Abstract
Hydrolyzed collagen (HC) from defatted Asian sea bass skin was prepared by different enzymatic hydrolysis processes. For one-enzyme hydrolysis, papain (0.3 unit per g dry matter, DM) at 40 °C for 90 min or Alcalase (0.2 or 0.3 unit per g DM) at 50 °C for 90 min were used. The two-enzyme hydrolysis was accomplished with papain at 0.3 unit per g DM (P0.3), followed by Alcalase hydrolysis at 0.2 or 0.3 units per g DM (A0.2 or A0.3, respectively). HC prepared using the P0.3 + A0.3 process showed higher peptide yield, recovery and imino acid content in addition to stronger ABTS, DPPH radical scavenging activities and ferric reducing antioxidant power than other hydrolysis processes. HC obtained from the P0.3 + A0.3 process (at 125–500 μg mL−1) induced MRC-5 fibroblast proliferation and augmented migration and lamellipodia formation in the cells. Peptides with average molecular weight of 750 Da exhibited the highest ABTS radical scavenging activity while the 4652 Da fraction had the lowest. Thus, HC can be considered as a suitable ingredient to formulate functional products for skin nourishment and wound healing.
Hydrolyzed collagen (HC) from sea bass skin prepared using papain and Alcalase had antioxidant potency and could enhance MRC-5 cell proliferation and lamellipodia formation. HC can be used as a nutraceutical or functional food ingredient.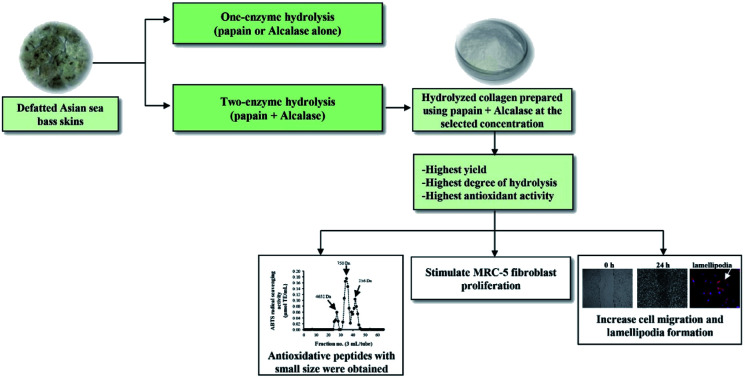
1. Introduction
Bioactive peptides from fish byproducts prepared using enzymatic hydrolysis have been associated with health-promotion since they possess various activities such as antioxidant, anti-hypertension and wound-healing.1 Nevertheless, differences in the bioactivity of peptides mostly depend on protein substrate and type of protease used, which cause variations in size, amino acid composition and sequence of the resultant peptides.2 Qiu et al.3 found that hydrolyzed collagen (HC) from skipjack tuna scale prepared by Alcalase hydrolysis showed a high radical scavenging activity. HC from tuna blood prepared using Neutrase had higher antioxidant activities and ACE-inhibitory activity when compared to those produced by other processes.4 Apart from the type of enzyme used, the hydrolysis sequence also affected the size as well as bioactivities of peptides obtained. For example, Yarnpakdee et al.5 documented that the size of peptides derived from Nile tilapia meat was effectively reduced by two-step hydrolysis process, in which Alcalase was used with subsequent hydrolysis by papain. Also, HC from salmon skin prepared with Alcalase and Flavourzyme hydrolysis had higher dipeptidyl peptidase IV (DPP-IV) inhibitory activity, when compared to those from Alcalase or Promod 144MG hydrolysis.6 Benjakul et al.2 reported that the use of papain, followed by Alcalase was the most effective in producing antioxidative peptides with small size from sea bass skin.
Generally, Asian sea bass skin (ASB-S) could yield bioactive peptides via enzymatic hydrolysis.1 HC from ASB-S had anti-inflammatory activity in lipopolysaccharide-treated raw 264.7 macrophage cells.7 Sea bass skin-derived peptides containing Gly–Leu–Phe–Gly–Pro–Arg showed the highest ABTS radical scavenging activity (ABTS-RS-A), which was reported by Sae-leaw et al.8 Recently, Chotphruethipong et al.9 documented that HC from defatted ASB-S prepared by papain hydrolysis had ability to inhibit reactive oxygen species (ROS) in skin cells. Also, the HC promoted cell proliferation and cell migration of fibroblast cells.10,11 Although HC from defatted ASB-S has several advantages, the peptide size should be more reduced and bioactivity need to be further improved. Previous studies showed that the use of the commercial protease hydrolysis could reduce the size of peptides and improve bioactivity of HC from sea bass skin effectively.2,12 New peptides with strong bioactivities, especially antioxidant and wound healing, could be produced to serve as potential functional ingredient for food supplement formulation. Therefore, the present study aimed to examine the effect of two commercial proteases and the sequence of enzyme hydrolysis on production of HC from defatted ASB-S as well as antioxidant, cell proliferation and would healing activities.
2. Materials and methods
2.1. Chemicals, cell line and Asian sea bass skin
Alcalase and papain were purchased from Siam Victory Chemicals Co, Ltd (Bangkok, Thailand). Porcine pancreas lipase (PPL), rhodamine phalloidin, protein markers and other chemicals were supplied by Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). Antibiotics, fetal bovine serum (FBS) and Dulbecco's Modified Eagle Medium (DMEM) were obtained from Gibco (Carlsbad, CA, USA). MRC-5 human lung fibroblast cells were procured from ATCC (Bethesda, MD, USA).
Asian sea bass skin were obtained from King-fisher holding, Co., Ltd, Songkhla, Thailand. The fish were raised in a farm in Kho Yor, Songkhla, in which the controlled aquaculture system was implemented for fish and environmental welfare.
2.2. Enzyme assays
PPL activity was measured following the protocol of Chotphruethipong et al.1 One unit of activity was defined as the amount of PPL that produced 1 μmol p-nitrophenol (p-NP) per min. Activities of papain (pH 7.0, 40 °C) and Alcalase (pH 8.0, 50 °C) were assayed for 15 min using casein as substrate.1,2 One unit of activity was defined as the amount of papain or Alcalase that liberates 0.01 μmol of tyrosine equivalent per min (μmol Tyr equiv. min−1).
2.3. Preparation and pretreatment of skin using pulsed electric field (PEF)
The skins (2 × 2 cm2) were first treated with 0.1 M NaOH and subsequently washed with tap water as described by Chotphruethipong et al.,1 prior to PEF pretreatment.13 Electric field intensity at 24 kV cm−1 with 72 ms pulses was used. Pulse duration, specific energy input and pulse repetition times were 0.1 ms, 135 kJ kg−1 and 20 ms, respectively. When PEF treatment was done, the skins were swollen using 0.05 M citric acid for 2 h, followed by washing until neutral pH was obtained.13
2.4. Defatting of PEF-treated skin using PPL in combination with vacuum impregnation (VI)
Swollen skins (200 g) were mixed with 1 L of PPL solution containing 42.36 unit per g (dry matter, DM) in a vacuum chamber.14 VI process was performed for 4 cycles under 100 kPa pressure as described by Chotphruethipong et al.14 The defatted swollen skin was used for preparing HC.
2.5. Preparation of HC by different hydrolysis processes
Defatted skins were subjected to hydrolysis using different enzymatic processes. Before hydrolysis, the swollen skins were added to water at the ratio of 1 : 5 (w/v) and then adjusted to pH 7.0 (for papain hydrolysis) or pH 8.0 (for Alcalase hydrolysis). For one-step hydrolysis, Alcalase (0.2 or 0.3 units per g DM) or papain (0.3 unit per g DM) was added. Hydrolysis was conducted at 40 °C for 90 min for papain or at 50 °C for 90 min for Alcalase using a temperature-controlled water bath (Model W350, Memmert, Schwabach, Germany). The conditions used for papain or Alcalase hydrolysis were chosen from previous study.1 Subsequently, termination of the reaction (90 °C, 15 min) was carried out. The papain hydrolysis was named as ‘P0.3’ while those of Alcalase hydrolysis at 0.2 and 0.3 units were named as ‘A0.2’ or ‘A0.3’, respectively. For two-step hydrolysis, the mixture obtained from papain hydrolysis at 0.3 unit per g DM was inactivated and further hydrolyzed using Alcalase (0.2 or 0.3 unit per g DM) at 50 °C for 90 min.2 The two-step processes using papain and Alcalase at different levels were referred to as ‘P0.3 + A0.2’ and ‘P0.3 + A0.3’. After enzyme was inactivated, the reaction mixtures were each centrifuged (9000×g at 4 °C), followed by lyophilization of the supernatant.1
2.5.1. Analyses
Yield and recovery
Extraction yield1 and recovery2 of HCs were calculated and reported.
Determination of α-amino group content (AAGC)
The total AAGC in HC samples was determined using TNBS reagent12 and expressed as mmol glycine equivalent (GE) per g sample.
Proximate analysis
HC samples were analyzed for protein, fat and ash contents using analytical method no. of 920.153, 960.39 and 928.08, respectively.15 The conversion factor used for calculation of protein content was 5.55 based on FAO/WHO.16 The data was reported as % dry weight basis.
Determination of antioxidative activities
ABTS and DPPH radical scavenging activities (ABTS-RS-A and DPPH-RS-A), and ferric reducing antioxidant power (FRAP) were determined and reported as μmol Trolox equivalents (TE) per g dry sample.17 Metal chelating activity (MCA) was examined and expressed as μmol EDTA equivalents (EE) per g dry sample.9 Ascorbic acids at a concentration of 25 mg mL−1 (based on the preliminary study) was used as positive control for all assays.
Process rendering the highest yield, recovery, AAGC and antioxidant activities was chosen for the following experiments.
2.6. Impact of the selected HC on proliferation and migration of MRC-5 fibroblast cells
2.6.1. Cell culture
The MRC-5 cells were cultured in complete DMEM medium containing 10% FBS and antibiotics.18
2.6.2. Cell proliferation assays
HC solutions at different concentrations (0, 125, 250 and 500 μg mL−1) were tested for the effect on cell proliferation using the protocol of Ritto et al.19 The levels without cytotoxicity were selected for following studies.
2.6.3. Cell migration assays
MRC-5 cells (5 × 105 cells per well) were first seeded in 6-well plates and wound was made by scratching as tailored by Chotphruethipong et al.10 Cells were treated with HC solutions for 0 and 24 h. Wound gap was measured using a microscope (Olympus IX70 with DP50, Shinjuku-ku, Tokyo, Japan) and calculated with the aid of ImageJ 1.41 software (NIH, Bethesda, MD, USA).18 The result was expressed as % wound area as detailed by Chotphruethipong et al.10
2.6.4. Lamellipodia formation
The method of Chotphruethipong et al.10 was adopted for monitoring lamellipodia formation. MRC-5 cells (5 × 103 cells per well) were seeded in 24-well plates and treated with HC at the selected levels for 0 h and 24 h. Thereafter, cells were stained with rhodamine phalloidin (10 μg mL−1) and morphology of cells was visualized by a microscope (Olympus IX70 with DP50, Shinjuku-ku, Tokyo, Japan).10 Lamellipodia formation was reported as relative fluorescence intensity to that of day 0.16
2.7. Molecular weight (MW) distribution
Sephadex G-25 gel filtration column (GE Healthcare Bio-Science AB, Uppsala, Sweden) was used for estimating the MW of peptides present in the selected HC. The fractions (3 mL) were pooled and tested for ABTS-RS-A. The MW of the selected fraction was calculated in comparison with those of protein markers, including blue dextran (2 000 000 Da), insulin chain B (3495.89 Da), vitamin B12 (1355.4 Da), glycine–tyrosine (238.25 Da) and tyrosine (181.2 Da).1
2.8. Amino acid analysis
Amino acid composition of the selected HC was determined11 using an amino acid analyzer (MLC-703; Atto Co., Tokyo, Japan).
2.9. Statistical analysis
All the experiments were run in triplicate and analysis of variance (ANOVA) was conducted for all the data. Proximate analysis was run in duplicate. The Duncan's multiple range test was used for mean comparison. Statistical Package for Social Science (SPSS 11.0 for windows, SPSS Inc., Chicago, IL, USA) was used for data analysis.
3. Results and discussion
3.1. Effect of protease types and hydrolysis processes on yield, recovery, and α-amino group content of hydrolyzed collagen (HC)
3.1.1. Yield and recovery
Yields of HC prepared using two types of proteases and different hydrolysis processes are presented in Table 1. Overall, higher yield of HC was found when two-step hydrolysis was conducted (p < 0.05), especially when high concentration of Alcalase was used. It was noted that the use of papain at 0.3 unit per g DM, followed by Alcalase at 0.3 unit per g DM (P0.3 + A0.3) increased the cleavage of skin proteins to a higher degree than other processes. During pre-incubation (40 °C, 90 min), heat induced the breakdown of the hydrogen bonds that stabilize tropocollagen in the defatted swollen skin. As a result, peptides could be released by trypsin present in porcine pancreas lipase with ease,1 which could loosen the skin matrix. Therefore, addition of papain in the first step of hydrolysis led to more collagen hydrolysis. Subsequently, the released protein and peptide substrates from papain hydrolysis were further hydrolyzed by Alcalase in the second step of hydrolysis. This sequential enzyme treatment contributed to higher cleavage of peptides with concomitant increase in yield. The results are similar to data reported by Benjakul et al.2 who prepared HC from sea bass skin without descaling using 3% papain hydrolysis prior to 2% Alcalase hydrolysis, in which a high extraction yield (49.64%) was attained. Nevertheless, HC yield obtained from P0.3 + A0.3 treatment (20%) in the present work was lower than the previously reported 49.64%. This is likely due to differences in the type or treatment of raw material used for HC production.
Yield, recovery and α-amino group content of hydrolyzed collagen (HC) from defatted Asian sea bass skin prepared using different hydrolysis processesa.
| Samples | Yield (%) | Recovery (%) | α-Amino group content (mmol GE/g sample) |
|---|---|---|---|
| A0.2 | 12.19 ± 0.94D | 16.16 ± 0.44E | 0.12 ± 0.00CD |
| A0.3 | 15.87 ± 0.08B | 17.90 ± 0.95D | 0.13 ± 0.00C |
| P0.3 | 14.42 ± 0.52C | 19.54 ± 0.17C | 0.11 ± 0.00D |
| P0.3 + A0.2 | 16.91 ± 0.02B | 21.55 ± 0.22B | 0.21 ± 0.00B |
| P0.3 + A0.3 | 20.00 ± 0.37A | 25.99 ± 0.28A | 0.22 ± 0.00A |
Values are mean ± SD (n = 3). Different superscripts in the same column indicate significant difference (p < 0.05). A0.2 or A0.3: HC prepared from defatted skin using Alcalase at 0.2 or 0.3 units per g dry matter at 50 °C for 90 min; P0.3: HC prepared from defatted skin using papain at 0.3 unit per g dry matter at 40 °C for 90 min; P0.3 + A0.2 or P0.3 + A0.3: HC prepared from defatted skin using papain at 0.3 unit per g dry matter, followed by hydrolysis using Alcalase at 0.2 or 0.3 unit per g dry matter, respectively.
For recovery, HC prepared using different enzymatic hydrolysis processes are shown in Table 1. The results were in tandem with yield values (Table 1), in which HC prepared using two-enzyme hydrolysis showed the higher recovery as compared to that prepared using one-enzyme. The results suggest that the two-enzyme hydrolysis using papain, followed by Alcalase cleaved more peptide bonds and loosened the skin matrix to a higher degree, leading to the liberation of peptides containing Hyp as ascertained by the increased recovery (Table 1). When comparing HC obtained from P0.3 + A0.2 process with P0.3 + A0.3 process, higher recovery was found for the latter (p < 0.05), suggesting that the increased level of Alcalase augmented recovery of the resulting HC.
3.1.2. The α-amino group content (AAGC)
AAGC in HC samples prepared using different enzymatic hydrolysis processes is depicted in Table 1. Lower AAGC was observed for HCs prepared using one-enzyme hydrolysis when compared to the two-enzyme counterpart (p < 0.05), regardless of enzyme concentration. Generally, two-enzyme hydrolysis using papain, followed by Alcalase (P + A) hydrolysis increased the cleavage of peptide bonds in protein substrates of defatted swollen skin as evidenced by the increased AAGC. Nevertheless, higher AAGC was attained for HC prepared using P + A hydrolysis process with increasing level of Alcalase (0.3 unit). The results suggest that Alcalase concentration directly affected AAGC of HC. The results were in tandem with the yield and recovery (Table 1) values, suggesting that hydrolysis with P0.3 + A0.3 protocol drastically augmented the cleavage of peptide bonds in HC. Commonly, papain was able to hydrolyze peptide bonds that involve glycine, basic amino acids or leucine,1 while Alcalase prefers to cleave peptide bonds made by hydrophobic residues.20 With papain hydrolysis, swollen skin might be more loosened and some peptides, particularly those with hydrophobic amino acids were released or exposed during incubation (40 °C, 90 min). Papain has been known to have the broad specificity toward the peptide hydrolysis.21 Subsequently, those substrates were further hydrolyzed by Alcalase added in the second step of hydrolysis, leading to increased AAGC. For HCs prepared using one-enzyme hydrolysis, higher AAGC was found for Alcalase when compared to that obtained from papain hydrolysis at the same level of addition (p < 0.05). Alcalase was reported to preferably cleave the peptides containing Ser, Asn, His and hydrophobic amino acids.22 Types of enzyme used generally had the effect on degree of hydrolysis of skin protein. Nevertheless, there was no difference in AAGC for HCs prepared using Alcalase hydrolysis at both levels tested (p > 0.05). Type of enzyme used was therefore an essential factor affecting degree of hydrolysis of HC from defatted ASB-S owing to their complementary specificity toward protein substrates.
3.2. Chemical compositions of defatted Asian sea bass skin HC powders
All samples had the similar protein content (96.58 ± 0.22–96.81 ± 0.34% dry weight basis) and fat content (0.08 ± 0.00–0.083 ± 0.00% dry weight basis) (data not shown). HC powder obtained from two-step hydrolysis (3.32 ± 0.12–3.34 ± 0.10% dry weight basis) showed slightly higher ash content as compared with that obtained from one-step hydrolysis (3.11 ± 0.08–3.14 ± 0.12% dry weight basis).
3.3. Antioxidant activities of defatted Asian sea bass skin HC as influenced by protease types and hydrolysis processes
Among samples tested, the HC prepared using Alcalase hydrolysis exhibited higher antioxidant activities than that prepared with papain (p < 0.05) as shown in Table 2. The use of Alcalase for preparing HC produced antioxidative peptides more effectively than papain. This may be because the two enzymes had varied influences on the resulting antioxidative peptides in HC such as differences in chain length as well as amino acid composition and sequence.1 Thus, the differences in ability in providing proton and electron as well as chelating metal ions were observed among HC prepared using the two enzymes. When considering Alcalase during one-enzyme hydrolysis, the antioxidant activities increased as the enzyme level was enhanced (p < 0.05). The results suggested increased liberation of antioxidative peptides at higher enzyme levels, except for MCA that had no difference (p > 0.05), regardless of enzyme level used. With the two-enzyme hydrolysis, HC prepared using P0.3 + A0.3 process had higher ABTS-RS-A, FRAP and DPPH-RS-A when compared to those prepared using P0.3 + A0.2 process (p < 0.05). Thus, sequential hydrolysis with different levels of proteases had marked impact on the generation of peptides with different antioxidant activities. When compared with ascorbic acid, HCs prepared using one/two steps possessed stronger ABTS-RS-A and MCA than those of ascorbic acid (p < 0.05), indicating that HCs were the potent antioxidants capable of scavenging ABTS˙+ radical and chelating metal ions. In addition, DPPH-RSA of HCs prepared using two-step hydrolysis was higher than ascorbic acid (p < 0.05). This result suggested that process used could be the vital factor governing antioxidant activity of the resulting HCs. Nevertheless, HCs had a weaker FRAP than ascorbic acid, which has been known as the powerful reducing agent.23 Sai-Ut et al.24 prepared HC from unicorn leatherjacket skin using Bacillus amyloliquefaciens H11 protease. The resulting HC showed greater ABTS-RS-A and FRAP as compared to that of HC prepared using Alcalase. Karnjanapratum and Benjakul25 used purified glycyl endopeptidase for producing unicorn leatherjacket skin HC, which showed FRAP and ABTS˙+ radical quenching efficacies. Since HC prepared using P0.3 + A0.3 enzyme hydrolysis process rendered the highest yield, recovery, AAGC and antioxidant activities, it was selected for further studies.
Antioxidative activities of HC from defatted Asian sea bass skin prepared using different hydrolysis processesa.
| Samples | ABTS radical scavenging activity (μmol TE/g sample) | DPPH radical scavenging activity (μmol TE/g sample) | FRAP (μmol TE/g sample) | Metal chelating activity (μmol EE/g sample) |
|---|---|---|---|---|
| A0.2 | 241.79 ± 0.64C | 2.87 ± 0.24D | 0.16 ± 0.01E | 8.23 ± 0.12C |
| A0.3 | 274.4 ± 44.04C | 3.80 ± 0.16C | 0.26 ± 0.01D | 8.20 ± 0.52C |
| P0.3 | 142.97 ± 7.85D | 2.23 ± 0.14E | 0.15 ± 0.01E | 6.77 ± 0.25D |
| P0.3 + A0.2 | 628.85 ± 9.12B | 8.52 ± 0.25A | 0.31 ± 0.01C | 22.28 ± 0.83A |
| P0.3 + A0.3 | 650.20 ± 8.36A | 8.97 ± 0.14A | 0.36 ± 0.01B | 20.94 ± 0.13B |
| Ascorbic acid | 7.74 ± 0.50E | 5.73 ± 0.48B | 13.14 ± 0.37A | 0.34 ± 0.10E |
Values are mean ± SD (n = 3). Different superscripts in the same column indicate significant difference (p < 0.05). Ascorbic acid at 25 mg mL−1 was used as a positive control.
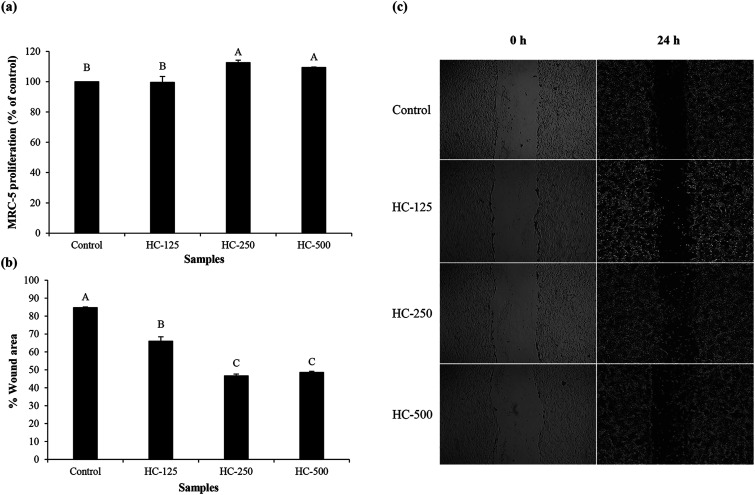
3.4. MRC-5 fibroblast cell proliferation
The effect of HC prepared using P0.3 + A0.3 process at different levels on growth of MRC-5 cells is presented in Fig. 1a. All the tested HC levels had no cytotoxicity towards MRC-5 cells. Moreover, similar proliferation was observed between HC at 250 and 500 μg mL−1 (p > 0.05). It has been suggested that ASB-S HC could stimulate MRC-5 multiplication because of the high content of hydrophobic amino acids (AAs),1,10 which were reported to promote fibroblast proliferation.26 Glycine, alanine and proline were found as the dominant AAs in ASB-S HC in addition to hydroxyproline (Table 1). Ohara et al.27 previously reported that Pro–Hyp and Pro–Hyp–Gly peptides were able to stimulate fibroblast proliferation. These peptides were also detected in sea bass skin HC, which more likely increased proliferation of fibroblast cells.8 Since all levels tested had no cytotoxicity, they were chosen for cell migration and lamellipodia formation studies.
Fig. 1. Effect of the selected HC from defatted Asian sea bass skin on proliferation of MRC-5 fibroblast cells (a), cell migration (b) and cell morphology (c). Bars represent standard deviation (n = 3). Different uppercase letters on bars indicate significant differences (p < 0.05).
3.5. Cell migration and lamellipodia formation of MRC-5 fibroblast cells
The impact of different HC levels on migration of fibroblast cells is shown in Fig. 1b and c. After 24 h of incubation, the increased cell migration was observed, especially when HC at level of 250–500 μg mL−1 was used (p < 0.05), in which the highest effectiveness in wound healing (50–52%) was achieved, as compared to the control (without HC). HC prepared from P0.3 + A0.3 process had lower efficiency in wound healing than HC prepared from P0.3 process, in which the wound gap was closed by 60–65%.10 Differences in wound healing effectiveness might be due to the variations in sequence and amino acid composition as affected by enzyme types, hydrolysis processes and material used. For example, HC prepared by P0.3 + A0.3 enzyme hydrolysis process had lower glycine content than the one prepared by P0.3 treatment (21.74 g/100 g).10 For the wound healing process, glycine is known to play a key role in tissue regeneration.28 Apart from glycine, hydroxyproline in HC is an important amino acid for tissue granulation.29 Commonly, collagen is produced by fibroblast cell, which plays a crucial role in generation of granulation tissue and leads to a decrease in wound area.30 Recently, Chotphruethipong et al.1 revealed that HC from defatted sea bass skin also induced collagen production in fibroblast cells, which is plausibly related with the decreased wound gap.
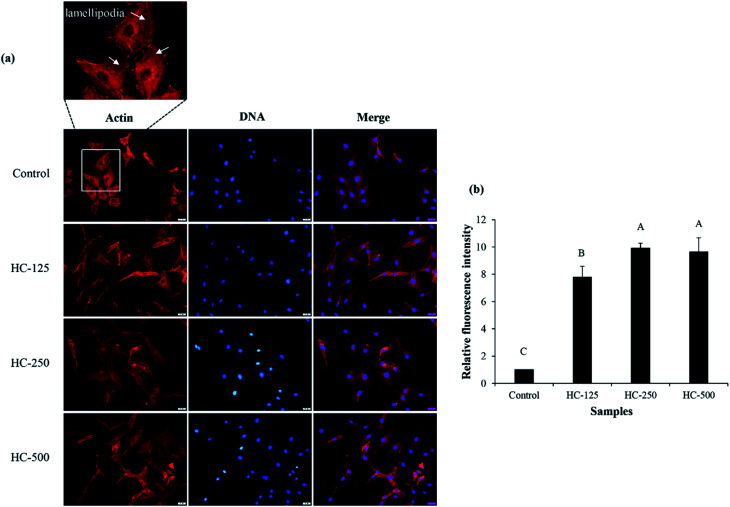
Lamellipodia formation by fibroblast cells after treatment with different HC levels is illustrated in Fig. 2. The ASB-S HC significantly enhanced lamellipodia formation (p < 0.05) as evidenced by the increase in fluorescence intensity when compared to the control (Fig. 2a and b). The results showed that the HC treatment augmented lamellipodia formation or migration of MRC-5 cells, resulting in the observed decreased wound gap (Fig. 1b). However, no difference in intensity was found in cells treated with HC in the range of 250–500 μg mL−1 (p > 0.05), which might be due to increased peptide–peptide interactions via hydrogen bond or hydrophobic bonds, leading to lower wound healing activity. In general, lamellipodia is a cytoskeleton actin related with cell migration, cell division and wound-healing process.31 Augmented formation of lamellipodia indicates that the ASB-S HC has a positive role in wound healing.
Fig. 2. Effect of the selected HC from defatted Asian sea bass skin on lamellipodia formation of MRC-5 fibroblast cells (a) and relative fluorescence intensity of lamellipodia after treatment with the selected HC at various levels (b). Bars represent standard deviation (n = 3). Different uppercase letters on bars indicate significant differences (p < 0.05).
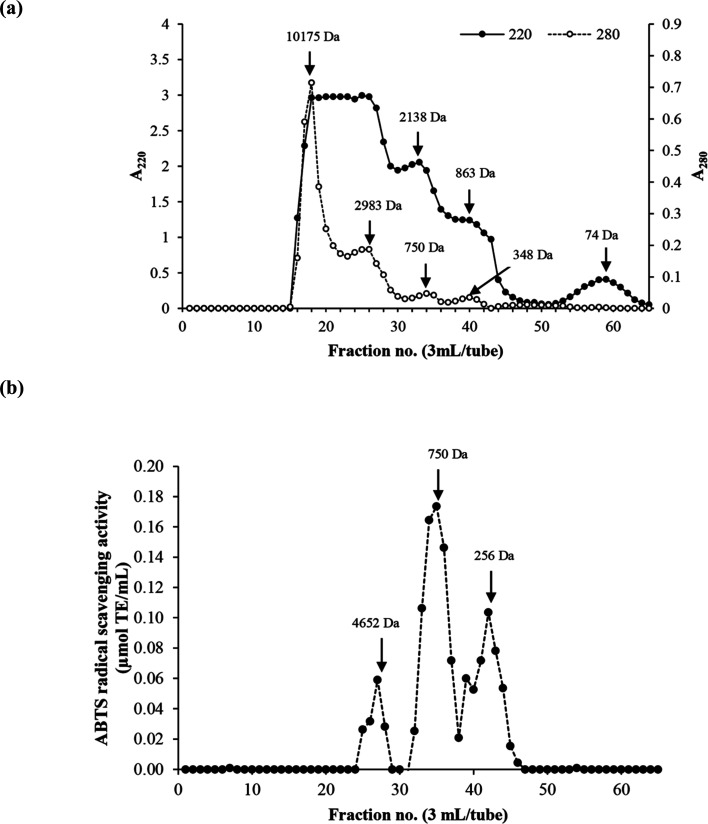
3.6. Molecular weight (MW) distribution of the selected HC
M W distribution of the selected HC (P0.3 + A0.3 process) contained four major peptide peaks with high levels of aromatic AAs (A280) while A220 is based on peptide bond absorption (Fig. 3a). MW of peptides ranged from 74 Da to 10 175 Da. The peptide fraction with average MW of 750 Da had the highest antioxidant activity, followed by the 256 Da peptides (Fig. 3b). The MW range of peptides present in the HC from P0.3 + A0.3 process as shown in Fig. 3a is smaller than previously reported for the P0.3 process alone.10 Antioxidant activity of peptides is governed by MW, AA composition, sequence and chain length, which could be responsible for the observed activities of the peptide peaks as shown in Fig. 3b.5 The antioxidant results are related with AAGC (Table 1), confirming that peptides with small sizes (256 and 750 Da) are more potent when compared to the 4652 Da peptides. Chotphruethipong et al.10 noted that peptides with MW < 4050 Da from defatted ASB-S exhibited high ABTS-RS-A using papain at 0.3 unit per g DM. Antioxidative peptides with small size likely increased the antioxidant and wound-healing activities of the HC. In general, reactive oxygen species (ROS) cause skin cell damage and inhibit the healing process via disruption of DNA and cell membrane.32 ROS might be eliminated by antioxidative peptides in ASB-S HC, especially those rich in hydrophobic AAs. Chotphruethipong et al.9 have previously revealed that ASB-S HC had ability to scavenge ROS, which led to ameliorated skin cell damage and could have contributed to the decreased wound gap (Fig. 1b).
Fig. 3. Elution profiles (a) of the selected HC using Sephadex™ G-25 gel filtration chromatography and ABTS radical scavenging activity (b).
3.7. Amino acid (AA) composition of the selected HCs
AA compositions of HC obtained from P0.3 + A0.3 process are presented in Table 3. HC had glycine as the dominant AA. Alanine and proline were also found at high levels, followed by hydroxyproline and glutamine/glutamic acid. HC had low contents of tryptophan, and tyrosine. Moreover, the imino acid content of HC obtained from the P0.3 + A0.3 process (224.90 residues/1000 residues) was higher than 185 residues/1000 residues reported for HC from P0.3 hydrolysis.10 Coincidentally, the recovery of HC prepared from the P0.3 + A0.3 process is higher than that prepared by P0.3 process (Table 1). Differences in AA composition might be due to different enzymatic hydrolysis processes used, including type of enzyme. Apart from the aforementioned AAs, hydrophobic AAs were found at a high content in the HC sample, which have been associated with several antioxidative and biological peptides.1,10,11 Hydrophobic AAs obtained from HC prepared by the P0.3 + A0.3 process were approximately 56.90% of total AAs. The presence of hydrophobic AAs, including Leu, Pro, Gly, Val and Ala could have contributed to the augmented antioxidant and wound-healing activities of the HC prepared by the P0.3 + A0.3 protocol.11,33
Amino acid composition of hydrolyzed collagen from defatted Asian sea bass skin prepared using P0.3 + A0.3 processa.
| Amino acid | Residues/1000 residues |
|---|---|
| Alanine (Ala) | 144.91 |
| Arginine (Arg) | 97.24 |
| Asparagine/asparatic acid (Asn/Asp) | 50.30 |
| Cysteine (Cys) | 8.04 |
| Glutamine (Gln)/glutamic acid (Gln/Glu) | 81.41 |
| Glycine (Gly) | 202.55 |
| Histidine (His) | 18.40 |
| Isoleucine (Ile) | 8.74 |
| Leucine (Leu) | 19.42 |
| Lysine (Lys) | 28.83 |
| Hydroxylysine (Hylys) | 9.95 |
| Methionine (Met) | 13.94 |
| Phenylalanine (Phe) | 14.21 |
| Hydroxyproline (Hyp) | 84.73 |
| Proline (Pro) | 140.18 |
| Serine (Ser) | 28.68 |
| Threonine (Thr) | 23.40 |
| Tyrosine (Tyr) | 5.67 |
| Valine (Val) | 19.01 |
| Tryptophan (Trp) | 0.39 |
| Total | 1000 |
| Imino acid (Hyp + Pro) | 224.90 |
P0.3 + A0.3 (0.3 unit of papain + 0.3 unit of Alcalase).
4. Conclusion
The use of a two-enzyme hydrolysis process with papain at 0.3 unit per g DM at 40 °C for 90 min, followed by Alcalase at 0.3 unit at 50 °C for 90 min increased yield, recovery and peptide content of HC from defatted ASB-S. This HC contained peptides with antioxidant activities, stimulated cell proliferation and migration as well as enhanced lamellipodia formation in MRC-5 cells, which reflected the better wound-healing effect. In addition, small peptides (average MW < 750 Da) with high imino acids and hydrophobic amino acids were determined to be present in the HC from P0.3 + A0.3 process. Therefore, the HC produced from P0.3 + A0.3 process could serve as a promising active ingredient in the functional food and nutraceutical industry, mostly for skin nourishment or wound healing.
Conflicts of interest
There is no conflict of interest.
Supplementary Material
Acknowledgments
This research was supported by National Research Council of Thailand (NRCT) under International Research Network (IRN) program and National Science and Technolgy Development Agency (Grant No. P-20-52297). Financial support from Prince of Songkla University under Prachayacharn grant (Grant No. AGR6402088N) was also acknowledged.
References
- Chotphruethipong L. Aluko R. E. Benjakul S. J. Food Biochem. 2019a;43:1–13. doi: 10.1111/jfbc.12825. [DOI] [PubMed] [Google Scholar]
- Benjakul S. Karnjanapratum S. Visessanguan W. Waste Biomass Valori. 2018a;9:549–559. [Google Scholar]
- Qiu Y.-T. Wang Y.-M. Yang X.-R. Zhao Y.-Q. Chi C.-F. Wang B. Mar. Drugs. 2019;17:565. doi: 10.3390/md17100565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongkonkamthorn N. Malila Y. Yarnpakdee S. Makkhun S. Regenstein J. M. Wangtueai S. Processes. 2020;8:1–22. doi: 10.3390/pr8111518. [DOI] [Google Scholar]
- Yarnpakdee S. Benjakul S. Kristinsson H. G. Kishimura H. J. Food Sci. Technol. 2015;52:3336–3349. doi: 10.1007/s13197-014-1394-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harnedy P. A. Parthsarathy V. McLaughlin C. M. O'Keeffe M. B. Allsopp P. J. McSorley E. M. O'Harte F. P. FitzGerald R. J. Food Res. Int. 2018;106:598–606. doi: 10.1016/j.foodres.2018.01.025. [DOI] [PubMed] [Google Scholar]
- Sae-leaw T. O'Callaghan Y. C. Benjakul S. O'Brien N. M. J. Food Sci. Technol. 2016;51:1545–1551. doi: 10.1111/ijfs.13123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sae-leaw T. Karnjanapratum S. O’Callaghan Y. C. O’Keeffe M. B. FitzGerald R. J. O’Brien N. M. Benjakul S. J. Food Biochem. 2017;41:1–11. doi: 10.1111/jfbc.12350. [DOI] [Google Scholar]
- Chotphruethipong L. Sukketsiri W. Battino M. Benjakul S. RSC Adv. 2021;11:2175–2184. doi: 10.1039/D0RA07135H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chotphruethipong L. Sukketsiri W. Aluko R. E. Sae-leaw T. Benjakul S. J. Food Sci. Technol. 2020:1–11. doi: 10.1007/s13197-020-04566-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjakul S. Karnjanapratum S. Visessanguan W. J. Food Sci. Technol. 2018b;53:1871–1879. [Google Scholar]
- Senphan T. Benjakul S. J. Funct. Foods. 2014;6:147–156. doi: 10.1016/j.jff.2013.10.001. [DOI] [Google Scholar]
- Chotphruethipong L. Aluko R. E. Benjakul S. J. Food Sci. 2019a;84:1799–1805. doi: 10.1111/1750-3841.14687. [DOI] [PubMed] [Google Scholar]
- Chotphruethipong L. Aluko R. E. Benjakul S. Process Biochem. 2019b;86:58–64. [Google Scholar]
- AOAC, Official Method of Analysis of AOAC International, The Association of Official Analytical Chemists, Virginia, 7th edn, 2000 [Google Scholar]
- FAO/WHO, Energy and Protein Requirements. FAO Nutrition Meetings Report Series No. 52, WHO Technical Report Series No. 522, 1973 [PubMed] [Google Scholar]
- Chotphruethipong L. Benjakul S. Kijroongrojana K. J. Food Biochem. 2017;41:1–10. doi: 10.1111/jfbc.12379. [DOI] [Google Scholar]
- Singkhorn S. Tantisira M. H. Tanasawet S. Hutamekalin P. Wongtawatchai T. Sukketsiri W. Phytother Res. 2018;32:1397–1403. doi: 10.1002/ptr.6075. [DOI] [PubMed] [Google Scholar]
- Ritto D. Tanasawet S. Singkhorn S. Klaypradit W. Hutamekalin P. Tipmanee V. Sukketsiri W. Nutr. Res. Pract. 2017;11:275. doi: 10.4162/nrp.2017.11.4.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiansilakul Y. Benjakul S. Shahidi F. J. Food Biochem. 2007;31:266–287. doi: 10.1111/j.1745-4514.2007.00111.x. [DOI] [Google Scholar]
- Karnjanapratum S. Benjakul S. Kishimura H. Tsai Y.-H. Food Chem. 2013;141:4138–4145. doi: 10.1016/j.foodchem.2013.07.001. [DOI] [PubMed] [Google Scholar]
- Mazloomi S. N. Mora L. Aristoy M. Mahoonak A. S. Ghorbani M. Houshmand G. Toldrá F. Foods. 2020;9:1217. doi: 10.3390/foods9091217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J. Cullen J. J. Buettner G. R. Biochim. Biophys. Acta. 2012;1826:443–457. doi: 10.1016/j.bbcan.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sai-Ut S. Benjakul S. Sumpavapol P. Kishimura H. J. Food Process. Preserv. 2015;39:394–403. doi: 10.1111/jfpp.12244. [DOI] [Google Scholar]
- Karnjanapratum S. Benjakul S. Int. Aquat. Res. 2015;7:101–114. doi: 10.1007/s40071-014-0088-0. [DOI] [Google Scholar]
- Ray J. Baird A. Gage F. H. Proc. Natl. Acad. Sci. U.S.A. 1997;94:7047–7052. doi: 10.1073/pnas.94.13.7047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara H. Ichikawa S. Matsumoto H. Akiyama M. Fujimoto N. Kobayashi T. Tajima S. J. Dermatol. 2010;37:330–338. doi: 10.1111/j.1346-8138.2010.00827.x. [DOI] [PubMed] [Google Scholar]
- de Sousa Sá O. M. Lopes N. N. F. Alves M. T. S. Caran E. M. M. Nutrients. 2018;10:1–11. doi: 10.3390/nu10101485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak B. Anderson M. Pereira L. P. Fitoterapia. 2007;78:540–544. doi: 10.1016/j.fitote.2007.06.008. [DOI] [PubMed] [Google Scholar]
- Guo S. a. DiPietro L. A. J. Dent. Res. 2010;89:219–229. doi: 10.1177/0022034509359125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedeva I. Koripelly G. Caballero D. Chièze L. Guichard B. Romain B. Pencreach E. Lehn J.-M. Carlier M.-F. Riveline D. Nat. Commun. 2013;4:1–11. doi: 10.1038/ncomms3165. [DOI] [PubMed] [Google Scholar]
- Banerjee P. Suguna L. Shanthi C. Amino Acids. 2015;47:317–328. doi: 10.1007/s00726-014-1860-6. [DOI] [PubMed] [Google Scholar]
- Intarasirisawat R. Benjakul S. Wu J. Visessanguan W. J. Funct. Foods. 2013;5:1854–1862. doi: 10.1016/j.jff.2013.09.006. [DOI] [Google Scholar]