Abstract
A cefotaxime-resistant, ceftazidime-susceptible Escherichia coli isolate was obtained from a patient with sepsis in 1997, from which a β-lactamase with a pI of 8.1 was cloned. Cephaloridine and cefotaxime relative hydrolysis rates were 167 and 81, respectively (penicillin G rate = 100), whereas ceftazidime hydrolysis was not detected. The nucleotide sequence revealed a bla gene related to that coding for CTX-M-3. Despite 21 nucleotide substitutions, only 2 determined amino acid changes (Ala27Val and Arg38Gln). The amino acid sequence identity between this enzyme, designated CTX-M-10, and the chromosomal β-lactamase of Kluyvera ascorbata was 81%.
Plasmid-mediated expanded-spectrum β-lactamases (ESBLs) have been responsible for several outbreaks of enterobacteria resistant to expanded-spectrum cephalosporins since they were first reported in 1987 (7, 21). To date, the ESBLs found most frequently are those derived from the common TEM-1 and SHV-1 broad-spectrum β-lactamases (8, 24). These enzymes are characterized as conferring resistance, to a greater or lesser extent, to all expanded-spectrum cephalosporins but are most frequently associated with a higher level of resistance to ceftazidime than to cefotaxime. In 1992, a novel type of ESBL was reported, which in contrast to those found previously, determined high-level resistance to cefotaxime but did not significantly affect ceftazidime (3). ESBLs belonging to this new type, named cefotaximases (CTX-M), have caused outbreaks of cefotaxime-resistant enterobacteria mainly in South America, Eastern Europe, and Japan (6, 11, 14, 15; M. F. Galas, M. J. Rapoport, F. G. Pasteran, R. G. Melano, A. E. Petroni, P. G. Ceriana, W. Group, and M. A. Rosi, Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1474, 1999).
In 1997 a cefotaxime-resistant, ceftazidime-susceptible Escherichia coli isolate (97/38582) was obtained from blood and urine cultures from a patient with sepsis. DNA sequencing, isoelectric focusing (IEF), and determination of the resistance phenotype and antibiotic hydrolysis profile were performed. The strain harbored a novel CTX-M β-lactamase closely related to CTX-M-3, which was previously found in Citrobacter freundii (11).
Identification and initial antibiotic susceptibility testing were performed with the semiautomatic PASCO system (Difco, Detroit, Mich.). MICs of amoxicillin, amoxicillin-clavulanate, ticarcillin, piperacillin, piperacillin-tazobactam, cefazolin, cefuroxime, cefpodoxime, cefoxitin, cefotaxime, ceftazidime, cefepime, aztreonam, and imipenem were determined by agar dilution according to the NCCLS guidelines (16). Additionally, MICs of cefuroxime, cefpodoxime, cefotaxime, and ceftazidime plus a 4-μg/ml fixed concentration of clavulanate were determined. The inoculum effect on antibiotic MICs was determined using a 103-CFU/ml inoculum (low inoculum) and a 107-CFU/ml inoculum (high inoculum) in addition to the 105-CFU/ml standard inoculum. The manufacturers provided all antibiotics as powders.
Conjugation experiments were performed by filter mating using E. coli JMG21 (MC4100 with kanamycin and nalidixic acid resistance markers) (12) as the recipient. Transconjugants were selected on Mueller-Hinton agar plates (MHAP) with 100 and 50 μg of ampicillin and kanamycin per ml, respectively. Plasmid DNA from E. coli clinical strain 97/38582 (High Pure plasmid isolation kit; Boehringer Manheim, Indianapolis, Ind.) was transformed into E. coli MC4100 competent cells. Selection of transformants was performed both on MHAP with 100 μg of ampicillin per ml and on MHAP with 2 μg of cefotaxime per ml. Plasmid DNAs obtained from strain 97/38582 and plasmid pBGS18− (kanamycin resistance marker) (22) were digested with EcoRI and BamHI. After enzyme inactivation, the digestion products were mixed, ligated overnight with T4 DNA ligase, and transformed into MC4100 competent cells. E. coli MC4100 transformants harboring the pBGS18− plasmid containing the cloned β-lactamase gene were selected on MHAP with 2 and 50 μg of cefotaxime and kanamycin per ml, respectively. This plasmid was used as the DNA template, which was sequenced on both strands by the method of Sanger et al. (19). Sequencing was performed with a Big DyeDeoxyTerminator cycle sequencing kit (Perkin-Elmer, Foster City, Calif.) and specific primers for the coding sequence. The DNA sequence was analyzed in an automatic DNA sequencer (377 Abi-Prism: Perkin-Elmer).
IEF was performed by applying the supernatant of a crude sonic extract to Phast gels (pH gradient of 3 to 9) in a PhastSystem apparatus (Pharmacia AB, Uppsala, Sweden). β-Lactamases with known pIs were focused in parallel as controls. Gels were stained with 500 μg of nitrocefin (Oxoid, Basingstoke, Hampshire, England) per ml to identify the β-lactamase bands. An exponentially growing culture of the E. coli MC4100 strain harboring the cloned β-lactamase was sonicated, partially purified through a Sephadex G 100 column (Pharmacia AB), and concentrated with a Minicon concentrator (Amicon, Danvers, Mass.). Hydrolysis rates were determined spectrophotometrically (UVIKON 940; Kontron, Schlieren, Switzerland) in duplicate experiments at 25°C in 0.05 M phosphate buffer (pH 7.4) during 10 min using a 100 μM concentration of each antibiotic. Kinetic parameters were determined in duplicate experiments based on the initial steady-state rates using at least five different substrate concentrations (Lineweaver-Burk transformation). Fifty percent inhibitory concentrations (IC50s) were determined for clavulanic acid, sulbactam, and tazobactam. The enzyme and inhibitor were preincubated for 10 min at 25°C before addition of nitrocefin (100 μM, final concentration). Hydrolysis was measured spectrophotometrically at 482 nm, and IC50s were determined graphically.
IEF of the E. coli clinical strain 97/38582 crude sonic extract revealed two β-lactamases with pIs of 5.4 and 8.1. Both β-lactamases were cotransferred by conjugation into the JMG21 E. coli strain. A few transformants obtained on MHAP with ampicillin carried only the pI 5.4 β-lactamase (suggestive of TEM-1), and those selected on MHAP with cefotaxime carried only the pI 8.1 β-lactamase, suggesting that the two β-lactamases were encoded by different plasmids. Enzymatic restriction of the pBGS18− plasmid harboring the pI 8.1 β-lactamase revealed an insert of approximately 3 kb.
MICs of several antibiotics against the E. coli clinical strain 97/38582, the transconjugant, and the MC4100 strain with the pI 8.1 cloned β-lactamase are shown in Table 1. β-Lactam MICs against the transformant carrying only the pI 5.4 β-lactamase resembled those expected against a TEM-1 β-lactamase-producing E. coli strain (data not shown). Interestingly, a high resistance level was found for cefotaxime, while MICs of ceftazidime were only slightly increased. Clavulanate restored the MIC to the basal value in all combinations tested. A high inoculum effect was observed with all cephalosporins except ceftazidime. For example, when the inoculum was increased from 103 to 107 CFU/ml, the cefepime MICs increased from 2 to 512 μg/ml. Hydrolysis rates and kinetic parameters for penicillin G, cephaloridine, cefuroxime, cefotaxime, ceftazidime, and cefepime are shown in Table 2. The hydrolysis rates for cephalosporins were high compared to that for penicillin G, with the exception of ceftazidime, for which hydrolysis could not be detected.
TABLE 1.
MICs of different antibiotics for E. coli 97/38582, E. coli MC4100, the E. coli JMG21 (modified MC4100) transconjugant, and E. coli MC4100 with the cloned β-lactamase
| Antimicrobial agent | MIC (μg/ml) for strain:
|
|||||
|---|---|---|---|---|---|---|
| 97/38582 (clinical
strain)a
|
MC4100b | JMG21 (transconjugant)b | MC4100 (pBctx-M-10)b | |||
| 103 | 105 | 107 | ||||
| Amoxicillin | 256 | 256 | >512 | 4 | 256 | 256 |
| Amoxicillin-clavulanatec | 8/4 | 8/4 | 8/4 | 2/1 | 4/2 | 8/4 |
| Ticarcillin | >512 | >512 | >512 | 4 | >512 | 512 |
| Piperacillin | 64 | 128 | >512 | 1 | 32 | 32 |
| Piperacillin-tazobactamd | 1/4 | 2/4 | 2/4 | 1/4 | 2/4 | 2/4 |
| Cefazolin | 128 | 512 | >512 | 4 | 128 | 512 |
| Cefuroxime | 512 | 512 | >512 | 2 | 512 | >512 |
| Cefuroxime plus clavulanatee | 2 | 2 | 4 | 2 | 4 | 4 |
| Cefpodoxime | 32 | 32 | >512 | ≤1 | 16 | 256 |
| Cefpodoxime plus clavulanatee | ≤1 | ≤1 | ≤1 | ≤1 | ≤1 | 2 |
| Cefoxitin | 4 | 4 | 8 | 2 | 4 | 4 |
| Cefotaxime | 8 | 8 | 256 | ≤0.12 | 4 | 128 |
| Cefotaxime plus clavulanatee | 0.2 | 0.25 | 0.5 | ≤0.12 | ≤0.12 | 0.5 |
| Ceftazidime | 0.5 | 0.5 | 1 | 0.25 | 0.25 | 2 |
| Ceftazidime plus clavulanatee | ≤0.12 | ≤0.12 | ≤0.12 | ≤0.12 | ≤0.12 | ≤0.12 |
| Aztreonam | 1 | 2 | 64 | ≤0.12 | 2 | 16 |
| Cefepime | 2 | 4 | 512 | ≤0.12 | 2 | 32 |
| Imipenem | ≤0.1 | ≤0.12 | ≤0.12 | ≤0.12 | ≤0.12 | 0.25 |
The inoculum effect was tested using 103, 105, and 107 CFU per spot.
At 105 CFU per spot.
2:1 concentration ratio.
Tazobactam was at a fixed concentration of 4 μg/ml.
Clavulanate was at a fixed concentration of 4 μg/ml.
TABLE 2.
Kinetic parameters of CTX-M-10
| Antibiotic | Relative hydrolysis ratea (%) | Relative Vmax (%) | Km (μM) | Relative Vmax/Km ratio | IC50 (μM) |
|---|---|---|---|---|---|
| Penicillin G | 100 | 100 | 34 | 100 | |
| Cephaloridine | 167 | 200 | 96 | 71 | |
| Cefuroxime | 95 | 100 | 38 | 88 | |
| Cefotaxime | 81 | 50 | 28 | 61 | |
| Ceftazidime | NHb | NDc | ND | ND | |
| Cefepime | 5 | ND | ND | ND | |
| Clavulanic acid | 0.006 | ||||
| Tazobactam | 0.0009 | ||||
| Sulbactam | 0.32 |
Hydrolysis rates (micromoles per minute per microliter) were determined using a 100 μM concentration of each substrate; relative values were determined by considering the penicillin G rate to be 100.
NH, no hydrolysis detected.
ND, not done.
DNA sequencing of the 3-kb fragment carrying the β-lactamase gene revealed an 873-bp open reading frame. The deduced amino acid sequence revealed a 291-amino-acid protein with the typical motifs of serine β-lactamases. Nucleotide sequence analysis showed that this bla gene was related to those coding for the CTX-M-3 (11) and CTX-M-1 (4) β-lactamases, with 21 and 23 nucleotide substitutions, respectively. The deduced amino acid sequence of this new β-lactamase, designated CTX-M-10, revealed that despite the high number of nucleotide substitutions, only two and six of them determined amino acid changes compared with the CTX-M-3 (Ala27Val and Arg38Gln) and the CTX-M-1 (Ala27Val, Arg38Gln, Asp114Asn, Ser140Ala, Val177Ala, and Asn288Asp) enzymes, respectively. Amino acid numbers were assigned as described by Ambler et al. (1). Sequence identity with other CTX-M β-lactamases is far lower than that with CTX-M-1 or CTX-M-3; CTX-M-2, CTX-M-5, and CTX-M-8 were the next three most related CTX-M β-lactamases, and each had only 81% amino acid identity to CTX-M-10 (4, 5, 6). This result suggests the existence of different subgroups with different phylogenetic origins within the CTX-M β-lactamase group.
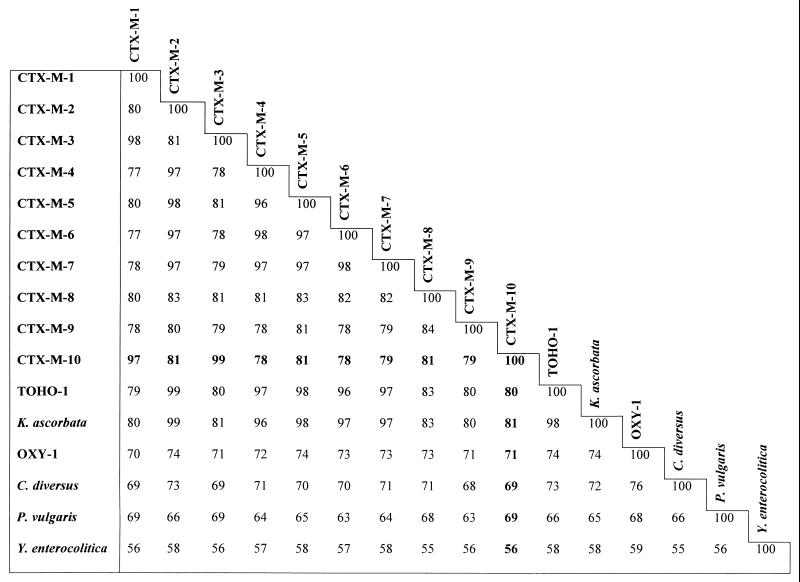
To date, CTX-M enzymes have been thought to be derived from the chromosomal β-lactamase of Klebsiella oxytoca, although a direct relationship is not likely, given a sequence identity lower than 80%. The amino acid sequence identities between this new β-lactamase and chromosomal enzymes from Kluyvera ascorbata (C. Humeniuk et al., unpublished data [GenBank accession number CAB59824]), K. oxytoca (2), and Citrobacter diversus (17) were 81, 71, and 69%, respectively (Fig. 1). Therefore, if this β-lactamase is derived from one of the known sequenced chromosomal enzymes, K. ascorbata is the most likely candidate. In fact, the CTX-M-2 β-lactamase differs from that of K. ascorbata in only two amino acids, making the direct relationship clearly established.
FIG. 1.
Percent identity between CTX-Ms and related chromosomal class A β-lactamases CTX-M-2 (4), CTX-M-3 (11), CTX-M-4 (9), CTX-M-5 (6), CTX-M-6 (10), CTX-M-7 (previously designated CTX-M-5) (10), CTX-M-8 (5), and CTX-M-9 (18), and chromosomal β-lactamases of Kluyvera ascorbata (C. Humeniuk et al., unpublished data [GenBank accession number CAB59824]), Klebsiella oxytoca (OXY-1) (2), Proteus vulgaris (23), and Yersinia enterocolitica (20).
The β-lactam resistance phenotypes conferred by CTX-M-10 and the closely related CTX-M-3 β-lactamase (11) in their respective conjugation recipient strains are very similar. As found for CTX-M-3, for CTX-M-10, MICs of cefotaxime are at least 8 times greater than those of ceftazidime, which in both cases remain in the susceptible category. Both amino acid substitutions (Ala27Val and Arg38Gln) are located in the mature protein that starts at residue 25 (4). Residue 27 has been found to be nonvisible in the recently crystallized Toho-1 β-lactamase, but residue 38 was found to be solvent oriented (13). Both amino acids are divergent in CTX-M β-lactamases. Alanine 27 in CTX-M-1 and CTX-M-3 is replaced by an aspargine in CTX-M-2 and closely related CTX-M β-lactamases such as TOHO-1, CTX-M-4, CTX-M-5, and CTX-M-6, whereas it is replaced by a serine in CTX-M-9 and by a valine in CTX-M-10 (4, 6, 9, 10, 14, 18). On the other hand, arginine 38 of CTX-M-1 and CTX-M-3 is replaced by a lysine in CTX-M-2 and closely related CTX-Ms and by a glutamine in CTX-M-10 (4, 11).
Several E. coli, Klebsiella pneumoniae, and Enterobacter cloacae isolates from different patients at our hospital, as well as five isolates (four E. coli and one Salmonella) with similar susceptibility profiles from four other Spanish hospitals, were found to harbor a β-lactamase with the same 8.1 pI value. Moreover, in a 10-year survey approximately 50% of E. coli isolates harboring ESBLs at our institution produced a CTX-M-like β-lactamase with the same 8.1 pI value (data not shown), suggesting that CTX-M-10 may be widely distributed. Different outbreaks involving CTX-M β-lactamase-producing Enterobacteriaceae have been reported. CTX-M β-lactamases are now recognized as an important cause of cefotaxime resistance in Enterobacteriaceae. In Poland, several E. coli and C. freundii isolates from the same institution were found to harbor the CTX-M-3 β-lactamase (11). In Argentina, the CTX-M-2 β-lactamase is widely distributed and is currently the ESBL most frequently found in K. pneumoniae (69% of all ESBLs found) (Galas et al., 39th ICAAC) as well as in Enterobacter, Citrobacter, Serratia, Morganella, and Providencia (E. G. Pasteran, R. G. Melano, M. F. Galas, M. M. Rodriguez, W. Group, and M. A. Rosi, Abstr., 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr 1475, 1999).
It is presently not clear whether isolates harboring CTX-M β-lactamases should be considered resistant to all cephalosporins as recommended for ESBLs (16). In vitro susceptibility testing shows that MICs of ceftazidime are not much increased in isolates harboring these β-lactamases, and a significant inoculum effect is not found (Table 1). Moreover, the enzymatic hydrolysis profile demonstrates that ceftazidime is highly stable to hydrolysis by CTX-M β-lactamases. Finally, the chromosomal β-lactamases from which CTX-M is thought to be derived (from K. ascorbata, K. oxytoca, and C. diversus), even when hyperproduced, hardly affect ceftazidime and are routinely reported with the NCCLS criteria as being susceptible to this antibiotic. Determination of whether to report isolates harboring CTX-M β-lactamases as susceptible or resistant to ceftazidime can only be done with clinical experience.
In summary, we describe the characterization of a novel cefotaxime-hydrolyzing β-lactamase, CTX-M-10, that is closely related to CTX-M-3 and is widely distributed in our institution.
Nucleotide sequence accession number.
The GenBank accession number for the CTX-M-10 β-lactamase is AF255298.
Acknowledgments
We thank L. de Rafael for English correction and critical reading and J. Blázquez for suggestions.
REFERENCES
- 1.Ambler R P, Coulson A F W, Frere J M, Ghuysen J M, Joris B, Forsman M, Levesque R C, Tiraby G, Waley S G. A standard numbering scheme for the class A β-lactamases. Biochem J. 1991;276:269–270. doi: 10.1042/bj2760269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arakawa Y, Ohta M, Kido N, Mori M, Ito H, Komatsu T, Fujii Y, Kato N. Chromosomal β-lactamase of Klebsiella oxytoca, a new class A enzyme that hydrolyzes broad-spectrum β-lactam antibiotics. Antimicrob Agents Chemother. 1989;33:63–70. doi: 10.1128/aac.33.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barthelemy M, Peduzzi J, Bernard H, Tancrede C, Labia R. Close amino acid sequence relationship between the new plasmid-mediated extended-spectrum β-lactamase MEN-1 and chromosomally encoded enzymes of Klebsiella oxytoca. Biochim Biophys Acta. 1992;1122:15–22. doi: 10.1016/0167-4838(92)90121-s. [DOI] [PubMed] [Google Scholar]
- 4.Bauernfeind A, Stemplinger I, Jungwirth R, Ernst S, Casellas J M. Sequences of β-lactamase genes encoding CTX-M-1 (MEN-1) and CTX-M-2 and relationship of their amino acid sequences with those of other β-lactamases. Antimicrob Agents Chemother. 1996;40:509–513. doi: 10.1128/aac.40.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonnet R, Sampaio J L M, Labia R, Champs C D, Sirot D, Chanal C, Sirot J. A novel CTX-M β-lactamase (CTX-M-8) in cefotaxime-resistant Enterobacteriaceaeisolated in Brazil. Antimicrob Agents Chemother. 2000;44:1936–1942. doi: 10.1128/aac.44.7.1936-1942.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bradford P A, Yang Y, Sahm D, Grope I, Gardovska D, Storch G. CTX-M-5, a novel cefotaxime-hydrolyzing β-lactamase from an outbreak of Salmonella typhimuriumin Latvia. Antimicrob Agents Chemother. 1998;42:1980–1984. doi: 10.1128/aac.42.8.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brun-Buisson C, Legrand P, Philippon A, Montravers F, Ansquer M, Duval J. Transferable enzymatic resistance to third-generation cephalosporins during nosocomial outbreaks of multiresistant Klebsiella pneumoniae. Lancet. 1987;ii:302–306. doi: 10.1016/s0140-6736(87)90891-9. [DOI] [PubMed] [Google Scholar]
- 8.Chanal C, Sirot D, Romaszko J P, Bret L, Sirot J. Survey of prevalence of extended spectrum β-lactamases among Enterobacteriaceae. J Antimicrob Chemother. 1996;38:127–132. doi: 10.1093/jac/38.1.127. [DOI] [PubMed] [Google Scholar]
- 9.Gazouli M, Tzelepi E, Sidorenko S V, Tzouvelekis L S. Sequence of the gene encoding a plasmid-mediated cefotaxime-hydrolizing class A beta-lactamase (CTX-M-4): involvement of serine 237 in cephalosporin hydrolysis. Antimicrob Agents Chemother. 1998;42:1259–1262. doi: 10.1128/aac.42.5.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gazouli M, Tzelepi E, Markogiannakis A, Legakis N J, Tzouvelekis L S. Two novel plasmid-mediated cefotaxime-hydrolyzing β-lactamases (CTX-M-5 and CTX-M-6) from Salmonella typhimurium. FEMS Microbiol Lett. 1998;165:289–293. doi: 10.1111/j.1574-6968.1998.tb13159.x. [DOI] [PubMed] [Google Scholar]
- 11.Gniadkowski M, Schneider I, Palucha A, Jungwirth R, Mikiewicz B, Bauernfeind A. Cefotaxime-resistant Enterobacteriaceaeisolates from a hospital in Warsaw, Poland: identification of a new CTX-M-3 cefotaxime-hydrolyzing β-lactamase that is closely related to the CTX-M-1/MEN-1 enzyme. Antimicrob Agents Chemother. 1998;42:827–832. doi: 10.1128/aac.42.4.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gómez-Gómez J M, Blázquez J, Espinosa de los Monteros L E, Baquero M R, Baquero F, Martinez J L. In vitro plasmid-encoded resistance to quinolones. FEMS Microbiol Lett. 1997;154:271–276. doi: 10.1111/j.1574-6968.1997.tb12655.x. [DOI] [PubMed] [Google Scholar]
- 13.Ibuka A, Taguchi A, Ishiguro M, Fushinobu S, Ishii Y, Kamitori S, Okuyama K, Yamaguchi K, Konno M, Matsuzawa H. Crystal structure of the E166A mutant of extended-spectrum β-lactamase Toho-1 at 1.8 A resolution. J Mol Biol. 1999;285:2079–2087. doi: 10.1006/jmbi.1998.2432. [DOI] [PubMed] [Google Scholar]
- 14.Ishii I, Ohno A, Taguchi H, Imajo S, Ishiguro M, Matsuzawa H. Cloning and sequence of the gene encoding a cefotaxime-hydrolyzing class A β-lactamase isolated from E. coli. Antimicrob Agents Chemoher. 1995;39:2269–2275. doi: 10.1128/aac.39.10.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma L, Ishii Y, Ishiguro M, Matsuzawa M, Yamaguchi K. Cloning and sequencing of the gene encoding Toho-2, a class A β-lactamase preferentially inhibited by tazobactam. Antimicrob Agents Chemother. 1998;42:1181–1186. doi: 10.1128/aac.42.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Aproved standard M7–A5. Wayne, Pa: National Committee for Clinical Laboratory Standards; 2000. [Google Scholar]
- 17.Perilli M, Franceschini N, Segatore B, Amicosante G, Oratore A, Duez C, Joris B, Frere J M. Cloning and nucleotide sequencing of the gene encoding the beta-lactamase from Citrobacter diversus. FEMS Microbiol Lett. 1991;67:79–84. doi: 10.1016/0378-1097(91)90448-j. [DOI] [PubMed] [Google Scholar]
- 18.Sabate M, Tarrago R, Navarro F, Miro E, Verges C, Barre J, Prats G. Cloning and sequence of the gene encoding a novel cefotaxime-hydrolyzing β-lactamase (CTX-M-9) from Escherichia coliin Spain. Antimicrob Agents Chemother. 2000;44:1970–1973. doi: 10.1128/aac.44.7.1970-1973.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanger T, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seoane A, García Lobo J M. Nucleotide sequence of a new class A beta-lactamase gene from the chromosome of Yersinia enterocolitica: implications for the evolution of class A β-lactamases. Mol Gen Genet. 1991;228:215–220. doi: 10.1007/BF00282468. [DOI] [PubMed] [Google Scholar]
- 21.Sirot D, Sirot J, Labia R, Monard A, Courvalin P, Darfeuille-Michaud A, Perroux R, Cluzel R. Transferable resistance to third-generation cephalosporins in clinical isolates of Klebsiella pneumoniae: identification of CTX-1, a novel β-lactamase. J Antimicrob Chemother. 1987;20:323–334. doi: 10.1093/jac/20.3.323. [DOI] [PubMed] [Google Scholar]
- 22.Spratt B G, Hedge P J, Heesen S T, Edelman A, Broome-Smith J K. Kanamycin-resistant vectors that are analogues of plasmids pUC8, pUC9, pEMBL8 and pEMBL9. Gene. 1986;41:337–342. doi: 10.1016/0378-1119(86)90117-4. [DOI] [PubMed] [Google Scholar]
- 23.Tamaki M, Nukaga M, Sawai T. Replacement of serine 237 in class A beta-lactamase of Proteus vulgarismodifies its unique substrate specificity. Biochemistry. 1994;33:10200–10206. doi: 10.1021/bi00199a049. [DOI] [PubMed] [Google Scholar]
- 24.Yuan M, Aucken H, Hall L M, Pitt T L, Livermore D M. Epidemiological typing of Klebsiellaewith extended-spectrum β-lactamases from European intensive care units. J Antimicrob Chemother. 1998;41:527–539. doi: 10.1093/jac/41.5.527. [DOI] [PubMed] [Google Scholar]