Abstract
Suboptimal sleep patterns predict poorer cognitive function in older adults and induce inflammatory responses. Inflammation could also adversely affect cognitive function. This study explored whether systemic inflammation may be one biological mechanism through which sleep influences cognitive performance. Participants were 4877 men and women from the English Longitudinal Study of Ageing who were followed-up for 8 years starting at wave 4 (2008-09), through wave 6 (2012-13), and until wave 8 (2016-17). Sleep quality and duration were measured with self-reported questionnaires. Cognitive function was assessed objectively with tests of verbal fluency, memory (immediate and delayed recall) and time orientation. Analyses were stratified by sex and adjusted for socio-economic circumstances, health behaviours, limiting long-standing illness, medication, depressive symptoms, and baseline inflammation and cognition. In men, in comparison with optimal sleep duration, short sleep (≤6 h: β = −0.343, C.I. −0.611 to −0.076; >6-7 h: β = −0.263, C.I. −0.506 to −0.020) and long sleep (β = −0.536, C.I. −1.019 to −0.053) measured at baseline predicted lower scores in delayed memory recall at follow-up. In women, sleep duration was unrelated to cognitive performance at follow-up, and in both sexes, there was no relationship between sleep quality and follow-up cognitive performance. There was no evidence of mediating effects of inflammatory markers in the relationship between sleep measures and cognitive performance in both sexes. In conclusion, baseline short and long sleep duration is associated with follow-up cognitive performance in older men, but we found no evidence of any mediating effects of inflammation.
Keywords: sleep quality, sleep duration, cognitive function, inflammation, longitudinal, ageing
1. Introduction
Poor sleep becomes increasingly prevalent with advancing age (Crowley, 2011), which adversely affects numerous aspects of physical and mental health. For example, too short (typically defined as ≤5–6 h per night), too long (typically defined as >8 h per night) sleep, and sleep of poor quality increase the risk of cardiovascular outcomes and mortality (Cappuccio, Cooper, D’elia, Strazzullo, & Miller, 2011; Sofi et al., 2014), type 2 diabetes, obesity and hypertension (Jike, Itani, Watanabe, Buysse, & Kaneita, 2018; Schmid, Hallschmid, & Schultes, 2015), as well as subsequent depression (Koyanagi et al., 2014; Zhai, Zhang, & Zhang, 2015). This is particularly relevant for older adults, whose risk of physical and mental diseases and disability increases substantively with advancing age (Franco et al., 2009).
Growing evidence seems to suggest that unhealthy sleep patterns also have an adverse impact on cognitive function. Briefly, in the Whitehall II study, middle-aged adults, whose sleep duration either increased or decreased from 7-8 hours between baseline and follow-up assessments had lower performance on a number of cognitive tests such as reasoning, phonemic, and semantic fluency approximately 5 years later (Ferrie et al., 2011). Becoming either a short or long sleeper, and extremes of sleep duration (≤5 or ≥9 hours), were also linked to poorer overall cognitive performance in the Nurses’ Health Study (Devore et al., 2014). Similarly, a 22.5-year follow-up of the Finnish Twin Cohort revealed that participants with short and long sleep hours had poorer performance on cognitive tests, based on cognitive domains such as orientation, short- and long-term memory and attention (Virta et al., 2013). However, a recent prospective analysis of the Doetinchem Cohort Study showed that long, but not short, sleep was predictive of a lower global cognitive function, memory and flexibility (van Oostrom, Nooyens, van Boxtel, & Verschuren, 2018). Long and short sleep durations have also been found associated with lower cognitive function cross-sectionally (Blackwell et al., 2011; Faubel et al., 2009; Miller, Wright, & Cappuccio, 2014).
Sleep duration does not capture other issues with sleep prevalent in older adults, such as difficulties with falling or staying asleep. To date, however, only a small number of studies explored the prospective relationship between sleep disturbances with cognitive function. For example, Virta et al. (2013) found that poor sleep quality predicted lower cognitive function scores at a 22.5-year follow-up. Cross-sectional studies in Denmark, the US, and the UK (Gadie, Shafto, Leng, & Kievit, 2017; Nebes, Buysse, Halligan, Houck, & Monk, 2009; Waller et al., 2016) found that disturbances of sleep measured with the Pittsburgh Sleep Quality Index (PSQI) were associated with a reduced performance on a range of cognitive tests. In contrast, Blackwell et al. (2011) and Saint Martin et al. (2012) reported no associations between the PSQI and cognitive function. Another cross-sectional analysis of the English Longitudinal Study of Ageing (ELSA) revealed that greater sleep disturbances were found among participants with the highest scores on cognitive function tests (Miller at al., 2014).
Although the evidence is limited, the few published studies that used objective sleep monitoring have also suggested that sleep is linked to cognitive function. For example, a cross-sectional investigation of the Rotterdam Study, which measured sleep with actigraphy, reported that longer sleep latencies were associated with poorer word memory recall and verbal fluency (Luik et al., 2015). In the Osteoporotic Fractures in Men Study, which recorded sleep with in-home polysomnography, Blackwell et al. (2011) found that older men who spent more time awake after initially following asleep, and those with longer sleep duration, had lower scores on cognitive function tests than good sleepers. A follow-up analysis of these data revealed that reduced time spent in REM sleep, and extended time spent in stage 1 sleep, which are often found in older people, both predicted worse performance in cognitive function approximately 3.4 years later (Song et al., 2015).
Experimental studies have also shown that sleep deprivation and disturbance of circadian rhythms, frequently reported in ageing adults, are associated with diminished cognitive function (Franzen, Siegle, & Buysse, 2008; Santhi et al., 2016). Furthermore, deeper slow-wave sleep and sleep spindles appear particularly important for cerebral plasticity underlying learning and memory consolidation; yet they both decline with advancing age (Fogel et al., 2012).
Taken together, the current evidence suggests that suboptimal sleep patterns predict poorer performance on cognitive tests in older adults. Several mechanisms have been proposed as possible mediators in this relationship, and there is a growing recognition that inflammation is one probable pathway through which sleep can adversely affect subsequent cognitive processes (Irwin & Vitiello, 2019; Yaffe, Falvey, & Hoang, 2014). Firstly, older age is associated with greater concentrations of inflammatory markers, which have been suggested to be a consequence of declining levels of sex hormones, and an increase in visceral adipose tissue (see Singh & Newman, 2010, for a detailed review). Secondly, a meta-analysis of 72 observational studies comprising of over 50,000 adults reported that individuals with too short and poor sleep quality have elevated markers of inflammation (Irwin, Olmstead, & Carroll, 2016). Thirdly, there is also some, albeit still limited, evidence that raised levels of inflammatory markers predict follow-up cognitive impairment (Bettcher & Kramer, 2014; Irwin & Vitiello, 2019). Specifically, relative to older adults with low levels of inflammation, those with high levels have been found to have a reduced volume of the hippocampus and medial temporal lobes. Inflammation also exerts a deleterious effect on vascular permeability, endothelial function, and microvascular structure, which subsequently affect white matter (see Bettcher & Kramer, 2014, for a review on this topic). Recently, a prospective analysis of ELSA revealed that higher concentrations of CRP and fibrinogen both predicted worse performance on episodic memory tests (Tampubolon, 2016). However, a different analysis of ELSA found no link between baseline CRP and a follow-up memory score 10 years later (Lassale et al., 2018).
Very few studies to date have attempted to integrate the evidence on sleep, inflammation and follow-up cognitive function. There is some evidence from animal research (Zhu et al., 2012) and clinical populations (Haensel et al., 2009) lending tentative support for the mediating role of inflammatory factors, but a 2-year follow-up of the Singapore-Longitudinal Aging Brain Study showed that CRP was unrelated to subsequent cognitive performance or sleep (Lo, Loh, Zheng, Sim, & Chee, 2014). More longitudinal studies are needed to explore the relationship between baseline sleep, inflammation and follow-up cognition.
In light of the limitations of the literature discussed above, the main aim of this study was to test whether inflammatory markers mediate the association between self-reported sleep and follow-up cognitive performance. Based on the available evidence, we hypothesised that the association between baseline sleep and follow-up cognition would be stronger in women than in men, due to the association between sleep measures and inflammatory markers being potentially stronger in women. Specifically, population-based studies have found that women, when compared with men, appear to be more vulnerable to the effects of poor sleep quality and too short sleep duration, and show higher increases in inflammatory markers such as interleukin-6 and C-reactive protein (Irwin, 2015; Irwin et al., 2016; Irwin & Vitiello, 2019). Previous research has also demonstrated evidence for sex differences in the relationship between sleep and cognitive function, in both young and older adults (De Frias, Nilsson, & Herlitz, 2006; Santhi et al., 2016). Therefore all our analyses were stratified by sex.
2. Method
2.1. Participants and procedures
This article is based on data from the English Longitudinal Study of Ageing (ELSA) (Steptoe, Breeze, Banks, & Nazroo, 2012), which is a nationally representative study of men and women aged 50 years and older living in England. In ELSA, data are collected biannually in participants’ home using a computer-assisted personal interview (CAPI) and a nurse visit a few days later, during which blood and other medical information are obtained.
Analyses presented here are based on participants from waves 4 (2008-09), 6 (2012-13) and 8 (2016-17). Wave 4 is used here as the baseline since this was when sleep measures were introduced. In ELSA, bloods are collected only during the nurse visits that are available at every other wave, and, to date, included waves 2, 4, 6 and 8; therefore, inflammatory markers described in our analyses were selected from waves 4 and 6. Wave 8 was chosen as this is the most recent data available in ELSA that contain cognitive function. Inflammation measured at wave 6, adjusted by baseline inflammation at wave 4, was considered as a mediating factor in our analysis. This is an intermediate time point of assessment selected temporally in the middle of our study period between exposure (wave 4) and outcome (wave 8). We selected this temporal order to investigate the deleterious effects of poor sleep quality, or too short and/or too long sleep, subsequent inflammatory markers measured four years later, on follow-up cognition, measured another four years later. Inflammation at wave 8 was not considered in our analyses due to limited blood data available at that wave.
When we tried to restrict our analytical sample to participants who provided data on sleep, inflammation (CRP and fibrinogen), cognitive function and covariates at baseline, CRP and fibrinogen at wave 6, and cognitive measures at wave 8 this reduced the sample to N=2549. This would have reduced the statistical power and posed a threat of type II error; we would have also been prevented from running sex-stratified analyses. The differences between participants with complete data on all variables and those with missing data on one or more variable are detailed in the statistical approach section below. We, therefore, decided to restrict our analytical sample to participants with complete baseline data on all covariates, sleep measures, inflammatory factors and cognitive function variables (N=4877). However, as part of our sensitivity analyses, we repeated our mediation models on participants with complete data on waves 4, 6 and 8.
Verbal informed consent was obtained from all participants, and ethical approval was issued by the National Research Ethics Service.
2.2. Measures
2.2.1. Socio-demographic measures
All measures described here were obtained during the CAPI. Socio-economic circumstances were estimated by total household wealth taking into account financial wealth (e.g., savings), the value of any property (less mortgage), and the value of any business assets and physical wealth (e.g., artwork), net of debt. Wealth was divided into quintiles for these analyses. Educational attainment was categorised into: “degree-level education”, “lower than degree education”, “GCE (General certificate of education) A-level equivalent education”, “up to GCE/O-level education”, “foreign or other qualification” and “no formal qualifications”. Age was included as one of our socio-demographic measures as well. These variables were selected based on previous research findings indicating their relevance to sleep (Arber, Bote, & Meadows, 2009; Stranges et al., 2008), cognitive function (Cagney & Lauderdale, 2002), and inflammation (Koster et al., 2006).
2.2.2. Sleep measures
Sleep quality was indexed with three questions from the Jenkins Sleep Problems Scale (Jenkins, Stanton, Niemcryk, & Rose, 1988): difficulties falling asleep, waking up several times a night, and waking up in the morning feeling tired. Participants answered these questions with regards to the past month (anchored at 1 = “not during the past month” to 4 = “three or more times a week”). Scores were averaged (range 1-4) with higher scores indicating poorer sleep quality. The Cronbach’s alpha at wave 4 for this analytical sample was 0.60.
Sleep duration was measured by asking participants about their average sleep duration on a weeknight. For the analyses described here, sleep duration was categorised into “≤6 h” (short sleep duration), “>6-7 h”, “>7-8 h” (optimal sleep duration) and “>8 h” (long sleep duration).
2.2.3. Biological data
Blood samples were obtained during the nurse visit. Blood samples were not taken from participants who had clotting disorders, or who were taking anticoagulant medication. C-reactive protein was analysed using the N Latex C-reactive protein mono immunoassay on the Behring Nephelometer II analyser. Quality control results showed target values from 1.50/1.45 (low concentrations) to 48.00/48.10 (high concentrations) mg/l. The acceptable range was 1.2 to 1.7 for low concentrations (standard deviation (SD 0.005) and 44.0 to 52.0 for high concentrations (SD 1.66). Fibrinogen concentrations were quantified using a modification of the Clauss thrombin clotting method on the Organon Teknika MDA 180 analyser. According to external quality assessments, target values were between 1.49 to 2.07 mmol/l, and the assessed values ranged from 1.49 to 1.98 mmol/l (Craig, Deverill, Pickering, 2006). All blood samples were analysed in the Royal Victoria Infirmary laboratory in Newcastle upon Tyne, UK.
2.2.4. Cognitive function measures
Cognitive function was measured with verbal fluency, memory as well as orientation, which are the main cognitive measures administered in ELSA at each wave (Steptoe et al., 2012).
Verbal fluency was measured with animal naming, a task reflecting executive function. The test measures how quickly participants can think of words from a particular category, counting how many distinct elements from the animal kingdom (real or mythical, excluding repetitions or proper nouns) the respondent can name within one minute. This test requires self-initiated activity, organisation, abstraction and set-shifting abilities (Steel, Huppert, McWilliams, & Melzer, 2004). In our study, the total number of correctly named animals represented the measure of verbal fluency, with a higher score representing a superior executive function.
Memory was measured with a word recall test. A random ten-word list was read by a computer with the speed of one word every two seconds. Participants were required to remember as many words as they could, immediately and after a short delay. We used the independent measures of immediate and delayed recall (with scores ranging on each task from 0 to 10), with higher scores indicating better immediate and delayed memory.
Time orientation was assessed using questions relating to day and date from the Mini-Mental State Examination (Folstein, Folstein, & McHugh, 1975). Higher scores reflect superior performance.
2.2.5. Health-related variables
Participants were requested to indicate whether they had any chronic illnesses or disability. Those who indicated having an illness/disability were further asked whether their condition(s) limited their activities. The response was then stratified into “yes” for limiting long-standing illness, and “no” for the absence of limiting long-standing illness. Information on limiting long-standing conditions was included in our analysis because of the well-established links with sleep (Zee & Turek, 2006), cognitive function (Juster, McEwen, & Lupien, 2010), and inflammation (Singh & Newman, 2010). Medication use was self-reported in this study. Specifically, high blood pressure and cholesterol medication data were obtained by asking participants if they were taking high blood pressure, and medication to lower cholesterol level, respectively. Arthritis and joint pain medication were collected by asking whether paracetamol was suggested as first medication (at wave 4 in ELSA there is no question about anti-inflammatory medication for arthritis). Responses to these questions were categorised into “yes” and “no” categories. To measure depression medication participants were requested to indicate whether they have taken medication in last 2 years or had counselling; possible responses included “medication”, “counselling”, “medication and counselling” or “none”. For the purpose of our analyses these responses were categorised into “yes” for depression treatment and “no” for its absence. Height and weight were assessed by the nurse and were used to calculate body mass index (BMI, kg/m2). Data on smoking were collected by asking respondents whether they have ever smoked, and those who responded positively were further asked to state if they still smoked. Responses were classified into “no” for never/past smokers and “yes” for current smokers. Physical activity was indexed by asking whether respondents participated in mild, moderate and vigorous physical activity. For the analyses described here, we categorised responses into “moderate or vigorous physical activity less than once per week” and “moderate or vigorous physical activity at least once per week”. Alcohol consumption was measured by asking respondents how many times they had an alcoholic drink in the last 12 months. In this article responses were categorised into “5-6 days a week or daily” and “less than daily”. The above health-related variables were selected for our analyses because of their well-documented associations with sleep (Stranges et al., 2008), cognitive function (Sabia et al., 2008), and inflammation (McDade, Hawkley, & Cacioppo, 2006).
Depressive symptoms were assessed with an 8-item version of the Centre for Epidemiologic Studies Depression scale (CES-D) initially modified for the Health and Retirement Study in the US (Steffic, 2000). For our analyses, the item concerning sleep was removed from the CES-D, to avoid the issue of shared variance with the measure of sleep quality. The 7-item scale was answered with a “yes” or “no” response. Scores were totalled (range 0-7) and greater scores were reflective of higher depressive symptoms. The Cronbach’s alpha for the scale at wave 4 was 0.80. We included depressive symptoms in our study since they are closely linked with sleep (Sofi et al., 2014; Koyanagi et al., 2014), cognitive function (Singh-Manoux et al., 2010), and inflammation (Howren, Lamkin, & Suls, 2009).
2.3. Statistical analysis
Data were inspected descriptively and graphically. Descriptive statistics were calculated with t-tests and chi-square tests, as appropriate. All analyses were performed using IBM’s Statistical Package for the Social Sciences (SPSS), version 26.
Fibrinogen was normally distributed, but CRP data were skewed, and their distribution was normalised using log-transformation prior to analysis. In this article, CRP was treated as a continuously distributed measure. C-reactive protein levels of >10 mg/L are indicative of an acute inflammatory response or infection (Pearson, Mensah, Alexander, 2003), and values above this cut-off point were therefore excluded from analysis.
In comparison with participants who had complete data on all variables used in our analyses (waves 4, 6 and 8, N=2549), those with missing information were older (P<0.001), more likely to be in the lowest wealth quintile (P<0.001), and to have no formal qualifications (P<0.001). With regards to health-related variables, participants with missing data were less likely to exercise moderately and vigorously more than once per week (P<0.001), and they were more likely to currently smoke (P<0.001). They also had a slightly higher BMI (P=0.005), were more likely to have elevated depressive symptoms (P<0.001), report a limiting chronic illness (P<0.001), and be on medication for high blood pressure (P<0.001), and high cholesterol (P=0.040).
All analyses were adjusted for age, wealth, educational attainment, smoking status, alcohol consumption, physical activity, BMI, limiting long-standing illness, depressive symptoms, depression treatment and medication for high blood pressure, cholesterol, and arthritis since these are related to sleep and cognitive function. We also adjusted for baseline CRP and fibrinogen, and the corresponding test on cognitive performance.
Mediation analysis was performed using the PROCESS package (version 3) for SPSS (Hayes, 2018). Baseline sleep measure at wave 4 was the exposure factor, an inflammatory variable at wave 6 was the potential mediator, and cognitive function at wave 8 was the outcome variable. The total effect (c, see Tables 2–5) represents the sum of the direct and indirect effect of sleep on cognitive function. The direct effect (c’, see Tables 2–5) is the effect of sleep on cognitive function after adjustment for an inflammatory factor. Finally, the indirect effect (ab, see Tables 2–5) is the effect of sleep on cognitive function through an inflammatory factor (Field, 2018). See Fig. 1 below depicting the conceptual model tested in our study. Separate analyses were performed for each mediator (CRP and fibrinogen), and sleep quality and duration.
Table 2.
Summary of mediation analysis for sleep quality, CRP and cognitive function variables.
| Exposure variable (wave 4) | Mediating variable (wave 6) | Outcome variable (wave 8) | Fully Adjusted Mediation Analysis (Coefficient β, 95 % CI) | Fully Adjusted Mediation Analysis (Coefficient β, 95 % CI) | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Women | Men | |||||||
|
| ||||||||
| Sleep quality | CRP | Cognitive function | Indirect effect (ab)* | Total effect (c) | Direct effect (c’) | Indirect effect (ab)* | Total effect (c) | Direct effect (c’) |
| Verbal fluency | −0.001 (−0.016, 0.011) | 0.359 (−0.032, 0.748) | 0.360 (−0.030, 0.750) | 0.000 (−0.017, 0.017) | 0.370 (−0.108, 0.849) | 0.370 (−0.109, 0.849) | ||
| Verbal memory: immediate recall | −0.000 (−0.004, 0.003) | 0.026 (−0.072, 0.123) | 0.026 (−0.072, 0.124) | 0.000 (−0.004, 0.003) | −0.015 (−0.128, 0.099) | −0.014 (−0.128, 0.100) | ||
| Verbal memory: delayed recall | −0.001 (−0.005, 0.003) | 0.037, (−0.082, 0.155) | 0.037, (−0.081, 0.155) | −0.000 (−0.005, 0.004) | 0.008 (−0.129, 0.145) | 0.008 (−0.129, 0.145) | ||
| Time orientation | −0.000 (−0.001, 0.001) | 0.039 (−0.001, 0.079) | 0.039 (−0.001, 0.079) | −0.000 (−0.004, 0.003) | −0.016 (−0.069, 0.037) | −0.016 (−0.069, 0.037) | ||
CRP = C-reactive protein; CI = confidence interval.
For indirect effect bias-corrected 95% confidence intervals are reported. Significant effects are denoted in bold.
Table 5.
Summary of mediation analysis for sleep duration, fibrinogen and cognitive function variables.
| Exposure variable (wave 4) | Mediating variable (wave 6) | Outcome variable (wave 8) | Fully Adjusted Mediation Analysis (Coefficient β, 95 % CI)* | Fully Adjusted Mediation Analysis (Coefficient β, 95 % CI)* | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Women | Men | |||||||
|
| ||||||||
| Sleep duration categories | Fibrinogen | Cognitive function | Indirect effect (ab) | Total effect (c) | Direct effect (C) | Indirect effect (ab) | Total effect (c) | Direct effect (c’) |
| <6 | Verbal fluency | −0.013 (−0.065, 0.024) | −0.055 (−0.870, 0.760) | −0.043 (−0.858, 0.773) | 0.003 (−0.031, 0.041) | −0.109 (−1.049, 0.831) | −0.113 (−1.053, 0.828) | |
| >6-7 | −0.005 (−0.040, 0.026) | 0.551 (−0.250, 1.352) | 0.555 (−0.246, 1.357) | −0.000 (−0.034, 0.026) | 0.008 (−0.844, 0.860) | 0.008 (−0.844, 0.860) | ||
| >7-8 | Reference | Reference | Reference | Reference | Reference | Reference | ||
| >8 | −0.016 (−0.085, 0.035) | 0.407 (−0.916, 1.729) | 0.423 (−0.900, 1.746) | 0.007 (−0.052, 0.078) | −0.910 (−2.606, 0.786) | −0.917 (−2.613, 0.780) | ||
| <6 | Verbal memory: immediate recall | −0.004 (−0.017, 0.005) | −0.186 (−0.389, 0.017) | −0.182 (−0.385, 0.021) | 0.001 (−0.007, 0.011) | −0.178 (−0.400, 0.044) | −0.179 (−0.401, 0.043) | |
| >6-7 | −0.001 (−0.011, 0.007) | 0.035 (−0.165, 0.235) | 0.036 (−0.164, 0.236) | −0.000 (−0.008, 0.007) | −0.079 (−0.280, 0.122) | −0.079) (−0.280, 0.122) | ||
| >7-8 | Reference | Reference | Reference | Reference | Reference | Reference | ||
| >8 | −0.005 (−0.022, 0.007) | −0.010 (−0.340, 0.320) | −0.006 (−0.336, 0.324) | 0.002 (−0.011, 0.019) | −0.335 (−0.734, 0.065) | −0.336 (−0.736, 0.064) | ||
| <6 | Verbal memory: delayed recall | −0.002 (−0.016, 0.009) | 0.003 (−0.243, 0.249) | 0.005 (−0.241, 0.252) | 0.000 (−0.010, 0.009) | −0.343 (−0.611,−0.076) | −0.344 (−0.611, −0.076) | |
| >6-7 | −0.001 (−0.011, 0.007) | 0.184 (−0.059, 0.426) | 0.184 (−0.058,0.427) | 0.000 (−0.008, 0.008) | −0.263 (−0.506,−0.020) | −0.263 (−0.506,−0.020) | ||
| >7-8 | Reference | Reference | Reference | Reference | Reference | Reference | ||
| >8 | −0.003 (−0.022, 0.011) | −0.026 (−0.426, 0.373) | −0.023 (−0.423, 0.376) | 0.000 (−0.017, 0.017) | −0.536 (−1.019, −0.053) | −0.536 (−1.019, −0.053) | ||
| <6 | Time orientation | 0.001 (−0.002, 0.005) | 0.057 (−0.026, 0.139) | 0.056 (−0.026, 0.139) | −0.001 (−0.006, 0.003) | −0.067 (−0.171, 0.037) | −0.066 (−0.170, 0.038) | |
| >6-7 | 0.000 (−0.002, 0.003) | 0.034 (−0.046, 0.115) | 0.034 (−0.047,0.115) | 0.000 (−0.004, 0.004) | −0.002 (−0.096, 0.092) | −0.002 (−0.096, 0.092) | ||
| >7-8 | Reference | Reference | Reference | Reference | Reference | Reference | ||
| >8 | 0.001 (−0.003, 0.006) | −0.121 (−0.254, 0.013) | −0.121 (−0.255, 0.012) | −0.001 (−0.011, 0.006) | −0.125 (−0.312, 0.062) | −0.124 (−0.311, 0.064) | ||
CI = confidence interval.
For indirect effect bias-corrected 95% confidence intervals are reported. Significant effects are denoted in bold
Fig 1.
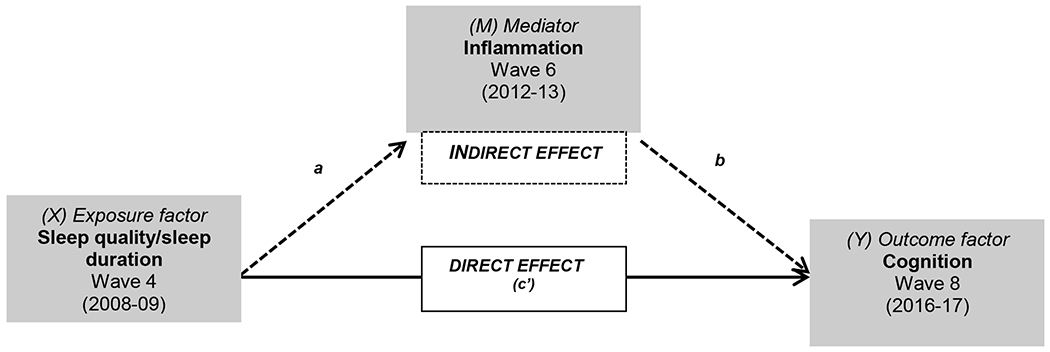
A conceptual figure of the mediation analysis of inflammatory markers (wave 6) between baseline sleep quality/duration (wave 4) and follow-up cognitive functioning (wave 8).
We also performed a number of sensitivity analyses. All mediation analyses were repeated in the sample with complete data on all variables used in the study (N=2549). These are reported in the sensitivity analysis section, while tables are presented as supplementary material. Further sensitivity analyses were conducted after excluding participants with CRP > 3 at baseline, as to ensure that the significant effects of baseline sleep on inflammation at wave 6 were not driven by inflammation at baseline. Finally, all mediation analyses were additionally repeated in the entire sample; these models were adjusted for sex, and all other covariates, rather than being stratified by sex (see supplementary material for tables).
For all analyses, results are presented as unstandardized coefficients and 95% confidence intervals (95% C.I.). For indirect effects, 95% confidence intervals (C.I.) were calculated using bootstrapping, with 5000 bias-correcting (Bc) bootstrap samples (5000 is a default option in the PROCESS package version 3) (Hayes, 2018). As recommended by Field (2018) and Hayes (2018) we used 95% C.I. (for direct and total effects) and Bc 95% C.I., (for indirect effects), rather than p-values, to infer whether the observed effects were statistically significant.
3. Results
3.1. Baseline participants’ characteristics
Men were more likely than women to have a degree and had slightly more wealth (see Table 1). In terms of health-related variables, considerably more men than women drank alcohol daily, but more women reported a long-standing limiting and had higher levels of inflammatory markers. Women also reported more sleep and depressive symptoms than men, and more women were short sleepers. In terms of medication, more women than men were receiving treatment for depressive symptoms and arthritis. However, women performed better on all cognitive tests included in the study.
Table 1.
Baseline participants characteristics (N=4877).
| Variable | Mean (SD)/N (%) | ||
|---|---|---|---|
|
| |||
| Men (N=2203) | Women (N=2674) | P-value | |
| Age | 65.4 (8.8) | 65.8 (9.3) | 0.101 |
| Education attainment | <0.001 | ||
| Degree | 548 (24.9) | 396 (14.8) | |
| Less than a degree | 447 (20.3) | 341 (12.8) | |
| GCE A-level/equivalent | 203 (9.2) | 219 (8.2) | |
| Up to GCE/O-level | 490 (22.2) | 676 (25.3) | |
| Foreign or other qualification | 95 (4.3) | 271 (10.1) | |
| No formal qualification | 420 (19.1) | 771 (28.8) | |
| Wealth quintiles | 0.001 | ||
| Poorest quintile | 273 (12.4) | 391 (14.6) | |
| 2nd quintile | 366 (16.6) | 517 (19.3) | |
| 3rd quantile | 447 (20.3) | 561 (21.0) | |
| 4th quantile | 530 (24.1) | 563 (21.1) | |
| Richest quintile | 587 (26.6) | 642 (24.0) | |
| Current smoking status | 0.157 | ||
| No | 1949 (88.5) | 2330 (87.1) | |
| Yes | 254 (11.5) | 344 (12.9) | |
| Alcohol consumption | <0.001 | ||
| 5-6 days a week or daily | 640 (29.1) | 467 (17.5) | |
| Less than daily | 1563 (70.9) | 2207 (82.5) | |
| Moderate or vigorous physical activity | <0.001 | ||
| Less than once per week | 582 (26.4) | 939 (35.1) | |
| At least once per week | 1621 (73.6) | 1735 (64.9) | |
| BMI (kg/m2) | 28.0 (4.3) | 28.0 (5.5) | 0.596 |
| Limiting long-standing illness | 0.001 | ||
| Yes | 587 (26.6) | 829 (31.0) | |
| No | 1616 (73.4) | 1845 (69.0) | |
| Cholesterol medication | 0.136 | ||
| Yes | 463 (21.0) | 516 (19.3) | |
| No | 1740 (79.0) | 2158 (80.7) | |
| High blood pressure medication | 0.256 | ||
| Yes | 668 (30.3) | 771 (28.8) | |
| No | 1535 (69.7) | 1903 (71.2) | |
| Arthritis/joint pain medication | 0.012 | ||
| Yes | 45 (2.0) | 86 (3.2) | |
| No | 2158 (98.0) | 2588 (96.8) | |
| Depressive symptoms (CES-D) | 0.6 (1.3) | 1.1 (1.7) | <0.001 |
| Depression treatment | 0.005 | ||
| Yes | 61 (2.8) | 114 (4.3) | |
| No | 2142 (97.2) | 2560 (95.7) | |
| Sleep quality | 2.1 (0.8) | 2.4 (0.9) | <0.001 |
| Sleep duration | <0.001 | ||
| ≤6 h | 608 (27.6) | 919 (34.4) | |
| >6-7 h | 780 (35.4) | 834 (31.2) | |
| >7-8 h | 673 (30.5) | 736 (27.5) | |
| >8 h | 142 (6.4) | 185 (6.9) | |
| CRP 1 | 3.0 (4.5) | 3.5 (4.7) | 0.001 |
| Fibrinogen | 3.3 (0.5) | 3.4 (0.5) | <0.001 |
| Verbal fluency | 21.8 (6.6) | 21.1 (6.4) | <0.001 |
| Verbal memory | |||
| Immediate recall | 5.9 (1.6) | 6.1 (1.7) | <0.001 |
| Delayed recall | 4.5 (1.9) | 4.9 (2.0) | <0.001 |
| Time orientation | 3.78 (0.5) | 3.82 (0.5) | 0.006 |
SD = standard deviation; GCE = General Certificate of Education; BMI = body mass index; CES-D = Center for Epidemiologic; CRP = C-recatve protein;
untransformed data.
3.2. Sleep quality, CRP and cognitive function
In women, baseline sleep quality did not predict follow-up performance on verbal fluency, immediate and delayed recall, or time orientation. There was no evidence that CRP had any mediating effect in these relationships (see Table 2).
In men, sleep quality was not predictive of any cognitive function test 8 years later, and there was also no evidence of any mediating effect of CRP.
3.3. Sleep quality, fibrinogen and cognitive function
In women, sleep quality did not predict any cognitive function test, and there was no evidence of any mediating effects of fibrinogen (see Table 3).
Table 3.
Summary of mediation analysis for sleep quality, fibrinogen and cognitive function variables.
| Exposure variable (wave 4) | Mediating variable (wave 6) | Outcome variable (wave 8) | Fully Adjusted Mediation Analysis (Coefficient β, 95 % CI)* | Fully Adjusted Mediation Analysis (Coefficient β, 95 % CI)* | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Women | Men | |||||||
|
| ||||||||
| Sleep quality | Fibrinogen | Cognitive function | Indirect effect (ab) | Total effect (c) | Direct effect (c’) | Indirect effect (ab) | Total effect (c) | Direct effect (c’) |
| Verbal fluency | 0.007 (−0.011, 0.034) | 0.367 (−0.014, 0.748) | 0.361 (−0.021, 0.742) | −0.000 (−0.018, 0.017) | 0.375 (−0.101, 0.851) | 0.375 (−0.101, 0.852) | ||
| Verbal memory: immediate recall | 0.003 (−0.002, 0.010) | 0.042 (−0.053, 0.138) | 0.040 (−0.055, 0.135) | −0.000 (−0.005, 0.004) | −0.056 (−0.168, 0.057) | −0.055 (−0.168, 0.057) | ||
| Verbal memory: delayed recall | 0.001 (−0.004, 0.009) | 0.054 (−0.061, 0.170) | 0.053 (−0.062, 0.168) | 0.000 (−0.004, 0.005) | 0.010 (−0.127, 0.146) | 0.010 (−0.127, 0.146) | ||
| Time orientation | −0.000 (−0.003, 0.001) | 0.036 (−0.002, 0.075) | 0.037 (−0.002, 0.075) | 0.000 (−0.002, 0.003) | −0.013 (−0.066, 0.039) | −0.013 (−0.066, 0.039) | ||
CI = confidence interval.
For indirect effect bias-corrected 95% confidence intervals are reported. Significant effects are denoted in bold.
In men, as already reported with regards to CRP, sleep quality was unrelated to follow-up cognitive function, and there was no evidence of any mediating effects of fibrinogen.
3.4. Sleep duration, CRP and cognitive function
As shown in Table 4, in women sleep duration was unrelated to follow-up cognitive function, and there was no evidence of any mediating effect of CRP.
Table 4.
Summary of mediation analysis for sleep duration, CRP and cognitive function variables.
| Exposure variable (wave 4) | Mediating variable (wave 6) | Outcome variable (wave 8) | Fully Adjusted Mediation Analysis (Coefficient β, 95 % CI)* | Fully Adjusted Mediation Analysis (Coefficient β, 95 % CI)* | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Women | Men | |||||||
|
| ||||||||
| Sleep duration categories | CRP | Cognitive function | Indirect effect (ab) | Total effect (c) | Direct effect (c’) | Indirect effect (ab) | Total effect (c) | Direct effect (c’) |
| <6 | Verbal fluency | 0.003 (−0.022, 0.037) | −0.015 (−0.846, 0.817) | −0.018 (−0.850, 0.814) | 0.001 (−0.031, 0.034) | −0.048 (−0.989, 0.893) | −0.049 (−0.991, 0.892) | |
| >6-7 | 0.005 (−0.024, 0.043) | 0.589 (−0.224, 1.402) | 0.584 (−0.229,1.397) | 0.001 (−0.032, 0.036) | 0.118 (−0.733, 0.969) | 0.117 (−0.735, 0.968) | ||
| >7-8 | Reference | Reference | Reference | Reference | Reference | Reference | ||
| >8 | 0.007 (−0.035, 0.060) | 0.188 (−1.160, 1.537) | 0.182 (−1.167, 1.531) | 0.002 (−0.058, 0.064) | −0.859 (−2.558, 0.840) | −0.861 (−2.561, 0.839) | ||
| <6 | Verbal memory: immediate recall | 0.001 (−0.006, 0.009) | −0.200 (−0.408, 0.008) | −0.201 (−0.409, 0.007) | −0.000 (−0.007, 0.007) | −0.134 (−0.357, 0.090) | −0.134 (−0.357, 0.090) | |
| >6-7 | 0.001 (−0.007, 0.011) | 0.056 (−0.148, 0.260) | 0.056 (−0.149, 0.260) | −0.000 (−0.008, 0.007) | −0.064 (−0.266, 0.138) | −0.063 (−0.265, 0.139) | ||
| >7-8 | Reference | Reference | Reference | Reference | Reference | Reference | ||
| >8 | 0.001 (−0.019, 0.14) | −0.080 (−0.418, 0.258) | −0.081 (−0.419, 0.257) | −0.000 (−0.013, 0.012) | −0.345 (−0.747, 0.058) | −0.344 (−0.747, 0.058) | ||
| <6 | Verbal memory: delayed recall | 0.001 (−0.006, 0.014) | −0.007 (−0.259, 0.245) | −0.008 (−0.260, 0.244) | −0.000 (−0.010, 0.008) | −0.317 (−0.585, −0.048) | −0.316 (−0.585, −0.047) | |
| >6-7 | 0.002 (−0.006, 0.015) | 0.197 (−0.050, 0.443) | 0.195 (0.052, 0.441) | −0.001 (−0.010, 0.007) | −0.222 (−0.465, 0.021) | −0.222 (−0.465,0.021) | ||
| >7-8 | Reference | Reference | Reference | Reference | Reference | Reference | ||
| >8 | 0.003 (−0.009, 0.020) | −0.119 (−0.527, 0.290) | −0.121 (−0.530, 0.287) | −0.001 (−0.017, 0.013) | −0.485 (−0.970, −0.001) | −0.485 (−0.970, 0.001) | ||
| <6 | Time orientation | 0.000 (−0.002, 0.003) | 0.069 (−0.017, 0.154) | 0.068 (−0.017, 0.154) | −0.001 (−0.008, 0.005) | −0.069 (−0.173, 0.035) | −0.068 (−0.172, 0.036) | |
| >6-7 | 0.000 (−0.002, 0.004) | 0.055 (−0.028, 0.139) | 0.055 (−0.029, 0.138) | −0.002 (−0.009, 0.004) | −0.023 (−0.117, 0.071) | −0.022 (−0.116, 0.072) | ||
| >7-8 | Reference | Reference | Reference | Reference | Reference | Reference | ||
| >8 | 0.001 (−0.003, 0.006) | −0.095 (−0.233, 0.044) | −0.096 (−0.234, 0.043) | −0.003 (−0.014, 0.006) | −0.142 (−0.329, 0.046) | −0.138 (−0.325, 0.049) | ||
CRP = C-reactive protein; CI = confidence interval.
For indirect effect bias-corrected 95% confidence intervals are reported. Significant effects are denoted in bold.
In men, however, both short (≤6 h) (total effect: β = −0.317, C.I. −0.585 to −0.048) and long sleep duration (total effect: β = −0.485, C.I. −0.970 to −0.001) were predictive of a lower score on the delayed recall test, when compared with optimal sleep (>7-8 h), but this was not mediated by CRP levels in either of the models. Sleep duration was unrelated to tests of verbal fluency, immediate recall and time orientation, and CRP had no mediating effect on the relationship between sleep hours and these cognitive scores.
3.5. Sleep duration, fibrinogen and cognitive function
In women, there was no evidence that sleep duration was associated with cognitive function 8 years later (see Table 5), and sleep duration did not have any impact on follow-up cognition through fibrinogen as well.
In men, in comparison with optimal sleep duration (>7-8 h), those sleeping ≤6 hours (total effect: β = −0.343, C.I −0.611 to −0.076) and >6-7 hours (total effect: β = −0.263, C.I. − 0.506 to −0.020) had worst performance on the delayed recall test at follow-up. Long sleep at baseline was also predictive on lower performance on this cognitive test (total effect: β = −0.536, C.I. −1.019 to −0.053). However, fibrinogen concentrations did not mediate these associations.
3.6. Sensitivity analyses
3.6.1. Sleep quality, CRP and cognitive function
In contrast to main analyses, in women, baseline (poorer) sleep quality was associated with higher performance on the verbal fluency test at follow-up (total effect: β = 0.388, C.I. 0.001 to 0.774) in the models relating CRP. Sleep quality was also predictive of better time orientation at follow-up (total effect: β = 0.044, C.I. 0.007 to 0.081). There was no evidence of any mediating effects of inflammation in both models (see Supplementary Table 1). In a further analysis excluding participants with CRP > 3 at baseline, sleep quality was no longer a predictor of verbal fluency (total effect: β = 0.262, C.I. −0.222 to 0.745), or time orientation at follow-up (total effect: β = 0.039, C.I. −0.007 to 0.085), potentially as a result from a loss of power. Sleep quality was unrelated to performance on immediate or delayed recall memory tests.
In men, baseline sleep quality was unrelated to cognition at follow-up, and there was no evidence of any mediation by CRP (See Supplementary Table 1).
3.6.2. Sleep quality, fibrinogen and cognitive function
In women, sleep quality was associated with time orientation at follow-up in the models with fibrinogen (total effect: β = 0.044, C.I. 0.007 to 0.081), but there was no evidence of mediating effects of this inflammatory marker (see Supplementary Table 2). There was also no prospective association between sleep quality and other cognitive tests.
In men, baseline sleep quality was unrelated to cognition at follow-up, and there was no evidence of any mediation by fibrinogen (See Supplementary Table 2).
3.6.3. Sleep duration, CRP and cognitive function
In women, baseline sleep duration was unrelated to cognitive scores at follow-up in the models with CRP, and there was no evidence of any mediating effects of inflammation (See Supplementary Table 3).
In men, results regarding sleep duration were in line with those obtained in our main analyses. Namely, in comparison with the >7-8 h sleep category, men sleeping <6 hours (total effect: β = −0.328, .C.I. −0.598 to −0.058) and >8 hours (total effect: β = −0.515, C.I. −0.998 to −0.032) had lower scores on the delayed recall test in the model with CRP (see Supplementary Table 3). In a further analysis excluding participants with CRP > 3 at baseline, men sleeping <6 hours (total effect: β = −0.462, C.I. −0.758 to −0.145) as well as >8 hours (total effect: β = −0.706, C.I. −1.303 to −0.109) had lower performance on the delayed recall test. In neither of the models was there any evidence of mediating effects of CRP.
3.6.4. Sleep duration, fibrinogen and cognitive function
In women, baseline sleep duration was unrelated to cognitive scores at follow-up in the models with fibrinogen, and there was no evidence of any mediating effects of inflammation (See Supplementary Table 4).
In men, those sleeping ≤6 hours (total effect: β = −0.327, C.I. −0.597 to −0.057) and >8 hours (total effect: β = −0.509, C.I. −0.992 to −0.026), in comparison with the >7-8 h sleep category, also had poorer performance on the delayed recall test at follow-up. However, there was no evidence of any mediating effects of fibrinogen (see Supplementary Table 4). Baseline sleep duration was unrelated to the other three cognitive tests measured in our study, and there was no evidence of any mediating effects of inflammation.
3.6.5. All sample analyses
In the analyses based on the entire sample, sleep quality at baseline predicted better performance on the verbal fluency test at follow-up in the models with CRP (total effect: β = 0.348, C.I 0.048 to 0.649) and fibrinogen (total effect: β = 0.345, C.I. 0.049 to 0.640), but there was no evidence of any mediating effects by these inflammatory markers. There was also no prospective association between sleep quality and other cognitive tests in models with CRP or fibrinogen (see Supplementary Table 5 and 6).
In comparison with optimal sleep (>7-8 h), short sleep duration (≤6 h) predicted lower performance on the immediate recall test at follow-up in the models with CRP (total effect: β = −0.184, C.I. −0.335 to −0.032) and fibrinogen (total effect: β = −0.197, C.I. −0.347 to −0.048). There was no evidence of any mediating effects by inflammatory makers in these models. Long sleep (>8 h), when compared with the >7-8h category, was predictive of lower performance on the time orientation test at follow-up in the models with CRP (total effect: β = −0.124, C.I. −0.236 to −0.013) and fibrinogen (total effect: β = −0.128, C.I. −0.237 to −0.019) (see Supplementary Table 7 and 8). As with other models, these prospective associations were not mediated by inflammation. Sleep duration did not predict performance on the verbal fluency or delayed recall test at follow-up.
4. Discussion
We found that men reporting short (≤6 h, and >6-7 h) and long (>8 h) sleep hours at baseline were more likely to have lower scores on verbal memory at follow-up, especially in delayed recall, when compared with the optimal sleep category (>7-8 h). Sleep measures were unrelated to follow-up performance on various cognitive tests in women. None of the associations between sleep measures and follow-up cognitive performance reported here were mediated by inflammatory factors; thus our research hypothesis was unsupported by our data.
Our analyses revealed that men, but not women, in the ≤6 h, >6-7 h and >8 h sleep categories had lower scores on the delayed recall test 8 years later. The same pattern of results was confirmed in our sensitivity analyses restricted to participants with complete data on all variables used in the study (N=2549). This supports the hypothesis that sleep plays an active role in memory consolidation (Kreutzmann, Havekes, Abel, & Meerlo, 2015), and shows for the first time, to the best of our knowledge, the importance of sleep for delayed memory in older adults. Most previous studies looking at sleep and cognitive function did not look separately at delayed or immediate recall, but used an overall memory score, or computed a global cognitive function index from various cognitive tests. Our overall findings are in line with a number of these studies, which were based on samples of similar age to our participants (approximately 65 years old). For example, an earlier cross-sectional analysis of ELSA found that short sleep and long sleep were associated with lower scores on cognition, albeit only among those aged 64 or younger (Miller et al., 2014). Regarding prospective findings, our study supports data obtained by Virta et al. (2013), whereby short and long sleep predicted lower cognitive scores in men and women aged 65 years on average. We also corroborate a study in which short sleep predicted a decline in cognitive performance of older Chinese men and women (Lo et al. (2014). In the Nurses’ Health Study, composed solely of female participants, short and long sleep also predicted poor cognitive performance (Devore et al., 2014). This is at odds with our results since we only found this relationship in men, but not women. Finally, our finding that short and long sleep predict lower scores on delayed memory recall, when compared with optimal sleep duration, only partly supports recent prospective analysis of the Doetinchem Cohort Study (van Oostrom et al., 2018) in which short sleep was unrelated to subsequent cognitive performance.
One possibility why baseline short and long sleep duration predicted lower performance on the delayed memory recall test in men, but not women, at follow-up, could be because we lacked statistical power to also detect this relationship in women. This does not seem to be the case since, in our data, more women than men were short sleepers. However, in our study men had lower scores on all cognitive tests including the delayed recall memory test. Notably, sex differences in cognition have been reported previously, including in ageing adults (De Frias et al., 2006). Although we adjusted our analysis for baseline cognition and other confounders that could potentially impact follow-up cognition, we cannot rule out the possibility that the association between baseline sleep duration and follow-up cognition was a result of a third unmeasured variable.
In our analyses based on the main analytical sample, sleep quality was unrelated to follow-up condition in women. However, in the sensitivity models conducted on the sample with complete data on all variables used in the study (N=2549) women with poorer sleep quality performed better on follow-up tests of verbal fluency and time orientation (in men sleep quality did not predict cognitive performance in any models tested for this article). These findings corroborate a cross-sectional analysis of ELSA (Miller et al., 2014). This is, however, in contrast with the study by Virta et al. (2013) where disturbed sleep was linked to worst cognitive performance approximately 22 years later. We do not have a strong explanation for this rather counterintuitive finding. One possibility could be that participants who perform better on cognitive function tests were also able to record their sleep more accurately, given that prior evidence suggested that poor cognitive function has been found to adversely influence the level of agreement between self-reported and actigraphy-based sleep data in older adults (Van Den Berg et al., 2008). However, while a poor cognitive function may impact on how sleep is reported if both measurements are taken at the same time, as was done by Van Den Berg and colleagues (2008), this is unlikely to be the case in our study in which baseline sleep and follow-up cognition were measured 8 years apart. Another possibility explaining why only women, but not men, with poorer sleep quality performed better on follow-up cognitive measures could be due to the way women self-report their sleep quality. Indeed, women have been found to report poorer sleep quality even though objectively they appear to have healthier sleep than men; there is also some evidence that women’s self-reports of sleep are influenced by their higher perceived need of sleep, and greater awareness of or vigilance to their bodies (Bixler et al., 2009; Ursin, Bjorvatn, & Holsten, 2005; Van Den Berg et al., 2008). It would, therefore, be interesting to see if the same pattern of findings would be revealed in analyses based on objective measures of sleep; but these are not yet available in ELSA.
In our analyses based on the entire sample findings from the sex-stratified analyses were only partially supported. Poor sleep quality was predictive of better performance on the verbal fluency test at follow-up. This mirrors our finding for women only in the sensitivity analyses based on the sample restricted to participants with complete data on all variables used in the study, but not in the main models. Another possible explanation as to why sleep quality was inversely associated with the follow-up score on the verbal fluency test could be due to a third unmeasured variable, or due to the relatively modest reliability of the sleep scale used in ELSA, which we discuss below. In terms of sleep duration, short sleep (≤6 h), when compared with the >7-8 h sleep category, predicted worst performance on the immediate recall memory test, while long sleep was associated with a lower score on the time orientation test at follow-up. These results, to some extent, support our findings in men obtained in the main as well as sensitivity analyses, where short and long sleep hours predicted lower scores on the delayed recall test at follow-up.
Several experimental studies of sleep deprivation conducted in rodents and humans have documented the importance of sleep duration for effective memory consolidation. Sleep quality also appears to be important, and clinical data from patients with insomnia suggest that poor sleep quality correlates with a diminished sleep-related consolidation of declarative memory (Kreutzmann et al., 2015). Hypoxia and disturbed neuronal activity are also plausible mechanisms through which too short or poor quality sleep might impair cognitive functioning in older adults. Circadian rhythms affect activity in frontal, thalamic, and hypothalamic regions of the brain, impairing learning and memory (Yaffe et al., 2014). Furthermore, as far as memory consolidation is concerned, human neuroimaging studies suggest that the brain region that appears to be particularly sensitive to the consequences of sleep loss or its poor quality is the hippocampus (Kreutzmann et al., 2015). Relatedly, the glymphatic system, which removes neurotoxic waste products, including β-amyloid, and soluble proteins and metabolites from the central nervous system, is another plausible mechanism linking sleep with cognitive health given that it is turned on predominantly during sleep, and largely inactive during wake hours. As the overall seep quality deteriorates in ageing adults, this is likely to have an adverse effect on the efficiency of the glymphatic system, and subsequently cognitive function (Jessen, Munk, Lundgaard, & Nedergaard, 2015).
In our data, the prospective association between sleep quality and duration with follow-up cognitive function was not mediated by inflammatory factors, namely CRP and fibrinogen. In a study of older Chinese adults CRP was also unrelated to sleep and cognitive function (Lo et al., 2014), albeit the authors did not perform a mediation analysis, so our findings cannot be directly compared with that data. Greater levels of inflammatory factors have been found associated with reduced cognitive function in patients with obstructive sleep apnoea (Haensel et al., 2009), and in animal studies of acute sleep deprivation (Zhu et al., 2012). This raises the possibility that more severe sleep disturbances than reported by our participants are needed to raise inflammation, which could then adversely affect cognition. More studies are warranted to explore if inflammation may be translating the deleterious impact of aberrant sleep on follow-up cognition in community-dwelling older adults.
The strengths of our study include a prospective design of (older) adults who are chosen to be representative of men and women living in England. Since a large number of data are collected as part of ELSA, this minimises the risk that participants are aware of researchers’ interests such as sleep and cognitive function described here.
Our findings must be interpreted in light of the limitations of our data. Sleep was measured by self-report, and estimations of sleep quality (Jackowska, Dockray, Hendrickx, & Steptoe, 2011) and duration (Lauderdale, Knutson, Yan, Liu, & Rathouz, 2008) are imprecise when compared with sleep data measured objectively. Factors that may influence people’s perceptions of sleep include, for example, age, fewer years of education and work stress (Jackowska et al., 2011; Lauderdale et al., 2008). In addition, the reliability of the three sleep items was lower (Cronbach’s alpha 0.60) than the recommended threshold of 0.75 in the literature (Christmann & Van Aelst, 2006). This may be explained by the fact that these items were derived from a sleep scale that consists of 4, not 3 items. We also do not have information about sleep disorders, such as sleep apnoea, insomnia and sedative hypnotics medication, since these were not collected in wave 4 of ELSA that was our baseline, but all analyses were adjusted for the presence of limiting long-standing illness, smoking, BMI and depressive symptoms, which are relevant for these disorders. Attrition is a well-known issue in longitudinal studies including ELSA, and participants excluded from our analyses were older, more likely to have no formal qualifications; they also reported more depressive symptoms and limiting long-standing illnesses. Furthermore, at the time of these analyses, ELSA did not have up-to-date mortality records. Moreover, a common issue associated with longitudinal studies of ageing is the potential survivor effect. Lastly, the participants’ age captured by this analytical sample is relatively young (mean age 65), and possibly more fit cognitively compared to a population sample of much older participants, considering that the subtle signs of cognitive impairment seem to exhibit more often after the age of 65. Therefore, the effect of sleep on impaired cognitive function may have been less evident within this study and further investigation of older populations cohorts should be considered.
In conclusion, our study adds to the body of evidence that adverse sleep patterns are prospectively associated with cognitive performance in middle-aged and elderly participants. In men, short and long sleep was associated with lower scores on the delayed recall memory test in comparison with optimal sleep duration. However, inflammatory factors did not mediate the prospective association between sleep and cognitive performance reported here. Nevertheless, this work opens up possibilities for exploring other mechanistic ways in which a range of modifiable risk factors, such as good sleep, might enhance cognitive functioning in people at older ages, for the possible improvement of health and social function in the later stages of life.
Supplementary Material
Highlights.
8-year follow-up of sleep, cognition and inflammation in older men and women.
Short and long sleep predicted lower scores on the delayed memory test in men.
No evidence of mediating effects of inflammation was found.
Acknowledgments
Funding
Marta Jackowska (author 1) is a senior lecturer at University of Roehampton and did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sector for the work on this manuscript. Dorina Cadar (author 2) is a Senior Research Fellow at University College London and receives funding from the National Institute on Aging (grants 5218182, RO1AG7644-01A1 and RO1AG017644). The English Longitudinal Study of Ageing is funded by the National Institute on Aging (Grant RO1AG7644) and by a consortium of UK government departments coordinated by the Economic and Social Research Council (ESRC) and the Office for National Statistics. The English Longitudinal Study of Ageing (ELSA) was developed by a team of researchers based at University College London, the Institute for Fiscal Studies and the National Centre for Social Research. The data are linked to the UK Data Archive and freely available through the UK data services and can be accessed here: https://discover.ukdataservice.ac.uk.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Competing Interest
Both authors declare that they have no conflict of interest concerning this article.
References
- Arber S, Bote M, & Meadows R (2009). Gender and socio-economic patterning of self-reported sleep problems in Britain. Social science & medicine, 68(2), 281–289. 10.1016/j.socscimed.2008.10.016 [DOI] [PubMed] [Google Scholar]
- Bettcher BM, & Kramer JH (2014). Longitudinal Inflammation, Cognitive Decline, and Alzheimer’s Disease: A Mini-Review. Clinical Pharmacology & Therapeutics, 96(4), 464–469. 10.1038/clpt.2014.147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bixler EO, Papaliaga MN, Vgontzas AN, LIN HM, Pejovic S, Karataraki M, … & Chrousos GP (2009). Women sleep objectively better than men and the sleep of young women is more resilient to external stressors: effects of age and menopause. Journal of sleep research, 18(2), 221–228. 10.1111/i.1365-2869.2008.00713.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell T, Yaffe K, Ancoli-Israel S, Redline S, Ensrud KE, Stefanick ML, … & Osteoporotic Fractures in Men (MrOS) Study Group. (2011). Association of sleep characteristics and cognition in older community-dwelling men: the MrOS sleep study. Sleep, 34(10), 1347–1356. 10.5665/SLEEP.1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagney KA, & Lauderdale DS (2002). Education, wealth, and cognitive function in later life. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 57(2), 163–172. 10.1093/geronb/57.2.P163 [DOI] [PubMed] [Google Scholar]
- Cappuccio FP, Cooper D, D’elia L, Strazzullo P, & Miller MA (2011). Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. European heart journal, 32(12), 1484–1492. 10.1093/eurheartj/ehr007 [DOI] [PubMed] [Google Scholar]
- Craig R, Deverill C, Pickering K (2006). Quality control of blood, saliva and urine analytes. In: Spronston KJ & Mindell J (Eds.), Health Survey for England 2004, Methodology and Documentation (vol. 2, pp. 34–41). London: The Information Centre. [Google Scholar]
- Christmann A, & Van Aelst S (2006). Robust estimation of Cronbach’s alpha. Journal of Multivariate Analysis, 97(7), 1660–1674. 10.1016/j.jmva.2005.05.012 [DOI] [Google Scholar]
- Crowley K (2011). Sleep and sleep disorders in older adults. Neuropsychology review, 21(1), 41–53. 10.1007/s11065-010-9154-6 [DOI] [PubMed] [Google Scholar]
- De Frias CM, Nilsson LG, & Herlitz A (2006). Sex differences in cognition are stable over a 10-year period in adulthood and old age. Aging, Neuropsychology, and Cognition, 13(3-4), 574–587. 10.1080/13825580600678418 [DOI] [PubMed] [Google Scholar]
- Devore EE, Grodstein F, Duffy JF, Stampfer MJ, Czeisler CA, & Schernhammer ES (2014). Sleep duration in midlife and later life in relation to cognition. Journal of the American Geriatrics Society, 62(6), 1073–1081. 10.1111/jgs.12790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faubel R, López-GarcÍa E, Guallar-CastillÓn P, Graciani A, Banegas JR, & RodrÍguez-Artalejo F (2009). Usual sleep duration and cognitive function in older adults in Spain. Journal of sleep research, 18(4), 427–435. 10.1111/i.1365-2869.2009.00759.x [DOI] [PubMed] [Google Scholar]
- Ferrie JE, Shipley MJ, Akbaraly TN, Marmot MG, Kivimäki M, & Singh-Manoux A (2011). Change in sleep duration and cognitive function: findings from the Whitehall II Study. Sleep, 34(5), 565–573. 10.1093/sleep/34.5.565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field A (2018). Discovering statistics using IBM SPSS statistics. London: Sage. [Google Scholar]
- Fogel S, Martin N, Lafortune M, Barakat M, Debas K, Laventure S, … & Carrier J (2012). NREM sleep oscillations and brain plasticity in aging. Frontiers in neurology, 3, 176. 10.3389/fneur.2012.00176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, & McHugh PR (1975). “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. Journal of psychiatric research, 12(3), 189–198. [DOI] [PubMed] [Google Scholar]
- Franco OH, Karnik K, Osborne G, Ordovas JM, Catt M, & van der Ouderaa F (2009). Changing course in ageing research: the healthy ageing phenotype. Maturitas, 63(1), 13–19. 10.1016/j.maturitas.2009.02.006 [DOI] [PubMed] [Google Scholar]
- Franzen PL, Siegle GJ, & Buysse DJ (2008). Relationships between affect, vigilance, and sleepiness following sleep deprivation. Journal of sleep research, 17(1), 34–41 . 10.1111/j.1365-2869.2008.00635.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadie A, Shafto M, Leng Y, & Kievit RA (2017). How are age-related differences in sleep quality associated with health outcomes? An epidemiological investigation in a UK cohort of 2406 adults. BMJ open, 7(7), e014920. 10.1136/bmjopen-2016-014920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haensel A, Bardwell WA, Mills PJ, Loredo JS, Ancoli-Israel S, Morgan EE, … & Dimsdale JE (2009). Relationship between inflammation and cognitive function in obstructive sleep apnea. Sleep and Breathing, 13(1), 35–41. 10.1007/s11325-008-0198-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF (2018). Introduction to Mediation, Moderation, and Conditional Process Analysis. A Regression-Based Approach (2nd edition). London: Guilford Press. [Google Scholar]
- Howren MB, Lamkin DM, & Suls J (2009). Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosomatic medicine, 71(2), 171–186. doi: 10.1097/PSY.0b013e3181907c1b [DOI] [PubMed] [Google Scholar]
- Irwin MR (2015). Why sleep is important for health: a psychoneuroimmunology perspective. Annual review of psychology, 66, 143–172. 10.1146/annurev-psych-010213-115205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Olmstead R, & Carroll JE (2016). Sleep disturbance, sleep duration, and inflammation: a systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biological psychiatry, 80(1), 40–52. 10.1016/j.biopsych.2015.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, & Vitiello MV (2019). Implications of sleep disturbance and inflammation for Alzheimer’s disease dementia. The Lancet Neurology, 18, 296–306. 10.1016/S1474-4422(18)30450-2 [DOI] [PubMed] [Google Scholar]
- Jackowska M, Dockray S, Hendrickx H, & Steptoe A (2011). Psychosocial factors and sleep efficiency: discrepancies between subjective and objective evaluations of sleep. Psychosomatic Medicine, 73(9), 810–816. 10.1097/PSY.0b013e3182359e77 [DOI] [PubMed] [Google Scholar]
- Jenkins CD, Stanton BA, Niemcryk SJ, & Rose RM (1988). A scale for the estimation of sleep problems in clinical research. Journal of clinical epidemiology, 41(4), 313–321. 10.1016/0895-4356(88)90138-2 [DOI] [PubMed] [Google Scholar]
- Jessen NA, Munk ASF, Lundgaard I, & Nedergaard M (2015). The glymphatic system: a beginner’s guide. Neurochemical research, 40(12), 2583–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jike M, Itani O, Watanabe N, Buysse DJ, & Kaneita Y (2018). Long sleep duration and health outcomes: A systematic review, meta-analysis and meta-regression. Sleep Medicine Reviews, 39, 25–36. 10.1016/j.smrv.2017.06.011 [DOI] [PubMed] [Google Scholar]
- Juster RP, McEwen BS, & Lupien SJ (2010). Allostatic load biomarkers of chronic stress and impact on health and cognition. Neuroscience & Biobehavioral Reviews, 35(1), 2–16. 10.1016/j.neubiorev.2009.10.002 [DOI] [PubMed] [Google Scholar]
- Karlamangla AS, Miller-Martinez D, Aneshensel CS, Seeman TE, Wight RG, & Chodosh J (2009). Trajectories of cognitive function in late life in the United States: demographic and socioeconomic predictors. American journal of epidemiology, 170(3), 331–342. 10.1093/aje/kwp154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster A, Bosma H, Penninx BW, Newman AB, Harris TB, Van Eijk JTM, … & Ayonayon HN (2006). Association of inflammatory markers with socioeconomic status. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 61(3), 284–290. 10.1093/gerona/61.3.284 [DOI] [PubMed] [Google Scholar]
- Koyanagi A, Garin N, Olaya B, Ayuso-Mateos JL, Chatterji S, Leonardi M, … & Haro JM (2014). Chronic conditions and sleep problems among adults aged 50 years or over in nine countries: a multi-country study. PloS one, 9(12), e114742. 10.1371/journal.pone.0138261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreutzmann JC, Havekes R, Abel T, & Meerlo P (2015). Sleep deprivation and hippocampal vulnerability: changes in neuronal plasticity, neurogenesis and cognitive function. Neuroscience, 309,173–190. 10.1016/j.neuroscience.2015.04.053 [DOI] [PubMed] [Google Scholar]
- Lassale C, Batty GD, Steptoe A, Cadar D, Akbaraly TN, Kivimäki M, & Zaninotto P (2018). Association of 10-Year C-reactive protein trajectories with markers of healthy aging: Findings from the English Longitudinal Study of Aging. The Journals of Gerontology: Series A, 74(2), 195–203. 10.1093/gerona/gly028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauderdale DS, Knutson KL, Yan LL, Liu K, & Rathouz PJ (2008). Self-reported and measured sleep duration: how similar are they?. Epidemiology, 19(6), 838–845. https://dx.doi.org/10.1097%2FEDE.0b013e318187a7b0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luik AI, Zuurbier LA, Hofman A, Van Someren EJ, Ikram MA, & Tiemeier H (2015). Associations of the 24-h activity rhythm and sleep with cognition: a population-based study of middle-aged and elderly persons. Sleep medicine, 16(7), 850–855. 10.1016/j.sleep.2015.03.012 [DOI] [PubMed] [Google Scholar]
- Lo JC, Loh KK, Zheng H, Sim SK, & Chee MW (2014). Sleep duration and age-related changes in brain structure and cognitive performance. Sleep, 37(7), 821–821. 10.5665/sleep.3832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDade TW, Hawkley LC, & Cacioppo JT (2006). Psychosocial and behavioral predictors of inflammation in middle-aged and older adults: the Chicago health, aging, and social relations study. Psychosomatic medicine, 68(3), 376–381.doi: 10.1097/01.psy.0000221371.43607.64 [DOI] [PubMed] [Google Scholar]
- Miller MA, Wright H, Ji C, & Cappuccio FP (2014). Cross-sectional study of sleep quantity and quality and amnestic and non-amnestic cognitive function in an ageing population: The English Longitudinal Study of Ageing (ELSA). PLoS One, 9(6), e100991. 10.1371/journal.pone.0100991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebes RD, Buysse DJ, Halligan EM, Houck PR, & Monk TH (2009). Self-reported sleep quality predicts poor cognitive performance in healthy older adult. The Journals of Gerontology: Series B, 64(2), 180–187. 10.1093/geronb/gbn037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson TA, Mensah GA, Alexander RW (2003). Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation, 107, 499–511. 10.1161/01.CIR.0000052939.59093.4 [DOI] [PubMed] [Google Scholar]
- Sabia S, Nabi H, Kivimäki M, Shipley MJ, Marmot MG, & Singh-Manoux A (2009). Health behaviors from early to late midlife as predictors of cognitive function: The Whitehall II study. American journal of epidemiology, 170(4), 428–437. 10.1093/aje/kwp161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint Martin M, Sforza E, Barthélémy JC, Thomas-Anterion C, & Roche F (2012). Does subjective sleep affect cognitive function in healthy elderly subjects? The Proof cohort. Sleep medicine, 13(9), 1146–1152. 10.1016/j.sleep.2012.06.021 [DOI] [PubMed] [Google Scholar]
- Santhi N, Lazar AS, McCabe PJ, Lo JC, Groeger JA, & Dijk DJ (2016). Sex differences in the circadian regulation of sleep and waking cognition in humans. Proceedings of the National Academy of Sciences, 113(19), E2730–E2739. 10.1073/pnas.1521637113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid SM, Hallschmid M, & Schultes B (2015). The metabolic burden of sleep loss. The lancet Diabetes & endocrinology, 3(1), 52–62. 10.1016/S2213-8587(14)70012-9 [DOI] [PubMed] [Google Scholar]
- Singh T, & Newman AB (2011). Inflammatory markers in population studies of aging. Ageing research reviews, 10(3), 319–329. 10.1016/j.arr.2010.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh-Manoux A, Akbaraly TN, Marmot M, Melchior M, Ankri J, Sabia S, & Ferrie JE (2010). Persistent depressive symptoms and cognitive function in late midlife: the Whitehall II study. The Journal of clinical psychiatry, 71(10), 1379. doi: 10.4088/JCP.09m05349gry [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofi F, Cesari F, Casini A, Macchi C, Abbate R, & Gensini GF (2014). Insomnia and risk of cardiovascular disease: a meta-analysis. European journal of preventive cardiology, 21(1), 57–64.https://doi.org/10.1177%2F2047487312460020 [DOI] [PubMed] [Google Scholar]
- Song Y, Blackwell T, Yaffe K, Ancoli-Israel S, Redline S, Stone KL (2015). Relationships between sleep stages and changes in cognitive function in older men: the MrOS Sleep Study. Sleep, 38, 411–421. 10.5665/sleep.4500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffick DE (2000). Documentation of affective functioning measures in the Health and Retirement Study. Ann Arbor, MI: HRS Health Working Group. [Google Scholar]
- Steptoe A, Breeze E, Banks J, & Nazroo J (2012). Cohort profile: the English longitudinal study of ageing. International journal of epidemiology, 42(6), 1640–1648. 10.1093/ije/dys168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranges S, Dorn JM, Shipley MJ, Kandala NB, Trevisan M, Miller MA, … & Cappuccio FP (2008). Correlates of short and long sleep duration: a cross-cultural comparison between the United Kingdom and the United States: the Whitehall II Study and the Western New York Health Study. American journal of epidemiology, 168(12), 1353–1364. 10.1093/aje/kwn337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steel N, Huppert F, McWilliams B, Melzer D Physical and cognitive function. (2003). In: Marmot M Banks J Blundell R Lessof C & Nazroo J (Eds.), Health, wealth and lifestyles of the older population in England: The 2002 English Longitudinal Study of Ageing (pp. 249–71). London: IFS. [Google Scholar]
- Tampubolon G (2016). Repeated systemic inflammation was associated with cognitive deficits in older Britons. Alzheimer’s & Dementia: Diagnosis, Assessment & Disease Monitoring, 3, 1–6. 10.1016/j.dadm.2015.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursin R, Bjorvatn B, & Holsten F (2005). Sleep duration, subjective sleep need, and sleep habits of 40-to 45-year-olds in the Hordaland Health Study. Sleep, 28(10), 1260–1269. 10.1093/sleep/28.10.1260 [DOI] [PubMed] [Google Scholar]
- Van Den Berg JF, Van Rooij FJ, Vos H, Tulen JH, Hofman A, Miedema HM, … & Tiemeier H (2008). Disagreement between subjective and actigraphic measures of sleep duration in a population-based study of elderly persons. Journal of sleep research, 17(3), 295–302. 10.1111/j.1365-2869.2008.00638.x [DOI] [PubMed] [Google Scholar]
- van Oostrom SH, Nooyens AC, van Boxtel MP, & Verschuren WM (2018). Long sleep duration is associated with lower cognitive function among middle-age adults-the Doetinchem Cohort Study. Sleep medicine, 41, 78–85. 10.1016/j.sleep.2017.07.029 [DOI] [PubMed] [Google Scholar]
- Virta JJ, Heikkilä K, Perola M, Koskenvuo M, R†ih† I, Rinne JO, & Kaprio J (2013). Midlife sleep characteristics associated with late life cognitive function. Sleep, 36(10), 1533–1541. 10.5665/sleep.3052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller KL, Mortensen EL, Avlund K, Osler M, Fagerlund B, Lauritzen M, & Jennum P (2016). Subjective sleep quality and daytime sleepiness in late midlife and their association with age-related changes in cognition. Sleep medicine, 17, 165–173. 10.1016/j.sleep.2015.01.004 [DOI] [PubMed] [Google Scholar]
- Yaffe K, Falvey CM, & Hoang T (2014). Connections between sleep and cognition in older adults. The Lancet Neurology, 13(10), 1017–1028. 10.1016/S1474-4422(14)70172-3 [DOI] [PubMed] [Google Scholar]
- Zee PC, & Turek FW (2006). Sleep and health: everywhere and in both directions. Archives of internal medicine, 166(16), 1686–1688. doi: 10.1001/archinte.166.16.1686 [DOI] [PubMed] [Google Scholar]
- Zhai L, Zhang H,& Zhang D (2015). Sleep duration and depression among adults: A meta-analysis of prospective studies. Depression and anxiety, 32(9), 664–670. 10.1002/da.22386 [DOI] [PubMed] [Google Scholar]
- Zhu B, Dong Y, Xu Z, Gompf HS, Ward SA, Xue Z, … & Xie Z (2012). Sleep disturbance induces neuroinflammation and impairment of learning and memory. Neurobiology of disease, 48(3), 348–355. 10.1016/j.nbd.2012.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


