Abstract
We have characterized a recently rediscovered chemosensory structure at the rear of the mandibular mucosa in the mouse oral cavity originally reported in the 1980s. This consists of unorganized taste buds, not contained within troughs, associated with the ducts of an underlying minor salivary gland. Using whole-mount preparations of transgenic mice expressing green fluorescent protein under the promoter of taste-signaling-specific genes, we determined that the structure contains taste bud clusters and salivary gland orifices at the rear of each mandible, distal to the last molar and anterior to the ascending ramus. Immunohistochemical analysis shows in the retromolar taste buds expression of the taste receptors Tas2R131 and T1R3 and taste cascade molecules TrpM5, PLCβ2, and GNAT3, consistent with type II taste cells, and expression of GAD1, consistent with type III taste cells. Furthermore, the neuronal marker, calcitonin gene-related peptide, in retromolar mucosa tissue wrapping around TrpM5+ taste buds was observed. RT–PCR showed that retromolar taste buds express all 3 mouse tas1r genes, 28 of the 35 tas2r genes, and taste transduction signaling genes gnat3, plcb2, and trpm5, making the retromolar taste buds similar to other lingual and palate taste buds. Finally, histochemistry demonstrated that the mandibular retromolar secretory gland is a minor salivary gland of mucous type. The mandibular retromolar taste structure may thus play a role in taste sensation and represent a potential novel pharmacological target for taste disorders.
Keywords: chemosensory, minor salivary gland, retromolar, taste buds, taste receptors
Introduction
The gustatory system is composed of chemoreceptor cells in the oral cavity with several cranial nerves that ultimately transfer this information to the gustatory cortex. The chemoreceptor cells responsible for taste are found in taste buds contained on the tongue. Mammals have 3 different types of papillae assembled in specific parts of the tongue (Mistretta 1991). The fungiform papillae are distributed on the anterior two-thirds of the tongue and are innervated by a branch of the facial nerve (N. VII) known as the chorda tympani (Kinnamon 2008). The circumvallate (CV) papillae, the largest of the lingual papillae, and the foliate papillae, located on the posterolateral tongue bilaterally, are localized on the distal third of the tongue. The CV and foliate papillae contain the majority of the lingual taste buds and line both sides of the lateral trench walls (Tomiyama 1977; Bartoshuk 1991; Jung et al. 2004; Finger 2005; Sohn et al. 2011). Taste buds are also located in the soft palate, epiglottis, pharynx, and the esophagus (Lalonde and Eglitis 1961; Miller and Spangler 1982; Witt 2015). Gustatory information from the CV and foliate papillae is transmitted by the glossopharyngeal nerve (N. IX) to the nucleus of the solitary tract (NTS) in the brain stem (Purves et al. 2018), and from there, the information is relayed to the thalamus, cortex, and ultimately forebrain (Norgren and Leonard 1973).
In rodents, each taste bud is composed of 50–80 elongate, mature taste cells consisting of several different categories of cells, generally classified as types I, II, III, and IV (Finger 2005; Chaudhari and Roper 2010). Type II cells, which make up one-third of the cells in a taste bud, have spherical nuclei and function as chemosensory receptors for sugars, amino acids, and/or bitter stimuli. Type II cells express G protein-coupled taste receptors (T1Rs, T2Rs) and their downstream effectors, including the G-protein gustducin (GNAT; subunits α, ß, γ), phospholipase C subtype beta 2 (PLCβ2), and transient receptor potential cation channel subfamily M member 5 (TrpM5) (McLaughlin et al. 1992; Wong et al. 1996; Huang et al. 1999; Chandrashekar et al. 2000; Mueller et al. 2005). Type III cells, termed presynaptic cells, detect sour taste, have prominent dense-core vesicles, form morphologically identifiable synapses with postsynaptic structures, and express molecules associated with vesicular exocytosis (Yee et al. 2001; Finger 2005; DeFazio et al. 2006; Roper and Chaudhari 2017).
The von Ebner glands, serous-secreting minor salivary glands, are found underlying the CV and foliate papillae (Gurkan and Bradley 1987). The von Ebner gland possesses ducts exiting into the base of the troughs between the CV and foliate papillae (Hand and Frank 2015) and secretes serous contents into the base of the moats around these papillae. The arrangement of these glands around the CV papillae provides a continuous flow of fluid over the great number of taste buds lining the sides of the papillae and is important for enhancing taste sensation and aiding in digestion.
In contrast to fungiform, foliate, and CV papillae, Iida’s research group, using light and scanning electron microscopy, first described the presence of “taste bud papillae” around the orifice of a “molar gland” of the retromolar mucosa in mice, rats, and hamsters (Iida et al. 1983). Miller and Smith (1984) also described the presence of taste bud papillae on the “buccal wall lateral to the foliate papillae.” This retromolar mucosa location containing taste bud papillae was later physiologically characterized by Travers and Norgren (1995) for its orosensory response capabilities. They observed activation of gustatory neurons in the rostral division of the NTS when stimulating the retromolar region, as well as when stimulating anterior tongue, foliate papillae, nasoincisor duct, and soft palate. Although they found that stimulation of the retromolar mucosa was the least effective for gustatory neurons in the rostral NTS, this was nonetheless the first report of neuronal response from the retromolar taste mucosa. In humans, the minor salivary gland in this retromolar region has been described as the mucous type (Iwanaga et al. 2018) and is a known site for tumors to occur (Horta et al. 2016). However, no reports have associated human taste bud papillae with this retromolar salivary gland.
Because only a few reports describe the anatomical location of the retromolar taste buds and associated salivary gland (Iida et al. 1983, Miller and Smith 1984) and its physiological sensory response to chemical stimulation (Travers and Norgren 1995), several questions remain unanswered. First, it is unknown if this mandibular retromolar chemosensory structure contains taste cells and, if so, the types of taste receptors and downstream signaling elements present. Second, although existing research in humans suggests the retromolar salivary gland is probably of mucous type, its nature remains undetermined. Third, it is not clear if the retromolar taste buds are sensory and part of the gustatory response, nor is their functional relation to the underlying salivary gland. Our study focused on 1) describing the anatomical location of the taste buds and associated salivary gland of the mandibular retromolar chemosensory structure, 2) characterizing the molecular expression of taste signaling cascade elements in the retromolar taste buds, and 3) determining the secretory type of the retromolar salivary gland.
To visualize and anatomically characterize the retromolar taste buds, we used transgenic mice expressing green fluorescent protein (GFP) under the promoter of taste-signaling-specific genes. To determine the molecular expression of taste receptors and elements of the taste signaling cascade in retromolar taste buds, we used RT–PCR and immunohistochemistry. Then, to describe the anatomical location and the secretory nature of the retromolar salivary gland, we used hematoxylin and eosin (H&E) and periodic acid Schiff (PAS) staining in serial sections of oral mucosa. We found that the mandibular retromolar chemosensory structure is composed of unorganized taste buds clusters surrounding salivary gland orifices and located bilaterally at the rear of the mandibular oral mucosa, distal to the last molar and anterior to the ascending ramus of the mandible. The retromolar taste buds express taste signaling elements, including taste receptors, similarly to other lingual and palate taste buds, and the retromolar salivary gland is of mucous type. Future studies involving physiology, biochemistry, and psychophysics will be necessary to test for a sensory function of the mandibular retromolar chemosensory structure, to determine the composition of the retromolar salivary gland secretions, and to characterize in human the orosensory responsiveness to chemical stimulation of the retromolar mucosal region.
Materials and methods
Animals
All experimental procedures were approved by the Animal Care and Use Committee of the Monell Chemical Senses Center. Adult transgenic mice used for this study expressed one of the following, all in the C57BL/6 background: TrpM5-GFP; taste receptor type 1 member 3 (T1R3)-GFP (Clapp et al. 2006); α subunit of the G-protein gustducin (Gnat3)-GFP (Wong et al. 1999); glutamate decarboxylase 1 (GAD1)-GFP (Chattopadhyaya et al. 2004); or type 2 taste receptor 131 (Tas2R131)-GFP (Voigt et al. 2012).
Anatomical characterization of the retromolar taste papilla
Mice were perfusion-fixed in 4% paraformaldehyde in 0.1 M phosphate buffer (PB). Tissues were postfixed (4 °C, 60 min) and cryoprotected in 20% sucrose-PB (4 °C, overnight). Tissue was prepared in two different ways: whole-mount of the molar and retromolar area of the mandible and maxilla and transversal serial sections of the tongue, including the mandibular molar and retromolar mucosae. Whole-mounts were prepared from all the available GFP transgenic animals and mounted flat on a concave glass slide for perpendicular observation of the taste buds and salivary gland orifices using an upright fluorescent microscope (Olympus BX63). Serial sections were prepared using TrpM5-GFP mice and cryostat sections (14–16 μm) were collected and dried onto Superfrost Plus slides (Fisher Scientific). Slides were rinsed 3 times in 0.1 M phosphate-buffered saline (PBS; 150 mM sodium chloride, 25 mM sodium phosphate dibasic anhydrous, 75 mM sodium phosphate monobasic monohydrate; pH 7.2) before being coverslipped with Fluromount G (Southern Biotechnology Associates). Images were collected with an upright Olympus BX63 fluorescent microscope, and brightness, contrast, and gamma were adjusted postacquisition in Adobe Photoshop (Adobe Systems) to approximate the appearance of the original histological samples.
Immunohistochemistry
Mice were perfusion-fixed in 4% paraformaldehyde in 0.1 M PB. Tissues were postfixed (4 °C, 60 min) and cryoprotected in 20% sucrose-PB (4 °C, overnight). Cryostat sections (14–16 μm) were collected and dried onto Superfrost Plus slides (Fisher Scientific). Slides were rinsed in 0.1 M PBS, and nonspecific binding was blocked for 1 h in blocking solution (2% normal goat or donkey serum, 1% bovine serum albumin, 0.3% Triton in PBS). Next, the slides were incubated overnight with primary antibodies in blocking solution (Table 1). Following incubation with the primary antibodies, the tissue samples were rinsed with PBS (3 × 20 min) and incubated for 2 h with fluorescent secondary antibodies (Table 2). The slides then were washed 2× 10 min in 0.1 M PBS and once for 10 min in 0.05 M PB before being coverslipped with Fluromount G (Southern Biotechnology Associates). All images were collected with a Leica TCS SP2 laser scanning confocal microscope (Leica Microsystems) using UV, Ar, GeNe, and HeNe lasers, as well as appropriate excitation spectra. Scanware software (Leica Microsystems) was used to acquire z-series stacks captured at a step size of 0.25–0.35 μm. For each image, channels were merged to produce the composite image using the native acquisition software for each device. Brightness, contrast, and gamma were adjusted postacquisition in Adobe Photoshop (Adobe Systems) to approximate the appearance of the original histological samples.
Table 1.
Primary antibodies used in the immunohistochemistry experiments
| Antisera against | Marker for | Company, catalog no., lot no. | Host; dilution |
|---|---|---|---|
| GNAT3 (α-gustducin) | G-protein subunit in Type II taste cells | Santa Cruz Biotechnology, cat. no. sc-395, lot no. J0615 | Rabbit; 1:1000 |
| PLCβ2 | Transduction component in Type II taste cells | Santa Cruz Biotechnology, cat. no. sc-206, lot no. L0715 | Rabbit; 1:500 |
| CGRP | Peptidergic nocioceptor | Sigma–Aldrich; ref. no. C8198, lot no. 066M4836V | Rabbit; 1:5000 |
Table 2.
Secondary antisera used in the immunohistochemistry experiments
| Primary antibody | Secondary antibody | Antisera against (company) | Dilution |
|---|---|---|---|
| GNAT3 (α-gustducin) | Alexa Fluor 568 | Donkey anti-rabbit (Invitrogen) | 1:400 |
| PLCβ2 | Alexa Fluor 568 | Donkey anti-rabbit (Invitrogen) | 1:400 |
| CGRP | Alexa Fluor 568 | Donkey anti-rabbit (Invitrogen) | 1:400 |
Retromolar tissue biopsy and RT–PCR
RNAseZap (Invitrogen, Waltham, MA) was used on all instruments and surfaces before biopsy removal to avoid RNase contamination. Tissues containing the retromolar taste buds and CV papillae were isolated from C57BL/6 wild-type mice after they were anesthetized with isoflurane and sacrificed by cervical dislocation. CV papillae and the retromolar epithelium containing the taste bud clusters around the salivary gland orifices (located distally to the third molar) were removed using a scalpel and smooth-grip forceps under microscopic visualization. CV papillae were included as positive control for taste signaling transduction 35 TAS2R receptors and their downstream signaling effectors (Lossow et al. 2016, Yoshida et al. 2018). The specimens were immediately placed in fresh lysis buffer containing 1% β-mercaptoethanol on ice. Sample lysis, homogenization, and RNA purification were completed according to the manufacturer’s instructions using the Invitrogen PureLink RNA Mini Kit. RNA from mouse CV papillae and retromolar tissue was extracted according to the manufacturer’s instructions using the Invitrogen PureLink RNA Mini Kit (Waltham, MA). To validate the integrity of the extracted RNA, the concentration and quality of the RNA samples were analyzed in the 2200 Bioanalyzer (Agilent, Santa Clara, CA), and RT–PCR for β-actin was performed for both CV papillae and retromolar samples. DNase I treatment and reverse transcription (cDNA synthesis) were performed using Invitrogen SuperScript IV Vilo Master Mix with ezDNASE. Reactions were set up in which the reverse transcriptase enzyme was omitted as a control to detect for possible genomic DNA contamination. The following PCR conditions were used: 94 °C for 3 min, followed by 35 cycles of 94 °C for 30 s, annealing at 57 °C for 30 s, extension at 72 °C for 30 s, and concluding with a 72 °C final extension for 7 min. Amplified sequences were visualized by gel electrophoresis in 2% agarose gels. RT–PCR primer sequences are listed in Table 3.
Table 3.
Primers used for the RT–PCR experiments
| Primer name | Sequence (5′−3′) | #BP | Primer name | Sequence (5′−3′) | #BP |
|---|---|---|---|---|---|
| Gnat3_131F | GAGAGCAAGGAATCAGCCAG | 121 | Tas2r121_658F | CGAGACCCCAGCACTAAAGC | 230 |
| Gnat3_252R | GTGCTTTTCCCAGATTCACC | Tas2r121_888R | CATCACCCAAAGACTGGCTTG | ||
| Trpm5_952F | TTCCCCAGCGAGTGTTTCTC | 269 | Tas2r122_181F | CAACAATTGCTGGTGCCTCT | 590 |
| Trpm5_1221R | CCATTCCACGTCCCCATTGA | Tas2r122_771R | GGAGCTTGCCACAATAAGCA | ||
| Plcb2_1954F | CCTGGAGGTGACAGCTTATGA | 124 | Tas2r123_102F | AGTGAACATCATGGACTGGGT | 147 |
| Plcb2_2078R | GCTCCGTGAAGGAAGAGACA | Tas2r123_249R | TCTCCTAGGCAAATGTGGGC | ||
| Actb_251F | GGTCAGAAGGACTCCTATGTGG | 102 | Tas2r124_393F | GCTTCTGGGAAGCTTGGTGT | 288 |
| Actb_353R | TGTCGTCCCAGTTGGTAACA | Tas2r124_681R | ATTTCTGTGGGCCGTAGCAC | ||
| Tas2r102_26F | AGGCGACGCTGTTATATGCC | 328 | Tas2r125_666F | CACCACCACAGCTGCACATA | 269 |
| Tas2r102_354R | AAGCCAGAGGCTGAAGTGAC | Tas2r125_935R | CAGGGAACCAACATCCGTACA | ||
| Tas2r103_565F | ACCCCATTCGCTGTGTCTTT | 312 | Tas2r126_140F | TCCTCTTCAGTTTGGGCACC | 284 |
| Tas2r103_877R | AGGCTTGCCTCAGCTTACTG | Tas2r126_424R | CGGACACCAAGATAGAGCCC | ||
| Tas2r104_434F | TTCCGCTAGCTGTGAAGGTC | 447 | Tas2r129_671F | TTGCAGATGCCCACATCAGA | 147 |
| Tas2r104_881R | AGTGCCCTCATAGTGGCTTG | Tas2r129_818R | GCTGCAACAATCTCGCAGAA | ||
| Tas2r105_294F | GTTTGCCACCAGCCTAAGCA | 212 | Tas2r130_37F | GCTGTTGGTGAGGCCTTAGT | 509 |
| Tas2r105_506R | TCCCAGTACATCTCCGAGGTC | Tas2r130_546R | GACAGAGGCATGTCCAGCTT | ||
| Tas2r106_2F | TGCTGACTGTAGCAGAAGGA | 132 | Tas2r131_314F | CCCACATTTCCCATCCCCTT | 304 |
| Tas2r106_134R | AAGCCAGCTGTGGAGAACTT | Tas2r131_618R | GTCAAGGCTTCGGGAGTGTT | ||
| Tas2r107_119F | GCTCGGAGTTTTAGGGGACA | 754 | Tas2r134_457F | ATGGCGGCCTGTGAAAACTA | 206 |
| Tas2r107_873R | AGAGGCATGTGGCTGTCAAA | Tas2r134_663R | GTGAGCCTGGGTGCTGTAAT | ||
| Tas2r108_112F | AGTCGCAGAATTGCCTCTCC | 576 | Tas2r135_543F | GAGTGGCCATCAACCTTGGA | 287 |
| Tas2r108_688R | GCCTCATAGCACCCATGTGA | Tas2r135_830R | GCAGAACTGAGTACCAGCGT | ||
| Tas2r109_587F | CTGTCCCCGTTGTTTTGTCC | 328 | Tas2r136_712F | CCCAGTGCTTCAACCCACAT | 251 |
| Tas2r109_915R | CAACACAGAGAGAGAGGCGT | Tas2r136_963R | CCAGAACCTTGCTCTCACCT | ||
| Tas2r110_700F | CAGGTCAATGCCAAACCACC | 269 | Tas2r137_19F | ACAAGCAAGGATCAGGGTGG | 638 |
| Tas2r110_969R | GCACCTCAGACAATGCAACA | Tas2r137_657R | CAGAAGGTAGGCAACCAGGG | ||
| Tas2r113_632F | ATATGCAGCACACCGCCAAA | 179 | Tas2r138_618F | AGCTTTCCTGGTTTCCTCGG | 365 |
| Tas2r113_811R | CCAGAGCCCAGACAAACAAA | Tas2r138_983R | GGAGGAACCTTGTGGACTGG | ||
| Tas2r114_7F | AGCACAATGGAAGGTGTCCT | 615 | Tas2r139_3F | GGCTCAACCCAGCAACTACT | 429 |
| Tas2r114_622R | GCCTGCGATGTCTCCAAAGT | Tas2r139_432R | CCACAGAAGCCAGGGCATTA | ||
| Tas2r115_692F | AGACTGTGGTTGCCTTCCTC | 230 | Tas2r140_665F | CCAGCACCACAGCCCATATT | 182 |
| Tas2r115_922R | AGGTTTTCTCACGCTTGCAC | Tas2r140_847R | TTAGGACACAAGAGTGGCCC | ||
| Tas2r116_569F | TTGCTGTGTCACTGGTCACT | 115 | Tas2r143_99F | AGAGTGGATGAGGAACCGGA | 584 |
| Tas2r116_684R | TCTGATGTGGGCCTTAGTGC | Tas2r143_683R | GCCATGGTATGTGCCTGAGT | ||
| Tas2r117_92F | ATGGGTTCATGGTCCTGGTC | 468 | Tas2r144_651F | CTCACTCAAGAGGCACACCC | 106 |
| Tas2r117_560R | AACACCTGCCTGTGACACTT | Tas2r144_757R | TGAGAGAGTGGCTGGTCGAT | ||
| Tas2r118_127F | TCACCGGTGGAGACGATTCT | 229 | Tas1r1_918 F | CTCCACGTACATCACCAATGT | 499 |
| Tas2r118_356R | CTCAGCCAGAGGAAGATGGG | Tas1r1_1416R | CAGTGAGGCAGAACCAATGA | ||
| Tas2r119_276F | TCTGGTTTGCCACATGGCTT | 382 | Tas1r2_42F | AGGCGAGGACACTCCATTTG | 372 |
| Tas2r119_658R | GGCATGTCTGCTAGGTTCCC | Tas1r2_413R | GGATGGGCAGGAAGTCATCTAT | ||
| Tas2r120_282F | CACTTGGCTGGGGACCATAC | 387 | Tas1r3_781F | CGCCAAGTGAACCAAAGTAAAG | 496 |
| Tas2r120_669R | GTGGACCATGGTGCTCTGAT | Tas1r3_1276R | CTGATACGTGGCAATGTGAGA |
#BP, number of base pairs (band size); tas2rs, bitter taste receptors; tas1rs, sweet/umami taste receptors; gnat3, gustducin; plcb2, plcβ2; trpm5, trpm5; and actb, β-actin.
Histochemistry
H&E and PAS staining from frozen tissue sections were used to determine the cellular composition and the secretory type of the retromolar salivary gland. Mandibular retromolar mucosal tissue containing the retromolar salivary gland was dissected, postfixed (4 °C, 60 min), and cryoprotected in 20% sucrose-PB (4 °C, overnight). Cryostat sections (12 μm) were collected and dried onto Superfrost Plus slides (Fisher Scientific). For H&E staining, slides were placed in slide holder (Electron Microscope Sciences; cat. no. 62543-06) and stained with Mayer’s hematoxylin (Sigma; cat. no. MHS-32) for 10 min in the dark followed by 5-min wash with cool running distilled water. Slides were dipped in alcoholic eosin (Sigma; cat. no. HT110115) 12 times, followed by distilled water. Slides were then dehydrated in graded alcohol (50%, 70%, 95%, and 100% ethyl alcohol) and then dipped in xylene (Sigma; cat. no. 534056), air-dried, mounted with mounting medium (Southern Biotech; cat. no. 0100-01), and coverslipped (VWR; cat. no. 48393-106). PAS staining was performed as of manufacturer recommended protocol. Briefly, the 12-μm-thick frozen sections were air-dried, immersed in periodic acid solution for 5 min, and rinsed 4 times with distilled water. Slides were then stained in Schiff’s solution for 15 min and rinsed once in hot running tap water, followed by one wash in distilled water at room temperature. Slides were then stained in Mayer’s hematoxylin (Sigma; cat. no. MHS-32) for 3 min in the dark and then with bluing reagent for 30 s, followed by rinse with distilled water. Slides were then dehydrated in graded alcohol and mounted.
Results
Anatomical location of the mandibular retromolar chemosensory structure
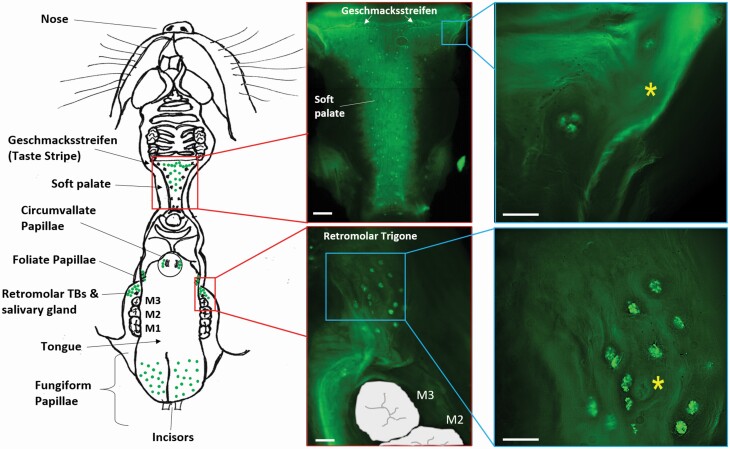
Consistent with previous reports (Iida et al. 1983, Miller and Smith 1984), our data confirmed the presence of taste buds in the mandibular retromolar mucosa lateral to the lingual foliate papillae. Our whole-mount tissue preparation from transgenic mice expressing GFP under the trpm5 promoter (Clapp et al. 2006) showed the presence of single and clustered GFP-positive taste buds (Figure 1, lower right inset) surrounding an orifice connected to the underlying secretory gland. This mandibular retromolar chemosensory structure and secretory gland are positioned at the rear of each mandible, distal to the last molar, and anterior to the ascending ramus (Figure 1, schematic; Figure 2, top). The orifice of the retromolar secretory gland is composed of epithelium arranged in concentric circles (Figure 1, lower right inset, asterisks; Figure 2, top right).
Figure 1.
Location of the retromolar chemosensory structure in the oral cavity. (Left) Schematic drawing of the mouse oral cavity. At the top is the palate and maxillary oral epithelium; at the bottom is the tongue and mandibular mucosa. Green dots represent the location of taste buds throughout the oral cavity. The retromolar chemosensory structure, posterior to the third molar (M3), is composed of taste buds (TBs) and secretory ducts from an underlying salivary gland. (Center) Whole-mount preparations of palate and retromolar mucosa from TrpM5-GFP mice (areas shown by red boxes at left) showing the taste bud (GFP-expressing) from a top view. (Right) Magnified areas of the palate and retromolar mucosa (locations indicated by blue boxes at left) both showing taste buds in the vicinity (palate) or surrounding (retromolar chemosensory structure) a ductal orifice (yellow asterisks) of the secretory gland laying below the mucosa. Scale bars = 100 µm.
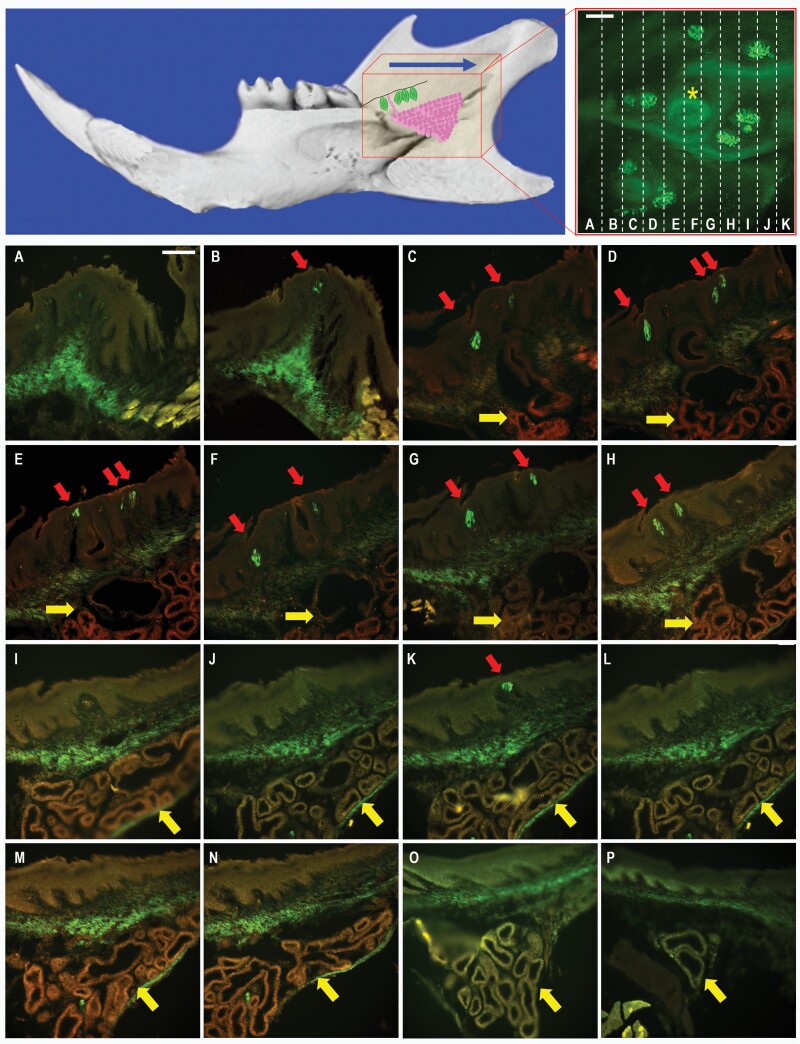
Figure 2.
Transversal serial sections of tongue and surrounding buccal mucosa. (Top left) Micro-CT picture image of the mouse mandible with a schematic representation of the retromolar chemosensory structure, showing the location of TrpM5-GFP+ taste buds (green) and associated minor salivary gland (magenta). The red box represents the area sectioned, starting posterior of the third molar and extending distally through the tongue (blue arrow). (Top right) Micrograph of the retromolar mucosa with schematic of serial sectioning (dashed lines, A–K) of the chemosensory structure with taste buds (GFP) and ductal orifice (yellow asterisk). Scale bar = 50 µm. (A–P) Serial sections of the retromolar chemosensory structure. Red arrows indicate locations of taste buds in the mucosal epithelium (B–H, K). Taste buds first appear distal to the third molar and surround the ductal orifice and secretory duct (C–G) of the underlying salivary gland (C–P). The minor salivary gland, indicated by yellow arrows, is first visible in panel C and terminates in (P). Muscle, connective tissue, and the gland show a certain level of autofluorescence that is also present in wild-type mice (see Supplementary Figure 1). Scale bar = 100 µm.
The CV and foliate papillae contain highly organized taste buds situated within troughs of the papillae (Choi et al. 2015, Roper and Chaudhari 2017); in contrast, the retromolar taste buds, sometimes found singly or in clusters, appear to be randomly distributed in the epithelium surrounding the orifice of the retromolar secretory gland duct (Figures 1 and 2), with the taste bud apex oriented toward the oral cavity perpendicular to the epithelium, similar to the palatal taste buds (Figure 1). Since gustatory papillae and the von Ebner salivary glands are closely associated to facilitate detection of taste compounds (Hand 1970, Gurkan and Bradley 1987, Sbarbati et al. 1999, Hand and Frank 2015), and because the retromolar chemosensory structure has taste buds surrounding orifices of a secretory duct, we hypothesized that the retromolar chemosensory structure is also associated with a secretory gland, probably a minor salivary gland.
To characterize the morphology and spatial organization of the mandibular retromolar chemosensory structure, we performed serial sectioning of oral cavity tissue from TrpM5-GFP mice that included the posterior portion of the tongue and the retromolar portion of the mandibular epithelium. Tissue sections were cut transversally to obtain sections of the tongue and retromolar mucosa on the same plane. Tissue collection started distal to the last mandibular molar and extended through the posterior part of the tongue (Figure 2, top). Looking inside the oral cavity from the mouth, the retromolar chemosensory structure appears first on the serial sections as taste buds, endogenously expressing GFP, situated on the superficial mucosal epithelium posterior to the third molar (Figure 2B, red arrow). The tissue sections present some autofluorescence in muscular and connective tissue structures, as shown in our serial section of retromolar mucosa from C57BL6/J wild-type mice (Supplementary Figure 1, top). After a few sections, a glandular structure (Figure 2C–P, yellow arrow) and associated duct (Figure 2C–G) appear underneath the taste-bud-containing epithelium. Posterior to the orifice, more taste buds are visible on the mucosal epithelium (Figure 2C–K, arrows), and the secretory glandular structure extends in the retromolar trigone until disappearing (Figure 2P). After further sectioning of the lingual epithelium, the foliate papillae will be visible, and once the retromolar secretory gland is long gone, the CV finally appears on the serial sections (not shown). Figure 1 (left) schematically represents the spatial distribution observed in the serial sections of the 3 chemosensory structures, with the retromolar taste buds on each side of the mandibular mucosa appearing first, followed by the foliate and then the CV papillae, with the retromolar chemosensory structure and foliate papillae in close proximity to the food during mastication and the CV more distal before the swallowing process begins. This arrangement resembles a funnel shape, with the retromolar taste buds and foliate papillae at the widening of the funnel and the CV at the narrower position.
Molecular expression of taste signaling cascade elements in the retromolar taste buds
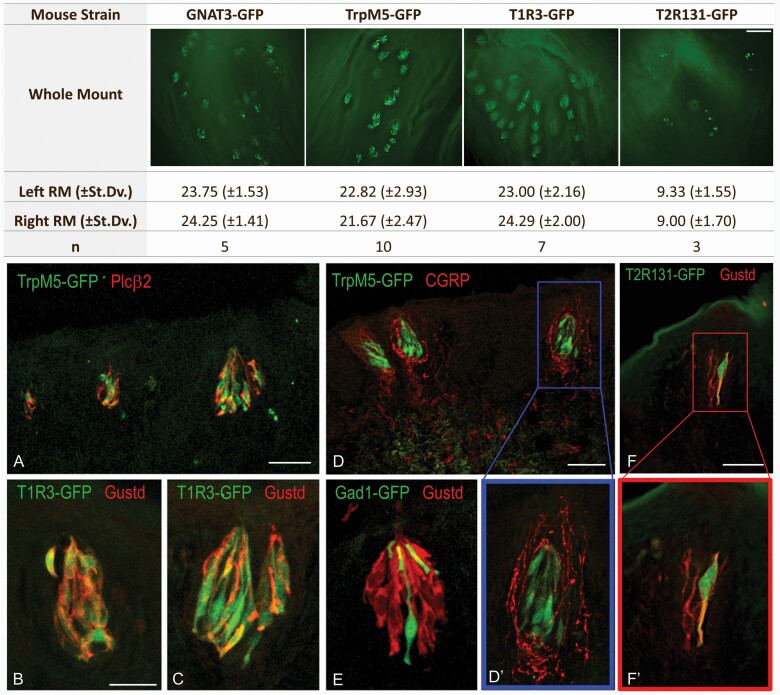
To determine the expression pattern of the retromolar taste buds for elements of the taste signaling transduction cascade, we used whole-mount preparations of the retromolar trigone mucosa of transgenic mice expressing GFP under promoters for trpm5 (n = 10), gnat3 (n = 5), tas1r3 (n = 7), and tas2r131 (n = 3) (Figure 3, top). An average of 23.19 ± 2.20 taste buds on the left and 23.40 ± 1.96 taste buds on the right mandibular mucosae expressed trpm5, gnat3, and tas1r3. In contrast, in Tas2R131-GFP mice we observed approximately 9 taste buds on each side (Figure 3, top right), indicating that not all retromolar taste buds express this particular bitter receptor.
Figure 3.
Quantification of taste buds of the retromolar chemosensory structure and immunohistochemistry for markers of the taste signaling transduction cascade. (Top) Quantification of the retromolar taste buds in transgenic mice expressing GFP under the promoter for gnat3, trpm5, tas1r3, or tas2r131 (mean ± SD). Representative fluorescent micrographs are shown for each GFP-transgenic strain. Scale bar = 100 µm. (Bottom) Immunohistochemical analysis of chemosensory markers in transgenic mice expressing GFP under the promoter for trpm5, tas1r3, tas2r131 (all markers for type II taste cells), and gad1 (marker for type III taste cells). Sections are stained with primary antibodies against Gnat3 or PLCβ2 as markers for type II taste cells and CGRP as marker of peptidergic nociceptive nerve fibers. PLCβ2 immunoreactivity (red) is visible in TrpM5-GFP+ taste bud cells (A). Gnat3 (Gustd; red) co-stains most of the T1R3-GFP+ cells (B and C) and all the Tas2R131-GFP-positive cells (F–F’) but does not colocalize with GAD1-GFP+ cells (E). TrpM5-GFP+ taste buds show perigemmal CGRP-immunoreactive (red) peptidergic innervations (D–D’). (D’) and (F’) are magnified micrographs of (D) and (F). Scale bars = B, 50 µm for panels B, C, E, D’, F’; 100 µm in A, D, and F.
To characterize the chemosensory profile of the retromolar taste buds, we used immunohistochemistry on tissue sections obtained from TrpM5-, T1R3-, Tas2R131-, or GAD1-GFP mice. Tissue sections were stained with primary antibodies specific for the taste chemosensory marker PLCβ2 or GNAT3. We observed GFP-positive retromolar taste bud cells in the TrpM5-, Tas2R131-, and T1R3-GFP mice immunoreactive for PLCβ2 or GNAT3, with morphological features of elongated cells with large round nuclei consistent with type II taste cells (Figure 3A–C and F,F’), consistent with staining patterns previously described in type II taste cells of the CV and foliate papillae (Tizzano et al. 2008). Tissue sections from GAD1-GFP mice showed the presence of GFP-positive type III taste cells in the retromolar taste buds that never coexpress the type II taste cell marker GNAT3 (Figure 3E).
Furthermore, we characterized retromolar taste buds from the TrpM5-GFP mouse for their association with sensory innervation. Using a specific antibody against calcitonin gene-related peptide (CGRP), a marker of peptidergic polymodal nociceptive sensory innervations, we observed immunoreactive fibers for CGRP in the retromolar mucosa tissue wrapping around TrpM5-GFP-positive taste buds (Figure 3D and D’), consistent with observations in previous studies of CGRP-immunoreactive nerve fibers densely distributed in the connective tissue core of the CV and foliate papillae (Montavon and Lindstrand 1991, Kusakabe et al. 1998).
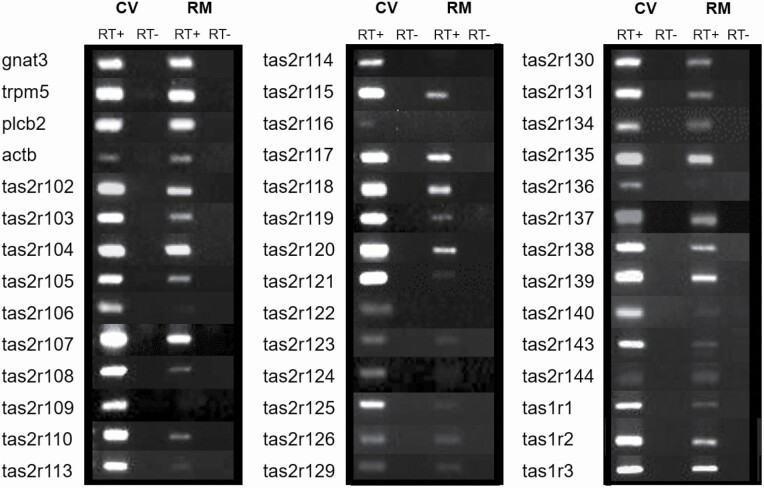
Because previous studies have shown that the retromolar mucosa responds to a taste mixture of sucrose, quinine hydrochloride, NaCl, and HCl (Travers and Norgren 1995), we used RT–PCR to determine whether the mandibular retromolar taste buds express taste receptors (tas1rs and tas2rs) and downstream genes of the canonical taste signaling cascade (gnat3, plcb2, and trpm5). Our results show that the retromolar taste buds express all 3 mouse tas1r genes, as well as the taste transduction signaling genes gnat3, plcb2, and trpm5 (Figure 4), which confirms our immunohistochemistry results for Gnat3 and PLCβ2 immunostaining in TrpM5- and T1R3-GFP mice (Figure 3A–C). Unlike the CV papillae, not all the tas2r genes are expressed in the mandibular retromolar taste buds, with 28 of the 35 mouse tas2r genes present in the CV taste tissue expressed in the retromolar taste mucosa (Figure 4) (Lossow et al. 2016, Yoshida et al. 2018). Our RT–PCR experiment for tas2r genes confirms the presence of tas2r131 observed in the immunohistochemistry for Gnat3 in Tas2R131-GFP animals (Figure 3F and F’).
Figure 4.
Expression analysis of the retromolar chemosensory structure for sweet, umami, and bitter taste receptor and downstream signaling effector genes. The retromolar taste buds (RM) express all 3 mouse tas1r genes, as well as the taste transduction signaling cascade genes gnat3, plcb2, and trpm5. Unlike in the circumvallate papilla (CV), only 28 of the 35 tas2r genes are expressed in the retromolar taste mucosa tissue. The CV was used as positive control for all the genes. RT+ and RT− = RNA samples transcribed in the presence or absence (negative control) of reverse transcription enzyme, respectively. actb = β-actin as housekeeping gene. Table 3 lists band sizes and primer sequences.
Determining the secretory type of the retromolar salivary gland
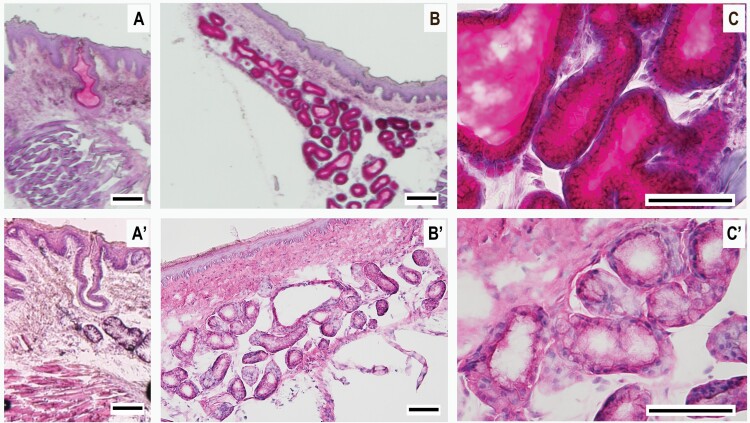
We characterized the cellular composition and secretory type of the mandibular retromolar gland using H&E and PAS histochemical staining. These experiments demonstrated that the mandibular retromolar secretory gland is a minor salivary gland of mucous type. With H&E staining, we observed that the retromolar salivary gland contains acini consisting of light-stained mucous cells with nuclei positioned at the base of the acinus cells (Figure 5A’–C’, Supplementary Figure 3, bottom) compared with the darker staining typical of the serous von Ebner gland cells (Supplementary Figure 2). The terminal secretory portion of the retromolar salivary gland acini passes into narrow glandular tubules, which form the excretory intercalated duct that empties into a reservoir. The mucin secretion then reaches the surface epithelium through a secretory duct ending in an epithelial orifice located between the taste buds of the mandibular retromolar taste structure (Figs. 2C–G and 5A,A’). With PAS staining, we determined that the salivary secretions of the acinus cells and salivary ducts are composed of mucin glycoprotein, as evidenced by the cell and duct content stained magenta (Figure 5A–C, Supplementary Figure 3, top), confirming the findings of the previous H&E experiments that reveal the retromolar salivary gland as mucous type.
Figure 5.
H&E and PAS histochemical staining of the retromolar mucous salivary gland and salivary duct. Micrographs of salivary gland tissue sections from posterior lingual tissue and retromolar mucosa with H&E and PAS histochemical staining. (A–C) The acinus cells of the salivary gland and the salivary secretions in the ducts are composed of mucin glycoprotein, as evidenced by the magenta color, a subproduct of the histochemical reaction of the PAS staining methodology. (A’–C’) Serial sections of the retromolar chemosensory structure stained with H&E, confirming that the retromolar salivary gland is of mucous type. Acini comprise light-stained mucous cells with nuclei positioned at the base, compared with the darker staining typical of the serous von Ebner gland cells (Supplementary Figure 2). Visible in (A) and (A’) the secretory duct ending in an epithelial orifice located between the taste buds of the mandibular retromolar taste structure. (A), (B), (A’), and (B’) are at 10× magnification, whereas C and C’ are at 40×. The seriate sections of the entire retromolar salivary gland are available in Supplementary Figure 3. Scale bars = 100µm.
Discussion
We have used histochemical and RT–PCR techniques to examine a retromolar chemosensory structure in the rodent mandibular mucosa previously characterized using light and electron microscopy (Iida et al. 1983, Miller and Smith 1984). We confirmed the presence and location of this mandibular retromolar chemosensory structure and closely characterized its taste buds and the mucous minor salivary gland using transgenic mouse models, biochemistry, histochemistry, and molecular biology techniques. The number of taste buds we found in TrpM5-, Gnat3-, and T1R3-GFP transgenic mice averaged 23.19 ± 2.20 on the left and 23.40 ± 1.96 on the right mandibular mucosae, for a total of ~46 taste buds for the two sides together, comparable to the total number of 42 ± 6 retromolar taste buds reported by Iida research group (Iida et al. 1983).
The mandibular retromolar taste buds are located on the surface of the epithelium, adjacent but posterior to the last molar, distributed around the ductal orifice of the underlying salivary gland, suggesting a close relationship between these taste buds and the salivary secretions. The overall morphology of the mandibular retromolar taste buds is similar to that of CV and foliate papilla taste buds (Hand 1970, Hand and Frank 2015); however, in contrast to CV and foliate taste buds, the retromolar taste buds are not contained within troughs of the papillae. Instead, they rest on the basal lamina of the epithelium, with inner taste pores oriented toward the oral cavity, thus resembling taste buds found on the palate (Miller 1977), pharynx, and larynx (Travers and Nicklas 1990).
Our immunohistochemistry results obtained from T1R3-GFP, TrpM5-GFP, and GAD1-GFP mice show that the retromolar taste buds are composed of different cell types, including type II and III taste cells, suggesting that they are indeed chemosensory in function. Travers and Norgren (1995) reported that the rat retromolar region is responsive to gustatory stimulation. When applying a mix of sucrose, sodium chloride, hydrogen chloride, and quinine hydrochloride to the retromolar region, they observed activation of the gustatory neurons of the NST. In support of these functional data and our immunohistochemical characterization, our RT–PCR analysis revealed that retromolar taste bud tissue expresses all 3 tas1r and 28 of the 35 tas2r genes and their downstream signaling mediators (gnat3, plcb2, and trpm5). The expression of sweet, umami, and bitter taste receptors on mandibular retromolar taste buds supports the hypothesis of a chemosensory function for this structure and may augment the taste detection from other regions of the oral cavity. However, the full complement of tas2r genes is not expressed in the retromolar taste buds, so they may serve a more specialized function than the T2Rs of the CV and foliate papillae and other oral taste buds.
Our immunohistochemistry revealed that CGRP is expressed in the sensory neurons innervating the retromolar taste buds, similar to what was found in other lingual taste buds. Indeed, immunohistochemistry revealed that CGRP and TRPV1 coexist in the sensory neurons innervating the CV papillae (Ishida et al. 2002). Several studies point to a role for CGRP as an efferent transmitter in the peripheral taste organs (Holzer 1988, Maggi and Meli 1988, Wang et al. 1995, Simon et al. 2003). More recently, CGRP has been implicated to be an inhibitory transmitter that shapes peripheral taste signals via serotonergic signaling during processing gustatory information in taste buds (Huang and Wu 2015). The extensive CGRP-immunoreactive innervation of the retromolar taste buds may also contribute to specific chemical sensitivities relating to the taste in the retromolar chemosensory structure. In addition, CGRP would be still capable of evoking nociceptive responses to chemical irritants as part of a common chemical sense.
Interestingly, the distribution of oral taste buds in the posterior portion of the oral cavity resembles a V-like shape comprising, proximal-laterally, the mandibular retromolar chemosensory structure, followed by the foliate papillae, and converging more distal-centrally with the CV papillae. This distribution would allow for more efficient detection of food during mastication. As food is masticated by the molars, it is pushed posteriorly into the retromolar trigone mucosa. The retromolar taste buds and associated mucous salivary gland may function as an accessory chemosensory organ in the oral cavity and augment the function of the gustatory lingual papillae and associated von Ebner secretory glands in facilitating taste sensation and digestion, as well as in evaluating the chemical composition of potential nutrients or toxins before swallowing a bolus of food.
Although the von Ebner glands associated with CV and foliate papillae secrete serous fluid containing digestive enzymes into the papillae troughs (Hand 1970, Hand and Frank 2015), our H&E and PAS staining experiments demonstrate that the mandibular retromolar salivary gland secretion is composed of mucin and possibly other glycoproteins. Since the secretory ducts of the retromolar salivary gland open directly onto the surface of the oral mucosa posterior to the third molars, the gland secretion may moisten the retromolar mucosa and lubricate the food.
It is possible that there is a connection between the mandibular retromolar chemosensory structure and a few clinical reports of temporary taste changes (dysgeusia), following surgical extraction of the third molar (Shafer et al. 1999; Akal et al. 2004; Anand et al. 2018; Albuquerque et al. 2019). It is still not clear if these taste changes, observed in some patients (Shafer et al. 1999; Akal et al. 2004; Anand et al. 2018; Albuquerque et al. 2019), but not in others (Ridaura-Ruiz et al. 2012), may be a consequence of the surgical procedure. The retromolar tissue is often disturbed to efficiently remove the tooth, and it is possible that damage to the retromolar taste bud structure, its innervation, and/or the associated salivary gland may occur during the extraction procedure. Moreover, in these studies, different tastants at different concentrations were used to test sensory sensitivity, which could explain the divergent results.
Because taste abnormalities can lead to a decrease in quality of life, weight loss, malnutrition, and certain diseases and conditions (Bromley 2000), further studies are needed to determine whether the retromolar chemosensory structure is present in humans, whether it plays a role in taste sensation, and whether third molar extraction and other oral surgical procedures may affect normal gustatory function. Future studies involving physiology, biochemistry, and psychophysics experiments will be necessary to determine the complete composition of the retromolar salivary gland secretion and whether the retromolar chemosensory structure plays a functional role in gustation.
Supplementary material
Supplementary data are available at Chemical Senses online.
Supplementary Figure 1. Autofluorescence in retromolar mucosa sections of a C57BL6/J wild-type mouse. Fluorescent micrographs (magnifications of 10×, 20×, and 40×) taken in the red and green channels showing autofluorescence in retromolar mucosa tissue sections of both C57BL5/J wild-type mice (top) and TrpM5-GFP mice (bottom). Autofluorescence is present mostly in the connective, glandular, and muscular tissues in both mouse strains. True GFP expression is present only in the retromolar taste buds of the TrpM5-GFP mouse (arrows). Scale bars = 50 µm.
Supplementary Figure 2. H&E and PAS histochemical staining of the von Ebner serous glands. Micrographs (magnifications of 10×, 20×, and 40×) of salivary gland tissue sections from posterior lingual tissue stained with H&E and PAS. Top. The von Ebner secretory gland, located beneath the circumvallate papillae (green box), is of serous type, as shown by the darker PAS staining typical of serous salivary gland cells. Bottom. This is confirmed in the H&E staining, where most of the gland shows a darker staining typical of serous cells (yellow dotted line), although a few acini cells are mucous type (blue dotted line). Scale bars = 50µm.
Supplementary Figure 3. H&E and PAS histochemical staining of the retromolar mucous salivary gland (Serial section). A–P. Serial sections of the retromolar chemosensory structure stained with PAS staining methodology. A’–P’. Serial sections of the retromolar chemosensory structure stained with H&E staining methodology. Scale bars = 100 µm.
Acknowledgments
We thank Dr. Susan Travers for her help in providing us with insightful materials on previous reports of retromolar taste papillae. We are also very grateful to the German Institute of Human Nutrition Potsdam-Rehbrücke (Germany) as the source of the Tas2R131-GFP mouse strain.
Contributor Information
Quan T Nguyen, Monell Chemical Senses Center, 3500 Market Street, Philadelphia, PA 19104, USA.
Grace E Beck Coburn, Department of Endodontics, The Robert Schattner Center, University of Pennsylvania, School of Dental Medicine, 240 South 40th Street, Philadelphia, PA 19104-6030, USA.
Amber Valentino, Monell Chemical Senses Center, 3500 Market Street, Philadelphia, PA 19104, USA.
Bekir Karabucak, Department of Endodontics, The Robert Schattner Center, University of Pennsylvania, School of Dental Medicine, 240 South 40th Street, Philadelphia, PA 19104-6030, USA.
Marco Tizzano, Monell Chemical Senses Center, 3500 Market Street, Philadelphia, PA 19104, USA; Department of Endodontics, The Robert Schattner Center, University of Pennsylvania, School of Dental Medicine, 240 South 40th Street, Philadelphia, PA 19104-6030, USA.
Funding
This work was supported by Monell Institutional funds to M.T. and G20OD020296 “Improvements to the animal facility HVAC system at the Monell Chemical Senses Center.”
Conflict of interest
The authors declare that there is no conflict of interest in the publication of this manuscript.
References
- Akal UK, Kucukyavuz Z, Nalcaci R, Yilmaz T. 2004. Evaluation of gustatory function after third molar removal. Int J Oral Maxillofac Surg. 33:564–568. [DOI] [PubMed] [Google Scholar]
- Albuquerque AFM, Soares ECS, De barros silva PG, De lima BB. Carvalho FSR, Ribeiro TR, De Sa Cavalcante D, Costa FWG. 2019. Clinical investigation of gustatory and neurosensory alterations following mandibular third molar surgery: an observational prospective study. Clin Oral Investig. 23:2941–2949. [DOI] [PubMed] [Google Scholar]
- Anand R, Shankar DP, Manodh P, Devadoss P, Aparna M, Neelakandan RS. 2018. Short-term evaluation of gustatory changes after surgical removal of mandibular third molar-a prospective randomized control trial. J Oral Maxillofac Surg. 76:258–266. [DOI] [PubMed] [Google Scholar]
- Bartoshuk L. 1991. Losses of taste in regions of the tongue’s surface. Appetite. 17:69. [DOI] [PubMed] [Google Scholar]
- Bromley SM. 2000. Smell and taste disorders: a primary care approach. Am Fam Phys. 61:427–436, 438. [PubMed] [Google Scholar]
- Chandrashekar J, Mueller KL, Hoon MA, Adler E, Feng L, Guo W, Zuker CS, Ryba NJ. 2000. T2Rs function as bitter taste receptors. Cell. 100:703–711. [DOI] [PubMed] [Google Scholar]
- Chattopadhyaya B, Di Cristo G, Higashiyama H, Knott GW, Kuhlman SJ, Welker E, Huang ZJ. 2004. Experience and activity-dependent maturation of perisomatic GABAergic innervation in primary visual cortex during a postnatal critical period. J Neurosci. 24:9598–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhari N, Roper SD. 2010. The cell biology of taste. J Cell Biol. 190:285–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi M, Lee WM, Yun SH. 2015. Intravital microscopic interrogation of peripheral taste sensation. Sci Rep. 5:8661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp TR, Medler KF, Damak S, Margolskee RF, Kinnamon SC. 2006. Mouse taste cells with G protein-coupled taste receptors lack voltage-gated calcium channels and SNAP-25. BMC Biol. 4:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defazio RA, Dvoryanchikov G, Maruyama Y, Kim JW, Pereira E, Roper SD, Chaudhari N. 2006. Separate populations of receptor cells and presynaptic cells in mouse taste buds. J Neurosci. 26:3971–3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger TE. 2005. Cell types and lineages in taste buds. Chem Senses. 30(Suppl 1):i54–i55. [DOI] [PubMed] [Google Scholar]
- Gurkan S, Bradley RM. 1987. Autonomic control of von Ebner’s lingual salivary glands and implications for taste sensation. Brain Res. 419:287–293. [DOI] [PubMed] [Google Scholar]
- Hand AR. 1970. The fine structure of von Ebner’s gland of the rat. J Cell Biol. 44(2):340–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand AR, Frank ME. 2015. Fundamentals of oral histology and physiology. Ames (IA): John Wiley & Sons. [Google Scholar]
- Holzer P. 1988. Local effector functions of capsaicin-sensitive sensory nerve endings: involvement of tachykinins, calcitonin gene-related peptide and other neuropeptides. Neuroscience. 24:739–68. [DOI] [PubMed] [Google Scholar]
- Horta R, Nascimento R, Silva A, Amarante J. 2016. The retromolar trigone: anatomy, cancer treatment modalities, reconstruction, and a classification system. J Craniofac Surg. 27:1070–1076. [DOI] [PubMed] [Google Scholar]
- Huang L, Shanker YG, Dubauskaite J, Zheng JZ, Yan W, Rosenzweig S, Spielman AI, Max M, Margolskee RF. 1999. Ggamma13 colocalizes with gustducin in taste receptor cells and mediates IP3 responses to bitter denatonium. Nat Neurosci. 2:1055–1062. [DOI] [PubMed] [Google Scholar]
- Huang AY, Wu SY. 2015. Calcitonin gene-related peptide reduces taste-evoked atp Secretion from mouse taste buds. J Neurosci. 35:12714–12724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida M, Yoshioka I, Muto H. 1983. Taste bud papillae on the retromolar mucosa of the rat, mouse and golden hamster. Acta Anat (Basel). 117:374–381. [DOI] [PubMed] [Google Scholar]
- Ishida Y, Ugawa S, Ueda T, Murakami S, Shimada S. 2002. Vanilloid receptor subtype-1 (VR1) is specifically localized to taste papillae. Brain Res Mol Brain Res. 107:17–22. [DOI] [PubMed] [Google Scholar]
- Iwanaga J, Nakamura K, Alonso F, Kirkpatrick C, Oskouian RJ, Watanabe K, Tubbs RS. 2018. Anatomical study of the so-called “retromolar gland”: distinguishing normal anatomy from oral cavity pathology. Clin Anat. 31:462–465. [DOI] [PubMed] [Google Scholar]
- Jung HS, Akita K, Kim JY. 2004. Spacing patterns on tongue surface-gustatory papilla. Int J Dev Biol. 48:157–161. [DOI] [PubMed] [Google Scholar]
- Kinnamon S. 2008. 4.10 – taste transduction. San Diego (CA): Academic Press. [Google Scholar]
- Kusakabe T, Matsuda H, Gono Y, Furukawa M, Hiruma H, Kawakami T, Tsukuda M, Takenaka T. 1998. Immunohistochemical localisation of regulatory neuropeptides in human circumvallate papillae. J Anat. 192(Pt 4):557–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalonde ER, Eglitis JA. 1961. Number and distribution of taste buds on the epiglottis, pharynx, larynx, soft palate and uvula in a human newborn. Anat. Rec. 140:91–95. [DOI] [PubMed] [Google Scholar]
- Lossow K, Hubner S, Roudnitzky N, Slack JP, Pollastro F, Behrens M, Meyerhof W. 2016. Comprehensive analysis of mouse bitter taste receptors reveals different molecular receptive ranges for orthologous receptors in mice and humans. J Biol Chem. 291:15358–15377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi CA, Meli A. 1988. The sensory-efferent function of capsaicin-sensitive sensory neurons. Gen Pharmacol. 19:1–43. [DOI] [PubMed] [Google Scholar]
- Mclaughlin SK, Mckinnon PJ, Margolskee RF. 1992. Gustducin is a taste-cell-specific G protein closely related to the transducins. Nature. 357:563–569. [DOI] [PubMed] [Google Scholar]
- Miller IJ, Jr. 1977. Gustatory receptors of the palate. In: Katsuki Y, Stakagi MS, Yoomura ND, editors. Food intake and chemical senses. Tokyo (Japan): Jpn. Sci. Soc. [Google Scholar]
- Miller IJ Jr., Smith DV. 1984. Quantitative taste bud distribution in the hamster. Physiol Behav. 32:275–285. [DOI] [PubMed] [Google Scholar]
- Miller IJ, Jr, Spangler KM. 1982. Taste bud distribution and innervation on the palate of the rat. Chem Senses. 7:99–108. [Google Scholar]
- Mistretta CM. 1991. Developmental neurobiology of the taste system. In: Getchell TV, Doty RL, Bartoshuk LM, Snow JB, editors. Taste and smell in health and disease. New York: Raven Press. [Google Scholar]
- Montavon P, Lindstrand K. 1991. Immunohistochemical localization of neuron-specific enolase and calcitonin gene-related peptide in rat taste papillae. Regul Pept. 36:219–233. [DOI] [PubMed] [Google Scholar]
- Mueller KL, Hoon MA, Erlenbach I, Chandrashekar J, Zuker CS, Ryba NJ. 2005. The receptors and coding logic for bitter taste. Nature. 434:225–259. [DOI] [PubMed] [Google Scholar]
- Norgren R, Leonard CM. 1973. Ascending central gustatory pathways. J Comp Neurol. 150:217–237. [DOI] [PubMed] [Google Scholar]
- Purves D, Augustine GJ, Fitzpatrick D, Hall WC, Lamantia A-S, White LE, Mooney RD, Platt ML. 2018. The chemical senses. In: Purves D, Augustine GJ, Fitzpatrick D, Hall WC, Lamantia A-S, White LE, Mooney RD, Platt ML, editors. Neuroscience. New York: Oxford University Press. [Google Scholar]
- Ridaura-Ruiz L, Figueiredo R, Valmaseda-Castellón E, Berini-Aytés L, Gay-Escoda C. 2012. Sensibility and taste alterations after impacted lower third molar extractions. A prospective cohort study. Med Oral Patol Oral Cir Bucal. 17(5):e759–e764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper SD, Chaudhari N. 2017. Taste buds: cells, signals and synapses. Nat Rev Neurosci. 18:485–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sbarbati A, Crescimanno C, Osculati F. 1999. The anatomy and functional role of the circumvallate papilla/von Ebner gland complex. Med Hypotheses. 53:40–44. [DOI] [PubMed] [Google Scholar]
- Shafer DM, Frank ME, Gent JF, Fischer ME. 1999. Gustatory function after third molar extraction. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 87:419–428. [DOI] [PubMed] [Google Scholar]
- Simon SA, Liu L, Erickson RP. 2003. Neuropeptides modulate rat chorda tympani responses. Am J Physiol Regul Integr Comp Physiol. 284:R1494–R505. [DOI] [PubMed] [Google Scholar]
- Sohn WJ, Gwon GJ, An CH, Moon C, Bae YC, Yamamoto H, Lee S. Kim JY. 2011. Morphological evidences in circumvallate papilla and von Ebners’ gland development in mice. Anat Cell Biol. 44:274–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tizzano M, Dvoryanchikov G, Barrows JK, Kim S, Chaudhari N, Finger TE. 2008. Expression of Galpha14 in sweet-transducing taste cells of the posterior tongue. BMC Neurosci. 9:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomiyama H. 1977. A quantitative evaluation of the area and the number of the somata in the human circumvallate taste bud by electron microscope (author’s transl). Nihon Jibiinkoka Gakkai Kaiho. 80:1352–1328. [DOI] [PubMed] [Google Scholar]
- Travers SP, Nicklas K. 1990. Taste bud distribution in the rat pharynx and larynx. Anat Rec. 227:373–379. [DOI] [PubMed] [Google Scholar]
- Travers SP, Norgren R. 1995. Organization of orosensory responses in the nucleus of the solitary tract of rat. J Neurophysiol. 73:2144–2162. [DOI] [PubMed] [Google Scholar]
- Voigt A, Hubner S, Lossow K, Hermans-Borgmeyer I, Boehm U, Meyerhof W. 2012. Genetic labeling of Tas1r1 and Tas2r131 taste receptor cells in mice. Chem Senses. 37:897–911. [DOI] [PubMed] [Google Scholar]
- Wang Y, Erickson RP, Simon SA. 1995. Modulation of rat chorda tympani nerve activity by lingual nerve stimulation. J Neurophysiol. 73:1468–1483. [DOI] [PubMed] [Google Scholar]
- Witt MRK. 2015. Anatomy of the tongue and taste buds. In: Doty RL, editor. Handbook of olfaction and gustation. New Jersey: Wiley. [Google Scholar]
- Wong GT, Gannon KS, Margolskee RF. 1996. Transduction of bitter and sweet taste by gustducin. Nature. 381:796–800. [DOI] [PubMed] [Google Scholar]
- Wong GT, Ruiz-Avila L, Margolskee RF. 1999. Directing gene expression to gustducin-positive taste receptor cells. J Neurosci. 19:5802–5809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee CL, Yang R, Bottger B, Finger TE, Kinnamon JC. 2001. “Type III” cells of rat taste buds: immunohistochemical and ultrastructural studies of neuron-specific enolase, protein gene product 9.5, and serotonin. J Comp Neurol. 440:97–108. [DOI] [PubMed] [Google Scholar]
- Yoshida R, Takai S, Sanematsu K, Margolskee RF, Shigemura N, Ninomiya Y. 2018. Bitter taste responses of gustducin-positive taste cells in mouse fungiform and circumvallate papillae. Neuroscience. 369:29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.