Abstract
The lipid formulation of amphotericin B, Amphotec (ABCD), has not been used intrathecally. After a single intrathecal dose or after four doses, conventionally formulated deoxycholate amphotericin B (AMB) (Fungizone) resulted in higher levels of amphotericin B in the cerebrospinal fluid of rabbits than did ABCD. Clinically and histologically, ABCD was about threefold less toxic than AMB after a single dose and 3- to 30-fold less toxic after multiple dosing. These data are encouraging for the potential use of ABCD as an intrathecal treatment.
Fungal meningitis caused by Coccidioides immitis causes severe morbidity and is universally fatal if left untreated. Oral treatment with fluconazole or itraconazole has been used, but is not curative (3). Prolonged intrathecal therapy with amphotericin B deoxycholate sometimes produces cure, whereas intravenous therapy is ineffective in treating meningeal disease. Treatment can be limited by associated toxicities whether given intravenously or intrathecally (5, 6).
With lipid-based formulations of amphotericin B, amphotericin B-associated toxicity is reduced after intravenous infusions (1, 9–12). However, no data are available on the toxicities of a lipid-based amphotericin B preparation when administered intrathecally. The objective in these studies was to compare the neuropharmacology and neurotoxicity of a lipid-formulation of amphotericin B with that of conventionally formulated deoxycholate amphotericin B (AMB) (Fungizone) after intrathecal administration to rabbits. The lipid-formulation used was Amphotec (ABCD) (Alza Corp., Menlo Park, Calif.); ABCD is a colloidal dispersion of cholesteryl sulfate and amphotericin B (4, 7).
Sampling of CSF.
Male New Zealand White rabbits (body weight, ca. 4 kg) were obtained from Kralek Farms, Turlock, Calif., and maintained under clean conditions. All animals had access to appropriate food and water ad libitum. The method used to sample and to inject into the cerebrospinal fluid (CSF) was direct puncture of the cisterna magna, similar to that described previously (19).
Single-dose study.
This experiment examined the pharmacokinetics of ABCD or AMB (Pharma-Tek, Inc., Huntington, N.Y.) in the CSF after a single intrathecal dosage. Four dose concentrations (0.0015, 0.015, 0.045, and 0.135 mg of amphotericin B per kg of body weight) were studied in two rabbits each. Both drugs were reconstituted per the manufacturers' instructions and further diluted in sterile 5% dextrose-water to be given in 0.2 or 0.25 ml. At time zero, CSF was taken and the drug was administered. CSF samples were taken at 6, 24, 48, and 72 h postdose. In addition, a 0.5- to 1.0-ml sample of blood was collected by phlebotomy from an ear vein at 6 h postdose.
Multiple-dose study.
This study compared the relative toxicity of AMB and ABCD upon multiple dosing using doses found to be nontoxic in the single-dose study. All four doses of ABCD and 0.0015- or 0.015-mg/kg AMB were tested. The four doses were given 24 h apart to each of two rabbits through direct cisternal puncture. Twenty-four hours after a dose and just prior to the next dose, a sample of spinal fluid was taken. CSF samples were also taken at 24, 48, and 72 h after the final dose. All samples were stored at −80°C until used for bioassay determination of amphotericin B concentration.
Animals exhibiting signs of discomfort requiring analgesic intervention were given buprenorphine subcutaneously at a dosage of 0.008 mg/kg twice daily. All animals were examined twice daily and evaluated for clinical signs of neurotoxicity.
After the last sampling time, all animals were euthanatized using a saturated solution of pentobarbital given intravenously. A blunt dissection of the brain and spinal cord was done, and tissue samples were removed for histopathological assessment. Tissues were placed into 10% buffered formalin; paraffin-embedded and hematoxylin-and-eosin-stained sections were examined by an observer who was blinded to the treatment and clinical signs of the animals.
Bioassay of amphotericin B.
CSF and serum samples were analyzed by bioassay for the concentration of amphotericin B present. A bioassay, with characteristics described previously (13, 16), was done using Paecilomyces variotti as the indicator organism as described (8, 18) and using AMB deoxycholate as the standard prepared in either serum or CSF. The lower limit of detection this assay was 0.015 to 0.031 μg/ml. Standard curves for amphotericin B were prepared by quadratic regression analysis (GBStat, version 6.0; Dynamic Microsystems, Silver Spring, Md.); the concentration of amphotericin B in each sample was estimated from the regression.
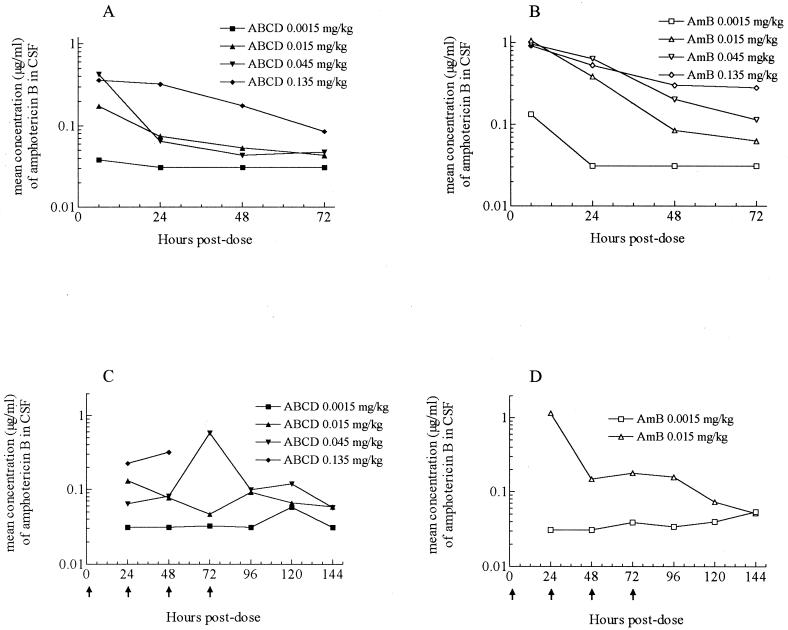
Figure 1 shows that the levels of amphotericin B in the CSF after a single dosage were higher for AMB than for ABCD, especially at 6 and 24 h postdose. The concentrations declined from the 6 h point to 24 h for both drugs at dosages of ≤0.045 mg/kg. Estimates of half-life (t1/2) (based on the data points from the descending limb of the curve) would be <18 h for ABCD and about 18 to 24 h for AMB. However, the profiles observed from the CSF of the animals receiving the 0.135-mg/kg doses show that the t1/2s of both drugs appear more extended after a higher dose (for ABCD, >30 h; for AMB, about 48 h), which might reflect binding to tissues or proteins and subsequent release into the CSF, effectively prolonging the t1/2. These data points appear to fit a two-compartment model of pharmacokinetics. Amphotericin B was detected in the serum (0.033 μg/ml) at 6 h in a single animal given 0.135-mg/kg AMB.
FIG. 1.
Mean concentrations of amphotericin B given as ABCD or AMB in the CSF of two rabbits each after a single dose (A and B) or four doses (C and D). Dosages were as given in the figure. The arrows in panels C and D indicate the times of dosing.
The comparative toxicity was determined by clinical observations (Table 1) and histopathological assessment of the brain. Weight loss occurred in 75% of the animals independent of drug or dosage. Body temperature findings showed minimal changes after ABCD treatment. Mild to moderate fever occurred at 6 h postdose in one or both animals given AMB at ≥0.015 mg/kg; hypothermia occurred in a single animal given AMB at 0.015 mg/kg at 24 h and both given AMB at 0.135 mg/kg at 24 or 48 h postdose.
TABLE 1.
Signs and symptoms of neurological effects observed in rabbits given AMB or ABCD intrathecallya
| Drug and concn (mg/kg) | Hyperthermia or hypothermia
|
Weight loss
|
Mobility problem
|
Convulsion or tremor
|
Paresis or paralysis
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| SD | MD | SD | MD | SD | MD | SD | MD | SD | MD | |
| ABCD | ||||||||||
| 0.0015 | −/− | −/− | +/− | +/− | −/− | −/− | −/− | −/− | −/− | −/− |
| 0.015 | −/− | −/− | −/− | +/+ | −/− | +/+ | −/− | −/− | −/− | −/− |
| 0.045 | +/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− |
| 0.135* | +/− | +/+ | +/− | +/+ | +/+ | +/+ | −/− | +/− | +/− | +/+ |
| AMB | ||||||||||
| 0.0015 | −/− | −/− | +/− | −/− | +/− | −/− | −/− | −/− | −/− | −/− |
| 0.015 | +/+ | +/+ | +/+ | +/− | +/+ | +/+ | −/− | +/+ | −/− | +/+ |
| 0.045 | +/− | +/+ | +/− | +/+ | +/− | |||||
| 0.135 | +/+ | +/− | +/+ | +/− | +/− | |||||
SD indicates results from the single-dose study, and MD indicates results from the multiple-dose study. A + indicates that these signs and symptoms occurred at some point for that rabbit during the study. A +/+ indicates that they occurred in both rabbits, +/− indicates the only one rabbit of the two showed that sign or symptom sign, and −/− indicates that neither rabbit showed that sign or symptom. ∗, animals died or were euthanatized 24 h after 2nd or 3rd dose.
Neurological manifestations of convulsion, paralysis, and loss of mobility showed more dose-dependent effects (Table 1). Only one animal given 0.045-mg/kg ABCD and one given 0.135-mg/kg ABCD showed any of these signs. In contrast, one or both animals given these doses of AMB showed one or more of these clinical signs; one rabbit given 0.135-mg/kg AMB showed convulsion and paralysis. On a comparative basis, ABCD at the 0.135-mg/kg dose seemed about equivalent to the 0.045-mg/kg dose of AMB in causing dose-dependent neurological signs.
Histologically, a range of tissue damage was observed. Normal brains were observed in the rabbits given ABCD at 0.0015, 0.015, and 0.045 mg/kg. In comparison, one rabbit given AMB at 0.0015 mg/kg had some meningeal hemorrhage; at 0.015-mg/kg AMB one had normal tissues and the other had multiple acute and subacute areas of necrosis, with some hemorrhage in the brain stem and cerebellum. Both given 0.045-mg/kg AMB had mild meningitis and areas of focal necrosis in the brain stem or cerebellum. Most severe was the ischemia noted in all of the animals at the highest dose of either drug. Areas of acute ischemia on the cerebellar surface occurred in animals given ABCD at 0.135 mg/kg. Both had foci of acute ischemia and necrosis in different areas (i.e., cerebellum, pons, midbrain, and cerebral hemispheres). Taken together with the clinical signs, these results indicate that after a single dose, ABCD was approximately threefold less toxic than AMB.
Multiple-dose study.
The results of the multiple-dose studies again showed that the concentrations of amphotericin B in the CSF were generally higher after treatment with AMB than with ABCD (Fig. 1). Both gave a prolonged, steady level after the cessation of dosing, indicating that amphotericin B might be being released from a tissue reservoir. Interestingly, accumulation of amphotericin B during and after the dosing was not apparent with either formulation.
Similar to the single-dose study, the clinical signs were most severe in animals given the highest total dosage of amphotericin B (Table 1). ABCD was well tolerated up to the 0.045-mg/kg dose, and AMB was well tolerated only at 0.0015 mg/kg. ABCD was found to be lethal after two or three doses at 0.135 mg/kg. The severity of the clinical signs increased after the second dose, with paralysis, reduced awareness, and hypothermia most prevalent. In contrast, only one animal given a lower dose of ABCD showed some loss of mobility, and none showed abnormalities of body temperature. Those animals given the 0.0015-mg/kg dose of AMB showed no prominent clinical signs, whereas those given 0.015 mg/kg did (Table 1). Changes in body weight were not associated with drug or dosage.
The histological assessments indicated that the rabbits with the most severe clinical signs had areas of infarct. Other than some vessel congestion, slight subarachnoid hemorrhage, and a recent focal midbrain hemorrhage in one animal, no pathological abnormalities were observed in animals given 0.0015-mg/kg ABCD or AMB or 0.015-mg/kg ABCD. In contrast, both rabbits given 0.015-mg/kg AMB had large and acute infarcts either in the hippocampus, midbrain, cerebellum, and/or temporal lobe. Animals given 0.135-mg/kg ABCD had extensive subarachnoid hemorrhage and acute hemorrhagic infarcts in the cerebellum and other sites. The pathogenesis of the infarcts is unclear, and no embolic material was observed in the vessels. They could possibly have been due to vasospasm resulting from amphotericin B in the subarachnoid space (2). Trauma did not appear to be the cause, since the incidence and extent of the infarcts generally correlated with the drug dose.
Taken together, the comparative toxicity of ABCD and AMB would appear to have changed from that determined in the single-dose study, where ABCD was about threefold less toxic. In the multiple-dose study ABCD would appear to be between 3- and 30-fold less toxic than AMB. Thus, when given on a daily schedule, ABCD was much better tolerated than was AMB.
We sought to use dosages in rabbits relevant to those given intrathecally to humans. For humans the intrathecal dose can range from 0.01 to 1 mg, which would be 0.0001 to 0.014 mg/kg for a 70-kg person. A desired or typical dosage is 0.5 mg, which equates to 0.007 mg per kg of body weight. Thus, our dose of 0.015 mg/kg is close to a dose of 1 mg for a 70-kg human, and our 0.135-mg/kg dose is about 19-fold higher than the typical 0.5-mg dose given intrathecally to a human.
The aims of this study were twofold in comparing intrathecal administration of ABCD with AMB. The studies overall showed that intrathecally administered ABCD was less toxic than conventionally formulated deoxycholate amphotericin B, which is in accord with the comparative toxicity found in rodents after systemic administration and clinically in humans after intravenous administration (1, 11, 14, 17). Similarly, our results show that the pharmacokinetics in CSF indicated that at equivalent doses of amphotericin B, ABCD levels were generally lower.
Intravenous administration of AMB often results in adverse events, which often can be ameliorated by premedication (5). Similarly, adverse events occur after intrathecal administration and include headache, delerium, arachnoiditits, paresthesias, meningitis, etc. (2, 5). Information on induced histological changes of the central nervous system is limited, but one study done in rats indicated a gliosis and neuronal dropout occurring after a single dose (15). Our studies indicate that animals given a single high or multiple low doses of AMB had resultant histopathological changes that reflected severity of the clinical signs; e.g., animals showing mobility problems or paresis were most likely to have areas of infarct in the brain. Animals given ABCD were less likely to have severe histopathological changes. However, the two rabbits given ABCD that succumbed to lethal toxicity in the multiple-dose study had histological findings consistent with amphotericin B toxicity and similar to animals given AMB.
These studies indicate ABCD is less toxic than conventional amphotericin B when given intrathecally. However, the pharmacokinetics assays show that when given as ABCD, amphotericin B is present in lower concentrations in CSF after dosing. This might require higher doses to be administered to achieve levels above the MIC for the infecting organism. Alternatively, the lower CSF levels may reflect higher levels in meninges and brain. Regardless, these data are encouraging for the potential use of ABCD for intrathecal treatment of severe fungal meningitis.
Acknowledgments
We thank R. Ramirez, Y. Yao, S. Royaltey, and M. Martinez for their assistance during these studies.
These studies were funded in part by Sequus Pharmaceuticals, Inc.
REFERENCES
- 1.Allende M C, Lee J W, Francis P, Garrett K, Dollenberg H, Berenguer J, Lyman C A, Pizzo P A, Walsh T J. Dose-dependent antifungal activity and nephrotoxicity of amphotericin B colloidal dispersion in experimental pulmonary aspergillosis. Antimicrob Agents Chemother. 1994;38:518–522. doi: 10.1128/aac.38.3.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carnevale N T, Galgiani J N, Stevens D A, Herrick M K, Langston J W. Amphotericin B-induced myelopathy. Arch Intern Med. 1980;140:1189–1192. [PubMed] [Google Scholar]
- 3.Dewsnup D H, Galgiani J N, Graybill J R, Diaz M, Rendon A, Cloud G A, Stevens D A. Is it ever safe to stop azole therapy for Coccidioides immitis meningitis? Ann Intern Med. 1996;124:305–310. doi: 10.7326/0003-4819-124-3-199602010-00004. [DOI] [PubMed] [Google Scholar]
- 4.Fielding R M, Smith P C, Wang L H, Porter J, Guo L S. Comparative pharmacokinetics of amphotericin B after administration of a novel colloidal delivery system, ABCD, and a conventional formulation to rats. Antimicrob Agents Chemother. 1991;35:1208–1213. doi: 10.1128/aac.35.6.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gallis H A, Drew R H, Pickard W W. Amphotericin B: 30 years of clinical experience. Rev Infect Dis. 1990;12:308–329. doi: 10.1093/clinids/12.2.308. [DOI] [PubMed] [Google Scholar]
- 6.Groll A H, Piscitelli S C, Walsh T J. Clinical pharmacology of systemic antifungal agents: a comprehensive review of agents in clinical use, current investigational compounds, and putative targets for antifungal drug development. Adv Pharmacol. 1998;44:343–495. doi: 10.1016/s1054-3589(08)60129-5. [DOI] [PubMed] [Google Scholar]
- 7.Guo L S S, Fielding R M, Lasic D D, Hamilton R L, Mufson D. Novel antifungal drug delivery: stable amphotericin B-cholesteryl sulfate discs. Int J Pharm. 1991;75:45–54. [Google Scholar]
- 8.Hanson L H, Perlman A M, Clemons K V, Stevens D A. Synergy between cilofungin and amphotericin B in a murine model of candidiasis. Antimicrob Agents Chemother. 1991;35:1334–1337. doi: 10.1128/aac.35.7.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herbrecht R. Safety of amphotericin B colloidal dispersion. Eur J Clin Microbiol Infect Dis. 1997;16:74–80. doi: 10.1007/BF01575124. [DOI] [PubMed] [Google Scholar]
- 10.Hiemenz J W, Walsh T J. Lipid formulations of amphotericin B: recent progress and future directions. Clin Infect Dis. 1996;22(Suppl. 2):S133–144. doi: 10.1093/clinids/22.supplement_2.s133. [DOI] [PubMed] [Google Scholar]
- 11.Hostetler J S, Clemons K V, Hanson L H, Stevens D A. Efficacy and safety of amphotericin B colloidal dispersion compared with those of amphotericin B deoxycholate suspension for treatment of disseminated murine cryptococcosis. Antimicrob Agents Chemother. 1992;36:2656–2660. doi: 10.1128/aac.36.12.2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Janoff A S, Boni L T, Popescu M C, Minchey S R, Cullis P R, Madden T D, Taraschi T, Gruner S M, Shyamsunder E, Tate M W, Mendelsohn R, Bonner D. Unusal lipid structures selectively reduce the toxicity of amphotericin B. Proc Natl Acad Sci USA. 1988;85:6122–6126. doi: 10.1073/pnas.85.16.6122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Odds F C, Dupont B, Rinaldi M G, Stevens D A, Warnock D W, Woestenborghs R. Bioassays for itraconazole blood levels: an interlaboratory collaborative study. J Antimicrob Chemother. 1999;43:723–727. doi: 10.1093/jac/43.5.723. [DOI] [PubMed] [Google Scholar]
- 14.Oppenheim B A, Herbrecht R, Kusne S. The safety and efficacy of amphotericin B colloidal dispersion in the treatment of invasive mycoses. Clin Infect Dis. 1995;21:1145–1153. doi: 10.1093/clinids/21.5.1145. [DOI] [PubMed] [Google Scholar]
- 15.Reuhl K R, Vapiwala M, Ryzlak M T, Schaffner C P. Comparative neurotoxicities of amphotericin B and its mono-methyl ester derivative in rats. Antimicrob Agents Chemother. 1993;37:419–428. doi: 10.1128/aac.37.3.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rex J H, Hanson L H, Amantea M A, Stevens D A, Bennett J E. Standardization of a fluconazole bioassay and correlation of results with those obtained by high-pressure liquid chromatography. Antimicrob Agents Chemother. 1991;35:846–850. doi: 10.1128/aac.35.5.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanders S W, Buchi K N, Goddard M S, Lang J K, Tolman K G. Single-dose pharmacokinetics and tolerance of a cholesteryl sulfate complex of amphotericin B administered to healthy volunteers. Antimicrob Agents Chemother. 1991;35:1029–1034. doi: 10.1128/aac.35.6.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tucker R M, Williams P L, Arathoon E G, Stevens D A. Treatment of mycoses with itraconazole. Ann N Y Acad Sci. 1988;544:451–470. doi: 10.1111/j.1749-6632.1988.tb40443.x. [DOI] [PubMed] [Google Scholar]
- 19.Williams P L, Sobel R A, Sorensen K N, Clemons K V, Shuer L M, Royaltey S S, Yao Y, Pappagianis D, Lutz J E, Reed C, River M E, Lee B C, Bhatti S O, Stevens D A. A model of coccidioidal meningoencephalitis and cerebrospinal vasculitis in the rabbit. J Infect Dis. 1998;178:1217–1221. doi: 10.1086/515689. [DOI] [PubMed] [Google Scholar]