INTRODUCTION
Dementia with Lewy bodies (DLB) is the second most common neurodegenerative dementia in older people after Alzheimer’s disease (AD), accounting for 10-15% of all dementia cases1. Clinically, DLB manifests as progressive cognitive decline, typically in conjunction with REM sleep behavior disorder, cognitive fluctuations, parkinsonism, and visual hallucinations1. Pathologically, DLB is characterized by progressive accumulation and aggregation of the synaptic protein alpha-synuclein (α-syn) in Lewy bodies and Lewy neurites in the brainstem, limbic, and neocortical regions2. Improved biomarkers for DLB could aid in earlier and more accurate differential diagnosis compared to other overlapping neurodegenerative conditions such as Parkinson’s and AD1. The purpose of this study is to explore the viscoelastic changes in lobar regions of the brain associated with DLB. In this study we considered 13 DLB subjects to evaluate the feasibility of lobar regional stiffness changes as a marker of DLB in a sample with sufficient power to detect effects comparable to those found in AD.
MATERIALS AND METHODS
Study participants:
This study was approved by our Institutional Review Boards and written informed consent was obtained from the volunteers and/or their proxies before performing the experiments. Fifty-seven participants were recruited, consisting of 44 cognitively unimpaired controls (CU) with age 56-87 years, and 13 patients with clinically probable Dementia with Lewy bodies (DLB) of age 56-75 years. A power calculation was performed to determine that the minimum sample size should be 12 in each group. To compute this sample size, we assumed the effect size observed in the temporal lobes of our previous AD study (the smallest of the statistically significant effect in that study, Δμ=0.11 kPa with pooled standard deviation of 0.10 kPa)3, in order to achieve a power of 80% at a significance level of 0.05. Control data were taken from previous studies4. Patients with probable DLB were diagnosed according to 4th Consortium Criteria for DLB1.
MRE Data Acquisition:
All CU and 10 of the DLB participants were scanned on an HDxt (GE, Waukesha, WI) 3T Magnetic Resonance scanner using a modified flow-compensated, spin-echo, echo planar imaging sequence. Data from 3 DLB participants were acquired on a Discovery MR750w system (GE, Waukesha, WI). MRE data were acquired with 60-Hz motion and 3-mm isotropic resolution. A complete methodology for both MRE and anatomical image acquisition was described previously5.
Direct inversion Lobar Analysis:
For direct comparison to our previous studies, MRE data were processed using the direct inversion-based pipeline described previously for measuring regional brain stiffness4. The median stiffness in 8 regions was calculated for each subject, including cerebrum, frontal lobes, occipital lobes, parietal lobes, temporal lobes, deep gray matter/white matter (insula, deep gray nuclei and white matter tracts), cerebellum and the brain stem.
Statistical analysis:
We tested the hypothesis that DLB and CU participants had significantly different stiffness in each region by two-sample t-test while fixing the effects of age, sex, and scanner. P-values less than 0.05 were considered significant.
RESULTS
The stiffness estimates over the 8 lobar regions are summarized in Figure 1 and Table 1. No significant differences were detected between DLB and control groups. Stiffness significantly decreased with age in 7 regions (P<0.0001 for cerebrum, frontal, occipital, parietal and temporal lobes, P=0.003 for deep GM/WM and P=0.002 for cerebellum). A trend towards decrease in stiffness with age was observed for brain stem with P=0.057. The results were unaffected when only age-matched controls were considered in the analysis.
FIGURE 1:
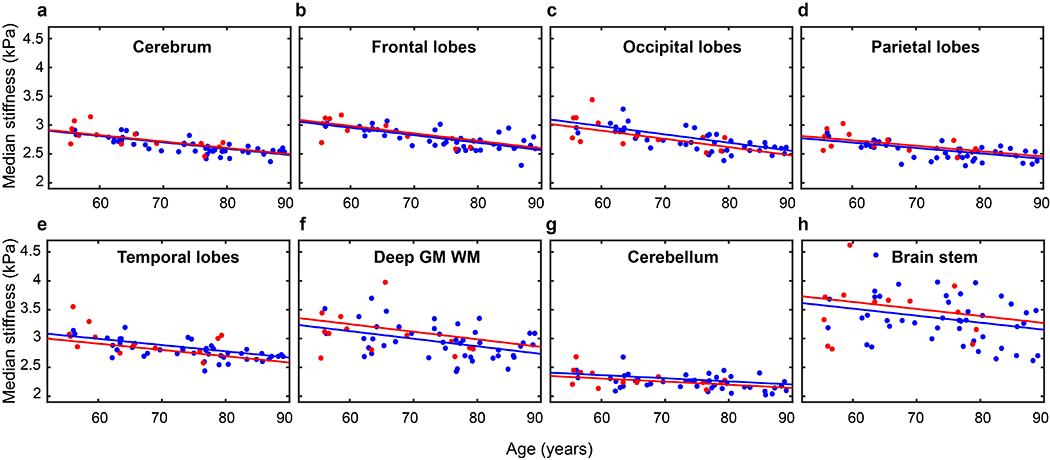
Median stiffness versus age in cognitively unimpaired (CU) (blue) and dementia with Lewy bodies (DLB) subjects (red) in the regions (a) cerebrum (b) frontal lobes (c) occipital lobes (d) parietal lobes (e) temporal lobes (f) deep grey matter (GM) and white matter (WM) (g) cerebellum (h) brainstem. Multiple linear regression lines representing stiffness versus age are shown (CU in blue, DLB in red).
Table 1:
Parameter estimates of multiple linear regression models of DI median stiffness for both controls and DLB participants. Stiffness = Intercept +age slope (age)+sex bias (sex)+group slope (group)+scanner bias (scanner), where group = 0 for controls and 1 for DLB patients, sex = 0 for female and 1 for male.
| Brain region | Group slope (kPa) (P value) | Age slope (kPa/year) (P value) | Sex bias (kPa) (P value) | Overall F-test P value | R2 |
|---|---|---|---|---|---|
| Cerebrum | 0.057 (P=0.20) |
−0.011 (P <0.0001) |
−0.043 (P=0.15) |
<0.0001 | 0.61 |
| Frontal lobes | 0.052 (P=0.34) |
−0.013 (P <0.0001) |
−0.02 (P=0.58) |
<0.0001 | 0.53 |
| Occipital lobes | 0.009 (P=0.89) |
−0.014 (P <0.0001) |
−0.087 (P=0.05) |
<0.0001 | 0.53 |
| Parietal lobes | 0.074 (P=0.14) |
−0.009 (P <0.0001) |
−0.035 (p=0.30) |
<0.0001 | 0.48 |
| Temporal lobes | 0.007 (P=0.89) |
−0.011 (P <0.0001) |
−0.092 (P=0.01) |
<0.0001 | 0.60 |
| Deep GM/WM | −0.087 (P=0.46) |
−0.013 (P=0.003) |
0.033 (P=0.68) |
0.012 | 0.21 |
| Cerebellum | 0.037 (P=0.45) |
−0.005 (P=0.002) |
−0.09 (P=0.005) |
0.0001 | 0.34 |
| Brain stem | 0.134 (P=0.45) |
−0.012 (P=0.057) |
−0.018 (P=0.88) |
0.20 | 0.11 |
DISCUSSION
We investigated the impact of DLB on the mechanical properties of the brain using methods previously applied to the study of AD and FTD3, 6. Across the eight regions of interest, no regions indicated statistically significant changes in stiffness compared to the CU group. The absence of stiffness change in probable DLB may be explained by limited cell death and localization of pathology to presynpatic terminals7, whereas macroscopic tissue mechanical properties are thought to reflect the rigidity and tension of the extracellular matrix-cytoskeleton network8. However, multiple linear regression analysis has shown that the median stiffness for 7 lobar regions decreased with age with P<0.01. These results are consistent with our aging study of the brain4. Brainstem stiffness was included in the analysis for the first time given the widespread distribution of Lewy bodies in the brainstem in DLB patients. Brainstem stiffness estimates did not show statistically significant changes in DLB compared to CU, however a trend towards decrease in stiffness with age was observed.
Reliable and objective diagnostic criteria and biomarkers are needed in DLB due to significant overlap with Parkinson’s and AD. Currently, DAT imaging, 123Iodine-MIBG myocardial scintigraphy, CT, MRI, FDG-PET imaging are used as sensitive and specific biomarkers of DLB1, 9. Preservation of hippocampal and medial temporal lobe volumes observed through structural MRI is one of the most robust biomarkers in differentiating DLB from AD10. Consistent with other modalities, any effect of DLB on brain mechanical properties appears small. Though we cannot rule out that DLB is associated with stiffness alterations below the detection limit of this methodology, even if detected by another approach, it is unlikely that these alterations would be reliable at the level of an individual.
The limitations of this study will be the subject of future investigation. Though the sample size was computed to detect effects comparable to those observed in AD, this sample may be too small to detect effects of DLB. Also, given that half or more of DLB patients have amyloid or tau pathology, further investigation is needed to assess the differential effect of these various pathologies. Future studies that include a greater number of DLB participants, biomarker assessments of AD pathology, higher resolution MRE imaging protocols along with more sensitive inversions may allow differentiation from other types of dementia.
In conclusion, after controlling for effects of age, brain stiffness in DLB patients is not substantially different from control participants using previously established methods. This relatively small effect compared to AD and FTD may reflect a differential impact of α-syn pathology on brain mechanics as compared to tau pathology.
GRANT SUPPORT
This research was supported by grants from the NIH (http://www.nih.gov/), EB027064, EB001981, U01 NS100620 and P50 AG062677.
Footnotes
CONFLICT OF INTEREST
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests. J.H., R.L.E., A.M., M.C.M. and Mayo Clinic have a financial conflict of interest related to this research. R.C.P., has financial conflict of interests in Roche Inc., Merck Inc., Biogen Inc., Consultant and Genentech Inc., DSMB. This research has been reviewed by the Mayo Clinic Conflict of Interest Review Board and is being conducted in compliance with Mayo Clinic Conflict of Interest policies.
REFERENCES
- 1.McKeith IG, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology 2017;89:88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferman TJ, Aoki N, Crook JE, et al. The limbic and neocortical contribution of α-synuclein, tau, and amyloid β to disease duration in dementia with Lewy bodies. Alzheimers Dement 2018;14:330–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murphy MC, Huston Iii J, Jack CR Jr, et al. Decreased brain stiffness in Alzheimer’s disease determined by magnetic resonance elastography. Journal of Magnetic Resonance Imaging 2011;34:494–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arani A, Murphy MC, Glaser KJ, et al. Measuring the effects of aging and sex on regional brain stiffness with MR elastography in healthy older adults. Neuroimage 2015;111:59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murphy MC, Cogswell PM, Trzasko JD, et al. Identification of Normal Pressure Hydrocephalus by Disease-Specific Patterns of Brain Stiffness and Damping Ratio. Investigative Radiology 2020;55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huston J 3rd, Murphy MC, Boeve BF, et al. Magnetic resonance elastography of frontotemporal dementia. Journal of magnetic resonance imaging : JMRI 2016;43:474–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schulz-Schaeffer WJ. The synaptic pathology of alpha-synuclein aggregation in dementia with Lewy bodies, Parkinson’s disease and Parkinson’s disease dementia. Acta neuropathologica 2010;120:131–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reiter N, Roy B, Paulsen F, Budday S. Insights into the Microstructural Origin of Brain Viscoelasticity. Journal of Elasticity 2021. [Google Scholar]
- 9.Kantarci K, Lowe VJ, Boeve BF, et al. Multimodality imaging characteristics of dementia with Lewy bodies. Neurobiology of aging 2012;33:2091–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burton EJ, Barber R, Mukaetova-Ladinska EB, et al. Medial temporal lobe atrophy on MRI differentiates Alzheimer’s disease from dementia with Lewy bodies and vascular cognitive impairment: a prospective study with pathological verification of diagnosis. Brain 2009;132:195–203. [DOI] [PubMed] [Google Scholar]


