Summary
Loss-of-function variants in PHD Finger Protein 8 (PHF8) cause Siderius X-linked intellectual disability (ID) syndrome, hereafter called PHF8-XLID. PHF8 is a histone demethylase that is important for epigenetic regulation of gene expression. PHF8-XLID is an under-characterized disorder with only five previous reports describing different PHF8 predicted loss-of-function variants in eight individuals. Features of PHF8-XLID include ID and craniofacial dysmorphology. In this report we present 16 additional individuals with PHF8-XLID from 11 different families of diverse ancestry. We also present five individuals from four different families who have ID and a variant of unknown significance in PHF8 with no other explanatory variant in another gene. All affected individuals exhibited developmental delay and all but two had borderline to severe ID. Of the two who did not have ID, one had dyscalculia and the other had mild learning difficulties. Craniofacial findings such as hypertelorism, microcephaly, elongated face, ptosis, and mild facial asymmetry were found in some affected individuals. Orofacial clefting was seen in three individuals from our cohort, suggesting that this feature is less common than previously reported. Autism spectrum disorder and attention deficit hyperactivity disorder, which were not previously emphasized in PHF8-XLID, were frequently observed in affected individuals. This series expands the clinical phenotype of this rare ID syndrome caused by loss of PHF8 function.
Keywords: PHF8, X-linked intellectual disability, orofacial clefting, epigenetic gene regulation, histone demethylation
Hemizygous loss-of-function variants in PHF8 cause X-linked intellectual disability (PHF8-XLID) and craniofacial dysmorphology. There are only 5 reports describing 8 adult or adolescent individuals with PHF8-XLID currently in the literature. We describe 11 new variants and 16 additional individuals to expand the phenotype of this rare genetic syndrome.
Introduction
Control of gene expression occurs through the interaction of a repertoire of regulatory factors. Gene regulation is complex, and is affected by a number of systems, including DNA binding proteins, enzymes that modify DNA bases, microRNA, and a large family of histone modification enzymes.1 The interaction between DNA and histones may be regulated by methylation of arginine2,3 and lysine amino acids4 as well as a diverse array of other histone modifications.5, 6, 7 Histone methylation status is controlled by enzymes that may methylate or demethylate and the methylation status affects binding of other proteins to specific regions of the histone tails. Loss-of-function variants of PHF8 (MIM: 300560), along with a number of other genes involved with regulation of histone methylation, have been associated with intellectual disability (ID) and developmental disorders in humans.8,9
PHF8 belongs to a sequence-related group of nuclear proteins that function in epigenetic gene regulation.10 PHF8 contains 1,024 amino acids and has multiple domains including a zinc-finger plant homeodomain (PHD), a Jumanji (JmjC) domain, several nuclear localization signal domains, and a serine-rich region.11, 12, 13 The PHD domain recognizes methylated histones, and the JmjC domain has catalytic histone demethylation activity.14 PHF8 has dual functions. The PHD domain preferentially recognizes trimethylated H3K4 (H3K4me3),15 an epigenetic marker typically associated with active transcription and the catalytic JmjC domain is responsible for preferential demethylation of H3K9me2.14,16,17 In this way, an epigenetic marker associated with transcriptional repression is removed. By recognizing a mark of transcriptional activation and removing an adjacent modification associated with transcriptional repression, PHF8 plays a role in switching genes from a repressed state to an active state. PHF8 has also been shown to demethylate H3K9me1.
PHF8 orthologs have been studied in various model systems that show that it plays diverse roles in gene regulation, growth, and development. Examples include regulation via RNA polymerase II,15 regulation of ribosomal gene transcription,16 development of colorectal cancer,18 and migration of endothelial cells.19 Loss of Phf8 in mouse embryonic stem cells (mESCs), neural progenitor cells, and mouse embryonic fibroblasts causes a severe reduction in proliferation of these cell types.20 Loss of Phf8 also causes changes in stem cell differentiation. Phf8 knockout in mESCs shifts differentiation toward cardiac and mesodermal cell fate.21
Various neuronal functions for PHF8 have been elucidated. JMJD-1.2, the Caenorhabditis elegans ortholog for human PFH8, is required for correct axon guidance.22 In addition, Phf8 null mice were shown to have impaired learning and memory.23 Interestingly, a previous study found that mice deficient in Phf8 had a resilience to anxiety and depression but no difference in learning and memory, possibly due to using a different background strain.20 Other studies demonstrate that Phf8 plays a role in astrocyte differentiation,24 and also the regulation of neuronal differentiation through the retinoic acid receptor.25
In humans, PHF8 is X-linked and its loss of function leads to developmental delay and dysmorphology. The first report of PHF8-XLID was enabled by linkage analysis, which implicated genes in the Xp11.3-q21.3 region in midline development, orofacial clefting, and ID in three related individuals.26 This initial discovery led to the eponym of Siderius X-linked mental retardation syndrome (MIM: 300263), which is hereafter called PHF8-X-linked ID (PHF8-XLID). Six years later, the three individuals described in the initial report by Siderius et al., were found to have a 12-base pair deletion affecting the splice donor site for intron 8 of PHF8, and causing a frameshift of the encoded protein. In addition, an unrelated individual with a stop-gain variant in exon 7 of PHF8 was described.11 In 2007, one additional individual was described.27 To our knowledge, there are only two additional reports describing individuals with pathogenic PHF8 variants, but photographic documentation of the phenotype was not included.12,13
Here, we describe 11 additional variants and 16 individuals affected with predicted PHF8 loss of function. We also describe five additional individuals with four different PHF8 variants of unknown significance (VUS). Identification of families from clinicians and researchers worldwide was enabled by MatchMaker Exchange (MME),28 GeneMatcher,29 MyGene2,30 Deciphering Developmental Disorders,31 and Decipher.32
Materials and methods
We evaluated the facial dysmorphology, development, and behavioral characteristics among a cohort of 16 individuals who were identified to have predicted loss-of-function variants in PHF8 after either exome, X-exome, or gene panel sequencing. The five individuals with a PHF8 VUS had next-generation sequencing-based testing. Three of the VUS were identified by trio exome sequencing, and one by analysis of an ID/autism spectrum disorder (ASD) gene panel. The parents of all affected individuals gave written permission to publish clinical information. Written permission to publish photographs was obtained from the parents of all affected individuals except for individuals 13, 14, and 21, who are not included in the image panels. All investigators who enrolled participants in this study had ethical oversight from their respective institutional review boards.
Results
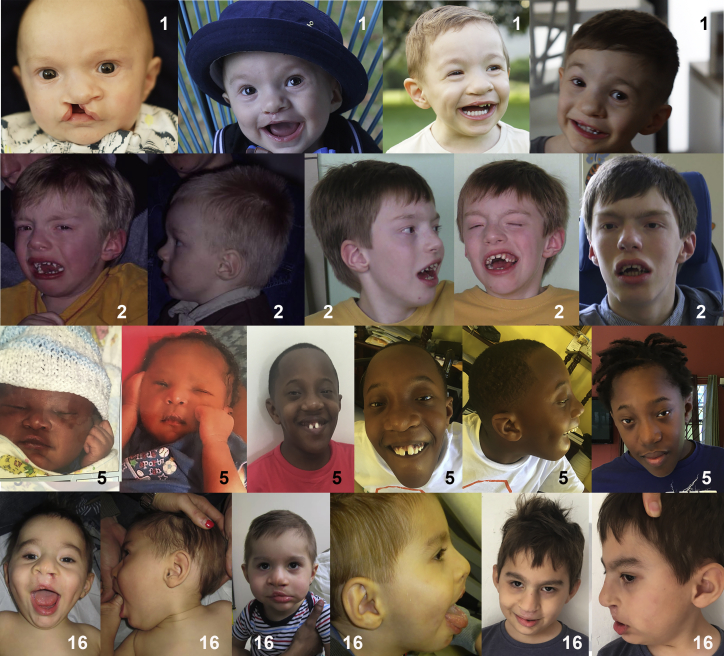
We identified 16 male individuals of various ancestries who harbor predicted loss-of-function variants in PHF8. Of the individuals in this cohort, nine were of Western European ancestry, three were Moroccan, two were Asian-Indian, and one was Afro-Caribbean. Photographs of the affected individuals show the wide range of facial dysmorphology caused by disruption of PHF8 (Figure 1). In some individuals, photographs showing the evolution of the facial dysmorphology with age were available. Two of these individuals have an elongated face that tended to become more apparent with age (Figure 2).
Figure 1.
Facial features of individuals with predicted loss-of-function variants leading to PHF8-XLID
Photographs of individuals 3, 4, 6, 7, 8, 9, 10, 11, 12, and 15.
Figure 2.
Evolution of the facial features of individuals affected with PHF8-XLID
Photographs of individuals 1, 2, 5, and 16.
Features of affected individuals
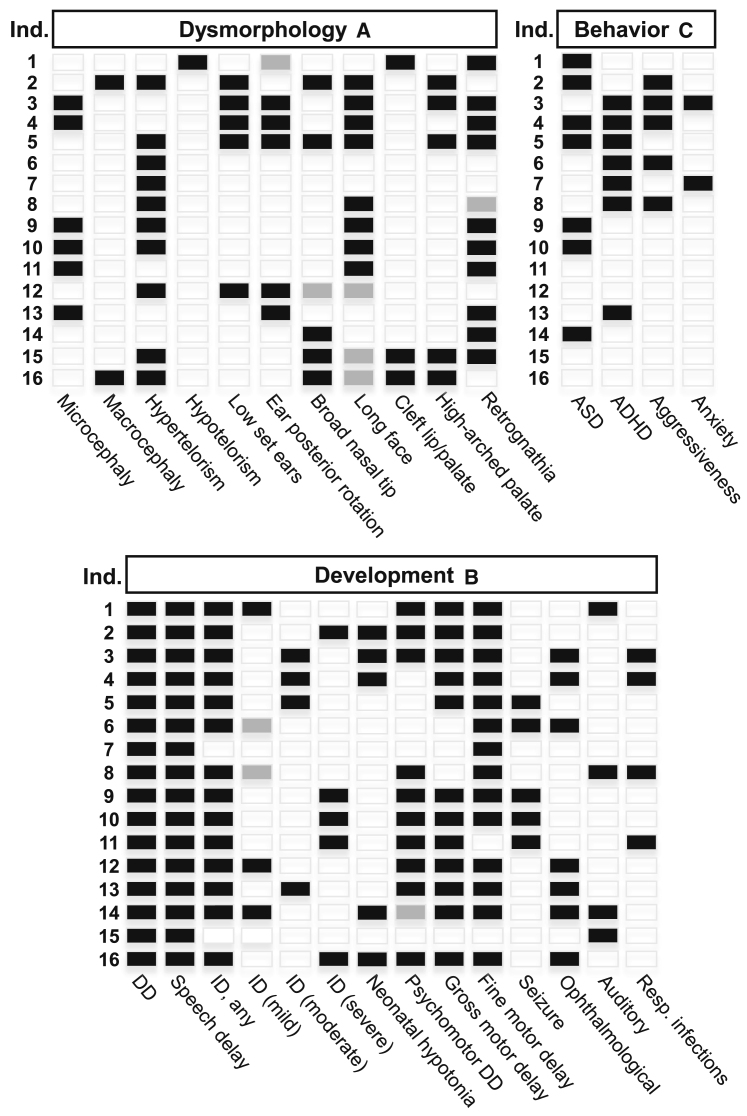
Our cohort consisted of 16 individuals with predicted loss-of-function variants in PHF8. From measurements obtained at the most recent evaluation, six of these individuals had microcephaly, two had macrocephaly, and the remainder had head circumference within the normal range. Hypertelorism was observed in 11 individuals and hypotelorism was seen in one. Five individuals had low-set ears and, of these, four had posterior angulation of the ear. An elongated face was seen in eight individuals, and a borderline long face was seen in an additional three. When photographic evidence was available, the facial elongation appeared to become more pronounced with age. Retrognathia was seen in 10 individuals with a borderline presentation in one more. A broad nasal tip was observed in five individuals. Cleft lip/palate was seen in three individuals, two of which also had a high-arched palate. A high-arched palate in the absence of frank orofacial clefting was seen in an additional three others.
All affected individuals exhibited developmental delay (DD) and speech delay. All except two individuals had some degree of ID. There was a wide range of ID: borderline mild in two, mild in three, moderate in four, and severe in five. One individual had language delay thought to be due to hearing loss, but otherwise he did not have ID. The other individual who was reported to not have ID had dyscalculia. Psychomotor DD was seen in 12 affected individuals. Gross motor delay was seen in 12, and fine motor delay was seen in 14. Five individuals were reported to have recurrent seizures.
Ophthalmological and auditory abnormalities were also seen among individuals in our cohort, albeit never in the same individual. Six individuals had notable ophthalmologic features, which included unexplained unilateral squint, myopia, astigmatism, and hypermetropia. Auditory abnormalities were observed less frequently than vision problems, and included one individual who required ventilation tubes, one who was hearing impaired, one with unilateral sensorineural hearing loss, and one with progressive bilateral conductive hearing loss. The reported age at walking for 13 of the 16 individuals was delayed with a mean of 20 months and standard deviation of 7 months. Two individuals were not able to walk, and had to use a wheelchair. Birth length was available from seven individuals and the mean was 48.25 cm (50th centile); birth weight was 2.68 kg (10th centile), and birth head circumference from six individuals had a mean of 33.4 cm (25th centile).
Seven of the individuals in our cohort were diagnosed with ASD. Seven individuals carried a diagnosis of attention deficit hyperactivity disorder (ADHD), while only two individuals were diagnosed with both ASD and ADHD. The Autism Diagnostic Observation Schedule was used to diagnose ASD in two individuals, and clinical impressions or unspecified testing was used for the others. Some individuals were not tested for ADHD, three because of severe ID. Several were not tested for ASD because it was not clinically indicated. Aggressive behavior was documented in five individuals, and two individuals were noted to have anxiety. The distribution of the craniofacial dysmorphology features, developmental findings, and behavioral aspects of the affected individuals is shown in Figure 3.
Figure 3.
Features of individuals with PHF8-XLID
(A) Characteristic dysmorphology features.
(B) Delay and neurological features.
(C) Behavioral characteristics. Partially filled in boxes (gray) indicate borderline feature.
Developmental regression, or loss of previously attained milestones, was not seen in any individual within the cohort of individuals who have a predicted PHF8 loss-of-function variant. Feeding issues at infancy were observed in 10 of the 16 individuals and included inability to breast feed, requirement for percutaneous endoscopic gastrostomy, failure to thrive, typical problems associated with cleft lip/palate, and excessive drooling. In early childhood one individual developed gastroesophageal reflux disease, another was noted to have poor chewing ability and to prefer soft foods.
Brain MRI was obtained for six different individuals and significant findings were observed in five. Individual 2 showed normal brain MRI findings at age 3 years. Individual 3 had a mildly increased intensity of the subarachnoid space and high signal in the white matter around the posterior horns of the lateral ventricles. Individuals 9 and 10, who are monozygotic twins with consanguineous parents, had polymicrogyria and cortical dysplasia upon MRI at age 11 years. Individual 11 had an abnormal signal intensity in the striatum, normal cerebrum, normal gyri, and abnormal signal intensity in the caudate nucleus and globus pallidus, but the thalamus was normal. Individual 16 had tied cranio-occipital malformations without the typical Chiari malformation, but with a hypoplasia posterior fossa and a thin corpus callosum. A compilation of the collected clinical data for each individual in this cohort is in Table S1. Clinical descriptions for a subset of these individuals may be found in the supplemental material.
PHF8 VUS
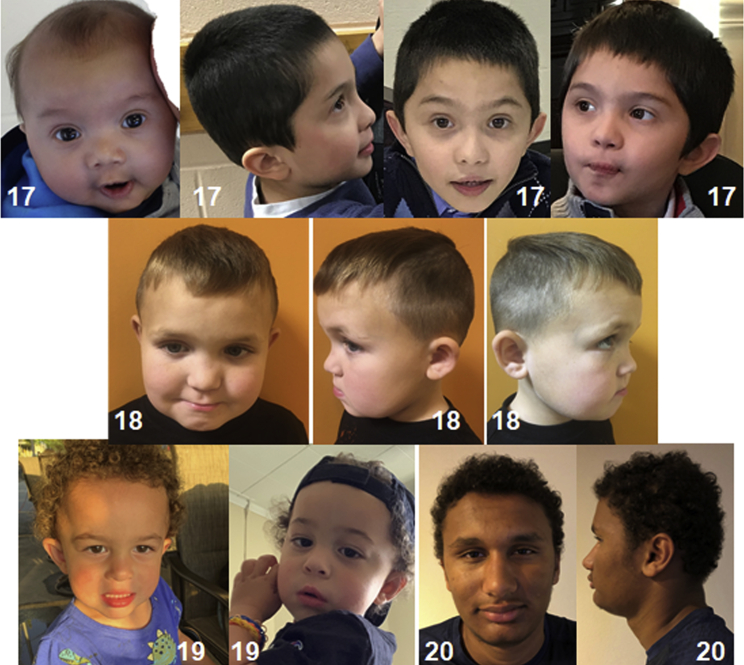
We also present five male individuals (17–21 years) who were identified with a hemizygous VUS in PHF8 following either exome sequencing or gene panel analysis. No other explanatory variant was identified for these individuals. All had mild to severe ID, and two exhibited regression of previously attained milestones. As with the individuals in the pathogenic/likely pathogenic cohort, the facial features of the individuals with PHF8 VUS are heterogeneous. Orofacial clefting was not observed in any of these individuals (Figure 4). Overall, the clinical phenotype of the individuals with VUS appears to show a broad range of phenotypic variability similar to that seen in patients with predicted loss-of-function variants.
Figure 4.
Facial features of individuals who have intellectual disability and VUS in PHF8
Photographs of individuals 17, 18, 19, and 20.
Genetic investigations
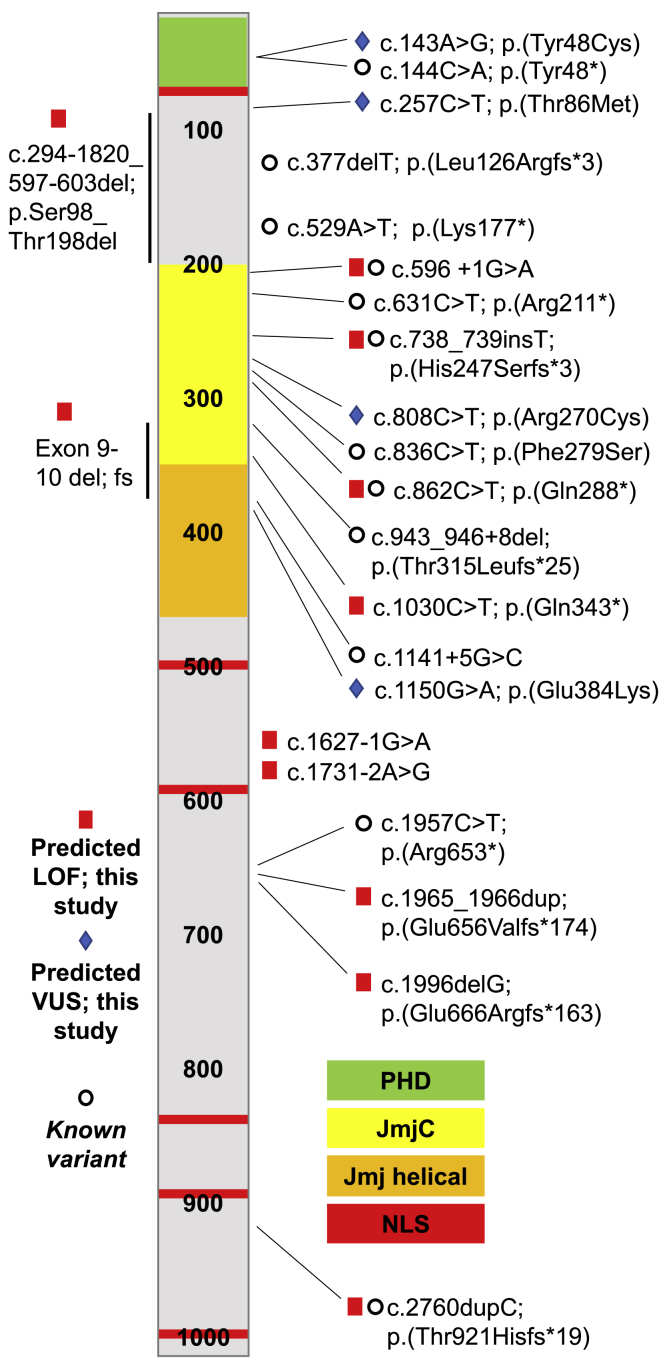
All PHF8 variants in our cohort were identified by next-generation sequencing (NGS). One individual was diagnosed by NGS of a custom panel of genes known to be associated with ID, two via a panel designed to detect genes associated with cleft lip/palate, and one individual was identified by X-exome sequencing. The remainder were identified via exome sequencing. In three families, additional affected individuals were identified following diagnosis in a sibling or a nephew. Of the 12 mothers who had children affected with PHF8-XLID, 10 were shown to be unaffected carriers and 2 did not have a PHF8 variant suggesting de novo inheritance. Skewed X-inactivation was tested in 3 of the 10 healthy carrier mothers. In two of the mothers, completely skewed X-inactivation was observed and, in the third, the test was uninformative. A schematic of PHF8 with the placement of previously reported variants and the variants reported here is shown in Figure 5. Of the variants we describe here, four were previously reported in large-scale sequencing studies.9,33 In addition, several other predicted loss-of-function PHF8 variants were described in other next-generation sequencing-based studies,9,34,35 but detailed clinical information was not included. Previously reported PHF8 loss-of-function variants,9,11, 12, 13,26,27,33, 34, 35 the loss-of-function variants described in this study, and the VUS we report are listed in Table 1.
Figure 5.
Schematic of PHF8 protein structure annotated with variants
Known PHF8 variants are indicated with open circles; predicted loss-of-function PHF8 variants are indicated with red squares, and predicted PHF8 VUS are indicated with blue diamonds. Protein domains of PHF8 are color coded. PHD, plant homology domain; JmjC, jumonji C domain; NLS, nuclear localization signal.
Table 1.
Known variants in PHF8
| Ind. | var # | PHF8 NM_015107.2 Variant | Protein consequence | Variant effect | Inh. | Variant first identified by | Source |
|---|---|---|---|---|---|---|---|
| This study (predicted loss of function) | |||||||
| 1 | 1 | del exons 9-10 | p.Gly316_Arg380del | Intragenic del with frameshift | Maternal | Gene panel (ID) | This study |
| 2 | 2 | c.596+1G>A | Unknown | Splice site | de novo | Trio exome, DDD | |
| 3, 4 | 3 | c.862C>T | p.(Gln288∗) | Stop gain | Maternal | Quad exome, DDD | |
| 5 | 4 | c.1996delG | p.(Glu666Argfs∗163) | Frameshift | Maternal | Proband exome | |
| 6,7,8 | 5 | c.1030C>T | p.(Gln343∗) | Stop gain | Maternal | X-exome | |
| 9,10,11 | 6 | c.1731-2A>G | Unknown | Splice site | Maternal | Trio exome | |
| 12 | 7 | c.1627-1G>A | Unknown | Splice site | de novo | Trio exome | |
| 13 | 8 | c.738_739insT | p.(His247Serfs∗3) | Frameshift | Maternal | Trio exome, DDD | |
| 14 | 9 | c.2760dupC | p.(Thr921Hisfs∗19) | Frameshift | Maternal | Trio exome | |
| 15 | 10 | c.1965_1966dup | p.(Glu656Valfs∗174) | Frameshift | de novo | Gene panel (CL/P) | |
| 16 | 11 | c.294-1820_597-603del | p.Ser98_Thr198del | Deletion | Maternal | Gene panel (CL/P) | |
| This study (variants of unkwown significance) | |||||||
| 17 | 12 | c.143A>G | p.(Tyr48Cys) | Missense | Maternal | Trio exome | This study |
| 18,19 | 13 | c.257C>T | p.(Thr86Met) | Missense | Maternal | Trio exome | |
| 20 | 14 | c.808C>T | p.(Arg270Cys) | Missense | Maternal | Trio ID gene panel | |
| 21 | 15 | c.1150G>A | p.(Glu384Lys) | Missense | Maternal | Trio exome | |
| PHF8 variants in previously described indviduals | |||||||
| 23 | c.144C>A | p.(Tyr48∗) | Stop gain | Maternal | XLID panel (NGS) | Ibarluzea et al.13 | |
| 25,26 | c.836C>T | p.(Phe279Ser) | Missense | Maternal | Sanger seq | Koivisto et al.27 | |
| 27 | c.529A>T | p.(Lys177∗) | Stop gain | Not noted | Sanger seq | Abidi et al.12 | |
| 28 | c.631C>T | p.(Arg211∗) | Stop gain | Maternal | Sanger seq | Laumonnier et al.11 | |
| 29,30,31 | c.943_946+8del | p.(Thr315Leufs∗25) | Frameshift | Maternal | Linkage analysis | Siderius et al.26 | |
| PHF8 variants described in large-scale exome sequencing projects | |||||||
| na | c.596+1G>A | Unknown | Splice site | Not noted | Exome | Faundes et al.9 | |
| na | c.738_739insT | p.His247Serfs∗3 | Frameshift | Not noted | Exome | ||
| na | c.862C>T | p.(Gln288∗) | Stop gain | Not noted | Exome | ||
| na | c.1957C>T | p.(Arg653∗) | Stop gain | Not noted | Exome | ||
| na | c.377delT | p.(Leu126Argfs∗3) | Frameshift | Not noted | Exome | Posey et al.34 | |
| na | c.2760dupC | p.(Thr921Hisfs∗19) | Frameshift | Not noted | Exome | Retterer et al.33 | |
| na | c.1141+5G>C | Unknown | Splice site | Not noted | Exome | Redin et al.35 | |
The table includes the variants described in this study, variants described in previously published reports, and variants described in large-scale sequencing projects. All variants were coded according to the NM_015107.2 PHF8 transcript.
Most affected individuals had previous genetic testing. Microarray was obtained for 13, fragile-X syndrome testing was obtained for 10, and karyotype was obtained for 8 individuals. Angelman syndrome was suspected and tested for in three individuals, and one was tested for myotonic dystrophy. One individual underwent testing via a gene panel for ID and DD which was uninformative. Two individuals underwent metabolic testing, which included analysis of amino acids, organic acids, glycosaminoglycans, oligosaccharides, acylcarnitines, and very long chain fatty acids. The previous genetic and biochemical testing obtained for the individuals in this cohort is in Table S2.
Of the individuals with PHF8 VUS, three had trio exome sequencing, one had targeted analysis of PHF8, because of the known PHF8 variant in a maternal half-brother, and one had a trio ID/ASD gene panel. In all cases, the PHF8 VUS was inherited from an unaffected carrier mother. None of the mothers were tested for skewed X-inactivation.
Discussion
The five previously published reports describing PHF8-XLID include photographic documentation of six individuals, and written descriptions of two more. All six of the individuals who were described with photographs were either adult or in late adolescence, so descriptions of PHF8-XLID in young children has previously not been available. Six of the eight previously reported individuals exhibited facial clefting and this was viewed as a defining characteristic feature of PHF8-XLID. It is likely that this is because cleft lip/palate was a screening criterion combined with ID in the first four reports.11,12,26,27 In the Ibarluzea 2020 report, orofacial clefting was not used as one of the screening criteria,13 so a predicted PHF8 loss-of-function variant was detected from a panel of genes known to cause X-linked ID. In our cohort, only 3 of the 16 patients with predicted loss-of-function variants had a cleft lip/palate and none of the individuals with VUS had a cleft lip/palate, consistent with a less severe phenotype. While cleft lip/palate can be caused by pathogenic variants in PHF8, it does not appear to be a consistent diagnostic feature of PHF8-XLID.
Unbiased screening by either exome, X-exome, or ID gene panel sequencing reveals the broad range of phenotypic heterogeneity and variable expressivity displayed by individuals who harbor PHF8 loss-of-function variants. The only clinical feature common to all individuals was DD and speech delay. ID was seen in all except for two individuals, and the severity ranged from borderline mild to severe. Within our cohort of individuals who had predicted PHF8 loss-of-function variants, there were three sets of siblings. The clinical features within these sibling pairs was relatively consistent compared with the overall cohort. For instance, individuals 6 and 7 had either mild or no ID, individuals 3 and 4 had moderate ID, and individuals 9 and 10, who are consanguineous identical twins, had severe ID. In addition many other clinical features were similar within these sibling pairs.
All except one variant described in the previous reports of PHF8-XLID were predicted to cause truncation of PHF8 and disruption of the catalytic JmjC domain. The one exception was a missense variant leading to a Phe279Ser substitution in the encoded protein.27 In vitro functional studies demonstrated that the Phe279Ser variant abolished the lysine demethylase activity of the encoded protein,36 suggesting that aberrant epigenetic regulation of gene expression leads to the disorder. Five of the loss-of-function variants we describe (in seven individuals) are located outside of the JmjC domain. All of these variants either lead to a frameshift or are predicted to affect splicing. Notably, the individuals who we describe with predicted loss-of-function variants outside of the JmjC domain do not have different clinical features or phenotypic severity than those with variants within the JmjC domain. Notably, one of the individuals with a loss-of-function variant outside of the JmjC domain is one of the three patients in our cohort with a cleft lip/palate. Four of the variants we identified were found by either an ID gene panel, a cleft lip/palate panel, or X-exome sequencing. Since exome sequencing was not used for these individuals, it is possible that they may have additional but unknown variants that contribute to their phenotype.
A variant predicted to cause an in-frame deletion of a serine amino acid at position 969 of PHF8 was reported in two individuals and a carrier mother.37 However, we did not include Ser969del as a deleterious variant in this study for three reasons. First, it is abundantly found in gnomAD in both heterozygous males and homozygous females.38 The second reason is that it is annotated as benign or likely benign in ClinVar.39 Finally, functional studies were not done to characterize this variant.
Our cohort includes five individuals with four previously unreported missense variants. The functional implication is currently unknown and future study of these VUS could allow us to better understand the importance of other PHF8 domains. Functional studies to assess PHF8 activity would be useful to help characterize these missense variants. Three reports of microdeletion or unspecified mutation within the Xp11.22 region describe individuals with phenotypes similar to PHF8-XLID. Although the deletions in these patients spanned multiple genes, their phenotypic features are similar to that seen in individuals with predicted loss of function of only PHF8.40, 41, 42
The methylcytosine epigenetic signatures of various genes involved in histone methylation have been established.43 For example, the epi-signature of Claes-Jensen syndrome due to pathogenic loss of function of the X-linked gene KDM5C is significantly different between unaffected individuals, carrier mothers, and affected males.44 However, a recognizable epi-signature was not observed from an analysis of nine males affected with PHF8-XLID. It is possible that a larger sample size is needed, or that DNA samples from a tissue other than blood might be needed to uncover a potentially altered methylation pattern for this disorder.45
Overall, PHF8-XLID appears to be characterized by DD, ID, and craniofacial anomalies. Cleft lip or palate is found in a much smaller percentage of affected individuals who were found through unbiased sequencing. The phenotypic variability does not appear to be linked to the variant location in individuals who harbor a null allele. Future studies aimed at evaluating the cause of the variability would be helpful to predict disease severity and developmental progression as well as the interpretation of VUS in PHF8.
Ethical oversight
This study was performed in accordance with the ethical standards detailed in the Declaration of Helsinki. The parents or guardians of all participants provided written informed consent for inclusion in this study.
Web resources
OMIM, http://www.omim.org/
Acknowledgments
Sequencing was provided by the University of Washington Center for Mendelian Genomics (UW-CMG) and was funded by NHGRI and NHLBI grants UM1 HG006493 and U24 HG008956, by the Office of the Director, NIH under Award Number S10OD021553. We thank the patients and their families for participating in this study. The DDD study presents independent research commissioned by the Health Innovation Challenge Fund [grant number HICF-1009-003]. This study makes use of DECIPHER (http://decipher.sanger.ac.uk), which is funded by Wellcome. See Nature PMID: 25533962 or www.ddduk.org/access.html for full acknowledgement.
Declaration of interest
J.J., J.Z., A.T., R.P., and K.M. are employees of GeneDx, Inc. All other authors have no conflict of interest to declare.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xhgg.2022.100102.
Contributor Information
Andrew K. Sobering, Email: andrew.sobering@uga.edu.
Elizabeth J. Bhoj, Email: bhoje@chop.edu.
Supplemental information
Data and code availability
The published article includes a supplemental table with the full clinical dataset generated and analyzed during the study. All exome sequencing data are not publicly available due to privacy or ethical restrictions.
References
- 1.Ahsendorf T., Müller F.J., Topkar V., Gunawardena J., Eils R. Transcription factors, coregulators, and epigenetic marks are linearly correlated and highly redundant. PLoS One. 2017;12:1–25. doi: 10.1371/journal.pone.0186324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Di Lorenzo A., Bedford M.T. Histone arginine methylation. FEBS Lett. 2011;585:2024–2031. doi: 10.1016/j.febslet.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang J., Jing L., Li M., He L., Guo Z. Regulation of histone arginine methylation/demethylation by methylase and demethylase (Review) Mol. Med. Rep. 2019;19:3963–3971. doi: 10.3892/mmr.2019.10111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Husmann D., Gozani O. Histone lysine methyltransferases in biology and disease. Nat. Struct. Mol. Biol. 2019;26:880–889. doi: 10.1038/s41594-019-0298-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor B.C., Young N.L. Combinations of histone post-translational modifications. Biochem. J. 2021;478:511–532. doi: 10.1042/BCJ20200170. [DOI] [PubMed] [Google Scholar]
- 6.Ryu H.Y., Hochstrasser M. Histone sumoylation and chromatin dynamics. Nucleic Acids Res. 2021;49:6043–6052. doi: 10.1093/nar/gkab280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Villaseñor R., Baubec T. Regulatory mechanisms governing chromatin organization and function. Curr. Opin. Cell Biol. 2021;70:10–17. doi: 10.1016/j.ceb.2020.10.015. [DOI] [PubMed] [Google Scholar]
- 8.Vallianatos C.N., Iwase S. Disrupted intricacy of histone H3K4 methylation in neurodevelopmental disorders. Epigenomics. 2015;7:503–519. doi: 10.2217/epi.15.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faundes V., Newman W.G., Bernardini L., et al. Histone lysine methylases and demethylases in the landscape of human developmental disorders. Am. J. Hum. Genet. 2018;102:175–187. doi: 10.1016/j.ajhg.2017.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Piazza R., Magistroni V., Redaelli S., et al. SETBP1 induces transcription of a network of development genes by acting as an epigenetic hub. Nat. Commun. 2018;9:2192. doi: 10.1038/s41467-018-04462-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laumonnier F., Holbert S., Ronce N., et al. Mutations in PHF8 are associated with X linked mental retardation and cleft lip/cleft palate. J. Med. Genet. 2005;42:780–786. doi: 10.1136/jmg.2004.029439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abidi F.E., Miano M.G., Murray J.C., Schwartz C.E. A novel mutation in the PHF8 gene is associated with X-linked mental retardation with cleft lip/cleft palate. Clin. Genet. 2007;72:19–22. doi: 10.1088/1367-2630/15/1/015008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ibarluzea N., de la Hoz A.B., Villate O., et al. Targeted next-generation sequencing in patients with suggestive X-linked intellectual disability. Genes (Basel) 2020;11:1–22. doi: 10.3390/genes11010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horton J.R., Upadhyay A.K., Qi H.H., Zhang X., Shi Y., Cheng X. Enzymatic and structural insights for substrate specificity of a family of jumonji histone lysine demethylases. Nat. Struct. Mol. Biol. 2010;17:38–43. doi: 10.1038/nsmb.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fortschegger K., de Graaf P., Outchkourov N.S., van Schaik F.M.A., Timmers H.T.M., Shiekhattar R. PHF8 targets histone methylation and RNA polymerase II to activate transcription. Mol. Cell Biol. 2010;30:3286–3298. doi: 10.1128/MCB.01520-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feng W., Yonezawa M., Ye J., Jenuwein T., Grummt I. PHF8 activates transcription of rRNA genes through H3K4me3 binding and H3K9me1/2 demethylation. Nat. Struct. Mol. Biol. 2010;17:445–451. doi: 10.1038/nsmb.1778. [DOI] [PubMed] [Google Scholar]
- 17.Yu L., Wang Y., Huang S., et al. Structural insights into a novel histone demethylase PHF8. Cell Res. 2010;20:166–173. doi: 10.1038/cr.2010.8. [DOI] [PubMed] [Google Scholar]
- 18.Lv Y., Shi Y., Han Q., Dai G. Histone demethylase PHF8 accelerates the progression of colorectal cancer and can be regulated by miR-488 in vitro. Mol. Med. Rep. 2017;16:4437–4444. doi: 10.3892/mmr.2017.7130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gu L., Hitzel J., Moll F., et al. The histone demethylase PHF8 is essential for endothelial cell migration. PLoS One. 2016;11:1–15. doi: 10.1371/journal.pone.0146645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walsh R.M., Shen E.Y., Bagot R.C., et al. Phf8 loss confers resistance to depression-like and anxiety-like behaviors in mice. Nat. Commun. 2017;8:1–11. doi: 10.1038/ncomms15142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang Y., Hong Y.Z., Bai H.J., et al. Plant homeo domain finger protein 8 regulates mesodermal and cardiac differentiation of embryonic stem cells through mediating the histone demethylation of pmaip1. Stem Cell. 2016;34:1527–1540. doi: 10.1002/stem.2333. [DOI] [PubMed] [Google Scholar]
- 22.Riveiro A.R., Mariani L., Malmberg E., et al. JMJD-1.2/PHF8 controls axon guidance by regulating Hedgehog-like signaling. Development. 2017;144:856–865. doi: 10.1242/dev.142695. [DOI] [PubMed] [Google Scholar]
- 23.Chen X., Wang S., Zhou Y., et al. Phf8 histone demethylase deficiency causes cognitive impairments through the mTOR pathway. Nat. Commun. 2018;9:114. doi: 10.1038/s41467-017-02531-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iacobucci S., Padilla N., Gabrielli M., et al. The histone demethylase PHF8 regulates astrocyte differentiation and function. Development. 2021;148:1–14. doi: 10.1242/dev.194951. [DOI] [PubMed] [Google Scholar]
- 25.Qiu J., Shi G., Jia Y., et al. The X-linked mental retardation gene PHF8 is a histone demethylase involved in neuronal differentiation. Cell Res. 2010;20:908–918. doi: 10.1038/cr.2010.81. [DOI] [PubMed] [Google Scholar]
- 26.Siderius L.E., Hamel B.C.J., van Bokhoven H., et al. X-linked mental retardation associated with cleft lip/palate maps to Xp11.3-q21.3. Am. J. Med. Genet. 1999;85:216–220. doi: 10.1002/(SICI)1096-8628(19990730)85:3<216. [DOI] [PubMed] [Google Scholar]
- 27.Koivisto A.M., Ala-Mello S., Lemmelä S., Komu H.A., Rautio J., Järvelä I. Screening of mutations in the PHF8 gene and identification of a novel mutation in a Finnish family with XLMR and cleft lip/cleft palate. Clin. Genet. 2007;72:145–149. doi: 10.1111/j.1399-0004.2007.00836.x. [DOI] [PubMed] [Google Scholar]
- 28.Philippakis A.A., Azzariti D.R., Beltran S., et al. The matchmaker Exchange: a platform for rare disease gene discovery. Hum. Mutat. 2015;36:915–921. doi: 10.1002/humu.22858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sobreira N., Schiettecatte F., Valle D., Hamosh A. GeneMatcher: a matching tool for connecting investigators with an interest in the same gene. Hum. Mutat. 2015;36:928–930. doi: 10.1002/humu.22769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sobreira N., Arachchi H., Buske O.J., et al. Matchmaker exchange. Curr. Protoc. Hum. Genet. 2017;95:9.31.1–9.31.15. doi: 10.1002/cphg.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fitzgerald T.W., Gerety S.S., Jones W.D., et al. Large-scale discovery of novel genetic causes of developmental disorders. Nature. 2015;519:223–228. doi: 10.1038/nature14135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Firth H.V., Richards S.M., Bevan A.P., et al. DECIPHER: database of chromosomal imbalance and phenotype in humans using ensembl resources. Am. J. Hum. Genet. 2009;84:524–533. doi: 10.1016/j.ajhg.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Retterer K., Juusola J., Cho M.T., et al. Clinical application of whole-exome sequencing across clinical indications. Genet. Med. 2016;18:696–704. doi: 10.1038/gim.2015.148. [DOI] [PubMed] [Google Scholar]
- 34.Posey J.E., Harel T., Liu P., et al. Resolution of disease phenotypes resulting from multilocus genomic variation. N. Engl. J. Med. 2017;376:21–31. doi: 10.1056/nejmoa1516767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Redin C., Gérard B., Lauer J., et al. Efficient strategy for the molecular diagnosis of intellectual disability using targeted high-throughput sequencing. J. Med. Genet. 2014;51:724–736. doi: 10.1136/jmedgenet-2014-102554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Loenarz C., Ge W., Coleman M.L., et al. PHF8, a gene associated with cleft lip/palate and mental retardation, encodes for an Nε-dimethyl lysine demethylase. Hum. Mol. Genet. 2010;19:217–222. doi: 10.1093/hmg/ddp480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nava C., Lamari F., Héron D., et al. Analysis of the chromosome X exome in patients with autism spectrum disorders identified novel candidate genes, including TMLHE. Transl. Psychiatry. 2012;2:1–12. doi: 10.1038/tp.2012.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.gnomAD. https://gnomad.broadinstitute.org/variant/X-53966800-TGAG-T?dataset=gnomad_r2_1
- 39.ClinVar. https://www.ncbi.nlm.nih.gov/clinvar/variation/445714/
- 40.Qiao Y., Liu X., Harvard C., et al. Autism-associated familial microdeletion of Xp11.22. Clin. Genet. 2008;74:134–144. doi: 10.1111/j.1399-0004.2008.01028.x. [DOI] [PubMed] [Google Scholar]
- 41.De Wolf V., Crepel A., Schuit F., et al. A complex Xp11.22 deletion in a patient with syndromic autism: exploration of FAM120C as a positional candidate gene for autism. Am. J. Med. Genet. Part. A. 2014;164(12):3035–3041. doi: 10.1002/ajmg.a.36752. [DOI] [PubMed] [Google Scholar]
- 42.Raynaud M., Gendrot C., Dessay B., et al. X-linked mental retardation with neonatal hypotonia in a French family (MRX15): gene assignment to Xp11.22-Xp21.1. Am. J. Med. Genet. 1996;64:97–106. doi: 10.1002/(SICI)1096-8628(19960712)64:1<97. [DOI] [PubMed] [Google Scholar]
- 43.Aref-Eshghi E., Rodenhiser D.I., Schenkel L.C., et al. Genomic DNA methylation signatures enable concurrent diagnosis and clinical genetic variant classification in neurodevelopmental syndromes. Am. J. Hum. Genet. 2018;102:156–174. doi: 10.1016/j.ajhg.2017.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schenkel L.C., Aref-Eshghi E., Skinner C., et al. Peripheral blood epi-signature of Claes-Jensen syndrome enables sensitive and specific identification of patients and healthy carriers with pathogenic mutations in KDM5C. Clin. Epigenet. 2018;10:1–11. doi: 10.1186/s13148-018-0453-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aref-Eshghi E., Kerkhof J., Pedro V.P., et al. Evaluation of DNA methylation episignatures for diagnosis and phenotype correlations in 42 mendelian neurodevelopmental disorders. Am. J. Hum. Genet. 2020;106:356–370. doi: 10.1016/j.ajhg.2020.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The published article includes a supplemental table with the full clinical dataset generated and analyzed during the study. All exome sequencing data are not publicly available due to privacy or ethical restrictions.