Abstract
Herein, the skin whitening effect of the fermentation residue of Saccharomyces cerevisiae was investigated. The fermentation residue showed radical scavenging activity and attenuated tyrosinase activity. Furthermore, the fermentation residue of S. cerevisiae significantly suppressed melanin generation in B16F10 cells. Interestingly, the sample-containing formulation exhibited increased skin whitening activity compared with that by the control formulation in a clinical study. Notably, the endogenous tyrosinase expression was not altered by the fermentation residue of S. cerevisiae; however, the enzymatic activity of tyrosinase was inhibited. Furthermore, the sample did not change TRP1 and TRP2 expression in B16F10 cells. Thus, the fermentation residue of S. cerevisiae was assumed to directly suppress the tyrosinase enzyme. It was confirmed that the fermentation residue of S. cerevisiae was a competitive inhibitor of tyrosinase. Taken together, the fermentation residue of S. cerevisiae could be a novel skin whitening agent originating from the traditional Korean liquor production process.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10068-022-01062-7.
Keywords: Fermentation residue, Skin whitening, Saccharomyces cerevisiae, Tyrosinase inhibition
Introduction
Melanin is a biological pigment that distributed in skin, hair, eyes, feather, and soil (Roulin et al., 2008; Vinther, 2015). In the human skin, melanin is produced from melanocyte, which presents in stratum basale (Joshi et al., 2007). The production of melanin in melanocytes is precisely regulated by the intracellular signaling pathways. After stimulation of melanocortin-1-receptor (MC1R) by α-MSH, which is secreted by keratinocytes surrounding melanocytes, cyclic AMP (cAMP) is increased, and this increased cAMP activates transcription of micorphthalmia-associated transcription factor (MITF) (D'Orazio et al., 2006; Hirobe, 2011). Consequently, the critical enzymes for melanin production, tyrosinase, tyrosinase-related protein 1 (TRP-1), and tyrosinase-related protein 2 (TRP-2), were stimulated. Tyrosinase plays critical role in melanin production by dual pathways. The first action of tyrosinase is the catalyzation mechanism from tyrosine to DOPA by hydroxylation. The second mechanism of tyrosinase is the oxidation of DOPA to making dopaquinone (del Marmol and Beermann, 1996). On the other hand, TRP-1 and -2 have their functions for melanin production, such as dopacrome tautomerase activity of TRP2 (del Marmol and Beermann, 1996; Tsukamoto et al., 1992). Hence, the regulation of melanin-producing mechanisms by synthetic or natural agents could be a good strategy for the development skin whitening agents.
According to 2020 annual report of L'Oréal, one of the biggest global cosmetic industries, the world cosmetic market size has been increased (L'Oréal, 2015). From 2011 to 2019, the average growth of the worldwide cosmetics market was reached at 4.5%. Many researchers have tried to find skin whitening agents. Among them, glucosamine and retinol are well-known skin whitening agents (Niwano et al., 2018; Sato et al., 2008). Those skin whitening agents directly target various steps for melanin production, melanogenesis. In the case of arbutin and kojic acid directly inhibit tyrosinase enzyme, which is a crucial protein for melanin production (Couteau and Coiffard, 2016; Jin et al., 1999). On the other hand, vitamin C accelerates epidermis turnover and inhibits oxidative reactions essential for melanin production (Couteau and Coiffard, 2016; Gupta et al., 2006). Moreover, linoleic acid shows inhibitory activity on melanosome transfer (Briganti et al., 2003). Although arbutin and kojic acid reveal skin whitening activity the side effects of those agents have been reported such as liver cancer (Takizawa et al., 2004). Therefore, it has been required to develop a novel skin whitening agent from safe resources.
During the process of the Korean liquor production, the carbon source such as smashed sweet potatoes is saccharified and fermented by Saccharomyces cerevisiae, Saccharomyces coreanus, Aspergillusflavus, Aspergillus nomius, and Aspergillus parasiticus. The fermentation products are distilled to obtain alcohol (Eom et al., 2021; Zhang et al., 2011). After this process, huge amounts of fermentation residue are discarded, even though it contains lots of beneficial compounds such as phenolics (Mateus et al., 2017; Shen et al., 2018; Yang and Ling, 1989). Hence, many researchers have tried to find the way to apply the fermentation residues. Recently, we reported immune-enhancing activity of polysaccharide extract of by-products of Korean liquor fermented by S. cerevisiae (Eom et al., 2021). According to this report, RAW264.7 cells treated with the by-products increased nitric oxide secretion and several immune-enhancing-related proteins expression. Moreover, proliferation of mouse spleen cells was increased by the treatment of by-products of Korean liquor fermented by S. cerevisiae.
We studied about skin whitening activity of fermentation residue of S. cerevisiae. Indeed, several previous reports have demonstrated the skin whitening activity of fermentation products of specific microorganisms. The ferment lysate of Bifidobacterium longum Reuter showed anti-aging, moisturizing and anti-acne activity (Dreno et al., 2020; Gueniche et al., 2010), and the skin whitening activity and antioxidative activity of the fermentation product of Galactomyces was reported (Miyamoto et al., 2021). Similar to previous literatures, we found skin whitening activity of fermentation residue of S. cerevisiae. The skin whitening activity of fermentation residue of S. cerevisiae was confirmed in human skin trial. Interestingly, it is confirmed that the enzyme activity of tyrosinase was significantly reduced by the fermentation residue of S. cerevisiae, but not tyrosinase expression in melanin-producing cells. Collectively, we suggest the fermentation residue of S. cerevisiae as a novel skin whitening agent by directly inhibiting tyrosinase enzyme activity.
Materials and methods
Reagents
DPPH was purchased from Cayman (MI, USA). Vitamin C, mushroom tyrosinase, L-DOPA, Folin-Ciocalteus phenol reagent (FC reagent), gallic acid, quercetin, α-MSH, and phenazine methosulfate (PMS) were obtained from Sigma-Aldrich (MO, USA). Cell lysis buffer was purchased from Cell Signaling Technology (MA, USA). Reporter lysis buffer and MTS (CellTiter 96® AQueous MTS Reagent Powder) were acquired from Promega (Madison, WI, USA). Monoclonal anti-tyrosinase was purchased from Invitrogen (Carlsbad, CA, USA). Monoclonal anti-TRP-1 antibody was purchased from Santa Cruz Biotechnology (TX, USA). Polyclonal anti-TRP-2 antibody was obtained from Abcam (Cambridge, UK). Pierce BCA Protein Assay Kit was purchased from Thermo Fisher Scientific (Waltham, MA, USA). Dipeptides (Tyr-Pro, Tyr-Glu, Val-Tyr, Phe-Lys) were obtained from Peptron (Daejeon, KR).
Fermentation residue preparation
Saccharomyces cerevisiae GNIA2 (KCCM12665P) which is trifluoro-dl-leucine (TFL) mutant of S. cerevisiae 88-4 (KCCM11456P) isolated from nuruk (Korean-style bran koji) was obtained from the Korea Food Research Institute (Wanju, Korea). The production of fermented liquor using S. cerevisiae 88-4 was performed as previously described (Chin et al., 2021). Sweet potato was used as the raw material, and fermentation was maintained at 25 °C for 15 days. The fermented sweet potato was distilled at 760 mm Hg, 70–100 °C until the final alcohol content amounted to 45%. To obtain the fermentation residue of S. cerevisiae, the supernatant of fermentation residue was separated by centrifugation. The supernatant was then freeze-dried to obtain powder.
DPPH assay
A solution of 0.2 mM DPPH in 80% methanol was prepared. The solution was stirred for 20 min. Then, 80 µL of fermentation residue of S. cerevisiae (250, 500, 1000, and 2000 µg/mL) was mixed with 80 µL of methanolic DPPH solution. After the mixture was shaken vigorously for 30 min in the dark, the absorbance at 517 nm was measured using a microplate reader. DPPH radical scavenging activity (%) was calculated against fermentation residue of S. cerevisiae untreated control after attenuation with DPPH untreated control. Vitamin C was used as a positive control.
Mushroom tyrosinase activity assay
The tyrosinase activity assay was performed using a previously described method, with slight modifications (Song et al., 2020). Briefly, 80 µL of DW, 80 µL of mushroom tyrosinase (0.02 mg/mL), and 160 µL of fermentation residue of S. cerevisiae were added to a 24 well plate. The plates were incubated at 25 °C for 10 min. Then, 40 µL of L-DOPA (1 mM) was added to the wells. After incubation at 25 °C for 30 min, the dopachrome product was measured at 475 nm.
Total phenolic contents (TPC)
The total phenolic content was determined using Folin-Ciocalteu reagent (Singleton, 1985). Briefly, 40 µL of fermentation residue of S. cerevisiae (1 mg/mL), 20 µL of Folin-Ciocalteu reagent (1 N), and 60 µL of 20% (w/v) Na2CO3 were added to a 96-well plate. After 30 min of incubation at room temperature, the absorbance was measured at 700 nm. The concentration of total phenolic compounds in the fermentation residue of S. cerevisiae was expressed as gallic acid equivalent in milligrams per gram (mg GAE/g) by referencing the gallic acid standard curve.
Total flavonoid contents (TFC)
The total flavonoid content was determined using a previously published method, with slight modifications (Jang et al., 2017). The fermentation residue of S. cerevisiae (25 L, 1 mg/mL) was added to a 96-well plate. Then, 125 µL of DW and 8 µL of NaNO2 (1 M) were added. After the mixture was shaken vigorously for 5 min, 15 µL of 10% (w/v) AlCl3 was added, and the mixture was shaken vigorously for 6 min. Then, 50 µL of NaOH (1 M) and 27 µL of DW were added. After the mixture was shaken vigorously for 6 min in the dark, the absorbance at 510 nm was measured. The concentration of total flavonoid compounds in the fermentation residue of S. cerevisiae was expressed as quercetin equivalent in milligrams per gram (mg QE/g) by referencing the quercetin standard curve.
Cell culture
Murine melanoma B16F10 cells were obtained from the Korean Cell Line Bank (Seoul, Korea) and maintained in Dulbecco’s modified Eagle medium (DMEM) (GIBCO, Waltham, MA, USA) supplemented with 10% (v/v) fetal bovine serum (FBS) (GIBCO, Waltham, MA, USA) and 1% penicillin–streptomycin–neomycin (Gibco, Waltham, MA, USA) at 37 °C in a 5% CO2 incubator.
Cell viability assay
The MTS assay was used to measure the cytotoxicity of the fermentation residue on human melanoma B16F10 cells. Cells were seeded in 96-well plates. After the cells were confluence, the cells were treated fermentation residue of S. cerevisiae (62.5–500 µg/mL) for 24 h, and 0.2% (w/v) MTS solution including PMS was treated to the cells for 30 min. Cell viability was estimated by measuring the absorbance at 490 nm wavelength.
Melanin contents
B16F10 cells were seeded at a density of 2.5 × 105 cells per well into six-well plates. After overnight incubation, the cells were pretreated with fermentation residue of S. cerevisiae (62.5–500 µg/mL) for 1 h. Then, 100 nM of α-MSH was added and further incubated for 72 h. After the cells were harvested, the cells were centrifuged at 11,000 rpm for 10 min and the pellets were collected. The pellets were dissolved in 1 N NaOH by boiling for 20 min at 90 °C. Melanin production was estimated by measuring the absorbance at 490 nm.
Cellular tyrosinase assay
B16F10 cells were seeded at a density of 2.5 × 105 cells per well into 6-well plates. After overnight incubation, the cells were pretreated with fermentation residue of S. cerevisiae (62.5–500 µg/mL) for 1 h. Then, 100 nM of α-MSH was added and further incubated for 72 h. After the cells were dissolved in 1 × reporter lysis buffer, centrifuged at 11,000 rpm for 10 min, and the supernatants were collected. Then, 40 µL of supernatant and 100 µL of L-DOPA (10 mM) were reacted to a 96-well plate at 37 °C for 2 h. Cellular tyrosinase activity was estimated by measuring the absorbance at 490 nm.
Western blot analysis
B16F10 cells were lysed with 1 × cell lysis buffer and quantified using the Pierce BCA Protein Assay Kit. Proteins were separated by 10% sodium dodecyl sulfate acrylamide gels based on molecular weight and then transferred to PVDF membranes. The membranes were blocked with bovine serum albumin solution for 1 h and incubated with the indicated primary antibody at 4 °C for 12 h. After reacting with horseradish peroxidase-conjugated secondary antibody for 1 h, the intensity of protein expression was visualized using a chemiluminescence reader (LuminoGraph III Lite, ATTO).
Lineweaver–Burk plot
Tyrosinase inhibition by fermentation residue of S. cerevisiae (500 µg/mL) was estimated by measuring the absorbance at 490 nm with increasing concentrations of L-DOPA. The absorbance data were analyzed using a Lineweaver–Burk plot to investigate the inhibition type of the fermentation residue. The final concentration of tyrosinase was 67 µg/mL, and the final concentration of L-DOPA was increased 0.13 mM to 4.17 mM. The effect of dipeptides (0.05 mM) on tyrosinase enzyme was estimated using 4 µg/mL tyrosinase and 0.8 mM to 3.2 mM L-DOPA condition.
Subjects and treatments
The skin whitening effect of fermentation residue was assessed in a double-blind clinical trial in 10 women over the age of 20 years (approved by the Korea Institute of Dermatological Sciences Institutional Review Board; IRB number KIDSIRB-2021-223). The base formulation was prepared using purified water, polyacrylic acid, 1,2-hexanediol, and sodium hydroxide. The fermentation residue had a final concentration of 0.01% (v/v). The subject applied the fermentation residue formula to one side of their face once a day for six weeks. The other side of the face was treated once a day with only the base formulation as a control. The use of other cosmetics was not allowed within this period.
Evaluation of skin condition
Skin conditions were evaluated according to the standard operating protocol of the Korea Institute of Dermatological Sciences (Seoul, Korea) and was performed at 22 ± 2 °C and 50 ± 5% humidity. The skin state of each subject was investigated 30 min after washing the face using SkinColorCatch (Delfin Technologies, Kuopio, Finland), and a survey was conducted thereafter. The scores were determined by the L* value (brightness) and measured immediately before the test and at 3 and 6 weeks later. Skin brightness was evaluated according to the standard operating protocol of the Korea Institute of Dermatological Sciences (Seoul, Korea) and was performed at 22 ± 2 °C and 50 ± 5% humidity.
Quantitative analysis of fermentation residues
The fermentation residues were analyzed using a liquid chromatography-tandem mass spectrometry (LC–MS/MS) system. A highly sensitive tandem mass spectrometry system, comprising a Triple TOF 5600 + (AB Sciex, USA) and an Ultimate3000 (Thermo Scientific, USA) equipped with a Waters Cortex C18 (2.1 mm × 150 mm, 1.6 µm), was used. The substances in the sample were separated with buffer A (0.1% formic acid v/v in water) and buffer B (0.1% formic acid v/v in acetonitrile) with a gradient as follows: 0 min (99% A, 1% B), 10 min (95% A, 5% B), 30 min (70% A, 30% B), 50 min (0% A, 100% B), and 55 min (99% A, 1% B). Gas and voltage setting were adjusted as required. Information-dependent acquisition (IDA) was performed, with an MS-TOF scan from an m/z of 30–2,000. The substances in the sample were ionized in both positive and negative electrospray ionization (ESI) mode. A calibrated delivery system was set as follows: nitrogen gas for nebulization at 50 psi, heater gas pressure at 50 psi, curtain gas at 25 psi, temperature of 500 °C, and ion spray voltage at 5.5 kV in positive ion mode, and 4.5 kV in negative ion mode. The optimized declustering potential (DP) and collision energy (CE) were set at 60 eV and 10 eV in positive ion mode, and to − 60 eV and − 10 eV in negative ion mode, respectively. A sweeping collision energy setting at 35/ − 35 eV ± 15 eV was applied for collision-induced dissociation (CID).
Statistical analysis
All experiments were performed at least three times, and data are expressed as mean ± standard deviation. Statistical significance was evaluated using Student’s t-test in SPSS program (IL, USA), and significant differences were expressed as p < 0.05.
Results and discussion
Antioxidative activity and total phenolics & flavonoid contents of fermentation residue of S. cerevisiae
To investigate the antioxidant activity of the fermentation residue of S. cerevisiae, the DPPH radical scavenging activity was confirmed. As shown in Fig. 1B, the antioxidative activity of the fermentation residue of S. cerevisiae increased in a dose-dependent manner. Next, we analyzed the total phenolic and flavonoid contents of the fermentation residue of S. cerevisiae. In Table 1, it was estimated that 21.32 ± 0.14 (mg GAE/g) and 12.96 ± 0.04 (mg QE/g) were present in the fermentation residue of S. cerevisiae.
Fig. 1.
Effect of fermentation residue of S. cerevisiae on DPPH radical scavenging. (A) schematic diagram of fermentation residue of S. cerevisiae preparation. (B) Various concentrations of fermentation residue of S. cerevisiae and 1000 μg/mL of vitamin C were used in a DPPH assay, performed as described in the Materials and Methods. Data represent the mean ± SD. (**) p < 0.01 and (***) p < 0.001 versus the untreated control (Student’s t-test)
Table 1.
Total phenolics content, Total flavonoid content of fermentation residue of S. cerevisiae
| Total phenolics content (mg GAE/g) | 21.32 ± 0.14 |
| Total flavonoid content (mg QE/g) | 12.96 ± 0.04 |
As shown in Fig. 1, the DPPH radical scavenging activity of the fermentation residue of S. cerevisiae was evaluated by DPPH assay, showing that the fermentation residue of S. cerevisiae is a good antioxidant. Furthermore, we investigated the phenolic and flavonoid contents using TPC and TFC assays. These phenolic compounds and antioxidants are involved in health improvement, such as anticancer (Borek, 2004) and anti-inflammatory activities (Geronikaki and Gavalas, 2006). Phenolic compounds are known as great antioxidants (RiceEvans et al., 1997), while flavonoids can also activate antioxidant enzymes (Prochazkova et al., 2011).
Cytotoxicity of fermentation residue of S. cerevisiae and the effect of fermentation residue of S. cerevisiae on skin whitening activity
To investigate the cytotoxicity of the fermentation residue of S. cerevisiae, an MTS assay was conducted in B16F10 cells. Various concentrations (0 µg/mL, 62.5 µg/mL, 125 µg/mL, 250 µg/mL, and 500 µg/mL) of fermentation residue of S. cerevisiae was treated to B16F10 cells. The results show that the fermentation residue of S. cerevisiae had no effect on the viability of B16F10 cells up to 500 µg/mL (Fig. 2A). To investigate the skin whitening potential of the fermentation residue of S. cerevisiae, we measured the effect of the fermentation residue of S. cerevisiae on mushroom tyrosinase activity. Notably, mushroom tyrosinase activity was significantly reduced by the fermentation residue of S. cerevisiae (Fig. 2B). To confirm the skin whitening effect of the fermentation residue of S. cerevisiae in cells, we investigated the inhibitory effect of the fermentation residue of S. cerevisiae on cellular tyrosinase activity. Indeed, the fermentation residue of S. cerevisiae significantly inhibited cellular tyrosinase (Fig. 2C). Melanin content was also evaluated to verify the skin whitening effect of the fermentation residue of S. cerevisiae. Melanin content was dose-dependently inhibited by the fermentation residue of S. cerevisiae (Fig. 2D). Thus, it can be noted that the fermentation residue of S. cerevisiae possesses skin whitening activity.
Fig. 2.
Fermentation residue of S. cerevisiae reduce melanin contents in B16F10 cells through the inhibition of tyrosinase activity. (A) Cytotoxic effect of fermentation residue of S. cerevisiae on B16F10 cells measured by MTS assay. (B) The effect of fermentation residue of S. cerevisiae on tyrosinase activity was estimated using a mushroom tyrosinase assay. (C) The effect of fermentation residue of S. cerevisiae on cellular tyrosinase activity in α-MSH-induced B16F10 cell was estimated using a cellular tyrosinase assay. (D) The effect of fermentation residue of S. cerevisiae on melanin contents in α-MSH-induced B16F10 cell was estimated using a melanin contents assay. Data are presented as mean ± SD. (*) p < 0.05 and (***) p < 0.001 versus control group (Student’s t-test)
Fermentation residue of S. cerevisiae directly reduces tyrosinase enzyme activity
To determine the underlying mechanisms of the fermentation residue of S. cerevisiae for skin whitening activity, we examined the expression of proteins related to melanin synthesis, such as TRP-1 and TRP-2. Interestingly, we found that treatment with fermentation residue of S. cerevisiae did not affect the expression of tyrosinase, TRP-1, and TRP-2 in B16F10 cells. Because the enzymatic activity of tyrosinase and melanin production were attenuated by the fermentation residue of S. cerevisiae, we thought that the fermentation residue of S. cerevisiae was a direct enzyme inhibitor of tyrosinase. The initial velocity of the tyrosinase-L-DOPA reaction was measured using 0.067 µg/mL of tyrosinase and various concentrations of L-DOPA and the presence and absence of fermentation residue of S. cerevisiae. The results of the Lineweaver–Burk plot showed that there were insignificant differences in Vmax values and significant differences in Km values between fermentation residues of S. cerevisiae treated and untreated graphs (Fig. 3B). This implies that the fermentation residue of S. cerevisiae is a competitive inhibitor of tyrosinase.
Fig. 3.
Fermentation residue of S. cerevisiae directly reduce tyrosinase enzyme activity. (A) Tyrosinase, TRP1, TRP2 protein expression were evaluated by western blot analysis. B16F10 cells were treated with fermentation residue for 1 h followed by α-MSH for 24 h. Data are representative of three independent experiments, which gave similar results. (B) Effect of fermentation residue of S. cerevisiae on mushroom tyrosinase activity was analyzed by Lineweaver–Burk plots. The initial velocity was expressed as increased absorbance over 1 min. Data are presented as mean ± SD
Fermentation residue of S. cerevisiae has skin whitening effect on human skin
The skin whitening activity of the fermentation residue of S. cerevisiae was confirmed in a clinical trial. The study was performed using a double-blind test. The test [0.01% (v/v) containing the fermentation residue of S. cerevisiae] and control (without fermentation residue of S. cerevisiae) samples were prepared and treated to each side of the face for 6 weeks. Skin brightness was assessed by the L* value using a SkinColorCatch (Delfin Technologies) device. Increased L* values were observed in the test sample group after 3 weeks of treatment (Fig. 4A).
Fig. 4.
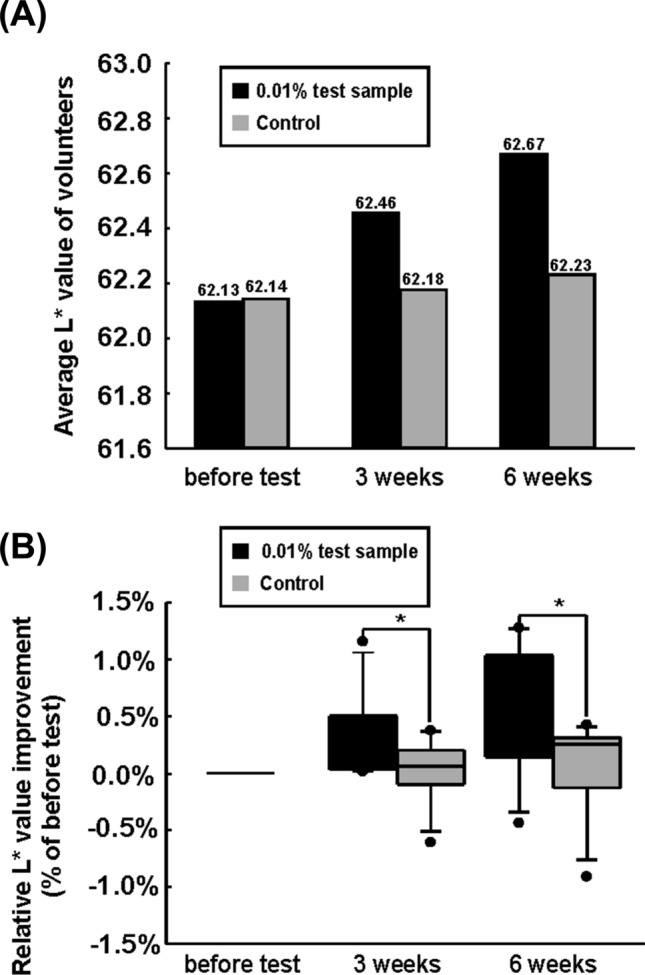
Skin whitening effect of fermentation residue of S. cerevisiae. (A) Human facial skin brightness affected by fermented residue of S. cerevisiae treatment were estimated. The vertical axis expresses the skin brightness (L* value) of the subjects (n = 11). (B)The increase in L* value was reanalyzed. Improvement in L* values are displayed in the y-axis. (*) p < 0.05 versus control group (Student’s t-test)
The L* values of the fermentation residue of the S. cerevisiae formulation group (0.53%) were significantly higher than that of the control group (0.05%) after 3 weeks. In addition, the L* values of the fermentation residue of the S. cerevisiae formulation group (0.87%) were higher than that of the control group (0.15%) after 6 weeks (Fig. 4B). During the test, skin irritation and formulation stability surveys were conducted. Results show that erythema, pain, edema, burning sensation, keratinization, tight-skin feeling, pruritus, and skin tingling did not occur during the test (Table 2).
Table 2.
Skin irritation and formulation stability survey (N = 11)
| Category | 3 weeks | 6 weeks | ||
|---|---|---|---|---|
| Numbers | Percentage (%) | Numbers | Percentage (%) | |
| Erythema | 0 | 0 | 0 | 0 |
| Edema | 0 | 0 | 0 | 0 |
| Keratinization | 0 | 0 | 0 | 0 |
| Pruritus | 0 | 0 | 0 | 0 |
| Pain | 0 | 0 | 0 | 0 |
| Burning sensation | 0 | 0 | 0 | 0 |
| Tight-skin feeling | 0 | 0 | 0 | 0 |
The food industry, including traditional liquor companies, spend a lot of money in food waste disposal every year (Badgett and Milbrandt, 2021; Han and Shin, 2004; Russ and Meyer-Pittroff, 2004). The by-products of the manufacturing process are treated as food waste (Morales-Polo et al., 2018; Russ and Meyer-Pittroff, 2004). Food waste requires an appropriate process to prevent environmental pollution and often incur significant costs in landfill tipping fees (Badgett and Milbrandt, 2021; Morales-Polo et al., 2018). If these by-products can be used to produce appropriate products such as cosmetics, the company could save not only the cost of the material but also the costs of waste disposal. Therefore, the utilization of by-products such as fermentation residue could be a tremendous profit for the food industry. To utilize these fermentation residues as cosmetic compounds, we investigated the skin whitening function of the fermentation residue of S. cerevisiae from traditional liquor production. In conclusion, fermentation residue of S. cerevisiae could be an effective and efficient skin whitening agent.
Qualitative analysis of fermentation residue of S. cerevisiae
To identify the substances involved in skin whitening effect, the fermentation residue was analyzed by LC–MS/MS. The substances with a mass accuracy score of 0.8 or higher were listed in Table S1. Among the substances, short-length peptides and amino acids were dominant, and dipeptides were the most abundant with 67 kinds. It is presumed that these substances were liberated by hydrolysis of S. cerevisiae cells and sweet potato proteins during distillation process. Unlike single substances such as vitamin C and albutin, peptides have a wide variety of length and amino acid composition, and therefore studies on the skin whitening effect have been relatively few. It was reported that 32 types out of 400 types of dipeptides showed more than 50% of melanogenesis inhibitory effect in an experiment using B16 malignant melanoma cell (Park, 2010). Among the 32 types of dipeptides, 15 dipeptides (Ile-Phe, Val-Phe, Ile-Ile, Val-Trp, Asn-Leu, Tyr-Phe, Val-Tyr, Phe-Lys, Val-Pro, Tyr-Glu, Ser-Ile, Tyr-Pro, Pro-Phe, Val-Ala, and Gly-Leu) were found in the fermentation residue of this study, and therefore, these are probably involved in the skin whitening effect. Polyphenolic compounds in sweet potato have also been reported to exhibit a skin whitening effect by inhibiting tyrosinase activity (Krochmal-Marczak et al., 2021; Ohgidani et al., 2012). As shown in Table S1, several polyphenols including caffeic acid and ferulic acid derived from sweet potato were detected in the fermentation residue. The polyphenols have been reported to have not only antioxidant activity, but also anti-melanogenic effects (Ohgidani et al., 2012). Thus, the polyphenols may also have contributed to the whitening effect of the fermentation residue.
Dipeptides in fermentation residue of S. cerevisiae directly reduces tyrosinase enzyme activity
To determine the active compounds of fermentation residue of S. cerevisiae, we checked the effect of dipeptide which originated from our sample on tyrosinase enzyme activity. We selected four dipeptides (Tyr-Pro, Tyr-Glu, Val-Tyr, Phe-Lys) which already reported as active dipeptides on tyrosinase enzyme (Tseng et al., 2015) among the 15 types of dipeptides identified in LC–MS/MS in Supplementary Fig. 2. We analyzed the initial velocity of the tyrosinase-L-DOPA reaction using 4 μg/mL of tyrosinase and various concentrations of L-DOPA and the presence and absence of 4 types of dipeptides. The results of the Lineweaver–Burk plot showed that Km values were up-regulated by 4 types of dipeptides, but Vmax values were not affected. This implies that the 4 types of dipeptides are competitive inhibitors of tyrosinase same as fermentation residue of S. cerevisiae and might be the active compounds of fermentation residue of S. cerevisiae.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (Nos. 2020R1C1C1004670 and 2018M3C1B505214813).
Declarations
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Dong-Uk Jo and Young-Wook Chin have contributed equally to this work.
Contributor Information
Dong-Uk Jo, Email: headack@naver.com.
Young-Wook Chin, Email: ywchin@kfri.re.kr.
Yongeun Kim, Email: yongeunkim7@gmail.com.
Kyung-Tack Kim, Email: tack@kfri.re.kr.
Tae-Wan Kim, Email: ktwco@kfri.re.kr.
Tae-Gyu Lim, Email: tglim@sejong.ac.kr.
References
- Badgett A, Milbrandt A. Food waste disposal and utilization in the United States: A spatial cost benefit analysis. Journal of Cleaner Production. 2021;314:128057. doi: 10.1016/j.jclepro.2021.128057. [DOI] [Google Scholar]
- Borek C. Dietary antioxidants and human cancer. Integrative Cancer Therapies. 2004;3:333–341. doi: 10.1177/1534735404270578. [DOI] [PubMed] [Google Scholar]
- Briganti S, Camera E, Picardo M. Chemical and instrumental approaches to treat hyperpigmentation. Pigment Cell Research. 2003;16:101–110. doi: 10.1034/j.1600-0749.2003.00029.x. [DOI] [PubMed] [Google Scholar]
- Chin YW, Lee S, Yu HH, Yang SJ, Kim TW. Combinatorial effects of protective agents on survival rate of the yeast starter, Saccharomyces cerevisiae 88-4, after Freeze-Drying. Microorganisms. 9 (2021) [DOI] [PMC free article] [PubMed]
- Couteau C, Coiffard L. Overview of skin whitening agents: Drugs and cosmetic products. Cosmetics. 2016;3:27. doi: 10.3390/cosmetics3030027. [DOI] [Google Scholar]
- D'Orazio JA, Nobuhisa T, Cui RT, Arya M, Spry M, Wakamatsu K, Igras V, Kunisada T, Granter SR, Nishimura EK, Ito S, Fisher DE. Topical drug rescue strategy and skin protection based on the role of Mc1r in UV-induced tanning. Nature. 2006;443:340–344. doi: 10.1038/nature05098. [DOI] [PubMed] [Google Scholar]
- del Marmol V, Beermann F. Tyrosinase and related proteins in mammalian pigmentation. FEBS Letters. 1996;381:165–168. doi: 10.1016/0014-5793(96)00109-3. [DOI] [PubMed] [Google Scholar]
- Dreno B, Araviiskaia E, Kerob D, Andriessen A, Anfilova M, Arenbergerova M, Barrios OFL, Mokos ZB, Haedersdal M, Hofmann MA, Khamaysi Z, Kosmadaki M, Lesiak A, Roo E, Zbranca-Toporas A, Wiseman MC, Zimmo S, Guerin L, Fabbrocini G. Nonprescription acne vulgaris treatments: Their role in our treatment armamentarium-An international panel discussion. Journal of Cosmetic Dermatology. 2020;19:2201–2211. doi: 10.1111/jocd.13497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eom SJ, Kim TW, Kim S, Kim JH, Park JT, Lee NH, Choi YS, Kang MC, Song KM. Immune-enhancing effects of polysaccharide extract of by-products of Korean liquor fermented by Saccharomyces cerevisiae. International Journal of Biological Macromolecules. 2021;188:245–252. doi: 10.1016/j.ijbiomac.2021.08.044. [DOI] [PubMed] [Google Scholar]
- Geronikaki AA, Gavalas AM. Antioxidants and inflammatory disease: synthetic and natural antioxidants with anti-inflammatory activity. Combinatorical Chemistry and High Throughput Screening. 2006;9:425–442. doi: 10.2174/138620706777698481. [DOI] [PubMed] [Google Scholar]
- Gueniche A, Bastien P, Ovigne JM, Kermici M, Courchay G, Chevalier V, Breton L, Castiel-Higounenc I. Bifidobacterium longum lysate, a new ingredient for reactive skin. Experimental Dermatology. 2010;19:E1–E8. doi: 10.1111/j.1600-0625.2009.00932.x. [DOI] [PubMed] [Google Scholar]
- Gupta AK, Gover MD, Nouri K, Taylor S. The treatment of melasma: A review of clinical trials. Journal of the American Academy of Dermatology. 2006;55:1048–1065. doi: 10.1016/j.jaad.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Han SK, Shin HS. Performance of an innovative two-stage process converting food waste to hydrogen and methane. Journal of the Air & Waste Management Association. 2004;54:242–249. doi: 10.1080/10473289.2004.10470895. [DOI] [PubMed] [Google Scholar]
- Hirobe T. How are proliferation and differentiation of melanocytes regulated? Pigment Cell Melanoma Research. 2011;24:462–478. doi: 10.1111/j.1755-148X.2011.00845.x. [DOI] [PubMed] [Google Scholar]
- Jang M, Lee YC, Hong HD, Rhee Y, Lim TG, Kim KT, Chen F, Kim HJ, Cho CW. Anti-oxidative and anti-inflammatory activities of devil's club (Oplopanax horridus) leaves. Food Science and Biotechnology. 2017;26:213–220. doi: 10.1007/s10068-017-0029-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin YH, Lee SJ, Chung MH, Park JH, Park YI, Cho TH, Lee SK. Aloesin and arbutin inhibit tyrosinase activity in a synergistic manner via a different action mechanism. Archives of Pharmacal Research. 1999;22:232–236. doi: 10.1007/BF02976355. [DOI] [PubMed] [Google Scholar]
- Joshi PG, Nair N, Begum G, Joshi NB, Sinkar VP, Vora S. Melanocyte-keratinocyte interaction induces calcium signalling and melanin transfer to keratinocytes. Pigment Cell Research. 2007;20:380–384. doi: 10.1111/j.1600-0749.2007.00397.x. [DOI] [PubMed] [Google Scholar]
- Krochmal-Marczak B, Zagorska-Dziok M, Michalak M, Kieltyka-Dadasiewicz A. Comparative assessment of phenolic content, cellular antioxidant, antityrosinase and protective activities on skin cells of extracts from three sweet potato (Ipomoea batatas (L.) Lam.) cultivars. Journal of King Saud University Science. 33 (2021)
- L'Oréal. The world of beauty in 2020 https://www.loreal-finance.com/en/annual-report-2020/cosmetics-market-2-1-0/. (2015)
- Mateus MM, Ventura P, Rego A, Mota C, Castanheira I, Bordado JM, dos Santos RG. Acid liquefaction of potato (Solanum tuberosum) and sweet potato (Ipomoea batatas) cultivars peels pre-screening of antioxidant activity/total phenolic and sugar contents. Bioresources. 2017;12:1463–1478. doi: 10.15376/biores.12.1.1463-1478. [DOI] [Google Scholar]
- Miyamoto K, Dissanayake B, Omotezako T, Takemura M, Tsuji G, Furue M. Daily fluctuation of facial pore area, roughness and redness among young Japanese women; Beneficial effects of galactomyces ferment filtrate containing antioxidative skin care formula. Journal of Clinical Medicine. 10(11): 2502 (2021) [DOI] [PMC free article] [PubMed]
- Morales-Polo C, Cledera-Castro MD, Moratilla Soria BY. Reviewing the anaerobic digestion of food waste: From waste generation and anaerobic process to its perspectives. Applied Sciences-Basel. 8(10): 1084 (2018)
- Niwano T, Terazawa S, Sato Y, Kato T, Nakajima H, Imokawa G. Glucosamine abrogates the stem cell factor plus endothelin-1-induced stimulation of melanogenesis via a deficiency in MITF expression due to the proteolytic degradation of CREB in human melanocytes. Archives of Dermatological Research. 2018;310:625–637. doi: 10.1007/s00403-018-1850-8. [DOI] [PubMed] [Google Scholar]
- Ohgidani M, Komizu Y, Goto K, Ueoka R. Antimelanogenic and antioxidative effects of residual powders from Shochu distillation remnants. Food Chemistry. 2012;132:2140–2143. doi: 10.1016/j.foodchem.2011.12.049. [DOI] [Google Scholar]
- Park SH, Lee HK, Choi HR, Republic of Korea, WO2011126163A1 (2011.10.13).
- Prochazkova D, Bousova I, Wilhelmova N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia. 2011;82:513–523. doi: 10.1016/j.fitote.2011.01.018. [DOI] [PubMed] [Google Scholar]
- RiceEvans CA, Miller J, Paganga G. Antioxidant properties of phenolic compounds. Trends in Plant Science. 1997;2:152–159. doi: 10.1016/S1360-1385(97)01018-2. [DOI] [Google Scholar]
- Roulin A, Almasi B, Rossi-Pedruzzi A, Ducrest AL, Wakamatsu K, Miksik I, Blount JD, Jenni-Eiermann S, Jenni L. Corticosterone mediates the condition-dependent component of melanin-based coloration. Animal Behaviour. 2008;75:1351–1358. doi: 10.1016/j.anbehav.2007.09.007. [DOI] [Google Scholar]
- Russ W, Meyer-Pittroff R. Utilizing waste products from the food production and processing industries. Critical Reviews in Food Science and Nutrition. 2004;44:57–62. doi: 10.1080/10408690490263783. [DOI] [PubMed] [Google Scholar]
- Sato K, Morita M, Ichikawa C, Takahashi H, Toriyama M. Depigmenting mechanisms of all-trans retinoic acid and retinol on B16 melanoma cells. Bioscience, Biotechnology and Biochemistry. 2008;72:2589–2597. doi: 10.1271/bbb.80279. [DOI] [PubMed] [Google Scholar]
- Shen YX, Sun HY, Zeng HY, Prinyawiwatukul W, Xu WQ, Xu ZM. Increases in phenolic, fatty acid, and phytosterol contents and anticancer activities of sweet potato after fermentation by Lactobacillus acidophilus. Journal of Agricultural and Food Chemistry. 2018;66:2735–2741. doi: 10.1021/acs.jafc.7b05414. [DOI] [PubMed] [Google Scholar]
- Singleton VL. Citation classic - colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Current Contents/Agriculture Biology & Environmental Sciences. 18–18 (1985)
- Song YR, Lim WC, Han A, Lee MH, Shin EJ, Lee KM, Nam TG, Lim TG. Rose petal extract (Rosa gallica) exerts skin whitening and anti-skin wrinkle effects. Journal of Medicinal Food. 2020;23:870–878. doi: 10.1089/jmf.2020.4705. [DOI] [PubMed] [Google Scholar]
- Takizawa T, Imai T, Onose J, Ueda M, Tamura T, Mitsumori K, Izumi K, Hirose M. Enhancement of hepatocarcinogenesis by kojic acid in rat two-stage models after initiation with N-bis(2-hydroxypropyl)nitrosamine or N-diethylnitrosamine. Toxicology Science. 2004;81:43–49. doi: 10.1093/toxsci/kfh195. [DOI] [PubMed] [Google Scholar]
- Tseng TS, Tsai KC, Chen WC, Wang YT, Lee YC, Lu CK, Don MJ, Chang CY, Lee CH, Lin HH, Hsu HJ, Hsiao NW. Discovery of potent cysteine-containing dipeptide inhibitors against tyrosinase: A comprehensive investigation of 20 x 20 dipeptides in inhibiting dopachrome formation. Journal of Agricultural and Food Chemistry. 2015;63:6181–6188. doi: 10.1021/acs.jafc.5b01026. [DOI] [PubMed] [Google Scholar]
- Tsukamoto K, Jackson IJ, Urabe K, Montague PM, Hearing VJ. A second tyrosinase-related protein, TRP-2, is a melanogenic enzyme termed DOPAchrome tautomerase. The EMBO Journal. 1992;11:519–526. doi: 10.1002/j.1460-2075.1992.tb05082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinther J. A guide to the field of palaeo colour: Melanin and other pigments can fossilise: Reconstructing colour patterns from ancient organisms can give new insights to ecology and behaviour. Bioessays. 2015;37:643–656. doi: 10.1002/bies.201500018. [DOI] [PubMed] [Google Scholar]
- Yang SS, Ling MY. Tetracycline production with sweet-potato residue by solid-state fermentation. Biotechnology and Bioengineering. 1989;33:1021–1028. doi: 10.1002/bit.260330811. [DOI] [PubMed] [Google Scholar]
- Zhang LA, Zhao H, Gan MZ, Jin YL, Gao XF, Chen QA, Guan JF, Wang ZY. Application of simultaneous saccharification and fermentation (SSF) from viscosity reducing of raw sweet potato for bioethanol production at laboratory, pilot and industrial scales. Bioresource Technology. 2011;102:4573–4579. doi: 10.1016/j.biortech.2010.12.115. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





