Abstract
Background
Previous studies have recognized temporal muscle thickness (TMT) as a prognostic marker in glioblastoma, but clinical implementation is hampered due to studies’ heterogeneity and lack of established cutoff values. The aim of this study was to assess the validity of recent proposed sex-specific TMT cutoff values in a real-world population of genotyped primary glioblastoma patients.
Methods
We measured TMT in preoperative MR images of 328 patients. Sex-specific TMT cutoff values were used to divide patients into “at risk of sarcopenia” or “normal muscle status”. Kaplan-Meier analyses and stepwise multivariate Cox-Regression analyses were used to assess the association with overall survival (OS) and progression-free survival (PFS). The association with occurrence of complications and discontinuation of glioblastoma treatment was investigated using odds ratios (OR).
Results
Patients at risk of sarcopenia had a significantly higher risk of progression and death than patients with normal muscle status, which remained significant in the multivariate analyses (OS HR = 1.437; 95%CI: 1.046–1.973; P = .025 and PFS HR = 1.453; 95%CI: 1.037–2.036; P = .030). Patients at risk of sarcopenia also had a significantly higher risk of early discontinuation of treatment (OR = 2.45; 95%CI: 1.011–5.952; P = .042) and a significantly lower chance of receiving second-line treatment (OR = 0.23; 95%CI: 0.09–0.60; P = .001). There was no association with the occurrence of complications.
Conclusions
Our study confirms external validity of the use of proposed sex-specific TMT cutoff values as an independent prognostic marker in newly diagnosed glioblastoma patients. This simple, noninvasive marker could improve patient counseling and aid in treatment decision processes or trial stratification.
Keywords: glioblastoma, imaging marker, sarcopenia, survival, temporal muscle thickness
Key Points.
TMT is an independent prognostic marker in newly diagnosed glioblastoma patients.
With sex-specific TMT cutoff values, patients at risk of sarcopenia can be identified.
Importance of the Study.
Temporal muscle thickness has been shown to be a surrogate marker for sarcopenia, which is known to have a negative impact on the outcome of cancer patients. Although others have investigated TMT as objective prognostic marker in glioblastoma, clinical implementation of TMT in routine clinical setting has been limited so far due to inconsistent cutoff values, varying study sample sizes, missing molecular tumor data, and low percentages of bilateral measurements. By using sex-specific cutoff values based on a normative reference population, a recent study in a large trial population paved the way to facilitate and implement TMT measurement into clinical workflow for assessment of sarcopenia in glioblastoma patients. Our study confirms its applicability in a nonstudy population, which is important for external validity since clinical trial patients often significantly differ from real-world patients. This simple, noninvasive marker could improve patient counseling and aid in treatment decision processes or trial stratification.
Glioblastoma is the most common primary brain cancer in adults.1 Despite surgical resection, chemotherapy, and radiotherapy, median survival after diagnosis is only 15 months.2 Half of the patients do not complete the optimal treatment regimen due to disease progression or toxicities. Patients’ frailty is a key factor negatively influencing survival, alongside with older age, less extensive tumor resection, corticosteroid treatment at baseline and the absence of promoter methylation of the O6-methylguanine-DNA-methyltransferase (MGMT) gene.3 Although standard instruments to assess patients’ clinical condition such as the Karnofsky Performance Status (KPS) and Eastern Cooperative Oncology Group (ECOG) Performance Status are simple and useful, they are subject to bias and limitations in intra- or interobserver variability.4
Sarcopenia is a key feature of cancer-associated cachexia, and an independent marker for clinical outcomes including postoperative complications, chemotherapy dose-limiting toxicity, and overall survival (OS).5–7 Temporal muscle thickness (TMT) might serve as a marker for patients’ frailty, since it is proven to correlate with lumbar skeletal muscle mass,8 which in turn, is a surrogate marker for sarcopenia in several solid cancers.9 TMT may thus be a novel imaging marker for patient frailty and survival prognosis, which could provide a more informed pretreatment management plan and improve patient counseling early in the diagnostic process. For example, if clinically validated, TMT measurement can be a practical and objective tool to assess frailty and suitability for more aggressive versus reduced-intensity therapy, such as shorter courses of radiotherapy or hypofractionated chemoradiotherapy instead of the standard Stupp protocol.2,10 Since Furtner et al. reported that a reduced TMT is negatively associated with survival in recurrent glioblastoma,11 several other groups have investigated TMT as an objectively assessable prognostic imaging marker in glioblastoma.12–17 However, implementation of TMT measurement in routine clinical setting has been limited so far. Studies have solely shown that there is a negative association between reduced TMT and survival, but with lack of established cutoff values, varying study sample size, high percentages of missing molecular tumor data, and low percentages of bilateral measurements due to previous tumor surgery.11–17 In a powerful attempt to facilitate implementation of TMT measurement in the clinical setting, a very recent hallmark study of Furtner et al. on behalf of the EORTC Brain tumor group,18 proposed and used sex-specific TMT cutoff values 2.5 standard deviation below a normative reference population.19 This is in line with a recommendation of the European Working Group on Sarcopenia in Older People (EWGSOP).20 In a large sample of 755 newly diagnosed glioblastoma patients enrolled in two clinical trials they found that patients at risk of sarcopenia at baseline, defined as TMT below the sex-specific cutoff, had a significantly higher risk of progression and death than patients with normal muscle status.18 In addition, the extent of TMT loss over time showed a significant inverse correlation with median OS time in patients at risk of sarcopenia. However, solely patients with an ECOG score of 0-1 (fully active or only restricted in physically strenuous activity) were included and often clinical trial participants significantly differ from real-world patients which could limit external validity.21,22
The aim of this study was to assess the validity of the proposed sex-specific cutoff values in a large sample of a real-world population of genotyped primary glioblastoma patients. In addition, we investigated if there is an association between TMT and the occurrence of complications or discontinuation of glioblastoma treatment.
Materials and Methods
Patient Selection
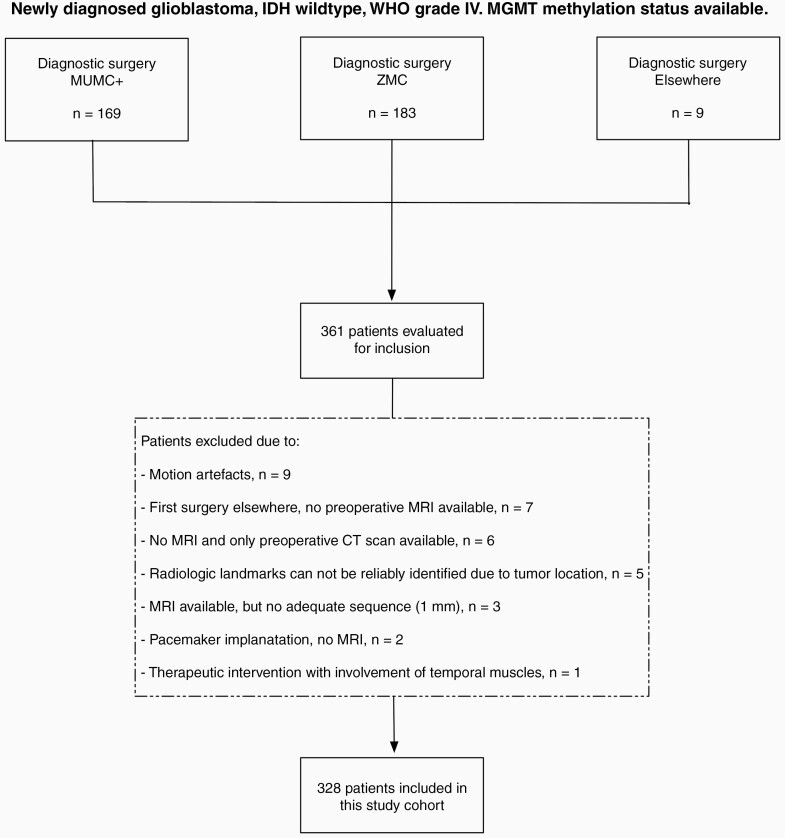
A Dutch multicenter retrospective study was performed at the Maastricht University Medical Center + (MUMC+) and Zuyderland Medical Center (ZMC). From an existing genotyped glioma database covering routine clinical diagnostics, data from 361 newly diagnosed glioblastoma patients diagnosed or treated in MUMC+ or ZMC between 2006–2020 were retrieved. We selected those cases with high-grade histology and with full information on isocitrate dehydrogenase gene 1 or 2 (IDH1/2), 1p/19q copy number status, and MGMT methylation status available. Only patients with glioblastoma (WHO grade 4), IDH wildtype, both MGMT hypermethylated or unmethylated were selected. Because of the influence of surgery on the TMT measurements, we decided to include only patients with adequate preoperative brain Magnetic Resonance (MR) imaging for this validation study. The specifics of the case selection are shown in the flowchart (Figure 1).
Figure 1.
Flowchart of the selection process.
Patient characteristics and clinical data were collected from medical records. The following characteristics and data were used for this study: gender, age at diagnosis, ECOG Performance Status at baseline (score 0–4), corticosteroid use at baseline (yes/no), type of surgery (biopsy only or resection), initial glioblastoma treatment and second-line treatment (type of treatment, duration of treatment, early discontinuation of Stupp2 treatment yes or no), the occurrence of clinically relevant thrombopenia during chemotherapy treatment (defined as platelet count levels <100.0 x 10e9/L), the occurrence of venous thromboembolism during treatment or follow up, any infection requiring treatment during the first year after diagnosis, hospital admissions during the first year after diagnosis, OS and progression-free survival (PFS). OS was defined as time from date of diagnosis (set as date of diagnostic surgery) to date of death or date of last follow up for patients still alive. PFS was defined as time from date of diagnosis to date of disease progression or date of death from any cause, whichever occurred first, or date of last follow up for patients still alive without disease progression. Disease progression was based on radiological and clinical evaluations rated by a multidisciplinary tumor board.
Assessment of Temporal Muscle Thickness
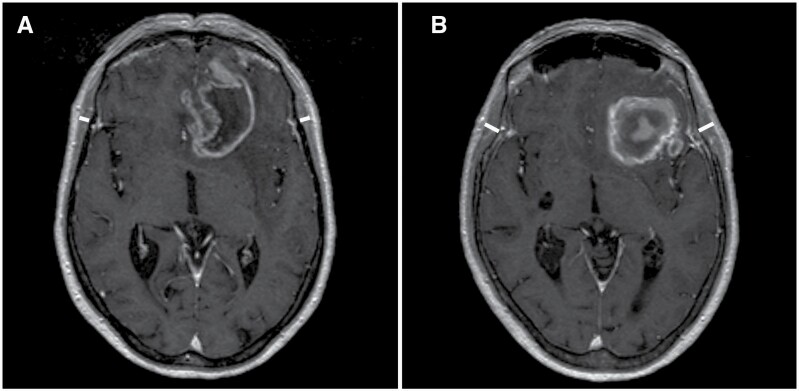
TMT measurements were performed on axial isotropic (1x1x1mm) contrast-enhanced T1-weighted MR images, which were routinely performed for neurosurgery navigation on the same day or one day before surgery. In 33 patients (9.1%) these measurements were not possible, due to varying reasons (See Figure 1). In the remaining 328 patients, the TMT was measured perpendicular to the long axis of the temporal muscle at the level of the Sylvian fissure (anterior-posterior hallmark) and the orbital roof (craniocaudal landmark). The MR plane was oriented parallel to the anterior commissure-posterior commissure line (Figure 2).23 The measurements were assessed on the left and right sides separately and were further summed and divided by 2, resulting in a mean TMT per patient. In case of unilateral oppression of the Sylvian fissure, for example, by tumor or edema, the measurement was taken parallel to the opposite side. MR scans were assessed by two independent reviewers (MB and RB), trained by a board-certified neuroradiologist (AP) prior to the study. Preoperative images from ZMC were reviewed by RB (n = 164) and the remaining preoperative MR images by MB (n = 164). Both were blinded to patient characteristics and outcome measures. When there was doubt about correct measurements, this was reviewed and resolved by AP (n = 3).
Figure 2.
Axial T1-weighted contrast-enhanced cranial MR images representing TMT assessment (A) in a 70-year-old female patient at risk of sarcopenia (mean TMT 4.9 mm) with an overall survival of 4.2 months in comparison to (B) a 70-year-old female patient with normal muscle status (mean TMT 7.9 mm) with an overall survival of 16.9 months.
We used the proposed sex-specific TMT cutoff value from Furtner et al., set at ≤6.3 mm for men and ≤5.2 mm for women, to divide our patients into two groups.18 These cutoff values are previously defined as 2.5 standard deviation (SD) below the mean TMT value of a normative reference population, to identify patients at risk of sarcopenia.19 Patients with TMT values below or equal to the sex-specific cutoff were classified as patients “at risk of sarcopenia”, whereas patients with TMT values above the sex-specific cutoff were classified as patients with “normal muscle status”.
This study was approved by the institutional review boards (reference number: METC2019-1396) of the participating centers and conducted in accordance with the Helsinki declaration and national legislation.
Statistical Analysis
Statistical analysis was performed using SPSS version 27.0 (IBM Corporation, Armonk, NY, USA). A P-value of < .05 was considered statistically significant. Descriptive statistics were calculated for all 361 patients: the 33 excluded patients, the 51 patients at risk of sarcopenia, and the 277 patients with normal muscle status. The statistical significance of differences between these groups was assessed using the Pearson Chi-Square test or Fisher’s Exact Test (whichever was appropriate according to the number of cases) for categorical variables, and the Mann-Whitney U test for continuous variables. Kaplan-Meier analysis was used to calculate the OS curve, and the log-rank test was applied to investigate differences in OS between patients at risk of sarcopenia and patient with normal muscle status. This procedure was repeated for PFS. Next, univariate Cox-Regression analysis was used to calculate the hazard ratio (HR) for the association between TMT below or equal to the sex-specific cutoff and OS. Thereafter, multivariate Cox-Regression analysis was conducted using an automated stepwise forward selection procedure with a threshold P-value of .05 to asses independent prognostic variables affecting overall survival in our patients. The following variables, known to affect survival in glioblastoma patients, were included: age at diagnosis, gender, preoperative TMT, MGMT methylation status, surgery type, glioblastoma treatment, corticosteroid use at baseline, and ECOG Performance Score. To assess the association between TMT and PFS, the abovementioned univariate and multivariate Cox-Regression were repeated for PFS. Given the detrimental effect of increasing age on survival in patients with glioblastoma and the occurrence of primary sarcopenia during aging, we additionally investigated age-related differences in the association between TMT and survival. We compared patients of 70 years of age or older at diagnosis (“elderly group”) with patients younger than 70 years of age at diagnosis (“younger group”). The abovementioned procedures were repeated for both age groups independently.
Results
Cohort Characteristics
The final cohort for this study consisted of 328 patients (Figure 1). Their characteristics are listed in Table 1. Characteristics did not significantly differ between in- and excluded patients, except for glioblastoma treatment (Supplementary Table 1). At the time of analysis 309 patients (94.2%) were deceased. All patients had measurements for both right and left TMT. The mean TMT value of all patients was 7.6 mm (SD1.8 mm). In male patients, the mean TMT was significantly higher than in female patients (8.3 mm, SD1.7 mm versus 6.6 mm, SD1.6 mm, P = .000). Sex-specific TMT cutoff values were used to separate the cohort into patients at risk of sarcopenia (n = 51, 15.5%) and patients with normal muscle status (n = 277, 84.5%). The characteristics of these two groups are shown in Table 2, revealing that there were significantly more female patients in the at risk of sarcopenia group and that patients at risk were significantly older and had lower OS and PFS. These differences were accounted for in the multivariate Cox-Regression analysis.
Table 1.
Patient Characteristics
| Variables | Study cohort (n = 328) |
|---|---|
| Gender, n (%) Male Female |
205 (62.5) 123 (37.5) |
| Age at diagnosis, years Mean (SD) Median (range) |
63.1 (11.0) 64.7 (21.0–88.0) |
| Type of surgery, n (%) Biopsy Resection |
152 (46.3) 176 (53.7) |
| MGMT hypermethylation, n (%) Yes No |
123 (37.5) 205 (62.5) |
| ECOG score at baseline, n (%) 0 or 1 ≥ 2 Missing |
235 (71.6) 91 (27.7) 2 (0.6) |
| Corticosteroid use at baseline, n (%) Yes No |
239 (72.9) 89 (27.1) |
| Glioblastoma treatment, n (%) Stupp protocol completed Stupp protocol not completed Radiotherapy only Chemotherapy only Chemoradiation, 12 cycles No treatment Missing |
113 (34.5) 108 (32.9) 33 (10.1) 21 (6.4) 2 (0.6) 46 (14.0) 5 (1.5) |
| Overall survival, months All patients, mean (SD) Treated patients (all treatments), mean (SD) No treatment, mean (SD) |
12.0 (10.4) 13.7 (10.2) 2.0 (1.7) |
| Progression-free survival, months Mean (SD) |
8.5 (7.2) |
n, number; SD, standard deviation; MGMT, O6-methylguanine-DNA-methyltransferase, ECOG, Eastern Cooperative Oncology Group Performance Status
Table 2.
Characteristics of Patients at Risk of Sarcopenia Versus Patients With Normal Muscle Status
| Variables | Patients at risk of sarcopenia (n = 51) | Patients with normal muscle status (n = 277) | P-value |
|---|---|---|---|
| Gender, n (%) Male Female |
25 (49.0) 26 (51.0) |
180 (65.0) 97 (35.0) |
.030 |
| Age at diagnosis, years Mean (SD) Median (range) |
67.1 (9.0) 66.8 (48.9-85.3) |
62.3 (11.2) 64.3 (21.0-88.0) |
.008 |
| Type of surgery, n (%) Biopsy Resection |
28 (54.9) 23 (45.1) |
124 (44.8) 153 (55.2) |
.182 |
| MGMT hypermethylation, n (%) Yes No |
16 (31.4) 35 (68.6) |
107 (38.6) 170 (61.4) |
.325 |
| ECOG score at baseline, n (%) 0 or 1 ≥ 2 Missing |
34 (66.7) 16 (31.4) 1 (1.9) |
201 (72.6) 75 (27.1) 1 (0.3) |
.484 |
| Corticosteroid use at baseline, n (%) Yes No |
39 (76.5) 12 (23.5) |
200 (72.2) 77 (27.8) |
.529 |
| Temporal muscle thickness at baseline, millimeter Mean (SD) Median (range) |
4.9 (0.8) 4.9 (3.4–6.3) |
8.1 (1.5) 8.0 (5.3–12.4) |
.000 |
| Overall survival*, months Mean (SD) |
9.4 (10.0) | 12.4 (10.4) | .006 |
| Progression-free survival*, months Mean (SD) |
6.7 (8.2) | 8.8 (7.0) | .007 |
* Both treated and untreated patients
n, number; SD, standard deviation; MGMT, O6-methylguanine-DNA-methyltransferase; ECOG, Eastern Cooperative Oncology Group Performance Status
Association Between TMT and Overall Survival
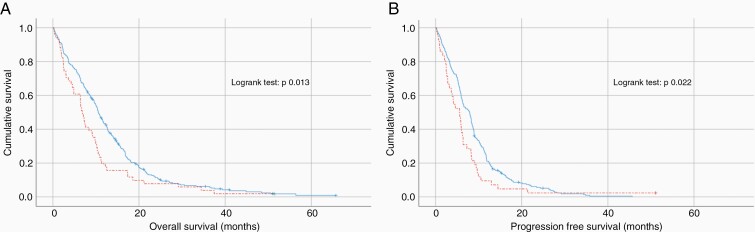
Overall survival in patients at risk of sarcopenia was significantly lower compared to patients with normal muscle status (9.4 months, SD10.0 versus 12.4 months, SD10.4, P = .006). Kaplan-Meier survival curves for OS of patients at risk of sarcopenia (mean TMT equal to or below sex-specific cutoffs) and patients with normal muscle status (mean TMT above the sex-specific cutoffs) are shown in Figure 3A, revealing a significant shorter OS in patients at risk of sarcopenia (log-rank test P = .013). Univariate Cox-Regression analysis showed a HR of 1.465 (95% CI 1.081–1.987; P = .014 for patients at risk of sarcopenia. Stepwise multivariate Cox-Regression analysis in 322 patients (6 patients had missing values for treatment and or ECOG score, including 2 at risk of sarcopenia) showed that TMT remained an independent predictor of overall survival (HR 1.437; 95% CI 1.046–1.974; P = .025). The significant independent explanatory variables in the final model after 5 steps are listed in Table 3.
Figure 3.
Kaplan-Meier survival curves for OS (A) and PFS (B), of patients at risk of sarcopenia (mean TMT equal or below the sex-specific cutoffs, dashed line), and patients with normal muscle status (mean TMT above the sex-specific cutoffs, straight line).
Table 3.
Significant Variables in the Final Model of the Stepwise Forward Multivariate Cox-Regression Analyses for Overall Survival (OS) and Progression-free Survival (PFS)
| HR | 95% CI | P-value | |||
|---|---|---|---|---|---|
| Lower bound | Upper bound | ||||
|
OS
(N = 322) |
No glioblastoma treatment | 10.463 | 6.646 | 16.473 | .000 |
| ECOG score ≥ 2 at baseline |
1.791 | 1.322 | 2.426 | .000 | |
| Only biopsy at (diagnostic) surgery | 1.526 | 1.182 | 1.970 | .001 | |
| No MGMT hypermethylation | 1.384 | 1.084 | 1.767 | .009 | |
| At risk of sarcopenia at baseline* | 1.437 | 1.046 | 1.973 | .025 | |
|
PFS
(N = 304) |
No glioblastoma treatment | 8.346 | 5.445 | 12.793 | .000 |
| ECOG score ≥ 2 at baseline |
1.567 | 1.141 | 2.152 | .005 | |
| No MGMT hypermethylation | 1.445 | 1.129 | 1.849 | .003 | |
| At risk of sarcopenia at baseline* | 1.453 | 1.037 | 2.036 | .030 |
* Temporal muscle thickness equal to or below the sex-specific cutoff value
N, number; CI, confidence interval; HR, hazard ratio; ECOG, Eastern Cooperative Oncology Group Performance Status; MGMT, O6-methylguanine-DNA-methyltransferase
Association Between TMT and Progression-Free Survival
In 23 patients PFS status was unknown, leaving 305 patients for this analysis (42 at risk of sarcopenia and 263 with normal muscle status). Progression-free survival in patients at risk of sarcopenia was significantly lower compared to patients with normal muscle status (6.7 months, SD8.2 versus 8.8 months, SD7.0, P = .007). Kaplan-Meier survival curves for PFS of patients at risk of sarcopenia and patients with normal muscle status are shown in Figure 3B, revealing a significant shorter PFS in patients at risk of sarcopenia (log-rank test P = .022). Univariate Cox-Regression analysis showed a HR of 1.472 (95% CI 1.055–2.054; P = .023) for patients at risk of sarcopenia. Stepwise multivariate Cox-Regression analysis in 304 patients (1 patient with normal muscle status had a missing value for ECOG score) showed that TMT remained an independent predictor of PFS (HR 1.453; 95% CI 1.037–2.036; P = .030). The significant independent explanatory variables in the final model after 4 steps are listed in Table 3.
Age-Related Differences in the Association Between TMT and Survival
The characteristics of both age groups are shown in Supplementary Table 2, 237 patients were at diagnosis younger than 70 years of age and 91 older. Patients in the elderly group were less likely to be male, had more often diagnostic biopsies instead of resections, and received treatment less often. As expected, mean OS and PFS were shorter in the elderly group (8.4 months SD8 versus 13.3 months, SD10.9, P = .000 and 6.4 months, SD8.0 versus 9.3 months, SD 7.2, P = .000, respectively), with a significant lower TMT (6.9 mm, SD1.8 mm versus. 7.9 mm, SD1.8 mm, P = .000). In the elderly group, more patients were at risk of sarcopenia (24%) compared to the younger group (12%). In the younger age group, but not in the elderly group, OS and PFS in patients at risk of sarcopenia were significantly lower compared to patients with normal muscle status. Kaplan-Meier survival curves are shown in Supplementary Figure 1. In the younger age group, univariate Cox-Regression analysis for OS showed a HR of 1.677 (95% CI 1.131–2.486; P = .010) for patients at risk of sarcopenia, and TMT remained an independent predictor of OS in the Stepwise multivariate Cox-Regression analysis (HR 1.77; 95% CI 1.196–2.708; P = .005). In the elderly group, no significant differences in OS and PFS in patients at risk of sarcopenia versus patients with normal muscle status were found (OS 9.2 months, SD12.2 versus 8.2 months, SD 6.2, P = .481, PFS 7.4 months, SD11.9 versus 6.1 months SD 4.6, P = .391). Details of the Cox-Regression analysis are listed in Supplementary Table 3.
Association Between TMT and Occurrence of Complications
An overview of the most important complications is given in Supplementary Table 4. As for some patients, it was not clear if they had or had not experienced a specific complication, they were excluded from the analysis on that complication. Obviously, the occurrence of thrombopenia during chemotherapy treatment could only be analyzed in the subpopulation receiving chemotherapy. The most common complication was one or more hospital admissions during the first year after diagnosis (43.6%), followed by clinically relevant thrombopenia during chemotherapy treatment (33.3%), any infection requiring treatment during the first year after diagnosis (23.0%), and the development of venous thromboembolism during treatment or follow up (15.2%). The comparison of patients at risk of sarcopenia with the patients with normal muscle status regarding the occurrence of one or a combination of complications failed to show any significant association (Supplementary Table 4).
Association Between TMT and Discontinuation of Glioblastoma Treatment
Of the 221 patients starting Stupp protocol, almost half (48.9%) did not complete treatment due to disease progression or toxicities. Comparison of patients at risk of sarcopenia (17/25 = 68.0% discontinued) with the patients with normal muscle status (91/196 = 46.4% discontinued) revealed that the patients at risk of sarcopenia had a significantly higher risk of early discontinuation of Stupp treatment (OR 2.45; 95% CI 1.011–5.952; P = .042).
Association Between TMT and Start of Second-Line Treatment at Recurrence
Of 315 patients with complete data on this topic, 98 (31.1%) received a second-line therapy at disease progression or recurrence. Comparison of patients at risk of sarcopenia (5/46 = 10.9% received second-line treatment) with the patients with normal muscle status (93/269 = 34.6% received second-line treatment) revealed that the patients at risk of sarcopenia had a significantly lower chance of receiving a second-line treatment at disease progression or recurrence (OR 0.23; 95% CI: 0.09–0.60, P = .001).
Discussion
Our results confirm the prognostic role of TMT as an independent predictor of survival in a real-world population of genotyped primary glioblastoma patients, using proposed sex-specific cutoff values. In addition, we found that glioblastoma patients at risk of sarcopenia had a significantly higher risk of early discontinuation of Stupp treatment and also received a second-line treatment at recurrence significantly less frequent.
Although previous studies have recognized that TMT is associated with the outcome of patients in glioblastoma11–17 as well as other diseases,24 clinical implementation was hampered due to the lack of established cutoff values and shortcomings in the previous studies. By using sex-specific cutoff values based on a normative reference population and recommendations of the EWGSOP, Furtner et al. paved the way to facilitate and implement TMT measurement into clinical workflow for assessment of sarcopenia in glioblastoma patients.18 Our study confirms its applicability in a nonstudy population, which is important for external validity since clinical trial patients are selected, for example, upon their performance status and often significantly differ from real-world patients.21,22 In our study, patients at risk of sarcopenia had a significant shorter OS compared to patients with a normal muscle status, independent of other well-known prognostic factors. This negative association was also true for PFS. Mean OS of the whole study population was 12 months (SD 10.4), which seems rather low compared to data from prospective clinical trials.2,18 This is presumably caused by the real-world setting of our study in which we also included patients with low performance score (28% ECOG ≥ 2), typically excluded from clinical trials. Likely, this led to a relatively high percentage (14%) of patients not receiving any treatment after surgery due to clinical deterioration, greatly diminishing mean survival rates. However, we think our study group is representative of a high-grade glioma population. For example, when we compare the subset of patients who started standard chemoradiotherapy treatment protocol (n = 229), mean OS of this subgroup was 14.9 months (SD 10.8), 15.2 months in patients with normal muscle status, and 10 months in patients at risk of sarcopenia. These survival rates are comparable to OS times found in prospective clinical trials.2,18 An interesting finding is that age seems to influence the prognostic impact of TMT in our study population. In elderly patients, defined as 70 years of age or above at time of diagnosis, TMT was not a significant prognostic factor for survival, as it was for younger patients. However, some caution should be taken in interpreting these results, since some bias could be introduced by the small sample size of the elderly group (n = 91). A possible explanation for the lack of association between survival and TMT in the elderly group could be the high percentage of elderly patients who underwent biopsy (64%) or didn’t receive any treatment (24%). Especially the latter has such a detrimental effect on survival, which could overshadow other variables with smaller magnitude effects on survival such as TMT. Additionally, primary sarcopenia progresses with age, which is reflected by the lower mean TMT in the elderly group, making the intra-group differences smaller. This may also affect the detectability of significant effects on survival in already a small subgroup. Prospective studies with larger samples of elderly patients are needed to confirm the clinical value of TMT measurement as a prognostic marker also in the elderly population.
Since all patients in our study had, besides MGMT methylation status, a known IDH status, compared to 45% in the analysis of Furtner et al. we were able to integrate the molecular profile of all patients in the analysis. By doing so, we were able to confirm that TMT is independent of established prognostic molecular markers such as MGMT methylation and IDH mutation status. Another important difference between our study and the study by Furtner et al. is that we also included patients with an ECOG score of 2 or more (28%). Besides that, 14% of our patients eventually received no treatment, mostly due to deterioration shortly after surgery. In addition, we used preoperative MR images, making bilateral measurements possible, whereas Furtner et al. used postoperative images before initiation of study treatment. This could be an explanation for the slightly higher mean TMT found in our study compared to Furtner et al. (7.6 mm vs 6.7 mm), since we observed a difference between mean left TMT (8.0 mm) and mean right TMT (7.2 mm). Another explanation could be the higher percentage of males in our study (63% vs. 55%), with males having a higher mean TMT in general compared to females. Females seem to be at higher risk for sarcopenia and with the higher mean TMT in our study, this might elucidate the observed lower percentage of patients at risk of sarcopenia in our study (16% vs. 32%). However, despite these differences, we were able to replicate the findings of Furtner et al. We believe that this makes the proposed cutoff values even more robust and also applicable in a real-world clinical setting.
Sarcopenia is increasingly recognized as an important topic in medical research, but as with TMT, many research findings have not yet been translated into clinical practice.20 Sarcopenia’s etymological origins are two Greek words: sarx for flesh and penia for reduced or deficiency. It can be classified as primary (age-related) or secondary sarcopenia (disease-related, such as cancer).25 Sarcopenia has been mostly studied in patients with solid tumors and correlated with mortality, complications of cancer surgery, chemotherapy toxicity,5–7 and lowered quality of life.26 Computed tomography (CT) analysis of the muscle composition at the lumbar 3rd vertebrae has been shown to give practical and precise measures of body composition, correlated with prognosis.27 A previous study by Leitner et al. showed a high correlation between lumbar skeletal muscle area and TMT.8 This supports TMT as an adequate surrogate marker for sarcopenia in glioblastoma patients, where abdominal CT is not routinely performed. By identifying patients at risk of sarcopenia, appropriate patient education and interventions could be implemented. For example, for the management of sarcopenia there is a strong recommendation that individuals with sarcopenia should be enrolled in a resistance exercise program. Although evidence is less strong, it is also advisable to stimulate the use of a protein-rich diet (1 to 1.5 g/day) or protein supplementation.25 Stimulating exercise and giving appropriate nutritional advice in patients at risk of sarcopenia based on TMT measurement, might improve the percentage of patients completing treatment or receiving a second-line treatment and ultimately also improves survival. In addition, TMT measurement could aid in treatment selection, for example, more aggressive versus reduced-intensity therapies, and patient stratification in clinical trials as an objectively assessable parameter in regard to the patient’s physical condition. However, prospective clinical validation of our results is necessary to fast-forward its implementation in daily decision making.
Our study has some limitations. First, muscle thickness was assessed only once by a single observer. To increase concordance between measurement techniques, both observers were trained by an experienced neuroradiologist. The fact that there was no significant difference in mean TMT between the two assessors, presumably reflects adequate training. In addition, we relied on previously published data that showed a high inter-rater and intra-rater agreement of TMT assessment.8,19 To further reduce the risk of bias, the observers were blinded to all clinical and demographic data. All patients had bilateral measurements, limiting possible measurement errors compared to unilateral measurements in previous studies. In our experience, the measurement of TMT is a fast technique that can be trained in an efficient and nontime-consuming manner, smoothing its more widespread implementation in clinical care.
An additional challenge is the relatively small size of the temporal muscle, making some measurement inaccuracies inevitable. With bilateral measurements, these differences are likely diminished and with the rise of automatic segregation programs, inaccuracies might be overcome in the future. A second possible limitation is that we did not have full information on ethnicity of the patients, although most patients were Caucasian. The sex-specific cutoffs we used are based on normative reference values of a healthy Caucasian population. Additional studies are needed with patients of other ethnicities to confirm the use of the TMT cutoffs in a more worldwide setting. Lastly, due to the retrospective design, muscular strength or biochemical markers could not be considered. In addition to unraveling the influence of glioblastoma-related catabolic, paraneoplastic, or inflammatory processes, it is important for clinical implementation to confirm that patients labeled at risk for sarcopenia based on TMT measurements eventually develop sarcopenia throughout the disease and treatment course. Although Furtner et al.,18 showed that the extent of TMT loss over time showed an inverse correlation with OS times in patients at risk for sarcopenia at baseline but not in patients with normal muscle status at baseline, prospective studies are needed to confirm the association in time between TMT, sarcopenia and physical fitness in GBM patients. Ultimately, by doing so more efficient preventive individualized exercise or nutritional strategies can be developed for glioblastoma patients at risk of sarcopenia.
Conclusion
Our study confirms external validity of the use of proposed sex-specific TMT cutoff values in newly diagnosed adult glioblastoma patients as an independent predictor of survival in real-world patients. In addition, glioblastoma patients at risk of sarcopenia had a significantly higher risk of early discontinuation of their Stupp treatment, and also received a second-line treatment at recurrence significantly less frequently. Although further prospective studies are needed to clarify the underlying pathophysiology and optimal treatment strategy, this simple, noninvasive marker could improve patient counseling, aid in treatment decision processes, or trial stratification, and is applicable in daily routine practice.
Supplementary Material
Funding
This work was not funded.
Conflict of interest statement. Authors report no relevant disclosures.
Authorship statement. M.P.G.B. design, data collection, analysis and interpretation of data, writing first draft. R.B. data collection, analysis and interpretation of data, writing manuscript. A.C.H.W. design, interpretation of data, writing manuscript. S.M.H.H. data collection, interpretation of data, writing manuscript. R.C.O.S.P. design, interpretation of data, writing manuscript. D.B.P.E. design, interpretation of data, writing manuscript. L.A. design, interpretation of data, writing manuscript. J.B. analysis and interpretation of data, writing manuscript. E.P.M.R. analysis and interpretation of data, writing manuscript. M.V. data collection, interpretation of data, writing manuscript. M.H.M.E.A. design, interpretation of data, writing manuscript. A.H. design, interpretation of data, writing manuscript. A.A.P. design, data collection, analysis and interpretation of data, writing manuscript
References
- 1. Tan AC, Ashley DM, Lopez GY, et al. Management of glioblastoma: state of the art and future directions. CA Cancer J Clin. 2020;70(4):299–312. [DOI] [PubMed] [Google Scholar]
- 2. Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 3. Gorlia T, van den Bent MJ, Hegi ME, et al. Nomograms for predicting survival of patients with newly diagnosed glioblastoma: prognostic factor analysis of EORTC and NCIC trial 26981-22981/CE.3. Lancet Oncol. 2008;9(1):29–38. [DOI] [PubMed] [Google Scholar]
- 4. Kelly CM, Shahrokni A. Moving beyond Karnofsky and ECOG Performance Status Assessments with New Technologies. J Oncol 2016;2016:6186543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kawamura T, Makuuchi R, Tokunaga M, et al. Long-term outcomes of gastric cancer patients with preoperative sarcopenia. Ann Surg Oncol. 2018;25(6):1625–1632. [DOI] [PubMed] [Google Scholar]
- 6. Olson B, Edwards J, Stone L, et al. Association of sarcopenia with oncologic outcomes of primary surgery or definitive radiotherapy among patients with localized oropharyngeal squamous cell carcinoma. JAMA Otolaryngol Head Neck Surg. 2020;146(8):714–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Prado CM, Baracos VE, McCargar LJ, et al. Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin Cancer Res. 2009;15(8):2920–2926. [DOI] [PubMed] [Google Scholar]
- 8. Leitner J, Pelster S, Schopf V, et al. High correlation of temporal muscle thickness with lumbar skeletal muscle cross-sectional area in patients with brain metastases. PLoS One. 2018;13(11):e0207849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shachar SS, Williams GR, Muss HB, Nishijima TF. Prognostic value of sarcopenia in adults with solid tumours: a meta-analysis and systematic review. Eur J Cancer. 2016;57:58–67. [DOI] [PubMed] [Google Scholar]
- 10. Perry JR, Laperriere N, O’Callaghan CJ, et al. Short-Course Radiation plus Temozolomide in Elderly Patients with Glioblastoma. N Engl J Med. 2017;376(11):1027–1037. [DOI] [PubMed] [Google Scholar]
- 11. Furtner J, Genbrugge E, Gorlia T, et al. Temporal muscle thickness is an independent prognostic marker in patients with progressive glioblastoma: translational imaging analysis of the EORTC 26101 trial. Neuro Oncol. 2019;21(12):1587–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. An G, Ahn S, Park JS, Jeun SS, Hong YK. Association between temporal muscle thickness and clinical outcomes in patients with newly diagnosed glioblastoma. J Cancer Res Clin Oncol. 2021;147(3):901–909. [DOI] [PubMed] [Google Scholar]
- 13. Huq S, Khalafallah AM, Ruiz-Cardozo MA, et al. A novel radiographic marker of sarcopenia with prognostic value in glioblastoma. Clin Neurol Neurosurg. 2021;207:106782. [DOI] [PubMed] [Google Scholar]
- 14. Liu F, Xing D, Zha Y, et al. Predictive value of temporal muscle thickness measurements on cranial magnetic resonance images in the prognosis of patients with primary glioblastoma. Front Neurol. 2020;11:523292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Muglia R, Simonelli M, Pessina F, et al. Prognostic relevance of temporal muscle thickness as a marker of sarcopenia in patients with glioblastoma at diagnosis. Eur Radiol. 2021;31(6):4079–4086. [DOI] [PubMed] [Google Scholar]
- 16. Yan OY, Teng HB, Fu SN, Chen YZ, Liu F. Temporal muscle thickness is an independent prognostic biomarker in patients with glioma: analysis of 261 cases. Cancer Manag Res. 2021;13:6621–6632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yesil Cinkir H, Colakoglu Er H. Is temporal muscle thickness a survival predictor in newly diagnosed glioblastoma multiforme? Asia Pac J Clin Oncol. 2020;16(5):e223–e227. [DOI] [PubMed] [Google Scholar]
- 18. Furtner J, Weller M, Weber M, et al. Temporal muscle thickness as a prognostic marker in newly diagnosed glioblastoma patients: translational imaging analysis of the CENTRIC EORTC 26071-22072 and CORE trials. Clin Cancer Res. 2022;28(1):129–136. [DOI] [PubMed] [Google Scholar]
- 19. Steindl A, Leitner J, Schwarz M, et al. Sarcopenia in neurological patients: standard values for temporal muscle thickness and muscle strength evaluation. J Clin Med 2020;9(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kennedy-Martin T, Curtis S, Faries D, Robinson S, Johnston J. A literature review on the representativeness of randomized controlled trial samples and implications for the external validity of trial results. Trials. 2015;16:495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rogers JR, Liu C, Hripcsak G, Cheung YK, Weng C. Comparison of clinical characteristics between clinical trial participants and nonparticipants using electronic health record data. JAMA Netw Open. 2021;4(4):e214732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Furtner J, Berghoff AS, Albtoush OM, et al. Survival prediction using temporal muscle thickness measurements on cranial magnetic resonance images in patients with newly diagnosed brain metastases. Eur Radiol. 2017;27(8):3167–3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Katsuki M, Yamamoto Y, Uchiyama T, Wada N, Kakizawa Y. Clinical characteristics of aneurysmal subarachnoid hemorrhage in the elderly over 75; would temporal muscle be a potential prognostic factor as an indicator of sarcopenia? Clin Neurol Neurosurg. 2019;186:105535. [DOI] [PubMed] [Google Scholar]
- 25. Bauer J, Morley JE, Schols A, et al. Sarcopenia: a time for action. An SCWD position paper. J Cachexia Sarcopenia Muscle. 2019;10(5):956–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Beaudart C, Biver E, Reginster JY, et al. Validation of the SarQoL(R), a specific health-related quality of life questionnaire for Sarcopenia. J Cachexia Sarcopenia Muscle. 2017;8(2):238–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kim EY, Kim YS, Park I, et al. Prognostic significance of CT-Determined sarcopenia in patients with small-cell lung cancer. J Thorac Oncol. 2015;10(12):1795–1799. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.