Abstract
Although the cerebellum has been consistently noted in the process of cognition, the pathophysiology of this link is still under exploration. Cerebellar stroke, in which the lesions are focal and limited, provides an appropriate clinical model disease for studying the role of the cerebellum in the cognitive process. This review article targeting the cerebellar stroke population (1) describes a cognitive impairment profile, (2) identifies the cerebellar structural alterations linked to cognition, and (3) reveals possible mechanisms of cerebellar cognition using functional neuroimaging. The data indicates the disruption of the cerebro-cerebellar loop in cerebellar stroke and its contribution to cognitive dysfunctions. And the characteristic of cognitive deficits are mild, span a broad spectrum, dominated by executive impairment. The consideration of these findings could contribute to deeper and more sophisticated insights into the cognitive function of the cerebellum and might provide a novel approach to cognitive rehabilitation. The goal of this review is to spread awareness of cognitive impairments in cerebellar disorders.
1. Introduction
For many decades, the cerebellum has been considered a pure control machine for physical movement. However, several studies have shown the effect of the cerebellum on cognition and affect [1]. For the first time, Schmahmann et al. described a series of cognitive and behavioral dysfunctional symptoms in 20 patients with focal cerebellar lesions and summarized them with the term “cerebellar cognitive affective syndrome” (CCAS) [2]. The concept of CCAS is a milestone in the study of cerebellar cognition. In the past two decades, the role of the cerebellum in cognition has been widely verified in patients with different types of cerebellar disorders [3–5]. Cerebellar stroke, in which the lesion is confined to the cerebellum and not complicated by cerebral abnormities such as atrophy and hydrocephalus, provides an appropriate clinical model for studying the role of the cerebellum in the cognitive process. In addition, the distribution of cerebellar infarction lesions varied by different vascular territories, which was applicable to studying the cognitive topography of the cerebellum. Beyond physical disability, cognitive impairment has been recognized in cerebellar stroke patients, with approximately 64% of whom developed cognitive impairment and 24% of whom fulfilled diagnostic criteria for dementia [6].
In the current review, we aim to describe the cognitive profile following cerebellar damage, reveal the cognitive topography of the cerebellum, and explore the effect of the cerebral-cerebellar loop on cognition through the model of cerebellar stroke.
2. The Cognitive Deficit in Patients with Cerebellar Stroke
CCAS has been observed in patients with cerebellar infarction [7], which is characterized by executive function disturbances such as poor planning, perseveration of shifting set, abstract reasoning, and verbal fluency; visual-spatial disorganization and impaired visual-spatial memory; dysprosodia such as language difficulties, mild anomia, and agrammatism; and personality change characterized by a flattening or blunting affect, disinhibited, and inappropriate behavior [2].
2.1. Visual-Spatial Cognition
Spatial ability refers to the capacity to understand, reason about, and remember the spatial relations among objects or space. Botez described that a patient with a left superior cerebellar artery infarct did worse in picture arrangement, Benton's judgment of line orientation, and Hooper's visual organization test [8]. This is the first time visual-spatial deficits have been reported in isolated cerebellar infarction patients. Since then, several studies have noted impaired performance on a range of visual-spatial measures in patients with cerebellar stroke, including the block design tasks from the Wechsler Intelligence Scale [9, 10], copying and recall of Rey-Osterrieth complex figure [11], letter cancellation test [12], “blank clock” test [6], character-line bisection task [13], and mental rotation tasks [14]. These observations are suggestive of abnormalities in the field of visuoconstruction, visual attention, visuospatial planning, and the actualization of visual concepts. Furthermore, consistent with the contralateral connections between the cerebellum and the cerebral cortex, studies have found that patients with left cerebellar lesions are more likely to have deficits in visual-spatial tasks [15, 16] implying a possible link between lateralization of damage and spatial information processing.
2.2. Language
Early in the twentieth century, Holmes (1922) described the effects on the speech of cerebellar damage, a condition later to become known as ataxic dysarthria [17], which is commonly explained by the uncoordinated muscle movement of the articulatory organs. With the development of neurolinguistics tests, such as the Boston Diagnostic Aphasia Examination (BDAE) and the Aachener Aphasia test (AAT), high-level and subtle language impairment secondary to cerebellar damage began to be discovered gradually. Marien described a patient with ischemic infarction in the vascular territory of the right cerebellar superior artery (SCA). The patient presented with normal performance on standard neuropsychological tests and intact conversational skills but performed significantly poorer than norms in tasks of verbal fluency (phonemic and semantic), word stem completion, and oral naming speed [18]. By summarizing his symptoms, Marien proposed the concept of cerebellar-induced aphasia, which is characterized by nonfluent aphasia, including reduced speech initiation, decreased dynamics of language, word-finding disturbances, marked agrammatism, and reading and writing difficulties [18].
The evidence of language dysfunctions in cerebellar disorders comes from several case-series analyses and case reports. The disability in verbal fluency and semantic access are considered as prominent language symptoms following cerebellar infarction [19]. In word generation and retrieval tasks, patients had slowed language production and problems detecting their own error. Another common language symptom is grammatical impairment. Silveri (1996) reported a male following right cerebellar infarction presented motor aphasia for the first time [20]. The patient manifested sentence production deficit but did not reveal cortical abnormality that could account for the behavior [20]. The underlying mechanism remains to be elucidated, and experts supposed that the disruption of the connection between the cerebellum and frontal cortex contributes to language impairments in cerebellar infarction. Other language deficits including transcortical sensory aphasia [19], impaired reading and writing [6], spatial dysgraphia [21], and lexical-semantic retrieval functions of nonnative languages [22] were also revealed in stroke patients whose lesions were confined to the cerebellum.
2.3. Working Memory
Working memory (WM) is the ability that allows information to be maintained temporarily and manipulated online during diverse cognitive demands and is the central executive function. Previous studies have revealed the impaired function of WM in patients following an isolated cerebellar infarction [9, 23, 24]. Ravizza et al. investigated that selective damage in verbal WM occurred secondary to cerebellar disorders, but articulatory rehearsal strategies were unaffected, supporting motor problems did not implicate the impaired WM system [23]. WM comprises an attentional control system, a central executive, and two subsidiary systems for the storage of visuospatial and verbal material [25, 26]. By means of comparisons between two traditional and presumably less demanding “short-term memory” tasks, digit and word span, and the more recent and demanding listening span task, patients with cerebellar infarction only perform significantly worse in respect of the listening span task, suggesting impairment of the central executive domains of WM [9]. The result was consistent with a previous functional magnetic imaging study, which provided evidence that the cerebellum participates in an amodal bilateral neuronal network representing the central executive during working memory n-back tasks [27].
2.4. Executive Function
Executive functions, which define the ability to orchestrate different cognitive tasks to achieve a specific goal, are not a separate concept. These cognitive abilities are required for adapting to changes in the environment, for example, the capacity to plan, anticipate results, and focus resources appropriately to objectives, as well as the ability to keep attention for lengthy periods of time while distracted by adverse surroundings [28]. Five processes were distinguished in previous studies: attention and inhibition, task management, planning, monitoring the contents of working memory, and encoding [29, 30]. Executive deficits have been reported in a variety of studies of patients following cerebellar infarction, including effects on attention [6, 31], sequencing [32, 33], inhibition of inappropriate responses [34], task planning [6, 28], integration, and organization [35], using standard neuropsychological tasks such as reversed digit span, category switching, Trails A and B, Go/No-Go test, and the Stroop color-word interference.
The term “executive function” has long been used synonymously with the term “frontal lobe function.” [36] Executive disturbances are the most prominent symptom of CCAS. The ascending cerebellar projections to the frontoparietal cortex and the feedback loops may be the neural substrates of cerebellar involvement in the processing of execution.
2.5. Neuropsychiatric Features
In addition to language, executive, and visuospatial impairments, other psychological deficits following cerebellar disorders have been reported [37]. The list of cognitive functions that are impaired as a result of cerebellar dysfunction is expansive, including source memory [38], which is the ability to remember original contextual (i.e., temporal and spatial) features of an event or information, metalinguistics ability to understand metaphorical expressions or construct sentences with pragmatic quality [39], social cognition such as face emotion recognition [40], procedural learning [41], spatial-temporal confusion [42], and loss of emotions [43].
Cognitive impairments after isolated cerebellar stroke span a broad spectrum and are mild and transient [44]. A few studies conducted on the subacute or chronic period have not detected any significant deficit in cerebellar cognition [6]. Furthermore, cognitive disorders in cerebellar infarctions may recover in time, which means that the prognosis is good [45, 46].Traditional neuropsychological tests, which may detect well-defined cognitive profiles caused by supratentorial cerebral damage, are sometimes inadequately sensitive to identify “subclinical” abnormalities that might occur as a result of cerebellar diseases [47].The use of specific tests to detect CCAS may be critical in understanding cognitive changes following cerebellar disorders. The CCAS scale, which was developed in 2018, is an easily applicable bedside test to detect CCAS in clinical practice [48].The scale is a 10-item battery including significantly abnormal cognitive tests between patients and healthy controls: semantic fluency, phonemic fluency, category switching, verbal memory, digit span forward and backward, cube drawing, similarities, and Go/No-Go test. A pass/fail judgment is established for each test based on a threshold score, and one, two, or three and more failed subtests were defined as possible, probable, or definite CCAS, respectively. The latter study showed the area under the receiver operating characteristic (ROC) curves was 0.84 in isolated cerebellar infarction, indicating the strong diagnostic value of CCAS scale [49].
3. Cerebellar Lesion Location Determines Functional Deficits
Lesion-deficit studies in patients with focal cerebellar infarction provide pivotal insights into structure-function correlations. Studies found that lesion size was not associated with cognitive outcomes. Even very large lesions did not produce significant impairment in cognitive performance if they did not extend into the specific site of the cerebellum [14, 50]. This evidence hypothesis shows a strict localization of functions in the cerebellum. And a motor-cognitive dichotomy has been well-recognized: tasks with a significant motor component are impacted by a lesion in the anterior lobe, whereas performance on cognitive tasks with limited motor demands is more affected by lesions in the cerebellar posterior lobe regions [14, 51], suggesting an explanation of dissociation between motor deficits with preserved cognition and cognitive deficits without ataxia in patients with cerebellar disorders [42, 52]. Previous studies indicated that patients with posterior inferior cerebellar artery (PICA) lesions damaged cognitive function than those with superior cerebellar artery (SCA) lesions, which were manifested as motor dysfunction [10, 32]. There are other publications, however, which come to a different conclusion: no obvious differences in cognitive functions were found between patients with infarction of the PICA and SCA [12, 34]. Because the common SCA territory includes the anterior lobe as well as a portion of the posterior lobe, cognitive dysfunctions in SCA patients with extended posterior lesions may not be unexpected.
For the vague identification of the different cerebellar regions divided by vascular territory, medial/lateral, anterior/posterior, or vermis/hemisphere, Larsell et al. (1972) constructed a detailed atlas of the human cerebellum [53]. The atlas divided the human cerebellum into ten regions marked with Roman numerals from the anterior (regions I-V) to the posterior (regions VI-X), which demonstrates the details of the cerebellar cortex within the three cardinal planes in Talairach proportional stereotaxic space and provides a more contemporary and accessible cerebellar nomenclature (Figure 1). Damage to regions VI and VII has been observed to be associated with impaired cognitive performance [51], matching the functional topography of the cerebellum in healthy controls using task-based and resting-state functional magnetic resonance imaging (fMRI) [54–57].
Figure 1.
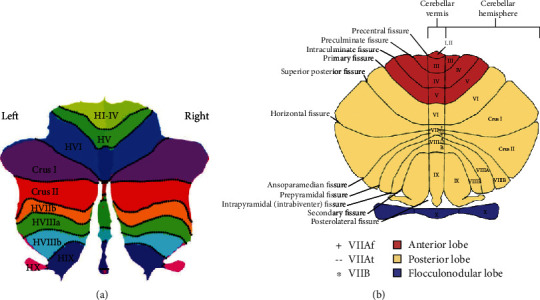
Flattened representation and illustration of the cerebellum and its major fissures, lobes, and lobules. Description: (a) Flattened representation of the human cerebellum developed by Diedrichsen et al. [105] In contrast to the vermis parts in the middle of the flat map, H stands for “hemispheric.” (b) The anterior lobe is colored red; the posterior lobe is cream and the flocculonodular lobe is purple. In the lobule VII, the VIIAf at the vermis expands in the hemisphere to become the Crus I. The lobule VIIAt at the vermis merges with the Crus II in the hemisphere, whereas the lobule VIIB retains its structural integrity both at the vermis and in the hemispheres. Author's diagram adapted from Schmahmann et al. [106] and first published in D'Mello et al. [107].
3.1. The Theory of Universal Cerebellar Transform
The theory of universal cerebellar transform (UCT) indicates that the cerebellum has a consistent internal structure and works as a modulator to optimize performance according to context [58]. The strict localization of functions in the cerebellum results from the heterogeneity of cerebellar connections with extracerebellar structures rather than variations in the cerebellar microstructure itself [59]. Functional neuroimaging studies confirmed that the cerebellum has extensive connectivity with different cerebral areas: the cerebellar posterior lobe connects with the prefrontal, posterior parietal, superior temporal, and limbic cortices, while the anterior lobe connects with the primary motor and premotor cortex [15, 60–62].Furthermore, resting-state functional connectivity analysis shows that the cerebellum can be divided into elaborate functional regions based on the patterns of anatomical connectivity between different regions of the cerebellum and association areas of the cerebral cortex [15, 56, 63] (Figure 2). Various psychological deficits following different cerebellar lesions are assumed to be a result of the interruption of different cerebro-cerebellar cognitive loops: prefrontal cortical associated cerebellar areas in relation to executive control, parietal cortical areas with respect to visuospatial function, and frontotemporal regions in relation to linguistic function.
Figure 2.
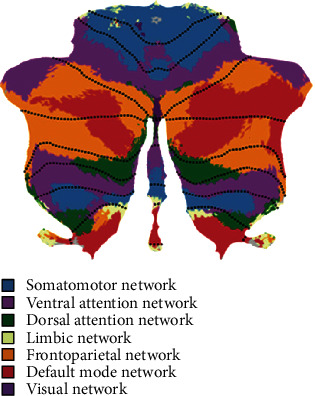
A map of the human cerebellum based on functional connectivity to seven major networks in the cerebrum. Description: Author's diagram adapted from Schmahmann et al. [108] and first published in Buckner et al. [61]
3.2. Functional Topography of Cerebellum
Voxel-based lesion-symptom mapping (VLSM) is an imaging method that analyzes the relationship between brain lesions and behavioral performance on a voxel-by-voxel basis [64]. This method offers better resolution than grouping stroke lesions by the affected artery or conducting region of interest analyses. With the use of VLSM, cerebellar functional topography can be described well in the disease model of cerebellar stroke for its confined lesions.
Consistent with traditional group analyses, the study of VLSM further confirmed damage in the posterior lobe, especially in the region of VI and VII that produced the cognitive impairment [12, 49, 51]. Richter et al. firstly used this technique in patients following isolated cerebellum infarction to reveal functional regions underpinning cognition in the cerebellum and demonstrated that impaired performance in a verbal fluency task was associated with the lesion of the right hemispheric region Crus II [12].Stoodly et al. (2016) found patients with damage to cerebellar lobules III–VI had worse ataxia symptoms, while posterior cerebellar damage involving lobules VII and VIII was a risk factor for cognitive deficits, which further validated the anterior-sensorimotor/posterior-cognitive dichotomy in the cerebellum (Figure 3). In addition, different locations of lesions were found to lead to significantly poorer scores on particular cognitive tasks, such as language (right Crus I and II extending through IX), spatial (bilateral Crus I, Crus II, and right lobule VIII), and executive function (lobules VII–VIII) [51].Chirino-Pérez et al. recently conducted a support vector regression-based multivariate VLSM study in 22 patients with chronic isolated cerebellar strokes and used the CCAS scale to detect cognition damage more sensitively. They found global cognition impairment was associated with damage to the right lateral posterior lobe of the cerebellum, particularly in region VI and Crus I [49]. The subanalyses of this study also revealed that semantic fluency, category switching, and cube drawing were impaired severely when damage was involved right VI, VIIb, Crus I, and Crus II [49].
Figure 3.
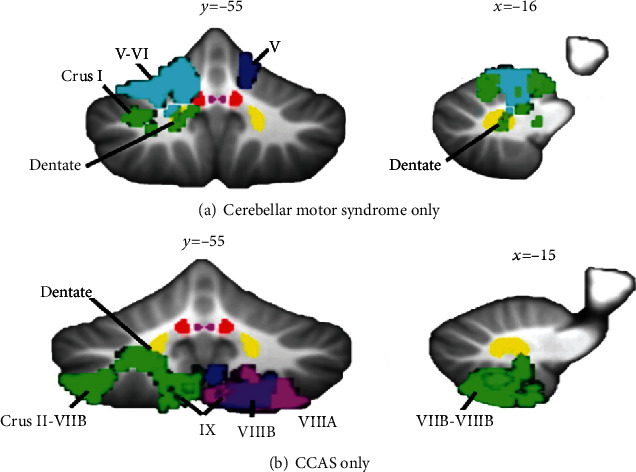
Lesion symptom mapping in patients with cerebellar infarction. Description: (a) Lesions of lobules IV–V of the anterior lobe extending into adjacent lobule VI produce the cerebellar motor syndrome of ataxia but not cerebellar cognitive affective syndrome (CCAS). (b) Lesions confined to posterior lobe lobules Crus II through lobule IX produce the cerebellar cognitive affective syndrome but no motor ataxia. Different colors represent the lesions of individual patients. Author' s diagram developed by Stoodley et al. [51].
In addition, some other studies of VLSM using specific cognitive tasks focused on exploring a link between specific cognitive impairment and lesion location in patients with cerebellar infarction. Those studies observed that impaired phonemic fluency correlated with lesions in right Crus II, IX, and X and the deep nuclei; [65] visual attention deficit correlated with lesions of the pyramid of the vermis, the culmen, and partly the inferior semilunar lobule; [66] visuomotor rotation adaptation damage correlated with lesions in region VI; [67] impaired spatial and temporal visual attention correlated with the left posterior cerebellar region Crus II; [31]and difficulties in the recognition of emotion from voices (emotional prosody) correlated with lesions in the right region VIIb and VIII, and Crus I and II [68, 69]. More details are provided in Table 1. Studies of VLSM make an effort to identify cerebellar regions which are crucial to the presence of cognitive dysfunction and describe a precise cerebellum cognitive functional topography. The conclusion of those studies is consistent with studies on healthy subjects [54, 70].
Table 1.
Voxel-based lesion-symptom mapping studies of cognitive function in patients following cerebellar infarction.
| Number | Studies | Scanner | Subjects | Task/clinical performance | Result |
|---|---|---|---|---|---|
| 1 | Richter (2007) | 3T | 21 patients vs. 25 controls | Verb generation task, neglect tests (letter cancellation, line bisection), visual extinction test, verbal fluency task | Impaired performance in the verbal fluency task correlated with lesions in the right region Crus II |
| 2 | Baier (2010) | 3T | 26 patients vs. 15 controls | Covert visual attention task | Impaired covert visual attentional processes correlated with lesions in vermal structures such as the pyramid |
| 3 | Stoodley (2016) | 3T | 18 patients vs. norms | Wechsler Adult Intelligence Test-3, Trails A and B, Wisconsin Card Sorting Task, Wechsler Memory Scale, fluency task, Boston Naming Test, Benton Judgment of Line Orientation, mental rotation and Rey figure task | Cognitive impairment correlated with lesions in posterior lobe. More specifically, lesions of right Crus I and II extending through IX lead to poorer scores on language, lesions of bilateral Crus I, Crus II and right region VIII associate with spatial, and lesions of region VII–VIII associate with executive function |
| 4 | Kim (2017) | 3T | 24 patients vs. norms | Geriatric Depression Scale | Lesions in left VI, VIIb, VIII, Crus I, and Crus II are related with severity of depressive symptoms |
| 5 | Thomasson (2019) | 3T | 15 patients vs. 15 controls | Emotional prosody recognition task | Emotional misattributions correlated with lesions in right region VIIb, VIII and IX; and rhythm discrimination correlated with lesions in region VIIb |
| 6 | Pérez (2021) | 3T | 22 patients vs. 22 controls | Montreal Cognitive Assessment, cerebellar cognitive affective syndrome scale(CCAS-s) | Lesions in right region VI and Crus I are related with poor performance of CCAS-s, semantic fluency subtest, and cube drawing subtest; lesion in right region VIIb, Crus I, and Crus II are related with poor category switching score |
| 7 | Craig (2021) | 1.5 T | 14 patients vs. 24 controls | Reflexive and voluntary covert attention task, attentional blink task, sustained attention to response task | Deficits in spatial and temporal visual attention correlated with lesions of left Crus II |
| 8 | Thomasson (2021) | 1.5T | 24 patients vs. 24 controls | Emotional prosody recognition task | Emotional misattributions correlated with lesions in the right region VIIb, VIII, Crus I and II |
4. Cerebro-Cerebellar Loop: Evidence from Neuroimaging
The cognitive deficits following cerebellar damage were held to result from the disrupted connectivity between the posterior cerebellum lobe and cerebral association areas [71]. Anatomic and imaging studies have indicated that the cerebro-cerebellar loop consists of afferent inputs through cortico-ponto-cerebellar projections and an efferent pathway through the cerebello-thalamic-cortical [72]. At the cellular level, there exists a direct excitatory loop from the cortex through the cerebellar nucleus dentatus and an inhibitory input through the Purkinje cells in the cerebellar cortex.
Studies employing fMRI in cerebellar stroke patients further provide evidence for a cortico-cerebellar connection as the functional substrate of cognition. Ziemus et al. explored the activation pattern changes during an n-back working memory task in patients with isolated cerebellar infarct: compared with healthy controls, bilateral increased BOLD activations in the ventrolateral prefrontal cortex, dorsolateral prefrontal cortex, parietal cortex, presupplementary motor area, and anterior cingulate were found in cerebellar patients during the task [35]. Wang et al. revealed that the abnormal alterations in the right posterior cingulate gyrus, bilateral median cingulate and paracingulate gyri, and right precuneus may play a core role in the cognitive impairment following cerebellar infarctions using diffusion tensor imaging [73]. Fan et al. found that the lower fractional amplitude of low-frequency fluctuation in the left hippocampus and right cingulate gyrus is related to poor cognitive performance in patients with acute posterior cerebellar infarction [74].
4.1. Crossed Cerebello-Cerebral Diaschisis and Lateralization
Crossed cerebello-cerebral diaschisis (CCD) has been described in patients with focal cerebellar lesions showing decreased cerebral perfusion and metabolism contralateral to cerebellar lesions [75].The cellular and molecular events of Wallerian degeneration that spread over the cerebello-cerebral tracts distant from the primary cerebellar lesions are speculated to be a possible mechanism for the phenomenon [76, 77]. Functional neuroimaging studies using single photon emission computed tomography (SPECT), positron emission tomography (PET), and near-infrared spectroscopy have demonstrated that cerebral hypometabolism and hypoperfusion may contribute to cognitive dysfunction in cerebellar infarction [8, 18, 78–81]. A quantified SPECT study showed that, in the absence of any structural damage in the supratentorial brain regions, contralateral hypoperfusion in the left medial frontal lobe secondary to the cerebellar lesion may explain frontal lobe symptoms such as executive dysfunction, apathy, and disinhibition [79].
For the crossing of cerebello-cortico-cerebellar connections, the functional lateralization of cerebellar disorders is currently being considered. Clinical studies showed patients with cerebellar infarction lesions in the right posterior lobe manifested poorer cognitive performance than in left-lateralized regions [49, 50].In addition, studies also indicated the cognitive characteristics of different cerebellar hemispheric infarcts: language dysfunctions often follow damage to the right cerebellar hemisphere, whereas visual-spatial disability can result from left cerebellar hemisphere lesions [51, 82]. However, the side of the lesion showed no significant effect on cognitive performance in other studies [12, 32]. Two possible reasons may help explain this inconsistent conclusion. One hypothesis is that, as bilateral cortical activation was observed during linguistic and spatial tasks [83, 84], cerebral cortex functions are not always completely lateralized. An alternative interpretation is that cerebellar reserve, the capacity of the cerebellum to compensate for tissue damage or loss of function by the formation of new synaptic connections with cerebral cortical neurons [85], can prompt changes in connectivity between distinct networks and lead to reorganization of cerebellar functional topography [86].
5. Theory of Cerebellar Cognitive Function
Dysmetria of thought (DoT) is proposed as a fundamental framework attempting to explain the cognitive symptoms in patients with focal cerebellar lesions [87]. The intact cerebellar function facilitates actions harmonious with the goal, appropriate to the context, and judged accurately and reliably according to the strategies mapped out prior to and during the behavior. It is hypothesized that the prefrontal discharges are regulated and modulated rather than generated, by cerebellar structures. Cognitive dysfunctions following cerebellar disorders are associated not with the death of cortical neurons but with “discordance” in their operation, which explains why cognitive impairments after focal cerebellar disorders in adults are mild or transient.
Other theories which are compatible with the DoT theory on how the cerebellum modulates cognitive function have been inspired by anatomical and physiological studies. These include the theories of biological clock [88], timing machine [89], error detection [90], sequence learning [91], automatization [92], dynamic state monitoring [93], neuronal machine [94], and implementing supervised learning using computational and engineering organizational principles [95].
6. Future Perspective
CCAS identifies the key characteristics of cerebellar patients' cognitive and emotional impairments. Aside from deficits of executive functions, visuospatial cognition, linguistic functions, and personality changes in cerebellar disorders, the involvement of the cerebellum in metalanguage and social cognition has also been discovered [39, 40]. Metalinguistic abilities include explicit awareness of abstract language representations. Patients with cerebellar lesions may have defects in perceiving ambiguities, formulating intelligible statements for a specific context, inferring logically, and comprehending figurative language, in contrast to the grammatical and semantic abilities that have been retained [39]. Social cognition is the process of observing and understanding the behavior and mental state of others, including oneself, in response to nonverbal or verbal stimuli [96]. Distortions in social cognition are usually regarded as the underlying dysfunction causing severe malfunctions in social and affective function. Mirroring, mentalizing, and abstract judgment are three sets of categories of social cognition. According to an activation likelihood estimation meta-analysis, the cerebellum is not responsible for any specific function but rather increases the efficiency with which other neocortical regions accomplish their own processes [97]. The role of the cerebellum in metalanguage and social cognition is consistent with the unifying framework of the UCT and the DoT and provides new insights into the nature of the cognitive impairments in patients with the CCAS.
Besides the cerebellar functional topography mentioned above, a recent resting-state imaging analysis revealed novel functional properties of cerebellar double motor representation (lobules I-VI and VIII) and triple nonmotor representation (lobules VI/Crus I, Crus II/VIIB, and IX/X) [63]. The first cerebellar motor representation that targets the primary motor cortex is engaged in motor control, whereas the second cerebellar motor representation that targets regions surrounding the precentral gyrus is more important for movement planning rather than movement execution [55]. The three cognitive representations are considered in the same way: the relationship between the first and second motor representations is similar to that of the first/second and third cognitive representations [57, 63]. And future studies are needed to explore what different functions are enabled by the different cognitive representations and what the differing consequences of lesions to the different representations are. Also, experts indicated that functional subdivisions did not align with lobular borders, which are often utilized to summarize functional data; they suggested that the novel parcellation serves as a functional atlas should be performed for future neuroimaging research [56]. Due to the localization and diversity of the lesions, cerebellar stroke could be a good disease model to demonstrate a more detailed and specific cerebellar functional topography by using fMRI techniques.
Due to the role in motor and nonmotor function, the cerebellum is attracting scientists interested in basic and clinical research of neuromodulation. Transcranial direct current stimulation (tDCS) and repetitive transcranial magnetic stimulation (rTMS) of the cerebellum can modify cognitive function, and targeted stimulation to narrow areas within the cerebellum produces differential effects on cognitive tasks such as language, memory and learning, and visuospatial orientation [98, 99]. Previous research has confirmed the validity of cerebellar noninvasive stimulation. A previous study reported that a patient with a left cerebellar stroke showed improvement on tasks modeling procedural learning after administration of rTMS to the unaffected right cerebellar hemispheres [41]. In addition, cerebellum-targeted rehabilitation exercises have provided a realistic opportunity for intervention in mental diseases such as autism spectrum disorders, affective disorders, and psychotic spectrum disorders, as well as Alzheimer's disease and aphasia [100, 101]. However, because of the highly convoluted nature of the cerebellar cortex, effects of noninvasive cerebellar brain stimulation are difficult to anticipate, and the robustness and replicability of previous findings will need to be evaluated before any recommendations on these forms of therapy can be made [102]. More research should be done to standardize the stimulation paradigms of cerebellum-targeted brain stimulation [103, 104].
7. Conclusion
Cerebellar involvement in cognition has long been a research topic but is gaining increasing clinical attention. This review provides new details about how cognitive dysfunctions manifest in cerebellar stroke. Evidence from neuroimaging and patient populations suggests that the posterolateral cerebellum contributes to cognitive processing and demonstrates a detailed functional topography using VLSM. In addition, the disruption of the cerebro-cerebellar loop has been considered as the mechanism of CCAS. The application of the tools of contemporary cognitive neuroscience may allow us to understand the cerebellum's role in cognition and emotion more in-depth. And new opportunities may also be possible for rehabilitation intervention in neuropsychiatry by targeting focal areas in the cerebellar node of the distributed cerebro-cerebellar networks subserving human cognition and emotion.
Acknowledgments
We are grateful to the patients and their families for their continued support of our research. Y. Z is supported by the National Natural Science Foundation of general project (81972144). Y.C. is supported by the National Natural Science Foundation of China Youth Program (82001124) and an early career research fellowship funded by Beijing Tiantan Hospital, Capital Medical University (2020MP06).
Contributor Information
Yu Chen, Email: sherryyu.chen@ucsf.edu.
Yumei Zhang, Email: zhangyumei95@aliyun.com.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
Yu Chen and Yumei Zhang contributed equally to this work.
References
- 1.Koziol L. F., Budding D., Andreasen N., et al. Consensus paper: the cerebellum’s role in movement and cognition. The Cerebellum . 2014;13(1):151–177. doi: 10.1007/s12311-013-0511-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmahmann J. D., Sherman J. C. The cerebellar cognitive affective syndrome. Brain . 1998;121(4):561–579. doi: 10.1093/brain/121.4.561. [DOI] [PubMed] [Google Scholar]
- 3.Stezin A., Bhardwaj S., Hegde S., et al. Cognitive impairment and its neuroimaging correlates in spinocerebellar ataxia 2. Parkinsonism & Related Disorders . 2021;85:78–83. doi: 10.1016/j.parkreldis.2021.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maas R., Killaars S., van de Warrenburg B., Schutter D. J. L. G. The cerebellar cognitive affective syndrome scale reveals early neuropsychological deficits in SCA3 patients. Journal of Neurology . 2021;268(9):3456–3466. doi: 10.1007/s00415-021-10516-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmadian N., van Baarsen K., van Zandvoort M., Robe P. A. The cerebellar cognitive affective syndrome-a meta-analysis. The Cerebellum . 2019;18(5):941–950. doi: 10.1007/s12311-019-01060-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalashnikova L. A., Zueva Y. V., Pugacheva O. V., Korsakova N. K. Cognitive impairments in cerebellar infarcts. Neuroscience and Behavioral Physiology . 2005;35(8):773–779. doi: 10.1007/s11055-005-0123-0. [DOI] [PubMed] [Google Scholar]
- 7.Bolceková E., Mojzeš M., Van Tran Q., et al. Cognitive impairment in cerebellar lesions: a logit model based on neuropsychological testing. Cerebellum & Ataxias . 2017;4(1):p. 13. doi: 10.1186/s40673-017-0071-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Botez-Marquard T., Léveillé J., Botez M. I. Neuropsychological functioning in unilateral cerebellar damage. Canadian Journal of Neurological Sciences . 1994;21(4):353–357. doi: 10.1017/S0317167100040956. [DOI] [PubMed] [Google Scholar]
- 9.Malm J., Kristensen B., Karlsson T., Carlberg B., Fagerlund M., Olsson T. J. N. Cognitive impairment in young adults with infratentorial infarcts. Neurology . 1998;51(2):433–440. doi: 10.1212/WNL.51.2.433. [DOI] [PubMed] [Google Scholar]
- 10.Exner C., Weniger G., Irle E. Cerebellar lesions in the PICA but not SCA territory impair cognition. Neurology . 2004;63(11):2132–2135. doi: 10.1212/01.WNL.0000146197.44568.CD. [DOI] [PubMed] [Google Scholar]
- 11.Hoffmann M., Schmitt F. Cognitive impairment in isolated subtentorial stroke. Acta Neurologica Scandinavica . 2004;109(1):14–24. doi: 10.1034/j.1600-0404.2003.00169.x. [DOI] [PubMed] [Google Scholar]
- 12.Richter S., Gerwig M., Aslan B., et al. Cognitive functions in patients with MR-defined chronic focal cerebellar lesions. Journal of Neurology . 2007;254(9):1193–1203. doi: 10.1007/s00415-006-0500-9. [DOI] [PubMed] [Google Scholar]
- 13.Kim E. J., Choi K. D., Han M. K., et al. Hemispatial neglect in cerebellar stroke. Journal of the Neurological Sciences . 2008;275(1-2):133–138. doi: 10.1016/j.jns.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 14.Schmahmann J. D., Macmore J., Vangel M. Cerebellar stroke without motor deficit: clinical evidence for motor and non-motor domains within the human cerebellum. Neuroscience . 2009;162(3):852–861. doi: 10.1016/j.neuroscience.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Reilly J. X., Beckmann C. F., Tomassini V., Ramnani N., Johansen-Berg H. Distinct and overlapping functional zones in the cerebellum defined by resting state functional connectivity. Cerebral Cortex . 2010;20(4):953–965. doi: 10.1093/cercor/bhp157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kellermann T., Regenbogen C., De Vos M., Mossnang C., Finkelmeyer A., Habel U. Effective connectivity of the human cerebellum during visual attention. The Journal of Neuroscience . 2012;32(33):11453–11460. doi: 10.1523/JNEUROSCI.0678-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown J. R., Darley F. L., Aronson A. E. Ataxic dysarhria. International Journal of Neurology . 1970;7(2):302–318. [PubMed] [Google Scholar]
- 18.Mariën P., Saerens J., Nanhoe R., et al. Cerebellar induced aphasia: case report of cerebellar induced prefrontal aphasic language phenomena supported by SPECT findings. Journal of the Neurological Sciences . 1996;144(1-2):34–43. doi: 10.1016/S0022-510X(96)00059-7. [DOI] [PubMed] [Google Scholar]
- 19.Mariën P., Baillieux H., De Smet H. J., et al. Cognitive, linguistic and affective disturbances following a right superior cerebellar artery infarction: a case study. Cortex . 2009;45(4):527–536. doi: 10.1016/j.cortex.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 20.Silveri M., Leggio M., Molinari M. J. N. The cerebellum contributes to linguistic production: a case of agrammatic speech following a right cerebellar lesion. Neurology . 1994;44(11):2047–2050. doi: 10.1212/WNL.44.11.2047. [DOI] [PubMed] [Google Scholar]
- 21.Silveri M. C., Misciagna S., Leggio M. G., Molinari M. Spatial dysgraphia and cerebellar lesion: a case report. Neurology . 1997;48(6):1529–1532. doi: 10.1212/WNL.48.6.1529. [DOI] [PubMed] [Google Scholar]
- 22.Mariën P., van Dun K., Van Dormael J., et al. Cerebellar induced differential polyglot aphasia: a neurolinguistic and fMRI study. Brain and Language . 2017;175:18–28. doi: 10.1016/j.bandl.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 23.Ravizza S. M., McCormick C. A., Schlerf J. E., Justus T., Ivry R. B., Fiez J. A. Cerebellar damage produces selective deficits in verbal working memory. Brain . 2006;129(2):306–320. doi: 10.1093/brain/awh685. [DOI] [PubMed] [Google Scholar]
- 24.Hokkanen L. S., Kauranen V., Roine R. O., Salonen O., Kotila M. Subtle cognitive deficits after cerebellar infarcts. European Journal of Neurology . 2006;13(2):161–170. doi: 10.1111/j.1468-1331.2006.01157.x. [DOI] [PubMed] [Google Scholar]
- 25.Jarrold C., Baddeley A. D. Short-term memory for verbal and visuospatial information in down’s syndrome. Cognitive Neuropsychiatry . 1997;2(2):101–122. doi: 10.1080/135468097396351. [DOI] [PubMed] [Google Scholar]
- 26.Constantinidis C., Klingberg T. The neuroscience of working memory capacity and training. Nature Reviews Neuroscience . 2016;17(7):438–449. doi: 10.1038/nrn.2016.43. [DOI] [PubMed] [Google Scholar]
- 27.Küper M., Kaschani P., Thürling M., et al. Cerebellar fMRI activation increases with increasing working memory demands. The Cerebellum . 2016;15(3):322–335. doi: 10.1007/s12311-015-0703-7. [DOI] [PubMed] [Google Scholar]
- 28.Manes F., Villamil A., Ameriso S., Roca M., Torralva T. "Real life" executive deficits in patients with focal vascular lesions affecting the cerebellum. Journal of the Neurological Sciences . 2009;283(1-2):95–98. doi: 10.1016/j.jns.2009.02.316. [DOI] [PubMed] [Google Scholar]
- 29.Smith E. E., Koeppe R. A., Awh E., Minoshima S., Mintun M. A. Spatial working memory in humans as revealed by PET. Nature . 1993;363(6430):623–625. doi: 10.1038/363623a0. [DOI] [PubMed] [Google Scholar]
- 30.Smith E. E., Jonides J. Storage and executive processes in the frontal lobes. Science . 1999;283(5408):1657–1661. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- 31.Craig B. T., Morrill A., Anderson B., Danckert J., Striemer C. L. Cerebellar lesions disrupt spatial and temporal visual attention. Cortex . 2021;139:27–42. doi: 10.1016/j.cortex.2021.02.019. [DOI] [PubMed] [Google Scholar]
- 32.Tedesco A. M., Chiricozzi F. R., Clausi S., Lupo M., Molinari M., Leggio M. G. The cerebellar cognitive profile. Brain . 2011;134(12):3672–3686. doi: 10.1093/brain/awr266. [DOI] [PubMed] [Google Scholar]
- 33.Silveri M., Di Betta A., Filippini V., Leggio M. Verbal short-term store-rehearsal system and the cerebellum. Evidence from a patient with a right cerebellar lesion. Brain . 1998;121(11):2175–2187. doi: 10.1093/brain/121.11.2175. [DOI] [PubMed] [Google Scholar]
- 34.Neau J. P., Arroyo-Anllo E., Bonnaud V., Ingrand P., Gil R. Neuropsychological disturbances in cerebellar infarcts. Acta Neurologica Scandinavica . 2000;102(6):363–370. doi: 10.1034/j.1600-0404.2000.102006363.x. [DOI] [PubMed] [Google Scholar]
- 35.Ziemus B., Baumann O., Luerding R., et al. Impaired working-memory after cerebellar infarcts paralleled by changes in BOLD signal of a cortico-cerebellar circuit. Neuropsychologia . 2007;45(9):2016–2024. doi: 10.1016/j.neuropsychologia.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 36.Mayes A. R., Downes J. J. What do theories of the functional deficit(s) underlying amnesia have to explain? Memory . 1997;5(1-2):3–36. doi: 10.1080/741941147. [DOI] [PubMed] [Google Scholar]
- 37.Schmahmann J., Weilburg J., Sherman J. The neuropsychiatry of the cerebellum - insights from the clinic. Cerebellum . 2007;6(3):254–267. doi: 10.1080/14734220701490995. [DOI] [PubMed] [Google Scholar]
- 38.Tamagni C., Mondadori C., Valko P., Brugger P., Schuknecht B., Linnebank M. Cerebellum and source memory. 2010;63(4):234–236. doi: 10.1159/000282277. [DOI] [PubMed] [Google Scholar]
- 39.Guell X., Hoche F., Schmahmann J. D. Metalinguistic deficits in patients with cerebellar dysfunction: empirical support for the dysmetria of thought theory. Cerebellum . 2015;14(1):50–58. doi: 10.1007/s12311-014-0630-z. [DOI] [PubMed] [Google Scholar]
- 40.Hoche F., Guell X., Sherman J., Vangel M., Schmahmann J. J. C. Cerebellar contribution to social cognition. The Cerebellum . 2016;15(6):732–743. doi: 10.1007/s12311-015-0746-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Torriero S., Oliveri M., Koch G., et al. Cortical networks of procedural learning: evidence from cerebellar damage. Neuropsychologia . 2007;45(6):1208–1214. doi: 10.1016/j.neuropsychologia.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 42.Paulus K., Magnano I., Conti M., et al. Pure post-stroke cerebellar cognitive affective syndrome: a case report. Neurological Sciences . 2004;25(4):220–224. doi: 10.1007/s10072-004-0325-1. [DOI] [PubMed] [Google Scholar]
- 43.Annoni J., Ptak R., Caldara-Schnetzer A., Khateb A., Pollermann B. Z. Decoupling of autonomic and cognitive emotional reactions after cerebellar stroke. Annals of Neurology . 2003;53(5):654–658. doi: 10.1002/ana.10549. [DOI] [PubMed] [Google Scholar]
- 44.Alexander M. P., Gillingham S., Schweizer T., Stuss D. T. Cognitive impairments due to focal cerebellar injuries in adults. Cortex . 2012;48(8):980–990. doi: 10.1016/j.cortex.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 45.Nickel A., Cheng B., Pinnschmidt H., et al. Clinical outcome of isolated cerebellar stroke-a prospective observational study. Frontiers in Neurology . 2018;9:p. 580. doi: 10.3389/fneur.2018.00580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Erdal Y., Perk S., Keskinkılıc C., Bayramoglu B., Mahmutoglu A. S., Emre U. The assessment of cognitive functions in patients with isolated cerebellar infarctions: a follow-up study. Neuroscience Letters . 2021;765, article 136252 doi: 10.1016/j.neulet.2021.136252. [DOI] [PubMed] [Google Scholar]
- 47.Noroozian M. The role of the cerebellum in cognition: beyond coordination in the central nervous system. Neurologic Clinics . 2014;32(4):1081–1104. doi: 10.1016/j.ncl.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 48.Hoche F., Guell X., Vangel M. G., Sherman J. C., Schmahmann J. D. The cerebellar cognitive affective/Schmahmann syndrome scale. Brain . 2018;141(1):248–270. doi: 10.1093/brain/awx317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chirino-Pérez A., Marrufo-Meléndez O., Muñoz-López J., et al. Mapping the Cerebellar Cognitive Affective Syndrome in Patients with Chronic Cerebellar Strokes. The Cerebellum . 2021;2022(21):208–218. doi: 10.1007/s12311-021-01290-3. [DOI] [PubMed] [Google Scholar]
- 50.Shin M. A., Park O. T., Shin J. H. Anatomical correlates of neuropsychological deficits among patients with the cerebellar stroke. Annals of Rehabilitation Medicine . 2017;41(6):924–934. doi: 10.5535/arm.2017.41.6.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stoodley C. J., MacMore J. P., Makris N., Sherman J. C., Schmahmann J. D. Location of lesion determines motor vs. cognitive consequences in patients with cerebellar stroke. NeuroImage: Clinical . 2016;12:765–775. doi: 10.1016/j.nicl.2016.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Richter S., Aslan B., Gerwig M., et al. Patients with chronic focal cerebellar lesions show no cognitive abnormalities in a bedside test. Neurocase . 2007;13(1):25–36. doi: 10.1080/13554790601186942. [DOI] [PubMed] [Google Scholar]
- 53.Schmahmann J. D., Doyon J., McDonald D., et al. Three-dimensional MRI atlas of the human cerebellum in proportional stereotaxic space. NeuroImage . 1999;10(3):233–260. doi: 10.1006/nimg.1999.0459. [DOI] [PubMed] [Google Scholar]
- 54.Stoodley C. J., Schmahmann J. D. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. NeuroImage . 2009;44(2):489–501. doi: 10.1016/j.neuroimage.2008.08.039. [DOI] [PubMed] [Google Scholar]
- 55.Stoodley C., Valera E., Schmahmann J. J. N. Functional topography of the cerebellum for motor and cognitive tasks: an fMRI study. NeuroImage . 2012;59(2):1560–1570. doi: 10.1016/j.neuroimage.2011.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.King M., Hernandez-Castillo C., Poldrack R., Ivry R., Diedrichsen J. Functional boundaries in the human cerebellum revealed by a multi-domain task battery. 2019;22(8):1371–1378. doi: 10.1038/s41593-019-0436-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guell X., Gabrieli J. D. E., Schmahmann J. D. Triple representation of language, working memory, social and emotion processing in the cerebellum: convergent evidence from task and seed-based resting-state fMRI analyses in a single large cohort. NeuroImage . 2018;172:437–449. doi: 10.1016/j.neuroimage.2018.01.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schmahmann J. D. Disorders of the cerebellum: ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. The Journal of Neuropsychiatry and Clinical Neurosciences . 2004;16(3):367–378. doi: 10.1176/jnp.16.3.367. [DOI] [PubMed] [Google Scholar]
- 59.Diedrichsen J., King M., Hernandez-Castillo C., Sereno M., Ivry R. J. N. Universal transform or multiple functionality? Understanding the contribution of the human cerebellum across task domains. Neuron . 2019;102(5):918–928. doi: 10.1016/j.neuron.2019.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bernard J. A., Seidler R. D., Hassevoort K. M., et al. Resting state cortico-cerebellar functional connectivity networks: a comparison of anatomical and self-organizing map approaches. Frontiers in Neuroanatomy . 2012;6:p. 31. doi: 10.3389/fnana.2012.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Buckner R., Krienen F., Castellanos A., Diaz J., Yeo B. T. T. The organization of the human cerebellum estimated by intrinsic functional connectivity. Journal of Neurophysiology . 2011;106(5):2322–2345. doi: 10.1152/jn.00339.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Middleton F., Strick P. L. Cerebellar projections to the prefrontal cortex of the primate. Journal of Neuroscience . 2001;21(2):700–712. doi: 10.1523/JNEUROSCI.21-02-00700.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guell X., Schmahmann J., Gabrieli J., Ghosh S. S. Functional gradients of the cerebellum. eLife . 2018;7, article e36652 doi: 10.7554/eLife.36652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bates E., Wilson S. M., Saygin A. P., et al. Voxel-based lesion-symptom mapping. Nature Neuroscience . 2003;6(5):448–450. doi: 10.1038/nn1050. [DOI] [PubMed] [Google Scholar]
- 65.Urban P. P., Marx J., Hunsche S., et al. Cerebellar speech representation: lesion topography in dysarthria as derived from cerebellar ischemia and functional magnetic resonance imaging. Archives of Neurology . 2003;60(7):965–972. doi: 10.1001/archneur.60.7.965. [DOI] [PubMed] [Google Scholar]
- 66.Baier B., Dieterich M., Stoeter P., Birklein F., Muller N. G. Anatomical correlate of impaired covert visual attentional processes in patients with cerebellar lesions. The Journal of Neuroscience . 2010;30(10):3770–3776. doi: 10.1523/JNEUROSCI.0487-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Burciu R., Reinold J., Rabe K., et al. Structural correlates of motor adaptation deficits in patients with acute focal lesions of the cerebellum. Experimental Brain Research . 2014;232(9):2847–2857. doi: 10.1007/s00221-014-3956-3. [DOI] [PubMed] [Google Scholar]
- 68.Thomasson M., Benis D., Saj A., et al. Sensory contribution to vocal emotion deficit in patients with cerebellar stroke. NeuroImage: Clinical . 2021;31, article 102690 doi: 10.1016/j.nicl.2021.102690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thomasson M., Saj A., Benis D., Grandjean D., Assal F., Peron J. Cerebellar contribution to vocal emotion decoding: insights from stroke and neuroimaging. Neuropsychologia . 2019;132, article 107141 doi: 10.1016/j.neuropsychologia.2019.107141. [DOI] [PubMed] [Google Scholar]
- 70.Chen S. H., Ho M. H., Desmond J. E. A meta-analysis of cerebellar contributions to higher cognition from PET and fMRI studies. Human Brain Mapping . 2014;35(2):593–615. doi: 10.1002/hbm.22194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schmahmann J. D., Pandya D. N. The cerebrocerebellar system. International Review of Neurobiology . 1997;41:31–60. doi: 10.1016/S0074-7742(08)60346-3. [DOI] [PubMed] [Google Scholar]
- 72.Schmahmann J. D. The cerebrocerebellar system: anatomic substrates of the cerebellar contribution to cognition and emotion. International Review of Psychiatry . 2001;13(4):247–260. doi: 10.1080/09540260120082092. [DOI] [Google Scholar]
- 73.Wang D., Yao Q., Yu M., et al. Topological disruption of structural brain networks in patients with cognitive impairment following cerebellar infarction. Frontiers in Neurology . 2019;10:p. 759. doi: 10.3389/fneur.2019.00759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fan L., Hu J., Ma W., Wang D., Yao Q., Shi J. Altered baseline activity and connectivity associated with cognitive impairment following acute cerebellar infarction: a resting-state fMRI study. Neuroscience Letters . 2019;692:199–203. doi: 10.1016/j.neulet.2018.11.007. [DOI] [PubMed] [Google Scholar]
- 75.Broich K., Hartmann A., Biersack H., Horn R. Crossed cerebello-cerebral diaschisis in a patient with cerebellar infarction. Neuroscience Letters . 1987;83(1-2):7–12. doi: 10.1016/0304-3940(87)90207-2. [DOI] [PubMed] [Google Scholar]
- 76.Strother M., Buckingham C., Faraco C., et al. Crossed cerebellar diaschisis after stroke identified noninvasively with cerebral blood flow-weighted arterial spin labeling MRI. European Journal of Radiology . 2016;85(1):136–142. doi: 10.1016/j.ejrad.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.van Niftrik C., Sebök M., Muscas G., et al. Investigating the association of wallerian degeneration and diaschisis after ischemic stroke with BOLD cerebrovascular reactivity. Frontiers in Physiology . 2021;12, article 645157 doi: 10.3389/fphys.2021.645157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Saita K., Ogata T., Watanabe J., et al. Contralateral cerebral hypometabolism after cerebellar stroke: a functional near-infrared spectroscopy study. Journal of Stroke and Cerebrovascular Diseases . 2017;26(4):e69–e71. doi: 10.1016/j.jstrokecerebrovasdis.2017.01.014. [DOI] [PubMed] [Google Scholar]
- 79.Baillieux H., De Smet H. J., Dobbeleir A., Paquier P. F., De Deyn P. P., Marien P. Cognitive and affective disturbances following focal cerebellar damage in adults: a neuropsychological and SPECT study. Cortex . 2010;46(7):869–879. doi: 10.1016/j.cortex.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 80.Fujii M., Tanigo K., Yamamoto H., et al. A case of dysgraphia after cerebellar infarction where functional NIRS guided the task aimed at activating the hypoperfused region. Case Reports in Neurological Medicine . 2021;2021:9. doi: 10.1155/2021/6612541.6612541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Botez M. I., Léveillé J., Lambert R., Botez T. Single photon emission computed tomography (SPECT) in cerebellar disease: cerebello-cerebral diaschisis. European Neurology . 2004;31(6):405–412. doi: 10.1159/000116705. [DOI] [PubMed] [Google Scholar]
- 82.Stoodley C. J., Schmahmann J. D. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex . 2010;46(7):831–844. doi: 10.1016/j.cortex.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Knecht S., Deppe M., Dräger B., et al. Language lateralization in healthy right-handers. Brain . 2000;123(1):74–81. doi: 10.1093/brain/123.1.74. [DOI] [PubMed] [Google Scholar]
- 84.Ferrara K., Seydell-Greenwald A., Chambers C., Newport E., Landau B. Development of bilateral parietal activation for complex visual-spatial function: evidence from a visual-spatial construction task. Developmental Science . 2021;24(4, article e13067) doi: 10.1111/desc.13067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Keller A., Arissian K., Asanuma H. Formation of new synapses in the cat motor cortex following lesions of the deep cerebellar nuclei. Experimental Brain Research . 1990;80(1):23–33. doi: 10.1007/BF00228843. [DOI] [PubMed] [Google Scholar]
- 86.Conrad J., Habs M., Ruehl M., et al. Structural reorganization of the cerebral cortex after vestibulo-cerebellar stroke. NeuroImage: Clinical . 2021;30, article 102603 doi: 10.1016/j.nicl.2021.102603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schmahmann J. D. Dysmetria of thought: clinical consequences of cerebellar dysfunction on cognition and affect. Trends in Cognitive Sciences . 1998;2(9):362–371. doi: 10.1016/S1364-6613(98)01218-2. [DOI] [PubMed] [Google Scholar]
- 88.Braitenberg V., Heck D., Sultan F. The detection and generation of sequences as a key to cerebellar function: experiments and theory. Behavioral and Brain Sciences . 1997;20(2):229–245. doi: 10.1017/S0140525X9700143X. [DOI] [PubMed] [Google Scholar]
- 89.Ivry R., Keele S. W. Timing functions of the cerebellum. Journal of Cognitive Neuroscience . 1989;1(2):136–152. doi: 10.1162/jocn.1989.1.2.136. [DOI] [PubMed] [Google Scholar]
- 90.Fiez J., Petersen S., Cheney M. Impaired non-motor learning and error detection associated with cerebellar damage. A single case study. Brain . 1992;115(1):155–178. doi: 10.1093/brain/115.1.155. [DOI] [PubMed] [Google Scholar]
- 91.Molinari M., Leggio M. G., Solida A., et al. Cerebellum and procedural learning: evidence from focal cerebellar lesions. Brain . 1997;120(10):1753–1762. doi: 10.1093/brain/120.10.1753. [DOI] [PubMed] [Google Scholar]
- 92.Doyon J., Laforce R., Jr., Bouchard G., et al. Role of the striatum, cerebellum and frontal lobes in the automatization of a repeated visuomotor sequence of movements. Neuropsychologia . 1998;36(7):625–641. doi: 10.1016/S0028-3932(97)00168-1. [DOI] [PubMed] [Google Scholar]
- 93.Paulin M. J. B. The role of the cerebellum in motor control and perception. Brain, Behavior and Evolution . 2004;41(1):39–50. doi: 10.1159/000113822. [DOI] [PubMed] [Google Scholar]
- 94.Ito M. Cerebellar circuitry as a neuronal machine. Progress in Neurobiology . 2006;78(3-5):272–303. doi: 10.1016/j.pneurobio.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 95.Raymond J., Medina J. F. Computational principles of supervised learning in the cerebellum. Annual Review of Neuroscience . 2018;41(1):233–253. doi: 10.1146/annurev-neuro-080317-061948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Van Overwalle F. Social cognition and the brain: a meta-analysis. Human Brain Mapping . 2009;30(3):829–858. doi: 10.1002/hbm.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Van Overwalle F., Baetens K., Mariën P., Vandekerckhove M. Social cognition and the cerebellum: a meta-analysis of over 350 fMRI studies. NeuroImage . 2014;86:554–572. doi: 10.1016/j.neuroimage.2013.09.033. [DOI] [PubMed] [Google Scholar]
- 98.Grimaldi G., Argyropoulos G. P., Boehringer A., et al. Non-invasive cerebellar stimulation--a consensus paper. Cerebellum . 2014;13(1):121–138. doi: 10.1007/s12311-013-0514-7. [DOI] [PubMed] [Google Scholar]
- 99.Pope P. A., Miall R. C. Restoring cognitive functions using non-invasive brain stimulation techniques in patients with cerebellar disorders. Frontiers in Psychiatry . 2014;5:p. 33. doi: 10.3389/fpsyt.2014.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Brady R. O., Jr., Gonsalvez I., Lee I., et al. Cerebellar-prefrontal network connectivity and negative symptoms in schizophrenia. American Journal of Psychiatry . 2019;176(7):512–520. doi: 10.1176/appi.ajp.2018.18040429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Woodward N., Cascio C. J. Resting-state functional connectivity in psychiatric disorders. JAMA Psychiatry . 2015;72(8):743–744. doi: 10.1001/jamapsychiatry.2015.0484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Miterko L., Baker K., Beckinghausen J., et al. Consensus paper: experimental neurostimulation of the cerebellum. Cerebellum . 2019;18(6):1064–1097. doi: 10.1007/s12311-019-01041-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cassani R., Novak G., Falk T., Oliveira A. A. Virtual reality and non-invasive brain stimulation for rehabilitation applications: a systematic review. Journal of neuroengineering and rehabilitation . 2020;17(1):p. 147. doi: 10.1186/s12984-020-00780-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Butti N., Biffi E., Genova C., et al. Virtual Reality Social Prediction Improvement and Rehabilitation Intensive Training (VR-SPIRIT) for paediatric patients with congenital cerebellar diseases: study protocol of a randomised controlled trial. Trials . 2020;21(1):p. 82. doi: 10.1186/s13063-019-4001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Diedrichsen J., Zotow E. Surface-based display of volume-averaged cerebellar imaging data. PLoS One . 2015;10(7, article e0133402) doi: 10.1371/journal.pone.0133402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gordon N. The cerebellum and cognition. European Journal of Paediatric Neurology . 2007;11(4):232–234. doi: 10.1016/j.ejpn.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 107.D'Mello A., Stoodley C. J. Cerebro-cerebellar circuits in autism spectrum disorder. Frontiers in Neuroscience . 2015;9:p. 408. doi: 10.3389/fnins.2015.00408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Schmahmann J., Guell X., Stoodley C., Halko M. The theory and neuroscience of cerebellar cognition. Annual Review of Neuroscience . 2019;42(1):337–364. doi: 10.1146/annurev-neuro-070918-050258. [DOI] [PubMed] [Google Scholar]


