Highlights
-
•
TSPAN31 is rarely studied in human malignant tumors and a little study had explored its function and mechanism.
-
•
This study found that the expression level of TSPAN31 was related to the clinical characteristics and prognosis of patients with gastric cancer.
-
•
This study found that TSPAN31 can regulate the proliferation, migration and apoptosis of gastric cancer cells.
-
•
This study found that expression level of TSPAN31, METTL1 and CCT2 was positive correlation in gastric cancer cells.
-
•
This study found that down-regulation of TSPAN31 could be partially reversed the promoting effect of high expression of METTL1 or CCT2 on malignant phenotype of gastric cancer cells.
Keywords: Gastric cancer, TSPAN31, Cell proliferation, Cell migration
Abstract
Gastric cancer (GC) is one of the most common human malignancies worldwide, but the molecular mechanism of GC has not been fully elucidated. Tetraspanin 31 (TSPAN31) has been rarely studied in human malignant tumors. This study aimed to investigate the effects of TSPAN31 on GC. We analyzed GC tissues through high-throughput sequencing technology and chose TSPAN31 with high expression. The expression of TSPAN31 in GC was analyzed through bioinformatics website and qRT-PCR. The protein level of TSPAN31 in GC tissues was determined by western blot and immunochemistry. The proliferation, migration, and apoptosis of GC cells were detected by the cell counting kit-8, transwell, and apoptosis experiments. METTL1 and CCT2 that may co-express with TSPAN31 were predicted by the GEPIA database, and analyzed the correlation between the expression levels of TSPAN31, METTL1 and CCT2. The results shows TSPAN31 was highly expressed in GC tissues, and high expression of TSPAN31 was found to result in poor prognosis of patients with GC. TSPAN31 could regulate the proliferation, migration and apoptosis of GC cells. The relative expression levels of TSPAN31, METTL1 and CCT2 in GC were positively correlated. Low expression of TSPAN31 could partially reverse the effect of high expression of METTL1 and CCT2 on the tumor progression of GC cells. In conclusion, TSPAN31 was highly expressed in GC tissues and led to poor prognosis of patients with GC. TSPAN31 may regulate the proliferation, migration, and apoptosis of GC cells. This regulatory mechanism may be achieved through co-expression with METTL1 and CCT2.
Background
Gastric cancer (GC) is globally one of the most common malignant tumors of the human digestive tract. In 2020, there were 1.089 million new cases of GC and 769,000 deaths worldwide, accounting for 5.6% and 7.7% of all cancer cases and deaths, respectively [1]. Early diagnosis of GC is often difficult due to unclear or lack of specific symptoms, and it is usually diagnosed in the advanced stage once detected [2]. Although the continuous advancement of medical science has led to immense progress in the diagnosis and therapy of GC, the overall survival rate of patients with GC is still a cause of concern [3]. Therefore, it is particularly important to detect genes that are closely related to GC development and have diagnostic and therapeutic significance. These genes can be used as new targets to achieve early detection, diagnosis, and treatment of GC.
Tetraspanins (TSPANs) are a family of small transmembrane proteins [4] that participate in a variety of biological processes such as cell migration, cell signal transduction, and transmembrane transport [5], [6], [7]. Structurally, TSPAN consists of four transmembrane segments, a small extracellular domain, and a large extracellular loop. The intracellular domain, including the N-terminal and C-terminal tails, is relatively small and contains palmitoyl cysteine. Except for a small variable region located in the extracellular loop, the homology between homotypes is highly conserved [8]. Tetraspanin 31 (TSPAN31) is a member of the TSPAN protein family and was first reported by Jankowski in 1994 [9]. In recent years, the research on the role of TSPAN31 in human malignant tumors has gradually increased. A previous study reported that the expression of TSPAN31 is significantly upregulated in osteosarcoma and may be closely related to tumor growth and metastasis [10]. It is also worth noting that TSPAN31 is a natural antisense transcript of CDK4, and studies have revealed that TSPAN31 regulates tumor progression by regulating the expression of CDK4 in cervical cancer and liver cancer [11,12]. Previous studies have found that some members of the TSPAN protein family are abnormally expressed in gastric cancer. For example, DErrico and Cho et al. found that the expressions of TSPAN4, TSPAN9, TSPAN28 and TSPAN29 in gastric cancer patients were higher than those in the normal population, and Chen et al. found that TSPAN7 was low expressed in gastric cancer, then Qi et al. found that TSPAN31 expression level was elevated in colorectal cancer. In this study, Qi et al. investigated the biological function of TSPAN7 in liver cancer and found that TSPAN7 may play a role of tumor suppressor gene in liver cancer [13]. However, to date, no studies have reported the role of TSPAN31 in GC.
Qi et al. [13] found no significant difference in TSPAN31 expression in gastric cancer from oncomine database. But in the early stage of the present study, we found that TSPAN31 was abnormally highly expressed in GC tissues through high-throughput sequencing. In addition, we also found in TCGA database that the expression level of TSPAN31 in gastric cancer tissues was higher than that in normal tissues. We speculated that the differences in TSPAN31 expression levels might be caused by different data sets. As a starting point, we hypothesized that TSPAN31 potentially plays an oncogene role in GC development. The present study investigated the biological function and molecular mechanisms of TSPAN31 in GC through a series of in vivo and in vitro experiments. The biological functions and molecular mechanisms of TSPAN31 reported in this article are expected to provide new ideas for the diagnosis and treatment of GC.
Methods
Sample collection
Three pairs of GC tissues and corresponding normal tissues were collected to supply Quantitative Proteomics Research by (TMT)Tandem Mass Tag labeling Strategy, TMT labelling of trypsin-digested proteins was performed according to the manufacturer's instructions (TMT-10plex kit, Thermo Fisher, China). Equal volumes of all samples were mixed, concentrated in a vacuum concentrator and acidified with TFA for LC/MS/MS analysis. Samples were separated by nano-HPLC (Ultimate RSLC 3000, Thermo Fisher) using reversed phase C18 columns and 420 min gradients. The eluate was directly introduced into an Orbitrap Fusion Tribrid mass spectrometer (Thermo Fisher) equipped with nano-ESI source. Samples were analyzed using a collision-induced dissociation (combined ion trap-CID/high resolution-HCD) MS/MS strategy for peptide identification and reporter ion quantification. A total of 37 pairs of GC tissues and corresponding normal tissues from patients admitted to the Second Affiliated Hospital of Nanjing Medical University were collected in this study. All patients did not receive radiotherapy or chemotherapy before the operation. After obtaining the tissues in vitro, they were quickly stored in a refrigerator at -80 °C and then stored in liquid nitrogen. Tumor pathological classification and staging standards were implemented in accordance with American Joint Committee on Cancer (AJCC) staging standards. This study was approved by the ethical review committee of the Second Affiliated Hospital of Nanjing Medical University, and all patients provided their informed consent for sample collection. The ethics of this study conformed to the Declaration of Helsinki.
Cell culture
Human normal gastric mucosal cells GES-1 and gastric cancer cell lines AGS, HGC-27, MGC-803, BGC-823, and SGC-7901 were obtained from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). All cells were cultured in RPMI-1640 medium containing 10% fetal bovine serum (FBS) and double antibiotic (penicillin 100U/ mL, streptomycin 100 μg/ml). All cells were cultured in a cell incubator at 37 °C and 5% CO2.
Cell transfection
Small interfering RNAs (si-TSPAN31#1 and si-TSPAN31#2) and negative control (si-NC) against TSPAN31 were purchased from RiboBio (Guangzhou, Guangdong, China). Interference plasmid (shRNA) and overexpression vectors (OEs) of TSPAN31, METTL1, and CCT2 were obtained from GenePharma (Shanghai, China). For siRNA, when the cell fusion rate reached 70%, Lipofectamine 3000 (Invitrogen, Shanghai, China) was used for cell transfection according to the manufacturer's instructions. The cells were exchanged for 6 h after transfection, and the transfected cells were digested after 24–48 h for subsequent cell experiments. For OEs, when the cell fusion rate reached 70%, lentiviral vectors were added according to the product instructions, and fluorescent staining cells were observed to adhere to more than 60% under fluorescence microscope. All cells were cultured in a cell incubator at 37 °C and 5% CO2.
Quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from tissues or cells using Trizol reagent (Invitrogen, Shanghai, China) according to the manufacturer's protocol. A NanoDrop 2000 microplate reader (Thermo Fisher Scientific, Shanghai, China) was used to measure RNA concentration, and reverse transcription of RNA into cDNA was performed using the PrimeScript RT kit (Takara, Dalian, China) according to the manufacturer's instructions. SYBR® Green Master Mix (Takara, Dalian, China) was used to detect the CT value of the gene in the cell, with GAPDH as an internal reference. The primer sequences are shown in Table 1. The expression of the target gene was calculated according to the 2−ΔΔCt method, and the experiment and technique were repeated three times.
Table 1.
Primer sequence for qRT-PCR.
| Gene | Primer sequence | |
|---|---|---|
| TSPAN31 | Forward | 5’- CTGCTCCAAGAATGCGCTTTG -3’ |
| Reverse | 5’- CAATGACTCCGCCGATGATGT-3’ | |
| METTL1 | Forward | 5’-GGCAACGTGCTCACTCCAA-3’ |
| Reverse | 5’-CACAGCCTATGTCTGCAAACT-3’ | |
| CCT2 | Forward | 5’-GCACTACCTCTGTTACCGTTTT-3’ |
| Reverse | 5’-CTTCTCTCCAACCCGCTATGA-3’ | |
| GAPDH | Forward | 5’-CGGAGTCAACGGATTTGGTCGTAT-3’ |
| Reverse | 5’-AGCCTTCTCCATGGTGGTGAAGAC-3’ |
Note: METTL1, methyltransferase-like1; CCT2,T-complex protein-1 ring complex2; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Immunochemistry
GC tissue sections were dewaxed, hydrated, and immersed in methanol containing 0.3% hydrogen peroxide for 30 min. The tissues were washed three times with PBS and blocked with 1% blocking serum for 30 min, followed by overnight incubation with primary antibodies TSPAN31(Invitrogen, China), which were diluted 1000 times by antibody dilution(Invitrogen, China). The slides were washed three times with PBS, incubated with biotinylated sheep anti-rat IgG for 15 min, and then washed three times with PBS again. Finally, the slides were incubated with diaminobenzidine (DAB) for 10 min to observe the peroxidase reaction. Each experiment was repeated once and the technique was repeated three times.
Cell counting kit-8 (CCK-8) assay
The GC cells transfected for 48 h were collected by trypsin digestion and seeded onto a 96-well plate at the cell concentration of 4 × 103 cells per well. The medium in the 96-well plate was aspirated at 0, 24, 48,and 72h. The CCK-8 kit (Beyotime Biotechnology,Shanghai,China) was used according to the manufacturer's instructions, wherein 10 μL CCK-8 reagent and 90 μL RPMI-1640 medium were added to each well, and the plate was incubated in a cell incubator for 2 h. Following the incubation period, the absorbance was read at 450 nm by using a microplate reader. The experiment was repeated three times. Each experiment and technique were repeated three times.
Wound healing
After transfection, the GC cells were seeded onto a 6-well plate. When the cell adhesion was above 80%, a sterile pipette tip was used to manually create a wound, and the cells were washed with RPMI 1640 medium without FBS. The 6-well plate was then observed under a microscope, and images were acquired. Image Pro Plus 6.0 software was used for quantitative analysis. Each experiment and technique were repeated three times.
Transwell assay
The Transwell assay was performed in a 24-well plate with an 8 mm pore size chamber. A 100 µL of the transfected GC cells was inoculated in the upper chamber containing RPMI-1640 medium without FBS at the concentration of 1 × 105 cells per well. In the lower chamber, 600 μL of RPMI-1640 medium containing 10% FBS was added. After culturing for 24 h in a cell cabinet at 37 °C and 5% CO2, the upper cavity was wiped with a cotton swab from the culture dish for nonmigrated cells. The cells were then fixed with 4% paraformaldehyde for 10 min at room temperature and stained with 0.1% Crystal Violet for 20 min at room temperature. The number of cells in five fields of each chamber was counted. Each experiment and technique were repeated three times.
Apoptosis experiment
GC cells transfected for 48 h were incubated in 6-well plates at the concentration of 2 × 105 cells per well. The cells were then collected and washed twice with PBS at 4 ℃. The annexin V-FITC/propidium iodide (PI) apoptosis detection kit (BD Biosciences, San Jose, CA, USA) was used for apoptosis analysis in accordance with the manufacturer's instructions. The GC cells were stained with FITC and PI, and FACScan (BD Biosciences) was used for fluorescence-activated cell sorting analysis. Each experiment and technique were repeated three times.
Western blot assay
Proteins were extracted from GC cells by using RIPA lysis buffer, and the protein content was then determined using a protein detection kit (Takara, Dalian, China). The target protein was separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and loaded on a polyvinylidene fluoride (PVDF) membrane. The membrane was then blocked with Tris buffer salt solution containing 5% skimmed milk and 0.1% TBST for 1h at room temperature. METTL1, CCT2 and internal reference GAPDH antibodies were purchased from Abcam (Shanghai, China). All antibodies were diluted 1000 times with antibody diluent (Abcam, Shanghai, China). Specific primary antibodies in TBST containing 5% non-fat milk were added, and the membrane was blocked overnight at 4 ℃. The membrane was then washed and incubated with secondary antibodies. Each experiment was repeated three times and the technique was repeated once.
Tumor bearing experiment
The nude mice used in the experiment were SPF 4-weeks-old BALB/C nude mice of Shanghai Institute of zoology, Chinese Academy of Sciences. The transfected gastric cancer cells AGS were subcutaneously injected into the immunodeficient mice in the experimental group and the control group, respectively, there were 3 nude mice in each group, the number of injected cells was 1 × 106 per nude mouse. After 28 days of tumor formation, the tumor was removed and the volume was measured and the volume was measured. All animal experiments comply with the ARRIVE guidelines and should be carried out in accordance with the National Research Council's Guide for the Care and Use of Laboratory Animals. The experiment was repeated once and the technique was repeated three times.
Statistical analysis
SPSS 16.0 software and GraphPad 7.0 software were used for statistical analysis of the study data. The data were expressed as the mean±standard deviation (mean±SD). For experimental results of normal distribution, student's t-test was used for comparing between the two groups and Pearson's correlation was used for the correlation analysis. Analysis of variance (ANOVA) was used to compare the differences between two groups or more. When multiple groups of data do not meet the normality and homogeneity of variance, we can adopt Kruskal-Wallis single-factor ANOVA analysis and two-way ANOVA analysis for the data that meet the normal distribution. P < 0.05 was considered to be statistically significant.
Results
TSPAN31 is highly expressed in GC cells
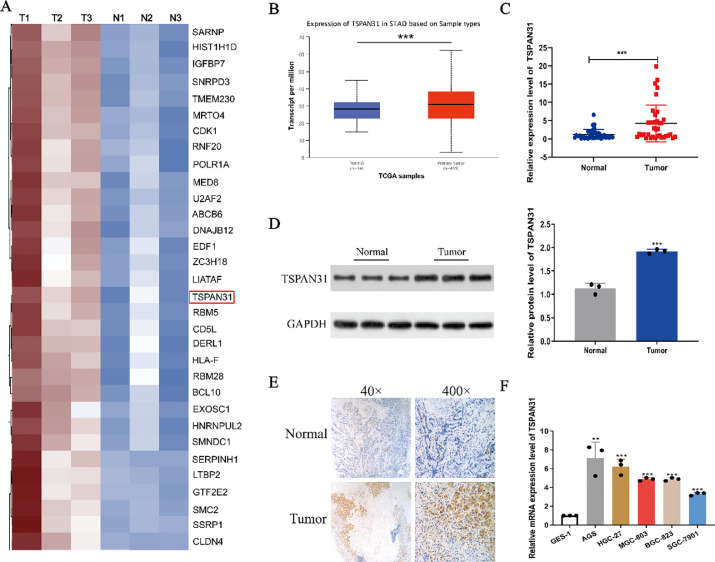
First, we screened abnormally expressed genes in GC tissues through the high-throughput sequencing technique and Proteins with expression differential multiple greater than 1.2 times (up-expression or down-expression) and P value (T test) less than 0.05 as screening criteria were considered as differentially expressed proteins, among which TSPAN31 was highly expressed in GC tissues was 3.33 times than normal tissues(The multiples of gene expression fold change are shown in supplementary document 2). Futher more, TSPAN31 has not been studied in gastric cancer. Hence, this gene was selected for further studies (Fig. 1A). Western blot assay and immunohistochemistry methods were used to determine the protein level of TSPAN31 in GC tissues. The results showed that the TSPAN31 protein expression level in GC tissues was significantly higher than that in normal tissues (Fig. 1D and E). According to the UALCAN database, the expression level of TSPAN31 was significantly higher in GC tissues than in normal tissues (Fig. 1B). Subsequently, we detected the expression of TSPAN31 in the 37 pairs of GC tissues, GC cell lines (AGS, HGC-27, MGC-803, BGC-823, and SGC-7901), and GES-1 cells. The results showed that TSPAN31 was upregulated in GC tissues or GC cells as compared to that in normal tissues or GES-1 cells (Fig. 1C and F).
Fig. 1.
TSPAN31 is highly expressed in GC. (A) High-throughput sequencing analysis of differentially expressed genes in gastric cancer (GC) tissues. (B) The UALCAN database was used to analyze the expression level of TSPAN31 in GC tissues. (C) Determination of the expression level of TSPAN31 in GC tissues by qRT-PCR. (D) Analysis of the protein level of TSPAN31 in GC tissues by western blot assay. (E) Determination of the protein level of TSPAN31 in GC tissues by immunohistochemistry. (F) Analysis of the expression level of TSPAN31 in GC cells by qRT-PCR (⁎⁎P < 0.01; ⁎⁎⁎P < 0.001).
TSPAN31 expression is related to the prognosis of patients with GC
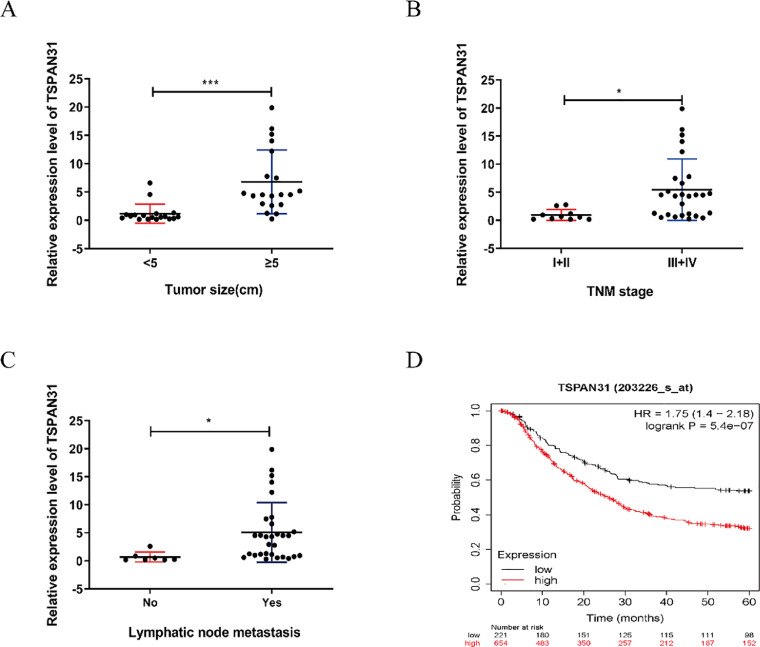
We investigated the association between TSPAN31 expression and the clinicopathological characteristics of patients with GC. The results showed that higher TSPAN31 expression tended to correlate with larger tumor size, more advanced TNM stage, and higher lymphatic metastasis rate (Table 2; Fig. 2A–C). The Kaplan-Meier plotter (KM-plotter) database (https://kmplot.com/analysis/) was used to analyze the influence of TSPAN31 expression level on the overall survival rate of patients with GC. As shown in Fig. 2D, a negative association was found between TSPAN31 expression level and the overall survival rate of patients with GC.
Table 2.
Relationship between TSPAN31 expression and the clinical pathological characteristics of GC patients (n = 37).
| Clinic pathological features | NO. of cases | TSPAN31 expression | p - value | ||
|---|---|---|---|---|---|
| Low(n = 18) | High(n = 19) | ||||
| Gender | Male | 9 | 3 | 6 | P = 0.269 |
| Female | 28 | 15 | 13 | ||
| Age | ≤60 | 12 | 6 | 6 | P = 0.641 |
| > 60 | 25 | 12 | 13 | ||
| Tumor size | <5 | 17 | 12 | 5 | P = 0.003 |
| ≥5 | 20 | 6 | 14 | ||
| Differentiation | Well | 14 | 5 | 9 | P = 0.557 |
| Poor | 23 | 13 | 10 | ||
| TNM stage | Ⅰ/Ⅱ | 10 | 7 | 3 | P = 0.015 |
| Ⅲ/Ⅳ | 27 | 11 | 16 | ||
| Lymph node metastasis | Negative | 7 | 6 | 1 | P < 0.001 |
| Positive | 30 | 12 | 18 | ||
Fig. 2.
TSPAN31 expression is related to the prognosis of patients with GC. (A) Relationship between TSPAN31 expression level and tumor size in patients with GC. (B) Relationship between TSPAN31 expression level and TNM stage in patients with GC. (C) Relationship between TSPAN31 expression level and TNM stage in patients with GC. (D) Effect of TSPAN31 on the overall survival rate of patients with GC (*P < 0.05; ⁎⁎⁎P < 0.001).
TSPAN31 can promote proliferation of GC cells and inhibit their apoptosis
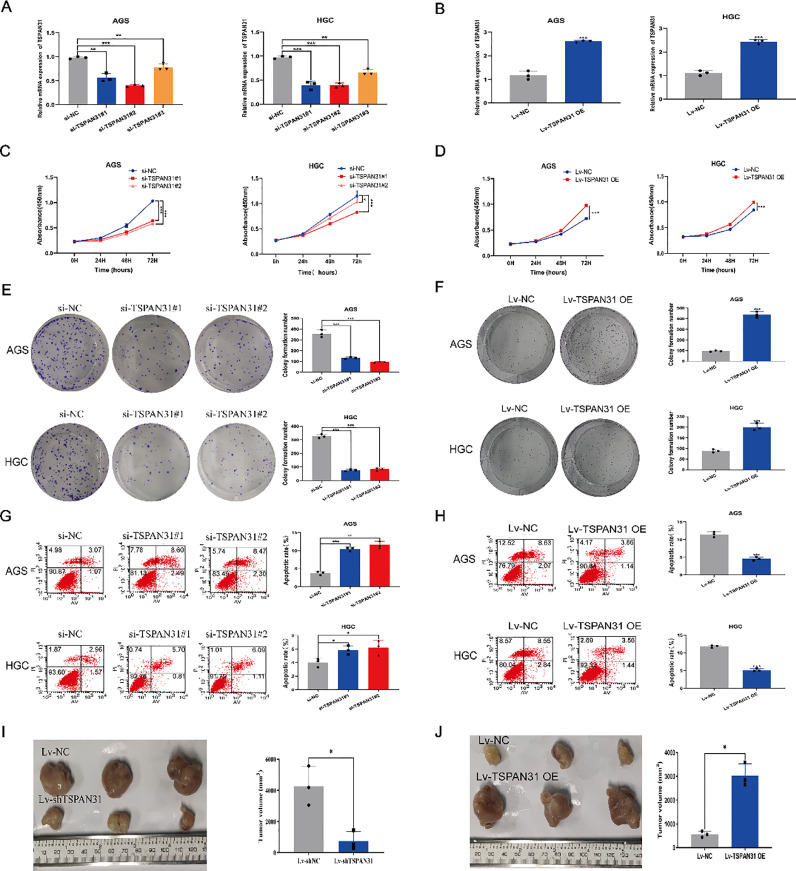
Transfection efficiency was verified by qRT-PCR as shown in Fig. 3A and B. The expression level of TSPAN31 in GC cells was significantly changed after transfection with si-TSPAN31 or Lv-TSPAN31 OE. The CCK-8 assay showed that the downregulation of TSPAN31 expression inhibited and the upregulation of TSPAN31 expression promoted the proliferation of GC cells (Fig. 3C and D). We then tested the clonogenic of GC cells through the clone formation experiment. The results showed the same trends as those observed in the CCK-8 assay (Fig. 3E and F). Subsequently, the apoptosis level was detected by flow cytometry, and it was found that the apoptotic level of GC cells was significantly increased following the upregulation of TSPAN31 expression and decreased following the downregulation of TSPAN31 expression (Fig. 3G and H). Regarding whether TSPAN31 could affect tumor growth in vivo, the results showed that the tumor diameter of nude mice transfected with Lv-shTSPAN31 was significantly smaller than that of the control group (Fig. 3I), while the tumor diameter of nude mice transfected with Lv-TSPAN31 OE was significantly larger than that of nude mice transfected with Lv-NC (Fig. 3J). These results indicated that TSPAN31 aggressively promoted GC proliferation and apoptosis in vitro and promote GC tumor growth in vivo.
Fig. 3.
TSPAN31 regulates the proliferation, tumor growth, and apoptosis of GC cells. (A and B) Determination of the expression level of TSPAN31 in GC cells transfected with siTSPAN31 and Lv-TSPAN31 OE by qRT-PCR. (C and D) The optical density (OD value) of GC cells transfected with si-TSPAN31 and Lv-TSPAN31 OE was measured at 450 nm by the CCK-8 assay. (E and F) The clonogenic of GC cells transfected with si-TSPAN31 and Lv-TSPAN31 OE was determined by the clone formation assay. (G and H) The apoptosis level of GC cells transfected with si-TSPAN31 and Lv-TSPAN31 OE was determined by the apoptosis assay. (I and J) The effect of si-TSPAN31 and LV-TSPAN31 OE transfection on GC tumor growth was detected by tumor bearing experiment in nude mice (*P < 0.05; ⁎⁎P < 0.01; ⁎⁎⁎P < 0.001).
TSPAN31 can promote migration and invasion of GC cells
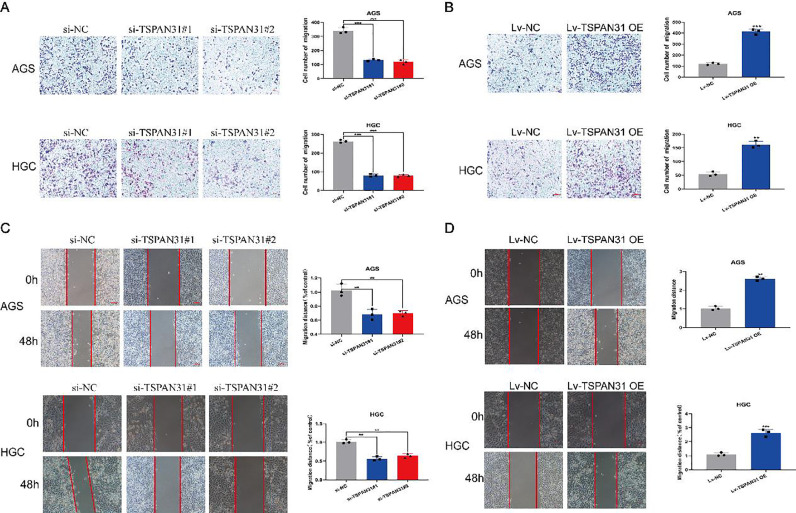
Next, we performed a wound healing assay and the Transwell assay to investigate whether TSPAN31 played a role in GC cell migration and invasion. The Transwell assay showed that the downregulation of TSPAN31 expression drastically inhibited the migration and invasion ability of GC cells, while overexpression of TSPAN31 had the opposite effect on GC cells (Fig. 4A and B). The results of the wound healing assay are consistent with those of the Transwell assay (Fig. 4C and D). These results indicated that TSPAN31 promoted migration and invasion of GC cells.
Fig. 4.
TSPAN31 regulates the migration of GC cells. (A and B) The longitudinal migration capacity of GC cells transfected with siTSPAN31 and Lv-TSPAN31 OE was determined by the Transwell assay (magnification: 100×). (C and D) The lateral migration capacity of GC cells transfected with siTSPAN31 and Lv-TSPAN31 OE was determined by the wound healing assay (magnification: 100×) (*P < 0.05; ⁎⁎P < 0.01; ⁎⁎⁎P < 0.001).
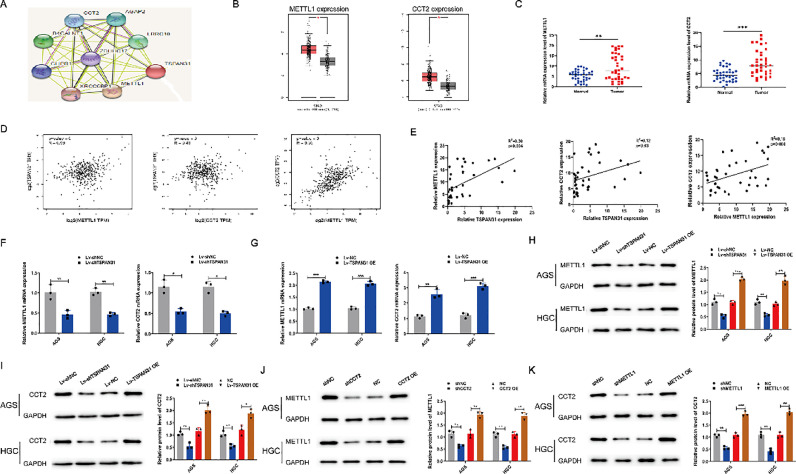
TSPAN31 and METTL1/CCT2 expression levels are positively correlated
The String database (https://string-db.org/) predicted that METTL1 and CCT2 may be co-expressed with TSPAN31 (Fig. 5A). We then analyzed the expression levels of METTL1 and CCT2 in GC tissues through the GEPIA database (http://gepia.cancer-pku.cn/) and in the 37 paired GC tissues. The results showed that the expression levels of METTL1 and CCT2 in GC tissues were significantly higher than those in normal tissues (Fig. 5B and C). We also found that the expression level of TSPAN31 and METTL1 or TSPAN31 and CCT2 was positively correlated through the analysis of the GEPIA database and the present study data (Fig. 5D and E). We used qRT-PCR to determine the mRNA expression levels of METTL1 and CCT2 in GC cell lines with overexpression or knockdown of TSPAN31. The results revealed that the overexpression of TSPAN31 increased the mRNA expression level of METTL1 and CCT2, while the knockdown of TSPAN31 had an opposite effect (Fig. 5F and G). We then performed western blot assay after TSPAN31 downregulation and found that the protein expression levels of METTL1 and CCT2 were significantly downregulated, and after the upregulation of TSPAN31 expression, the protein expression levels of METTL1 and CCT2 were also significantly upregulated (Fig. 5H and I). Moreover, we found that the expression levels of METTL1 and CCT2 showed identical results with TSPAN31 expression (Fig. 5J and K). These results suggest that TSPAN31 may regulate METTL1 and CCT2, respectively, but METTL1 and CCT2 expression levels are only positively correlated in gastric cancer, but the specific relationship between the two is still unclear and needs further study.
Fig. 5.
TSPAN31 and METTL1/CCT2 expression levels are positively correlated. (A) The String database was used to predict the genes that might be co-expressed with TSPAN31. (B) The expression levels of METTL1 and CCT2 in GC were analyzed by the GEPIA database. (C) The expression levels of METTL1 and CCT2 in 37 pairs of GC tissues were detected by qRT-PCR. (D) The relationship between the expression levels of TSPAN31 and METTL1, TSPAN31 and CCT2, and METTL1 and CCT2 in GC was determined by the GEPIA database. (E) The correlation of the expression levels between TSPAN31 and METTL1, TSPAN31 and CCT2, and METTL1 and CCT2 in GC was analyzed by Pearson's correlation method. (F and G) After the downregulation or upregulation of TSPAN31 in GC cells, the expression levels of METTL1 and CCT2 were detected by qRT-PCR. (H and I) Western blot was used to detect the protein expression levels of METTL1 and CCT2 after TSPAN31 downregulation or upregulation. (J) The protein expression level of METTL1 after GC cells were transfected with shCCT2 or CCT2 OE (K) The protein expression level of CCT2 after GC cells were transfected with sh METTL1 or METTL1 OE (*P < 0.05;⁎⁎P < 0.01; ⁎⁎⁎P < 0.001).
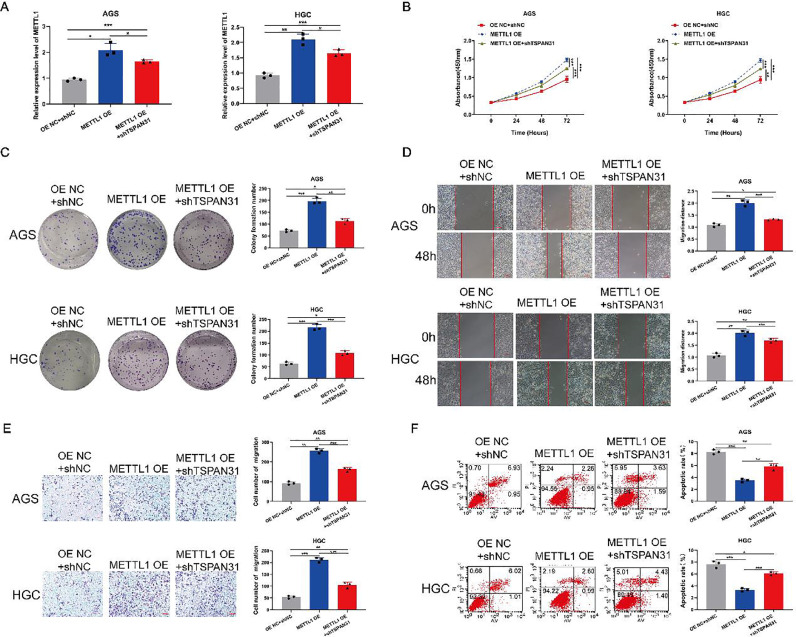
Low expression of TSPAN31 can partially reverse the inhibitory effect of high expression of METTL1 on the malignant phenotype of GC cells
We co-transfected GC cells with METTL1 OE and shTSPAN31 and tested the transfection efficiency by qRT-PCR. The mRNA expression level of METTL1 in GC cells co-transfected with METTL1 OE and shTSPAN31 was higher than that of the control but lower than that in cells transfected with METTL1 OE alone (Fig. 6A). The CCK-8 assay showed that the OD value of GC cells co-transfected with METTL1 OE and shTSPAN31 at 450 nm was higher than that of the control but lower than that of cells transfected with METTL1 OE (Fig. 6B). The colony formation assay revealed that the number of colonies of GC cells co-transfected with METTL1 OE and shTSPAN31 was higher than that of the control but lower than that of cells transfected with METTL1 OE (Fig. 6C). The Transwell assay and the scratch test showed that the migration ability of GC cells co-transfected with METTL1 OE and shTSPAN31 was higher than that of the control but lower than that of cells transfected with METTL1 OE (Fig. 6D and E). The apoptosis experiments showed that the apoptotic level of cells co-transfected with METTL1 OE and shTSPAN31 was lower than that of the control but higher than that of cells transfected with METTL1 OE (Fig. 6F).
Fig. 6.
Low expression of TSPAN31 can partially reverse the inhibitory effect of high expression of METTL1 on the malignant phenotype of GC cells. (A) The mRNA expression of METTL1 after GC cells were co-transfected with METTL1 OE and shTSPAN31 was determined by qRT-PCR. (B) The OD value of GC cells co-transfected with METTL1 OE and shTSPAN31 was determined at 450 nm by the CCK-8 assay. (C) The clonogenic of GC cells co-transfected with METTL1 OE and shTSPAN31 was detected by the clone formation assay. (D) The lateral migration capacity of GC cells co-transfected with METTL1 OE and shTSPAN31 was determined by the wound healing assay (magnification: 100×). (E) The longitudinal migration capacity of GC cells co-transfected with METTL1 OE and shTSPAN31 was determined by the Transwell assay (magnification: 100×). (F) The apoptosis level of GC cells co-transfected with METTL1 OE and shTSPAN31 was determined by the apoptosis assay (*P < 0.05; ⁎⁎P < 0.01; ⁎⁎⁎P < 0.001).
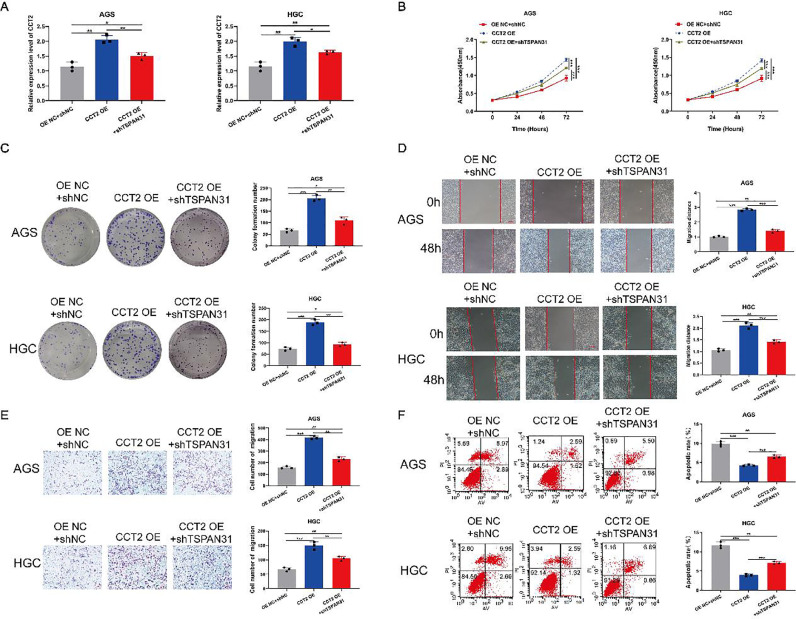
Low expression of TSPAN31 can partially reverse the inhibitory effect of high expression of CCT2 on the malignant phenotype of GC cells
We co-transfected GC cells with CCT2 OE and shTSPAN31 and tested the transfection efficiency by qRT-PCR. The mRNA expression level of CCT2 in GC cells co-transfected with CCT2 OE and shTSPAN31 was higher than that of the control but lower than that of cells transfected with CCT2 OE alone (Fig. 7A). The CCK-8 assay showed that the OD value of GC cells co-transfected with CCT2 OE and shTSPAN31 at 450 nm was higher than that of the control but lower than that of cells transfected with CCT2 OE (Fig. 7B). The colony formation assay revealed that the number of colonies of GC cells co-transfected with CCT2 OE and shTSPAN31 was higher than that of the control but lower than that of cells transfected with CCT2 OE (Fig. 7C). The Transwell assay and the scratch test showed that the migration ability of GC cells co-transfected with CCT2 OE and shTSPAN31 was higher than that of the control but lower than that of cells transfected with CCT2 OE (Fig. 7D and E). The apoptosis experiments showed that the apoptotic level of cells co-transfected with CCT2 OE and shTSPAN31 was lower than that of the control but higher than that of cells transfected with CCT2 OE (Fig. 7F).
Fig. 7.
Low expression of TSPAN31 can partially reverse the inhibitory effect of high expression of CCT2 on the malignant phenotype of GC cells. (A) The mRNA expression of CCT2 after GC cells were co-transfected with CCT2 OE and shTSPAN31 was determined by qRT-PCR. (B) The OD value of GC cells co-transfected with CCT2 OE and shTSPAN31 was measured at 450 nm by the CCK-8 assay. (C) The clonogenic of GC cells co-transfected with CCT2 OE and shTSPAN31 was determined by the clone formation assay. (D) The lateral migration capacity of GC cells co-transfected with CCT2 OE and shTSPAN31 was measured by the wound healing assay (magnification: 100×). (E) The longitudinal migration capacity of GC cells co-transfected with CCT2 OE and shTSPAN31 was determined by the Transwell assay (magnification: 100×). (F) The apoptosis level of GC cells co-transfected with CCT2 OE and shTSPAN31 was determined by the apoptosis assay (*P < 0.05; ⁎⁎P < 0.01; ⁎⁎⁎P < 0.001).
Discussion
As one of the most common human malignant tumors, GC shows characteristics of high heterogeneity, strong invasion ability, and metastasis. Distant metastasis and chemotherapy resistance are the main reasons for increased mortality of patients with GC [14]. Presently, the main treatment for early GC is usually surgery and chemotherapy. The effect of postoperative chemotherapy may affect the prognosis and survival rate of patients with cancer [15]. Most malignancies may eventually metastasize, Once tumor metastasis occurs, the prognosis of these patients becomes worse [16,17]. Therefore, it is essential to find new biomarkers that indicate the progression of GC and provide new targets for the diagnosis and treatment of GC.
In recent years, several studies have shown that the TSPAN family plays an important biological role in GC. Qi et al. reported that TSPAN9 played the role of a tumor suppressor gene in GC and regulated the migration and invasion of GC cells, which was closely related to the expression of EMILIN1. EMILIN1 can synergistically promote the tumor suppressive effect of TSPAN9 [18]. Lu et al. showed that TSPAN1 was the target of miR-573 in GC and regulated the proliferation and migration of GC cells [19]. Moreover, Cai et al. found that the high expression of TSPAN-1 is associated with the TNM staging of advanced gastric cancer and the tumor diameter, influences the survival prognosis, and may involve the processes of angiogenesis and epithelial-mesenchymal transition [20]. Li et al. indicated that TSPAN9 inhibited the proliferation, migration, and invasion of SGC7901 cells through the ERK1/2 pathway [21]. These studies indicated that TSPAN protein family regulates the progression of gastric cancer to varying degrees and is closely related to the clinical characteristics and prognosis of gastric cancer patients. Our study also showed that TSPAN31 may be a new oncogene in the genesis and progression of GC; however, its function and mechanism have not yet been fully clarified. We initially found that the expression level of TSPAN31 in GC was abnormally increased and affected the prognosis of patients with GC. The downregulation of TSPAN31 expression inhibited the proliferation and migration of GC cells and promoted cell apoptosis, while the overexpression of TSPAN31 promoted the malignant phenotype of GC cells; this finding indicated that TSPAN31 regulated the proliferation, migration, and apoptosis levels of GC cells. We further searched the GEPIA database (http://gepia.cancer-pku.cn/) and found that TSPAN31 was co-expressed with METTL1 and CCT2 in GC; moreover, a series of in vitro experiments confirmed that the expression levels of TSPAN31 and METTL1 or CCT2 were significantly positively correlated, which indicated that TSPAN31 may regulate GC development through METTL1/CCT2. A previous study showed that many cytogenetic abnormalities in the region of chromosome 12q13-q15, mainly the amplification of chromosome 12, were closely related to the development of well-differentiated lipoma. Many genes with carcinogenic potential have been detected in the region of chromosome 12q14-q15, including MDM2, CDK4, HMGA2, and TSPAN31. METTL1 is a flanking gene of CDK4 and CCT2 is a flanking gene of MDM2 [22], [23], [24], [25]. TSPAN31 may regulate the malignant phenotype of GC cells through regulate expression of METTL1 and CCT2. The abnormal expression of m6A-related genes in GC, such as FTO and WTAP, is closely related to the prognosis of patients with GC [26]. Through the bioinformatics website (http://rna.sysu.edu.cn/rmbase/), we further found that TSPAN31 had a methylation site with a high predictive score on chromosome 12, and we speculated that TSPAN31 may be regulated by some m6A-related genes in GC and played a relative biological role. TSPAN31 is the natural antisense transcript of CDK4, and natural antisense transcripts may play a variety of regulatory functions through transcriptional interference at the transcriptional level [27]. The PI3K/Akt/mTOR pathway is the key upstream regulator of CDK4 [28]. Previous studies have found that PI3K/Akt/mTOR can participate in the malignant progression of gastric cancer through Long noncoding RNA TPPO-AS1/Mir-126-5p/BRCC3 axis [29]. It has also been found that PI3K/Akt/mTOR can regulate the proliferation, migration and invasion of gastric cancer through m6A modification mediated by methyltransferase METTL14 [30], Shu et al. found that BCAT1 is involved in the angiogenesis and tumorigenesis of gastric cancer by activating the PI3K/AKT/mTOR pathway [31]. These results suggest that PI3K/Akt/mTOR also plays a vital biological role in gastric cancer. The effect of TSPAN31 on the malignant phenotype of GC is probably regulated by the PI3K/Akt/mTOR pathway.
METTL1 (methyltransferase-like 1) is a tRNA and miRNA modification enzyme that catalyzes the 7-methylguanosine (m7G) modification of tRNA and miRNA in mammalian cells [32,33]. In recent years, several studies have been conducted to elucidate the role of METTL1 in human malignant tumors. Previous studies have shown that METTL1 played an important biological function in human malignancies such as liver cancer [34], colorectal cancer [35], and lung cancer [36], including cell proliferation, cell migration, cell invasion, and cell apoptosis. In the present study, we found that METTL1 was highly expressed in GC and participated in the regulation of the proliferation, migration, and apoptosis of GC cells. This regulatory ability may be mediated by TSPAN31.
CCT (T-complex protein-1 ring complex) is an important eukaryotic chaperonin protein with a molecular weight of 1 MDa; it contains a double ring structure, and each ring has a central hole [37]. Each loop contains eight homologous but different structural subunits (CCT1-8). Each subunit is approximately 60 kDa in size and contains three domains: apical, intermediate, and equatorial [38,39]. Previous studies have shown that each subunit of CCT played an independent function [40]. Among these subunits, CCT2 is a molecular chaperone that helps many proteins to fold correctly and is particularly important for maintaining the dynamic balance of cell stability [41]. Previous studies have reported that CCT2 was highly correlated with tumorigenesis, tumor progression, and prognosis of several human malignant tumors, including liver cancer and colorectal cancer [42,43]. However, few studies have been conducted on the role of CCT2 in GC. The present study found that CCT2 was involved in the regulation of proliferation, migration, and apoptosis of GC cells. We also found that this regulatory mechanism may be closely related to the expression level of TSPAN31.
The biological functions and related molecular mechanisms of METTL1 and CCT2 in GC, however, for the same molecule, the predicted results of these databases may be different, mainly due to the differences in data source, sample size and cutoff value, the effect of TSPAN31 on the overall survival rate of gastric cancer patients remains to be further explored. Our study showed that TSAPN31, METTL1, and CCT2 played the function of oncogenes in GC and their regulation of the malignant progression of GC may be achieved through mutual expression. Moreover, TSPAN31 was found to may regulate METTL1 or CCT2 in malignant phenotypes of GC. This may be related to the fact that METTL1 and CCT2 are flanking genes of oncogenes which are enriched on chromosome 12q14-q15. However, the mechanism of interaction of TSAPN31, METTL1, and CCT2 has not been thoroughly investigated in this study and should be further explored in future studies.
Conclusion
In conclusion, TSPAN31 was highly expressed in GC. The high expression of TSPAN31 in GC tissues led to poor prognosis of patients with GC, as it could promote the malignant phenotype of GC. TSPAN31 expression was significantly positively correlated with the expression levels of METTL1 and CCT2 in GC. Thus, the TSPAN31/METTL1/CCT2 pathway may co-regulate tumor progression in GC.
Funding information
The Open Project of The Key Laboratory of Modern Toxicology of Ministry of Education, Nanjing Medical University (NMUMT202004); General project of Natural Science Foundation of the Jiangsu Higher Education Institutions (20KJB320015); Science and Technology Development Fund of Nanjing Medical University(NMUB2019049); Youth talent support program of Nanjing City during the 13th Five-Year Plan Period (QRX 17208).
CRediT authorship contribution statement
Xiang Ma: Visualization, Funding acquisition, Formal analysis, Writing – original draft. Shipei Qiu: Formal analysis, Writing – original draft, Methodology. Xin Tang: Methodology. Qingyu Song: Resources. Pengchao Wang: Resources. Jiawei Wang: Formal analysis. Qingcheng Xia: Resources. Zijun Wang: Formal analysis. Qinghong Zhao: Visualization, Funding acquisition. Ming Lu: Visualization, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
We sincerely thank all editors and reviewers for their helpful comments on this article
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.tranon.2022.101423.
Contributor Information
Qinghong Zhao, Email: njzhqh@sina.com.
Ming Lu, Email: lumingmd1983@126.com.
Appendix. Supplementary materials
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Eloranta S., Smedby K.E., Dickman P.W. Andersson TM. cancer survival statistics for patients and healthcare professionals - a tutorial of real-world data analysis. J. Intern. Med. 2021;289(1):12–28. doi: 10.1111/joim.13139. [DOI] [PubMed] [Google Scholar]
- 3.Kino H., Nakano M., Kanamori A., Suzuki T., Kaneko Y., Tsuchida C., et al. Gastric adenocarcinoma of the fundic gland type after endoscopic therapy for metachronous gastric cancer. Intern. Med. 2018;57(6):795–800. doi: 10.2169/internalmedicine.9359-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang S., Yuan S., Dong M., Su J., Yu C., Shen Y., et al. The phylogenetic analysis of tetraspanins projects the evolution of cell-cell interactions from unicellular to multicellular organisms. Genomics. 2005;86(6):674–684. doi: 10.1016/j.ygeno.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 5.Hemler M.E. Tetraspanin functions and associated microdomains. Nat. Rev. Mol. Cell Biol. 2005;6(10):801–811. doi: 10.1038/nrm1736. [DOI] [PubMed] [Google Scholar]
- 6.Charrin S., Jouannet S., Boucheix C., Rubinstein E. Tetraspanins at a glance. J. Cell Sci. 2014;127(17):3641–3648. doi: 10.1242/jcs.154906. Pt. [DOI] [PubMed] [Google Scholar]
- 7.Lang T., Hochheimer N. Tetraspanins. Curr. Biol. 2020;30(5):R204–R2R6. doi: 10.1016/j.cub.2020.01.007. [DOI] [PubMed] [Google Scholar]
- 8.Seigneuret M., Delaguillaumie A., Lagaudriere-Gesbert C., Conjeaud H. Structure of the tetraspanin main extracellular domain. A partially conserved fold with a structurally variable domain insertion. J. Biol. Chem. 2001;276(43):40055–40064. doi: 10.1074/jbc.M105557200. [DOI] [PubMed] [Google Scholar]
- 9.Jankowski S.A., Mitchell D.S., Smith S.H., Trent J.M., Meltzer P.S. SAS, a gene amplified in human sarcomas, encodes a new member of the transmembrane 4 superfamily of proteins. Oncogene. 1994;9(4):1205–1211. [PubMed] [Google Scholar]
- 10.Ragazzini P., Gamberi G., Pazzaglia L., Serra M., Magagnoli G., Ponticelli F., et al. Amplification of CDK4, MDM2, SAS and GLI genes in leiomyosarcoma, alveolar and embryonal rhabdomyosarcoma. Histol. Histopathol. 2004;19(2):401–411. doi: 10.14670/HH-19.401. [DOI] [PubMed] [Google Scholar]
- 11.Xia Y., Deng Y., Zhou Y., Li D., Sun X., Gu L., et al. TSPAN31 suppresses cell proliferation in human cervical cancer through down-regulation of its antisense pairing with CDK4. Cell Biochem. Funct. 2020;38(5):660–668. doi: 10.1002/cbf.3526. [DOI] [PubMed] [Google Scholar]
- 12.Wang J., Zhou Y., Li D., Sun X., Deng Y., Zhao Q. TSPAN31 is a critical regulator on transduction of survival and apoptotic signals in hepatocellular carcinoma cells. FEBS Lett. 2017;591(18):2905–2918. doi: 10.1002/1873-3468.12737. [DOI] [PubMed] [Google Scholar]
- 13.Qi Y., Li H., Lv J., Qi W., Shen L., Liu S., et al. Expression and function of transmembrane 4 superfamily proteins in digestive system cancers. Cancer Cell Int. 2020;20:314. doi: 10.1186/s12935-020-01353-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sakamoto J., Morita S., Kodera Y., Rahman M., Nakao A. Adjuvant chemotherapy for gastric cancer in Japan: global and Japanese perspectives. Cancer Chemother. Pharmacol. 2004;54(1):S25–S31. doi: 10.1007/s00280-004-0883-1. Suppl. [DOI] [PubMed] [Google Scholar]
- 15.Robb W.B., Messager M., Goere D., Pichot-Delahaye V., Lefevre J.H., Louis D., et al. Predictive factors of postoperative mortality after junctional and gastric adenocarcinoma resection. JAMA Surg. 2013;148(7):624–631. doi: 10.1001/jamasurg.2013.63. [DOI] [PubMed] [Google Scholar]
- 16.Li P., Wu F., Zhao H., Dou L., Wang Y., Guo C., et al. Analysis of the factors affecting lymph node metastasis and the prognosis of rectal neuroendocrine tumors. Int. J. Clin. Exp. Pathol. 2015;8(10):13331–13338. [PMC free article] [PubMed] [Google Scholar]
- 17.Cho J.H., Lee Y.S., Sun D.I., Kim M.S., Cho K.J., Nam I.C., et al. Prognostic impact of lymph node micrometastasis in oral and oropharyngeal squamous cell carcinomas. Head Neck. 2016;38(1):E1777–E1782. doi: 10.1002/hed.24314. Suppl. [DOI] [PubMed] [Google Scholar]
- 18.Qi Y., Lv J., Liu S., Sun L., Wang Y., Li H., et al. TSPAN9 and EMILIN1 synergistically inhibit the migration and invasion of gastric cancer cells by increasing TSPAN9 expression. BMC Cancer. 2019;19(1):630. doi: 10.1186/s12885-019-5810-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu Z., Luo T., Nie M., Pang T., Zhang X., Shen X., et al. TSPAN1 functions as an oncogene in gastric cancer and is downregulated by miR-573. FEBS Lett. 2015;589(15):1988–1994. doi: 10.1016/j.febslet.2015.05.044. [DOI] [PubMed] [Google Scholar]
- 20.Cai Y., Zheng M., Zhao Z., Huang H., Fu W., Xu X. Expression of Tspan-1 gene in patients with advanced gastric cancer. Oncol. Lett. 2017;14(3):2996–3000. doi: 10.3892/ol.2017.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li P.Y., Lv J., Qi W.W., Zhao S.F., Sun L.B., Liu N., et al. Tspan9 inhibits the proliferation, migration and invasion of human gastric cancer SGC7901 cells via the ERK1/2 pathway. Oncol. Rep. 2016;36(1):448–454. doi: 10.3892/or.2016.4805. [DOI] [PubMed] [Google Scholar]
- 22.Mandahl N., Hoglund M., Mertens F., Rydholm A., Willen H., Brosjo O., et al. Cytogenetic aberrations in 188 benign and borderline adipose tissue tumors. Genes Chromosomes Cancer. 1994;9(3):207–215. doi: 10.1002/gcc.2870090309. [DOI] [PubMed] [Google Scholar]
- 23.Forus A., Weghuis D.O., Smeets D., Fodstad O., Myklebost O., van Kessel A.G. Comparative genomic hybridization analysis of human sarcomas: I. occurrence of genomic imbalances and identification of a novel major amplicon at 1q21-q22 in soft tissue sarcomas. Genes Chromosomes Cancer. 1995;14(1):8–14. doi: 10.1002/gcc.2870140103. [DOI] [PubMed] [Google Scholar]
- 24.Hameed M. Pathology and genetics of adipocytic tumors. Cytogenet. Genome Res. 2007;118(2-4):138–147. doi: 10.1159/000108294. [DOI] [PubMed] [Google Scholar]
- 25.Tap W.D., Eilber F.C., Ginther C., Dry S.M., Reese N., Barzan-Smith K., et al. Evaluation of well-differentiated/de-differentiated liposarcomas by high-resolution oligonucleotide array-based comparative genomic hybridization. Genes Chromosomes Cancer. 2011;50(2):95–112. doi: 10.1002/gcc.20835. [DOI] [PubMed] [Google Scholar]
- 26.Guan K., Liu X., Li J., Ding Y., Li J., Cui G., et al. Expression status and prognostic value Of M6A-associated genes in gastric cancer. J. Cancer. 2020;11(10):3027–3040. doi: 10.7150/jca.40866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosikiewicz W., Makalowska I. Biological functions of natural antisense transcripts. Acta Biochim. Pol. 2016;63(4):665–673. doi: 10.18388/abp.2016_1350. [DOI] [PubMed] [Google Scholar]
- 28.Hamilton E., Infante J.R. Targeting CDK4/6 in patients with cancer. Cancer Treat. Rev. 2016;45:129–138. doi: 10.1016/j.ctrv.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 29.Hu Y., Zhang Y., Ding M., Xu R. Long noncoding RNA TMPO-AS1/miR-126-5p/BRCC3 axis accelerates gastric cancer progression and angiogenesis via activating PI3K/Akt/mTOR pathway. J. Gastroenterol. Hepatol. 2021;36(7):1877–1888. doi: 10.1111/jgh.15362. [DOI] [PubMed] [Google Scholar]
- 30.Liu X., Xiao M., Zhang L., Li L., Zhu G., Shen E., et al. The m6A methyltransferase METTL14 inhibits the proliferation, migration, and invasion of gastric cancer by regulating the PI3K/AKT/mTOR signaling pathway. J. Clin. Lab. Anal. 2021;35(3):e23655. doi: 10.1002/jcla.23655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shu X., Zhan P.P., Sun L.X., Yu L., Liu J., Sun L.C., et al. BCAT1 Activates PI3K/AKT/mTOR pathway and contributes to the angiogenesis and tumorigenicity of gastric cancer. Front. Cell Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.659260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin S., Liu Q., Lelyveld V.S., Choe J., Szostak J.W., Gregory RI. Mettl1/Wdr4-Mediated m(7)G tRNA methylome is required for normal mRNA translation and embryonic stem cell self-renewal and differentiation. Mol. Cell. 2018;71(2):244–255. doi: 10.1016/j.molcel.2018.06.001. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pandolfini L., Barbieri I., Bannister A.J., Hendrick A., Andrews B., Webster N., et al. METTL1 promotes let-7 MicroRNA processing via m7G methylation. Mol. Cell. 2019;74(6):1278–1290. doi: 10.1016/j.molcel.2019.03.040. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tian Q.H., Zhang M.F., Zeng J.S., Luo R.G., Wen Y., Chen J., et al. METTL1 overexpression is correlated with poor prognosis and promotes hepatocellular carcinoma via PTEN. J. Mol. Med. (Berl) 2019;97(11):1535–1545. doi: 10.1007/s00109-019-01830-9. [DOI] [PubMed] [Google Scholar]
- 35.Liu Y., Zhang Y., Chi Q., Wang Z., Sun B. Methyltransferase-like 1 (METTL1) served as a tumor suppressor in colon cancer by activating 7-methyguanosine (m7G) regulated let-7e miRNA/HMGA2 axis. Life Sci. 2020;249 doi: 10.1016/j.lfs.2020.117480. [DOI] [PubMed] [Google Scholar]
- 36.Wang C., Wang W., Han X., Du L., Li A., Huang G. Methyltransferase-like 1 regulates lung adenocarcinoma A549 cell proliferation and autophagy via the AKT/mTORC1 signaling pathway. Oncol. Lett. 2021;21(4):330. doi: 10.3892/ol.2021.12591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu J., Liu T., Rios Z., Mei Q., Lin X., Cao S. Heat shock proteins and cancer. Trends Pharmacol. Sci. 2017;38(3):226–256. doi: 10.1016/j.tips.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 38.Kalisman N., Adams C.M., Levitt M. Subunit order of eukaryotic TRiC/CCT chaperonin by cross-linking, mass spectrometry, and combinatorial homology modeling. Proc. Natl. Acad. Sci. U. S. A. 2012;109(8):2884–2889. doi: 10.1073/pnas.1119472109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leitner A., Joachimiak L.A., Bracher A., Monkemeyer L., Walzthoeni T., Chen B., et al. The molecular architecture of the eukaryotic chaperonin TRiC/CCT. Structure. 2012;20(5):814–825. doi: 10.1016/j.str.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kubota H., Hynes G., Carne A., Ashworth A., Willison K. Identification of six Tcp-1-related genes encoding divergent subunits of the TCP-1-containing chaperonin. Curr. Biol. 1994;4(2):89–99. doi: 10.1016/s0960-9822(94)00024-2. [DOI] [PubMed] [Google Scholar]
- 41.Zou Q., Yang Z.L., Yuan Y., Li J.H., Liang L.F., Zeng G.X., et al. Clinicopathological features and CCT2 and PDIA2 expression in gallbladder squamous/adenosquamous carcinoma and gallbladder adenocarcinoma. World J. Surg. Oncol. 2013;11:143. doi: 10.1186/1477-7819-11-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yokota S., Yamamoto Y., Shimizu K., Momoi H., Kamikawa T., Yamaoka Y., et al. Increased expression of cytosolic chaperonin CCT in human hepatocellular and colonic carcinoma. Cell Stress Chaperones. 2001;6(4):345–350. doi: 10.1379/1466-1268(2001)006<0345:ieoccc>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qian-Lin Z., Ting-Feng W., Qi-Feng C., Min-Hua Z., Ai-Guo L. Inhibition of cytosolic chaperonin CCTzeta-1 expression depletes proliferation of colorectal carcinoma in vitro. J. Surg. Oncol. 2010;102(5):419–423. doi: 10.1002/jso.21625. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.