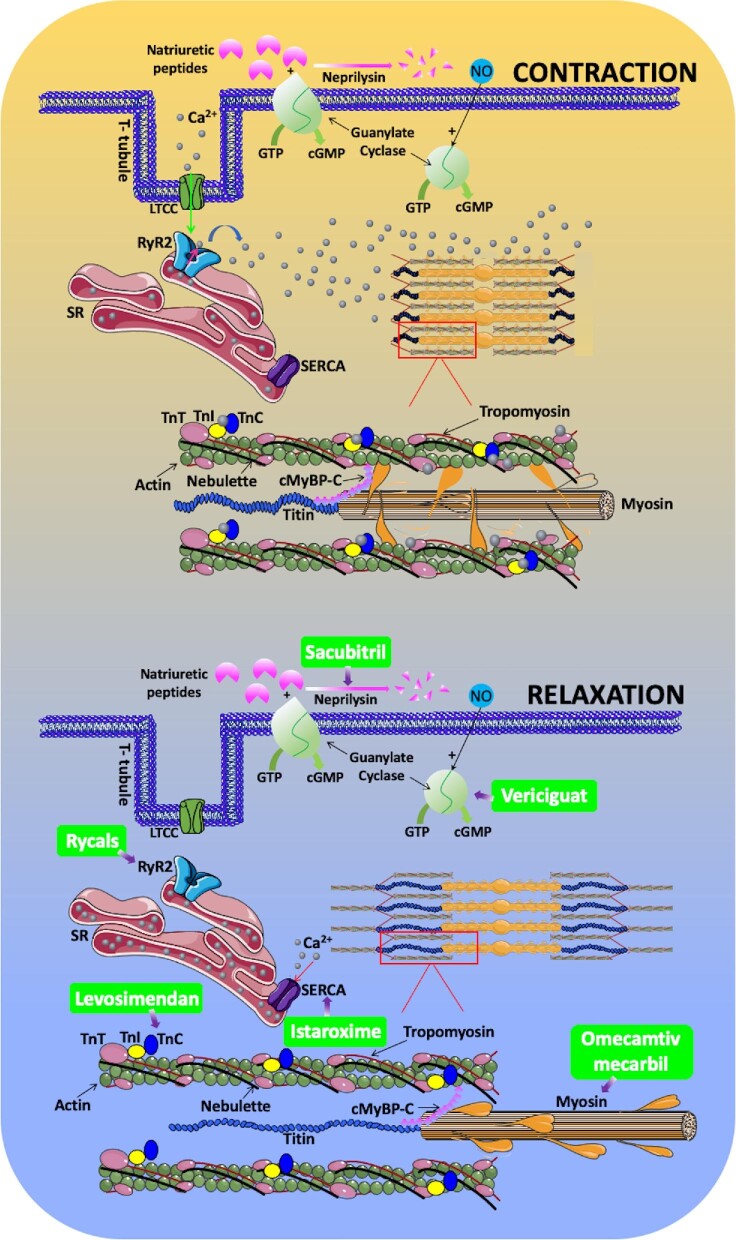
Excitation-contraction coupling (ECC) converts electrical stimuli to mechanical responses. Herein we provide a concise summary of the most updated insights on, and nomenclature of, the main actors involved in cardiac ECC, posing the basis for pharmacologic interventions in heart failure (HF) and arrhythmias. Recently introduced therapeutic strategies targeting myocardial ECC are appraised as well, specifying their molecular mechanism of action.
Excitation-contraction coupling starts with the entry of Ca2+ into the cardiomyocyte through the L-type Ca2+ channels (LTCC, also known as dihydropyridine channel); this step triggers Ca2+-induced Ca2+-release from the sarcoplasmic reticulum (SR) by activating type 2 ryanodine receptor Ca2+ release channel (RyR2).1 Ca2+-releasing units consisting of closely (∼15 nm) approximated LTCCs (on T-tubules, which are invaginations of the sarcolemma with a diameter of ∼200 nm that form a highly branched network) and RyRs (on the SR) are known as dyadic clefts or dyads; within each dyad, ∼25 LTCCs and ∼100 RyRs are closely associated (∼1:4 ratio). Junctophilin-2 is a membrane-binding protein that is responsible for the localization of LTCCs in close proximity to RyR2. Mutations in the joining region of Junctophilin-2 cause T-tubule remodeling and dyad loss with subsequent asynchronous Ca2+-release after β-adrenergic stimulation.2
LTCC contains the α1 subunit, a tetramer of four six-transmembrane domains forming the pore, and auxiliary subunits (α2δ, β, γ). The α1 subunit, known as Ca2+-voltage-gated channel subunit α1 (CaV1), has four isoforms: α1S (CaV1.1), α1C (CaV1.2), α1D (CaV1.3), and α1F (CaV1.3). Two groups of Ca2+-voltage-gated channels complete the family: CaV2 (P-type, N-type, R-type), and CaV3 (T-type).
RyR2, a tetrameric intracellular Ca2+-release channel located on the SR, can be modulated by post-translational modifications and by interactions with a number of other proteins, including calmodulin, striated muscle enriched protein kinase (SPEG), calstabin2, triadin/calsequestrin, calcineurin, and transmembrane protein 38A (TMEM-38A). Small molecules known as Rycals have been shown to stabilize RyRs, preventing the pathologic intracellular Ca2+ leak,1 defined as inappropriate release of Ca2+ from the SR (e.g. during the diastolic phase).
The release of Ca2+ through RyRs increases its concentration in the dyad from ∼100 nMol to >∼100 µMol. How does this increase in Ca2+ concentration lead to contraction? Contraction of the cardiomyocyte relies on the synchronized movements of the main constituents of the sarcomere (the smallest functional unit of the striated muscle), with the mechanic translocation of the myosin thick filament respect to the thin filament, which is composed by a double-stranded actin polymer, two continuous tropomyosin polymers, and troponin (Tn) complex (Figure 1).
Figure 1.
Current pharmacologic strategies targeting the major players involved in cardiac excitation-contraction coupling. cGMP: 3′,5′-cyclic guanosine monophosphate (both the membrane-bound and the soluble forms are depicted); cMyBP-C: cardiac myosin binding protein-C; GTP: guanosine triphosphate; NO: nitric oxide; RyR2: ryanodine receptor; SERCA: sarco/endoplasmic-reticulum Ca2+-ATPase; SR: sarcoplasmic reticulum; Tn: troponin.
The Tn complex is formed by three distinct proteins (TnC, TnT, and TnI), encoded for by different genes that are expressed in cardiac and skeletal muscle (but not in smooth muscle). TnC is responsible for Ca2+ binding, which triggers conformational modifications of the Tn complex that cause tropomyosin to move deeper into the actin groove, exposing the myosin-binding sites that are covered in the relaxed state; while TnC in skeletal muscle has four Ca2+ binding sites, in cardiac muscle there are only three binding sites. The Ca2+ sensitizer levosimendan has been shown to interact with cardiac TnC.3 TnT interacts with tropomyosin and helps its positioning on actin; mutations in cardiac TnT are known to cause human dilated cardiomyopathy. TnI has an inhibitory role in tropomyosin/TnT interaction and alterations in its methylation at sites R74/R79 and R146/R148 have been linked to hypertrophic cardiomyopathy.
Tropomyosins are integral components of actin filaments consisting of rod-shaped coiled-coil dimers (four genes are responsible for generating more than 40 isoforms) that lie along actin. Interaction occurs along the length of the actin filament, with dimers aligning in a head-to-tail fashion. In mammals, four genes (TPM1, TPM2, TPM3, and TPM4) are responsible for generating more than forty different tropomyosin isoforms. Mutations in TPM1 have been associated with hypertrophic cardiomyopathy, dilated cardiomyopathy, and left ventricular non-compaction cardiomyopathy.
Myosin is a mechanochemical protein that converts chemical energy into mechanical force. The human genome contains >40 different myosin genes whose protein products share the basic properties of actin binding, ATP hydrolysis, and force transduction. By promoting the interaction between myosin and actin, omecamtiv mecarbil4 has been recently shown to significantly increase myocardial force production, although the exact underlying molecular mechanisms are not fully clear5; specifically, the GALACTIC-HF trial4 has revealed that in patients with HF and a reduced ejection fraction, those who received omecamtiv mecarbil, had a lower risk of a composite of HF or cardiovascular death than those who received placebo.4
After cytosolic Ca2+ has activated the contractile units, it is rapidly extruded from the cytosol in preparation for the following cycle; the main efflux mechanisms in cardiomyocytes are the sarco/endoplasmic reticulum Ca2+ ATPase (SERCA) pump and the Na+/Ca2+ exchanger (NCX).
Sarco/endoplasmic reticulum Ca2+ ATPase is responsible for the reuptake of Ca2+ from cytosol to SR. Three genes are present in vertebrates (ATP2A1-3), coding for three isoforms (SERCA-1–3); alternative splicing results in a total of ten different proteins: SERCA-1a/b, SERCA-2a/b, SERCA-3a/b/c/d/e/f. Sarco/endoplasmic reticulum Ca2+ ATPase is regulated by a number of different proteins, including phospholamban (ventricles and slow-twitch muscles), and sarcolipin (fast-twitch muscles and atria). Istaroxime is a Na+/K+ ATPase inhibitor with the distinctive property of increasing SERCA2a activity,6 most likely by relieving the inhibitory effect of phospholamban, thereby improving Ca2+ handling and diastolic dysfunction.
NCX, a.k.a. solute carrier family 8 (SLC8), is a low-affinity, high-capacitance antiporter that mediates the electrogenic exchange of three Na+ ions with one Ca2+ ion.
Other important components of the ECC machinery, for which therapeutic approaches are not (yet) available include cMyBP-C, titin, and nebulette.
cMyBP-C, a myosin-associated protein that binds at 43 nm intervals along the myosin thick filament backbone, influences contractile mechanics and myofilament orientation. Mutations of cMyBP-C have been linked to familial hypertrophic cardiomyopathy.
Titin is a giant protein (>1 µm) responsible for passive muscle elasticity, acting as a molecular spring, comprising 244 domains that unfold when the protein is stretched and refold once tension is removed. Titin mutations are among the most common causes of adult dilated cardiomyopathy.
The nebulin family includes five members: nebulin, nebulette, N-RAP, LASP-1, and LASP-2. Nebulin (∼800 kDa) extends along the thin filament of the skeletal muscle and consists of 185 copies of a 35 amino acid sequence (‘nebulin-repeat’) containing the actin-binding SDxxYK motif. Nebulette, a smaller protein (∼116 kDa, 23 nebulin repeats), replaces nebulin in cardiac muscle.7 Nebulin-related anchoring protein (NRAP) contains 46 nebulin repeats, while LASP-1 (not present in cardiomyocytes), and LASP-2, contain only two and three nebulin repeats, respectively.
We conclude this brief excursus presenting a molecular pathway that, although not being inherently involved in ECC, has been recently successfully targeted in HF: the generation of 3ʹ,5ʹ-cyclic guanosine monophosphate (cGMP), which has anti-inflammatory and anti-fibrotic properties. Sacubitril inhibits the degradation (mediated by neprilysin) of natriuretic peptides—which are known to activate the membrane-bound form of the enzyme responsible for cGMP production, guanylyl cyclase—and its association with valsartan has been shown to be effective in HF.8,9 Vericiguat, instead, is a stimulator of the soluble form of guanylate cyclase, stabilizing its nitrosyl-heme interaction10. In patients with high-risk HF, the incidence of death from cardiovascular causes or hospitalization for HF was shown to be lower among those who received vericiguat than among those who received placebo.10
Contributor Information
Urna Kansakar, Departments of Medicine (Cardiology) and Molecular Pharmacology, Einstein College of Medicine, Wilf Family Cardiovascular Research Institute, Einstein-Sinai Diabetes Research Center (ES-DRC), Fleisher Institute for Diabetes and Metabolism (FIDAM), Einstein Institute for Aging Research, 1300 Morris PARK AVENUE, New York City, 10461, NY, USA.
Fahimeh Varzideh, Departments of Medicine (Cardiology) and Molecular Pharmacology, Einstein College of Medicine, Wilf Family Cardiovascular Research Institute, Einstein-Sinai Diabetes Research Center (ES-DRC), Fleisher Institute for Diabetes and Metabolism (FIDAM), Einstein Institute for Aging Research, 1300 Morris PARK AVENUE, New York City, 10461, NY, USA.
Stanislovas S Jankauskas, Departments of Medicine (Cardiology) and Molecular Pharmacology, Einstein College of Medicine, Wilf Family Cardiovascular Research Institute, Einstein-Sinai Diabetes Research Center (ES-DRC), Fleisher Institute for Diabetes and Metabolism (FIDAM), Einstein Institute for Aging Research, 1300 Morris PARK AVENUE, New York City, 10461, NY, USA.
Jessica Gambardella, Departments of Medicine (Cardiology) and Molecular Pharmacology, Einstein College of Medicine, Wilf Family Cardiovascular Research Institute, Einstein-Sinai Diabetes Research Center (ES-DRC), Fleisher Institute for Diabetes and Metabolism (FIDAM), Einstein Institute for Aging Research, 1300 Morris PARK AVENUE, New York City, 10461, NY, USA; International Translational Research and Medical Education (ITME) Consortium and Department of Advanced Biomedical Sciences, “Federico II” University, Via Sergio Pansini, 5, 80131, Naples, Italy.
Bruno Trimarco, International Translational Research and Medical Education (ITME) Consortium and Department of Advanced Biomedical Sciences, “Federico II” University, Via Sergio Pansini, 5, 80131, Naples, Italy.
Gaetano Santulli, Departments of Medicine (Cardiology) and Molecular Pharmacology, Einstein College of Medicine, Wilf Family Cardiovascular Research Institute, Einstein-Sinai Diabetes Research Center (ES-DRC), Fleisher Institute for Diabetes and Metabolism (FIDAM), Einstein Institute for Aging Research, 1300 Morris PARK AVENUE, New York City, 10461, NY, USA; International Translational Research and Medical Education (ITME) Consortium and Department of Advanced Biomedical Sciences, “Federico II” University, Via Sergio Pansini, 5, 80131, Naples, Italy.
Conflict of interest: None declared.
References
- 1. Gambardella J, Trimarco B, Iaccarino G, Santulli G. New insights in cardiac calcium handling and excitation-contraction coupling. Adv Exp Med Biol 2018;1067:373–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gross P, Johnson J, Romero CM, Eaton DM, Poulet C, Sanchez-Alonso J, Lucarelli C, Ross J, Gibb AA, Garbincius JF, Lambert J, Varol E, Yang Y, Wallner M, Feldsott EA, Kubo H, Berretta RM, Yu D, Rizzo V, Elrod J, Sabri A, Gorelik J, Chen X, Houser SR. Interaction of the joining region in junctophilin-2 with the L-Type Ca(2+) channel is pivotal for cardiac dyad assembly and intracellular Ca(2+) dynamics. Circ Res 2021;128:92–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Burkhoff D, Borlaug BA, Shah SJ, Zolty R, Tedford RJ, Thenappan T, Zamanian RT, Mazurek JA, Rich JD, Simon MA, Chung ES, Raza F, Majure DT, Lewis GD, Preston IR, Rich S. Levosimendan improves hemodynamics and exercise tolerance in PH-HFpEF: results of the randomized placebo-controlled HELP trial. JACC Heart Fail 2021;9:360–370. [DOI] [PubMed] [Google Scholar]
- 4. Teerlink JR, Diaz R, Felker GM, McMurray JJV, Metra M, Solomon SD, Adams KF, Anand I, Arias-Mendoza A, Biering-Sorensen T, Bohm M, Bonderman D, Cleland JGF, Corbalan R, Crespo-Leiro MG, Dahlstrom U, Echeverria LE, Fang JC, Filippatos G, Fonseca C, Goncalvesova E, Goudev AR, Howlett JG, Lanfear DE, Li J, Lund M, Macdonald P, Mareev V, Momomura SI, O'Meara E, Parkhomenko A, Ponikowski P, Ramires FJA, Serpytis P, Sliwa K, Spinar J, Suter TM, Tomcsanyi J, Vandekerckhove H, Vinereanu D, Voors AA, Yilmaz MB, Zannad F, Sharpsten L, Legg JC, Varin C, Honarpour N, Abbasi SA, Malik FI, Kurtz CE, Investigators G-H . Cardiac myosin activation with omecamtiv mecarbil in systolic heart failure. N Engl J Med 2021;384:105–116. [DOI] [PubMed] [Google Scholar]
- 5. Woody MS, Greenberg MJ, Barua B, Winkelmann DA, Goldman YE, Ostap EM. Positive cardiac inotrope omecamtiv mecarbil activates muscle despite suppressing the myosin working stroke. Nat Commun 2018;9:3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Torre E, Arici M, Lodrini AM, Ferrandi M, Barassi P, Hsu SC, Chang GJ, Boz E, Sala E, Vagni S, Altomare C, Mostacciuolo G, Bussadori C, Ferrari P, Bianchi G, Rocchetti M. SERCA2a stimulation by istaroxime improves intracellular Ca2+ handling and diastolic dysfunction in a model of diabetic cardiomyopathy. Cardiovasc Res 2021:cvab123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vejandla RM, Orgil BO, Alberson NR, Li N, Munkhsaikhan U, Khuchua Z, Martherus R, Azeloglu EU, Xu F, Lu L, Towbin JA, Purevjav E. Deficiency in nebulin repeats of sarcomeric nebulette is detrimental for cardiomyocyte tolerance to exercise and biomechanical stress. Am J Physiol Heart Circ Physiol 2021;320:H2130–H2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. DeVore AD, Braunwald E, Morrow DA, Duffy CI, Ambrosy AP, Chakraborty H, McCague K, Rocha R, Velazquez EJ, Investigators P-H . Initiation of angiotensin-neprilysin inhibition after acute decompensated heart failure: secondary analysis of the open-label extension of the PIONEER-HF trial. JAMA Cardiol 2020;5:202–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jackson AM, Jhund PS, Anand IS, Dungen HD, Lam CSP, Lefkowitz MP, Linssen G, Lund LH, Maggioni AP, Pfeffer MA, Rouleau JL, Saraiva JFK, Senni M, Vardeny O, Wijkman MO, Yilmaz MB, Saito Y, Zile MR, Solomon SD, McMurray JJV. Sacubitril-valsartan as a treatment for apparent resistant hypertension in patients with heart failure and preserved ejection fraction. Eur Heart J 2021;42:3741–3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Armstrong PW, Pieske B, Anstrom KJ, Ezekowitz J, Hernandez AF, Butler J, Lam CSP, Ponikowski P, Voors AA, Jia G, McNulty SE, Patel MJ, Roessig L, Koglin J, O'Connor CM, Group VS . Vericiguat in patients with heart failure and reduced ejection fraction. N Engl J Med 2020;382:1883–1893. [DOI] [PubMed] [Google Scholar]