Abstract
We evaluated the efficacy of voriconazole, a new broad-spectrum triazole antifungal compound, in the treatment of murine pulmonary blastomycosis. Since mice metabolize voriconazole rapidly, we took advantage of our previous observation that administration of grapefruit juice to mice resulted in suitable serum voriconazole concentrations so that treatment studies with mice could be done (A. M. Sugar and X.-P. Liu, Med. Mycol. 38:209–121, 2000). Our results show that voriconazole prolonged survival in a dose-dependent fashion and that the fungal burden in the lungs was decreased by voriconazole administered at 40 mg/kg of body weight/day. Voriconazole should be studied in humans with blastomycosis.
Blastomycosis is a fungal infection that occurs primarily in specific areas of the United States. While several antifungals have been useful in the treatment of patients with the disease, including itraconazole, amphotericin B, and fluconazole, additional options would be welcome. In the study described here, we used a well-described murine model of pulmonary blastomycosis to evaluate the antifungal efficacy of voriconazole, a new broad-spectrum triazole antifungal drug (5–7, 10).
Early work with voriconazole documented that serum voriconazole concentrations were very low to undetectable in mice. This necessitated a switch to other animals, such as guinea pigs, in order to continue the preclinical development of voriconazole. Unfortunately, guinea pigs are resistant to the development of pulmonary blastomycosis. We then found that grapefruit juice could increase the concentrations of voriconazole in serum in the mouse to suitable levels to conduct treatment studies (14). In the present study, we made use of this pharmacokinetic interaction to study the efficacy of voriconazole in the treatment of murine pulmonary blastomycosis.
Male specific-pathogen-free BALB/cByJ mice were obtained from Jackson Laboratories. Mice were housed at 10 mice per cage. They were fed mouse chow and water ad libitum. Five days before infection, the water was replaced with grapefruit juice in the water bottle and was provided to the mice ad libitum. On average, mice ingested 2 to 3 ml of grapefruit juice/day, as determined in our previous study (14).
Blastomyces dermatitidis ATCC 26199 was obtained as a new culture from the American Type Culture Collection (Manassas, Va.) and was maintained in the yeast form in 1% sterile milk in yeast nitrogen broth at −70°C. Prior to an experiment, a loopful was plated on blood agar and the plate was incubated at 37°C for 4 to 6 days. A 4- to 6-day growth from a subculture of this was used to prepare inocula for infection of mice.
The susceptibility of the yeast form of B. dermatitidis to voriconazole was tested according to NCCLS guidelines (11), using the microtiter plate format. The (MIC) was read at 48 h and was defined as the first clear well. The minimal fungicidal concentration (MFC) was obtained by culturing the entire contents of the clear wells, with the well showing no growth representing the MFC.
The pulmonary infection model used in our previous studies was used in this study (9). Mice were lightly anesthetized with halothane. Approximately 5 × 103 or 5 × 104 CFU of B. dermatitidis yeasts/0.05 ml was placed on the nares of the mice, which were held in the upright position. Following aspiration of the droplet, the mice were replaced in their cages. Ten mice per group were used to determine survival, and additional mice (up to four mice) were used for lung cultures in each experiment.
Voriconazole was obtained as the powder from Pfizer Central Research (Groton, Conn.). Stock suspensions were prepared in 4% polyethylene glycol (PEG) 400. Control mice received PEG 400 only.
Treatment was begun 5 days following infection and was continued for 21 days. Voriconazole was administered once daily by oral gavage. Some groups were treated with voriconazole three times per week, and this is noted in the text. Mice were observed twice daily, deaths were recorded, and moribund mice unable to eat or drink were killed. The experiment was terminated on day 45.
At specified time points, mice were killed, the lungs were removed and homogenized, and appropriate dilutions were cultured on Sabouraud dextrose agar plates. These plates were incubated for 5 days at 37°C, and colonies were then counted.
Mice were bled approximately 4 h following dosing on day 11 of the study and on day 23, 2 days after therapy was completed. Blood was allowed to clot at room temperature and centrifuged, and the sera were stored at −20°C until the assay was performed.
Voriconazole standards (twofold dilutions ranging from 0.6125 to 10 μg/ml) were prepared in PEG 400 from a stock solution of 5,000 μg/ml. The stock solution was diluted in PEG 400 to a concentration of 100 μg/ml. Subsequent dilutions were made in mouse serum. Candida kefyr from a 2- to 7-day-old yeast nitrogen agar plate was used to inoculate 5 ml of yeast nitrogen broth, and this was incubated for 5 to 6 h at 37°C on a roller drum. Yeast nitrogen agar was melted and kept in a water bath at 48°C. The yeast suspension was adjusted to a number 2 McFarland standard. A 500-μl aliquot of this yeast suspension was added to the agar (50 ml), and the components were mixed and poured into petri dishes. Wells that accommodated 50-μl volumes were formed in the agar. Sera obtained from voriconazole-treated mice were assayed in duplicate on each plate. Zone sizes were read after 24 h of incubation at 35°C. The correlation coefficient was ≥0.99.
Comparisons of the Kaplan-Meier survival curves for each treatment group were performed by using the log rank statistic. Colony counts in the lungs were compared by using the Student t test. Significance was defined as a P value of ≤0.05. These analyses were performed on a personal computer with a commercially available statistics program (SPSS, Chicago, Ill.).
The MIC and MFC for the yeast form of B. dermatitidis ATCC 26199 were 2 and 64 μg/ml, respectively.
In the first experiment, mice were infected with 5 × 103 B. dermatitidis yeasts. As shown in Fig. 1, all control mice had died by day 2, with a median survival time (MST) of 21 days. Voriconazole treatment resulted in improved survival in a dose-response fashion, with MSTs of 21, 28, and 35 days with doses of 1, 5, and 20 mg/kg of body weight/day, respectively (voriconazole at 20 mg/kg/day versus the control and voriconazole at 1 mg/kg/day, P < 0.03; voriconazole at 20 mg/kg/day versus voriconazole at 5 mg/kg/day, not significant; voriconazole at 1 mg/kg/day compared to the control, P > 0.05; voriconazole at 5 mg/kg/day compared to the control, P < 0.05).
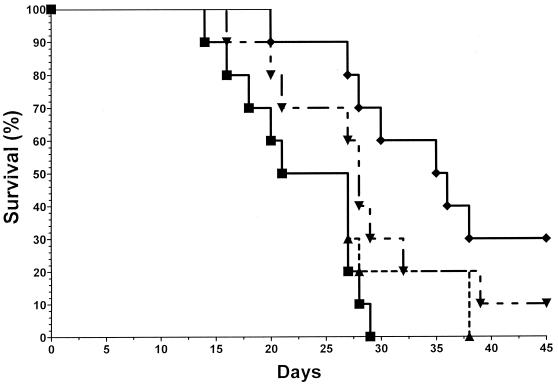
FIG. 1.
Mice were infected with 5 × 103 CFU of B. dermatitidis yeasts and were treated with diluent (no drug; ■) or voriconazole at 1 (▴), 5 (▾), or 20 (⧫) mg/kg/day for 21 days. All mice in the control group died by day 29. In contrast, voriconazole provided protection in a dose-response fashion.
In a follow-up experiment, mice were infected with 5 × 104 yeasts and were treated with voriconazole at 0, 1, 5, or 20 mg/kg/day, and one group was treated with 20 mg/kg three times weekly (on Monday, Wednesday, and Friday) for 21 days. As in the previous experiment, voriconazole afforded improved survival in a dose-response fashion, with MSTs of 22, 22, 25, 39, and 28 days, respectively. The highest dose of voriconazole, 20 mg/kg/day, was significantly more efficacious than any of the other treatments administered (P < 0.01).
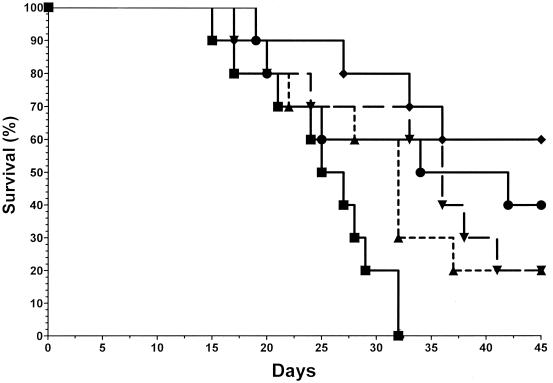
In a third experiment, mice were infected with 5.0 × 104 yeasts, and the results were similar to those described above for the higher doses of voriconazole being studied. All control mice died by day 32 (MST = 25 day) (Fig. 2). The lowest dose used in this experiment, 5 mg/kg/day, prolonged the MST slightly to 32 days, and the overall survival rate was 20%. However, this was not significantly different from the values seen for the control group (P = 0.063). In contrast, the three other treatment groups had significantly improved survivals and MSTs compared to those for the controls, with 20% (MST, 36 days), 60% (MST, >45 days), and 40% (MST, 34 days) survival rates observed for mice treated with 20 mg/kg/day, 40 mg/kg/day, or 40 mg/kg three times weekly, respectively (P ≤ 0.025 for all groups compared to the results for the control). The best results were obtained with the 40-mg/kg/day dosing regimen.
FIG. 2.
Mice were infected with 5 × 104 CFU of B. dermatitidis yeasts and were treated with diluent (no drug; ■) or voriconazole at 5 (▴), 20 (▾), or 40 (⧫) mg/kg/day or 40 mg/kg three times per week (●) for 21 days. All mice in the control group died by day 32. In contrast, voriconazole provided protection in a dose-response fashion.
Serum voriconazole concentrations were measured in this experiment. As shown in Table 1, voriconazole was detectable in serum obtained on day 11 of therapy and on day 23, 2 days following discontinuation of therapy. No differences were seen in mice treated with 5 or 20 mg/kg/day, but treatment with 40 mg/kg/day resulted in higher levels in serum. Notably, when voriconazole was administered three times weekly, the concentration in serum at the end of the experiment (2.8 μg/ml) was higher than that during therapy (1.6 μg/ml).
TABLE 1.
Serum voriconazole concentrationsa
| Dose | Serum voriconazole concn (μg/ml)
|
|
|---|---|---|
| Day 11 | Day 23 | |
| 5 mg/kg/day | 1.6 ± 0.2 | 2.2 ± 0.1 |
| 20 mg/kg/day | 1.6 ± 0.2 | 1.7 ± 0.1 |
| 40 mg/kg/day | 4.7 ± 0.2 | 4.4 ± 0.1 |
| 40 mg/kg three times/wk | 1.6 ± 0.2 | 2.8 ± 0.5 |
Values are means ± standard deviations of values obtained from six mice at each time point. Blood was obtained on day 11 (midtreatment, approximately 4 h after dosing) and day 23, 2 days after treatment was stopped. Day 11 was Thursday, and thus, mice in the group that received voriconazole at 40 mg/kg three times per week (on Mondays, Wednesdays, and Fridays) received voriconazole approximately 24 h earlier.
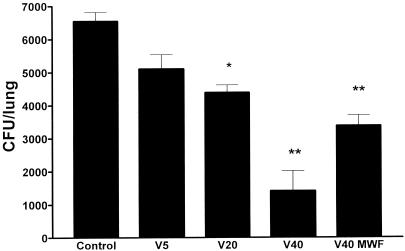
In this experiment, lung cultures were also done. As shown in Fig. 3, voriconazole at 40 mg/kg/day produced a significant suppression in the numbers of CFU compared to those for the controls and the groups treated with at voriconazole 5 and 20 mg/kg/day (P = 0.003). Suppression of colony counts was similar in mice treated with 20 mg/kg/day or 40 mg/kg three times weekly.
FIG. 3.
Numbers of CFU recovered from culture of the left lung on day 23, 1 day after therapy was discontinued. ∗, P < 0.05 compared to control; ∗∗, P < 0.01 compared to control. Voriconazole at 40 mg/kg/day (V40) resulted in greater suppression of CFU than voriconazole at 20 mg/kg/day (V20) (P = 0.003). V5, voriconazole at 5 mg/kg/day; V40 MWF, voriconazole at 40 mg/kg three times a week (Mondays, Wednesdays, and Fridays).
The results obtained in this study with a well-described murine model of blastomycosis demonstrates that voriconazole is efficacious in this setting. While measurable concentrations of voriconazole were detectable in the mice that were receiving grapefruit juice, the optimal concentration in serum for the best efficacy is not known. Therefore, over interpretation of our results with respect to lung sterilization or overall survival in treated mice is not warranted. The data do show that voriconazole prolongs survival in a dose-dependent manner and that fungal burden in the lungs is decreased. Whether higher doses, more frequent administration, or longer duration of therapy would improve the activity of voriconazole in this model, either in survival or in sterilization of organs, is not known.
Previous work from our laboratory with this same murine model of pulmonary blastomycosis showed that fluconazole given at 25 mg/kg twice daily resulted in 100% survival but that no decrease in colony counts in the lung occurred (9). Itraconazole at 150 mg/kg/day divided into two doses afforded a survival rate of 30% when it was delivered in PEG 400, with minimal effects on the colony counts in the lungs (13). However, itraconazole absorption is not optimal when the compound is suspended in PEG, and blood itraconazole levels were not determined in that study. In contrast, amphotericin B at 1 mg/kg/day provided complete protection in terms of survival and sterilized the lungs of these mice (13). Thus, voriconazole, especially when it is administered at 40 mg/kg/day, compares favorably with the other azoles. Further formal study of the pharmacokinetics of voriconazole in mice would help provide a more completely understanding of the optimal dosing of the drug for various mycoses in mice and would allow more detailed comparisons of the efficacy of voriconazole with those of other antifungal compounds.
Itraconazole has become the major antifungal drug for use in the oral treatment of blastomycosis in humans (3). However, absorption of itraconazole capsules is erratic, and drug interactions may be problematic in certain patients. Fluconazole, while effective in this murine model in terms of prolongation of survival (9), has not been as effective as itraconazole in the clinical situation when prescribed at doses of 200 to 400 mg/day (3). Therefore, new antifungal drugs would be useful in providing options to clinicians who must treat patients with this mycosis. The data presented in this paper suggest that voriconazole compares favorably to itraconazole (1) in the murine model of pulmonary blastomycosis and that studies in the treatment of human blastomycosis are warranted.
It should be stressed that voriconazole was administered once daily for 7 days/week or only 3 days/week. The optimal regimen for achievement of maximal concentrations of voriconazole in serum in mice is not known. Serum voriconazole concentrations appeared to increase over the course of the experiment in mice treated three times weekly. However, the initial measurement was obtained about 24 h after administration of the dose, thereby reflecting the effects of drug metabolism and redistribution over that period of time. The peak concentration of voriconazole in mice dosed daily or thrice weekly is not known and was not studied here. Given the complicated effects of voriconazole metabolism in mice and the compounding effects of grapefruit juice, more detailed investigation of the pharmacokinetics of this azole in the mouse is needed.
Finally, the mechanism of action of grapefruit juice in increasing serum voriconazole concentrations is not known but presumably involves inhibition of cytochrome P450 enzymes in the intestines and/or liver (2, 4). Given that grapefruit juice decreases serum itraconazole concentrations in humans by a mechanism that remains to be determined (12), that voriconazole is structurally related to itraconazole, and that there is an important pharmacokinetic interaction between voriconazole and grapefruit juice in the mouse, studies of this potential interaction in humans are warranted. Patients taking voriconazole should be instructed to avoid drinking grapefruit juice (8) until further information concerning the potential interaction is available.
Acknowledgments
This work was supported in part by the Collaborative Medical Mycology Research Program (Pfizer, Inc., Roerig Division, and the Section of Infectious Diseases, Department of Medicine, Boston University School of Medicine).
Footnotes
Publication 018 from the Collaborative Medical Research Program.
REFERENCES
- 1.Arathoon E G, Brummer E, Stevens D A. Efficacy of itraconazole in blastomycosis in a murine model and comparison with ketoconazole. Mycoses. 1989;32(Suppl. 1):109–112. doi: 10.1111/j.1439-0507.1989.tb02300.x. [DOI] [PubMed] [Google Scholar]
- 2.Bailey D G, Kreeft J H, Munoz C, Freeman D J, Bend J R. Grapefruit juice-felodipine interaction: effect of naringin and 6′,7′-dihydroxybergamottin in humans. Clin Pharmacol Ther. 1998;64:248–256. doi: 10.1016/S0009-9236(98)90173-4. [DOI] [PubMed] [Google Scholar]
- 3.Bradsher R W. Therapy of blastomycosis. Semin Respir Infect. 1997;12:263–267. [PubMed] [Google Scholar]
- 4.Chan W K, Nguyen L T, Miller V P, Harris R Z. Mechanism-based inactivation of human cytochrome P450 3A4 by grapefruit juice and red wine. Life Sci. 1998;62:L135–L142. doi: 10.1016/s0024-3205(98)00013-7. [DOI] [PubMed] [Google Scholar]
- 5.Cuenca-Estrella M, Rodriguez-Tudela J L, Mellado E, Martinez-Suarez J V, Monzon A. Comparison of the in-vitro activity of voriconazole (UK-109, 496), itraconazole and amphotericin B against clinical isolates of Aspergillus fumigatus. J Antimicrob Chemother. 1998;42:531–533. doi: 10.1093/jac/42.4.531. [DOI] [PubMed] [Google Scholar]
- 6.Espinel-Ingroff A. In vitro activity of the new triazole voriconazole (UK-109,496) against opportunistic filamentous and dimorphic fungi and common and emerging yeast pathogens. J Clin Microbiol. 1998;36:198–202. doi: 10.1128/jcm.36.1.198-202.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghannoum M A, Okogbule-Wonodi I, Bhat N, Sanati H. Antifungal activity of voriconazole (UK-109,496), fluconazole and amphotericin B against hematogenous Candida krusei infection in neutropenic guinea pig model. J Chemother. 1999;11:34–39. doi: 10.1179/joc.1999.11.1.34. [DOI] [PubMed] [Google Scholar]
- 8.Lundahl J U, Regardh C G, Edgar B, Johnsson G. The interaction effect of grapefruit juice is maximal after the first glass. Eur J Clin Pharmacol. 1998;54:75–81. doi: 10.1007/s002280050424. [DOI] [PubMed] [Google Scholar]
- 9.Lyman C A, Sugar A M, Diamond R D. Comparative activities of UK-49,858 and amphotericin B against Blastomyces dermatitidis infections in mice. Antimicrob Agents Chemother. 1986;29:161–162. doi: 10.1128/aac.29.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marco F, Pfaller M A, Messer S, Jones R N. In vitro activities of voriconazole (UK-109,496) and four other antifungal agents against 394 clinical isolates of Candida spp. Antimicrob Agents Chemother. 1998;42:161–163. doi: 10.1128/aac.42.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing for yeasts. Proposed standard. Document M27-P. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1992. [Google Scholar]
- 12.Penzak S R, Gubbins P O, Gurley B J, Wang P L, Saccente M. Grapefruit juice decreases the systemic availability of itraconazole capsules in healthy volunteers. Ther Drug Monit. 1999;21:304–309. doi: 10.1097/00007691-199906000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Sugar A M, Liu X-P. Treatment of murine pulmonary blastomycosis with SCH 51048, a broad-spectrum triazole antifungal agent. Antimicrob Agents Chemother. 1995;39:996–997. doi: 10.1128/aac.39.4.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sugar A M, Liu X-P. Effect of grapefruit juice on serum voriconazle concentrations in the mouse. Med Mycol. 2000;38:209–212. doi: 10.1080/mmy.38.3.209.212. [DOI] [PubMed] [Google Scholar]