Graphical abstract
Abbreviations: IMS, ion mobility spectrometry; DTIMS, drift tube ion mobility spectrometry; TWIMS, traveling wave ion mobility spectrometry; FAIMS, field asymmetric ion mobility spectrometry; DMS, differential mobility spectrometry; IM-MS, ion mobility-mass spectrometry; CCS, collision cross section; TIMS, trapped ion mobility spectrometry; CIMS, cyclic ion mobility spectrometry; SLIM, structures for lossless ion manipulations
Keywords: Glucocorticoids, Ion mobility spectrometry, Ion mobility-mass spectrometry
Highlights
-
•
Liquid chromatography-ion mobility-mass spectrometry (LC-IM-MS) for glucocorticoids.
-
•
Determination of collision cross sections (CCS) for isomers.
-
•
Different cation adducts shifted mobility and improved IM separation.
-
•
Changing drift gas (He, Ar, CO2) shifted mobility and improved resolution.
Abstract
Introduction
Ion mobility-mass spectrometry (IM-MS) is an emerging technique in the -omics fields that has broad potential applicability to the clinical lab. As a rapid, gas-phase structure-based separation technique, IM-MS offers promise in isomer separations and can be easily combined with existing LC-MS methods (i.e., LC-IM-MS). Several experimental conditions, including analyte cation adducts and drift composition further provide a means to tune separations for global and/or targeted applications.
Objectives
The primary objective of this study was to demonstrate the utility of IM-MS under a range of experimental conditions for detection of glucocorticoids, and specifically for the separation of several isomeric pairs.
Methods
LC-IM-MS was used to characterize 16 glucocorticoids including three isomer pairs: cortisone/prednisolone, betamethasone/dexamethasone, and flunisolide/triamcinolone acetonide. Collision cross section (CCS) values were measured for all common adducts (e.g., protonated and sodiated) using both step-field and single-field methods. Alternative alkali, alkaline earth, and transition metals were introduced, such that their adducts could also be measured. Finally, four different drift gases (helium, nitrogen, argon, and carbon dioxide) were compared for their relative separation capability.
Results
LC-IM-MS offered a robust, multidimensional separation technique that allowed for the 16 glucocorticoids to be analyzed and separated in three-dimensions (retention time, CCS, and m/z). Despite the relatively modest resolution of isomer pairs under standard conditions (i.e., nitrogen drift gas, sodiated ions, etc.), improvements were observed for alkaline earth and transition metals (notable barium adducts) and in carbon dioxide drift gas.
Conclusion
In summary, LC-IM-MS offers potential as a clinical method due to its ease of coupling with traditional LC-MS methods and its promise for tuning separations to better resolve targeted and/or global isomers in complex biological samples.
1.1. Introduction
Ion mobility spectrometry (IMS) is an emerging analytical technique in the -omics fields (e.g., metabolomics, proteomics, etc.)[1] with potential application to the clinical lab.[2] IMS is a gas-phase separation technique based on ion movement through a buffer gas in the presence of an electric field. Separation occurs based on differences in ions’ size, shape, and charge. Experimental variables include the composition, pressure, and temperature of the buffer gas environment, as well as the nature of the electric field. Traditional time dispersive IMS experiments, referred to as drift tube IMS (DTIMS), utilize a static electric field that moves ions through a drift device at a constant drift velocity.[3] Ions of various size/shape will experience a differing number of collisions with buffer gas molecules, thereby traveling more or less slowly and resulting in differences in drift time measured at a detector; this is somewhat analogous to the way ion mass-to-charge (m/z) is measured in a time-of-flight mass spectrometer. Other time dispersive IMS methods include the common traveling wave IMS (TWIMS), in which a dynamic waveform is applied to the electrodes of the mobility cell, thereby propelling the ions. In contrast, high-field asymmetric waveform IMS (FAIMS), also referred to as differential mobility spectrometry (DMS), applies an alternating high/low electric field to electrodes perpendicular to ion motion through the separation cell. Ions undergo a “zig-zag” motion between these electrodes, with their net movement dependent on differences in their mobility in the high vs. low field.[4], [5], [6] Ultimately, ions will collide with either electrode, however a compensation voltage can be applied to one of the electrodes and scanned to allow for sequential transmission of ions of different mobilities; this is more analogous to the way that a mass-selective quadrupole operates as an ion filter.
These various IMS techniques have seen increased usage in biomedical applications, especially when coupled with mass spectrometry (i.e., IM-MS). One of the advantages of IMS is its relatively rapid analytical timescale, with measurements made on the order of tens of milliseconds. This conveniently allows for “nesting” of IMS between chromatographic separations (minutes/hours) and fast acquisition time-of-flight MS (hundreds of microseconds), This produces multidimensional separations without sacrificing time of analysis relative to conventional LC-MS measurements. Furthermore, measurement of an ion’s mobility can be used to derive its collision cross section (CCS), a property characteristic of a given ion under consistent experimental conditions. Over time, growing CCS databases, complementing existing databases with retention time and/or mass spectral information, have increased confidence of identification for unknowns in complex samples. Lastly, because IMS separates based on size/shape differences (in contrast to MS-based separation by mass) it provides the potential for differentiation of isomers that may otherwise be challenging.
IM-MS (often coupled with chromatography) has seen application across numerous clinically relevant biomolecular classes, including metabolites, lipids, carbohydrates, and steroids. The latter includes the various classes of endogenous steroids (e.g., androgens, estrogens, mineralocorticoids, etc.), exogenous anabolic agents, and analogs such as cholesterol, Vitamin D metabolites, and bile acids. Addition of IM to existing LC-MS workflows provides a third separation dimension and measurand (CCS) that can improve confidence in identification and reduce false positives. These CCS values can be used in conjunction with computational modeling to elucidate gas-phase structures of biomolecules and their complexes.[7], [8], [9] Furthermore, recent advances in computational modeling approaches, including machine learning, have prompted population of theoretical/predictive CCS databases that could be used to further identify unknowns even in the absence of previously measured chemical standards.[10], [11], [12], [13].
One of the challenges with current IMS approaches is the relatively limited resolving power of most commercial platforms, making separation of structurally similar compounds (i.e., stereoisomers) difficult. Aside from development of higher resolution IMS instruments (e.g., trapped IMS (TIMS),[14], [15], [16] cyclic IMS (CIMS),[17], [18], [19] and structures for lossless ion manipulations (SLIM)[20], [21], [22]), many experimental strategies have been undertaken to augment separation of these challenging cases. Most notably, these have included changes to ionic structure/conformation and drift gas environment (e.g., temperature, gas composition, volatile solvent modifiers, etc.). First, simple chemical modifications have been demonstrated to improve resolution of various isomeric steroids; these have included derivatization with p-toluenesulfonyl isocyanate,[23] ozonolysis,[24] and the Paternò-Büchi reaction.[25] Investigation of ion adducts as alternatives to the most common protonated/deprotonated/sodiated has led to interesting separation results, with examples of alkali, alkaline earth, and transition metal complexes as well as negatively charged species, such as chlorides.[5], [16], [26], [27], [28], [29] Complexation with other steroids or with larger molecules, such as cyclodextrins and crown eithers (themselves including stereochemical differences), have also promoted improved differentiation.[7], [20], [29] Second, modifications of the mobility separation environment have also been examined. Historically, IM-MS measurements have been performed in either helium or, more recently, nitrogen buffer gas maintained at either roughly atmospheric pressure or 1–4 Torr. However, measurements in other gases, such as argon, nitrous oxide, carbon dioxide, and sulfur hexafluoride, have been tested for potential analytical gains.[23], [26], [30] Furthermore, modification by volatile solvent addition (especially in the case of atmospheric pressure FAIMS/DMS) has provided some interesting improvements in resolution.[31], [32].
Herein, we demonstrate a comprehensive study of various ion mobility conditions, including alternative ion adducts and drift gases, for their potential utility in targeted analysis of glucocorticoids, a clinically significant group of compounds involved in several biological processes including inflammation and the immune response.
2.1. Materials and methods
2.1.1. Chemicals and reagents
Glucocorticoid standards were purchased as powders from Cayman Chemical (Ann Arbor, MI) and prepared as 1 mg/mL stock solutions in acetonitrile. Working standards were prepared as 10 µg/mL solutions in 50:50 (v/v) water (0.1% formic acid)/methanol. All solvents were Fisher Scientific Optima LC-MS grade (Pittsburgh, PA). All cations were purchased from Fisher Scientific as their acetate salts and prepared as 1 mg/mL stock solutions in water or 50:50 (v/v) water (0.1% formic acid)/methanol, depending on solubility. These cations were then added to working solutions at 10 µg/mL. All ultrahigh purity drift gases (helium, nitrogen, argon, and carbon dioxide) were purchased from nexAir (Melbourne, FL).
2.1.2. LC-IM-MS instrumentation
All samples were analyzed using an Agilent 6560 IM-QTOF (Santa Clara, CA). Each measurement was performed with the corresponding drift gas maintained at approximately 4 Torr and 25 °C. Other relevant instrument parameters can be found in the Supporting Information Table S1. Chromatographic separations were performed using Agilent 1290 Infinity II UHPLC (Santa Clara, CA) coupled to the 6560 IM-QTOF. Samples were injected (10 µL) onto an Agilent ZORBAX Extend-C18 column (2.1 × 50 mm, 1.8 μm) maintained at 30 °C. Mobile phase A was water (0.1% formic acid) and mobile phase B was methanol. The flow rate was maintained at 0.400 mL/min with gradient conditions shown in Table S2. Multiplexing (4-bit) was enabled for all LC-IM-MS measurements, which allowed for a maximum ion funnel trap fill time of 3900 μs; the release time was 150 μs.
2.1.3. CCS measurements
Samples were infused at 15 μL/min via syringe pump into an Agilent Jetstream ESI source operated in either positive or negative mode. Accurate step-field DTCCSXX measurements (where the subscript ‘XX’ indicates the specific gas used for each measurement) were performed using the step-field method with five field strength steps (30 s each) ranging from 13.5 to 18.6 V/cm for all gases except helium, for which the field range was lowered to 9.6–14.7 V/cm to prevent discharge in the drift tube. The effective drift tube length was measured using the method from the McLean group’s Collision Cross Section Compendium Reporting Guidelines; this value was used to adjust experimentally obtained DTCCSXX values. Triplicate measurements were made in all cases. Single-field DTCCSXX measurements [33] were performed following chromatographic separation at a drift tube field strength of 18.6 V/cm for all gases except helium, for which measurements were made at 14.7 V/cm. The Agilent Tune Mix reference standard was used to create a calibration line with slope (beta) and intercept (tfix) to convert drift time to DTCCSXX. Reference CCS values for the Tune Mix ions in He, Ar, and CO2 were found in work by Morris et al.[34].
2.1.4. Data processing
All data was processed using Agilent MassHunter 10.0 software (IM-MS Browser and Qualitative Analysis) and homebuilt software for high-throughput extraction of raw data plots. All multiplexed data was demultiplexed using the standard Agilent De-Multiplexing Tool.
3.1. Results and discussion
3.1.1. IM-MS analysis of glucocorticoids
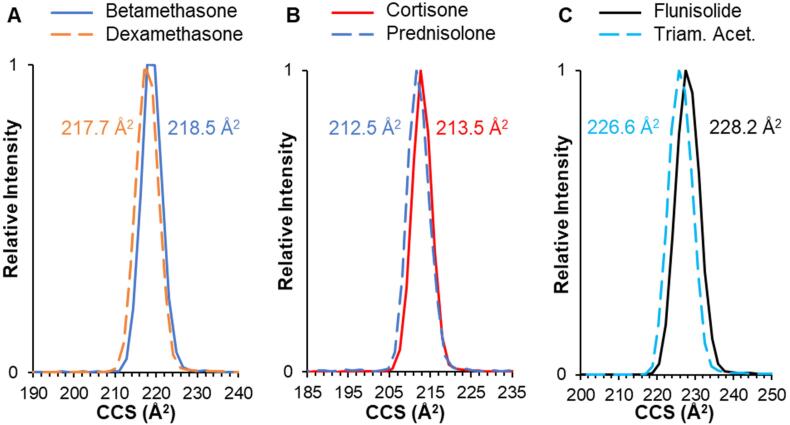
We analyzed 16 glucocorticoids using IM-MS, most of which are included in the 2021 WADA Prohibited List. These compounds ranged in molecular weight from 358 to 540 Da and were observed primarily as the sodiated species, [M + Na]+. The protonated species [M + H]+ was not observed for many of the compounds under these experimental conditions; relative abundances for the protonated species (when observed) are included in Table S3. Collision cross sections (CCS) were measured using the established step-field method and those values are displayed in Table 1. CCS ranged from 185 to 240 Å2 for protonated and 213–264 Å2 for sodiated species; these values were generally reproducible with standard deviation of ≤ 1.5 Å2 for most of the compounds as sodium adducts. The combination of high-resolution accurate mass and CCS can be used to improve confidence when identifying these compounds in complex biological mixtures, such as urine extractions. Of particular interest is the IM separation of isomers, as they display identical molecular weight and are often indistinguishable by MS/MS fragmentation pattern. These included three isomer pairs: prednisolone/cortisone (C21H28O5), betamethasone/dexamethasone (C22H29FO5), and flunisolide/triamcinolone acetonide (C24H31FO6). As can be observed in Fig. 1, these isomers are not well resolved as protonated/sodiated species under standard IM conditions (i.e., nitrogen drift gas at ∼ 25 °C), with differences (by ΔCCS) of 0.4%, 0.5%, and 0.7%, respectively. This presents a challenge for definitively identifying these species in a mixture if they are not chromatographically resolved first.
Table 1.
Step-field DTCCSN2 values for protonated/sodiated ions of all glucocorticoids analyzed with theoretical m/z values for each adduct. Isomers are highlighted in color.
| Glucocorticoid | Formula |
[M + H]+ m/z |
[M + H]+ DTCCSN2 (Å2) |
[M + Na]+ m/z |
[M + Na]+ DTCCSN2 (Å2) |
|---|---|---|---|---|---|
| Prednisone | C21H26O5 | 359.186 | 185.7 ± 1.9 | 381.168 | 213.2 ± 0.1 |
| Prednisolone | C21H28O5 | 361.202 | 188.3 ± 0.7 | 383.183 | 213.5 ± 0.6 |
| Cortisone | C21H28O5 | 361.202 | --- | 383.183 | 212.5 ± 0.5 |
| Hydrocortisone | C21H30O5 | 363.217 | 189.0 ± 0.7 | 385.199 | 213.6 ± 1.2 |
| Methylprednisolone | C22H30O5 | 375.217 | --- | 397.199 | 216.9 ± 1.7 |
| Betamethasone | C22H29FO5 | 393.208 | --- | 415.190 | 218.5 ± 0.6 |
| Dexamethasone | C22H29FO5 | 393.208 | --- | 415.190 | 217.7 ± 0.9 |
| Triamcinolone | C21H27FO6 | 395.187 | --- | 417.160 | 216.5 ± 1.0 |
| Mometasone | C22H28Cl2O4 | 427.144 | --- | 449.126 | 222.4 ± 1.3 |
| Budesonide | C25H34O6 | 431.243 | --- | 453.225 | 233.7 ± 1.7 |
| Triamcinolone Acetonide | C24H31FO6 | 435.218 | 209.7 ± 2.9 | 457.200 | 228.2 ± 0.2 |
| Flunisolide | C24H31FO6 | 435.218 | --- | 457.200 | 226.6 ± 1.3 |
| Deflazacort | C25H31NO6 | 442.223 | 206.0 ± 1.1 | 464.205 | 214.3 ± 0.9 |
| Fluticasone Propionate | C25H31F3O5S | 501.192 | 173.3 ± 0.1 | 523.174 | 181.4 ± 0.1 |
| Fluticasone Furoate | C27H29F3O6S | 539.172 | --- | 561.154 | 231.4 ± 0.1 |
| Ciclesonide | C32H44O7 | 541.317 | 240.1 ± 3.7 | 563.299 | 264.6 ± 2.6 |
Fig. 1.
IM separation and CCS measurements for the sodiated species, [M + Na]+, of three isomer groups: (A) betamethasone/dexamethasone, (B) cortisone/prednisolone, and (C) flunisolide/triamcinolone acetonide.
3.1.2. LC-IM-MS analysis of glucocorticoids
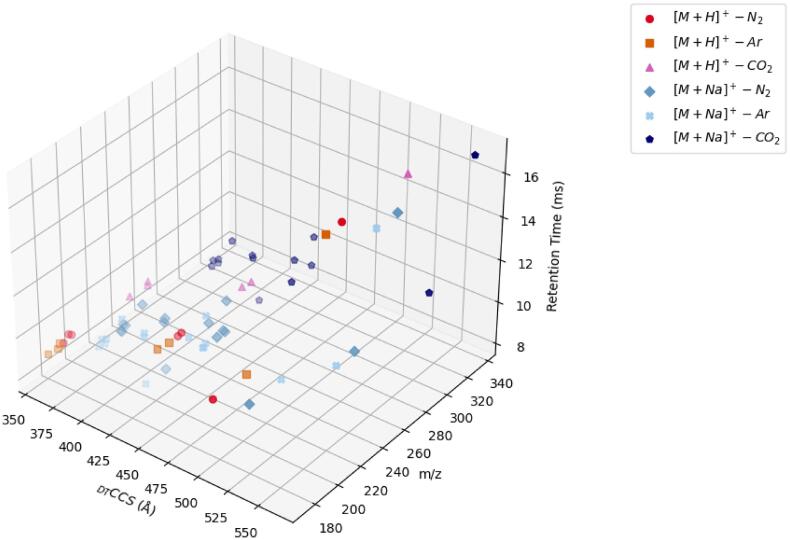
An advantage of IM is its acquisition speed (≤60 ms per drift spectrum), which allows for nesting in conventional LC-MS methods (i.e., LC-IM-MS). However, although the previously described step-field method provides the most accurate direct measurement of CCS, the need to step through multiple electric fields means that each measurement can take several minutes. Because this can’t be performed on a chromatographic timescale, the single-field method has been adopted by ‘calibrating’ the CCS scale relative to a set of known CCS standards (i.e., the Agilent Tune Mix ions). To demonstrate the agreement between the two methods, we injected individual standards of each glucocorticoid using a routine LC-IM-MS method with a 20 min gradient. Table S4 lists DTCCSN2 for all protonated and sodiated species measured using the single-field method during triplicate injections with LC-IM-MS. The agreement between these two CCS measurement methods is demonstrated by the small ΔCCS, and confirms that reliable values can be obtained for targeted and/or new/unidentified glucocorticoids in a complex sample. Fig. 2 further shows a three-dimensional (3D) plot of chromatographic retention time (min) vs. m/z vs. DTCCSN2 for all protonated and sodiated species of all glucocorticoids analyzed. This plot demonstrates the power of using multidimensional separations, mainly the ability to separate these targeted compounds using any one of the three different separation modes. It should also be noted that although a traditional 20 min gradient was employed here, the ultimate goal would be to couple a rapid (≤2 min) LC step for sample cleanup, while relying on IM separation to differentiate between the isomers.
Fig. 2.
Three-dimensional plot of chromatographic retention time vs. m/z vs. DTCCSN2 for protonated and sodiated species for all sixteen glucocorticoids analyzed by LC-IM-MS.
Additionally, all glucocorticoids were measured in negative ion mode by LC-IM-MS. However, low ionization efficiency resulted in poor abundance for these species (when detected), and as such they were not explored further.
3.1.3. Other cation adducts
Biomolecules are often detected in positive mode ESI-MS as protonated [M + H]+ or sodiated [M + Na]+ species due to the addition of acid into solvents/mobile phases or ubiquitous sodium, respectively. Other less commonly observed adducts include lithiated [M + Li]+ and potassiated [M + K]+ species, which might result from salts present in solvents or intentional addition to improve ionization efficiency and/or fragmentation pattern. Because several studies have demonstrated the effect on gas-phase structure of various cation adducts, we investigated the IM separation of alkali, alkaline earth, and first-row transition metals for these isomeric glucocorticoids.
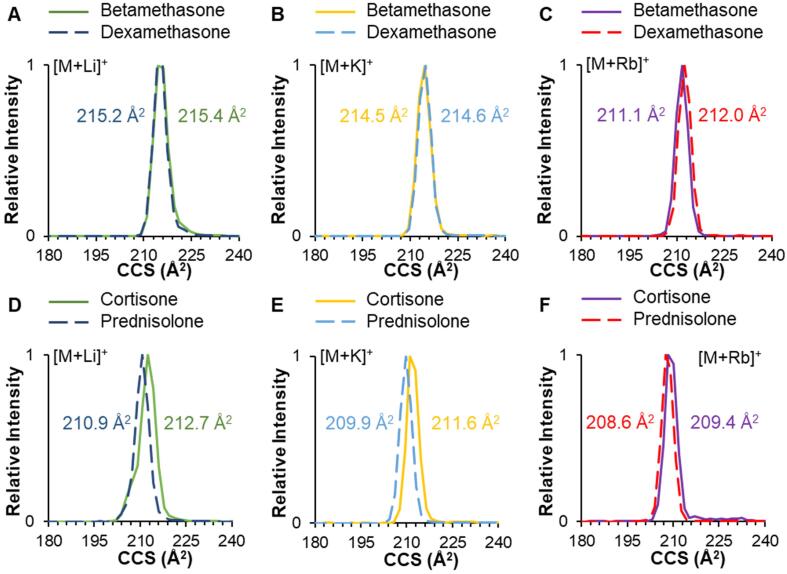
Alkali metal adducts investigated include lithium, potassium, rubidium, and cesium (in addition to sodium). In contrast to the minimal separation observed previously (Fig. 1) for the sodiated isomers, these adducts provided slightly improved resolution for isomers. Fig. 3 demonstrates IM separation of lithium, potassium, and rubidium adducts for both betamethasone/dexamethasone and cortisone/prednisolone. Although baseline resolution was not achieved with these adducts, some relative improvements in separation were observed; specifically, separation improved to 0.5% for betamethasone/dexamethasone as [M + Rb]+, and to 0.9% for cortisone/prednisolone as [M + Li]+. Values for all additional alkali metal adducts are listed in Table S5.
Fig. 3.
IM separation and CCS measurements for isomers betamethasone and dexamethasone as (A) [M + Li]+, (B) [M + K]+, and (C) [M + Rb]+; and for cortisone and prednisolone as (D) [M + Li]+, (E) [M + K]+, and (F) [M + Rb]+.
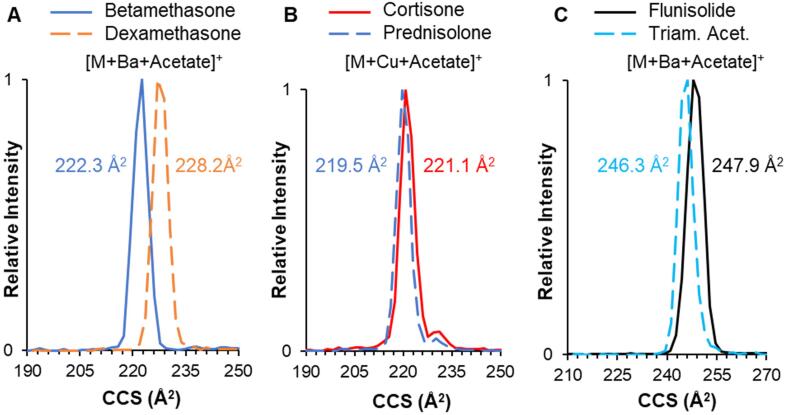
Based on the minor separation observed for alkali metals, we further investigated alkaline earth metals (magnesium, calcium, strontium, and barium) and first-row transition metals (iron, cobalt, nickel, copper, and zinc). It should be noted that the predominant ion for each of these additions (which were introduced as acetate salts) was the doubly charged metal with a single acetate counter anion, observed as a singly charged ion (i.e., [M + X + Acetate]+). Despite periodic trends (e.g., atomic mass, ionic radius, etc.) there were no observed patterns in CCS within either group. However, several cation adducts did have positive effects on separations, such as those shown in Fig. 4. For example, both betamethasone dexamethasone and flunisolide/ triamcinolone acetonide were considerably more resolved as barium adducts. Values for all additional alkaline earth and transition metal adducts are listed in Tables S6-S7.
Fig. 4.
IM separation and CCS measurements for isomers (A) betamethasone/dexamethasone as [M + Ba + Acetate]+; (B) cortisone/prednisolone as as [M + Cu + Acetate]+; and (C) flunisolide/triamcinolone acetonide as as [M + Ba + Acetate]+.
3.1.4. IM drift gas composition
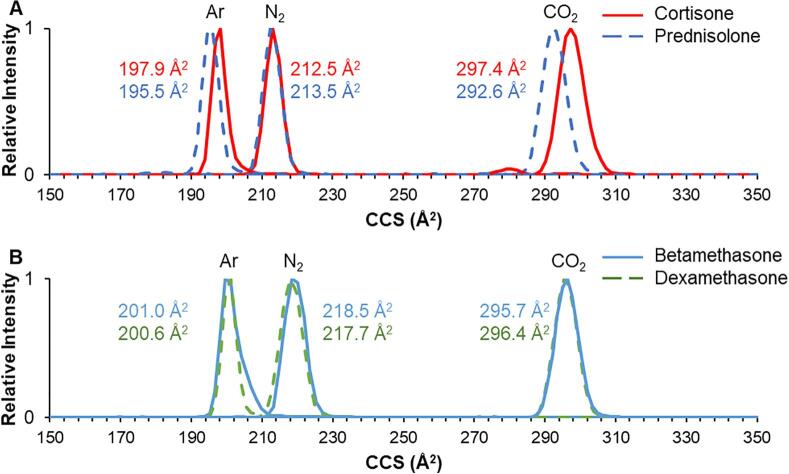
Lastly, we investigated the potential for improved separation by changing the IM drift gas. Drift gases explored included helium, argon, and carbon dioxide. Changes to the gas composition affect CCS based on their mass (as reduced mass with the analyte ion), molecular shape (monoatomic, linear diatomic, etc.), and their polarizability. In comparison with the standard nitrogen conditions, separations in helium yielded expectedly lower CCS and even less separation amongst isomer pairs. Separations in argon yielded slightly lower CCS (owing to the monoatomic nature), but did yield some moderate improvements in resolution for some isomer pairs, such as cortisone/prednisolone (Fig. 5A). Furthermore, ΔCCS increased to 1.6% for this pair in carbon dioxide gas, which resulted in considerably improved separation. This trend was not, however, observed for all isomer pairs, as betamethasone and dexamethasone remained challenging to resolve under all drift gas conditions. Larger gases, such as sulfur hexafluoride, could potentially be used in an attempt to separate these epimers. CCS values for all glucocorticoids in each of the four gases are included in Tables S8-S9.
Fig. 5.
IM separation and CCS measurements in argon, nitrogen, and carbon dioxide for the [M+Na]+ ion of isomers (A) cortisone/prednisolone, and (B) betamethasone/dexamethasone.
Although sampling of the entire parameter space (i.e., measurement of CCS for every cation adduct in all drift gas conditions) was outside of the scope of the present work, the simplest cation adducts, [M + Li]+ and [M + K]+, were analyzed in He, Ar, and CO2 for comparison with the protonated and sodiated results. Several interesting observations were made, which may prompt further investigation of these conditions. Specifically, the potassiated adducts of prednisolone and cortisone were well resolved in Ar, with CCS of 226.9 and 217.9 Å2, respectively (Table S8). Additionally, their lithiated adducts also showed improved resolution in CO2, with CCS of 290.2 and 278.6 Å2, respectively (Table S9). However, neither of the remaining isomer pairs showed any improvement by incorporating lithium/potassium into analysis with the other drift gases; as such, the other cations (e.g., Sr, Ba, etc.) will likely provide the best resolution for these species in future studies.
Finally, it should be noted that the ultimate goals of incorporating IM into current clinical LC-MS/MS methods are not only qualitative (separation/identification), but also quantitative. Our group has recently demonstrated targeted quantitation of steroids in a biological matrix using LC-IM-MS with limits of detection <600 pg/mL.[35] This work was achieved using multiplexed acquisition, which involves injection of multiple ion packets per mobility frame to maximize IM duty cycle and improve sensitivity.[36] Achieving IM separation of isomers/isobars, the primary focus of this current work, will further allow for reduction of chemical noise due to potentially coeluting species, and provide a future avenue by which to perform targeted quantitative clinical measurements using LC-IM-MS/MS.
4.1. Conclusion
We have demonstrated LC-IM-MS to be a robust, multidimensional technique with promise for broad applicability in the clinical lab. CCS were successfully measured for abundant ions of all glucocorticoids under standard operating conditions. These values could be used in future analysis of glucocorticoids by IM-MS to improve confidence in identification and/or quantification. Furthermore, CCS can be measured accurately on a chromatographic timescale, making it amenable for LC-IM-MS workflows to analyze complex biological samples. Challenging isomer separations were aided by experimental changes to the drift tube gas composition and introduction of alternative metal salts (alkali, alkaline earth, and transition metals); simple changes that could be implemented in future protocols. Overall, LC-IM-MS has a bright future in the clinical lab.
IRB and ethics statement
No human subjects, human-derived materials, or human medical records were used in this work.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could affect the work described in this article.
Acknowledgements
Financial support for this work was provided by Agilent Technologies (Global Academic Research Support Program #2624930) and Florida Institute of Technology startup funds. This project was also supported in part by funding from the Partnership for Clean Competition Research Collaborative. The content of this publication does not necessarily reflect the views or policies of Research Collaborative.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jmsacl.2022.03.003.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Chouinard C.D., Nagy G., Smith R.D., Baker E.S. Ion Mobility-Mass Spectrometry in Metabolomic. Lipidom. Proteom. Analys. 2019 [Google Scholar]
- 2.Chouinard C.D., Wei M.S., Beekman C.R., Kemperman R.H.J., Yost R.A. Ion mobility in clinical analysis: Current progress and future perspectives. Clin. Chem. 2016;62:124–133. doi: 10.1373/clinchem.2015.238840. [DOI] [PubMed] [Google Scholar]
- 3.May J.C., McLean J.A. Ion mobility-mass spectrometry: Time-dispersive instrumentation. Anal. Chem. 2015;87:1422–1436. doi: 10.1021/ac504720m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arthur K.L., Turner M.A., Brailsford A.D., Kicman A.T., Cowan D.A., Reynolds J.C., Creaser C.S. Rapid Analysis of Anabolic Steroid Metabolites in Urine by Combining Field Asymmetric Waveform Ion Mobility Spectrometry with Liquid Chromatography and Mass Spectrometry. Anal. Chem. 2017;89:7431–7437. doi: 10.1021/acs.analchem.7b00940. [DOI] [PubMed] [Google Scholar]
- 5.Wei M.S., Kemperman R.H.J., Palumbo M.A., Yost R.A. Separation of Structurally Similar Anabolic Steroids as Cation Adducts in FAIMS-MS. J. Am. Soc. Mass Spectrom. 2020;31:355–365. doi: 10.1021/jasms.9b00127. [DOI] [PubMed] [Google Scholar]
- 6.Guddat S., Thevis M., Kapron J., Thomas A., Schänzer W. Application of FAIMS to anabolic androgenic steroids in sport drug testing. Drug Test. Anal. 2009;1:545–553. doi: 10.1002/dta.73. [DOI] [PubMed] [Google Scholar]
- 7.Chouinard C.D., Cruzeiro V.W.D., Roitberg A.E., Yost R.A. Experimental and Theoretical Investigation of Sodiated Multimers of Steroid Epimers with Ion Mobility-Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2017;28 doi: 10.1007/s13361-016-1525-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Graton J., Hernández-Mesa M., Normand S., Dervilly G., Le Questel J.Y., Le Bizec B. Characterization of Steroids through Collision Cross Sections: Contribution of Quantum Chemistry Calculations. Anal. Chem. 2020;92:6034–6042. doi: 10.1021/acs.analchem.0c00357. [DOI] [PubMed] [Google Scholar]
- 9.Thevis M., Dib J., Thomas A., Höppner S., Lagojda A., Kuehne D., Sander M., Opfermann G., Schänzer W. Complementing the characterization of in vivo generated N-glucuronic acid conjugates of stanozolol by collision cross section computation and analysis. Drug Test. Anal. 2015;7:1050–1056. doi: 10.1002/dta.1907. [DOI] [PubMed] [Google Scholar]
- 10.Zheng X., Aly N.A., Zhou Y., Dupuis K.T., Bilbao A., Paurus V.L., Orton D.J., Wilson R., Payne S.H., Smith R.D., Baker E.S. A structural examination and collision cross section database for over 500 metabolites and xenobiotics using drift tube ion mobility spectrometry. Chem. Sci. 2017;8:7724–7736. doi: 10.1039/c7sc03464d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou Z., Tu J., Zhu Z.J. Advancing the large-scale CCS database for metabolomics and lipidomics at the machine-learning era. Curr. Opin. Chem. Biol. 2018;42:34–41. doi: 10.1016/j.cbpa.2017.10.033. [DOI] [PubMed] [Google Scholar]
- 12.May J.C., Morris C.B., McLean J.A. Ion mobility collision cross section compendium. Anal. Chem. 2017;89:1032–1044. doi: 10.1021/acs.analchem.6b04905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hernández-Mesa M., Le Bizec B., Monteau F., García-Campaña A.M., Dervilly-Pinel G. Collision Cross Section (CCS) Database: An Additional Measure to Characterize Steroids. Anal. Chem. 2018;90:4616–4625. doi: 10.1021/acs.analchem.7b05117. [DOI] [PubMed] [Google Scholar]
- 14.N.R. Oranzi, R.H.J. Kemperman, M.S. Wei, V.I. Petkovska, S.W. Granato, B. Rochon, J. Kaszycki, A. La Rotta, K. Jeanne Dit Fouque, F. Fernandez-Lima, R.A. Yost, Measuring the integrity of gas-phase conformers of sodiated 25-hydroxyvitamin D3 by drift tube, travelling wave, trapped, and high-field asymmetric ion mobility. Anal. Chem. 91, acs.analchem.8b05723 (2019). 10.1021/acs.analchem.8b05723. [DOI] [PubMed]
- 15.Delvaux A., Rathahao-Paris E., Alves S. An emerging powerful technique for distinguishing isomers: Trapped ion mobility spectrometry time-of-flight mass spectrometry for rapid characterization of estrogen isomers. Rapid Commun. Mass Spectrom. 2020;34 doi: 10.1002/rcm.8928. [DOI] [PubMed] [Google Scholar]
- 16.Cole R.B., Bayat P., Murray J.S., Albers C., Brombach D. “Conformation pinning” by anion attachment enabling separation of isomeric steroid monomers by ion mobility spectrometry. J. Mass Spectrom. 2020;55(12) [Google Scholar]
- 17.McCullagh M., Giles K., Richardson K., Stead S., Palmer M. Investigations into the performance of travelling wave enabled conventional and cyclic ion mobility systems to characterise protomers of fluoroquinolone antibiotic residues. Rapid Commun. Mass Spectrom. 2019;33:11–21. doi: 10.1002/rcm.8371. [DOI] [PubMed] [Google Scholar]
- 18.Ujma J., Ropartz D., Giles K., Richardson K., Langridge D., Wildgoose J., Green M., Pringle S. Cyclic Ion Mobility Mass Spectrometry Distinguishes Anomers and Open-Ring Forms of Pentasaccharides. J. Am. Soc. Mass Spectrom. 2019;30:1028–1037. doi: 10.1007/s13361-019-02168-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williamson D.L., Bergman A.E., Nagy G. Investigating the Structure of α/β Carbohydrate Linkage Isomers as a Function of Group I Metal Adduction and Degree of Polymerization as Revealed by Cyclic Ion Mobility Separations. J. Am. Soc. Mass Spectrom. 2021;32(10):2573–2582. doi: 10.1021/jasms.1c00207. [DOI] [PubMed] [Google Scholar]
- 20.Chouinard C.D., Nagy G., Webb I.K., Garimella S.V.B., Baker E.S., Ibrahim Y.M., Smith R.D. Rapid Ion Mobility Separations of Bile Acid Isomers Using Cyclodextrin Adducts and Structures for Lossless Ion Manipulations. Anal. Chem. 2018;90(18):11086–11091. doi: 10.1021/acs.analchem.8b02990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chouinard C.D., Nagy G., Webb I.K., Shi T., Baker E.S., Prost S.A., Liu T., Ibrahim Y.M., Smith R.D. Improved Sensitivity and Separations for Phosphopeptides using Online Liquid Chromotography Coupled with Structures for Lossless Ion Manipulations Ion Mobility-Mass Spectrometry. Anal. Chem. 2018;90:10889–10896. doi: 10.1021/acs.analchem.8b02397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagy G., Chouinard C.D., Attah I.K., Webb I.K., Garimella S.V.B., Ibrahim Y.M., Baker E.S., Smith R.D. Distinguishing enantiomeric amino acids with chiral cyclodextrin adducts and structures for lossless ion manipulations. Electrophoresis. 2018;39(24):3148–3155. doi: 10.1002/elps.201800294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahonen L., Fasciotti M., Gennäs G.B.af., Kotiaho T., Daroda R.J., Eberlin M., Kostiainen R. Separation of steroid isomers by ion mobility mass spectrometry. J. Chromatogr. A. 2013;1310:133–137. doi: 10.1016/j.chroma.2013.08.056. [DOI] [PubMed] [Google Scholar]
- 24.Maddox S.W., Fraser Caris R.H., Baker K.L., Burkus-Matesevac A., Peverati R., Chouinard C.D. Ozone-Induced Cleavage of Endocyclic C═C Double Bonds within Steroid Epimers Produces Unique Gas-Phase Conformations. J. Am. Soc. Mass Spectrom. 2020;31(2):411–417. doi: 10.1021/jasms.9b00058. [DOI] [PubMed] [Google Scholar]
- 25.Maddox S.W., Olsen S.S.H., Velosa D.C., Burkus-Matesevac A., Peverati R., Chouinard C.D. Improved Identification of Isomeric Steroids Using the Paternò-Büchi Reaction with Ion Mobility-Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2020;31 doi: 10.1021/jasms.0c00215. [DOI] [PubMed] [Google Scholar]
- 26.Chouinard C.D., Beekman C.R., Kemperman R.H.J., King H.M., Yost R.A. Ion mobility-mass spectrometry separation of steroid structural isomers and epimers. Int. J. Ion Mobil. Spectrom. 2017;20(1-2):31–39. [Google Scholar]
- 27.Chouinard C.D., Cruzeiro V.W.D., Kemperman R.H.J., Oranzi N.R., Roitberg A.E., Yost R.A. Cation-dependent conformations in 25-hydroxyvitamin D3-cation adducts measured by ion mobility-mass spectrometry and theoretical modeling. Int. J. Mass Spectrom. 2018;432:1–8. doi: 10.1016/j.ijms.2018.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rister A.L., Martin T.L., Dodds E.D. Application of Group I Metal Adduction to the Separation of Steroids by Traveling Wave Ion Mobility Spectrometry. J. Am. Soc. Mass Spectrom. 2019;30:248–255. doi: 10.1007/s13361-018-2085-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rister A.L., Martin T.L., Dodds E.D. Formation of multimeric steroid metal adducts and implications for isomer mixture separation by traveling wave ion mobility spectrometry. J. Mass Spectrom. 2019;54:429–436. doi: 10.1002/jms.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kurulugama R.T., Darland E., Kuhlmann F., Stafford G., Fjeldsted J. Evaluation of drift gas selection in complex sample analyses using a high performance drift tube ion mobility-QTOF mass spectrometer. Analyst. 2015;140:6834–6844. doi: 10.1039/c5an00991j. [DOI] [PubMed] [Google Scholar]
- 31.Ruskic D., Klont F., Hopfgartner G. Clustering and Nonclustering Modifier Mixtures in Differential Mobility Spectrometry for Multidimensional Liquid Chromatography Ion Mobility-Mass Spectrometry Analysis. Anal. Chem. 2021;93:6638–6645. doi: 10.1021/acs.analchem.0c04889. [DOI] [PubMed] [Google Scholar]
- 32.Coughlan N.J.A., Liu C., Lecours M.J., Campbell J.L., Hopkins W.S. Preferential Ion Microsolvation in Mixed-Modifier Environments Observed Using Differential Mobility Spectrometry. J. Am. Soc. Mass Spectrom. 2019;30:2222–2227. doi: 10.1007/s13361-019-02332-1. [DOI] [PubMed] [Google Scholar]
- 33.Stow S.M., Causon T.J., Zheng X., Kurulugama R.T., Mairinger T., May J.C., Rennie E.E., Baker E.S., Smith R.D., McLean J.A., Hann S., Fjeldsted J.C. An Interlaboratory Evaluation of Drift Tube Ion Mobility-Mass Spectrometry Collision Cross Section Measurements. Anal. Chem. 2017;89:9048–9055. doi: 10.1021/acs.analchem.7b01729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morris C.B., May J.C., Leaptrot K.L., McLean J.A. Evaluating Separation Selectivity and Collision Cross Section Measurement Reproducibility in Helium, Nitrogen, Argon, and Carbon Dioxide Drift Gases for Drift Tube Ion Mobility-Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2019;30:1059–1068. doi: 10.1007/s13361-019-02151-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Velosa D.C., Rivera M.E., Neal S.P., Olsen S.S.H., Burkus-Matesevac A., Chouinard C.D. Toward Routine Analysis of Anabolic Androgenic Steroids in Urine Using Ion Mobility-Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2021 doi: 10.1021/jasms.1c00231. [DOI] [PubMed] [Google Scholar]
- 36.May J.C., Knochenmuss R., Fjeldsted J.C., McLean J.A. Resolution of Isomeric Mixtures in Ion Mobility Using a Combined Demultiplexing and Peak Deconvolution Technique. Anal. Chem. 2020;92:9482–9492. doi: 10.1021/acs.analchem.9b05718. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.