Abstract
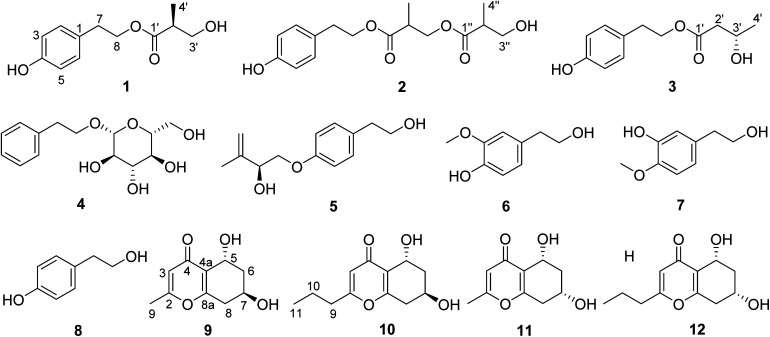
Three new phenolic metabolites, daldispols A–C (1–3), two new chromone derivatives, (5R,7R)-5,7-dihydroxy-2-methyl-5,6,7,8-tetrahydro-4H-chromen-4-one (9) and (5R,7R)-5,7-dihydroxy-2-propyl-5,6,7,8-tetrahydro-4H-chromen-4-one (10), together with five known phenolic compounds (4–8) and two known chromone compounds (11 and 12) were isolated from the endolichenic fungus Daldinia sp. CPCC 400770. Their structures were elucidated on the basis of spectroscopic methods, electronic circular dichroism (ECD), and comparison with reported data. Compounds 1, 3, 4, 9, and 11 exhibited significant anti-influenza A virus (IAV) activities with IC50 values of 12.7, 6.4, 12.5, 16.1, and 9.0 μM, respectively, and compound 8 displayed significant anti-ZIKV activity with inhibitory ratio of 42.7% at 10 μM. The results demonstrated that the fungus Daldinia sp. CPCC 400770 might be a rich source for discovering anti-IAV secondary metabolites as potential novel leading compounds.
Eight phenols including three new ones (1–3) and four chromones including two new ones (9 and 10) were isolated from endolichenic fungus Daldinia sp. CPCC 400770, and some of them showed significant antiviral activities.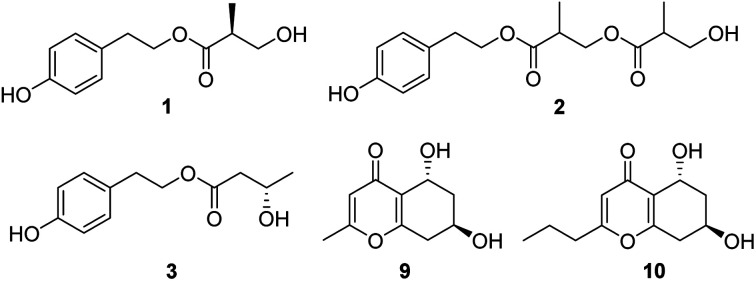
Introduction
Endolichenic fungi, inhabiting asymptomatically in the thalli of lichen, have been demonstrated to be a valuable source for the discovery of novel and bioactive natural products, and have attracted considerable attention from chemists and biologists.1–5 In recent years, a variety of secondary metabolites with impressive biological activities have been reported in endolichenic fungi.6–12 The fungus Daldinia species was widely distributed around the world, and a total of 47 taxa in Daldinia were recognized by 2014,13 however, only a limited number of secondary metabolites were reported from endolichenic fungus Daldinia species found in lichen.14–16 The fungus Daldinia sp. CPCC 400770 was isolated from lichen, collected from Yunnan province, China, and the crude extract of this strain showed antiviral and antibacterial activities in preliminary bioactive assays. Our prior work on endolichenic fungi Daldinia sp. CPCC 400770 afforded two new cyclopentenones, daldispones A and B, which showed significant anti-influenza A virus and moderate antibacterial activities.17 In our continuing search for various bioactive secondary metabolites from this fungus, its extract was further investigated, which led to the isolation of five new compounds, including three phenols, daldispols A–C (1–3), two chromones, (5R,7R)-5,7-dihydroxy-2-methyl-5,6,7,8-tetrahydro-4H-chromen-4-one (9) and (5R,7R)-5,7-dihydroxy-2-propyl-5,6,7,8-tetrahydro-4H-chromen-4-one (10), along with five known phenolic compounds (4–8) and two known chromone compounds (11 and 12) (Fig. 1). Details of the isolation, structure elucidation, and biological activities of these metabolites are reported herein.
Fig. 1. Structures of compounds 1–12.
Results and discussion
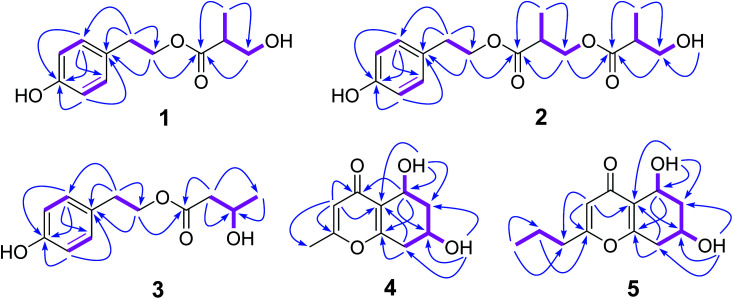
Compound 1 was isolated as a colourless oil, and the molecular formula was established to be C12H16O4 by HRESIMS with m/z 247.0930 [M + Na]+ (calcd 247.0946). The 1H NMR spectrum (Table 1) of 1 displayed the presence of AA′BB′ system aromatic protons at δH 7.03 (2H, d, J = 8.4 Hz) and 6.67 (2H, d, J = 8.4 Hz), two pair of ortho-coupled methylenes at δH 4.14 (2H, td, J = 12.6, 1.8 Hz) and 2.75 (2H, t, J = 12.6), one oxygenated methylene at δ 3.51 (1H, m) and 3.40 (1H, m), one methine at δH 2.48 (1H, m), and one methyl at δH 0.99 (3H, d, J = 7.2 Hz). The 13C NMR and DEPT spectra (Table 1) of 1 displayed 12 carbons, including three nonprotonated carbons (δC 174.4, 155.8, and 127.9, including one ketone carbonyl and one oxygenated aromatic), five methine carbons (δC 129.8, 129.8, 115.1, 115.1, and 42.1, including four aromatic and one aliphatic), three methylene carbons (δC 64.6, 63.3, and 33.6, including two oxygenated aliphatic), and one methyl carbons (δC 13.5). In the HMBC spectrum (Fig. 2), the cross peaks from H-2 to C-3, C-4, C-6, and C-7, from H-3 to C-1, C-4, and C-5, from H-7 to C-1, C-2, C-6, and C-8, indicated the presence of p-hydroxyphenyl ethanol group; the correlations from H-3′ to C-1′, C-2′, and C-4′, from H-4′ to C-1′, C-2′, and C-3′, along with the with 1H–1H COSY correlations (Fig. 2) of H-4′/H-2′/H-3′/3′-OH established the 3-hydroxy-2-methylpropionic acid substituent. Furthermore, the critical HMBC correlations from H-8 to C-1′ determined the location of 3-hydroxy-2-methylpropionic acid group. The chiral center (C-2′) was far from chromophore group, and have no effect on the ECD spectrum of 1, so the absolute configuration of 1 was not determined by ECD spectrum. Since compound 1 possess only one chiral carbon, its optical rotation can be used as an important evidence to determine the absolute configuration. The specific optical rotation of 1 {[α]20D +11.3 (c 0.12, MeOH)} was opposite to that reported of (R)-3-hydroxy-2-methylpropionic acid ethyl ester {[α]20D −15.8 (c 1.04, MeOH)},18 which established the S configuration of C-2′. Therefore, compound 1 was determined as daldispol A.
1H (600 MHz) and 13C NMR (150 MHz) data of compounds 1–3 in DMSO-d6.
| No. | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
| δ C | δ H (J in Hz) | δ C | δ H (J in Hz) | δ C | δ H (J in Hz) | |
| 1 | 127.9, C | 127.8, C | 127.8, C | |||
| 2 | 129.8, CH | 7.03, d, (8.4) | 129.7, CH | 7.02, d, (8.4) | 129.7, CH | 7.02, d, (8.4) |
| 3 | 115.1, CH | 6.67, d, (8.4) | 115.1, CH | 6.67, d, (8.4) | 115.1, CH | 6.67, d, (8.4) |
| 4 | 155.8, C | 155.9, C | 155.9, C | |||
| 5 | 115.1, CH | 6.67, d, (8.4) | 115.1, CH | 6.67, d, (8.4) | 115.1, CH | 6.67, d, (8.4) |
| 6 | 129.8, CH | 7.03, d, (8.4) | 129.7, CH | 7.02, d, (8.4) | 129.7, CH | 7.02, d, (8.4) |
| 7 | 33.6, CH2 | 2.75, t, (7.2) | 33.5, CH2 | 2.76, t, (7.2) | 33.6, CH2 | 2.75, t, (7.2) |
| 8 | 64.6, CH2 | 4.13, td, (7.2, 1.8) | 65.0, CH2 | 4.16, t, (7.2) | 64.6, CH2 | 4.13, t, (7.2) |
| 1′ | 174.4, C | 173.1, C | 171.5, C | |||
| 2′ | 42.1, CH | 2.48, m | 38.4, CH | 2.76, m | 44.0, CH2 | 2.33, dd, (14.4, 7.2) |
| 2.30, dd, (14.4, 6.0) | ||||||
| 3′ | 63.3, CH2 | 3.51, m | 64.9, CH2 | 4.10, dd, (10.8, 6.6) | 63.4, CH | 3.96, m |
| 3.40, m | 4.07, dd, (10.8, 5.4) | |||||
| 4′ | 13.5, CH3 | 0.99, d, (7.2) | 13.4, CH3 | 1.06, d, (7.2) | 23.3, CH3 | 1.06, d, (6.0) |
| 1′′ | 174.2, C | |||||
| 2′′ | 42.0, CH | 2.48, m | ||||
| 3′′ | 63.2, CH2 | 3.51, m | ||||
| 3.41, m | ||||||
| 4′′ | 13.4, CH3 | 1.00, d, (7.2) | ||||
| 3′′-OH | 4.76, t, (6.0) | 4.76, t, (6.0) | 4.71, br s | |||
Fig. 2. Key HMBC (arrows) and COSY (bold lines) correlations of compounds 1–5.
Compound 2 was obtained as a colourless oil, and its molecular formula of C16H22O6 was determined by the HRESIMS peak at m/z 333.1293 [M + Na]+ (calcd 333.1314). Comparison of 1H and 13C NMR spectroscopic data (Table 1) of 2 with those of 1 indicated that 2 showed the additional presence of one carbonyl (δC 173.1), one methine [δH 2.75 (1H, m); δC 38.9], one oxygenated methylene [δH 4.10 (1H, dd, J = 10.8, 6.6 Hz), 4.07 (1H, dd, J = 10.8, 5.4 Hz); δC 65.3], and one methyl [δH 1.06 (1H, d, J = 7.2 Hz); δC 13.8], constructing another 3-hydroxy-2-methylpropanoic acid group, which is further confirmed by with 1H–1H COSY correlations (Fig. 2) of H-4′/H-2′/H-3′ and the HMBC correlations (Fig. 2) from H-3′ to C-1′, C-2′, and C-4′, from H-4′ to C-1′, C-2′, and C-3′. Moreover, the crucial HMBC for H-8/C-1′ and H-3′/C-1′′ established the structure of 2. The absolute configuration of 2 was not ascertained. Thus, compound 2 was elucidated as daldispol B.
Compound 3 was isolated as a colourless oil. The molecular formula C12H16O4 of 3 was established by HR-ESI-MS at m/z 247.0932 [M + Na]+ (calcd 247.0946), which is the same as that of 1. The general features of 1H and 13C NMR spectra (Table 1) of 3 were very similar to those of 1. The obvious differences included the presence of one oxygenated methine [δH 3.96 (1H, m); δC 63.4] and one methylene [δH 2.33 (1H, dd, J = 14.4, 7.2 Hz), 2.30 (1H, dd, J = 14.4, 6.0 Hz); δC 44.0] in 3 instead of a methine [δH 2.48 (1H, m); δC 42.6] and a oxygenated methylene [δH 3.51 (1H, m), 3.40 (1H, m); δC 63.7] in 1. In addition, the 1H–1H COSY correlations (Fig. 2) of H-2′/H-3′/H-4′ and the HMBC correlations (Fig. 2) from H-2′ to C-1′, C-3′, and C-4′, from H-4′ to C-2′ and C-3′ indicated the presence of 3-hydroxybutanoic acid moiety, and its location was further confirmed by the critical HMBC between H-8 and C-1′. The specific optical rotation of 3 {[α]20D −2.1 (c 0.19, CHCl3)} was the similar as that reported of (3S)-4-hydroxyphenethyl-3-hydroxy-5-phenylpentanoate {[α]24D −4.7 (c 0.738, CHCl3)},19 indicating the S configuration of C-3′. Accordingly, compound 3 was established as daldispol C.
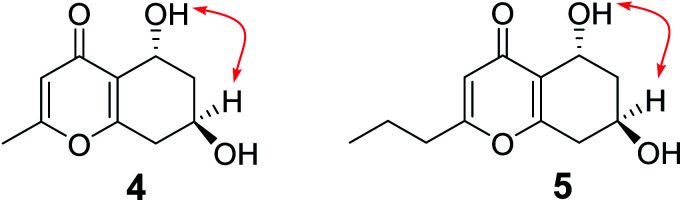
Compound 9 was obtained as a brown oil. HRESIMS data of 9 showed an [M + H]+ ion at m/z 197.0800 (calcd 197.0814), consistent to the molecular formula C10H12O4 with five degrees of unsaturation. The 1H NMR and HSQC spectra (Table 2) of 9 indicated the presence of one olefinic proton at δH 6.10 (1H, s), two oxygenated methines at δH 4.73 (1H, dd, J = 8.4, 4.2 Hz) and 4.14 (1H, m), two methylene at δH 2.80 (1H, dd, J = 17.4, 5.4 Hz), 2.38 (1H, ddd, J = 17.4, 9.0 Hz), 1.89 (1H, ddd, J = 12.6, 3.6, 3.6 Hz), and 1.52 (1H, ddd, J = 12.6, 10.8, 4.2 Hz), and one methyl at δH 2.22 (1H, s). The 13C NMR and DEPT spectra (Table 2) showed 10 carbon resonances, which consisted of four nonprotonated carbons (δC 177.5, 165.5, 162.6, and 122.2, including one ketone carbonyl and two oxygenated olefinic), three methine carbons (δC 113.1, 61.1, and 60.0, including one olefinic and two oxygenated), two methylene carbons (δC 39.2 and 36.3), and one methyl carbon (δC 19.2). The HMBC correlations (Fig. 2) from H-3 to C-2, C-4, C-4a, and C-9, from H-5 to C-4, C-4a, C-7, and C-8a, from H-8 to C-4a, C-6, C-7 and C-8a, from 5-OH to C-4a, C-5, and C-6, from 7-OH to C-6, C-7, and C-8, together with the 1H–1H COSY correlations (Fig. 2) of H-5/H-6/H-7/H-8 established the planar structure of 9, which is the same as known compound, (5R,7S)-5,7-dihydroxy-2-methyl-5,6,7,8-tetrahydro-4H-chromen-4-one (11).20 In the NOE experiment (Fig. 3), the enhancement of H-7 was not observed when H-5 was irradiated, and irradiation of 5-OH resulted in the enhancement of H-5 and H-7, indicating the opposite side of H-5 and H-7. In the ECD spectrum, the positive Cotton effect around 242 nm was similar to that of known compound 11, suggesting the 5R, 7R configurations of 9. Therefore, the structure of 9 was determined to be (5R,7R)-5,7-dihydroxy-2-methyl-5,6,7,8-tetrahydro-4H-chromen-4-one.
1H (600 MHz) and 13C NMR (150 MHz) data of compounds 9 and 10 in DMSO-d6.
| No. | 9 | 10 | ||
|---|---|---|---|---|
| δ C | δ H (J in Hz) | δ C | δ H (J in Hz) | |
| 2 | 165.5, C | 168.4, C | ||
| 3 | 113.1, CH | 6.10 (1H, s) | 112.5, CH | 6.09, s |
| 4 | 177.5, C | 177.6, C | ||
| 4a | 122.2, C | 122.4, C | ||
| 5 | 60.0, CH | 4.73, dd, (4.2, 4.2, 3.6) | 60.0, CH | 4.74, ddd, (4.2, 3.6, 3.6) |
| 6 | 39.2, CH2 | 1.89, ddd, (12.6, 3.6, 3.6) | 39.4, CH2 | 1.89, ddd, (12.6, 3.6, 3.6) |
| 1.52, ddd, (12.6, 10.8, 4.2) | 1.53, ddd, (12.6, 10.8, 3.6) | |||
| 7 | 61.1, CH | 4.14, (m) | 61.1, CH | 4.13, m |
| 8 | 36.3, CH2 | 2.80, dd, (17.4, 5.4) | 36.3, CH2 | 2.80, dd, (17.4, 6.0) |
| 2.38, ddd, (17.4, 9.0) | 2.39, dd, (17.4, 9.0) | |||
| 8a | 162.6, C | 162.7, C | ||
| 9 | 19.2, CH3 | 2.22, s | 34.4, CH2 | 2.46, t, (7.2) |
| 10 | 19.7, CH2 | 1.60, m | ||
| 11 | 13.2, CH3 | 0.91, t, (7.2) | ||
| 5-OH | 4.84, d, (4.2) | 4.84, d, (4.2) | ||
| 7-OH | 5.03, d, (4.2) | 5.02, d, (4.8) | ||
Fig. 3. Key NOE correlations of compounds 9 and 10.
Compound 10 was isolated as a brown oil, and possessed the molecular formula C12H16O4 as supported by the HRESIMS ion peak at m/z 225.1111 [M + H]+ (calcd 225.1127). The 1H and 13C NMR spectroscopic data (Table 2) of 10 closely resembled those of 9, except for the presence of additional two methylenes [δH 2.46 (2H, t, J = 7.2 Hz), 1.60 (2H, m); δC 34.4, 19.7]. The 1H–1H COSY correlations (Fig. 2) of H-9/H-10/H-11 and the HMBC correlations (Fig. 2) from H-11 to C-9 and C-10, from H-9 to C-2, C-3, C-10, and C-11, confirmed the propyl group attached to C-2. Interpretation of its 1D and 2D NMR data established the planar structure of 10, which is the same as the known compound, (5R,7S)-5,7-dihydroxy-2-propyl-5,6,7,8-tetrahydro-4H-chromen-4-one (12).20 The relative configuration was established by the NOESY correlation (Fig. 3) between H-7 and 5-OH. In the ECD spectrum, positive Cotton effect at 241 nm were nearly identical to that of known compound 12, indicating the 5R, 7R configurations of 10. Thus, the structure of 10 was identified as (5R,7R)-5,7-dihydroxy-2-propyl-5,6,7,8-tetrahydro-4H-chromen-4-one.
The known metabolites were identified as 2-phenylethyl-β-d-glucopyranoside (4),21 stachyline C (5),22 3-methoxy-4-hydroxy-phenylethanol (6),23 3-hydroxy-4-methoxy-phenylethanol (7),24p-hydroxyphenethyl alcohol (8),25 (5R,7S)-5,7-dihydroxy-2-methyl-5,6,7,8-tetrahydro-4H-chromen-4-one (11),20 (5R,7S)-5,7-dihydroxy-2-propyl-5,6,7,8-tetrahydro-4H-chromen-4-one (12)20 by comparison of their NMR and MS data with those reported.
Compounds 1–12 were evaluated for anti-influenza A virus (IAV), anti-Zika virus (ZIKV), and antibacterial activities. Compounds 1, 3, 4, 9, and 11 displayed significant anti-influenza A virus (IAV) activities as shown in Table 3, and compound 8 exhibited anti-ZIKV activity with inhibitory ratio of 42.7% at 10 μM (Table S1†). Compounds 1–12 were tested for antibacterial activities against Staphylococcus aureus ATCC 29213 and Escherichia coli ATCC 25922. All compounds showed no antibacterial activities against Staphylococcus aureus ATCC 29213 and Escherichia coli ATCC 25922 at the concentration of 32 μg ml−1.
The anti-IAV activities of 1, 3, 4, 9, and 11.
| Compound | IC50 (μM) | CC50 (μM) |
|---|---|---|
| 1 | 12.7 | >100 |
| 3 | 6.4 | >100 |
| 4 | 12.5 | >100 |
| 9 | 16.1 | >100 |
| 11 | 9.0 | >100 |
| Ribavirin | 21.7 | >100 |
Conclusions
In conclusion, eight phenol derivatives including three new phenols, daldispols A–C (1–3), four chromone derivatives including two new ones, (5R,7R)-5,7-dihydroxy-2-methyl-5,6,7,8-tetrahydro-4H-chromen-4-one (9) and (5R,7R)-5,7-dihydroxy-2-propyl-5,6,7,8-tetrahydro-4H-chromen-4-one (10) were isolated from endolichenic fungus Daldinia sp. CPCC 400770. Their structures were determined by a detailed interpretation of the spectroscopic data and ECD spectra. Compounds 1, 3, 4, 9, and 11 displayed significant anti-influenza A virus (IAV) activities with IC50 values of 12.7, 6.4, 12.5, 16.1, and 9.0 μM, respectively, which were all stronger than the positive control, ribavirin (IC50 = 21.7 μM). Compound 8 displayed anti-ZIKV activity with inhibitory ratio of 42.7% at 10 μM, which is similar to positive control (ribavirin). Structure–activity relationship analysis for chromone analogues (9–11) indicated that the substitution group at C-2 play an important role on anti-IAV activity, and the 7-OH substitution have little effect on anti-IAV activity. Our finding suggested that the genus Daldinia might be an important source for antiviral natural products.
Experimental
General experimental procedures
Optical rotations were recorded on an Autopol IV automatic polarimeter with a 10 cm glass microcell at 20 °C (Rudolph Research Analytical, NJ, USA). The CD spectra were measured on a JASCO J-815 spectropolarimeter using CH3OH as the solvent at room temperature (Jasco Corporation, Tokyo, Japan). UV spectra were obtained in CH3OH on a Persee TU-1901 UV-Vis spectrometer (Beijing Purkinje General Instrument Co., Ltd., Beijing, China). IR spectra were acquired on a PerkinElmer FT-IR/NIR spectrometer (PerkinElmer, Waltham, MA, USA). 1D and 2D NMR spectra were performed at 600 MHz for 1H NMR and 150 MHz for 13C NMR on Bruker ARX-600 spectrometer using solvent signals (DMSO-d6: δH 2.50, δC 39.5) as the internal standard (Bruker, Switzerland). Chemical shifts (δ) are given in ppm, and coupling constants (J) are given in hertz (Hz). ESIMS data were recorded on a Thermo LTQ mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA). HRESIMS data were measured using a Thermo LTQ Orbitrap XL mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA). Column chromatography (CC) were carried out with silica gel (200–300 mesh, Qingdao Marine Chemical Inc. Qingdao, PR China). Analytical TLC was carried out on pre-coated silica gel GF254 plates (Qingdao Marine Chemical Industry, Qingdao, China), and spots were visualized under UV light or by spraying with 10% H2SO4 in 90% EtOH followed by heating at 120 °C.
Fungal material
The fungal strain Daldinia sp. CPCC 400770 was isolated from lichen, collected in Laojun mountain, Dali Bai autonomous prefecture, Yunnan province, China, in 2012, and identified using morphological and molecular (ITS1-5.8S-ITS2 rRNA gene sequence) analyses by China Pharmaceutical Culture Collection. The strain was deposited at the China Pharmaceutical Culture Collection (no. CPCC 400770), Institute of Medicinal Biotechnology, Chinese Academy of Medical Sciences and Peking Union Medical College.
Fermentation, extraction and isolation
The fungal strain was spread onto slants of modified potato dextrose agar (PDA) medium (potato 200 g, glucose 20 g, distilled water 1 l, KH2PO4 3 g, MgSO4 0.73 g, vitamin B1 10 mg, agar 8.0 g, pH 6.0–6.3), and incubated at 28 °C for 8–10 days. The agar plugs were inoculated into 250 ml Erlenmeyer flasks containing 50 ml of sterile fermentation medium (glucose 2.0%, corn steep liquor 1.0%, soymeal 0.2%, dextrin 1.0%, peptone 0.5%, (NH4)2SO4 0.05%, pH 6.0) at 30 °C on a rotary shaker (200 rpm) for 5 days to prepare the seed culture. Fermentation was carried out in Fernbach flasks (500 ml), each containing 80 g of rice. Distilled H2O (120 ml) was added to each flask, and the contents were soaked overnight before autoclaving at 121 °C for 30 min. After cooling to room temperature, each flask was inoculated with 10 ml of the seed culture, and incubated at 30 °C for 40 days.
The fermented material (35 flasks) was extracted repeatedly with EtOAc (3 × 3 l), and the organic solvent was evaporated to dryness under vacuum to yield the crude extract (51.4 g), which was initially subjected to silica gel column chromatography (CC) eluting with petroleum ether–acetone gradient (95 : 5–0 : 100, v/v) to produce eighteen fractions (Fr.1–Fr.18). Fraction Fr.5 (6.6 g) was isolated by medium pressure liquid chromatography (MPLC) eluting with CH3CN–H2O gradient (30 : 70–100 : 0, v/v) to afford 12 fractions (Fr.5.1–Fr.5.12), fraction Fr.5.4 (24 mg) was purified by reversed-phase semi-preparative HPLC eluting with CH3CN–H2O (30 : 70, v/v) at 4 ml min−1 to obtain 5 (5.1 mg). Fraction Fr.6 (2.0 g) was separated via MPLC eluting with CH3CN–H2O gradient (10 : 90–100 : 0, v/v) to yield 13 fractions (Fr.6.1–Fr.6.13), fraction Fr.6.4 (210 mg) was further purified by semi-preparative HPLC eluting with CH3OH–H2O (20 : 80, v/v) at 4 ml min−1 to give 6 (4.2 mg), 7 (5.2 mg), and 8 (8.5 mg), fraction Fr.6.5 (250 mg) was further purified by semi-preparative HPLC eluting with CH3CN–H2O (20 : 80, v/v) at 4 ml min−1 to afford 1 (54 mg) and 3 (5.0 mg), fraction Fr.6.6 (72 mg) was further purified by semi-preparative HPLC eluting with CH3CN–H2O (30 : 70, v/v) at 4 ml min−1 to afford 2 (8.9 mg). Fraction Fr.9 (600 mg) was isolated via MPLC eluting with CH3CN–H2O gradient (10 : 90–100 : 0, v/v) to yield 10 fractions (Fr.9.1–Fr.9.10), fraction Fr.9.2 (28 mg) was further purified by semi-preparative HPLC eluting with CH3CN–H2O (20 : 80, v/v) at 4 ml min−1 to give 12 (2.7 mg). Fraction Fr.10 (300 mg) was isolated by MPLC eluting with CH3CN–H2O gradient (10 : 90–100 : 0, v/v) to yield 9 fractions (Fr.10.1–Fr.10.9), fraction Fr.10.1 (5 mg) was further purified by semi-preparative HPLC eluting with CH3CN–H2O (15 : 85, v/v) at 4 ml min−1 to give 10 (3.7 mg). Fraction Fr.11 (150 mg) was purified by reversed-phase semi-preparative HPLC eluting with CH3CN–H2O gradient (10 : 90–100 : 0, v/v) at 4 ml min−1 to obtain 11 (3.0 mg). Fraction Fr.13 (190 mg) was purified by reversed-phase semi-preparative HPLC eluting with CH3OH–H2O (10 : 90, v/v) at 4 ml min−1 to produce 9 (4.0 mg). Fraction Fr.14 (100 mg) was purified by reversed-phase semi-preparative HPLC eluting with CH3CN–H2O (15 : 85, v/v) at 4 ml min−1 to yield 4 (4.1 mg).
Daldispol A (1)
Brown oil; [α]20D +11.3 (c 0.12, MeOH); UV (MeOH) λmax (log ε): 227 (3.05), 278 (0.88) nm; IR (νmax): 3366, 2975, 1710, 1615, 1516, 1458, 1230, 1187, 1172, 1031, and 828 cm−1; ESIMS m/z 225.1 [M + H]+; HRESIMS m/z 247.0930 [M + Na]+ (calcd for C12H16O4Na, 247.0946); 1H and 13C NMR data, see Table 1.
Daldispol B (2)
Brown oil; [α]20D +27.0 (c 0.15, MeOH); UV (MeOH) λmax (log ε): 212 (3.56), 227 (3.99), 278 (1.61) nm; IR (νmax): 3395, 2975, 1716, 1615, 1517, 1462, 1186, 1035, and 827 cm−1; ESIMS m/z 311.2 [M + H]+; HRESIMS m/z 333.1293 [M + Na]+ (calcd for C16H22O6Na, 333.1314); 1H and 13C NMR data, see Table 1.
Daldispol C (3)
Brown oil; [α]20D −2.1 (c 0.19, CHCl3); UV (MeOH) λmax (log ε): 214 (3.35), 227 (3.73), 278 (1.47) nm; IR (νmax): 3346, 2971, 1716, 1615, 1516, 1450, 1235, 1173, 1066, and 832 cm−1; ESIMS m/z 225.1 [M + H]+; HRESIMS m/z 247.0932 [M + H]+ (calcd for C12H16O4Na, 247.0946); 1H and 13C NMR data, see Table 1.
(5R,7R)-5,7-Dihydroxy-2-methyl-5,6,7,8-tetrahydro-4H-chromen-4-one (9)
Brown oil; [α]20D −25 (c 0.08, MeOH); UV (MeOH) λmax (log ε): 214 (2.19), 247 (2.70) nm; IR (νmax): 3364, 2959, 1659, 1579, 1430, 1343, 1262, 1086, 1060, 1031, 951, 872, and 799 cm−1; CD (MeOH) Δε (nm): +3.40 (242), −0.74 (293.5); ESIMS m/z 197.1 [M + H]+; HRESIMS m/z 197.0800 [M + H]+ (calcd for C10H13O4, 197.0814); 1H and 13C NMR data, see Table 2.
(5R,7R)-5,7-Dihydroxy-2-propyl-5,6,7,8-tetrahydro-4H-chromen-4-one (10)
Brown oil; [α]20D −3.8 (c 0.08, MeOH); UV (MeOH) λmax (log ε): 208 (1.32), 248 (0.41) nm; IR (νmax): 3335, 2960, 2925, 1732, 1659, 1596, 1444, 1262, 1075, 1032, and 802 cm−1; CD (MeOH) Δε (nm): +0.44 (241); ESIMS m/z 225.1 [M + H]+; HRESIMS m/z 225.1111 [M + H]+ (calcd for C12H17O4, 225.1127); 1H and 13C NMR data, see Table 2.
Anti-IAV activity assays26
293T-Gluc cells (5 × 105 cells per ml) were seeded in the wells of 96-well plate at 100 μl per well. After an 24 h incubation at 37 °C and 5% CO2, cells were cultured to 90% confluence. For evaluation assay of antivirals, 1 μl of each tested compound (DMSO and ribavirin were used as negative and positive controls, respectively) was added to cells and incubated for 2 h prior to infection, after which cells were infected with influenza A/H1N1/WSN/33 virus at an MOI of 0.05. After a further incubation for 24 h at 37 °C, the cell supernatant was collected and measured for Gluc activity.
Anti-ZIKV activity assays27
Cell viability was assessed by Cell Counting Kit-8 (CCK-8; Beyotime). Typically, 8 × 104 cells per ml Vero E6 cells were seeded per well and grown for 24 h. The compound was added to the cells, after which cells were infected with ZIKV at multiplicity of infection (MOI) of 0.05. Ribavirin and DMSO were used as positive and negative controls, respectively. After incubation at 37 °C for 96 h, the supernatants were replaced with 110 μl fresh medium containing 10 μl CCK-8 reagent. After incubation for 1.5 h at 37 °C with 5% CO2, the absorbance at 450 nm was subsequently measured using EnSpire 2300 Multilable Reader.
Antibacterial activity assays28
The minimal inhibitory concentrations (MICs) of the isolated compounds were determined by the broth microdilution method in 96-well culture plates according to Clinical and Laboratory Standards Institute. Organisms used in this study included strains from ATCC collection, and levofloxacin was used as positive control. The final concentrations of compounds ranged from 0.125 to 32 μg ml−1. Culture plates were incubated at 37 °C for 18 h. The MICs were defined as the lowest concentration that prevented visible growth of the bacteria.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
This research was funded by the National Natural Science Foundation of China (82073744, 81402835), the National Mega-project for Innovative Drugs (2019ZX09721001-004-006), CAMS Innovation Fund for Medical Sciences (2016-12M-3-14 and 2020-I2M-2-010), and the National Microbial Resource Center (NMRC-2020-3), the Non-profit Central Research Institute Fund of iCAMS (2020-PT310-003 and 2019PT350004).
Electronic supplementary information (ESI) available: HRESIMS, IR, UV, 1D and 2D NMR for 1–3, 9 and 10; ECD spectra of 9 and 10; antiviral activities of 1–12. See DOI: 10.1039/d1ra03754d
Notes and references
- Calcott M. J. Ackerley D. F. Knight A. Keyzers R. A. Owen J. G. Chem. Soc. Rev. 2018;47:1730–1760. doi: 10.1039/C7CS00431A. [DOI] [PubMed] [Google Scholar]
- Gao H. Zou J. Li J. Zhao H. Stud. Nat. Prod. Chem. 2016;48:347–397. [Google Scholar]
- Hellog J. J. Huzefa Raja H. A. Phytochem. Rev. 2017;16:271–293. doi: 10.1007/s11101-016-9473-1. [DOI] [Google Scholar]
- Masters K. S. Bräse S. Chem. Rev. 2012;112:3717–3776. doi: 10.1021/cr100446h. [DOI] [PubMed] [Google Scholar]
- Agrawal S. Deshmukh S. K. Reddy M. S. Prasas R. Goel M. S. Afr. J. Bot. 2020;134:163–186. doi: 10.1016/j.sajb.2019.12.008. [DOI] [Google Scholar]
- Chen M. Wang R. Zhao W. Yu L. Zhang C. Chang S. Li Y. Zhang T. Xing J. Gan M. Feng F. Su S. Org. Lett. 2019;21:1530–1533. doi: 10.1021/acs.orglett.9b00385. [DOI] [PubMed] [Google Scholar]
- Li W. Gao W. Zhang M. Li Y. L. Li L. Li X. B. Chang W. Q. Zhao Z. T. Lou H. X. J. Nat. Prod. 2016;79:2188–2194. doi: 10.1021/acs.jnatprod.6b00197. [DOI] [PubMed] [Google Scholar]
- Xie F. Luan X. Y. Gao Y. Xu K. Lou H. X. J. Nat. Prod. 2020;83:1623–1633. doi: 10.1021/acs.jnatprod.0c00108. [DOI] [PubMed] [Google Scholar]
- Xu K. Gao Y. Li Y. L. Xie F. Zhao Z. T. Lou H. X. J. Nat. Prod. 2018;81:2041–2049. doi: 10.1021/acs.jnatprod.8b00362. [DOI] [PubMed] [Google Scholar]
- Yuan W. H. Teng M. T. Yun Y. F. Jiang N. Ma L. Sun S. S. Yuan B. Tang J. Wu Q. Y. Li Q. Zhang P. Morris-Natschke S. L. Lee K. H. J. Nat. Prod. 2020;83:1716–1720. doi: 10.1021/acs.jnatprod.0c00024. [DOI] [PubMed] [Google Scholar]
- Kithsiri Wijeratne E. M. Gunaherath G. M. K. B. Chapla V. M. Tillotson J. de la Cruz F. Kang M. U'Ren J. M. Araujo A. R. Arnold A. E. Chapman E. Leslie Gunatilaka A. A. J. Nat. Prod. 2016;79:340–352. doi: 10.1021/acs.jnatprod.5b00986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K. Li G. Zhu R. Xie F. Li Y. Yang W. Xu L. Shen T. Zhao Z. Lou H. Phytochemistry. 2020;170:112191. doi: 10.1016/j.phytochem.2019.112191. [DOI] [PubMed] [Google Scholar]
- Stadler M. Læssøe T. Fournier J. Decock C. Schmieschek B. Tichy H. V. Peršoh D. Stud. Mycol. 2014;77:1–143. doi: 10.3114/sim0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X. Yang C. Meng Q. Liu L. Fu S. J. Chin. Chem. Soc. 2021;68:678–681. doi: 10.1002/jccs.202000274. [DOI] [Google Scholar]
- Manthrirathna M. A. T. P. Kandiah R. Gunasekera D. S. Samanthi K. A. U. Welideniya D. T. Maduranga H. A. K. Paranagama P. A. J. Natl. Sci. Found. Sri Lanka. 2020;48:143–148. doi: 10.4038/jnsfsr.v48i2.10095. [DOI] [Google Scholar]
- Jeong G. S. Kang M. G. Han S. A. Noh J. I. Park J. E. Nam S. J. Park D. Yee S. T. Kim H. J. Fungi. 2021;7:84. doi: 10.3390/jof7020084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu G. Cai G. Wang Y. Li L. Bai J. Zhang T. Cen S. Zhang D. Yu L. J. Antibiot. 2021;74:215–218. doi: 10.1038/s41429-020-00384-0. [DOI] [PubMed] [Google Scholar]
- Jeulin S. Ayad T. Ratovelomanana-Vidal V. Genêt J. P. Adv. Synth. Catal. 2007;349:1592–1596. doi: 10.1002/adsc.200700025. [DOI] [Google Scholar]
- Mapunya M. B. Hussein A. A. Rodriguez B. Lall N. Phytomedicine. 2011;18:1006–1012. doi: 10.1016/j.phymed.2011.03.013. [DOI] [PubMed] [Google Scholar]
- Luo Y. P. Song X. P. Zheng C. J. Chen G. Y. Luo X. X. Han J. X. Chem. Biodiversity. 2020;17:e1900547. doi: 10.1002/cbdv.201900547. [DOI] [PubMed] [Google Scholar]
- Horo I. Kocabaş F. Alankuş-Çalışkan Ö. Özgökçe F. Khan I. A. Bedir E. Nat. Prod. Commun. 2016;11:1847–1850. doi: 10.1177/1934578X1601101218. [DOI] [PubMed] [Google Scholar]
- Almeida C. Part N. Bouhired S. Kehraus S. König G. M. J. Nat. Prod. 2011;74:21–25. doi: 10.1021/np1005345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng W. S. Su F. Y. Zheng X. K. Li Y. J. Chin. Pharm. J. 2011;46:496–499. [Google Scholar]
- Zhang X. Gao H. Wang N. L. Yao X. S. Chin. Tradit. Herb. Drugs. 2006;37:652–655. [Google Scholar]
- Chang D. D. Dai H. F. Zuo W. J. Mei W. L. Chin. J. Antibiot. 2012;37:421–424. [Google Scholar]
- Gao Q. Wang Z. Liu Z. Li X. Zhang Y. Zhang Z. Cen S. Acta Pharm. Sin. B. 2014;4:301–306. doi: 10.1016/j.apsb.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui X. Zhou R. Huang C. Zhang R. Wang J. Zhang Y. Ding J. Li X. Zhou J. Cen S. Front. Pharmacol. 2020;11:514313. doi: 10.3389/fphar.2020.514313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI), Performance Standards for Antimicrobial Susceptibility Testing; Twenty-sixth Informational Supplement, CLSI document M100-S26, Wayne, PA, 2016 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.