Abstract
Background
The treatment success rate of drug-resistant (DR) tuberculosis is alarmingly low. Therefore, more effective and less complex regimens are urgently required.
Methods
We compared the efficacy of an all oral DR tuberculosis drug regimen consisting of bedaquiline (25 mg/kg), delamanid (2.5 mg/kg), and linezolid (100 mg/kg) (BDL) on the mycobacterial load in the lungs and spleen of tuberculosis-infected mice during a treatment period of 24 weeks. This treatment was compared with the standard regimen of isoniazid, rifampicin, pyrazinamide, and ethambutol (HRZE). Relapse was assessed 12 weeks after treatment. Two logistic regression models were developed to compare the efficacy of both regimens.
Results
Culture negativity in the lungs was achieved at 8 and 20 weeks of treatment with BDL and HRZE, respectively. After 14 weeks of treatment only 1 mouse had relapse in the BDL group, while in the HRZE group relapse was still observed at 24 weeks of treatment. Predictions from the final mathematical models showed that a 95% cure rate was reached after 20.5 and 28.5 weeks of treatment with BDL and HRZE, respectively.
Conclusion
The BDL regimen was observed to be more effective than HRZE and could be a valuable option for the treatment of DR tuberculosis.
Keywords: tuberculosis, early bactericidal activity, treatment efficacy, pharmacokinetics, pharmacodynamics, BDL
The bedaquiline, delamanid, and linezolid regimen significantly reduced treatment time compared with isoniazid, rifampicin, pyrazinamide, and ethambutol. Delamanid is a good alternative to pretomanid, and bedaquiline is the main contributor to treatment efficacy during the early phase of therapy.
It is estimated that in 2018 10 million people contracted tuberculosis, drug-resistant (DR) tuberculosis in 500 000 [1]. The treatment duration of DR tuberculosis is ≥9 months, and frequently >1.5 years, requiring a combination of many different drugs. Even with this intensive therapy, the treatment success rate for DR tuberculosis is only 54% in case of multidrug-resistant (MDR) and 30% for extensively-drug resistant (XDR) tuberculosis [1]. Therefore shorter, less toxic, and more effective regimens are required.
Despite the urgent need for new treatments, only 3 new tuberculosis drugs have become commercially available in the last 4 decades. Bedaquiline is the first member of a new class of drugs called diarylquinolines. Its mechanism of action relies on blocking a proton pump of Mycobacterium tuberculosis that is required for adenosine triphosphate synthase, resulting in the loss of energy production and cell death [2]. Delamanid and pretomanid belong to another novel class of drugs, the nitroimidazoles. Exposure to delamanid blocks methoxy-mycolic and keto-mycolic acid synthesis causing destabilization of the mycobacterial cell wall [3]. In addition to these new tuberculosis drugs, linezolid has recently been repurposed as a core second-line agent for MDR tuberculosis and belongs to the oxazolidinones, which inhibit protein synthesis [4].
Each of the drugs mentioned above possesses different mechanisms of antimycobacterial action. Therefore, by combining the 3 orally administered drugs bedaquiline, delamanid, and linezolid (BDL) we aimed to provide further evidence that this regimen can shorten the duration of DR tuberculosis treatment. Experiments were performed in our previously validated mouse tuberculosis model using a drug-sensitive clinical M. tuberculosis strain [5, 6], and compared it with the standard regimen of isoniazid, rifampicin, pyrazinamide, and ethambutol (HRZE). We used a drug-sensitive strain, because no “gold standard” DR tuberculosis regimen has yet been defined, enabling the comparison of the performance of the BDL regimen with that of the classic tuberculosis drug regimen HRZE, as well as with other studies in this field [7]. The treatment-shortening potential of the BDL regimen is supported by a hollow-fiber model study, indicating the synergistic activity of the combination of bedaquiline and linezolid against M. tuberculosis in various metabolic states [8].
A previous in vivo study investigated the combination of bedaquiline, pretomanid, and linezolid (BPaL) in a mouse tuberculosis model, showing good efficacy [7]. Subsequently, this particular combination was assessed in the Nix-TB trial, with promising results regarding efficacy and safety in patients with either MDR or XDR tuberculosis [9]. Studying the efficacy of delamanid in this DR tuberculosis drug regimen (as a replacement for pretomanid) is of interest, because some mutations in the deazaflavin-dependent nitroreductase enzyme are associated with resistance to pretomanid but do not seem to affect susceptibility to delamanid [10]. This is particularly relevant, as such a mutation was already found to be present in a clinical M. tuberculosis Beijing strain without the isolate ever being exposed to either pretomanid or delamanid [10]. In this respect, delamanid could be a viable alternative for pretomanid in the BPaL regimen in cases of pretomanid resistance.
METHODS
Animals
Specified pathogen-free female BALB/c mice were obtained from Charles River. Only female mice were used since some studies have shown sex differences in pharmacokinetic profiles in mice [11]. Animals were 13–15 weeks old at the start of the experiments, with experimental protocols adhering to the rules specified in the Dutch Animal Experimentation Act—concordant with the European Union animal directive 2010/63/EU (license nos. 117-14-04 and AVD1010020173687).
Bacterial Strain and Tuberculosis Drugs
Experiments were performed using the drug-sensitive M. tuberculosis Beijing VN 2002-1585 genotype strain [6] with MICs of 0.125 mg/L for isoniazid, 0.25 mg/L for rifampicin, 5 mg/L for ethambutol [12], 0.06 mg/L for bedaquiline, 0.015 mg/L for delamanid, and 0.25 mg/L for linezolid. MICs were determined according to Clinical and Laboratory Standards Institute standards [13]. The antibiotic drugs used were prepared as described elsewhere (Supplement 1) [5, 14].
Experimental Setup
Using previously described power calculations (Supplement 2) [5], a total of 378 mice were needed, 210 for efficacy assessment and 168 for pharmacokinetic analysis. Mice were infected with M. tuberculosis suspensions as described elsewhere [15]. In short, a suspension of M. tuberculosis, stored at −80°C, was defrosted at room temperature and centrifuged for 10 minutes at 14 000g. The mycobacterial pellet was resuspended in phosphate-buffered saline and centrifuged again for 1 minute at 1900g to eliminate any aggregated bacteria. The mycobacterial suspension was then diluted in phosphate-buffered saline to obtain the intended bacterial load. Mice under anesthesia were infected via intratracheal instillation with a suspension containing 1.2 (range, 0.8–2.0) × 105 colony-forming units (CFUs) of M. tuberculosis, followed by inhalation to ensure the formation of a bilateral tuberculosis infection. Therapy was started 2 weeks after infection. Mice were checked daily and were euthanized when humane end points (instability, dark eyes, decreased response to stimuli) were reached.
To assess the dose-response of monotherapy, mice were exposed to 0.5×, 1×, or 2× the human pharmacokinetic equivalent doses (HED) of bedaquiline (ie, 12.5, 25, or 50 mg/kg) and delamanid (1.25, 2.5, and 5 mg/kg) and 0.25×, 0.5×, or 1× the HED of linezolid (25, 50 and 100 mg/kg). Mice intended for combination therapy were divided into 2 groups. The first group received a combination of 25-mg/kg bedaquiline, 2.5-mg/kg delamanid, and 100-mg/kg linezolid (BDL). The second group received standard (HRZE) therapy, that is, 25-mg/kg isoniazid, 10-mg/kg rifampicin, 150-mg/kg pyrazinamide, and 100-mg/kg ethambutol. All drugs were administered orally 5 times per week, using a feeding cannula and a total drug combination volume of 0.2 mL per day.
Pharmacokinetic Analyses
To quantify the drug concentrations, 2 blood samples per mouse were obtained via venous tail puncture. Samples were taken after 4 weeks of treatment and for a period of 24 hours. These 24-hour samples were taken in duplicate 0.25, 0.5, 0.75, 1, 1.5, 2, 3, 4, 6, 8, 12, and 24 hours after drug administration. Blood was collected in microcentrifuge tubes containing ethylenediaminetetraacetic acid and centrifuged at 10 000g for 5 minutes to obtain plasma, which was stored at −80°C. Methods for drug quantification by liquid chromatography–tandem mass spectrometry can be found in Supplementary File 3. Pharmacokinetic-pharmacodynamic parameters were determined using Prism 8 software (GraphPad).
Mycobacterial Load (Efficacy) Assessment
Mice receiving bedaquiline monotherapy were euthanized 0, 1, 2, or 4 weeks after the start of treatment (n = 3 per dose per time point). Mice receiving delamanid or linezolid monotherapy were euthanized 0, 1, 2, or 3 weeks after the start of treatment (n = 3 per dose per time point) because they reached humane end points before week 4 owing to severe tuberculosis infection. After euthanasia, the mycobacterial load was measured by CFU counting for assessment of early bactericidal activity (EBA). For CFU counts, the lungs and spleen were removed aseptically and homogenized, followed by serial dilution and culture. To prevent drug carryover, therapy was stopped 72 hours before euthanasia of the mice, with samples cultured on 7H10 Middlebrook agar containing activated charcoal.
Mice receiving combination therapy (total n = 90) were euthanized at the start of treatment (n = 3) and—to assess the CFU count immediately after treatment—after a treatment duration of 8, 12, 16, 20, or 24 weeks (n = 3 per time point). To assess relapse, mice were also euthanized 12 weeks after treatment was stopped, after a treatment duration of 8, 10, 12, 14, 16, 18, 20, 22, or 24 weeks (n = 3 per time point), as described previously (Supplement 4) [5].
Statistical Analysis
The CFU counts per milliliter were log10 transformed before analysis and multiplied by 2.3 and 2.1 for lung and spleen, respectively. Group mean CFU counts were compared 3 or 4 weeks after the start of monotherapy (along with the control), using 1-way analysis of variance with a Dunnett posttest to assess monoactivity. Differences in CFU count between the BDL and HRZE control groups were assessed at the start of treatment using unpaired 2-sample t test. The difference in cumulative time to relapse between both combination therapy groups was assessed using the log-rank test. The statistical significance level adopted was P < .05. Analyses were performed using Prism 8 software (GraphPad).
A logistic regression model was used to predict the treatment lengths required to reach 85%, 90%, and 95% cure rates in mice treated with either the BDL or the HRZE regimen. CFU counts were transformed into a binary outcome variable, describing relapse or no relapse. Relapse was defined as a positive culture 12 weeks after discontinuation of treatment and no relapse (ie, cure) as a negative culture result. To describe the relationship between probability of relapse and treatment length, 2 logistic regression models were developed, 1 for each regimen. During model development, all parameters were estimated simultaneously using NONMEM software (version 7.4) [16]. Model development is described in Supplement 5.
To compare the efficacies of BDL and HRZE regimens, the cure rate at any treatment length, as well as the minimum treatment lengths needed to reach 85%, 90%, and 95% cure, were predicted based on the final model for each regimen. Because the observed data consisted of 3 mice per time point, we could create cure rates of only 0%, 33%, 67% and 100% and used mathematical modeling to generate continuous cure rates between 0% and 100%. While the probability of relapse was modeled in the logistic regression model, the probability of no relapse (ie, cure) was used in the simulations.
Because the probability of cure is the complementary to the probability of relapse, the probability of cure (Pcure) was obtained by subtracting the probability of relapse (Prelapse) from 1, as described in Equation 1 in Supplementary File 5. The proportion of cured mice was then simulated at each time point. Predicted cure rates were simulated for treatment lengths between 0 and 30 weeks in 0.5-week increments for 1000 mice per arm and time point to achieve a high resolution in the predictions. Samples were randomly drawn from a uniform distribution, using Monte Carlo sampling.
RESULTS
Pharmacokinetic Analyses
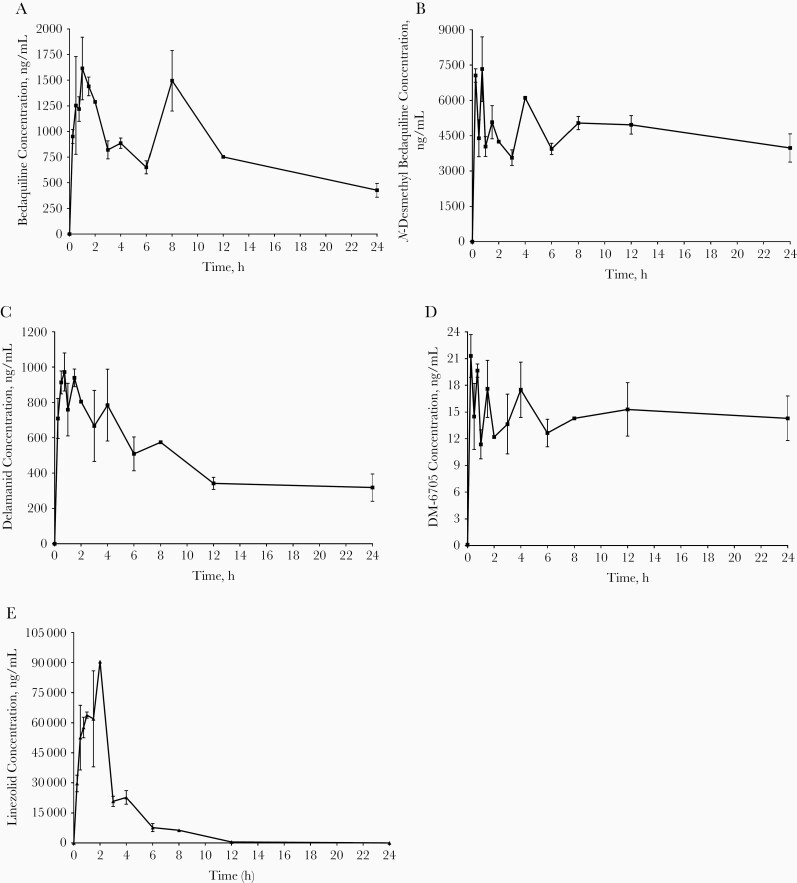
Plasma concentration-time profiles and pharmacokinetic parameters of the BDL combination and the metabolites of bedaquiline and delamanid are shown in Figure 1 and Table 1.
Figure 1.
Mice were treated with 25-mg/kg bedaquiline, 2.5-mg/kg delamanid, and 100-mg/kg linezolid 5 times per week. Bedaquiline (A), N-desmethyl bedaquiline (B), delamanid (C), DM-6705 (D), and linezolid © plasma concentration-time profiles after 4 weeks of bedaquiline, delamanid, and linezolid therapy in tuberculosis-infected BALB/c mice. Plasma concentrations are plotted as mean and standard errors of the mean for 2 mice per study drug per time point.
Table 1.
Results of Pharmacokinetic Analysis (n = 2 per Time Point)
| Drug | T max, h | C max, range, ng/mL | AUC0–24, mean (SEM), ng/mL⋅h) |
|---|---|---|---|
| Bedaquiline (25 mg/kg) | 1.00 | 1920–1310 | 19 661 (1121) |
| N-Desmethyl-bedaquiline | 0.75 | 5960–8700 | 111 273 (6312) |
| Delamanid (2.5 mg/kg) | 0.75 | 864–1080 | 11 234 (841.7) |
| DM-6705 | 0.25 | 18.9–23.7 | 352.4 (34.9) |
| Linezolid (100 mg/kg) | 2.00 | 90 500a | 251 269 (14 931) |
Abbreviations: AUC0–24, 24-hour area under the receiver operating characteristic curve; Cmax, maximum plasma concentration; SEM, standard error of the mean; Tmax, time to maximum plasma concentration.
aFor linezolid, the drug concentration was assessed in only 1 mouse.
Pharmacodynamic Analyses—Mycobacterial Load Assessment
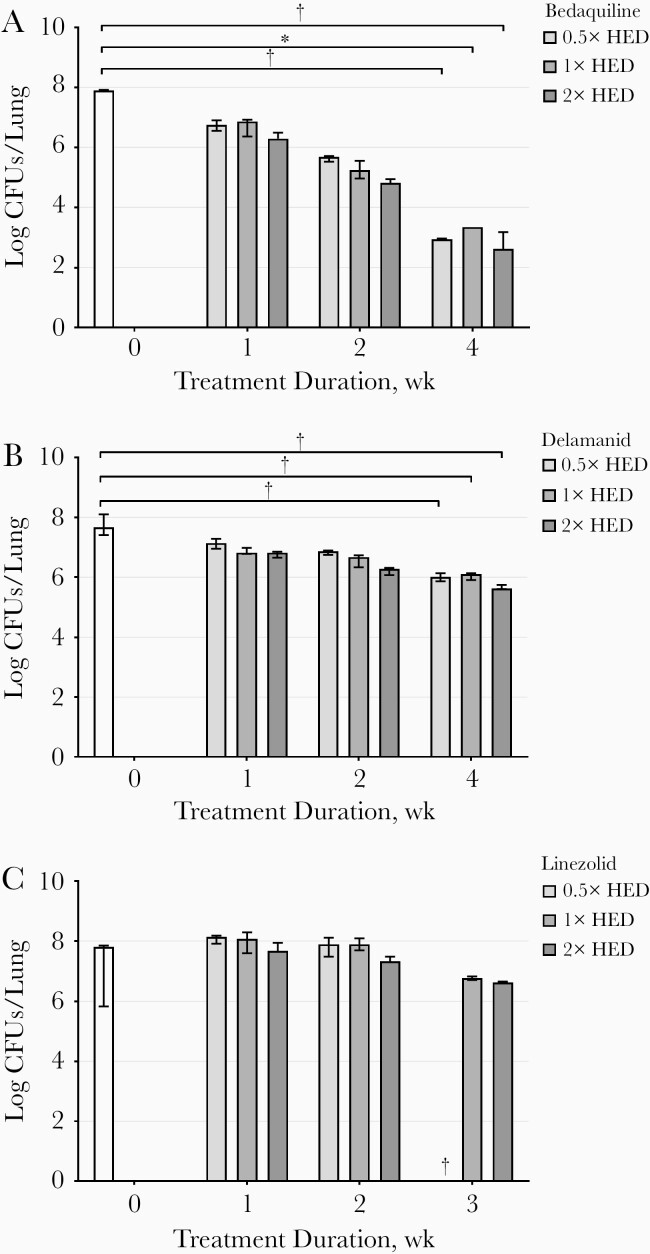
During monotherapy, bedaquiline was well tolerated. However, mice treated with delamanid or linezolid showed stress during therapy, and all mice receiving monotherapy of 25-mg/kg linezolid were euthanized before 3 weeks of treatment as humane end points were reached. The mycobacterial loads in the lungs during 3 or 4 weeks of treatment with bedaquiline, delamanid, and linezolid are presented in Figure 2. The median total amount of mycobacteria in the lungs at the start of treatment with bedaquiline was 7.89 log10 CFUs (range, 7.87–7.92 log10 CFUs), which declined significantly (P < .01) after 4 weeks of treatment to 4.96, 4.55, and 5.28 log10 CFU, at 0.5×, 1×, and 2× the HED, respectively. Delamanid significantly reduced the CFU count in the lungs of mice after 3 weeks of treatment and at all doses tested (approximately 2 log10 CFUs were observed for all doses; P < .001). Linezolid did not have a significant effect on the CFU count after 3 weeks of treatment (P = .63). Results in the spleen were comparable for all drugs (data not shown).
Figure 2.
Mycobacterial load in lungs expressed as median and range (error bars) of the colony-forming units (CFUs) (n = 3), at weeks 0, 1, 2, and 3 or 4 weeks after treatment with bedaquiline (A), delamanid (B), or linezolid (C). White bars represent control mice; bars from light to dark gray represent mice treated with 0.5×, 1×, and 2× the human pharmacokinetic equivalent dose (HED). *P < .01; **P < .001 (1-way analysis of variance). †Three mice were euthanized before planned as humane end points had been reached. Results for 1 mouse treated with 0.5×, 2 treated with 1×, and 1 treated with 2× the HED for bedaquiline are missing in week 4, since no undiluted samples were analyzed and 10× diluted samples of lungs and spleen did not show any CFUs.
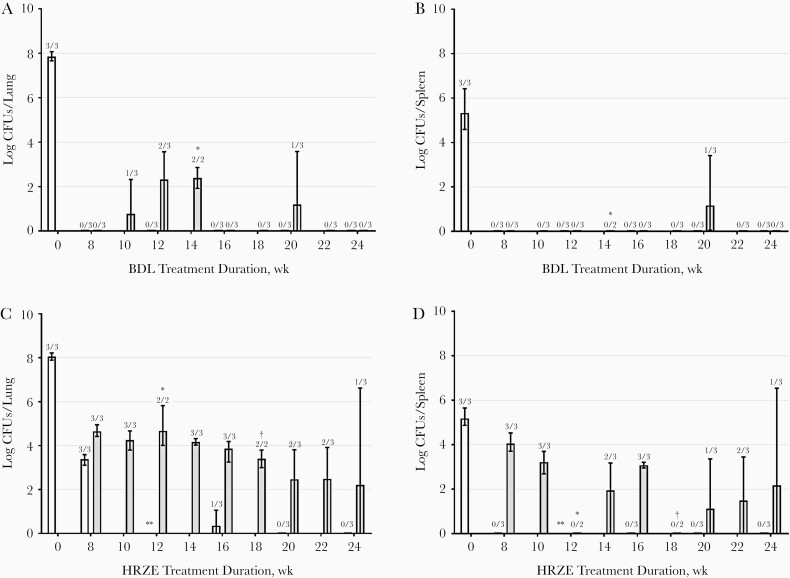
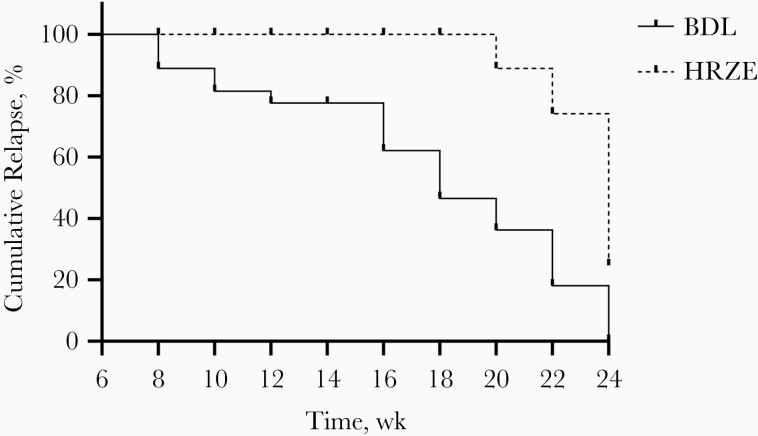
Both BDL and HRZE combination treatments were well tolerated. The mycobacterial load in lungs and spleen are presented in Figure 3. The median total amounts of mycobacteria were 7.80 log10 CFUs (range, 7.66–8.07) in the lungs at the start of BDL treatment and 8.03 log10 CFUs (7.89–8.22) at the start of HRZE treatment (P = .26). At 8 weeks of treatment with BDL, culture negativity in the lungs and spleens was achieved. As of 16 weeks of treatment, no relapse was observed in the BDL group, except in 1 mouse at week 20. In HRZE-treated mice, culture negativity in the lungs was achieved after 20 weeks of treatment. However, mice in the HRZE treatment arm relapsed even after 24 weeks of treatment. Results for the spleen showed a similar pattern. Significantly fewer relapse episodes were observed in the BDL group than in the HRZE group (P < .001) (Figure 4).
Figure 3.
Mycobacterial load in lung (A, C) and spleen (B, D) expressed as medians and ranges (error bars) of the colony-forming units (CFUs) per organ. Results at weeks 8, 12, 16, 20, and 24 of treatment are expressed as white bars, and relapse assessment was performed 12 weeks after a treatment duration of 8, 10, 12, 14, 16, 18, 20, 22, and 24 weeks, expressed as gray bars. Mice were treated with bedaquiline, delamanid, and linezolid (BDL) (A, B) or isoniazid, rifampicin, pyrazinamide, and ethambutol (HRZE) (C, D). Numbers above bars are the numbers of culture-positive mice of total numbers of mice at that time point. *CFU counting was not performed since the culture plates were contaminated. †One mouse reached humane end points before planned euthanasia.
Figure 4.
Cumulative relapse of mice treated with bedaquiline, delamanid, and linezolid (BDL) or isoniazid, rifampicin, pyrazinamide, and ethambutol (HRZE) between 8 and 24 weeks. Solid line represents BDL group; dotted line, HRZE group.
Pharmacodynamic Analyses—Modeling
For the BDL regimen, none of the assessed functions that related treatment length and probability of relapse (linear, the maximum reachable probability of cure [Emax] and sigmoidal Emax function (Supplementary File 6)) led to a significant improvement in model fit compared with the base model (P < .05). Therefore, a “surge” function was evaluated (in addition to the already assessed functions) as graphic analysis revealed an initial increase, followed by a decrease, in the probability of relapse as a function of treatment length (Figure 3C). This use of a surge function resulted in a statistically significant improvement in model fit compared with the base model (P < .001) and was consequently retained in the final model relating length of BDL treatment with the probability of relapse. For the HRZE regimen, the probability of relapse in relation to different treatment lengths was best described by a sigmoidal Emax relationship. The model parameter estimates and parameter uncertainties are described in Supplementary File 6.
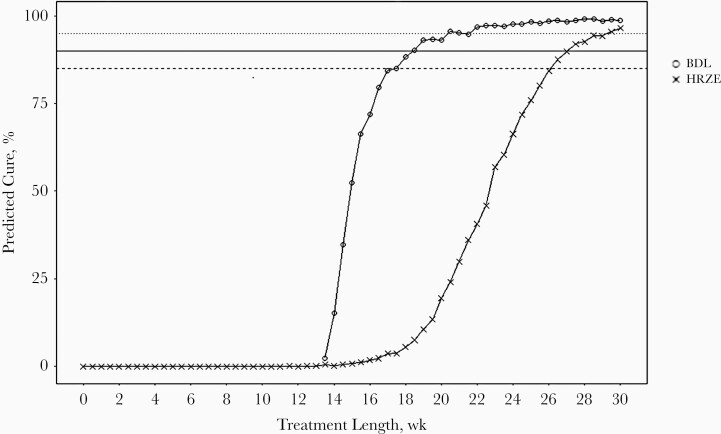
The required treatment lengths for the BDL regimen to reach cure rates of 85%, 90%, and 95%, were predicted to be 17.5, 18.5, and 20.5 weeks, respectively. For the HRZE regimen, simulations of the predicted cure rates at different treatment lengths showed that a 95% cure rate was reached after 28.5 weeks of treatment, and for cure rates of 85% and 90%, 26.0 and 27.0 weeks, respectively. A 95% cure rate was thus predicted to be reached 8 weeks faster in mice treated with the BDL regimen than in those treated with the HRZE regimen, which corresponds to a 28.1% decrease in treatment length. The predicted cure rates at different treatment lengths for both BDL and HRZE regimens are shown in Figure 5 and Table 2.
Figure 5.
Model-predicted cure rates at different treatment lengths for both bedaquiline, delamanid, and linezolid (BDL) and isoniazid, rifampicin, pyrazinamide, and ethambutol (HRZE) regimens. Dashed line represents 85%, solid line 90% and dotted line 95% cure rate. HRZE regimen included 25-mg/kg isoniazid, 10-mg/kg rifampicin, 150-mg/kg pyrazinamide, and 100-mg/kg ethambutol. BDL regimen included 25-mg/kg bedaquiline, 2.5-mg/kg delamanid, and 100-mg/kg linezolid.
Table 2.
Model Predicted Treatment Lengths Required to Reach 85%, 90%, and 95% by Regimen
| Regimena | Minimal Treatment Length Required to Achieve Cure Rate, wk | ||
|---|---|---|---|
| 85% Cure Rate | 90% Cure Rate | 95% Cure Rate | |
| BDL | 17.5 | 18.5 | 20.5 |
| HRZE | 26.0 | 27.0 | 28.5 |
Abbreviations: BDL, bedaquiline, delamanid, and linezolid; HRZE, isoniazid, rifampicin, pyrazinamide, and ethambutol.
aBDL included 25-mg/kg bedaquiline, 2.5-mg/kg delamanid, and 100-mg/kg linezolid. HRZE included 25-mg/kg isoniazid, 10-mg/kg rifampicin, 150-mg/kg pyrazinamide, and 100-mg/kg ethambutol 5 times per week.
DISCUSSION
The current study indicated a higher efficacy for the BDL combination treatment regimen, than for the standard HRZE regimen, when using a mouse tuberculosis model and the clinically relevant M. tuberculosis Beijing genotype strain [6]. In the early phase of treatment, efficacy appeared to be mainly bedaquiline driven, as illustrated by the EBA results obtained using the 3 drugs separately as monotherapy. The observed dose-dependent activity of bedaquiline was comparable to other murine studies [17, 18]. Delamanid showed only minor bactericidal activity, and linezolid no bactericidal activity in the first 3 weeks of monotherapy, and mice were consequently euthanized 1 week before schedule. The limited EBA of delamanid is in line with other published murine studies. For example, 1 study showed a decrease of 2.5 log10 CFUs/mL after 28 days of treatment with a dose of 2.5-mg/kg delamanid [3], and in another study a dose of 100 mg/kg (40 times as much as in our study) reduced the CFU count in the lungs by 1 log10 after 4 weeks of treatment [19]. In line with the present study findings, linezolid showed no bactericidal activity in previous murine studies [20].
Our results indicated that a minimal treatment length of 20.5 weeks was predicted to be required in order to reach a 95% cure rate using the BDL regimen. This was 8 weeks shorter than for the HRZE regimen. Interestingly, 2 other murine studies assessed the efficacy of the combination of bedaquiline, pretomanid, and linezolid (BPaL), showing good results for this combination regimen [7, 21]. Xu et al showed that the addition of pretomanid increased the bactericidal activity of the bedaquiline-linezolid combination and prevented the emergence of bedaquiline resistance [21].
Although the individual contribution of delamanid to the total efficacy of the BDL regimen was not assessed, it could be speculated that the same effect holds true for the contribution of delamanid in the current combination regimen. Furthermore, the additional value of delamanid is supported by a study in patients with MDR tuberculosis showing increased sputum conversion when delamanid was added to a backbone treatment regimen [22]. Moreover, the combination of bedaquiline and delamanid also appears to be promising in patients with tuberculosis. In studies reviewing the activity of these drugs in combination, 84% sputum conversion after 4 months of treatment [23] and 88% culture negativity after 24 weeks of treatment was observed when both drugs were added to a backbone regimen (92% of these patients also received linezolid) [24]. Therefore, it is reasonable to assume that the BDL combination may also be a potent tuberculosis drug regimen for patients, which would be in line with the first results of the Nix-TB trial studying the efficacy of BPaL in patients with MDR and XDR tuberculosis [9].
As such, delamanid seems to be a reasonable alternative to pretomanid in specific situations such as pretomanid resistance [10, 25]. Other murine studies showed robust efficacy of the combination of bedaquiline and pretomanid combined with moxifloxacin and pyrazinamide (BPaMZ) with a relapse-free survival of only 2 months of treatment [21, 26]. Given the results of our present study, it would be interesting to study whether the substitution of pretomanid for delamanid in the BPaMZ regimen results in similar efficacy.
In the present study, the EBA of linezolid as monotherapy was low, prompting questions about the contribution of this drug to the total efficacy of the BDL regimen. However, Tasneen et al [7] revealed the treatment shortening potential of linezolid in the BPaL regimen by showing that the addition of linezolid to the combination of bedaquiline and pretomanid resulted in no relapse in mice after 3 months of treatment, while mice treated with only bedaquiline and pretomanid relapsed after 4 months of treatment. These findings further strengthen the hypothesis that EBA should not be solely used as a guide for regimen efficacy [27]. In addition, the efficacy of linezolid was assessed in patients with XDR tuberculosis. The treatment success rate in the linezolid therapy group was significantly higher (70%) than in the nonlinezolid group (34%) [28]. Therefore, despite the disappointing performance of linezolid in terms of EBA, it appears to be a valuable component of the drug combination studied in present publication.
Our pharmacokinetic results for bedaquiline, delamanid, and linezolid fell within the range of those reported in previous murine tuberculosis studies [3, 29–31] and were also comparable to human pharmacokinetic findings [22, 32–34]. Our bedaquiline concentration-time curve showed a remarkable second peak after 8 hours of treatment. This second peak has not been observed in other murine studies [3, 29], although it has been reported in a human pharmacokinetic study [35]. In this clinical study the double peak was explained by better absorption after food intake, which has also been shown in another study for rifapentine [36].
We also assessed the concentration versus time curves of the major metabolites of bedaquiline and delamanid, N-monodesmethyl bedaquiline (M2) and DM-6705 (M1), respectively. In humans, it is assumed that M2 does not have a significant effect on the total efficacy of bedaquiline since its exposure is 4–5 times lower, and the antimicrobial activity is 3–6-fold lower, compared with the parent compound [18]. However, Rouan et al [18] showed that the exposure of M2 in mice (in terms of area under the receiver operating characteristic curve [AUC]) was 3 times higher than with bedaquiline, and in our study it was actually 5 times higher. Because the AUC is the driver of efficacy for bedaquiline, and M2 plus bedaquiline has an additive effect, the efficacy of bedaquiline in mice might be overestimated when translated to humans. However, this effect is assumed to be limited owing to the lower antimicrobial activity of M2 [37].
In our study, the exposure of M1 was 32 times lower compared with delamanid, which is in line with the findings of Sasahara et al [31]. Their study showed that, after repeated drug administration, metabolite exposure in humans was much higher compared with mice and rats. However, in our study, we reasoned that this finding did not lead to an underestimation of the effect of delamanid, since antimicrobial activity of the 3 major metabolites of delamanid (DM-6704, 6705, and 6706) was previously reported to be poor, with MICs ranging from 6.25 to 50 mg/L [38].
A limitation of this study was that it was powered on 3 mice every 4 weeks for CFU assessment immediately after treatment and 3 mice every 2 weeks to asses relapse, as based on previous modeling experience [5]. Unexpectedly, we observed zero relapse after week 8, while in the following 6 weeks the proportion of relapsing mice increased. Subsequently, after 14 weeks of treatment, no mice relapsed, except 1 mouse in week 20. Although this could be a sampling artifact, this event resulted in an incomplete fit of the BDL regimen to the sigmoidal Emax model. This issue was overcome by using a surge model, which enabled the assessment of expected treatment duration of the BDL regimen in relation to HRZE. Another limitation was the use of a drug-sensitive strain to assess this DR tuberculosis regimen. However, many previous studies have also used drug sensitive tuberculosis strains to model DR tuberculosis treatment regimens [7, 21, 26] and are now evaluated in clinical trials, and some of them already showing a good translation to clinical practice [9].
In conclusion, BDL seems to be a promising combination for the treatment of DR tuberculosis. Furthermore, since all 3 drugs are commercially available, this combination could be readily implemented in clinical practice after assessment in clinical studies and might be a good alternative for BPaL when pretomanid is not available for inclusion in combined tuberculosis treatment regimens.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank John Hays for his writing assistance and Gerjo de Knegt, Marian ten Kate. And Heleen van der Spek for their technical assistance. Research was conducted on behalf of the PreDiCT-TB Consortium (http://predict-tb.eu).
Financial support. This work was supported by the Innovative Medicines Initiative Joint Undertaking (no. 115337), which is supported by the European Union’s Seventh Framework Programme (grant FP7/2007–2013) and European Federation of Pharmaceutical Industries and Associations companies.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: Netherlands Centre for One Health—Anti-Microbial Resistance symposium, Nijmegen, the Netherlands, 12 October 2018.
Contributor Information
Elise D Pieterman, Department of Medical Microbiology and Infectious Diseases, Erasmus University Medical Center, Rotterdam, the Netherlands.
Lina Keutzer, Department of Pharmaceutical Biosciences, Uppsala University, Uppsala, Sweden.
Aart van der Meijden, Department of Medical Microbiology and Infectious Diseases, Erasmus University Medical Center, Rotterdam, the Netherlands.
Sanne van den Berg, Department of Medical Microbiology and Infectious Diseases, Erasmus University Medical Center, Rotterdam, the Netherlands.
Han Wang, Center for Discovery and Innovation, Hackensack Meridian Health , Nutley, New Jersey, USA.
Matthew D Zimmerman, Center for Discovery and Innovation, Hackensack Meridian Health , Nutley, New Jersey, USA.
Ulrika S H Simonsson, Department of Pharmaceutical Biosciences, Uppsala University, Uppsala, Sweden.
Hannelore I Bax, Department of Medical Microbiology and Infectious Diseases, Erasmus University Medical Center, Rotterdam, the Netherlands; Department of Internal Medicine, Section of Infectious Diseases, Erasmus University Medical Center, Rotterdam, the Netherlands.
Jurriaan E M de Steenwinkel, Department of Medical Microbiology and Infectious Diseases, Erasmus University Medical Center, Rotterdam, the Netherlands.
References
- 1. World Health Organization. Global tuberculosis report. Geneva: World Health Organization; 2019.
- 2. Worley MV, Estrada SJ. Bedaquiline: a novel antitubercular agent for the treatment of multidrug-resistant tuberculosis. Pharmacotherapy 2014; 34:1187–97. [DOI] [PubMed] [Google Scholar]
- 3. Matsumoto M, Hashizume H, Tomishige T, et al. OPC-67683, a nitro-dihydro-imidazooxazole derivative with promising action against tuberculosis in vitro and in mice. PloS Med 2006; 3:e466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization. WHO consolidated guidelines on drug-resistant tuberculosis treatment. Geneva: World Health Organization; 2019.
- 5. Mourik BC, Svensson RJ, de Knegt GJ, et al. Improving treatment outcome assessment in a mouse tuberculosis model. Sci Rep 2018; 8:5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. de Steenwinkel JE, ten Kate MT, de Knegt GJ, et al. Drug susceptibility of Mycobacterium tuberculosis Beijing genotype and association with MDR TB. Emerg Infect Dis 2012; 18:660–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tasneen R, Betoudji F, Tyagi S, et al. Contribution of oxazolidinones to the efficacy of novel regimens containing bedaquiline and pretomanid in a mouse model of tuberculosis. Antimicrob Agents Chemother 2016; 60:270–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. de Miranda Silva C, Hajihosseini A, Myrick J, et al. Effect of linezolid plus bedaquiline against Mycobacterium tuberculosis in log phase, acid phase, and nonreplicating-persister phase in an in vitro assay. Antimicrob Agents Chemother 2018; 62:00856–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Conradie F, Diacon AH, Ngubane N, et al. ; Nix-TB Trial Team . Treatment of highly drug-resistant pulmonary tuberculosis. N Engl J Med 2020; 382:893–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee BM, Almeida DV, Afriat-Jurnou L, et al. The evolution of nitroimidazole antibiotic resistance in Mycobacterium tuberculosis. bioRxiv 2019:631127. [Google Scholar]
- 11. Soldin OP, Mattison DR. Sex differences in pharmacokinetics and pharmacodynamics. Clin Pharmacokinet 2009; 48:143–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. de Steenwinkel JE, ten Kate MT, de Knegt GJ, et al. Consequences of noncompliance for therapy efficacy and emergence of resistance in murine tuberculosis caused by the Beijing genotype of Mycobacterium tuberculosis. Antimicrob Agents Chemother 2012; 56:4937–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Woods GL, Brown-Elliott BA, Conville PS. Susceptibility testing of mycobacteria, nocardiae, and other aerobic actinomycetes: approved standard M24-A. Vol. 26. Wayne, PA: National Committee for Clinical Laboratory Standards, 2003. [PubMed] [Google Scholar]
- 14. Pieterman ED, Te Brake LHM, de Knegt GJ, et al. Assessment of the additional value of verapamil to a moxifloxacin and linezolid combination regimen in a murine tuberculosis model. Antimicrob Agents Chemother 2018; 62:01354–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. De Steenwinkel JE, De Knegt GJ, Ten Kate MT, et al. Immunological parameters to define infection progression and therapy response in a well-defined tuberculosis model in mice. Int J Immunopathol Pharmacol 2009; 22:723–34. [DOI] [PubMed] [Google Scholar]
- 16. Beal SL, Boeckmann A, Bauer R. NONMEM 7.4 users guides. Gaithersburg, MD: ICON plc; 1989.
- 17. Andries K, Verhasselt P, Guillemont J, et al. A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis. Science 2005; 307:223–7. [DOI] [PubMed] [Google Scholar]
- 18. Rouan MC, Lounis N, Gevers T, et al. Pharmacokinetics and pharmacodynamics of TMC207 and its N-desmethyl metabolite in a murine model of tuberculosis. Antimicrob Agents Chemother 2012; 56:1444–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Upton AM, Cho S, Yang TJ, et al. In vitro and in vivo activities of the nitroimidazole TBA-354 against Mycobacterium tuberculosis. Antimicrob Agents Chemother 2015; 59:136–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Williams KN, Stover CK, Zhu T, et al. Promising antituberculosis activity of the oxazolidinone PNU-100480 relative to that of linezolid in a murine model. Antimicrob Agents Chemother 2009; 53:1314–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xu J, Li SY, Almeida DV, et al. Contribution of pretomanid to novel regimens containing bedaquiline with either linezolid or moxifloxacin and pyrazinamide in murine models of tuberculosis. Antimicrob Agents Chemother 2019; 63:00021–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gler MT, Skripconoka V, Sanchez-Garavito E, et al. Delamanid for multidrug-resistant pulmonary tuberculosis. N Engl J Med 2012; 366:2151–60. [DOI] [PubMed] [Google Scholar]
- 23. Sarin R, Vohra V, Singla N, et al. Early efficacy and safety of bedaquiline and delamanid given together in a “salvage regimen” for treatment of drug-resistant tuberculosis. Indian J Tuberc 2019; 66:184–8. [DOI] [PubMed] [Google Scholar]
- 24. Hafkin J, Hittel N, Martin A, Gupta R. Compassionate use of delamanid in combination with bedaquiline for the treatment of multidrug-resistant tuberculosis. Eur Respir J 2019; 53:1801154. [DOI] [PubMed] [Google Scholar]
- 25. Wen S, Jing W, Zhang T, et al. Comparison of in vitro activity of the nitroimidazoles delamanid and pretomanid against multidrug-resistant and extensively drug-resistant tuberculosis. Eur J Clin Microbiol Infect Dis 2019; 38:1293–6. [DOI] [PubMed] [Google Scholar]
- 26. Li SY, Tasneen R, Tyagi S, et al. Bactericidal and sterilizing activity of a novel regimen with bedaquiline, pretomanid, moxifloxacin, and pyrazinamide in a murine model of tuberculosis. Antimicrob Agents Chemother 2017; 61:00913–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Andries K, Gevers T, Lounis N. Bactericidal potencies of new regimens are not predictive of their sterilizing potencies in a murine model of tuberculosis. Antimicrob Agents Chemother 2010; 54:4540–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tang S, Yao L, Hao X, et al. Efficacy, safety and tolerability of linezolid for the treatment of XDR-TB: a study in China. Eur Respir J 2015; 45:161–70. [DOI] [PubMed] [Google Scholar]
- 29. Irwin SM, Prideaux B, Lyon ER, et al. Bedaquiline and pyrazinamide treatment responses are affected by pulmonary lesion heterogeneity in Mycobacterium tuberculosis infected C3HeB/FeJ mice. ACS Infect Dis 2016; 2:251–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Grossman TH, Shoen CM, Jones SM, Jones PL, Cynamon MH, Locher CP. The efflux pump inhibitor timcodar improves the potency of antimycobacterial agents. Antimicrob Agents Chemother 2015; 59:1534–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sasahara K, Shimokawa Y, Hirao Y, et al. Pharmacokinetics and metabolism of delamanid, a novel anti-tuberculosis drug, in animals and humans: importance of albumin metabolism in vivo. Drug Metab Dispos 2015; 43:1267–76. [DOI] [PubMed] [Google Scholar]
- 32. Rustomjee R, Diacon AH, Allen J, et al. Early bactericidal activity and pharmacokinetics of the diarylquinoline TMC207 in treatment of pulmonary tuberculosis. Antimicrob Agents Chemother 2008; 52:2831–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dietze R, Hadad DJ, McGee B, et al. Early and extended early bactericidal activity of linezolid in pulmonary tuberculosis. Am J Respir Crit Care Med 2008; 178:1180–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McGee B, Dietze R, Hadad DJ, et al. Population pharmacokinetics of linezolid in adults with pulmonary tuberculosis. Antimicrob Agents Chemother 2009; 53:3981–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McLeay SC, Vis P, van Heeswijk RP, Green B. Population pharmacokinetics of bedaquiline (TMC207), a novel antituberculosis drug. Antimicrob Agents Chemother 2014; 58:5315–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zvada SP, Van Der Walt JS, Smith PJ, et al. Effects of four different meal types on the population pharmacokinetics of single-dose rifapentine in healthy male volunteers. Antimicrob Agents Chemother 2010; 54:3390–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. NDA 204-384. US Food and Drug Administration Website. 2012. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Anti-InfectiveDrugsAdvisoryCommittee/UCM329260.pdf.
- 38. European Medicines Agency. London, UK: Assessment report deltyba.2014.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.