Abstract
Background
Estimates of the severity of the SARS-CoV-2 omicron variant (B.1.1.529) are crucial to assess the public health impact associated with its rapid global dissemination. We estimated the risk of SARS-CoV-2-related hospitalisations after infection with omicron compared with the delta variant (B.1.617.2) in Denmark, a country with high mRNA vaccination coverage and extensive free-of-charge PCR testing capacity.
Methods
In this observational cohort study, we included all RT-PCR-confirmed cases of SARS-CoV-2 infection in Denmark, with samples taken between Nov 21 (date of first omicron-positive sample) and Dec 19, 2021. Individuals were identified in the national COVID-19 surveillance system database, which included results of a variant-specific RT-PCR that detected omicron cases, and data on SARS-CoV-2-related hospitalisations (primary outcome of the study). We calculated the risk ratio (RR) of hospitalisation after infection with omicron compared with delta, overall and stratified by vaccination status, in a Poisson regression model with robust SEs, adjusted a priori for reinfection status, sex, age, region, comorbidities, and time period.
Findings
Between Nov 21 and Dec 19, 2021, among the 188 980 individuals with SARS-CoV-2 infection, 38 669 (20·5%) had the omicron variant. SARS-CoV-2-related hospitalisations and omicron cases increased during the study period. Overall, 124 313 (65·8%) of 188 980 individuals were vaccinated, and vaccination was associated with a lower risk of hospitalisation (adjusted RR 0·24, 95% CI 0·22–0·26) compared with cases with no doses or only one dose of vaccine. Compared with delta infection, omicron infection was associated with an adjusted RR of hospitalisation of 0·64 (95% CI 0·56–0·75; 222 [0·6%] of 38 669 omicron cases admitted to hospital vs 2213 [1·5%] of 150 311 delta cases). For a similar comparison by vaccination status, the RR of hospitalisation was 0·57 (0·44–0·75) among cases with no or only one dose of vaccine, 0·71 (0·60–0·86) among those who received two doses, and 0·50 (0·32–0·76) among those who received three doses.
Interpretation
We found a significantly lower risk of hospitalisation with omicron infection compared with delta infection among both vaccinated and unvaccinated individuals, suggesting an inherent reduced severity of omicron. Our results could guide modelling of the effect of the ongoing global omicron wave and thus health-care system preparedness.
Funding
None.
Introduction
On Nov 26, 2021, the SARS-CoV-2 omicron variant (B.1.1.529) was designated a variant of concern (VOC) by WHO.1 2 days later, on Nov 28, 2021, the first two omicron cases were identified in Denmark in travellers returning from South Africa. Since this first detection, the omicron variant has spread rapidly in Denmark and comprised around 93% of the daily detected cases by Jan 3, 2022.2
The combination of mutations that define the omicron variant has been found to increase transmissibility compared with the delta variant (B.1.617.2) and is associated with marked immune escape, resulting in higher rates of reinfection and lower protection against infection by vaccination compared with delta.3, 4 With the rapid global spread of omicron, it is of critical public health importance to determine whether the omicron variant is associated with less severe disease than the delta variant. In South Africa, increased rates of hospitalisation were observed with rising case numbers at the epicentre of omicron infection, but the distribution of inpatients showed a skew towards younger populations, and these patients had shorter lengths of hospital stay and generally reduced oxygen requirements compared with hospitalised patients with COVID-19 in previous waves.5 However, this difference might be attributed to both a higher level of natural immunity in the population after extensive transmission of other variants in previous waves, as well as vaccine-induced immunity after the rollout of the vaccination programme. Conclusions on altered severity of omicron cannot be reached based on these findings alone. Therefore, observational studies at the individual level are needed to assess the severity of omicron relative to delta, taking previous infection and vaccination status into account. Furthermore, to detect severity the studies’ capacity to obtain variant information for all or representative severe or hospitalised cases, either by variant PCR or whole-genome sequencing, is crucial.
Research in context.
Evidence before this study
There is global concern about the rapid spread of the SARS-CoV-2 omicron variant (B.1.1.529); however, the risk of hospitalisation associated with this new variant of concern is unclear. We searched PubMed and medRxiv with the following terms, with no date or language restrictions: (“Omicron” OR “B.1.1.529”) AND (“hospitalisation” OR “hospital admission”). The search was last updated on Jan 31, 2022. Published studies from the UK, Scotland, South Africa, Canada, Norway, the USA, and Portugal showed that patients infected with the omicron variant were less likely to be admitted to a hospital.
Added value of this study
Using nationwide data from health-care registers in a country with a high national vaccination coverage (>77% for at least two doses and >40% for three doses on Dec 27, 2021) and one of Europe's highest capacities for detecting the omicron variant, it was possible to estimate the risk of hospitalisation associated with omicron infection compared with delta variant (B.1.617.2) infection, taking vaccination status and previous infection into account.
Implications of all the available findings
Our findings show that patients infected with the SARS-CoV-2 omicron variant were less likely to be admitted to hospital, with two-thirds of the risk observed in patients infected with the delta variant. However, in our cohort, patients who were infected with the omicron variant were younger and had fewer comorbidities than those infected with the delta variant. The rapid global spread of the omicron variant already challenges public health systems worldwide and, in some countries, also the health-care systems. Information on the documented severity of the omicron variant compared with the delta variant is crucial to understanding the public health consequences of the viral evolution of the omicron variant and to inform modelling studies and public health planning. Our analysis should be repeated at a later timepoint when omicron spread has affected also older age groups within the population.
Reports in late December, 2021, took previous infection or vaccination into account, but had limited follow-up for hospitalisations and included cases suspected to be omicron (eg, based on S-gene dropout analyses). A report from Imperial College London, UK, found that omicron cases had a reduced risk of emergency department attendance (hazard ratio [HR] 0·80, 95% CI 0·75–0·85) and a reduced reduced risk of hospitalisation (a stay of ≥1 nights; 0·55, 0·51–0·59).6 Another report from the UK Health Security Agency based on 431 attendances to the emergency department and 70 hospital admissions with omicron found that the risk of presentation to emergency care or hospital admission with omicron was reduced compared with delta (HR 0·62, 95% CI 0·55–0·69). The risk of hospital admission with omicron was lower than that of hospital admission with delta (0·38, 95% CI 0·30–0·50).7
A report on a nested case-control study from Scotland based on 15 suspected omicron hospitalisations suggested that omicron was associated with a lower risk of COVID-19 hospitalisation than was delta (HR 0·32, 95% CI 0·19–0·52).8 A matched cohort study from Canada reported a lower risk of hospitalisation or death for omicron relative to delta (0·41, 0·30–0·55; 53 hospitalisations and three deaths of suspected or confirmed omicron cases), but did not consider previous infection.9 A study from South Africa also on suspected omicron found a reduced risk of admission to hospital for omicron relative to other SARS-CoV-2 variants (odds ratio 0·2, 95% CI 0·1–0·3).10
During January, 2022, studies from Norway (HR 0·27, 95% CI 0·20–0·36), the USA (0·48, 0·36–0·64), the Western Cape in South Africa (0·56, 0·34–0·91), and Portugal (0·25, 0·15–0·43) reported varying risk reductions for admission to hospital based on suspected omicron cases or based on whole-genome sequencing for a small proportion of all SARS-CoV-2 cases in the period of omicron emergence.11, 12, 13, 14
Denmark has one of the highest RT-PCR testing capacities per capita in the world and until Dec 19, 2021, all positive RT-PCR tests were screened with an omicron-specific RT-PCR test, which has been validated using whole-genome sequencing.15, 16 Using information from whole-genome sequencing and national registers, we previously described an increased risk of hospitalisation associated with the alpha variant (B.1.1.7) of SARS-CoV-2 compared with the previous variants and for the delta variant compared with the alpha variant.17, 18
In this study, we aimed to estimate the risk of SARS-CoV-2-related hospital admission associated with infection with the omicron variant compared with the delta variant overall and stratified by vaccination status.
Methods
Study design and population
In this observational cohort study, we included all RT-PCR-confirmed cases of SARS-CoV-2 infection in Denmark, with samples taken between Nov 21 (date of first omicron-positive sample) and Dec 19, 2021. Omicron cases were defined as cases with a valid positive omicron-specific RT-PCR test result. All analyses were based on information from the national COVID-19 surveillance system database, which was updated and extracted on Jan 4, 2022. The study size was determined based on the available samples in the study period. This study was done with use of administrative register data. According to Danish law, ethics approval is not needed for such research.
Data sources
We obtained data from the National Microbiology Database for all individuals tested with SARS-CoV-2 by RT-PCR in Denmark since March 1, 2020,16, 19, 20 and data from other national registers, available in the national COVID-19 surveillance system database at Statens Serum Institut (SSI; Copenhagen, Denmark), described in detail elsewhere.16 Briefly, the surveillance system links individual-level information daily between national registers and databases by the use of the unique personal identification number of all Danish citizens, thereby centralising surveillance information from the National Patient Register (inpatient and outpatient diagnoses, admission and discharge dates),21 the National Vaccination Registry,22 the Civil Registration System (vital status and previous and current addresses),23 and variant-specific RT-PCR test results from the National Microbiology Database.16, 19, 20
In Denmark, health-care personnel (who are routinely tested for SARS-CoV-2) and individuals with symptoms suggestive of COVID-19 who were seen by a doctor were tested for SARS-CoV-2 by RT-PCR in regional clinics connected with the ten Danish departments of clinical microbiology (CMDs), which serve public and private hospitals and primary care clinics, as well as long-term care facilities with suspected or ongoing outbreaks. This workflow is referred to as the health-care track.
Additionally, a centralised high-throughput public COVID-19 test laboratory—the Test Center Denmark (TCDK)—was established by the end of April, 2020, at SSI. TCDK offers free RT-PCR testing to close contacts of cases, asymptomatic individuals, and those with mild COVID-19 symptoms. This workflow is referred to as the community track. All tests are offered as part of a universal tax-funded health-care system and provided free-of-charge to citizens. Test timeslots at TCDK are publicly available and can be booked online.
The Danish COVID-19 testing strategy includes testing of close contacts of confirmed cases on days 4 and 6 after exposure, and screening tests in certain settings (eg, workplaces and primary schools). Overall, around 25% of the Danish population was PCR tested at least once a week during the study period. The test capacity was very high in both workflow tracks. By Dec 26, 2021, 12·2% of the Danish population had tested positive for SARS-CoV-2 by PCR test since the start of the epidemic. The testing strategy was supplemented by an extensive antigen-testing programme with an even higher weekly test frequency, but according to national recommendations a positive antigen test must be confirmed with an RT-PCR test.
Surveillance of SARS-CoV-2 variants in Denmark is based on both extensive whole-genome sequencing and variant-specific RT-PCR testing. For surveillance, the TCDK (the community track) provides a maximum capacity of 15 000 whole-genome sequencing tests per week (when capacity is exceeded samples are picked using an algorithm that ensures representativeness). When new VOCs are detected, variant-specific RT-PCR tests are developed to track the VOC. For the omicron variant, an RT-PCR test using the L452 marker (estimated specificity 99·99% based on retrospective analysis) was developed and implemented by the TCDK by Dec 1, 2021 (hereafter known as TCDK vPCR).24 In eight of ten local CMDs (ie, the health-care track), similar omicron-specific vPCR solutions have been set up (hereafter CMD vPCR) and documented; the remaining two of ten local CMDs account for less than 7% of RT-PCR tests done by CMDs.
Samples from cases found to be RT-PCR-positive for the omicron variant, either by TCDK or CMD vPCR, were subsequently subjected to whole-genome sequencing according to capacity.
For the TCDK vPCR, we defined a confirmed omicron case as a case testing positive with RT-PCR targeting the wild type sequence L452, as described elsewhere.24 The delta variant is detected by the 452R substitution and was the only predominant variant in Denmark (>99%)25 in the period before occurrence of omicron; individuals testing positive for the 452R substitution were therefore considered to be infected with the delta VOC.26 Similar descriptions for the CMD vPCR are not described in detail in this study, but are available on request.
In the community track, more than 95% of all positive samples were screened with the TCDK omicron vPCR from Nov 24, 2021. The screening was discontinued on Dec 20, 2021, because of a rapidly increasing number of cases. Afterwards, around 2000 daily RT-PCR-positive tests were screened using an algorithm that secures representativeness. In the health-care track, a proportion of data on negative and inconclusive omicron CMD vPCR results were at the time of writing only registered in the National Microbiology Database from a subset of CMDs, whereas positive omicron results were received from all eight CMDs where omicron-specific vPCR solutions were present. Furthermore, omicron screening after Dec 20, 2021, was limited to specific patient groups. For these reasons, the study population is limited to primary samples before Dec 20, 2021.
COVID-19 hospital admission (primary outcome)
In the Danish national COVID-19 surveillance system, a COVID-19 hospital admission (ie, a hospitalisation) is identified using three different data sources. First, the National Patient Register, which includes any contact to a Danish hospital longer than 12 h, supplemented by the second data source, consisting of twice-daily data snapshots of hospitalisations in each region, as there can be several days’ delay in the National Patient Register.21 Snapshot data also include admissions shorter than 12 h on the condition that a bed is assigned to the patient. Furthermore, all samples from hospitalised patients are confirmed as SARS-CoV-2 positive in the National Microbiology Database. A COVID-19 hospitalisation is defined as a hospitalisation that occurs within 14 days of the first sample with a positive SARS-CoV-2 RT-PCR test or during an ongoing hospitalisation using the three data sources.27 As the primary outcome, we included hospitalised cases with a first positive SARS-CoV-2 RT-PCR test within 14 days before or up to 48 h after admission to hospital (hence forward denoted as a COVID-19 hospital admission or hospitalisation, or a SARS-CoV-2-related hospitalisation). We did not include cases who tested positive 48 h after admission, as these patients might have been infected at the hospital. The study included data with the latest possible admission to hospital for COVID-19 on Jan 4, 2022.
A recent study28 has validated the definition of COVID-19 hospitalisation used in the national COVID-19 surveillance system, and a script algorithm was developed that either defines the main diagnosis of the hospitalisation as a COVID-19 diagnosis (International Classification of Diseases, tenth revision codes DB342A, DB972A, DB972B, and DB948A), a respiratory diagnosis, an observational diagnosis, or other diagnoses, by using the information from the National Patient Registry when it is complete up to 2 months after the last discharge of interest. We used this script to likewise categorise diagnoses based on registry data updated on Feb 2, 2022, and for sensitivity analyses of the primary outcome, by restricting analyses to COVID-19 hospitalisations so far registered with COVID-19 diagnoses.
Covariates
We included possible confounders (covariates) suspected to be associated with severity of COVID-19 or associated with both SARS-CoV-2 infection variant and hospitalisation. The basic covariates at time of sampling were sex, age, calendar week, geographical region of sampling (capital, central Denmark, north Denmark, Zealand, or southern Denmark), comorbidities (diabetes, adiposity, cancer, neurological diseases, nephrological diseases, haematological diseases, cardiac diseases, respiratory disorder, immunological diseases, and other comorbid diseases based on the latest 5 years of admission diagnoses),29 reinfection status (previous SARS-CoV-2 infection >60 days previously, confirmed by RT-PCR as registered in the National Microbiology Database since March, 2020), and SARS-CoV-2 vaccination status at infection based on the Danish Vaccination Register30 (unvaccinated or none or only one dose: no vaccination or less than 14 days after first dose; first vaccination or less than 14 days after second dose; second vaccination: 14 days after second dose or less than 14 days after third vaccination [or booster]; third vaccination: 14 days after third dose). Most of the Danish population was vaccinated with the Cominarty (Pfizer-BioNTech; BNT162b2) vaccine as the primary dose (84%), followed by Spikevac (Moderna; mRNA-1273; 12%), Vaxzevria (Oxford-AstraZeneca; ChAdOx1; 3%), and the Johnson & Johnson (Ad26.COV2.S; JNJ-78436735; VAC31518; 1%) vaccine. Cross-vaccination with another mRNA vaccine as the second or third dose has only been recommended for those who received Vaxzevria and Johnson & Johnson as the primary dose. Reinfections were defined as an RT-PCR-positive test 60 days or more after the first RT-PCR-positive test. Additional covariates for later sensitivity analyses included test track (health-care track or community track) and ethnicity (second generation, both parents born abroad, Danish-born, or born abroad).31 Information on intensive care unit (ICU) treatment was obtained from the National Patient Register.21
Statistical analysis
We estimated the risk ratios (RRs) of SARS-CoV-2-related hospitalisation (primary outcome) by omicron variant infection compared with delta variant infection using a log-linear Poisson regression model with robust SEs using the PROC GENMOD procedure in SAS, version 9.4. Poisson regression with robust SEs was chosen as a more robust way to perform log-linear binomial regression.32 We adjusted the RRs for the basic covariates of sex, age (0–9 years, 10–19 years, 20–29 years, 30–39 years, 40–49 years, 50–59 years, 60–69 years, 70–79 years, 80–89 years, and ≥90 years), period as a continuous variable (calendar week [of 2021] of the sample date; 46+47, 48, 49, and 50), number of comorbidities in the past 5 years (0 vs ≥1), and previous SARS-CoV-2 infection (reinfection yes vs no). To evaluate collinearity and contributions to confounding from each of these a-priori decided basic covariates included in the main model, we subsequently excluded each basic covariate one at a time in a model always including variant (omicron vs delta) and keeping the remaining variables in the model. We checked for substantial changes in the parameter estimates or the SEs when excluding each basic covariate, to evaluate the contribution of each covariate to the model. To evaluate residual confounding by age in the main analysis, tighter categories of age (5-year age-groups) were used for adjustment in sensitivity analyses, and to evaluate effect modification by age an interaction term was included in the model. Associations between omicron infection and risk of hospitalisation within strata of COVID-19 vaccination status were estimated by including interaction terms in the model, and tests for differences in association between vaccination categories were done as tests for interaction. RRs for vaccinated individuals compared with non-vaccinated or one-dose vaccinated individuals with full protection more than 14 days after injection were estimated in the model used to estimate the overall RR for omicron compared with delta. All p values from the Poisson regression model with robust SEs were estimated using score tests. Differences in proportions were evaluated using χ2 tests, and differences in median age were evaluated using a Wilcoxon test. Missing values occurred for name of region of sample only and were excluded (0·5% of cases).
Role of the funding source
There was no funding source for this study. The study was done by the governmental institution SSI as a health authority task, and SSI had a role in study design, data collection, data analysis, data interpretation, and writing of the report.
Results
Between Nov 21 and Dec 19, 2021, 2722 (1·4%) of 192 610 RT-PCR primary positive individuals in Denmark were excluded because of an inconclusive omicron vPCR test, and 908 (0·5%) were excluded because of missing name of which region the positive sample was from, leaving 188 980 (98·1%) eligible for inclusion in the study (appendix p 2). 38 669 (20·5%) of 188 980 were infected with omicron (as verified for 35 904 cases by TCDK vPCR and 2765 cases by CMD vPCR), and 2435 (1·3%) were hospitalised, of whom 222 (9·1%) were infected with omicron (table 1 ). The proportion of individuals with variants other than omicron and delta was extremely low during the study period, as described elsewhere.25
Table 1.
Characteristics of 188 980 cases of RT-PCR-confirmed SARS-CoV-2 between Nov 21 and Dec 19, 2021, in Denmark
|
SARS-CoV-2 infection |
COVID-19 hospitalisation |
|||
|---|---|---|---|---|
| Omicron (N=38 669) | Delta (N=150 311) | Omicron (N=222) | Delta (N=2213) | |
| COVID-19 vaccination (status at infection)* | ||||
| None or only one dose | 7266 (18·8%) | 69 885 (46·5%) | 54 (24·3%) | 997 (45·1%) |
| Two doses | 29 922 (77·4%) | 77 441 (51·5%) | 145 (65·3%) | 1041 (47·0%) |
| Three doses | 1481 (3·8%) | 2985 (2·0%) | 23 (10·4%) | 175 (7·9%) |
| Sex | ||||
| Female | 19 432 (50·3%) | 75 723 (50·4%) | 135 (60·8%) | 1117 (50·5%) |
| Male | 19 237 (49·7%) | 74 588 (49·6%) | 87 (39·2%) | 1096 (49·5%) |
| Age, years | ||||
| Median, IQR | 29·0 (20·0–45·0) | 31·0 (11·0–48·0) | 38·5 (27·0–59·0) | 56·0 (35·0–74·0) |
| 0–9 | 2118 (5·5%) | 26 883 (17·9%) | 11 (5·0%) | 128 (5·8%) |
| 10–19 | 6783 (17·5%) | 26 865 (17·9%) | 20 (9·0%) | 71 (3·2%) |
| 20–29 | 11 187 (28·9%) | 18 689 (12·4%) | 42 (18·9%) | 177 (8·0%) |
| 30–39 | 5772 (14·9%) | 20 152 (13·4%) | 41 (18·5%) | 288 (13·0%) |
| 40–49 | 5515 (14·3%) | 23 650 (15·7%) | 31 (14·0%) | 258 (11·7%) |
| 50–59 | 4704 (12·2%) | 17 136 (11·4%) | 23 (10·4%) | 279 (12·6%) |
| 60–69 | 1845 (4·8%) | 9938 (6·6%) | 21 (9·5%) | 288 (13·0%) |
| 70–79 | 557 (1·4%) | 5451 (3·6%) | 19 (8·6%) | 396 (17·9%) |
| 80–89 | 156 (0·4%) | 1326 (0·9%) | 11 (5·0%) | 280 (12·7%) |
| ≥90 | 32 (0·1%) | 221 (0·1%) | 3 (1·4%) | 48 (2·2%) |
| Number of comorbidities | ||||
| 0 | 33 782 (87·4%) | 126 164 (83·9%) | 137 (61·7%) | 1082 (48·9%) |
| ≥1 | 4887 (12·6%) | 24 147 (16·1%) | 85 (38·3%) | 1131 (51·1%) |
| Reinfection with SARS-CoV-2 | ||||
| No | 36 527 (94·5%) | 148 425 (98·7%) | 211 (95·0%) | 2177 (98·4%) |
| Yes | 2142 (5·5%) | 1886 (1·3%) | 11 (5·0%) | 36 (1·6%) |
| Test track | ||||
| Community | 35 904 (92·8%) | 137 199 (91·3%) | 119 (53·6%) | 1133 (51·2%) |
| Health care | 2765 (7·2%) | 13 112 (8·7%) | 103 (46·4%) | 1080 (48·8%) |
Data are n (%), unless otherwise specified.
None or only one dose: tested positive and not vaccinated, or tested positive up to 14 days after first vaccination, more than 14 days after first vaccination, or up to 14 days after second vaccination (if any); two doses: tested positive more than 14 days after second vaccination and up to 14 days after third vaccination (if any); three doses: tested positive more than 14 days after third vaccination (if any).
124 313 (65·8%) of 188 980 individuals were vaccinated (any dose received), and 4028 (2·1%) were reinfected. 5398 (4·3%) of 124 313 vaccinated individuals had received a booster dose, 107 684 (86·6%) a second dose, 11 231 (9·0%) a first dose only, and all at least one dose. Most people were vaccinated with Cominarty (106 877 [86·0%]) or Spikevax (11 903 [9·6%]), and a smaller proportion with the Vaxzevria or Johnson & Johnson vaccine (5533 [4·5%]). Being infected when fully vaccinated with two or three doses was associated with an overall reduced risk of hospitalisation after infection (overall RR 0·24, 95% CI 0·22–0·26; among individuals with omicron 0·29, 0·21–0·39; among individuals with delta 0·24, 0·22–0·27) compared with being infected and unvaccinated (ie, none or only one dose). The result was similar for any dose of vaccine versus no doses.
Overall, the proportion of omicron cases increased during the study period from 14 (0·1%) in week 46 and week 47 to 32 916 (43·2%) in week 50 (appendix p 3). A higher proportion of individuals were infected with omicron than with delta after second and third vaccination, but the proportion was lower for omicron compared with delta in the unvaccinated group (table 1). Furthermore, compared with individuals with delta, individuals with omicron were younger, had less comorbidities, and were more often previously infected with SARS-CoV-2. Among hospitalised cases, a similar pattern was observed for omicron compared with delta with regards to vaccination status, age, comorbidity, and reinfection, whereas omicron was more common among women than men (table 1). Additionally, the length of stay was shorter for individuals hospitalised with omicron (median 15 h, IQR 6–45) compared with individuals hospitalised with delta (38 h, 10–124), although this was based on incomplete data on discharge truncated on Feb 2, 2022. For further characteristics of cases, (eg, numbers by sample date and admission date, and vaccination status by age, reinfection status, and length of hospital stay) see the appendix (pp 3–4).
In both crude and adjusted analyses, there was a lower risk of hospitalisation associated with omicron infection (crude RR 0·39, 95% CI 0·34–0·45; adjusted RR 0·64, 0·56–0·75; 222 [0·6%] of 38 669 omicron cases) compared with delta infection (2213 [1·5%] of 150 311 delta cases; table 2 ). Formally, by interaction test, vaccination status did not modify this association (p=0·15). Omicron was associated with a reduced RR of hospitalisation among unvaccinated individuals and those who received only one dose (0·57, 0·44–0·75), those who received two doses (0·71, 0·60–0·86), and those who received three doses (0·50, 0·32–0·76) compared with delta infection (table 2).
Table 2.
RR of hospitalisation by infection with the SARS-CoV-2 omicron variant compared with the delta variant according to COVID-19 vaccination status among 188 980 cases between Nov 21 and Dec 19, 2021, in Denmark
| Hospitalised (n=2435) | Not hospitalised (n=186 545) | Crude RR (95% CI) | p value | Adjusted RR (95% CI) | p value | ||
|---|---|---|---|---|---|---|---|
| Overall infection with SARS-CoV-2 variant | |||||||
| Delta | 2213/150 311 (1·5%) | 148 098/150 311 (98·5%) | 1 (ref) | <0·0001 | 1 (ref) | <0·0001 | |
| Omicron | 222/38 669 (0·6%) | 38 447/38 669 (99·4%) | 0·39 (0·34–0·45) | .. | 0·64 (0·56–0·75) | .. | |
| By vaccination status | |||||||
| None or only one dose | .. | .. | .. | 0·024 | .. | 0·15 | |
| Delta | 997/69 885 (1·4%) | 68 888/69 885 (98·6%) | 1 (ref) | .. | 1 (ref) | .. | |
| Omicron | 54/7266 (0·7%) | 7212/7266 (99·3%) | 0·52 (0·40–0·68) | .. | 0·57 (0·44–0·75) | .. | |
| Two doses | .. | .. | .. | .. | .. | .. | |
| Delta | 1041/77 441 (1·3%) | 76 400/77 441 (98·7%) | 1 (ref) | .. | 1 (ref) | .. | |
| Omicron | 145/29 992 (0·5%) | 29 777/29 992 (99·5%) | 0·36 (0·30–0·43) | .. | 0·71 (0·60–0·86) | .. | |
| Three doses | |||||||
| Delta | 175/2985 (5·9%) | 2810/2985 (94·1%) | 1 (ref) | .. | 1 (ref) | .. | |
| Omicron | 23/1481 (1·6%) | 1458/1481 (98·4%) | 0·26 (0·17–0·41) | .. | 0·50 (0·32–0·76) | .. | |
Data are n/N (%), unless otherwise indicated. p values in the stratified analysis are tests for interaction terms. Adjusted RRs were adjusted for the basic (a priori) covariates of sex, age (10-year groups), sample period as a continuous variable (week 46+47, 48, 49, or 50), region (capital, central Denmark, north Denmark, Zealand, or southern Denmark), comorbidities in the preceding 5 years (none vs one or more), and previous SARS-CoV-2 infection more than 60 days earlier confirmed by RT-PCR. RR=risk ratio.
We considered possible waning of vaccine effect over time by stratifying the RR for hospital admission of omicron relative to delta by time since vaccination with two doses, and by time since vaccination with three doses. The RR for omicron hospitalisation with 4 months or less since dose two was higher (RR 1·20, 95% CI 0·88–1·65) than the RR for omicron hospitilisation with more than 4 months since dose two (0·59, 0·47–0·73; p=0·0071). For time since dose three, the RR with 2 months or less since dose three tended to be lower (0·43, 0·24–0·76) than the RR with 2 months or more since dose three (0·66, 0·34–1·27; p=0·21).
To evaluate to what degree the basic covariates chosen a priori confounded the observed reduction in RR for hospitalisation for omicron compared with delta, we excluded each adjustment covariate one at a time. Only age had a strong confounding effect on the main estimate (appendix p 5). This analysis was also done with stratification for vaccination status (none or one dose, two doses, or three doses).
In sensitivity analyses presented in the appendix (pp 6–7), we observed that the main estimates in table 2 were not substantially affected by alternative adjustment for age. However, for stratification by age in four groups, the RR for hospitalisation with omicron for individuals aged 0–19 years was 1·59 (95% CI 1·09–2·32) compared with hospitalisation for delta in the same age group. Since the incidence of SARS-CoV-2 in this period increased specifically among those aged 0–2 years,33 we stratified this RR further by age in groups of 0–2 years, 3–11 years, 12–15 years, and 16–19 years, and observed RRs of 1·18 (95% CI 0·59–2·33), 0·90 (0·36–2·23), 2·48 (0·98–2·33), and 1·26 (0·62–2·53), respectively. In the largest age group of 20–39 years, the RR among non-vaccinated individuals was 0·53 (0·36–0·80) and thus compatible with the main estimate for the unvaccinated group (and those who received one dose only; 0·57, 0·44–0·75; appendix p 5). Among unvaccinated individuals without reinfection there was also no substantially different RR compared with the main estimate (0·57, 0·43–0·76, 48 omicron hospitalisations), whereas numbers were small among unvaccinated individuals with reinfection (0·80, 0·31–2·03, six omicron hospitalisations).
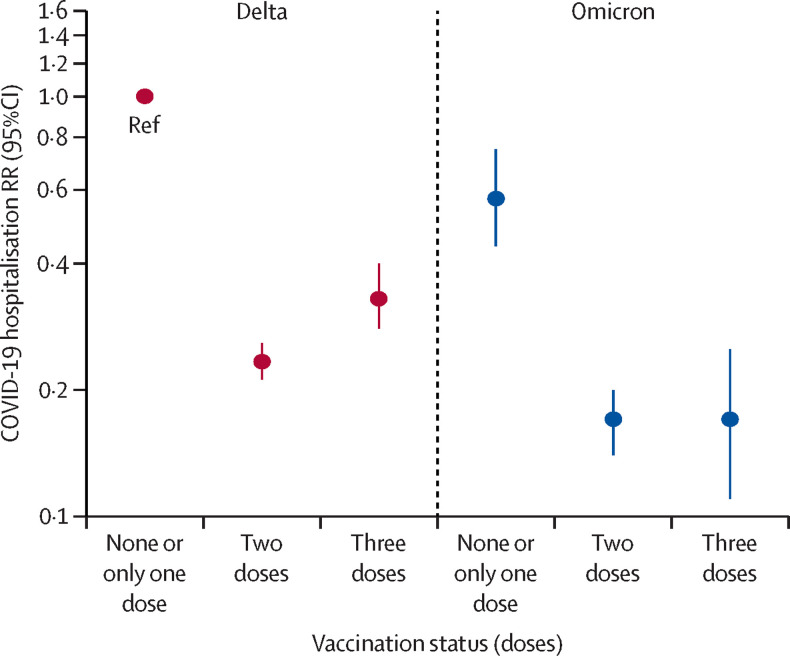
Because the analysis stratified by vaccine status in table 2 is restricted to show an analysis of the difference between delta variant and omicron variant hospitalisation risk within each vaccination group, we also did the analysis by comparing hospitalisation risk across vaccination groups, with delta infections in unvaccinated individuals as a reference (table 3 , figure ). The entire group of vaccinated individuals had a lower risk of hospitalisation compared with the unvaccinated group with delta infections. Among unvaccinated people (and those who received only one dose), the risk of hospitalisation was lower for omicron infection compared with delta infection (RR 0·57, 95% CI 0·44–0·75). The risk was even lower for omicron infection after second (0·17, 0·14–0·20) or third vaccination (0·17, 0·11–0·25), as it was for delta infection after second (0·23, 0·21–0·26) or third vaccination (0·33, 0·28–0·40), compared with unvaccinated individuals with delta infection. In the first 2 months after third vaccination, the RR for hospitalisation after delta infection was 0·30 (0·25–0·37) and after 2 months was 0·42 (0·32–0·54; data not shown).
Table 3.
RR of hospitalisation due to infection with SARS-CoV-2 omicron variant according to COVID-19 vaccination status, with unvaccinated individuals with delta infection as a reference, among 188 980 cases between Nov 21 and Dec 19, 2021, in Denmark
|
Not hospitalised |
Hospitalised |
Crude RR |
Adjusted RR |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Delta (n=148 098) | Omicron (n=38 447) | Delta (n=2213) | Omicron (n=222) | Delta | Omicron | p value | Delta | Omicron | p value | |
| None or one dose | 68 888/76 100 (90·5%) | 7212/76 100 (9·5%) | 997/1051 (94·9%) | 54/1051 (5·1%) | 1 (ref) | 0·52 (0·40–0·68) | <0·0001 | 1 (ref) | 0·57 (0·44–0·75) | <0·0001 |
| Two doses | 76 400/106 177 (72·0%) | 29 777/106 177 (28·0%) | 1041/1186 (87·8%) | 145/1186 (12·2%) | 0·94 (0·86–1·03) | 0·34 (0·29–0·40) | .. | 0·23 (0·21–0·26) | 0·17 (0·14–0·20) | .. |
| Three doses | 2810/4268 (65·8%) | 1458/4268 (34·2%) | 175/198 (88·4%) | 23/198 (11·6%) | 4·11 (3·51–4·81) | 1·09 (0·72–1·64) | .. | 0·33 (0·28–0·40) | 0·17 (0·11–0·25) | .. |
Data are n/N (%) or RR (95% CI). p values in the stratified analysis are tests for interaction terms. Adjusted RRs were adjusted for the basic (a priori) covariates sex, age (10-year groups), sample period as a continuous variable (week 46+47, 48, 49, or 50), region (capital, central Denmark, north Denmark, Zealand, or southern Denmark), comorbidities in the preceding 5 years (none vs one or more), and previous SARS-CoV-2 infection more than 60 days earlier. RR=risk ratio.
Figure.
RR of hospitalisation by infection with the SARS-CoV-2 omicron variant according to COVID-19 vaccination status, with unvaccinated individuals with delta infection as the reference, among 188 980 cases between Nov 21 and Dec 19, 2021, in Denmark.
Ref indicates the reference group. RR=risk ratio.
We also had information on ICU treatment and observed that six (2·7%) of 222 hospitalised omicron cases had been in ICU treatment versus 180 (8·1%) of 2213 hospitalised delta cases. Two (4·9%) of 41 patients with omicron infection who tested positive more that 48 h after admission to hospital (ie, otherwise defined as non-hospitalised cases in all analyses) had been treated in the ICU versus 23 (8·2%) of 281 patients with delta infection. Additional analyses were not done for the ICU data due to restricted statistical power.
Discussion
In this study, we found that infection with the SARS-CoV-2 omicron variant was associated with an adjusted RR of hospital admission of 0·64 (95% CI 0·56–0·75) compared with that of delta infection. This finding is in line with studies from the UK, Scotland, South Africa, Canada, Norway, the USA, and Portugal, as well as experimental studies,34, 35 and suggests that omicron is associated with an inherently lower risk of severe disease (and admission to hospital) compared with delta. This reduced risk was observed across unvaccinated people (and those who received only one vaccine dose), as well as individuals who received two and three doses of vaccine.
The lower risk of hospitalisation among individuals with omicron infection was less pronounced in the population that had received two doses of vacine than among non-vaccinated individuals and those who had received one vaccination only. This finding is probably explained by a higher and longer lasting protection against hospitalisation for the delta variant (the reference group) than omicron after two doses of vaccine, as vaccine effectiveness has been reported to decline at a faster rate for omicron compared with delta after the second vaccine dose.36 This interpretation is further supported by the difference (although not statistically significant) in hospital admission in the first 4 months after the second dose of vaccine, which could be ascribed to the fact that the vaccine masks the severity after delta infection more than after omicron infection. This point is also supported by an analysis by Ferguson and colleagues showing that after two doses of Cominarty there was no difference in hospitalisation risk for delta infection compared with omicron infection.6 However, with a longer follow-up period, the reduced severity associated with omicron infection becomes apparent in the population of double-vaccinated individuals.6 Furthermore, the notion of reduced severity associated with omicron infection is supported by our crude observations of a shorter length of stay and a reduced probability of being transferred to the ICU.
When we used the unvaccinated population of individuals with delta infection as a reference group, we found a reduced risk of hospitalisation among vaccinated people for both delta and omicron infection. Similarly, among people with omicron, vaccination also reduced the risk of hospitalisation, which underlines the importance of continued rollout of vaccination programmes to mitigate the public health impact of SARS-CoV-2.
Omicron has spread rapidly worldwide and is the dominant variant in many countries, including Denmark, the UK, and South Africa (>90% of cases).37, 38, 39 In two risk assessments related to the spread of omicron, the European Centre for Disease Prevention and Control (ECDC) stated that a multi-layered approach is needed to reduce the impact of omicron, comprising both increased and timely uptake of booster vaccination coupled with non-pharmaceutical interventions.40, 41 The ECDC recommended on Dec 15, 2021, that immediate planning should be considered to increase health-care capacity to treat the expected higher number of hospitalised cases,40 while with later observed staff shortages in the ongoing omicron waves, emphasis is also on the importance of prevention and control of infections in health-care settings, to protect people with underlying heath conditions.41 To assess the impact of such measures, comparative data on the severity of omicron relative to delta are crucial. Our finding of an intrinsic lower risk of hospitalisation for omicron compared with delta is consistent with studies from other countries. However, even if the risk of hospitalisation is lower for omicron compared with delta, the extensive spread due to higher transmissibility could rapidly outweigh any potential benefits of reduced severity, and raise questions over whether omicron represents an important step towards the end of the pandemic. Nevertheless, it is reassuring that we found a lower risk of hospitalisation with omicron among individuals who had received both second and booster vaccine doses.
The strengths of the present study include access to national health registers and SARS-CoV-2 laboratory data. Denmark has one of the highest PCR test capacities in the world, with a weekly testing rate increasing from 215 per 1000 people in calendar week 47 to 270 per 1000 people in calendar week 50, 2021. Until Dec 19, 2021, the aim was to screen all RT-PCR-positive tests with the omicron-specific vRT-PCR (eg, >95% of samples in the community track were screened). The PCR testing capacity is complemented by extensive antigen testing with around a million additional weekly tests in week 47 increasing to 1·7 million in week 50. The extensive testing scheme in Denmark leads to a low proportion of undetected cases. Repeated seroprevalence studies in Denmark have estimated that the number of undetected cases per detected case decreased from seven to eight in August, 2020, to less than one in June, 2021,42 and there was an increase in seroprevalence among unvaccinated individuals from 2·2% to 8·6%.
Our study has some limitations. We did not have access to complete results from omicron vPCR tests for RT-PCR-positive cases in the health-care track; however, we assume these are delta infections because eight of the ten CMDs, covering more than 93% of all samples, confirmed that they screened all RT-PCR positives and reported all omicron-positive cases to the surveillance system during the period. If omicron is underreported among hospitalised individuals, we could theoretically underestimate the hospitalisation risk of omicron relative to delta.
Some patients might be admitted to hospital with and not because of SARS-CoV-2 infection, as all acutely admitted patients are screened for SARS-CoV-2 infection on admission. Such misclassification of our outcome could differ for omicron versus delta, especially if there is increased community transmission because of omicron. In a first sensitivity analysis, we excluded patients who did not have COVID-19-specific diagnoses, and in a second, we excluded cases with admission to hospital shorter than 12 h, which might be more likely to be associated with less severe disease that is not associated with COVID-19. Both of these analyses produced estimates similar to that of the main analysis. More studies are needed to describe the clinical course of patients admitted to hospital with omicron infection compared with delta infection.
Furthermore, in the first 2 weeks after the first detected case of omicron in Denmark, intensified contact tracing of confirmed omicron cases was done, where all close contacts and close contacts of close contacts were isolated and recommended to be tested on day 4 and day 6 after potential exposure, regardless of vaccination status. This strategy could result in more asymptomatic or milder cases being detected for omicron compared with delta. However, by far, most omicron cases were detected in the later period of the study, and when restricting our analysis to this period (Dec 5–19, 2021) similar results were observed (appendix p 6), which mean this is unlikely to have affected the results.
We observed a different age distribution among omicron cases and delta cases, with a relatively higher proportion of omicron cases in those aged 20–29 years, and a lower proportion of cases in those aged 0–9 years and 60–79 years compared with delta cases. All Danish schoolchildren were home schooled from Dec 15, 2021, which might have prevented omicron transmission among school-aged children. We expect the reason for the high proportion of omicron-infected young adults to be a combination of contact patterns, risk behaviour, and waning vaccine immunity towards omicron, as most of this age group in Denmark received only two doses of vaccine, with the second dose received by the end of August, 2021. As both vaccination status and hospitalisation risk are strongly associated with age, we considered this an important confounder in the study. In fact, our evaluation of confounding suggests other considered factors than age had smaller confounding effects. Furthermore, we adjusted for age using 10-year age groups, but found similar results when adjusting for age in 5-year age groups or age as a continuous variable. Taken together, these additional results do not support that our results are strongly confounded.
We found a higher proportion of reinfections among omicron cases compared with delta cases, probably because of immune escape. This theory is in reasonable agreement with UK weekly reinfection rates of three to four versus one for omicron versus delta up to calendar week 48 in 2021.43 According a UK report, reinfection was associated with a 55–70% reduction in hospitalisation risk compared with primary infection.7 If reinfections are undetected among omicron infections, this could bias the results towards a lower risk of hospitalisation among omicron cases. Given the high testing rate throughout the COVID-19 pandemic in Denmark, combined with high vaccination coverage and a strategy in which all close contacts of confirmed cases have been recommended to get PCR tested on days 4 and 6, regardless of vaccination status, we believe Denmark has a lower rate of undetected cases than other countries. We adjusted for reinfection in the analyses, and in sensitivity analyses we excluded all reinfections and also stratified by reinfections among all individuals and among unvaccinated (and those who received one dose of vaccine) individuals only, and found similar results for all these analyses. These results, in combination with the likely low number of undetected cases in Denmark, suggest that our results cannot be explained by more undetected reinfections among omicron cases.42
In conclusion, our analysis shows that omicron infection is associated with a reduced risk of hospitalisation compared with delta infection in the unvaccinated population (and among those who have received one dose of vaccine only). This finding supports that omicron has an inherently lower severity compared with delta in immunologically naive individuals. A similar difference was observed in the vaccinated population. We observed a substantially reduced risk of hospitalisation for individuals with omicron infection after both the second and third vaccination dose compared with unvaccinated individuals with delta infection, and with unvaccinated individuals with omicron infection. These findings underline the importance of continued rollout of vaccination programmes during the ongoing omicron wave to mitigate risks of high hospitalisation burden. Our findings support hospital preparedness and modelling of the projected impact of ongoing waves and the spread of omicron globally.
Data sharing
The datasets analysed in this study can be found in the Danish National COVID-19 Surveillance System database at Statens Serum Institut (Copenhagen, Denmark), which is available for research with permission from the Danish Data Protection Agency and Danish Health Data Authority.
To request data access see https://sundhedsdatastyrelsen.dk/da/forskerservice
Declaration of interests
We declare no competing interests.
Contributors
All authors contributed to either the conception and design of the study, acquisition of data, or data analysis and interpretation. All authors had access to the underlying data and PB, JW, AC (from the study group), KS, EM, SG (from the study group), and MV verified all data. TGK, PB, and JW drafted the manuscript and all authors provided critical revisions and final approval for the decision to submit for publication. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Contributor Information
the Omicron-Delta study group:
Sofie Marie Edslev, Raphael Niklaus Sieber, Anna Cäcilia Ingham, Maria Overvad, Mie Agermose Gram, Frederikke Kristensen Lomholt, Louise Hallundbæk, Caroline Hjorth Espensen, Sophie Gubbels, Marianne Karakis, Karina Lauenborg Møller, Stefan Schytte Olsen, Zitta Barrella Harboe, Caroline Klint Johannesen, Maarten van Wijhe, Jon Gitz Holler, Ram Benny Christian Dessau, Martin Barfred Friis, David Fuglsang-Damgaard, Mette Pinholt, Thomas Vognbjerg Sydenham, John Eugenio Coia, Ea Sofie Marmolin, Anders Fomsgaard, Jannik Fonager, Morten Rasmussen, and Arieh Cohen
Supplementary Material
References
- 1.WHO Classification of omicron (B.1.1.529): SARS-CoV-2 variant of concern. Nov 26, 2021. https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1.1.529)-sars-cov-2-variant-of-concern
- 2.Espenhain L, Funk T, Overvad M, et al. Epidemiological characterisation of the first 785 SARS-CoV-2 omicron variant cases in Denmark, December 2021. Euro Surveill. 2021;26 doi: 10.2807/1560-7917.ES.2021.26.50.2101146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.UK Health Security Agency SARS-CoV-2 variants of concern and variants under investigation in England. Technical briefing 31. Dec 10, 2021. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1042367/technical_briefing-31-10-december-2021.pdf
- 4.Pulliam JRC, van Schalkwyk C, Govender N, et al. Increased risk of SARS-CoV-2 reinfection associated with emergence of omicron in South Africa. medRxiv. 2022 doi: 10.1101/2021.11.11.21266068. published online March 6. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abdullah F, Myers J, Basu D, et al. Decreased severity of disease during the first global omicron variant COVID-19 outbreak in a large hospital in Tshwane, South Africa. Int J Infect Dis. 2022;116:38–42. doi: 10.1016/j.ijid.2021.12.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferguson N, Ghani A, Hinsley W, Volz E. Report 50—hospitalisation risk for omicron cases in England. Dec 22, 2021. https://www.imperial.ac.uk/mrc-global-infectious-disease-analysis/covid-19/report-50-severity-omicron/
- 7.UK Health Security Agency SARS-CoV-2 variants of concern and variants under investigation in England. Technical briefing 33. Dec 23, 2021. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1043807/technical-briefing-33.pdf
- 8.Sheikh A, Kerr S, Woolhouse M, Mcmenamin J, Robertson C. Severity of omicron variant of concern and vaccine effectiveness against symptomatic disease: national cohort with nested test negative design study in Scotland. Dec 22, 2021. https://www.research.ed.ac.uk/en/publications/severity-of-omicron-variant-of-concern-and-vaccine-effectiveness- [DOI] [PMC free article] [PubMed]
- 9.Ulloa AC, Buchan SA, Daneman N, Brown KA. Estimates of SARS-CoV-2 omicron variant severity in Ontario, Canada. JAMA. 2022 doi: 10.1001/jama.2022.2274. published online Feb 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolter N, Jassat W, Walaza S, et al. Early assessment of the clinical severity of the SARS-CoV-2 omicron variant in South Africa. medRxiv. 2021 doi: 10.1101/2021.12.21.21268116. published online Dec 21. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Veneti L, Bøås H, Bråthen Kristoffersen A, et al. Reduced risk of hospitalisation among reported COVID-19 cases infected with the SARS-CoV-2 omicron BA.1 variant compared with the delta variant, Norway, December 2021 to January 2022. Euro Surveill. 2022;27 doi: 10.2807/1560-7917.ES.2022.27.4.2200077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewnard JA, Hong VX, Patel MM, Kahn R, Lipsitch M, Tartof SY. Clinical outcomes among patients infected with omicron (B.1.1.529) SARS-CoV-2 variant in southern California. medRxiv. 2022 doi: 10.1101/2022.01.11.22269045. published online March 7. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hussey H, Davies M-A, Heekes A, et al. Assessing the clinical severity of the omicron variant in the Western Cape Province, South Africa, using the diagnostic PCR proxy marker of RdRp target delay to distinguish between omicron and delta infections—a survival analysis. medRxiv. 2022 doi: 10.1101/2022.01.13.22269211. published online Jan 14. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peralta-Santos A, Rodrigues EF, Moreno J, et al. Omicron (BA.1) SARS-CoV-2 variant is associated with reduced risk of hospitalization and length of stay compared with delta (B.1.617.2) medRxiv. 2022 doi: 10.1101/2022.01.20.22269406. published online Jan 25. (preprint). [DOI] [Google Scholar]
- 15.Our World in Data World map: total tests performed relative to the size of the population. https://ourworldindata.org/coronavirus-testing#world-map-total-tests-performed-relative-to-the-size-of-population
- 16.Schønning K, Dessau RB, Jensen TG, et al. Electronic reporting of diagnostic laboratory test results from all healthcare sectors is a cornerstone of national preparedness and control of COVID-19 in Denmark. APMIS. 2021;129:438–451. doi: 10.1111/apm.13140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bager P, Wohlfahrt J, Fonager J, et al. Risk of hospitalisation associated with infection with SARS-CoV-2 lineage B.1.1.7 in Denmark: an observational cohort study. Lancet Infect Dis. 2021;21:1507–1517. doi: 10.1016/S1473-3099(21)00290-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bager P, Wohlfahrt J, Rasmussen M, Albertsen M, Krause TG. Hospitalisation associated with SARS-CoV-2 delta variant in Denmark. Lancet Infect Dis. 2021;21 doi: 10.1016/S1473-3099(21)00580-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Voldstedlund M, Haarh M, Mølbak K. The Danish Microbiology Database (MiBa) 2010 to 2013. Euro Surveill. 2014;19 doi: 10.2807/1560-7917.es2014.19.1.20667. [DOI] [PubMed] [Google Scholar]
- 20.Statens Serum Institut MiBa, HAIBA og det digitale infektionsberedskab. Nov 5, 2021. https://www.ssi.dk/sygdomme-beredskab-og-forskning/miba-haiba-og-det-digitale-infektionsberedskab
- 21.Lynge E, Sandegaard JL, Rebolj M. The Danish National Patient Register. Scand J Public Health. 2011;39(suppl):30–33. doi: 10.1177/1403494811401482. [DOI] [PubMed] [Google Scholar]
- 22.Statens Serum Institut Det Danske Vaccinationsregister (DDV) https://www.ssi.dk/vaccinationer/boernevaccination/vaccinationsdaekning-og-aarsraporter/det-danske-vaccinationsregister-ddv
- 23.Pedersen CB. The Danish Civil Registration System. Scand J Public Health. 2011;39(suppl):22–25. doi: 10.1177/1403494810387965. [DOI] [PubMed] [Google Scholar]
- 24.Spiess K, Gunalan V, Marving E, et al. Rapid surveillance platforms for key SARS-CoV-2 mutations in Denmark. medRxiv. 2021 doi: 10.1101/2021.10.25.21265484. published online Oct 26. (preprint). [DOI] [Google Scholar]
- 25.Danish Covid-19 Genome Consortium Genomic overview of SARS-CoV-2 in Denmark. https://www.covid19genomics.dk/statistics
- 26.Global Initiative on Sharing of Avian Influenza Data (GISAID) GISAID—Initiative. https://www.gisaid.org/ [DOI] [PMC free article] [PubMed]
- 27.Statens Serum Institut Notat om kategorisering af covid-19-relaterede indlaeggelser. 2021. https://covid19.ssi.dk/datakilder-og-definitioner
- 28.Statens Serum Institut Fokusrapport om COVID-19-relaterede hospitalsindlæggelser under SARS-CoV-2-epidemien den 6. januar 2022. https://covid19.ssi.dk/analyser-og-prognoser/fokusrapporter
- 29.Emborg HD, Krause TG, Nielsen L, et al. Influenza vaccine effectiveness in adults 65 years and older, Denmark, 2015/16—a rapid epidemiological and virological assessment. Euro Surveill. 2016;21 doi: 10.2807/1560-7917.ES.2016.21.14.30189. [DOI] [PubMed] [Google Scholar]
- 30.Grove Krause T, Jakobsen S, Haarh M, Mølbak K. The Danish vaccination register. Euro Surveill. 2012;17:2. doi: 10.2807/ese.17.17.20155-en. [DOI] [PubMed] [Google Scholar]
- 31.Statens Serum Institut Status på COVID-19-smitte blandt etniske minoriteter i Danmark. 2020. https://www.ssi.dk/aktuelt/nyheder/2020/status-pa-covid-19-smitte-blandt-etniske-minoriteter-i-danmark
- 32.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162:199–200. doi: 10.1093/aje/kwi188. [DOI] [PubMed] [Google Scholar]
- 33.Statens Serum Institut COVID-19 Dashboard. https://experience.arcgis.com/experience/aa41b29149f24e20a4007a0c4e13db1d
- 34.Halfmann PJ, Iida S, Iwatsuki-Horimoto K, et al. SARS-CoV-2 omicron virus causes attenuated disease in mice and hamsters. Nature. 2022;603:687–692. doi: 10.1038/s41586-022-04441-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shuai H, Chan JF-W, Hu B, et al. Attenuated replication and pathogenicity of SARS-CoV-2 B.1.1.529 omicron. Nature. 2022;603:693–699. doi: 10.1038/s41586-022-04442-5. [DOI] [PubMed] [Google Scholar]
- 36.Gardner BJ, Kilpatrick AM. Estimates of reduced vaccine effectiveness against hospitalization, infection, transmission and symptomatic disease of a new SARS-CoV-2 variant, omicron (B.1.1.529), using neutralizing antibody titers. medRxiv. 2021 doi: 10.1101/2021.12.10.21267594. published online Dec 12. [DOI] [Google Scholar]
- 37.Statens Serum Institut Rapport om omikronvarianten. Jan 7, 2022. https://files.ssi.dk/covid19/omikron/statusrapport/rapport-omikronvarianten-07012022-27nk
- 38.UK Health Security Agency Omicron daily overview. 31 December 2021. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1044522/20211231_OS_Daily_Omicron_Overview.pdf
- 39.Global Initiative on Sharing of Avian Influenza Data (GISAID) GISAID—hCov19 variants. https://www.gisaid.org/hcov19-variants/
- 40.European Centre for Disease Prevention and Control Assessment of the further emergence of the SARS-CoV-2 omicron VOC in the context of the ongoing delta VOC transmission in the EU/EEA, 18th update. Dec 15, 2021. https://www.ecdc.europa.eu/en/publications-data/covid-19-assessment-further-emergence-omicron-18th-risk-assessment
- 41.European Centre for Disease Prevention and Control Assessment of the further spread and potential impact of the SARS-CoV-2 omicron variant of concern in the EU/EEA, 19th update. Jan 27, 2022. https://www.ecdc.europa.eu/en/publications-data/covid-19-omicron-risk-assessment-further-emergence-and-potential-impact
- 42.Statens Serum Institut Covid-19: Den Nationale Prævalensundersøgelse. July 21, 2021. https://covid19.ssi.dk/-/media/cdn/files/praevalensundersoegelse_runde5.pdf?la=da
- 43.UK Health Security Agency Weekly national influenza and COVID-19 surveillance report. Week 1 report (up to week 52 data) Jan 6, 2022. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1045027/Weekly_Flu_and_COVID-19_report_w1.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analysed in this study can be found in the Danish National COVID-19 Surveillance System database at Statens Serum Institut (Copenhagen, Denmark), which is available for research with permission from the Danish Data Protection Agency and Danish Health Data Authority.
To request data access see https://sundhedsdatastyrelsen.dk/da/forskerservice