Abstract
The pharmacokinetics of the antifungal echinocandin-lipopeptide caspofungin (MK-0991) in plasma were studied in groups of three healthy rabbits after single and multiple daily intravenous administration of doses of 1, 3, and 6 mg/kg of body weight. Concentrations were measured by a validated high-performance liquid chromatography method and fitted into a three-compartment open pharmacokinetic model. Across the investigated dosage range, caspofungin displayed dose-independent pharmacokinetics. Following administration over 7 days, the mean peak concentration in plasma (Cmax) ± standard error of the mean increased from 16.01 ± 0.61 μg/ml at the 1-mg/kg dose to 105.52 ± 8.92 μg/ml at the 6-mg/kg dose; the mean area under the curve from 0 h to infinity rose from 13.15 ± 2.37 to 158.43 ± 15.58 μg · h/ml, respectively. The mean apparent volume of distribution at steady state (Vdss) was 0.299 ± 0.011 liter/kg at the 1-mg/kg dose and 0.351 ± 0.016 liter/kg at the 6-mg/kg dose (not significant [NS]). Clearance (CL) ranged from 0.086 ± 0.017 liter/kg/h at the 1-mg/kg dose to 0.043 ± 0.004 liter/kg/h at the 6-mg/kg dose (NS), and the mean terminal half-life was between 30 and 34 h (NS). Except for a trend towards an increased Vdss, there were no significant differences in pharmacokinetic parameters in comparison to those after single-dose administration. Caspofungin was well tolerated, displayed linear pharmacokinetics that fit into a three-compartment pharmacokinetic model, and achieved sustained concentrations in plasma that were multiple times in excess of reported MICs for susceptible opportunistic fungi.
Caspofungin (MK-0991) is a novel, investigational parenteral antifungal agent that belongs to a new generation of semisynthetic cyclic lipopeptides of the echinocandin family. It acts by noncompetitive inhibition of the synthesis of 1, 3-β-d-glucan, an essential homopolysaccharide in the cell wall of many pathogenic fungi (11, 13). Similar to other current investigational echinocandin derivatives, caspofungin has potent and fungicidal in vitro activity against most clinically relevant Candida species without cross-resistance to currently approved antifungal agents, and it has cell-wall damaging effects on several Aspergillus species (3, 6, 8, 9, 15, 16, 20). The drug has demonstrated very promising activity in infection models of oropharyngeal (A. M. Flattery, G. K. Abruzzo, J. G. Smith, C. J. Gill, H. Rosen, H. Kropp, and K. Bartizal, Abstr. 36th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-40, 1996) and disseminated (1, 10; K. Bartizal, J. G. Smith, C. J. Gill, A. M. Flattery, L. Kong, C. Leighton, J. Stone, D. Cylc, A. Yuan, and G. K. Abruzzo, Abstr. 36th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-80, 1997) candidiasis and significantly prolonged the survival in mouse models of disseminated (1; K. Bartizal et al., Abstr. 36th Intersci. Conf. Antimicrob. Agents Chemother.) and pulmonary (E. M. Bernard, T. Ishimaru, and D. Armstrong, Abstr. 36th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-39, 1996) aspergillosis, both in healthy and immunocompromised animals, respectively. In addition to its antifungal activity, caspofungin was also effective as a preventive and therapeutic modality against Pneumocystis carinii pneumonia in dexamethasone-immunocompromised rodents (19). Little is known, however, about the pharmacokinetics of caspofungin. The purpose of this study was to characterize the pharmacokinetics of caspofungin in plasma in the rabbit, to compare them with those of other echinocandins, and to provide the basis for the investigation of the concentration-response relationships of caspofungin in experimental rabbit models of invasive fungal infections.
Study drug.
Caspofungin (MK-0991; Merck & Co., Rahway, N.J.) was provided as a lyophilized powder and dissolved according to the recommendations of the manufacturer in sterile water to produce a 50-mg/ml stock solution that was maintained at −70° C. Prior to use, the drug was freshly diluted with sterile water to 10-, 5-, and 2-mg/ml solutions for the 6-, 3-, and 1 mg/kg dosage group, respectively. The reconstituted drug was administered at ambient temperature as a slow intravenous bolus over 1 min through the indwelling catheter.
Animals.
Healthy female New Zealand White rabbits (Hazleton, Denver, Pa.) weighing 2.5 to 3.5 kg were used in all experiments. They were individually housed and maintained with water and standard rabbit feed ad libitum according to National Institutes of Health Guidelines for Laboratory Animal Care (4). Vascular access was established in each rabbit ≥72 h prior to experimentation by the surgical placement of a subcutaneous silastic central venous catheter as previously described (21).
Single-dose studies.
Three groups of three rabbits were studied by single-dose studies. Animals received caspofungin at either 1, 3, or 6 mg/kg of body weight as a single steady intravenous bolus over 1 min. Plasma samples were drawn immediately before administration, immediately after administration (maximum concentration of drug in plasma [Cmax]), and then at 10 and 30 min and 1, 2, 4, 8, 12, 18, 24, 48, 72, and 96 h postdosing.
Multiple-dose studies.
A different set of three groups of three rabbits were studied by multiple-dose studies. Animals received caspofungin at either 1, 3, or 6 mg/kg of body weight daily as an intravenous bolus over 1 min for a total of seven doses. Plasma samples were drawn immediately before administration of the seventh dose, immediately after administration (Cmax), and then at 10 and 30 min and 1, 2, 4, 8, 12, 18, 24, 48, 72, and 96 h postdosing. Hepatic and renal toxicities were monitored in plasma 24 h after the last drug dose and compared to normal values. All animals were clinically evaluated each day and weighed before the first dose and at the end of the study.
Processing of blood samples.
Blood samples were collected in heparinized syringes. Plasma was immediately separated by centrifugation and stored at −80°C until shipment in dry ice to the laboratories of Merck, Sharp & Dohme-Chibret, Riom, France, for assay.
Analytical method.
Drug levels in plasma were determined after solid-phase extraction and dilution in mobile phase by reversed-phase high-performance liquid chromatography. The mobile phase consisted of acetonitrile–0.01 M KH2PO4 (60:40, vol/vol), pH 3. Separation was achieved using a Brownlee Cyano column (220 by 4.6 mm [inner diameter]; particle size, 5 μm; Perkin-Elmer, Norwalk, Conn.). Caspofungin was detected by fluorometric detection (excitation at 224 nm; emission at, 302 nm).
Quantitation was based on an internal standard method using the semisynthetic echinocandin L-733,560 as the internal standard. Eight-point standard curves (range of concentrations: 0.15 to 10 μg/ml) were linear with r2 values greater then 0.998. The lower limit of quantification (LLQ) was 0.15 μg/ml. Accuracies were within ±15% and intra- and interday variability (precision) was <5%.
Pharmacokinetic data analysis.
Pharmacokinetic parameters for caspofungin were determined using compartmental analysis. Experimental plasma concentration-time data were fitted to a three-compartment open model with intravenous bolus input and linear first-order elimination from the central compartment using iterative weighted nonlinear least-squares regression in the Adapt II (5) computer program. Model selection was guided by Akaike's information criterion (23). The model fit the data well, with r2 values for the individual fits ranging from 0.974 to 0.999 (mean, 0.991). The regression lines through the plot of observed versus estimated concentrations did not differ from the line of identity, and no bias was observed. Cmax values were determined as model-estimated concentrations immediately after bolus administration, and AUC0–∞ values were calculated from estimated plasma concentration profiles using the trapezoidal rule and extrapolation to infinity by standard techniques. Dose linearity after single and after multiple dosing was determined by comparison of the dose-normalized area under the curve from 0 h to infinity (AUC0–∞) across dosage levels by analysis of variance (ANOVA) and linear regression analysis. Accumulation was assessed for each dosage level by comparing the mean AUC between doses after multiple dosing as an approximation of AUC between doses at steady state with the mean AUC0–∞ after single dosing.
Statistical analysis.
Differences between the means of pharmacokinetic parameters across dosage levels were evaluated by ANOVA with Bonferroni's correction for multiple comparisons. Student's or Welch's t test was used in addition for comparison of pharmacokinetic parameters after single dosing with those after multiple dosing. A two-tailed P value of <0.05 was considered statistically significant.
Pharmacokinetics in plasma.
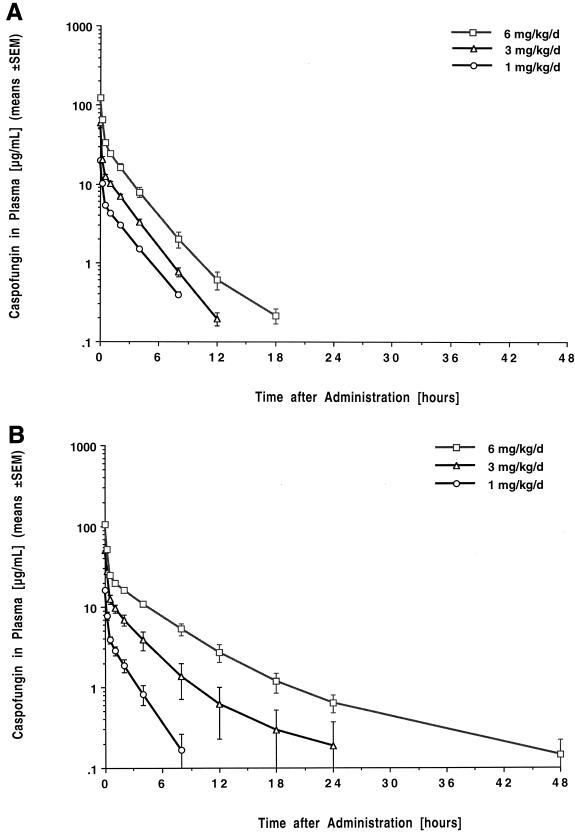
The estimated plasma concentration-versus-time profiles of caspofungin are shown in Fig. 1, and the corresponding mean compartmental pharmacokinetic parameters are listed in Table 1. Administration of caspofungin at single doses of 1, 3, and 6 mg/kg resulted in escalating peak levels in plasma that ranged from 20.02 ± 1.18 to 123.4 ± 5.17 μg/ml (means ± standard errors of the means). The drug exhibited a rapid initial distribution phase, followed by a second, somewhat slower distribution-elimination phase, and a prolonged elimination phase with a mean terminal half-life ranging from 26 to 31 h. Mean plasma levels fell below LLQ in a dose-dependent manner after 8, 12, and 18 h postdosing. Caspofungin demonstrated linear pharmacokinetics in plasma with no changes in dose-normalized AUC0–∞ or total clearance (CL) across the investigated dosage range. The apparent volume of distribution at steady state (Vss) was comparatively small and independent of the dosage.
FIG. 1.
Concentration-versus-time plots after single dosing (A) and after multiple daily dosing over seven days (B) with 1, 3, and 6 mg of caspofungin per kg, respectively. Each point plots the mean concentration ± standard error of the mean (error bars) for three rabbits each at that time.
TABLE 1.
Estimated compartmental pharmacokinetic parameters of caspofungin in plasmaa
| Drug dose (mg/kg) | Cmax (μg/ml) | Cmin (24 h) (μg/ml) | AUC0–∞ (μg/ml · h) | Vss (liter/kg) | CL (liter/h/kg) | t1/2αb (h) | t1/2β (h) | t1/2γ∗ (h) |
|---|---|---|---|---|---|---|---|---|
| Single dose | ||||||||
| 1 | 20.02 ± 1.18 | 0.00 ± 0.00 | 20.57 ± 0.89 | 0.239 ± 0.011 | 0.050 ± 0.002 | 0.09 ± 0.00 | 2.00 ± 0.06 | 31.61 ± 0.12 |
| 3 | 60.47 ± 8.49 | 0.00 ± 0.00 | 47.14 ± 3.60 | 0.277 ± 0.033 | 0.068 ± 0.005 | 0.05 ± 0.00 | 1.84 ± 0.07 | 30.23 ± 1.19 |
| 6 | 123.40 ± 5.17 | 0.10 ± 0.00 | 119.30 ± 10.91 | 0.275 ± 0.006 | 0.053 ± 0.006 | 0.11 ± 0.01 | 1.85 ± 0.14 | 26.19 ± 1.29 |
| Multiple dose | ||||||||
| 1 | 16.01 ± 0.61 | 0.00 ± 0.00 | 13.15 ± 2.37 | 0.299 ± 0.011 | 0.086 ± 0.017 | 0.09 ± 0.01 | 1.62 ± 0.21 | 31.87 ± 0.18 |
| 3 | 51.05 ± 4.59 | 0.18 ± 0.18 | 63.70 ± 21.34 | 0.378 ± 0.050 | 0.065 ± 0.015 | 0.09 ± 0.01 | 2.20 ± 0.37 | 34.77 ± 3.09 |
| 6 | 105.52 ± 8.92 | 0.63 ± 0.26 | 158.43 ± 15.58 | 0.351 ± 0.016 | 0.043 ± 0.004 | 0.10 ± 0.00 | 3.53 ± 0.48 | 30.86 ± 0.96 |
All values represent the means ± standard errors of the means of values three rabbits each. Abbreviations: Cmin(24 h), plasma concentration at the end of the dosing interval (24 h); t1/2α distributional half-life; t1/2β, apparent elimination half-life; t1/2γ, terminal elimination half-life.
P ⩽ 0.05 for the comparison among dosage groups by ANOVA.
After multiple dosing over 7 days, peak concentrations in plasma were not significantly different from those observed after administration of a single dose. Mean levels in plasma fell below the LLQ in a dose-dependent manner after 8, 24, and 48 h. Levels in plasma at the end of the dosing interval were below the LLQ in all rabbits receiving the 1-mg/kg dose and in two of three rabbits receiving the 3-mg/kg dose, respectively. There were no significant differences in AUC, CL, and half-life compared to those after single dosing. The Vss, however, showed a trend towards larger values after multiple dosing (P < 0.05, P = 0.16, and P < 0.05 for the 1-, 3-, and 6-mg/kg dosages, respectively, by t test only). No differences in dose-normalized AUC0–∞ across the investigated dosage range were noted by ANOVA and linear regression, indicating dose-independent, linear pharmacokinetics of the compound also after multiple dosing.
Blood urea nitrogen, serum creatinine, bilirubin, and alanine aminotransferase levels determined after 7 days of treatment with caspofungin were within the range of normal values determined in 24 healthy, drug-naive animals. Infusion-related toxicity or other clinical abnormalities, including abnormal weight changes, were not observed.
The results of this study demonstrate linear pharmacokinetics of caspofungin over the investigated dosage range of 1 to 6 mg/kg/day, with dose-proportional increases in the AUC0–∞ with increasing dosage. Plasma concentration data fitted well into a three-compartment open pharmacokinetic model that revealed a prolonged terminal elimination half-life in the range of 30 to 35 h. There were no significant differences in pharmacokinetic parameters between single-dose and multiple-dose administration except for a trend towards an increased V after multiple dosing. The investigated dosages achieved sustained concentrations in plasma that were multiple times in excess of MICs reported for susceptible opportunistic fungi (8, 15). Caspofungin was well tolerated without evidence of renal or hepatic toxicities.
The favorable pharmacokinetic profile of caspofungin stands in marked contrast to that of cilofungin, the first echinocandin derivative that had entered clinical development. The pharmacokinetics of this drug in the rabbit were characterized by a very rapid elimination from the bloodstream via first-order kinetics, and its antifungal efficacy in vivo was limited. Increased antifungal efficacy, particularly in the brain, could only be achieved through intermittent and continuous infusion of daily dosages of as much as 180 mg/kg that elicited nonlinear saturation kinetics (14, 22).
The pharmacokinetics of caspofungin in plasma in the rabbit appear somewhat different from those of the investigational echinocandin VER-002 (v-echinocandin; formerly LY303366) in the same species. In comparison to caspofungin, at similar dosages, VER-002 exhibited an approximately twofold faster clearance and twofold lower Cmax and AUC values but a three- to fourfold larger V (18; A. H. Groll, D. Mickiene, V. Petraitis, R. Petraitiene, A. Field-Ridley, M. Candelario, J. Bacher, C. McMillian, S. C. Piscitelli, and T. J. Walsh, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. J-59, 1998.). It is yet unknown whether these subtle differences in the pharmacokinetics of both compounds also result in different pharmacodynamics.
Similar to cilofungin (22), VER-002 (17), and the original diamino analog L-733,560 (2), the in vitro fungicidal activity of caspofungin against Candida spp. appears to be concentration dependent (7). The implications of these observations remain to be investigated in vivo.
The low apparent Vss in the rabbit is reflective of the extensive protein binding of caspofungin across all species (12; J. A. Stone, S. D. Holland, W. D. Ju, Z. Zhang, M. Schwartz, V. L. Hoagland, K. E. Mazina, T. L. Hunt, and S. Waldman, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. C-51, 1998). Tissue distribution studies with radiolabeled drug in mice following intraperitoneal administration revealed preferential distribution to liver, kidney, and small intestine and a slower CL from all tissues than from plasma (12). The slow equilibration rates of most tissues is consistent with the existence of a terminal elimination phase beyond the dosing interval of 24 h and the increasing Vss after multiple dosing in our study and may be important for the dynamics of the drug against infections located in tissues. Indeed, infection models investigating cilofungin and VER-002 have demonstrated a correlation of antifungal efficacy with concentrations in tissue, and time of exposure was important for achieving effective concentrations in tissue and antifungal efficacy (22; A. H. Groll, D. Mickiene, V. Petraitis, R. Petraitiene, C. McMillian, S. Piscitelli, and T. J. Walsh, Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 2001, 1999].
While caspofungin does not interact in a significant manner with the cytochrome P450 enzyme system, it undergoes substantial hepatic metabolism and biliary excretion (11). The experimental work with cilofungin indicates saturable elimination pathways for the class of echinocandins, either by saturable biliary excretion of the parent or by metabolite inhibition (14, 22). These circumstances may also be relevant to caspofungin at higher dosages or in more narrow dosing regimens and should specifically be considered when the drug is projected to be given concurrently with other drugs that may compete with its biliary elimination mechanisms. Thus, the potential of drug-drug interactions with drugs such as cyclosporine, amphotericin B, certain antineoplastic agents (anthracyclines, vinca alkaloids, cis-platinum, etoposide) and antibacterial agents (macrolides, metronidazole) needs to be carefully studied during preclinical development.
In conclusion, caspofungin displayed linear pharmacokinetics in plasma that fit into a three-compartment open pharmacokinetic model. No accumulation in plasma was observed after multiple dosing, and the compound was well tolerated without hepatic or renal laboratory toxicity. The characterization of the pharmacokinetics in the rabbit will aid in selecting appropriate dosing regimens in infection models and may be useful for the translation of the findings of these models into clinical studies.
Acknowledgments
We thank Jeffrey Grove at Merck, Sharp & Dohme-Chibret for expert assistance with the analytical assay.
REFERENCES
- 1.Abruzzo G K, Flattery A M, Gill C J, Kong L, Smith J G, Pikounis V B, Balkovec J M, Bouffard A F, Dropinski J F, Rosen H, Kropp H, Bartizal K. Evaluation of the echinocandin antifungal MK-0991 (L-743, 872): efficacies in mouse models of disseminated aspergillosis, candidiasis, and cryptococcosis. Antimicrob Agents Chemother. 1997;41:2333–2338. doi: 10.1128/aac.41.11.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartizal K, Scott T, Abruzzo G K, Gill C J, Pacholok C, Lynch L, Kropp H. In vitro evaluation of the pneumocandin antifungal agent L-733560, a new water-soluble hybrid of L-705589 and L-731373. Antimicrob Agents Chemother. 1995;39:1070–1076. doi: 10.1128/aac.39.5.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartizal K, Gill C J, Abruzzo G K, Flattery A M, Kong L, Scott P M, Smith J G, Leighton C E, Bouffard A, Dropinski J F, Balkovec J. In vitro preclinical evaluation studies with the echinocandin antifungal MK-0991 (L-743, 872) Antimicrob Agents Chemother. 1997;41:2326–2332. doi: 10.1128/aac.41.11.2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Committee on the Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council. Guide for the care and use of laboratory animals. Washington, D.C.: National Academy Press; 1996. [Google Scholar]
- 5.D'Argenio D Z, Schumitzky A. Adapt II user's guide. University of Southern CaliforniaLos Angeles, Calif: Biomedical Simulations Resource; 1990. [Google Scholar]
- 6.Del Poeta M, Schell W A, Perfect J R. In vitro antifungal activity of pneumocandin L-743,872 against a variety of clinically important molds. Antimicrob Agents Chemother. 1997;41:1835–1836. doi: 10.1128/aac.41.8.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ernst E J, Klepser M E, Ernst M E, Messer S A, Pfaller M A. In vitro pharmacodynamic properties of MK-0991 determined by time-kill methods. Diagn Microbiol Infect Dis. 1999;33:75–80. doi: 10.1016/s0732-8893(98)00130-8. [DOI] [PubMed] [Google Scholar]
- 8.Espinel-Ingroff A. Comparison of in vitro activities of the new triazole SCH56592 and the echinocandins MK-0991 (L-743,872) and LY303366 against opportunistic filamentous and dimorphic fungi and yeasts. J Clin Microbiol. 1998;36:2950–2956. doi: 10.1128/jcm.36.10.2950-2956.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franzot S P, Casadevall A. Pneumocandin L-743,872 enhances the activities of amphotericin B and fluconazole against Cryptococcus neoformans in vitro. Antimicrob Agents Chemother. 1997;41:331–336. doi: 10.1128/aac.41.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graybill J R, Najvar L K, Luther M F, Fothergill A W. Treatment of murine disseminated candidiasis with L-743,872. Antimicrob Agents Chemother. 1997;41:1775–1777. doi: 10.1128/aac.41.8.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Groll A H, Walsh T J. MK-0991. Curr Opin Investig Anti-Infect Drugs. 1999;1:334–335. [Google Scholar]
- 12.Hajdu R, Thompson R, Sundelof J G, Pelak B A, Bouffard F A, Dropinski J P, Kropp H. Preliminary animal pharmacokinetics of the parenteral antifungal agent MK-0991 (L-743,872) Antimicrob Agents Chemother. 1997;41:2339–2344. doi: 10.1128/aac.41.11.2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurtz M B, Douglas C M. Lipopeptide inhibitors of fungal glucan synthase. J Med Vet Mycol. 1997;35:79–86. doi: 10.1080/02681219780000961. [DOI] [PubMed] [Google Scholar]
- 14.Lee J W, Kelly P, Lecciones J, Coleman D, Gordee R, Pizzo P A, Walsh T J. Cilofungin ( LY121019) shows nonlinear plasma pharmacokinetics and tissue penetration in rabbits. Antimicrob Agents Chemother. 1990;34:2240–2245. doi: 10.1128/aac.34.11.2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marco F, Pfaller M A, Messer S A, Jones R N. Activity of MK-0991 (L-743,872), a new echinocandin, compared with those of LY303366 and four other antifungal agents tested against blood stream isolates of Candida spp. Diagn Microbiol Infect Dis. 1998;32:33–37. doi: 10.1016/s0732-8893(98)00050-9. [DOI] [PubMed] [Google Scholar]
- 16.Nelson P W, Lozano-Chiu M, Rex J H. In vitro growth-inhibitory activity of pneumocandins L-733,560 and L-743,872 against putatively amphotericin B- and fluconazole-resistant Candida isolates: influence of assay conditions. J Med Vet Mycol 1997. 1997;35:285–287. [PubMed] [Google Scholar]
- 17.Petraitiene R, Petraitis V, Groll A H, Candelario M, Sein T, Bell A, Peter J, Lyman C A, Schaufele R L, McMillian C L, Bacher J, Walsh T J. Antifungal efficacy, safety, and single-dose pharmacokinetics of LY30366, a novel echinocandin, in experimental disseminated candidiasis in persistently neutropenic rabbits. Antimicrob Agents Chemother. 1999;43:2148–2155. doi: 10.1128/aac.43.9.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petraitis V, Petraitiene R, Groll A H, Bell A, Callender D P, Candelario M, Lyman C A, Bacher J, Walsh T J. Efficacy of LY-303366 against invasive pulmonary aspergillosis. Antimicrob Agents Chemother. 1998;42:2898–2905. doi: 10.1128/aac.42.11.2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Powles M A, Liberator P, Anderson J, Karkhanis Y, Dropinski J F, Bouffard F A, Balkovec J M, Fujioka H, Aikawa M, McFadden D, Schmatz D. D. Efficacy of MK-991 (L-743,872), a semisynthetic pneumocandin, in murine models of Pneumocystis carinii. Antimicrob Agents Chemother. 1998;42:1985–1989. doi: 10.1128/aac.42.8.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vazquez J A, Lynch M, Boikov D, Sobel J D. In vitro activity of a new pneumocandin antifungal, L-743,872, against azole-susceptible and -resistant Candida species. Antimicrob Agents Chemother. 1997;41:1612–1614. doi: 10.1128/aac.41.7.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walsh T J, Bacher P, Pizzo P A. Chronic silastic central venous catheterization for induction, maintenance, and support of persistent granulocytopenia in rabbits. Lab Anim Med. 1988;38:467–470. [PubMed] [Google Scholar]
- 22.Walsh T J, Lee J W, Kelly P, Bacher J, Lecciones J, Thomas V, Lyman C, Coleman D, Gordee R, Pizzo P A. Antifungal effects of the nonlinear pharmacokinetics of cilofungin, a 1, 3-β-glucan synthetase inhibitor, during continuous and intermittent intravenous infusions in treatment of experimental disseminated candidiasis. Antimicrob Agents Chemother. 1991;35:1321–1328. doi: 10.1128/aac.35.7.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamaoka K, Nakagawa T, Uno T. Application of Akaike's information criterion in the evaluation of linear pharmacokinetic equations. J Pharmacokinet Biopharm. 1978;6:165–175. doi: 10.1007/BF01117450. [DOI] [PubMed] [Google Scholar]