Abstract
Background
While Pseudomonas aeruginosa (Pa) eradication regimens have contributed to a decline in Pa prevalence in people with cystic fibrosis (CF), this antibiotic exposure might increase the risk of acquisition of drug-resistant organisms. This study evaluated the association between antipseudomonal antibiotic exposure intensity and acquisition risk of drug-resistant organisms among children with CF and new Pa infection.
Methods
We utilized data from the Early Pseudomonas Infection Control Clinical Trial (EPIC CT), a randomized controlled trial comparing Pa eradication strategies in children with CF and new Pa. The exposure was the number of weeks of oral or inhaled antipseudomonal antibiotics or ever versus never treatment with intravenous antipseudomonal antibiotics during the 18 months of EPIC CT participation. Primary outcomes were risks of acquisition of several respiratory organisms during 5 years of follow-up after EPIC CT estimated using Cox proportional hazards models separately for each specific organism.
Results
Among 249 participants, there was no increased acquisition risk of any organism associated with greater inhaled antibiotic exposure. With each additional week of oral antibiotics, there was an increased hazard of Achromobacter xylosoxidans acquisition (HR, 1.24; 95% CI: 1.02–1.50; P = .03). Treatment with intravenous antibiotics was associated with an increased hazard of acquisition of multidrug-resistant Pa (HR, 2.47; 95% CI: 1.28–4.78; P = .01) and MRSA (HR, 1.57; 95% CI: 1.03–2.40; P = .04).
Conclusions
Results from this study illustrate the importance of making careful antibiotic choices to balance the benefits of antibiotics in people with CF while minimizing risk of acquisition of drug-resistant organisms.
Keywords: cystic fibrosis, antibiotics, pulmonary exacerbations, Pseudomonas aeruginosa
This study examined the association between antipseudomonal antibiotic exposure and acquisition risk of drug-resistant organisms in children with cystic fibrosis and new Pseudomonas. We found no risk associated with inhaled antibiotics but an increased risk of several organisms with increasing oral or intravenous antibiotic exposure.
Cystic fibrosis (CF) is a life-shortening genetic disease characterized by chronic endobronchial infection and inflammation resulting in progressive obstructive lung disease and bronchiectasis [1, 2]. Chronic airway infection with Pseudomonas aeruginosa (Pa), in particular, has been associated with decline in lung function, increased frequency of pulmonary exacerbations, and shortened survival [3–5]. In an attempt to delay or prevent chronic airway infection, Pa eradication protocols have become standard of care when new Pa is detected [6–10]. In the United States, these regimens typically include inhaled tobramycin [11] with or without oral ciprofloxacin.
While widespread adoption of Pa eradication protocols has contributed to a decreased incidence and prevalence of Pa infection in people with CF [12], this antibiotic exposure might have unintended consequences, including selection for multidrug-resistant Pa or other drug-resistant respiratory organisms. For example, in clinical trials of inhaled tobramycin for chronic Pa infection, participants randomized to inhaled tobramycin had higher rates of Aspergillus fumigatus compared with those randomized to placebo [13, 14]. A key unanswered question is whether the intensity of exposure to antipseudomonal antibiotics in children with new Pa is associated with risk of acquisition of drug-resistant organisms.
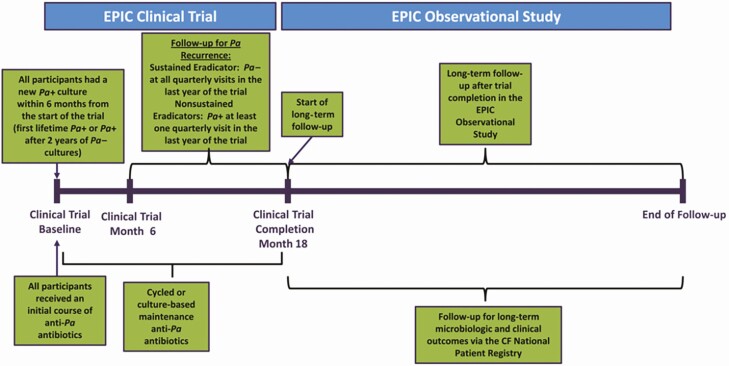
The Early Pseudomonas Infection Control (EPIC) Clinical Trial (EPIC CT) was a multicenter, randomized, controlled 18-month trial in which children with CF ages 1 to 12 years with new isolation of Pa were randomized to cycled therapy (tobramycin inhalation solution [TIS] for 28 days every quarter, with oral ciprofloxacin or oral placebo for the first 14 days) or culture-based therapy (the same treatments only during quarters with positive Pa cultures) [15]. Additional courses of antibiotics prescribed clinically were also captured. Participants received long-term follow-up (up to 5 years) in the EPIC Observational Study (EPIC OBS), in which the EPIC CT was nested. Because of the wide range of antibiotic exposures rigorously recorded in a clinical trial setting and standardized long-term (5-year) follow-up, this cohort provides a unique opportunity to evaluate the association between intensity of antibiotic exposure and emergence of drug-resistant respiratory organisms among children with CF with new isolation of Pa.
This study aims to evaluate the association between the intensity of antipseudomonal antibiotic exposure (oral, inhaled, and intravenous [IV]) during the 18-month EPIC CT with subsequent risk of acquisition of multidrug-resistant Pa, methicillin-resistant Staphylococcus aureus (MRSA), and other respiratory organisms. We hypothesized that greater intensity of antipseudomonal antibiotic exposure during the EPIC CT period would be associated with a greater hazard of acquisition of these organisms during the subsequent 5 years.
METHODS
This is a secondary analysis of the EPIC CT with 5-year follow-up through the EPIC OBS study. As previously described, the EPIC CT enrolled 304 US children with CF aged 1 to 12 years who had new-onset Pa detected within 6 months prior to enrollment (either as first lifetime Pa infection or after 2 years of respiratory cultures negative for Pa) between 2004 and 2008 [8]. All participants received an initial course of TIS for 28 days and were then randomized to 1 of 2 study arms: (1) children randomized to “cycled therapy” received 28 days of TIS every quarter (90-day period) irrespective of routine quarterly respiratory culture results (ie, 6 courses of TIS over the 18-month trial period) or (2) children randomized to “culture-based therapy” received TIS only if Pa was isolated from a quarterly respiratory culture. Participants were further randomized to receive oral ciprofloxacin or placebo for 14 days each time they received TIS (Figure 1). All clinically prescribed antibiotics (oral, inhaled, or IV) were also recorded. The EPIC OBS study provided 5 years of follow-up, including respiratory culture results as recorded in the Cystic Fibrosis Foundation Patient Registry (CFFPR).
Figure 1.
Overview of the study design of the EPIC Clinical Trial and EPIC Observational Study (reproduced with permission). Abbreviations: CF, Cystic Fibrosis; EPIC, Early Pseudomonas Infection Control. (Mayer-Hamblett N, Kloster M, Rosenfeld M, Gibsons RL, Retsch-Bogart GZ, Emerson J, Thompson V, Ramsey BW. Impact of Sustained Eradication of New Pseudomonas aeruginosa Infection on Long-term Outcomes in Cystic Fibrosis. Clinical Infectious Diseases 2015; 61(5): 707-715.)
Inhaled and oral antibiotic exposure intensity was defined as the cumulative number of weeks of inhaled or oral antipseudomonal antibiotics (separately), while, due to the distribution of exposure in the cohort, IV antibiotic exposure was classified as ever/never (ie, at least 1 course vs no exposure). Antibiotic exposure was assessed during the 18-month EPIC CT period only, including study drug and clinically prescribed antipseudomonal antibiotics. Antibiotic exposure data were not collected during the 5-year EPIC OBS follow-up period. The primary outcomes were acquisition of the following organisms during the 5-year EPIC OBS follow-up period: multidrug-resistant Pa (MDR-Pa), MRSA, Achromobacter xylosoxidans, Stenotrophomonas maltophilia, Aspergillus fumigatus, and Burkholderia cepacia complex species (BCC). Among all respiratory cultures collected during EPIC CT, 94% were obtained from the oropharynx and 6% were expectorated sputum samples.
Clinical and demographic characteristics were described using summary statistics. Risk of respiratory organism acquisition was assessed in separate models for each organism. Since we were interested in treatment-emergent (ie, incident) acquisition of each organism, we created separate organism-specific cohorts, each composed of children who never cultured that organism during the EPIC CT period. Each antibiotic route (inhaled, oral, IV) was included in these models as an independent covariate. We used Prentice, Williams, and Peterson Total Time multiple-failure Cox proportional hazards models [16] to allow for multiple positive cultures for each organism during follow-up, and each model was stratified by failure number to allow for the baseline hazard to be different for each subsequent infection by the same organism. Analysis time was the total time since the end of participation in the EPIC CT. Models were adjusted for age, sex, race (non-Hispanic White vs other) and cystic fibrosis transmembrane conductance regulator (CFTR) mutation (Phe508del homozygous, Phe508del heterozygous, other). Models with and without quadratic terms for cumulative weeks of antibiotics were fit and their goodness of fit compared using log-likelihood ratios. Only results of models without quadratic terms are reported, as quadratic terms did not substantially improve model fit.
Analyses were performed with Stata 16.1 (StataCorp). Each study was approved by the Institutional Review Board at Seattle Children’s Hospital and participating sites, and informed consent was obtained from the parent/guardian of all participants.
RESULTS
Among the 304 children enrolled in the EPIC CT, 249 (82%) completed the trial (observed for the full 18-month EPIC CT period) and were followed in EPIC OBS for a median (interquartile range) of 68.7 (67.4–69.8) months; these individuals comprised our study cohort. The average age at enrollment was 5.8 (standard deviation, 3.6) years, and 55% of participants were Phe508del homozygous. There were no significant differences between study arms with respect to demographic or clinical characteristics (Table 1).
Table 1.
Demographic and Clinical Characteristics of the Study Cohorts
| Characteristic | Total (N = 249) | Cycled TSI With Ciprofloxacin (n = 61) | Cycled TSI With Placebo (n = 61) | Culture-Based TSI With Ciprofloxacin (n = 59) | Culture-Based TSI With Placebo (n = 68) | P a |
|---|---|---|---|---|---|---|
| Age, mean (SD), years | 5.8 (3.6) | 6.3 (3.3) | 5.8 (3.6) | 5.2 (3.6) | 6.0 (3.8) | .4 |
| Sex (female), n (%) | 126 (50.6) | 27 (44.3) | 34 (55.7) | 31 (52.5) | 34 (50.0) | .6 |
| Race (non-Hispanic White), n (%) | 238 (95.6) | 60 (98.4) | 54 (88.5) | 59 (100.0) | 65 (95.6) | .4 |
| Phe508del, n (%) | .2 | |||||
| Homozygous | 130 (54.6) | 28 (47.5) | 28 (49.1) | 31 (55.4) | 43 (65.2) | |
| Heterozygous | 92 (38.7) | 29 (49.2) | 24 (42.1) | 19 (33.9) | 20 (30.3) | |
| Other | 16 (6.7) | 2 (3.4) | 5 (8.8) | 6 (10.7) | 3 (4.5) | |
| BMI percentile, mean (SD) | 55.1 (24.7) | 52.4 (26.1) | 60.3 (22.1) | 54.6 (26.7) | 53.4 (23.8) | .3 |
Abbreviations: BMI, body mass index; CFTR, cystic fibrosis transmembrane conductance regulator; SD, standard deviation; TSI, tobramycin solution for inhalation.
aFrom analysis of variance (ANOVA) for continuous variables (age, body mass index) and chi-square test for categorical variables (sex, race, and CFTR genotype).
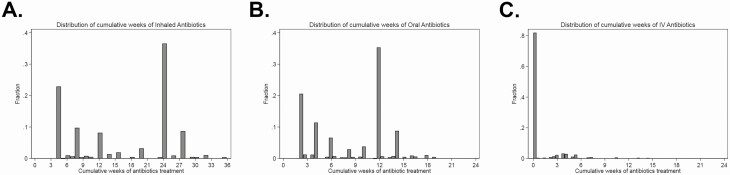
The distribution of cumulative weeks of inhaled, oral, and IV antibiotic exposure during the 18-month clinical trial period is shown in Figure 2. The EPIC CT study design ensured a broad distribution of exposure to inhaled and oral antibiotics, whereas IV antibiotics were prescribed clinically. Among the 249 participants, 40 (16.0%) were prescribed at least 1 course of IV antibiotics during the 18-month EPIC CT period.
Figure 2.
Distribution of cumulative weeks of (A) inhaled, (B) oral, and (C) IV antibiotic exposure during EPIC CT. Abbreviations: EPIC CT, Early Pseudomonas Infection Control Clinical Trial; IV, intravenous.
The number of participants in each organism-specific cohort and the number acquiring each organism during follow-up are shown in Table 2. Participants were least likely to have positive cultures for MDR-Pa or BCC both during trial participation and follow-up, and most likely to have positive cultures for MRSA and S. maltophilia.
Table 2.
Number of Trial Participants Eligible for Each Study Cohort (Not Mutually Exclusive) and Number With Incident Infection
| Organism | Number at risk (Excludes People Who Had a Given Organism During the 18-Month EPIC CT) | Number With Incident Infection During 5-Year Follow-up (%) |
|---|---|---|
| MRSA | 147 | 55 (37.4) |
| MDR-Pa | 235 | 18 (8.1) |
| Achromobacter xylosoxidans | 212 | 30 (14.2) |
| Stenotrophomonas maltophilia | 152 | 67 (44.1) |
| Aspergillus fumigatus | 185 | 64 (34.6) |
| BCC | 232 | 17 (7.3) |
Abbreviations: BCC, Burkholderia cepacia complex species; EPIC CT, Early Pseudomonas Infection Control Clinical Trial; MDR-Pa, multidrug-resistant Pseudomonas aeruginosa; MRSA, methicillin-resistant Staphylococcus aureus.
In Cox proportional hazards models, there was no increased risk of acquisition of any respiratory organism with each additional week of inhaled antibiotic exposure (Table 3), although there did appear to be a decreased risk of acquisition of A. xylosoxidans (hazard ratio, .90; 95% confidence interval [CI]: .82–.98; P = .02). With respect to oral antibiotics, with each additional week of exposure there was a 24% increased hazard of A. xylosoxidans acquisition (95% CI: 1.02–1.50; P = .03). In terms of IV antibiotics, the risk of acquisition of MDR-Pa was 2.47-fold greater (95% CI: 1.28–4.78; P = .01) and of MRSA was 1.57-fold greater (95% CI: 1.03–2.40; P = .04) among children prescribed IV antibiotics compared with receiving no IV antibiotics.
Table 3.
Association of Cumulative Antimicrobial Exposure and Risk of Treatment-Emergent Respiratory Organism Acquisition
| Organism | Hazard Ratioa | 95% CI | P |
|---|---|---|---|
| MDR-Pa | |||
| Inhaled | 1.06 | (.95–1.18) | .32 |
| Oral | .91 | (.74–1.12) | .38 |
| IV (ever vs never) | 2.47 | (1.28–4.78) | .01 |
| MRSA | |||
| Inhaled | 1.04 | (.99–1.09) | .14 |
| Oral | .94 | (.85–1.05) | .28 |
| IV (ever vs never) | 1.57 | (1.03–2.40) | .04 |
| Achromobacter xylosoxidans | |||
| Inhaled | .90 | (.82–.98) | .02 |
| Oral | 1.24 | (1.02–1.50) | .03 |
| IV (ever vs never) | .94 | (.49–1.82) | .86 |
| Stenotrophomonas maltophilia | |||
| Inhaled | .99 | (.94–1.05) | .75 |
| Oral | 1.04 | (.92–1.18) | .52 |
| IV (ever vs never) | .77 | (.48–1.24) | .29 |
| Aspergillus fumigatus | |||
| Inhaled | 1.09 | (.98–1.21) | .10 |
| Oral | .84 | (.69–1.03) | .10 |
| IV (ever vs never) | .92 | (.59–1.45) | .72 |
| BCC | |||
| Inhaled | 1.04 | (.95–1.12) | .41 |
| Oral | .89 | (.77–1.04) | .14 |
| IV (ever vs never) | .63 | (.10–4.09) | .60 |
Abbreviations: BCC, Burkholderia cepacia complex species; CFTR, ; CI, confidence interval; IV, intravenous; MDR-Pa, multidrug-resistant Pseudomonas aeruginosa; MRSA, methicillin-resistant Staphylococcus aureus.
aAdjusted for age, sex, race (non-Hispanic White vs other), CFTR genotype, and other antibiotic routes of administration (ie, inhaled antibiotic analysis additionally adjusted for oral and IV use).
DISCUSSION
This secondary analysis of 249 children with new Pa infection enrolled in the EPIC CT found no increased risk of acquisition of drug-resistant organisms associated with greater inhaled antibiotic exposure, but an increased risk of MDR-Pa and MRSA acquisition associated with IV antibiotic exposure. The widespread adoption of antibiotic treatment protocols to eradicate Pa has led to a decreased prevalence of chronic Pa infection and likely contributed to the improving lung function and survival of people with CF over the last 2 decades [3–5, 17]. In the United States, standard eradication regimens typically include inhaled antibiotics, although oral and IV anti–Pa antibiotics are also utilized [18]. Thus, people with CF without chronic Pa infection are exposed to more anti–Pa antibiotics than in earlier eras, potentially increasing the risk of acquiring MDR-Pa or other drug-resistant respiratory organisms. The design of the EPIC randomized controlled trial of Pa eradication regimens with subsequent follow-up in EPIC OBS offers a unique opportunity to evaluate the risks of drug-resistant organism acquisition associated with antibiotic exposure. All antibiotic exposures (study drugs and clinically prescribed) were rigorously captured during EPIC CT participation and the long-term (5-year) follow-up rate was high (249 of the original 304 trial participants). Inhaled and oral antibiotic exposure during clinical trial participation was predominantly determined by assigned study arm, minimizing indication bias.
In our cohort, the incidence of MDR-Pa was low; 8% of participants acquired new MDR-Pa during the 5 years following clinical trial participation. In contrast, the incidences of S. maltophilia, MRSA, and Aspergillus acquisition were higher (44%, 37%, and 34%, respectively, during the 5-year follow-up), similar to age-specific trends in the CFFPR [17]. Reassuringly, we found no increased risk of acquisition of any organism with each additional week of exposure to inhaled antibiotics. In terms of oral anti–Pa antibiotics, with each additional week of exposure we did find an increased risk of acquisition of A. xylosoxidans, although not of any other organism. Intravenous antibiotic exposure was the only route associated with increased risk of acquisition of MDR-Pa and MRSA. These risks were substantial: exposure to IV antibiotics was associated with greater than double the risk of acquiring MDR-Pa and a greater than 50% increased risk of acquiring MRSA. No route was associated with an increased risk of S. maltophilia, BCC, or Aspergillus acquisition. The reason for our observation of an apparent mild protective effect of inhaled antibiotics on the risk of A. xylosoxidans acquisition is unclear and this result should be interpreted with great caution. To our knowledge, no prior studies have identified an association between increased antibiotic exposure and A. xylosoxidans acquisition.
The mechanisms by which antipseudomonal antibiotics select for drug-resistant Pa in the CF airway have been well described [19, 20]. Antipseudomonal antibiotics may promote the growth of other organisms in the CF airway through 2 mechanisms. First, if Pa is eradicated, interspecies competition between Pa and other organisms will be minimized and the physicochemical milieu of the CF airway may be altered, promoting growth of other organisms. Second, a number of Pa exoproducts have been shown to decrease the susceptibility of S. aureus to clinically relevant antibiotics. For example, inhibition of electron transport by Pa-produced phenazines and 2-heptyl-4-hydroxyquinoline n-oxide (HQNO) can decrease S. aureus susceptibility to tobramycin, ciprofloxacin, and vancomycin [19]. Growth of S. aureus in the presence of Pa exoproducts can promote the growth of small colony variants, which are slow-growing and drug-resistant due to electron transport defects [20]. These phenomena have been evaluated mostly in terms of interactions between Pa and S. aureus in the CF airway, but similar mechanisms may apply to other CF respiratory organisms.
To our knowledge, this is the first study to evaluate the long-term (5-year) risk of acquisition of drug-resistant organisms associated with Pa eradication regimens beyond the clinical trial period. The Early Inhaled Tobramycin for Eradication (ELITE) trial (which randomized participants with new Pa acquisition to 28 vs 56 days of TIS) did not demonstrate any difference in the emergence of CF respiratory organisms between arms over 2 years following enrollment [6]. The Optimizing Treatment for Early Pa Infection in Cystic Fibrosis (OPTIMIZE) trial that randomized children with CF and new-onset Pa infection to TIS and either azithromycin or placebo similarly did not find a difference in CF organism emergence between treatment arms (median follow-up, 11.8 months) [21]. Finally, the TORPEDO CF study comparing prolonged oral antibiotics (12 weeks) with 14 days of IV antibiotics for Pa eradication (in addition to 12 weeks of inhaled colistin in both arms) did not find a difference between arms in rates of isolation of MRSA, BCC, or A. fumigatus (15-month follow-up) [22]. Our results are similar to those of the ELITE and OPTIMIZE studies but differ from those of the TORPEDO study, in which exposure to IV antibiotics was not associated with an increased risk of acquisition of drug-resistant organisms. Potential reasons for these discrepant results include slightly different eligibility criteria for the 2 trials, different underlying rates of MRSA in the United States relative to the United Kingdom and Italy, and longer-term follow-up in our study.
While our study has unique strengths discussed above, important limitations must also be acknowledged. First, we defined the antibiotic exposure as occurring during the 18 months of clinical trial participation, observing for the outcome (acquisition of new organisms) during the subsequent 5 years. While this design ensured that the outcome never preceded the exposure, any associations between antibiotic exposure and acquisition of a respiratory organism does not imply causation. We did not have access to treatment-adherence data, and thus these results are based on assumed completion of each treatment regimen during the study period. We did not have data on antibiotic exposure prior to clinical trial enrollment. In addition, we were not able to account for any ongoing exposure to antipseudomonal antibiotics during the follow-up period, as acute courses of oral and inhaled antibiotics are not recorded on a granular level in the CFFPR. Since the EPIC CT showed no difference in Pa eradication rates or chronic Pa infection between study arms, it is unlikely that antibiotic exposure during follow-up differed systematically between different arms, but this possibility cannot be entirely ruled out. Since IV antibiotics were prescribed clinically, sicker participants might have been more likely to receive IV antibiotics (indication bias) not only during enrollment in the clinical trial but during the 5-year follow-up. We classified IV antibiotic exposure as ever/never, but it is possible that some of the observed association between exposure to IV antibiotics during the clinical trial and acquisition of resistant organisms may have been due to repeated, unmeasured exposure to IV antibiotics during the follow-up period. Furthermore, given the possible indication bias in the prescription of IV antibiotics, unmeasured confounders may have contributed to the observed association between IV antibiotic exposure and risk of pathogen acquisition.
As the majority of our cohort (94%) were unable to expectorate, we evaluated the results of both oropharyngeal (OP) and sputum cultures (as is standard of care in the United States). Oropharyngeal cultures have been shown to have better specificity than sensitivity for the presence of lower airway Pa and S. aureus [23]; diagnostic accuracy for other organisms is not known. Thus, the inclusion of OP culture results introduced some misclassification of the outcome. We also evaluated only conventional culture results; evaluating the effect of antipseudomonal antibiotics on the respiratory microbiome was beyond the scope of this analysis. Our sample size of 249 may have limited our power to detect associations. Our cohort was younger than 12 years of age at enrollment with new isolation of Pa. Thus, our results should not be generalized to older people with CF or those with chronic Pa infection. Our study was also conducted in the pre-CFTR modulator era. While highly effective modulator therapy is likely to reduce exposure to antipseudomonal antibiotics overall, it seems unlikely that it would affect the risk of drug-resistant organism acquisition associated with antibiotic exposure.
In conclusion, this study found no increased risk of respiratory organism acquisition associated with inhaled antibiotic exposure. As inhaled antibiotics are the most common route prescribed to eradicate Pa, this result is reassuring. We did detect an increased risk of A. xylosoxidans acquisition with each additional week of oral antibiotics. In the EPIC CT, the addition of oral ciprofloxacin to inhaled tobramycin did not demonstrate significant benefit as compared with inhaled tobramycin alone. Our finding provides additional rationale against adding oral antibiotics to inhaled antibiotics for first-line treatment of new Pa. We also found an increased risk of MDR-Pa and MRSA acquisition among people who received IV antibiotics. Results from this study illustrate the importance of making careful antibiotic choices in order to balance the benefits of antibiotics in people with CF while minimizing the risk of acquisition of drug-resistant organisms.
Notes
Acknowledgments. We thank the Cystic Fibrosis (CF) Foundation for the use of CF Foundation Patient Registry data to conduct the study. Additionally, we thank the patients, care providers, and clinic coordinators at CF centers throughout the United States for their contributions to the CF Foundation Patient Registry. We also thank the EPIC CT investigators and research staff who support this project.
Financial support. This work was supported by The Cystic Fibrosis Foundation (grant numbers EPIC0K0, OBSERV13K0, and COGEN18Y7).
Potential conflicts of interest. R. L. G. reports grant support from Vertex Pharmaceuticals, and is co-investigator on a Clinical Core for the National Institutes of Health (NIH), outside the submitted work. N. M. H. reports grants from the Cystic Fibrosis Foundation and NIH, outside the submitted work. W. J. M. reports a Data Safety Monitoring Board infrastructure grant and personal fees (on-call retainer for CFF DSM; CFF Comparative Effectiveness/Patient Registry Committee) from the Cystic Fibrosis Foundation, grants from NIH (Tucson Children’s Respiratory Study, Senior Investigator; ORBEX clinical trial, Site Investigator, Senior Investigator CCC; PARK Clinical Trial Investigator; Community Asthma Improvement Program, Senior Investigator), and speaker fees from the American College of Chest Physicians and the American Thoracic Society, outside the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Jonathan D Cogen, Department of Pediatrics, University of Washington School of Medicine, Seattle, Washington, USA; Seattle Children’s Research Institute, Seattle, Washington, USA.
Frankline M Onchiri, Seattle Children’s Research Institute, Seattle, Washington, USA.
Nicole Mayer Hamblett, Department of Pediatrics, University of Washington School of Medicine, Seattle, Washington, USA; Seattle Children’s Research Institute, Seattle, Washington, USA; Department of Biostatistics, University of Washington School of Medicine, Seattle, Washington, USA.
Ronald L Gibson, Department of Pediatrics, University of Washington School of Medicine, Seattle, Washington, USA; Seattle Children’s Research Institute, Seattle, Washington, USA.
Wayne J Morgan, Department of Pediatrics, The University of Arizona, Tucson, Arizona, USA.
Margaret Rosenfeld, Department of Pediatrics, University of Washington School of Medicine, Seattle, Washington, USA; Seattle Children’s Research Institute, Seattle, Washington, USA.
References
- 1. Stoltz DA, Meyerholz DK, Welsh MJ. Origins of cystic fibrosis lung disease. N Engl J Med 2015; 372:351–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cantina AM, Hart D, Konstan MW, Chmiel JF. Inflammation in cystic fibrosis lung disease: pathogenesis and therapy. J Cyst Fibros 2015; 14:419–30. [DOI] [PubMed] [Google Scholar]
- 3. Emerson J, Rosenfeld M, McNamara S, Ramsey B, Gibson RL. Pseudomonas aeruginosa and other predictors of mortality and morbidity in young children with cystic fibrosis. Pediatr Pulmonol 2002; 34:91–100. [DOI] [PubMed] [Google Scholar]
- 4. Murray TS, Egan M, Kazmierczak BI. Pseudomonas aeruginosa chronic colonization in cystic fibrosis patients. Curr Opin Pediatr 2007; 19:83–8. [DOI] [PubMed] [Google Scholar]
- 5. Sanders DB, Bittner RC, Rosenfeld M, Hoffman LR, Redding GJ, Goss CH. Failure to recover to baseline pulmonary function after cystic fibrosis pulmonary exacerbation. Am J Respir Crit Care Med 2010; 182:627–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ratjen F, Munck A, Kho P, Angyalosi G; ELITE Study Group . Treatment of early Pseudomonas aeruginosa infection in patients with cystic fibrosis: the ELITE trial. Thorax 2010; 65:286–91. [DOI] [PubMed] [Google Scholar]
- 7. Proesmans M, Vermeulen F, Boulanger L, Verhaegen J, De Boeck K. Comparison of two treatment regimens for eradication of Pseudomonas aeruginosa infection in children with cystic fibrosis. J Cyst Fibros 2013; 12:29–34. [DOI] [PubMed] [Google Scholar]
- 8. Treggiari MM, Retsch-Bogart G, Mayer-Hamblett N, et al. ; Early Pseudomonas Infection Control (EPIC) Investigators . Comparative efficacy and safety of 4 randomized regimens to treat early Pseudomonas aeruginosa infection in children with cystic fibrosis. Arch Pediatr Adolesc Med 2011; 165:847–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cohen-Cymberknoh M, Gilead N, Gartner S, et al. Eradication failure of newly acquired Pseudomonas aeruginosa isolates in cystic fibrosis. J Cyst Fibros 2016; 15:776–82. [DOI] [PubMed] [Google Scholar]
- 10. Blanchard AC, Horton E, Stanojevic S, Taylor L, Waters V, Ratjen F. Effectiveness of a stepwise Pseudomonas aeruginosa eradication protocol in children with cystic fibrosis. J Cyst Fibros 2017; 16:395–400. [DOI] [PubMed] [Google Scholar]
- 11. Mogayzel PJ Jr, Naureckas ET, Robinson KA, et al. ; Cystic Fibrosis Foundation Pulmonary Clinical Practice Guidelines Committee . Cystic Fibrosis Foundation pulmonary guideline. Pharmacologic approaches to prevention and eradication of initial Pseudomonas aeruginosa infection. Ann Am Thorac Soc 2014; 11:1640–50. [DOI] [PubMed] [Google Scholar]
- 12. Salsgiver EL, Fink AK, Knapp EA, et al. Changing epidemiology of the respiratory bacteriology of patients with cystic fibrosis. Chest 2016; 149:390–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Burns JL, Van Dalfsen JM, Shawar RM, et al. Effect of chronic intermittent administration of inhaled tobramycin on respiratory microbial flora in patients with cystic fibrosis. J Infect Dis 1999; 179:1190–6. [DOI] [PubMed] [Google Scholar]
- 14. Harun SN, Holford NHG, Grimwood K, Wainwright CE, Hennig S; Australasian Cystic Fibrosis Bronchoalveolar Lavage (ACFBAL) Study Group . Pseudomonas aeruginosa eradication therapy and risk of acquiring Aspergillus in young children with cystic fibrosis. Thorax 2019; 74:740–8. [DOI] [PubMed] [Google Scholar]
- 15. Treggiari MM, Rosenfeld M, Mayer-Hamblett N, et al. ; EPIC Study Group . Early anti-pseudomonal acquisition in young patients with cystic fibrosis: rationale and design of the EPIC clinical trial and observational study. Contemp Clin Trials 2009; 30:256–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Therneau TM, Grambsch PM. Modeling survival data: extending the Cox model. New York: Springer; 2000. [Google Scholar]
- 17.Cystic Fibrosis Foundation. Cystic Fibrosis Foundation patient registry 2018 annual data report. Bethesda, MD: Cystic Fibrosis Foundation; 2019. [Google Scholar]
- 18. Talwalkar JS, Murray TS. The approach to Pseudomonas aeruginosa in cystic fibrosis. Clin Chest Med 2016; 37:69–81. [DOI] [PubMed] [Google Scholar]
- 19. Pang Z, Raudonis R, Glick BR, Lin TJ, Cheng Z. Antibiotic resistance in Pseudomonas aeruginosa: mechanisms and alternative therapeutic strategies. Biotechnol Adv 2019; 37:177–92. [DOI] [PubMed] [Google Scholar]
- 20. Stefani S, Campana S, Cariani L, et al. Relevance of multidrug-resistant Pseudomonas aeruginosa infections in cystic fibrosis. Int J Med Microbiol 2017; 307:353–62. [DOI] [PubMed] [Google Scholar]
- 21. Mayer-Hamblett N, Retsch-Bogart G, Kloster M, et al. ; OPTIMIZE Study Group . Azithromycin for early pseudomonas infection in cystic fibrosis. The OPTIMIZE randomized trial. Am J Respir Crit Care Med 2018; 198:1177–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hewer SCL, Smyth AR, Brown M, et al. ; TORPEDO-CF Study Group . Intravenous versus oral antibiotics for eradication of Pseudomonas aeruginosa in cystic fibrosis (TORPEDO-CF): a randomised controlled trial. Lancet Respir Med 2020; 8:975–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rosenfeld M, Emerson J, Accurso F, et al. Diagnostic accuracy of oropharyngeal cultures in infants and young children with cystic fibrosis. Pediatr Pulmonol 1999; 28:321–8. [DOI] [PubMed] [Google Scholar]