Abstract
In the middle of November 2021, Omicron (B.1.1.529), a novel variant of SARS-CoV-2 was identified in South Africa. Owing to continuous increasing cases with rapid transmissibility and immune evasion, the World Health Organization (WHO) has categorized this strain as a variant of concern (VOC). In total, over 60 mutations have been identified in Omicron (BA.1) and latterly, its three sub-lineages (BA.1.1, BA.2, and BA.3) have also been found with additional mutations and pathogenicity. The highly contagious Omicron causes less severe sickness than Delta, but it is still dangerous for those who have not been vaccinated. Following the unique identification of the Omicron variant, a fresh debate has erupted regarding the natural vaccines. A number of experts believe that Omicron can work as a natural vaccine, because it is similar to live attenuated vaccines in certain ways. Additionally, it was highlighted that the high rate of antibody generation in individuals cured of Omicron provide suggestive evidence in favor of those researchers who claimed Omicron acts as natural vaccine. Some disagreements also noted, as it also has tremendous health effects and high infection rate, as similar to the prior variants. This review summarizes the contradictory scenario among the scientists about Omicron variant.
Keywords: Omicron, Delta, VOC, Mutation, Natural vaccine, SARS-CoV-2
1. Introduction
Coronavirus (CoV) is a single-stranded, enclosed positive sense RNA virus with a spherical or pleomorphic shape that contains spike-like projections on its surface that give it a crown-like appearance [1]. The SARS epidemic of 2002–2003 was triggered by the SARS coronavirus (SARS-CoV), which first appeared in southern China in 2002. In 2012, the MERS-CoV became known in the Arabian Peninsula as a severe human disease [2]. The story of SARS-CoV-2 begins on December 31st, 2019, when the Wuhan Municipal Health Commission in China identified a huge number of pneumonia cases in Wuhan, Hubei Province [3,4]. After several investigation processes and case studies, the Director General of the World Health Organization (WHO) declared the new coronavirus (2019-nCoV or SARS-CoV-2) outbreak a Public Health Emergency of International Concern (PHEIC) on January 30, 2020 [5]. According to the report by WHO on January 30th, a total of 7818 SARS-CoV-2 infected confirmed cases were documented globally, with 7736 positive cases in China and only 82 cases reported outside of China among the 18 countries (WHO 2019-nCoV2020). At this moment, worldwide about 472,850,919 COVID-19 positive cases have been detected, along with the 6,107,123 number of individual death cases [6]. Many efforts have been made to develop COVID-19 vaccinations in order to prevent a pandemic with the spike (S) protein of SARS-CoV-2 [7]. Random mutations accumulate in any virus due to natural selection. The S1 subunit of the 'S' protein has been found to be the hotspot of mutations in terms of virulence, mode of transmission, and host immune evasion. This could have a lot of therapeutic implications along with the development of several variants. WHO use Greek alphabets to rename the most widely transmitted variants of SARS-CoV-2, i.e., Alpha (α) for B.1.1.7 variant, Beta (β) for B.1.351, Gamma (γ) for P.1, Delta (δ) for B.1.617.2 which was identified for the first time in the U.K., South Africa, Brazil and India respectively [8,9]. In the second wave of COVID-19 outbreaks that have scattered across the world, the most contagious mutant Delta variant (B.1.617.2) and Delta Plus (AY. 1) strains were detected for the first time in India in late 2020. The Delta plus strain is a mutation of Delta, identified as a “variant of concern” (VOC) with increased transmissibility, greater binding to lung cell receptors, and possibly lower monoclonal antibody (mAb) response than Delta [10]. From the prior reported information, a common K417 N mutation was identified between the Delta plus (AY.1) and Beta variant (B.1.351) which is responsible for the neutralization resistance. Additionally, another sub-lineage of the Delta variant was identified in Vietnam, designated as Delta-V, which may have contributed to the increased number of cases at that time. After the identification, this variant was highlighted as a hybrid virus due to the mutations found in the 'S' protein of the Alpha (B.1.1.7) variant. As per the GISAID (Global Initiative on Sharing All Influenza Data) analysis, it was revealed that, the Vietnamese Delta variant contains deletion mutations at positions H67, V70, and/or Y144, which are also present in the spike of the Alpha (B.1.1.7) variant [11]. Next, in mid-November 2021, the Omicron (B.1.1.529), a new variant of SARS-CoV-2, was discovered for the first time in Gauteng, South Africa [12]. The Omicron variant has already been detected in 87 nations on December 16th, 2021 [13]. This mutant strain of SARS-CoV-2 possesses 30 mutations in the 'S' protein, whereas 15 mutations have been identified in its receptor binding domain (RBD) [14]. The number of Omicron cases increased massively due to its high transmission rate, which may be due to its host immune evasion property [13]. It has a shorter incubation period of only three days [15]. Since the virus replicates in the upper respiratory tract, it causes less lung damage than prior strains. Several researchers think that the high transmission rate with very mild pathogenicity of the Omicron variant may build herd immunity, giving hope for the end of the pandemic [16]. The mild form of pathogenic characteristics of this variant includes sore throat, throat pain, headache, myalgia, fatigue, and fever which are commonly found in ‘cold and flu’. There is no evidence of a loss of taste or fragrance like other variants of SARS-CoV-2. All of them are milder symptoms that do not require hospitalisation; only a few patients have worse symptoms that necessitate hospitalisation [17]. Although contrary studies have discovered that a large proportion (21%) of hospitalized patients from the African community were infected with the Omicron type of SARS-CoV-2 and had a catastrophic clinical outcome [18]. According to an ICMR study, individuals infected with Omicron have a robust immune response capable of neutralizing not only Omicron but also other harmful variants of SARS-CoV-2, lowering the risk of re-infection with the Delta variant and thus displacing it as the dominant strain [19]. Because the Omicron wave is so new, there is no convincing data on the level of infection-induced immunity, but according to Schulze ZurWiesch, it will be similar to other variations. That indicates that if someone has been infected with Omicron in the last several weeks, the individual is likely safe for the next few months. However, because Omicron has a faster rate of transmission than prior strains, larger antibody levels are required to avoid infection [20]. Throughout the history of infectious disease outbreaks, their emergence and spread have had significant and long-lasting societal consequences [21]. Major pandemics and epidemics such as plague, cholera, flu, SARS-CoV, and MERS-CoV have already had an impact on human civilization [22]. In history, rapid vaccination along with acquiring herd immunity limited the rapid spread of prior pandemics, although some cases still occur, but they are not as virulent as before. In the case of Spanish Flu, which broke out in the year 1918, almost 35% of the world population was infected before its conversion to endemic by 1920. In this case, a weaker version of mutant strain spared rapidly with mild and symptoms like common cold. Here also, the second wave was most powerful among the three consecutive waves. This similar infectivity pattern has been discovered for existing COVID-19 diseases, whereas Omicron appears to be a weaker version of SARS-CoV-2, with the hope of ending the current pandemic [23]. In this present review, efforts have been made to understand the nature of Omicron variant in a more precise way and also whether it could be considered Mother Nature's own way of approaching mass vaccination through herd immunity and pandemic control.
2. The journey of Omicron
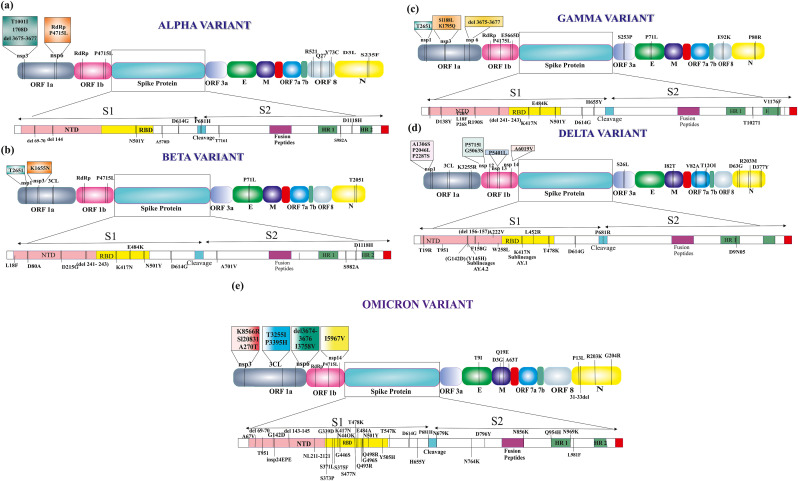
SARS-CoV-2 variants have been identified worldwide to date, including Alpha (α) (B.1.1.7), Beta (β) (B.1.351), Gamma (γ) (P.1), Delta (δ) (B.1.617.2), and Omicron (B.1.1.529), with varying characteristics and protein sequences (Fig. 1 ) [24]. As per genome sequencing, the Omicron belongs to Pango line B.1.1.529, Next strain branch 21k, and GISAID branch GR/484A [25]. Many non-synonymous mutations, including those in spike, are responsible for disease severity, transmissibility, and immune evasion. In total, over 60 alterations, deletions, and insertion mutations have been discovered in the Omicron variation [26]. According to the structural analysis of SARS-CoV-2 variants, four 'S' proteins are found in the alpha variant, six in beta, eight in gamma, ten in Delta, and lastly, thirty 'S' proteins in Omicron [24]. The envelope (E) protein has one mutation (T9I) and the membrane (M) protein has three mutations (D3G, Q19E and A63T), whereas the nucleocapsid (N) protein also has three mutations (P13L, R203K and G204R). Omicron is more transmissible than prior variants because of triple mutations in the furin-like cleavage site (FCS), such as H655Y, N679K, and P681H [26]. The RBD of the 'S' protein found in the Omicron variant has 15 amino acid changes, some of which have been seen in prior variants such as with Alpha: N501Y, Beta: K417 N, E484K, N501Y, Gamma: K417T, E484K, N501Y, and Delta: L452R, T478K are common [25]. For angiotensin-converting enzyme 2 (ACE2), RBD of Omicron exhibits a 2–2.5-fold higher binding affinity than prototype SARS-CoV-2 due to T478K, Q493K, and Q498R [27]. According to the reports of Kannan et al. [28] a total of 30 numbers (A67V, T95I, Y145del, G339D, N440K, G446S, S477 N, T478K, E484A, Q493R, G496S, Q498R, N501Y, Y505H, T547K, D614G, H655Y, N679K, P681H, N764K, D796Y, N856K, Q954H, N969K, L981F, L212I, S371L, S373P, S375F, K417 N) of >50% prevalence mutation were identified as signature mutation in case of Omicron variant. When compared to the prior existing SARS-CoV-2 variations, 23 number (A67V, Y145del, G339D, N440K, G446S, E484A, Q493R, G496S, Q498R, Y505H, T547K, H655Y, N679K, N764K, D796Y, N856K, Q954H, N969K, L981F, L212I, S371L, S373P, S375F) of these hallmark mutations of Omicron variant were identified as unique mutations. Moreover, they also identified nine more additional mutations (ORF1a:K856R, ORF1a:L2084I, ORF1a:A2710T, ORF1a:T3255I, ORF1a:P3395H, ORF1a:I3758V, ORF1b:P314L, ORF1b:I1566V, and ORF9b:P10S) in the Omicron infected individual's (N = 70) gene sequencing. Among these additional mutations, only two mutations i.e. ORF1a:T3255I and ORF1b: P314L were previously identified in Delta and Delta Plus variants of SARS-CoV-2 with >40% prevalence rate as compared to the >80% prevalence of Omicron variant. This report also suggested that 46 distinct mutations in the ORF1a, ORF1b, and S genes of the SARS-CoV-2 Omicron variant, with more than 50% frequency. Conclusively, these investigations revealed that multiple mutations are localised to the area of the 'S' protein that is the primary target of antibodies, implying that the mutations of the Omicron variant may alter the antibody binding affinities to the 'S' protein [28]. This variant was reported for the first time in South Africa on November 24th, 2021, and WHO has classified it as a VOC after two days of its detections [29]. Omicron has a few deletion and more than 30 mutations, with several of them overlapping, including in the Alpha, Beta, Gamma, or Delta [30]. The Omicron has spread over Australia, Austria, the Czech Republic, Canada, Italy, Denmark, France, the Netherlands, and the United Kingdom by the 29th of November 2021 [31]. On December 1, 2021, in the United States, the first Omicron case was identified [32]. In China, the first and second Omicron cases were reported on December 9th and December 10th, respectively [33]. As of December 28th, 2021, there are 53,695 number of Omicron cases found worldwide, with the biggest numbers found in the United Kingdom (34,573), the United States (8,311), South Africa (1,643), Australia (859), Denmark (2,001), Canada (586), Belgium (609), and Switzerland (471), among others in a short period of time. According to WHO, the Omicron virus was transmitted in 149 countries on January 6, 2022 [31]. In between 22 January 2022, 0.5 million positive cases and 115 deaths were identified worldwide due to the Omicron infection [34]. As of April 11, 2022, there were 1,029,448 confirmed cases in the UK, 808,648 in the United States, 182311 in Denmark and 179,499 in Germany. These are the top 4 most affected countries by the Omicron variant [35]. Based on the PCR analysis of the Omicron variant, it was reported that one of the three target genes is missing (known as S gene dropout or S gene target failure) and in the absence of sequencing confirmation, this test can be used as a marker for this variation [36]. This mutant variant has improved immune tolerance and the ability to evade antibodies developed by existing vaccinations. Mutations in the Omicron variant, particularly in the 'S' protein, increase transmissibility and immune evasion, and it becomes resistant to neutralizing antibodies (nAbs) in previously vaccinated people [37]. mAbs such as Bamlanivimab and the Rockefeller University antibody C144 have low effectiveness against Omicron and REGN-COV2 (Casirivimab and Imdevimab), whereas the Rockefeller University antibody C135 is still found to be effective against Omicron [38].
Fig. 1.
The schematic presentation of region specific mutational landscape of the different variants of SARS-CoV-2. The several mutations especially in the 'S' protein and ORF domain of the different VOCs i.e. Alpha (a), Beta (b), Gamma (c), Delta (d) and Omicron (e) are shown in this diagram.
This new VOC contains more than 30 numbers of mutations in the 'S' protein domain (Ala67Val, Δ69-70, Thr95Ile, Gly142Asp, Δ143-145, Δ211, Leu212Ile, Gly339Asp, Ser371Leu, Ser373Pro, Ser375Phe, Lys417Asn, Asn440Lys, Gly446Ser, Ser477Asn, Thr478Lys, Glu484Ala, Gln493Arg, Gly496Ser, Gln498Arg, Asn501Tyr, Tyr505His, Thr547Lys, Asp614Gly, His655Tyr, Asn679Lys, Pro681His, Asn764Lys, Asp796Tyr, Asn856Lys, Gln954His, Asn969Lys, and Leu981Phe), whereas the 15 numbers of identified mutations (residues 319–541) reside in the RBD [39,40]. A computational comparison of the Omicron and Delta variant of SARS-CoV-2 revealed that Omicron has a higher affinity for ACE2 receptors than the Delta variant. A recent in silico analysis demonstrated that the mutations Q493R, N501Y, S371L, S373P, S375F, Q498R, and T478K contribute to greater affinity for ACE2 receptors [34]. Mutations such as Asn501Tyr, N501Y, D614G, N501Y, L452R, and K417 significantly boost ACE2 receptor binding capabilities, which may aid in viral transmission and result in a greater infection rate [34,41,42]. N501Y is related with enhanced transmissibility, other mutations also improve spike affinity for ACE2, such as the L452R in the B.1.429 lineage, which provide stronger ACE2 interaction [43]. Kinase like PI3K and AKT are important for signalling when SARS-CoV-2 enters the body. In strain that have N501Y mutation, a molecular docking analysis found that epidermal growth factor receptor could be another possible acceptor. Because cancer patients have a lot of kinases, lineages that have the N501Y mutation might be more harmful [44]. Additionally, His655Tyr is proximal to the FCS, which may facilitate spike cleavage and transmission. The alterations at positions N679K and P681H, which are also present in Alpha and Delta variants, may also contribute to the virus's transmission capabilities [12,42]. In order for viruses to reproduce, they need RNA polymerase (Nsp12) and nonstructural protein 14 (Nsp14), but it is not established that mutations in these parts of Omicron could lead to higher mutation rates. Omicron also have mutations in the nucleocapsid protein (R203K and G204R), which are not unique to the Omicron but are linked to increased sub-genomic RNA expression and viral replication [34].
3. Sub-variants of Omicron
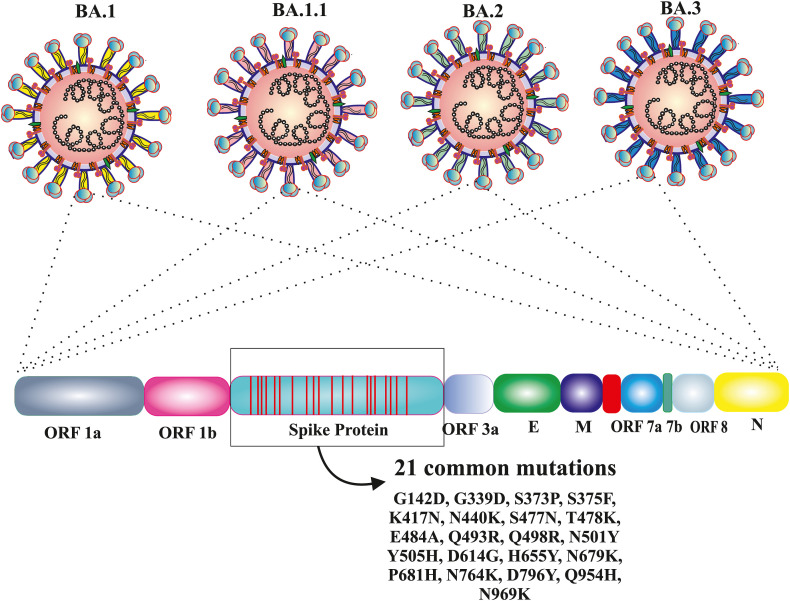
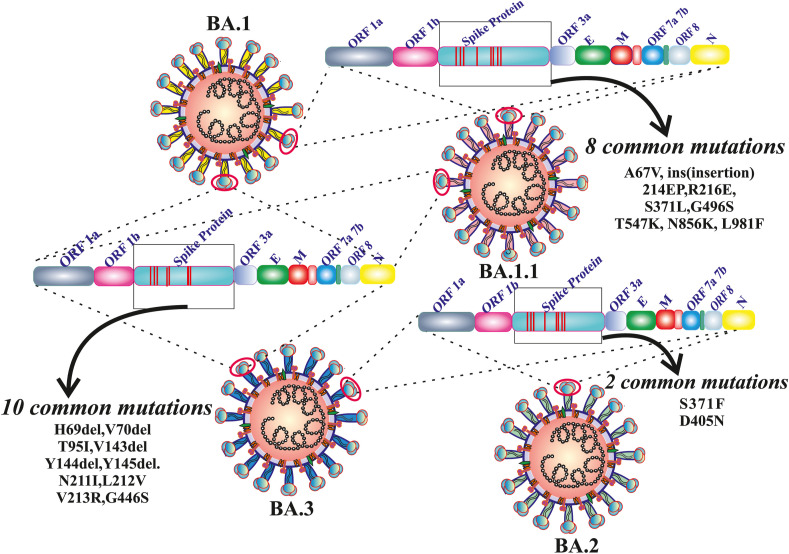
Omicron (BA.1) and three sub-lineages such as BA.1.1, BA.2 and BA.3 of Omicron are closely related variants with a common ancestor. Based on various mutational patterns, Omicron was divided into different lineages: BA.1, BA.1.1, BA.2, and BA.3 each have 39 mutations, 40 mutations (BA.1+ spike R346K), 31 mutations, and 34 mutations, respectively [45]. As of February 10th, 2022, BA.1 has been discovered in 135 countries, BA.1.1 and BA.2 are found in 69 countries, and BA.3 in 16 countries. There are 21 mutations that are common to all of them (G142D, G339D, S373P, S375F, K417 N, N440K, S477 N, T478K, E484A, Q493R, Q498R, N501Y, Y505H, D614G, H655Y, N679K, P681H, N764K, D796Y, Q954H, and N969K) (Fig. 2 ). The N501Y and Q498R along with the 21 mutations, improve the ACE2 receptor binding as well as the H655Y, N679K, and P681H mutations, which are known to boost spike cleavage and the spread of viruses [46]. There are eight most prevalent mutations (A67V, ins (insertion) 214 EP, R216E, S371L, G496S, T547K, N856K, L981F) found between BA.1 and BA.1.1, two mutations (S371F and D405 N) have been found in BA.2 and BA.3 and 10 most prevalent mutations (H69del, V70del, T95I, V143del, Y144del, Y145del. N211I, L212V, V213R, G446S) found in BA.1.1, BA.1 and BA.3 (Fig. 3 ). BA.1.1, BA.2, and BA.3 are more transmissible than Omicron (BA.1) and Delta. When compared to the Delta variant, it shows less mortality across the world [45]. In the 'S' protein region of BA.1 significant 16 mutations were identified, i.e., ins214EPE, S371L, G496S, T547K, N856K, L981F, A67V, H69del, V70 del, T95I, V143del, Y144del, Y145del, N211I, L212Idel, and G446S; similarly 10 signature mutations (T19I, L24S, P25del, P26del, A27S, V213G, T376A, and R408S) in the BA.2 variant of Omicron. The difference in the number of mutations in the spike protein domain of BA.1 and BA.2 could explain why BA.1 spreads faster than BA.2 [46]. Due to the removal of six mutations (ins214EPE, S371L, G496S, T547K, N856K, and L981F) from BA.1 or the gain of two mutations (S371F and D405 N) from BA.2, the BA.3 lineage generated the fewest cases of these three lineages [46]. The BA.1.1 now accounts for 30% of Omicron sequences worldwide, with 30–45% in South Africa, the United Kingdom, and the United States. The BA.2 sub-lineage, accounts for just 10% of all Omicron sequences worldwide. However, in nations like Denmark, India, and South Africa, it is not only rising, but it is also becoming the dominating form [47]. According to WHO, BA.1 replicates in the lower respiratory tract whereas the Delta in the upper respiratory tract. That's the reason for high transmission and less severity in the case of BA.1 than in the case of Delta. According to WHO, between January 30th, 2022, the BA.2 sub-lineages spread in 57 countries, and nearly 50,000 positive cases were found up to February 3rd, 2022. As per the report of the UK Health Security Agency, in England, the growth rate of BA.2 is faster than BA.1. At least 400 occurrences of the BA.3 sub-lineage had been found around the world on February 3, 2022, while it had apparently spread to at least 19 nations (Table 1 ) [48].
Fig. 2.
Number of common mutations in 'S' protein region among the Omicron variant of SARS-CoV-2 and its own sub-lineages. Here, 21 common mutations are represented among the Omicron (BA.1) and its sub-lineages BA.1.1, BA.2, BA.3 respectively.
Fig. 3.
Representation of several common mutations amid the new variant of concern BA.1 (Omicron) and its sub-lineages i.e. BA.1.1, BA.2 and BA.3. Eight common mutations are found in 'S' protein domain of BA.1 and BA.1.1. Similarly, ten types of identical mutations also observed among the BA.1, BA.1.1 and BA.3. Between the BA.2 and BA.3 more other two types of significant common mutations are identified.
Table 1.
This table summarizes the characteristics of the new Variant of Concern (Omicron: BA.1) and its sub-lineages (BA.1.1, BA.2 and BA.3). Additionally, very brief information about vaccine efficacy and combined antibiotic potency against BA.1 and its respective sub-lineages is provided.
| Omicron and its sub lineages | Pango lineages | Molecular weight | No of mutations | Unique mutation | Remarks | Reference |
|---|---|---|---|---|---|---|
| Omicron (BA.1) | B.1.1.529.1 | 141,328.11 | 39 | – | BA.1 is more likely to infect and reproduce in the upper respiratory tract than delta variant, whereas delta is more likely to infect and multiply in the lower respiratory tract. Early results revealed that the two-dose COVID vaccination schedule was less effective than prior variations against milder sickness caused by BA.1. It has been discovered that administering a third “booster” dose provides higher protection. Ronapreve (a monoclonal antibody combination of casirivimab and imdevimab) was reported to have decreased efficacy against BA.1. Another antibody therapy, sotrovimab, seems to retain anti-BA.1 spike protein action. The antiviral molnupiravir was also found to retain activity against BA.1 in six preclinical tests; however clinical trials have not yet been conducted. | [48,49] |
| BA.1.1 | B.1.1.529.1.1 | 141300.09 | 40 | One (R346K) | S309 (the precursor of sotrovimab), which has been shown to have lower neutralizing activity against omicron/BA.1.1. The combination of REGN10987 and REGN10933 (marketed as casirivimab) inhibited omicron/BA.2 but did not inhibit omicron/BA.1 or omicron/BA.1.1. | [50,51] |
| BA.2 | B.1.1.529.2 | 141,185.78 | 31 | Eight (T19I, L24del (deletion), P25del, P26del, A27S, V213G, T376A, R408S | The UKHSA's preliminary investigations showed no evidence that COVID vaccines were less effective against symptomatic disease when compared to BA.1. For, BA.2 vaccination efficacy against symptomatic infection was reported to be 13% at least 25 weeks after two doses (versus 9% for BA.1). This jumped to 70% two weeks following a third booster dose (versus 63% for BA.1). | [48] |
| BA.3 | B.1.1.529.3 | 140900.61 | 34 | One (R408S) | Some researchers have hypothesized that the reason for BA.3's delayed spread may be due to the fact that it does not contain the six extra mutations that BA.1 does. Best of our knowledge nothing is yet know about COVID vaccine effectiveness against this omicron sub-variant. | [46] |
4. Delta vs Omicron: genomic configuration and infectivity pattern
In late 2020, the Delta (B.1.617.2) variant was reported for the first time in India [52]. On November 24th, the Omicron (B.1.1.529) variant was reported for the first time in South Africa, and WHO on November 26th, 2021, called this variant “VOC” for the first time [53]. On January 21, 2022, 3.23 million new infections through Omicron were observed all around the world. The peak infection rate of Omicron is four times greater than the Delta wave [54]. The ‘S’ protein has 30 mutations in the Omicron variant, half of which are present in the RBD. The viral factors RBD T470-T478 loop and Y505 have been identified for the specific detection of SARS-CoV-2 RBD by ACE2. In Delta and Omicron variations, T478 is a common and frequent mutation. In a comparison of Delta and Omicron, the existence of multiple mutations in the RBD of the ‘S’ protein in Omicron indicates that it may be immunologically susceptible to antibody-mediated protection. The molecular weight of Delta and Omicron is 140,986.31 and 141,328.11. Omicron has a greater proportion of alpha helix structure than Delta, although it has a shorter strand and random coil structure, and in secondary structure prediction, the RBD of Omicron has a higher alpha helix composition than the Delta variant. Although beta strands are more susceptible to mutations than alpha helices, Omicron has a slightly higher random composition [55]. Omicron adopts a new strategy to enter the host without the help of the transmembrane serine protease 2 (TMPRSS2), so there is a lower or no dependency on the TMPRS22 route for replication, so the involvement of the lungs is limited [56]. Omicron binds to ACE2 more strongly than Delta, resulting in higher infection rates. Due to the presence of a mutation in the RBD, Omicron's interactions with ACE2 are also different from prior ones, which improve its interaction with ACE and antibody escape [34]. The Omicron has a less disordered area in the complete ‘S’ protein than the Delta, and the RBD shows a disorder-to-order transition. In Delta variant-RBD, the disorder prediction ranges from 469 to 471, containing residues EIY, and no disorder residues are expected in this area in Omicron-RBD [55]. Omicron results in high transmissibility and increased re-infection rates. The Delta, which is the most common in the world, is also known for its strong infectivity. One is a combination of deletions and the other one is insertion, as well as high mutation in the S1/RBD and S2 spike areas, and a set of highly conserved point replacements shows exceptional penetration in each of them [57]. It has higher amino acid compositions for arginine (Arg), glutamic acid (Glu), lysine (Lys) and aspartic acid (Asp) than the Delta, which suggests that the charged residues in Omicron are higher, that are visible to a greater level and lead to the development of salt bridges. When the Omicron spike protein is compared to the Delta, the increased amino acid composition of phenylalanine (F) and isoleucine (I) indicates that, the presence of hydrophobic amino acids inside the protein core may explain why the Omicron spike protein has more of them. When compared to the Delta, the Omicron has a lower proportion of polar amino acids, including asparagine (N) and glutamine (Q). Solvent cannot reach these residues because they are located within the protein core [55]. The nature of the Omicron reveals that it is a milder variation than Delta, yet it transmits at a faster rate. In terms of symptoms, a runny nose, headache, weariness, and sore throat are four very typical indicators in both forms. Chills and fever are only experienced on rare occasions. Sneezing and a loss of smell and taste can occur when infected with the Delta strain. Sneezing is prevalent in Omicron, although loss of smell and taste is uncommon. A common cold is also common in Delta, but not in Omicron [58]. In South Africa, the first female patient infected with Omicron was discovered to have symptoms including fatigue, pain, and aches, but no change in cough, smell, or taste [59]. The symptoms are milder, and the condition is less severe than Delta, leading in fewer hospitalizations and fatalities. The high mutation rate in Omicron indicates that the variation spreads more rapidly than in Delta. Omicron has a rate of infection approximately 2.7–3.7 times that of Delta in vaccinated and boosted individuals. However, no noticeable difference in infection rates was observed in unvaccinated patients. Additional factors that may contribute to the variant's increased transmissibility include Omicron's three-day incubation period, as opposed to Delta's four-day incubation period. This could mean that individual infected with Omicron have less time to take precautions to protect others [60]. Recently, a highly transmissible SARS-CoV-2 double variant dubbed Delmicron was identified, which was most likely produced by the Delta and Omicron variants, and a flu virus with SARS-CoV-2 named Flurona causes double infection. SARSCoV2 infections are typically caused by a single mutant strain, however two strains can co-infect, but this is a highly improbable occurrence. The combination of Delta and Omicron, as well as any possible double variations, could result in an increase in global hazard. Both strains may have switched genes, resulting in a more hazardous variant [61].
5. COVID-19 vaccine strategy and its efficacy against Omicron
It is crucial to look into the different aspects of the effectiveness of vaccines, especially when comparing countries that have administered different types of vaccines. The neutralization capacity of Omicron was found to be reduced when the titers of nAbs in sera from vaccinated people were examined [56]. Existing COVID-19 vaccine protection will not be wiped out, and boosters should help to enhance immunity against Omicron, according to research conducted in South Africa, Germany, and Sweden, as well as by the Pfizer–BioNTech collaboration, despite the fact that they seem to reduce the likelihood of hospitalisation or death. Just a few months after the second dose, the protection given by two doses of a messenger RNA (mRNA) vaccine diminishes to less than 40%. On the other hand, a third, “booster” dose seems to be helpful. According to a recent study, two weeks after the third booster shot, the protection against infection and serious disease increases from 60 to 70% [62,63]. Vaccination causes a temporary increase in the number of immune cells producing antibodies, which then gradually decreases. This leaves a tiny pool of memory B and T cells in the body, which become activated during re-infection. The booster dose can cause the proliferation of B cells and also re-increase antibody levels against the infection. Their numbers will eventually drop over time, but the pool of memory B cells will be higher than before, which results in a faster, more powerful response to subsequent encounters. The booster dose also promotes the affinity maturation in which the B cells are triggered by the vaccine, travel to the lymph node and generate more powerful antibodies against the pathogen [64]. Individuals with two doses of Pfizer generate significant immunity against Omicron, which declines 40 fold from the prior variant. The South African data showed that only two doses of Pfizer were not enough to protect against Omicron, but the addition of a booster shot or a third dose of BNT162b2 caused an increase in the titer of nAb by 25 times more than the level of two vaccine doses to protect against the prior strain [65]. Two doses of the vaccine induce CD8+ T cell protection against 80% of the epitopes that are not mutated in the Omicron variant. A third dose significantly raises CD8+ T cell numbers against numerous “S" protein epitopes, which are thought to be linked to disease protection. Two doses of Pfizer vaccine exhibit significantly reduced neutralization titers against the Omicron, but the addition of one dose of vaccine improves the immunity against it. The third dose of the vaccine generates high and long lasting protection against Omicron [66]. The Omicron was resistant to two doses of the ChAdOx1 vaccination, and the vaccine's ability to protect against Omicron became about 71.4% after the third dose of BNT162b2, which was administered based on two doses of ChAdOx1. But when an individual was administered two doses of BNT162b2, the vaccine's efficacy was 88%. Then it began to decline 2–9 weeks after the second dose, and then continued to decline. The vaccine effectiveness against Omicron dropped from 48.5% to 34.2% from 10–14 weeks–25 weeks after the second dose of vaccination. Then the third dose of BNT162b2 was administered. Based on two doses of BNT162b2, the vaccine effectiveness against Omicron became high, exceeding 75.5% after two weeks [25]. After four months of administering two doses of Pfizer mRNA vaccine, survival rates decline by around 40%, which is better than zero, and implies that the presence of some protection and the efficiency of the booster dose of vaccination is nearly 80%. Despite the fact that the booster was generated by the original virus, these findings suggest that strengthening the immune system after a halt promotes immunity against new versions both quantitatively and qualitatively [65]. After 14 days of administration of two doses of BBIBPCorV inactivated vaccines, the geometric mean titres (GMTs) against Omicron were 6.04. However, in 80% of the samples, neutralization activity against Omicron was below the quantifiable limit. On day 14 following booster immunization, the neutralization titre against Omicron was 48.73, and on day 28 following booster dosage, the GMTs against Omicron stayed constant or increased to 47.69%. Following booster vaccination, 100% of subjects had positive Omicron neutralization activity [67]. According to comparable statistics from South Africa and the United Kingdom, the individual receives two doses of vaccine, which provides up to 70% protection against hospitalisation and can be extended to 90% with a booster dose [68]. This demonstrates that a third vaccination dosage, a booster dose injection, may improve Omicron neutralization activity in individuals who have already received two doses of mRNA vaccines (Pfizer, ChAdOx1, BNT162b2) or inactivated vaccines (BBIBPCorV) [25]. The neutralization activity of covalescent plasma decreased by 8.4 fold but in case of other VOCs and VOIs (Variant of Interest) the activity decreased by 1.2–4.5 folds in comparison with D614G. The neutralization potency estimation of some mAb drugs on Omicron reveled that majority of mAbs (LY-CoV555, LY-CoV016, REGN10933, REGN10987, AZD1061) were completely ineffective against Omicron variant (IC50 > 6.5 mg/L) whereas only two mAbs (IC50: 0.181 mg/L for Vir-7831; 0.287 mg/μl for DXP-604) neutralization ability noted against Omicron. Delta variant can be prevented by imdevimab and casirivimab but Omicron can resist them. Only three out of 29 RBM-directed mAbs maintained their In vitro neutralizing effect against Omicron, including the ACE2 imitating S2K146 mAb. These findings suggest that the amount of Omicron-mediated immune evasion represents a significant antigenic shift in SARS-CoV-2 [25]. According to recent studies, serum from both convalescent and fully vaccinated patients (BNT162b2, mRNA-1273, Ad26.COV2.5 or ChAdOx1-nCoV19, Sputnik V or BBIBP-CorV) has very low to undetectable levels of mAbs against Omicron [14]. Several investigations also show that Omicron avoids binding and neutralization by the majority of therapeutic SARS-CoV-2 mAbs, with the exception of some widely neutralizing mAbs like sotrovimab [69,70]. Cao et al. [70] revealed that 85% of 247 human RBD-targeted mAbs fail to bind Omicron. Despite the fact that nAb can let viruses get through to people who have been vaccinated and are on the road to recovery, it's important to note that pre-existing cellular and innate immunity, non-nAb, and residual nAb are still likely to keep people from getting very sick [14].
6. Learning from past pandemic and its association with Omicron
Through history, infectious diseases with pandemic potential have arisen and spread on a regular basis. The COVID-19 pandemic is wreaking havoc around the world right now, and it is expected to continue to do so. Plague, cholera, influenza, and coronavirus infections are just a few examples of major pandemics that have had a significant influence on humanity over the course of recorded history [22]. According to the World Health Organization, plague has been classified as a re-emerging infectious disease since the 1990s [71]. Following the initial pandemic, plague outbreaks occurred every 8–12 years for the next two centuries before mysteriously disappearing [22]. Due to its quick spread, high fatality rate without prompt treatment, and capacity to disrupt society and healthcare systems, plague should now be considered a neglected human hazard [72]. Both the Spanish flu and swine flu were caused by the influenza A H1N1 virus. When the swine flu first arrived in 2009, no one was immune. The virus was a subtype of the H1N1 influenza virus that caused the 1918 flu pandemic. By the winter of 2009, a large proportion of the population had been infected or vaccinated, resulting in extensive herd immunity. As a result, by August 2010, the pandemic was over. However, the virus has become endemic and has developed into a new seasonal strain of the common flu. In the United States, influenza and HIV continue to kill people; an endemic of sickness isn't exactly a happy ending [73]. SARS-CoV-2 and the Spanish Flu triggered the first and second waves of infection, which followed a similar pattern. There was a slight initial wave, but a huge and fatal second wave. When the Spanish flu virus mutated after two waves, it became so mild that people who were infected only displayed symptoms similar to a cold. Thus, the third wave was less potent and dealt less damage than the previous two. In India, there were no more waves after that one. As a result, the Omicron may be able to put an end to this pandemic. After three waves, the Spanish flu became endemic within two years due to the modified strain like Omicron, which was also likely less severe and more contagious, and which may eventually be responsible for the pandemic's conclusion [74]. Due to the pandemic's lower virulence and higher transmission rate, the causal symptoms grew weaker at the pandemic's end. The earlier contagious variety could be suppressed or neutralised by a higher transmission rate [74]. Although Omicron remains hazardous in some people, such as those who have not been vaccinated or are immune compromised, hospitalisation and fatality rates have dropped in comparison to previous strains of the virus. The great majority of the world's population would be either vaccinated or infected. As a result, the global population would acquire herd immunity [73]. The strong immunity against SARS-CoV-2 that can provide the strongest protection against the infection is referred to as hybrid immunity, and after getting the vaccine, those who are exposed to the virus have a lower risk of becoming ill as a result [75]. In the event of a pandemic outbreak, a comprehensive public health response, including vaccination, is almost certainly needed. It is possible that effective vaccines can save lives and restrict the spread of disease if they are used alongside other pandemic response strategies such as surveillance, communication plans, quarantine, and disease treatment. However, not all diseases have vaccines and even those that do face considerable obstacles to their use in a pandemic [76]. For millennia, epidemic diseases known as “pandemics” have ravaged human populations around the world, claiming the lives of hundreds of millions of people. Between 25 and 75 million people died in Europe during the 1300s as a result of bubonic plague, commonly known as the “Black Death,” according to historians [77]. Until the 1660s, waves of the disease swept across Europe, with the largest outbreak being in England. Smallpox, on the other hand, ravaged the world for millennia before being declared eradicated in 1980 [78]. An estimated 40–70 million people died as a result of the influenza epidemic that swept the world in 1918–19. In 1957–58, 1968, and 2009, less severe pandemics of influenza broke out [79]. Vaccines against the circulating viruses were developed in the last three cases, but experts disagree on their effectiveness in slowing disease spread. While human cases of human-to-human transmission of the H5N1 influenza virus known as bird flu have occurred since 2003, the virus has not evolved to move between humans. In the event that H5N1 becomes human transmissible, public health officials are keeping a close eye on the situation. The government of the United States has an H5N1 vaccination on hand, but its efficacy against new strains of the virus is uncertain [80].
7. Is Omicron acts as a natural vaccine?
Regardless of whether Omicron functions as a natural vaccination or not, the ongoing debut is still underway. While some specialists have asserted that Omicron can operate as a natural vaccination, other groups of scientists have expressed scepticism about this claim [81]. The Omicron variant spreads quickly and efficiently, causes less severe disease than prior versions, and may offer some protection against other variants like Delta [39,62]. This could be the conclusion of the pandemic and the beginning of an endemic phase, with the pandemic predicted to conclude in 2022 [82]. All immunizations aim to prime the immune system in order to fight an infectious organism. Certain vaccines, such as the Pfizer-BioNTech and Moderna vaccines, administer a specific portion of viruses into the host body, while others, such as chicken pox, measles, mumps, and rubella, administer a weakened form of viruses into the host cell body. Both types of vaccine elicit an immune response [83,84]. Due to a considerable number of changes in the RBD of SARS-CoV-2, the Omicron displayed a stronger affinity for human ACE2 than the Delta and also showed a higher potential for transmission [55]. Omicron is 1.5–2 times more infectious than Delta, which explains Omicron's global dominance [85]. The more and faster spread of Omicron is thought to be rapidly displacing the dangerous Delta [86]. Individuals infected with Omicron develop a strong immune response that can neutralize Omicron along with other VOCs. This suggests that the Omicron-induced immune response could effectively neutralize the Delta, reducing the re-infection with Delta and thereby displacing the Delta as the dominant strain [87]. Omicron may act as a vaccine because it allows a large number of people to effectively establish herd immunity against the Omicron, halting transmission and bringing the COVID-19 pandemic to an end, in the same way that vaccination develops herd immunity [88]. High levels of antibodies have been discovered in those who have been cured of Omicron, which combats viruses, and the vaccine also does the same things. However, according to some reports, the antibodies produced by Omicron infection are so potent that no vaccine can produce them [89]. When the body triggers an immune response to re-infection by a virus due to the antibody that remains in the body for at least 450 days after infection, this is referred to as a “natural vaccine” or “natural immunity” from COVID-19. According to some people, Omicron does the same [90]. Omicron has spread like wildfire, but most cases are mild, and according to some doctors and virologists, it could result in major immunity and work as a natural vaccine. This variant has the potential to increase immunity without causing major sickness. Omicron is similar to these live attenuated vaccines in certain ways because it generates a milder infection and triggers a strong immune response against viruses [91]. Genetic modifications of Omicron make it more resistant to being captured by antibodies produced by the immune system. This aids the virus's spread until it reaches a point where it becomes self-defeating if the virus is too adept at spreading and producing sickness. The virus becomes so weak that it acts as a vaccine itself [92]. According to Delta patients, this reduced severity may be due in part to high levels of individual's immunity resulting from natural infection and/or vaccination. The high population immunity resulting from earlier infection and/or immunisation may protect the population from serious disease [17]. Omicron may be the safest SARS-CoV-2 strain to infect of all the strains that have infected patients in the previous two years because it does not cause the severe illness. If vaccinated persons become infected with Omicron and develop immunity, that immunity can be paired with immunity against the previous variant to create a threshold level of herd immunity, allowing 70–90% of people to be recovered from or vaccinated against COVID-19 [93,94]. Also, according to some experts, considering Omicron as a natural vaccine is a dangerous idea. It creates complacency and is based more on pandemic fatigue and incapacity to do more than on current data and that Omicron is not a vaccine; no matter how light it is, because this variant has resulted in deaths and hospitalizations [95]. If the variant is allowed to spread, then it may be mutated and also infect again, meaning the pandemic will persist for a long time. The virus is continually changing and finding new methods to spread. The more it spreads, the more likely it is to mutate and create new outbreaks of cases. So it is preferable to halt transmission and eliminate any potential for mutation [96]. Natural infection of Omicron, in comparison to vaccination, cannot protect the population against death or hospitalisation from any of the Alpha, Beta, Gamma, or Delta [97].
Apart from the ongoing debate over Omicron's potential as a natural vaccination, Delmicron (or Deltacron) recently raised a serious concern. The Cypriot Laboratory of Biotechnology and Molecular Virology at the University of Cyprus published the first-ever information on the recombination of Delta and Omicron variants [98]. According to the EMERGEN consortium, this recombinant virus has been spreading in numerous regions of France since early January 2022. Denmark and the Netherlands have also uncovered genomes with similar characteristics. GISAID, the world's largest collection of novel coronavirus genome sequences, just published information regarding a unique coronavirus recombination event. Numerous verified Delmicron cases have been reported recently in various parts of the world. While the COVID-19 situation in India is now quiet, the national government has warned of an approaching wave due to the virus's spread in other countries. In India, at least 568 cases of Delta and Omicron recombinant (Deltacron) have been reported. Karnataka leads the way with 221 such occurrences, followed by Tamil Nadu with 90, Maharashtra with 66, Gujarat with 33, West Bengal with 32, Telangana with 25 and Delhi with 20 [99]. In mid November 2021, another SARS-CoV-2 strain designated as IHU (B.1.640.2) was identified in 12 French individuals for the first time at the IHU Mediterranee Infection Institute in Marseille [100]. The IHU variation has 46 mutations and 37 deletions, and the ‘S' protein itself contains 14 amino acid substitutions with N501Y and E484K, as well as 9 deletions [101]. Furthermore, in Israel, it has been verified that a pregnant lady was infected with both seasonal flu and COVID-19 at the same time. Florona is the scientific term for this type of co-infection caused by COVID-19 and influenza, which is not an issue of novel version of SARS-CoV-2 [102]. A 21-year-old Egyptian woman also faced co-infection of COVID-19 and H1N1 at same time. This co-infection caused pneumonia, lung damage, cardiac damage, sepsis, and brain, heart, and muscle inflammation [100]. Recently, WHO announced the identification of the second recombinant virus XE, which is a combination strain of two Omicron sub-variants, BA.1 and BA.2. Numerous international health organisations have expressed alarm about the virus's high transmissibility, which makes it extremely contagious and could result in a new high risk [103]. This recombinant XE variation was detected for the first time in the United Kingdom around the end of January 2022. The XE variety has been found in India, Thailand, and New Zealand as well. In the United Kingdom, around 637 confirmed instances of XE have been reported, whereas in India, the first positive XE case was found in Mumbai on April 6, 2022 [104]. WHO identified more recombinant viruses, XD and XF along with XE. The French Delta and BA.1 lineages are now known as XD which contains the 'S' protein of BA.1 as well as the genome of Delta variant. XF is a BA.1 and Delta lineage from the United Kingdom. It contains 'S' protein and structural proteins of BA.1 and genome of Delta but only the 5′ part its genome [103]. As a result of this cumulative evidence, it is too difficult to assert whether the Omicron wave is the beginning of the end of this pandemic or something more to be added in COVID-19 history.
8. Conclusion
Omicron has more than 60 mutations than earlier variants, including at least 30 mutations in the “S" protein and 15 mutations in RBD, resulting in quick attachment to human cells and escape immunity. It is assume that, Omicron cases will increase dramatically due to high disease transmission but will be less severe among hospitalized patients than Delta. Due to two to three times higher transmission rates than Delta, more people will infected and the Delta variant fades away gradually, which might be the end phase of a pandemic or the starting phase of an endemic. As there are numerous similarities between the Omicron and previous pandemics, such as low virulence, high transmission rate, and typical symptoms, the Omicron may decline in the same way through vaccination. But the neutralization capacity of the existing COVID-19 vaccination against Omicron was found to be reduced, so administration of another dose, such as a booster dose, can improve the immunity and prevent hospitalisation. In some aspects, Omicron is similar to previous live attenuated vaccines since it causes a milder illness and elicits a strong immune response against the virus. So, initially it was thought it may be able to act as a natural vaccine, but later a large portion of experts opposed this idea. Accruing herd immunity and hybrid immunity, along with weaker variants and rapid vaccination, may be helpful for the conversion of a pandemic to an endemic. In very recent times, according to some experts, Omicron may not be the end of the pandemic because a super variant, Delmicron, a combination of previous Delta and Omicron variants, is being identified in some places around the world. If it exerts pathogenicity like Delta and transmissibility rate like Omicron, then it will become more severe due to a mixture of both “VOCs”. The new super variant may then become new severe threat to mankind.
Funding
There was no funding for this study.
Authorship contribution
SD, SS and JB: acquisition of information, drafted the article, designed the figures, and final approval of the version to be submitted. SKD and SSK: the conception and design of the study, revising it critically for important intellectual content, designed the figures, and final approval of the version to be submitted. JB, AP and BG: interpretation of data, and revising it critically for important intellectual content. All the authors gave final approval of the version to be submitted.
Declaration of competing interest
Authors have no conflict of interest.
Acknowledgements
The University of Gour Banga is greatly acknowledged for providing the opportunity to work in the Department of Physiology.
Abbreviation
- ACE2
Angiotensin-converting enzyme 2
- CoV
Coronavirus
- COVID-19
Coronavirus disease
- E Protein
Envelope Protein
- FCS
Furin-like cleavage site
- GMTs
Geometric mean titres
- HCoV
Human coronaviruses
- M Protein
Membrane Protein
- mAb
Monoclonal antibodie
- MERS-CoV
Middle Eastern respiratory syndrome coronavirus
- mRNA
Messenger RNA
- N Protein
Nucleocapsid Protein
- nAbs
Neutralizing antibodies
- PHEIC
Public Health Emergency of International Concern
- RBD
Receptor Binding DomainS protein: Spike Protein
- S protein
Spike protein
- SARS-HCoV
Severe Acute Respiratory Syndrome human coronavirus
- TMPRSS2
Transmembrane serine protease 2
- VOC
Variant of Concern
- VOIs
Variant of Interest
References
- 1.Kumar D., Malviya R., Sharma P.K. Corona virus: a review of COVID-19. EJMO. 2020;4(1):8–25. doi: 10.14744/ejmo.2020.51418. [DOI] [Google Scholar]
- 2.Da Silva P.G., Mesquita J.R., Nascimento M.D., Ferreira V.A. Viral, host and environmental factors that favor anthropozoonotic spillover of coronaviruses: an opinionated review, focusing on SARS-CoV, MERS-CoV and SARS-CoV-2. Sci Total Environ. 2021 Jan 1;141483:750. doi: 10.1016/j.scitotenv.2020.141483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P. A novel coronavirus from patients with pneumonia in China. N Engl J Med. 2019 doi: 10.1056/NEJMoa2001017. 2020 Jan 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Samanta S., Banerjee J., Rahaman S.N., Ali K.M., Ahmed R., Giri B., Pal A., Dash S.K. Alteration of dietary habits and lifestyle pattern during COVID-19 pandemic associated lockdown: an online survey study. Clin Nutr ESPEN. 2022 Feb 16 doi: 10.1016/j.clnesp.2022.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jee Y. WHO international health regulations emergency committee for the COVID-19 outbreak. Epidemiol Health. 2020;42 doi: 10.4178/epih.e2020013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.2022. https://www.worldometers.info/coronavirus/
- 7.Dhama K., Sharun K., Tiwari R., Dadar M., Malik Y.S., Singh K.P., Chaicumpa W. COVID-19, an emerging coronavirus infection: advances and prospects in designing and developing vaccines, immunotherapeutics, and therapeutics. Hum Vaccines Immunother. 2020 Jun 2;16(6):1232–1238. doi: 10.1080/21645515.2020.1735227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khateeb J., Li Y., Zhang H. Emerging SARS-CoV-2 variants of concern and potential intervention approaches. Crit Care. 2021 Dec;25(1):1–8. doi: 10.1186/s13054-021-03662-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Das S., Kar S.S., Samanta S., Banerjee J., Giri B., Dash S.K. Immunogenic and reactogenic efficacy of Covaxin and Covishield: a comparative review. Immunol Res. 2022 Feb 22:1–27. doi: 10.1007/s12026-022-09265-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malabadi R.B., Kolkar K.P., Meti N.T., Chalannavar R.K. Outbreak of coronavirus (SARS-CoV-2) Delta variant (B. 1.617. 2) and Delta plus (AY. 1) with fungal infections, mucormycosis: herbal medicine treatment. Int J Res Sci Innovat. 2021;8(6):59–70. 2021g. [Google Scholar]
- 11.Arora P., Kempf A., Nehlmeier I., Graichen L., Sidarovich A., Winkler M.S., Schulz S., Jäck H.M., Stankov M.V., Behrens G., Pöhlmann S. Delta variant (B. 1.617. 2) sublineages do not show increased neutralization resistance. Cell Mol Immunol. 2021 Nov;18(11):2557–2559. doi: 10.1038/s41423-021-00772-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thakur V., Kanta Ratho R. OMICRON (B. 1.1. 529): a new SARS-CoV-2 Variant of Concern mounting worldwide fear. J Med Virol. 2021 Dec 22 doi: 10.1002/jmv.27541. [DOI] [PubMed] [Google Scholar]
- 13.Patil B.B. Omicron: the tsunami?? Indian J Health Sci Biomd Res (KLEU) 2022 Jan 1;1(1):15. doi: 10.4103/kleuhsj.kleuhsj_76_22. [DOI] [Google Scholar]
- 14.Flemming A. Omicron, the great escape artist. Nat Rev Immunol. 2022 Jan 11 doi: 10.1038/s41577-022-00676-6. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brandal L.T., MacDonald E., Veneti L., Ravlo T., Lange H., Naseer U., Feruglio S., Bragstad K., Hungnes O., Ødeskaug L.E., Hagen F. Outbreak caused by the SARS-CoV-2 omicron variant in Norway, November to December 2021. Euro Surveill. 2021 Dec 16;26(50):2101147. doi: 10.2807/1560-7917.ES.2021.26.50.2101147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kozlov M. Omicron's feeble attack on the lungs could make it less dangerous. Nature. 2022:177. doi: 10.1038/d41586-022-00007-8. [DOI] [PubMed] [Google Scholar]
- 17.Wolter N., Jassat W., Walaza S., Welch R., Moultrie H., Groome M., Amoako D.G., Everatt J., Bhiman J.N., Scheepers C., Tebeila N. Early assessment of the clinical severity of the SARS-CoV-2 Omicron variant in South Africa. medRxiv. 2021 Jan 1 doi: 10.1101/2021.12.21.21268116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nealon J., Cowling B.J. Omicron severity: milder but not mild. Lancet. 2022 Jan 19 doi: 10.1016/S0140-6736(22)00056–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.2022. https://economictimes.indiatimes.com/industry/healthcare/biotech/healthcare/immune-responseinducedbyomicroneffectivelyneutralisedeltavarianticmrstudy/articleshow/89136215.cms?from=mdr
- 20.https://www.dw.com/en/omicron-is-natural-immunity-better-than-a-vaccine/a-60425426
- 21.Huremović D. Springer; Cham: 2019. Brief history of pandemics (pandemics throughout history). InPsychiatry of pandemics; pp. 7–35. [DOI] [Google Scholar]
- 22.Piret J., Boivin G. Pandemics throughout history. Front Microbiol. 2021;3594 doi: 10.3389/fmicb.2020.631736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.2022. https://www.outlookindia.com/website/story/india-news-will-omicron-end-covid-pandemic-like-a-mild-variant-did-to-spanish-flu-100-years-back/407090
- 24.Ettaboina S.K., Nakkala K., Laddha K.S. A mini review on SARS-COVID-19-2 omicron variant (B. 1.1. 529) SciMed J. 2021 Dec 30;3(4):399–406. doi: 10.28991/SciMedJ-2021-0304-10. [DOI] [Google Scholar]
- 25.Tian D., Sun Y., Xu H., Ye Q. The emergence and epidemic characteristics of the highly mutated SARS-CoV-2 Omicron variant. J Med Virol. 2022 Feb 3 doi: 10.1002/jmv.27643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He X., Hong W., Pan X., Lu G., Wei X. SARS-CoV-2 Omicron variant: characteristics and prevention. MedComm. 2021 Dec 16 doi: 10.1002/mco2.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Woo H.G., Shah M. Omicron: a heavily mutated SARS-CoV-2 variant exhibits stronger binding to ACE2 and potently escape approved COVID-19 therapeutic antibodies. bioRxiv. 2021 Jan 1 doi: 10.1101/2021.12.04.471200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kannan S.R., Spratt A.N., Sharma K., Chand H.S., Byrareddy S.N., Singh K. Omicron SARS-CoV-2 variant: unique features and their impact on pre-existing antibodies. J Autoimmun. 2022 Jan 1;102779:126. doi: 10.1016/j.jaut.2021.102779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.2021. https://timesofindia.indiatimes.com/world/rest-of-world/covid-19-how-omicron-uses-spike-protein-to-enter-cells/articleshow/88056481.cms
- 30.Karim S.S., Karim Q.A. Omicron SARS-CoV-2 variant: a new chapter in the COVID-19 pandemic. Lancet. 2021 Dec 11;398(10317):2126–2128. doi: 10.1016/S0140-6736(21)02758-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saxena S.K., Kumar S., Ansari S., Paweska J.T., Maurya V.K., Tripathi A.K., Abdel-Moneim A.S. Transmission dynamics and mutational prevalence of the novel SARS-CoV-2 Omicron Variant of Concern. J Med Virol. 2022 Jan 20 doi: 10.1002/jmv.27611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.COVID C Team R. SARS-CoV-2 B. 1.1. 529 (omicron) variant—United States, December 1–8, 2021. MMWR (Morb Mortal Wkly Rep) 2021 Dec 17;70(50):1731. doi: 10.15585/mmwr.mm7050e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tan Z., Chen Z., Yu A., Li X., Feng Y., Zhao X., Xu W., Su X. The first two imported cases of SARS-CoV-2 omicron variant—Tianjin municipality, China, December 13, 2021. China CDC Weekly. 2022 Jan 28;4(4):76. doi: 10.46234/ccdcw2021.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khandia R., Singhal S., Alqahtani T., Kamal M.A., Nahed A., Nainu F., Desingu P.A., Dhama K. Emergence of SARS-CoV-2 Omicron (B. 1.1. 529) variant, salient features, high global health concerns and strategies to counter it amid ongoing COVID-19 pandemic. Environ Res. 2022 Jan 29;112816 doi: 10.1016/j.envres.2022.112816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.2022. https://www.statista.com/statistics/1279100/number-omicron-variant-worldwide-by-country/
- 36.Choudhary O.P., Manish Dhawan P. Omicron variant (B. 1.1. 529) of SARS-CoV-2: threat assessment and plan of action. Int J Surg. 2022 Jan;106187:97. doi: 10.1016/j.ijsu.2021.106187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kannan S., Sheeza A. Omicron (B. 1.1. 529)-variant of concern-molecular profile and epidemiology: a mini review. Eur Rev Med Pharmacol Sci. 2021 Dec 1;25(24):8019–8022. doi: 10.26355/eurrev_202112_27653. [DOI] [PubMed] [Google Scholar]
- 38.Cascella M., Rajnik M., Aleem A., Dulebohn S.C., Di Napoli R. Features, evaluation, and treatment of coronavirus (COVID-19) Statpearls [internet] 2022 Feb 5 [PubMed] [Google Scholar]
- 39.Kupferschmidt K., Vogel G. How bad is Omicron? Some clues are emerging. Science. 2021 Dec 10;374(6573):1304–1305. doi: 10.1126/science.acx9782. [DOI] [PubMed] [Google Scholar]
- 40.Saxena S.K., Kumar S., Ansari S., Paweska J.T., Maurya V.K., Tripathi A.K., Abdel-Moneim A.S. Characterization of the novel SARS-CoV-2 Omicron (B. 1.1. 529) variant of concern and its global perspective. J Med Virol. 2022 Apr;94(4):1738–1744. doi: 10.1002/jmv.27524. [DOI] [PubMed] [Google Scholar]
- 41.Ali F., Kasry A., Amin M. The new SARS-CoV-2 strain shows a stronger binding affinity to ACE2 due to N501Y mutant. Med Drug Discov. 2021 Jun 1;100086:10. doi: 10.1016/j.medidd.2021.100086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Poudel S., Ishak A., Perez-Fernandez J., Garcia E., León-Figueroa D.A., Romaní L., Bonilla-Aldana D.K., Rodriguez-Morales A.J. Highly mutated SARS-CoV-2 Omicron variant sparks significant concern among global experts–What is known so far? Trav Med Infect Dis. 2022 Jan;102234:45. doi: 10.1016/j.tmaid.2021.102234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gong S.Y., Chatterjee D., Richard J., Prévost J., Tauzin A., Gasser R., Bo Y., Vézina D., Goyette G., Gendron-Lepage G., Medjahed H. Contribution of single mutations to selected SARS-CoV-2 emerging variants spike antigenicity. Virology. 2021 Nov 1;563:134–145. doi: 10.1016/j.virol.2021.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kazybay B., Ahmad A., Mu C., Mengdesh D., Xie Y. Omicron N501Y mutation among SARS-CoV-2 lineages: insilico analysis of potent binding to tyrosine kinase and hypothetical repurposed medicine. Trav Med Infect Dis. 2022 Jan 1;102242:45. doi: 10.1016/j.tmaid.2021.102242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kumar S., Karuppanan K., Subramaniam G. Omicron (BA. 1) and sub-variants (BA. 1, BA. 2 and BA. 3) of SARS-CoV-2 spike infectivity and pathogenicity: a comparative sequence and structural-based computational assessment. bioRxiv. 2022 Jan 1 doi: 10.1101/2022.02.11.480029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Desingu P.A., Nagarajan K., Dhama K. Emergence of Omicron third lineage BA. 3 and its importance. J Med Virol. 2022 Jan 18 doi: 10.1002/jmv.27601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Iketani S., Liu L., Guo Y., Liu L., Chan J.F., Huang Y., Wang M., Luo Y., Yu J., Chu H., Chik K.K. Antibody evasion properties of SARS-CoV-2 Omicron sublineages. Nature. 2022 Mar 3:1. doi: 10.1038/s41586-022-04594-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mahase E. Covid-19: what do we know about omicron sublineages? bmj. 2022 Feb 11;376 doi: 10.1136/bmj.o358. [DOI] [PubMed] [Google Scholar]
- 49.Wilkinson E. Covid-19: we have good treatments for omicron, but questions remain, say doctors. 2022. [DOI] [PubMed]
- 50.Takashita E., Kinoshita N., Yamayoshi S., Sakai-Tagawa Y., Fujisaki S., Ito M., Iwatsuki-Horimoto K., Halfmann P., Watanabe S., Maeda K., Imai M. Efficacy of antiviral agents against the SARS-CoV-2 omicron subvariant BA. 2. N Engl J Med. 2022 Mar 9 doi: 10.1056/NEJMc2201933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Uraki R, Kiso M, Imai M, Yamayoshi S, Ito M, Ujie M, Furusawa Y, Iwatsuki-Horimoto K, Sakai-Tagawa Y, Kawaoka Y. Therapeutic efficacy of antibodies and antivirals against a SARS-CoV-2 Omicron variant. 10.21203/rs.3.rs-1240227/v1. [DOI] [PubMed]
- 52.Mlcochova P., Kemp S.A., Dhar M.S., Papa G., Meng B., Ferreira I.A., Datir R., Collier D.A., Albecka A., Singh S., Pandey R. SARS-CoV-2 B. 1.617. 2 Delta variant replication and immune evasion. Nature. 2021 Nov;599(7883):114–119. doi: 10.1038/s41586-021-03944-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mohapatra R.K., Sarangi A.K., Kandi V., Azam M., Tiwari R., Dhama K. Omicron (B. 1.1. 529 variant of SARS-CoV-2); an emerging threat: current global scenario. J Med Virol. 2021 Dec 29 doi: 10.1002/jmv.27561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Daria S., Islam M.R. The SARS-CoV-2 omicron wave is indicating the end of the pandemic phase but the COVID-19 will continue. J Med Virol. 2022 Jan 30 doi: 10.1002/jmv.27635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kumar S., Thambiraja T.S., Karuppanan K., Subramaniam G. Omicron and Delta variant of SARS-CoV-2: a comparative computational study of spike protein. J Med Virol. 2022 Apr;94(4):1641–1649. doi: 10.1002/jmv.27526. [DOI] [PubMed] [Google Scholar]
- 56.Arora S., Grover V., Saluja P., Algarni Y.A., Saquib S.A., Asif S.M., Batra K., Alshahrani M.Y., Das G., Jain R., Ohri A. Literature review of omicron: a Grim Reality amidst COVID-19. Microorganisms. 2022 Feb 16;451(2):10. doi: 10.3390/microorganisms10020451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Papanikolaou V., Chrysovergis A., Ragos V., Tsiambas E., Katsinis S., Manoli A., Papouliakos S., Roukas D., Mastronikolis S., Peschos D., Batistatou A. From Delta to Omicron: S1-RBD/S2 mutation/deletion equilibrium in SARS-CoV-2 defined variants. Gene. 2022 Jan 4;146134 doi: 10.1016/j.gene.2021.146134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.2022. https://timesofindia.indiatimes.com/lifestyle/healthfitness/healthnews/coronavirusomicronsymptomsvsdeltasymptomshowomicronsymptomsdifferfromdeltaandpreviouscovidvariants/photostory/89115711.cms
- 59.Bhoye S, Marakwad T. A comparative characteristics of both variants of severe Acute respiratory syndrome coronavirus-2 (SARS-CoV-2): Delta and Omicron.
- 60.2022. https://www.health.com/condition/infectious-diseases/coronavirus/omicron-vs-delta
- 61.Colson P., Fournier P.E., Delerce J., Million M., Bedotto M., Houhamdi L., Yahi N., Bayette J., Levasseur A., Fantini J., Raoult D. Culture and identification of a Deltamicron SARS-CoV-2 in a three cases cluster in southern France. medRxiv. 2022 Jan 1 doi: 10.1101/2022.03.03.22271812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Callaway E. Omicron likely to weaken COVID vaccine protection. Nature. 2021;600(7889):367–368. doi: 10.1038/d41586-021-03672-3. [DOI] [PubMed] [Google Scholar]
- 63.Willyard C. What the Omicron wave is revealing about human immunity. Nature. 2022 Feb 1;602(7895):22–25. doi: 10.1038/d41586-022-00214-3. [DOI] [PubMed] [Google Scholar]
- 64.Callaway E. COVID vaccine boosters: the most important questions. Nature. 2021;596(7871):178–180. doi: 10.1038/d41586-021-02158-6. [DOI] [PubMed] [Google Scholar]
- 65.2021. https://directorsblog.nih.gov/2021/12/14/the-latest-on-the-omicron-variant-and-vaccine-protection/
- 66.2021. https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-provide-update-omicron-variant
- 67.Ai J., Zhang H., Zhang Y., Lin K., Zhang Y., Wu J., Wan Y., Huang Y., Song J., Fu Z., Wang H. Omicron variant showed lower neutralizing sensitivity than other SARS-CoV-2 variants to immune sera elicited by vaccines after boost. Emerg Microb Infect. 2021 Dec 23:1–24. doi: 10.1080/22221751.2021.2022440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.2022. https://www.ema.europa.eu/en/news/preliminary-data-indicate-covid-19-vaccines-remain-effective-against-severe-disease-hospitalisation
- 69.Cameroni E., Bowen J.E., Rosen L.E., Saliba C., Zepeda S.K., Culap K., Pinto D., VanBlargan L.A., De Marco A., di Iulio J., Zatta F. Broadly neutralizing antibodies overcome SARS-CoV-2 Omicron antigenic shift. Nature. 2022 Feb;602(7898):664–670. doi: 10.1038/s41586-021-04386-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cao Y., Wang J., Jian F., Xiao T., Song W., Yisimayi A., Huang W., Li Q., Wang P., An R., Wang Y. Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. Nature. 2022 Feb;602(7898):657–663. doi: 10.1038/s41586-021-04385-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.2017. https://www.who.int/news-room/fact-sheets/detail/plague
- 72.Vallès X., Stenseth N.C., Demeure C., Horby P., Mead P.S., Cabanillas O., Ratsitorahina M., Rajerison M., Andrianaivoarimanana V., Ramasindrazana B., Pizarro-Cerda J. Human plague: an old scourge that needs new answers. PLoS Neglected Trop Dis. 2020 Aug 27;14(8) doi: 10.1371/journal.pntd.0008251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.2022. https://connect.uclahealth.org/2022/01/13/when-does-a-pandemic-become-endemic-and-what-is-the-difference/
- 74.2022. https://www.outlookindia.com/website/story/india-news-will-omicron-end-covid-pandemic-like-a-mild-variant-did-to-spanish-flu-100-years-back/407090
- 75.2022. https://www.medicalnewstoday.com/articles/vaccination-gives-hybrid-immunity-to-recovered-covid-19-patients
- 76.Samanta S., Banerjee J., Kar S.S., Ali K.M., Giri B., Pal A., Dash S.K. Awareness, knowledge and acceptance of COVID-19 vaccine among the people of West Bengal, India: a web-based survey. Vacunas. 2022 Feb 2 doi: 10.1016/j.vacun.2022.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Huremović D. Springer; Cham: 2019. Brief history of pandemics (pandemics throughout history). InPsychiatry of pandemics; pp. 7–35. [DOI] [Google Scholar]
- 78.Krylova O., Earn D.J. Patterns of smallpox mortality in London, England, over three centuries. PLoS Biol. 2020 Dec 21;18(12):e3000506. doi: 10.1371/journal.pbio.3000506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lemon SM, Mahmoud A, Mack A, Knobler SL, editors. The threat of pandemic influenza: are we ready? workshop summary. [PubMed]
- 80.Peiris J.M., De Jong M.D., Guan Y. Avian influenza virus (H5N1): a threat to human health. Clin Microbiol Rev. 2007 Apr;20(2):243–267. doi: 10.1128/CMR.00037-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.2022. https://www.deccanherald.com/science-and-environment/does-omicron-act-as-a-natural-vaccine-heres-what-experts-say-1067121.html
- 82.Alexandridi M., Mazej J., Palermo E., Hiscott J. The coronavirus pandemic–2022: viruses, variants & vaccines. Cytokine & growth factor reviews. 2022 Feb 12. [DOI] [PMC free article] [PubMed]
- 83.Mascellino M.T., Di Timoteo F., De Angelis M., Oliva A. Overview of the main anti-SARS-CoV-2 vaccines: mechanism of action, efficacy and safety. Infect Drug Resist. 2021;3459:14. doi: 10.2147/IDR.S315727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hajj Hussein I., Chams N., Chams S., El Sayegh S., Badran R., Raad M., Gerges-Geagea A., Leone A., Jurjus A. Vaccines through centuries: major cornerstones of global health. Front Public Health. 2015 Nov 26;269:3. doi: 10.3389/fpubh.2015.00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lundberg A.L., Lorenzo-Redondo R., Ozer E.A., Hawkins C.A., Hultquist J.F., Welch S.B., Prasad P.V., Oehmke J.F., Achenbach C.J., Murphy R.L., White J.I. Has omicron changed the evolution of the pandemic? JMIR Public Health Surveil. 2022 Jan 31;8(1):e35763. doi: 10.2196/35763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Del Rio C., Omer S.B., Malani P.N. Winter of Omicron—the evolving COVID-19 pandemic. JAMA. 2022 Jan 25;327(4):319–320. doi: 10.1001/jama.2021.24315. [DOI] [PubMed] [Google Scholar]
- 87.Yadav P.D., Sapkal G.N., Sahay R.R., Potdar V.A., Deshpande G.R., Patil D.Y., Nyayanit D.A., Shete A.M., Shastri J., Awate P., Malhotra B. Substantial immune response in Omicron infected breakthrough and unvaccinated individuals against SARS-CoV-2 variants of concern. J Infect. 2022 Jan 1 doi: 10.1016/j.jinf.2022.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.2022. https://sc.edu/uofsc/posts/2022/01/02_conversation_omicron.php#.Yj1ydufMJPZ
- 89.2022. https://www.dnaindia.com/analysis/report-dna-special-is-omicron-the-natural-vaccine-for-covid-19-2926780
- 90.2022. https://timesofindia.indiatimes.com/life-style/health-fitness/health-news/coronavirus-couldomicronvariantactasanaturalvaccinewetrytofindout/photostory/88647115.cms?picid=88647126
- 91.Sigal A. Milder disease with Omicron: is it the virus or the pre-existing immunity? Nat Rev Immunol. 2022 Jan 19:1–3. doi: 10.1038/s41577-022-00678-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang C., Han J. Will the COVID-19 pandemic end with the Delta and Omicron variants? Environ Chem Lett. 2022 Jan 15 doi: 10.1007/s10311-021-01369-7. 1–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bugalia S., Tripathi J.P., Wang H. Mutations make pandemics worse or better: modeling SARS-CoV-2 variants and imperfect vaccination. 2022 Jan 17. arXiv preprint arXiv:2201.06285. [DOI] [PubMed]
- 94.2022. https://time.com/6141679/omicron-end-covid-19/
- 95.2022. https://timesofindia.indiatimes.com/life-style/health-fitness/health-news/coronavirus-could-omicron-variant-act-as-a-natural-vaccine-wetrytofindout/photostory/88647115.cms
- 96.2022. https://www.thehealthsite.com/news/exclusive-omicron-is-not-a-natural-vaccine-covid-cannot-be-treated-like-seasonal-flu-says-who-expert-859623/
- 97.2022. https://www.dnaindia.com/health/report-does-omicron-infection-act-as-natural-vaccine-against-covid-19-experts-refute-rumours-2926574
- 98.Cooperman Y., Allegretti G. Is Deltacron a thing? Maybe not. https://ro.co/health-guide/deltacron/
- 99.2022. https://www.downtoearth.org.in/news/health/covid-19-update-deltacron-omicronsubvariant-2-looming-threats-82032
- 100.Mohapatra R.K., Sarangi A.K., Kandi V., Azam M., Tiwari R., Dhama K. Omicron (B. 1.1. 529 variant of SARS-CoV-2); an emerging threat: current global scenario. J Med Virol. 2021 Dec 29 doi: 10.1002/jmv.27561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Colson P., Delerce J., Burel E., Dahan J., Jouffret A., Fenollar F., Yahi N., Fantini J., La Scola B., Raoult D. Emergence in Southern France of a new SARS-CoV-2 variant of probably Cameroonian origin harbouring both substitutions N501Y and E484K in the spike protein. medRxiv. 2021 Jan 1 doi: 10.1101/2021.12.24.21268174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ghaznavi H., Shirvaliloo M., Sargazi S., Mohammadghasemipour Z., Shams Z., Hesari Z., Shahraki O., Nazarlou Z., Sheervalilou R., Shirvalilou S. SARS-CoV-2 and influenza viruses: strategies to Cope with Co-infection and Bioinformatics perspective. Cell Biol Int. 2022 Mar 24 doi: 10.1002/cbin.11800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.https://www.livemint.com/science/news/xe-recombinants-virus-10-covid-symptoms-to-watch-out-for-with-mutant-omicron-strain-circulating11649063446647.html
- 104.https://www.ndtv.com/india-news/indias-first-case-of-coronavirus-variant-xereported-from-mumbai-2866489