on plants under drought conditions.
| Stress Conditions | nZnO Concentrations; Primary (PS) or Hydrodynamic Size (HS) | Plant Species | Application Method | Ameliorative Effects | Ref. |
|---|---|---|---|---|---|
| 30% of total moisture for 3 days. | Not stated | Triticum aestivum L. | Seed priming for 4 h | Increased leaf RWC, chlorophyll and carotenoids levels, and antioxidant enzyme activities (SOD and CAT). Decreased lipid peroxidation | [91] |
| 40% field capacity | 1, 3 and 5 mg L−1; PS 18 nm |
Sorghum bicolor | Soil amended | Increased grain yield | [37] |
| 40% field capacity for 210 days | 2.17 mg kg−1; not stated |
Triticum aestivum L. | Soil amended | Increased chlorophyll content and grain nutrient | [92] |
| 6 days at 45% (soil water content). | 100 mg L−1; PS 20 nm |
Zea mays L. | Seed priming | Increased root and shoot height, fresh and dry weight, as well as sugars, protein, amino acids (tryptophane) and proline. Improvement of antioxidant enzyme activities and gene relative expression (SOD, POD, APX and CAT). Decreased H2O2 production | [93] |
| 12 days | 25 and 100 mg L−1; PS 50 nm |
Cucumis sativus L. | Foliar application 3 time a week, for two weeks | Increased shoot fresh and dry weight, root dry weight and length, leaf RWC, chlorophylls, carotenoids, protein content, net CO2 assimilation rate, stomatal conductance, transpiration rate, intercellular CO2 concentration, Fv/Fm, qP and ΦPSII. Accumulation of proline, glycine betaine, free amino acids, and sugars. Improvement of antioxidant enzyme activities (SOD, POD, APX, CAT, GR, DHAR and MDHAR) and PAL activity. Increased total phenols, flavonoids, ascorbate (AsA) and glutathione. Decreased O2−• and H2O2 production, lipid peroxidation, electrolyte leakage and NPQ | [80] |
| 60% of crop evapotranspiration (ETc) for 5 months. | 50 and 100 mg L−1; not stated |
Solanum melongena L. | Foliar application 2 times | Increased leaf RWC and Fv/Fm. Improved membrane stability | [90] |
Table 4.
Effects of nZnO on plants under salinity conditions.
| Stress Conditions | nZnO Concentrations; Primary (PS) or Hydrodynamic Size (HS) | Plant Species | Application Method | Ameliorative Effects | Ref. |
|---|---|---|---|---|---|
| Irrigated in the beginning with 150 mM NaCl | 20, 40 and 60 mg L−1; PS 21.3 nm |
Lupinus termis Forssk. | Seed priming for 12 h | Increased proline, protein and free amino acids, sugars, chlorophylls and carotenoids, total phenols and AsA. Improved the antioxidant enzyme activities (SOD, POD, APX and CAT). Decreased lipid peroxidation | [89] |
| Irrigated in the beginning with 108 mM NaCl | 10 mg L−1; PS 30 nm |
Brassica napus L. | Three foliar applications at 50, 65, and 80 days after sowing | Increased carotenoids, proline and sugars. Improved the activity of antioxidant enzymes (SOD, POD, and CAT), and the pool of ASA and total phenolic compounds. Decreased H2O2 production, lipid peroxidation and membrane leakage | [98] |
| Salinized drainage water | 50, 100 and 150 mg L−1; PS < 100 nm |
Mangifera indica L. | Two foliar applications (full bloom and 1 month after) | Enhanced leaf NPK content, total carbohydrates and proline. Increased the activities of the antioxidant enzymes SOD, POX, and CAT | [97] |
| <30 ds/m NaCl | 12, 15 and 20 mg L−1; PS 4.50–5.80 nm |
Solanum tuberosum L. | Soil amended, 15 days before planting, and 20, 35, 45 and 70 days after planting | Increased plant height, fresh and dry weight, and leaf RWC. Improved the net CO2 assimilation rate, stomatal conductance, intercellular CO2 concentration and WUE. Increased the levels of chlorophyll, proline, phytohormones (GA) and leaf nutrients (N, P, K, Ca, Na, Zn and B). Decreased the transpiration rate and the levels of ABA | [96] |
| 150 mM NaCl | 10, 50, 100 mg L−1; not stated |
Lycopersicon esculentum Mill. | Soil amended at the time of transplanting (15 days after sowing) | Increase the shoot and root fresh and dry weight, and length, leaf area, protein content, proline, and chlorophyll. Improved the net CO2 assimilation rate, stomatal conductance, transpiration rate and intercellular CO2 concentration. Improved the activity of the antioxidant enzymes (SOD, POD and APX). | [94] |
| 150 mM NaCl applied 30 days after sowing | 50 mg/L; not stated |
Linum usitatissimum L. | Foliar application 60 days after sowing | Increased shoot fresh and dry weight, root dry weight and length, leaf area, and leaf nutrients (C, K and Ca). Improved net CO2 assimilation rate, stomatal conductance, intercellular CO2 concentration, WUE, Fv/Fm, qP and ΦPSII. Increased the levels of chlorophylls, proline, carbohydrates, NR, carbonic anhydrase, and the activity of antioxidant enzymes (SOD, POD, and CAT). Decreased the O2− and H2O2 production, lipid peroxidation. | [95] |
| 150 mM NaCl for 7 days | 25, 50 and 100 mg L−1; not stated |
Brassica napus L. | Seed priming for 8 h | Increased sugar, soluble protein and SOD activity | [99] |
Concerning drought stress, positive effects of nZnO treatments (seed priming, soil amendment, or foliar application) on wheat, cucumber, and aubergine plants were reported, increasing water availability, both stages of photosynthesis, light-dependent and independent reactions, and photosynthetic pigments [80,90,91,92]. nZnO stimulated carbohydrate (e.g., leaf sugar levels) and amino acid (e.g., proline, glycine betaine, and free amino acids) metabolism and increased shoot and root growth (length, fresh and dry weight) in maize and cucumber plants [80,93]. Furthermore, nZnO seems to induce a strong boost of the antioxidant system, upregulating the expression and activity of several antioxidant enzymes (SOD, POD, APX, CAT, glutathione reductase (GR), dehydroascorbate reductase (DHAR), monodehydroascorbate reductase (MDHAR), and phenylalanine ammonia lyase (PAL) activity) and non-enzymatic antioxidants (ascorbate, glutathione, total phenols, and flavonoids) [80,91,93] leading to lower oxidative stress due to less lipid peroxidation, membrane leakage, and O2−• and H2O2 accumulation under drought stress conditions [80,90,91,93]. A study conducted with sorghum plants also showed that nZnO application in soil improves grain yield [37] and in wheat grains enhances nutrient levels under drought conditions [92].
Under salinity conditions (30–150 mM), different nZnO applications (soil amendment or foliar spray) induced positive effects in potato, tomato, and flax plants (Table 4), with improvements at the shoot and root growth attributes (length, fresh and dry weight), leaf area, photosynthetic parameters (including both light-dependent and independent reactions), chlorophyll, protein, and proline [94,95,96]. In these species, nZnO treatment increased leaf nutrient uptake and stimulated the antioxidant system (antioxidant enzymes), leading to lower oxidative stress (reduction of lipid peroxidation, O2−• and H2O2 production). In addition, Mahmoud et al. [96] verified an increase of the RWC and gibberellic acid (GA) levels in salt-stressed potato plants. Furthermore, nZnO foliar application stimulated the carbohydrate and amino acid metabolism in mango plants watered with salinized drainage water [97]. These authors also reported an enhancement of nutrient levels and antioxidant capacity (antioxidant enzymes SOD, POX, and CAT).
nZnO foliar application improved the levels of carotenoids, proline, sugars, and antioxidants (enzymes, ascorbate, and total phenolic compounds) in salt-stressed rapeseed plants [98]. These authors reported that nZnO decreased the levels of oxidative stress (H2O2 production, lipid peroxidation, and membrane leakage) in this species caused by salinity (108 mM). In Lupinus exposed to 150 mM NaCl, seed priming with nZnO increased protein, sugars, free amino acids, including proline, and pigments levels, as well as antioxidant enzyme activities (SOD, POD, APX and CAT) and non-enzymatic antioxidants (total phenols and ascorbate) resulting in lower levels of lipid peroxidation [89]. Furthermore, rapeseed seeds primed with nZnO lead to improved levels of sugars, soluble protein, proline, and increased activity of SOD under 150 mM NaCl [99]. These authors also verified that these NPs upregulated the gene BnPER (peroxiredoxin antioxidant family gene) in priming seeds, contributing to oxidative damage reduction under salinity.
3.2. Environmental Contaminants
Climatic stresses combined or separated with xenobiotics induce major damage in sensitive crops, making them major threats to food security and the financial safety of farmers [100]. Soil contamination by metals, in particular heavy metals, is an environmental problem worldwide, with a negative impact on agriculture as a consequence of their phytotoxicity, low mobility, non-biodegradable nature, and high persistence [101,102]. Moreover, the contamination of soils by metals is a health problem due to the trophic transfer in the food chain and the bioaccumulation and biomagnification of metals [103].
The consumption of crops contaminated with metals causes risks to human health and animals, as some of these elements are not essential to humans (e.g., Pb, Cd, As, Hb, Al), and others, despite having functional roles in humans (e.g., Zn, Fe, Mn, Mg, Cr, Ni, Cu, Mo, Se), can have adverse health outcomes at high doses. Some of the putative effects may include alterations in reproductive health [104], impacts on the nervous system, the induction of carcinogenesis, oxidative stress, and loss of cellular functions [105,106].
In crops, multiple effects have been described to be induced by metal exposure, frequently dependent on the dose, exposure period, crop species and/or genotype, and soil physicochemical characteristics (e.g., pH) [107]. Impairments in plant morphology, physiology, biochemistry, and adjustments in plant metabolism are transversal to a high number of metal contaminants, as well as their cytotoxicity and genotoxicity [107,108].
Some of the metals of great environmental concern include chromium (Cr), cadmium (Cd), lead (Pb), arsenic (As), and aluminum (Al). Cr—in particular, its hexavalent form (VI) [107]—can reduce the germination rate [109], impair root development, reduce plant biomass/growth and yield [109,110,111], damage membranes [110], impair photosynthesis [111,112,113], and induce chlorosis and necrosis [108,114], oxidative stress [110,113,115], and ultrastructural changes [116], as well as DNA damage [107,117]. Cd can also inhibit photosynthesis [118,119], induce reactive oxygen species (ROS) overproduction and oxidative stress [120], and induce genotoxicity and cytotoxicity [121]. Pb is described as being able to impair germination and plant growth [122], even under low concentrations [123], decrease the net photosynthetic rate and effective PSII photosynthetic efficiency [124], impair the Calvin cycle [125], and induce DNA damage [126,127,128] and antioxidant response due to redox homeostasis loss [128,129]. Al, which represents 7% of the soil matter of the Earth’s crust, can severely impair crop development and yield in acidic environments [130,131], can change root ultrastructure and development [132,133,134,135], induce nutrient imbalances by limiting the availability of minerals such as Mg, Ca, and K [136,137,138], negatively affect photochemical and non-photochemical phases of photosynthesis [139,140,141], and can increase ROS production [142,143,144,145]. Arsenic (As) is a persisting metalloid in the environment and promotes ROS production and oxidative damage [146], including cell membrane and DNA damages, and alters photosynthesis and nutrient supply [147]. Likewise, copper (Cu), despite not being a xenobiotic due to its role as a micronutrient, may impair plant growth and photosynthetic processes [148], alter root ultrastructure [149], and induce oxidative stress [150].
Both metallic and metal oxide nanomaterials, including SiO2, TiO2, Fe2O3, Fe2O4, ZnO, Mn3O4, and CeO2, have been described to mitigate, at some level, the toxicity of metals in plants. Nevertheless, the benefits of these materials are frequently linked with low doses, whereas higher concentrations are more prone to induce toxicity [100,151], highlighting the need to optimize the dose when it is intended to use this kind of materials in agriculture.
3.2.1. Ameliorative Effects of nTiO2 in Plants Exposed to Environmental Contaminants
nTiO2 has been described, during the last half decade, to be able to ameliorate the toxic effects of several environmental contaminants, including Cd, Cu, Pb, Al, Sb, As, 2,4-dichlorophenoxyacetic acid, and tetracycline, although most works focus on Cd toxicity (Table 5 and Table 6). In the majority of works, it is not stated whether the crystalline phase of nTiO2 is considered, making it impossible to establish a relationship between the crystalline phase and the effects reported.
Table 5.
Ameliorative effects of nTiO2 application against the phytotoxicity of Cadmium (Cd).
| Salt; Concentration | nTiO2 Crystalline Phase; Concentrations Used; Primary (PS) or Hydrodynamic Size (HS) | Plant Species | Application Method | Ameliorative Effects | Ref. |
|---|---|---|---|---|---|
| CdCl2; 50 mg kg−1 |
Not stated; 40, 80, 160 mg L−1; PS < 100 nm | Coriandrum sativum L. | Seed priming (24 h) | Decreased Cd uptake and improved germination rate, plant growth and biomass; increased pigment contents; improved gas exchange parameters; increased CAT, SOD and APX activity; increased proline level; decreased MDA content and electrolyte leakage; improved seed yield | [164] |
| Cd(NO3)2; 13.95 mg kg−1 |
Not stated; 100–1000 mg k−1; PS 15–40 nm | Trifolium repens | In soil (80 d) | Increased plant length and biomass | [153] |
| CdCl2; 10 mg kg−1 |
Not stated; 100, 200 mg L−1; PS 100 nm | Vigna unguiculata | Foliar spray in 21 days-old plants | Increased chlorophyll b; decreased in Cd uptake and translocation; MDA decrease; stimulated the antioxidant enzyme activity; increased Zn, Mn and Co in seeds | [162] |
| 1.03, 2.46, 5.06 mg kg−1 | Not stated; 50, 100, 500 mg kg−1; PS 20–40 nm | Oryza sativa L. | In soil (30, 60, 90 d) | Increased the plant height in tillering and booting growth stages; decreased MDA content and the activity of antioxidant enzymes, mostly when plants were treated with the higher doses of TiO2 | [152] |
| 50 µM | Anatase; 100; 250 mg L−1; PS 6.5 nm; HS 310–421 nm in foliar spay; HS 700–1880 nm in hydroponics | Zea mays L. | Foliar spray in 19 days-old plants (for 14 days evenly) and hydroponic system | Foliar spray: increased the membrane integrity (250 mg L−1); decreased Cd content in roots (100 mg L−1) and shoots (both); downregulated amino acid metabolic pathways Hydroponics: increased membrane integrity (250 mg L−1); decreased Cd content in roots (250 mg L−1); upregulated carbohydrates metabolic pathways |
[157] |
| 8.5 mg L−1 | Sodium dodecyl benzene sulfonate-coated and uncoated nTiO2; 100, 200, 500, 1000 mg L−1; HS 260–350 nm | Triticum aestivum L. | Seedlings In petri dishes with moistened filter paper (5 days) | The highest doses increased root length | [165] |
| CdCl2; 10, 20 mg L−1 |
Not stated; 10, 100, 1000 mg L−1; PS 18–166 nm | Oryza sativa L. | Hydroponic system (10 days) | Stimulated plant growth; decreased Cd uptake; stimulated the net photosynthetic rate and chlorophyll content; decreased the MDA and modulated the antioxidant response | [158] |
| CdCl2; 100 mg kg−1 |
Not stated; 100, 200, 300 mg kg−1; PS < 100 nm | Glycine max L. | In soil (30–60 days after sowing) | Decreased proline content; increased protein content; increased chlorophyll b content | [154] |
| Contaminated soil; 7.86 mg kg−1 |
5, 10, 20, 30 mg L−1; PS 20–30 nm; | Oryza sativa L. | Foliar spray at 26, 33 and 40 d after sowing | Stimulated plant growth; promoted gas exchange; increased chlorophyll contents; decreased MDA, electrolyte and H2O2 contents in both roots and leaves; stimulated antioxidant enzyme activities; decreased Cd accumulation and translocation | [163] |
Table 6.
Ameliorative effects of nTiO2 application against the phytotoxicity of several environmental contaminants.
| Contaminant | Salt; Concentration | nTiO2 Crystalline Phase; Concentrations Used; Primary (PS) or Hydrodynamic Size (HS) | Plant Species | Application Method | Ameliorative Effects | Ref. |
|---|---|---|---|---|---|---|
| Cu | CuSO4·5H2O; 1, 2 mg L−1 |
Anatase; 10 mg L−1; HS 374 nm (1 h in suspension); HS 1064 nm (48 h in suspension) | Glycine max L. | Hydroponic system (6 days) | Decreased the translocation factor of Cu | [159] |
| Pb | Pb(NO3); 10 mg kg−1 |
P25; 5 mg kg−1; HS ~130 nm | Lactuca sativa L. | In soil (12 days) | Decreased the relative membrane permeability; increased pigment contents; promoted gas exchange, including the net photosynthetic rate | [155] |
| Al | AlCl3·6H2O; 50 mg kg−1 |
P25; 5 mg kg−1; HS ~130 nm | Lactuca sativa L. | In soil (12 days) | Decreased the relative membrane permeability; promoted gas exchange; increased the effective efficiency of photosystem II | [155] |
| Sb | K2H2Sb2O7⋅4H2O; not stated |
Not stated; 100–250 mg kg−1; PS 15–40 nm | Sorghum bicolor | In soil (80 days) | Increased the germination rate | [156] |
| As | Sodium arsenate; 10 µmol L−1 |
Not stated; Chemical NPs: 2500 mg L−1; HS 64.3 nm. Green NPs: 1000 mg L−1; HS 53.2 nm | Vigna radiata L. | Seeds treated prior germination and during the germination period | Increased biomass and seedling length; decreased H2O2 and MDA contents; increased the protein content, and gene expression of SOD and CAT | [166] |
| 2,4-Dichloro phenoxyacetic acid | 1000 µM | Not stated; PS < 100 nm; HS 260 nm | Azolla pinnata R.Br | Pre-treatment with TiO2 (3 days) followed by exposure to 2,4-D in a hydroponic system | Modulated K, N, P accumulation; increased biomass; increased the activity of the enzyme invertase; promoted the nitrogen metabolism | [167] |
| Tetracycline | 1, 5, 10 mg L−1 | Rutile; 40, 100, 200 mg L−1; PS 5–15 nm | Arabidopsis thaliana L. | Hydroponic system (12 days) | Increased fresh biomass (40 mg L−1); altered the activity of antioxidant enzymes; changed the expression of genes encoding GST, MDHAR, GR, SiR, APR, APT | [161] |
| 5–20 mg L−1 | Anatase; 500, 1000, 2000 mg L−1; PS 10–25 nm | Oryza sativa L. | Hydroponic system (10 d) | Increased shoot and root biomass; decreased tetracycline content in shoots and roots; modulated nutrient accumulation; decreased antioxidant enzymes activity; showed antagonist effect with tetracycline | [160] |
Concerning the nTiO2 application method, two main routes are used: (1) via roots in a solid matrix, by mixing nTiO2 powder [152] with the soil/substrate or by spiking with nTiO2 suspensions [153,154,155,156], or in hydroponic systems using NP suspensions [157,158,159,160,161]; (2) via leaves by foliar spray with nTiO2 suspensions [157,162,163]. Besides, seed priming was analyzed by Sardar et al. [164], whereas Dai et al. [165] applied nTiO2 during the seedling stage in petri-dishes with moistened paper, and Katiyar et al. [166] treated the seeds and seedlings with nTiO2 suspensions, both simultaneously with the contaminant.
Besides the application methods, the NP concentrations and size used, as well as the treatment period, are also very diverse, with concentrations ranging from 50 to 500 mg kg−1 in soil experiments, 10 to 2000 mg L−1 in hydroponic systems, and 10 to 200 mg L−1 in foliar application, whereas the treatment period may range from 24 h to 90 days (Table 5 and Table 6). Nevertheless, despite these differences, it is evident that nTiO2 application may alleviate toxic symptoms induced by several contaminants.
Under Cd, soil amendment with nTiO2 improved several physiological attributes in white clover [153], rice [152], and soybean [154]. In white clover, nTiO2 (500 mg kg−1) stimulated plant growth with the increase of plant length and biomass [153]. Similarly, nTiO2 (500 mg kg−1) application in rice improved plant growth and also decreased MDA content simultaneously with the decrease of CAT, SOD, and POD activity [152]. In soybean, nTiO2 (100–300 mg kg−1) increased the protein and chlorophyll b contents and decreased the proline content [154]. Furthermore, in rice, but using a hydroponic system, nTiO2 treatments (10–1000 mg L−1) showed some potential to stimulate plant growth, reducing the MDA content, modulating the antioxidant response, altering the levels of some phytohormones (indole-3-acetic acid, isopentenyl adenosine, methyl jasmonate, and zeatin riboside), and stimulating photosynthesis by increasing the net photosynthetic rate and the chlorophyll content [158]. In addition, and in contrast to what was described by Zhang et al. [152], in this case, the Cd uptake decreased. Root application of nTiO2 using a hydroponic system in maize also decreased Cd content in roots (250 mg L−1), likewise increasing the membrane integrity and upregulating the carbohydrate metabolic pathways [157].
The foliar spray with nTiO2 also shows potential in mitigating Cd phytotoxicity. In cowpea [162] and rice [157,163], leaf application of nTiO2 decreased Cd uptake and increased membrane integrity. In both species, the increment of pigment levels (Chl b in cowpea and Chl a, b and carotenoids in maize) was also reported, as well as the stimulation of the antioxidant response by increasing the activity of antioxidant enzymes [162,163]. Besides the pigment levels, nTiO2 also stimulated the photosynthesis in maize by improving the gas exchange parameters, including the net photosynthetic rate, transpiration rate, and stomatal conductance, which may have contributed to the observed stimulation of plant growth [163]. Interestingly, the mitigating effects observed by Rizwan et al. [163] were induced despite using 10 times lower concentrations of nTiO2 than Ogunkunle et al. [162] and Lian et al. [157].
At the seedling stage, sodium dodecyl benzene sulfonate-coated and uncoated nTiO2 (1000 mg L−1) increased the root length of wheat exposed to 8.5 mg L−1 of Cd [165]. Finally, the priming of coriander seeds [164] with nTiO2 (40, 80 and 160 mg L−1) also showed promising results and a potential strategy to mitigate Cd phytotoxicity (50 mg kg−1) once: it enhanced the germination rate, plant growth, and biomass; increased the pigment levels (80 mg L−1); stimulated the non-photochemical phase of photosynthesis by increasing the intercellular CO2 content, stomatal conductance, transpiration rate, and net photosynthetic rate; stimulated an antioxidant response by increasing the activity of the enzymes CAT, SOD and APX, and the proline content; improved membrane integrity; improved the seed yield; and decreased Cd uptake.
Under Cu and using a hydroponic system, nTiO2 (10 mg L−1) decreased the translocation factor of Cu in soybean [159]. In lettuce grown in soil, Mariz-Ponte et al. [155] reported ameliorative effects induced by nTiO2 (5 mg kg−1) amendment in the presence of Pb or Al. In both cases, nTiO2 decreased the relative membrane permeability and promoted the intercellular CO2 content, stomatal conductance, transpiration rate, and net photosynthetic rate. Furthermore, under Pb, nTiO2 also increased the pigment content (chlorophyll a, b and carotenoids), whereas in the presence of Al, it enhanced the effective efficiency of photosystem II [155]. nTiO2 (2500 mg L−1) also showed positive effects to mitigate As effects in mung bean by upregulating the expression of antioxidant enzymes (SOD and CAT), which may have conferred greater protection against oxidative damages, as supported by the decrease of MDA content and ROS levels (H2O2 and O2−•) [166].
Besides metal contaminants, nTiO2 was able to mitigate some effects of 2,4-dichlorophenoxyacetic acid (2,4-D), a systemic herbicidal, and tetracycline, an antibiotic widely used in agriculture and livestock industries. In the case of 2,4-D, nTiO2 increased the biomass, promoted the accumulation of N and K, despite reducing P content, upregulated the activity of soluble invertase to values closer to the control (despite decreasing the amount of cell wall bounded invertase), and promoted the nitrogen metabolism by increasing nitrate reductase and glutamine 2-oxoglutarate amino transferase activities [167]. Concerning the tetracycline, nTiO2 application alleviated the negative effects on plant/pod biomass in Arabidopsis [161] and rice [160] grown in hydroponics. Furthermore, in both species, nTiO2 modulated the antioxidant response, altering the activity of antioxidant enzymes, and in Arabidopsis, it upregulated the expression of adenyltransferase (APT), adenosine-5′-phosphosulfate reductase (APR), and sulfite reductase (SiR) in the roots. It is worth mentioning that in rice, nTiO2 significantly decreased the levels of tetracycline in shoots and roots and altered the nutrient content, showing a trend to increase P, S, and Zn [160].
Taking into consideration all the works presented in Table 5 and Table 6, it seems that nTiO2 has the capability to decrease metals and non-metals contaminants uptake and translocation to shoots when the NPs are applied as suspensions to the leaves/seeds or are added to a nutritive solution together with the contaminant (hydroponic system). When nTiO2 is applied to the soil, it looks to promote metal uptake, nevertheless without increasing its toxicity. In fact, an overall mitigation of toxic symptoms was observed. In both cases, an antagonistic effect is reported, as was particularly stated by Mariz-Ponte et al. [155] and Ma et al. [160], reducing metal phytotoxicity and enhancing plant performance. The mechanism underlying these results may be related to a reduction in contaminant bioavailability due to their immobilization by nTiO2 [10,156,160].
3.2.2. Ameliorative Effects of nZnO in Plants Exposed to Environmental Contaminants
The studies available using nZnO as a strategy to cope with the adverse effects of environmental contaminants in plants, despite being limited and fractionated, unveil their potential to improve plant physiology under stress conditions. Similar to nTiO2, nZnO was mostly explored to ameliorate Cd toxic effects, despite a couple of studies existing with As, Pb, Cu, and Co (Table 7 and Table 8). Most of the works focus on plant growth, ROS production, oxidative stress, and the antioxidant response, and show the capability of nZnO to modulate those physiological processes under metal stress.
Table 7.
Ameliorative effects of nZnO application against the phytotoxicity of Cadmium (Cd).
| Salt; Concentration |
nZnO Concentration; Primary (PS) or Hydrodynamic Size (HS) | Plant Species | Application Method | Ameliorative Effects | Ref. |
|---|---|---|---|---|---|
| CdCl2; 0.8 mM |
50 mg L−1; not stated | Oryza sativa L. | Foliar spray at 30 to 35 days after sowing | Improved plant length and biomass; Higher chlorophyll index; improved the gas exchange, including the net CO2 assimilation rate; reduced ROS accumulation and MDA content; promoted CAT activity; increased proline levels; promoted essential nutrient uptake; decreased Cd accumulation | [168] |
| Contaminated soil; 7.67 mg kg−1 | 25, 50, 100 mg L−1; PS 20–30 nm | Triticum aestivum L. | Foliar spray at two, three, four and five weeks after sowing | Enhanced plant growth; increased grain dry weight; increased pigment content; decreased MDA, electrolyte leakage and H2O2 content; upregulated SOD and CAT activity; decreased Cd accumulation and transfer to shoots, and Cd bioavailability | [171] |
| 7.86 mg kg−1 | 50, 75, 100 mg L−1; PS 20–30 nm | Zea mays L. | Foliar spray in 32 days-old plants | Increases shoot and root dry weight; enhanced photosynthetic pigment content and gas exchange related parameters; decreased MDA and membrane permeability; promoted CAT, APX and POD activity; decreased Cd accumulation | [170] |
| Contaminated soil; 7.38 mg kg−1 | 25, 50, 75, 100 mg L−1; PS 20–30 nm; | Triticum aestivum L. | Seed priming | Stimulated plant growth; promoted gas exchange; increased pigment contents; decreased electrolyte leakage; increased SOD and G-POX activities; decreased Cd content and bioavailability | [172] |
| Contaminated soil; 7.38 mg kg−1 | 50, 75, 100 mg L−1; PS 20–30 nm; | Orysa sativa L. | Foliar spray after 14, 21, 38 and 35 days after transplantation | Increased shoot length and dry weight, and root dry weight; increased chlorophyll a and b contents; stimulated gas exchange; decreased Cd uptake and translocation | [169] |
| Contaminated soil; 7.38 mg kg−1 | 25, 50, 75, 100 mg L−1; PS 20–30 nm; | Triticum aestivum L. | In soil; Foliar spray after 2, 4, 6 and 8 weeks after sowing | Promoted plant growth; increased grain dry weight; increased chlorophyll a and b, and carotenoid contents; stimulated gas exchange; electrolyte leakage decrease; SOD and G-POX activities increase; decreased Cd uptake and translocation, and Cd accumulation in grains | [73] |
| CdCl2; 50 mg L−1 |
25 mg L−1; PS 2–64 nm | Leucaena leucocephala (Lam.) de Wit | Hydroponic system (15 days) | Promoted plant growth; increased chlorophyll a and b, and carotenoid contents; increased total soluble protein levels; SOD, CAT and G-POX activities increase; decrease of DHA damage | [61] |
Table 8.
Ameliorative effects of nZnO application against the phytotoxicity of environmental contaminants.
| Contaminant | Salt | Salt Concentration | nTiO2 Crystalline Phase; Concentrations Used; Primary (PS) or Hydrodynamic Size (HS) | Plant Species | Application Method | Ameliorative Effects | Ref. |
|---|---|---|---|---|---|---|---|
| Pb | PbNO3 | 100 mg L−1 | 25 mg L−1; PS 2–64 nm | Leucaena leucocephala (Lam.) de Wit | Hydroponic system (15 d) | Promoted plant growth; increased chlorophyll a and b, and carotenoid contents; decreased MDA content; increased total soluble protein levels; SOD, CAT and G-POX activities increase; decrease of DHA damage | [61] |
| Cd + Cr + Pb | Irrigation with contaminated wastewater | 3.1 mg kg−1 + 39.5 mg kg−1 + 14.4 mg kg−1 | 60 mg L−1; not stated | Helianthus annuus L. | Foliar spray: 25 and 45 days after sowing | Improved plant height, leaf area and seed yield; increased proline content; Increased oil yield; decreased Cd, Pb and Cr content in plants; decreased Cr and Pb soil bioavailability | [174] |
| Cd + Pb | CdSO4 | 1 mg L−1 + Pb(NO3)2; 100 mg L−1 | Polyvinylpyrrolidone coated NPs; 100 mg L−1; HS > 600 nm |
Spinaciae oleracea L.; Petroselinum sativum Hoffm.; Coriandrum sativum L. |
Hydroponic system (15 days) | Decreased Cd content in shoots and of Pb in roots of cilantro; decreased Cd and Pb content in parley and spinach roots; increased Fe content in parsley and spinach; increased Zn content in all species | [173] |
| As | NaAsO2 | 2 mg L−1 | 10, 20, 50, 100, 200 mg L−1; PS 20–30 nm | Oryza sativa L. | Germination and seedling growth in petri dishes | The lowest doses enhanced seedling length and the pigment content, and stimulated the antioxidant response by increasing the activity of SOD and CAT; decreased MDA content and As accumulation and translocation | [146] |
| As(V); Na2HAsO4 | 25 µM | 25 µM (2.0345 mg L−1); PS 20 nm | Glycine max L. | Hydroponic system (10 days) | Increased root and shoot dry weight; decreased As content in roots and shoots; decreased ROS (H2O2 and O2−•) and MDA in roots and shoots; enhanced the activity of antioxidant enzymes (SOD, G-POX, CAT, APX, GR); upregulated the expression of defense- and detoxification-encoding genes; increased GSH/GSSH, and AsA, proline and glycine betaine contents | [175] | |
| Co | CoCl2 | 300 µM | 500 mg L−1; PS 20 nm | Zea mays L. | Seed priming | Enhanced shoot/root length and biomass; decreased Co bioaccumulation; increased the chlorophyll contents; increased Fv/Fm and gas exchange related parameters, including net CO2 assimilation rate; decreased MDA; stimulated the activity of antioxidant enzymes; promoted essential nutrient uptake; restored the ultrastructure of cell organelles, cell guards and stomatal aperture | [176] |
| Cu | CuSO4·5H2O | 100 mg kg−1 | 50 mg L−1; not stated | Solanum lycopersicum L. | Foliar spray at 35 days after sowing | Enhanced plant biomass, length, and leaf area; increased chlorophyll index; promoted the gas exchange, including the net CO2 assimilation rate; induced an antioxidant response, by increasing antioxidant enzyme activity, proline content; decreased ROS and Cu accumulation | [177] |
Cd phytotoxicity was alleviated in rice [168,169], maize [170], and wheat [73,171] plants sprayed with nZnO suspensions (25–100 mg L−1). In all these species, plant growth improvements were described, such as plant length and biomass; promotion of gas exchange, including the net CO2 assimilation rate; an increase of pigment contents; and a decrease of Cd uptake and/or translocation. In addition, the stimulation of the antioxidant response with the upregulation of antioxidant enzymes (CAT, SOD, G-POX) [73,168,170,171] and proline [168] was reported, together with the reduction of ROS and MDA content and decrease of electrolyte leakage. Similar responses were obtained in wheat after seed priming with nZnO (25–100 mg L−1) [172] and when nZnO (25 mg L−1) was supplemented in a hydroponics system in Leucaena seedlings [61].
In Leucaena, nZnO induced the enhancement of soluble protein and genomic alterations (presence of new DNA bands and/or absence of normal bands in the RAPD pattern of the exposed plants); nevertheless, in contrast to the previous works, in this case, it augmented the Cd accumulation [61]. Besides Cd, Venkatachalam et al. [61] also conducted the same experiment under Pb stress, with similar ameliorative results as those obtained for Cd. Under combined exposure of low Cd concentration (1 mg L−1) and high Pb (100 mg L−1), the amendment with polyvinylpyrrolidone-coated nZnO (100 mg L−1) decreased Cd content in shoots of cilantro, parsley, and spinach, whereas it increased the Pb levels in cilantro and did not affect the Pb content in parsley and spinach [173]. In roots, Sharifan et al. [173] described that Cd content decreased in parsley and spinach and that Pb was significantly reduced in all three species. The authors attributed the Cd and Pb mitigation to the adsorption of metals onto the nZnO surface, despite its overall significance possibly being affected by the nZnO surface charge plus the presence of roots exudates [173]. Additionally, nZnO altered the dynamic translocation and uptake of essential minerals such as Cu, Fe, and Zn: Fe increased in shoots of parsley and spinach; Zn increased in all species and both organs; and Cu decreased in cilantro shoots [173]. Furthermore, under combined stress, sunflower plants irrigated with heavy-metal-contaminated wastewater (mainly Cr, Cd, and Pb) showed improved performance when foliar sprayed with nZnO (60 mg L−1), as well as a decrease in metal content [174].
Under As, nZnO amendment (10–200 mg L−1, with higher effects when were used 10–50 mg L−1 nZnO) promoted the rice seedlings’ growth, increasing root and shoot biomass, enhanced the chlorophyll levels, and upregulated the activity of CAT and SOD [146]. Furthermore, nZnO reduced the MDA content, as well the levels of As in both roots and shoots, whereas it increased the Zn concentration. In soybean plants, nZnO (2 mg L−1) amendment of the nutritive solution containing As (V) reduced several cellular toxicants such as ROS (H2O2 and O2−•), MDA, and oxidized glutathione [175]. On the other hand, in both roots and shoots, several antioxidant pathways were activated, which included the upregulation of the expression of detoxification-encoding genes (GmSOD, GmG-POX, GmAPX, GmCAT, GmGR, GmGST) and the activity of antioxidant enzymes (SOD, G-POX, CAT, APX, GR); the increase of compatible organic solutes (proline and glycine betaine) levels and GmP5CS expression; and the stimulation of the ascorbate-glutathione cycle [175].
The single work under cobalt (Co) stress revealed several beneficial effects in maize induced by seed priming with nZnO (500 mg L−1) [176]. The pre-treatment with nZnO enhanced maize growth (length and biomass), promoted Zn uptake while reducing Co levels in shoots and roots, and increased chlorophyll contents, which may have contributed to the detected improvement of Fv/Fm. Besides, seed priming reduced the damage induced by Co in guard cells and restored, at some level, the stomatal aperture, as well the chloroplast and thylakoid ultrastructure. These changes may be the reflection of oxidative stress mitigation, as proven by MDA reduction and the superior activity of antioxidant enzymes, and together be responsible for the restoration of gas exchange, including the net CO2 assimilation rate [176].
Finally, Cu phytotoxicity was reduced in tomato plants when foliar sprayed with nZnO (50 mg L−1) [177]. Treated plants showed lower Cu content, superior length and biomass, higher chlorophyll index, and superior fluorescence of chlorophyll a, with the increase of Fv/Fm. However, concerning photosynthesis, nZnO improved the net photosynthetic rate, internal CO2 content, stomatal conductance, and transpiration rate, and promoted carbonic anhydrase activity. Seed priming also promoted an antioxidant response with the upregulation of the activities of several enzymes (CAT, APX, and SOD) and the increase of proline, which may have contributed to control ROS production/scavenge (reflect of O2−• and H2O2 reduction) and reduce the oxidative stress (MDA decrease) [177].
4. Protective Effects of ZnO and TiO2 against Biotic Stress
Plants are affected by numerous pathogens that are able to induce diseases and diminish plant performance and yield. Crop production is globally affected by pests and phytopathogens such as viruses, bacteria, and fungi, with losses reaching up to 40% of crop local or global production [178,179,180], and thus affecting global food security. NM have been explored as a sustainable alternative to the conventional synthetic agrochemicals, which lack selectivity and sensitivity and are a threat to the environment and human health. This nano-based approach shows desirable properties for agro-application, such as slow and controlled release of active compounds, low cost, efficient drug delivery, multi-site mode of action, ameliorative effects, antimicrobial and/or fungicidal activity, among others [32]. Hence, NM are promising strategies for both plant health monitoring and disease management in smart agriculture. When NM became to be explored for agricultural proposes, these materials were mostly synthesized by conventional methods. Nevertheless, as their potential was revealed, emerged bio-based synthetic methods, where NM were prepared from plants and microbes, as an environmentally-friendly alternative to chemical synthesis with promising results in agricultural fields, such as in crop diseases management [181].
NP induce the generation of ROS, such as hydroxyl, hydroperoxyl, peroxyl, alkoxyl and carbon dioxide radicals, superoxide anions, hydrogen peroxide, and carbonate, and nonradicals, such as ozone, nitric oxide, peroxy nitrite, hypobromous acid, hypochlorite, and organic peroxides [25,182], increasing the level of oxidative stress. Moreover, oxidative stress induces single and double-strand breaks and lesions on nitrogen base and pentose sugar [182], cell damage, injury of cell membrane with leakage of cytoplasmic material, proteins and nuclei acids [183,184]. The accumulation of NP in the membrane of bacteria or fungi induce alterations in cell membrane permeability, leading to disturbances in the proton motive force [182]. Several metal and oxide-NM show direct action against bacteria, fungi, and viruses and even nematodes. Among them are silver (nAg), gold (nAu), cupper (nCu), and nickel (nNi) NP, as well as nZnO (Table 9), nTiO2 (Table 10), copper oxide (nCuO), aluminum oxide (Al2O3), iron oxide (nFe2O3), and magnesium oxide (nMgO) NP (for review see [32]).
Table 9.
Beneficial application of nZnO on plant diseases management.
| NM | Size; Concentrations |
Disease Management | Causal Organism | Targeted Plant | Application Method | Outcome | Ref. |
|---|---|---|---|---|---|---|---|
| nZnO doped with Fe, Mn, Cu or Ni | Not stated; 5, 10 mg L−1 | White spot | Pantoea ananatis | Zea mays L. | Leaf spray | Antibacterial activity (nZnO doped with Fe or Mn); reduced disease progression (all). | [189] |
| nZnO | ≤40 nm; 0.01% | Bacterial blight diseases complex | Meloidogyne incógnita + Pseudomonas syringae pv. pisi | Pisum sativum L. | Seed priming; Foliar spray | Antibacterial activity; nematocidal activity Increased plant growth and pigment content; reduced the disease index; reduced galling and nematode populations. |
[188] |
| nZnO | 16–31 nm; 500 mg kg−1 | Bacterial wilt | Ralstonia solanacearum | Solanum lycopersicum L. | Soil amendment | Enhanced plant growth; decreased MDA content; increased plenylalanine ammonia lyase and POD activity; Increased the richness and diversity of soil microbial communities | [185] |
| CuZn@DEG and ZnO@PEG | 35 nm and 18 nm; 50–1400 µg mL−1 |
Lettuce drop | Botrytis cinerea; Sclerotinia sclerotiorum | Lactuca sativa L. | Foliar spray | Antifungal activity; reduced the disease index; improved photosynthesis | [193] |
| nZnO | 9–32 nm; 18 µg mL−1 |
Bacterial wilt | Ralstonia solanacearum | Solanum lycopersicum L. | Soil amendment | Antibacterial activity; stimulated plant growth; reduced bacterial soil population; decreased disease severity | [190] |
| nZnO | 23.44 nm; 100, 1500, 3000 mg L−1 | Fungal wilt | Fusarium oxysporum | Solanum lycopersicum | Foliar spray | Antifungal activity; impaired disease development; promoted plant growth | [194] |
| nZnO | 56.1–110.0 nm; 16.0 μg mL−1 |
Bacterial leaf blight | Xanthomonas oryzae pv. oryzae | Oryza sativa L. | Foliar spray | Antibacterial activity; decreased the percentage disease leaf area; improved plant growth | [183] |
| nZnO | 25–450 nm; 7.5 × 10−3 M |
Gray mold | Botrytis cinerea | Fragaria × ananassa | Foliar and fruit spay | Antifungal activity; reduced disease incidence; improved crop production; increased fruit shelf-life | [198] |
| nZnO | 74.68 nm; 100 μg mL−1 |
Mosaic disease | Tobacco mosaic virus | Solanum lycopersicum L. | Foliar spray | Induced systemic acquired resistance (SAR) and reduction of viral accumulation levels and of disease severity; increased plant growth; up-regulated the transcriptional levels of PAL, PR-1, CHS, and POD genes. | [199] |
Table 10.
Beneficial application of nTiO2 on plant disease management.
| Size; Concentration |
Disease Management | Causal Organism | Targeted Plant | Application Method | Outcome | Ref. |
|---|---|---|---|---|---|---|
| Not stated; 0.5 g L−1 |
Cercospora leaf spot | Cercospora beticola | Beta vulgaris L. | Foliar spray | Antifungal agent; reduced leaf spots; increased growth and yield |
[202] |
| 10–50 nm; 1.6% |
Angular leaf spot of cucumber and downy mildew disease | P. syringae pv. lachrymans and Pseudomonas cubensis | Cucumis sativus L. | Foliar spray | Antibacterial activity; decreased leaf lesions; improved photosynthesis and chlorophyll levels |
[205] |
| 20 nm; 0.02–0.0007% |
Root-knot | Meloidogyne incognita | Solanum lycopersicum L. | Soil | Nematocidal activity Reduced plant weight, and root and stem length |
[207] |
| 700 × 900 nm; 150 μM |
Broad bean stain disease | Broad bean stain virus (BBSV) | Vicia faba L. | Foliar spray | Antiviral activity; reduced stain virus accumulation in leaves; growth improvement |
[208] |
| Not stated; 1000, 100, and 250 ppm |
Tomato psyllid | Bactericera cockerelli | Solanum lycopersicum L. | Foliar spray | Insecticidal effect; increased insect mortality. |
[209] |
4.1. nZnO Potential for Crop Disease Control
nZnO shows antimicrobial activity to plant pathogens, including bacteria and fungi, as well as the nematode Meloidogyne incognita [1,185,186,187,188] (Table 9).
In particular, nZnO doped with Fe and Mn showed antibacterial activity against Pantoea ananatis, and in the pathosystem P. anantis—corn, nZnO doped with Fe, Mn, Cu, or Ni reduced the diseases progression when the NM were foliar sprayed to plants before and after plant inoculation with the bacteria [189]. In the bacterial blight diseases complex of pea, caused by M. incognita and Pseudomonas syringae pv. pisi, nZnO was able to reduce the index diseases and galling population and improve plant growth and pigment content [188]. In tomato, soil amendment with nZnO reduced the diseases incidence induced by Ralstonia solanacearum and stimulated plant growth and the antioxidant response (with the decrease of MDA content) and improved soil microbial community [185] (Table 9).
nZnO synthesized from a flower extract presented antibacterial activity against R. solanacearum and decreased the bacterial wilt disease in tomato [190], whereas nZnO synthesized from Citrus medica peel extracts showed antimicrobial activity against Streptomyces sannanesis, Bacillus subtilis, Pseudomonas aeruginosa, Salmonella enterica, Candida albicans, and Aspergillus niger [191] (Table 9). Biogenic nZnO NPs synthesized from Trichoderma harzianum, Trichoderma reesei, and co-culture [192], or from Paenibacillus polymyxa strain Sx3 [183], showed antibacterial activity against Xanthomonas oryzae pv. oryzae, responsible for the bacterial leaf blight diseases in rice. Besides, Ogunyemi et al. [183] reported a decrease of bacterial leaf blight diseases in plants foliar sprayed with biogenic nZnO, together with the improvement of plant growth.
CuZn@DEG and ZnO@PEG nanoflowers showed antifungal activity against Botrytis cinerea and Sclerotinia sclerotiorum, and in lettuce plants inoculated with S. sclerotiorum, both NM reduced the disease index and improved the net photosynthesis, photosynthetic quantum yield, and photosynthetic efficiency [193]. The antifungal activity of nZnO was also evaluated against Fusarium oxysporum in tomato plants, decreasing the diseases incidence and severity and improving plant growth [194].
Biogenically synthesized nZnO (Table 9) using lemon peel extract showed antifungal activity against Alternaria citri, responsible for citrus black rot disease [195], whereas using leaf extract of Cinnamomum camphora (L.) Presl, nZnO presented antifungal activity against Alternaria alternate, responsible for early blight disease in Solanum lycopersicum [184]. In A. alternate, nZnO induced an excessive accumulation of MDA and caused the damage of the cell membrane, leading to the leakage of protein and nucleic acid [184]. In addition, ZnO bio-synthesized using Penicillium chrysogenum showed antifungal activity against Fusarium solani, Fusarium oxysporum, Sclerotium sclerotia, and Aspergillus terreus [196]. An innovative approach in plant defense is the photoactivation of nZnO, and using this approach, it was possible to inactivate Escherichia coli B. and F. oxysporum in contaminated seeds [197], whereas in strawberry, photoactivated nZnO reduced B. cinerea incidences, promoted crop production, and increased fruit shelf-life [198].
Concerning plant diseases induced by viruses, Abdelkhalek et al. [199] reported the decrease of Tobacco mosaic virus diseases incidence in tomato plants after being sprayed with green-synthesized nZnO. These particles improved plant growth and upregulated tomato-innate defense genes (PAL, PR-1, CHS, and POD) [199].
4.2. nTiO2 Potential for Crop Diseases Control
The photocatalytic activity of nTiO2 contributes to its antifungal and antibacterial activity [1]. For instance, Sar et al. [200] highlighted the antifungal activity of nTiO2 (anatase; 3–12 nm; 50, 100, 150, and 200 ppm) against F. oxysporum f. sp. radices lycopersici and F. oxysporum f. sp. lycopersici. Application of nTiO2 (10–100 nm; 20, 40, 60 and 80 mg L−1) in wheat plants reduced the severity of the diseases caused by the fungus Bipolaris sorokiniana [201]. Similarly, Hamza et al. [202] demonstrated that these NPs can control Cercospora beticola infection in sugar beet (Table 10). Boxi et al. [203] demonstrated that nTiO2 at 0.75 and 0.43 mg/plate induces a growth inhibitory effect in two potent phytopathogens: F. solani, which causes Fusarium wilt diseases in potato and tomato plants, and Venturia inaequalis, which is responsible for apple scab disease. nTiO2 foliar application in cucumber (1.6%) and poinsettia and geranium (25 and 75 mM) showed antibacterial action against the pathogenic P. syringae pv. lachrymans and Pseudomonas cubensis and Xanthomonas hortorum pv. pelargonii, Xanthomonas axonopodis pv. poinsettiicola [204,205] (Table 10). The antibacterial activity of TiO2 (0.5 mol L−1) against the bacteria Dickeya dadantii, which causes the stem and root rot diseases in sweet potato, was reported by Hossain et al. [206]. Similarly, nTiO2 has a strong antimicrobial activity against nematodes and viruses [32]. Ardakani [207] found nematocidal activity of nTiO2 against the root-knot nematode M. incognita in tomato plants. nTiO2 can also control the pathogenic activities of the virus Turnip mosaic in tobacco plants by limiting the replication of DNA. In faba bean plants, the foliar treatment with nTiO2 helped to control the spread of the broad bean stain virus [208]. An insecticide effect of nTiO2 was also observed in tomato plants infected with Bactericera cockerelli Sulc [209]. The nTiO2 treatment induced a high insecticidal effect after 24 h, with a mortality around 93% for the concentrations above 100 ppm.
5. Conclusions
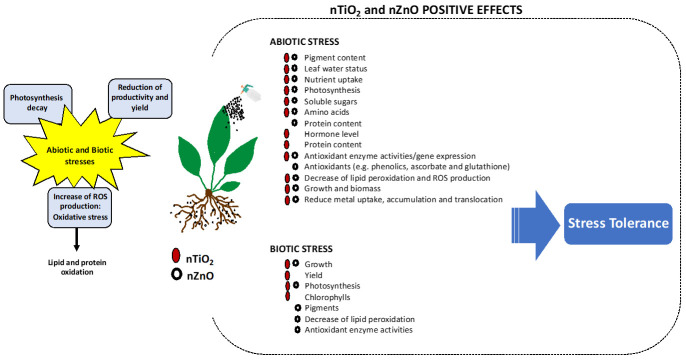
In the last years, nanotechnology has gained much attention in the agro-food system, mostly due to the potential to increase plant performance, enhancing tolerance to biotic and abiotic stresses. In this review, we highlighted the most recent studies on the application of NPs, particularly nZnO and nTiO2, in several species exposed to the most common climatic stresses, such as drought and salinity, as well as environmental contaminants, such as heavy metals, and phytopathogens and pests (Figure 1). The beneficial effects of nZnO and nTiO2 on plants exposed to these stressors at the molecular, metabolic, and physiological levels are well demonstrated in several works performed under controlled and field conditions. These effects can already depend on several factors such as the type of NP used, method of application, concentration, and the type and extent of stress exposure. In general, these NPs show the potential to improve plant performance and may represent a sustainable strategy to alleviate the negative impacts of (a)biotic stresses in agricultural species (Figure 1).
Figure 1.
Representative scheme illustrating the positive effects of nZnO and nTiO2 application on plant physiology reported for plants grown under abiotic and biotic conditions.
Author Contributions
Conceptualization, S.S.; Formal analysis, S.S. and M.C.D.; Funding acquisition, A.M.S.S.; Investigation, S.S. and M.C.D.; Writing—original draft, S.S. and M.C.D.; Writing—review and editing, S.S., M.C.D. and A.M.S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financed by Fundação para a Ciência a Tecnologia (FCT) and Ministério da Educação e Ciência through national funds and co-funding by the FEDER, within the PT2020 Partnership Agreement, and COMPETE 2010, within the projects of the CEF UI0183—UID/BIA/04004/2020, and LAQV-REQUIMTE UIDB/50006/2020. The FCT supported the research contracts of M.C. Dias (SFRH/BPD/100865/2014) and S. Silva (SFRH/BPD/74299/2010) in the scope of the framework contract foreseen in the numbers 4, 5, and 6 of article 23, of the Decree-Law 57/2016, of 29 August, changed by Law 57/2017, of 19 July.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
This is a review article; however, data used and/or analyzed during the review are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Elmer W., White J.C. The future of nanotechnology in plant pathology. Annu. Rev. Phytopathol. 2018;56:111–133. doi: 10.1146/annurev-phyto-080417-050108. [DOI] [PubMed] [Google Scholar]
- 2.El Gamal A.Y., Tohamy M.R., Abou-Zaid M.I., Atia M.M., El Sayed T., Farroh K.Y. Silver nanoparticles as a viricidal agent to inhibit plant-infecting viruses and disrupt their acquisition and transmission by their aphid vector. Arch. Virol. 2022;167:85–97. doi: 10.1007/s00705-021-05280-y. [DOI] [PubMed] [Google Scholar]
- 3.Landa P. Positive effects of metallic nanoparticles on plants: Overview of involved mechanisms. Plant Physiol. Biochem. 2021;161:12–24. doi: 10.1016/j.plaphy.2021.01.039. [DOI] [PubMed] [Google Scholar]
- 4.Sundaria M.N., Singh P.M., Upreti R.P., Chauhan J.R.P., Jaiswal A.J.P., Kumar A. Seed priming with iron oxide nanoparticles triggers iron acquisition and biofortification in wheat (Triticum aestivum L.) grains. J. Plant Growth Regul. 2019;38:122–131. doi: 10.1007/s00344-018-9818-7. [DOI] [Google Scholar]
- 5.An J., Hu P., Li F., Wu H., Shen Y., White J.C., Tian X., Li Z., Giraldo J.P. Emerging investigator series: Molecular mechanisms of plant salinity stress tolerance improvement by seed priming with cerium oxide nanoparticles. Environ. Sci. Nano. 2020;7:2214–2228. doi: 10.1039/D0EN00387E. [DOI] [Google Scholar]
- 6.Khan I., Raza M.A., Awan S.A., Shah G.A., Rizwan M., Ali B., Tariq R., Hassan M.J., Alyemeni M.N., Brestic M., et al. Amelioration of salt induced toxicity in pearl millet by seed priming with silver nanoparticles (AgNPs): The oxidative damage, antioxidant enzymes and ions uptake are major determinants of salt tolerant capacity. Plant Physiol. Biochem. 2020;156:221–232. doi: 10.1016/j.plaphy.2020.09.018. [DOI] [PubMed] [Google Scholar]
- 7.Kalwani M., Chakdar H., Srivastava A., Pabbi S., Shukla P. Effects of nanofertilizers on soil and plant-associated microbial communities: Emerging trends and perspectives. Chemosphere. 2021;287:132107. doi: 10.1016/j.chemosphere.2021.132107. [DOI] [PubMed] [Google Scholar]
- 8.Kumari B., Singh D. A review on multifaceted application of nanoparticles in the field of bioremediation of petroleum hydrocarbons. Ecol. Eng. 2016;97:98–105. doi: 10.1016/j.ecoleng.2016.08.006. [DOI] [Google Scholar]
- 9.Fajardo C., Costa G., Nande M., Martín C., Martín M., Sánchez-Fortún S. Heavy metals immobilization capability of two iron-based nanoparticles (nZVI and Fe3O4): Soil and freshwater bioassays to assess ecotoxicological impact. Sci. Total Environ. 2019;656:421–432. doi: 10.1016/j.scitotenv.2018.11.323. [DOI] [PubMed] [Google Scholar]
- 10.Peikam E.N., Jalali M. Application of three nanoparticles (Al2O3, SiO2 and TiO2) for metal-contaminated soil remediation (measuring and modeling) Int. J. Environ. Sci. Technol. 2019;16:7207–7220. doi: 10.1007/s13762-018-2134-8. [DOI] [Google Scholar]
- 11.Khan M.N., Mobin M., Abbas Z.K., Al-Mutairi K., Siddiqui Z. Role of nanomaterials in plants under challenging environments. Plant Physiol. Biochem. 2017;110:194–209. doi: 10.1016/j.plaphy.2016.05.038. [DOI] [PubMed] [Google Scholar]
- 12.Etesami H., Fatemi H., Rizwan M. Interactions of nanoparticles and salinity stress at physiological, biochemical and molecular levels in plants: A review. Ecotoxicol. Environ. Saf. 2021;225:112769. doi: 10.1016/j.ecoenv.2021.112769. [DOI] [PubMed] [Google Scholar]
- 13.Singh H., Sharma A., Bhardwaj S.K., Arya S.K., Bhardwaj N., Khatri M. Recent advances in the applications of nano-agrochemicals for sustainable agricultural development. Environ. Sci. Process. Impacts. 2020;23:213–239. doi: 10.1039/D0EM00404A. [DOI] [PubMed] [Google Scholar]
- 14.Ahmed B., Rizvi A., Syed A., Jailani A., Elgorban A.M., Khan M.S., Al-Shwaiman H.A., Lee J. Differential bioaccumulations and ecotoxicological impacts of metal-oxide nanoparticles, bulk materials, and metal-ions in cucumbers grown in sandy clay loam soil. Environ. Pollut. 2021;289:117854. doi: 10.1016/j.envpol.2021.117854. [DOI] [PubMed] [Google Scholar]
- 15.Acharya A., Pal P.K. Agriculture nanotechnology: Translating research outcome to field applications by influencing environmental sustainability. NanoImpact. 2020;19:100232. doi: 10.1016/j.impact.2020.100232. [DOI] [Google Scholar]
- 16.García-Sánchez S., Gala M., Žoldák G. Nanoimpact in plants: Lessons from the transcriptome. Plants. 2021;10:751. doi: 10.3390/plants10040751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rizwan M., Ali S., Qayyum M.F., Ok Y.S., Adrees M., Ibrahim M., Rehman M.Z.U., Farid M., Abbas F. Effect of metal and metal oxide nanoparticles on growth and physiology of globally important food crops: A critical review. J. Hazard. Mater. 2017;322:2–16. doi: 10.1016/j.jhazmat.2016.05.061. [DOI] [PubMed] [Google Scholar]
- 18.Tripathi D.K., Shweta , Singh S., Singh S., Pandey R., Singh V.P., Sharma N.C., Prasad S.M., Dubey N.K., Chauhan D.K. An overview on manufactured nanoparticles in plants: Uptake, translocation, accumulation and phytotoxicity. Plant Physiol. Biochem. 2017;110:2–12. doi: 10.1016/j.plaphy.2016.07.030. [DOI] [PubMed] [Google Scholar]
- 19.Zuverza-Mena N., Martínez-Fernández D., Du W., Hernandez-Viezcas J.A., Bonilla-Bird N., López-Moreno M.L., Komárek M., Peralta-Videa J.R., Gardea-Torresdey J.L. Exposure of engineered nanomaterials to plants: Insights into the physiological and biochemical responses-A review. Plant Physiol. Biochem. 2017;110:236–264. doi: 10.1016/j.plaphy.2016.05.037. [DOI] [PubMed] [Google Scholar]
- 20.Feregrino-Perez A.A., Magaña-López E., Guzmán C., Esquivel K. A general overview of the benefits and possible negative effects of the nanotechnology in horticulture. Sci. Hortic. 2018;238:126–137. doi: 10.1016/j.scienta.2018.03.060. [DOI] [Google Scholar]
- 21.Shakiba S., Astete C.E., Paudel S., Sabliov C.M., Rodrigues D.F., Louie S.M. Emerging investigator series: Polymeric nanocarriers for agricultural applications: Synthesis, characterization, and environmental and biological interactions. Environ. Sci. Nano. 2020;7:37–67. doi: 10.1039/C9EN01127G. [DOI] [Google Scholar]
- 22.Vega-Vásquez P., Mosier N.S., Irudayaraj J. Nanoscale drug delivery systems: From medicine to agriculture. Front. Bioeng. Biotechnol. 2020;8:79. doi: 10.3389/fbioe.2020.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Camara M.C., Campos E.V.R., Monteiro R.A., Pereira A.D.E.S., Proença P.L.D.F., Fraceto L.F. Development of stimuli-responsive nano-based pesticides: Emerging opportunities for agriculture. J. Nanobiotechnol. 2019;17:1–19. doi: 10.1186/s12951-019-0533-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh A., Tiwari S., Pandey J., Lata C., Singh I.K. Role of nanoparticles in crop improvement and abiotic stress management. J. Biotechnol. 2021;337:57–70. doi: 10.1016/j.jbiotec.2021.06.022. [DOI] [PubMed] [Google Scholar]
- 25.Khan M., Khan A.U., Hasan M.A., Yadav K.K., Pinto M., Malik N., Yadav V.K., Khan A.H., Islam S., Sharma G.K. Agro-nanotechnology as an emerging field: A novel sustainable approach for improving plant growth by reducing biotic stress. Appl. Sci. 2021;11:2282. doi: 10.3390/app11052282. [DOI] [Google Scholar]
- 26.Ali S., Mehmood A., Khan N. Uptake, translocation, and consequences of nanomaterials on plant growth and stress adaptation. J. Nanomater. 2021;2021:1–17. doi: 10.1155/2021/6677616. [DOI] [Google Scholar]
- 27.Zhao L., Lu L., Wang A., Zhang H., Huang M., Wu H., Xing B., Wang Z., Ji R. Nano-biotechnology in agriculture: Use of nanomaterials to promote plant growth and stress tolerance. J. Agric. Food Chem. 2020;68:1935–1947. doi: 10.1021/acs.jafc.9b06615. [DOI] [PubMed] [Google Scholar]
- 28.Rajput V., Minkina T., Kumari A., Harish , Singh V.K., Verma K.K., Mandzhieva S., Sushkova S., Srivastava S., Keswani C. Coping with the challenges of abiotic stress in plants: New dimensions in the field application of nanoparticles. Plants. 2021;10:1221. doi: 10.3390/plants10061221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raliya R., Saharan V., Dimkpa C., Biswas P. Nanofertilizer for precision and sustainable agriculture: Current state and future perspectives. J. Agric. Food Chem. 2018;66:6487–6503. doi: 10.1021/acs.jafc.7b02178. [DOI] [PubMed] [Google Scholar]
- 30.Adisa I.O., Pullagurala V.L.R., Peralta-Videa J.R., Dimkpa C.O., Elmer W.H., Gardea-Torresdey J.L., White J.C. Recent advances in nano-enabled fertilizers and pesticides: A critical review of mechanisms of action. Environ. Sci. Nano. 2019;6:2002–2030. doi: 10.1039/C9EN00265K. [DOI] [Google Scholar]
- 31.Zhu Y., Xu F., Liu Q., Chen M., Liu X., Wang Y., Sun Y., Zhang L. Nanomaterials and plants: Positive effects, toxicity and the remediation of metal and metalloid pollution in soil. Sci. Total Environ. 2019;662:414–421. doi: 10.1016/j.scitotenv.2019.01.234. [DOI] [PubMed] [Google Scholar]
- 32.Kumar A., Choudhary A., Kaur H., Guha S., Mehta S., Husen A. Potential Applications of Engineered Nanoparticles in Plant Disease Management: A Critical Update. Chemosphere. 2022;295:133798. doi: 10.1016/j.chemosphere.2022.133798. [DOI] [PubMed] [Google Scholar]
- 33.Elmer W.H., White J.C. The use of metallic oxide nanoparticles to enhance growth of tomatoes and eggplants in disease infested soil or soilless medium. Environ. Sci. Nano. 2016;3:1072–1079. doi: 10.1039/C6EN00146G. [DOI] [Google Scholar]
- 34.Cai L., Chen J., Liu Z., Wang H., Yang H., Ding W. Magnesium Oxide Nanoparticles: Effective Agricultural Antibacterial Agent Against Ralstonia solanacearum. Front. Microbiol. 2018;9:790. doi: 10.3389/fmicb.2018.00790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumari M., Pandey S., Mishra S.K., Giri V.P., Agarwal L., Dwivedi S., Pandey A.K., Nautiyal C.S., Mishra A. Omics-Based Mechanistic Insight into the Role of Bioengineered Nanoparticles for Biotic Stress Amelioration by Modulating Plant Metabolic Pathways. Front. Bioeng. Biotechnol. 2020;8:242. doi: 10.3389/fbioe.2020.00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cao Z., Stowers C., Rossi L., Zhang W., Lombardini L., Ma X. Physiological effects of cerium oxide nanoparticles on the photosynthesis and water use efficiency of soybean (Glycine max (L.) Merr.) Environ. Sci. Nano. 2017;4:1086–1094. doi: 10.1039/C7EN00015D. [DOI] [Google Scholar]
- 37.Dimkpa C.O., Singh U., Bindraban P.S., Elmer W.H., Gardea-Torresdey J.L., White J.C. Zinc oxide nanoparticles alleviate drought-induced alterations in sorghum performance, nutrient acquisition, and grain fortification. Sci. Total Environ. 2019;688:926–934. doi: 10.1016/j.scitotenv.2019.06.392. [DOI] [PubMed] [Google Scholar]
- 38.Wang M., Chen L., Chen S., Ma Y. Alleviation of cadmium-induced root growth inhibition in crop seedlings by nanoparticles. Ecotoxicol. Environ. Saf. 2012;79:48–54. doi: 10.1016/j.ecoenv.2011.11.044. [DOI] [PubMed] [Google Scholar]
- 39.Alabdallah N.M., Hasan M., Hammami I., Alghamdi A.I., Alshehri D., Alatawi H.A. Green synthesized metal oxide nanoparticles mediate growth regulation and physiology of crop plants under drought stress. Plants. 2021;10:1730. doi: 10.3390/plants10081730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ze Y., Liu C., Wang L., Hong M., Hong F. The regulation of TiO2 nanoparticles on the expression of light-harvesting complex ii and photosynthesis of chloroplasts of Arabidopsis thaliana. Biol. Trace Elem. Res. 2010;143:1131–1141. doi: 10.1007/s12011-010-8901-0. [DOI] [PubMed] [Google Scholar]
- 41.Yuan S.-J., Chen J.-J., Lin Z.-Q., Li W.-W., Sheng G.-P., Yu H.-Q. Nitrate formation from atmospheric nitrogen and oxygen photocatalysed by nano-sized titanium dioxide. Nat. Commun. 2013;4:2249. doi: 10.1038/ncomms3249. [DOI] [PubMed] [Google Scholar]
- 42.Abdel Latef A.A.H., Srivastava A.K., El-Sadek M.S.A., Kordrostami M., Tran L.S.P. Titanium dioxide nanoparticles improve growth and enhance tolerance of broad bean plants under saline soil conditions. Land Degrad. Dev. 2018;29:1065–1073. doi: 10.1002/ldr.2780. [DOI] [Google Scholar]
- 43.Szymańska R., Kołodziej K., Ślesak I., Zimak-Piekarczyk P., Orzechowska A., Gabruk M., Żądło A., Habina-Skrzyniarz I., Knap W., Burda K., et al. Titanium dioxide nanoparticles (100–1000 mg/l) can affect vitamin E response in Arabidopsis thaliana. Environ. Pollut. 2016;213:957–965. doi: 10.1016/j.envpol.2016.03.026. [DOI] [PubMed] [Google Scholar]
- 44.Pejam F., Ardebili Z.O., Ladan-Moghadam A., Danaee E. Zinc oxide nanoparticles mediated substantial physiological and molecular changes in tomato. PLoS ONE. 2021;16:e0248778. doi: 10.1371/journal.pone.0248778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Šebesta M., Kolenčík M., Sunil B.R., Illa R., Mosnáček J., Ingle A.P., Urík M. Field application of ZnO and TiO2 nanoparticles on agricultural plants. Agronomy. 2021;11:2281. doi: 10.3390/agronomy11112281. [DOI] [Google Scholar]
- 46.Andrejic G., Gajic G., Prica M., Dzeletovic Z., Rakic T. Zinc accumulation, photosynthetic gas exchange, and chlorophyll a fluorescence in Zn-stressed Miscanthus × giganteus plants. Photosynthetica. 2018;56:1249–1258. doi: 10.1007/s11099-018-0827-3. [DOI] [Google Scholar]
- 47.Siddiqi K.S., Husen A. Plant response to engineered metal oxide nanoparticles. Nanoscale Res. Lett. 2017;12:1–18. doi: 10.1186/s11671-017-1861-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hezaveh T.A., Pourakbar L., Rahmani F., Alipour H. Interactive effects of salinity and ZnO nanoparticles on physiological and molecular parameters of rapeseed (Brassica napus L.) Commun. Soil Sci. Plant Anal. 2019;50:698–715. doi: 10.1080/00103624.2019.1589481. [DOI] [Google Scholar]
- 49.Zakharova O.V., Gusev A.A. Photocatalytically active zinc oxide and titanium dioxide nanoparticles in clonal micropropagation of plants: Prospects. Nanotechnol. Russ. 2019;14:311–324. doi: 10.1134/S1995078019040141. [DOI] [Google Scholar]
- 50.Waani S.P.T., Irum S., Gul I., Yaqoob K., Khalid M.U., Ali M.A., Manzoor U., Noor T., Ali S., Rizwan M., et al. TiO2 nanoparticles dose, application method and phosphorous levels influence genotoxicity in Rice (Oryza sativa L.), soil enzymatic activities and plant growth. Ecotoxicol. Environ. Saf. 2021;213:111977. doi: 10.1016/j.ecoenv.2021.111977. [DOI] [PubMed] [Google Scholar]
- 51.Silva S., de Oliveira J.M.F., Dias M.C., Silva A., Santos C. Antioxidant mechanisms to counteract TiO2-nanoparticles toxicity in wheat leaves and roots are organ dependent. J. Hazard. Mater. 2019;380:120889. doi: 10.1016/j.jhazmat.2019.120889. [DOI] [PubMed] [Google Scholar]
- 52.Tolaymat T., Genaidy A., Abdelraheem W., Dionysiou D., Andersen C. The effects of metallic engineered nanoparticles upon plant systems: An analytic examination of scientific evidence. Sci. Total Environ. 2017;579:93–106. doi: 10.1016/j.scitotenv.2016.10.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hou J., Wu Y., Li X., Wei B., Li S., Wang X. Toxic effects of different types of zinc oxide nanoparticles on algae, plants, invertebrates, vertebrates and microorganisms. Chemosphere. 2018;193:852–860. doi: 10.1016/j.chemosphere.2017.11.077. [DOI] [PubMed] [Google Scholar]
- 54.Silva S., Craveiro S.C., Oliveira H., Calado A.J., Pinto R.J., Silva A.M., Santos C. Wheat chronic exposure to TiO2-nanoparticles: Cyto- and genotoxic approach. Plant Physiol. Biochem. 2017;121:89–98. doi: 10.1016/j.plaphy.2017.10.013. [DOI] [PubMed] [Google Scholar]
- 55.Song C., Huang M., White J.C., Zhang X., Wang W., Sarpong C.K., Jamali Z.H., Zhang H., Zhao L., Wang Y. Metabolic profile and physiological response of cucumber foliar exposed to engineered MoS2 and TiO2 nanoparticles. NanoImpact. 2020;20:100271. doi: 10.1016/j.impact.2020.100271. [DOI] [Google Scholar]
- 56.Wu B., Zhu L., Le X.C. Metabolomics analysis of TiO2 nanoparticles induced toxicological effects on rice (Oryza sativa L.) Environ. Pollut. 2017;230:302–310. doi: 10.1016/j.envpol.2017.06.062. [DOI] [PubMed] [Google Scholar]
- 57.Moghadam A.V., Iranbakhsh A., Saadatmand S., Ebadi M., Ardebili Z.O. New insights into the transcriptional, epigenetic, and physiological responses to zinc oxide nanoparticles in Datura stramonium; potential species for phytoremediation. J. Plant Growth Regul. 2022;41:271–281. doi: 10.1007/s00344-021-10305-6. [DOI] [Google Scholar]
- 58.Yusefi-Tanha E., Fallah S., Rostamnejadi A., Pokhrel L.R. Zinc oxide nanoparticles (ZnONPs) as a novel nanofertilizer: Influence on seed yield and antioxidant defense system in soil grown soybean (Glycine max cv. Kowsar) Sci. Total Environ. 2020;738:140240. doi: 10.1016/j.scitotenv.2020.140240. [DOI] [PubMed] [Google Scholar]
- 59.Zoufan P., Baroonian M., Zargar B. ZnO nanoparticles-induced oxidative stress in Chenopodium murale L., Zn uptake, and accumulation under hydroponic culture. Environ. Sci. Pollut. Res. 2020;27:11066–11078. doi: 10.1007/s11356-020-07735-2. [DOI] [PubMed] [Google Scholar]
- 60.Wang X., Li Q.Q., Pei Z.M., Wang S. Effects of zinc oxide nanoparticles on the growth, photosynthetic traits, and antioxidative enzymes in tomato plants. Biol. Plant. 2018;62:801–808. doi: 10.1007/s10535-018-0813-4. [DOI] [Google Scholar]
- 61.Venkatachalam P., Jayaraj M., Manikandan R., Geetha N., Rene E.R., Sharma N., Sahi S. Zinc oxide nanoparticles (ZnONPs) alleviate heavy metal-induced toxicity in Leucaena leucocephala seedlings: A physiochemical analysis. Plant Physiol. Biochem. 2017;110:59–69. doi: 10.1016/j.plaphy.2016.08.022. [DOI] [PubMed] [Google Scholar]
- 62.García-Gómez C., Obrador A., González D., Babín M., Fernández M.D. Comparative study of the phytotoxicity of ZnO nanoparticles and Zn accumulation in nine crops grown in a calcareous soil and an acidic soil. Sci. Total Environ. 2018;644:770–780. doi: 10.1016/j.scitotenv.2018.06.356. [DOI] [PubMed] [Google Scholar]
- 63.Silva S., Oliveira H., Silva A.M.S., Santos C. The cytotoxic targets of anatase or rutile plus anatase nanoparticles depend on the plant species. Biol. Plant. 2017;61:717–725. doi: 10.1007/s10535-017-0733-8. [DOI] [Google Scholar]
- 64.Azarin K., Usatov A., Minkina T., Plotnikov A., Kasyanova A., Fedorenko A., Duplii N., Vechkanov E., Rajput V.D., Mandzhieva S., et al. Effects of ZnO nanoparticles and its bulk form on growth, antioxidant defense system and expression of oxidative stress related genes in Hordeum vulgare L. Chemosphere. 2021;287:132167. doi: 10.1016/j.chemosphere.2021.132167. [DOI] [PubMed] [Google Scholar]
- 65.Chemingui H., Smiri M., Missaoui T., Hafiane A. Zinc oxide nanoparticles induced oxidative stress and changes in the photosynthetic apparatus in fenugreek (Trigonella foenum graecum L.) Bull. Environ. Contam. Toxicol. 2019;102:477–485. doi: 10.1007/s00128-019-02590-5. [DOI] [PubMed] [Google Scholar]
- 66.Chen J., Dou R., Yang Z., You T., Gao X., Wang L. Phytotoxicity and bioaccumulation of zinc oxide nanoparticles in rice (Oryza sativa L.) Plant Physiol. Biochem. 2018;130:604–612. doi: 10.1016/j.plaphy.2018.08.019. [DOI] [PubMed] [Google Scholar]
- 67.García-Gómez C., Obrador A., González D., Babín M., Fernández M.D. Comparative effect of ZnO NPs, ZnO bulk and ZnSO4 in the antioxidant defences of two plant species growing in two agricultural soils under greenhouse conditions. Sci. Total Environ. 2017;589:11–24. doi: 10.1016/j.scitotenv.2017.02.153. [DOI] [PubMed] [Google Scholar]
- 68.Silva S., Oliveira H., Craveiro S.C., Calado A.J., Santos C. Pure anatase and rutile + anatase nanoparticles differently affect wheat seedlings. Chemosphere. 2016;151:68–75. doi: 10.1016/j.chemosphere.2016.02.047. [DOI] [PubMed] [Google Scholar]
- 69.Intergovernmental Panel on Climate Change (IPCC) In: Climate Change 2014 Impacts, Adaptation, and Vulnerability. Part A: Global and Sectoral Aspects. Field C.B., editor. Cambridge University Press; Cambridge, UK: 2014. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. [Google Scholar]
- 70.Holman I., Hess T., Rey D., Knox J. A Multi-level framework for adaptation to drought within temperate agriculture. Front. Environ. Sci. 2021;8:9871. doi: 10.3389/fenvs.2020.589871. [DOI] [Google Scholar]
- 71.Naumann G., Cammalleri C., Mentaschi L., Feyen L. Increased economic drought impacts in Europe with anthropogenic warming. Nat. Clim. Chang. 2021;11:485–491. doi: 10.1038/s41558-021-01044-3. [DOI] [Google Scholar]
- 72.Ma Y., Dias M.C., Freitas H. Drought and salinity stress responses and microbe-induced tolerance in plants. Front. Plant Sci. 2020;11:591911. doi: 10.3389/fpls.2020.591911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hussain A., Ali S., Rizwan M., Zia ur Rehman M.Z., Javed M.R., Imran M., Chatha S.A.S., Nazir R. Zinc oxide nanoparticles alter the wheat physiological response and reduce the cadmium uptake by plants. Environ. Pollut. 2018;242:1518–1526. doi: 10.1016/j.envpol.2018.08.036. [DOI] [PubMed] [Google Scholar]
- 74.Mohammadi R., Maali-Amiri R., Mantri N. Effect of TiO2 nanoparticles on oxidative damage and antioxidant defense systems in chickpea seedlings during cold stress. Russ. J. Plant Physiol. 2014;61:768–775. doi: 10.1134/S1021443714050124. [DOI] [Google Scholar]
- 75.Mohammadi R., Maali-Amiri R., Abbasi A. Effect of TiO2 Nanoparticles on chickpea response to cold stress. Biol. Trace Elem. Res. 2013;152:403–410. doi: 10.1007/s12011-013-9631-x. [DOI] [PubMed] [Google Scholar]
- 76.Qi M., Liu Y., Li T. Nano-TiO2 Improve the photosynthesis of tomato leaves under mild heat stress. Biol. Trace Elem. Res. 2013;156:323–328. doi: 10.1007/s12011-013-9833-2. [DOI] [PubMed] [Google Scholar]
- 77.Hassan N.S., El Din T.A.S., Hendawey M.H., Borai I.H., Mahdi A.A. Magnetite and zinc oxide nanoparticles alleviated heat stress in wheat plants. Curr. Nanomater. 2018;3:32–43. doi: 10.2174/2405461503666180619160923. [DOI] [Google Scholar]
- 78.Alkharabsheh H.M., Seleiman M.F., Hewedy O.A., Battaglia M.L., Jalal R.S., Alhammad B.A., Schillaci C., Ali N., Al-Doss A. Field crop responses and management strategies to mitigate soil salinity in modern agriculture: A review. Agronomy. 2021;11:2299. doi: 10.3390/agronomy11112299. [DOI] [Google Scholar]
- 79.Etesami H., Noori F. Saline Soil-Based Agriculture by Halotolerant Microorganisms. Springer Science and Business Media LLC; Berlin/Heidelberg, Germany: 2019. Soil salinity as a challenge for sustainable agriculture and bacterial-mediated alleviation of salinity stress in crop plants; pp. 1–22. [Google Scholar]
- 80.Ghani M.I., Saleem S., Rather S.A., Rehmani M.S., Alamri S., Rajput V.D., Kalaji H.M., Saleem N., Sial T.A., Liu M. Foliar application of zinc oxide nanoparticles: An effective strategy to mitigate drought stress in cucumber seedling by modulating antioxidant defense system and osmolytes accumulation. Chemosphere. 2021;289:133202. doi: 10.1016/j.chemosphere.2021.133202. [DOI] [PubMed] [Google Scholar]
- 81.Mustafa H., Ilyas N., Akhtar N., Raja N.I., Zainab T., Shah T., Ahmad A., Ahmad P. Biosynthesis and characterization of titanium dioxide nanoparticles and its effects along with calcium phosphate on physicochemical attributes of wheat under drought stress. Ecotoxicol. Environ. Saf. 2021;223:112519. doi: 10.1016/j.ecoenv.2021.112519. [DOI] [PubMed] [Google Scholar]
- 82.Faraji J., Sepehri A. Exogenous nitric oxide improves the protective effects of TiO2 nanoparticles on growth, antioxidant system, and photosynthetic performance of wheat seedlings under drought stress. J. Soil Sci. Plant Nutr. 2020;20:703–714. doi: 10.1007/s42729-019-00158-0. [DOI] [Google Scholar]
- 83.Faraji J., Sepehri A. Ameliorative effects of TiO2 nanoparticles and sodium nitroprusside on seed germination and seedling growth of wheat under PEG-stimulated drought stress. J. Seed Sci. 2019;41:309–317. doi: 10.1590/2317-1545v41n3213139. [DOI] [Google Scholar]
- 84.Karvar M., Azari A., Rahimi A., Maddah-Hosseini S., Ahmadi-Lahijani M.J. Titanium dioxide nanoparticles (TiO2-NPs) enhance drought tolerance and grain yield of sweet corn (Zea mays L.) under deficit irrigation regimes. Acta Physiol. Plant. 2021;44:1–14. doi: 10.1007/s11738-021-03349-4. [DOI] [Google Scholar]
- 85.Kiapour H., Moaveni P., Habibi D., Sani B. Evaluation of the application of gibberellic acid and titanium dioxide nanoparticles. Inter. J. Agron. Agric. Res. 2015;6:138–150. [Google Scholar]
- 86.Sheikhalipour M., Esmaielpour B., Gohari G., Haghighi M., Jafari H., Farhadi H., Kulak M., Kalisz A. Salt stress mitigation via the foliar application of chitosan-functionalized selenium and anatase titanium dioxide nanoparticles in stevia (Stevia rebaudiana Bertoni) Molecules. 2021;26:4090. doi: 10.3390/molecules26134090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Karami A., Sepehri A. Nano titanium dioxide and nitric oxide alleviate salt induced changes in seedling growth, physiological and photosynthesis attributes of barley. Zemdirb.-Agric. 2018;105:123–132. doi: 10.13080/z-a.2018.105.016. [DOI] [Google Scholar]
- 88.Aghdam M.T.B., Mohammadi H., Ghorbanpour M. Effects of nanoparticulate anatase titanium dioxide on physiological and biochemical performance of Linum usitatissimum (Linaceae) under well-watered and drought stress conditions. Braz. J. Bot. 2016;39:139–146. doi: 10.1007/s40415-015-0227-x. [DOI] [Google Scholar]
- 89.Latef A.A.H.A., Abu Alhmad M.F., Abdelfattah K.E. The possible roles of priming with ZnO Nanoparticles in mitigation of salinity stress in lupine (Lupinus termis) plants. J. Plant Growth Regul. 2016;36:60–70. doi: 10.1007/s00344-016-9618-x. [DOI] [Google Scholar]
- 90.Semida W., Abdelkhalik A., Mohamed G., El-Mageed T.A., El-Mageed S.A., Rady M., Ali E. Foliar application of zinc oxide nanoparticles promotes drought stress tolerance in eggplant (Solanum melongena L.) Plants. 2021;10:421. doi: 10.3390/plants10020421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Taran N., Storozhenko V., Svietlova N., Batsmanova L., Shvartau V., Kovalenko M. Effect of zinc and copper nanoparticles on drought resistance of wheat seedlings. Nanoscale Res. Lett. 2017;12:60. doi: 10.1186/s11671-017-1839-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dimkpa C.O., Andrews J., Sanabria J., Bindraban P.S., Singh U., Elmer W.H., Gardea-Torresdey J.L., White J.C. Interactive effects of drought, organic fertilizer, and zinc oxide nanoscale and bulk particles on wheat performance and grain nutrient accumulation. Sci. Total Environ. 2020;722:137808. doi: 10.1016/j.scitotenv.2020.137808. [DOI] [PubMed] [Google Scholar]
- 93.Sun L., Song F., Zhu X., Liu S., Liu F., Wang Y., Li X. Nano-ZnO alleviates drought stress via modulating the plant water use and carbohydrate metabolism in maize. Arch. Agron. Soil Sci. 2021;67:245–259. doi: 10.1080/03650340.2020.1723003. [DOI] [Google Scholar]
- 94.Faizan M., Bhat J.A., Chen C., Alyemeni M.N., Wijaya L., Ahmad P., Yu F. Zinc oxide nanoparticles (ZnO-NPs) induce salt tolerance by improving the antioxidant system and photosynthetic machinery in tomato. Plant Physiol. Biochem. 2021;161:122–130. doi: 10.1016/j.plaphy.2021.02.002. [DOI] [PubMed] [Google Scholar]
- 95.Singh P., Arif Y., Siddiqui H., Sami F., Zaidi R., Azam A., Alam P., Hayat S. Nanoparticles enhances the salinity toxicity tolerance in Linum usitatissimum L. by modulating the antioxidative enzymes, photosynthetic efficiency, redox status and cellular damage. Ecotoxicol. Environ. Saf. 2021;213:112020. doi: 10.1016/j.ecoenv.2021.112020. [DOI] [PubMed] [Google Scholar]
- 96.Mahmoud A.W.M., Abdeldaym E.A., Abdelaziz S.M., El-Sawy M.B.I., Mottaleb S.A. Synergetic effects of zinc, boron, silicon, and zeolite nanoparticles on confer tolerance in potato plants subjected to salinity. Agronomy. 2019;10:19. doi: 10.3390/agronomy10010019. [DOI] [Google Scholar]
- 97.Elsheery N.I., Helaly M.N., El-Hoseiny H.M., Alam-Eldein S.M. Zinc oxide and silicone nanoparticles to improve the resistance mechanism and annual productivity of salt-stressed mango trees. Agronomy. 2020;10:558. doi: 10.3390/agronomy10040558. [DOI] [Google Scholar]
- 98.Farouk S., Al-Amri S.M. Exogenous zinc forms counteract nacl-induced damage by regulating the antioxidant system, osmotic adjustment substances, and ions in canola (Brassica napus L. cv. Pactol) plants. J. Soil Sci. Plant Nutr. 2019;19:887–899. doi: 10.1007/s42729-019-00087-y. [DOI] [Google Scholar]
- 99.El-Badri A.M., Batool M., Mohamed I.A., Khatab A., Sherif A., Wang Z.K., Salah A., Nishawy E., Ayaad M., Kuai J., et al. Modulation of salinity impact on early seedling stage via nano-priming application of Zinc oxide on rapeseed (Brassica napus, L.) Plant Physiol. Biochem. 2021;166:376–392. doi: 10.1016/j.plaphy.2021.05.040. [DOI] [PubMed] [Google Scholar]
- 100.Banerjee A., Roychoudhury A. Explicating the cross-talks between nanoparticles, signaling pathways and nutrient homeostasis during environmental stresses and xenobiotic toxicity for sustainable cultivation of cereals. Chemosphere. 2021;286:131827. doi: 10.1016/j.chemosphere.2021.131827. [DOI] [PubMed] [Google Scholar]
- 101.Ennaji W., Barakat A., El Baghdadi M., Rais J. Heavy metal contamination in agricultural soil and ecological risk assessment in the northeast area of Tadla plain, Morocco. J. Sediment. Environ. 2020;5:307–320. doi: 10.1007/s43217-020-00020-9. [DOI] [Google Scholar]
- 102.Kim H., Lee M., Lee J.-H., Kim K.-H., Owens G., Kim K.-R. Distribution and extent of heavy metal(loid) contamination in agricultural soils as affected by industrial activity. Appl. Biol. Chem. 2020;63:1–8. doi: 10.1186/s13765-020-00517-x. [DOI] [Google Scholar]
- 103.Ali H., Khan E. Trophic transfer, bioaccumulation, and biomagnification of non-essential hazardous heavy metals and metalloids in food chains/webs—Concepts and implications for wildlife and human health. Hum. Ecol. Risk Assess. Int. J. 2019;25:1353–1376. doi: 10.1080/10807039.2018.1469398. [DOI] [Google Scholar]
- 104.Ma Y., He X., Qi K., Wang T., Qi Y., Cui L., Wang F., Song M. Effects of environmental contaminants on fertility and reproductive health. J. Environ. Sci. 2019;77:210–217. doi: 10.1016/j.jes.2018.07.015. [DOI] [PubMed] [Google Scholar]
- 105.Jaishankar M., Tseten T., Anbalagan N., Mathew B.B., Beeregowda K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014;7:60–72. doi: 10.2478/intox-2014-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Engwa G.A., Ferdinand P.U., Nwalo F.N., Unachukwu M.N. Mechanism and health effects of heavy metal toxicity in humans. In: Karcioglu O., Arslan B., editors. Poisoning in the Modern World—New Tricks for an Old Dog? IntechOpen; London, UK: 2019. [DOI] [Google Scholar]
- 107.Shahid M., Shamshad S., Rafiq M., Khalid S., Bibi I., Niazi N.K., Dumat C., Rashid M.I. Chromium speciation, bioavailability, uptake, toxicity and detoxification in soil-plant system: A review. Chemosphere. 2017;178:513–533. doi: 10.1016/j.chemosphere.2017.03.074. [DOI] [PubMed] [Google Scholar]
- 108.Srivastava D., Tiwari M., Dutta P., Singh P., Chawda K., Kumari M., Chakrabarty D. Chromium stress in plants: Toxicity, tolerance and phytoremediation. Sustainability. 2021;13:4629. doi: 10.3390/su13094629. [DOI] [Google Scholar]
- 109.Hafiz M.F., Ma L. Effect of chromium on seed germination, early seedling growth and chromium accumulation in tomato genotypes. Acta Physiol. Plant. 2021;43:1–11. doi: 10.1007/s11738-021-03267-5. [DOI] [Google Scholar]
- 110.Singh D., Sharma N.L., Singh C.K., Yerramilli V., Narayan R., Sarkar S.K., Singh I. Chromium (VI)-induced alterations in physio-chemical parameters, yield, and yield characteristics in two cultivars of mungbean (Vigna radiata L.) Front. Plant Sci. 2021;12:5129. doi: 10.3389/fpls.2021.735129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Singh D., Sharma N.L., Singh C.K., Sarkar S.K., Singh I., Dotaniya M.L. Effect of chromium (VI) toxicity on morpho-physiological characteristics, yield, and yield components of two chickpea (Cicer arietinum L.) varieties. PLoS ONE. 2020;15:e0243032. doi: 10.1371/journal.pone.0243032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mathur S., Kalaji H.M., Jajoo A. Investigation of deleterious effects of chromium phytotoxicity and photosynthesis in wheat plant. Photosynthetica. 2016;54:185–192. doi: 10.1007/s11099-016-0198-6. [DOI] [Google Scholar]
- 113.Tripathi A., Tripathi D.K., Chauhan D.K., Kumar N. Chromium (VI)-induced phytotoxicity in river catchment agriculture: Evidence from physiological, biochemical and anatomical alterations in Cucumis sativus (L.) used as model species. Chem. Ecol. 2016;32:12–33. doi: 10.1080/02757540.2015.1115841. [DOI] [Google Scholar]
- 114.Sharma A., Kapoor D., Wang J., Shahzad B., Kumar V., Bali A.S., Jasrotia S., Zheng B., Yuan H., Yan D. Chromium bioaccumulation and its impacts on plants: An overview. Plants. 2020;9:100. doi: 10.3390/plants9010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kabir A.H. Biochemical and molecular changes in rice seedlings (Oryza sativa L.) to cope with chromium stress. Plant Biol. 2016;18:710–719. doi: 10.1111/plb.12436. [DOI] [PubMed] [Google Scholar]
- 116.Daud M.K., Ali S., Variath M.T., Khan M., Jamil M., Ahmad M., Zhu S.J. Chromium (VI)-induced leaf-based differential physiological, metabolic and microstructural changes in two transgenic cotton cultivars (J208, Z905) and their hybrid line (ZD14) J. Plant Growth Regul. 2021;41:391–403. doi: 10.1007/s00344-021-10310-9. [DOI] [Google Scholar]
- 117.Rodriguez E., Azevedo R., Fernandes P., Santos C. Cr(VI) induces dna damage, cell cycle arrest and polyploidization: A flow cytometric and comet assay study in Pisum sativum. Chem. Res. Toxicol. 2011;24:1040–1047. doi: 10.1021/tx2001465. [DOI] [PubMed] [Google Scholar]
- 118.Küpper H., Parameswaran A., Leitenmaier B., Trtílek M., Šetlík I. Cadmium-induced inhibition of photosynthesis and long-term acclimation to cadmium stress in the hyperaccumulator Thlaspi caerulescens. New Phytol. 2007;175:655–674. doi: 10.1111/j.1469-8137.2007.02139.x. [DOI] [PubMed] [Google Scholar]
- 119.Mobin M., Khan N.A. Photosynthetic activity, pigment composition and antioxidative response of two mustard (Brassica juncea) cultivars differing in photosynthetic capacity subjected to cadmium stress. J. Plant Physiol. 2007;164:601–610. doi: 10.1016/j.jplph.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 120.Sabir A., Naveed M., Bashir M.A., Hussain A., Mustafa A., Zahir Z.A., Kamran M., Ditta A., Núñez-Delgado A., Saeed Q., et al. Cadmium mediated phytotoxic impacts in Brassica napus: Managing growth, physiological and oxidative disturbances through combined use of biochar and Enterobacter sp. MN17. J. Environ. Manag. 2020;265:110522. doi: 10.1016/j.jenvman.2020.110522. [DOI] [PubMed] [Google Scholar]
- 121.Monteiro C., Santos C., Pinho S., Oliveira H., Pedrosa T., Dias M.C. Cadmium-Induced Cyto- and Genotoxicity are Organ-Dependent in Lettuce. Chem. Res. Toxicol. 2012;25:1423–1434. doi: 10.1021/tx300039t. [DOI] [PubMed] [Google Scholar]
- 122.Ahmad M.S.A., Ashraf M., Tabassam Q., Hussain M., Firdous H. Lead (Pb)-induced regulation of growth, photosynthesis, and mineral nutrition in maize (Zea mays L.) plants at early growth stages. Biol. Trace Elem. Res. 2011;144:1229–1239. doi: 10.1007/s12011-011-9099-5. [DOI] [PubMed] [Google Scholar]
- 123.Pourrut B., Shahid M., Dumat C., Winterton P., Pinelli E. Lead uptake, toxicity, and detoxification in plants. In: Whitacre D., editor. Reviews of Environmental Contamination and Toxicology. Volume 213. Springer; New York, NY, USA: 2011. Continuation of Residue Reviews. [DOI] [PubMed] [Google Scholar]
- 124.Silva S., Pinto G., Santos C. Low doses of Pb affected Lactuca sativa photosynthetic performance. Photosynthetica. 2017;55:50–57. doi: 10.1007/s11099-016-0220-z. [DOI] [Google Scholar]
- 125.Rodriguez E., Santos M.D.C., Azevedo R., Correia C., Moutinho-Pereira J., de Oliveira J.M.F., Dias M.C. Photosynthesis light-independent reactions are sensitive biomarkers to monitor lead phytotoxicity in a Pb-tolerant Pisum sativum cultivar. Environ. Sci. Pollut. Res. 2014;22:574–585. doi: 10.1007/s11356-014-3375-9. [DOI] [PubMed] [Google Scholar]
- 126.Pourrut B., Jean S., Silvestre J., Pinelli E. Lead-induced DNA damage in Vicia faba root cells: Potential involvement of oxidative stress. Mutat. Res. Toxicol. Environ. Mutagen. 2011;726:123–128. doi: 10.1016/j.mrgentox.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 127.Silva S., Silva P., Oliveira H., Gaivão I., Matos M., Pinto-Carnide O., Santos C. Pb low doses induced genotoxicity in Lactuca sativa plants. Plant Physiol. Biochem. 2017;112:109–116. doi: 10.1016/j.plaphy.2016.12.026. [DOI] [PubMed] [Google Scholar]
- 128.López-Orenes A., Santos C., Dias M.C., Oliveira H., Ferrer M.Á., Calderón A.A., Silva S. Genotoxicity and cytotoxicity induced in Zygophyllum fabago by low Pb doses depends on the population’s redox plasticity. Horticulturae. 2021;7:455. doi: 10.3390/horticulturae7110455. [DOI] [Google Scholar]
- 129.Dias M.C., Mariz-Ponte N., Santos C. Lead induces oxidative stress in Pisum sativum plants and changes the levels of phytohormones with antioxidant role. Plant Physiol. Biochem. 2019;137:121–129. doi: 10.1016/j.plaphy.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 130.Silva S. Aluminium Toxicity Targets in Plants. J. Bot. 2012;2012:1–8. doi: 10.1155/2012/219462. [DOI] [Google Scholar]
- 131.Lei Y., Riaz M., Liu J., Min Y., Jiang C.C. The aluminum tolerance and detoxification mechanisms in plants; recent advances and prospects. Crit. Rev. Environ. Sci. Technol. 2020;52:1491–1527. [Google Scholar]
- 132.Silva S., Santos C., Matos M., Pinto-Carnide O. Al toxicity mechanism in tolerant and sensitive rye genotypes. Environ. Exp. Bot. 2012;75:89–97. doi: 10.1016/j.envexpbot.2011.08.017. [DOI] [Google Scholar]
- 133.Silva S., Rodriguez E., Pinto-Carnide O., Martins-Lopes P., Matos M., Guedes-Pinto H., Santos C. Zonal responses of sensitive vs. tolerant wheat roots during Al exposure and recovery. J. Plant Physiol. 2012;169:760–769. doi: 10.1016/j.jplph.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 134.Lin Y.-H., Chen J.-H. Effects of aluminum on the cell morphology in the root apices of two pineapples with different Al-resistance characteristics. Soil Sci. Plant Nutr. 2019;65:353–357. doi: 10.1080/00380768.2019.1625286. [DOI] [Google Scholar]
- 135.Zeng C.-Q., Liu W.-X., Hao J.-Y., Fan D.-N., Chen L.-M., Xu H.-N., Li K.-Z. Measuring the expression and activity of the CAT enzyme to determine Al resistance in soybean. Plant Physiol. Biochem. 2019;144:254–263. doi: 10.1016/j.plaphy.2019.09.026. [DOI] [PubMed] [Google Scholar]
- 136.Chauhan D.K., Yadav V., Vaculík M., Gassmann W., Pike S., Arif N., Singh V.P., Deshmukh R., Sahi S., Tripathi D.K. Aluminum toxicity and aluminum stress-induced physiological tolerance responses in higher plants. Crit. Rev. Biotechnol. 2021;41:715–730. doi: 10.1080/07388551.2021.1874282. [DOI] [PubMed] [Google Scholar]
- 137.Silva S., Pinto-Carnide O., Martins-Lopes P., Matos M., Guedes-Pinto H., Santos C. Differential aluminium changes on nutrient accumulation and root differentiation in an Al sensitive vs. tolerant wheat. Environ. Exp. Bot. 2010;68:91–98. doi: 10.1016/j.envexpbot.2009.10.005. [DOI] [Google Scholar]
- 138.Fan Y., Ouyang Y., Pan Y., Hong T., Wu C., Lin H. Effect of aluminum stress on the absorption and transportation of aluminum and macronutrients in roots and leaves of Aleurites montana. For. Ecol. Manag. 2020;458:1–9. doi: 10.1016/j.foreco.2019.117813. [DOI] [Google Scholar]
- 139.Cárcamo M.P., Reyes-Díaz M., Rengel Z., Alberdi M., Omena-Garcia R.P., Nunes-Nesi A., Inostroza-Blancheteau C. Aluminum stress differentially affects physiological performance and metabolic compounds in cultivars of highbush blueberry. Sci. Rep. 2019;9:1–13. doi: 10.1038/s41598-019-47569-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Silva S., Pinto G., Dias M.C., Correia C.M., Moutinho-Pereira J., Pinto-Carnide O., Santos C. Aluminium long-term stress differently affects photosynthesis in rye genotypes. Plant Physiol. Biochem. 2012;54:105–112. doi: 10.1016/j.plaphy.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 141.Gavassi M.A., Dodd I.C., Puértolas J., Silva G.S., Carvalho R.F., Habermann G. Aluminum-induced stomatal closure is related to low root hydraulic conductance and high ABA accumulation. Environ. Exp. Bot. 2020;179:104233. doi: 10.1016/j.envexpbot.2020.104233. [DOI] [Google Scholar]
- 142.Yamamoto Y. Aluminum toxicity in plant cells: Mechanisms of cell death and inhibition of cell elongation. Soil Sci. Plant Nutr. 2019;65:41–55. doi: 10.1080/00380768.2018.1553484. [DOI] [Google Scholar]
- 143.Silva S., Pinto G., Correia B., Pinto-Carnide O., Santos C. Rye oxidative stress under long term Al exposure. J. Plant Physiol. 2013;170:879–889. doi: 10.1016/j.jplph.2013.01.015. [DOI] [PubMed] [Google Scholar]
- 144.Sun C., Liu L., Zhou W., Lu L., Jin C., Lin X. Aluminum induces distinct changes in the metabolism of reactive oxygen and nitrogen species in the roots of two wheat genotypes with different aluminum resistance. J. Agric. Food Chem. 2017;65:9419–9427. doi: 10.1021/acs.jafc.7b03386. [DOI] [PubMed] [Google Scholar]
- 145.Ranjan A., Sinha R., Sharma T.R., Pattanayak A., Singh A.K. Alleviating aluminum toxicity in plants: Implications of reactive oxygen species signaling and crosstalk with other signaling pathways. Physiol. Plant. 2021;173:1765–1784. doi: 10.1111/ppl.13382. [DOI] [PubMed] [Google Scholar]
- 146.Wu F., Fang Q., Yan S., Pan L., Tang X., Ye W. Effects of zinc oxide nanoparticles on arsenic stress in rice (Oryza sativa L.): Germination, early growth, and arsenic uptake. Environ. Sci. Pollut. Res. 2020;27:26974–26981. doi: 10.1007/s11356-020-08965-0. [DOI] [PubMed] [Google Scholar]
- 147.Bali A.S., Sidhu G.P.S. Arsenic acquisition, toxicity and tolerance in plants—From physiology to remediation: A review. Chemosphere. 2021;283:131050. doi: 10.1016/j.chemosphere.2021.131050. [DOI] [PubMed] [Google Scholar]
- 148.Mir A.R., Alam P., Hayat S. Effect of different levels of soil applied copper on the morpho-physiological, photochemical, and antioxidant system of Brassica juncea. J. Soil Sci. Plant Nutr. 2021;21:3477–3492. doi: 10.1007/s42729-021-00621-x. [DOI] [Google Scholar]
- 149.Ambrosini V.G., Rosa D.J., de Melo G.W.B., Zalamena J., Cella C., Simão D.G., da Silva L.S., dos Santos H.P., Toselli M., Tiecher T.L., et al. High copper content in vineyard soils promotes modifications in photosynthetic parameters and morphological changes in the root system of ‘Red Niagara’ plantlets. Plant Physiol. Biochem. 2018;128:89–98. doi: 10.1016/j.plaphy.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 150.Girotto E., Ceretta C.A., Rossato L.V., Farias J.G., Brunetto G., Miotto A., Tiecher T.L., de Conti L., Lourenzi C.R., Schmatz R., et al. Biochemical changes in black oat (avena strigosa schreb) cultivated in vineyard soils contaminated with copper. Plant Physiol. Biochem. 2016;103:199–207. doi: 10.1016/j.plaphy.2016.02.030. [DOI] [PubMed] [Google Scholar]
- 151.Rastogi A., Zivcak M., Sytar O., Kalaji H.M., He X., Mbarki S., Brestic M. Impact of metal and metal oxide nanoparticles on plant: A critical review. Front. Chem. 2017;5:78. doi: 10.3389/fchem.2017.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhang W., Long J., Geng J., Li J., Wei Z. Impact of titanium dioxide nanoparticles on Cd phytotoxicity and bioaccumulation in rice (Oryza sativa L.) Int. J. Environ. Res. Public Health. 2020;17:2979. doi: 10.3390/ijerph17092979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zand A.D., Tabrizi A.M., Heir A.V. Application of titanium dioxide nanoparticles to promote phytoremediation of Cd-polluted soil: Contribution of PGPR inoculation. Bioremediat. J. 2020;24:171–189. doi: 10.1080/10889868.2020.1799929. [DOI] [Google Scholar]
- 154.Singh J., Lee B.K. Influence of nano-TiO2 particles on the bioaccumulation of Cd in soybean plants (Glycine max): A possible mechanism for the removal of Cd from the contaminated soil. J. Environ. Manag. 2016;170:88–96. doi: 10.1016/j.jenvman.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 155.Mariz-Ponte N., Dias C.M., Silva A.M., Santos C., Silva S. Low levels of TiO2-nanoparticles interact antagonistically with Al and Pb alleviating their toxicity. Plant Physiol. Biochem. 2021;167:1–10. doi: 10.1016/j.plaphy.2021.07.021. [DOI] [PubMed] [Google Scholar]
- 156.Zand A.D., Tabrizi A.M., Heir A.V. Co-application of biochar and titanium dioxide nanoparticles to promote remediation of antimony from soil by Sorghum bicolor: Metal uptake and plant response. Heliyon. 2020;6:e04669. doi: 10.1016/j.heliyon.2020.e04669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lian J., Zhao L., Wu J., Xiong H., Bao Y., Zeb A., Tang J., Liu W. Foliar spray of TiO2 nanoparticles prevails over root application in reducing Cd accumulation and mitigating Cd-induced phytotoxicity in maize (Zea mays L.) Chemosphere. 2019;239:124794. doi: 10.1016/j.chemosphere.2019.124794. [DOI] [PubMed] [Google Scholar]
- 158.Ji Y., Zhou Y., Ma C., Feng Y., Hao Y., Rui Y., Wu W., Gui X., Le V.N., Han Y., et al. Jointed toxicity of TiO2 NPs and Cd to rice seedlings: NPs alleviated Cd toxicity and Cd promoted NPs uptake. Plant Physiol. Biochem. 2017;110:82–93. doi: 10.1016/j.plaphy.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 159.Xiao Y., Du Y., Xiao Y., Zhang X., Wu J., Yang G., He Y., Zhou Y., Peijnenburg W.J., Luo L. Elucidating the effects of TiO2 nanoparticles on the toxicity and accumulation of Cu in soybean plants (Glycine max L.) Ecotoxicol. Environ. Saf. 2021;219:112312. doi: 10.1016/j.ecoenv.2021.112312. [DOI] [PubMed] [Google Scholar]
- 160.Ma C., Liu H., Chen G., Zhao Q., Eitzer B., Wang Z., Cai W., Newman L.A., White J.C., Dhankher O.P., et al. Effects of titanium oxide nanoparticles on tetracycline accumulation and toxicity in Oryza sativa (L.) Environ. Sci. Nano. 2017;4:1827–1839. doi: 10.1039/C7EN00280G. [DOI] [Google Scholar]
- 161.Liu H., Ma C., Chen G., White J.C., Wang Z., Xing B., Dhankher O.P. Titanium dioxide nanoparticles alleviate tetracycline toxicity to Arabidopsis thaliana (L.) ACS Sustain. Chem. Eng. 2017;5:3204–3213. doi: 10.1021/acssuschemeng.6b02976. [DOI] [Google Scholar]
- 162.Ogunkunle C.O., Odulaja D.A., Akande F.O., Varun M., Vishwakarma V., Fatoba P.O. Cadmium toxicity in cowpea plant: Effect of foliar intervention of nano-TiO2 on tissue Cd bioaccumulation, stress enzymes and potential dietary health risk. J. Biotechnol. 2020;310:54–61. doi: 10.1016/j.jbiotec.2020.01.009. [DOI] [PubMed] [Google Scholar]
- 163.Rizwan M., Ali S., Rehman M.Z.U., Malik S., Adrees M., Qayyum M.F., Alamri S.A., Alyemeni M.N., Ahmad P. Effect of foliar applications of silicon and titanium dioxide nanoparticles on growth, oxidative stress, and cadmium accumulation by rice (Oryza sativa) Acta Physiol. Plant. 2019;41:1–12. [Google Scholar]
- 164.Sardar R., Ahmed S., Yasin N.A. Titanium dioxide nanoparticles mitigate cadmium toxicity in Coriandrum sativum L. through modulating antioxidant system, stress markers and reducing cadmium uptake. Environ. Pollut. 2021;292:118373. doi: 10.1016/j.envpol.2021.118373. [DOI] [PubMed] [Google Scholar]
- 165.Dai C., Shen H., Duan Y., Liu S., Zhou F., Wu D., Zhong G., Javadi A., Tu Y.-J. TiO2 and SiO2 nanoparticles combined with surfactants mitigate the toxicity of Cd2+ to wheat seedlings. Water Air Soil Pollut. 2019;230:232. doi: 10.1007/s11270-019-4297-4. [DOI] [Google Scholar]
- 166.Katiyar P., Yadu B., Korram J., Satnami M.L., Kumar M., Keshavkant S. Titanium nanoparticles attenuates arsenic toxicity by up-regulating expressions of defensive genes in Vigna radiata L. J. Environ. Sci. 2020;92:18–27. doi: 10.1016/j.jes.2020.02.013. [DOI] [PubMed] [Google Scholar]
- 167.De A.K., Ghosh A., Debnath S.C., Sarkar B., Saha I., Adak M.K. Modulation of physiological responses with TiO2 nano-particle in Azolla pinnata R.Br. under 2,4-D toxicity. Mol. Biol. Rep. 2018;45:663–673. doi: 10.1007/s11033-018-4203-y. [DOI] [PubMed] [Google Scholar]
- 168.Faizan M., Bhat J.A., Hessini K., Yu F., Ahmad P. Zinc oxide nanoparticles alleviates the adverse effects of cadmium stress on Oryza sativa via modulation of the photosynthesis and antioxidant defense system. Ecotoxicol. Environ. Saf. 2021;220:112401. doi: 10.1016/j.ecoenv.2021.112401. [DOI] [PubMed] [Google Scholar]
- 169.Ali S., Rizwan M., Noureen S., Anwar S., Ali B., Naveed M., Abd Allah E.F., Alqarawi A.A., Ahmad P. Combined use of biochar and zinc oxide nanoparticle foliar spray improved the plant growth and decreased the cadmium accumulation in rice (Oryza sativa L.) plant. Environ. Sci. Pollut. Res. 2019;26:11288–11299. doi: 10.1007/s11356-019-04554-y. [DOI] [PubMed] [Google Scholar]
- 170.Rizwan M., Ali S., ur Rehman M.Z., Adrees M., Arshad M., Qayyum M.F., Ali L., Hussain A., Chatha S.A.S., Imran M. Alleviation of cadmium accumulation in maize (Zea mays L.) by foliar spray of zinc oxide nanoparticles and biochar to contaminated soil. Environ. Pollut. 2019;248:358–367. doi: 10.1016/j.envpol.2019.02.031. [DOI] [PubMed] [Google Scholar]
- 171.Adrees M., Khan Z.S., Hafeez M., Rizwan M., Hussain K., Asrar M., Alyemeni M.N., Wijaya L., Ali S. Foliar exposure of zinc oxide nanoparticles improved the growth of wheat (Triticum aestivum L.) and decreased cadmium concentration in grains under simultaneous Cd and water deficient stress. Ecotoxicol. Environ. Saf. 2021;208:111627. doi: 10.1016/j.ecoenv.2020.111627. [DOI] [PubMed] [Google Scholar]
- 172.Rizwan M., Ali S., Ali B., Adrees M., Arshad M., Hussain A., ur Rehman M.Z., Waris A.A. Zinc and iron oxide nanoparticles improved the plant growth and reduced the oxidative stress and cadmium concentration in wheat. Chemosphere. 2019;214:269–277. doi: 10.1016/j.chemosphere.2018.09.120. [DOI] [PubMed] [Google Scholar]
- 173.Sharifan H., Moore J., Ma X. Zinc oxide (ZnO) nanoparticles elevated iron and copper contents and mitigated the bioavailability of lead and cadmium in different leafy greens. Ecotoxicol. Environ. Saf. 2020;191:110177. doi: 10.1016/j.ecoenv.2020.110177. [DOI] [PubMed] [Google Scholar]
- 174.Seleiman M.F., Alotaibi M.A., Alhammad B.A., Alharbi B.M., Refay Y., Badawy S.A. Effects of ZnO nanoparticles and biochar of rice straw and cow manure on characteristics of contaminated soil and sunflower productivity, oil quality, and heavy metals uptake. Agronomy. 2020;10:790. doi: 10.3390/agronomy10060790. [DOI] [Google Scholar]
- 175.Zeeshan M., Hu Y.X., Iqbal A., Salam A., Liu Y.X., Muhammad I., Ahmad S., Khan A.H., Hale B., Wu H.Y., et al. Amelioration of AsV toxicity by concurrent application of ZnO-NPs and Se-NPs is associated with differential regulation of photosynthetic indexes, antioxidant pool and osmolytes content in soybean seedling. Ecotoxicol. Environ. Saf. 2021;225:112738. doi: 10.1016/j.ecoenv.2021.112738. [DOI] [PubMed] [Google Scholar]
- 176.Salam A., Khan A.R., Liu L., Yang S., Azhar W., Ulhassan Z., Zeeshan M., Wu J., Fan X., Gan Y. Seed priming with zinc oxide nanoparticles downplayed ultrastructural damage and improved photosynthetic apparatus in maize under cobalt stress. J. Hazard. Mater. 2021;423:127021. doi: 10.1016/j.jhazmat.2021.127021. [DOI] [PubMed] [Google Scholar]
- 177.Faizan M., Bhat J.A., Noureldeen A., Ahmad P., Yu F. Zinc oxide nanoparticles and 24-epibrassinolide alleviates Cu toxicity in tomato by regulating ROS scavenging, stomatal movement and photosynthesis. Ecotoxicol. Environ. Saf. 2021;218:112293. doi: 10.1016/j.ecoenv.2021.112293. [DOI] [PubMed] [Google Scholar]
- 178.Nazarov P.A., Baleev D.N., Ivanova M.I., Sokolova L.M., Karakozova M.V. Infectious plant diseases: Etiology, current status, problems and prospects in plant protection. Acta Nat. 2020;12:46–59. doi: 10.32607/actanaturae.11026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Savary S., Willocquet L., Pethybridge S.J., Esker P., McRoberts N., Nelson A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019;3:430–439. doi: 10.1038/s41559-018-0793-y. [DOI] [PubMed] [Google Scholar]
- 180.Food and Agriculture Organization (FAO) International Year of Plant Health—Protecting Plants, Protecting Life. [(accessed on 1 December 2021)]. Available online: https://www.fao.org/plant-health-2020/home/en/
- 181.Xu L., Zhu Z., Sun D.-W. Bioinspired Nanomodification Strategies: Moving from Chemical-Based Agrosystems to Sustainable Agriculture. ACS Nano. 2021;15:12655–12686. doi: 10.1021/acsnano.1c03948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.De Filpo G., Palermo A.M., Rachiele F., Nicoletta F.P. Preventing fungal growth in wood by titanium dioxide nanoparticles. Int. Biodeterior. Biodegrad. 2013;85:217–222. doi: 10.1016/j.ibiod.2013.07.007. [DOI] [Google Scholar]
- 183.Ogunyemi S.O., Zhang M., Abdallah Y., Ahmed T., Qiu W., Ali A., Yan C., Yang Y., Chen J., Li B. The bio-synthesis of three metal oxide nanoparticles (ZnO, MnO2, and MgO) and Their Antibacterial Activity Against the Bacterial Leaf Blight Pathogen. Front. Microbiol. 2020;11:1–14. doi: 10.3389/fmicb.2020.588326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zhu W., Hu C., Ren Y., Lu Y., Song Y., Ji Y., Han C., He J. Green synthesis of zinc oxide nanoparticles using Cinnamomum camphora (L.) Presl leaf extracts and its antifungal activity. J. Environ. Chem. Eng. 2021;9:106659. doi: 10.1016/j.jece.2021.106659. [DOI] [Google Scholar]
- 185.Jiang H., Lv L., Ahmed T., Jin S., Shahid M., Noman M., Osman H.-E.H., Wang Y., Sun G., Li X., et al. Effect of the nanoparticle exposures on the tomato bacterial wilt disease control by modulating the rhizosphere bacterial community. Int. J. Mol. Sci. 2021;23:414. doi: 10.3390/ijms23010414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Thounaojam T.C., Meetei T.T., Devi Y.B., Panda S.K., Upadhyaya H. Zinc oxide nanoparticles (ZnO-NPs): A promising nanoparticle in renovating plant science. Acta Physiol. Plant. 2021;43:1–22. doi: 10.1007/s11738-021-03307-0. [DOI] [Google Scholar]
- 187.Şahin B., Soylu S., Kara M., Türkmen M., Aydin R., Çetin H. Superior antibacterial activity against seed-borne plant bacterial disease agents and enhanced physical properties of novel green synthesized nanostructured ZnO using Thymbra spicata plant extract. Ceram. Int. 2021;47:341–350. doi: 10.1016/j.ceramint.2020.08.139. [DOI] [Google Scholar]
- 188.Kashyap D., Siddiqui Z.A. Effect of Zinc Oxide Nanoparticles and Rhizobium leguminosarum on growth, photosynthetic pigments and blight disease complex of pea. Gesunde Pflanz. 2021;74:29–40. doi: 10.1007/s10343-021-00586-y. [DOI] [Google Scholar]
- 189.Mamede M.C., Mota R.P., Silva A.C.A., Tebaldi N.D. Nanoparticles in inhibiting Pantoea ananatis and to control maize white spot. Ciênc. Rural. 2022;52:1–7. doi: 10.1590/0103-8478cr20210481. [DOI] [Google Scholar]
- 190.Khan R.A.A., Tang Y., Naz I., Alam S.S., Wang W., Ahmad M., Najeeb S., Rao C., Li Y., Xie B., et al. Management of Ralstonia solanacearum in tomato using ZnO nanoparticles synthesized through Matricaria chamomilla. Plant Dis. 2021;105:3224–3230. doi: 10.1094/PDIS-08-20-1763-RE. [DOI] [PubMed] [Google Scholar]
- 191.Keerthana P., Vijayakumar S., Vidhya E., Punitha V.N., Nilavukkarasi M., Praseetha P.K. Biogenesis of ZnO nanoparticles for revolutionizing agriculture: A step towards anti -infection and growth promotion in plants. Ind. Crop. Prod. 2021;170:113762. doi: 10.1016/j.indcrop.2021.113762. [DOI] [Google Scholar]
- 192.Shobha B., Lakshmeesha T.R., Ansari M.A., Almatroudi A., Alzohairy M.A., Basavaraju S., Alurappa R., Niranjana S.R., Chowdappa S. Mycosynthesis of ZnO nanoparticles using Trichoderma spp. isolated from rhizosphere soils and its synergistic antibacterial effect against Xanthomonas oryzae pv. oryzae. J. Fungi. 2020;6:181. doi: 10.3390/jof6030181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Tryfon P., Kamou N.N., Mourdikoudis S., Karamanoli K., Menkissoglu-Spiroudi U., Dendrinou-Samara C. CuZn and ZnO nanoflowers as nano-fungicides against Botrytis cinerea and Sclerotinia sclerotiorum: Phytoprotection, translocation, and impact after foliar application. Materials. 2021;14:7600. doi: 10.3390/ma14247600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.González-Merino A.M., Hernández-Juárez A., Betancourt-Galindo R., Ochoa-Fuentes Y.M., Valdez-Aguilar L.A., Limón-Corona M.L. Antifungal activity of zinc oxide nanoparticles in Fusarium oxysporum-Solanum lycopersicum pathosystem under controlled conditions. J. Phytopathol. 2021;169:533–544. doi: 10.1111/jph.13023. [DOI] [Google Scholar]
- 195.Sardar M., Ahmed W., Al Ayoubi S., Nisa S., Bibi Y., Sabir M., Khan M.M., Ahmed W., Qayyum A. Fungicidal synergistic effect of biogenically synthesized zinc oxide and copper oxide nanoparticles against Alternaria citri causing citrus black rot disease. Saudi J. Biol. Sci. 2021;29:88–95. doi: 10.1016/j.sjbs.2021.08.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Mohamed A.A., Abu-Elghait M., Ahmed N.E., Salem S.S. Eco-friendly mycogenic synthesis of ZnO and CuO nanoparticles for in vitro antibacterial, antibiofilm, and antifungal applications. Biol. Trace Elem. Res. 2021;199:2788–2799. doi: 10.1007/s12011-020-02369-4. [DOI] [PubMed] [Google Scholar]
- 197.Zudyte B., Luksiene Z. Visible light-activated ZnO nanoparticles for microbial control of wheat crop. J. Photochem. Photobiol. B Biol. 2021;219:112206. doi: 10.1016/j.jphotobiol.2021.112206. [DOI] [PubMed] [Google Scholar]
- 198.Luksiene Z., Rasiukeviciute N., Zudyte B., Uselis N. Innovative approach to sunlight activated biofungicides for strawberry crop protection: ZnO nanoparticles. J. Photochem. Photobiol. B Biol. 2020;203:111656. doi: 10.1016/j.jphotobiol.2019.111656. [DOI] [PubMed] [Google Scholar]
- 199.Abdelkhalek A., Al-Askar A.A. Green Synthesized ZnO Nanoparticles Mediated by Mentha Spicata Extract Induce Plant Systemic Resistance against Tobacco Mosaic Virus. Appl. Sci. 2020;10:5054. doi: 10.3390/app10155054. [DOI] [Google Scholar]
- 200.Sar T.A.I., Unal M. Inhibitory effect of antifungal activity of Titanium Dioxide (TiO2) nanoparticles on some pathogenic Fusarium isolates; Proceedings of the International Workshop Plant Health: Challenges and Solutions; Antalya, Turkey. 23–28 April 2017; p. 78. [Google Scholar]
- 201.Satti S.H., Raja N.I., Javed B., Akram A., Mashwani Z.-U.-R., Ahmad M.S., Ikram M. Titanium dioxide nanoparticles elicited agro-morphological and physicochemical modifications in wheat plants to control Bipolaris sorokiniana. PLoS ONE. 2021;16:e0246880. doi: 10.1371/journal.pone.0246880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Hamza A., El-Mogazy S., Derbalah A. Fenton reagent and titanium dioxide nanoparticles as antifungal agents to control leaf spot of sugar beet under field conditions. J. Plant Prot. Res. 2016;56:270–278. doi: 10.1515/jppr-2016-0040. [DOI] [Google Scholar]
- 203.Boxi S.S., Mukherjee K., Paria S. Ag doped hollow TiO2 nanoparticles as an effective green fungicide against Fusarium solani and Venturia inaequalis phytopathogens. Nanotechnology. 2016;27:1–13. doi: 10.1088/0957-4484/27/8/085103. [DOI] [PubMed] [Google Scholar]
- 204.Norman D.J., Chen J. Effect of foliar application of titanium dioxide on bacterial blight of geranium and xanthomonas leaf spot of poinsettia. HortScience. 2011;46:426–428. doi: 10.21273/HORTSCI.46.3.426. [DOI] [Google Scholar]
- 205.Cui H., Ping Z., Gu W., Jiang J. Application of anatase TiO2 sol derived from peroxotitannic acid in crop diseases control. NSTI Nanotech. 2009;2:286–289. [Google Scholar]
- 206.Hossain A., Abdallah Y., Ali A., Masum M.I., Li B., Sun G., Meng Y., Wang Y., An Q. Lemon-fruit-based green synthesis of zinc oxide nanoparticles and titanium dioxide nanoparticles against soft rot bacterial pathogen Dickeya dadantii. Biomolecules. 2019;9:863. doi: 10.3390/biom9120863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Ardakani A.S. Toxicity of silver, titanium and silicon nanoparticles on the root-knot nematode, Meloidogyne incognita, and growth parameters of tomato. Nematology. 2013;15:671–677. doi: 10.1163/15685411-00002710. [DOI] [Google Scholar]
- 208.Elsharkawy M.M., Derbalah A. Antiviral activity of titanium dioxide nanostructures as a control strategy for broad bean strain virus in faba bean. Pest Manag. Sci. 2019;75:828–834. doi: 10.1002/ps.5185. [DOI] [PubMed] [Google Scholar]
- 209.Gutierrez-Ramirez J.A., Betancourt-Galindo R., Aguirre-Uribe L.A., Cerna-Chavez E., Sandoval-Rangel A., Castro-del Angel E., Chacon-Hernandez J.C., Garcia-Lopez J.I., Hernandez-Juarez A. Insecticidal effect of zinc oxide and titanium dioxide nanoparticles against Bactericera cockerelli Sulc. (Hemiptera: Triozidae) on tomato Solanum lycopersicum. Agronomy. 2021;11:1460. doi: 10.3390/agronomy11081460. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This is a review article; however, data used and/or analyzed during the review are available on request from the corresponding author.